Abstract
Mutations in WNK4 protein kinase cause pseudohypoaldosteronism type II (PHAII), a genetic disorder that is characterized by renal NaCl and K+ retention leading to hypertension and hyperkalemia. Consistent with this, WNK4 is known to regulate several renal tubule transporters, including the NaCl cotransporter, NCC, and the K+ channel, ROMK, but the mechanisms are incompletely understood, and the role of the kinase activity in its actions is highly controversial. To assay WNK4 kinase activity, we have now succeeded in expressing and purifying full-length, enzymatically active WNK4 protein from HEK293 cells. We show that full-length wild-type WNK4 phosphorylates oxidative stress response kinase 1 (OSR1) and Ste20/SPS1-related proline/alanine-rich kinase (SPAK) in vitro. Introducing the PHAII-associated mutations, E559K, D561A, and Q562E, into our protein had no significant effect on this phosphorylation. We conclude that PHAII is unlikely to be caused by abnormal WNK4 kinase activity. We also made the intriguing observation that inactivating mutations of the WNK4 kinase domain did not completely abolish in vitro phosphorylation of OSR1/SPAK. Led by this, we identified a novel 40-kDa kinase that associates specifically with the COOH-terminal half of WNK4 and is able to phosphorylate both WNK4 and SPAK/OSR1. We suggest that this 40-kDa kinase functions in the WNK4 signal transduction pathway and may mediate some of the physiological actions attributed to WNK4.
Keywords: protein kinase, pseudohypoaldosteronism type II, in-gel phosphorylation
pseudohypoaldosteronism type II (PHAII) is an autosomal dominant disorder characterized by salt-sensitive hypertension and hyperkalemia, renal tubular acidosis, and suppressed plasma renin activity. The pathogenesis of PHAII is likely to be increased transcellular and paracellular NaCl reabsorption and increased potassium secretion in the distal nephron (6, 9, 10). In PHAII patients, mutations have been identified in WNK1 and WNK4, two homologous members of the WNK (With No lysine [K]) gene family of protein kinases, so named because they lack a lysine residue within the β3 strand of their putative kinase domain that is highly conserved in all other protein kinases (14). WNK4 is expressed in the renal distal convoluted tubule and cortical collecting duct, primarily at the tight junction (14).
Several important functions for WNK4 in the kidney have been identified. WNK4 inhibits the thiazide-sensitive NaCl cotransporter, NCC, and the renal K+ channel, ROMK (4, 15, 20). PHAII mutations in WNK4 simultaneously relieve inhibition of NCC and enhance inhibition of ROMK, possibly explaining the hypertension and hyperkalemia in this disease. WNK4 also increases paracellular chloride permeability in renal epithelial cells, possibly by phosphorylating tight junction proteins of the claudin family (3, 19). This effect is stimulated by PHAII mutations. How WNK4 mediates these and other functions is incompletely understood. Furthermore, the molecular mechanism by which PHAII mutations modulate WNK4 function and how this relates (if at all) to the kinase function of WNK4 are essentially unknown. Evidence for a role of WNK4 kinase activity in any of its functional effects has been largely indirect and remains highly controversial. For example, Wilson et al. (15) found that the mutation, D318A, which is predicted to abolish kinase activity, abrogates the ability of WNK4 to inhibit NCC. However, Yang et al. (21) did not observe this and instead found that even a COOH-terminal fragment of WNK4 lacking the kinase domain could inhibit NCC.
The biochemical characteristics of WNK4 are poorly studied, and its structure has not yet been elucidated. No group has been able to express significant amounts of full-length WNK4 protein for biochemical analysis. So far, all studies of WNK4 kinase activity have used various short polypeptides encompassing the putative kinase domain, expressed either in bacteria or insect cells. These studies have yielded conflicting results. Wang et al. (13) found no kinase activity using histone as a substrate, while Yamauchi et al. (19) found that WNK4 phosphorylates claudin-4 and Lenertz et al. (5) found that it phosphorylates WNK1. More recently, Vitari et al. (11) found that the WNK4 kinase domain phosphorylates the Ste20-related kinases, STE20/SPS1-related proline/alanine-rich kinase (SPAK) and oxidative stress-responsive kinase-1 (OSR1) but has negligible kinase activity against myelin basic protein (MBP), histone, or claudin-4. Because of the lack of a full-length protein preparation, it has not been possible to assess the effect of PHAII mutations in the middle of the protein on the kinase domain, which is near the NH2 terminal.
To study the molecular mechanism by which WNK4 influences its targets, we expressed and purified full-length and enzymatically active wild-type and PHAII mutant WNK4 proteins using a tandem affinity purification protocol and assayed them for in vitro phosphorylation of OSR1 and SPAK. We find that the full-length protein does indeed have robust kinase activity and that the PHAII mutations have no significant effects on this activity. Furthermore, we discovered a potentially novel protein kinase that strongly associates with WNK4 and may mediate some of its effects.
MATERIALS AND METHODS
WNK4 expression constructs.
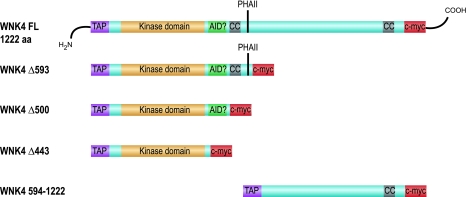
The open reading frame from mouse WNK4 (IMAGE clone 4234311) was cloned into the pNTAP vector (Stratagene) so that a tandem affinity purification (TAP) tag consisting of a streptavidin-binding peptide and a calmodulin-binding peptide was fused in-frame to its NH2 terminal and a c-myc epitope tag to the COOH terminal. The following constructs were generated by PCR and/or site-directed mutagenesis: 1) kinase-inactivating mutations, K183M, D318A, and a double mutation of K183M and D318A (KD), 2) PHAII-associated mutations, E559K, D561A, and Q562E, 3) inactivating mutation of the SPAK/OSR1-binding site, F997A (converts RFQV motif to RAQV), 4) COOH terminal truncated WNK4 constructs with truncations at amino acid 443 (Δ443), 500 (Δ500), and 593 (Δ593; see Fig. 4), and 5) NH2 terminal truncated WNK4 construct, containing amino acids 594–1222 (Fig. 4).
