Abstract
Although interleukin (IL)-4 and IL-13 participate in allergic inflammation and share a receptor subunit (IL-4Rα), differential functions for these cytokines have been reported. Therefore, we compared cells expressing type I and II IL-4 receptors with cells expressing only type II receptors for their responsiveness to these cytokines. IL-4 induced highly efficient, γC-dependent tyrosine phosphorylation of insulin receptor substrate 2 (IRS-2), whereas IL-13 was less effective, even when phosphorylation of signal transducer and activator of transcription 6 (STAT6) was maximal. Only type I receptor-γC+ signaling induced efficient association of IRS-2 with p85 or GRB2. IL-4 signaling through type I receptor complexes induced more robust expression of a subset of genes associated with alternatively activated macrophages than did IL-13, despite equivalent activation of STAT6. Thus, IL-4 activates signaling pathways through the type I receptor complex, qualitatively differently from IL-13, which cooperate to induce optimal gene expression.
INTRODUCTION
Interleukin (IL)-4 and IL-13 are both members of the short four-helix bundle family of cytokines (1–3). They share approximately 25% sequence identity (4) and are closely linked on human chromosome 5 (5) and mouse chromosome 11. IL-4 and IL-13 are known as T-helper type 2 (Th2) cytokines (6) because they are produced by Th2 cells in response to antigen-receptor engagement. They are also produced by natural killer (NK) T cells, and by mast cells and basophils upon cross-linkage of the high affinity receptor for immunoglobulin E (IgE) (7). IL-4 and IL-13 are also secreted by stimulated eosinophils ((8–11), reviewed in (12)) and are produced by macrophages (13). These cytokines can elicit similar responses in vitro and when exogenously introduced in vivo. However, there is growing evidence that the two cytokines mediate distinct physiologic functions, with IL-4 being involved in Th2 development and IL-13 being responsible for effector activities, such as regulating airway hypersensitivity, collagen production, and mucus hypersecretion (14). Because therapeutic strategies targeting IL-4 and IL-13 or their signaling pathways are currently being developed to treat allergy and asthma, understanding their precise mechanisms of action and contributions to allergic inflammation is essential.
A functional IL-4 receptor is composed of two transmembrane proteins (7). The IL-4Rα chain binds IL-4 with high affinity (Kd 20–300 pM), leading to dimerization with either the common gamma chain (γC) or with IL-13Rα1 to form the type I or type II receptor complexes, respectively. In cells of hematopoietic lineage, expression of both IL-4Rα and γ c leads to the assemblage of the type I receptor, whereas the type II receptor is generally the exclusive IL-4 receptor found in nonhematopoietic cells. Both receptor types are generally found in cells of myeloid origin and in human B cells. IL-13 binds to IL-13Rα1 with moderate affinity (Kd 2–10 nM), inducing heterodimerization with IL-4Rα to form a high affinity complex (Kd 200 pM). Indeed, whereas the overall equilibrium binding affinity of IL-13 for the type II receptor complex is similar to that of IL-4 for the type I or type II receptors, the energetic contribution of the individual chains to affinity differ for these cytokines (15, 16). Although IL-13 also binds to IL-13Rα2 with high affinity (Kd 200 pM), this interaction fails to activate the Janus kinase (JAK)-signal transducer and activator of transcription (STAT) pathway (17) but this is controversial (18).
The signaling pathways activated by IL-4 and IL-13 have been examined in vitro. IL-4Rα associates with JAK1 whereasγC associates exclusively with JAK3; IL-13Rα1 interacts with TYK2 and JAK2 (7, 19–21). Thus, JAK3 is activated by IL-4 through the type I receptor, but not by IL-4 or IL-13 through the type II receptor (22). Activation of the JAK kinases by receptor engagement and heterodimerization leads to phosphorylation of tyrosine residues in the cytoplasmic domain of IL-4Rα (7). These phosphotyrosines then act as docking sites for adaptor and signaling molecules containing protein tyrosine–binding domains (PTBs) such as insulin receptor substrate (IRS)-2, IRS-1, downstream of kinase (Dok)-1 or Dok-2, and for those proteins containing Src homology 2 (SH2) domains such as STAT6. STAT6 plays a central role in gene regulation and the allergic responses regulated by IL-4 or IL-13, including the differentiation of Th2 cells, IgE production, and chemokine and mucus production at sites of allergic inflammation, whereas the role of IRS-2 has not been fully elucidated (23–25).
Many cell types respond to IL-4 and IL-13 with activation of signaling pathways and a biologic response in vitro (5). Because IL-4 and IL-13 can both use the type II receptor complex, the molecular basis for the separation of functional responses between the two cytokines is unclear. The relative abundance of the three receptor subunits (IL-4Rα, γC, and IL-13Rα1) influences the overall capacities of IL-4 and IL-13 to mediate phosphorylation of STAT6 and gene activation, providing a potential mechanism through which cells may adjust their relative responsiveness to the two cytokines (26).
In this study, we found that IL-4 induced highly efficient tyrosine phosphorylation of the signaling adaptor molecule IRS-2 in human monocytic cell lines and primary murine macrophages, which depended on the presence of the γC receptor subunit. The ability of IL-13 to stimulate tyrosine phosphorylation of IRS-2 was significantly less than that achieved by IL-4 stimulation in either cell type under conditions in which phosphorylation of STAT6 was equivalent. These results suggest that IL-4 signaling through type I IL-4 receptor, in addition to being generally more potent than that of IL-13, selectively induced efficient tyrosine phosphorylation of IRS-2. The presence of the γC subunit was responsible for this selectivity. In addition, signaling by IL-4 through the type I receptor complex resulted in the induction of expression of genes that encode markers of alternatively activated macrophages (arginase I, FIZZ1, Ym1; reviewed in (27)) to a much greater degree than that by IL-13, even under conditions of comparable states of STAT6 activation. These signaling differences between the two cytokines may explain the differences in their functional effects on the various key cell types involved in the pathogenesis of allergic disease. Appreciation of the subtle differences between the actions of IL-4 and IL-13 will enable a better understanding of cellular responses during allergic inflammation and a more targeted approach to strategies aimed at blocking inflammation.
RESULTS
IL-4 and IL-13 signaling in human cell lines
To compare the signaling responses of cells to IL-4 with those to IL-13, we performed experiments with a human monocytic cell line that expresses both type I and type II receptors (U937) and another that expresses only type II receptors (THP-1) (28). Analysis by flow cytometry revealed a substantial difference in the abundance of the γC component of the type I receptor on the surface of these two cell types (Fig. 1). The average mean fluorescence intensity (MFI) of γC staining on the surface of U937 cells was 73 ± 5, whereas that for THP-1 cells was the same as the nonspecific control (7 ± 1). The abundance of the other receptor components (IL-4Rα and IL-13Rα1) was only moderately different between the two cell populations, and IL-13Rα2 was not detectable on either cell type (Fig. 1). These data indicated that both type I and type II IL-4 receptors were found on U937 cells whereas only type II receptors were found on THP-1 cells.
Fig. 1.
Abundance of receptor chains on U937 and THP-1 cells. Equivalent numbers of cells were incubated with specific antibodies (filled histograms) against human γC, IL-4Rα, IL-13Rα1, and IL-13Rα2 or with the corresponding isotype-matched controls (open histograms) as indicated and were analyzed by flow cytometry. Representative histogram plots from one experiment for each receptor subunit are shown. The mean fluorescence intensity of the positive peaks are shown.
We stimulated these cell lines with various concentrations of human IL-4 or IL-13 and examined the tyrosine phosphorylation status of STAT6 and insulin receptor substrate 2 (IRS-2), two major signaling molecules that are activated in response to IL-4Rα signaling. Analysis of Western blots of immunoprecipitated STAT6 and IRS-2 showed that both IL-4 and IL-13 stimulated the phosphorylation of tyrosine residues in STAT6 in U937 and THP-1 cells (Fig. 2A). As noted previously for various cell types (15, 26), we found that ~3 to 10-fold more IL-13 than IL-4 was required to reach a plateau of STAT6 phosphorylation in U937 cells. However, both cytokines stimulated similar degrees of tyrosine phosphorylation of STAT6 at concentrations greater than 10 ng/mL. In contrast, there was a much more substantial difference in their relative abilities to induce phosphorylation of tyrosine residues in IRS-2. IL-4 stimulated tyrosine phosphorylation of IRS-2 in U937 at low concentrations (0.33 ng/mL, reaching maximal levels at 3.3 ng/mL), whereas IL-13 elicited substantially less phosphorylation of IRS-2 at all concentrations tested. In THP-1 cells, phosphorylation of IRS-2 was weak in response to either IL-4 or IL-13, although STAT6 was equivalently phosphorylated by both cytokines (Fig. 2A, 2B). Indeed, IL-13 was more potent than IL-4 at stimulating the phosphorylation of STAT6 in THP-1 cells at high concentrations.
Fig. 2.
IL-4 and IL-13 signaling through the type I and type II IL-4 receptors in U937 and THP-1 cells. (A) U937 and THP-1 cells were treated with various concentrations of human IL-4 or IL-13, as indicated, for 30 minutes. Cell lysates were prepared and used in immunoprecipitation assays with anti-STAT6 or anti-IRS-2 antibodies, which were analyzed by Western blotting with anti-phosphotyrosine antibodies. After stripping, the blots were incubated with anti-STAT6 or anti-IRS-2 antibodies, as indicated. Autoradiograms of a representative experiment out of 3 independent experiments are shown. (B) Autoradiograms shown above in A were scanned and densitometry was performed on the bands with NIH Image software. The relative densities of bands corresponding to phosphorylated proteins were divided by the densities of bands corresponding to total proteins, as indicated. (C) U937 and THP-1 cells were treated with increasing concentrations of IL-4 or 100 ng/mL IGF-I for 30 minutes, as indicated. For IRS-2, cell lysates were analyzed as described for panel A. For analysis of STAT6, Western blots of total lysates were incubated with antiphospho-STAT6-Tyr641 antibody. After stripping, the blots were incubated with anti-STAT6 antibody. The gap in the U937 blot (left-hand side) indicates other lanes on the SDS-PAGE gel that are not shown here. The samples are from the same exposure of the same blot. Autoradiograms of a representative experiment out of 2 independent experiments are shown.
