Abstract
Disruption of cell contact sites in renal epithelial cells contributes to organ dysfunction after ischemia. We hypothesized that heat shock protein 27 (Hsp27), a known cytoprotectant protein, preserves cell architecture and cell contact site function during ischemic stress. To test this hypothesis, renal epithelial cells were subjected to transient ATP depletion, an in vitro model of ischemia-reperfusion injury. Compared with control, selective Hsp27 overexpression significantly preserved cell-cell junction function during metabolic stress as evidenced by reduced stress-mediated redistribution of the adherens junction protein E-cadherin, higher transepithelial electrical resistance, and lower unidirectional flux of lucifer yellow. Hsp27 overexpression also preserved paxillin staining within focal adhesion complexes and significantly decreased cell detachment during stress. Surprisingly, Hsp27, an F-actin-capping protein, only minimally reduced stress induced actin cytoskeleton collapse. In contrast to Hsp27 overexpression, siRNA-mediated knockdown had the opposite effect on these parameters. Since ischemia activates c-Src, a tyrosine kinase that disrupts both cell-cell and cell-substrate interactions, the relationship between Hsp27 and c-Src was examined. Although Hsp27 and c-Src did not coimmunoprecipitate and Hsp27 overexpression failed to inhibit whole cell c-Src activation during injury, manipulation of Hsp27 altered active c-Src accumulation at cell contact sites. Specifically, Hsp27 overexpression reduced, whereas Hsp27 knockdown increased active p-416Src detected at contact sites in intact cells as well as in a purified cell membrane fraction. Together, this evidence shows that Hsp27 overexpression prevents sublethal REC injury at cell contact sites possibly by a c-Src-dependent mechanism. Further exploration of the biochemical link between Hsp27 and c-Src could yield therapeutic interventions for ameliorating ischemic renal cell injury and organ dysfunction.
Keywords: ischemia, renal failure, acute kidney injury, ATP depletion, renal epithelial cells
ischemic renal failure is a common cause of morbidity and mortality that lacks an effective treatment. Ischemia targets the proximal tubule epithelial cells and causes both sublethal and lethal injury, resulting in organ failure (17–19, 30). Sublethal injury presents with disruption of epithelial cell architecture that precedes and may in fact contribute to cell death (17–19, 22, 30). Both cell deenergization in vitro (induced by exposure to metabolic inhibitors) and renal ischemia in vivo induce early, dramatic, potentially reversible changes in the integrity of the actin cytoskeleton, disruption of cell-cell contact sites and loss of cell adhesion to the substratum (5, 18, 27, 37). As a consequence, cell polarity and vectoral solute transport are compromised and tight junction function is altered which results in an increase in paracellular solute leak. Following the disruption of cell-cell and cell-extracellular matrix (ECM) junctions, viable epithelial cells are shed in the tubular lumen (22). Each of these defects in renal cell function is believed to contribute to the fall in glomerular filtration rate that accompanies ischemic acute renal failure. In intact organisms and in culture, viable cells that originate from the proximal tubule have been harvested after an ischemic insult, suggesting that cell death is not a prerequisite for detachment (22).
Since heat shock protein 27 (Hsp27), an inducible stress protein, acts partly as an actin-capping protein and prevents stress-mediated F-actin degradation (11, 23, 35), it has been assumed that most of the cytoprotective effects of Hsp27 on cell architecture is related to its inhibition of F-actin collapse during stress (26, 28, 31). Whether Hsp27 also protects cell contact sites has not been previously examined. In the present study, we demonstrate that Hsp27 ameliorates sublethal changes in renal epithelial cell contact structure during metabolic stress and this structural protection correlates with functional parameters.
Metabolic stress in vitro or transient ischemia in vivo activates Src, a tyrosine kinase that rapidly accumulates at both the cell-cell and cell-ECM target sites (27). Contact site function is disrupted by Src-mediated phosphorylation of proteins within the focal adhesion complex and adherens junction (29). We have previously shown that pharmacological inhibition of Src with 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine (PP2) (27) as well as prior heat stress (5), a nonspecific inducer of HSPs, preserve renal epithelial cell adherens junction function and promote cell attachment (5, 27). Here, we show that Hsp27 overexpression replicates the protective effects of heat stress and Src inhibition in cells subjected to ATP depletion. In the present study, we provide novel evidence that Hsp27 preserves cell architecture by preventing p-416Src accumulation at the cell membrane, thus describing a new mechanism by which Hsp27 prevents sublethal injury in renal tubular cells during metabolic stress.
MATERIALS AND METHODS
Materials and reagents.
All reagents were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise specified.
Cell culture.
Previously characterized proximal tubular epithelial cell lines derived from the immortalized mouse (BUMPT) (27) and from the normal human kidney (HK-2; catalog no. CRL-2190, ATCC) were used for all experiments simultaneously.
Metabolic stress.
Cells were incubated for 10–30 min at 37°C in glucose-free medium (DMEM, 23800-014, Invitrogen) that contained 5 mM sodium cyanide and 5 mM 2-deoxy-d-glucose as previously described by our laboratory (14, 24). This maneuver results in ATP depletion to <10% of baseline ATP content and is reversible with the removal of cyanide and the addition of exogenous glucose (33). In control, parallel medium changes were performed using DMEM.
Selective Hsp27 overexpression.
