Abstract
The lipid A of LPS activates TLR45 through an interaction with MD-2 and the degree of lipid A acylation affects TLR4 responsiveness. Two TLR4 single nucleotide polymorphisms (SNPs) (Asp299Gly and Thr399Ile) have been associated with LPS-hyporesponsiveness. We hypothesized that the combination of hypoacylation and these SNPs would exhibit a compounded effect on TLR4 signaling. HEK293T transfectants expressing wild-type (WT) or polymorphic TLR4 were stimulated with E. coli (predominantly hexaacylated lipid A) or S. flexneri 2a (a mixture of hexaacylated, pentaacylated, and predominantly tetraacylated lipid A) LPS, or hexaacylated vs. pentaacylated synthetic lipid As. NF-κB-reporter activity was significantly lower in response to S. flexneri 2a than E. coli LPS, and further decreased in polymorphic transfectants. Neither hexaacylated nor pentaacylated synthetic lipid A induced NF-κB activity in WT transfectants under the identical transfection conditions used for LPS; however, increasing human MD-2 expression rescued responsiveness to hexaacylated lipid A only, while murine MD-2 was required to elicit a response to pentaacylated lipid A. Adherent PBMC of healthy volunteers were also compared for LPS-induced TNF-α, IL-6, IL-1β, and IL-10 production. Cytokine levels were significantly lower (~20–90%) in response to S. flexneri than to E. coli LPS/lipid A and PBMC from polymorphic individuals secreted decreased cytokine levels in response to both LPS types and failed to respond to pentaacylated lipid A. Thus, the combination of acylation state and host genetics may significantly impact vaccine immunogenicity and/or efficacy, whether LPS is an integral component of a whole organism vaccine or included as an adjuvant.
Keywords: TLR4, LPS, lipid A, SNPs, inflammation, human, cytokines, Shigella
Introduction
In response to infection, the capacity of the host to mount an effective, early innate immune response is a key determinant of disease resistance or susceptibility. The outer membrane of Gram negative bacteria is comprised predominantly of endotoxic lipopolysaccharide (LPS). LPS elicits a wide variety of biological activities in the host (1). For most LPS species, TLR4 acts as the “pattern recognition receptor” that senses LPS, and initiates intracellular signaling leading to an inflammatory response (2, 3). LPS is comprised of three covalently linked regions: a repeating O-polysaccharide side chain (which confers serologic specificity to the organism), a more conserved “core” oligosaccharide that, in turn, is covalently linked to a hydrophobic glycolipid portion, lipid A. Lipid A is responsible for the biological activity of LPS and has been termed the “endotoxic principle” of LPS (4). Proinflammatory signal initiation by LPS depends upon the interaction of the TLR4 receptor complex (i.e., TLR4, MD-2, and CD14) with the lipid A moiety of LPS. Structural variations within lipid A, such as the number and positions of acyl chains or the number and position of charged groups on the sugar backbone of the lipid A molecule, greatly influence this interaction (5). Using infrared attenuated total reflectance spectroscopy, Seydel et al. (6) showed that the endotoxic effect of lipid A portion of LPS directly correlated with, and strongly depends upon, the tilt angle of the sugar backbone with respect to the membrane surface, and the number and distribution of the hydrocarbon chains.
It also has been shown that only those LPS molecules comprised of a hexaacylated lipid A moiety with a conical/concave shape are highly active biologically (5, 6). In contrast, lipid A molecules that are either tetraacylated or pentaacylated exhibit a cylindrical molecular shape and greatly attenuated endotoxic activity, and may even exhibit antagonistic activity (6, 7). For example, Hajjar et al. found that a hexaacylated Pseudomonas aeruginosa LPS was much more stimulatory in cells expressing human TLR4 than pentaacylated LPS prepared from a laboratory strain (8). Therefore, in response to a Gram negative bacterial infection or vaccine, or in response to a vaccine in which a TLR4 agonist is present as an adjuvant, the relative contribution of TLR4-mediated signaling to the initial host innate immune response may differ depending on the acylation state of the TLR4 agonist. The induction of a proinflammatory cytokine milieu and up-regulation of co-stimulatory molecules induced by LPS activation of TLR4 impacts greatly on the subsequent acquired immune response. In this context, structural variations such as the degree of acylation within the lipid A moiety could contribute significantly toward determining the immunogenicity and/or efficacy of a specific Gram negative vaccine. It has been previously shown that the E. coli LPS is comprised mainly of hexaacylated lipid A, while Shigella flexneri LPS has been reported to be predominantly pentaacylated (9). Therefore, it would be anticipated that the overall TLR4 response to S. flexneri 2a LPS would be less than that of cells stimulated with E. coli LPS.
