Abstract
20-Hydroxyeicosatetraenoic acid (20-HETE) has been reported to promote mitogenicity in a variety of cell types, including renal epithelial cells. However, the signal transduction pathways activated by 20-HETE have not been fully defined. The present study evaluated the effects of 20-HETE and its more stable agonist analogs 20-hydroxyeicosa-5(Z),14(Z)-dienoic acid (5,14-20-HEDE) and N-[20-hydroxyeicosa-5(Z),14(Z)-dienoyl]glycine (5,14-20-HEDGE) on the Raf/MEK/ERK and phosphatidylinositol 3-kinase (PI3K)-Akt pathway in LLC-PK1 renal epithelial cells. 20-HETE (20 μM) increased phosphorylation of Raf-1 (2.5 ± 0.2-fold), MEK1/2 (6.3 ± 1.6-fold), and ERK1/2 (5.8 ± 0.3-fold) compared with vehicle-treated cells. Similarly, the 20-HETE analogs also strongly activated ERK1/2 in a Raf-1- and MEK1/2-dependent manner. Moreover, 5,14-20-HEDE increased Akt phosphorylation by 2.2 ± 0.3-fold. 20-HETE and 5,14-20-HEDE also promoted activation (Y1086) of epidermal growth factor receptor (EGFR; Y1086) by 1.9 ± 0.2- and 2.5 ± 0.2-fold, respectively. These effects were completely blocked by the EGFR inhibitor EKB-569 (0.1 μM). Moreover, EKB-569 (0.1 μM), as well as a c-Src inhibitor, SKI-606 (0.05 μM), completely abolished the 20-HETE-mediated activation of the Raf/MEK/ERK and PI3K-Akt pathways. Blockade of PKC with bisindolylmaleimide I had no effect on 20-HETE-induced ERK1/2 activation. This study demonstrated that 20-HETE activated the Raf/MEK/ERK and Akt pathways in renal epithelial cells secondary to the activation of c-Src and EGFR.
Keywords: epithelial cell proliferation, epidermal growth factor receptor, cell signaling
20-hydroxyeicosatetraenoic acid (20-HETE) is a lipid metabolite of arachidonic acid that is produced by ω-hydroxylase enzymes of the cytochrome P-450 4A (CYP4A) and 4F families (21). 20-HETE has long been known to play an integral role in the regulation of renal function, including renal vascular tone (14), tubuloglomerular feedback (49), autoregulation of renal blood flow (12, 13, 48), tubular transport (6, 30, 38, 45), and mitogenesis (4, 18, 23, 44).
There has been renewed interest in 20-HETE because of its potential as a novel therapeutic target in the treatment of acute kidney injury (32), cancer (8–10), and polycystic kidney disease (PKD) (28, 29). Regner et al. (32) showed that acute administration of a 20-HETE mimetic resulted in increased renal protection following ischemia-reperfusion injury. In other studies, 20-HETE was found to play an important role as a mitogen to promote aberrant growth and proliferation in cancer cell lines in vitro (8, 9) and cystic epithelial collecting duct cells in animal models of PKD in vitro and in vivo (28, 29).
The mitogenic role of 20-HETE was initially documented in proximal tubular cells (18) and vascular smooth muscle cells (23) after agonist stimulation (18, 23). Lin et al. (18) reported that epidermal growth factor (EGF) stimulated the formation of 20-HETE in primary cultures of rat proximal tubular cells, and inhibitors of 20-HETE synthesis attenuated the proliferative effect of EGF. Similarly, Muthalif et al. (23) reported that 20-HETE synthesis inhibitors attenuated the mitogenic actions of ANG II on vascular smooth muscle. More recent studies showed that inhibition of 20-HETE synthesis attenuated the growth of U251 glioma and 9L gliosarcoma cells (8–10), and the reduced kidney size and cyst development in autosomal recessive PKD (28, 29). Despite the importance of 20-HETE in mediating abnormal cell growth in proliferative diseases, as well as promoting cell survival in acute kidney injury, very little is known about the signal transduction pathways mediating these actions of 20-HETE.
One candidate pathway involved in cellular proliferation and survival is Raf/MEK/ERK (31). We and others found that inhibition of 20-HETE synthesis reduced ERK1/2 activation (9, 10, 28, 29), but the mechanism by which 20-HETE stimulated this protein complex remains to be established. Studies have implicated an interaction between receptor and nonreceptor tyrosine kinases to mediate the functions of 20-HETE in the kidney (7, 39). These tyrosine kinases, specifically EGF receptor (EGFR), are known to play an important role in mediating growth and cell proliferation through the activation of the Raf/MEK/ERK pathway in PKD and various epithelial cancers (22, 33, 40, 46). To further validate the potential interaction of 20-HETE with tyrosine kinases, recent studies showed that inhibition of 20-HETE synthesis reduced EGFR activation, which was associated with reduced proliferative activity (9, 10, 28).
For these reasons, the present study was designed to expand our knowledge regarding the signal transduction pathways involving 20-HETE. Western blot analysis was performed to examine whether 20-HETE and newly designed 20-HETE agonist analogs activate downstream signaling cascades, including Raf/MEK/ERK and Akt, through an interaction with EGFR and Src kinase pathways in a renal epithelial cell line.
MATERIALS AND METHODS
Drugs.
20-HETE and the 20-HETE agonist analogs 20-hydroxyeicosa-5(Z),14(Z)-dienoic acid (5,14-20-HEDE) and N-[20-hydroxyeicosa-5(Z),14(Z)-dienoyl]glycine (5,14-20-HEDGE) were synthesized by Dr. John R. Falck (University of Texas Southwestern). Indomethacin [a nonselective cyclooxygenase (COX) inhibitor] was purchased from Sigma-Aldrich (St. Louis, MO); bisindolylmaleimide I (a PKC inhibitor), U0126 (an MEK1/2 inhibitor), and wortmannin [a phosphatidylinositol 3-kinase (PI3K) inhibitor] from Cell Signaling Technology (Danvers, MA); and human EGF from BD Biosciences (San Jose, CA). SKI-606 (a selective Src kinase inhibitor) and EKB-569 (a selective EGFR inhibitor) were generously provided by Wyeth Pharmaceuticals (Pearl River, NY).
Animals.
Male Sprague-Dawley (SD) rats (250–350 g body wt) were purchased from a commercial vendor (Taconic, Hudson, NY). All protocols were approved by the Institutional Animal Care and Use Committee at the Medical College of Wisconsin. At 90 min after they were treated with vehicle (0.1 M Na2HPO4 buffer) or 5,14-20-HEDE (30 mg/kg sc), the animals were anesthetized with pentobarbital sodium (60 mg/kg), and both kidneys were harvested. The renal cortex was separated from the renal medulla, and the tissue was immediately frozen in liquid nitrogen and stored at −80°C until protein extraction was performed.
Cell lines and media.