Wild-type human Hsp27 expression was increased in renal cells using a previously characterized adenoviral construct (16). Control cells were infected with an empty vector (AdTR5/GFP). Infection efficiency was >90–99% as determined by direct visualization of green fluorescent protein in cells infected with 40–100 multiplicity of infection. Cells were infected with the adenovirus for 16 h at 37°C in DMEM supplemented with 2% FBS followed by a 24-h washout period, during which the medium was replaced with fresh DMEM containing 10% FBS. Increased Hsp27 expression was confirmed by immunoblot analysis. To reproduce the physiological cellular response to stress, Hsp27 expression was increased by the adenovirus to the same level as that afforded by sublethal heat stress (43.0°C × 45 min) (32) followed by a 16-h recovery (10).
RNA interference.
Cells were transfected with small interference (si)RNA against Hsp27 (sc-29350) or control siRNA (sc-37007) from Santa Cruz Biotechnology (Santa Cruz, CA) as per the manufacturer's protocol. Briefly, subconfluent (50–60%) cells grown in antibiotic-free medium were transfected using siRNA Transfection Medium (sc-36868) and Transfection Reagent (sc-29528) at an siRNA concentration of 10 nM. After 8 h, cells were washed and cultured for 72 h in complete medium. This method achieved 60–80% knockdown.
Immunoblot analysis.
Analysis was performed as described previously (24). Commercially available antibodies were used to detect Hsp27 (1:1,000 dilution, catalog no. SPA-803, StressGen Biotechnologies, Victoria BC, Canada, or catalog number sc-1048, Santa Cruz Biotechnology); c-Src (1:1,000, sc-18, Santa Cruz Biotechnology); p-Src (1:1,000, Tyr416, catalog no. 2101, Cell Signaling); and Na-K-ATPase (1:1,000, catalog no. sc-28800, Santa Cruz Biotechnology). Secondary antibodies conjugated to horseradish peroxidase (Jackson ImmunoResearch Laboratories, West Grove, PA) were used in combination with a chemiluminescence detection method (GE Healthcare-Amersham Pharmacia, Piscataway, NJ) to visualize specific protein bands.
Immunocytochemistry.
Cells were grown to confluence on collagen- coated coverslips. The cells were fixed with 3.7% paraformaldehyde in Tris-buffered saline (TBS) for 10 min at 4°C followed by washing three times, for 5 min each, with PBS. Then, monolayers were permeabilized with 0.2% Triton X-100 for 10 min followed by blocking with 5% BSA (ABO1099–00100, American Bioanalytical) in PBS for 30 min at room temperature. Then, cells were incubated with primary antibodies in 1% BSA in PBS overnight at 4°C (paxillin, 1:100, 05–417, Upstate/Cell Signaling); E-cadherin (1:50, M1060, Takara Bio); pan-cadherin (1:100, ab6528, Abcam); and p-Src (Tyr416, 1:50, catalog no. 2101, Cell Signaling). After washing the cells three times with PBS, monolayers were incubated with appropriate fluorescent-labeled secondary antibodies (Alexa Fluor Dyes, Molecular Probes/Invitrogen). Cells were photographed using a Nikon Optiphot immunofluorescence microscope (×40 objective) and RT Diagnostic Instrument Spot digital camera. Fluorescein phalloidin (F432, Invitrogen) was used as previously described by our laboratory (5).
Cell fractionation.
Confluent cells in 100-mm plates were used for membrane separation using a commercially available kit: Qiagen Qproteome Cell Compartment Kit (catalog no. 37502, Santa Clarita, CA).
Transcellular electrical resistance.
Transepithelial electrical resistance (TER), a marker of tight junction integrity, was measured with an epithelial voltohmmeter (World Precision Instruments, New Haven, CT) in confluent cell monolayers grown on permeable filters (Transwell-3460, Costar) incubated in DMEM in 12-well trays. To control for intrinsic resistance of the filters, periodic measurements of electrical resistance were obtained across cell-free inserts equilibrated in DMEM. The electrical resistance of the filter was then subtracted from all subsequent measurements. Measurements were taken in duplicate in three filters for each condition in at least three separate experiments done on different days (n = 9) (5).
Transcellular permeability.
Unidirectional flux of lucifer yellow, a fluorescent, fluid-phase marker (molecular weight 482) was serially measured across confluent epithelial monolayers grown on permeable supports (Transwell-3460, Costar) and maintained at 37°C throughout the study. At the onset of the experiment, 1 mg/ml lucifer yellow was added to the apical solution. The intact cell monolayers in each insert well prevent free diffusion of lucifer yellow to the bottom wells. The entire volume of the basolateral compartment (1 ml) was sampled at 20-min intervals and then replaced with fresh medium. Apical solution samples were also obtained at the same intervals as for the basolateral solution. The concentration of lucifer yellow in each compartment was determined by fluorescence spectrofluorometry at an excitation wavelength of 425 nm and an emission of 535 nm. The emission intensity of a standard curve was linear over the range of samples tested and exhibited an r value of >0.95. Permeabilities were determined as previously described (5, 13): permeability (cm/s) = flux (mg/s)/[ΔC (mg/ml) × A (cm2)], where C is concentration and A is surface area.
Cell detachment.
Confluent HK-2 cell monolayers were used, and individually detached cells were counted as previously described by our laboratory (15).
Densitometric and statistical analysis.
After digitizing of the immunoblot image (Hewlett-Packard, Desk Scan II), band densities were quantified using NIH ImageQuant Software. Data are expressed as means ± SE. Comparison of two groups was performed using a two-tailed Student's t-test. Results involving more than one group were compared using two-way ANOVA and were then analyzed with the Fisher's post hoc test. A result was considered significant if P < 0.05.
