Abstract
The intrarenal renin-angiotensin system (RAS) plays a key role in the development of diabetic nephropathy. Recently, we showed that combination therapy with an AT1 receptor blocker (ARB) and an activated vitamin D analog produced excellent synergistic effects against diabetic nephropathy, as a result of blockade of the ARB-induced compensatory renin increase. Given the diversity of vitamin D analogs, here we used a pro-drug vitamin D analog, doxercalciferol (1α-hydroxyvitamin D2), to further test the efficacy of the combination strategy in long-term treatment. Streptozotocin-induced diabetic DBA/2J mice were treated with vehicle, losartan, doxercalciferol (0.4 and 0.6 μg/kg), or losartan and doxercalciferol combinations for 20 wk. Vehicle-treated diabetic mice developed progressive albuminuria and glomerulosclerosis. Losartan alone moderately ameliorated kidney injury, with renin being drastically upregulated. A similar therapeutic effect was seen with doxercalciferol alone, which markedly suppressed renin and angiotensinogen expression. The losartan and doxercalciferol combination most effectively prevented albuminuria, restored glomerular filtration barrier structure, and dramatically reduced glomerulosclerosis in a dose-dependent manner. These effects were accompanied by blockade of intrarenal renin upregulation and ANG II accumulation. These data demonstrate an excellent therapeutic potential for doxercalciferol in diabetic renal disease and confirm the concept that blockade of the compensatory renin increase enhances the efficacy of RAS inhibition and produces synergistic therapeutic effects in combination therapy.
Keywords: renin-angiotensin system, compensatory renin increase, albuminuria, glomerulosclerosis
diabetic nephropathy (DN) is the most common renal complication of diabetes mellitus and a leading cause of end-stage renal disease, accounting for 44% of new cases in 2005 (9). It is well established that the renin-angiotensin system (RAS) is a major mediator of progressive renal injury. Since renal interstitial angiotensin (ANG) II levels are much higher than in the plasma (28), the local RAS in the kidney is believed to play the major damaging role in diabetic nephropathy. Kidney cells, including mesangial cells and podocytes, are able to synthesize all components of the RAS, including renin, the (pro)renin receptor, angiotensinogen (AGT), and ANG II receptors independently of the systemic RAS, making the kidney capable of maintaining a high level of local ANG II. Intrarenal renin and AGT levels are induced in diabetic animals (4, 48). In vitro studies showed that when exposed to high glucose levels, mesangial cells and podocytes increase renin and ANG II production (13, 38, 42). Intrarenal ANG II promotes the progression of renal injury via multiple pathways that increase glomerular permeability, induce oxidative stress, and promote the synthesis of profibrotic and proinflammatory factors and extracellular matrix (8, 15). The consequence of the progression of diabetic renal injury is the development of proteinuria, glomerulosclerosis, and tubulointerstitial fibrosis, which are the pathogenic hallmarks of diabetic nephropathy.
1,25-Dihydroxyvitamin D3 [1,25(OH)2D3], the active hormonal metabolite of vitamin D, has been shown to function as a key negative endocrine regulator of the RAS (24). 1,25(OH)2D3 suppresses renin gene transcription (43). Genetic null mutant mice lacking the vitamin D receptor (VDR) or Cyp27B1, the enzyme catalyzing 1,25(OH)2D3 biosynthesis, develop hyperreninemia, high blood pressure, and cardiac hypertrophy (19, 24, 41, 47). In the diabetic state, VDR knockout mice develop more severe nephropathy than wild-type mice because of more robust activation of the intrarenal RAS (44). Therefore, vitamin D plays an important protective role in the renal and cardiovascular systems via regulation of the RAS. Pharmacologically, low-calcemic vitamin D analogs are able to inhibit renin expression in animals (14, 32) and improve cardiac functions in animal models and humans in part by targeting the cardiac RAS (6). These studies provide a basis for vitamin D analogs to be used as renin synthesis inhibitors to control the RAS for therapeutic purposes.
At present, the first-line treatment of diabetic nephropathy is to inhibit the RAS using ANG-converting enzyme inhibitors (ACEIs), ANG II type 1 receptor blockers (ARBs), and renin enzymatic inhibitors. These drugs have been shown to reduce the progression of glomerulosclerosis, tubulointerstitial fibrosis, and proteinuria in humans (3, 7, 21, 29). However, because of the disruption of the feedback inhibitory loop in renin production, the common problem for all classes of RAS inhibitors, including the new renin enzymatic inhibitor aliskiren (5), is the appearance of a compensatory renin increase after drug use (27). This increase in renin activity stimulates the conversion of ANG I and ultimately ANG II, leading to reduction in the efficacy of RAS inhibition. In addition, the increased renin levels can also act through the (pro)renin receptor and cause renal and cardiovascular damage independently of ANG II. To tackle the problem of a compensatory renin rise, we recently performed a study using an AT1 blocker (losartan) in combination with an activated vitamin D analog (paricalcitol) to treat diabetic nephropathy in diabetic mice. This combination achieved excellent therapeutic results in preventing renal injury as a result of blocking the compensatory renin increase by the vitamin D analog (46). Given the diversity of vitamin D analogs, however, the effectiveness of the combination strategy needed to be verified. In the present study, we used a pro-drug vitamin D analog, doxercalciferol (1α-hydroxyvitamin D2), to reexamine the therapeutic efficacy of the combination strategy in a type 1 diabetes model. In contrast to paricalcitol (19-nor-1,25-dihydroxyvitamin D2), doxercalciferol is a pro-drug that is activated by the hepatic 25-hydroxylase in the body. Here, we report that doxercalciferol has excellent therapeutic potential against renal injury either alone or in combination with an ARB.
EXPERIMENTAL DESIGNS AND METHODS
Animals and treatment.
DBA/2J mice (Jackson Laboratory, Bar Harbor, ME) were fed the 2018 Teklad rodent diet containing 1.5 IU/g vitamin D3 and 38 μg/g cholecalciferol and exposed to a 12:12-h light-dark cycle. Eight-week-old male DBA/2J mice were made diabetic by intraperitoneal (ip) injection of freshly prepared streptozotocin (STZ; dissolved in 10 mM citrate buffer, pH 4.2), which was given at a dose of 35 mg·kg−1·day−1 for 5 consecutive days. Three weeks after STZ injection (week 0), the mice were randomly separated into six groups and treated, respectively, with vehicle (V; ip injection, 3× per week); doxercalciferol at 0.4 (DL; low dose) and 0.6 (DH; high dose) μg/kg (dissolved in propylene glycol:H2O = 80:20, ip injection, 3× per week); losartan (L; dissolved in drinking water at 0.025 mg/ml); or a combination of losartan and doxercalciferol at the two doses (L+DL and L+DH). Control mice (C) were nondiabetic mice without STZ or any drug treatment. Blood glucose levels were monitored with the CONTOUR blood glucose-monitoring system (Bayer). Spot urine was collected at various time points, and urinary albumin and creatinine levels were determined using commercial kits as reported previously (44). All mice were killed at 23 wk after the start of STZ treatment, and serum and kidneys were immediately harvested for a variety of biochemical, histological, and molecular analyses. In an additional experiment, animals were killed at 7 wk after STZ injection. The animal study protocols were approved by the Institutional Animal Care and Use Committee at The University of Chicago.