Just as there are variations in the microbial structures that contribute to the host response, the innate immune response is also subject to genetic regulation. TLR4 is a highly conserved transmembrane receptor for LPS. Arbour et al. (10) identified two TLR4 SNPs that encode single amino acid substitutions, Asp299Gly (D299G) and Thr399Ile (T399I), in the ectodomain of human TLR4. These two polymorphisms were identified in ~6% and ~3% of individuals, respectively, and may be expressed concurrently (10). Inheritance of these SNPs, singly or together, was associated with an LPS-hyporesponsive phenotype in human airway epithelial cells and alveolar macrophages, and both SNPs were associated with a significantly blunted response to inhaled LPS. Kiechl et al. (11) reported that subjects who expressed the Asp299Gly SNP were more susceptible to Gram negative bacterial infections, and Lorenz et al. (12) reported that expression of the TLR4 Asp299Gly variant was associated with septic shock patients, and that septic shock patients with Asp299Gly or Thr399Ile forms exhibited a higher incidence of Gram negative infection. Collectively, these data support the hypothesis that faulty TLR4 signaling fails to induce a sufficiently robust inflammatory response to control infection.
We recently reported that expression of TLR4 molecules that carry the Asp299Gly and/or Thr399Ile extracellular polymorphisms in HEK293T cells results in mitigated host responses to three structurally unrelated TLR4 agonists: Gram negative LPS, the fusion (F) protein of RSV, and Chlamydia heat shock protein 60 (3). In all cases, the pattern of responsiveness to these three agonists was similar: transfectants expressing either the Asp299Gly or Thr399Ile were less responsive than cells expressing WT TLR4, but more responsive than HEK293T cells expressing the doubly mutated TLR4 vector (i.e., both SNPs are expressed on the same chromosomal homolog). Molecular modeling revealed that the amino acids located in positions 299 and 399 of TLR4 lie on the same extracellular “face” of the TLR4 protein and that expression of the polymorphic amino acids might create steric and charge alterations that could potentially alter its interaction with ligand or common co-receptor molecules (3, 13, 14).
In this study, we sought to extend our previous findings by analyzing the capacity of two species of LPS or synthetic lipid A that differ in the degree of acylation to modulate the host innate immune response in cells that express either WT or polymorphic TLRs. To this end, we tested highly purified LPS preparations derived from E. coli and S. flexneri 2a, as well as synthetic hexaacylated and pentaacylated lipid A molecules, for their capacity to elicit TLR4-mediated responses, first in a transient transfection system in HEK293T cells (3), and then in human PBMC derived from WT (non-polymorphic) and polymorphic individuals. The rationale for using Shigella flexneri 2a was based on the facts that (i) S. flexneri 2a is the single most important S. flexneri subserotype that causes shigellosis in developing countries, and (ii) attenuated S. flexneri 2a vaccines are at an advanced stage of development and currently being evaluated in clinical trials (15). In contrast to a previous report (9), we demonstrate that the S. flexneri 2a LPS used in this study is comprised of a mixture of hexaacylated, pentaacylated, and predominantly tetraacylated lipid A-containing LPS species. The lipid A acylation state for the S. flexneri 2a strain used in this study differs from the wild-type S. flexneri serotype 5a that expressed mainly hexaacylated lipid A-containing LPS species, as reported by D’Hauteville et al. (16). Consistent with previous observations, S. flexneri 2a LPS elicited significantly diminished TLR4-mediated signaling when compared to E. coli LPS (shown to possess predominantly hexaacylated lipid A-containing LPS), and the response to both LPS preparations was further diminished in cells expressing the TLR4 polymorphic variants. Interestingly, the response to synthetic hexaacylated lipid A by WT transfectants required much higher human MD-2 expression than for E. coli LPS, yet no response to pentaacylated lipid A was detected unless rescued by murine MD-2. Taken collectively, these data support the hypothesis that both the structural makeup of the LPS, in concert with inherited structural modifications to TLR4, can combine to affect significantly the outcome of the host response to infection by Gram negative organisms or in vaccines that contain intrinsic or extrinsic TLR4 agonists in a significant number of individuals.