RESULTS
Hsp27 preserves epithelial barrier function during sublethal injury.
To assess the cytoprotective effects of Hsp27 on sublethal cell injury, HK-2 and BUMPT cells treated with Hsp27 adenovirus or Hsp27 siRNA were subjected to metabolic stress achieved by 30-min ATP depletion followed by 30 min recovery. Given that results obtained with HK-2 and BUMPT cells were similar, only results with HK-2 cells are shown.
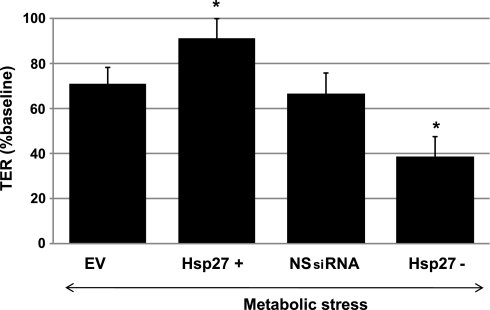
In HK-2 cells, transient ATP depletion causes structural changes, including disruption of the actin cytoskeleton, cell-cell junction, and focal adhesion integrity (5, 27). To evaluate the integrity of cell-cell contact sites, TER was measured before and after metabolic stress in confluent cell monolayers grown on semipermeable filters. At baseline there was no statistically significant difference in TER between groups. In HK-2 cells, baseline TER was 217 ± 20 Ω·cm2 in the empty vector group (EV), 214 ± 18 Ω·cm2 in the nonspecific siRNA group (NSsiRNA), 221 ± 18 Ω·cm2 in the Hsp27-overexpressing cells, and 214 ± 21 Ω·cm2 in the Hsp27 knockdown cells. After transient metabolic stress, a fall in TER was observed in all groups (Fig. 1). However, Hsp27 overexpression significantly preserved TER, while Hsp27 knockdown enhanced the loss of TER compared with controls (Fig. 1).
Fig. 1.
Effect of heat shock protein 27 (Hsp27) expression on cell-cell junction integrity during sublethal injury. Transepithelial resistance (TER), an estimate of adherens junction integrity, was measured after 30 min of ATP depletion followed by 30-min recovery in empty vector (EV)- or nonspecific small interference (si)RNA (NSsiRNA)-treated, Hsp27-overexpressing (Hsp27+) or Hsp27 knockdown (Hsp27−) cell monolayers. TER is expressed as a percentage of baseline. Measurements were taken in duplicate in 3 filters for each condition in at least 3 separate experiments done on different days. Values are means ± SE (n = 9). *P < 0.05 vs. EV or NSsiRNA.
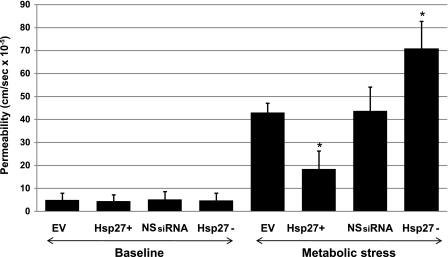
Measurement of unidirectional flux of lucifer yellow, a fluorescent, fluid-phase marker, was used to confirm that an alteration in TER was associated with physiological disruption of the tight junction. Lucifer yellow flux across confluent cell monolayers, an estimate of permeability, was relatively low in all four groups before stress (Fig. 2, baseline). In control cells (EV and NSsiRNA), metabolic stress caused a massive (8- to 10-fold) increase in lucifer yellow permeability. Hsp27 upregulation decreased permeability by 60%, whereas Hsp27 knockdown increased permeability by 40–50%, suggesting that Hsp27 partially regulates transepithelial permeability during stress (Fig. 2).
Fig. 2.
Effect of Hsp27 expression on epithelial barrier function during sublethal injury. Unidirectional lucifer yellow flux, an estimate of permeability, was measured across monolayers grown on semipermeable supports, in EV- or NSsiRNA-treated or Hsp27+ or Hsp27− cells after 30 min of ATP depletion followed by 30-min recovery. Baseline represents cell monolayers not subjected to injury. Measurements were taken in duplicates in 3 filters for each condition in at least 3 separate experiments done on different days. Values are means ± SE (n = 6). *P < 0.05 vs. EV or NSsiRNA.
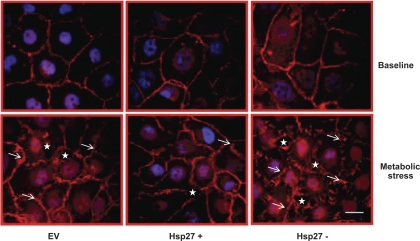
To confirm the effect of Hsp27 on the morphological integrity of cell-cell contact sites, immunocytochemical staining of E-cadherin, an adherens junction protein, was assessed in confluent monolayers. Metabolic stress caused breaks (Fig. 3, arrow) in the linear distribution of E-cadherin and resulted in complete separation of cell-cell contact sites (Fig. 3, star) that positively correlated with a decrease in TER and increase in lucifer yellow permeability. Hsp27 overexpression clearly diminished, whereas Hsp27 knockdown enhanced these untoward changes (Fig. 3).
Fig. 3.
Effect of Hsp27 expression on adherence junction morphology during sublethal injury. Immunocytochemical staining of E-cadherin (red) assessing the morphological integrity of adherence junctions at baseline and after metabolic stress in EV-treated or Hsp27+ or Hsp27− cells is shown. The arrow indicates breaks in the linear distribution of E-cadherin, and the star indicates separation of cell-cell contact sites. Nuclei are stained with DAPI (blue). Scale bar = 5 μm. EV- and NSsiRNA-treated cells were not visually distinguishable (NSsiRNA not shown). Images represent random fields from 3 independently repeated experiments.