Western blotting.
Kidneys were homogenized in Laemmli buffer, followed by 5 min of boiling and centrifugation to obtain the supernatant. Protein concentrations were determined using a Bio-Rad Protein Assay kit. Proteins were separated by SDS-PAGE and transferred onto Immobilon membranes. Western blotting was carried out as previously described (23).
RT-PCR.
Total cellular RNAs were isolated using TRIzol reagents (Invitrogen, Carlsbad, CA). First-strand cDNAs were synthesized from 2 μg of total RNAs in a 20-μl reaction using Moloney murine leukemia virus reverse transcriptase and hexanucleotide random primers. The first-strand cDNAs served as the template for the PCR performed using a Bio-Rad DNA Engine (Bio-Rad). Real-time RT-PCR was performed with the Applied Biosystems 7900 Real Time PCR System using a SYBR green PCR reagent kit (Applied Biosystems, Foster City, CA) as described previously (44). GAPDH and β-2 microglobulin (B2M) served as the internal controls. The PCR primers used in this study were as described previously (44, 46).
Histology and immunostaining.
Freshly dissected kidneys were fixed overnight with 4% formaldehyde in PBS (pH 7.2), processed, embedded in paraffin, and cut into 4-μm sections. Renal sections were stained with periodic acid-Schiff (PAS). Semiquantitative scoring of glomerular sclerosis was performed using a five-grade method described previously (36, 44). For immunostaining, sections were first boiled in 10 mM Na citrate solution (pH 6.0) for 10 min to retrieve the antigens before antibody staining. The sections were stained with primary antibodies [against MOMA (Chemicon), WT1, and ANG I/ANG II (Santa Cruz Biotechnology), or fibronectin (FN; Sigma)], followed by incubation with horseradish peroxidase-conjugated or Cy3-conjugated second antibody. Antigens were visualized with a substrate diaminobenzidine kit (Vector Laboratories, Burlingame, CA) or with a fluorescence microscope.
Electron microscopy.
Tissue blocks of kidney cortex were fixed in 2% glutaraldehyde in 0.1 M phosphate buffer, pH 7.4, and then postfixed in 1% osmium tetroxide. Electron microscopic analyses and measurement of glomerular basement membrane (GBM) thickness were performed as described previously (44).
Statistical analysis.
Data values are presented as means ± SE. Statistical comparisons were made using Student's t-test, with P < 0.05 being considered significant.
RESULTS
Combination therapy prevents albuminuria.
We used the STZ-induced type 1 diabetic model to evaluate the therapeutic capacity of doxercalciferol on diabetic nephropathy. Male mice in the DBA/2J background were used as they are susceptible to hyperglycemia-induced renal injury (31). Three weeks after STZ treatment (week 0), we randomized the mice into six groups (n = 6–7/group; see Animals and treatment) and treated them, respectively, with vehicle, losartan, a low dose (0.4 μg/kg) and a high dose (0.6 μg/kg) of doxercalciferol, or a losartan and doxercalciferol combination at these two doses for 20 wk, with nondiabetic mice receiving no treatment serving as the control. Unexpectedly, two to three weeks into the drug treatment, all mice in the DH group died without a clear cause. However, all the other groups of mice, including the L+DH group, survived well to the end of the treatment at 23 wk without apparent health problems. Repeated experiments using exactly the same experimental condition with the 0.6 μg/kg dose indicated that the animal mortality seen in the study was likely an accident unrelated to doxercalciferol treatment (see below).
As shown in Table 1, blood glucose levels in all diabetic groups rose to ∼400 mg/dl within 3 wk of STZ treatment and continued to increase with time to >600 mg/dl, and all drug treatments had no effects on the blood glucose levels in these mice (Table 1). Therefore, the effect resulting from the drug treatment is unlikely to be mediated through targeting the pancreas or by reducing hyperglycemia.
Table 1.
Blood glucose levels during the treatment period
|
Blood Glucose, mg/dl |
|||||||
|---|---|---|---|---|---|---|---|
| Treatment | Week −3 | Week 0 | Week 4 | Week 8 | Week 12 | Week 16 | Week 20 |
| Control (n = 6) | 127±5 | 130±4 | 111±9 | 133±8 | 101±10 | 123±6 | 113±4 |
| Vehicle (n = 6) | 126±9 | 446±41 | 541±24 | >600 | >600 | >600 | >600 |
| Losartan (n = 7) | 128±9 | 439±44 | 546±38 | >600 | >600 | >600 | >600 |
| DL (n = 7) | 127±6 | 409±54 | 508±56 | >600 | >600 | >600 | >600 |
| L+DL (n = 7) | 126±3 | 391±34 | 527±45 | >600 | >600 | >600 | >600 |
| DH (n = 7) | 125±8 | 409±38 | All died | ||||
| L+DH (n = 7) | 126±7 | 399±45 | 461±46 | 543±58 | >600 | >600 | >600 |
Values are means ± SE; n = no. of mice. DL and DH, doxercalciferol at 0.4 and 0.6 μg/kg; L+DL and L+DH, losartan+doxercalciferol at the 2 doses, respectively; control, nondiabetic.
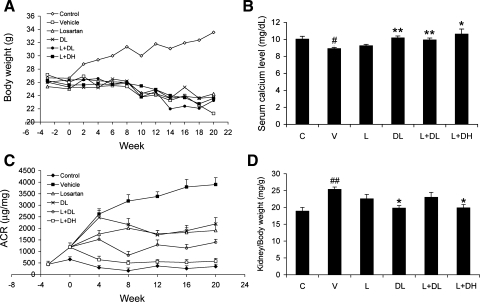
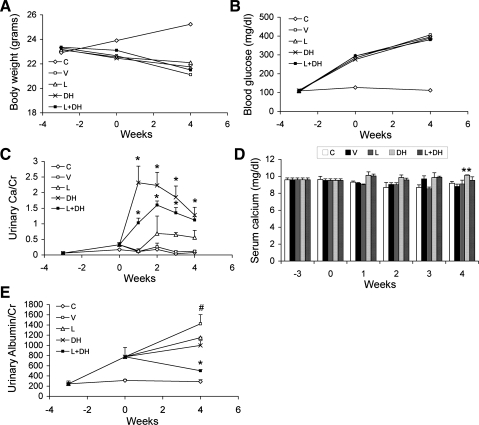
As shown in Fig. 1, in contrast to the nondiabetic mice, all STZ-treated diabetic mice failed to gain weight during the 23-wk treatment period (Fig. 1A). Although DL and the two combinations slightly raised serum calcium levels in these mice, the levels still fell within the physiological range and were not significantly different from nondiabetic control mice (Fig. 1B), consistent with the low-calcemic nature of doxercalciferol. Serum 25-hydroxyvitamin D levels were similar in all groups of mice and were in the range of 100–120 nmol/l, except in the L+DH group, which was ∼150 nmol/l. As expected, the V-treated diabetic mice developed time-dependent progressive albuminuria, and the urinary albumin-to-creatinine ratio (ACR) increased more than sevenfold over baseline at week 20 (Fig. 1C). L and DL alone had similar inhibitory effects on the development of albuminuria; both moderately prevented the progression of the ACR increase. The most striking effect on the prevention of albuminuria was seen with the combination therapy, and the effect was dose dependent. While L+DL showed better effect than L or DL alone, it was L+DH cotreatment that almost completely prevented the development of albuminuria, with the ACR of the diabetic mice being maintained at a level similar to that of nondiabetic mice during the entire treatment period (Fig. 1C).