WNK4 protein expression and TAP.
Plasmid DNA was transfected into HEK293, Cos-7, or Chinese hamster ovary cells (CHO) cells in 10-cm plates using Lipofectamine 2000 (Invitrogen). Cells were harvested 24 h posttransfection by scraping into 1 ml of lysis buffer (InterPlay Mammalian TAP System, Stratagene) supplemented with Complete Mini protease inhibitor cocktail (Roche) and incubating for 1 h at 4°C. Lysates were cleared by 10-min centrifugation at 16,000 g. TAP-tagged protein was recovered according to manufacturer's protocol. In brief, TAP-WNK4 was precipitated from the supernatant with 50 μl streptavidin resin slurry for 2 h at 4°C. Precipitates were washed twice with streptavidin-binding buffer (supplemented with protease inhibitors and β-mercaptoethanol) and TAP-WNK4 was eluted into 100 μl streptavidin elution buffer. Eluates were diluted to 500 μl with calmodulin-binding buffer and TAP-WNK4 was precipitated again with 25 μl calmodulin resin slurry. Precipitates were washed twice with calmodulin-binding buffer and TAP-WNK4 was eluted in 50 μl of calmodulin elution buffer. Where indicated, the regular protocol (Reg) was replaced with a high-salt and detergent (HSD) protocol introducing the following modifications: 1) kit-provided lysis buffer was replaced with a modified lysis buffer used by Vitari et al. (11) containing 50 mM Tris·HCl, pH 7.5, 1 mM EDTA, 1 mM EGTA, 1% NP-40, 1 mM Na3VO4, 50 mM NaF, 5 mM sodium pyrophosphate, 0.27 M sucrose, and 1 mM DTT and 2) four extra 5- min washes of the streptavidin resin precipitate in the modified lysis buffer supplemented with 0.15 M NaCl were performed.
Streptavidin and Western blots.
Polyacrylamide protein gels were electrophoretically transferred to PVDF membrane and blocked with Odyssey blocking reagent (Li-Cor Biosciences). To detect the COOH-terminal epitope tag, the membranes were immunoblotted with mouse anti-c-myc as the primary antibody at 1:500 dilution (Invitrogen), and Alexa Fluor 680 goat anti-mouse IgG secondary antibody at 1:5,000 (Invitrogen), and then detected with an Odyssey infrared imaging system (Li-Cor Biosciences). To detect the NH2-terminal streptavidin-binding peptide tag, membranes were probed with streptavidin-horseradish peroxidase conjugate at 1:5,000 (Invitrogen), and then incubated with ECL chemiluminescent substrate (GE Healthcare) and detected by autoradiography.
In vitro (in-solution) kinase assay.
GST-OSR1 and GST-SPAK were used as the substrates. These were prepared by glutathione-sepharose affinity purification from BL21 Escherichia coli cells transformed with pGEX-6P constructs encoding glutathione S-transferase fusion protein of kinase-inactive OSR1-D164A or SPAK-D212A [gifts from D. Alessi (11)]. Ten microliters of TAP-WNK4 (0.5 μg) were mixed with 10 μl of 2× reaction buffer [40 mM HEPES, pH 8, 24 mM MgCl, 2 mM DTT, 0.2 mg/ml bovine serum albumin, 100 μM Na2ATP, 1 μCi/μl γ32P-ATP, and 0.5 μg/μl (5 μM) GST-OSR1 or GST-SPAK]. Phosphorylation was carried out for 30 min at 30°C and the reaction was stopped by boiling the samples in SDS sample buffer (62.5 mM Tris·HCl, pH 6.8, 2% SDS, 20% glycerol, 0.01% Bromophenol Blue). Phosphorylated proteins were separated in parallel with prestained protein marker on 7.5% polyacrylamide gels and phosphorylation was visualized by autoradiography of dried gels. Phosphate incorporation into protein bands, excised from the gel, was quantified on a Beckmann LS6000 liquid scintillation counter.
In-gel kinase assay.
This was adapted from Wooten (16). A 7.5% polyacrylamide separating gel was cast with 1% glycerol and with substrate [OSR1 or SPAK at 0.2 mg/ml in separating gel buffer (375 mM Tris·HCl, pH 8.8, 0.1% SDS)] copolymerized into gel. Kinase samples were boiled for 3 min in SDS sample buffer and then separated on the substrate containing gel. SDS was removed from the gel by four washes with SDS removal solution I (50 mM Tris·HCl, pH 8, and 20% 2-propanol) and three washes in SDS removal solution II (50 mM Tris·HCl, pH 8, and 1 mM DTT) for a total of 2 h. Proteins were denatured for 2 h in denaturation solution (50 mM Tris·HCl, pH 8, 20 mM DTT, and 6 M guanidine·HCl) and then renatured overnight in renaturation solution (50 mM Tris·HCl, pH 8, 5 mM DTT, 0.04% Tween 20, 100 mM NaCl, and 5 mM MgCl2). Phosphorylation was carried out by submerging the gel in kinase buffer (25 mM HEPES, pH 7.4, 20 mM MgCl2, 1 mM MnCl2, 5 mM NaF, 100 μM Na3VO4, 2.5 μg/ml pNPP, 1 mM DTT, 50 μM Na2ATP, and 20 μCi/ml γ32P-ATP) for 2 h at 30°C, after which excess γ32P-ATP was removed with gel wash solution (5% trichloro-acetic acid and 1% sodium pyrophosphate) for 1 h. Phosphorylated bands were visualized by autoradiography of the dried gel.
