Abstract
Polybrominated diphenyl ethers (PBDEs) are brominated flame retardants that persist in the environment and are present in geographically widespread fish species. PBDE concentrations can be particularly high in resident Chinook salmon (Onchorhynchus tshawytscha) in the Puget Sound, Washington. Although PBDE residues in salmon and other fish are often dominated by lower brominated congeners, these congeners are not produced commercially in the greatest quantity, suggesting bioaccumulation of the lower molecular weight PBDEs or debromination of more fully brominated congeners. We determined the capacity of Chinook liver fractions to debrominate 2,2′,4,4′,5-pentabromodiphenyl ether (BDE 99), a model PBDE congener readily debrominated by common carp (Cyprinus caprio). Liver subcellular fractions from two strains of Chinook were incubated with BDE 99 prior to liquid/liquid extraction followed by gas chromatography/mass spectrometry analysis (GC/MS analysis) to identify metabolites and debromination products. In contrast to common carp, debromination of BDE 99 to BDE 47 (2,2′,4,4′-tetrabromodiphenyl ether) was not observed in microsomal fractions from either strain of Chinook salmon. However, Chinook salmon liver microsomes from both Chinook strains slowly debrominated BDE 99 to BDE 49 (2,2′,4,5′-tetrabromodiphenyl ether), a unique debromination product whose formation has not been reported in other fish. Three-year old males belonging to a Rapid River Spring Chinook salmon genetic strain showed a somewhat greater microsomal debromination capacity than older hatchery returning male Chinook, but were still inefficient in the debromination of BDE 99 relative to carp. Microsomal debromination of BDE 99 to BDE 49 was not NADPH-dependent, indicating a lack of cytochrome P450 involvement. By contrast, omission of the reductant dithiothreitol (DTT) from Chinook microsomal preparations resulted in a lack of BDE 99 debromination, suggesting the involvement of a microsomal reductase(s) or deiodinase (DI). Cytosolic fractions from Chinook salmon and Common carp debrominated BDE 99 to BDE 49 in vitro. However, carp cytosolic enzymes preferentially formed BDE 47. In summary, our data indicate significant differences among teleosts with respect to efficiency and metabolite profiles of BDE 99 debromination, and suggest that the high concentrations of BDE 47 in resident Chinook salmon from the Puget Sound are not a result of hepatic metabolism of BDE 99. The results of our study also suggest the involvement of an unidentified hepatic reductase or DI in PBDE debromination in fish.
Keywords: Chinook salmon, polybrominated diphenyl ethers, in vitro metabolism
1. Introduction
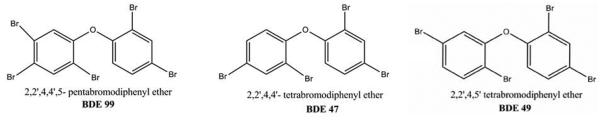
Polybrominated diphenyl ethers (PBDEs) are flame retardants added to consumer products including electronics, polyurethane foam and plastics. PBDEs can leach from commercial products into the aquatic environment due to the fact that they are not chemically bound to these commercial products during manufacturing processes (de Wit, 2002). Recently, the use of two commercial PBDE mixtures (Penta- and Octa- BDE) has been banned due to their environmental persistence and potential toxicity. Accordingly, the only commercial mixture still in use in the United States is Deca BDE, which consists primarily of the fully brominated diphenyl ether, BDE 209. Although Penta and Octa BDE mixtures are no longer produced or used commercially in the United States, tetra-, penta- and octa- brominated BDE congeners are still detected in human and wildlife samples (Law et al., 2006; McKinney et al., 2006a; Hayward et al., 2007; Gauthier et al., 2008). The congeners typically detected in the greatest quantity in humans and in wildlife tissues are the tetrabrominated PBDEs, and specifically, BDE 47 (2,2′,4,4′-tetrabromodiphenyl ether) (Birnbaum and Staskal, 2004; Hites, 2004). Illustrated in Figure 1 are PBDE congeners of particular relevance to the present study.
Figure 1.
PBDE congeners of relevance to the present study.
Several studies have reported high concentrations (1-4 ng/g) of PBDEs in farm-raised and wild salmon, as well as in other fish species (Hale et al., 2001; Boon et al., 2002; Hites et al., 2004b; Tittlemier et al., 2004; Stone, 2006; Hayward et al., 2007; Jenssen et al., 2007; Pulkrabova et al., 2007). These studies consistently indicate that that BDE 47 is a predominant residue and can comprise up to 70% of the total fish body burden of PBDEs. PBDEs in wild Chinook salmon from British Columbia have been reported to be higher than those from farmed or wild salmon (Hites et al., 2004b). Interestingly, the PBDE levels in Chinook salmon from British Columbia were far lower than levels detected in salmon from the Puget Sound using similar methodologies (PSAT, 2007). In a different study, PBDE concentrations in another Washington state salmonid, rainbow trout (Onchorhynchus mykiss), were relatively similar to those in Puget Sound Chinook (Johnson and Olson, 2001). However, little is known regarding the sources and mechanisms underlying PBDE bioaccumulation in salmonids. Common carp collected from field sites in the Pacific Northwest typically exhibit one- to two- orders of magnitude less accumulation of penta BDE, suggesting differences in PBDE congener bioaccumulation patterns, or differences in ability to metabolize penta BDEs relative to salmon (Johnson and Olson, 2001). Carp readily debrominate BDE 99 (2,2′,4,4′5 pentabromodiphenyl ether) to BDE 47 (Stapleton et al., 2004b; Benedict et al., 2007) but there are likely species differences in debromination among fish. For example, rainbow trout do not efficiently debrominate certain PBDEs relative to carp (Stapleton et al., 2006). Furthermore, mammals have the ability to metabolize PBDEs to hydroxylated metabolites (McKinney et al., 2006b). Although hydroxylated PBDE metabolites have been detected in some fish species, it is not clear that the source of these metabolites occurs as a result of in vivo metabolism or bioaccumulation via environmental or dietary sources (Valters et al., 2005).
