Abstract
Pharmacological and physiological phenomena suggest that cells somewhere inside the central nervous system are responsive to aldosterone. Here, we present the fundamental physiological limitations for aldosterone action in the brain, including its limited blood-brain barrier penetration and its substantial competition from glucocorticoids. Recently, a small group of neurons with unusual sensitivity to circulating aldosterone were identified in the nucleus of the solitary tract. We review the discovery and characterization of these neurons, which express the enzyme 11β-hydroxysteroid dehydrogenase type 2, and consider alternative proposals regarding sites and mechanisms for mineralocorticoid action within the brain.
Keywords: sodium deficiency, glucocorticoids, mineralocorticoids, nucleus of the solitary tract, blood-brain barrier, 11β-hydroxysteroid dehydrogenase
sodium is a ubiquitous ion of paramount importance for many physiological functions. Tight regulation of the amount and concentration of sodium in the body is necessary for life and indispensable for growth and is achieved by the coordinated activities of neural and endocrine control systems. From the promotion of sodium reabsorption in the kidney to the regulation of sodium appetite in the brain, an intricate network of regulatory systems maintains body sodium balance.
Aldosterone is a key player in this control network, although traditionally it was considered simply an endocrine mediator for enhancing sodium reabsorption across certain epithelia. Now it is known that this hormone can influence nonepithelial tissues, including the brain, where it may promote short-term physiological and behavioral adaptations in response to low-sodium conditions as well as chronic, maladaptive responses in pathophysiological states (29).
Over the past few decades, various sites of aldosterone action within the central nervous system (CNS) have been proposed or presumed with very little evidence. In this review, we highlight a series of recent investigations leading to the discovery and characterization of the first cells in the brain with established sensitivity to aldosterone.
SEARCH FOR ALDOSTERONE-SENSITIVE CELLS IN THE BRAIN
Central mineralocorticoid phenomena.
Five years ago, we initiated a search for aldosterone-sensitive neurons in the brain. Our interest in this topic stemmed from the possibility that aldosterone and other mineralocorticoid receptor (MR) agonists elevate blood pressure by acting directly within the CNS. A central pressor action of aldosterone was suggested by the finding that small doses (ineffective when infused systemically) could elevate blood pressure after chronic infusion into the cerebral ventricles (69, 95, but see Ref. 112). Conversely, central administration of an MR antagonist can block the central pressor effect of aldosterone (73), transiently reduce blood pressure in normotensive rats (143, 173), and attenuate the hypertension produced by chronic high-dose mineralocorticosteroid infusions (92, 172) or a high-sodium diet in Dahl salt-sensitive rats (72). Interestingly, psychological stress appears to play a major role in these cardiovascular effects, because, in some experiments involving central MR manipulation, cardiovascular effects appear only when blood pressure is recorded in restrained animals (tail-cuff measurement), and not in unrestrained, freely moving animals (34, 171).
In addition to blood pressure, salt intake is increased by aldosterone and other MR agonists (46, 48, 146, 182–184). In rats, exogenous mineralocorticoids can stimulate sodium appetite with high selectivity relative to other hormones, such as angiotensin II, which nonselectively increase the ingestion of water and salt (for review see Ref. 62). Conversely, MR antagonists can reduce sodium appetite (47, 152, 166, 170). Aldosterone has been reported to influence other behavioral and autonomic functions as well, including mood, appetite, exploratory behavior, and baroreceptor function (36, 82, 124, 175, 177, 187).
Hypothesized site(s) of action for aldosterone in the brain.
Despite these well-documented phenomena, very little was known about the site(s) of action of aldosterone inside the brain until recently. The aforementioned observations suggest that aldosterone-sensitive cells, possibly neurons, exist somewhere inside the brain, at a site that is relatively accessible to circulating aldosterone. In adrenalectomized weanling rats, large doses of radiolabeled aldosterone or corticosterone revealed binding sites in a variety of brain regions (14), later corroborated by the identification of widespread mRNA expression for the MR (9). Some investigators predicted that aldosterone target cells would be found in the so-called anteroventral third ventricular (AV3V) region of the hypothalamus (70) because of this region's importance in thirst and salt-sensitive hypertension (17). Until recently, however, the location(s) and identity of aldosterone-sensitive brain cells remained purely speculative.
ROADBLOCKS TO THE IDENTIFICATION OF ALDOSTERONE-SENSITIVE CELLS IN THE BRAIN
Poor penetration of the blood-brain barrier.
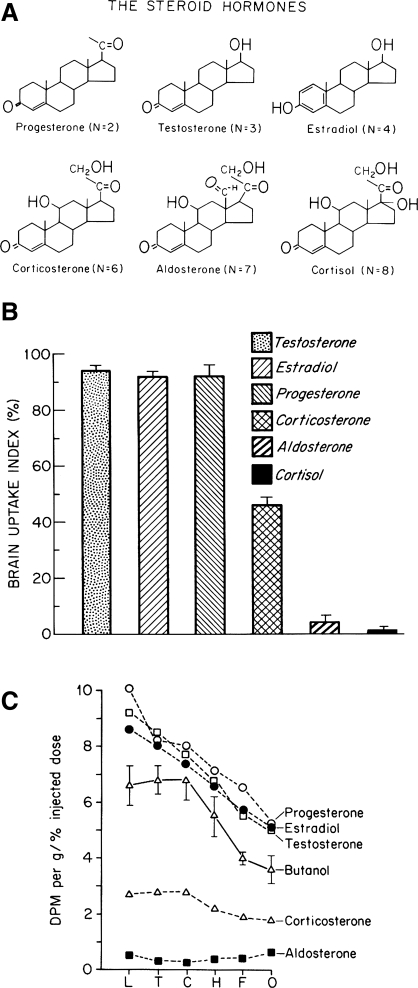
One basic and often-ignored roadblock to identifying aldosterone-sensitive brain cells is aldosterone's surprisingly poor penetration of the blood-brain barrier (BBB) (49, 136, 138). Despite its lipophilicity, the marginal brain penetration of aldosterone and certain other steroids (Fig. 1 ) is due, at least in part, to a protein transporter situated within the BBB, mdr1 (also known as P-glycoprotein), which pumps certain substrates back across the cerebral vascular endothelium and into the blood (139, 167, 168). In vitro, aldosterone binds avidly to the MR in brain tissue homogenates (13, 14, 27, 106), and it does cross the BBB in detectable amounts (188, 191). Also, infusion of large systemic doses of radiolabeled aldosterone can produce nuclear labeling in the MR binding sites left vacant throughout the brain by eliminating competition from endogenous corticosteroids (14, 15). However, relative to endogenous corticosterone, the brain contains minute amounts of aldosterone (188, 191). When its uptake and MR binding are compared between the brain and other organs, or with the brain uptake and MR binding of another ligand (corticosterone), the substantially reduced BBB penetration of aldosterone is readily apparent (49, 135, 137, 188).
Fig. 1.
Blood-brain barrier (BBB) penetration by aldosterone is substantially less than by most other steroid hormones, as first demonstrated by Pardridge and Mietus (136, 138). Using the Oldendorf technique (132), they observed that reduced brain uptake of certain steroid hormones was associated with their increased molecular polarity, as reflected by the hydrogen bonding number, as shown in A, where n is the sum of 2 per -OH (hydroxyl) group and 1 per =O (ketone) group (in solution, aldosterone exists in equilibrium between 2 states: a predominant hemiacetal with n = 6 and an 18-aldehyde with n = 7). B: only a small fraction of labeled aldosterone (n = 6–7) or cortisol (n = 8) enters the brain, whereas corticosterone (the primary glucocorticoid in rodents; n = 6) exhibits substantial penetration of the BBB. “Brain uptake index” of 100% represents BBB penetration of a freely diffusible reference molecule, butanol (138). C: brain uptake of aldosterone [disintegrations per minute (dpm) per gram] was low in every BBB-protected region tested. L, inferior and superior colliculi; T, thalamus and hypothalamus; C, caudate-putamen; H, hippocampus; F, frontal cortex; O, olfactory bulb (136). [Reproduced with permission from Pardridge (135) and Pardridge and Mietus (136, 138).]
A direct consequence of aldosterone's limited BBB penetration is that the vast majority of cells in the brain are exposed to only a small fraction of its typically subnanomolar concentration in the blood plasma. This limitation, which compounds aldosterone's already uphill battle against other, more prevalent corticosteroids (see below), implies that if the brain does contain any aldosterone-sensitive cells, they are probably located in one of its few sites with an incomplete BBB.
MR occupation by other corticosteroids.
Another major roadblock to identification of aldosterone-sensitive cells in the brain stems from the fact that the MR binds with high affinity to multiple adrenal corticosteroids, not just aldosterone (13, 106, 145, 157). A wide variety of sites throughout the brain express the MR, but, in the vast majority of these cells, adrenal glucocorticoids are its primary occupants. Similar to aldosterone, which circulates at nanomolar and subnanomolar concentrations, corticosterone (in rodents) and cortisol (in humans) bind the MR with subnanomolar affinity, yet they circulate at 1,000-fold higher (micromolar) concentrations. In vitro experiments suggest that the aldosterone-MR interaction is 10-fold more potent in stimulating transcriptional changes (9), but even after accounting for >90% plasma protein binding, free glucocorticoid concentrations remain 100-fold higher. Thus the vast majority of MRs are occupied by glucocorticoids, making the vast majority of MR-expressing cells effectively blind to the relatively small fluctuations in the concentration of aldosterone within its physiological range.
The degree to which this glucocorticoid-predominant occupation of the MR causes receptor activation under various conditions is a separate issue (51), as are the unexplored species differences related to the greatly reduced BBB penetration of cortisol (the primary glucocorticoid in humans) relative to corticosterone (Fig. 1). For the purposes of the present review, the most important implication of this arrangement is that mere identification of the expression of the MR, particularly in the brain, does not tell us whether a cell is sensitive to aldosterone.
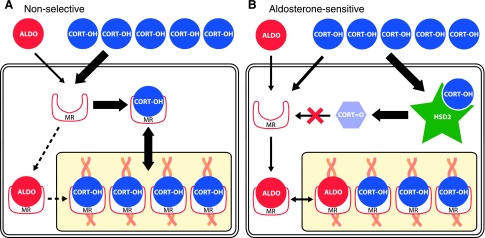
Aldosterone sensitivity, therefore, requires a mechanism that substantially decreases MR occupation by glucocorticoids, creating a window of opportunity for aldosterone (Fig. 2). The fact that a compound in liquorice produces mineralocorticoid-like physiological changes by altering urinary glucocorticoid metabolism (163), combined with a similar metabolic phenotype observed in patients with the heritable syndrome of apparent mineralocorticoid excess (162, 169), led to the discovery that the indispensable mechanism for cellular aldosterone sensitivity is the enzymatic oxidation of glucocorticoids (corticosterone and cortisol) to inactive 11-oxo metabolites (11-dehydrocorticosterone and cortisone) (42, 52). This paradigm-shifting development suggested that aldosterone-sensitive cells could be identified unambiguously by colocalization of MR expression with whatever enzyme catalyzes this reaction.
Fig. 2.
In most mineralocorticoid receptor (MR)-expressing cells (A), the primary ligand is corticosterone or cortisol (both designated “CORT”). These glucocorticoids circulate in the blood plasma at 1,000-fold higher concentrations than aldosterone (ALDO). This systemic imbalance between CORT and ALDO is further exaggerated in the brain, at least in rodents, where only a minute fraction of circulating aldosterone penetrates the BBB (49, 136, 138). Thus, in most MR-expressing cells, basal occupation of this receptor by glucocorticoids prevents the binding of aldosterone, which despite an equivalent binding affinity, may be 10-fold more potent in producing transcriptional activation once it is bound to the MR (9). In contrast, cells that express 11β-hydroxysteroid dehydrogenase type 2 (HSD2; B) can convert a large fraction of intracellular CORT to an 11-oxo metabolite with greatly reduced MR-binding affinity. Aldosterone is not a substrate for HSD2, so this mechanism confers cellular sensitivity to physiological concentrations of this hormone. HSD2 is only a partial filter, leaving a significant amount of unaltered corticosterone able to bind the MR (49, 54), probably because of its greater affinity for the MR (Km <1 nM) than HSD2 (Km ∼10 nM in rat).
HSD1 vs. HSD2.
Initially, some confusion arose because of the assumption that a previously identified liver enzyme, now known as HSD1, mediates the 11-dehydrogenation of glucocorticoids in vivo (2, 42). This assumption was based on the in vitro, low-affinity 11-dehydrogenase activity of HSD1 in tissue homogenates (108, 144). “Dehydrogenase” turned out to be somewhat of a misnomer, because in vivo this enzyme mediates the reverse reaction (11-oxidation), mediating the reactivation of inactive metabolites into potent glucocorticoids (104, 144). The expression and in vitro activity of HSD1 were identified in a variety of tissues, including virtually every region of the brain, where its existence was interpreted as evidence for a large array of potential target sites for aldosterone (93, 109, 122, 123).
However, the existence of HSD1 in brain sites such as the hippocampus seemed at odds with previous data showing a lack of 11-dehydrogenase activity and a lack of MR selectivity for aldosterone in tissue from this region (42, 52). Subsequently, Naray-Fejes-Toth and others (121, 126, 128, 129) demonstrated the existence of a separate enzyme, with cofactor requirements and kinetics that closely matched the high-affinity 11-dehydrogenase activity identified in the kidney. This enzyme, termed HSD2, was isolated, cloned in multiple species, and found to be necessary for reducing intracellular glucocorticoid levels in aldosterone-sensitive epithelia (3, 103, 126).
HSD2 in developing and adult rat brains.
Armed with the knowledge that HSD2 is the key to identifying which MR-expressing cells are aldosterone-sensitive, the expression of this enzyme was mapped in developing and adult animals (18, 20, 28, 37, 149, 194). These initial efforts produced disparate results for a variety of reasons, including low-resolution tissue sampling, probes designed against mRNA from separate species (149), large-scale changes in HSD2 expression during embryonic and neonatal development (37, 148), and, possibly, intrinsic species differences as well.