Taken together, these results demonstrate that Hsp27 ameliorates metabolic stress-induced disruption in epithelial barrier function, a hallmark of sublethal injury.
Hsp27 preserves cell attachment to the ECM during sublethal injury.
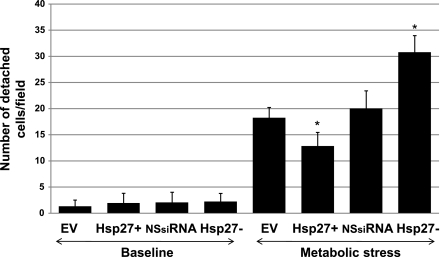
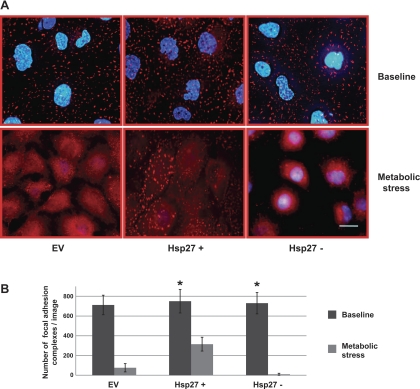
Transient ATP depletion decreases cell adherence to the substratum by disrupting the focal adhesion complexes. Focal adhesions form a structural link between the extracellular matrix and the actin cytoskeleton and also represent important sites of signal transduction (6, 8, 35). To evaluate cell adherence to the extracellular surface, the number of detached cells was counted before and after metabolic stress (Fig. 4). Compared with ATP-replete cells (baseline, Fig. 4), metabolic stress increased the number of detached cells by four- to fivefold. In contrast, Hsp27 overexpression significantly diminished, whereas Hsp27 knockdown significantly increased the number of detached cells. To visualize the morphological integrity of focal adhesions, immunocytochemical staining of paxillin, a key focal adhesion-associated protein, was performed (Fig. 5A). As expected, paxillin localizes to discrete structures in confluent monolayers of HK-2 cells at baseline (Fig. 5A). After metabolic stress, however, focal adhesions are disassembled and paxillin is diffusely redistributed into the cytosol. In the Hsp27-overexpressing cells subjected to stress, most focal adhesions remain intact (Fig. 5A, bottom middle), whereas Hsp27 knockdown aggravates focal adhesion disruption and increases cell detachment from the monolayer (Fig. 5A, bottom right). Almost half of the focal adhesion complexes remained intact after stress in the Hsp27-overexpressing cells, whereas Hsp27 knockdown resulted in almost complete disruption of most adhesion complexes (Fig. 5B).
Fig. 4.
Effect of Hsp27 expression on cell detachment during sublethal injury. Number of detached cells per high power field of EV- or NSsiRNA-treated or Hsp27+ or Hsp27− cells is shown. Baseline represents cell monolayers not subjected to injury. Values are means ± SE of 10 random fields/sample in 3 separate studies. *P < 0.05 vs. EV or NSsiRNA.
Fig. 5.
Effect of Hsp27 expression on focal adhesion complex integrity during sublethal injury. A: immunocytochemical staining of paxillin (red) assessing the integrity of the focal adhesion complexes at baseline (top) and after metabolic stress (bottom) in EV-treated or Hsp27+ or Hsp27− cells. Nuclei are stained with DAPI (blue). EV- and NSsiRNA-treated cells were not visually distinguishable (NSsiRNA not shown). Images represent random fields from 3 independently repeated experiments. Scale bar = 5 μm. B: number of intact focal adhesion complexes per field counted with Image J. Minimum particle size: 50 pixels2. Data are means ± SE and represent 5 random fields from 3 separate experiments; (n = 15). *P < 0.05 vs. EV.
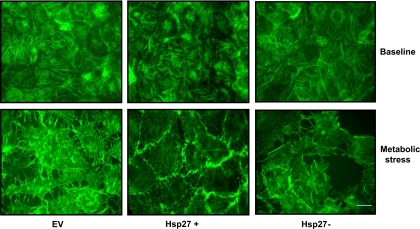
Hsp27 ameliorates disruption of the actin cytoskeleton during sublethal injury.
The role of Hsp27 in regulating changes in the actin cytoskeleton during metabolic stress was investigated in intact cells stained with fluorescent phalloidin. As expected, regular arrays of large actin stress fibers were visible at baseline (5) (Fig. 6, top). In contrast, metabolic stress severely disrupted filamentous F-actin fibers (Fig. 6, bottom). Hsp27 knockdown resulted in more severe disruption of the F-actin cytoskeleton and produced large aggregates composed of depolymerized actin (Fig. 6, bottom right). In Hsp27-overexpressing cells, modest preservation of stress fibers was apparent, as were numerous short F-actin fragments that persisted despite stress (Fig. 6, bottom middle). Interestingly, relative accumulation of stress fibers at the junctional regions in the Hsp27-overexpressing cells was observed after metabolic stress, suggesting that Hsp27 overexpression preserves both the junctional complexes and the actin cytoskeleton associated with it.
Fig. 6.