RESULTS
Full-length WNK4 phosphorylates OSR1 in vitro.
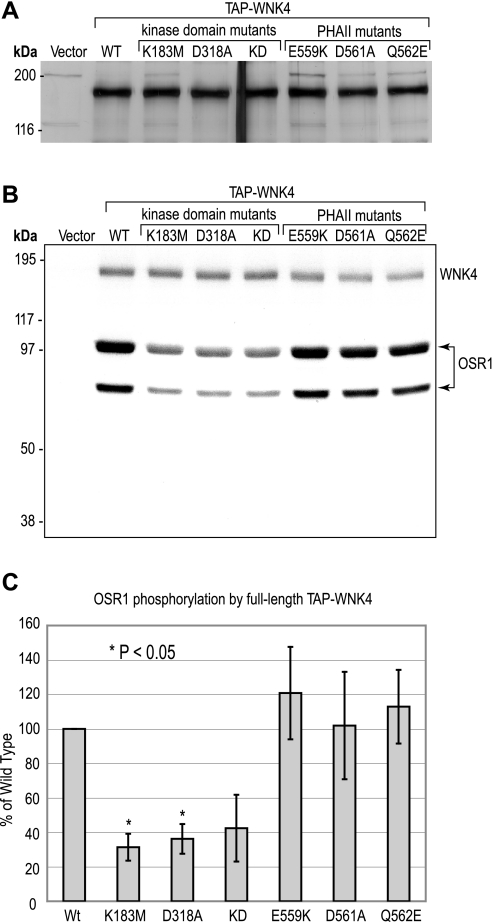
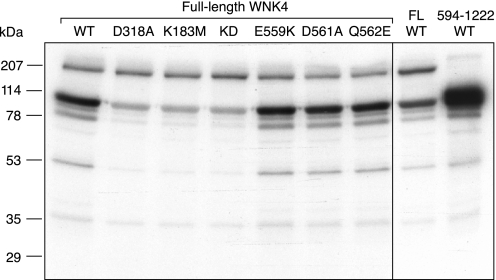
We expressed full-length mouse WNK4 (1,222 amino acids) by transient expression in HEK293 cells. We initially attempted to purify it by immunoprecipitation but found that such preparations were heavily contaminated with nonspecific kinases. To minimize copurification of nonspecifically interacting proteins, we fused a TAP tag to the NH2 terminal of WNK4 and recovered the protein using a TAP protocol, a method designed to prepare relatively pure protein complexes (8). Unless otherwise stated, we used a modified protocol that included supplemental HSD washes aimed at dissociating weakly interacting proteins. The purified preparation contained a single predominant band by SDS-polyacrylamide gel electrophoresis that migrated with an apparent molecular weight of roughly 170 kDa (Fig. 1A) as has been previously observed for WNK4 (15). To determine whether our full-length WNK4 protein was kinase active, we assayed it for in vitro phosphorylation of the previously reported WNK4 substrate, OSR1, in an in-solution assay. OSR1 was found to be strongly phosphorylated by full-length, wild-type WNK4 (Fig. 1B). WNK4 harboring inactivating mutations of the kinase domain, K183M, D318A, and the double mutant, KD, phosphorylated OSR1 significantly less than the wild-type protein. Quantitation of the specific activity of WNK4 showed that it was roughly threefold higher for wild-type than for either kinase domain mutant (Fig. 1C). This shows that full-length WNK4 has intrinsic kinase activity and that OSR1 is a target of full-length WNK4 phosphorylation.
Fig. 1.
Expression and in vitro kinase assay of full-length (FL) WNK4. A: wild-type (WT) and mutant WNK4 proteins, expressed and recovered from HEK293 cells with the high-salt and detergent (HSD) protocol, were separated by SDS-PAGE (0.5 μg of protein/lane) and the gel was silver-stained. As a negative control for WNK4 expression, a lysate from HEK293 cells transfected with vector alone was also treated with the HSD protocol and included in the SDS-PAGE (vector). B: WT and mutant WNK4 proteins (0.5 μg of protein/sample), purified with the HSD protocol, were incubated with kinase-inactive OSR1 (D164A) in the presence of γ32P-ATP. In vitro phosphorylated OSR1 was visualized after SDS-PAGE by 1 h of autoradiography. To exclude OSR1 autophosphorylation, the mock protein preparation from cells transfected with vector alone was incubated with OSR1 in the presence of γ32P-ATP (vector). C: OSR1 phosphorylation was quantified by scintillation counting of phosphorylated bands (both bands of OSR1 doublet), excised from the dried gel. OSR1 phosphorylation is plotted as the percent 32P incorporation compared with WT (means ± SD, n = 3 independent experiments) and statistical significance was determined by repeated-measures ANOVA. PHAII, pseudohypoaldosteronism type II.
In vitro phosphorylation is not affected by PHAII-associated mutations E559K, D561A, and Q562E.
The effect of introducing PHAII-associated mutations, E559K, D561A, and Q562E, on WNK4 phosphorylation of OSR1 in vitro was then measured. All three PHAII mutants phosphorylated OSR1 robustly and there was no statistically significant difference from wild-type WNK4 (Fig. 1, B and C), suggesting that change in WNK4 kinase activity is not the primary pathophysiological mechanism of PHAII.