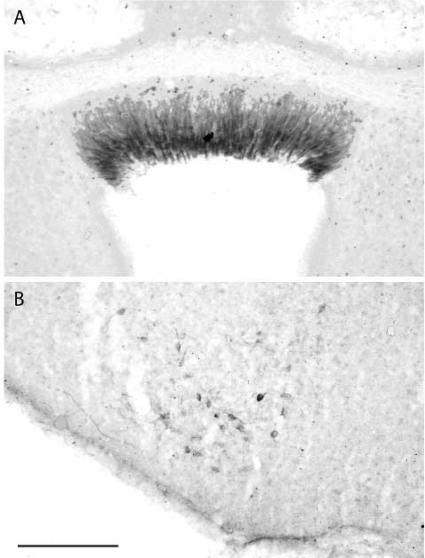
Some initial reports failed to detect HSD2 expression anywhere in the brain (20, 28, 149). Using a more sensitive and species-appropriate probe and examining a full series of tissue sections throughout the brain, however, Roland and colleagues (150) showed that, in the rat brain, HSD2 is expressed in a select few sites: the nucleus of the solitary tract (NTS), the subcommissural organ, and the ventromedial nucleus of the hypothalamus (Figs. 3 and 4). In embryos and neonatal animals, subsequent investigators observed strong expression of HSD2 mRNA in a number of other sites (18, 37, 59, 127, 148). This widespread developmental pattern of HSD2 expression in the brain becomes restricted to just a few small populations of cells in adult rats (148, 150) and, possibly, to just the NTS in mice (84, 85).
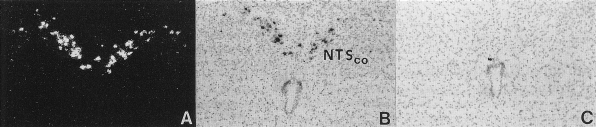
Fig. 3.
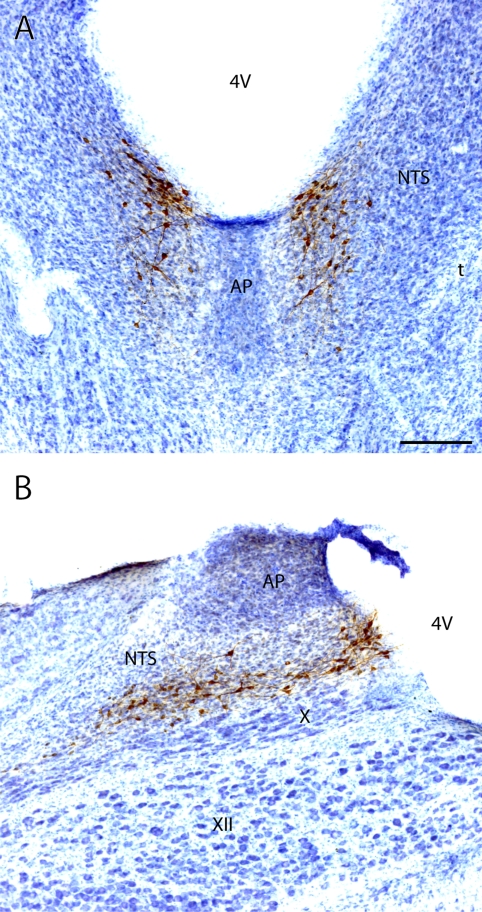
Prominent expression of HSD2 mRNA in a group of cells within the caudal nucleus of the solitary tract (NTS). A and B: autoradiographic in situ hybridization signal for HSD2 mRNA (antisense probe) with dark- and brightfield optics, respectively. C: lack of hybridization signal (sense probe used as a control). [Reproduced with permission from Roland et al. (150).]
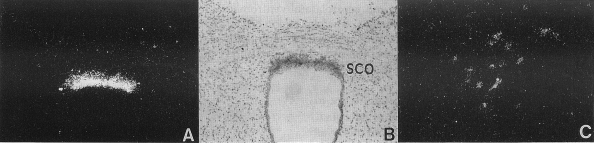
Fig. 4.
A and B: strong hybridization signal in a glial structure known as the subcommissural organ (SCO) with dark- and brightfield optics, respectively. C: weak expression in the caudal, ventrolateral subdivision of the ventromedial nucleus of the hypothalamus (VMHvl). [Reproduced with permission from Roland et al. (150).]
Despite the identification of HSD2-expressing brain sites, much confusion remained as to which, if any, cells in the adult brain are aldosterone-sensitive. One study (148) reported weak labeling for HSD2 in a handful of brain sites (locus ceruleus, dorsal raphe, thalamus, and amygdala), where it was not detected in prior or subsequent mapping studies (85, 150). More importantly, no attempt was made in any of these studies to colocalize MR in HSD2-expressing cells. In fact, multiple investigators speculated, on the basis of prior data, that no MR was present in most or all of these sites; they concluded, instead, that HSD2 may simply dampen activation of the glucocorticoid receptor (GR) in these cells (29, 37, 148, 150, 154).
DISCOVERY OF ALDOSTERONE-SENSITIVE NEURONS IN THE NTS
The search renewed.
Our approach began with a reanalysis of the cellular distribution of MR protein throughout the CNS (55). Reflecting the well-known difficulty in generating an MR antibody with reasonable sensitivity and selectivity (66), previous attempts at mapping MR-like immunoreactivity in the brain (4, 5) corresponded rather poorly with the established pattern of mRNA expression (9). After finding similarly dissimilar, nonspecific, or absent labeling with a variety of commercially available anti-MR antisera, we obtained an antiserum generated in the laboratory of Mitsuhiro Kawata with high selectivity for MR vs. GR; this antiserum produced labeling in the hippocampus that closely approximated the known pattern of mRNA expression (90).
Using the Kawata antiserum, we found particularly intense nuclear MR in the indusium griseum, mixed nuclear-cytoplasmic labeling in the hippocampus, and moderately elevated labeling in a number of other sites, including the supraoptic, paraventricular, and arcuate nuclei of the hypothalamus, cerebellar Purkinje cells, and all brain stem motor nuclei, similar to the locations of MR mRNA identified by in situ hybridization (9, 15, 65). Contrary to earlier predictions (70), we did not find evidence for elevated MR expression in the AV3V region of the hypothalamus relative to the low-level cytoplasmic labeling in neurons throughout the brain (55). In general, virtually every neuron in the brain exhibited a mild-to-moderate degree of cytoplasmic, reticulated MR-like immunoreactivity (for examples, see nonnuclear labeling outside HSD2 neurons in Figs. 5D and 7; see also Figs. 4 and 5 of Ref. 81), although the extent to which this ubiquitous, low-level immunoreactivity represents actual MR protein is unclear.
Fig. 5.
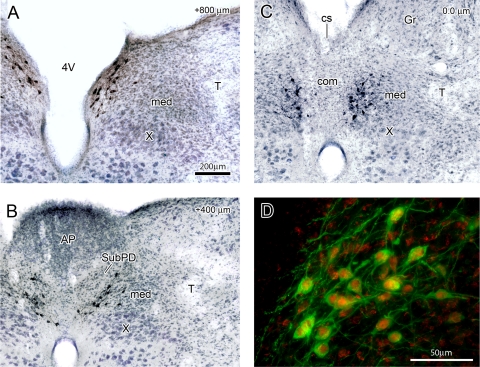
A–C: immunoreactivity for HSD2 (stained in black), which labels the aldosterone-sensitive subpopulation of neurons in the rat NTS, shown as a transverse (coronal) section at 3 characteristic rostrocaudal levels through the caudal NTS. A: just rostal to the area postrema (AP). B: a level containing the AP. C: where clusters of HSD2 neurons lie just ventral and caudal to the AP, spanning the medial (med) and commissural (com) NTS subnuclei. Sections were counterstained to reveal surrounding cytoarchitecture (blue), and distance rostral to calamus scriptorius (cs, in C) is shown at top right in A–C. D: rostral cluster of HSD2 neurons lining the 4V as in A. Note colocalization of cytoplasmic HSD2 (green) with nuclear immunoreactivity for the MR (red). Animal used in D was treated with aldosterone to maximize MR nuclear translocation (as described in Ref. 54). Gr, gracile nucleus; T, solitary tract; X, dorsal motor nucleus of the vagus nerve. [Reproduced with permission from Geerling and Loewy (62).]
Fig. 7.
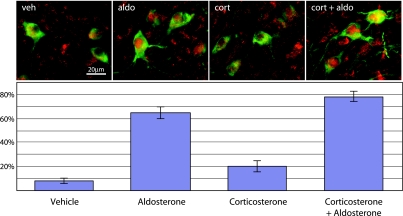
Within HSD2 neurons in the NTS, nuclear translocation of the MR is sensitive to and largely selective for physiological concentrations of blood-borne aldosterone. Adrenalectomized rats were treated with vehicle, aldosterone (74.4 ± 10.2 ng/dl), corticosterone (7,900 ± 1,400 ng/dl), or corticosterone + aldosterone. For each group, average percentage of HSD2-immunoreactive neurons (green) that exhibited dense, nuclear-filling MR immunoreactivity (red; nuclear MR represents activated receptors that have moved from the cytoplasm into the nucleus after agonist binding) is shown. Both aldosterone-treated groups exhibited a significant elevation relative to groups treated with vehicle or corticosterone alone, and addition of corticosterone to aldosterone did not produce significantly greater MR activation than aldosterone alone. Corticosterone alone produced a small, but significant, increase relative to vehicle. [Reproduced with permission from Geerling et al. (54).]
One group of cells, however, stood out from the rest because of their dense nuclear immunolabeling. In the brain stem, we found a small cluster of MR-immunoreactive nuclei in the caudal NTS (55). This caudal, medial region of the NTS had been noted to exhibit MR mRNA hybridization in a previous study (9), so we hypothesized that the nuclear pattern of immunoreactivity here marked actual MR protein within a novel population of aldosterone-sensitive cells.
In retrospect, this initial observation was somewhat serendipitous: on further inspection, animals with minimal or undetectable aldosterone levels do not exhibit this pattern of dense nuclear MR immunoreactivity (54). The brain sections in which these nuclei were initially found were taken from rats that had been anesthetized and perfused immediately on arrival from an animal supplier, suggesting that they were somewhat hypovolemic (with elevated aldosterone production) after many hours in transit.
NEURONS FORMING A SMALL SUBPOPULATION OF THE NTS ARE ALDOSTERONE-SENSITIVE AND ALDOSTERONE-SELECTIVE
As discussed above, the first step in determining whether a group of MR-expressing cells might be aldosterone-sensitive is to test whether they also express HSD2. Immunofluorescence staining for HSD2 in the NTS revealed abundant cytoplasmic labeling surrounding the nuclear-concentrated MR immunoreactivity in the same subpopulation of cells (Figs. 5 and 6). These cells demonstrate the same distinctive NTS distribution as the pattern of HSD2 mRNA first identified by Roland et al. (150; compare Fig. 5B with Fig. 3, A and B).
Fig. 6.
Rostrocaudal distribution of aldosterone-sensitive neurons (labeled by immunoreactivity for HSD2, stained brown) in the NTS in a dorsal horizontal section (A) and in a parasagittal section (B) through the caudal medulla. Each section was counterstained (blue) to reveal surrounding cytoarchitecture. HSD2 neurons form a dense rostral cluster abutting the 4V, as shown in A and extend caudally in an elongated group just dorsal to the dorsal motor nucleus of the vagus nerve (X), as shown in B. XII, hypoglossal motor nucleus. [Reproduced with permission from Geerling and Loewy (62).]
By revealing their characteristic dendritic morphology (Figs. 5 and 6), HSD2 immunolabeling also revealed that these cells are neurons, not glia. Interestingly, their cell bodies and dendritic arbors occupy a subregion of the NTS with a diminished BBB extending ventrally beneath the area postrema (16, 79). This location may afford them more exposure to circulating aldosterone than other MR-expressing cells in the CNS, potentially explaining the ability of this hormone to exert central effects, despite its poor penetration of the BBB.
After colocalization of the MR and HSD2 in these putative aldosterone-sensitive neurons, it became of interest whether they would exhibit any functional response to circulating aldosterone. The pronounced nuclear translocation of steroid receptors in response to agonist binding (44, 90) allowed us to address this question using a simple morphological assay. After rats were adrenalectomized (to eliminate endogenous corticosteroid production), very few HSD2-expressing neurons in the NTS contained nuclear-concentrated MR. In contrast, in animals infused with aldosterone (with or without corticosterone), the percentage of HSD2 neurons with nuclear-localized MR was greatly elevated relative to that in animals treated with vehicle or corticosterone alone (Fig. 7).
In these experiments, a dose of aldosterone was selected to produce plasma concentrations (average ∼75 ng/dl produced by 36 μg/day minipump infusion) between baseline levels and the amounts stimulated by dietary sodium deprivation in rats (100–300 ng/dl) (38); in humans, these concentrations would be in the pathophysiological range (118). The dose of corticosterone (subcutaneous 25% continuous-release pellets) was chosen to produce a plasma concentration (∼8,000 ng/dl) that is well within the physiological range for this hormone and ∼100-fold higher than the aldosterone infusion; the reduced aldosterone concentrations of adult humans result in an aldosterone-to-cortisol ratio typically closer to 1:1,000 (6). Interestingly, although it did not significantly alter aldosterone-induced nuclear translocation in these experiments, corticosterone produced a small, but significant, increase in MR nuclear translocation. This slight, residual effect of corticosterone in HSD2 neurons may be related to its higher affinity for the MR (Km ∼1 nM) (157) than for HSD2 (Km ∼10 nM in rat) (194), whereas in cells without HSD2, much lower concentrations of corticosterone produce complete nuclear translocation of MR (44). Thus our data confirmed that the HSD2 neurons in the NTS are selectively sensitive to aldosterone in vivo, at least in the binding and nuclear translocation of the MR.
Given that aldosterone gains access to the MR in these cells at physiological blood levels, the next logical question was: How does activation of the MR influence the activity of HSD2 neurons in the NTS? This question remains unanswered and largely unexplored. In hippocampal neurons tested in vitro, corticosterone and aldosterone produce a rapid, MR-mediated, nongenomic increase in the surface motility of synaptic glutamate receptors (GluR2), whereas corticosterone produces a delayed, GR-mediated genomic increase in surface expression of GluR2 and GluR1, coinciding with an increase in the amplitude of excitatory postsynaptic currents (78, 98). The relevance of delayed, MR-mediated transcriptional changes to neuronal function is less clear, and whether these or other mechanisms influence the postsynaptic dynamics of the aldosterone-sensitive HSD2 neurons in the NTS is an important topic for future exploration.