In the current study, we determined the ability of Chinook salmon to debrominate BDE 99 in vitro. The aforementioned congener is a major constituent of the commercial Penta BDE mixture, but is not typically detected in high concentrations in environmental media (Hites, 2004). Furthermore, BDE 99 can also serve as a prototypical model PBDE congener that undergoes metabolism in other species. We hypothesized that Chinook subcellular fractions would rapidly debrominate BDE 99 to lower molecular weight congeners, and in particular, to BDE 47.
2. Materials and Methods
2.1 Chemicals and Reagents
NADPH regenerating system was purchased from BD Biosciences (San Jose, CA). L-Glutathione reduced (GSH), phenylmethylsulphonylfluoride (PMSF) and bovine serum albumin were purchased from Sigma-Aldrich (St. Louis, MO). 1-chloro-2,4,dinitrobenzene (CDNB) and D,L-1,4,dithiothreitol (DTT) were purchased from Acros Chemical Inc. (Morris Plains, NJ). Potassium phosphate and glycerol were purchased from J.T. Baker (Phillipsburg, NJ). Protein assay dye reagent concentrate was purchased from BioRad (Hercules, CA). Tricaine (methanesulfonate, MS-222) was purchased from Argent Chemical Laboratories (Redmond, WA). BDE stocks (PBDE congeners 99,75, 71, 66, 49, 47 and hydroxylated BDE metabolite 6-OH-BDE 47) were purchased from Accustandard (New Haven, CT). Mono-fluorinated BDE 69 (4′fluoro-2,3′,4,6-tetrabromodiphenyl ether) and 160 (4′-fluoro-2,3,3′4,5,6-hexabromodiphenyl ether) was obtained from Chiron (Trondheim, Norway) and used as an internal and surrogate standards, respectively. All other solvents were of analytical grade and purchased from standard sources.
2.2 Animals and subcellular preparations
Three year-old male hatchery raised Rapid River Spring (RRS) Chinook were a generous gift of Dr. Penny Swanson at the NOAA Northwest Fisheries Science Center (Seattle, WA). Additional adult male Chinook livers used for comparative experiments were obtained from fish returning to the University of Washington hatchery (Seattle, WA). Sacrifice of fish was carried out either by blunt force to the head or a lethal overdose of tricaine (MS-222) followed by severing the spinal cord. All liver sections were rinsed immediately in ice-cold phosphate buffer (pH=7.4), snap frozen in liquid nitrogen, and stored at −80 °C. Frozen Common carp liver from an adult male fish was provided by Dr. Stapleton. Donated common carp was sacrificed similarly to the method described above for Chinook salmon. All animal procedures were conducted in accordance with approved animal care and use protocols.
Cytosolic and microsomal fractions were isolated by standard methods (McKinney et al., 2004) and typically included DTT in the homogenization, wash and resuspension buffers to preserve catalytic activity of reductases and deiodinases. With the exception of preliminary assay optimization studies and comparative studies with carp, most experiments involved pooling of livers from 5 fish. All buffers were made fresh daily. In some experiments, DTT was omitted in subcellular fractionations to assess the contribution of reductive enzymes that required this cofactor. The microsomal and cytosolic fractions were analyzed for protein concentrations and stored at −80 °C (Bradford, 1976).
2.3 Analysis of enzymatic activity of subcellular fractions
Microsomal integrity was determined using the NADPH-cytochrome C reductase assay (Sigma, St. Louis, MO) modified for a microplate reader. NADPH-cytochrome C reductase is an enzyme localized in the endoplasmic reticulum which transfers NADPH to oxygenases, including cytochrome P450s. The reduction of cytochrome C by NADPH cytochrome C reductase results in an increase in absorbance at 550 nm wavelength (Vermilion and Coon, 1974). Mean NADPH-cytochrome C reductase activity for Chinook microsomal fractions was 16 ±3 nmol, min−1, mg protein−1, and for carp microsomal fractions was 31 ±4 nmol, min−1, mg protein−1. Mean NADPH-cytochrome C reductase activity for Chinook salmon cytosolic fractions was 3 ±0.5 nmol, min−1, mg protein−1, and for carp cytosolic fractions was 6 ±0.9 nmol, min−1, mg protein−1. Cytosolic glutathione S-transferase activities were determined utilizing the broad GST substrate CDNB (Habig and Jakoby, 1981) modified for a microplate reader (Gallagher et al., 2000). Mean GST-CDNB activity for Chinook cytosolic fractions was 303 ±74 nmol, min−1, mg protein−1 and was 310 ±104 nmol, min−, mg protein−1 for carp cytosolic fractions. These aforementioned values are similar to cytosolic CDNB values from other salmonids (Trute et al., 2007), and validated the integrity of the cytosolic fractions.
2.4 In Vitro BDE 99 Metabolism
BDE 99 stock solutions were prepared at 50 μg/ml in isooctane. An aliquot of the stock solution was dried under a gentle stream of liquid nitrogen and brought to the appropriate working concentration in analytical grade acetone prior to in vitro incubations. All working stock solutions were made on the day of use. In addition, an aliquot of the spiking solution was transferred to GC auto sampler vials as a quality control for the mass of BDE congener added to each incubation. The final BDE 99 substrate concentrations were representative of those in previous fish PBDE metabolism studies (Stapleton et al., 2004b; Benedict et al., 2007) and included concentrations expected to generate potential dehalogenated metabolites at levels well above detection limits. BDE 99 concentrations ranged from 15 ng −1000 ng (0.03 μM - 1.8 μM) as specified in figure and table legends. Preliminary experiments included optimization of substrate concentrations and incubation times, and given the solubility limits of BDE 99. Based upon product formation over 0.5-24 hr, we selected 16 hours as an optimal incubation period for subsequent assays, as this time point was consistent with maximum dehalogenation and with linear product formation. All microsomal incubations were conducted in triplicate and included a “non-enzymatic” control (heat-inactivated microsomes) and an additional “negative control” reaction which contained carrier (0.5% acetone) in lieu of substrate. Typical incubations contained 5 μL of BDE 99 stock solution, 100 mM sodium phosphate buffer, 100 mM EDTA, 10 mM DTT, 3 mM NADPH regenerating system and 1 mg of microsomal protein for a total assay volume of 1 ml. The reaction mixtures were pre-incubated for five minutes at 25 °C in a shaking water bath (70 rpm) prior to addition of 60 μl of co-factor to initiate the reaction. In some experiments, enzymatic co-factors such as 3 mM NADPH regenerating system (necessary for cytochrome P450 activity), 10mM DTT (necessary for reductase or deiodinase activity), or 5 mM GSH (co-factor for GST) were excluded from the reactions. Presence or absence of these co-factors is noted in figures and tables. In other experiments, cytosolic fractions were subjected to dialysis for 24 hours in ice-cold buffer containing 10 mM potassium phosphate and 1 mM DTT to remove endogenous GSH (James et al., 1997; James et al., 1998). Some incubations were conducted at 15 °C to more closely simulate Chinook salmon ambient temperature . The product formation in these incubations was lower than that of the 25 °C incubations (see table 1 legend). For consistency, all other incubations were conducted at 25 °C. The incubations were stopped by the addition of 1 ml ice-cold methanol prior to transfer to 3 ml amber glass vials and storage at −20 °C.