Materials and Methods
Cells and reagents
HEK293T human embryonic kidney cells (ATCC, Rockville, MD, USA) were cultured in DMEM (BioWhittaker) supplemented with 10% FBS, 2 mM L-glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin. Protein free, Escherichia coli K235 LPS (17) and Shigella flexneri 2a (18, 19) were used in this study and synthetic hexaacylated lipid A and pentaacylated lipid A were synthesized as described elsewhere (20). The hexaacylated synthetic lipid A used in this study, (Compound 506, LA-15-PP), has the following structure: β(1–6) glucosamine disaccharide bisphosphorylated at the 1- and 4′-positions with the acylated pattern of 3′-position 14:0 [3-O (14:0)], 2′-position 14:0 [3-O (12:0)], 3-position 14:0 (3-OH) and 2-position 14:0 (3-OH) (21). The synthetic pentaacylated lipid A used in this study, LA-21-PP, has the following structure: 3′ position 14:0 (3-OH), 2′ position 14:0 [3-O (16:0)], 3 position 14:0 (3-OH), 2 position 14:0 (3-OH). The biological activity of this synthetic pentaacylated lipid A was described previously by Rietschel et al. (22). SuperFect transfection reagent was obtained from Qiagen. All expression plasmid constructs were prepared using EndoFree Plasmid Maxi Kit (Qiagen).
Plasmid constructs
The expression vector, pcDNA3-TLR4, that encodes an untagged wild-type TLR4, was kindly provided by Dr. Eva Lorenz (University of North Carolina, Chapel Hill, NC). Point mutations encoding an aspartic acid (Asp) to glycine (Gly) substitution at the amino acid position 299 (D299G) and a threonine (Thr) to isoleucine (Ile) substitution at amino acid 399 (T399I) in human TLR4 protein were created by site-directed mutagenesis using a QuikChange mutagenesis kit (Stratagene) according to the manufacturer’s instructions as described elsewhere (3).
Expression plasmids, pcDNA3-huCD14 and pEFBOS-HA-huMD-2, the ELAM-1 luciferase, pELAM-luc (NF-κB reporter), and pCMV1-β-galactosidase reporter plasmids were also kindly provided by Dr. Douglas Golenbock and have been described elsewhere (3, 23, 24). The murine MD-2 construct, pEFBOS-muMD-2, was kindly provided by Dr. Kensuke Miyake (University of Tokyo, Japan), and has been described elsewhere (25).
Mass spectrometry of lipid A species
Matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectra of the purified lipids were acquired in the positive-and negative-linear mode using an AXIMA-CFR mass spectrometer from Kratos Analytical (Manchester, UK). The AXIMA-CFR is equipped with a 337 nm nitrogen laser, a 20-kV extraction voltage, and time-delayed extraction. Saturated 6-aza-2-thiothymine (ATT) in 50% acetonitrile and 10% tribasic ammonium citrate (9:1, v/v) served as the matrix. Purified monophosphoryl lipid A was prepared by hydrolyzing the LPS in 0.1 N HCl at 100°C for 15 min. The lipid A was extracted as described previously (26, 27). The lipid A samples dissolved in chloroform/methanol (4:1, v/v) were deposited onto the sample plate with an equal portion of the matrix solution (0.8 μL). The sample mixtures were dried to room temperature prior to mass analysis. Hexaacylated lipid A 1,4′-bisphosphate from wild type E. coli (Sigma) served as an external standard for calibration.
Reporter assay
HEK293T cells were seeded into 12-well Costar plates (Corning Inc.) at a density of 2 × 105 cells/well and incubated overnight in a CO2 incubator. The next morning, cells were co-transfected for 3 hr with the optimized amounts of pcDNA3-TLR4, pcDNA3-huCD14 and pEFBOS-HA-huMD-2 (3) together with the NF-κB reporter (500 ng/well) and pCMV1-βGal (100 ng/well). The final DNA concentration was adjusted to 1.5 μg/well with the pcDNA3 blank vector (Invitrogen). The transfection was carried out with SuperFect transfection reagent (Qiagen). Cells were allowed to recover for 20 hr, washed with 1× PBS, and stimulated with E. coli or S. flexneri 2a LPS or hexaacylated or pentaacylated lipid A at the indicated concentrations for 5 hr. Cells were lysed in 1× reporter assay lysis buffer (Promega Corporation), and β-galactosidase (Tropix, Galacto-Light system) and luciferase (Promega, Luciferase assay system) activities were analyzed, using a Berthold LB 9507 Luminometer (Berthhold Technologies, Bad Wildbad, Germany). “Relative luciferase activity” was calculated by normalizing each sample’s luciferase activity for constitutive β-galactosidase activity measured within the same sample, and represented as Relative Luciferase Units (RLU) as described previously (3).