We have not yet tested the effects of aldosterone concentrations throughout its full physiological range (in rodents, prolonged dietary sodium deprivation or hyperkalemia produces plasma aldosterone concentrations >200 ng/dl), but moderate, short-term elevations in plasma aldosterone concentration (∼40–90 ng/day) do not increase the expression of one neuronal activity marker, c-Fos, in these neurons (J. C. Geerling, unpublished observations). If anything, lower doses slightly decrease the number of c-Fos-expressing HSD2 neurons in adrenalectomized animals (J. C. Geerling, unpublished observations), possibly as a consequence of the restoration of mineralocorticoid-dependent systemic sodium balance. Deoxycorticosterone, a less-potent MR agonist, produces a moderate increase in HSD2 neuron c-Fos activation when given in supraphysiological doses (2 mg/day for 7 days, a protocol used to stimulate sodium appetite) (54, 58). The mechanism(s) by which MR activation may influence the functionality of HSD2 neurons in the NTS (or MR-expressing neurons in general) remains an important topic for future investigation. Nonetheless, these findings and the activation of HSD2 neurons in adrenalectomized rats (see below) (54) indicate that whether or not aldosterone is capable of independently stimulating HSD2 neurons to fire, it is only one of the input signals they integrate to produce their unique pattern of activity.
Functional activation of HSD2 neurons in the NTS.
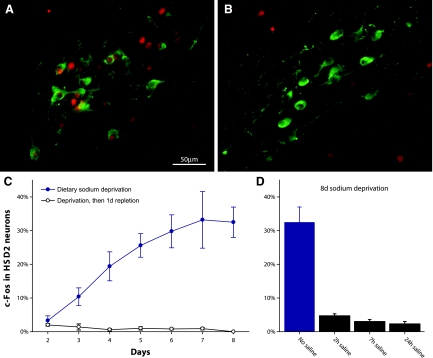
The HSD2 neurons in the NTS are the first phenotypically identified cells in the brain selectively activated in association with sodium deficiency. During dietary sodium deprivation, which is among the strongest physiological stimuli for aldosterone production (54, 61), the HSD2 neurons are activated in association with sodium appetite (Fig. 8). A variety of paradigms involving prolonged sodium deficiency and sodium appetite (dietary sodium deprivation, furosemide diuresis/natriuresis, and colloid-induced hypovolemia) produce a marked increase in nuclear c-Fos in these neurons. [c-Fos expression is a well-established indicator of neuronal activation (83, 151).] Importantly, however, HSD2 neuron activation in response to sodium deficiency does not require aldosterone or any other corticosteroid; adrenalectomized rats still exhibit increased c-Fos activation in these neurons after dietary sodium deprivation (54). Therefore, HSD2 neurons are not simple aldosterone detectors. Rather, they integrate multiple input signals associated with sodium deficiency. Besides aldosterone and various neural afferents (see below), these input signals remain to be discovered. One unexplored possibility is the sodium- and volume-sensitive hormone angiotensin II, for which receptor expression is abundant in this region of the NTS (120).
Fig. 8.
Aldosterone-sensitive HSD2 neurons in the NTS (green) are activated by chronic dietary sodium deprivation and then inactivated once salt is ingested. A: dramatic increase in percentage of HSD2 neurons expressing the neuronal activity marker c-Fos (red nuclear immunoreactivity) after 7 days of a sodium-free diet. B: virtual elimination of HSD2 neuronal activation (c-Fos immunoreactivity) when a sodium-deprived rat is switched to a high-sodium diet. C: progressive increase in percentage of HSD2 neurons exhibiting nuclear c-Fos immunoreactivity over 2–8 days of dietary sodium deprivation, paralleling a similar increase in sodium appetite (91, 164). Their activation was consistently eliminated in groups of control rats that were fed sodium-deficient chow but then switched to high-sodium chow for 24 h. D: rapid reversal of HSD2 neuronal activation by salt ingestion, resulting in a near-total loss of c-Fos immunoreactivity just 2 h after a drinking tube containing 3% NaCl solution was provided to salt-hungry rats (after 8 days of sodium-free diet). [Reproduced with permission from Geerling et al. (54).]
Initially, we focused on linking the HSD2 neurons to cardiovascular control during high-aldosterone states, such as primary aldosteronism and end-stage liver disease. However, even though some neurons in the NTS play well-established roles in cardiovascular regulation, the HSD2 neurons are located outside the distribution of baroreflex neurons that exhibit c-Fos activation during arterial hypertension (23, 181). Also, in contrast to numerous cardiovascular reflex neurons located in adjacent subnuclei of the NTS, the HSD2 neurons do not exhibit c-Fos activation, even after large decreases in blood pressure produced by hydralazine (J. C. Geerling, P. G. Guyenet, and A. D. Loewy, unpublished observations).
Instead, HSD2 neurons are situated within a subregion of the NTS that is innervated by subdiaphragmatic branches of the vagus nerve, which deliver information from the gut and related abdominal viscera (131). Yet, in contrast to most other neurons in this region of the NTS, HSD2 neurons are not activated after ingestion or intraperitoneal injection of hypertonic saline (60, 61). In fact, their activation during sodium deprivation or chronic mineralocorticoid treatment is abolished or attenuated by salt ingestion (58, 61). Thus, on the basis of available data in rats, the aldosterone-sensitive HSD2 neurons in the NTS exhibit a unique and highly selective activity profile that appears to be specifically associated with prolonged sodium deprivation.
Input-output connections of HSD2 neurons in the NTS.
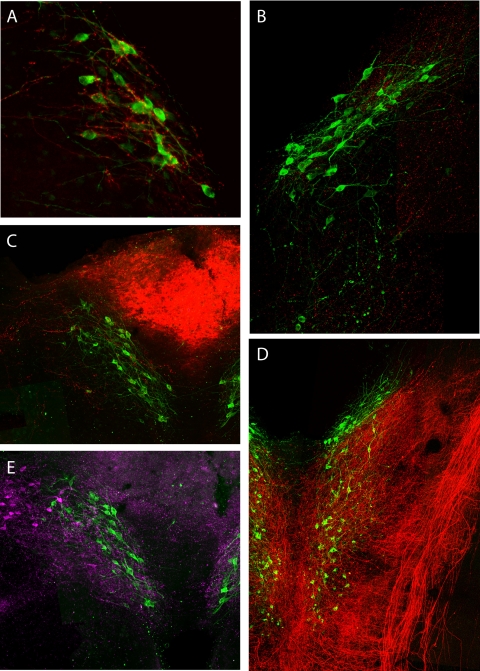
In addition to modulatory information provided by aldosterone (and perhaps other blood-borne mediators), HSD2 neurons receive neural input from a variety of sources. Most HSD2 neurons receive a small proportion of their input from as-yet-unidentified components of the vagus nerve (Fig. 9D) (159). They are heavily targeted by descending axonal projections from the central nucleus of the amygdala (Fig. 9A) (56), which is prominently involved in the regulation of sodium appetite (for review see Refs. 56, 62, 94). HSD2 neurons are also among the many NTS neurons innervated by the paraventricular nucleus of the hypothalamus (Fig. 9B) (J. C. Geerling, J. W. Shin, P. C. Chimenti, and A. D. Loewy, unpublished observations). Locally, HSD2 neurons are targeted by a group of neurons in the dorsomedial NTS that express neurotensin and receive input from neurons in the overlying area postrema (Fig. 9, C and E) (155). Presumably, these neural input connections are integrated with information from humoral modulators, including aldosterone, to shape the unusual activity profile of these neurons.
Fig. 9.
Aldosterone-sensitive neurons of the NTS (green, HSD2 immunoreactivity) integrate neural input from a wide variety of sources. A: central nucleus of the amygdala (medial subdivision). Axons and boutons labeled after tracer injection (red) densely entwine HSD2 neuronal somata and proximal dendrites. Transverse section. [Reproduced from Geerling and Loewy (56).] B: axons from the paraventricular nucleus of the hypothalamus (magenta) target many neuronal subpopulations within the NTS, including HSD2 neurons. Horizontal section. C: anterograde tracer injections into the AP (red) produce scattered anterograde labeling in the underlying NTS, including axons that target a minority of the HSD2 neurons. Transverse section. [Reproduced from Sequeira et al. (155).] D: filling the nodose ganglion (cell bodies of vagal afferents to the NTS) with an axonal tracer reveals dense projections covering most of the NTS, including a moderate input to HSD2 neurons caudal to the 4th ventricle. Horizontal section. [Reproduced from Shin et al. (159).] E: neurons in the dorsomedial NTS, which produce the neuropeptide neurotensin (magenta), densely innervate HSD2 neurons and other surrounding populations in their subregion of the NTS. Transverse section. [Reproduced from Sequeira et al. (155).]
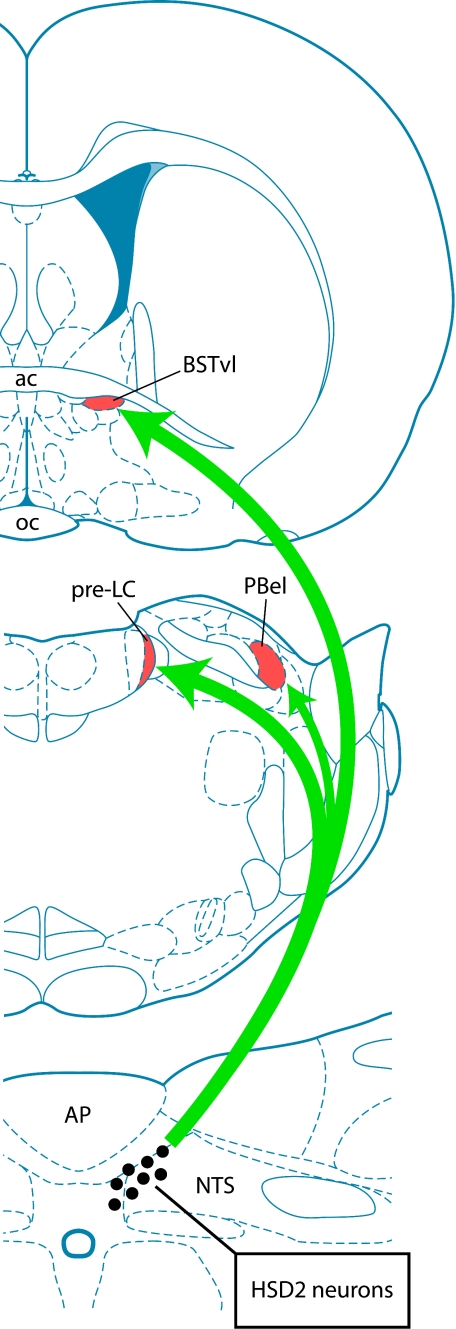
The output connections of HSD2 neurons are similarly unique and unexpected. Initially, we had anticipated that these aldosterone-sensitive neurons were involved primarily in the autonomic control of cardiovascular functions. However, despite hundreds of neural tracing experiments using traditional (monosynaptic) and viral (transneuronal) tracers, we were unable to find evidence for substantial efferent connections from these neurons to sympathetic and parasympathetic outflow sites in the hypothalamus, brain stem, spinal cord, and periphery (57). Instead, as shown in Fig. 10, the axons of HSD2 neurons extend to sites in the forebrain and forebrain-relay nuclei in the rostral brain stem, which have been implicated in behavioral changes related to sodium appetite (57, 62), reward (156), arousal (113), and mood (32, 153).
Fig. 10.
HSD2 neurons in the NTS project to a limited number of targets, including a pair of relay nuclei in the rostral, dorsolateral pons [the pre-locus ceruleus (pre-LC) and the innermost subdivision of the external lateral parabrachial nucleus (PBel)] as well as a small subregion of the ventrolateral bed nucleus of the stria terminalis (BSTvl). Other, more minor efferent target sites include the A8/A10 ventral tegmental area in the midbrain and the parasubthalamic nucleus in the caudal lateral hypothalamus (for details, see Ref. 57). ac, Anterior commissure; oc, optic chiasm.
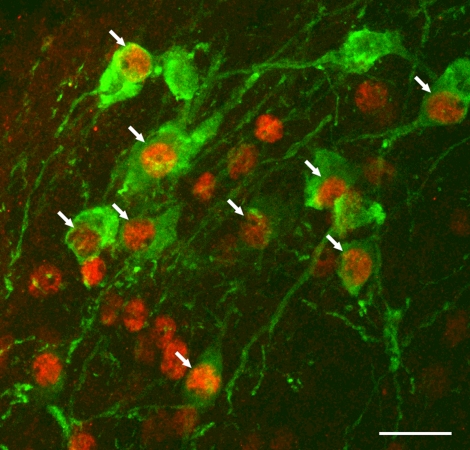
Their axons probably release the fast excitatory neurotransmitter glutamate, on the basis of the expression by HSD2 neurons of the transcription factor Phox2b, a selective marker for glutamatergic neurons in the NTS (Fig. 11) (53, 97), and on the basis of the concurrent increases in neuronal activation in their efferent target neurons during dietary sodium deprivation (61). It remains to be determined whether they express or corelease any other neuromodulators or synaptic transmitters. Besides their Phox2b expression, these neurons may represent a novel neuronal phenotype in the NTS, because HSD2 does not colocalize with a variety of other makers for peptidergic or monoaminergic neurons in this nucleus (55).
Fig. 11.
HSD2-immunoreactive neurons in the NTS (green) express the nuclear transcription factor Phox2b (red; arrows indicate colocalization). Other Phox2b-immunoreactive nuclei represent non-aldosterone-sensitive NTS neurons that are intermingled with the HSD2 group. Scale bar, 25 μm. [Reproduced with permission from Geerling et al. (53).]
Possible functions of the aldosterone-sensitive HSD2 neurons.
HSD2 neurons in the NTS integrate a wide variety of neural and humoral stimuli and generate an output signal that is selectively associated with physiological sodium deficiency. They then deliver this information to a unique network of subcortical neurons in the rostral brain stem and forebrain. It remains to be proven exactly what influence(s) they exert over any behavioral, neuroendocrine, or autonomic functions, although a few possibilities are suggested by the known functions of their efferent target sites and by the behavioral changes associated with elevated aldosterone.
The most obvious hypothesis, that these neurons stimulate sodium appetite, follows from a number of findings (for review see Ref. 62). 1) Mineralocorticoids selectively boost salt intake, and the HSD2 neurons in the NTS are the only definitively identified aldosterone target cells in the brain. 2) Their activity pattern appears to match the appearance and disappearance of sodium appetite; they are the only identified neurons with this property: activation specifically in association with sodium deprivation and then inactivation once salt is ingested (54, 58, 60, 61). 3) Most of the brain sites connected to the HSD2 neurons have been implicated in the stimulation or inhibition of sodium appetite (56, 57, 61; for review see Refs. 62 and 155). Despite a great deal of circumstantial evidence, however, firm conclusions await future experiments because of the anatomic complexity of this subregion of the NTS. This relatively small group of aldosterone-sensitive neurons is closely surrounded by a much larger population of neurons with the opposite pattern of activation (61), making traditional lesion or stimulation experiments in this region difficult to interpret. Nevertheless, the existence and characteristics of these neurons could help explain the paradoxical increases in salt intake in rats after systemic inhibition of HSD2 (31) and in a human with impaired HSD2 function (89), despite the concurrent sodium retention in each case, which should otherwise inhibit salt intake.