Effect of Hsp27 expression on the actin cytoskeleton during sublethal injury. Immunofluorescence micrographs of green fluorescent phalloidin staining of F-actin filaments at baseline (top) and after metabolic stress (bottom) in EV-treated or Hsp27+ or Hsp27− cells are shown. After stress, disruption of the F-actin cytoskeleton and formation of rhodamine-positive, actin-containing aggregates are evident. EV- and NSsiRNA-treated cells were not visually distinguishable (NSsiRNA not shown). Images represent random fields from 3 independently repeated experiments. Scale bar = 5 μm.
Hsp27-mediated cytoprotection involves Src.
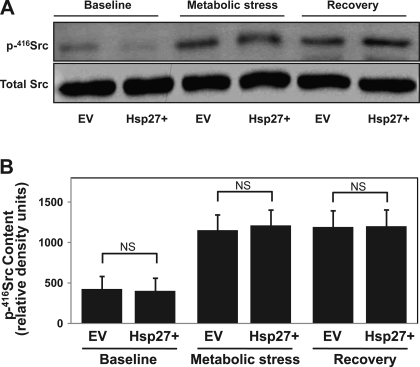
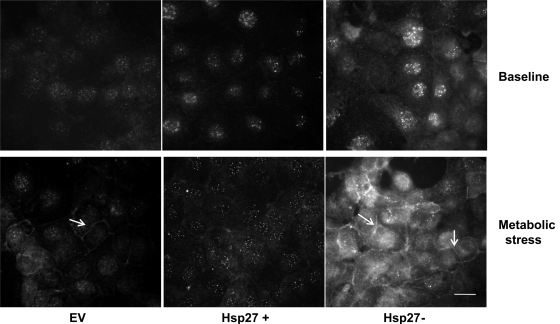
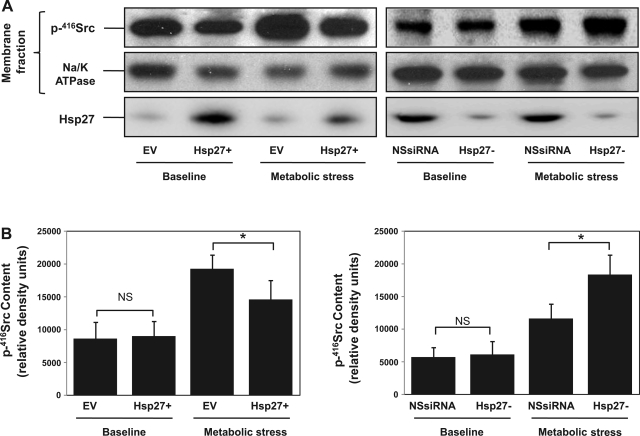
Metabolic stress in vitro and ischemic injury in vivo both phosphorylate and activate Src. This phosphorylation results in its accumulation at both cell-cell and cell-extracellular matrix contact sites (20, 27) where it disrupts adhesion function (29). In this study, we show (Figs. 1–6) that Hsp27 has similar protective effects on epithelial barrier function and cell attachment during metabolic stress as pharmacological inhibition of Src in our prior reports (5, 27), suggesting a link between the cytoprotection afforded by Hsp27 and Src inhibition. Estimating Src activation from whole cell lysate p416-Src content failed to reveal decreased Src activation in Hsp27-overexpressing cells (Fig. 7A). These results were confirmed in the densitometric analysis of three separate studies normalized to a loading control (Fig. 7B). Similarly, Hsp27 knockdown failed to alter p416-Src content in whole cell lysates (data not shown). Since several studies indicated that the subcellular localization of Src is crucial for regulating specific cellular processes such as cytoskeletal organization and cell contact site function (4), we used immunocytochemical staining to test whether Hsp27 alters the subcellular localization of p-416Src. At baseline, p-416Src is diffusely distributed in the cytosol (Fig. 8, top). After metabolic stress, however, p-416Src accumulation is detected in control cells at cell-cell contacts sites (EV; Fig. 8, bottom, arrow). Hsp27 overexpression diminished, whereas Hsp27 knockdown enhanced p-416Src accumulation at the cell-cell contact sites. A similar effect of Hsp27 on p-416Src accumulation in a purified cell membrane fraction was detected after stress (Fig. 9). Specifically, metabolic stress increased membrane-associated p-416Src content. Hsp27 overexpression decreased the amount of p-416Src in the membrane fraction, whereas Hsp27 knockdown increased membrane bound p-416Src (Fig. 9A, top). These results were confirmed in the densitometric analysis of three separate studies normalized to a loading control (Fig. 9). To determine whether Hsp27-mediated Src regulation requires interaction between the two proteins, bidirectional immunoprecipitation studies were performed. Minimal interaction was detected between Hsp27 and Src at baseline. Moreover, metabolic stress did not increase their interaction (data not shown).
Fig. 7.
Effect of Hsp27 expression on Src activation during sublethal injury. A: active (p-tyr416) Src content at baseline, after 30 min of metabolic stress and after 30 min of recovery in whole cell lysates from EV-treated or Hsp27+ cells. Total Src serves as a loading control. B: densitometric analysis of p-416Src content in whole cell lysates from 3 separate studies, normalized to Src loading control. Values are means ± SE (n = 3).
Fig. 8.
Effect of Hsp27 expression on p-416Src re-distribution to the cell membrane during sublethal injury. Immunofluorescence micrographs of p-416Src at baseline (top) and during metabolic stress (bottom) in EV-treated or Hsp27+ or Hsp27− cells are shown. The arrow indicates membrane-bound p-416Src. EV- and NSsiRNA-treated cells were not visually distinguishable (NSsiRNA not shown). Images represent random fields from 3 independently repeated experiments. Scale bar = 5 μm.
