Abstract
Adiponectin, an insulin-sensitizing factor secreted from adipose tissue, is decreased in individuals with type 2 diabetes (T2D) and increased in response to thiazolidinedione (TZD) therapy. Changes in its secretion and assembly into higher-order forms affect insulin sensitivity. To determine the relative potency of TZDs on intra-adipocyte multimerization and secretion of adiponectin, we assessed the impact of in vivo low- or high-dose rosiglitazone treatment alone or combined with metformin in subjects with T2D. T2D subjects received high-dose rosiglitazone (8 mg/day), high-dose metformin (2,000 mg/day), or low-dose combination rosiglitazone-metformin therapy (4 mg + 1,000 mg/day) for 4 mo. All subjects were then switched to high-dose rosiglitazone-metformin combination therapy (8 mg + 2,000 mg/day) for another 4 mo. Low-dose rosiglitazone increased serum adiponectin, whereas the high dose increased both adipocyte content and serum adiponectin levels. TZDs selectively increased the percentage of circulating adiponectin in the potent, high-molecular-weight (HMW) form. No TZD effects were evident on multimer distribution in the cell. Expression of the chaperone protein ERp44, which retains adiponectin within the cell, was decreased by TZD treatment. No changes occurred in Ero1-Lα expression. Metformin had no effect on any of these measures. Increases in adiponectin correlated with improvements in insulin sensitivity. In vivo, TZDs have apparent dose-dependent effects on cellular and secreted adiponectin. TZD-mediated improvements in whole body insulin sensitivity are associated with increases in circulating but not cellular levels of the HMW adiponectin multimer. Finally, TZDs promote the selective secretion of HMW adiponectin, potentially, in part, through decreasing the expression of the adiponectin-retaining protein ERp44.
Keywords: thiazolidinedione, adipocyte, ERp44, Ero1-Lα, chaperone proteins
as the prevalence of obesity continues to rise at an alarming rate, so do associated rates of obesity-related metabolic dysfunction; among these are coagulation abnormalities, dyslipidemia, hyperinsulinemia, glucose intolerance, and type 2 diabetes (13, 34). Although long recognized to play a role in fat metabolism, the endocrine role of adipose tissue is a relatively recent and important discovery, potentially providing a link between obesity and disorders of metabolism. Among the growing list of identified adipose endocrine products, adiponectin is uniquely related to changes in insulin sensitivity. Low levels are seen in obesity and insulin-resistant states and are predictive of type 2 diabetes (15), whereas high levels are associated with leanness and greater insulin sensitivity (28, 40) and reduced risk for development of type 2 diabetes (14).
Although synthesized as a 32-kDa monomeric protein, adiponectin undergoes extensive posttranslational processing and assembly into low-molecular-weight (LMW) trimers, middle-molecular-weight (MMW) heximers, and high-molecular-weight (HMW) complexes prior to secretion (38). In vitro, the HMW species appears to be the most biologically active form, possessing high-affinity binding and activation of AMPK in myocytes (10) and is required for insulin-sensitizing effects in hepatocytes (37). In vivo, increased ratios of HMW to total adiponectin and increased absolute amounts of HMW are related to antidiabetic and vascular protective effects of adiponectin (16, 21). Thiazolidinedione (TZD) therapy increases both total and HMW forms of circulating adiponectin (11, 33), suggesting the existence of a TZD-regulated secretory pathway. In support of this view, studies in 3T3-L1 cells have identified several endoplasmic reticulum (ER) chaperone proteins whose expression increases (Ero1-Lα) or decreases (ERp44) the secretion of adiponectin (25, 39). The mechanisms controlling adiponectin release in humans remain unclear.
The aim of the present study was to examine the impact of TZDs on circulating and cellular multimeric adiponectin and their relation to measures of insulin action. Toward this end, we studied the effect of varying doses of rosiglitazone alone or together with metformin on circulating total and multimeric adiponectin and compared these findings with similar measures of cellular adiponectin to identify processes subject to regulation. Second, we assessed changes in the expression of ER chaperone proteins ERp44 and Ero1-Lα to determine whether TZD-mediated changes in total and multimeric adiponectin paralleled changes in chaperone protein expression.
MATERIALS AND METHODS
Subjects.
51 patients met inclusion criteria, which included the following: type 2 diabetes as defined by the American Diabetes Association (1), age 20–75 yr, Hb A1c 5.8–9.5%, fasting glucose <225 mg/dl or <200 mg/dl if on medical therapy, and BMI from 23 to 47 kg/m2. Subjects were excluded if they were treated with more than one diabetic agent, previously treated with a TZD or received insulin as part of an outpatient regimen, were pregnant, or had uncontrolled hypertension, active cardiac disease, or other major illnesses.
The study was conducted in the Center for Metabolic Research in the Veterans Affairs San Diego Healthcare System (VASDHS) and was approved by the VASDHS Research and Development Committee of San Diego and the Human Research Protection Program of the University of California, San Diego. Prior to participation, written informed consent was obtained from all subjects.
Study design.
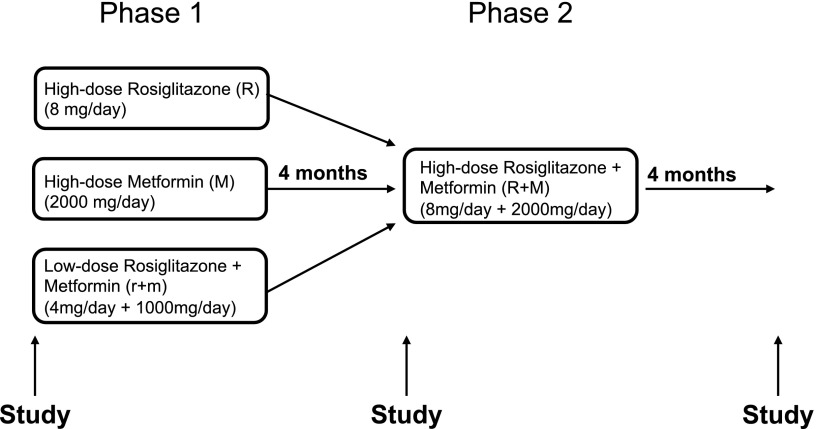
Following enrollment, those participants on an antidiabetic medication were asked to discontinue therapy for a 6-wk washout period. For phase I, all subjects were then randomized to one of three treatment arms: 1) high-dose rosiglitazone (4 mg twice daily), 2) high-dose metformin (1,000 mg twice daily), or 3) low-dose rosiglitazone + metformin combination (2 mg + 500 mg twice daily). Medications were started below target doses and then titrated up over the initial 2 wk to minimize potential side effects. Both subjects and investigators were blinded to treatment. Participants were placed on a standardized weight-maintaining diet and followed by a dietician monthly for adjustments in caloric intake. After 4 mo, subjects in all three treatment arms were converted and titrated up to phase II, an open-label high-dose rosiglitazone + metformin combination therapy (4 mg + 1,000 mg twice daily). Patients were then followed for an additional 4 mo (Fig. 1).
