Abstract
FAS-associated factor 1, FAF1, is an evolutionarily conserved protein that has several protein interaction domains. Although FAF1 was initially identified as a member of the FAS death-inducing signaling complex, subsequent work has revealed that FAF1 functions in diverse biological processes. FAF1 has been shown to play an important role in normal development and neuronal cell survival, whereas FAF1 down regulation may contribute to multiple aspects of tumorigenesis. In particular, there is compelling evidence implicating FAF1 as a tumor suppressor involved in the regulation of apoptosis and NF-κB activity, as well as in ubiquitination and proteasomal degradation. Here, we highlight FAF1's role in NF-κB signaling and postulate that this pathway has critical connotations for the pathogenesis and treatment of human cancers.
Keywords: TNF-α, NF-κB, anti-apoptosis, proteasomal degradation, ubiquitination, tumor suppressor gene, mesothelioma
FAS (TNFRSF6) is a member of the tumor necrosis factor (TNF) receptor super-family that interacts with FAS ligand to mediate programmed cell death or apoptosis in a number of tissues. The interaction of FAS with its ligand allows the formation of a death-inducing signaling complex (DISC).1 FAS-associated protein 1 (FAF1) was originally identified as an interactor of FAS that enhances apoptosis initiated through FAS antigen.2 The FAF1 gene encodes a ∼74-kDa protein composed of 650 amino acids, the amino terminal domain of which can bind to the cytoplasmic domain of FAS. Moreover, an ubiquitin-like domain localized to the carboxyl terminal region of FAF1 is required for proapoptotic activity.3 A smaller 40-kDa FAF1 protein has also been detected in certain tissues,4, 5 although it is not yet clear if the smaller form has alternate functions.
Here we present an overview of the current understanding of FAF1 function in various contexts. We highlight the role of FAF1 in NF-κB signaling and its potential role in oncogenesis, with particular emphasis on FAF1's involvement in the pathogenesis of mesothelioma, a form of cancer induced by asbestos exposure. Interestingly, FAF1 may have multiple roles in tumor development and progression, and it may also be an important factor in therapeutic response. Ongoing investigations are likely to provide important insights regarding the role of FAF in signaling pathways that are vital to both normal development and tumorigenesis.
FAF1 domains and interacting proteins
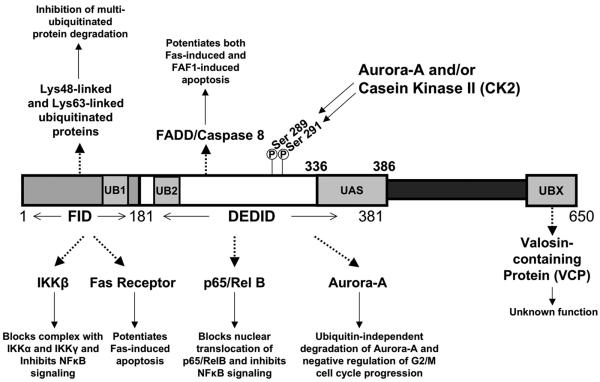
FAF1 is an evolutionarily conserved proapoptotic factor that contains multiple protein-interaction domains, including a FAS-interacting domain (FID), a death effector domain interacting domain (DEDID), and multi-ubiquitin-related domains (Figure 1). More recently, FAF1 has been shown to potentially interact with a variety of proteins mainly identified through two-hybrid screening or by overexpression and co-immunoprecipitation coupled with mass spectrometry experiments. Figure 1 and Table 1 illustrate the structural complexity and potentially diverse functions of FAF1. In addition to the interaction with FAS shown in Figure 2A, FAF1 has been shown to interact with FADD, caspase-8 and protein kinase CK2-β to facilitate apoptosis (Table 1). Moreover, there is persuasive evidence indicating that FAF1 can regulate NF-κB activity either through binding to RelA (p65) in the cytosol, suppression of IKK, or inhibition of other factors that activate NF-κB, such as pyrin-related proteins.6 A third group of interacting partners are related to FAF1's role in protein turnover. Interestingly, FAF1 may have tissue specific roles, as illustrated by the identification of FAF1-interacting proteins in neuronal cells.7
Figure 1.
Diagram of Proposed FAF1 Protein Domains and Binding Partners. FAF1 protein can be broken up into three domains based on sites of protein interactions. The N-terminus contains the FID (Fas-interacting domain) from amino acids 1 – 181. UB1, ubiquitin-homologous domain 1, spans amino acids 110 – 169 within the FID of FAF1. The DEDID (death effector domain-interacting domain) spans amino acids 181 – 381 and contains two phosphorylation sites located at serines 289 and 291 (Ser 289 and Ser 291). UB2, ubiquitin homologous domain 2, spans amino acids 205 – 260 within the DEDID of FAF1. UAS, ubiquitin associated domain, spans amino acids 336 – 386 within the DEDID through to the C-terminus of FAF1. UBX, ubiquitin-like regulatory X domain, spans amino acids 567 – 650 in the C-terminus of FAF1. Dotted arrows are used to depict protein-protein interactions, whereas solid arrows denote the cellular consequences of the protein-protein interactions. Protein-protein interactions are denoted in the specific domain required for that particular binding event, not the exact amino acid location where the interaction occurs.
Table 1.