Fig. 1.
Effect of drug treatments on body weight, serum calcium, albuminuria, and kidney mass in streptozotocin (STZ)-induced diabetic mice. Eight-week-old male DBA/2J mice were injected intraperitoneally with 35 mg/kg STZ for 5 consecutive days in the first week (week −3). Three weeks after STZ injection (week 0), the mice were treated with vehicle (V), losartan (L; 0.025 mg/ml), doxercalciferol (DL; 0.4 μg/kg), losartan and doxercalciferol at 0.4 μg/kg (L+DL), or losartan and doxercalciferol at 0.6 μg/kg (L+DH). Changes in body weight and urinary albumin levels were monitored for the following 20 wk. All mice were killed at week 20. Control (C) mice are nondiabetic mice without STZ injection or any drug treatment. A: body weight curves. B: serum calcium at the end of the treatments. C: changes in urinary albumin-to-creatinine ratio (ACR) over time. D: kidney mass-to-body weight ratio at the end of the treatments. Note the dramatic inhibition of albuminuria in the L+DL and L+DH groups. *P < 0.05, **P < 0.01 vs. V. #P < 0.05, ##P < 0.01 vs. C; n = 6–7.
The kidney is known to compensate for reduced renal function by increasing its mass. In diabetic mice, chronic hyperglycemia leads to renal hypertrophy. As shown in Fig. 1D, V-treated diabetic mice had significantly greater kidney mass than the nondiabetic controls; the drug treatments, particularly DL and L+DH, reduced or normalized the kidney size of the diabetic mice almost to the control level (Fig. 1D).
Combination therapy prevents podocyte loss and restores the glomerular filtration barrier.
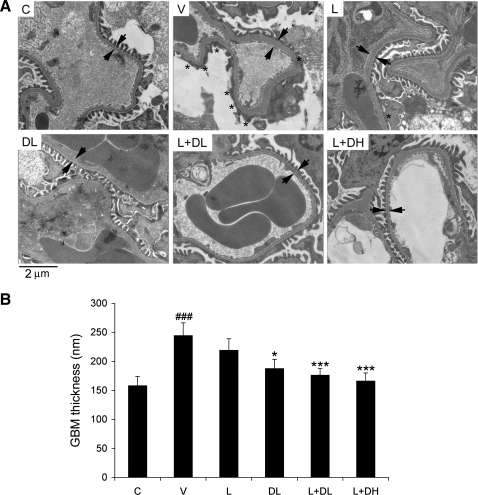
To explore the mechanism underlying the prevention of albuminuria, we examined the structure of the glomerular filtration barrier by electron microscopy. Nondiabetic control mice showed normal morphology in endothelial cells, GBM, and podocyte foot processes (Fig. 2A). In V-treated diabetic mice, chronic hyperglycemia caused a marked increase in the thickness of GBM and severe foot process effacement (Fig. 2A), consistent with the dramatic albuminuria seen in these mice. A moderate proliferation of the endothelial cells was also seen in these mice. Treatment with L or DL alone moderately reduced the increase in GBM thickness and foot process effacement (Fig. 2A); however, L+DL and L+DH treatment prevented the thickening of GBM and podocyte effacement and restored the glomerular filtration barrier structure (Fig. 2A). These observations were confirmed by quantification of GBM thickness in these mice on different treatments (Fig. 2B).
Fig. 2.
Transmission electron microscopy of the glomerular filtration barrier. A: kidney samples from nondiabetic control mice (C) and diabetic mice treated with V, L, DL, L+DL, or L+DH were subject to electron microscopic analyses. Shown is the structure of the glomerular filtration barrier. Stars indicate the areas of podocyte foot process effacement; arrow pairs point to both sides of the glomerular basement membrane (GBM). B: quantification of the thickness of the GBM from each group of mice as indicated. *P < 0.05, ***P < 0.001 vs. V. ###P < 0.001 vs. C; n = 3.
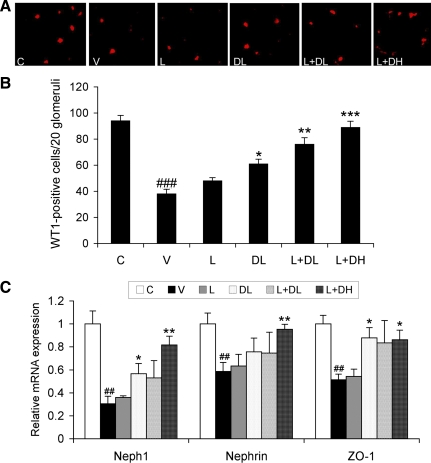
Diabetic nephropathy is known to be associated with loss of podocytes (40), which play a key role in the regulation of glomerular filtration. Immunostaining of the kidney sections with a podocyte-specific anti-WT1 antibody confirmed that in V-treated diabetic mice, WT1-positive cell number was markedly decreased in the glomerulus compared with the control (Fig. 3, A and B). Interestingly, the drug treatments, particularly with L+DL and L+DH, significantly prevented the reduction of WT1-positive cells in the diabetic mice (Fig. 3, A and B).
Fig. 3.
Podocyte number and expression of slit diaphragm proteins. A: immunostaining of podocytes. Kidney sections from nondiabetic control mice (C) and diabetic mice treated with V, L, DL, L+DL, or L+DH at 20 wk were stained with podocyte-specific anti-WT1 antibody. Shown are representatives of a single glomerulus from each group of mice as indicated. B: glomerular podocyte number. WT1-positive podocytes were counted in 20 glomeruli randomly selected from the immunostained sections of each group of mice. C: PCR quantitation of slit diaphragm proteins. Total RNAs were extracted from kidneys of the 6 groups of mice as indicated. The mRNA level of Neph-1, nephrin, and ZO-1 was determined by real-time RT-PCR. *P < 0.05, **P < 0.01, ***P < 0.001 vs. V. ##P < 0.01, ###P < 0.001 vs. C.