A 40-kDa kinase that phosphorylates OSR1 associates with WNK4.
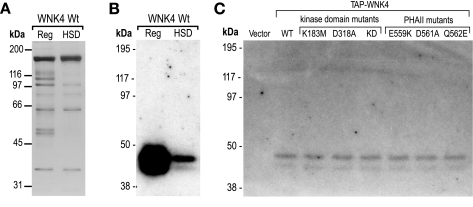
In our in-solution kinase assays, we made the interesting observation that despite single and even double inactivating mutations to the kinase domain of WNK4, there was substantial residual phosphorylation both of OSR1 and of WNK4 itself (lanes 2–4 in Fig. 1B). This indicates either that the mutations did not abolish WNK4 kinase activity completely, a possibility we considered unlikely, or that another kinase that copurified with WNK4 was responsible for the phosphorylation. To investigate the latter possibility, we set up an in-gel kinase assay with the purpose of identifying other components present in our WNK4 protein preparation that might have the ability to phosphorylate OSR1. Following a denaturing SDS-PAGE, the separated proteins were reconstituted to their native conformations in the separating gel and assayed for 32P-phosphorylation of OSR1 that had been copolymerized into the gel. A doublet band at roughly 40 kDa was detected in our TAP-WNK4 preparation (Fig. 2, A and B). No such band was seen in a preparation from vector-transfected cells (Fig. 2B), or if OSR1 was omitted from the gel (data not shown). This indicates that a 40-kDa kinase, that is able to phosphorylate OSR1, copurifies with WNK4 in HEK293 cells. The 40-kDa kinase was highly abundant in the WNK4 preparation using the regular TAP protocol (Reg) and was partly dissociated if we included a series of HSD washes, but complete removal was not possible. The introduction of PHAII mutations or inactivating mutations to the kinase domain of WNK4 did not alter the strength of the 40-kDa signal, indicating that the ability to associate with the 40-kDa kinase was not affected by these mutations (Fig. 2C). No OSR1 phosphorylation was detected at 170 kDa. We interpret this as indicating that full-length WNK4 cannot be refolded into an active conformation in this assay. The 40-kDa kinase copurified with WNK4 expressed in COS-7 and CHO cells, confirming that this kinase can be found in cells originating from a variety of organisms and tissues (Fig. 3).
Fig. 2.
In-gel kinase assay of WNK4. A: WT WNK4, expressed and purified from HEK293 cells, was recovered by regular (Reg) or HSD tandem affinity purification (TAP) protocol, separated by SDS-PAGE, and visualized by silver staining. B: protein samples from A were separated on a polyacrylamide gel in which OSR1-D164A had been copolymerized. After renaturation, in-gel phosphorylation was carried out in the presence of γ32P-ATP and phosphorylated bands were visualized by overnight autoradiography. C: WT and mutant WNK4, prepared by the HSD protocol, was assayed for in-gel phosphorylation of OSR1 as in A. A sample prep from cells transfected with vector alone was included as a negative control for OSR1 phosphorylation by nonspecific copurifying kinases (vector).
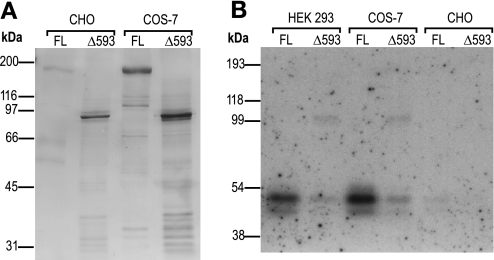
Fig. 3.
WNK4 expressed in different cell lines. A: Chinese hamster ovary cells (CHO) and African green monkey kidney cells (COS-7) were transfected as indicated with either FL or Δ593 truncated (Δ593) WNK4 constructs. Proteins were recovered by regular protocol and visualized on a silver-stained 7.5% polyacrylamide gel. B: in-gel phosphorylation with the indicated samples was carried out as described in Fig. 2. Phosphorylated bands were visualized by 3-h autoradiography.
COOH-terminal half of WNK4 mediates strong interaction with the 40-kDa kinase.
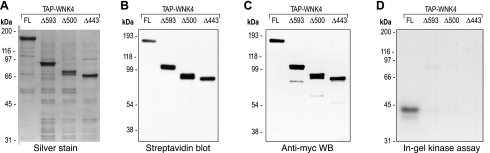
To localize the interaction site between WNK4 and the copurifying kinase, serial COOH-terminal truncations were introduced into the WNK4 open reading frame (Fig. 4), and the proteins were expressed in HEK293 cells and recovered by regular TAP (Fig. 5A). In-gel phosphorylation experiments with OSR1 as substrate showed a strong signal for the 40-kDa copurifying kinase for full-length WNK4, a marked reduction in the signal for Δ593 and Δ500, and no signal for Δ443 (Fig. 5D).
Fig. 4.
Diagram of the WNK4 truncation constructs. The Δ symbol followed by a number indicates the amino acid in the WNK4 polypeptide sequence after which the COOH terminal of the protein was truncated. FL denotes FL protein. TAP, tandem affinity purification tag; AID?, predicted autoinhibitory domain; CC, coiled-coil; PHAII, stretch of acidic amino acids where PHAII-associated mutations are located; c-myc, myc epitope tag.
Fig. 5.