FAF1 Protein Interactions.
| Interaction | Method of detection | Function | Reference |
|---|---|---|---|
| FAS | Yeast two-hybrid screening and overexpression in mouse L cells; overexpression in human BOSC23 cells |
Regulation of apoptosis | 2, 3 |
| FADD and caspase-8 | Co-immunoprecipitation, co-localization, and overexpression in Jurkat cells |
Regulation of apoptosis | 53 |
| Protein kinase casein kinase 2 beta (CK2beta) |
Yeast two-hybrid; immunoprecipitation and co- localization studies |
Regulation of apoptosis |
5, 12, 13, 54 |
| RelA (p65) subunit of NF-κB |
Overexpression and immunoprecipitation studies in HEK293 and NIH3T3 cells |
Inhibition of NF-κB by cytoplasmic retention of p65 |
22 |
| IκB kinase (IKK) | Immunoprecipitation, overexpression and siRNA studies in HEK293 cells |
Suppression of IKK activation linked to regulation of NF-κB |
23 |
| Pyrin domains of pyrin domain-containing Apaf1-like proteins (PYPAFs) |
Yeast two-hybrid screen, immunoprecipitation and co- expression in HEK 293 and HEK293T cells |
Inflammatory signaling associated with NF-κB |
6 |
| Ubiquitinated proteins and valosin-containing protein (VCP) |
Overexpression in HEK293T cells; protein dissociation of rat skeletal muscle and mass spectrometry |
Regulation of protein degradation in the ubiquitin-proteasome pathway |
9, 29 |
| Heat shock protein 70 (Hsp70) |
Co-immunoprecipitation, peptide mass fingerprinting, and co-localization in HEK293T cells |
Inhibition of Hsp70 chaperone of refolding denatured protein substrates |
55 |
| Mineralocorticoid receptor (MR) and glucocorticoid receptor (GR) |
Yeast two-hybrid screening and co-expression in HN9.10 mouse hippocampal cells |
Modulates transactivation potential; selectively stimulates MR- mediated transcription |
56 |
| Transient receptor potential vanilloid 1 receptor (TRPV1) |
Co-expression and immunoprecipitation from sensory neurons or HEK293T cells |
Modulates sensitivity to noxious stimuli (capsaicin, acid, heat) in sensory neurons |
7 |
Figure 2.
Diagram of the Proposed Role(s) of FAF1 in FasR/CD95, NF-κB and Proteasomal Signaling Pathways. A, FAF1 potentiates FasL-FasR/CD95-mediated apoptosis through interactions with both FasR/CD95 and DISC (death-inducing signaling complex) members FADD (Fas-associated death domain protein) and Caspase-8. B, FAF1 binds to Lys48- and Lys63-linked multi-ubiquitinated proteins and prevents their subsequent degradation by the 26S proteasome. C, FAF1 inhibits NF-κB signaling via two-independent mechanisms. As depicted in the right side of the panel, FAF1 directly inhibits NF-κB target gene transcription by binding to p65/RelA and retaining it in the cytoplasm. As shown in the left portion of this panel, FAF1 can also inhibit NF-κB signaling by preventing IKKα, IKKβ, and IKKγ/NEMO complex formation through a direct interaction with IKKβ, thus allowing for IκB-mediated degradation of p65/RelA and p50 transcription factors.
FAF1's diverse roles in apoptotic and survival signaling pathways are summarized in Table 2. Interestingly, FAF1 has been recently shown to mediate chemotherapy-induced apoptosis through the formation of death effector filaments (DEFs), structures associated with receptor-independent apoptosis.8 Overexpression of FAF1 enhanced DEF assembly and cell death induced by chemotherapeutic agents, whereas antisense FAF1 inhibited these processes. In particular, the interaction of the FAF1-death effector domain interacting domain (DEDID) with the death effector domains (DEDs) of FADD and caspase-8 resulted in enhanced DEF formation and apoptosis. Collectively, the data suggest a potential molecular mechanism for chemosensitization of tumor cells.
Table 2.
FAF1 Regulation of Apoptotic and Survival Pathways.
| Pathway | Context | Role of FAF1 | Reference |
|---|---|---|---|
| Chemotherapy-induced apoptosis via formation of death effector filaments (DEFs) |
FAF1 overexpression enhanced DEF assembly and cell death; antisense FAF1 inhibited DEF assembly and apoptosis; FAF1 co-localized with FADD and caspase-8 in DEFs in BOSC23 cells |
Chemosensitization by FAF1 expression |
8 |
| Aurora-A (Aur-A) phosphorylation of FAF1; proteasome-dependent degradation of Aur-A |
FAF1 mutants used to mimick or disrupt phosphorylation at S289 and S291 in HeLa cells |
Negative feedback regulation of Aur-A via phosphorylation of FAF1, Cross-talk between constituents of the cell cycle and apoptosis |
14 |
| Apoptotic peptidase activating factor 1 (Apaf1)-dependent caspase-3 activation |
Overexpression of FAF1 and mRNA down-regulation of FAF1 in proneuronal cells |
FAS-independent apoptosis; down- regulated FAF1 occurs in a caspase- and Apaf1-dependent manner |
19 |
| TNF-α/ NF-κB signaling | Re-expression of Faf1 in Faf1-deficient malignant mesothelioma (MM) cells or siRNA downregulation of Faf1 in Faf1-positive MM cells |
Down-regulation of Faf1 enhances NF-κB activity in asbestos- induced oncogenesis |
24 |
FAF1 involvement in ubiquitin-mediated protein turnover
FAF1 may serve as a scaffolding protein that regulates protein degradation in the ubiquitin-proteasome pathway,9 as depicted in Figure 2B. FAF1 contains various homologous ubiquitin-like domains, including two ubiquitin homologous domains (Ub's), one UAS (ubiquitin-associated) domain homologous with Caenorhabditis elegans open reading frame C281.1, and an UBX (ubiquitin-like regulatory X) domain homologous with proteins involved in the ubiquitin pathway. The 3-dimensional structure for both of the conserved UAS and UBX domains of FAF1 have been solved in solution using NMR.10 The N-terminal amino acid sequence of FAF1 also has similarity to an ubiquitin associated domain (UBA).9 Although the UBA domain of FAF1 is not fully conserved with other UBA domain sequences, the amino acid residues occupying the position on the hydrophobic surface patch in 3-D structure are well conserved.