Table 1.
Comparative debromination of BDE 99 to tetrabrominated congeners by Chinook salmon and common carp microsomal fractions.
| Percent Congener Formed (±SD)b |
|||
|---|---|---|---|
| Species | Incubation conditionsa | BDE 47 | BDE 49 |
| Chinook salmon |
+ NADPH (25 °C) | BDL | 2.1 (±0.2)% |
| − NADPHc (25 °C) | BDL | 2.7(±0.04)% | |
| −NADPH, − DTTc (25 °C) | BDL | BDL | |
| + NADPH (15 °C) | BDL | 1.4 (±0.1)% | |
| Common Carp |
+ NADPH (25 °C) | 53 (±7)% | BDL |
Chinook liver microsomes and carp liver microsomes were incubated with 0.9 μM BDE 99 and 10 mM DTT (except where noted) as described in materials and methods
Data represent mean ± standard deviation of technical triplicates. BDL= below detection limit. Percent recovery of surrogate was 87±8%.
Chinook microsomes for these incubations were prepared without DTT in the homogenenization, wash or resuspension buffers.
2.5 Extraction and Analysis of PBDE congeners
Samples were thawed and spiked with 50 ng of an internal standard (FBDE 160) and then extracted via liquid:liquid extraction using hexane (Stapleton et al., 2006). Prior to analysis on the GC/MS, 50 ng FBDE 69 (4′-fluoro-2,3′,4,6-tetrabromodiphenyl ether) was spiked into the sample to measure recovery of FBDE 160 (4′-fluoro-2,3,3′4,5,6-hexabromodiphenyl ether). All samples were analyzed using gas chromatography mass spectrometry operated in electron capture negative ionization mode (GC/ECNI-MS). Additionally, some samples were also analyzed using gas chromatography mass spectrometry operated in electron impact ionization mode (GC/EI-MS) to confirm the presence of unique debromination products. Extracts were routinely analyzed for a suite of 32 BDE congeners ranging from tri- to decaBDE using a PBDE calibration standard (Stapleton et al., 2007). A 0.25 mm (I.D.) × 15 m fused silica capillary column coated with 5% phenyl methylpolysiloxane (0.25 μm film thickness) was used for the separation of BDE congeners. Pressurized temperature vaporization (PTV) injection was employed in the GC. The inlet was set to 80 °C for 0.3 minutes and then a rapid 600°C/ min ramp to 275 °C was employed to efficiently transfer the samples to the head of the GC column. The oven temperature program was held at 40 °C for 1 min followed by a temperature ramp of 18 °C /min to 250 °C, followed by a temperature ramp of 1.5 °C /min to a temperature of 260 °C, followed by a final temperature ramp of 25 °C/min to 300 °C which was held for an additional 20 min. The transfer line temperature was maintained at 300 °C and the ion source was held at 200 °C. Tri- through octa-BDE congeners and both fluorinated BDE standards were quantified by monitoring bromide ions (m/z 79 and 81) in GC/ ECNI-MS and by monitoring tetrabrominated diphenyl ether ions (m/z 486 and 484) in GC/EI-MS.
Limits of detection (LOD) were determined by three times the standard deviation of the laboratory blanks. For congeners not detected in the blanks, the LOD was set at the lab limit of quantification (LOQ). Recovery of FBDE 160 averaged 87 ±12%. Percent of BDE 49 formation in Chinook samples was determined by dividing the moles of BDE 49 by the moles of BDE 99 in each incubation. In incubations using carp subcellular fractions, the percent of BDE 47 was determined by dividing the moles of BDE 47 detected in each sample by the initial moles of BDE 99. The aforementioned approach was used to account for the extensive debromination of BDE 99 by carp, in which case dividing by BDE 99 detected in each sample would not take into account the mass of BDE 99 debrominated. Data was not surrogate recovery corrected unless surrogate recovery rates fell below 70%. Surrogate recovery corrections are noted in the legends of figures where appropriate.
2.6 Statistical Analysis
The effect of time, substrate concentration, temperature and Chinook strain on formation of debromination products was analyzed using a one-way ANOVA with a Dunnett's test or Bonferroni's multiple comparisons post-hoc test, with differences considered significant at p≤0.05. Statistical analysis was carried out using GraphPad Prism Software version 5.00 for Windows (GraphPad Software, San Diego, CA).