Whether or not the HSD2 neurons increase sodium appetite, they may help generate the negative affective state associated with sodium deprivation and elevated aldosterone production. In rats, hedonic behaviors, such as sucrose ingestion and rewarding self-brain stimulation, are blunted by sodium depletion or chronic mineralocorticoid administration (77, 124). Humans, similarly, report pronounced gustatory anhedonia during chronic sodium deprivation (119). In rats, chronic systemic infusions of aldosterone increase anxiety-like behavior (82) and, in humans, cause a state of “malaise” in that lacks any apparent physiological basis (11). Primary aldosteronism is linked to an elevated rate of generalized anxiety disorder (160), and depressive symptoms, the presenting complaint for some patients with hyperaldosteronism, may remit after spironolactone treatment or unilateral adrenalectomy (101, 117). Elevated aldosterone production, particularly at night, has been identified in association with depression (43, 125).
In light of these findings, it may be worth noting that the HSD2 neurons represent a major input to the ventrolateral bed nucleus of the stria terminalis (BSTvl) (158), a key control point for generating negative affective states in a variety of experimental paradigms (32, 35, 153, 178). For example, BSTvl noradrenergic input from the A2 neurons in the NTS is responsible for the avoidant behaviors associated with opiate withdrawal (35, 178). The exclusively inhibitory neurons in the BSTvl represent the most concentrated source of input in the entire brain to the subgroup of wake- and activity-promoting neurons in the lateral hypothalamic area (LHA), which produce the neuropeptide orexin (189), suggesting a disynaptic route by which aldosterone, acting on the HSD2 neurons, may influence behavioral arousal.
As mentioned above, the HSD2 neurons do not appear to provide output connections to parts of the nervous system that directly increase blood pressure. The primary roles of neurons in most of their target sites are related to behavioral functions, not autonomic control. Some of their target sites do, however, exert parallel influences over various autonomic functions. For example, stimulating neurons in the BSTvl, or in a caudal region of the LHA that receives moderate input from the HSD2 neurons (57), produces a robust depressor response (25, 26). Part of the BSTvl depressor response may result from their direct inhibition of LHA orexin neurons, which provide sympathoexcitatory drive to autonomic sites in the brain stem and spinal cord (63, 100). Similarly, pressor or depressor responses can be elicited by stimulating various subregions of the pontine parabrachial region (22, 80), which could include the subnuclei targeted by HSD2 neurons in the NTS (Fig. 10). Stimulation of various subregions of the central nucleus of the amygdala, the lateral subdivision of which may receive parabrachial-relayed input from HSD2 neurons in the NTS (56), can produce gastrointestinal and cardiovascular (pressor and depressor) autonomic responses. Thus it is possible that the HSD2 neurons influence blood pressure and other autonomic variables indirectly, in parallel with behavioral changes, because of the strong links from brain regions that influence hedonic behaviors to brain stem and hypothalamic circuits that regulate autonomic functions. Nonetheless, on the basis of available neuroanatomic data, cardiovascular control does not appear to be the primary function of the HSD2 neurons. By extension, cardiovascular control may not be the primary role of aldosterone action in the brain, inasmuch as these are the only identified cells in the CNS for which there is definitive evidence of sensitivity to physiological levels of circulating aldosterone.
In summary, the aldosterone sensitivity, activational profile, and input-output connections of HSD2 neurons in the NTS suggest a number of potential functions related to sodium appetite, mood, and arousal. The process of characterizing these cells and the networks to which they are connected is in its infancy, but several intriguing possibilities have arisen. It is hoped that the next several years will yield exciting developments in our understanding of aldosterone-sensitive brain circuits.
OTHER BRAIN SITES WITH PURPORTED ALDOSTERONE SENSITIVITY
HSD2 expression outside the NTS.
Using a variety of immunohistochemical methods, we have searched the entire CNS for other sites of HSD2 expression in adult rats under a variety of physiological conditions. Outside the NTS, we detected labeling for this enzyme in only three sites: 1) a handful of neurons just rostral to the NTS, scattered along the fourth ventricular border in the medial vestibular nucleus, 2) the subcommissural organ (SCO), and 3) a caudal ventrolateral subdivision of the ventromedial nucleus of the hypothalamus (VMHvl). This pattern of HSD2 protein localization corresponds exactly with the distribution of HSD2 mRNA reported in rat brain by Roland et al. (compare Fig. 12 with Fig. 4) (150). In mice, HSD2 mRNA in the adult brain has been reported only in the NTS (84).
Fig. 12.
Outside the NTS, HSD2-immunoreactive cells in the adult rat brain exist in the SCO (A) and in the caudal VMHvl (B). All the columnar ependymal (nonneuronal) cells of the SCO are consistently and intensely immunoreactive for HSD2, whereas the VMHvl contains weaker immunoreactivity in a small subgroup of neurons. Scale bar, 200 μm. [Reproduced with permission from Geerling et al. (55).]
In HSD2-expressing cells outside the NTS, elevated immunoreactivity for MR protein was not evident, even in aldosterone-infused or sodium-deprived rats, beyond reticular cytoplasmic labeling produced with the Kawata MR antiserum, which is found in virtually every neuron in the brain (Figs. 5D and 7) and does not translocate into the nucleus in response to aldosterone or corticosterone treatment (54, 55). In a previous in situ hybridization study (9), the presence of MR mRNA was not reported in the SCO or VMHvl, so it remains unclear whether this pattern of immunoreactivity represents widespread, low-abundance MR expression or simply off-target cross-reactivity with this polyclonal antiserum. There is no evidence excluding the possibility that cells in one or more HSD2-expressing sites outside the NTS express functional MR, but on the basis of existing data, cells at these sites are at least qualitatively different from the HSD2 neurons in the NTS in terms of the amount of MR and/or its accessibility to circulating aldosterone. Similarly, HSD2-expressing cells in these other sites do not exhibit c-Fos labeling under conditions that produced robust activation in the NTS group (54).
Among these other sites, the sparse HSD2-expressing neurons near the medial vestibular nucleus were too few in number for systematic analysis (0–2 per section, scattered along a short expanse of the rostral medulla) (55, 150). Because of their morphology and location adjacent to the fourth ventricle, these neurons may be anatomic outliers related to the primary group of HSD2 neurons located nearby in the NTS.
The SCO, on the other hand, represents perhaps the most concentrated site of HSD2 expression in the adult rat brain (55, 148, 150). Its tightly packed columnar ependymal (nonneuronal) cells lie in the roof of the cerebral aqueduct beneath the posterior commissure. They secrete a glycoprotein thread that extends throughout the caudal ventricular system, known as Reissner's fiber. The SCO appears to degenerate in humans shortly after birth, but it may be important for facilitating the laminar flow of cerebrospinal fluid (CSF) through the cerebral aqueduct and has been implicated in the pathogenesis of congenital hydrocephalus (142). Its access to circulating aldosterone may be rather limited, because, unlike the brain's other circumventricular organs, it is protected by an intact BBB without fenestrated capillaries (40, 180). Exclusion of circulating aldosterone by the BBB may explain why we did not observe nuclear MR translocation here, in contrast to the NTS (54), despite the fact that cytoplasmic MR immunoreactivity appears increased in the SCO relative to other ventricular ependymal cells (J. C. Geerling, unpublished observations). Alternatively, the SCO could gain access to aldosterone via the CSF, where the aldosterone concentration may more closely approximate blood levels (96), possibly because of the absence of the mdr1 transporter from the blood-CSF barrier in the vasculature and epithelium of the choroid plexus (24, 161). Interestingly, long before the discovery of HSD2, the rat SCO was implicated in regulation of aldosterone production (134) and sodium excretion (39), but the physiological relevance of these findings is unclear.
The caudal VMHvl contains a small cluster of neurons that exhibit a weak HSD2 mRNA hybridization signal and scant immunoreactivity that is much less intense than that in the NTS and SCO (compare Figs. 4C and 12B with Figs. 3 and 4, A and B, and Fig. 5, A–C) (55, 150). The morphology of these cells is similar to that of HSD2 neurons in the NTS, and their distribution overlaps a comparable region with a compromised BBB (adjacent to the median eminence and arcuate nucleus) (16). This location may permit increased accessibility to circulating aldosterone, although we did not observe MR nuclear translocation in response to aldosterone infusion or sodium deprivation, and only weak cytoplasmic labeling for the MR was found in this region, similar to neurons throughout the rest of the brain (55). The VMHvl appears to be involved in reproductive behavior; many neurons in the VMHvl are notable for their strong estrogen receptor expression (141), activation during female sexual behavior (30), and axonal projections to the dorsal periaqueductal gray matter (21). The role of HSD2 in the VMHvl remains unknown.
Transient expression of HSD2 in the developing brain.
In embryos, even before the onset of MR expression, HSD2 can be found in many additional brain sites. Most of this HSD2 expression disappears around the time of birth (18, 37, 148). In the developing cerebellum of mice and rats, HSD2 expression persists for 1 or 2 wk after birth, protecting the normal proliferation of granule cell precursors from the growth inhibition and apoptotic effects of glucocorticoid exposure (84, 130). This GR-shielding mechanism may be similarly important for the normal development of other brain regions that exhibit transient HSD2 expression (154).
The transitory nature of HSD2 expression in many parts of the brain became a source of confusion recently in the anatomic characterization of a transgenic mouse engineered to express Cre recombinase under the control of the HSD2 promoter (59, 127). In HSD2-Cre mice crossed with a lacZ reporter strain, X-gal staining was used to visualize cells producing β-galactosidase (indicative of Cre-mediated recombination, either ongoing or earlier in their developmental lineage). In the brain, positive staining was observed in a large number of unexpected sites, including the pontine nuclei, superior colliculus, “hypothalamic region,” and “thalamic nuclei” (see Table 1 of Ref. 127). In the kidney, X-gal labeling was found in cells that contained HSD2 immunoreactivity, yet no such confirmatory data were provided for the brain.
A major problem with this approach is that Cre expression at any point in the developmental history of an animal results in irreversible recombination, permanently marking that cell and all its mitotic progeny (41). Although not emphasized explicitly by these investigators (but see Ref. 59), in these animals most transgene-reporter expression in the brain probably resulted from short-lived expression of HSD2 during embryogenesis.
Nonetheless, this anatomic study provides an interesting, retrospective point of view by revealing all postmitotic cells derived from any lineage that ever expressed HSD2. For example, most of the X-gal-positive sites in the brain stem and cerebellum shared a common origin, the math1/atoh1-expressing subset of cells in the rhombic lip (176), which suggests an important protective role for HSD2 in the development of brain stem and cerebellar neurons derived from this rhombencephalic lineage. However, in the absence of evidence for actual gene or protein expression in adult animals, these data should not be misinterpreted as evidence for a pattern of HSD2 in the adult brain beyond that supported by existing data (55, 148, 150).
Paraventricular nucleus of the hypothalamus.
Another proposed target site for aldosterone is the paraventricular nucleus of the hypothalamus (PVH). This brain region provides direct axonal projections to a variety of viscerosensory and autonomic motor nuclei in the brain stem and spinal cord (114) and plays a well-established role in maladaptive autonomic and neuroendocrine responses during certain pathophysiological states such as heart failure (192). The PVH exhibits a weak hybridization signal for MR mRNA (174, but see Ref. 9) and immunohistochemical labeling for MR protein (81), consistent with our own unpublished observations, but no HSD2 mRNA or protein could be detected by in situ hybridization (37, 148, 150) or immunohistochemistry (55).
The presence of HSD2 mRNA in the PVN was reported after RT-PCR amplification showed a 2.6-fold higher level of expression in tissue micropunches from this region than in tissue from the cerebral cortex (193). Unfortunately, no comparison was made with positive control tissues such as the kidney or brain sites with established HSD2 expression (NTS, SCO, and VMHvl), so the significance of this level of gene expression remains unclear. Also, HSD2 expression in the PVN cannot be detected using the most sensitive anatomic methods (37, 55, 148, 150), so it remains unknown which cell type(s) in the PVN produces HSD2 mRNA (possibilities include astrocytes, neurons, vascular smooth muscle cells, endothelial cells, ependymal cells, tanycytes, and microglia).
Nonetheless, pharmacological data have been advanced to support the possibility of functional HSD2 in the PVH. Building on the previous finding that blood pressure can be elevated by central infusion of glycyrrhizic acid (a nonspecific HSD inhibitor found in liquorice) or its synthetic derivative carbenoxolone (74), Zhang and colleagues (193) found acute (onset <10 min) increases in sympathetic outflow and PVH neuronal activity after intracerebroventricular administration of these compounds (193).
In the experiments of Zhang et al. (193), the concentrations of carbenoxolone and glycyrrhizic acid injected into the brain (13–35 and 10 mM, respectively) were more than three orders of magnitude higher than the concentrations necessary for inhibiting HSD2 and may directly activate MR (7, 8) and block local steroid metabolism (110). The effects of aldosterone or any other MR agonist were not tested, and experimental data from adrenalectomized control animals were not provided, so it remains unclear whether the rapid responses (onset <10 min) produced by these two compounds represent off-target drug effects or acute, nontranscriptional effects of MR activation by endogenous corticosteroids, as suggested by Zhang et al. In support of their conclusion, the response to ventricular administration of carbenoxolone was blocked by pretreatment with spironolactone, an MR antagonist (193). Testing the effects of aldosterone (the endogenous agonist proposed to stimulate HSD2-protected MR in the PVH under physiological conditions) and verifying the specificity of centrally infused HSD inhibitors in HSD2-knockout mice (12, 103) would be highly useful for establishing the physiological significance of these pharmacological findings.
Aldosterone synthesis inside the brain.