Importantly, UBA domain proteins can interact with ubiquitinated target proteins and regulate their proteolysis. The N-terminal UBA domain of FAF1 seems to be important for recruiting and stabilizing ubiquitinated proteasomal substrates, while the C-terminal UBX domain of FAF1 is known to interact with valosin-containing protein (Cdc48/VCP),9 an evolutionary conserved protein involved in various cellular processes including protein degradation, membrane fusion and chaperone activity.11 Notably, increased levels of VCP correlate with cancer, and VCP functions to protect cells from apoptosis by mediating and activating pro-survival Akt and NF-κB signaling pathways.11
Regulation of FAF1 by phosphorylation
FAF1 is modified post-translationally via phosphorylation at potential phosphorylation sites Y225, S270, S289, S291 and S320 (http://www.phosphosite.org). The protein kinase Casein Kinase 2 (CK2) has been shown to phosphorylate FAF1 at S289 and S291 to potentially regulate cell cycle progression, apoptosis, and intracellular location,5, 12, 13 whereas Aurora A (Aur-A) phosphorylates these same residues via a feedback loop that involves FAF1-mediated proteasome-dependent degradation of Aur-A.14
Importantly, CK2 and Aur-A have both been implicated in cancer. Elevated CK2 activity has been associated with malignant transformation and is associated with tumor aggressiveness.15 Although the exact oncogenic mechanism is incompletely understood, CK2 is postulated to protect cells from apoptosis by regulating tumor suppressor and oncogene activity. Aur-A is amplified in a variety of primary human tumors including gastric, breast and colorectal cancers, implying its involvement in oncogenesis and tumor progression.16 Under normal conditions, Aur-A expression is carefully regulated during cell cycle progression, and it is degraded via both ubiquitin-dependent and -independent proteolysis steps.17 Collectively, phosphorylation and regulation of FAF1 by these oncogenic kinases further implicate FAF1 in tumorigenesis.
FAF1 function in development and neuronal cell survival
Absence of Faf1 in gene-targeted mice results in embryonic lethality at the two-cell stage.18 Following the embryonic stages, Faf1 is expressed robustly in the brain and was found to enhance apoptosis through the mitochondrial/apoptosome pathway in neural precursor cells, suggesting a role of Faf1 as a proapoptotic factor in brain development.19 Moreover, after the induction of cell death, Faf1 itself is decreased in a caspase-dependent manner. Since Faf1 also potentiates caspase-3, a negative feedback loop was postulated to regulate Faf1 levels in neuronal cells.
Potentially relevant to this finding, FAF1 is the product of a gene at the PARK10 locus on human chromosome 1p32, a locus associated with late-onset Parkinson's Disease (PD).20 Recently, the role of FAF1 in neuronal cell death and PD was investigated. FAF1 levels were found to be significantly increased in the frontal cortex of PD patients.21 PD patients with Alzheimer's disease exhibited even higher frontal cortex levels of FAF1 than PD patients without Alzheimer's disease. It was further demonstrated that overexpression of FAF1 potentiated the toxic effects of PD stressors, including oxidative stress and proteasome inhibition, on cell survival. Thus, FAF1 upregulation may potentiate neural cell death in PD patients.
Role of FAF1 in regulating NF-κB signaling
Although FAF1 was initially discovered as a FAS receptor binding partner and a potentiator of FAS-induced apoptosis, FAF1 can also regulate cell survival through its effects of the NF-κB pathway (Figure 2C). FAF1 was first demonstrated to inhibit NF-κB activation induced by TNF-α, interleukin-1β (IL-1β), or lipopolysaccharide.22 Overexpression of FAF1 prevented translocation of the RelA subunit of NF-κB into the nucleus, thus decreasing its ability to regulate transcriptional targets of TNF-α.22 In addition to regulating RelA localization, FAF1 was later demonstrated to negatively regulate NF-κB activity via an alternate mechanism. FAF1 was shown to interact with IKKβ in response to TNF-α, IL-1β and lipopolysaccharide to suppress IKK activation.23 Overexpression of FAF1 reduced the level of IKKβ activity by inhibiting IKK complex formation, while conversely FAF1 depletion increased IKK activity in HEK293 cells. Together, these results indicate that FAF1 inhibits IKK activation and its downstream signaling by interrupting the assembly of the IKK complex through physical interaction with IKKβ. Thus, FAF1 can suppress NF-κB activity either through cytoplasmic retention of NF-κB p65, as we and others have demonstrated, or through the inhibition of IKK activation.22, 23
Recently, we identified recurrent hemizygous loss of the Faf1 gene in a mouse model of asbestos-induced malignant mesothelioma (MM),24 a highly aggressive tumor type that arises from the mesothelial cell lining of the pleura, heart and peritoneum. Although the genetic composition of MM in humans is typically complex and exhibits numerous chromosomal deletions, recurrent loss of CDKN2A/ARF, encoding the tumor suppressors p16INK4A and p14ARF, is well documented. Mice heterozygous (+/−) for the p19Arf locus (the murine homologue encodes a larger protein than that produced in humans) were found to be more susceptible to asbestos-induced MM than wild-type littermates.24 Tumors from p19Arf (+/−) mice did not exhibit the expected tumor suppressor loss profile, in that most MMs retained expression of p16Ink4a, p15Ink4b, Nf2 and Tp53. Using array-CGH (comparative genomic hybridization) analysis, we instead identified recurrent focal hemizygous losses of the Faf1 locus, on mouse chromosome 4, and down regulation of Faf1 protein.