Fig. 1.
Study design.
The same set of evaluations were performed at baseline, 4 mo (the completion of phase I), and 8 mo (end of phase II), including adipose tissue biopsy, blood collection for serum evaluation, and euglycemic hyperinsulinemic clamps. All procedures and laboratory evaluations were performed after a 10- to 12-h overnight fast.
Hyperinsulinemic euglycemic clamp.
In vivo insulin action was determined by performing a two-step 5-h hyperinsulinemic (60 mU·m−2·min−1 for 3 h, then 120 mU·m−2·min−1 for 2 h) euglycemic (5 mM) clamp as described previously (8, 30). The glucose disposal rates in each patient were determined from the values obtained during the steady-state periods for each insulin infusion, i.e., the values between the 140th and 180th minutes of the low-dose clamp and the 260th and 300th minute of the high-dose clamp.
Quantitative assay for circulating adiponectin.
Total adiponectin in serum was quantitated using a human cardiovascular disease Panel 1 LINCOplex kit (Millipore, Billerica, MA) according to the manufacturer's instructions, employing a Bio-Rad BioPlex 200 instrument. The intra- and interassay CVs were 8.4 and 8.1%, respectively.
Adipose tissue biopsy and preparation of human adipocytes.
Adipose tissue was obtained by needle biopsy of the lower subcutaneous abdominal depot using a 5-mm side-cutting needle. The adipose tissue biopsy was performed as previously described (6) prior to initiation of the hyperinsulinemic euglycemic clamp procedure. Isolated adipocytes were prepared by a modification (6) of the method of Rodbell (27). After digestion and filtration, the cells were washed twice in a buffer consisting of 150 mM NaCl, 5 mM KC1, 1.2 mM MgSO4, 1.2 mM CaC12, 2.5 mM NaH2PO4, 10 mM HEPES, and 2 mM pyruvate, pH 7.4, supplemented with 4% BSA. The cells were then washed twice in a buffer (HWS) consisting of 116 mM NaCl, 5 mM KCl, 0.5 mM MgSO4, 0.7 mM CaCl2, 25 mM HEPES, 5 mM glucose, and 2% BSA, pH 7.4 and resuspended at ∼5–10 × 104 cells/ml. Isolated cells were concentrated by centrifugation (50 g) and then rapidly washed twice in 17°C BSA-free HWS buffer, and proteins were extracted as previously described (31).
Protein expression.
Transfer and Western blotting of proteins were preformed using standard methods. Primary antibodies included monoclonal anti-human adiponectin (BD Bioscience, San Diego, CA) and polyclonal anti-ERO1-Lα (Cell Signaling, Danvers, MA). Polyclonal anti-ERp44 was a kind gift from Dr. Philipp Scherer (University of Texas Southwestern Medical Center, Dallas, TX). Secondary horseradish peroxidase-conjugated antibodies included anti-mouse and anti-rabbit IgG (Santa Cruz Biotechnology, Santa Cruz, CA). For antibody detection, SuperSignal (Pierce, Rockford, IL) was used for enhanced chemiluminescence, followed by densitometry and quantitation using ChemImager software (Alpha Innotech, San Leandro, CA).
Analysis of adiponectin multimerization.
Adiponectin in serum and adipocytes was analyzed by SDS-polyacrylamide gel electrophoresis followed by Western blotting under nonreducing conditions as reported previously (2). Serum (2 μl) or adipocyte extract (10 μg cell protein) were combined with Laemmli sample buffer (17) without added reducing agent and separated using Novex tris-acetate 3–8% polyacrylamide gradient gels (Invitrogen, Carlsbad, CA). Samples were kept at room temperature during preparation. Intra- and interassay CVs for detection of the HMW form were 7 and 12%, respectively.
The multimeric distribution of circulating adiponectin was quantitated using the Alpco adiponectin (multimeric) EIA kit (Alpco Diagnostics, Salem, NH) according to the manufacturer's instructions. Intra- and interassay CVs for the HMW form in the EIA were 6.0 and 8.5%, respectively.
Statistical analysis.
The baseline characteristics of the three treatment groups were compared by ANOVA. Paired Student's t-tests were used to compare values before and after treatments within groups. Linear regression analysis with Pearson coefficient was used for correlations. For subjects who withdrew from the study or were lost to follow-up after randomization, the last value was carried forward. Data are presented as means ± SE, with P < 0.05 considered statistically significant. All analyses were performed using Prism4 statistical software (GraphPad, San Diego, CA).
RESULTS
Subject characteristics.
Baseline clinical characteristics, including body weight, fasting glucose, and adiponectin levels, were comparable in all three treatment groups (Table 1). The other parameters measured at study initiation, including cellular and serum adiponectin, percent HMW, and ERp44 and Ero1-Lα expression in adipocytes also did not differ between groups at baseline (Table 1). Body weight and fasting glucose obtained at baseline and at the end of phase I, between baseline and the at end of phase II, and between the end of phase I and the end of phase II did not differ significantly between groups. There was also no significant effect of any of the treatments on body weight compared with baseline.
Table 1.