Fig. 9.
Effect of Hsp27 expression on p-416Src accumulation in the membrane fraction during sublethal injury. A, top: active p-416Src content in the membrane fraction at baseline and after metabolic stress from EV- and NSsiRNA-treated or Hsp27+ or Hsp27− cells. Middle: Na-K ATPase loading control. Bottom: Hsp27 content from whole cell lysate. Lanes contain 20 μg of total protein. B: densitometric analysis of p-416Src content in the membrane fraction from 3 separate studies, normalized to Na-K ATPase loading control. Values are means ± SE (n = 3). *P < 0.05.
DISCUSSION
Transient ischemia is a common cause of acute renal failure that leads to sublethal and lethal injury in proximal tubule epithelial cells, and ultimately organ dysfunction (17–19, 30). Sublethal injury is responsible for several specific pathophysiological and clinical changes during ischemic acute kidney injury (AKI) (18, 19, 22). Under physiological conditions, proximal tubule cells are columnar in shape with extensive polarity in the basolateral and apical surface membrane domains. Cells attach to their neighbors via junctional complexes, tight junctions, and adherens junctions as well as to the ECM via integrin adhesion complexes, called focal adhesion complexes. Transient ischemia results in loss of both cell-cell and cell-ECM attachments and disruption and dysregulation of the actin cytoskeleton that promote cell detachment. Dissociation of cellular junctions reduces cell-to-cell contacts and causes unregulated paracellular diffusion of water, ions, and macromolecules, thereby causing increased backleak, which reduces the glomerular filtration rate (1, 17, 19). Cellular junctional complex dissociation also allows membrane lipids and proteins to diffuse laterally into alternate membrane domains (17, 37). This loss of membrane polarity creates a more homogenous membrane surface without unique apical or basolateral membrane regions. The resulting nonpolarized renal epithelial cells no longer support vectoral solute and water reabsorption or secretion. These processes contribute significantly to pathophysiological consequences of ischemic AKI and are, in large part, secondary to alterations in cell adhesions and actin cytoskeleton structure.
Our in vitro sublethal ischemic injury model reproducibly mimics many of the characteristic morphological changes observed in HK-2 cells during in vivo renal ischemia, including disruption of cell-cell and cell-ECM contact sites (5, 22, 27, 36). Similarly, metabolic stress also induced cytoskeleton collapse and the accumulation of large, actin-containing aggregates. In this in vitro model, we demonstrate, that Hsp27, expressed to a physiological level that replicates heat preconditioning, markedly inhibits sublethal renal epithelial cell perturbations caused by transient metabolic stress (Figs. 1–6), and we correlate changes in cell structure with their function. Specifically, Hsp27 promotes the maintenance of adherens junctions and preserves epithelial barrier function during sublethal injury as assessed by TER and unidirectional lucifer yellow flux (Figs. 1–3). Hsp27 also protects focal adhesion integrity reflected by paxillin immunostaining and significantly decreases cell detachment during stress (Figs. 4 and 5). In addition to preserving contact sites, Hsp27 also modestly attenuated the collapse of the actin cytoskeleton induced by metabolic stress and selectively increased actin accumulation at cell-cell contact sites (Fig. 6). Importantly, Hsp27 knockdown had the opposite effect on each of these morphological and functional parameters (Figs. 1–6). Thus diverse methodological approaches indicate that Hsp27 modifies structural changes after sublethal injury. Improvement in cell-cell and cell-substrate interaction not only supports organ function but would preserve the attachment of viable renal cells known to be shed into the urine of both humans and rodents subjected to renal ischemia (22). Preservation of cell-cell and cell-substrate contact sites may attenuate some of the pathophysiological changes in the tubular epithelium that contribute to the loss of kidney function from an ischemic insult.
Cell-cell and cell-substrate interactions are fundamental for normal cellular homeostasis and influence crucial cellular processes such as growth, differentiation, wound healing, and migration (2, 4, 6, 9, 25, 29). It has long been recognized that Src prominently accumulates at cell-cell contact sites and focal adhesions (2, 4, 20). Several studies reported that expression of either v-Src or constitutively active Src perturbs the integrity of contact sites in epithelial cells (34). For example, Src has been shown to phosphorylate paxillin, a focal adhesion protein (2, 4, 34), and β-catenin, an adherens junction protein (7, 21). Paxillin serves as a docking protein that coordinates downstream signaling by recruiting signal molecules to focal adhesions. β-Catenin is connected to the actin cytoskeleton via E-cadherin and α-catenin, as well as other adherens junction proteins (3, 21). Src activation results in increased tyrosine phosphorylation of β-catenin, leading to rapid loosening of cell-cell contacts (3). In epithelial cells, β-catenin maintains cell architecture and is a major component of the Wnt signal transduction pathway that links two seemingly independent, but highly coordinated processes (12).
Given the role of Src in maintaining cell architecture, it is reasonable to hypothesize that Hsp27 protects cell contact sites and the actin cytoskeleton by either inhibiting Src activation or interfering with Src accumulation at its target sites. Manipulation of Hsp27 expression did not alter total active p-416Src content in whole cell lysates (Fig. 7). This could be explained by one of two factors. First, it is possible that detergent-exposed cell lysates do not contain the Src fraction that mediates alterations at the junction sites. Second, it is possible that only a small fraction of active p-416Src participates in these processes, and we are not able to detect even large differences in this smaller fraction while looking at the total active p-416Src content. The latter hypothesis appears to be far more likely since Hsp27 overexpression or knockdown significantly changes the amount of active p-416Src at the cell membrane, a primary site of its action (Figs. 8 and 9). We therefore propose that Hsp27 protects the cell architecture during transient metabolic stress by preventing Src recruitment to, and/or activation at, the cell contact sites and that Src likely serves as one of several key cellular signaling checkpoints where Hsp27 intervenes in the signal transduction cascade.