To test whether the IRS-2 pathway was functional in THP-1 cells, we stimulated the cells with insulin-like growth factor 1 (IGF-I), a potent activator of IRS-2 (Fig. 2C). IL-4 induced the tyrosine phosphorylation of IRS-2 in U937 cells similarly to that induced by IGF-I (ratio of p-IRS-2[IL-4] to p-IRS-2[IGF-I], 1.31), whereas in THP-1 cells, IL-4 was a much poorer stimulant of IRS-2 phosphorylation than was IGF-I (ratio of p-IRS-2[IL-4] to p-IRS-2[IGF-I], 0.2). As expected, IGF-I did not stimulate the phosphorylation of STAT6, whereas both IL-4 and IL-13 could. Thus, in the absence of γC and the type I receptor complex, activation of the IRS-2 pathway by IL-4, although not entirely absent, was considerably impaired. Because the dose-response curves for phosphorylation of STAT6 by IL-4 in U937 and THP-1 cells were similar (Fig. 2A, B), the impaired phosphorylation of IRS-2 by IL-4 and IL-13 in THP-1 cells cannot be explained by a dearth of functional receptors or JAKs. Because IGF-I-induced phosphorylation of IRS-2 was intact, it cannot be explained by a defect in the ability of IRS-2 to become phosphorylated. It was also not the result of a deficiency in the total abundance of cellular IRS-2, because stripping and reprobing the same Western blot did not show diminished total cellular IRS-2 (Fig. 2C).
Overexpression of γC in THP-1 cells enabled IL-4-stimulated phosphorylation of IRS-2
Because both IL-4 and IL-13 bind to and signal through the type II receptor, but only IL-4 binds to and signals through the type I receptor, it seemed likely that the different signaling responses to IL-4 and IL-13 in these cell lines resulted from the unique ability of IL-4 to use the type I IL-4 receptor. If the presence of type I receptors (γC+) was responsible for the difference in signaling between the two monocytic cell lines, then expression of the γC subunit and, therefore, the type I IL-4 receptor, on the surface of THP-1 cells should enable IL-4-induced phosphorylation of IRS-2. To this end, we transfected THP-1 cells with a linearized human γC-expressing plasmid and then selected stable antibiotic-resistant clones (Fig. 3). The abundance of γC in each clone was determined by flow cytometry and ranged from an MFI of 15 to 78. (Fig. 3A). We evaluated signaling responses in these clones (Fig. 3B, 3C) and found that the presence of the γC subunit in THP-1 cells enabled IL-4-stimulated phosphorylation of IRS-2 in the three clones with the highest abundance of γC (MFIs of 24 to 78), but not in the two clones in which the abundance of γC was only two-fold over background (MFI 15). However, IL-4 induced the tyrosine phosphorylation of STAT6 similarly in all five clones (Fig. 3B). We further compared the ability of IL-4 to stimulate tyrosine phosphorylation of IRS-2 with that of IL-13 and IGF-I in two of the γC-expressing clones. Both IL-4 and IGF-I stimulated tyrosine phosphorylation of IRS-2 whereas the IL-13 response was substantially lower (Fig. 3C). When expressed as a percentage of the maximal phosphorylation observed in each cell line, the percentage of total IRS-2 that was phosphorylated in response to IL-13 ranged from 28% in U937 cells to 30–50% in the γC-expressing clones, as compared to 100% for IL-4. Thus, efficient phosphorylation of IRS-2 induced by IL-4 correlated with the abundance of γC at the cell surface.
Fig. 3.
Ectopic expression of γC in THP-1 cells. (A) THP-1 cells were transfected with linearized plasmids encoding the human γC gene and neomycin resistance to allow selection of positive clones in media containing G418. Following 8 weeks of selection, clones were screened for the presence of surfaceγC by incubation with antibodies against human γC (filled histograms) or isotype-matched controls (open histograms) and were compared to the parental THP-1 cell line by flow cytometry. The mean fluorescence intensity (MFI) of the anti-γC-PE-stained sample is indicated in the upper right corner of each plot. (B) U937, THP-1, and THP-1 cells expressing human γC were treated with human IL-4 (10 ng/mL) as indicated for 30 minutes. Cell lysates were prepared and samples were immunoprecipitated with anti-IRS-2 or anti-STAT6 antibodies. Western blots were incubated with anti-phosphotyrosine antibodies. After stripping, the blots were incubated with anti-STAT6 or anti-IRS-2 antibodies, as indicated. Autoradiograms of a representative experiment out of 3 independent experiments are shown. Several molecular weight species of STAT6 commonly observed in macrophage cell lines are visible. Autoradiograms shown above were scanned and densitometry was performed on the major bands using NIH Image software. The relative densities of bands corresponding to phosphorylated proteins were divided by those corresponding to total proteins and the value is shown under the blots. (C) U937 and two THP-1 clones expressing human γC were treated with human IL-4 (10 ng/mL), human IL-13 (10 ng/mL), or human IGF-I (100 ng/mL) as indicated for 30 minutes. Analysis of phospho-IRS-2 and phospho-STAT6 was carried out as described in Fig. 2C. Densitometric analysis on autoradiograms was performed as described in the Materials and Methods and the data were expressed as a percentage of the maximal response for each cell type or clone. In this experiment the IGF-1-induced tyrosine phosphorylation of IRS-2 in U937 cells was weaker than usual as compared to the IL-4-induced response.
IL-4 and IL-13 signaling in primary bone marrow–derived macrophages
We extended our observations by comparing the responses of primary bone marrow derived macrophages (BMMs) from γC−/− mice to IL-4 and IL-13 with those of wild-type (WT) BMMs (Fig. 4). We chose to analyze macrophages because they typically express type I and type II receptors and because their development is unaffected by a deficiency in γC (29). Bone marrow was isolated from WT and γC−/− C57BL/6 mice and cultured in macrophage colony stimulating factor (M-CSF) for ten days. This protocol resulted in the growth of adherent macrophages (BMM) that were >90% CD11b+ by flow cytometry (data not shown). IL-4 and IL-13 signaling were assessed by immunoprecipitation and Western blotting experiments as described earlier. IL- 4 (20 ng/mL) stimulated strong tyrosine phosphorylation of IRS-2 in the WT (γC-positive) BMM, whereas the same concentration of IL-13 stimulated substantially less phosphorylation of IRS-2 (Fig. 4A, 4B). The difference between the IL-4- and IL-13-stimulated responses was consistent from experiment to experiment and statistically significant (Fig. 4B). In γC−/− BMMs, IL-4-stimulated phosphorylation of IRS-2 was low and similar to that of IL-13. However, both IL-4 and IL-13 (at 20 ng/mL) stimulated tyrosine phosphorylation of STAT6 in WT and γC−/− BMMs (Fig. 4A, 4B). Furthermore, the IRS-2 signaling pathway was intact in these cell preparations because IGF-I stimulated the tyrosine phosphorylation of IRS-2 in WT and γC−/− BMM equivalently (Fig. 4C).
Fig. 4.
Signaling in response to IL-4 and IL-13 in WT and γC−/− BMMs. (A) BMM were isolated and cultured from WT C57BL/6 and γC−/− mice as described in Materials and Methods. After ten days, the cells were stimulated with 20 ng/mL IL-4 or IL-13 for 30 minutes and cell lysates were collected and analyzed for IRS-2 and STAT6 phosphorylation as described in Fig. 1. Representative Western blot films are shown from a total of more than four independent experiments. (B) Densitometric analysis of Western blot films. Densitometric analysis was performed as described in Figure 1B. The amount of normalized phosphoprotein measured in response to IL-4 was set as the maximal response (= 100 %). n = 4, * P < 0.05. C. Western blot films showing IRS-2 phosphorylation in response to IGF-I in WT and γC−/− BMM. Cells were stimulated with 100 ng/mL mIGF-I or 20 ng/mL IL-4 and IL-13 and were analyzed for IRS-2 phosphorylation as described in Fig. 1.
In the experiments described thus far, cells were stimulated with a single concentration of cytokine and antibodies to IRS-2 and STAT6 were used for immunoprecipitations prior to incubating Western blots with pan antiphosphotyrosine antibodies (Fig. 4). As is shown in Fig. 4A (as well as in Fig. 2A, 2C, and Fig. 3B), the abundance of IRS-2 is exceedingly low (30, 31), making the immunoprecipitation step necessary to visualize phospho- or total IRS-2. However, STAT6 protein is sufficiently abundant in cells that its phosphorylation can be assessed directly on Western blots of total cell lysates or by flow cytometry. Using these approaches, it has been shown that IL-4 is ~10- to 100-fold more potent than IL-13 at stimulating the tyrosine phosphorylation of STAT6 in BMMs (26, 32). Therefore, we performed immunoprecipitations to analyze the ability of various concentrations of IL-4 and IL-13 to induce tyrosine phosphorylation of IRS-2, and assessed their ability to induce tyrosine phosphorylation of STAT6 by flow cytometry (intracellular staining for pSTAT6) or by analyzing Western blots of total lysates with an anti-STAT6-Tyr641 antibody (Fig. 5). We found that IL-4 was indeed more potent than IL-13 at stimulating the tyrosine phosphorylation of STAT6 by a factor of 3–100 fold. However, at concentrations of cytokine ≥20 ng/mL, the abundance of phosphorylated STAT6 in WT BMMs in response to either IL-4 or IL-13 was similar. In contrast, whereas IL-4 was consistently potent in stimulating phosphorylation of IRS-2, reaching a maximal level at ~3 ng/mL, higher concentrations of IL-13 did not induce potent tyrosine phosphorylation of IRS-2.