The signal transduction studies were performed using the LLC-PK1 porcine proximal tubular cell line (American Type Culture Collection, Rockville, MD). The cells were grown using α-MEM (Invitrogen, Carlsbad, CA) supplemented with glutamine (0.3 mg/ml), 10% fetal calf serum, penicillin (1 U/ml), and streptomycin (1 μg/ml). The cells were studied at a confluence of 75–85% in six-well plates. Before the start of the experiment, the cells were placed in serum-free medium for 3 h and then treated with vehicle (0.1% ethanol), 20 μM 20-HETE + 2 μM indomethacin to prevent metabolism of 20-HETE by COX, or the non-COX-metabolizable 20-HETE agonists 5,14-20-HEDE and 5,14-20-HEDGE at 20 μM. The cells were harvested in 1× RIPA buffer (Upstate, Temecula, CA) containing protease (Roche Applied Science, Indianapolis, IN) and phosphatase (Pierce Biotechnology, Rockford, IL) inhibitors 5, 15, 30, 60, and 180 min after stimulation.
Antibodies.
The following antibodies were purchased from Cell Signaling Technology: total Raf-1 (catalog no. 9422) and Raf-1 phosphorylated at S338 (p-Raf-1; catalog no. 9427), total MEK1/2 (catalog no. 9122) and MEK1/2 phosphorylated at S217/S221 (p-MEK1/2; catalog no. 9154), total ERK1/2 (catalog no. 4695) and ERK1/2 phosphorylated at T202/Y204 (p-ERK1/2; catalog no. 4377), total Akt (catalog no. 9272) and Akt phosphorylated at S473 (p-Akt; catalog no. 4051), and total EGFR (catalog no. 2232) and EGFR phosphorylated at Y1086 and Y1068 (p-EGFR; catalog nos. 2220 and 2236, respectively). Anti-β-actin antibody (catalog no. A5441) was purchased from Sigma. Antibodies specific for pan-Src (catalog no. 44656G) and c-Src phosphorylated at Y416 (p-c-Src; catalog no. 44-660G) were purchased from Invitrogen.
Western blot analysis.
Renal kidney tissue and LLC-PK1 cells were lysed in 1× RIPA buffer (Upstate) containing 0.5 M Tris·HCl (pH 7.4), 1.5 M NaCl, 2.5% deoxycholic acid, 10% NP-40, and 10 mM EDTA with protease inhibitors (Roche Applied Science) and phosphatase inhibitors (Pierce Biotechnology). The homogenate was centrifuged once at 3,000 g for 10 min and then twice at 10,000 g for 10 min. The cell culture lysates were centrifuged at 12,000 g for 5 min. The protein concentrations of the supernatants were measured using the Bradford method (Bio-Rad, Hercules, CA). Protein (10–25 μg) was reduced in LDS sample buffer (Invitrogen) and DTT. The reduced samples were separated on a 4–12% gradient gel (Invitrogen) and subsequently transferred to a supported nitrocellulose membrane (Bio-Rad). The membranes were blocked in a proprietary blocking buffer (Licor, Lincoln, NE) and blotted with the primary antibodies (1:1,000 dilution for phosphorylated and unphosphorylated forms of the various signaling molecules and 1:10,000 dilution for β-actin) overnight at 4°C. After subsequent washing steps with PBS-0.1% Tween 20, membranes were incubated with the secondary anti-rabbit or anti-mouse antibodies (1:2,000 dilution; Cell Signaling Technology) at room temperature for 1 h. The bands were detected on film after incubation in a chemiluminescent solution (GE Healthcare, Piscataway, NJ). All the Western blot images consist of bands run on the same gel, but, in some cases, we cropped the images to group the experimental bands together. In any of these cases, a white gap was added to the image to show that the image was noncontiguous. The densities of the bands obtained were determined using ImageJ software (National Institutes of Health). To minimize variability in the quantitative assessment in our study, each immunoblot represents sample data from a different individual experiment, and the densitometry values are a compilation of the immunoblots that were performed on at least two and up to eight different experiments in which triplicate samples for each treatment group were on each film.
Statistical analysis.
Values are means ± SE. The significance of differences in mean values between groups was tested by one-way ANOVA followed by a Student-Newman-Keuls post hoc test. A Student's t-test for unpaired observations was used to determine the significance of differences between two groups. P < 0.05 was considered to be significant.
RESULTS
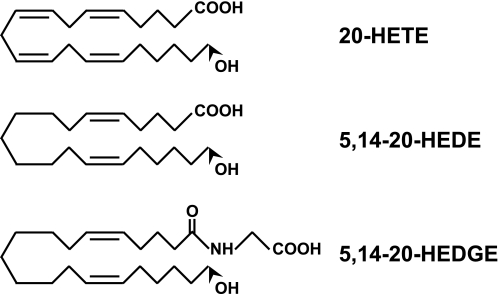
Schematic of chemical structures of 20-HETE and its agonist analogs.
The structures of 20-HETE and its analogs 5,14-20-HEDGE and 5,14-20-HEDE are depicted in Fig. 1. 5,14-20-HEDE and 5,14-20-HEDGE are partially saturated forms of 20-HETE in which two double bonds located between carbon positions 8–9 and 11–12 were removed to make these molecules resistant to metabolism by COX (1, 34). 5,14-20-HEDGE is further modified at its COOH terminus to make it more resistant to esterification and β-oxidation (1, 34). These compounds have been previously reported to act as 20-HETE agonists (1, 32, 43).
Fig. 1.
Chemical structure of 20-hydroxyeicosatetraenoic acid (20-HETE) and stable 20-HETE mimetics 20-hydroxyeicosa-5(Z),14(Z)-dienoic acid (5,14-20-HEDE) and N-[20-hydroxyeicosa-5(Z),14(Z)-dienoyl]glycine (5,14-20-HEDGE).
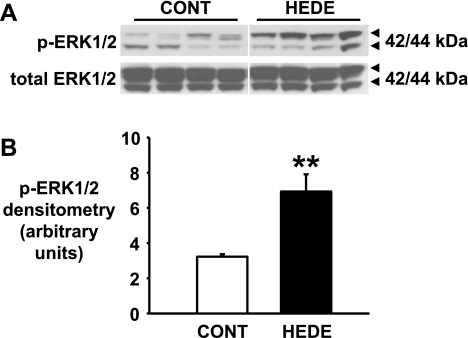
In vivo activation of ERK1/2 by administration of the 20-HETE analog 5,14-20-HEDE.
SD rats were treated with vehicle or 5,14-20-HEDE (30 mg/kg sc), and the kidneys were collected 90 min later. The activated (phosphorylated) form of ERK1/2 was significantly increased (P < 0.01) by 2.1 ± 0.3-fold (n = 4) in the renal medulla from 5,14-20-HEDE-treated compared with vehicle-injected rats (Fig. 2).