Baseline subject characteristics
| Rosiglitazone | Metformin | Rosi/Met | |
|---|---|---|---|
| Age, yr | 54.1±2.8 | 56.1±2.3 | 55.9±2.6 |
| Duration of DM, yr | 3.1±1.1 | 3.8±1.1 | 3.0±0.7 |
| Weight, kg | 95.8±4.5 | 105.6±5.2 | 99.7±4.5 |
| BMI, kg/m2 | 33.1±1.6 | 34.5±1.3 | 33.3±1.3 |
| Sex (male/female) | 12/2 | 14/2 | 13/3 |
| Previous DM therapies (no.) | |||
| None | 8 | 7 | 6 |
| Sulfonylurea | 1 | 3 | 3 |
| Metformin | 5 | 6 | 7 |
| FPG, mg/dl | 149±10.7 | 131±6.3 | 140±8.8 |
| OGTT AUC, mg·min·dl–1 | 10,189±634 | 11,651±699 | 12,323±741 |
| Serum adiponectin, μg/ml | 9.2±2.1 | 6.7±2.0 | 7.5±1.0 |
| Adipocyte adiponectin, AU/μg protein | 0.64±0.068 | 1.0±0.24 | 0.78±0.15 |
| Serum HMW adiponectin, %total | 34±7.4 | 24±6.4 | 25±4.5 |
| Adipocyte HMW adiponectin, %total | 44±8.2 | 52±2.8 | 49±2.9 |
| ERp44, AU/15 μg protein | 29±6.5 | 33±7.8 | 28±7.5 |
| Ero1-Lα, AU/15 μg protein | 9.3±2.1 | 11±2.7 | 8.3±1.8 |
Values are expressed as means ± SE; n = 14 Rosiglitazone, n = 16 Metformin, n = 16 Rosi/Met. DM, diabetes mellitus; HMW, high molecular weight; FPG, fasting splasma glucose; OGTT AUC, oral glucose tolerance test area under curve. See materials and methods for calculations. *P < 0.05 by ANOVA.
Treatment effects on insulin action.
Results of the effects of these treatment regimens on multiple aspects of glycemic control and insulin action are summarized here. In the 60 mU·m−2·min−1 hyperinsulinemic euglycemic clamp, primarily reflecting hepatic insulin sensitivity for suppression of glucose output (17), and in the 120 mU·m−2·min−1 hyperinsulinemic euglycemic clamp, reflecting primarily skeletal muscle-responsive glucose disposal (17, 18), low-dose rosiglitazone plus metformin combination therapy was as effective as high-dose rosiglitazone alone in improving insulin action (Table 2). Neither metformin alone nor metformin in combination with high-dose rosiglitazone had a significant effect on insulin action (Table 2).
Table 2.
Treatment effects on insulin action
| Rosiglitazone |
Metformin | Low-Dose Combination | ||||
|---|---|---|---|---|---|---|
| Treatment | GDR60 | GDR120 | GDR60 | GDR120 | GDR60 | GDR120 |
| Baseline | 3.0±0.4 | 5.7±0.6 | 2.8±0.4 | 5.3±0.4 | 3.3±0.5 | 6.1±0.4 |
| Phase I | 4.5±0.5* | 7.5±0.7* | 3.1±0.4 | 5.8±0.4 | 4.6±0.4* | 7.2±0.5* |
| Phase II | 4.7±0.5* | 7.6±0.7* | 4.1±0.4*† | 6.9±0.5*† | 4.8±0.5* | 7.6±0.5* |
Values are expressed as means ± SE in mg·kg–1·min–1. GDR, glucose disposal rates with 60 or 120 mU·m–2·min–1.
P < 0.05 vs. baseline;
P < 0.05 phase II vs. phase I.
Treatment effects on total adiponectin.
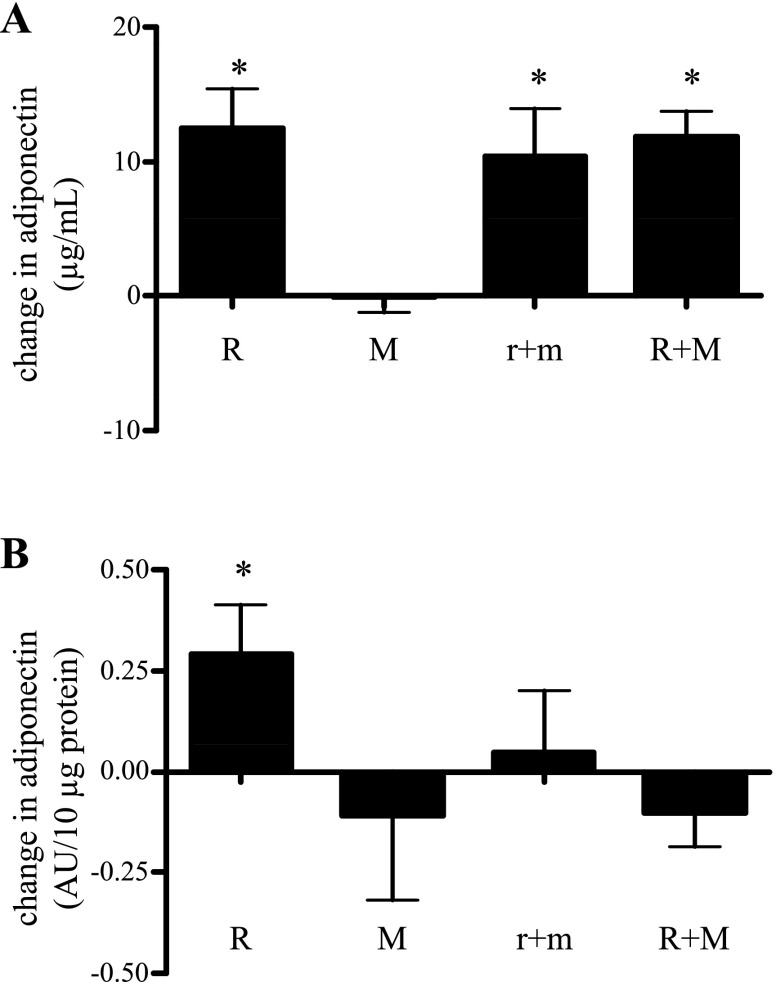
We next investigated whether the above changes in whole body insulin action, recorded after treatment, were related to changes in serum adiponectin. Low-dose rosiglitazone plus metformin and high-dose rosiglitazone plus metformin were equally effective in increasing serum adiponectin (Fig. 2A) by ∼50% over baseline. Intriguingly, a dose-response increase in serum adiponectin did not occur following conversion from low- to high-dose rosiglitazone plus metformin treatment. Hepatic insulin action (60 mU·m−2·min−1 clamp, Table 2) improved following both treatments. Treatment with metformin alone or in combination with high-dose rosiglitazone had no significant effect on measures of insulin action (Table 2) or on serum adiponectin levels (Fig. 2A).