Fig. 5.
Analysis of phosphoproteins in WT BMMs. (A) BMMs were isolated from WT C57BL/6 mice and cultured as described in Materials and Methods. After ten days, the cells were stimulated with IL-4 or IL-13 (20 ng/mL) for 30 minutes and cell lysates were collected and analyzed for IRS-2 and STAT6 phosphorylation by immunoprecipitation and Western blotting as described for Fig. 1. (B) BMMs were treated with various concentrations of IL-4 or IL-13 for 30 minutes. The analysis of tyrosine phosphorylation of IRS-2 and STAT6 was performed using the following methods: Graph 1 - densitometric analysis of IRS-2 phosphorylation by immunoprecipitation followed by analysis of Western blots with an anti-phosphotyrosine antibody. Graph 2 - densitometric analysis of pSTAT6 in total cell lysates by Western blotting with phospho-specific anti-STAT6 antibodies. Graph 3 - analysis of pSTAT6 by intracellular flow cytometry with PE-conjugated anti-pSTAT6 antibodies. These graphs are representative of 3 independent experiments.
Association of signaling molecules with phosphorylated IRS-2
IRS-2 is a large docking protein that contains >20 potential tyrosine phosphorylation sites (33), several of which have been characterized in detail (34–37). There are four potential phosphotyrosine sites in IRS-2 that can recruit the p85 subunit of phosphoinositide 3-kinase (PI3K) and only one for recruiting the cytosolic adadptor protein growth factor receptor-bound protein 2 (Grb2). To test whether the difference in degree of tyrosine phosphorylation of IRS-2 in response to IL-4 and IL-13 might reflect differential targeting of specific sites, we performed coprecipitation studies (Fig. 6). When we used anti-phosphotyrosine antibodies as the most sensitive probe for the 180 kD phospho-IRS-2, we found that both anti-p85 and anti-Grb2 antibodies coimmunoprecipitated phosphorylated IRS-2 from lysates of cells treated with IL-4. However, substantially less IRS-2 was coimmunoprecipitated by anti-p85 from lysates of cells treated with IL-13 (25% of the IL-4 response), and no detectable IRS-2 was coimmunoprecipitated by anti-Grb2 in cells stimulated with IL-13 (Fig. 6). Furthermore, the anti-IRS-2 precipitates contained a greater abundance of p85 and GRB2 protein in the IL-4 treated samples. As previously reported, the abundance of GRB2 found in anti-IRS precipitates of IL-4 treated samples is quite low (38). These results suggest that IL-4 signaling by the type I receptor complex specifically targets the phosphotyrosine that serves as the Grb2 docking site in IRS-2.
Fig. 6.
Association of pIRS-2 with the p85 subunit of PI3K and Grb2. (A) BMMs were isolated from WT C57BL/6 and γC−/− mice and cultured as described above. After ten days, the cells were stimulated with IL-4 or IL-13 (20 ng/mL) for 30 minutes and cell lysates were collected and precipitated with anti-IRS-2, anti-p85, or anti-Grb2 antibodies as indicated. Western blots were incubated with anti-phosphotyrosine antibody. The region of the blot containing the 180 kD, pIRS-2 is shown. The blots were stripped and incubated with anti-IRS-2, anti-p85 or anti-Grb2 antibodies as shown. (B) Densitometric quantitation of the phosphotyrosine Western blots shown in panel A. These data are representative of 3 independent experiments.
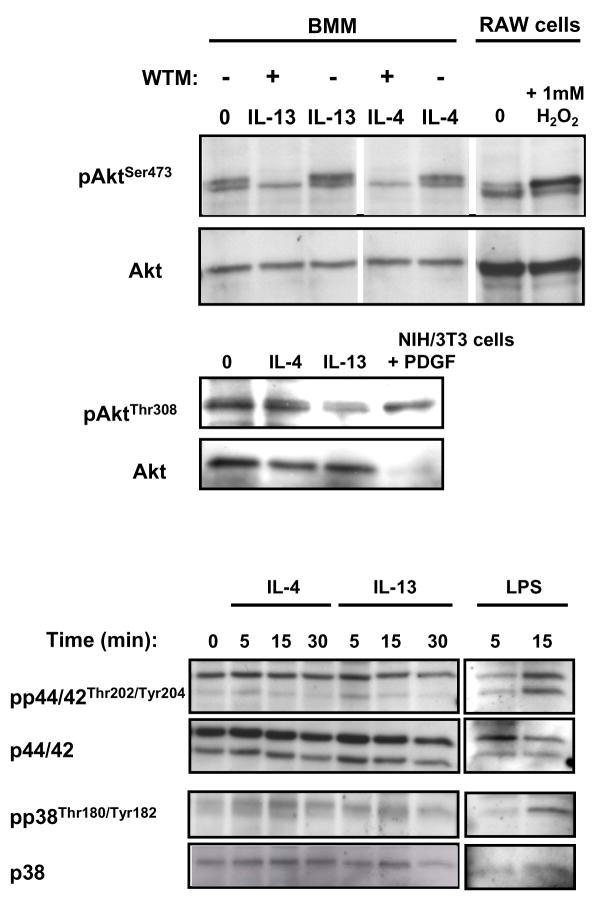
We further analyzed the ability of IL-4 and IL-13 to activate molecules that are classically downstream of PI3K and Grb2 in other growth factor signaling pathways, Akt and Map kinases respectively (Fig. 7). The M-CSF-cultured BMM demonstrated basal phosphorylation of Akt on Ser473 that was barely increased by IL-4 or IL-13. Treatment of the cells with wortmannin diminished the phosphorylation below the baseline suggesting that M-CSF induced the phosphorylation of Akt on Ser473. Neither IL-4 nor IL-13 induced the phosphorylation of Akt on Thr308. Similar to several previous reports (39–43), neither IL-4 nor IL-13 stimulated the phosphorylation of ERK1/2 or p38 in these primary BMM while LPS treatment was able to do so.
Fig. 7.
Analysis of downstream phosphorylation. (A) BMMs were isolated from WT C57BL/6 mice and cultured as described above. After ten days, the cells were stimulated with IL-4 or IL-13 (100 ng/mL) or LPS (100 ng/ml) for various times as indicated and cell lysates were collected. Where indicated the BMM were pre-treated with wortmannin as described in Materials and Methods. RAW264.7 macrophages were treated with 1 mM H2O2 for 15 minutes and used as a positive control. Western blots were probed with phosphospecific antibodies as indicated. The blots were stripped and reprobed for total protein as appropriate. These data are representative of 3 independent experiments.
Induction of genes characteristic of alternatively activated macrophages
To examine whether differential signaling through the type I receptor had any functional consequences for macrophages, we analyzed the expression of some of the characteristic and STAT6-dependent genes associated with the alternative macrophage phenotype (44). Total RNA was isolated from WT and γC−/− BMMs that had been treated for 6 and 24 hours with a high concentration of IL-4 or IL-13 (100 ng/mL). This concentration was chosen because we had observed that phosphorylation of STAT6 was both maximal and equivalently induced by IL-4 and IL-13, as shown by all three measurements (immunoprecipitation, phosphospecific Western blotting, and intracellular flow cytometric analysis), whereas IRS-2 phosphorylation was not. Expression of mRNAs encoding arginase I, found in inflammatory zone 1 (FIZZ1), Ym1, and macrophage mannose receptor (MMR) was analyzed by quantitative real-time reverse-transcriptase polymerase chain reaction (RT-PCR) assays. Total cell lysates were also harvested at 0, 6, 24, and 48 hours posttreatment and analyzed for the presence of arginase I and Ym1 proteins by Western blotting. The expression of Arginase I, FIZZ1, and Ym1 mRNAs was highly increased by IL-4 compared to that in untreated cells (Fig. 8A). This dramatic induction of gene expression by IL-4 was diminished in the γC−/− cells for all three genes. In contrast to IL-4, stimulation with IL-13 resulted in a more modest induction of expression of arginase I, FIZZ1, and Ym1 mRNAs in WT and γC−/− BMMs. However, the expression of MMR mRNA was comparably induced by both IL-4 and IL-13. We repeated our analysis of gene expression in multiple independent experiments. IL-4 induced significant expression of arginase I, FIZZ1 and Ym1 mRNA whereas the amount of mRNA induced by treatment with IL-13 was significantly less (Fig. 8A). The abundance of arginase I and Ym1 proteins was increased more slowly than their corresponding mRNA, as would be expected (Fig. 8B); clearly detectable proteins were observed only after 48 h of stimulation with cytokine. IL-4 was more potent than IL-13 at inducing the production of both arginase I and Ym1 proteins, which mirrored the mRNA expression data. IL-4 did not induce the production of arginase I or Ym1 in γC−/− BMMs, but IL-13 induced the production of both proteins, albeit at low abundance.
Fig. 8.
Induction of expression of genes characteristic of alternatively activated macrophages by IL-4 and IL-13 in WT and γC−/− BMM. (A) BMMs were isolated from WT C57BL/6 and γC−/− mice as described in the Materials and Methods. After ten days, cells were stimulated with 100 ng/mL IL-4 (black bars) or IL-13 (hatched bars) for 6 and 24 hours and total RNA was harvested. Complementary DNA was generated and real-time RT-PCR analysis was performed with specific primers for the named genes. The results are expressed as the fold-induction (2−ΔΔCt) of a given mRNA in cytokine-treated cells compared to that of unstimulated cells and normalized to HPRT, a housekeeping gene. The top panels show a representative of 3–5 individual experiments. The standard errors for the qPCR plate replicates were too small to appear on the graphic representation. In the bottom bar graphs, the average data from multiple individual experiments were expressed as a percentage of maximal induction (IL-4-stimulated value = 100%) at 24 hours. n = 3 – 5, * P < 0.05, ** P < 0.01, † P < 0.005, ‡ P < 0.001. (B) Production of arginase I and Ym1 protein in WT and γC knockout mice. Total cell lysates were harvested at the time points indicated and were analyzed by Western blotting as described in the Materials and Methods.