Murine macrophage cultures
Peritoneal exudate macrophages were obtained from 5–6 week old C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME) by lavage 5 days after i.p. injection of 3 ml sterile 3% thioglycollate broth (28). Cells were washed and resuspended in RPMI containing 2% FCS and standard supplements. Macrophages were plated in 24-well tissue culture dishes (2 × 106 cells/well) for supernatant collection. After overnight incubation to allow for adherence of macrophages, monolayers were washed to remove nonadherent cells and incubated with medium, LPS, or lipid A at the indicated concentrations in a final volume of 1 ml. Supernatants were harvested 24 hr after treatment and stored at −70°C. Murine IL-β, IFN-γ, RANTES, and TNF-α were detected using the antibody pairs and standards provided in the Quantikine M ELISA kit (R & D Systems). All experiments utilizing murine macrophages were carried out with institutional approval.
Subjects, isolation of PBMC, and genotyping of TLR4 Asp299Gly and Thr399Ile polymorphisms
Seventy-three healthy male and female volunteers, between 20 and 60 years of age, were recruited from the Baltimore-Washington area and University of Maryland at Baltimore campus (Baltimore, MD), to participate in this study. The purpose of the study was explained to the volunteers and they signed informed consent forms before blood donations. Study protocols were approved by the University of Maryland IRB. PBMC were isolated by density gradient centrifugation and cryopreserved in liquid N2 as previously described (29). DNA was extracted from PBMC according to a standard procedure (Puregene DNA Purification System). DNA samples were genotyped using a fluorogenic 5′-nuclease TaqMan assay in the ABI PRISM 7900HT Sequence Detection System (Applied Biosystems) (30). Thereafter, TaqMan-based results were validated for every DNA sample by sequencing (Biopolymer Core Facility, UMB) after labeling DNA with Big Dye Terminator Cycle Sequencing kit; v.3.1 (Applied Biosystems) as previously described (24).
PBMC cultures
PBMC prepared from 8 TLR4 polymorphic and 22 non-polymorphic volunteers were seeded at 2 × 106 cells into 24 well Costar tissue culture plates. Cells were allowed to adhere for 5 hrs at 37°C in 5% CO2. Non-adherent cells were removed by thorough washing with Hanks’ buffer. Adherent cells (predominantly monocytes) were stimulated for 18 hr with media alone, LPS or lipid A. Culture supernatants were harvested and stored at −80°C until analyzed for cytokines by BD™ Cytometric Bead Array (CBA BD Biosciences, San Diego, CA).
Cytokine bead assay
The Cytometric Bead Array is a multiplex, particle-based immunoassay that uses the sensitivity of amplified fluorescence detection by flow cytometry to measure soluble analytes. Four soluble analytes (IL-1β, IL-6, IL-10, and TNF-α) were measured using a Beckman Coulter Epics Elite ESP (Beckman Coulter, Hialeah, FL) flow cytometer and simple linear regression to determine absolute concentrations using the BD CBA Excel software. The limit of detection for the above mentioned cytokines was 10 pg/ml.
Statistical analyses
Distribution of TLR4 genotypes and alleles were determined by simple gene counting. Quantitative data of NF-κB activation in transfected cells are presented as mean ± standard error and were analyzed using a one-way ANOVA with repeated measures, followed by post-hoc comparisons using Tukey’s multiple paired comparison test (GraphPad PRISM 4 program for Windows). Non-parametric analysis of cytokine responses of human mononuclear cell cultures was performed using the Wilcoxon-sign ranked sum test for comparison of cytokines produced in response to E. coli vs Shigella flexneri 2a LPS in individual subjects (regardless of their TLR4 genotype) whereas the Mann-Whitney ranked sum test was employed to determine whether there were significant differences in cytokines elicited between TLR4 polymorphic vs. non-polymorphic PBMC to either E. coli or S. flexneri 2a LPS preparations (SigmaStat for Windows version 3.1, Systat Software).