Fig. 2.
Activation of ERK1/2 in renal medulla of Sprague-Dawley rats after 5,14-20-HEDE treatment. A: representative Western blot depicting ERK1/2 activation after 5,14-20-HEDE (HEDE) treatment (30 mg/kg sc). p-ERK1/2, phosphorylated ERK1/2; CONT, control. B: p-ERK1/2 densitometry (n = 4 individual animals). **Significant difference from control (P < 0.01).
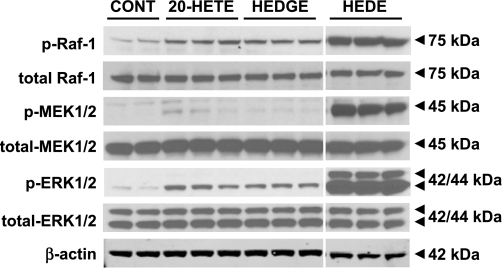
In vitro activation of the Raf/MEK/ERK pathway in LLC-PCK1 cells by 20-HETE and its analogs 5,14-20-HEDGE and 5,14-20-HEDE.
Because LLC-PK1 cells have been reported to produce very little, if any, 20-HETE (18, 25), they are a suitable model for use in determining the effects of exogenous administration of 20-HETE. Incubation with 20-HETE and 5,14-20-HEDGE for 3 h increased phosphorylation (activation) of ERK1/2 by 5.8 ± 0.3- and 9.2 ± 1.2-fold, respectively, compared with the levels detected in vehicle-treated (control) cells (Fig. 3). The increase in ERK1/2 phosphorylation in cells treated with equimolar amounts of 5,14-20-HEDE was 61.7 ± 3.2 and 55.5 ± 13.3 times greater than in cells treated with 20-HETE and 5,14-20-HEDGE, respectively (Fig. 3). The same pattern of ERK1/2 phosphorylation was also detectable after 1 h of incubation with 20-HETE (5.6 ± 1.4-fold, data not shown) and 5,14-20-HEDGE (6.3 ± 0.9-fold, data not shown). As described in methods, all the quantitation in Fig. 3, as well as all other figures, was performed on at least two and as many as eight different blots using triplicate samples from different experiments to ensure reproducibility of the results.
Fig. 3.
Activation of Raf/MEK/ERK pathway by 20-HETE and stable 20-HETE mimetics. Representative Western blot demonstrates activation of Raf/MEK/ERK pathway using specific antibodies. Cell lysates were obtained from LLC-PK1 cells incubated in serum-free media containing 20 μM 20-HETE, 20 μM 5,14-20-HEDGE (HEDGE), and 20 μM 5,14-20-HEDE for 3 h. Indomethacin (2 μM) was added to prevent COX-mediated metabolism 60 min before 20-HETE and agonist incubation. Changes in levels of p-ERK1/2, phosphorylated MEK1/2 (p-MEK1/2), and phosphorylated Raf-1 (p-Raf-1) in the presence of 20-HETE, 5,14-20-HEDGE, and 5,14-20-HEDE are shown. β-Actin was used as a loading control. Immunoblots represent 2–8 results for each antibody group.
As shown in Fig. 3, exposure of LLC-PK1 cells to 20-HETE, 5,14-20-HEDE, and 5,14-20-HEDGE for 3 h increased phosphorylation of Raf-1 and MEK1/2. Incubation with 20-HETE significantly increased the levels of activated MEK1/2 and Raf-1 (6.3 ± 1.6- and 2.5 ± 0.2-fold, respectively). Similar effects on the activation of MEK1/2 and Raf-1 (6.6 ± 1.4- and 2.7 ± 0.1-fold, respectively) were detected after stimulation of the cells with 5,14-20-HEDGE. On an equimolar basis, 5,14-20-HEDE strongly activated MEK1/2 and Raf-1 during the 3-h incubation compared with 20-HETE (11.0 ± 1.2- and 13.2 ± 1.7-fold, respectively) and 5,14-20-HEDGE (29.3 ± 3.3- and 11.7 ± 1.5-fold, respectively).
Temporal activation of the Raf/MEK/ERK pathway after stimulation with 5,14-20-HEDE.
Administration of 5,14-20-HEDE to LLC-PK1 cells for only 5 min increased the phosphorylation of Raf-1, MEK1/2, and ERK1/2 (2.2 ± 0.1-, 6.7 ± 0.5-, and 32.9 ± 3.6-fold, respectively) compared with control conditions (Fig. 4). Phosphorylation of the Raf/MEK/ERK pathway was maximally elevated 3 h after incubation with 5,14-20-HEDE.
Fig. 4.
Temporal activation of Raf/MEK/ERK pathway by 5,14-20-HEDE. Western blot analysis was performed to determine early activation of Raf/MEK/ERK pathway after incubation of LLC-PK1 cells with 20 μM 5,14-20-HEDE. Cells were collected 1 min, 5 min, 10 min, 20 min, and 3 h after addition of the drug. Cells were treated with vehicle for 5 min to use as control for comparison with 5,14-20-HEDE-treated cells. Blot was performed twice with samples from 2 different batches of cells studied in triplicate for each time point. β-Actin was used as a loading control.
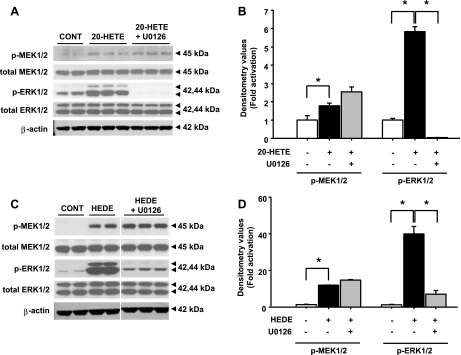
Effects of an MEK1/2 inhibitor on activation of the Raf/MEK/ERK pathway by 20-HETE and 5,14-20-HEDE.
Preincubation of the cells with U0126 (10 μM), a specific MEK1/2 inhibitor, attenuated the activation of ERK1/2 produced by 20-HETE. Phosphorylation of Raf-1 (data not shown) and MEK1/2 (Fig. 5, A and B) was not affected by U0126. Similar effects of 10 μM U0126 (Fig. 5, C and D) were observed in cells stimulated with 5,14-20-HEDE. Pretreatment of the cells with the MEK1/2 inhibitor also reduced ERK1/2 activation by 96.4 ± 0.3% (Fig. 5, C and D), and no change was detected in the phosphorylated levels of MEK1/2.
Fig. 5.
Effect of MEK inhibition on ERK1/2 activation mediated by 20-HETE and 5,14-20-HEDE. Western blot analysis was performed to determine changes in levels of phosphorylated and total forms of MEK1/2 and ERK1/2 after 3 h of incubation with 20 μM 20-HETE (A and B) and 20 μM 5,14-20-HEDE (C and D) in the presence or absence of 10 μM U0126. B and D: densitometry values for 20-HETE and 5,14-20-HEDE. All bands for each respective antibody were run on the same gel. Bands are separated to indicate that the blot was cropped to bring the most relevant grouping of bands together. Blots were performed twice with the lysates from 2 different batches of cells studied in triplicate for each treatment group. β-Actin was used as a loading control in A and C. *Significant difference between groups (P < 0.05).