3. Results
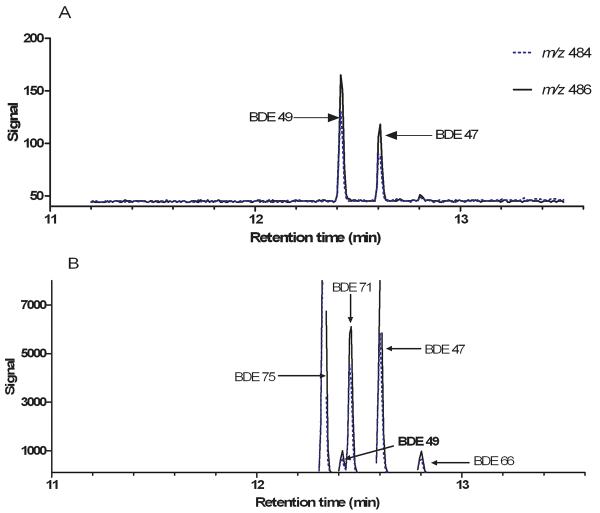
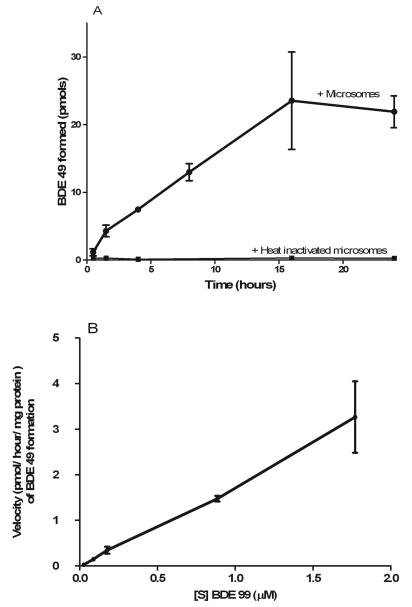
Incubation of Chinook salmon microsomal and cytosolic fractions in the presence of BDE 99 yielded BDE 49, a unique debromination product previously unreported in other fish species. Figure 2 illustrates a GC/EI-MS chromatogram of an incubation containing Chinook microsomes and a BDE standard for the purpose of confirming the formation of BDE 49 (largest peak, 12.6 min retention time). The formation of BDE 49 was detected in all in vitro incubations using Chinook microsomal or cytosolic fractions in the presence of BDE 99. By contrast, BDE 49 was not detected any of the negative control reactions or PBDE spiking solutions in the absence of enzyme. As observed in Figure 3A, the conversion of BDE 99 to BDE 49 occurred slowly (< 2 ρmols BDE 49 formed/hour/mg protein) with product formation diminishing after 16 hours of incubation. The average percent conversion of BDE 99 to BDE 49 at 30 minutes was 0.15%, but increased to 3.1% by 16 hours (Figure 3A). As observed in Figure 3B, the debromination reaction was linear with respect to product formation and substrate concentration over 24 hours using a range of substrate concentrations from 0.03 μM -1.8 μM. Substrate saturation was not observed at the highest concentration of BDE 99 tested (Figure 3B) and solubility limitations prevented higher substrate concentrations from being tested.
Figure 2.
GC/MS chromatogram illustrating (A) the presence of BDE 49 in Chinook salmon liver microsomal reactions incubated with 0.04 μM BDE 99, and (B) five different tetrabrominated congeners present in the BDE calibration standard. The retention times and ion ratios for BDE 49 and BDE 47 were identical in the Chinook salmon sample and the calibration standard. Samples were run by GC/ EI-MS monitoring m/z of 486 and 484 (M+).
Figure 3.
Effect of time (A) and substrate concentration (B) on the conversion of BDE 99 to BDE 49. In panel (A) the conversion of BDE 99 to BDE 49 occurred a 2 ρmol/hour/mg protein, linearly until 16 hours in Chinook microsomal fractions incubated with 0.9 μM BDE 99. The heat-inactivated controls did not contain detectable levels of BDE 49. In panel (B), we did not observe substrate saturation with increasing concentrations of BDE 99 (from 0.03 μM to 1.7 μM) over 24 hours. In both panels, the assays were conducted using Chinook salmon microsomes from an individual liver, and error bars represent mean ±SD for triplicate assays (n=3) and indicate technical variation.
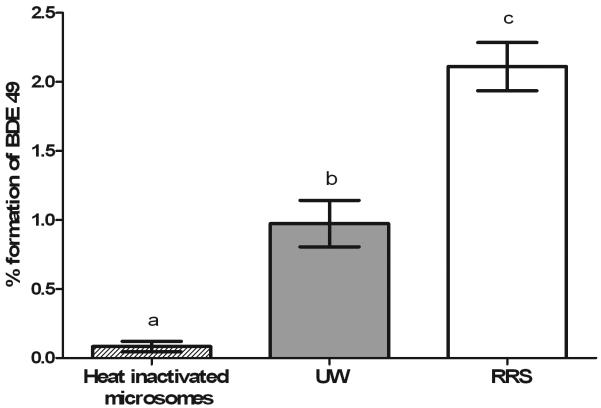
The effect of lowering the incubation temperature from 25 °C to 15 °C yielded a significant reduction in the rate of debromination of BDE 99 to BDE 49 (Table 1). Furthermore, microsomes prepared from RRS strain hatchery Chinook salmon had a significantly higher capacity to debrominate BDE 99 to BDE 49 relative to hatchery returning fish (Figure 4). Although BDE 47 was typically detected in extremely low concentrations in incubations containing BDE 99 and subcellular fractions, this congener was also detected at similar levels in the spiking and non-enzymatic controls, and was due to trace contamination. Furthermore, a subset of Chinook microsomal incubations was extracted and derivatized for measurement of hydroxylated metabolites using ethereal diazomethane and GC/ECNI-MS analysis. However, no hydroxylated metabolites were detected (data not shown). Consistent with earlier reports (Benedict et al., 2007), carp microsomal fractions serving as a positive control for BDE 99 debromination produced significant formation of BDE 47 over a 24 hour incubation (Table 1). Collectively, our data indicated that Chinook debrominate BDE 99 with much lower efficiency than carp, but preferentially debrominate BDE 99 to BDE 49, whereas carp preferentially debrominated BDE 99 to BDE 47.
Figure 4.
Comparative debromination of BDE 99 by two different Chinook salmon strains. Microsomal incubations with 0.9 μM BDE 99 confirm differences in debromination capacity between UW (late life stage, returning to UW hatchery) and RRS strain microsomes from 3 year old Chinook salmon (raised in a hatchery in Manchester, WA). Experiments were conducted using a pool of microsomes from 5 fish, with error bars reflecting mean ± standard deviation of three technical replicates. BDE 49 formation in the reactions containing heat-inactivated microsomes were significantly different than the respective RRS and UW control reactions at p<0.05.