The slit diaphragm is the key structure that controls protein filtration through the glomerular filtration barrier (18). We quantified the expression of the key structural proteins forming the slit diaphragm, including α-actinin-4, CD2AP, FAT-1, ZO-1, nephrin, Neph-1, and podocin, by real-time RT-PCR. Compared with the nondiabetic control, the mRNA levels of all these proteins were reduced in V-treated diabetic mice. Although all drug treatments had little effect on the decline of CD2AP, FAT-1, and podocin, the decline of Neph-1, nephrin, and ZO-1 was prevented by DL, L+DL, and L+DH to different extents (Fig. 3C). L+DH therapy almost completely restored the expression of these proteins, consistent with the magnitude of its effect on albuminuria.
Combination therapy inhibits glomerulosclerosis.
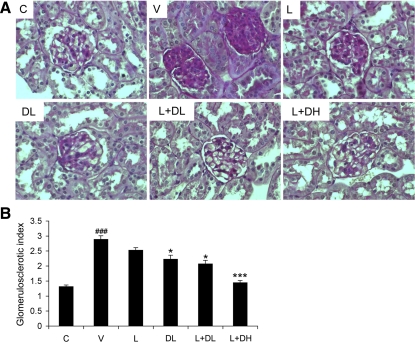
Glomerular sclerosis is another important feature of hyperglycemia-induced renal injury. We used PAS staining and semiquantitative scoring to assess glomerulosclerosis in the kidney. Compared with the control mice, V-treated diabetic mice showed marked glomerulosclerosis at week 20, with mesangial expansion and clearly increased accumulation of extracellular matrix (ECM) in the mesangium (Fig. 4A). Glomerulosclerosis was reduced by L or DL treatment alone, but L+DL and L+DH treatments had an even better inhibitory effect (Fig. 4A). A semiquantitative glomerulosclerotic index of kidney sections confirmed the histological data. V-treated mice showed the highest score, and all drug treatments led to a reduction in the index, with L+DL and L+DH showing the best prevention in a dose-dependent manner (Fig. 4B).
Fig. 4.
Effect of drug treatments on glomerulosclerosis. All mice were killed at week 20, and kidneys were harvested for histological analyses. All sections were stained with periodic acid-Schiff (PAS). A: representative glomerular morphology of nondiabetic control (C) and diabetic mice treated with V, L, DL, L+DL, or L+DH as indicated. Note the severe glomerular sclerosis in vehicle-treated kidneys and the normalization after the cotreatment. B: semiquantitative glomerulosclerotic score for all groups of mice as indicated. *P < 0.05, ***P < 0.001 vs. V. ###P < 0.001 vs. C.
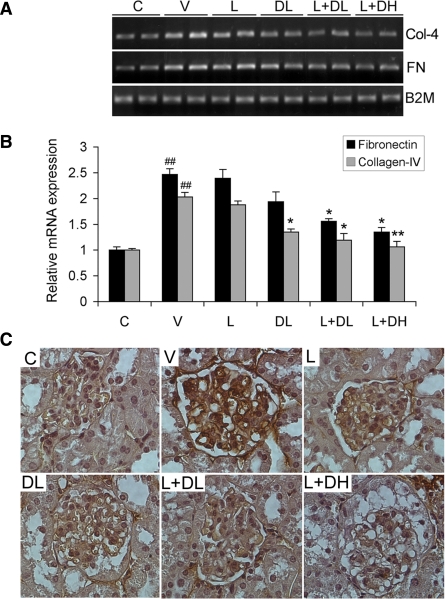
We further examined the extent of glomerulosclerosis by determining the expression of FN and collagen IV, two major ECM proteins, in the kidney by RT-PCR and immunostaining analyses. As shown in Fig. 5, V-treated mice showed marked increases in FN and collagen IV expression at the mRNA levels relative to the nondiabetic control (Fig. 5, A and B), and immunostaining revealed marked overproduction of FN in the glomerulus (Fig. 5C). The drug treatments, particularly DL, L+DL, and L+DH, prevented the upregulation of FN and collagen IV in the diabetic mice (Fig. 5, A and B). These treatments also clearly reduced FN in the glomerulus, as shown by immunostaining (Fig. 5C). These data are consistent with the antisclerotic effect of these drugs.
Fig. 5.
Expression of fibronectin (FN) and collagen IV. A: RT-PCR determination of FN and collagen IV (Col-4) mRNA expression in the kidney of all treatment groups as indicated. B2M, β-2 microglobulin. B: densitometric quantification of FN and Col-4 mRNA levels based on the RT-PCR data. C: glomerular immunostaining of FN. Kidney sections from nondiabetic control (C) and diabetic mice treated with V, L, DL, L+DL, or L+DH were immunostained with anti-FN antibody. *P < 0.05, **P < 0.01 vs. V. ##P < 0.01 vs. C; n = 6–7.
Combination therapy inhibits profibrotic and proinflammatory factors and macrophage infiltration.
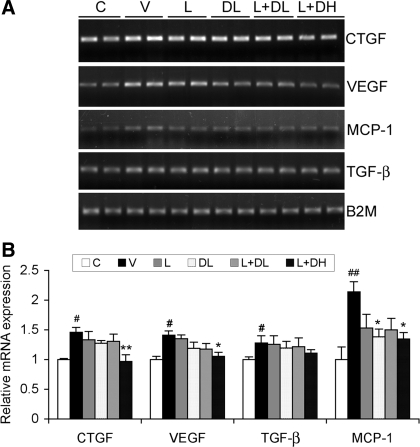
The downstream effectors of ANG II that mediate renal injury include profibrotic and proinflammatory cytokines. To determine the effect of the treatments on these factors, we measured the expression of connective tissue growth factor (CTGF), vascular endothelial growth factor (VEGF), transforming growth factor-β (TGF-β), and monocyte chemotactic protein-1 (MCP-1) in the kidney, which are known to be involved in development of diabetic renal damage. As shown in Fig. 6, in V-treated diabetic mice CTGF, VEGF, TGF-β, and MCP-1 were all increased as expected compared with the nondiabetic control. The induction of these factors was suppressed by the treatments to different extents, with L+DH showing the best inhibition (Fig. 6, A and B).
Fig. 6.
Expression of profibrotic and proinflammatory cytokines in the kidney. Total RNAs were extracted from kidneys of nondiabetic control mice (C) and diabetic mice treated with V, L, DL, L+DL, or L+DH as indicated. The mice were killed at week 20. A: RT-PCR analysis of connective tissue growth factor (CTGF), vascular endothelial growth factor (VEGF), monocyte chemotactic protein-1 (MCP-1), and transforming growth factor-β (TGF-β) expression using gene-specific primers. B: quantitative results of the RT-PCR data. *P < 0.05, **P < 0.01 vs. V. #P < 0.05, ##P < 0.01 vs. C; n = 6–7.