Effects of 20-HETE on phosphorylation of EGFR.
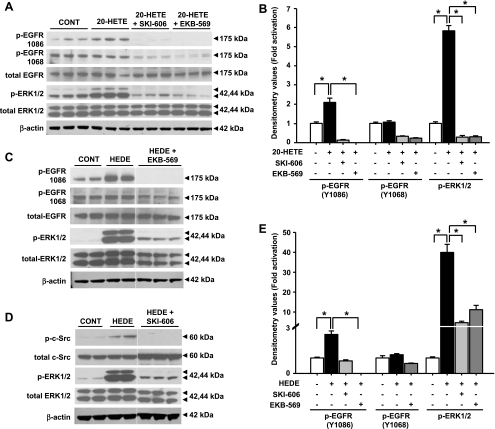
Stimulation of LLC-PK1 cells with 20-HETE (Fig. 6, A and B) and 5,14-20-HEDE (Fig. 6, C and E) increased phosphorylation (Y1086) of EGFR by 1.9 ± 0.2- and 2.5 ± 0.2-fold, respectively. No significant change in the phosphorylated (Y1068) form of EGFR was detected after treatment with 20-HETE or 5,14-20-HEDE (Fig. 6, A, B, C, and E).
Fig. 6.
Effect of c-Src and epidermal growth factor receptor (EGFR) inhibition on ERK1/2 activation mediated by 20-HETE and 5,14-20-HEDE. Western blot analysis was performed to determine changes in levels of phosphorylated and total forms of EGFR and ERK1/2 after 3-h incubation with 20 μM 20-HETE (A), EGFR and ERK1/2 after 3-h incubation with 20 μM 5,14-20-HEDE (C), and c-Src and ERK1/2 after 3-h incubation with 20 μM 5,14-20-HEDE (D) in the presence or absence of 0.05 μM SKI-606 or 0.1 μM EKB-569. B and E: band intensities for 20-HETE and 5,14-20-HEDE. β-Actin was used as a loading control in all groups. All bands for each respective antibody were run on the same gel and cropped only to group the relevant bands together. Each blot was performed twice with lysates from at least 2 different experiments with triplicate samples for each treatment group. *Significant difference between groups (P < 0.05).
Additional experiments were performed using the highly selective EGFR inhibitor EKB-569 to determine the role of EGFR in 20-HETE-mediated activation of the ERK pathway. The IC50 of EKB-569 to inhibit EGFR kinase activity in vitro is 38.5 ± 6.6 nM, whereas its effects on other tyrosine kinases requires 10-fold higher concentrations (42). Previous studies showed that 0.1 μM EKB-569 is highly selective for the EGFR (26, 42), and at 0.1 μM, EKB-569 has no effect on the activity of other tyrosine kinases, such as c-Src (data not shown). Pretreatment of the LLC-PK1 cells with 0.1 μM EKB-569 completely blocked phosphorylation (Y1086) of EGFR and the increase in ERK1/2 phosphorylation after stimulation of the cells with 20-HETE (Fig. 6, A and B). The 20-HETE-mediated increase in phosphorylation of Raf-1 and MEK1/2 was reduced to control (vehicle-treated) levels in the presence of EKB-569 (data not shown).
Similar effects of EKB-569 on activation of the Raf/MEK/ERK pathway were seen in cells treated with 5,14-20-HEDE. The activation of ERK1/2 significantly fell by 96 ± 0.3% (P < 0.05; Fig. 6, C and E), and the 5,14-20-HEDE-mediated increase in phosphorylation of Raf-1 and MEK1/2 returned to control levels (data not shown).
Effect of a c-Src inhibitor on 20-HETE- and 5,14-20-HEDE-mediated activation of ERK1/2.
SKI-606 is a selective inhibitor of Src family kinases, with an IC50 of 100 nM for inhibition of Src-dependent cell proliferation (2). The concentration used in our study (0.05 μM) was sufficient to block c-Src phosphorylation (Fig. 6D). Pretreatment of LLC-PK1 cells with SKI-606 markedly reduced ERK1/2 activation following stimulation of the cells with 20-HETE or 5,14-20-HEDE. SKI-606 blocked 20-HETE-mediated increase in ERK1/2 phosphorylation (Fig. 6, A and B) and significantly reduced 5,14-20-HEDE-mediated activation of ERK1/2 by 96 ± 0.6% (Fig. 6, D and E). The 20-HETE-mediated increase in activated Raf-1 and MEK1/2 was completely abolished by pretreatment with SKI-606 (data not shown).
Effect of a c-Src inhibitor on EGF-mediated activation of EGFR and ERK1/2.
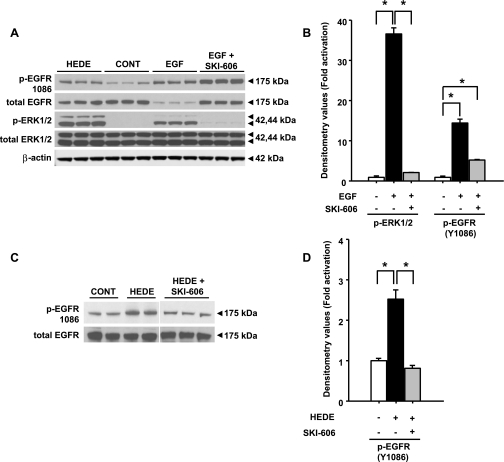
Stimulation of LLC-PK1 cells with 5,14-20-HEDE increased phosphorylation of EGFR at Y1086 (Fig. 7, A, C, and D) and ERK1/2 (Fig. 7A). Similar to 5,14-20-HEDE, EGF (0.02 μM) increased phosphorylation of EGFR at Y1086 and ERK1/2 (Fig. 7, A and B). In the presence of SKI-606, EGF-mediated activation of ERK1/2 significantly fell (P < 0.05) by 94% compared with the levels detected in cells treated with vehicle (Fig. 7, A and B). In the presence of SKI-606, EGF-mediated phosphorylation at Y1086 was preserved to a great extent and was significantly (P < 0.05) higher than the control (vehicle-treated) levels (Fig. 7, A and B). On the other hand, SKI-606 reduced 5,14-20-HEDE-mediated phosphorylation (Y1086) of EGFR to levels comparable to vehicle-treated control conditions (Fig. 7, C and D). Total EGFR levels remained the same in all groups other than the EGF-treated group, and this could be attributed to increased internalization and subsequent degradation of EGFR (3) (Fig. 7, A and C).
Fig. 7.