A series of experiments involving the removal of enzymatic co-factors for cytochrome P450s, glutathione S-transferases, reductases, and deiodinases was carried out to potentially identify the enzyme(s) responsible for BDE 99 debromination. As observed in Table 1, the absence of an NADPH regenerating system did not affect the capacity of Chinook microsomes to debrominate BDE 99. This observation was consistent with a similar study using carp microsomes (Benedict et al., 2007). In contrast, omission of the reductant DTT in microsomal preparations resulted in a complete lack of detectable debromination (Table 1). As observed in Table 2, Chinook cytosolic fractions debrominated BDE 99 to BDE 49, but with less efficiency than the microsomal fractions (Table 2). The removal of GSH and other low molecular weight compounds from Chinook cytosolic fractions by dialysis partially decreased (39% decrease) the conversion of BDE 99 to BDE 49. However, when GSH was added into the reactions, a similar decrease in BDE 99 debromination was observed (Table 2), suggesting that the partial reduction in BDE 99 debromination was not due to GST. Carp cytosolic fractions showed an extensive (> 50%) conversion of BDE 99 to BDE 47 relative to Chinook, but a similar potential to form similar amounts of BDE 49 (Table 2).
Table 2.
Comparative debromination of BDE 99 to tetrabrominated congeners by Chinook and carp cytosolic fractions.
| Amount of BDE 47 formed | Amount of BDE 49 formed | ||||
|---|---|---|---|---|---|
| Species | Cytosol incubation conditionsa |
Mean ρmols |
% formation (±SD) |
Mean ρmols |
% formation (±SD) |
| Chinook salmon |
Undialyzed + 5mM GSH |
BDL | n/a | 2.8 | 0.3 (±0.09)% |
| Undialyzed, no added GSH |
BDL | n/a | 2.5 | 0.3 (±0.03)% | |
| Dialyzed + 5mM GSH |
BDL | n/a | 1.7 | 0.2 (±0.03)% | |
| Dialyzed, no added GSH |
BDL | n/a | 1.7 | 0.2 (±0.03)% | |
| Common carpb |
Undialyzed + 5mM GSH |
561 | 62 (±2.7)% | 4.1 | 0.5 (±0.04)% |
| Undialyzed, no added GSH |
524 | 58 (±2.7)% | 4.1 | 0.5 (±0.08)% | |
| Dialyzed + 5mM GSH |
472 | 53 (±2.4)% | 3.9 | 0.4 (±0.05)% | |
| Dialyzed, no added GSH |
495 | 56 (±2.7)% | 3.8 | 0.4 (±0.03)% | |
Reactions contained a initial substrate concentration of 0.9 μM BDE 99 and were incubated for 16 hours.
Carp data has been surrogate recovery corrected because surrogate recovery in carp cytosolic experiment ranged from 60.5-101.5%.
4. Discussion
Previous reports have established that some fish species have the ability to debrominate BDE 99 (Stapleton et al., 2004b; Stapleton et al., 2004c; Stapleton et al., 2006; Benedict et al., 2007). In carp, BDE 47 is the major debromination product, typically accounting for >60% conversion of BDE 99 to BDE 47. Our study indicates that Chinook salmon also have the ability to debrominate BDE 99, but in contrast to cyprinids, this debromination pathway is relatively inefficient. Interestingly, prior studies have suggested that the PBDE congener profile detected in carp and other cyprinids is due to debromination of higher molecular weight congeners (Johnson and Olson, 2001). Collectively, these studies demonstrate extensive species differences with respect to their BDE 99 debromination capacity and profiles. Clearly, the results of the present study do not support the hypothesis that the relatively high residues of BDE 47 measured locally in Chinook salmon are due to debromination of BDE 99.
There are several other possible routes of BDE 47 accumulation on Chinook salmon, including both dietary transfer and recalcitrance of these compounds in waterways. Chinook salmon from British Columbia also have relatively high concentrations of PBDEs (Hites et al., 2004a), likely a result of their predation on small contaminated fish, including herring and sardines, as compared to other salmon species which feed mainly on invertebrates and zooplankton (Hites et al., 2004b; Quinn, 2005). It is possible that prey sources for Chinook have a debromination capacity for higher molecular weight congeners, and thus Chinook are accumulating high concentrations of lower molecular weight PBDEs via dietary consumption. Another possibility is that Chinook salmon in the Puget Sound bioaccumulate lower molecular weight tetra and penta brominated PBDEs due to the recalcitrance of these compounds. Higher molecular weight PBDE congeners can be broken down photolytically (Eriksson et al., 2004). Alternatively, there is increasing evidence to indicate the importance of microbial degradation or debromination of higher molecular weight congeners (Tokarz et al., 2008), which may be a particularly important mechanism leading to increased bioavailability of lower brominated congeners in aquatic environments. However, microbial degradation rates can be highly dependent upon the physicochemical characteristics of the aquatic system, and these processes have not been studied in detail in the Puget Sound region. The local “blackmouth” Chinook salmon, which have higher PBDE concentrations relative to other Chinook strains (PSAT, 2007), differ from their oceangoing relatives in that they reside in the Puget Sound for the majority of their adult life rather than migrate to the ocean (Quinn, 2005). Accordingly, the increased residence time in the Puget Sound may result in increased pollutant exposures associated with proximity to urbanized waterways and poor flushing of the Puget Sound relative to the ocean.
It is particularly interesting that the major BDE 99 debromination product detected in Chinook salmon microsomal fractions is BDE 49. Although this congener is detected in Chinook salmon from the Puget Sound, it is present in much lower concentrations than BDE 47 (PSAT, 2007). Although BDE 49 and BDE 47 are tetrabrominated diphenyl ethers, BDE 49 has bromine atoms at the 2,2′,4 and 5′ positions whereas, BDE 47 has bromine atoms at the 2,2′,4 and 4′ positions. Shao et al (2008) explored the toxicity of BDE 47 in trout gill and liver cells and found that BDE 47 may elicit toxicity via oxidative stress (Shao et al., 2008). In contrast, there is little information regarding the toxicity of BDE 49 relative to the other congeners. It is also interesting that a relatively small fraction of BDE 99 is converted to BDE 49 in carp cytosolic fractions, as this reaction has not been previously identified (Stapleton et al., 2004b; Stapleton et al., 2004c; Benedict et al., 2007). Furthermore, we observed some minor formation of BDE 28 and BDE 49 (<1% conversion, data not shown) in carp microsomal fractions in the presence of BDE 99. However, the potential formation of these minor metabolites was not above the assay detection limits and thus not conclusively verified in the present study.