Fig. 2.
Changes in circulating adiponectin levels (A) and adipocyte adiponectin protein content (B) following treatment with high-dose rosiglitazone (R), high-dose metformin (M), low-dose combined treatment (r+m), and following conversion of all subjects to high-dose combination (R+M) treatment. Results are expressed as absolute difference from baseline value (see Table 1) average + SE. For serum measurements, n = 10, 9, 11, and 34 for R, M, r+m, and R+M, respectively. For adipocytes, n = 10, 10, 13, and 33, respectively. *P < 0.05 vs. paired baseline value.
We hypothesized that increases in adipocyte content and/or cell secretion of adiponectin were responsible for the observed treatment-associated changes in serum adiponectin. Consistent with this hypothesis, we found an apparent dose-dependent response in cellular adiponectin to TZD treatment. Specifically, no change in cellular adiponectin was observed following low-dose rosiglitazone plus metformin, vs. an ∼45% increase in cellular adiponectin following high-dose rosiglitazone or high-dose rosiglitazone plus metformin combination treatment (Fig. 2B). Importantly, these changes in adipocyte adiponectin content were highly correlated to the observed changes in circulating adiponectin (r = 0.42, P = 0.039). No correlation was evident between low-dose rosiglitazone plus metformin cell content and serum adiponectin. Metformin had no independent effect to increase cellular adiponectin content. Specifically, there was no change in cell content of adiponectin in response to metformin alone and no difference in the augmentation of cellular adiponectin when high-dose metformin was added to high-dose rosiglitazone (Fig. 2B). Interestingly, adding high-dose metformin to high-dose rosiglitazone eliminated the augmentation of cellular adiponectin seen in subjects on high-dose rosiglitazone monotherapy.
Increases in serum total adiponectin, regardless of treatment, correlated with improvements in total body insulin action at both low (r = 0.42, P = 0.025) and high insulin infusion rates (r = 0.38, P = 0.043).
Adiponectin multimerization.
An increasing number of studies have reported an association between insulin sensitivity and HMW multimers of adiponectin, suggesting that this relationship may be stronger than that with total adiponectin (reviewed in Ref. 20). We sought next to determine whether TZDs preferentially affected the fraction of adiponectin present as HMW in serum or in the adipocyte. Following the analysis described by Pajvani et al. (21), we expressed HMW adiponectin as a percentage of total adiponectin and present comparative data on changes in cellular and serum adiponectin in response to the different treatment arms. The presence of multiple bands following electrophoresis under nonreducing conditions (Fig. 3) is in agreement with our previous work (2, 4, 19) and that of Waki et al. (36).
Fig. 3.
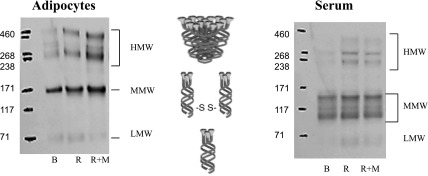
Representative Western blots of adiponectin multimerization in adipocyte (left) and serum (right) treatment effects. Samples were collected at baseline (B) and after R and R+M treatments. Samples are of electrophoresis under nonreducing conditions, as detailed in materials and methods.
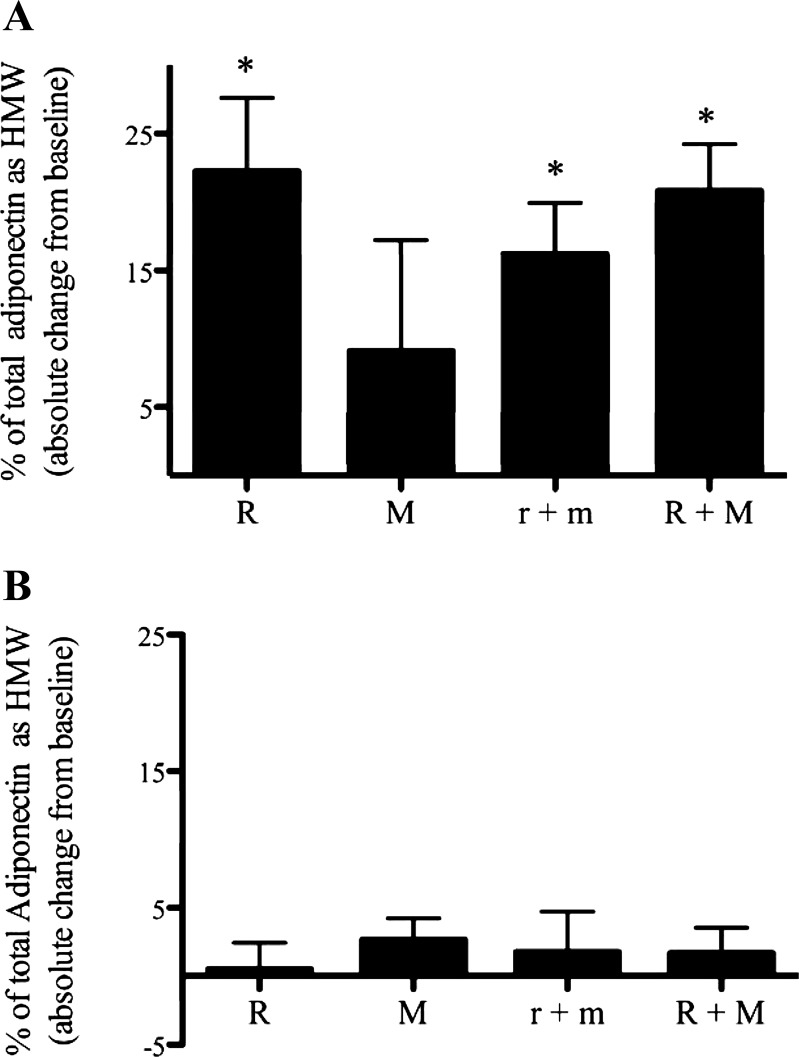
In the serum, all rosiglitazone treatment arms had an effect to increase the percentage of total adiponectin in the HMW form from less than 30% pretreatment to ∼50% (Fig. 4A). In contrast, no additional increase in the percentage of total adiponectin as HMW occurred following metformin monotherapy. Nor were changes in the ratio of HMW to total adiponectin observed following the conversion of low-dose combination to high-dose combination or addition of high-dose metformin to high-dose rosiglitazone (P = 0.4, both low-dose rosiglitazone + metformin vs. high-dose rosiglitazone + metformin and high-dose rosiglitazone vs. high-dose rosiglitazone + metformin).