Effect of c-Src inhibition in epidermal growth factor (EGF)-mediated activation of ERK1/2. Representative Western blot analysis for active (phosphorylated) and total EGFR and ERK1/2 protein was performed using LLC-PK1 cell lysates treated with 0.02 μM EGF (A) or 20 μM 5,14-20-HEDE (C) in the presence or absence of 0.05 μM SKI-606. B and D: band intensities for EGF and 5,14-20-HEDE. β-Actin was used as a loading control. All bands for each respective antibody were run on the same gel and cropped only to group the relevant bands together. *Significant difference between groups (P < 0.05).
Role of PKC in 20-HETE-mediated activation of ERK1/2.
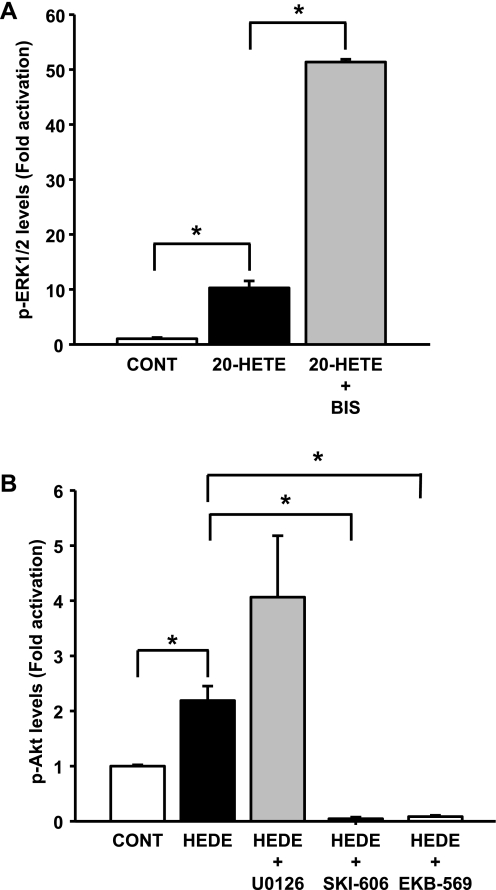
Preincubation of LLC-PK1 cells with a PKC inhibitor, bisindolylmaleimide I (4 μM), significantly increased (P < 0.05) 20-HETE-mediated ERK1/2 activation by fivefold (Fig. 8A). A similar result was observed in cells treated with the 20-HETE agonist 5,14-20-HEDE in the presence of the PKC inhibitor (data not shown).
Fig. 8.
Effect of PKC inhibition on 20-HETE-mediated ERK1/2 activation and effect of MEK1/2, c-Src, and EGFR inhibition on 5,14-20-HEDE-mediated activation of Akt. Western blot analyses were performed, and band intensities were graphed. A: changes in levels of phosphorylated form of ERK1/2 after 3-h incubation with 20 μM 20-HETE in the presence or absence of 4 μM bisindolylmaleimide I (BIS). B: changes in levels of phosphorylated form of Akt after 3-h incubation with 20 μM 5,14-20-HEDE in the presence or absence of an MEK (U0126, 10 μM), c-Src (SKI-606, 0.05 μM), or EGFR (EKB-569, 0.1 μM) inhibitor. *Significant difference between groups (P < 0.05).
Effect of 5,14-20-HEDE on the Akt pathway.
Akt phosphorylation was significantly increased (P < 0.05) by 2.2 ± 0.3-fold after 3 h of incubation with 20 μM 5,14-20-HEDE compared with levels in vehicle-treated LLC-PK1 cells (Fig. 8B). The phosphorylation of Akt by 5,14-20-HEDE was completely blocked by the addition of 0.05 μM SKI-606 (Fig. 8B), 0.1 μM EKB-569 (Fig. 8B), or 1 μM wortmannin (a PI3K inhibitor; data not shown). U0126 (10 μM), a specific MEK inhibitor, in the presence of 5,14-20-HEDE had no measureable effect on the activation of Akt (Fig. 8B).
DISCUSSION
The present study examined the effects of 20-HETE-mediated activation of the Raf/MEK/ERK signaling pathway in renal epithelial cells. Our results indicate that 20-HETE activated this pathway in LLC-PK1 cells through a mechanism that interacted with c-Src and EGFR. Phosphorylation (activation) of the ERK1/2 protein was increased in the renal medulla after administration of a stable 20-HETE agonist, 5,14-20-HEDE, to SD rats in vivo. Overall, the present findings are consistent with results from previous studies indicating that the ANG II-induced proliferation of vascular smooth muscle cells can be blocked by inhibitors of 20-HETE and was associated with activation of Ras and ERK1/2 (23, 24, 44). In our study, similar observations were made: not only 20-HETE, but also 20-HETE analogs, could activate the Raf/MEK/ERK pathway and, to a lesser extent, the Akt pathway in renal epithelial cells. Blockade of MEK1/2 function using U0126 completely blocked the ERK1/2 activation mediated by 20-HETE and 5,14-20-HEDE, providing support for the view that 20-HETE signaling in renal epithelial cells involves the activation of ERK1/2 through MEK1/2.
There remains a paucity of information regarding 20-HETE signaling and how it activates the Raf/MEK/ERK pathway, especially in renal epithelial cells. Several functions in mammalian cells are known to be regulated by tyrosine kinases, which can be broadly grouped into receptor and nonreceptor forms (37). In our study, using more specific inhibitors than previously available, we found that the receptor tyrosine kinase EGFR and the nonreceptor tyrosine kinase c-Src were involved in 20-HETE-mediated activation of the Raf/MEK/ERK pathway. 20-HETE was found to activate EGFR, which resulted in increased activation of downstream signaling cascades, specifically Raf/MEK/ERK. Activation of the Raf/MEK/ERK pathway was blocked by EKB-569, a highly specific inhibitor of EGFR that covalently binds to the receptor and blocks its function (42). The interaction of 20-HETE with EGFR has not been well characterized, but this finding is consistent with the results of some previous studies that documented a role for EGF in the synthesis and/or function of 20-HETE. For example, the vasoconstrictor effect of 20-HETE in rat renal microvessels and its ability to block the large-conductance K+ channels were attenuated by tyrosine kinase inhibitors (39). Moreover, blockade of 20-HETE formation attenuated growth responses to EGF in rat aortic vascular smooth muscle cells (23) and rat primary proximal tubular cells (18). Similar effects were found in U251 glioma cells, whereby inhibition of 20-HETE synthesis decreased the activation of EGFR and, subsequently, the proliferative actions of EGF (9). In autosomal recessive PKD models of aberrant epithelial cell proliferation, chronic administration of a 20-HETE synthesis inhibitor, HET-0016, inhibited proliferation of the cystic epithelium, and this was associated with reduced phosphorylation of EGFR and activation of the ERK1/2 pathway in cystic kidneys (28, 29).