Although MMs from asbestos-treated p19Arf (+/−) mice provided the first evidence that losses of Faf1 may be important in MM tumor formation, further analysis of MMs from wild-type littermates and human MMs showed that down regulation of Faf1 expression is not exclusive to p19Arf (+/−) mice and instead is a common occurrence in MMs generally.24 We observed down regulation of FAF1 protein levels in a high percentage of human MM cell lines and tumor specimens. Like human FAF1, we showed that mouse Faf1 regulates NF-κB activation induced by TNF-α, preventing translocation of p65/RelA into the nucleus. Both restoration of Faf1 expression in Faf1-deficient MM cells and knock down of Faf1 with siRNA in Faf1-positive MM cells had the predicted effect on NF-κB signaling. Thus, re-expression of Faf1 resulted in decreased nuclear p65 levels and reduced NF-κB luciferase activity in Faf1-deficient MM cells. Conversely, knock down of Faf1 in Faf1-positive MM cells resulted in up-regulation of NF-κB signaling. Therefore, we postulated that loss of Faf1 leading to deregulation of the NF-κB pathway might play an important role in the context of asbestos-induced MM.
Evidence for a more global role of FAF1 loss in human cancer
Since FAF1 is implicated as a pro-apoptotic factor that can potentiate and in some cases initiate cell death,4 functional loss of FAF1 may provide a pro-survival signal to cells in a disease state such as cancer. Importantly, loss or down regulation of FAF1 has been observed in various human cancers. For example, FAF1 expression was found to be down regulated in a high percentage of human gastric carcinomas.25 Moreover, genomic loss at the FAF1 locus has been reported in nearly 30% of uterine cervix carcinomas,26 and recurrent mono-allelic and homozygous deletion of FAF1 has been reported in mantle cell lymphoma.27 In addition, single nucleotide polymorphism (SNP) testing revealed that FAF1 may be associated with a genetic locus implicated in susceptibility to Crohn's disease, an inflammatory bowel disorder that conveys an increased risk for colorectal cancer.28
Although allelic losses of Faf1/FAF1 were associated with down regulated expression of the gene in our mouse model of MM, as well as in human uterine carcinoma and mantle cell lymphoma, it remains to be determined whether FAF1 levels may be down regulated by other mechanisms, such as epigenetic silencing, in other human cancers. Collectively, these studies suggest that loss of FAF1 is a common occurrence in some human cancers and implicate FAF1 as a tumor suppressor whose loss contributes to cancers of varying origins. Given its pro-apoptotic function, down regulation of FAF1 would thus be expected to promote the survival of a tumor cell as well as its resistance to the effects of anti-cancer therapies.
Importantly, NF-κB and ubiquitin proteasomal pathways are both negatively regulated by overexpression of FAF1,9, 22, 23, 29 and both of these pathways are currently being targeted pharmacologically as a chemotherapeutic approach to treat various human cancers.30-32 Inhibitors of both the NF-κB and proteasomal pathways have been evaluated preclinically for the treatment of MM,33-37 The NF-κB and ubiquitin proteasomal pathways have also been targeted therapeutically in gastric38, 39 and uterine carcinomas40 as well as in mantle cell lymphomas,41 all of which are cancers that exhibit frequent down regulation of FAF1.25, 42, 43 Thus, loss of FAF1 in cancer cells, leading to aberrant NF-κB and proteasomal signaling, could be exploited as a potential biomarker for responsiveness of these tumors to these types of pathway inhibitors.
Importance of NF-κB activity in oncogenesis
NF-κB was initially discovered as a transcription factor that bound an 11-base pair sequence in the enhancer of the kappa immunoglobulin light chain gene.44 Following this discovery, NF-κB signaling has been shown to regulate many normal biological processes such as inflammation and innate/adaptive immune responses and cellular processes such as proliferation and survival.31 Based on the involvement of NF-κB signaling in so many processes, it is perhaps not surprising that members and regulators of the NF-κB signaling pathway are deleted, mutated or amplified in many human diseases.45 Germane to this perspective, the NF-κB signaling axis is frequently dysregulated in human cancers and can consequently promote tumor cell survival and proliferation, as well as potentiate angiogenesis, metastasis and inflammation in established tumors.31 Interestingly, human cancers that frequently exhibit downregulated FAF1 levels, such as gastric and cervical carcinomas, MM and mantle cell lymphomas, also exhibit hyperactive NF-κB signaling,35, 41, 46-50 although a more comprehensive analysis is warranted to determine if a close correlation exists between FAF1 status and aberrant NF-κB signaling.
Our data suggest that FAF1 is frequently down regulated in MM, and this deficiency allows for aberrant NF-κB signaling. Germane to this, several groups have demonstrated that TNF-α signaling is important in mesothelial cell transformation and MM pathogenesis. For example, TNF-α has been shown to inhibit asbestos-induced cytotoxicity via an NF-κB-dependent pathway.51 A model was proposed whereby exposure to asbestos leads to the accumulation of macrophages and release of TNF-α, while asbestos simultaneously induces human mesothelial cells to secrete TNF-α. The release of TNF-α may then activate NF-κB to promote mesothelial cell survival and permit cells with asbestos-induced DNA damage to divide rather than undergo apoptosis.51 Based on this model, mesothelial cells deficient for FAF1 may exhibit stronger TNF-α-mediated NF-κB signaling and a survival advantage during initial exposure to asbestos, compared to mesothelial cells that retain FAF1 expression. NF-κB has also been shown to be a constitutive survival factor in transformed human mesothelial cells as well as in MM cells.35 Thus, MM cells that are deficient for FAF1 and exhibit increased NF-κB signaling may also have a distinct growth advantage when compared to cells that retain FAF1, although this has not been formally tested.