Fig. 4.
Changes in percent total adiponectin in the HMW form in serum (A) and adipocytes (B) following treatment as described in Fig. 1. Results are expressed as absolute difference from baseline value (see Table 1) and are average + SE; n = 8 each for R, M, and r+m; n = 24 for R+M. *P < 0.05 vs. paired baseline.
In distinction to what was seen in the serum, no treatment had any effect on the percentage of total adiponectin as HMW complexes contained within the adipocyte (Fig. 4B), although, when high-dose rosiglitazone was added to the high-dose metformin group, there was a strong tendency toward an increase in the percentage of cellular HMW adiponectin (P = 0.051; Fig. 4B). No changes in the percentage of total adiponectin as HMW complexes occurred in response to metformin monotherapy or following addition of high-dose metformin to high-dose rosiglitazone (Fig. 4B). Interestingly, at baseline, a greater percentage of adiponectin existed as HMW in cells (51 ± 2%, P < 0.05; n = 24) compared with circulation (26 ± 4%; n = 16), although in serum percent HMW increased to a value similar to that in cells after rosiglitazone treatment.
Adiponectin secretion.
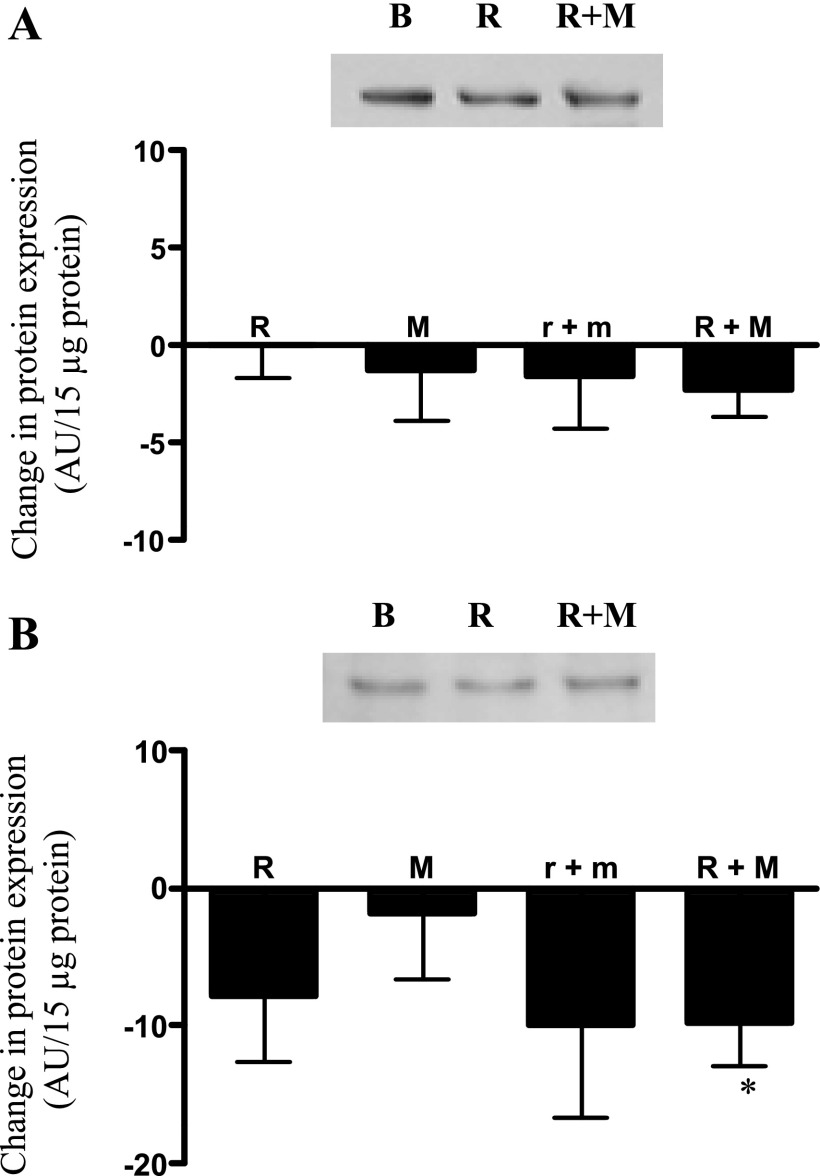
It has been postulated that secretion of properly assembled adiponectin multimers occurs through the coordinated actions of the ER chaperone proteins ERp44 (retention) and Erol-1α (release) (39). Although none of the treatments had any effect on adipocyte protein content of Ero1-Lα (Fig. 5A), there was a tendency for low-dose rosiglitazone plus metformin (P = 0.18) and high-dose rosiglitazone (P = 0.15) to reduce the protein expression of ERp44. The reduction in ERp44 protein expression became statistically significant (P < 0.05) upon conversion of all groups to high-dose rosiglitazone plus metformin treatment (Fig. 5B). Metformin alone or following addition to high-dose rosiglitazone had no effect on Erol-1α or ERp44 expression.
Fig. 5.
Changes in adipocyte protein expression of Ero1-Lα (A) and ERp44 (B) following treatment as described in Fig. 2. Results are expressed as absolute difference from baseline value (see Table 1). Representative autoradiograph is shown above data. Results are average + SE; n = 8 each for R, M, and r+m; n = 23 for R+M. *P < 0.05 vs. paired baseline.