Results
Chemical analysis of lipid A derived from E. coli K235 and S. flexneri 2a LPS
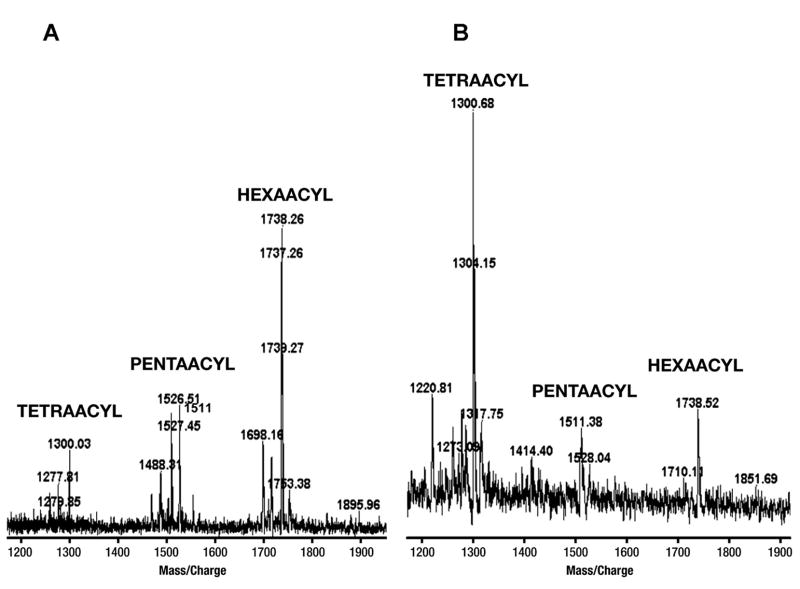
The E. coli K235 and S. flexneri 2a LPS preparations used for this study were subjected to lipid A analysis by thin layer chromatography (TLC) and mass spectroscopy (MS). Both TLC (data not shown) and MS revealed that while the lipid A from E. coli was predominantly hexaacylated (Fig. 1A), with lesser amounts of pentaacylated and tetraacylated species, the lipid A from S. flexneri 2a was a mixture comprised predominantly of tetraacylated species, with some pentaacylated lipid A and a small amount of hexaacylated lipid A (Fig. 1B). Although these results are consistent with those previously reported for the E. coli lipid A structure (9), they differ from a previous report that determined the lipid A structure of another S. flexneri (strain not designated) to be predominantly pentaacylated (9).
FIGURE 1.
Lipid A analysis of E. coli and S. flexneri 2a LPS preparations used in this study. Monophosphoryl lipid A prepared from the LPS of E. coli K235 and S. flexneri 2a was analyzed by mass spectrometry (A and B, respectively) as described in Materials and Methods. Analysis of the two LPS preparations by MS revealed that, while the lipid A from E. coli LPS was predominantly hexaacylated (A), the lipid A from S. flexneri 2a LPS was predominantly tetraacylated (where the myristoxymyristate was missing from the 3′ position of the glucosamine dissacharide), pentaacylated (the hydroxymyristate was missing from the 3-position) and hexaacylated forms were also present (B). This analysis was carried out on two separate preparations of S. flexneri 2a with similar results.