IL-4-induced tyrosine phosphorylation of IRS-2 leads to the recruitment and activation of phosphoinositide 3-kinase (PI3K) (33) and the production of phosphatidylinositol-3,4,5-trisphosphate [PI(3,4,5)P3]. PI(3,4,5)P3 in turn activates a myriad of signaling pathways and various transcription factors (45, 46). The IRS-2-PI3K pathway is one mechanism by which IL-4 is thought to mediate its antiapoptotic effects (47–50). Since the PI3K pathway was shown to regulate the STAT6-dependent induction of NFIL-3 by IL-4 in murine B-cells (43), we hypothesized that activation of this pathway might be a critical step linking signaling by IL-4 through type I receptors to the amplified induction of alternatively activated macrophage genes by IL-4 in BMMs. To test this hypothesis, we pretreated WT BMM for one hour with the specific PI3K inhibitor wortmannin (50 nM) and then stimulated the cells with 100 ng/mL of IL-4 or IL-13 for six and 24 hours. RNA was harvested and the expression of genes associated with alternatively activated macrophages was quantified (Fig. 9). Induction of the expression of FIZZ1 by IL-4 was suppressed by wortmannin at both time points; wortmannin reduced the abundance of FIZZ1 mRNA to that observed in IL-13-treated cells. The induction of expression of Ym1 and arginase I by IL-4 was barely affected by wortmannin. These results suggest that IL-4, acting through the type I receptor, can efficiently activate both STAT6- and IRS-2-dependent signaling pathways, whereas either IL-4 or IL-13, acting through the type II receptor, activates IRS-2 inefficiently. The integration of the STAT6 pathway with other pathways downstream of IRS-2, including the PI3K pathway, may lead to amplified expression of a subset of genes in alternatively activated macrophages.
Fig. 9.
Effect of wortmannin on the induction of FIZZ1, Ym1, and arginase 1 by IL-4. BMMs were isolated from WT C57BL/6 mice as described above. After ten days, the cells were treated with (filled circles) or without (open circles) 100 nM wortmannin for twenty minutes and were then stimulated with 100 ng/mL IL-4 or IL-13 (open triangles). Total RNA was harvested at six and 24 hours. cDNA was generated and real-time RT-PCR analysis with specific primers for the named genes was performed. The results are expressed as the fold-induction (2−ΔΔCt) of each specific mRNA in cytokine-treated cells compared to that of unstimulated cells and normalized to HPRT, a housekeeping gene. A representative experiment of two independent experiments is shown.
DISCUSSION
Because IL-4 and IL-13 activate STAT6 and share biological functions and receptor subunits, there has been much discussion about their relative importance and roles in both normal physiology and pathological situations, such as allergic inflammation (14). In this study, we undertook a careful side-by-side analysis of the signal transduction pathways activated by each cytokine through the receptor complex and the functional consequences of any differential signaling. We have shown that both IL-4 and IL-13 induce the phosphorylation of STAT6 but that IL-4 is typically more potent than IL-13. In addition to this dose-dependent difference in their activation of STAT6, we report that IL-4 strongly activates the IRS-2 pathway, with peak responses in the range of 1 to 3 ng/mL, but that IL-13 is a relatively poor activator of this pathway even at concentrations up to 200 ng/mL. Through experiments involving a human monocytic cell line that does not express γC and cells derived from γC-deficient mice, we found that the ability of IL-4 to efficiently induce phosphorylation of IRS-2 was dependent on the presence of the γC subunit on the cell surface, which enables the formation of type I IL-4 receptor complexes. Forced expression of γC in THP-1 cells, which normally lack γC, allowed for increased phosphorylation of IRS-2 in response to IL-4.
It has been suggested that the role of IL-4 is largely confined to the differentiation of Th2 cells, immunoglobulin (Ig) class switching (particularly to IgE), and initiation of the allergic immune response, whereas IL-13 is thought to act later as an “effector” cytokine targeting cells in the lung. This model is partly based on reports indicating the presence of larger amounts of IL-13 (51, 52) than of IL-4 (53, 54) in lung tissue and airway lavage. Our results suggest that due to the superior potency of IL-4 over IL-13 to activate STAT6 phosphorylation rapidly (15, 26), its higher affinity for its specific binding chain (KD 20–200 pM), and its superior ability to activate IRS-2, only very low concentrations of IL-4 would be required to elicit potent cellular responses. Much more IL-13 would usually be required to elicit similar responses, depending on the relative abundance of the three receptor chains on the responding cells. It is also clear that IL-13 alone cannot elicit all the key features of the late-phase of an allergic asthmatic response. It has been shown in many animal models that IL-4 is critical for the infiltration of eosinophils into the airway tissue [(55–58) and reviewed in (59)]. Furthermore, it was recently shown that the type II receptor complex is not required for eosinophilic infiltration of the airways or for the elaboration of several alternatively activated macrophage gene products (58, 60).
The relatively superior activation of the IRS-2 signal transduction pathway by IL-4, through the type I IL-4 receptor, and the enhanced association of IRS-2 with PI3K and Grb2, provides a molecular mechanism by which IL-4 can exert differential signaling when compared to that of IL-13. The mechanism by which the γC chain/type I receptor complex specifically enhances IRS-2 phosphorylation and targets the phosphorylation of the Grb2 docking site tyrosine on the IRS-2 molecule in response to IL-4 is unknown. One possibility is that the γC chain contains specific sequence motifs that would enhance the recruitment and activation of the IRS-2 signaling pathway. This possibility is supported by the observation that several other cytokines known to use the γC as part of their receptor complexes (IL-2, IL-7, IL-9, and IL-15) stimulate the phosphorylation of IRS molecules (61–63). Sequence alignment of human and murine γC with known IRS-2-docking sites found in other receptors (IL-4Rα and the insulin receptor), called the I4R motif, revealed a sequence in the intracellular domain of the γC subunit highly similar to the I4R motif, (GLXXXXQPXY compared to PLXXXXNPXY). This sequence is absent in IL-13Rα1, which suggests that the putative I4R motif in γC may act to enhance recruitment of IRS-2.
The type I IL-4 receptor complex recruits JAK3 by virtue of its constitutive association with the cytoplasmic domain of the γC subunit; the type II receptor does not interact with JAK3 (64). While it is generally thought that the JAK kinases do not encode any large degree of signaling specificity (65), we speculate the differences in signaling that we observed could be due to unique activity of JAK3. For example, JAK3 may preferentially phosphorylate the I4R motif of the IL-4Rα chain in type I receptor complexes whereas the other JAKs that are associated with the type II receptor complex (JAK1, Tyk2, and JAK2) may fail to do so. JAK3 may also be a better kinase for IRS-2 than the others and have a unique ability to discriminate between the multitude of potential tyrosine phosphorylation sites on IRS-2 and preferentially phosphorylate the tyrosines that serve as the p85 and Grb2-docking sites. It is also possible that the cytoplasmic domain of γC can recruit other molecules that might target the phosphorylation of the Grb2 tyrosine or mask phosphorylation at the other sites. Further study with in vitro experiments with purified activated JAK3 and its potential substrates will allow us to investigate these possibilities.
In terms of the functional impact of signaling through the type I IL-4 receptor, we have demonstrated that IL-4 signaling through type I IL-4 receptors and activation of IRS-2 in macrophages led to a dramatic induction of a particular subset of genes characteristic of alternatively activated macrophages (arginase I, FIZZ1, and Ym1). This induction was orders of magnitude more potent than that induced by signaling through the type II receptor, either by IL-4 or IL-13. In contrast, both IL-4 and IL-13 induced the expression of MMR mRNA similarly (two- or three-fold above control). This observation suggests that phosphorylation of IRS-2, recruitment of Grb2, activation of signaling molecules such as PI3K, transcription factors downstream of these molecules, or both substantially augment the transcription of the three hallmark genes (arginase I, FIZZ1, Ym1) but not of others, such as MMR.
The identity of the molecules downstream of IRS-2/PI3K and IRS-2/Grb2 with the capacity to influence the STAT6-depenent gene transcription are currently unknown. We did not observe a difference in Akt phosphorylation between IL-4 and IL-13 treated BMM nor did we observe an induction of ERK or p38 phosphorylation by these cytokines. These results are consistent with the finding that the IRS1/Grb2/SOS association does not lead to activation of MAP kinases (38). It has been proposed that the Grb2 adapter may interact with other molecules such as vav and dynamin leading to Grb2-dependent but MAP kinase-independent signaling pathways (66).