The present study also provided some insight into the origin of the Chinook salmon enzyme(s) mediating the debromination of BDE 99 to BDE 49. Our studies indicate that neither cytochrome P450s (CYPs) nor GSTs are carrying out these reactions in Chinook, at least under in vitro conditions. The aforementioned conclusions are based upon our studies involving the addition or omission of co-factors necessary for these enzyme systems. Furthermore, the fact that debromination of BDE 99 to BDE 49 by Chinook microsomes is still occurring at 16 hours in vitro, a time point at which CYPs tend to lose catalytic activity in vitro (Yamazaki et al., 1997), further supports that CYPs are not the enzyme responsible for debromination in Chinook. There is precedence for a GST-dependent reductive dechlorination reaction in other species, as Drosophila have the ability to reductively dechlorinate DTT (1,1,1-trichloro-2,2-bis-(pchlorophenyl)ethane) via GST (Tang and Tu, 1994). However, the results of our cytosolic GSH dialysis experiments clearly indicated that GST was not involved in the debromination of BDE 99 in Chinook cytosolic fractions. The fact that omission of DTT resulted in a loss of microsomal debromination of BDE 99 suggests that reductive enzymes or other proteins requiring DTT as a co-factor are important mediators of debromination. One particular enzyme that has been suggested to play a role in debromination of PBDEs by teleosts is thyroxine deiodinase (DI) (Stapleton et al., 2004a; Stapleton et al., 2006). These single pass, trans-endoplasmic reticulum membrane enzymes utilize DTT and are located in the microsomal, and possibly cytosolic fractions of tissues (Bianco and Larsen, 2005). It has been suggested that DIs mediate the debromination of BDE 99 to BDE 47 in carp liver microsomal fractions (Stapleton et al., 2004b). This hypothesis was further supported by the addition of a competitive substrate of deiodinase (reverse triiodothyronine) which significantly reduced, but did not totally inhibit, the debromination of BDE 99 to BDE 47 in carp liver (Benedict et al., 2007). The structure-activity debromination profiles of deiodinases are consistent with BDE 99 being a preferential substrate for these enzymes, at least with the production of certain dehalogenation products such as BDE 47 (Bianco and Larsen, 2005). Conclusions regarding the role of these enzymes in PBDE debromination in fish are hampered by the lack of specific inhibitors for fish DIs, as well as availability of recombinant cDNA- expressed proteins for use in enzymatic studies.
As discussed, a parallel experiment involving the extraction and analysis of polar PBDE metabolites from Chinook microsomal incubations was performed, but yielded negative results. In contrast, tissue fractions from mammalian species readily produce hydroxylated and glucuronidated metabolites (Hakk et al., 2002; Chen et al., 2006). In general, hydroxylated PBDE metabolites appear to be produced by CYPs dependent pathways (Hakk et al., 2002; Chen et al., 2006; McKinney et al., 2006b), suggesting that fish CYPs orthologues cannot utilize BDE 99 as a substrate. Collectively, neither in vivo nor in vitro studies have revealed the presence of hydroxylated metabolites using subcellular fractions prepared from carp (Stapleton et al., 2006; Benedict et al., 2007), rainbow trout (Stapleton et al., 2006) or Chinook salmon. Of interest would be comparative studies of hydroxylated PBDE metabolite formation by other phylogenetically diverse aquatic species.
5. Conclusions
Our studies indicate that two teleosts, common carp and Chinook salmon, have markedly different abilities to debrominate BDE 99. These differences are reflected in both debromination efficiencies and metabolite profiles. Our observations indicate that caution should be exercised when evaluating the role of metabolism in interpreting PBDE congener profiles in aquatic species. Although this study does not explain the origins of the high concentrations of BDE 47 observed in resident Chinook salmon from the Puget Sound, WA, it does support the notion that these lower brominated congeners are more resistant to environmental or biodegradation and thus lead to more bioaccumulation. The fact that BDE 49 has not been previously reported to be a debromination product of BDE 99 in fish underscores species differences in metabolism. From a chemical biotransformation standpoint, our study also raises interesting questions regarding the enzyme(s) responsible for debromination of BDE 99 in teleosts. The source of this metabolism may be a potentially unidentified reductase(s) or a thyroxine deiodinase. Finally, the fact that younger hatchery raised Chinook can debrominate BDE 99 to a greater extent than later life stage animals of a different genetic origin raises the possibility that PBDE metabolism may be to some extent, dependent upon age or genetic strain. Collectively, these studies indicate that BDE 99 and potentially other congeners have utility as tools to further our understanding of the biochemistry and metabolism of environmental substrates across species.
Acknowledgements
This work was supported by grants from the NOAA/NOS Oceans and Human Health Program (NA05NOS4781253) and the University of Washington Superfund Basic Research Program (NIEHS P42ES04696). SCT was the recipient of an NIEHS postdoctoral fellowship in Environmental Pathology and Toxicology (T32-ES07032). The authors would also like to acknowledge Dr. Penny Swanson at the NOAA Northwest Fisheries Science Center in Seattle, WA for donation of Rapid River Spring Chinook livers, as well as Sandy O'Neill at the Washington State Department of Ecology for technical discussions concerning PBDE levels in Puget Sound fish.