S. flexneri 2a LPS is less potent than E. coli LPS in TLR4-mediated activation of NF-κ B-luciferase reporter activity
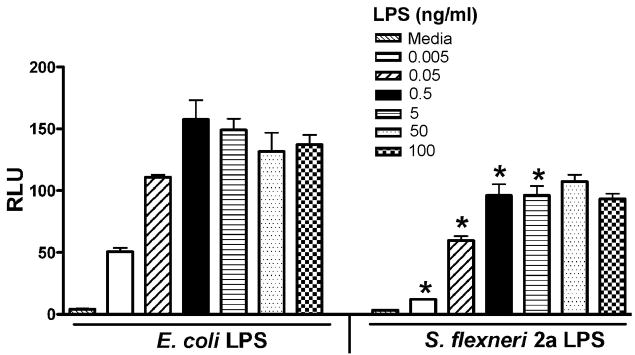
To compare the relative capacity of LPS derived from E. coli versus S. flexneri 2a to activate human TLR4, a transient transfection system in which HEK293T cells were transfected with human TLR4/MD-2/CD14 vectors, along with NF-κB luciferase and β-galactosidase reporter constructs, was initially utilized. Transfections were carried out using concentrations of expression constructs previously optimized for the measurement of TLR4-mediated NF-κB signaling in response to the identical preparation of E. coli K235 LPS used in this study (3). Since the HEK293T cells do not have endogenous TLR4, they respond to LPS only when transfected with the TLR4 complex (3). In multiple, side by side experiments, both E. coli LPS and S. flexneri 2a LPS induced dose-dependent NF-κB-driven luciferase activity; however, the S. flexneri 2a LPS was consistently less stimulatory when compared to the E. coli LPS at the same LPS concentrations (p < 0.05) (Fig. 2). These differences confirm and extend previous observations that predominantly hexaacylated, lipid A-containing LPS (e.g., E. coli) preparations are more stimulatory than LPS preparations that contain predominantly hypoacylated (e.g., pentaacylated and tetraacylated) lipid A species as contained in the S. flexneri 2a LPS (5, 6, 8).
FIGURE 2.
S. flexneri 2a LPS shows less activity than E. coli K235 LPS in the TLR4-mediated activation of NF-κB. Two × 105 cells/well HEK293T cells were co-transfected with untagged wild-type TLR4 (300 ng/well), huCD14 (30 ng/well) and huMD-2 (3 ng/well), along with the reporter constructs to obtain optimal E. coli LPS-induced activation. After overnight recovery, transfected cells were treated with the indicated concentrations of either E. coli K235 LPS or S. flexneri 2a LPS for 5 hr. Cells were washed twice with cold 1× PBS, lysed in 1× lysis buffer, and centrifuged to remove cell debris. Luciferase and β-galactosidase activities were measured in cell lysates as described in the Methods. A representative experiment is shown (n = 6). * p < 0.05 vs. E. coli LPS at the same concentration.
TLR4 signaling induced by hexaacylated lipid A, but not pentaacylated lipid A, requires higher expression levels of human MD-2 in HEK293T transfectants
To explore in greater detail the effects of acylation differences in lipid A on TLR4 signaling, we next compared the response of HEK293T cells transfected with the TLR4 signaling complex to stimulation by synthetic lipid A preparations that were either hexaacylated or pentaacylated. It has been previously reported that cylindrically shaped tetraacylated and pentaacylated lipid A species are less biologically active than conical/concave shaped hexaacylated lipid A (5–7). Therefore, we reasoned that the pentaacylated and hexaacylated species of lipid A that are present in the S. flexneri 2a LPS, and not the tetraacylated species, are primarily responsible for its observed biological activity. Therefore, in the next series of experiments, we compared pentaacylated and hexaacylated lipid A preparations to study the MD-2 requirement for biological activity. Initially, we stimulated HEK293T cells that had been transfected with expression vectors that encode WT human TLR4 (300 ng/well), huCD14 (30 ng/well), and huMD-2 (3 ng/well), along with the NF-κB reporter construct, each of which were titrated in our previous study to elicit optimal E. coli LPS-induced activation (3). However, under these identical transfection conditions where both E. coli and S. flexneri 2a LPS elicited robust NF-κB-driven luciferase activity (Fig. 2), neither synthetic hexaacylated nor pentaacylated lipid A preparations induced any measurable activity, even at a final lipid A concentration of 8 μg/ml (Fig. 3A). Based on our previous observations in murine macrophages that significantly higher concentrations of E. coli lipid A were required to drive cytokine secretion than E. coli LPS (31), and the observations of others that MD-2 greatly augments LPS signaling by TLR4 (32), we hypothesized that, perhaps, higher levels of human MD-2, in addition to higher concentrations of lipid A, might be necessary for lipid A to induce activity in this system. This was indeed the case for the hexaacylated lipid A. When the input human MD-2 expression vector concentration was increased 100-fold to 300 ng/well (Fig. 3B), we observed robust activation for the hexaacylated lipid A. In contrast, synthetic pentaacylated lipid A still failed to elicit any NF-κB activation, even when the MD-2 vector input concentration was increased to 600 ng/well (data not shown). This result clearly underscores the importance of structural modifications, e.g., the acylation state of the lipid A component of LPS in activating the TLR4-mediated NF-κB-driven gene activation, and the important role of MD-2 in this process.