How Hsp27 inhibits the accumulation of active Src at cell contact sites or antagonizes de novo p-416Src activation at these sites is presently unclear. Bidirectional coimmunoprecipitation did not detect interaction between Hsp27 and Src at baseline or after metabolic stress, even in cells with increased Hsp27 expression, making it unlikely that interaction between the two proteins is required. On the other hand, Hsp27 could prevent Src liberation from the cytosolic compartment during stress and/or could interfere with its trafficking to the cell contact sites. Alternatively, Hsp27 could prevent localized Src activation at the critical cell membrane sites by interacting with other signal transduction molecules and/or altering the function of phosphatases that regulate Src dephosphorylation. Although it would be informative to assess the effect of Hsp27 expression in cells subjected to Src knockdown, this maneuver decreases cell proliferation, making it difficult to achieve healthy confluent monolayers (36).
In summary, this study advances our understanding of the protective effects of Hsp27 during sublethal epithelial cell injury. We provide novel evidence that Hsp27 overexpression preserves cell architecture and prevents sublethal injury to HK-2 cells by preventing p-416Src redistribution to the cell membrane during metabolic stress. Dissecting the mechanism by which Hsp27 regulates Src at cell contact sites deserves further study since it may reveal potential targets for increasing cellular resistance to sublethal stress and could yield novel therapeutic interventions for preventing ischemic renal injury.
GRANTS
This work is supported by a National Kidney Foundation Fellowship Grant (A. Havasi) and National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-53387 (S. C. Borkan) and DK-52898 (J. H. Schwartz).
REFERENCES
- 1.Balda MS, Whitney JA, Flores C, Gonzalez S, Cereijido M, Matter K. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J Cell Biol 134: 1031–1049, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baruzzi A, Caveggion E, Berton G. Regulation of phagocyte migration and recruitment by Src-family kinases. Cell Mol Life Sci 65: 2175–2190, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Behrens J, Vakaet L, Friis R, Winterhager E, Van Roy F, Mareel MM, Birchmeier W. Loss of epithelial differentiation and gain of invasiveness correlates with tyrosine phosphorylation of the E-cadherin/beta-catenin complex in cells transformed with a temperature-sensitive v-SRC gene. J Cell Biol 120: 757–766, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bjorge JD, Jakymiw A, Fujita DJ. Selected glimpses into the activation and function of Src kinase. Oncogene 19: 5620–5635, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Borkan SC, Wang YH, Lieberthal W, Burke PR, Schwartz JH. Heat stress ameliorates ATP depletion-induced sublethal injury in mouse proximal tubule cells. Am J Physiol Renal Physiol 272: F347–F355, 1997. [DOI] [PubMed] [Google Scholar]
- 6.Brown MC, Perrotta JA, Turner CE. Serine and threonine phosphorylation of the paxillin LIM domains regulates paxillin focal adhesion localization and cell adhesion to fibronectin. Mol Biol Cell 9: 1803–1816, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen KH, Tung PY, Wu JC, Chen Y, Chen PC, Huang SH, Wang SM. An acidic extracellular pH induces Src kinase-dependent loss of beta-catenin from the adherens junction. Cancer Lett 267: 37–48, 2008. [DOI] [PubMed] [Google Scholar]
- 8.Gerthoffer WT, Gunst SJ. Invited review: focal adhesion and small heat shock proteins in the regulation of actin remodeling and contractility in smooth muscle. J Appl Physiol 91: 963–972, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Giancotti FG, Tarone G. Positional control of cell fate through joint integrin/receptor protein kinase signaling. Annu Rev Cell Dev Biol 19: 173–206, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Havasi A, Li Z, Wang Z, Martin JL, Botla V, Ruchalski K, Schwartz JH, Borkan SC. Hsp27 inhibits Bax activation and apoptosis via a phosphatidylinositol 3-kinase-dependent mechanism. J Biol Chem 283: 12305–12313, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Head MW, Goldman JE. Small heat shock proteins, the cytoskeleton, and inclusion body formation. Neuropathol Appl Neurobiol 26: 304–312, 2000. [DOI] [PubMed] [Google Scholar]
- 12.Jin T, George Fantus I, Sun J. Wnt and beyond Wnt: multiple mechanisms control the transcriptional property of beta-catenin. Cell Signal 20: 1697–1704, 2008. [DOI] [PubMed] [Google Scholar]
- 13.Leslie BR, Schwartz JH, Steinmetz PR. Coupling between Cl− absorption and HCO3− secretion in turtle urinary bladder. Am J Physiol 225: 610–617, 1973. [DOI] [PubMed] [Google Scholar]
- 14.Li F, Mao HP, Ruchalski KL, Wang YH, Choy W, Schwartz JH, Borkan SC. Heat stress prevents mitochondrial injury in ATP-depleted renal epithelial cells. Am J Physiol Cell Physiol 283: C917–C926, 2002. [DOI] [PubMed] [Google Scholar]