Pharmacologic agents that inhibit NF-κB signaling appear to preferentially kill tumor cells that have a dependence on NF-κB activity for survival not shared by normal cells. Cancer cells that exhibit down regulation of FAF1 and consequent aberrant NF-κB signaling, may be more responsive to NF-κB inhibition than those retaining FAF1 expression. Further rationale is provided by studies showing that cancer cells with either gain-of-function mutations in genes encoding positive regulators of NF-κB signaling or loss-of-function mutations in genes encoding negative regulators of NF-κB signaling are more responsive to NF-κB inhibition.31 Most of these genetic alterations result in activation of both the classical and alternative NF-κB signaling pathways. For example, multiple myeloma cells can harbor inactivating mutations of TRAF3 (TNFR-associated factor 3), a negative regulator of NF-κB signaling, and 90% of patients with low TRAF3 levels respond to bortezomib, with a significant increase in patient survival.52 Like TRAF3, FAF1 may represent a potential biomarker for tumor responsiveness to NF-κB inhibitors.
Issues to be resolved
FAF1 was only recently discovered and characterized as potent regulator of cell survival. As such, its role in the development of diseases such as Parkinson's and cancer is still relatively undefined. The recurrent loss/down regulation of FAF1 observed in certain human and murine cancers suggests that this pro-apoptotic factor is a tumor suppressor, although the frequency of FAF1 loss or down regulation in other human cancers requires further exploration. Furthermore, the link between FAF1 loss and aberrant NF-κB activity in these malignancies requires further investigation. For example, whether or not FAF1 status can predict tumor responsiveness to NF-κB pathway inhibitors remains unclear. In addition, FAF1 may also function in other signal transduction pathways, and how FAF1 loss may impinge on other pathways involved in cancer has yet to be determined. Given that FAF1 has multiple protein interacting domains, we postulate that loss of FAF1 function may contribute to diverse hallmarks of cancer, although its role(s) may be context dependent. Thus, future studies are needed to better understand the importance of FAF1 loss in various types of cancer, as well as its therapeutic implications.
Acknowledgements
This work was supported by NCI Grants CA-114047 (J.R.T.) and CA-06927 (Fox Chase Cancer Center), by an appropriation from the Commonwealth of Pennsylvania, and by a gift from Local No. 14 Mesothelioma Fund of the International Association of Heat and Frost Insulators & Allied Workers in memory of Hank Vaughan and Alice Haas. C.W.M was supported by NCI postdoctoral training grant CA-009035; D.A.A. was supported in part by the Liz Tilberis Scholar Program, funded by the Ovarian Cancer Research Fund, Inc.
Abbreviations
- Apaf1
apoptotic peptidase activating factor 1
- Aur-A
aurora A
- CK2
casein kinase 2
- CGH
Comparative Genomic Hybridization
- DEDID
death effector domain interacting domain
- DEFs
death effector filaments
- DISC
death-induced signaling complex
- FADD
Fas-associated protein with death domain
- FAF1
Fas (TNFRSF6)-associated Factor 1
- FAS
Fas (TNF receptor superfamily, member 6)
- FID
Fas-interacting domain
- GR
glucocorticoid receptor
- Hsp70
heat shock protein 70
- IKK
IκB kinase
- MM
malignant mesothelioma
- MR
mineralocorticoid receptor
- NF2
neurofibromatosis 2
- NF-κB
nuclear factor of kappa light polypeptide gene enhancer in B-cells
- NMR
nuclear magnetic resonance
- PD
Parkinson's disease
- PYPAFs
pyrin domain-containing Apaf1-like proteins
- RELA
v-rel reticuloendotheliosis viral oncogene homology A (avian)
- TNFα
tumor necrosis factor alpha
- TRAF3
TNFR (tumor necrosis factor receptor)-associated Factor 3
- TRPV1
transient teceptor potential vanilloid 1 receptor
- UAS
homologous with C. elegans open reading frame C281.1
- UBA
ubiquitin-associated domain
- UBX
ubiquitin-like regulatory X domain
- VCP
valosin-containing protein
References
- 1.Yu JW, Shi Y. FLIP and the death effector domain family. Oncogene. 2008;27:6216–27. doi: 10.1038/onc.2008.299. [DOI] [PubMed] [Google Scholar]
- 2.Chu K, Niu X, Williams LT. A Fas-associated protein factor, FAF1, potentiates Fas-mediated apoptosis. Proc Natl Acad Sci U S A. 1995:11894–8. doi: 10.1073/pnas.92.25.11894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ryu SW, Kim E. Apoptosis induced by human Fas-associated factor 1, hFAF1, requires its ubiquitin homologous domain, but not the Fas-binding domain. Biochem Biophys Res Commun. 2001;286:1027–32. doi: 10.1006/bbrc.2001.5505. [DOI] [PubMed] [Google Scholar]