At least a marginal level of gene expression for most steroid synthetic enzymes has been detected using RT-PCR amplification of mRNA in tissue from various regions of the brain (67, 68, 115, 116, 190). The low-level expression of some of these enzymes may be regulated in the brain with some semblance to their counterparts in the adrenal gland (185, 186). Much of the brain's steroid synthetic machinery is geared toward producing GABA receptor-modulatory neurosteroids (147), but minute fractions of corticosterone and aldosterone have been produced in vitro by incubation of brain tissue or cultured hippocampal neurons with their synthetic precursor deoxycorticosterone (67, 68), which is a much less polar steroid (n = 3, compare with others in Fig. 1) and freely penetrates the BBB (105). These findings suggest the possibility that the brain contains a paracrine system for aldosterone production, thereby curtailing its limited BBB penetration and, potentially, even overcoming the competition barrier presented by the 1,000-fold higher circulating glucocorticoid concentrations. The production of aldosterone in physiologically relevant quantities (exceeding the BBB-penetrant fraction of adrenal-derived aldosterone) would have profound implications for MR signaling in the brain, but a number of considerations have tempered enthusiasm for this provocative possibility.
First, the expression levels of most steroid synthetic enzymes are much lower in the brain than in the adrenal gland. This is particularly true for aldosterone synthase, the rate-limiting control point for aldosterone production in the adrenal cortex, where it is expressed at a >1,000-fold higher level (186, 190). Second, in contrast to the adrenal cortex, there is no consistent evidence for an anatomic distribution of cells that produce aldosterone synthase in the brain. To our knowledge, mRNA for this enzyme has not been found in the brain by in situ hybridization, and an attempt to localize this enzyme by immunohistochemical staining resulted in a labeling pattern inconsistent with RT-PCR results from the same study (115). Third, there are major species differences in aldosterone synthase expression in the brain. One group found evidence for expression in rat, but not mouse, brain (165), and a more recent report failed to identify expression anywhere in the human brain (116). Fourth, and perhaps most importantly, the small amount of aldosterone detected in the brains of adrenal-intact animals rises and falls in direct proportion to plasma aldosterone, and little or no aldosterone can be detected in the brain after adrenalectomy (71, 191). The low-picogram quantity of aldosterone remaining in the brains of some adrenalectomized animals (possibly representing tissue-bound, adrenal-derived aldosterone) decreases more than fivefold between postoperative days 2 and 7, and in contrast to earlier in vitro results, the provision of BBB-penetrant synthetic precursors (deoxycorticosterone or corticosterone) does not alter its abundance (71). These findings do not eliminate the possibility that a relevant amount of aldosterone is produced and rapidly cleared somewhere in the brain, in one or more extremely restricted sites, although this theory would seem difficult to reconcile with the widespread, minimal expression of rat aldosterone synthase found by RT-PCR and the lack of any evidence for a more concentrated site of expression with use of anatomic techniques.
Consistent with the possibility of physiologically relevant synthesis of aldosterone within the brain, however, the salt-induced hypertension of Dahl rats was prevented by intracerebroventricular infusion of trilostane, which inhibits 3β-HSD (3 synthetic steps upstream of aldosterone synthase) (75), or by certain doses of 19-ethynyldeoxycorticosterone, which inhibits aldosterone synthase (67). Also, central infusion of FAD286 (a relatively uncharacterized, benzonitrile-containing compound that reduces adrenal aldosterone production) (45) somehow attenuated the hypertension and sympathetic activation produced by direct infusion of hypertonic saline into the CSF (88).
As with aldosterone in the PVH, conclusions in this area are built largely on a foundation of RT-PCR and pharmacological data and would benefit greatly from more definitive hypothesis-testing experiments. For example, the specificity of the aforementioned drug effects could be tested in knockout mice that lack aldosterone synthase (111).
Where in the brain does the activation of MR increase blood pressure?
Despite the abundance of evidence that aldosterone increases blood pressure when infused directly into the brain, the relevance of this mechanism to cardiovascular control under physiological or even pathophysiological conditions remains uncertain. In addition to the inconvenient facts highlighted above, i.e., the poor BBB penetration of aldosterone, its uphill battle for MR in cells without HSD2, and the lack of HSD2 expression in hypothalamic sites with presumed aldosterone sensitivity in previous studies, a number of experimental findings suggest that the physiological significance of aldosterone's central pressor effect may have been overestimated.
For example, a concurrent central infusion of an equivalent dose of corticosterone diminishes the central pressor effect of aldosterone (76). This important finding suggests that the cells mediating this pressor effect express MR, but not HSD2, allowing corticosterone to block aldosterone from binding MR. In such cells, no physiologically relevant quantity of aldosterone could outcompete the much higher concentrations of circulating corticosterone, which more readily penetrates the BBB (Fig. 1). Unfortunately, studies demonstrating a pressor effect for aldosterone typically have not measured the central concentrations produced by intracerebroventricular infusion of aldosterone and corticosterone.
Also unmeasured in most of these studies were changes in the CSF electrolyte composition. Aldosterone and deoxycorticosterone decrease the CSF potassium and increase CSF sodium concentration (10, 102, 140), whereas spironolactone reduces CSF sodium concentration (33). These ionic changes are significant, because increasing CSF sodium concentration independently produces hypertension (19, 86, 87, 99), at least in part by stimulating osmo- and sodium-sensory neurons in the AV3V region of the hypothalamus (133), an effect that can be blocked by central infusion of benzamil, a sodium channel blocker (1). Conversely, restoration of CSF potassium by intracerebroventricular infusion attenuates the pressor effects of chronic mineralocorticoid treatment (102). Thus an altered ionic permeability of one or more central epithelia may play a major role in the central MR pressor effect. Multiple fluid interfaces in the brain remain relatively unexplored in this regard. These include the choroid plexus, through which blood plasma is filtered to form the CSF; the arachnoid granulations, where CSF is reabsorbed into the blood; the ventricular ependyma and pia, which separate the brain parenchyma from the CSF; and the entire capillary vascular endothelium, which is a key structural component of the BBB.
The only identified neurons with definitive sensitivity to physiological levels of circulating aldosterone (the HSD2 neurons in the NTS) do not seem likely to increase blood pressure in response to aldosterone. Available neuroanatomic evidence regarding the output connections of these neurons is not consistent with a major pressor role (if anything, they innervate primarily depressor sites) (25, 26, 57). Furthermore, the sensitivity of blood pressure to a central MR antagonist remains unchanged after vastly different dietary sodium regimens (8%, 0.4%, and 0% sodium for 3 wk) (143) that strongly influence aldosterone production and HSD2 neuron activation (50, 54). This finding suggests that aldosterone action in HSD2-expressing cells may not be involved, because any MR-mediated changes in these cells should be far more sensitive to receptor blockade when endogenous aldosterone production is maximal (the low-sodium condition).
On the other hand, the MR-dependent pressor effect of pharmacological HSD antagonists seems consistent with a pressor role for these neurons (74, 193). Blood pressure remains well compensated during a condition that potently activates HSD2 neurons in the NTS (sodium deprivation) (119), and one study even found an increase in blood pressure after chronic sodium deprivation in weanling rats (179). Thus HSD2 neurons in the NTS could somehow contribute to the compensatory support of blood pressure during hypovolemia via as-yet-undiscovered pathways.
A more relevant issue than whether aldosterone is involved is determination of the cells and mechanism(s) responsible for the pressor effects of central MR activation. Previous proposals were derived more from knowledge about the autonomic involvement of various brain sites than from any specific evidence for their aldosterone sensitivity (70, 107, 193). Nonetheless, ideas regarding the MR in the AV3V and forebrain circumventricular organs remain tenable, even if aldosterone is not the physiological agonist. Also, the finding that pressor and depressor responses to MR activation and inhibition appear primarily in combination with a psychological stressor (restraint) (34, 171) suggests the intriguing possibility that glucocorticoids decrease the activational threshold of MR-expressing neurons at limbic sites, such as the hippocampus, amygdala, and hypothalamus (9), which in turn drive coordinated behavioral and autonomic responses to stress.
In summary, various MR agonists, MR antagonists, and HSD antagonists clearly influence blood pressure when they are infused directly into the brain. A number of findings suggest that aldosterone itself, at physiological concentrations, may not directly influence the central control of blood pressure. The central sites and mechanisms of MR-mediated pressor responses remain poorly understood.
CONCLUSIONS
The HSD2 neurons of the NTS are the only cells in the CNS for which there is definitive evidence of physiological aldosterone sensitivity. The exact role of the aldosterone-MR interaction in modulating the neuronal activity of these cells remains unknown. These neurons integrate hormonal and neural information from a wide variety of sources and produce an unprecedented output signal that is selectively associated with systemic sodium deficiency. They deliver this output signal to other brain sites involved in behavioral functions, including appetite, mood, and arousal. Other potential sites and mechanisms for mineralocorticoid action in the CNS, as well as alternative functions for HSD2 in a handful of sites outside the NTS, remain active areas of investigation. Nonetheless, the aldosterone-sensitive HSD2 neurons in the NTS remain the only cells in the brain with demonstrated in vivo sensitivity to physiologically relevant concentrations of circulating aldosterone and the only phenotypically identified neurons known to be activated selectively in association with physiological sodium deficiency.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-25449 (A. D. Loewy) and the McDonnell Center for Systems Neuroscience, Washington University School of Medicine (J. C. Geerling).
REFERENCES
- 1.Abrams JM, Osborn JW. A role for benzamil-sensitive proteins of the central nervous system in the pathogenesis of salt-dependent hypertension. Clin Exp Pharmacol Physiol 35: 687–694, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agarwal AK, Monder C, Eckstein B, White PC. Cloning and expression of rat cDNA encoding corticosteroid 11β-dehydrogenase. J Biol Chem 264: 18939–18943, 1989. [PubMed] [Google Scholar]
- 3.Agarwal AK, Mune T, Monder C, White PC. NAD+-dependent isoform of 11β-hydroxysteroid dehydrogenase. Cloning and characterization of cDNA from sheep kidney. J Biol Chem 269: 25959–25962, 1994. [PubMed] [Google Scholar]
- 4.Agarwal MK, Mirshahi F, Mirshahi M, Rostene W. Immunochemical detection of the mineralocorticoid receptor in rat brain. Neuroendocrinology 58: 575–580, 1993. [DOI] [PubMed] [Google Scholar]
- 5.Ahima R, Krozowski Z, Harlan R. Type I corticosteroid receptor-like immunoreactivity in the rat CNS: distribution and regulation by corticosteroids. J Comp Neurol 313: 522–538, 1991. [DOI] [PubMed] [Google Scholar]
- 6.Ahokoski O, Virtanen A, Kairisto V, Scheinin H, Huupponen R, Irjala K. Biological day-to-day variation and reference change limits of serum cortisol and aldosterone in healthy young men on unrestricted diets. Clin Chem 45: 1097–1099, 1999. [PubMed] [Google Scholar]
- 7.Armanini D, Karbowiak I, Funder JW. Affinity of liquorice derivatives for mineralocorticoid and glucocorticoid receptors. Clin Endocrinol (Oxf) 19: 609–612, 1983. [DOI] [PubMed] [Google Scholar]
- 8.Armanini D, Karbowiak I, Krozowski Z, Funder JW, Adam WR. The mechanism of mineralocorticoid action of carbenoxolone. Endocrinology 111: 1683–1686, 1982. [DOI] [PubMed] [Google Scholar]
- 9.Arriza JL, Simerly RB, Swanson LW, Evans RM. The neuronal mineralocorticoid receptor as a mediator of glucocorticoid response. Neuron 1: 887–900, 1988. [DOI] [PubMed] [Google Scholar]