The regulation of STAT6 mediated transcription is complex and still relatively unclear. It has been reported that STAT6-mediated transcription is regulated by multiple uncharacterized serine/threonine kinase pathways (67) and that STAT6 cooperates with several other transcription factors including p300, C/EBPβ, p100, and CoaSt6 to mediate transcription (68). Interestingly, the induction of arginase I, FIZZ1, and Ym1appeared to be differentially sensitive to the inhibitory effects of wortmannin. These observations could be explained by a variable dependency on PI3K activity for transcriptional activation and indicates that there might be signaling molecules or transcription factors other than those downstream of PI3K that play a role in the expression of these genes. Induction of FIZZ1 expression by IL-4 appeared to be the most sensitive to wortmannin and was suppressed to levels similar to that induced by IL-13. This suggests that maximum transcriptional activity of this particular gene, although STAT6-dependent, is heavily dependent on PI3K-activated pathways, transcription factors, or both similar to the induction of NFIL3 by IL-4 in murine B-cells (43). We observed Akt phosporylation in the M-CSF-dependent BMM that was only modestly affected by IL-4 or IL-13. While Akt is a major target downstream of PI3K, it is not the only target (45) and it is possible that other targets downstream of PI3K participate in the regulation of the FIZZ1 promoter. The FIZZ1 promoter has been functionally analyzed (69) and it contains critical adjacent STAT6- and C/EBP-1-binding sites. Mutation of the C/EBP portion of the binding site greatly reduced the ability of FIZZ1 transcription to be induced by IL-4 even though the STAT6-binding site was still present. C/EBP requires the PI3K pathway for full expression and transcriptional activity (70–72), which might explain the acute sensitivity of IL-4-induced FIZZ1 expression to wortmannin. Detailed studies of the regulation of arginase I and Ym1 transcription would clarify the factors in addition to STAT6 that are activated and required by these genes for full expression.
It follows that macrophages will strongly express these genes (and perhaps others for which we did not screen) when exposed to an IL-4-containing environment, such as is found in the lung during allergic inflammation. Expression of arginase I, FIZZ1, and Ym1 by alternatively activated macrophages could worsen the process of allergic inflammation and exacerbate airway remodeling in a number of ways. Arginase I may participate in the proliferative changes and the transforming growth factor–β (TGF-β) -dependent fibrotic remodeling of the lung (73). Arginase I synthesizes ornithine from L-arginine, and excess L-ornithine (and proline) is taken up by fibroblasts and incorporated into collagen (74, 75). Ornithine is also a substrate for L-ornithine decarboxylase (ODC) resulting in the production of polyamines that act as growth factors necessary for cell division (76–78). An overabundance of arginase leads to the proliferation of vascular smooth muscle and endothelial cells (79, 80), which are both associated with chronic allergic remodeling. FIZZ1, also known as resistin-like moleculeα (RELMα), is a cysteine- containing secreted protein whose abundance is substantially increased in the bronchoalveolar lavage (BAL) fliud in models of murine allergic pulmonary inflammation (81). It is hypothesized to play a negative regulatory role in allergic inflammation, as it antagonizes the actions of nerve growth factor (NGF), which amplifies Th2 effector functions. In addition, FIZZ1 is implicated in mediating the deposition of extracellular matrix (82). Ym1 is an intracellular, soluble, chitinase-like lectin, which is thought to be involved in wound healing and degradation of worm eggs (83), as it binds chitin and other related glycan structures, and it also may act as a chemoattractant for eosinophils (84–86). Our data suggest that the considerable increased expression of arginase 1, FIZZ1, and Ym1 through the type I IL-4 receptor would substantially increase inflammation in the lung in an allergic inflammatory environment. Recent studies have demonstrated that the expression of these three alternatively activated macrophage genes is increased in the lungs of IL-13Rα1−/− mice during allergic lung inflammation, which suggests that the type I IL-4 receptor plays a major role in this feature of allergic lung pathology (58).
From the number of studies currently available, it is clear that both types of IL-4 receptor complexes play diverse roles in the regulation of allergic inflammation. The type II receptor complex is critical for airway hyperreactivity and goblet cell metaplasia (55, 56, 58, 60). Our study indicates that the type I, γC-containing, receptor can serve as an initiator of multiple signaling pathways (IRS-2-PI3K and IRS-2-Grb2) and that it can integrate with the STAT6 pathway to result in a dramatic amplification of a subset of macrophage genes. Further investigations of the molecular mechanisms and downstream signaling pathways involved, accompanied by in vivo experiments, will be required to fully elucidate the function type I IL-4 receptor signaling in the context of the allergic inflammatory response.
MATERIALS AND METHODS
Reagents
Recombinant murine and human IL-4, IL-13, and IGF-I were obtained from R&D Systems. Sodiumorthovanadate was obtained from ICN (Aurora, OH). Biotinylated anti-human IL-13Rα1 antibody was obtained from R&D Systems. Phycoerythrin (PE)-conjugated anti-IL-13Rα2 antibody was obtained from Cell Science (Canton, MA) and recognized IL-13Rα2 produced in response to treatment with IL-4 tumor necrosis factor α (TNFα). All PE-conjugated antibodies used for analysis of cells by flow cytometry were purchased from BD Pharmingen (San Jose, CA). Antibodies for immunoprecipitation (IP), analysis of Western blots, or both were purchased from the following companies: anti-IRS-2 (Upstate, Lake Placid, NY), anti-STAT6 (M-20 and S-20, Santa Cruz, Santa Cruz, CA), anti-human phospho-STAT6-Tyr641 (BD Transduction Laboratories), anti-phospho-Akt-Ser473 (Invitrogen, Carlsbad, CA), anti-mouse phospho-STAT6-Tyr641, anti-phospho-p38-Thr180/Tyr182, anti-p38, anti-phospho-ERK1/2-Thr202/Tyr204, anti-ERK1/2, anti-phospho-Akt-Thr308, and anti-Akt (Cell Signaling Technologies, Danvers, MA). Clean-Blot IP detection reagent (Thermo Fisher Scientific, Rockford, IL) was used in place of a secondary antibody for detection of Grb2 (~25 kD) to prevent detection of the light chain of the immunoprecipitating antibody.
Cell Culture
Human monocytic THP-1 cells were maintained in RPMI (BioWhittaker, Inc., Walkersville, MD) supplemented with 10% fetal bovine serum, 100 units/mL penicillin, 100 μg/mL streptomycin, 2 mM glutamine, 0.05 mM 2-mercaptoethanol and 4.5 g/L of glucose. Human monocytic U937 cells were maintained in RPMI supplemented with 10% fetal bovine serum, 100 units/mL penicillin, 100 μg/mL streptomycin, and 2 mM glutamine. Murine bone marrow-derived macrophages were generated from either WT C57BL/6 (Taconic Laboratories, Germantown, NY) or γC−/− (breeding pairs obtained from Taconic and bred in-house). Briefly, bone marrow was isolated from femurs and tibias of four- to six-week-old female mice.. To deplete adherent stromal cells, the harvested bone marrow was cultured overnight in α-10 medium [α-Minimal Essential Medium supplemented with penicillin, streptomycin, and glutamine (BioWhittaker) and 10% heat-inactivated fetal bovine serum (FBS; Invitrogen, Frederick, MD)] with 20 ng/mL of recombinant murine macrophage colony-stimulating factor [rmM-CSF, R&D Systems, Minneapolis, MN)]. Nonadherent cells were collected, red blood cell lysis was performed, and nonadherent mononuclear cellswere plated and cultured for ten days in the presence of 20 ng/mL of rmM-CSF to generate bone marrow-derived macrophages (BMMs).
Signaling experiments
Signaling experiments were performed as described previously (32, 49) with the following modifications. Cells were deprived of serum for two hours before the additionof the cytokine stimulus. Lysis buffer was composed of 50 mM Hepes, pH 8.0,50 mM NaCl, 1% NP-40, 5 mM ethylenediaminetetraacetic acid (EDTA), 10 mM sodium pyrophosphate, 50 mM NaF, 0.25% sodium deoxycholate, 1 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride (PMSF), 1 μg/ml pepstatin, 10 μg/ml leupeptin, and 100 μg/ml soy-bean trypsin inhibitor. For the analyses of serine/threonine phosphorylation, calyculin A (LC Laboratories, Woburn, MA) was included in the lysis buffer at 100 nM. ProteinG-agarose beads (Invitrogen) were used for immunoprecipitations. For detection of phosphotyrosines on Western blots, we used horseradish peroxidase (HRP)-congugated anti-phosphotyrosine antibody (PY-20, BD Biosciences) and HyGLO™ (Denville Scientific, Metuchen, NJ) or Amersham ECL (GE Healthcare, Piscataway, NJ) as a chemiluminescent substrate for visualization of membrane-bound proteins. Blots with darker protein bands were chosen for the representative figures.
Densitometric Analysis
Shorter exposures of films were chosen for densitometric analysis so that band intensities were in the linear range of the film. Films were scanned using a flat-bed scanner and the density of the bands on the captured image was analyzed using the NIH Image software (version 1.63f). The amount of phosphoprotein was calculated as a ratio of the density of the band of the phosphorylated form divided by the density of the band corresponding to the unphosphorylated form of the protein for normalization. For some experiments, the amount of phosphoprotein was calculated as a percentage of the maximal phosphoprotein signal. In these cases, the amount of induced phosphoprotein in response to a certain stimulus (usually IL-4 or IGF-I) was set to 100% (“maximal”) and the amount of induced phosphoprotein in response to the other stimuli was compared to that maximal value.
Flow cytometry
Cells were washed with FACS buffer [Dulbecco’s phosphate-buffered saline (DPBS) containing 0.1% bovine serum albumin (BSA) and 0.1% sodium azide)]three times and 3 × 105 cells were incubated either with specific PE-conjugated antibodies against the surface proteins of interest or isotype-matched PE-conjugated immunoglobulin as the negative control. An unstained sample of cells was also used as a negative control; propidium iodide (PI) was added to this sample to detect dead cells, so that only PI-negative live cells were included in the analysis. Cells were incubated with antibodies for 20 to 30 minutes on ice to allow binding, before an additional three washes to remove unbound antibody. Fluorescently labeled cells were analyzed using the FACSCalibur instrument and CellQuest software to generate FACS histograms showing the fluorescence intensity of the specific surface protein on live cells. The mean fluorescence intensities of these histograms were also measured by FACSCalibur/CellQuest software. For detection of intracellular phospho-STAT6, cells were fixed and permeabilized with 4% formaldehyde followed by 90% methanol at −20 °C for one hour or more. Fixed and permeabilized cells were washed three times with Triton buffer and incubation with the appropriate antibodies was performed for 30 minutes at room temperature in the dark. Unbound antibodies were removed by rinsing cells three times with Triton buffer and the fluorescently labeled cells were analyzed as described above.