- 4.Ryu SW, Chae SK, Lee KJ, Kim E. Identification and characterization of human Fas associated factor 1, hFAF1. Biochem Biophys Res Commun. 1999;262:388–94. doi: 10.1006/bbrc.1999.1217. [DOI] [PubMed] [Google Scholar]
- 5.Jensen HH, Hjerrild M, Guerra B, Larsen MR, Hojrup P, Boldyreff B. Phosphorylation of the Fas associated factor FAF1 by protein kinase CK2 and identification of serines 289 and 291 as the in vitro phosphorylation sites. Int J Biochem Cell Biol. 2001;33:577–89. doi: 10.1016/s1357-2725(01)00039-5. [DOI] [PubMed] [Google Scholar]
- 6.Kinoshita T, Kondoh C, Hasegawa M, Imamura R, Suda T. Fas-associated factor 1 is a negative regulator of PYRIN-containing Apaf-1-like protein 1. Int Immunol. 2006;18:1701–6. doi: 10.1093/intimm/dxl104. [DOI] [PubMed] [Google Scholar]
- 7.Kim S, Kang C, Shin CY, Hwang SW, Yang YD, Shim WS, et al. TRPV1 recapitulates native capsaicin receptor in sensory neurons in association with Fas-associated factor 1. J Neurosci. 2006;26:2403–12. doi: 10.1523/JNEUROSCI.4691-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park MY, Ryu SW, Kim KD, Lim JS, Lee ZW, Kim E. Fas-associated factor-1 mediates chemotherapeutic-induced apoptosis via death effector filament formation. Int J Cancer. 2005;115:412–8. doi: 10.1002/ijc.20857. [DOI] [PubMed] [Google Scholar]
- 9.Song EJ, Yim SH, Kim E, Kim NS, Lee KJ. Human Fas-associated factor 1, interacting with ubiquitinated proteins and valosin-containing protein, is involved in the ubiquitin-proteasome pathway. Mol Cell Biol. 2005;25:2511–24. doi: 10.1128/MCB.25.6.2511-2524.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buchberger A, Howard MJ, Proctor M, Bycroft M. The UBX domain: a widespread ubiquitin-like module. J Mol Biol. 2001;307:17–24. doi: 10.1006/jmbi.2000.4462. [DOI] [PubMed] [Google Scholar]
- 11.Braun RJ, Zischka H. Mechanisms of Cdc48/VCP-mediated cell death: from yeast apoptosis to human disease. Biochim Biophys Acta. 2008;1783:1418–35. doi: 10.1016/j.bbamcr.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 12.Guerra B, Boldyreff B, Issinger OG. FAS-associated factor 1 interacts with protein kinase CK2 in vivo upon apoptosis induction. Int J Oncol. 2001;19:1117–26. doi: 10.3892/ijo.19.6.1117. [DOI] [PubMed] [Google Scholar]
- 13.Olsen BB, Jessen V, Hojrup P, Issinger OG, Boldyreff B. Protein kinase CK2 phosphorylates the Fas-associated factor FAF1 in vivo and influences its transport into the nucleus. FEBS Lett. 2003;546:218–22. doi: 10.1016/s0014-5793(03)00575-1. [DOI] [PubMed] [Google Scholar]
- 14.Jang MS, Sul JW, Choi BJ, Lee SJ, Suh JH, Kim NS, et al. Negative feedback regulation of Aurora-A via phosphorylation of Fas-associated factor-1. J Biol Chem. 2008;283:32344–51. doi: 10.1074/jbc.M804199200. [DOI] [PubMed] [Google Scholar]
- 15.Duncan JS, Litchfield DW. Too much of a good thing: the role of protein kinase CK2 in tumorigenesis and prospects for therapeutic inhibition of CK2. Biochim Biophys Acta. 2008;1784:33–47. doi: 10.1016/j.bbapap.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 16.Katayama H, Brinkley WR, Sen S. The Aurora kinases: role in cell transformation and tumorigenesis. Cancer Metastasis Rev. 2003;22:451–64. doi: 10.1023/a:1023789416385. [DOI] [PubMed] [Google Scholar]
- 17.Meraldi P, Honda R, Nigg EA. Aurora kinases link chromosome segregation and cell division to cancer susceptibility. Curr Opin Genet Dev. 2004;14:29–36. doi: 10.1016/j.gde.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 18.Adham IM, Khulan J, Held T, Schmidt B, Meyer BI, Meinhardt A, et al. Fas-associated factor (FAF1) is required for the early cleavage-stages of mouse embryo. Mol Hum Reprod. 2008;14:207–13. doi: 10.1093/molehr/gan009. [DOI] [PubMed] [Google Scholar]
- 19.De Zio D, Ferraro E, D'Amelio M, Simoni V, Bordi M, Soroldoni D, et al. Faf1 is expressed during neurodevelopment and is involved in Apaf1-dependent caspase-3 activation in proneural cells. Cell Mol Life Sci. 2008;65:1780–90. doi: 10.1007/s00018-008-8075-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hicks AA, Petursson H, Jonsson T, Stefansson H, Johannsdottir HS, Sainz J, et al. A susceptibility gene for late-onset idiopathic Parkinson's disease. Ann Neurol. 2002;52:549–55. doi: 10.1002/ana.10324. [DOI] [PubMed] [Google Scholar]
- 21.Betarbet R, Anderson LR, Gearing M, Hodges TR, Fritz JJ, Lah JJ, et al. Fas-associated factor 1 and Parkinson's disease. Neurobiol Dis. 2008;31:309–15. doi: 10.1016/j.nbd.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park MY, Jang HD, Lee SY, Lee KJ, Kim E. Fas-associated factor-1 inhibits nuclear factor-kappaB (NF-kappaB) activity by interfering with nuclear translocation of the RelA (p65) subunit of NF-kappaB. J Biol Chem. 2004;279:2544–9. doi: 10.1074/jbc.M304565200. [DOI] [PubMed] [Google Scholar]