Expression and in-gel kinase assay of COOH-terminal truncated WNK4 proteins. A: FL and COOH-terminal truncated WNK4 proteins were expressed and recovered from HEK293 cells with the regular TAP protocol and then separated on a 7.5% polyacrylamide gel and visualized by silver staining. B: NH2 terminal of the WNK4 proteins shown in A was detected by a streptavidin blot. C: COOH terminal of the WNK4 proteins shown in A detected by immunoblotting with anti-myc antibody. D: assay of in-gel phosphorylation of OSR1-D164A by FL and COOH-terminal truncated WNK4 proteins. 32P-phosphorylated proteins were visualized by 3-h autoradiography.
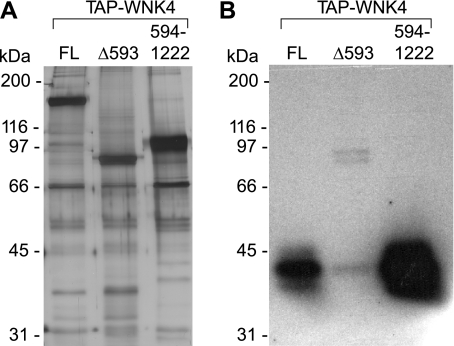
Our results suggest that the COOH-terminal amino acids 594–1222 are important for strong interaction with the copurifying kinase, but that amino acids 443–593 allow limited interaction to take place. To test this, an expression construct with just the COOH-terminal part of WNK4 (NH2-terminal TAP tag followed by amino acids 594–1222) was created (Fig. 4). To determine whether these residues alone are enough to coprecipitate the 40-kDa kinase, WNK4 594–1222, expressed and recovered from HEK293 cells (Fig. 6A), was assayed for in-gel phosphorylation of OSR1. Note that the entire gel is slightly darker than Fig. 5D, probably reflecting a somewhat higher specific activity of the 32P in this particular experiment. Nevertheless, it is very clear that the signal for the copurifying kinase is 20 times stronger in the sample containing WNK4 594–1222 compared with full-length WNK4 (Fig. 6B). Thus, the COOH-terminal part of WNK4 strongly interacts with the 40-kDa kinase.
Fig. 6.
Expression and in-gel kinase assay of NH2-terminal truncated WNK4 protein. A: FL and the indicated truncated WNK4 proteins were expressed and recovered from HEK293 cells with the regular TAP protocol and then separated on a 7.5% polyacrylamide gel and visualized by silver staining. B: assay of in-gel phosphorylation of OSR1-D164A by the NH2-terminal truncated WNK4 594–1222, compared with FL WNK4 and Δ593. 32P-phosphorylated proteins were visualized by 3-h autoradiography.
SPAK is also a substrate for both WNK4 and p40 phosphorylation.
SPAK shares many of the same properties as OSR1 and has also been proposed to be a substrate for WNK4. We therefore tested for phosphorylation of a GST fusion protein of kinase-inactive SPAK (D212A) in an in vitro in-solution kinase assay (Fig. 7). GST-SPAK has a predicted molecular mass of 88 kDa and normally runs as a doublet (∼90 and ∼70 kDa) on SDS-PAGE (Fig. 8A). In the presence of TAP-purified wild-type, full-length WNK4, the 90-kDa band was strongly phosphorylated (Fig. 7, left). This was markedly reduced, but not completely eliminated, in the kinase-inactive WNK4 single mutants, D318A and K183M, and the double mutant, KD. This suggests that both WNK4 and p40 are capable of phosphorylating SPAK and that the phosphorylation site present in the full-length SPAK construct is missing in the ∼70-kDa fragment. As with OSR1, we found that the level of phosphorylation of SPAK by the PHAII-associated WNK4 mutants, E559K, D561A, and Q562E, was identical to wild-type WNK4.
Fig. 7.
SPAK is a substrate of both WNK4 and p40. In vitro in-solution kinase assays were performed with the indicated WNK4 proteins expressed and purified from HEK293 cells, using a GST fusion protein of kinase-inactive SPAK (D212A) as the substrate (predicted molecular mass, 88 kDa). Left: FL constructs of WT WNK4 and the indicated kinase-inactive or PHAII mutants. Right: FL WT WNK4 compared with a COOH-terminal fragment (594–1222) of WT WNK4 lacking the kinase domain.
Fig. 8.
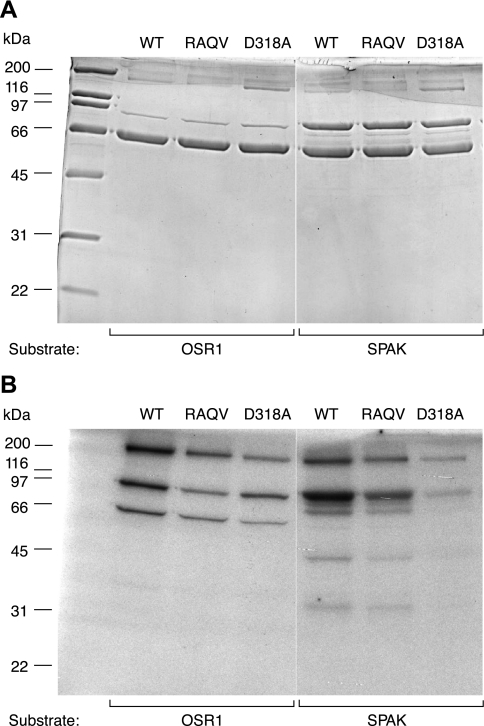
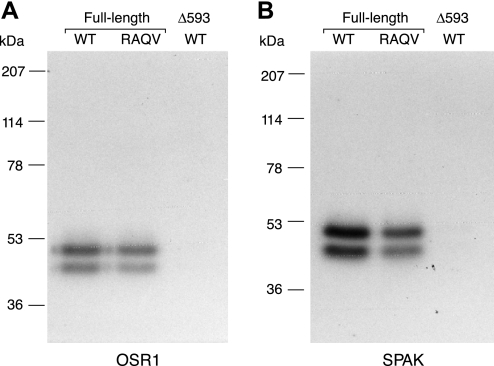
Role of the SPAK/OSR1-binding domain, RFQV, in WNK4. In vitro in-solution kinase assays were performed with the following TAP-purified FL WNK4 proteins: WT, a mutant in which the SPAK/OSR1-binding site was inactivated (RAQV), and a kinase-inactive mutant (D318A), using as substrates either OSR1 (lanes 2–4) or SPAK (lanes 5–7). A: Coomassie blue-stained gel. B: autoradiograph.