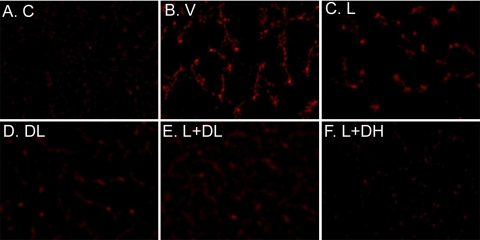
MCP-1-promoted monocyte/macrophage infiltration is well known to play an important role in diabetic renal injury (33). Therefore, we measured macrophage infiltration in the kidney by immunostaining using a macrophage-specific anti-MOMA antibody. Consistent with the expression of MCP-1, prominent macrophage recruitment was detected in the kidney of V-treated diabetic mice (Fig. 7B) relative to the control (Fig. 7A). Macrophage infiltration was reduced in different degrees in mice treated with L (Fig. 7C), DL (Fig. 7D), L+DL (Fig. 7E), and L+DH (Fig. 7F), with L+DH showing the most prominent inhibition. These results are well correlated with the expression profile of MCP-1 in these mice.
Fig. 7.
Effect of drug treatments on macrophage infiltration into the kidney. Nondiabetic mice (A) and diabetic mice treated with V (B), L (C), DL (D), L+DL (E), or L+DH (F) were killed at week 20, and kidney sections were stained with a macrophage-specific monoclonal anti-MOMA antibody.
Combination therapy synergistically inhibits the RAS.
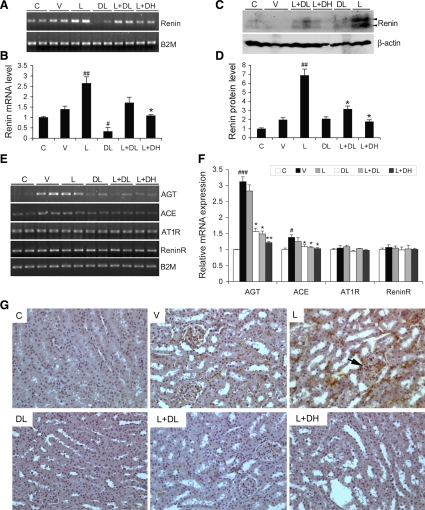
The study was designed based on the hypothesis that combination treatment would synergistically inhibit the RAS by blocking the compensatory renin increase; therefore, we evaluated the RAS in the kidney at week 20. As shown in Fig. 8, intrarenal renin was dramatically induced at both the mRNA (Fig. 8, A and B) and protein levels (Fig. 8, C and D) in mice treated with L, due to disruption of the feedback loop. DL treatment markedly suppressed renin expression (Fig. 8, A–D). Compared with L treatment, the L+DL or L+DH combination effectively blocked the induction of renin in a dose-dependent manner, with L+DH reducing renin almost to the control level (Fig. 8, A–D). We also checked the other components of the RAS. The renal expression of AGT, and to a lesser extent ACE, was significantly induced by hyperglycemia in V-treated mice, and the induction was not affected by L-treatment (Fig. 8, E and F). However, the induction of AGT and ACE was effectively prevented by DL, L+DL, or L+DH treatment (Fig. 8, E and F). On the other hand, the levels of (pro)renin receptor and AT1 receptor were not changed by either hyperglycemia or any of the treatments in diabetic mice relative to the nondiabetic control (Fig. 8, E and F).
Fig. 8.
Effect of drug treatments on the renin-angiotensin system (RAS) in the kidney. Total RNA or kidney lysates were obtained from nondiabetic mice (C) and diabetic mice treated with V, L, DL, L+DL, or L+DH at week 20. A and B: RT-PCR analysis (A) and quantification (B) of renin mRNA levels in the kidney of all mice. C and D: Western blot analyses (C) and quantification (D) of renin protein levels in the kidney of all mice as indicated. #P < 0.05, ##P < 0.01 vs. C. *P < 0.05 vs. L. E and F: RT-PCR determination of mRNA levels (E) and quantification (F) of angiotensinogen (AGT), angiotensin-converting enzyme (ACE), the (pro)renin receptor (ReninR), and AT1 receptor (AT1R) in the kidney. B2M served as an internal control. *P < 0.05, **P < 0.01 vs. L. #P < 0.05, ##P < 0.01 vs. C; n = 6–7. G: intrarenal ANG II accumulation determined by immunostaining. Nondiabetic mice (C) and diabetic mice treated with V, L, DL, L+DL, or L+DH were killed at week 20, and kidney sections from these mice were stained with an ANG I/II-specific antibody. Note strong staining in both the glomerular (indicated by an arrow) and tubular areas in L-treated kidney.
We further examined the levels of ANG II, the effector of the RAS that mediates renal damage, in the kidneys. Consistent with the expression of renin and AGT, immunostaining showed that intrarenal levels of ANG II were increased in V-treated mice (Fig. 8G) compared with the control, and the increase was even more dramatic in mice treated with L (Fig. 8G), as a consequence of a dramatic increase in both renin and AGT (Fig. 8, A, C, and E). Strong ANG II immunostaining was seen in both the glomerular and tubular areas. As expected, the ANG II accumulation in the kidney was basically blocked in mice treated with L+DL or L+DH (Fig. 8G). These data confirm that the combination strategy synergistically blocks the RAS in the kidney, as a result of the blockade of the compensatory renin increase. We hypothesize that this is the basis for the much better therapeutic effect seen with combination therapy.
Because the diabetic mice treated with the high-dose doxercalciferol (0.6 μg/kg) died unexpectedly without a clear cause, we repeated the experiment under exactly the same conditions, using the same doxercalciferol stock, to investigate whether this incident was related to doxercalciferol treatment. Again, 3 wk after STZ treatment (week 0), diabetic mice were treated with V, L, DH (0.6 μg/kg), or L+DH (n = 6–7/group), and this time none of the mice died. Since mice died within 2–3 wk of treatment in the first study, in the new experiment we monitored blood and urinary parameters weekly for the first 4 wk of treatment. As shown in Fig. 9, in contrast to nondiabetic control mice, all diabetic mice failed to gain weight (Fig. 9A) and developed the same degree of hyperglycemia (blood glucose ∼400 mg/dl) (Fig. 9B). Mice receiving doxercalciferol treatment (DH and L+DH groups) had marked increases in urinary calcium excretion (Fig. 9C). However, the serum calcium levels in these mice were maintained within the normal physiological range and were not significantly increased relative to nondiabetic control animals (Fig. 9D). Importantly, in contrast to the progressive albuminuria seen in V-treated mice, mice treated with L, DH, or L+DH showed different degrees of reduction in albuminuria, with a marked synergistic effect seen in the L+DH-treated mice (Fig. 9E). Therefore, this short-term study confirmed the findings of the first study and indicates that the animal mortality seen in the first study was an accident unrelated to doxercalciferol treatment.
Fig. 9.
New experiment to test the effect of high-dose (0.6 μg/kg) doxercalciferol. Two weeks after STZ treatment (week 0), diabetic mice were randomized (n = 6–7/group) and treated with V, L, DH, or L+DH, respectively. Control (C) mice were nondiabetic. The blood and urinary parameters were monitored weekly for 4 wk and plotted over time. A: growth curve. B: blood glucose levels. C: urinary calcium (Ca) excretion normalized to urinary creatinine (Cr) levels. D: serum calcium levels. E: urinary albumin levels normalized to Cr (ACR). *P < 0.05, **P < 0.01 vs. V. #P < 0.05 vs. C; n = 6–7.