Abbreviations
- NOAA
National Oceanographic and Atmospheric Administration
- GST
glutathione S-transferase
- CYP
cytochrome P450
- PBDE
polybrominated diphenyl ether
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Benedict R, Stapleton H, Letcher R, Mitchelmore C. Debromination of polybrominated diphenyl ether-99 (BDE-99) in carp (Cyprinus carpio) microflora and microsomes. Chemosphere. 2007;69:987–993. doi: 10.1016/j.chemosphere.2007.05.010. [DOI] [PubMed] [Google Scholar]
- Bianco A, Larsen P. Cellular and structural biology of the deiodinases. Thyroid. 2005;15:777–786. doi: 10.1089/thy.2005.15.777. [DOI] [PubMed] [Google Scholar]
- Birnbaum L, Staskal D. Brominated flame retardants: cause for concern? Environ Health Perspect. 2004;112:9–17. doi: 10.1289/ehp.6559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boon J, Lewis W, Tjoen-A-Choy M, Allchin C, Law R, De Boer J, Ten Hallers-Tjabbes C, Zegers B. Levels of polybrominated diphenyl ether (PBDE) flame retardants in animals representing different trophic levels of the North Sea food Web. Environ Sci Technol. 2002;36:4025–4032. doi: 10.1021/es0158298. [DOI] [PubMed] [Google Scholar]
- Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chen L, Lebetkin E, Sanders J, Burka L. Metabolism and disposition of 2,2′,4,4′,5-pentabromodiphenyl ether (BDE99) following a single or repeated administration to rats or mice. Xenobiotica. 2006;36:515–534. doi: 10.1080/00498250600674477. [DOI] [PubMed] [Google Scholar]
- De Wit C. An overview of brominated flame retardants in the environment. Chemosphere. 2002;46:583–624. doi: 10.1016/s0045-6535(01)00225-9. [DOI] [PubMed] [Google Scholar]
- Eriksson J, Green N, Marsh G, Bergman A. Photochemical decomposition of 15 polybrominated diphenyl ether congeners in methanol/water. Environ Sci Technol. 2004;38:3119–3125. doi: 10.1021/es049830t. [DOI] [PubMed] [Google Scholar]
- Gallagher E, Sheehy K, Lame M, Segall H. In vitro kinetics of hepatic glutathione Stransferase conjugation in largemouth bass and brown bullheads. Environmental Toxicology and Chemistry. 2000;19:319–326. [Google Scholar]
- Gauthier L, Hebert C, Weseloh D, Letcher R. Dramatic changes in the temporal trends of polybrominated diphenyl ethers (PBDEs) in herring gull eggs from the Laurentian Great Lakes: 1982-2006. Environ Sci Technol. 2008;42:1524–1530. doi: 10.1021/es702382k. [DOI] [PubMed] [Google Scholar]
- Habig W, Jakoby W. Assays for differentiation of glutathione S-transferases. Methods Enzymol. 1981;77:398–405. doi: 10.1016/s0076-6879(81)77053-8. [DOI] [PubMed] [Google Scholar]
- Hakk H, Larsen G, Klasson-Wehler E. Tissue disposition, excretion and metabolism of 2,2′,4,4′,5-pentabromodiphenyl ether (BDE-99) in the male Sprague-Dawley rat. Xenobiotica. 2002;32:369–382. doi: 10.1080/00498250110119117. [DOI] [PubMed] [Google Scholar]
- Hale R, La Guardia M, Harvey E, Mainor T, Duff W, Gaylor M. Polybrominated diphenyl ether flame retardants in Virginia freshwater fishes (USA) Environ Sci Technol. 2001;35:4585–4591. doi: 10.1021/es010845q. [DOI] [PubMed] [Google Scholar]
- Hayward D, Wong J, Krynitsky A. Polybrominated diphenyl ethers and polychlorinated biphenyls in commercially wild caught and farm-raised fish fillets in the United States. Environ Res. 2007;103:46–54. doi: 10.1016/j.envres.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Hites R. Polybrominated diphenyl ethers in the environment and in people: a meta-analysis of concentrations. Environ Sci Technol. 2004;38:945–956. doi: 10.1021/es035082g. [DOI] [PubMed] [Google Scholar]
- Hites R, Foran J, Carpenter D, Hamilton M, Knuth B, Schwager S. Global assessment of organic contaminants in farmed salmon. Science. 2004;303:226–229. doi: 10.1126/science.1091447. [DOI] [PubMed] [Google Scholar]
- Hites R, Foran J, Schwager S, Knuth B, Hamilton M, Carpenter D. Global assessment of polybrominated diphenyl ethers in farmed and wild salmon. Environ Sci Technol. 2004;38:4945–4949. doi: 10.1021/es049548m. [DOI] [PubMed] [Google Scholar]
- James M, Cornett R, Yan Z, Henderson G, Stacpoole P. Glutathione-dependent conversion to glyoxylate, a major pathway of dichloroacetate biotransformation in hepatic cytosol from humans and rats, is reduced in dichloroacetate-treated rats. Drug Metabolism and Deposition. 1997;25:1223–1227. [PubMed] [Google Scholar]
- James M, Sikazwe D, Gadagbui B. Isolation of a Pi class glutathione S-transferase (GST) from catfish intestinal mucosa. Marine Environmental Research. 1998;46:57–60. [Google Scholar]
- Jenssen B, Sørmo E, Baek K, Bytingsvik J, Gaustad H, Ruus A, Skaare J. Brominated flame retardants in North-East Atlantic marine ecosystems. Environ Health Perspect. 2007;115(Suppl 1):35–41. doi: 10.1289/ehp.9355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A, Olson N. Analysis and occurrence of polybrominated diphenyl ethers in Washington State freshwater fish. Archives of Environmental Contamination and Toxicology. 2001;41:339–344. doi: 10.1007/s002440010257. [DOI] [PubMed] [Google Scholar]
- Law R, Allchin C, De Boer J, Covaci A, Herzke D, Lepom P, Morris S, Tronczynski J, De Wit C. Levels and trends of brominated flame retardants in the European environment. Chemosphere. 2006;64:187–208. doi: 10.1016/j.chemosphere.2005.12.007. [DOI] [PubMed] [Google Scholar]