- 15.Mao H, Wang Y, Li Z, Ruchalski KL, Yu X, Schwartz JH, Borkan SC. Hsp72 interacts with paxillin and facilitates the reassembly of focal adhesions during recovery from ATP depletion. J Biol Chem 279: 15472–15480, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Martin JL, Hickey E, Weber LA, Dillmann WH, Mestril R. Influence of phosphorylation and oligomerization on the protective role of the small heat shock protein 27 in rat adult cardiomyocytes. Gene Expr 7: 349–355, 1999. [PMC free article] [PubMed] [Google Scholar]
- 17.Molitoris BA, Chan LK, Shapiro JI, Conger JD, Falk SA. Loss of epithelial polarity: a novel hypothesis for reduced proximal tubule Na+ transport following ischemic injury. J Membr Biol 107: 119–127, 1989. [DOI] [PubMed] [Google Scholar]
- 18.Molitoris BA, Leiser J, Wagner MC. Role of the actin cytoskeleton in ischemia-induced cell injury and repair. Pediatr Nephrol 11: 761–767, 1997. [DOI] [PubMed] [Google Scholar]
- 19.Molitoris BA, Marrs J. The role of cell adhesion molecules in ischemic acute renal failure. Am J Med 106: 583–592, 1999. [DOI] [PubMed] [Google Scholar]
- 20.Mukaiyama H, Nishimura T, Kobayashi S, Ozawa T, Kamada N, Komatsu Y, Kikuchi S, Oonota H, Kusama H. Synthesis and c-Src inhibitory activity of imidazo[1,5-a]pyrazine derivatives as an agent for treatment of acute ischemic stroke. Bioorg Med Chem 15: 868–885, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Piedra J, Miravet S, Castano J, Palmer HG, Heisterkamp N, Garcia de Herreros A, Dunach M. p120 Catenin-associated Fer and Fyn tyrosine kinases regulate beta-catenin Tyr-142 phosphorylation and beta-catenin-alpha-catenin Interaction. Mol Cell Biol 23: 2287–2297, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Racusen LC Epithelial cell shedding in acute renal injury. Clin Exp Pharmacol Physiol 25: 273–275, 1998. [DOI] [PubMed] [Google Scholar]
- 23.Schneider GB, Hamano H, Cooper LF. In vivo evaluation of hsp27 as an inhibitor of actin polymerization: hsp27 limits actin stress fiber and focal adhesion formation after heat shock. J Cell Physiol 177: 575–584, 1998. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz JH, Shih T, Menza SA, Lieberthal W. ATP depletion increases tyrosine phosphorylation of beta-catenin and plakoglobin in renal tubular cells. J Am Soc Nephrol 10: 2297–2305, 1999. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz MA, Schaller MD, Ginsberg MH. Integrins: emerging paradigms of signal transduction. Annu Rev Cell Dev Biol 11: 549–599, 1995. [DOI] [PubMed] [Google Scholar]
- 26.Shelden EA, Borrelli MJ, Pollock FM, Bonham R. Heat shock protein 27 associates with basolateral cell boundaries in heat-shocked and ATP-depleted epithelial cells. J Am Soc Nephrol 13: 332–341, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Sinha D, Wang Z, Price VR, Schwartz JH, Lieberthal W. Chemical anoxia of tubular cells induces activation of c-Src and its translocation to the zonula adherens. Am J Physiol Renal Physiol 284: F488–F497, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Smoyer WE, Ransom RF. Hsp27 regulates podocyte cytoskeletal changes in an in vitro model of podocyte process retraction. FASEB J 16: 315–326, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol 13: 513–609, 1997. [DOI] [PubMed] [Google Scholar]
- 30.Ueda N, Kaushal GP, Shah SV. Apoptotic mechanisms in acute renal failure. Am J Med 108: 403–415, 2000. [DOI] [PubMed] [Google Scholar]
- 31.Van Why SK, Mann AS, Ardito T, Thulin G, Ferris S, Macleod MA, Kashgarian M, Siegel NJ. Hsp27 associates with actin and limits injury in energy depleted renal epithelia. J Am Soc Nephrol 14: 98–106, 2003. [DOI] [PubMed] [Google Scholar]
- 32.Wang Y, Knowlton AA, Christensen TG, Shih T, Borkan SC. Prior heat stress inhibits apoptosis in adenosine triphosphate-depleted renal tubular cells. Kidney Int 55: 2224–2235, 1999. [DOI] [PubMed] [Google Scholar]
- 33.Wang YH, Borkan SC. Prior heat stress enhances survival of renal epithelial cells after ATP depletion. Am J Physiol Renal Fluid Electrolyte Physiol 270: F1057–F1065, 1996. [DOI] [PubMed] [Google Scholar]
- 34.Warren SL, Nelson WJ. Nonmitogenic morphoregulatory action of pp60v-src on multicellular epithelial structures. Mol Cell Biol 7: 1326–1337, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wei H, Campbell W, Vander Heide RS. Heat shock-induced cardioprotection activates cytoskeletal-based cell survival pathways. Am J Physiol Heart Circ Physiol 291: H638–H647, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Xing J, Zhang Z, Mao H, Schnellmann RG, Zhuang S. Src regulates cell cycle protein expression and renal epithelial cell proliferation via PI3K/Akt signaling-dependent and -independent mechanisms. Am J Physiol Renal Physiol 295: F145–F152, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zuk A, Bonventre JV, Brown D, Matlin KS. Polarity, integrin, and extracellular matrix dynamics in the postischemic rat kidney. Am J Physiol Cell Physiol 275: C711–C731, 1998. [DOI] [PubMed] [Google Scholar]