The mechanism by which 20-HETE activates EGFR in LLC-PK1 cells remains to be determined, but the present results are the first to indicate that 20-HETE, as well as the closely related mimetic 5,14-20-HEDE stimulated phosphorylation of EGFR at a specific residue, Y1086. The interaction of 20-HETE with EGFR (Y1086) is consistent with the results of our previous study, whereby chronic inhibition of 20-HETE synthesis with HET-0016 was associated with reduced phosphorylation of EGFR (Y1086) in cystic mouse kidneys (28). Similarly, in a study using human U251 cells (9), phosphorylation of EGFR at an alternate tyrosine residue (Y992) was markedly reduced in the presence of HET-0016. Although the exact tyrosine residue(s) activated by 20-HETE on EGFR has not been fully elucidated, it is well established that phosphorylated residues on EGFR serve as docking sites for signaling and adaptor molecules, leading to activation of downstream pathways (15). One such adaptor molecule is Grb2, which plays an important role in coupling Ras to the downstream Raf/MEK/ERK signaling pathway (15, 37). It was previously shown that Grb2 protein could directly bind to EGFR phosphorylated on Y1068 and Y1086 (27). Therefore, EGFR phosphorylated at Y1086 possesses the ability to transduce signals to the downstream Raf/MEK/ERK pathway to mediate 20-HETE activity. The other common route for activation of the Raf/MEK/ERK pathway independent of EGFR is stimulation of PKC (16). Even though some of the actions of 20-HETE on vascular reactivity have been linked to activation of PKC (16a), blockade of PKC with bisindolylmaleimide I in our study was not found to inhibit activation of ERK1/2 by 20-HETE in LLC-PK1 cells.
Because of our finding that 20-HETE interacts with EGFR in renal epithelial cells, we further investigated the role of c-Src in 20-HETE-mediated activation of the Raf/MEK/ERK pathway. c-Src and EGFR have been shown to reciprocally phosphorylate each other (35), and c-Src can be recruited to EGFR after the activation of EGFR to promote downstream signaling cascades (15). EGFR and c-Src have been shown to synergistically act to mediate EGF-induced proliferation (20). More recently, Sweeney et al. (41) demonstrated that there is a direct interaction with c-Src and activation of EGFR in two distinct models of PKD and that inhibition of c-Src with SKI-606 decreases activation of the Raf/MEK/ERK pathway. Our findings would be consistent with the view that c-Src mediates activation of EGFR (Y1086) in the presence of 20-HETE and that activation of the EGFR (Y1086) was not an autophosphorylation event. This possibility is further supported by previous findings that Y1086 is phosphorylated to a greater extent by c-Src than by autophosphorylation (19). It would appear that c-Src plays a role in the signal transduction of 20-HETE and that the signals from c-Src are upstream to EGFR phosphorylation following 20-HETE-mediated stimulation.
Apart from the Raf/MEK/ERK pathway, the PI3K-Akt pathway was also found to be activated in our study after 5,14-20-HEDE stimulation of the LLC-PK1 cells, and this activation was completely blocked by wortmannin, a specific inhibitor of PI3K, as well as by EGFR and c-Src inhibitors, EKB-569 and SKI-606, respectively. The activation of Akt by 20-HETE in the present study is consistent with the results from previous studies performed in aortic vascular smooth muscle cells (17) and bovine pulmonary artery endothelial cells (5). Similar to the Raf/MEK/ERK pathway, our studies showed that 20-HETE-mediated activation of Akt was strictly dependent on EGFR and c-Src signaling. These experiments demonstrated that the integration of 20-HETE signaling through the EGFR can result in activation of a multitude of distinct signaling pathways and produce various biological responses to this eicosanoid. One such biological response may contribute to the renal protective effect of 20-HETE after renal ischemia-reperfusion injury (32). This protection may partially involve the activation of Raf/MEK/ERK and PI3K-Akt pathways, which were shown to be protective against ischemia-reperfusion injury (11). On the contrary, we previously reported that overexpression of a 20-HETE-producing Cyp4a12 isoform was detrimental in LLC-PK1 cells after an in vitro protocol of ischemia-reperfusion (25). Because the genetically modified LLC-PK1 cells with the lentiviral vectors promoted constitutively, high-level production of 20-HETE (25), it is likely that there is a differential dose-dependent activation of signaling cascades resulting in the distinct biological functions of this eicosanoid, which may account for the paradoxical contradiction between these two studies.
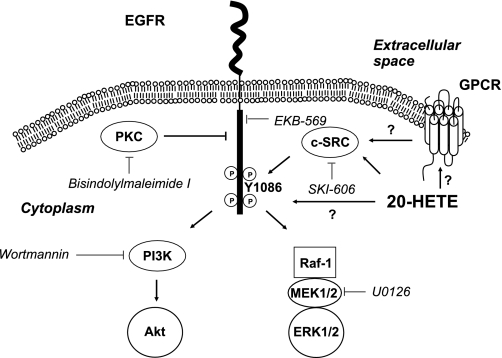
The results from our experiments are consistent with signaling characteristics of receptor tyrosine kinases (37). Our working hypothesis for 20-HETE signaling in renal epithelial cells is summarized in Fig. 9. Nonreceptor tyrosine kinase, c-Src, is activated by 20-HETE, which promotes phosphorylation of EGFR. Ultimately, this results in the sequential activation of the Raf/MEK/ERK cascade and, to a lesser extent, Akt, through the activation of PI3K via the activated EGFR. This may be a new paradigm by which 20-HETE signaling can be studied in proliferative disease cell types and provide the foundation for future studies to define the signaling pathways involved with 20-HETE and its agonist analogs. Moreover, c-Src activity is known to play an important role in coupling the G protein receptors to the mitogenic pathways, such as EGFR-mediated activation of the MAPK cascade (36, 37). Like some other lipid mediators such as PGE2 (47), interaction of 20-HETE with a G protein-coupled receptor might also be the initiation point for the signaling events mediated by 20-HETE.
Fig. 9.
Proposed mechanism of action of 20-HETE mediating activation of ERK1/2 and Akt in renal proximal tubular cells. PI3K, phosphatidylinositol 3-kinase; GPCR, G protein-coupled receptor.
In all, these studies provide a foundation for investigating the signal transduction pathways in cancer and other proliferative diseases in which 20-HETE production and/or activity is shown to be elevated.
GRANTS
The work in this manuscript was supported by grants awarded from the Polycystic Kidney Disease Foundation (F. Park), Advancing a Healthier Wisconsin (F. Park), Medical College of Wisconsin Clinical Translational Science Initiative (K. R. Regner), the Robert A. Welch Foundation (J. R. Falck), and National Institutes of Health Grants HL-36279 (R. J. Roman) and GM-312378 (J. R. Falck).