DISCUSSION
The synthesis and secretion of adiponectin is a complex process. Following translation, adiponectin undergoes a number of modifications, including hydroxylation, glycosylation, and assembly into trimers, heximers, and HMW forms (37, 38). Although poorly understood, it appears that the secretion of multimeric adiponectin is a regulated event and likely contributes importantly to the profile of circulating adiponectin, as interconversion of adiponectin isoforms does not appear to occur following secretion (29). A growing number of studies have suggested that the multimeric content, or more specifically the fraction of adiponectin released as HMW, is a better indicator of several aspects of adiponectin activity than is measurement of total adiponectin (9). In support of this view, the HMW fraction of adiponectin has been shown to be strongly correlated with insulin sensitivity (22), cardioprotection (35), and the anti-inflammatory effects of adiponectin (12). Unfortunately, despite its physiological importance, relatively little is known regarding the regulation of HMW adiponectin. A number of studies have identified multiple steps within the adiponectin biosynthetic pathway that are TZD responsive, including the secretion of HMW isoforms. Uncovering the mechanism by which TZDs augment circulating total and HMW are important first steps toward understanding global regulatory mechanisms governing adiponectin secretion. In this study, and similar to others (18, 36), we found that TZD treatment, even at low doses, resulted in a significant augmentation of serum adiponectin. The magnitude of this adiponectin response in newly diagnosed diabetes in the current study is less than what has been reported in established diabetics (36, 37). Importantly, and for the first time in human tissues, TZD treatment was shown to affect the expression of ERp44, an endoplasmic retention protein implicated in the regulated assembly and secretion of multimeric proteins (3).
In agreement with previous reports (32), we found that the TZD rosiglitazone increased serum adiponectin levels in type 2 diabetic subjects and that this increase correlated with an improvement in insulin sensitivity as measured by the glucose disposal rate during a hyperinsulinemic euglycemic clamp (24). In contrast, adiponectin levels were unchanged following treatment with metformin alone or metformin addition to high-dose rosiglitazone - supporting the view that improvements in glycemic control following metformin treatment are adiponectin independent. Serum adiponectin levels rose to their maximal level with even low doses of rosiglitazone in the majority of subjects, suggesting that many patients could benefit from submaximal TZD dosing.
Metformin therapy was not associated with any significant change in whole body insulin action or in circulating or cellular total or ratio of HMW adiponectin. No significant changes in whole body insulin action were evident following 4 mo of high-dose metformin monotherapy, nor were there further effects following the addition of metformin to high-dose rosiglitazone. Likewise, serum adiponectin levels remained unchanged following monotherapy with metformin, while adding metformin to high-dose rosiglitazone had no further effect. These results suggest that, with regard to insulin action and adiponectin, the comparison between low-dose rosiglitazone plus metformin treatment and high-dose rosiglitazone treatment becomes a de facto dose comparison of low- vs. high-dose rosiglitazone.
In this study, we found it intriguing that, although submaximal dosing of rosiglitazone increased serum adiponectin, it did so without a parallel change in cellular content, indicating that TZDs mediated a coupled increase in both the synthesis and secretion of adiponectin. In contrast, increases in serum adiponectin in response to high-dose rosiglitazone were accompanied by increases in cellular adiponectin, suggesting the existence of a saturable and rate-limiting step between the synthesis and secretion of adiponectin. We infer from this observation that TZD-mediated increases in adiponectin synthesis saturate the secretory pathway leading to accumulation of cellular adiponectin. We further suggest that the notable heterogeneity in interindividual serum adiponectin responses to TZD treatment (24, 41) may relate to the capacity and saturability of this pathway for adiponectin secretion.
TZD insulin-sensitizing effects have been attributed to increases in both circulating total and HMW adiponectin (22, 26). These increases in total and HMW adiponectin reflect in vitro direct effects of TZDs on human fat cells to increase total (23) and HMW (5) adiponectin. In nondiabetic subjects, the percentage of cellular adiponectin in the HMW form (∼26%) is less than circulating adiponectin as HMW (∼40%) (4). In contrast, in the diabetic subjects studied here, the opposite exists, with higher percentages of HMW in the cell (∼51%) compared with circulation (∼26%). These data point to an isolated impairment of HMW multimer secretion in type 2 diabetes. Rosiglitazone treatment resulted in a significant increase in the HMW fraction in circulation, to ∼50%, essentially returning the HMW distribution to that of nondiabetics. Consistent with our findings, Hammarstedt et al. (11) reported that short-term treatment of human subjects with pioglitazone resulted in an increase in %HMW in the circulation.
ER chaperone proteins ERp44 and Ero1-Lα have recently been identified in both 3T3-L1 cells and murine adipocytes as playing a role in the regulated assembly and secretion of adiponectin (39). ERp44 was shown to retain newly synthesized adiponectin monomers via thiol groups (thiol-mediated retention), facilitating their assembly into trimers, hexamers, and HMW oligomers. Ero1-Lα then displaces bound multimeric adiponectin, promoting its secretion. Little is known regarding thiol-mediated retention in human fat cells. In addition, the role of ER chaperone proteins ERp44 and Ero1-Lα in adiponectin secretion in human adipocytes has not been previously reported. Our finding that reductions in ERp44 expression are associated with increasing HMW secretion would be consistent with published data in 3T3-L1 cells (14) and the operation of this pathway in human adipocytes. One difference, however, in our report is that in human adipocytes we find a reduction, not an increase, in ERp44 expression in response to PPARγ agonism. We did not find a statistically significant change in Ero1-Lα expression in response to rosiglitazone treatment, unlike results in 3T3-L1 adipocytes (25). It is most likely that this could be reflective of a species difference in responsiveness or, alternatively, that a more sensitive method of detection or a greater sample size is required to assess Ero1-Lα expression in human adipocytes. We also note that statistically significant decreases in ERp44 were apparent only following conversion of all subjects to high-dose rosiglitazone plus metformin, suggesting that the sample size in each of the three individual treatment arms may have been too small to achieve statistical significance for this response.
There is a sexual dimorphism for circulating total and HMW adiponectin levels, with both being more elevated in females (29). Although the sex distribution in our study was similar between groups, the majority of the subjects were male, and the results of the study are therefore most likely reflective of adiponectin behavior in males. From the limited data available, the responses of circulating and cellular total and HMW adiponectin were similar in male and female subjects.
One limitation of this study is that only subcutaneous fat cell adiponectin content and chaperone protein expression were examined. Although serum adiponectin levels correlate with both subcutaneous and visceral adipose tissue mass, the correlation is stronger with visceral adiposity (7). However, our recently published studies of adipose tissue depot differences in adiponectin secretion raise the possibility that in obese diabetic subjects subcutaneous adipose tissue is not only an important source of adiponectin but is also highly responsive to TZD augmentation of adiponectin release, therefore contributing to the TZD-induced increase in circulating adiponectin seen in vivo.