- 10.Atarashi K, Matsuoka H, Takagi M, Yamada K, Hirata Y, Hayakawa H, Sugimoto T. Effects of intracerebroventricular infusion of aldosterone on blood pressure and sodium and potassium concentrations in cerebral spinal fluid in rats. Clin Exp Hypertens A 10 Suppl 1: 317–322, 1988. [DOI] [PubMed] [Google Scholar]
- 11.August JT, Nelson DH, Thorn GW. Response of normal subjects to large amounts of aldosterone. J Clin Invest 37: 1549–1555, 1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bailey MA, Paterson JM, Hadoke PW, Wrobel N, Bellamy CO, Brownstein DG, Seckl JR, Mullins JJ. A switch in the mechanism of hypertension in the syndrome of apparent mineralocorticoid excess. J Am Soc Nephrol 19: 47–58, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beaumont K, Fanestil DD. Characterization of rat brain aldosterone receptors reveals high affinity for corticosterone. Endocrinology 113: 2043–2051, 1983. [DOI] [PubMed] [Google Scholar]
- 14.Birmingham MK, Sar M, Stumpf WE. Localization of aldosterone and corticosterone in the central nervous system, assessed by quantitative autoradiography. Neurochem Res 9: 333–350, 1984. [DOI] [PubMed] [Google Scholar]
- 15.Birmingham MK, Stumpf WE, Sar M. Nuclear localization of aldosterone in rat brain cells assessed by autoradiography. Experientia 35: 1240–1241, 1979. [DOI] [PubMed] [Google Scholar]
- 16.Broadwell RD, Sofroniew MV. Serum proteins bypass the blood-brain fluid barriers for extracellular entry to the central nervous system. Exp Neurol 120: 245–263, 1993. [DOI] [PubMed] [Google Scholar]
- 17.Brody MJ Central nervous system and mechanisms of hypertension. Clin Physiol Biochem 6: 230–239, 1988. [PubMed] [Google Scholar]
- 18.Brown RW, Diaz R, Robson AC, Kotelevtsev YV, Mullins JJ, Kaufman MH, Seckl JR. The ontogeny of 11β-hydroxysteroid dehydrogenase type 2 and mineralocorticoid receptor gene expression reveal intricate control of glucocorticoid action in development. Endocrinology 137: 794–797, 1996. [DOI] [PubMed] [Google Scholar]
- 19.Bunag RD, Miyajima E. Sympathetic hyperactivity elevates blood pressure during acute cerebroventricular infusions of hypertonic salt in rats. J Cardiovasc Pharmacol 6: 844–851, 1984. [DOI] [PubMed] [Google Scholar]
- 20.Campbell LE, Yu M, Yang K. Ovine 11β-hydroxysteroid dehydrogenase type 2 gene predicts a protein distinct from that deduced by the cloned kidney cDNA at the C-terminus. Mol Cell Endocrinol 119: 113–118, 1996. [DOI] [PubMed] [Google Scholar]
- 21.Canteras NS, Simerly RB, Swanson LW. Organization of projections from the ventromedial nucleus of the hypothalamus: a Phaseolus vulgaris-leucoagglutinin study in the rat. J Comp Neurol 348: 41–79, 1994. [DOI] [PubMed] [Google Scholar]
- 22.Chamberlin NL, Saper CB. Topographic organization of cardiovascular responses to electrical and glutamate microstimulation of the parabrachial nucleus in the rat. J Comp Neurol 326: 245–262, 1992. [DOI] [PubMed] [Google Scholar]
- 23.Chan RK, Sawchenko PE. Organization and transmitter specificity of medullary neurons activated by sustained hypertension: implications for understanding baroreceptor reflex circuitry. J Neurosci 18: 371–387, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choudhuri S, Cherrington NJ, Li N, Klaassen CD. Constitutive expression of various xenobiotic and endobiotic transporter mRNAs in the choroid plexus of rats. Drug Metab Dispos 31: 1337–1345, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Ciriello J, Janssen SA. Effect of glutamate stimulation of bed nucleus of the stria terminalis on arterial pressure and heart rate. Am J Physiol Heart Circ Physiol 265: H1516–H1522, 1993. [DOI] [PubMed] [Google Scholar]
- 26.Ciriello J, Solano-Flores LP, Rosas-Arellano MP, Kirouac GJ, Babic T. Medullary pathways mediating the parasubthalamic nucleus depressor response. Am J Physiol Regul Integr Comp Physiol 294: R1276–R1284, 2008. [DOI] [PubMed] [Google Scholar]
- 27.Coirini H, Magarinos AM, De Nicola AF, Rainbow TC, McEwen BS. Further studies of brain aldosterone binding sites employing new mineralocorticoid and glucocorticoid receptor markers in vitro. Brain Res 361: 212–216, 1985. [DOI] [PubMed] [Google Scholar]
- 28.Cole TJ Cloning of the mouse 11β-hydroxysteroid dehydrogenase type 2 gene: tissue specific expression and localization in distal convoluted tubules and collecting ducts of the kidney. Endocrinology 136: 4693–4696, 1995. [DOI] [PubMed] [Google Scholar]
- 29.Connell JM, Davies E. The new biology of aldosterone. J Endocrinol 186: 1–20, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Coolen LM, Peters HJ, Veening JG. Fos immunoreactivity in the rat brain following consummatory elements of sexual behavior: a sex comparison. Brain Res 738: 67–82, 1996. [DOI] [PubMed] [Google Scholar]
- 31.Cooney AS, Fitzsimons JT. Increased sodium appetite and thirst in rat induced by the ingredients of liquorice, glycyrrhizic acid and glycyrrhetinic acid. Regul Pept 66: 127–133, 1996. [DOI] [PubMed] [Google Scholar]
- 32.Davis M, Shi C. The extended amygdala: are the central nucleus of the amygdala and the bed nucleus of the stria terminalis differentially involved in fear versus anxiety? Ann NY Acad Sci 877: 281–291, 1999. [DOI] [PubMed] [Google Scholar]
- 33.Davson H, Segal MB. The effects of some inhibitors and accelerators of sodium transport on the turnover of 22Na in the cerebrospinal fluid and the brain. J Physiol 209: 131–153, 1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Kloet ER, Van Acker SA, Sibug RM, Oitzl MS, Meijer OC, Rahmouni K, de Jong W. Brain mineralocorticoid receptors and centrally regulated functions. Kidney Int 57: 1329–1336, 2000. [DOI] [PubMed] [Google Scholar]
- 35.Delfs JM, Zhu Y, Druhan JP, Aston-Jones G. Noradrenaline in the ventral forebrain is critical for opiate withdrawal-induced aversion. Nature 403: 430–434, 2000. [DOI] [PubMed] [Google Scholar]
- 36.Devenport LD, Torres A, Murray CG. Effects of aldosterone and deoxycorticosterone on food intake and body weight. Behav Neurosci 97: 667–669, 1983. [DOI] [PubMed] [Google Scholar]
- 37.Diaz R, Brown RW, Seckl JR. Distinct ontogeny of glucocorticoid and mineralocorticoid receptor and 11β-hydroxysteroid dehydrogenase types I and II mRNAs in the fetal rat brain suggest a complex control of glucocorticoid actions. J Neurosci 18: 2570–2580, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Douglas J, Catt KJ. Regulation of angiotensin II receptors in the rat adrenal cortex by dietary electrolytes. J Clin Invest 58: 834–843, 1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dundore RL, Wurpel JN, Balaban CD, Keil LC, Severs WB. Central effects of aldosterone infused into the rat subcommissural organ region. Neurosci Res 1: 341–351, 1984. [DOI] [PubMed] [Google Scholar]
- 40.Duvernoy HM, Risold PY. The circumventricular organs: an atlas of comparative anatomy and vascularization. Brain Res Rev 56: 119–147, 2007. [DOI] [PubMed] [Google Scholar]
- 41.Dymecki SM, Kim JC. Molecular neuroanatomy's “three Gs”: a primer. Neuron 54: 17–34, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Edwards CR, Stewart PM, Burt D, Brett L, McIntyre MA, Sutanto WS, de Kloet ER, Monder C. Localisation of 11β-hydroxysteroid dehydrogenase—tissue specific protector of the mineralocorticoid receptor. Lancet 2: 986–989, 1988. [DOI] [PubMed] [Google Scholar]
- 43.Emanuele E, Geroldi D, Minoretti P, Coen E, Politi P. Increased plasma aldosterone in patients with clinical depression. Arch Med Res 36: 544–548, 2005. [DOI] [PubMed] [Google Scholar]
- 44.Fejes-Toth G, Pearce D, Naray-Fejes-Toth A. Subcellular localization of mineralocorticoid receptors in living cells: effects of receptor agonists and antagonists. Proc Natl Acad Sci USA 95: 2973–2978, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fiebeler A, Nussberger J, Shagdarsuren E, Rong S, Hilfenhaus G, Al-Saadi N, Dechend R, Wellner M, Meiners S, Maser-Gluth C, Jeng AY, Webb RL, Luft FC, Muller DN. Aldosterone synthase inhibitor ameliorates angiotensin II-induced organ damage. Circulation 111: 3087–3094, 2005. [DOI] [PubMed] [Google Scholar]
- 46.Fluharty SJ, Epstein AN. Sodium appetite elicited by intracerebroventricular infusion of angiotensin II in the rat. II. Synergistic interaction with systemic mineralocorticoids. Behav Neurosci 97: 746–758, 1983. [DOI] [PubMed] [Google Scholar]
- 47.Francis J, Weiss RM, Wei SG, Johnson AK, Beltz TG, Zimmerman K, Felder RB. Central mineralocorticoid receptor blockade improves volume regulation and reduces sympathetic drive in heart failure. Am J Physiol Heart Circ Physiol 281: H2241–H2251, 2001. [DOI] [PubMed] [Google Scholar]
- 48.Fregly MJ, Waters IW. Effect of mineralocorticoids on spontaneous sodium chloride appetite of adrenalectomized rats. Physiol Behav 1: 65–74, 1966. [Google Scholar]
- 49.Funder J, Myles K. Exclusion of corticosterone from epithelial mineralocorticoid receptors is insufficient for selectivity of aldosterone action: in vivo binding studies. Endocrinology 137: 5264–5268, 1996. [DOI] [PubMed] [Google Scholar]
- 50.Funder JW Central effects of mineralocorticoids on blood pressure: new answers, new questions. J Hypertens 20: 1715–1716, 2002. [DOI] [PubMed] [Google Scholar]
- 51.Funder JW Aldosterone, mineralocorticoid receptors and vascular inflammation. Mol Cell Endocrinol 217: 263–269, 2004. [DOI] [PubMed] [Google Scholar]
- 52.Funder JW, Pearce PT, Smith R, Smith AI. Mineralocorticoid action: target tissue specificity is enzyme, not receptor, mediated. Science 242: 583–585, 1988. [DOI] [PubMed] [Google Scholar]
- 53.Geerling JC, Chimenti PC, Loewy AD. Phox2b expression in the aldosterone-sensitive HSD2 neurons of the NTS. Brain Res 1226: 82–88, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Geerling JC, Engeland WC, Kawata M, Loewy AD. Aldosterone target neurons in the nucleus tractus solitarius drive sodium appetite. J Neurosci 26: 411–417, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Geerling JC, Kawata M, Loewy AD. Aldosterone-sensitive neurons in the rat central nervous system. J Comp Neurol 494: 515–527, 2006. [DOI] [PubMed] [Google Scholar]
- 56.Geerling JC, Loewy AD. Aldosterone-sensitive neurons in the nucleus of the solitary tract: bidirectional connections with the central nucleus of the amygdala. J Comp Neurol 497: 646–657, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Geerling JC, Loewy AD. Aldosterone-sensitive neurons in the nucleus of the solitary tract: efferent projections. J Comp Neurol 497: 223–250, 2006. [DOI] [PubMed] [Google Scholar]
- 58.Geerling JC, Loewy AD. Aldosterone-sensitive NTS neurons are inhibited by saline ingestion during chronic mineralocorticoid treatment. Brain Res 1115: 54–64, 2006. [DOI] [PubMed] [Google Scholar]
- 59.Geerling JC, Loewy AD. 11β-Hydroxysteroid dehydrogenase 2 vs. transgene: discrepant loci of expression in the adult brain. Am J Physiol Renal Physiol 293: F440–F443, 2007. [DOI] [PubMed] [Google Scholar]
- 60.Geerling JC, Loewy AD. Sodium depletion activates the aldosterone-sensitive neurons in the NTS independently of thirst. Am J Physiol Regul Integr Comp Physiol 292: R1338–R1348, 2007. [DOI] [PubMed] [Google Scholar]
- 61.Geerling JC, Loewy AD. Sodium deprivation and salt intake activate separate neuronal subpopulations in the nucleus of the solitary tract and the parabrachial complex. J Comp Neurol 504: 379–403, 2007. [DOI] [PubMed] [Google Scholar]
- 62.Geerling JC, Loewy AD. Central regulation of sodium appetite. Exp Physiol 93: 177–209, 2008. [DOI] [PubMed] [Google Scholar]
- 63.Geerling JC, Mettenleiter TC, Loewy AD. Orexin neurons project to diverse sympathetic outflow systems. Neuroscience 122: 541–550, 2003. [DOI] [PubMed] [Google Scholar]
- 65.Gerlach JL, McEwen BS. Rat brain binds adrenal steroid hormone: radioautography of hippocampus with corticosterone. Science 175: 1133–1136, 1972. [DOI] [PubMed] [Google Scholar]
- 66.Gomez-Sanchez CE, de Rodriguez AF, Romero DG, Estess J, Warden MP, Gomez-Sanchez MT, Gomez-Sanchez EP. Development of a panel of monoclonal antibodies against the mineralocorticoid receptor. Endocrinology 147: 1343–1348, 2006. [DOI] [PubMed] [Google Scholar]
- 67.Gomez-Sanchez CE, Zhou MY, Cozza EN, Morita H, Eddleman FC, Gomez-Sanchez EP. Corticosteroid synthesis in the central nervous system. Endocr Res 22: 463–470, 1996. [DOI] [PubMed] [Google Scholar]
- 68.Gomez-Sanchez CE, Zhou MY, Cozza EN, Morita H, Foecking MF, Gomez-Sanchez EP. Aldosterone biosynthesis in the rat brain. Endocrinology 138: 3369–3373, 1997. [DOI] [PubMed] [Google Scholar]
- 69.Gomez-Sanchez EP Intracerebroventricular infusion of aldosterone induces hypertension in rats. Endocrinology 118: 819–823, 1986. [DOI] [PubMed] [Google Scholar]
- 70.Gomez-Sanchez EP Central hypertensive effects of aldosterone. Front Neuroendocrinol 18: 440–462, 1997. [DOI] [PubMed] [Google Scholar]
- 71.Gomez-Sanchez EP, Ahmad N, Romero DG, Gomez-Sanchez CE. Is aldosterone synthesized within the rat brain? Am J Physiol Endocrinol Metab 288: E342–E346, 2005. [DOI] [PubMed] [Google Scholar]
- 72.Gomez-Sanchez EP, Fort C, Thwaites D. Central mineralocorticoid receptor antagonism blocks hypertension in Dahl S/JR rats. Am J Physiol Endocrinol Metab 262: E96–E99, 1992. [DOI] [PubMed] [Google Scholar]