Acknowledgments
The authors thank William E. Sweeney, Jr. (Department of Pediatrics, Medical College of Wisconsin), and Andrey Sorokin (Department of Medicine, Medical College of Wisconsin) for scientific discussions and critical analysis of the manuscript.
REFERENCES
- 1.Alonso-Galicia M, Falck JR, Reddy KM, Roman RJ. 20-HETE agonists and antagonists in the renal circulation. Am J Physiol Renal Physiol 277: F790–F796, 1999. [DOI] [PubMed] [Google Scholar]
- 2.Boschelli DH, Ye F, Wang YD, Dutia M, Johnson SL, Wu B, Miller K, Powell DW, Yaczko D, Young M, Tischler M, Arndt K, Discafani C, Etienne C, Gibbons J, Grod J, Lucas J, Weber JM, Boschelli F. Optimization of 4-phenylamino-3-quinolinecarbonitriles as potent inhibitors of Src kinase activity. J Med Chem 44: 3965–3977, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Carpenter G, Cohen S. 125I-labeled human epidermal growth factor. Binding, internalization, and degradation in human fibroblasts. J Cell Biol 71: 159–171, 1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen P, Guo M, Wygle D, Edwards PA, Falck JR, Roman RJ, Scicli AG. Inhibitors of cytochrome P450 4A suppress angiogenic responses. Am J Pathol 166: 615–624, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y, Medhora M, Falck JR, Pritchard KA Jr, Jacobs ER. Mechanisms of activation of eNOS by 20-HETE and VEGF in bovine pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol 291: L378–L385, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Escalante B, Erlij D, Falck JR, McGiff JC. Effect of cytochrome P450 arachidonate metabolites on ion transport in rabbit kidney loop of Henle. Science 251: 799–802, 1991. [DOI] [PubMed] [Google Scholar]
- 7.Good DW, George T, Wang DH. Angiotensin II inhibits HCO3− absorption via a cytochrome P-450-dependent pathway in MTAL. Am J Physiol Renal Physiol 276: F726–F736, 1999. [DOI] [PubMed] [Google Scholar]
- 8.Guo AM, Sheng J, Scicli GM, Arbab AS, Lehman NL, Edwards PA, Falck JR, Roman RJ, Scicli AG. Expression of CYP4A1 in U251 human glioma cell induces hyperproliferative phenotype in vitro and rapidly growing tumors in vivo. J Pharmacol Exp Ther 327: 10–19, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo M, Roman RJ, Falck JR, Edwards PA, Scicli AG. Human U251 glioma cell proliferation is suppressed by HET0016 [N-hydroxy-N′-(4-butyl-2-methylphenyl)formamidine], a selective inhibitor of CYP4A. J Pharmacol Exp Ther 315: 526–533, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Guo M, Roman RJ, Fenstermacher JD, Brown SL, Falck JR, Arbab AS, Edwards PA, Scicli AG. 9L gliosarcoma cell proliferation and tumor growth in rats are suppressed by N-hydroxy-N′-(4-butyl-2-methylphenol) formamidine (HET0016), a selective inhibitor of CYP4A. J Pharmacol Exp Ther 317: 97–108, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Hausenloy DJ, Yellon DM. Preconditioning and postconditioning: underlying mechanisms and clinical application. Atherosclerosis 204: 334–341, 2009. [DOI] [PubMed] [Google Scholar]
- 12.Imig JD, Falck JR, Inscho EW. Contribution of cytochrome P450 epoxygenase and hydroxylase pathways to afferent arteriolar autoregulatory responsiveness. Br J Pharmacol 127: 1399–1405, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Imig JD, Zou AP, Ortiz de Montellano PR, Sui Z, Roman RJ. Cytochrome P-450 inhibitors alter afferent arteriolar responses to elevations in pressure. Am J Physiol Heart Circ Physiol 266: H1879–H1885, 1994. [DOI] [PubMed] [Google Scholar]
- 14.Imig JD, Zou AP, Stec DE, Harder DR, Falck JR, Roman RJ. Formation and actions of 20-hydroxyeicosatetraenoic acid in rat renal arterioles. Am J Physiol Regul Integr Comp Physiol 270: R217–R227, 1996. [DOI] [PubMed] [Google Scholar]
- 15.Jorissen RN, Walker F, Pouliot N, Garrett TP, Ward CW, Burgess AW. Epidermal growth factor receptor: mechanisms of activation and signalling. Exp Cell Res 284: 31–53, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Kolch W, Heidecker G, Kochs G, Hummel R, Vahidi H, Mischak H, Finkenzeller G, Marme D, Rapp UR. Protein kinase Cα activates RAF-1 by direct phosphorylation. Nature 364: 249–252, 1993. [DOI] [PubMed] [Google Scholar]
- 16a.Lange A, Gebremedhin D, Narayanan J, Harder D. 20-Hydroxyeicosatetraenoic acid-induced vasoconstriction and inhibition of potassium current in cerebral vascular smooth muscle is dependent on activation of protein kinase C. J Biol Chem 272: 27345–27352, 1997. [DOI] [PubMed] [Google Scholar]
- 17.Li F, Malik KU. Angiotensin II-induced Akt activation is mediated by metabolites of arachidonic acid generated by CaMKII-stimulated Ca2+-dependent phospholipase A2. Am J Physiol Heart Circ Physiol 288: H2306–H2316, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Lin F, Rios A, Falck JR, Belosludtsev Y, Schwartzman ML. 20-Hydroxyeicosatetraenoic acid is formed in response to EGF and is a mitogen in rat proximal tubule. Am J Physiol Renal Fluid Electrolyte Physiol 269: F806–F816, 1995. [DOI] [PubMed] [Google Scholar]
- 19.Lombardo CR, Consler TG, Kassel DB. In vitro phosphorylation of the epidermal growth factor receptor autophosphorylation domain by c-Src: identification of phosphorylation sites and c-Src SH2 domain binding sites. Biochemistry 34: 16456–16466, 1995. [DOI] [PubMed] [Google Scholar]
- 20.Maa MC, Leu TH, McCarley DJ, Schatzman RC, Parsons SJ. Potentiation of epidermal growth factor receptor-mediated oncogenesis by c-Src: implications for the etiology of multiple human cancers. Proc Natl Acad Sci USA 92: 6981–6985, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maier KG, Roman RJ. Cytochrome P450 metabolites of arachidonic acid in the control of renal function. Curr Opin Nephrol Hypertens 10: 81–87, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Montagut C, Settleman J. Targeting the RAF-MEK-ERK pathway in cancer therapy. Cancer Lett. In press. [DOI] [PubMed]