Stable transfection of THP-1 with wild-type or mutant γC
THP-1 cells were co-transfected with a linearized plasmid encoding WT human γC [a generous gift from Dr. Warren Leonard, NHLBI, NIH, (87)] and a neomycin-resistance plasmid using the Amaxa nucleofection device. Stable clones were selected by growth in G418-containing medium and overexpression of the plasmid was verified by flow cytometry with specific antibodies to human γC as described above.
Real-time RT-PCR
Total RNA was isolated from BMM cultures with the RNeasy kit (Qiagen, Valencia, CA) according to the manufacturer’s protocol, and cDNA was generated using the SuperScript™ III First Strand Synthesis System (Invitrogen). Real-time PCR was performed with specific primer sets (synthesized by Invitrogen) by methods previously described (88) on an Applied Biosystems Inc. 7900HT machine. The relative abundance of mRNA for specific genes in treated cells are reported as the fold-induction over their abundance in untreated, control samples, with hypoxanthine guanine phosphoribosyl transferase (HPRT) as the internal reference gene [2−ΔΔCt method (89)].
Statistical Analysis
Average data are expressed as the mean ± SEM from three or more independent experiments. Statistical analysis was performed on the data with the Wilcoxon-Mann-Whitney Rank Sum test or Student’s t-test where appropriate. Statistical significance was reached when P < 0.05.
REFERENCES AND NOTES
- 1.Boulay JL, Paul WE. The interleukin-4 family of lymphokines. Curr Opin Immunol. 1992;4:294–298. doi: 10.1016/0952-7915(92)90079-t. [DOI] [PubMed] [Google Scholar]
- 2.Sprang SR, Bazan JF. Cytokine structural taxonomy and mechanisms of receptor engagement. Curr Opin Struct Biol. 1993;3:815–827. [Google Scholar]
- 3.Rozwarski DA, Gronenborn AM, Clore GM, Bazan JF, Bohm A, Wlodawer A, Hatada M, Karplus PA. Structural comparisons among the short-chain helical cytokines. Structure. 1994;2:159–173. doi: 10.1016/s0969-2126(00)00018-6. [DOI] [PubMed] [Google Scholar]
- 4.Minty A, Chalon P, Derocq JM, Dumont X, Guillemot JC, Kaghad M, Labit C, Leplatois P, Liauzun P, Miloux B, et al. Interleukin-13 is a new human lymphokine regulating inflammatory and immune responses. Nature. 1993;362:248–250. doi: 10.1038/362248a0. [DOI] [PubMed] [Google Scholar]
- 5.Chomarat P, Banchereau J. Interleukin-4 and interleukin-13: their similarities and discrepancies. Int Rev Immunol. 1998;17:1–52. doi: 10.3109/08830189809084486. [DOI] [PubMed] [Google Scholar]
- 6.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 7.Nelms K, Keegan AD, Zamorano J, Ryan JJ, Paul WE. The IL-4 receptor: signaling mechanisms and biologic functions. Annu Rev Immunol. 1999;17:701–738. doi: 10.1146/annurev.immunol.17.1.701. [DOI] [PubMed] [Google Scholar]
- 8.Sabin EA, Kopf MA, Pearce EJ. Schistosoma mansoni egg-induced early IL-4 production is dependent upon IL-5 and eosinophils. J Exp Med. 1996;184:1871–1878. doi: 10.1084/jem.184.5.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rumbley CA, Sugaya H, Zekavat SA, El Refaei M, Perrin PJ, Phillips SM. Activated eosinophils are the major source of Th2-associated cytokines in the schistosome granuloma. J Immunol. 1999;162:1003–1009. [PubMed] [Google Scholar]
- 10.Woerly G, Roger N, Loiseau S, Capron M. Expression of Th1 and Th2 immunoregulatory cytokines by human eosinophils. Int Arch Allergy Immunol. 1999;118:95–97. doi: 10.1159/000024038. [DOI] [PubMed] [Google Scholar]
- 11.Bandeira-Melo C, Hall JC, Penrose JF, Weller PF. Cysteinyl leukotrienes induce IL-4 release from cord blood-derived human eosinophils. J Allergy Clin Immunol. 2002;109:975–979. doi: 10.1067/mai.2002.124269. [DOI] [PubMed] [Google Scholar]
- 12.Jacobsen EA, Taranova AG, Lee NA, Lee JJ. Eosinophils: singularly destructive effector cells or purveyors of immunoregulation? J Allergy Clin Immunol. 2007;119:1313–1320. doi: 10.1016/j.jaci.2007.03.043. [DOI] [PubMed] [Google Scholar]
- 13.Shirey K, Cole LE, Keegan AD, Elkins KE, Vogel SN. Francisella tularensis LVS induces Macrophage Alternative Activation as a Survival Mechanism. Journal of Immunology. 2008 doi: 10.4049/jimmunol.181.6.4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wills-Karp M, Chiaramonte M. Interleukin-13 in asthma. Curr Opin Pulm Med. 2003;9:21–27. doi: 10.1097/00063198-200301000-00004. [DOI] [PubMed] [Google Scholar]
- 15.LaPorte SL, Juo ZS, Vaclavikova J, Colf LA, Qi X, Heller NM, Keegan AD, Garcia KC. Molecular and structural basis of cytokine receptor pleiotropy in the interleukin-4/13 system. Cell. 2008;132:259–272. doi: 10.1016/j.cell.2007.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andrews AL, Holloway JW, Holgate ST, Davies DE. IL-4 receptor alpha is an important modulator of IL-4 and IL-13 receptor binding: implications for the development of therapeutic targets. J Immunol. 2006;176:7456–7461. doi: 10.4049/jimmunol.176.12.7456. [DOI] [PubMed] [Google Scholar]
- 17.Kawakami K, Taguchi J, Murata T, Puri RK. The interleukin-13 receptor alpha2 chain: an essential component for binding and internalization but not for interleukin-13-induced signal transduction through the STAT6 pathway. Blood. 2001;97:2673–2679. doi: 10.1182/blood.v97.9.2673. [DOI] [PubMed] [Google Scholar]
- 18.Fichtner-Feigl S, Strober W, Kawakami K, Puri RK, Kitani A. IL-13 signaling through the IL-13alpha2 receptor is involved in induction of TGF-beta1 production and fibrosis. Nat Med. 2006;12:99–106. doi: 10.1038/nm1332. [DOI] [PubMed] [Google Scholar]
- 19.Boothby M, Mora AL, Stephenson LM. Lymphokine-dependent proliferation of T-lymphoid cells: regulated responsiveness and role in vivo. Crit Rev Immunol. 2001;21:487–522. [PubMed] [Google Scholar]
- 20.Pernis AB, Rothman PB. JAK-STAT signaling in asthma. J Clin Invest. 2002;109:1279–1283. doi: 10.1172/JCI15786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelly-Welch AE, Hanson EM, Boothby MR, Keegan AD. Interleukin-4 and interleukin-13 signaling connections maps. Science. 2003;300:1527–1528. doi: 10.1126/science.1085458. [DOI] [PubMed] [Google Scholar]
- 22.Keegan AD, Johnston JA, Tortolani PJ, McReynolds LJ, Kinzer C, O’Shea JJ, Paul WE. Similarities and differences in signal transduction by interleukin 4 and interleukin 13: analysis of Janus kinase activation. Proc Natl Acad Sci U S A. 1995;92:7681–7685. doi: 10.1073/pnas.92.17.7681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu J, Guo L, Watson CJ, Hu-Li J, Paul WE. Stat6 is necessary and sufficient for IL-4’s role in Th2 differentiation and cell expansion. J Immunol. 2001;166:7276–7281. doi: 10.4049/jimmunol.166.12.7276. [DOI] [PubMed] [Google Scholar]
- 24.Wurster AL, Withers DJ, Uchida T, White MF, Grusby MJ. Stat6 and IRS-2 cooperate in interleukin 4 (IL-4)-induced proliferation and differentiation but are dispensable for IL-4-dependent rescue from apoptosis. Mol Cell Biol. 2002;22:117–126. doi: 10.1128/MCB.22.1.117-126.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blaeser F, Bryce PJ, Ho N, Raman V, Dedeoglu F, Donaldson DD, Geha RS, Oettgen HC, Chatila TA. Targeted inactivation of the IL-4 receptor alpha chain I4R motif promotes allergic airway inflammation. J Exp Med. 2003;198:1189–1200. doi: 10.1084/jem.20030471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Junttila IS, Mizukami K, Dickensheets H, Meier-Schellersheim M, Yamane H, Donnelly RP, Paul WE. Tuning sensitivity to IL-4 and IL-13: Differential expression of IL-4Rα, IL-13Rα1 and γ c regulates relative cytokine sensitivity. Journal of Experimental Medicine. 2008 doi: 10.1084/jem.20080452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kreider T, Anthony RM, Urban JF, Jr, Gause WC. Alternatively activated macrophages in helminth infections. Curr Opin Immunol. 2007;19:448–453. doi: 10.1016/j.coi.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hart PH, Bonder CS, Balogh J, Dickensheets HL, Vazquez N, Davies KV, Finlay-Jones JJ, Donnelly RP. Diminished responses to IL-13 by human monocytes differentiated in vitro: role of the IL-13Ralpha1 chain and STAT6. Eur J Immunol. 1999;29:2087–2097. doi: 10.1002/(SICI)1521-4141(199907)29:07<2087::AID-IMMU2087>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 29.Andersson A, Grunewald SM, Duschl A, Fischer A, DiSanto JP. Mouse macrophage development in the absence of the common gamma chain: defining receptor complexes responsible for IL-4 and IL-13 signaling. Eur J Immunol. 1997;27:1762–1768. doi: 10.1002/eji.1830270725. [DOI] [PubMed] [Google Scholar]