- 73.Gomez-Sanchez EP, Fort CM, Gomez-Sanchez CE. Intracerebroventricular infusion of RU28318 blocks aldosterone-salt hypertension. Am J Physiol Endocrinol Metab 258: E482–E484, 1990. [DOI] [PubMed] [Google Scholar]
- 74.Gomez-Sanchez EP, Gomez-Sanchez CE. Central hypertensinogenic effects of glycyrrhizic acid and carbenoxolone. Am J Physiol Endocrinol Metab 263: E1125–E1130, 1992. [DOI] [PubMed] [Google Scholar]
- 75.Gomez-Sanchez EP, Samuel J, Vergara G, Ahmad N. Effect of 3β-hydroxysteroid dehydrogenase inhibition by trilostane on blood pressure in the Dahl salt-sensitive rat. Am J Physiol Regul Integr Comp Physiol 288: R389–R393, 2005. [DOI] [PubMed] [Google Scholar]
- 76.Gomez-Sanchez EP, Venkataraman MT, Thwaites D, Fort C. ICV infusion of corticosterone antagonizes ICV-aldosterone hypertension. Am J Physiol Endocrinol Metab 258: E649–E653, 1990. [DOI] [PubMed] [Google Scholar]
- 77.Grippo AJ, Moffitt JA, Beltz TG, Johnson AK. Reduced hedonic behavior and altered cardiovascular function induced by mild sodium depletion in rats. Behav Neurosci 120: 1133–1143, 2006. [DOI] [PubMed] [Google Scholar]
- 78.Groc L, Choquet D, Chaouloff F. The stress hormone corticosterone conditions AMPAR surface trafficking and synaptic potentiation. Nat Neurosci 11: 868–870, 2008. [DOI] [PubMed] [Google Scholar]
- 79.Gross PM, Wall KM, Pang JJ, Shaver SW, Wainman DS. Microvascular specializations promoting rapid interstitial solute dispersion in nucleus tractus solitarius. Am J Physiol Regul Integr Comp Physiol 259: R1131–R1138, 1990. [DOI] [PubMed] [Google Scholar]
- 80.Hade JS, Mifflin SW, Donta TS, Felder RB. Stimulation of parabrachial neurons elicits a sympathetically mediated pressor response in cats. Am J Physiol Heart Circ Physiol 255: H1349–H1358, 1988. [DOI] [PubMed] [Google Scholar]
- 81.Han F, Ozawa H, Matsuda K, Nishi M, Kawata M. Colocalization of mineralocorticoid receptor and glucocorticoid receptor in the hippocampus and hypothalamus. Neurosci Res 51: 371–381, 2005. [DOI] [PubMed] [Google Scholar]
- 82.Hlavacova N, Jezova D. Chronic treatment with the mineralocorticoid hormone aldosterone results in increased anxiety-like behavior. Horm Behav 54: 90–97, 2008. [DOI] [PubMed] [Google Scholar]
- 83.Hoffman GE, Smith MS, Verbalis JG. c-Fos and related immediate early gene products as markers of activity in neuroendocrine systems. Front Neuroendocrinol 14: 173–213, 1993. [DOI] [PubMed] [Google Scholar]
- 84.Holmes MC, Sangra M, French KL, Whittle IR, Paterson J, Mullins JJ, Seckl JR. 11β-Hydroxysteroid dehydrogenase type 2 protects the neonatal cerebellum from deleterious effects of glucocorticoids. Neuroscience 137: 865–873, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Holmes MC, Seckl JR. The role of 11β-hydroxysteroid dehydrogenases in the brain. Mol Cell Endocrinol 248: 9–14, 2006. [DOI] [PubMed] [Google Scholar]
- 86.Huang BS, Amin MS, Leenen FH. The central role of the brain in salt-sensitive hypertension. Curr Opin Cardiol 21: 295–304, 2006. [DOI] [PubMed] [Google Scholar]
- 87.Huang BS, Wang H, Leenen FH. Enhanced sympathoexcitatory and pressor responses to central Na+ in Dahl salt-sensitive vs. -resistant rats. Am J Physiol Heart Circ Physiol 281: H1881–H1889, 2001. [DOI] [PubMed] [Google Scholar]
- 88.Huang BS, White RA, Ahmad M, Jeng AY, Leenen FH. Central infusion of aldosterone synthase inhibitor prevents sympathetic hyperactivity and hypertension by central Na+ in Wistar rats. Am J Physiol Regul Integr Comp Physiol 295: R166–R172, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ingram MC, Wallace AM, Collier A, Fraser R, Connell JM. Sodium status, corticosteroid metabolism and blood pressure in normal human subjects and in a patient with abnormal salt appetite. Clin Exp Pharmacol Physiol 23: 375–378, 1996. [DOI] [PubMed] [Google Scholar]
- 90.Ito T, Morita N, Nishi M, Kawata M. In vitro and in vivo immunocytochemistry for the distribution of mineralocorticoid receptor with the use of specific antibody. Neurosci Res 37: 173–182, 2000. [DOI] [PubMed] [Google Scholar]
- 91.Jalowiec JE, Stricker EM. Sodium appetite in adrenalectomized rats following dietary sodium deprivation. J Comp Physiol Psychol 82: 66–77, 1973. [DOI] [PubMed] [Google Scholar]
- 92.Janiak PC, Lewis SJ, Brody MJ. Role of central mineralocorticoid binding sites in development of hypertension. Am J Physiol Regul Integr Comp Physiol 259: R1025–R1034, 1990. [DOI] [PubMed] [Google Scholar]
- 93.Jellinck PH, Monder C, McEwen BS, Sakai RR. Differential inhibition of 11β-hydroxysteroid dehydrogenase by carbenoxolone in rat brain regions and peripheral tissues. J Steroid Biochem Mol Biol 46: 209–213, 1993. [DOI] [PubMed] [Google Scholar]
- 94.Johnson AK, de Olmos J, Pastuskovas CV, Zardetto-Smith AM, Vivas L. The extended amygdala and salt appetite. Ann NY Acad Sci 877: 258–280, 1999. [DOI] [PubMed] [Google Scholar]
- 95.Kageyama Y, Bravo EL. Hypertensive mechanisms associated with centrally administered aldosterone in dogs. Hypertension 11: 750–753, 1988. [DOI] [PubMed] [Google Scholar]
- 96.Kageyama Y, Suzuki H, Saruta T. Presence of aldosterone-like immunoreactivity in cerebrospinal fluid in normotensive subjects. Acta Endocrinol 126: 501–504, 1992. [DOI] [PubMed] [Google Scholar]
- 97.Kang BJ, Chang DA, Mackay DD, West GH, Moreira TS, Takakura AC, Gwilt JM, Guyenet PG, Stornetta RL. Central nervous system distribution of the transcription factor Phox2b in the adult rat. J Comp Neurol 503: 627–641, 2007. [DOI] [PubMed] [Google Scholar]
- 98.Karst H, Berger S, Turiault M, Tronche F, Schutz G, Joels M. Mineralocorticoid receptors are indispensable for nongenomic modulation of hippocampal glutamate transmission by corticosterone. Proc Natl Acad Sci USA 102: 19204–19207, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kawano Y, Ferrario CM. Neurohormonal characteristics of cardiovascular response due to intraventricular hypertonic NaCl. Am J Physiol Heart Circ Physiol 247: H422–H428, 1984. [DOI] [PubMed] [Google Scholar]
- 100.Kayaba Y, Nakamura A, Kasuya Y, Ohuchi T, Yanagisawa M, Komuro I, Fukuda Y, Kuwaki T. Attenuated defense response and low basal blood pressure in orexin knockout mice. Am J Physiol Regul Integr Comp Physiol 285: R581–R593, 2003. [DOI] [PubMed] [Google Scholar]
- 101.Khurshid KA, Weaver ME. Conn's syndrome presenting as depression. Am J Psychiatry 162: 1226, 2005. [DOI] [PubMed] [Google Scholar]
- 102.Klarr SA, Keep RF, Betz AL. Chronic central potassium infusion prevents deoxycorticosterone-salt hypertension in rats. Am J Physiol Heart Circ Physiol 268: H646–H652, 1995. [DOI] [PubMed] [Google Scholar]
- 103.Kotelevtsev Y, Brown RW, Fleming S, Kenyon C, Edwards CR, Seckl JR, Mullins JJ. Hypertension in mice lacking 11β-hydroxysteroid dehydrogenase type 2. J Clin Invest 103: 683–689, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kotelevtsev Y, Holmes MC, Burchell A, Houston PM, Schmoll D, Jamieson P, Best R, Brown R, Edwards CR, Seckl JR, Mullins JJ. 11β-Hydroxysteroid dehydrogenase type 1 knockout mice show attenuated glucocorticoid-inducible responses and resist hyperglycemia on obesity or stress. Proc Natl Acad Sci USA 94: 14924–14929, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kraulis I, Foldes G, Traikov H, Dubrovsky B, Birmingham. Distribution, metabolism and biological activity of deoxycorticosterone in the central nervous system. Brain Res 88: 1–14, 1975. [DOI] [PubMed] [Google Scholar]
- 106.Krozowski ZS, Funder JW. Renal mineralocorticoid receptors and hippocampal corticosterone-binding species have identical intrinsic steroid specificity. Proc Natl Acad Sci USA 80: 6056–6060, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kumar NN, Goodchild AK, Li Q, Pilowsky PM. An aldosterone-related system in the ventrolateral medulla oblongata of spontaneously hypertensive and Wistar-Kyoto rats. Clin Exp Pharmacol Physiol 33: 71–75, 2006. [DOI] [PubMed] [Google Scholar]
- 108.Lakshmi V, Monder C. Purification and characterization of the corticosteroid 11β-dehydrogenase component of the rat liver 11β-hydroxysteroid dehydrogenase complex. Endocrinology 123: 2390–2398, 1988. [DOI] [PubMed] [Google Scholar]
- 109.Lakshmi V, Sakai RR, McEwen BS, Monder C. Regional distribution of 11β-hydroxysteroid dehydrogenase in rat brain. Endocrinology 128: 1741–1748, 1991. [DOI] [PubMed] [Google Scholar]
- 110.Latif SA, Conca TJ, Morris DJ. The effects of the licorice derivative, glycyrrhetinic acid, on hepatic 3α- and 3β-hydroxysteroid dehydrogenases and 5α- and 5β-reductase pathways of metabolism of aldosterone in male rats. Steroids 55: 52–58, 1990. [DOI] [PubMed] [Google Scholar]
- 111.Lee G, Makhanova N, Caron K, Lopez ML, Gomez RA, Smithies O, Kim HS. Homeostatic responses in the adrenal cortex to the absence of aldosterone in mice. Endocrinology 146: 2650–2656, 2005. [DOI] [PubMed] [Google Scholar]
- 112.Leon LA, McKinley MJ, McAllen RM, May CN. Aldosterone acts on the kidney, not the brain, to cause mineralocorticoid hypertension in sheep. J Hypertens 20: 1203–1208, 2002. [DOI] [PubMed] [Google Scholar]
- 113.Lu J, Sherman D, Devor M, Saper CB. A putative flip-flop switch for control of REM sleep. Nature 441: 589–594, 2006. [DOI] [PubMed] [Google Scholar]
- 114.Luiten PG, ter Horst GJ, Karst H, Steffens AB. The course of paraventricular hypothalamic efferents to autonomic structures in medulla and spinal cord. Brain Res 329: 374–378, 1985. [DOI] [PubMed] [Google Scholar]
- 115.MacKenzie SM, Clark CJ, Fraser R, Gomez-Sanchez CE, Connell JM, Davies E. Expression of 11β-hydroxylase and aldosterone synthase genes in the rat brain. J Mol Endocrinol 24: 321–328, 2000. [DOI] [PubMed] [Google Scholar]
- 116.MacKenzie SM, Dewar D, Stewart W, Fraser R, Connell JM, Davies E. The transcription of steroidogenic genes in the human cerebellum and hippocampus: a comparative survey of normal and Alzheimer's tissue. J Endocrinol 196: 123–130, 2008. [DOI] [PubMed] [Google Scholar]
- 117.Malinow KC, Lion JR. Hyperaldosteronism (Conn's disease) presenting as depression. J Clin Psychiatry 40: 358–359, 1979. [PubMed] [Google Scholar]
- 118.Mattsson C, Young WF Jr. Primary aldosteronism: diagnostic and treatment strategies. Nat Clin Pract Nephrol 2: 198–208; quiz, 191 p following 230, 2006. [DOI] [PubMed] [Google Scholar]
- 119.McCance RA Experimental human salt deficiency. Lancet 1: 823–830, 1936. [Google Scholar]
- 120.Mendelsohn FA, Quirion R, Saavedra JM, Aguilera G, Catt KJ. Autoradiographic localization of angiotensin II receptors in rat brain. Proc Natl Acad Sci USA 81: 1575–1579, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mercer WR, Krozowski ZS. Localization of an 11β-hydroxysteroid dehydrogenase activity to the distal nephron. Evidence for the existence of two species of dehydrogenase in the rat kidney. Endocrinology 130: 540–543, 1992. [DOI] [PubMed] [Google Scholar]
- 122.Moisan MP, Edwards CR, Seckl JR. Ontogeny of 11β-hydroxysteroid dehydrogenase in rat brain and kidney. Endocrinology 130: 400–404, 1992. [DOI] [PubMed] [Google Scholar]
- 123.Moisan MP, Seckl JR, Edwards CR. 11β-Hydroxysteroid dehydrogenase bioactivity and messenger RNA expression in rat forebrain: localization in hypothalamus, hippocampus, and cortex. Endocrinology 127: 1450–1455, 1990. [DOI] [PubMed] [Google Scholar]
- 124.Morris MJ, Na ES, Grippo AJ, Johnson AK. The effects of deoxycorticosterone-induced sodium appetite on hedonic behaviors in the rat. Behav Neurosci 120: 571–579, 2006. [DOI] [PubMed] [Google Scholar]
- 125.Murck H, Held K, Ziegenbein M, Kunzel H, Koch K, Steiger A. The renin-angiotensin-aldosterone system in patients with depression compared to controls—a sleep endocrine study. BMC Psychiatry 3: 15, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Naray-Fejes-Toth A, Colombowala IK, Fejes-Toth G. The role of 11β-hydroxysteroid dehydrogenase in steroid hormone specificity. J Steroid Biochem Mol Biol 65: 311–316, 1998. [DOI] [PubMed] [Google Scholar]
- 127.Naray-Fejes-Toth A, Fejes-Toth G. Novel mouse strain with Cre recombinase in 11β-hydroxysteroid dehydrogenase-2-expressing cells. Am J Physiol Renal Physiol 292: F486–F494, 2007. [DOI] [PubMed] [Google Scholar]
- 128.Naray-Fejes-Toth A, Rusvai E, Denault DL, St Germain DL, Fejes-Toth G. Expression and characterization of a new species of 11β-hydroxysteroid dehydrogenase in Xenopus oocytes. Am J Physiol Renal Fluid Electrolyte Physiol 265: F896–F900, 1993. [DOI] [PubMed] [Google Scholar]
- 129.Naray-Fejes-Toth A, Watlington CO, Fejes-Toth G. 11β-Hydroxysteroid dehydrogenase activity in the renal target cells of aldosterone. Endocrinology 129: 17–21, 1991. [DOI] [PubMed] [Google Scholar]
- 130.Noguchi KK, Walls KC, Wozniak DF, Olney JW, Roth KA, Farber NB. Acute neonatal glucocorticoid exposure produces selective and rapid cerebellar neural progenitor cell apoptotic death. Cell Death Differ 15: 1582–1592, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Norgren R, Smith GP. Central distribution of subdiaphragmatic vagal branches in the rat. J Comp Neurol 273: 207–223, 1988. [DOI] [PubMed] [Google Scholar]