In summary, our findings confirm reports that, in subjects with type 2 diabetes, TZD treatment is associated with a significant increase in circulating total and HMW adiponectin and that these changes are correlated with improvements in hepatic and skeletal muscle insulin sensitivity. Our studies further support the view that TZDs selectively regulate the formation and/or secretion of the HMW multimers and may implicate a role of thiol-mediated retention, via modulation of ERp44, in the regulated secretion of adiponectin in human fat cells. To our knowledge, this is the first study to provide information regarding the regulation of both serum and cellular adiponectin and its HMW isoform in response to TZDs in human diabetic subjects. Future research is needed to better understand the regulation of this pathway in human fat cells in type 2 diabetes.
GRANTS
This work was supported by grants from the Medical Research Service, Department of Veterans Affairs and VA San Diego Healthcare System (R. R. Henry). National Institute of Diabetes and Digestive and Kidney Disease Grants KO8-DK-061987 (S. A. Phillips) and RO1-DK-258291 (R. R. Henry), grants from the American Diabetes Association (R. R. Henry, T. P. Ciaraldi), GlaxoSmith Kline (R. R. Henry), and Grant M01-RR-00827 in support of the General Clinical Research Center from the General Clinical Research Branch, Division of Research Sources.
DISCLOSURES
R. R. Henry has received grant/research support, is on the speaker's bureau, and is a retained consultant for GlaxoSmithKline. R. R. Henry is also a stockholder in GlaxoSmithKline.
Acknowledgments
We thank Debra Armstrong and Paivi Burke for excellent nursing assistance.
Current Affiliation of L. Christianson: Sharp Health Care, San diego, CA.
REFERENCES
- 1.__________. Diagnosis and classification of diabetes mellitus. Diabetes Care 31, Suppl 1: S55–S60, 2008. [DOI] [PubMed] [Google Scholar]
- 2.Abbasi F, Chang SA, Chu JW, Ciaraldi TP, Lamendola C, McLaughlin T, Reaven GM, Reaven PD. Improvements in insulin resistance with weight loss, in contrast to rosiglitazone, are not associated with changes in plasma adiponectin or adiponectin multimeric complexes. Am J Physiol Regul Integr Comp Physiol 290: R139–R144, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Anelli T, Ceppi S, Bergamelli L, Cortini M, Masciarelli S, Valetti C, Sitia R. Sequential steps and checkpoints in the early exocytic compartment during secretory IgM biogenesis. EMBO J 26: 4177–4188, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aroda V, Ciaraldi TP, Chang SA, Dahan MH, Chang RJ, Henry RR. Circulating and cellular adiponectin in polycystic ovary syndrome: relationship to glucose tolerance and insulin action. Fertil steril 89: 1200–1208, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bodles AM, Banga A, Rasouli N, Ono F, Kern PA, Owens RJ. Pioglitazone increases secretion of high-molecular-weight adiponectin from adipocytes. Am J Physiol Endocrinol Metab 291: E1100–E1105, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Ciaraldi TP, Oh DK, Christiansen L, Nikoulina SE, Kong AP, Baxi S, Mudaliar S, Henry RR. Tissue-specific expression and regulation of GSK-3 in human skeletal muscle and adipose tissue. Am J Physiol Endocrinol Metab 291: E891–E898, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Cnop M, Havel PJ, Utzschneider KM, Carr DB, Sinha MK, Boyko EJ, Retzlaff BM, Knopp RH, Brunzell JD, Kahn SE. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia 46: 459–469, 2003. [DOI] [PubMed] [Google Scholar]
- 8.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol Endocrinol Metab Gastrointest Physiol 237: E214–E223, 1979. [DOI] [PubMed] [Google Scholar]
- 9.Fisher FF, Trujillo ME, Hanif W, Barnett AH, McTernan PG, Scherer PE, Kumar S. Serum high molecular weight complex of adiponectin correlates better with glucose tolerance than total serum adiponectin in Indo-Asian males. Diabetologia 48: 1084–1087, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Hada Y, Yamauchi T, Waki H, Tsuchida A, Hara K, Yago H, Miyazaki O, Ebinuma H, Kadowaki T. Selective purification and characterization of adiponectin multimer species from human plasma. Biochem Biophys Res Commun 356: 487–493, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Hammarstedt A, Sopasakis VR, Gogg S, Jansson PA, Smith U. Improved insulin sensitivity and adipose tissue dysregulation after short-term treatment with pioglitazone in non-diabetic, insulin-resistant subjects. Diabetologia 48: 96–104, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Hattori Y, Nakano Y, Hattori S, Tomizawa A, Inukai K, Kasai K. High molecular weight adiponectin activates AMPK and suppresses cytokine-induced NF-kappaB activation in vascular endothelial cells. FEBS Lett 582: 1719–1724, 2008. [DOI] [PubMed] [Google Scholar]
- 13.Hauner H Secretory factors from human adipose tissue and their functional role. Proc Nutr Soc 64: 163–169, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Heidemann C, Sun Q, van Dam RM, Meigs JB, Zhang C, Tworoger SS, Mantzoros CS, Hu FB. Total and high-molecular-weight adiponectin and resistin in relation to the risk for type 2 diabetes in women. Ann Int Med 149: 307–316, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hotta K, Funahashi T, Bodkin NL, Ortmeyer HK, Arita Y, Hansen BC, Matsuzawa Y. Circulating concentrations of the adipocyte protein adiponectin are decreased in parallel with reduced insulin sensitivity during the progression to type 2 diabetes in rhesus monkeys. Diabetes 50: 1126–1133, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi H, Ouchi N, Kihara S, Walsh K, Kumada M, Abe Y, Funahashi T, Matsuzawa Y. Selective suppression of endothelial cell apoptosis by the high molecular weight form of adiponectin. Circ Res 94: e27–31, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laemmli UK Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685, 1970. [DOI] [PubMed] [Google Scholar]