- 23.Muthalif MM, Benter IF, Karzoun N, Fatima S, Harper J, Uddin MR, Malik KU. 20-Hydroxyeicosatetraenoic acid mediates calcium/calmodulin-dependent protein kinase II-induced mitogen-activated protein kinase activation in vascular smooth muscle cells. Proc Natl Acad Sci USA 95: 12701–12706, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muthalif MM, Uddin MR, Fatima S, Parmentier JH, Khandekar Z, Malik KU. Small GTP binding protein Ras contributes to norepinephrine-induced mitogenesis of vascular smooth muscle cells. Prostaglandins Other Lipid Mediat 65: 33–43, 2001. [DOI] [PubMed] [Google Scholar]
- 25.Nilakantan V, Maenpaa C, Jia G, Roman RJ, Park F. 20-HETE-mediated cytotoxicity and apoptosis in ischemic kidney epithelial cells. Am J Physiol Renal Physiol 294: F562–F570, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nunes M, Shi C, Greenberger LM. Phosphorylation of extracellular signal-regulated kinase 1 and 2, protein kinase B, and signal transducer and activator of transcription 3 are differently inhibited by an epidermal growth factor receptor inhibitor, EKB-569, in tumor cells and normal human keratinocytes. Mol Cancer Ther 3: 21–27, 2004. [PubMed] [Google Scholar]
- 27.Okutani T, Okabayashi Y, Kido Y, Sugimoto Y, Sakaguchi K, Matuoka K, Takenawa T, Kasuga M. Grb2/Ash binds directly to tyrosines 1068 and 1086 and indirectly to tyrosine 1148 of activated human epidermal growth factor receptors in intact cells. J Biol Chem 269: 31310–31314, 1994. [PubMed] [Google Scholar]
- 28.Park F, Sweeney WE, Jia G, Roman RJ, Avner ED. 20-HETE mediates proliferation of renal epithelial cells in polycystic kidney disease. J Am Soc Nephrol 19: 1929–1939, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park F, Sweeney WE Jr, Jia G, Akbulut T, Mueller B, Falck JR, Birudaraju S, Roman RJ, Avner ED. Chronic blockade of 20-HETE synthesis reduces polycystic kidney disease in an orthologous rat model of ARPKD. Am J Physiol Renal Physiol 296: F575–F582, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quigley R, Baum M, Reddy KM, Griener JC, Falck JR. Effects of 20-HETE and 19(S)-HETE on rabbit proximal straight tubule volume transport. Am J Physiol Renal Physiol 278: F949–F953, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramos JW The regulation of extracellular signal-regulated kinase (ERK) in mammalian cells. Int J Biochem Cell Biol 40: 2707–2719, 2008. [DOI] [PubMed] [Google Scholar]
- 32.Regner KR, Zuk A, Van Why SK, Shames BD, Ryan RP, Falck JR, Manthati VL, McMullen ME, Ledbetter SR, Roman RJ. Protective effect of 20-HETE analogues in experimental renal ischemia-reperfusion injury. Kidney Int 75: 511–517, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene 26: 3291–3310, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Roman RJ P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev 82: 131–185, 2002. [DOI] [PubMed] [Google Scholar]
- 35.Roskoski R Src kinase regulation by phosphorylation and dephosphorylation. Biochem Biophys Res Commun 331: 1–14, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Rozengurt E Mitogenic signaling pathways induced by G protein-coupled receptors. J Cell Physiol 213: 589–602, 2007. [DOI] [PubMed] [Google Scholar]
- 37.Schlessinger J Cell signaling by receptor tyrosine kinases. Cell 103: 211–225, 2000. [DOI] [PubMed] [Google Scholar]
- 38.Schwartzman M, Ferreri NR, Carroll MA, Songu-Mize E, McGiff JC. Renal cytochrome P450-related arachidonate metabolite inhibits (Na+ + K+)ATPase. Nature 314: 620–622, 1985. [DOI] [PubMed] [Google Scholar]
- 39.Sun CW, Falck JR, Harder DR, Roman RJ. Role of tyrosine kinase and PKC in the vasoconstrictor response to 20-HETE in renal arterioles. Hypertension 33: 414–418, 1999. [DOI] [PubMed] [Google Scholar]
- 40.Sweeney WE, Avner ED. Molecular and cellular pathophysiology of autosomal recessive polycystic kidney disease (ARPKD). Cell Tissue Res 326: 671–685, 2006. [DOI] [PubMed] [Google Scholar]
- 41.Sweeney WE, von Vigier RO, Frost P, Avner ED. Src inhibition ameliorates polycystic kidney disease. J Am Soc Nephrol 19: 1331–1341, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Torrance CJ, Jackson PE, Montgomery E, Kinzler KW, Vogelstein B, Wissner A, Nunes M, Frost P, Discafani CM. Combinatorial chemoprevention of intestinal neoplasia. Nat Med 6: 1024–1028, 2000. [DOI] [PubMed] [Google Scholar]
- 43.Tunctan B, Korkmaz B, Buharalioglu CK, Firat SS, Anjaiah S, Falck J, Roman RJ, Malik KU. A 20-hydroxyeicosatetraenoic acid agonist, N-[20-hydroxyeicosa-5(Z),14(Z)-dienoyl]glycine, opposes the fall in blood pressure and vascular reactivity in endotoxin-treated rats. Shock 30: 329–335, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uddin MR, Muthalif MM, Karzoun NA, Benter IF, Malik KU. Cytochrome P-450 metabolites mediate norepinephrine-induced mitogenic signaling. Hypertension 31: 242–247, 1998. [DOI] [PubMed] [Google Scholar]
- 45.Wang W, Lu M. Effect of arachidonic acid on activity of the apical K+ channel in the thick ascending limb of the rat kidney. J Gen Physiol 106: 727–743, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wong KK Recent developments in anti-cancer agents targeting the Ras/Raf/MEK/ERK pathway. Recent Pat Anticancer Drug Discov 4: 28–35, 2009. [DOI] [PubMed] [Google Scholar]
- 47.Zhu T, Gobeil F, Vazquez-Tello A, Leduc M, Rihakova L, Bossolasco M, Bkaily G, Peri K, Varma DR, Orvoine R, Chemtob S. Intracrine signaling through lipid mediators and their cognate nuclear G-protein-coupled receptors: a paradigm based on PGE2, PAF, and LPA1 receptors. Can J Physiol Pharmacol 84: 377–391, 2006. [DOI] [PubMed] [Google Scholar]
- 48.Zou AP, Imig JD, Kaldunski M, Ortiz de Montellano PR, Sui Z, Roman RJ. Inhibition of renal vascular 20-HETE production impairs autoregulation of renal blood flow. Am J Physiol Renal Fluid Electrolyte Physiol 266: F275–F282, 1994. [DOI] [PubMed] [Google Scholar]
- 49.Zou AP, Imig JD, Ortiz de Montellano PR, Sui Z, Falck JR, Roman RJ. Effect of P-450 ω-hydroxylase metabolites of arachidonic acid on tubuloglomerular feedback. Am J Physiol Renal Fluid Electrolyte Physiol 266: F934–F941, 1994. [DOI] [PubMed] [Google Scholar]