- 30.Kornmann M, Maruyama H, Bergmann U, Tangvoranuntakul P, Beger HG, White MF, Korc M. Enhanced expression of the insulin receptor substrate-2 docking protein in human pancreatic cancer. Cancer Res. 1998;58:4250–4254. [PubMed] [Google Scholar]
- 31.Zamorano J, Kelly AE, Austrian J, Wang HY, Keegan AD. Costimulation of resting B lymphocytes alters the IL-4-activated IRS2 signaling pathway in a STAT6 independent manner: implications for cell survival and proliferation. Cell Res. 2001;11:44–54. doi: 10.1038/sj.cr.7290065. [DOI] [PubMed] [Google Scholar]
- 32.Moreno JL, Kaczmarek M, Keegan AD, Tondravi M. IL-4 suppresses osteoclast development and mature osteoclast function by a STAT6-dependent mechanism: irreversible inhibition of the differentiation program activated by RANKL. Blood. 2003;102:1078–1086. doi: 10.1182/blood-2002-11-3437. [DOI] [PubMed] [Google Scholar]
- 33.Sun XJ, Wang LM, Zhang Y, Yenush L, Myers MG, Jr, Glasheen E, Lane WS, Pierce JH, White MF. Role of IRS-2 in insulin and cytokine signalling. Nature. 1995;377:173–177. doi: 10.1038/377173a0. [DOI] [PubMed] [Google Scholar]
- 34.Sun XJ, Crimmins DL, Myers MG, Jr, Miralpeix M, White MF. Pleiotropic insulin signals are engaged by multisite phosphorylation of IRS-1. Mol Cell Biol. 1993;13:7418–7428. doi: 10.1128/mcb.13.12.7418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Skolnik EY, Lee CH, Batzer A, Vicentini LM, Zhou M, Daly R, Myers MJ, Jr, Backer JM, Ullrich A, White MF, et al. The SH2/SH3 domain-containing protein GRB2 interacts with tyrosine-phosphorylated IRS1 and Shc: implications for insulin control of ras signalling. EMBO J. 1993;12:1929–1936. doi: 10.1002/j.1460-2075.1993.tb05842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sugimoto S, Wandless TJ, Shoelson SE, Neel BG, Walsh CT. Activation of the SH2-containing protein tyrosine phosphatase, SH-PTP2, by phosphotyrosine-containing peptides derived from insulin receptor substrate-1. J Biol Chem. 1994;269:13614–13622. [PubMed] [Google Scholar]
- 37.Myers MG, Jr, Wang LM, Sun XJ, Zhang Y, Yenush L, Schlessinger J, Pierce JH, White MF. Role of IRS-1-GRB-2 complexes in insulin signaling. Mol Cell Biol. 1994;14:3577–3587. doi: 10.1128/mcb.14.6.3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pruett W, Yuan Y, Rose E, Batzer AG, Harada N, Skolnik EY. Association between GRB2/Sos and insulin receptor substrate 1 is not sufficient for activation of extracellular signal-regulated kinases by interleukin-4: implications for Ras activation by insulin. Mol Cell Biol. 1995;15:1778–1785. doi: 10.1128/mcb.15.3.1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Welham MJ, Duronio V, Schrader JW. Interleukin-4-dependent proliferation dissociates p44erk-1, p42erk-2, and p21ras activation from cell growth. J Biol Chem. 1994;269:5865–5873. [PubMed] [Google Scholar]
- 40.Foltz IN, Lee JC, Young PR, Schrader JW. Hemopoietic growth factors with the exception of interleukin-4 activate the p38 mitogen-activated protein kinase pathway. J Biol Chem. 1997;272:3296–3301. doi: 10.1074/jbc.272.6.3296. [DOI] [PubMed] [Google Scholar]
- 41.Foltz IN, Schrader JW. Activation of the stress-activated protein kinases by multiple hematopoietic growth factors with the exception of interleukin-4. Blood. 1997;89:3092–3096. [PubMed] [Google Scholar]
- 42.Hunt AE, Williams LM, Lali FV, Foxwell BM. IL-4 regulation of p38 MAPK signalling is dependent on cell type. Cytokine. 2002;18:295–303. doi: 10.1006/cyto.2002.1043. [DOI] [PubMed] [Google Scholar]
- 43.Canfield S, Lee Y, Schroder A, Rothman P. Cutting edge: IL-4 induces suppressor of cytokine signaling-3 expression in B cells by a mechanism dependent on activation of p38 MAPK. J Immunol. 2005;174:2494–2498. doi: 10.4049/jimmunol.174.5.2494. [DOI] [PubMed] [Google Scholar]
- 44.Mosser DM. The many faces of macrophage activation. J Leukoc Biol. 2003;73:209–212. doi: 10.1189/jlb.0602325. [DOI] [PubMed] [Google Scholar]
- 45.Chan TO, Rittenhouse SE, Tsichlis PN. AKT/PKB and other D3 phosphoinositide-regulated kinases: kinase activation by phosphoinositide-dependent phosphorylation. Annu Rev Biochem. 1999;68:965–1014. doi: 10.1146/annurev.biochem.68.1.965. [DOI] [PubMed] [Google Scholar]
- 46.Sale EM, Sale GJ. Protein kinase B: signalling roles and therapeutic targeting. Cell Mol Life Sci. 2008;65:113–127. doi: 10.1007/s00018-007-7274-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dancescu M, Rubio-Trujillo M, Biron G, Bron D, Delespesse G, Sarfati M. Interleukin 4 protects chronic lymphocytic leukemic B cells from death by apoptosis and upregulates Bcl-2 expression. J Exp Med. 1992;176:1319–1326. doi: 10.1084/jem.176.5.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Illera VA, Perandones CE, Stunz LL, Mower DA, Jr, Ashman RF. Apoptosis in splenic B lymphocytes. Regulation by protein kinase C and IL-4. J Immunol. 1993;151:2965–2973. [PubMed] [Google Scholar]
- 49.Zamorano J, Wang HY, Wang LM, Pierce JH, Keegan AD. IL-4 protects cells from apoptosis via the insulin receptor substrate pathway and a second independent signaling pathway. J Immunol. 1996;157:4926–4934. [PubMed] [Google Scholar]
- 50.Carey GB, Semenova E, Qi X, Keegan AD. IL-4 protects the B-cell lymphoma cell line CH31 from anti-IgM-induced growth arrest and apoptosis: contribution of the PI-3 kinase/AKT pathway. Cell Res. 2007;17:942–955. doi: 10.1038/sj.cr.2007.90. [DOI] [PubMed] [Google Scholar]
- 51.Berry MA, Parker D, Neale N, Woodman L, Morgan A, Monk P, Bradding P, Wardlaw AJ, Pavord ID, Brightling CE. Sputum and bronchial submucosal IL-13 expression in asthma and eosinophilic bronchitis. J Allergy Clin Immunol. 2004;114:1106–1109. doi: 10.1016/j.jaci.2004.08.032. [DOI] [PubMed] [Google Scholar]
- 52.Huang SK, Xiao HQ, Kleine-Tebbe J, Paciotti G, Marsh DG, Lichtenstein LM, Liu MC. IL-13 expression at the sites of allergen challenge in patients with asthma. J Immunol. 1995;155:2688–2694. [PubMed] [Google Scholar]
- 53.Kroegel C, Julius P, Matthys H, Virchow JC, Jr, Luttmann W. Endobronchial secretion of interleukin-13 following local allergen challenge in atopic asthma: relationship to interleukin-4 and eosinophil counts. Eur Respir J. 1996;9:899–904. doi: 10.1183/09031936.96.09050899. [DOI] [PubMed] [Google Scholar]
- 54.Jung T, Bews JP, Enssle KH, Wagner K, Neumann C, Heusser CH. Detection of and discrimination between total and free human interleukin-4 and free soluble interleukin-4 receptor by ELISA. J Immunol Methods. 1998;217:41–50. doi: 10.1016/s0022-1759(98)00084-2. [DOI] [PubMed] [Google Scholar]
- 55.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 56.Walter DM, McIntire JJ, Berry G, McKenzie AN, Donaldson DD, DeKruyff RH, Umetsu DT. Critical role for IL-13 in the development of allergen-induced airway hyperreactivity. J Immunol. 2001;167:4668–4675. doi: 10.4049/jimmunol.167.8.4668. [DOI] [PubMed] [Google Scholar]
- 57.Perkins C, Wills-Karp M, Finkelman FD. IL-4 induces IL-13-independent allergic airway inflammation. J Allergy Clin Immunol. 2006;118:410–419. doi: 10.1016/j.jaci.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 58.Munitz A, Brandt EB, Mingler M, Finkelman FD, Rothenberg ME. Distinct roles for IL-13 and IL-4 via IL-13 receptor alpha1 and the type II IL-4 receptor in asthma pathogenesis. Proc Natl Acad Sci U S A. 2008;105:7240–7245. doi: 10.1073/pnas.0802465105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moffatt JD. What targets have knockouts revealed in asthma? Pharmacol Ther. 2005;107:343–357. doi: 10.1016/j.pharmthera.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 60.Ramalingam TR, Pesce JT, Sheikh F, Cheever AW, Mentink-Kane MM, Wilson MS, Stevens S, Valenzuela DM, Murphy AJ, Yancopoulos GD, Urban JF, Jr, Donnelly RP, Wynn TA. Unique functions of the type II interleukin 4 receptor identified in mice lacking the interleukin 13 receptor alpha1 chain. Nat Immunol. 2008;9:25–33. doi: 10.1038/ni1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Johnston JA, Wang LM, Hanson EP, Sun XJ, White MF, Oakes SA, Pierce JH, O’Shea JJ. Interleukins 2, 4, 7, and 15 stimulate tyrosine phosphorylation of insulin receptor substrates 1 and 2 in T cells. Potential role of JAK kinases. J Biol Chem. 1995;270:28527–28530. doi: 10.1074/jbc.270.48.28527. [DOI] [PubMed] [Google Scholar]