- 23.Park MY, Moon JH, Lee KS, Choi HI, Chung J, Hong HJ, et al. FAF1 suppresses IkappaB kinase (IKK) activation by disrupting the IKK complex assembly. J Biol Chem. 2007;282:27572–5. doi: 10.1074/jbc.C700106200. [DOI] [PubMed] [Google Scholar]
- 24.Altomare DA, Menges CW, Pei J, Zhang L, Skele-Stump KL, Carbone M, et al. Activated TNF-alpha/NF-kappaB signaling via down-regulation of Fas-associated factor 1 in asbestos-induced mesotheliomas from Arf knockout mice. Proc Natl Acad Sci U S A. 2009;106:3420–5. doi: 10.1073/pnas.0808816106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bjørling-Poulsen M, Seitz G, Guerra B, Issinger OG. The pro-apoptotic FAS-associated factor 1 is specifically reduced in human gastric carcinomas. Int J Oncol. 2003;23:1015–23. [PubMed] [Google Scholar]
- 26.Hidalgo A, Baudis M, Petersen I, Arreola H, Piña P, Vázquez-Ortiz G, et al. Microarray comparative genomic hybridization detection of chromosomal imbalances in uterine cervix carcinoma. BMC Cancer. 2005:5–77. doi: 10.1186/1471-2407-5-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beà S, Salaverria I, Armengol L, Pinyol M, Fernández V, Hartmann EM, et al. Uniparental disomies, homozygous deletions, amplifications, and target genes in mantle cell lymphoma revealed by integrative high-resolution whole-genome profiling. Blood. 2009;113:3059–69. doi: 10.1182/blood-2008-07-170183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weersma RK, Stokkers PC, Cleynen I, Wolfkamp SC, Henckaerts L, Schreiber S, et al. Confirmation of multiple Crohn's disease susceptibility loci in a large Dutch-Belgian cohort. Am J Gastroenterol. 2009;104:630–8. doi: 10.1038/ajg.2008.112. [DOI] [PubMed] [Google Scholar]
- 29.Besche H, Haas W, Gygi S, Goldberg A. Isolation of mammalian 26S proteasomes and p97/VCP complexes using the ubiquitin-like domain from HHR23B reveals novel proteasome-associated proteins. Biochemistry. 2009 doi: 10.1021/bi802198q. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McConkey DJ, Zhu K. Mechanisms of proteasome inhibitor action and resistance in cancer. Drug Resist Updat. 2008;11:164–79. doi: 10.1016/j.drup.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 31.Baud V, Karin M. Is NF-kappaB a good target for cancer therapy? Hopes and pitfalls. Nat Rev Drug Discov. 2009;8:33–40. doi: 10.1038/nrd2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang Y, Kitagaki J, Wang H, Hou DX, Perantoni AO. Targeting the ubiquitin-proteasome system for cancer therapy. Cancer Sci. 2009;100:24–8. doi: 10.1111/j.1349-7006.2008.01013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun X, Gulyás M, Hjerpe A, Dobra K. Proteasome inhibitor PSI induces apoptosis in human mesothelioma cells. Cancer Lett. 2006;232:161–9. doi: 10.1016/j.canlet.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 34.Gordon GJ, Mani M, Mukhopadhyay L, Dong L, Yeap BY, Sugarbaker DJ, et al. Inhibitor of apoptosis proteins are regulated by tumour necrosis factor-alpha in malignant pleural mesothelioma. J Pathol. 2007;211:439–46. doi: 10.1002/path.2120. [DOI] [PubMed] [Google Scholar]
- 35.Sartore-Bianchi A, Gasparri F, Galvani A, Nici L, Darnowski JW, Barbone D, et al. Bortezomib inhibits nuclear factor-kappaB dependent survival and has potent in vivo activity in mesothelioma. Clin Cancer Res. 2007;13:5942–51. doi: 10.1158/1078-0432.CCR-07-0536. [DOI] [PubMed] [Google Scholar]
- 36.Borczuk AC, Cappellini GC, Kim HK, Hesdorffer M, Taub RN, Powell CA. Molecular profiling of malignant peritoneal mesothelioma identifies the ubiquitin-proteasome pathway as a therapeutic target in poor prognosis tumors. Oncogene. 2007;26:610–7. doi: 10.1038/sj.onc.1209809. [DOI] [PubMed] [Google Scholar]
- 37.Yuan BZ, Chapman JA, Reynolds SH. Proteasome Inhibitor MG132 Induces Apoptosis and Inhibits Invasion of Human Malignant Pleural Mesothelioma Cells. Tansl Oncol. 2008;1:129–40. doi: 10.1593/tlo.08133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fan XM, Wong BC, Wang WP, Zhou XM, Cho CH, Yuen ST, et al. Inhibition of proteasome function induced apoptosis in gastric cancer. Int J Cancer. 2001;93:481–8. doi: 10.1002/ijc.1373. [DOI] [PubMed] [Google Scholar]
- 39.Adachi M, Zhang Y, Zhao X, Minami T, Kawamura R, Hinoda Y, et al. Synergistic effect of histone deacetylase inhibitors FK228 and m-carboxycinnamic acid bis-hydroxamide with proteasome inhibitors PSI and PS-341 against gastrointestinal adenocarcinoma cells. Clin Cancer Res. 2004;10:3853–62. doi: 10.1158/1078-0432.CCR-03-0806. [DOI] [PubMed] [Google Scholar]