- McKinney M, Arukwe A, De Guise S, Martineau D, Beland P, Dallaire A, Lair S, Lebeuf M, Letcher R. Characterization and profiling of hepatic cytochromes P450 and phase II xenobiotic-metabolizing enzymes in beluga whales (Delphinapterus leucas) from the St. Lawrence River Estuary and the Canadian Arctic. Aquat Toxicol. 2004;69:35–49. doi: 10.1016/j.aquatox.2004.04.010. [DOI] [PubMed] [Google Scholar]
- McKinney M, Cesh L, Elliott J, Williams T, Garcelon D, Letcher R. Brominated flame retardants and halogenated phenolic compounds in North American west coast bald eaglet (Haliaeetus leucocephalus) plasma. Environ Sci Technol. 2006;40:6275–6281. doi: 10.1021/es061061l. [DOI] [PubMed] [Google Scholar]
- McKinney M, De Guise S, Martineau D, Beland P, Arukwe A, Letcher R. Biotransformation of polybrominated diphenyl ethers and polychlorinated biphenyls in beluga whale (Delphinapterus leucas) and rat mammalian model using an in vitro hepatic microsomal assay. Aquat Toxicol. 2006;77:87–97. doi: 10.1016/j.aquatox.2005.08.016. [DOI] [PubMed] [Google Scholar]
- PSAT, P.S.A.T. In: 2007 Puget Sound Update: Ninth Report of the Puget Sound Assessment and Monitoring Program. Team, P.S.A., editor. Olympia, WA: 2007. p. 260. [Google Scholar]
- Pulkrabova J, Hajslova J, Poustka J, Kazda R. Fish as biomonitors of polybrominated diphenyl ethers and hexabromocyclododecane in Czech aquatic ecosystems: pollution of the Elbe River basin. Environ Health Perspect. 2007;115(Suppl 1):28–34. doi: 10.1289/ehp.9354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn T. The Behavior and Ecology of Pacific Salmon and Trout. University of Washington Press; Seattle: 2005. [Google Scholar]
- Shao J, Eckert M, Lee L, Gallagher E. Comparative oxygen radical formation and toxicity of BDE 47 in rainbow trout cell lines. Mar Environ Res. 2008;66:7–8. doi: 10.1016/j.marenvres.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton H, Alaee M, Letcher R, Baker J. Debromination of the flame retardant decabromodiphenyl ether by juvenile carp (Cyprinus carpio) following dietary exposure. Environ Sci Technol. 2004;38:112–119. doi: 10.1021/es034746j. [DOI] [PubMed] [Google Scholar]
- Stapleton H, Brazil B, Holbrook R, Mitchelmore C, Benedict R, Konstantinov A, Potter D. In vivo and in vitro debromination of decabromodiphenyl ether (BDE 209) by juvenile rainbow trout and common carp. Environ Sci Technol. 2006;40:4653–4658. doi: 10.1021/es060573x. [DOI] [PubMed] [Google Scholar]
- Stapleton H, Keller J, Schantz M, Kucklick J, Leigh S, Wise S. Determination of polybrominated diphenyl ethers in environmental standard reference materials. Anal Bioanal Chem. 2007;387:2365–2379. doi: 10.1007/s00216-006-1054-5. [DOI] [PubMed] [Google Scholar]
- Stapleton H, Letcher R, Baker J. Debromination of polybrominated diphenyl ether congeners BDE 99 and BDE 183 in the intestinal tract of the common carp (Cyprinus carpio) Environ Sci Technol. 2004;38:1054–1061. doi: 10.1021/es0348804. [DOI] [PubMed] [Google Scholar]
- Stapleton H, Letcher R, Li J, Baker J. Dietary accumulation and metabolism of polybrominated diphenyl ethers by juvenile carp (Cyprinus carpio) Environ Toxicol Chem. 2004;23:1939–1946. doi: 10.1897/03-462. [DOI] [PubMed] [Google Scholar]
- Stone D. Polybrominated diphenyl ethers and polychlorinated biphenyls in different tissue types from chinook salmon (Oncorhynchus tshawytscha) Bull Environ Contam Toxicol. 2006;76:148–154. doi: 10.1007/s00128-005-0901-y. [DOI] [PubMed] [Google Scholar]
- Tang A, Tu C. Biochemical characterization of Drosophila glutathione S-transferases D1 and D21. J Biol Chem. 1994;269:27876–27884. [PubMed] [Google Scholar]
- Tittlemier S, Forsyth D, Breakell K, Verigin V, Ryan J, Hayward S. Polybrominated diphenyl ethers in retail fish and shellfish samples purchased from Canadian markets. J Agric Food Chem. 2004;52:7740–7745. doi: 10.1021/jf048665y. [DOI] [PubMed] [Google Scholar]
- Tokarz J, Ahn M, Leng J, Filley T, Nies L. Reductive debromination of polybrominated diphenyl ethers in anaerobic sediment and a biomimetic system. Environ Sci Technol. 2008;42:1157–1164. doi: 10.1021/es071989t. [DOI] [PubMed] [Google Scholar]
- Trute M, Gallis B, Doneanu C, Shaffer S, Goodlett D, Gallagher E. Characterization of hepatic glutathione S-transferases in coho salmon (Oncorhynchus kisutch) Aquat Toxicol. 2007;81:126–136. doi: 10.1016/j.aquatox.2006.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valters K, Li H, Alaee M, D' Sa I, Marsh G, Bergman A, Letcher R. Polybrominated diphenyl ethers and hydroxylated and methoxylated brominated and chlorinated analogues in the plasma of fish from the Detroit River. Environ Sci Technol. 2005;39:5612–5619. doi: 10.1021/es0506410. [DOI] [PubMed] [Google Scholar]
- Vermilion J, Coon M. Highly purified detergent-solubilized NADPH-cytochrome P-450 reductase from phenobarbital-induced rat liver microsomes. Biochem Biophys Res Commun. 1974;60:1315–1322. doi: 10.1016/0006-291x(74)90341-6. [DOI] [PubMed] [Google Scholar]
- Yamazaki H, Inoue K, Turvy CG, Guengerich FP, Shimada T. Effects of Freezing, Thawing, and Storage of Human Liver Samples on the Microsomal Contents and Activities of Cytochrome P450 Enzymes. Drug Metabolism and Disposition. 1997;25:168. [PubMed] [Google Scholar]