We also tested the WNK4 construct containing just the COOH-terminal half of the protein (594–1222). Since this construct is missing the WNK4 kinase domain, and since we already showed that it exhibits greatly enhanced interaction with p40 (Fig. 6), it allows us to assay p40 activity in an in-solution assay. Indeed, we found that in the presence of WNK4 594–1222, phosphorylation of SPAK was markedly increased compared with full-length WNK4 (Fig. 7, right), supporting the idea that p40 directly phosphorylates SPAK.
To confirm that p40 directly phosphorylates SPAK, we performed an in-gel kinase assay. As expected, a protein copurifying with wild-type WNK4, that migrates as a doublet at ∼40 kDa, was able to directly phosphorylate SPAK (Fig. 9B, lane 1). The negative control, the truncated form of WNK4 (Δ593) that lacks interaction with p40, showed no phosphorylated bands (Fig. 9B, lane 3).
Fig. 9.
Characterization of p40 activity by in-gel kinase assay. Either OSR1 (A) or SPAK (B) was copolymerized into the gel, through which FL WT, FL RAQV mutant, or the NH2-terminal fragment of WNK4 (Δ593) was electrophoresed and assayed in-gel in the presence of 32P.
Copurification of p40 with WNK4 does not require the RFQV motif.
The COOH-terminal half of WNK4 contains a RFQV motif that is known to bind to SPAK and OSR1. Mutation of this motif to RAQV completely abolishes SPAK/OSR1 binding (12). We tested whether copurification of p40 with WNK4 requires a functional SPAK/OSR1 binding site. As shown in Fig. 8, the TAP-purified complex of WNK4 RAQV was able to phosphorylate both OSR1 and SPAK in an in vitro in-solution kinase assay, albeit to a lesser degree than wild-type WNK4. Since the RAQV mutation is predicted to abolish direct interaction of WNK4 with SPAK/OSR1, this suggests that p40 mediates most of the phosphorylation in this case. This was confirmed by in-gel kinase assay, which showed the presence of p40 copurifying with both wild-type WNK4 and the RAQV mutant, as evidenced by phosphorylation at ∼40 kDa of both OSR1 and SPAK (Fig. 9, A and B, lanes 1 and 2).
DISCUSSION
Previous studies of WNK4 kinase activity all employed truncated forms of the protein in combination with either model substrates, such as MBP (1) and histone (13), or predicted physiological substrates, such as claudin 4 (19) and OSR1 (11). The results have been ambiguous and the only substrate that has been significantly phosphorylated in more than one study is OSR1 (1, 11). It is typically easier to express smaller truncated forms of large proteins like WNK4 and for in vitro activity studies it may also be desirable to remove potential autoinhibitory domains. To date, there are no reports where the full-length WNK4 protein has been successfully expressed and assayed.
We report here that full-length WNK4, expressed and recovered from HEK293 cells, specifically phosphorylates the substrate OSR1, providing the first evidence that full-length WNK4 has kinase activity. Working with the full-length protein allows us to observe intramolecular interactions and potential autoregulatory events that may be inadvertently abolished by truncation. The full-length WNK4 sequence includes a predicted autoinhibitory domain (13), but this does not prevent us from observing substrate phosphorylation. This agrees with the observation that full-length WNK1 also exhibits substrate and autophosphorylation in vitro (17) despite the presence of an autoinhibitory domain (18).
We observed residual OSR1 phosphorylation when assaying WNK4 harboring individual inactivating mutations to the kinase domain K183M and D318A (Fig. 1C). To address the possibility that these mutations were unable to completely abolish WNK4 kinase activity by themselves, we produced the double mutant KD, in which both mutations are combined. OSR1 phosphorylation was not reduced further, leading us to conclude that either mutation individually is probably sufficient to abolish kinase activity and that the residual signal stems from background phosphorylation by the copurifying kinase. Our results confirm observations made using truncated proteins that the D318A kinase domain mutation abolishes WNK4 kinase activity (11).
We observe a phosphorylated band at the expected molecular weight of WNK4 in our in vitro assay (Fig. 1B). The fact that the intensity of this band does not change when inactivating mutations are introduced into the kinase domain of WNK4 leads us to conclude that it does not represent significant autophosphorylation, but rather transphosphorylation of WNK4 by a copurifying kinase. It is possible that WNK4 autophosphorylates to some extent, but that the phosphorylation by the copurifying kinase is so much stronger that WNK4-specific activity cannot be detected over background, even when the protein is prepared with the HSD protocol. Moderate autophosphorylation at serine 332 has been reported for a kinase domain fragment of WNK4 (5).
Our findings exclude any strong activating or deactivating effect of PHAII mutations on WNK4 kinase activity (Fig. 1C). Because we only assayed phosphorylation of OSR1 and SPAK, we cannot discount potential effects on the interaction with and phosphorylation of other, as of yet undiscovered, physiological substrates. Nevertheless, it is much more likely that PHAII mutations affect WNK4 function in some other way, such as by altering its interaction with other proteins.