DISCUSSION
Recent studies have provided good evidence that vitamin D protects the kidney through suppressing the RAS (22), as 1,25(OH)2D3 downregulates renin gene transcription (24, 43). Given that the efficacy of the classic RAS inhibitors is often compromised by the compensatory increase in renin production, recently we developed a strategy to block the compensatory renin increase at the transcriptional level with an activated vitamin D analog used in combination with an ARB. The combination strategy produced excellent therapeutic synergism in the prevention of renal injury in an experimental diabetes model (46). The present study is a more comprehensive analysis of this therapeutic strategy using the vitamin D analog doxercalciferol, which was given at two doses for a longer treatment period. The advantage of this design is that it allows evaluation of dose-responsive effects of this pro-drug following a 20-wk treatment period vs. the previous study that was of a shorter duration (10 wk). Since diabetic nephropathy is a chronic kidney disorder, this long-term study allow for the development of prominent renal injury so that the therapeutic efficacy of the different treatments can be better evaluated. The comprehensive examination performed in this study of the RAS, including cytokine profiles and inflammatory status, defines the therapeutic properties of doxercalciferol in a diabetic setting.
The data show that long-term treatment with either doxercalciferol or losartan alone was able to attenuate diabetic renal injury. These two compounds target different steps of the RAS, namely, renin transcription and the AT1 receptor. Interestingly, in most parameters examined in this study, low-dose doxercalciferol had equal or better therapeutic efficacy compared with losartan; thus the therapeutic potential of doxercalciferol is at least comparable to that of losartan in renoprotection. Consistent with this finding, another vitamin D analog has been reported to reduce proteinuria in patients with chronic kidney disease (1). However, the comparable efficacy of vitamin D analogs vs. RAS inhibitors in monotherapy in human patients remains to be established. Long-term (20 wk) treatment with the low dose (0.4 μg/kg) of doxercalciferol had no apparent adverse effects on the overall health of the animals, as they survived well to the end of the study. Although diabetic mice receiving the high dose (0.6 μg/kg) of doxercalciferol alone unexpectedly died prematurely in the first experiment, this phenomenon was not seen in a repeated experiment, indicating that the occurrence was an isolated incident that was unrelated to doxercalciferol treatment. Importantly, the second short-term experiment basically confirmed the data obtained from the first experiment. It is worth noting that at both dose levels, doxercalciferol had no significant effects on the serum calcium status in the animals.
The combination of losartan and doxercalciferol had much better therapeutic effects against renal injury than doxercalciferol or losartan alone. The cotreatment dose dependently prevented albuminuria and glomerulosclerosis, which are the main pathological hallmarks of diabetic nephropathy. The development of albuminuria in the vehicle-treated diabetic mice was accompanied by severe structural distortion in the glomerular filtration barrier, GBM thickening, damage to podocyte foot processes, decrease in podocyte number, and decline in slit diaphragm protein expression. Most of these pathological changes were prevented by the combination therapy. The severe glomerulosclerosis seen in the diabetic mice was manifested by increased production of ECM proteins in the mesangium. Renal damage was also associated with a marked increase in profibrotic and proinflammatory factors and macrophage infiltration. Morphological and molecular changes in diabetic kidneys were virtually prevented by the cotreatment with losartan and doxercalciferol. Of note was that the protective effects on renal function were independent of glycemic control, indicating a direct effect on the kidney rather than affecting pancreatic insulin production or glucose metabolism.
Our study demonstrates that the molecular basis for the synergistic effect of combination strategy is effective suppression of the intrarenal RAS, the major mediator of diabetic renal injury. The dramatic induction of renin and ANG II in the kidney by losartan offsets its own therapeutic efficacy, but the induction of both was markedly suppressed in a dose-dependent manner when doxercalciferol was used together with losartan. Another key factor that contributes to ANG II accumulation is the induction of AGT in the kidney by hyperglycemia, and our data showed that doxercalciferol also blocked hyperglycemia-induced AGT expression. This clearly is part of the mechanism for the suppression of the intrarenal RAS. Our most recent data demonstrate that induction of AGT by high glucose in mesangial cells and podocytes is mediated by NF-κB, and the activation of NF-κB is inhibited by 1,25(OH)2D3 (12). Additionally, previous studies have also shown that 1,25(OH)2D3 or its analog suppresses renal proinflammatory cytokines by blocking NF-κB activity (35, 45).
The VDR is a ligand-activated transcription factor, through which 1,25(OH)2D3 regulates a large number of genes (16). Given the involvement of multiple gene products in the development of diabetic nephropathy, it is expected that vitamin D analogs or the combination therapy targets not only the RAS in therapeutic actions. Since ANG II is involved in additional mechanisms leading to renal injury (8), treatment may indirectly affect many genes, which leads to improvements in renal function. For example, TGF-β, a major profibrotic growth factor, is a downstream target of ANG II (49) and also upregulated by renin in mesangial cells through the (pro)renin receptor independently of ANG II (17). Both events are indirectly affected by vitamin D. However, MCP-1, a chemokine that promotes macrophage infiltration, is directly upregulated by ANG II (10) as well as downregulated by 1,25(OH)2D3 via NF-κB (45). Therefore, the amelioration of diabetic nephropathy is achieved likely through a complex network of regulatory events initiated by the treatment.
Mesangial cells and podocytes are known to play key roles in the development of diabetic nephropathy, and data from this study indicate that doxercalciferol targets both cell types. This conclusion is supported by early in vitro findings. Both mesangial cells and podocytes express the VDR, which is highly induced by 1,25(OH)2D3 (39, 45). We have shown that ECM proteins (FN, collagen IV) induced in mesangial cells and podocytes by high glucose are suppressed in the presence of 1,25(OH)2D3 (39, 45), whereas the slit diaphragm proteins such as nephrin are upregulated by 1,25(OH)2D3 in podocyte culture (34, 44). A variety of cytokines produced in mesangial cells and podocytes, such as TGF-β and MCP-1, are downregulated by 1,25(OH)2D3 (45, 46). The combination treatment reduced podocyte loss in the current animal model, consistent with a similar finding in a uremia model (20). Overall, the vitamin D analog most likely targets the local RAS in both mesangial cells and podocytes (25, 38, 42, 44) to ameliorate diabetic injury.