- 18.Maeda N, Takahashi M, Funahashi T, Kihara S, Nishizawa H, Kishida K, Nagaretani H, Matsuda M, Komuro R, Ouchi N, Kuriyama H, Hotta K, Nakamura T, Shimomura I, Matsuzawa Y. PPARgamma ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Diabetes 50: 2094–2099, 2001. [DOI] [PubMed] [Google Scholar]
- 19.O'Leary VB, Jorett AE, Marchetti CM, Gonzalez F, Phillips SA, Ciaraldi TP, Kirwan JP. Enhanced adiponectin multimer ratio and skeletal muscle adiponectin receptor expression following exercise training and diet in older insulin-resistant adults. Am J Physiol Endocrinol Metab 293: E421–E427, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Oh DK, Ciaraldi T, Henry RR. Adiponectin in health and disease. Diabetes Obes Metab 9: 282–289, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Pajvani UB, Du X, Combs TP, Berg AH, Rajala MW, Schulthess T, Engel J, Brownlee M, Scherer PE. Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin. Implications for metabolic regulation and bioactivity. J Biol Chem 278: 9073–9085, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Pajvani UB, Hawkins M, Combs TP, Rajala MW, Doebber T, Berger JP, Wagner JA, Wu M, Knopps A, Xiang AH, Utzschneider KM, Kahn SE, Olefsky JM, Buchanan TA, Scherer PE. Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J Biol Chem 279: 12152–12162, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Pereira RI, Leitner JW, Erickson C, Draznin B. Pioglitazone acutely stimulates adiponectin secretion from mouse and human adipocytes via activation of the phosphatidylinositol 3′-kinase. Life Sci 2008. [DOI] [PubMed]
- 24.Phillips SA, Ciaraldi TP, Kong AP, Bandukwala R, Aroda V, Carter L, Baxi S, Mudaliar SR, Henry RR. Modulation of circulating and adipose tissue adiponectin levels by antidiabetic therapy. Diabetes 52: 667–674, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Qiang L, Wang H, Farmer SR. Adiponectin secretion is regulated by SIRT1 and the endoplasmic reticulum oxidoreductase Ero1-L alpha. Mol Cell Biol 27: 4698–4707, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qurashi S, Mynarcik DC, McNurlan MA, Ahn H, Ferris R, Gelato MC. Importance of the high-molecular-mass isoform of adiponectin in improved insulin sensitivity with rosiglitazone treatment in HIV disease. Clin Sci (Lond) 115: 197–202, 2008. [DOI] [PubMed] [Google Scholar]
- 27.Rodbell M Metabolism of isolated fat cells. I. Effects of hormones on glucose metabolism and lipolysis. J Biol Chem 239: 375–380, 1964. [PubMed] [Google Scholar]
- 28.Satoh H, Nguyen MT, Trujillo M, Imamura T, Usui I, Scherer PE, Olefsky JM. Adenovirus-mediated adiponectin expression augments skeletal muscle insulin sensitivity in male Wistar rats. Diabetes 54: 1304–1313, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Schraw T, Wang ZV, Halberg N, Hawkins M, Scherer PE. Plasma adiponectin complexes have distinct biochemical characteristics. Endocrinology 149: 2270–2282, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sowell M, Mukhopadhyay N, Cavazzoni P, Carlson C, Mudaliar S, Chinnapongse S, Ray A, Davis T, Breier A, Henry RR, Dananberg J. Evaluation of insulin sensitivity in healthy volunteers treated with olanzapine, risperidone, or placebo: a prospective, randomized study using the two-step hyperinsulinemic, euglycemic clamp. J Clin Endocrinol Metab 88: 5875–5880, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Thies RS, Molina JM, Ciaraldi TP, Freidenberg GR, Olefsky JM. Insulin-receptor autophosphorylation and endogenous substrate phosphorylation in human adipocytes from control, obese, and NIDDM subjects. Diabetes 39: 250–259, 1990. [DOI] [PubMed] [Google Scholar]
- 32.Tiikkainen M, Hakkinen AM, Korsheninnikova E, Nyman T, Makimattila S, Yki-Jarvinen H. Effects of rosiglitazone and metformin on liver fat content, hepatic insulin resistance, insulin clearance, and gene expression in adipose tissue in patients with type 2 diabetes. Diabetes 53: 2169–2176, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Tonelli J, Li W, Kishore P, Pajvani UB, Kwon E, Weaver C, Scherer PE, Hawkins M. Mechanisms of early insulin-sensitizing effects of thiazolidinediones in type 2 diabetes. Diabetes 53: 1621–1629, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Trujillo ME, Scherer PE. Adipose tissue-derived factors: impact on health and disease. Endocr Rev 27: 762–778, 2006. [DOI] [PubMed] [Google Scholar]
- 35.von Eynatten M, Humpert PM, Bluemm A, Lepper PM, Hamann A, Allolio B, Nawroth PP, Bierhaus A, Dugi KA. High-molecular weight adiponectin is independently associated with the extent of coronary artery disease in men. Atherosclerosis 199: 123–128, 2008. [DOI] [PubMed] [Google Scholar]
- 36.Waki H, Yamauchi T, Kamon J, Ito Y, Uchida S, Kita S, Hara K, Hada Y, Vasseur F, Froguel P, Kimura S, Nagai R, Kadowaki T. Impaired multimerization of human adiponectin mutants associated with diabetes. Molecular structure and multimer formation of adiponectin. J Biol Chem 278: 40352–40363, 2003. [DOI] [PubMed] [Google Scholar]
- 37.Wang Y, Lam KS, Chan L, Chan KW, Lam JB, Lam MC, Hoo RC, Mak WW, Cooper GJ, Xu A. Post-translational modifications of the four conserved lysine residues within the collagenous domain of adiponectin are required for the formation of its high molecular weight oligomeric complex. J Biol Chem 281: 16391–16400, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Wang Y, Lam KS, Yau MH, Xu A. Post-translational modifications of adiponectin: mechanisms and functional implications. Biochem J 409: 623–633, 2008. [DOI] [PubMed] [Google Scholar]
- 39.Wang ZV, Schraw TD, Kim JY, Khan T, Rajala MW, Follenzi A, Scherer PE. Secretion of the adipocyte-specific secretory protein adiponectin critically depends on thiol-mediated protein retention. Mol Cell Biol 27: 3716–3731, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, Tataranni PA. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab 86: 1930–1935, 2001. [DOI] [PubMed] [Google Scholar]
- 41.Yu JG, Javorschi S, Hevener AL, Kruszynska YT, Norman RA, Sinha M, Olefsky JM. The effect of thiazolidinediones on plasma adiponectin levels in normal, obese, and type 2 diabetic subjects. Diabetes 51: 2968–2974, 2002. [DOI] [PubMed] [Google Scholar]