We found that a 40-kDa kinase, which strongly phosphorylates both OSR1 and SPAK, copurifies with WNK4 from HEK293 cells. We made extensive attempts to identify this protein but so far without success. The phosphorylated band corresponding to the 40-kDa kinase was greatly reduced when the COOH-terminal half of WNK4 was truncated, but the SDS-PAGE gel with full-length and truncated WNK4 in parallel lanes failed to show either Coomassie Blue- or silver-stained bands in the 40-kDa-size range that were stronger for the full-length protein than the truncated protein (Fig. 5A). Attempts at scaling up the amounts of WNK4 loaded on the gel and subsequently analyzing the 40-kDa-size range by the highly sensitive technique of nanoscale liquid chromatography and tandem mass spectrometry also failed to identify any candidate protein kinases in this region (data not shown). Several proteins were identified in the peptide mass fingerprinting experiments but they were either highly abundant cellular proteins (such as tubulin and Hsp70) or peptide fragments of WNK4 itself.
It is possible that the 40-kDa copurifying protein could represent a proteolytic cleavage product of our overexpressed mouse TAP-WNK4. We consider this less likely for two reasons. First, kinase domain mutations that inactivate WNK4 kinase activity did not abolish the 40-kDa signal detected in the in-gel kinase assay (Fig. 2C). Second, when we blotted the full-length protein with reagents that recognize either the NH2 terminus (streptavidin) or COOH terminus (anti-myc), the 40-kDa band was not labeled (Fig. 5, B and C). It is also possible that the 40-kDa kinase is a fragment of the endogenous human WNK4 expressed in HEK cells, that oligomerized with and hence copurified with our TAP-WNK4. However, in our mass spectrometry analyses, we detected numerous WNK4 peptides, but none were human WNK4 sequences.
Another possibility we considered is that p40 is a fragment of WNK1. WNK1 and WNK4 have been shown to bind to each other when coexpressed in Xenopus laevis oocytes (21), and WNK1 is known to be expressed endogenously in HEK293 cells (17). However, we consider it relatively unlikely that p40 is WNK1 for two reasons. First, although we too can detect endogenous WNK1 in HEK whole cell lysates by Western blots using anti-human WNK1 antibody (Alpha Diagnostics), we were never able to detect any endogenous WNK1 copurifying with our WNK4 (data not shown). This is consistent with the findings of Lenertz et al. (5), who were also unable to find evidence for a stable interaction between WNK1 and WNK4 either in vitro or in HEK cells. Second, we never observed a band at the predicted size for full-length WNK1 (250 kDa) on our 32P autoradiographs, either in our in-solution kinase assay (representing autophosphorylated WNK1) or our in-gel kinase assay (representing refolded WNK1 that is capable of transphosphorylating SPAK or OSR1). Thus, at present we cannot identify this kinase.
Despite the inability to identify the 40-kDa kinase, we can make several inferences about its characteristics. The kinase is likely to be ubiquitous as it is associated with WNK4 in cell lines derived from multiple different tissues (kidney and ovary) and organisms (human, monkey, and hamster). Its specific activity is likely to be very high since amounts of it too low to detect even on silver-stained gels are nevertheless sufficient to phosphorylate OSR1 and SPAK very strongly. The 40-kDa kinase probably is not autophosphorylated, nor is it phosphorylated by WNK4 or other WNK4-associated kinases, since no phosphorylated bands can be detected in the 40-kDa region in the in vitro kinase assay (Fig. 1B). This raises the intriguing question of how this kinase is activated. We postulate three possible explanations: 1) p40 may be constitutively active, 2) p40 may be activated by means other than phosphorylation, and 3) p40 may already have been fully phosphorylated in the cell. Since we do not dephosphorylate before we perform either of the 32P kinase assays, we would not be able to detect this nonradioactive form of phospho-p40.
We also know that p40 binds very strongly to WNK4 because we still detect the kinase after stringent washes with HSD. We mapped its interaction site on WNK4 to amino acids 594–1222 (Fig. 6). This region contains an RFxV consensus motif (996–999) which mediates its interaction with SPAK and OSR1 (7). However, we do not believe that the 40-kDa copurifying kinase represents endogenous full-length SPAK or OSR1 (which have considerably higher molecular weights) or proteolytic or anomalously migrating fragments. This is because we can still observe the 40-kDa kinase in a complex with WNK4, even when we mutate this motif to RAQV, which has previously been shown to completely abolish both its biochemical (12) and functional (2) interaction with SPAK/OSR1. Interestingly, we do see a slight reduction in the p40 signal when we carefully compare the RAQV mutant to wild-type WNK4 (lane 1 vs. 2 in both blots in Fig. 9), but at present we cannot explain this. The COOH-terminal half of WNK4 also contains a coiled-coil domain between residues 1110–1140, so potentially this could be involved in protein-protein interaction or protein oligomerization.
In summary, we demonstrated that full-length WNK4 protein has kinase activity against OSR1 and SPAK and that the PHAII mutations have no significant effects on this activity. Furthermore, we discovered a potentially novel ∼40-kDa protein kinase that associates with WNK4 and may mediate some of its effects.
GRANTS
These studies were supported by National Institutes of Health Grant DK-062283 (to A. Yu) and an American Heart Association Western States Affiliate Predoctoral Fellowship (to R. Ahlstrom).
Acknowledgments
We thank D. Alessi for kindly providing the OSR1 and SPAK expression constructs and Dr. J. Klein (University of Louisville), S. Bassilian, and Dr. J. Whitelegge (Pasarow Mass Spectrometry Laboratory at UCLA) for performing some of the preliminary mass spectrometry studies, and S. A. Kanzawa for technical assistance.