In both type 1 and type 2 diabetes, reduction of albuminuria is considered as a major target for renoprotective therapy, because it is a major indicator of a progressive decline of renal function in diabetic nephropathy (30) and thought to be the first step toward an inevitable progression of renal failure (26, 37). In a large cohort cross-sectional analysis of data from the Third National Health and Nutrition Examination Survey, vitamin D insufficiency was found to be associated with the increased prevalence of albuminuria (11), suggesting that vitamin D has an intrinsic antiproteinuric property. Pharmacologically, the antiproteinuric activity of the vitamin D analog has been reported in a retrospective analysis of patients with chronic kidney disease, and the beneficial effect of the vitamin D analog was seen even in subjects already treated with ACEI or ARB, indicating that vitamin D has additive effects to ACEI/ARB in decreasing proteinuria (1). More recently, a randomized double-blinded pilot trial with 24 patients with stage 2–3 chronic kidney disease reported significantly reduced albuminuria and inflammation status in subjects treated with paricaicitol for 1 mo, and these effects were independent of its effects on hemodynamics and parathyroid hormone suppression (2). In both the present and our previous studies with this experimental diabetes model (46), a complete prevention of albuminuria is the most dramatic effect of the combination therapy. Therefore, the synergistic therapy with vitamin D analogs and classic RAS inhibitors is worth further exploration with randomized controlled clinical trials in diabetic kidney disease.
GRANTS
This work was supported by a research grant from the Genzyme Corp. and National Heart, Lung, and Blood Institute Grant HL-085793.
DISCLOSURES
C. Arbeeny, S. Strugnell, and Y. Sabbagh are employees of the Genzyme Corp.
REFERENCES
- 1.Agarwal R, Acharya M, Tian J, Hippensteel RL, Melnick JZ, Qiu P, Williams L, Batlle D. Antiproteinuric effect of oral paricalcitol in chronic kidney disease. Kidney Int 68: 2823–2828, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Alborzi P, Patel NA, Peterson C, Bills JE, Bekele DM, Bunaye Z, Light RP, Agarwal R. Paricalcitol reduces albuminuria and inflammation in chronic kidney disease: a randomized double-blind pilot trial. Hypertension 52: 249–255, 2008. [DOI] [PubMed] [Google Scholar]
- 3.Andersen S, Tarnow L, Rossing P, Hansen BV, Parving HH. Renoprotective effects of angiotensin II receptor blockade in type 1 diabetic patients with diabetic nephropathy. Kidney Int 57: 601–606, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Anderson S, Jung FF, Ingelfinger JR. Renal renin-angiotensin system in diabetes: functional, immunohistochemical, and molecular biological correlations. Am J Physiol Renal Fluid Electrolyte Physiol 265: F477–F486, 1993. [DOI] [PubMed] [Google Scholar]
- 5.Azizi M, Menard J, Bissery A, Guyenne TT, Bura-Riviere A, Vaidyanathan S, Camisasca RP. Pharmacologic demonstration of the synergistic effects of a combination of the renin inhibitor aliskiren and the AT1 receptor antagonist valsartan on the angiotensin II-renin feedback interruption. J Am Soc Nephrol 15: 3126–3133, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Bodyak N, Ayus JC, Achinger S, Shivalingappa V, Ke Q, Chen YS, Rigor DL, Stillman I, Tamez H, Kroeger PE, Wu-Wong RR, Karumanchi SA, Thadhani R, Kang PM. Activated vitamin D attenuates left ventricular abnormalities induced by dietary sodium in Dahl salt-sensitive animals. Proc Natl Acad Sci USA 104: 16810–16815, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 345: 861–869, 2001. [DOI] [PubMed] [Google Scholar]
- 8.Carey RM, Siragy HM. The intrarenal renin-angiotensin system and diabetic nephropathy. Trends Endocrinol Metab 14: 274–281, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control. Centers for Disease Control and Prevention, National Diabetes Fact Sheet, 2007, http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2007.pdf.
- 10.Chen XL, Tummala PE, Olbrych MT, Alexander RW, Medford RM. Angiotensin II induces monocyte chemoattractant protein-1 gene expression in rat vascular smooth muscle cells. Circ Res 83: 952–959, 1998. [DOI] [PubMed] [Google Scholar]
- 11.de Boer IH, Ioannou GN, Kestenbaum B, Brunzell JD, Weiss NS. 25-Hydroxyvitamin D levels and albuminuria in the Third National Health and Nutrition Examination Survey (NHANES III). Am J Kidney Dis 50: 69–77, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Deb DK, Chen Y, Zhang Z, Zhang Y, Szeto FL, Wong KE, Kong J, Li YC. 1,25-Dihydroxyvitamin D3 suppresses high glucose-induced angiotensinogen expression in kidney cells by blocking the NF-κB pathway. Am J Physiol Renal Physiol 296: F1212–F1218, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Durvasula RV, Shankland SJ. Activation of a local renin angiotensin system in podocytes by glucose. Am J Physiol Renal Physiol 294: F830–F839, 2008. [DOI] [PubMed] [Google Scholar]
- 14.Fryer RM, Rakestraw PA, Nakane M, Dixon D, Banfor PN, Koch KA, Wu-Wong JR, Reinhart GA. Differential inhibition of renin mRNA expression by paricalcitol and calcitriol in C57/BL6 mice. Nephron Physiol 106: 76–81, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Gilbert RE, Krum H, Wilkinson-Berka J, Kelly DJ. The renin-angiotensin system and the long-term complications of diabetes: pathophysiological and therapeutic considerations. Diabet Med 20: 607–621, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Haussler MR, Whitfield GK, Haussler CA, Hsieh JC, Thompson PD, Selznick SH, Dominguez CE, Jurutka PW. The nuclear vitamin D receptor: biological and molecular regulatory properties revealed. J Bone Miner Res 13: 325–349, 1998. [DOI] [PubMed] [Google Scholar]
- 17.Huang Y, Wongamorntham S, Kasting J, McQuillan D, Owens RT, Yu L, Noble NA, Border W. Renin increases mesangial cell transforming growth factor-beta1 and matrix proteins through receptor-mediated, angiotensin II-independent mechanisms. Kidney Int 69: 105–113, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Kawachi H, Miyauchi N, Suzuki K, Han GD, Orikasa M, Shimizu F. Role of podocyte slit diaphragm as a filtration barrier. Nephrology (Carlton) 11: 274–281, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Kong J, Li YC. Effect of angiotensin II type I receptor antagonist and angiotensin-converting enzyme inhibitor on vitamin D receptor null mice. Am J Physiol Regul Integr Comp Physiol 285: R255–R261, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Kuhlmann A, Haas CS, Gross ML, Reulbach U, Holzinger M, Schwarz U, Ritz E, Amann K. 1,25-Dihydroxyvitamin D3 decreases podocyte loss and podocyte hypertrophy in the subtotally nephrectomized rat. Am J Physiol Renal Physiol 286: F526–F533, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, Ritz E, Atkins RC, Rohde R, Raz I. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 345: 851–860, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Li YC Renoprotective effects of vitamin D analogs. Kidney Int in press, 2009. [DOI] [PubMed]