- 40.Birle DC, Hedley DW. Suppression of the hypoxia-inducible factor-1 response in cervical carcinoma xenografts by proteasome inhibitors. Cancer Res. 2007;67:1735–43. doi: 10.1158/0008-5472.CAN-06-2722. [DOI] [PubMed] [Google Scholar]
- 41.Yang DT, Young KH, Kahl BS, Markovina S, Miyamoto S. Prevalence of bortezomib-resistant constitutive NF-kappaB activity in mantle cell lymphoma. Mol Cancer. 2008:7–40. doi: 10.1186/1476-4598-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hidalgo A, Baudis M, Petersen I, Arreola H, Pina P, Vazquez-Ortiz G, et al. Microarray comparative genomic hybridization detection of chromosomal imbalances in uterine cervix carcinoma. BMC Cancer. 2005:5–77. doi: 10.1186/1471-2407-5-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bea S, Salaverria I, Armengol L, Pinyol M, Fernandez V, Hartmann EM, et al. Uniparental disomies, homozygous deletions, amplifications, and target genes in mantle cell lymphoma revealed by integrative high-resolution whole-genome profiling. Blood. 2009;113:3059–69. doi: 10.1182/blood-2008-07-170183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sen R, Baltimore D. Inducibility of kappa immunoglobulin enhancer-binding protein Nf-kappa B by a posttranslational mechanism. Cell. 1986;47:921–8. doi: 10.1016/0092-8674(86)90807-x. [DOI] [PubMed] [Google Scholar]
- 45.Courtois G, Gilmore TD. Mutations in the NF-kappaB signaling pathway: implications for human disease. Oncogene. 2006;25:6831–43. doi: 10.1038/sj.onc.1209939. [DOI] [PubMed] [Google Scholar]
- 46.Sasaki N, Morisaki T, Hashizume K, Yao T, Tsuneyoshi M, Noshiro H, et al. Nuclear factor-kappaB p65 (RelA) transcription factor is constitutively activated in human gastric carcinoma tissue. Clin Cancer Res. 2001;7:4136–42. [PubMed] [Google Scholar]
- 47.Yeh PY, Chuang SE, Yeh KH, Song YC, Cheng AL. Involvement of nuclear transcription factor-kappa B in low-dose doxorubicin-induced drug resistance of cervical carcinoma cells. Biochem Pharmacol. 2003;66:25–33. doi: 10.1016/s0006-2952(03)00250-8. [DOI] [PubMed] [Google Scholar]
- 48.Lee BL, Lee HS, Jung J, Cho SJ, Chung HY, Kim WH, et al. Nuclear factor-kappaB activation correlates with better prognosis and Akt activation in human gastric cancer. Clin Cancer Res. 2005;11:2518–25. doi: 10.1158/1078-0432.CCR-04-1282. [DOI] [PubMed] [Google Scholar]
- 49.Venkatraman M, Anto RJ, Nair A, Varghese M, Karunagaran D. Biological and chemical inhibitors of NF-kappaB sensitize SiHa cells to cisplatin-induced apoptosis. Mol Carcinog. 2005;44:51–9. doi: 10.1002/mc.20116. [DOI] [PubMed] [Google Scholar]
- 50.Roue G, Perez-Galan P, Lopez-Guerra M, Villamor N, Campo E, Colomer D. Selective inhibition of IkappaB kinase sensitizes mantle cell lymphoma B cells to TRAIL by decreasing cellular FLIP level. J Immunol. 2007;178:1923–30. doi: 10.4049/jimmunol.178.3.1923. [DOI] [PubMed] [Google Scholar]
- 51.Yang H, Bocchetta M, Kroczynska B, Elmishad AG, Chen Y, Liu Z, et al. TNF-alpha inhibits asbestos-induced cytotoxicity via a NF-kappaB-dependent pathway, a possible mechanism for asbestos-induced oncogenesis. Proc Natl Acad Sci U S A. 2006;103:10397–402. doi: 10.1073/pnas.0604008103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Keats JJ, Fonseca R, Chesi M, Schop R, Baker A, Chng WJ, et al. Promiscuous mutations activate the noncanonical NF-kappaB pathway in multiple myeloma. Cancer Cell. 2007;12:131–44. doi: 10.1016/j.ccr.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ryu SW, Lee SJ, Park MY, Jun JI, Jung YK, Kim E. Fas-associated factor 1, FAF1, is a member of Fas death-inducing signaling complex. J Biol Chem. 2003;278:24003–10. doi: 10.1074/jbc.M302200200. [DOI] [PubMed] [Google Scholar]
- 54.Kusk M, Ahmed R, Thomsen B, Bendixen C, Issinger OG, Boldyreff B. Interactions of protein kinase CK2beta subunit within the holoenzyme and with other proteins. Mol Cell Biochem. 1999;191:51–8. [PubMed] [Google Scholar]
- 55.Kim HJ, Song EJ, Lee YS, Kim E, Lee KJ. Human Fas-associated factor 1 interacts with heat shock protein 70 and negatively regulates chaperone activity. J Biol Chem. 2005;280:8125–33. doi: 10.1074/jbc.M406297200. [DOI] [PubMed] [Google Scholar]
- 56.Obradovic D, Tirard M, Nemethy Z, Hirsch O, Gronemeyer H, Almeida OF. DAXX, FLASH, and FAF-1 modulate mineralocorticoid and glucocorticoid receptor-mediated transcription in hippocampal cells--toward a basis for the opposite actions elicited by two nuclear receptors? Mol Pharmacol. 2004;65:761–9. doi: 10.1124/mol.65.3.761. [DOI] [PubMed] [Google Scholar]