REFERENCES
- 1.Anselmo AN, Earnest S, Chen W, Juang YC, Kim SC, Zhao Y, Cobb MH. WNK1 and OSR1 regulate the Na+, K+, 2Cl− cotransporter in HeLa cells. Proc Natl Acad Sci USA 103: 10883–10888, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gagnon KB, England R, Delpire E. Volume sensitivity of cation-Cl− cotransporters is modulated by the interaction of two kinases: Ste20-related proline-alanine-rich kinase and WNK4. Am J Physiol Cell Physiol 290: C134–C142, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Kahle KT, Macgregor GG, Wilson FH, Van Hoek AN, Brown D, Ardito T, Kashgarian M, Giebisch G, Hebert SC, Boulpaep EL, Lifton RP. Paracellular Cl− permeability is regulated by WNK4 kinase: insight into normal physiology and hypertension. Proc Natl Acad Sci USA 101: 14877–14882, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kahle KT, Wilson FH, Leng Q, Lalioti MD, O'Connell AD, Dong K, Rapson AK, MacGregor GG, Giebisch G, Hebert SC, Lifton RP. WNK4 regulates the balance between renal NaCl reabsorption and K+ secretion. Nat Genet 35: 372–376, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Lenertz LY, Lee BH, Min X, Xu BE, Wedin K, Earnest S, Goldsmith EJ, Cobb MH. Properties of WNK1 and implications for other family members. J Biol Chem 280: 26653–26658, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Mayan H, Vered I, Mouallem M, Tzadok-Witkon M, Pauzner R, Farfel Z. Pseudohypoaldosteronism type II: marked sensitivity to thiazides, hypercalciuria, normomagnesemia, and low bone mineral density. J Clin Endocrinol Metab 87: 3248–3254, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Piechotta K, Lu J, Delpire E. Cation chloride cotransporters interact with the stress-related kinases Ste20-related proline-alanine-rich kinase (SPAK) and oxidative stress response 1 (OSR1). J Biol Chem 277: 50812–50819, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Rigaut G, Shevchenko A, Rutz B, Wilm M, Mann M, Seraphin B. A generic protein purification method for protein complex characterization and proteome exploration. Nat Biotechnol 17: 1030–1032, 1999. [DOI] [PubMed] [Google Scholar]
- 9.Schambelan M, Sebastian A, Rector FC Jr. Mineralocorticoid-resistant renal hyperkalemia without salt wasting (type II pseudohypoaldosteronism): role of increased renal chloride reabsorption. Kidney Int 19: 716–727, 1981. [DOI] [PubMed] [Google Scholar]
- 10.Take C, Ikeda K, Kurasawa T, Kurokawa K. Increased chloride reabsorption as an inherited renal tubular defect in familial type II pseudohypoaldosteronism. N Engl J Med 324: 472–476, 1991. [DOI] [PubMed] [Google Scholar]
- 11.Vitari AC, Deak M, Morrice NA, Alessi DR. The WNK1 and WNK4 protein kinases that are mutated in Gordon's hypertension syndrome phosphorylate and activate SPAK and OSR1 protein kinases. Biochem J 391: 17–24, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vitari AC, Thastrup J, Rafiqi FH, Deak M, Morrice NA, Karlsson HK, Alessi DR. Functional interactions of the SPAK/OSR1 kinases with their upstream activator WNK1 and downstream substrate NKCC1. Biochem J 397: 223–231, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Z, Yang CL, Ellison DH. Comparison of WNK4 and WNK1 kinase and inhibiting activities. Biochem Biophys Res Commun 317: 939–944, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Wilson FH, Disse-Nicodeme S, Choate KA, Ishikawa K, Nelson-Williams C, Desitter I, Gunel M, Milford DV, Lipkin GW, Achard JM, Feely MP, Dussol B, Berland Y, Unwin RJ, Mayan H, Simon DB, Farfel Z, Jeunemaitre X, Lifton RP. Human hypertension caused by mutations in WNK kinases. Science 293: 1107–1112, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Wilson FH, Kahle KT, Sabath E, Lalioti MD, Rapson AK, Hoover RS, Hebert SC, Gamba G, Lifton RP. Molecular pathogenesis of inherited hypertension with hyperkalemia: the Na-Cl cotransporter is inhibited by wild-type but not mutant WNK4. Proc Natl Acad Sci USA 100: 680–684, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wooten MW In-gel kinase assay as a method to identify kinase substrates. Sci STKE 2002: PL15, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Xu B, English JM, Wilsbacher JL, Stippec S, Goldsmith EJ, Cobb MH. WNK1, a novel mammalian serine/threonine protein kinase lacking the catalytic lysine in subdomain II. J Biol Chem 275: 16795–16801, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Xu BE, Min X, Stippec S, Lee BH, Goldsmith EJ, Cobb MH. Regulation of WNK1 by an autoinhibitory domain and autophosphorylation. J Biol Chem 277: 48456–48462, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Yamauchi K, Rai T, Kobayashi K, Sohara E, Suzuki T, Itoh T, Suda S, Hayama A, Sasaki S, Uchida S. Disease-causing mutant WNK4 increases paracellular chloride permeability and phosphorylates claudins. Proc Natl Acad Sci USA 101: 4690–4694, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang CL, Angell J, Mitchell R, Ellison DH. WNK kinases regulate thiazide-sensitive Na-Cl cotransport. J Clin Invest 111: 1039–1045, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang CL, Zhu X, Wang Z, Subramanya AR, Ellison DH. Mechanisms of WNK1 and WNK4 interaction in the regulation of thiazide-sensitive NaCl cotransport. J Clin Invest 115: 1379–1387, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]