- 62.Yin T, Keller SR, Quelle FW, Witthuhn BA, Tsang ML, Lienhard GE, Ihle JN, Yang YC. Interleukin-9 induces tyrosine phosphorylation of insulin receptor substrate-1 via JAK tyrosine kinases. J Biol Chem. 1995;270:20497–20502. doi: 10.1074/jbc.270.35.20497. [DOI] [PubMed] [Google Scholar]
- 63.Xiao H, Yin T, Wang XY, Uchida T, Chung J, White MF, Yang YC. Specificity of interleukin-2 receptor gamma chain superfamily cytokines is mediated by insulin receptor substrate-dependent pathway. J Biol Chem. 2002;277:8091–8098. doi: 10.1074/jbc.M106650200. [DOI] [PubMed] [Google Scholar]
- 64.Hofmann SR, Lam AQ, Frank S, Zhou YJ, Ramos HL, Kanno Y, Agnello D, Youle RJ, O’Shea JJ. Jak3-independent trafficking of the common gamma chain receptor subunit: chaperone function of Jaks revisited. Mol Cell Biol. 2004;24:5039–5049. doi: 10.1128/MCB.24.11.5039-5049.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen M, Cheng A, Chen YQ, Hymel A, Hanson EP, Kimmel L, Minami Y, Taniguchi T, Changelian PS, O’Shea JJ. The amino terminus of JAK3 is necessary and sufficient for binding to the common gamma chain and confers the ability to transmit interleukin 2-mediated signals. Proc Natl Acad Sci U S A. 1997;94:6910–6915. doi: 10.1073/pnas.94.13.6910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang S, Weinheimer C, Courtois M, Kovacs A, Zhang CE, Cheng AM, Wang Y, Muslin AJ. The role of the Grb2-p38 MAPK signaling pathway in cardiac hypertrophy and fibrosis. J Clin Invest. 2003;111:833–841. doi: 10.1172/JCI16290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pesu M, Takaluoma K, Aittomaki S, Lagerstedt A, Saksela K, Kovanen PE, Silvennoinen O. Interleukin-4-induced transcriptional activation by stat6 involves multiple serine/threonine kinase pathways and serine phosphorylation of stat6. Blood. 2000;95:494–502. [PubMed] [Google Scholar]
- 68.Goenka S, Cho SH, Boothby M. Collaborator of Stat6 (CoaSt6)-associated poly(ADP-ribose) polymerase activity modulates Stat6-dependent gene transcription. J Biol Chem. 2007;282:18732–18739. doi: 10.1074/jbc.M611283200. [DOI] [PubMed] [Google Scholar]
- 69.Stutz AM, Pickart LA, Trifilieff A, Baumruker T, Prieschl-Strassmayr E, Woisetschlager M. The Th2 cell cytokines IL-4 and IL-13 regulate found in inflammatory zone 1/resistin-like molecule alpha gene expression by a STAT6 and CCAAT/enhancer-binding protein-dependent mechanism. J Immunol. 2003;170:1789–1796. doi: 10.4049/jimmunol.170.4.1789. [DOI] [PubMed] [Google Scholar]
- 70.Han S, Ritzenthaler JD, Wingerd B, Roman J. Activation of peroxisome proliferator-activated receptor beta/delta (PPARbeta/delta) increases the expression of prostaglandin E2 receptor subtype EP4. The roles of phosphatidylinositol 3-kinase and CCAAT/enhancer-binding protein beta. J Biol Chem. 2005;280:33240–33249. doi: 10.1074/jbc.M507617200. [DOI] [PubMed] [Google Scholar]
- 71.Tomizawa M, Saisho H. Insulin-like growth factor (IGF)-II regulates CCAAT/enhancer binding protein alpha expression via phosphatidyl-inositol 3 kinase in human hepatoblastoma cell lines. J Cell Biochem. 2007;102:161–170. doi: 10.1002/jcb.21293. [DOI] [PubMed] [Google Scholar]
- 72.Cesena TI, Cui TX, Piwien-Pilipuk G, Kaplani J, Calinescu AA, Huo JS, Iniguez-Lluhi JA, Kwok R, Schwartz J. Multiple mechanisms of growth hormone-regulated gene transcription. Mol Genet Metab. 2007;90:126–133. doi: 10.1016/j.ymgme.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.King NE, Rothenberg ME, Zimmermann N. Arginine in asthma and lung inflammation. J Nutr. 2004;134:2830S–2836S. doi: 10.1093/jn/134.10.2830S. discussion 2853S. [DOI] [PubMed] [Google Scholar]
- 74.Kershenobich D, Fierro FJ, Rojkind M. The relationship between the free pool of proline and collagen content in human liver cirrhosis. J Clin Invest. 1970;49:2246–2249. doi: 10.1172/JCI106443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Albina JE, Abate JA, Mastrofrancesco B. Role of ornithine as a proline precursor in healing wounds. J Surg Res. 1993;55:97–102. doi: 10.1006/jsre.1993.1114. [DOI] [PubMed] [Google Scholar]
- 76.Pegg AE, McCann PP. Polyamine metabolism and function. Am J Physiol. 1982;243:C212–221. doi: 10.1152/ajpcell.1982.243.5.C212. [DOI] [PubMed] [Google Scholar]
- 77.Bacchi CJ, Garofalo J, Mockenhaupt D, McCann PP, Diekema KA, Pegg AE, Nathan HC, Mullaney EA, Chunosoff L, Sjoerdsma A, Hutner SH. In vivo effects of alpha-DL-difluoromethylornithine on the metabolism and morphology of Trypanosoma brucei brucei. Mol Biochem Parasitol. 1983;7:209–225. doi: 10.1016/0166-6851(83)90022-1. [DOI] [PubMed] [Google Scholar]
- 78.Van Voorhis WC. Therapy and prophylaxis of systemic protozoan infections. Drugs. 1990;40:176–202. doi: 10.2165/00003495-199040020-00002. [DOI] [PubMed] [Google Scholar]
- 79.Wei LH, Wu G, Morris SM, Jr, Ignarro LJ. Elevated arginase I expression in rat aortic smooth muscle cells increases cell proliferation. Proc Natl Acad Sci U S A. 2001;98:9260–9264. doi: 10.1073/pnas.161294898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li H, Meininger CJ, Kelly KA, Hawker JR, Jr, Morris SM, Jr, Wu G. Activities of arginase I and II are limiting for endothelial cell proliferation. Am J Physiol Regul Integr Comp Physiol. 2002;282:R64–69. doi: 10.1152/ajpregu.2002.282.1.R64. [DOI] [PubMed] [Google Scholar]
- 81.Holcomb IN, Kabakoff RC, Chan B, Baker TW, Gurney A, Henzel W, Nelson C, Lowman HB, Wright BD, Skelton NJ, Frantz GD, Tumas DB, Peale FV, Jr, Shelton DL, Hebert CC. FIZZ1, a novel cysteine-rich secreted protein associated with pulmonary inflammation, defines a new gene family. EMBO J. 2000;19:4046–4055. doi: 10.1093/emboj/19.15.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu T, Dhanasekaran SM, Jin H, Hu B, Tomlins SA, Chinnaiyan AM, Phan SH. FIZZ1 stimulation of myofibroblast differentiation. Am J Pathol. 2004;164:1315–1326. doi: 10.1016/S0002-9440(10)63218-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sandler NG, Mentink-Kane MM, Cheever AW, Wynn TA. Global gene expression profiles during acute pathogen-induced pulmonary inflammation reveal divergent roles for Th1 and Th2 responses in tissue repair. J Immunol. 2003;171:3655–3667. doi: 10.4049/jimmunol.171.7.3655. [DOI] [PubMed] [Google Scholar]
- 84.Owhashi M, Maruyama H, Nawa Y. Kinetic study of eosinophil chemotactic factor production with reference to eosinophilia and granuloma formation in mice infected with Schistosoma japonicum. Int J Parasitol. 1996;26:705–711. doi: 10.1016/0020-7519(96)00054-9. [DOI] [PubMed] [Google Scholar]
- 85.Owhashi M, Arita H, Niwa A. Production of eosinophil chemotactic factor by CD8+ T-cells in Toxocara canis-infected mice. Parasitol Res. 1998;84:136–138. doi: 10.1007/s004360050370. [DOI] [PubMed] [Google Scholar]
- 86.Falcone FH, Loke P, Zang X, MacDonald AS, Maizels RM, Allen JE. A Brugia malayi homolog of macrophage migration inhibitory factor reveals an important link between macrophages and eosinophil recruitment during nematode infection. J Immunol. 2001;167:5348–5354. doi: 10.4049/jimmunol.167.9.5348. [DOI] [PubMed] [Google Scholar]
- 87.Russell SM, Keegan AD, Harada N, Nakamura Y, Noguchi M, Leland P, Friedmann MC, Miyajima A, Puri RK, Paul WE, et al. Interleukin-2 receptor gamma chain: a functional component of the interleukin-4 receptor. Science. 1993;262:1880–1883. doi: 10.1126/science.8266078. [DOI] [PubMed] [Google Scholar]
- 88.Cuesta N, Salkowski CA, Thomas KE, Vogel SN. Regulation of lipopolysaccharide sensitivity by IFN regulatory factor-2. J Immunol. 2003;170:5739–5747. doi: 10.4049/jimmunol.170.11.5739. [DOI] [PubMed] [Google Scholar]
- 89.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.This work was supported by the following: AI018797 (S.N.V.), AI038985 and AI059775 (A.D.K.), and T32HL007698 (N.M.H.).