- 132.Oldendorf WH Measurement of brain uptake of radiolabeled substances using a tritiated water internal standard. Brain Res 24: 372–376, 1970. [DOI] [PubMed] [Google Scholar]
- 133.Orlov SN, Mongin AA. Salt-sensing mechanisms in blood pressure regulation and hypertension. Am J Physiol Heart Circ Physiol 293: H2039–H2053, 2007. [DOI] [PubMed] [Google Scholar]
- 134.Palkovits M, Monos E, Fachet J. The effect of subcommissural-organ lesions on aldosterone production in the rat. Acta Endocrinol 48: 169–176, 1965. [DOI] [PubMed] [Google Scholar]
- 135.Pardridge WM Transport of protein-bound hormones into tissues in vivo. Endocr Rev 2: 103–123, 1981. [DOI] [PubMed] [Google Scholar]
- 136.Pardridge WM, Mietus LJ. Regional blood-brain barrier transport of the steroid hormones. J Neurochem 33: 579–581, 1979. [DOI] [PubMed] [Google Scholar]
- 137.Pardridge WM, Mietus LJ. Transport of protein-bound steroid hormones into liver in vivo. Am J Physiol Endocrinol Metab Gastrointest Physiol 237: E367–E372, 1979. [DOI] [PubMed] [Google Scholar]
- 138.Pardridge WM, Mietus LJ. Transport of steroid hormones through the rat blood-brain barrier. Primary role of albumin-bound hormone. J Clin Invest 64: 145–154, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Parker RB, Yates CR, Laizure SC, Weber KT. P-glycoprotein modulates aldosterone plasma disposition and tissue uptake. J Cardiovasc Pharmacol 47: 55–59, 2006. [DOI] [PubMed] [Google Scholar]
- 140.Pennington GL, McKinley MJ. Reduction of cerebral NaCl concentration can abolish mineralocorticoid escape. Am J Physiol Renal Fluid Electrolyte Physiol 259: F839–F846, 1990. [DOI] [PubMed] [Google Scholar]
- 141.Pfaff D, Keiner M. Atlas of estradiol-concentrating cells in the central nervous system of the female rat. J Comp Neurol 151: 121–158, 1973. [DOI] [PubMed] [Google Scholar]
- 142.Picketts DJ Neuropeptide signaling and hydrocephalus: SCO with the flow. J Clin Invest 116: 1828–1832, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Rahmouni K, Barthelmebs M, Grima M, Imbs JL, De Jong W. Influence of sodium intake on the cardiovascular and renal effects of brain mineralocorticoid receptor blockade in normotensive rats. J Hypertens 20: 1829–1834, 2002. [DOI] [PubMed] [Google Scholar]
- 144.Rajan V, Edwards CR, Seckl JR. 11β-Hydroxysteroid dehydrogenase in cultured hippocampal cells reactivates inert 11-dehydrocorticosterone, potentiating neurotoxicity. J Neurosci 16: 65–70, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Reul JM, de Kloet ER. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology 117: 2505–2511, 1985. [DOI] [PubMed] [Google Scholar]
- 146.Rice KK, Richter CP. Increased sodium chloride and water intake of normal rats treated with desoxycorticosterone acetate. Endocrinology 33: 106–115, 1943. [Google Scholar]
- 147.Robel P, Baulieu EE. Neurosteroids. Biosynthesis and function. Trends Endocrinol Metab 5: 1–8, 1994. [DOI] [PubMed] [Google Scholar]
- 148.Robson AC, Leckie CM, Seckl JR, Holmes MC. 11β-Hydroxysteroid dehydrogenase type 2 in the postnatal and adult rat brain. Brain Res Mol Brain Res 61: 1–10, 1998. [DOI] [PubMed] [Google Scholar]
- 149.Roland BL, Krozowski ZS, Funder JW. Glucocorticoid receptor, mineralocorticoid receptors, 11β-hydroxysteroid dehydrogenase-1 and -2 expression in rat brain and kidney: in situ studies. Mol Cell Endocrinol 111: R1–R7, 1995. [DOI] [PubMed] [Google Scholar]
- 150.Roland BL, Li KX, Funder JW. Hybridization histochemical localization of 11β-hydroxysteroid dehydrogenase type 2 in rat brain. Endocrinology 136: 4697–4700, 1995. [DOI] [PubMed] [Google Scholar]
- 151.Sagar SM, Sharp FR, Curran T. Expression of c-Fos protein in brain: metabolic mapping at the cellular level. Science 240: 1328–1331, 1988. [DOI] [PubMed] [Google Scholar]
- 152.Sakai RR, Nicolaidis S, Epstein AN. Salt appetite is suppressed by interference with angiotensin II and aldosterone. Am J Physiol Regul Integr Comp Physiol 251: R762–R768, 1986. [DOI] [PubMed] [Google Scholar]
- 153.Schweimer J, Fendt M, Schnitzler HU. Effects of clonidine injections into the bed nucleus of the stria terminalis on fear and anxiety behavior in rats. Eur J Pharmacol 507: 117–124, 2005. [DOI] [PubMed] [Google Scholar]
- 154.Seckl JR 11β-Hydroxysteroid dehydrogenase in the brain: a novel regulator of glucocorticoid action? Front Neuroendocrinol 18: 49–99, 1997. [DOI] [PubMed] [Google Scholar]
- 155.Sequeira SM, Geerling JC, Loewy AD. Local inputs to aldosterone-sensitive neurons of the nucleus tractus solitarius. Neuroscience 141: 1995–2005, 2006. [DOI] [PubMed] [Google Scholar]
- 156.Shekhtman E, Geerling JC, Loewy AD. Aldosterone-sensitive neurons of the nucleus of the solitary tract: multisynaptic pathway to the nucleus accumbens. J Comp Neurol 501: 274–289, 2007. [DOI] [PubMed] [Google Scholar]
- 157.Sheppard KE, Funder JW. Equivalent affinity of aldosterone and corticosterone for type I receptors in kidney and hippocampus: direct binding studies. J Steroid Biochem 28: 737–742, 1987. [DOI] [PubMed] [Google Scholar]
- 158.Shin JW, Geerling JC, Loewy AD. Inputs to the ventrolateral bed nucleus of the stria terminalis. J Comp Neurol 511: 628–657, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Shin JW, Geerling JC, Loewy AD. Vagal innervation of the aldosterone-sensitive HSD2 neurons in the NTS. Brain Res 1249: 135–147, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sonino N, Fallo F, Fava GA. Psychological aspects of primary aldosteronism. Psychother Psychosom 75: 327–330, 2006. [DOI] [PubMed] [Google Scholar]
- 161.Soontornmalai A, Vlaming ML, Fritschy JM. Differential, strain-specific cellular and subcellular distribution of multidrug transporters in murine choroid plexus and blood-brain barrier. Neuroscience 138: 159–169, 2006. [DOI] [PubMed] [Google Scholar]
- 162.Stewart PM, Corrie JE, Shackleton CH, Edwards CR. Syndrome of apparent mineralocorticoid excess. A defect in the cortisol-cortisone shuttle. J Clin Invest 82: 340–349, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Stewart PM, Wallace AM, Valentino R, Burt D, Shackleton CH, Edwards CR. Mineralocorticoid activity of liquorice: 11β-hydroxysteroid dehydrogenase deficiency comes of age. Lancet 2: 821–824, 1987. [DOI] [PubMed] [Google Scholar]
- 164.Stricker EM, Thiels E, Verbalis JG. Sodium appetite in rats after prolonged dietary sodium deprivation: a sexually dimorphic phenomenon. Am J Physiol Regul Integr Comp Physiol 260: R1082–R1088, 1991. [DOI] [PubMed] [Google Scholar]
- 165.Stromstedt M, Waterman MR. Messenger RNAs encoding steroidogenic enzymes are expressed in rodent brain. Brain Res Mol Brain Res 34: 75–88, 1995. [DOI] [PubMed] [Google Scholar]
- 166.Sullivan MJ, Hasser EM, Moffitt JA, Bruno SB, Cunningham JT. Rats exhibit aldosterone-dependent sodium appetite during 24 h hindlimb unloading. J Physiol 557: 661–670, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ueda K, Okamura N, Hirai M, Tanigawara Y, Saeki T, Kioka N, Komano T, Hori R. Human P-glycoprotein transports cortisol, aldosterone, and dexamethasone, but not progesterone. J Biol Chem 267: 24248–24252, 1992. [PubMed] [Google Scholar]
- 168.Uhr M, Holsboer F, Muller MB. Penetration of endogenous steroid hormones corticosterone, cortisol, aldosterone and progesterone into the brain is enhanced in mice deficient for both mdr1a and mdr1b P-glycoproteins. J Neuroendocrinol 14: 753–759, 2002. [DOI] [PubMed] [Google Scholar]
- 169.Ulick S, Levine LS, Gunczler P, Zanconato G, Ramirez LC, Rauh W, Rosler A, Bradlow HL, New MI. A syndrome of apparent mineralocorticoid excess associated with defects in the peripheral metabolism of cortisol. J Clin Endocrinol Metab 49: 757–764, 1979. [DOI] [PubMed] [Google Scholar]
- 170.Vallee SM, Grillo CA, Gonzalez S, Cosen-Binker L, de Kloet ER, McEwen BS, De Nicola AF. Further studies in deoxycorticosterone acetate treated rats: brain content of mineralocorticoid and glucocorticoid receptors and effect of steroid antagonists on salt intake. Neuroendocrinology 61: 117–124, 1995. [DOI] [PubMed] [Google Scholar]
- 171.Van den Berg DT, de Jong W, de Kloet ER. Mineralocorticoid antagonist inhibits stress-induced blood pressure response after repeated daily warming. Am J Physiol Endocrinol Metab 267: E921–E926, 1994. [DOI] [PubMed] [Google Scholar]
- 172.Van den Berg DT, de Kloet ER, de Jong W. Central effects of mineralocorticoid antagonist RU-28318 on blood pressure of DOCA-salt hypertensive rats. Am J Physiol Endocrinol Metab 267: E927–E933, 1994. [DOI] [PubMed] [Google Scholar]
- 173.van den Berg DT, de Kloet ER, van Dijken HH, de Jong W. Differential central effects of mineralocorticoid and glucocorticoid agonists and antagonists on blood pressure. Endocrinology 126: 118–124, 1990. [DOI] [PubMed] [Google Scholar]
- 174.van Eekelen JA, Bohn MC, de Kloet ER. Postnatal ontogeny of mineralocorticoid and glucocorticoid receptor gene expression in regions of the rat tel- and diencephalon. Brain Res Dev Brain Res 61: 33–43, 1991. [DOI] [PubMed] [Google Scholar]
- 175.Veldhuis HD, De Kloet ER. Antagonistic effects of aldosterone on corticosterone-mediated changes in exploratory behavior of adrenalectomized rats. Horm Behav 17: 225–232, 1983. [DOI] [PubMed] [Google Scholar]
- 176.Wang VY, Rose MF, Zoghbi HY. Math1 expression redefines the rhombic lip derivatives and reveals novel lineages within the brainstem and cerebellum. Neuron 48: 31–43, 2005. [DOI] [PubMed] [Google Scholar]
- 177.Wang W Chronic administration of aldosterone depresses baroreceptor reflex function in the dog. Hypertension 24: 571–575, 1994. [DOI] [PubMed] [Google Scholar]
- 178.Wang X, Cen X, Lu L. Noradrenaline in the bed nucleus of the stria terminalis is critical for stress-induced reactivation of morphine-conditioned place preference in rats. Eur J Pharmacol 432: 153–161, 2001. [DOI] [PubMed] [Google Scholar]
- 179.Webb DJ, Clark SA, Brown WB, Fraser R, Lever AF, Murray GD, Robertson JI. Dietary sodium deprivation raises blood pressure in the rat but does not produce irreversible hyperaldosteronism. J Hypertens 5: 525–531, 1987. [DOI] [PubMed] [Google Scholar]
- 180.Weindl A, Joynt RJ. Barrier properties of the subcommissural organ. Arch Neurol 29: 16–22, 1973. [DOI] [PubMed] [Google Scholar]
- 181.Weston M, Wang H, Stornetta RL, Sevigny CP, Guyenet PG. Fos expression by glutamatergic neurons of the solitary tract nucleus after phenylephrine-induced hypertension in rats. J Comp Neurol 460: 525–541, 2003. [DOI] [PubMed] [Google Scholar]
- 182.Wolf G Sodium appetite elicited by aldosterone. Psychon Sci 1: 211–212, 1964. [Google Scholar]
- 183.Wolf G Effect of deoxycorticosterone on sodium appetite of intact and adrenalectomized rats. Am J Physiol 208: 1281–1285, 1965. [DOI] [PubMed] [Google Scholar]
- 184.Wolf G, Handal PJ. Aldosterone-induced sodium appetite: dose-response and specificity. Endocrinology 78: 1120–1124, 1966. [DOI] [PubMed] [Google Scholar]
- 185.Ye P, Kenyon CJ, Mackenzie SM, Nichol K, Seckl JR, Fraser R, Connell JM, Davies E. Effects of ACTH, dexamethasone, and adrenalectomy on 11β-hydroxylase (CYP11B1) and aldosterone synthase (CYP11B2) gene expression in the rat central nervous system. J Endocrinol 196: 305–311, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ye P, Kenyon CJ, MacKenzie SM, Seckl JR, Fraser R, Connell JM, Davies E. Regulation of aldosterone synthase gene expression in the rat adrenal gland and central nervous system by sodium and angiotensin II. Endocrinology 144: 3321–3328, 2003. [DOI] [PubMed] [Google Scholar]
- 187.Yee KM, Struthers AD. Aldosterone blunts the baroreflex response in man. Clin Sci (Lond) 95: 687–692, 1998. [DOI] [PubMed] [Google Scholar]
- 188.Yongue BG, Roy EJ. Endogenous aldosterone and corticosterone in brain cell nuclei of adrenal-intact rats: regional distribution and effects of physiological variations in serum steroids. Brain Res 436: 49–61, 1987. [DOI] [PubMed] [Google Scholar]
- 189.Yoshida K, McCormack S, Espana RA, Crocker A, Scammell TE. Afferents to the orexin neurons of the rat brain. J Comp Neurol 494: 845–861, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Yu L, Romero DG, Gomez-Sanchez CE, Gomez-Sanchez EP. Steroidogenic enzyme gene expression in the human brain. Mol Cell Endocrinol 190: 9–17, 2002. [DOI] [PubMed] [Google Scholar]
- 191.Yu Y, Wei SG, Zhang ZH, Gomez-Sanchez E, Weiss RM, Felder RB. Does aldosterone upregulate the brain renin-angiotensin system in rats with heart failure? Hypertension 51: 727–733, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Zhang ZH, Francis J, Weiss RM, Felder RB. The renin-angiotensin-aldosterone system excites hypothalamic paraventricular nucleus neurons in heart failure. Am J Physiol Heart Circ Physiol 283: H423–H433, 2002. [DOI] [PubMed] [Google Scholar]
- 193.Zhang ZH, Kang YM, Yu Y, Wei SG, Schmidt TJ, Johnson AK, Felder RB. 11β-Hydroxysteroid dehydrogenase type 2 activity in hypothalamic paraventricular nucleus modulates sympathetic excitation. Hypertension 48: 127–133, 2006. [DOI] [PubMed] [Google Scholar]
- 194.Zhou MY, Gomez-Sanchez EP, Cox DL, Cosby D, Gomez-Sanchez CE. Cloning, expression, and tissue distribution of the rat nicotinamide adenine dinucleotide-dependent 11β-hydroxysteroid dehydrogenase. Endocrinology 136: 3729–3734, 1995. [DOI] [PubMed] [Google Scholar]