- 23.Li YC, Bolt MJG, Cao LP, Sitrin MD. Effects of vitamin D receptor inactivation on the expression of calbindins and calcium metabolism. Am J Physiol Endocrinol Metab 281: E558–E564, 2001. [DOI] [PubMed] [Google Scholar]
- 24.Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D3 is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest 110: 229–238, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liebau MC, Lang D, Bohm J, Endlich N, Bek MJ, Witherden I, Mathieson PW, Saleem MA, Pavenstadt H, Fischer KG. Functional expression of the renin-angiotensin system in human podocytes. Am J Physiol Renal Physiol 290: F710–F719, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Mogensen CE, Christensen CK. Predicting diabetic nephropathy in insulin-dependent patients. N Engl J Med 311: 89–93, 1984. [DOI] [PubMed] [Google Scholar]
- 27.Muller DN, Luft FC. Direct renin inhibition with aliskiren in hypertension and target organ damage. Clin J Am Soc Nephrol 1: 221–228, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Nishiyama A, Seth DM, Navar LG. Renal interstitial fluid concentrations of angiotensins I and II in anesthetized rats. Hypertension 39: 129–134, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Parving HH, Persson F, Lewis JB, Lewis EJ, Hollenberg NK. Aliskiren combined with losartan in type 2 diabetes and nephropathy. N Engl J Med 358: 2433–2446, 2008. [DOI] [PubMed] [Google Scholar]
- 30.Perkins BA, Ficociello LH, Ostrander BE, Silva KH, Weinberg J, Warram JH, Krolewski AS. Microalbuminuria and the risk for early progressive renal function decline in type 1 diabetes. J Am Soc Nephrol 18: 1353–1361, 2007. [DOI] [PubMed] [Google Scholar]
- 31.Qi Z, Fujita H, Jin J, Davis LS, Wang Y, Fogo AB, Breyer MD. Characterization of susceptibility of inbred mouse strains to diabetic nephropathy. Diabetes 54: 2628–2637, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Qiao G, Kong J, Uskokovic M, Li YC. Analogs of 1alpha,25-dihydroxyvitamin D3 as novel inhibitors of renin biosynthesis. J Steroid Biochem Mol Biol 96: 59–66, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Sassy-Prigent C, Heudes D, Mandet C, Belair MF, Michel O, Perdereau B, Bariety J, Bruneval P. Early glomerular macrophage recruitment in streptozotocin-induced diabetic rats. Diabetes 49: 466–475, 2000. [DOI] [PubMed] [Google Scholar]
- 34.Takano Y, Yamauchi K, Hiramatsu N, Kasai A, Hayakawa K, Yokouchi M, Yao J, Kitamura M. Recovery and maintenance of nephrin expression in cultured podocytes and identification of HGF as a repressor of nephrin. Am J Physiol Renal Physiol 292: F1573–F1582, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Tan X, Wen X, Liu Y. Paricalcitol inhibits renal inflammation by promoting vitamin D receptor-mediated sequestration of NF-kappaB signaling. J Am Soc Nephrol 19: 1741–1752, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taneda S, Pippin JW, Sage EH, Hudkins KL, Takeuchi Y, Couser WG, Alpers CE. Amelioration of diabetic nephropathy in SPARC-null mice. J Am Soc Nephrol 14: 968–980, 2003. [DOI] [PubMed] [Google Scholar]
- 37.Viberti GC, Hill RD, Jarrett RJ, Argyropoulos A, Mahmud U, Keen H. Microalbuminuria as a predictor of clinical nephropathy in insulin-dependent diabetes mellitus. Lancet 1: 1430–1432, 1982. [DOI] [PubMed] [Google Scholar]
- 38.Vidotti DB, Casarini DE, Cristovam PC, Leite CA, Schor N, Boim MA. High glucose concentration stimulates intracellular renin activity and angiotensin II generation in rat mesangial cells. Am J Physiol Renal Physiol 286: F1039–F1045, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Wang Y, Zhou J, Minto AW, Hack BK, Alexander JJ, Haas M, Li YC, Heilig CW, Quigg RJ. Altered vitamin D metabolism in type II diabetic mouse glomeruli may provide protection from diabetic nephropathy. Kidney Int 70: 882–891, 2006. [DOI] [PubMed] [Google Scholar]
- 40.Wolf G, Chen S, Ziyadeh FN. From the periphery of the glomerular capillary wall toward the center of disease: podocyte injury comes of age in diabetic nephropathy. Diabetes 54: 1626–1634, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Xiang W, Kong J, Chen S, Cao LP, Qiao G, Zheng W, Liu W, Li X, Gardner DG, Li YC. Cardiac hypertrophy in vitamin D receptor knockout mice: role of the systemic and cardiac renin-angiotensin systems. Am J Physiol Endocrinol Metab 288: E125–E132, 2005. [DOI] [PubMed] [Google Scholar]
- 42.Yoo TH, Li JJ, Kim JJ, Jung DS, Kwak SJ, Ryu DR, Choi HY, Kim JS, Kim HJ, Han SH, Lee JE, Han DS, Kang SW. Activation of the renin-angiotensin system within podocytes in diabetes. Kidney Int 71: 1019–1027, 2007. [DOI] [PubMed] [Google Scholar]
- 43.Yuan W, Pan W, Kong J, Zheng W, Szeto FL, Wong KE, Cohen R, Klopot A, Zhang Z, Li YC. 1,25-Dihydroxyvitamin D3 Suppresses renin gene transcription by blocking the activity of the cyclic AMP response element in the renin gene promoter. J Biol Chem 282: 29821–29830, 2007. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Z, Sun L, Wang Y, Ning G, Minto AW, Kong J, Quigg RJ, Li YC. Renoprotective role of the vitamin D receptor in diabetic nephropathy. Kidney Int 73: 163–171, 2008. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Z, Yuan W, Sun L, Szeto FL, Wong KE, Li X, Kong J, Li YC. 1,25-Dihydroxyvitamin D3 targeting of NF-kappaB suppresses high glucose-induced MCP-1 expression in mesangial cells. Kidney Int 72: 193–201, 2007. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Z, Zhang Y, Ning G, Deb DK, Kong J, Li YC. Combination therapy with AT1 blocker and vitamin D analog markedly ameliorates diabetic nephropathy: blockade of compensatory renin increase. Proc Natl Acad Sci USA 105: 15896–15901, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou C, Lu F, Cao K, Xu D, Goltzman D, Miao D. Calcium-independent and 1,25(OH)2D3-dependent regulation of the renin-angiotensin system in 1alpha-hydroxylase knockout mice. Kidney Int 74: 170–179, 2008. [DOI] [PubMed] [Google Scholar]
- 48.Zimpelmann J, Kumar D, Levine DZ, Wehbi G, Imig JD, Navar LG, Burns KD. Early diabetes mellitus stimulates proximal tubule renin mRNA expression in the rat. Kidney Int 58: 2320–2330, 2000. [DOI] [PubMed] [Google Scholar]
- 49.Ziyadeh FN, Sharma K, Ericksen M, Wolf G. Stimulation of collagen gene expression and protein synthesis in murine mesangial cells by high glucose is mediated by autocrine activation of transforming growth factor-beta. J Clin Invest 93: 536–542, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]