FIGURE 3.
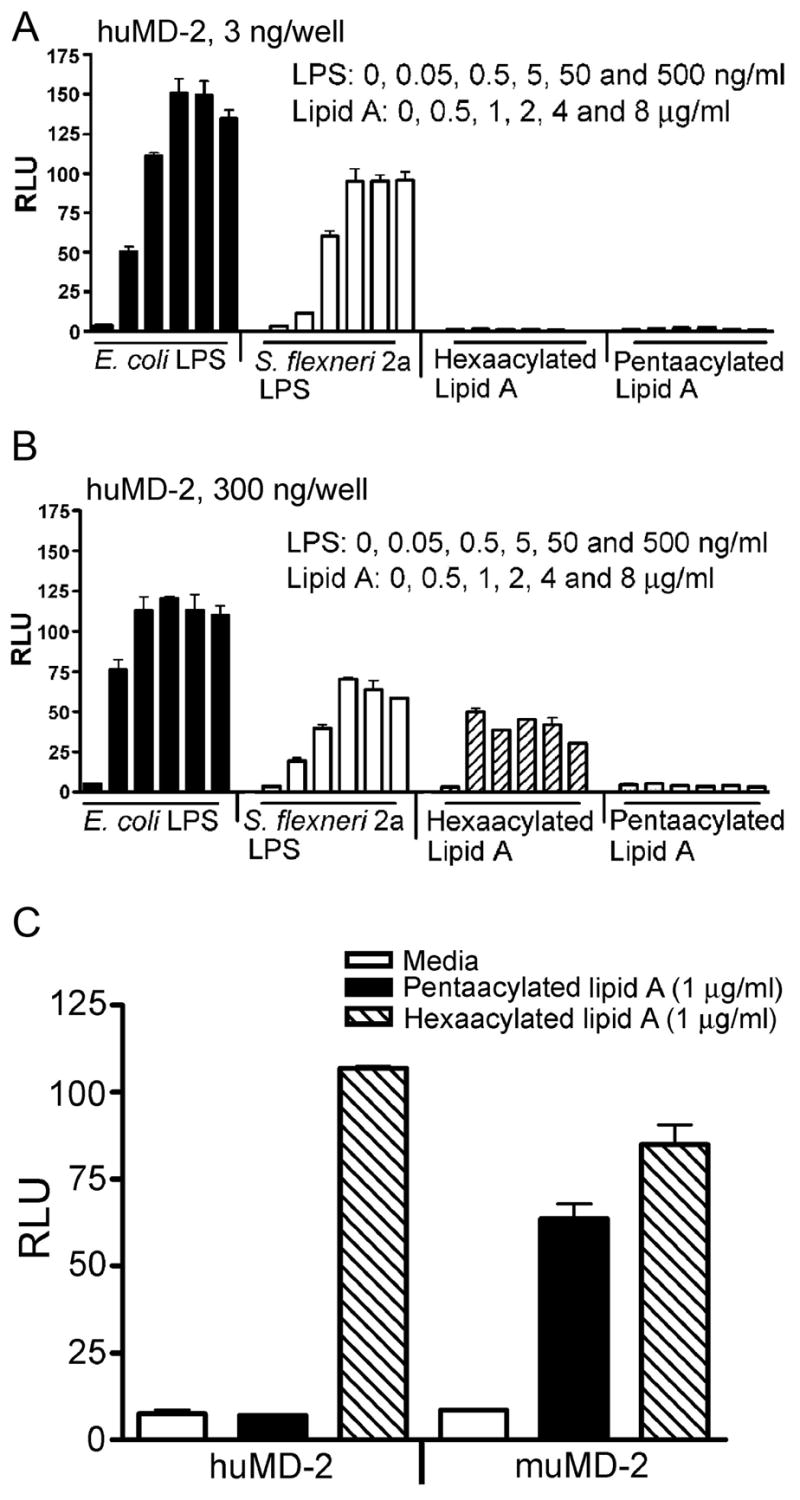
Signaling induced by hexaacylated lipid A through TLR4 requires higher expression levels of the co-receptor, MD-2. Two × 105 cells/well HEK293T cells were co-transfected with untagged wild-type TLR4 (300 ng/well), huCD14 (30 ng/well) and the indicated amounts of (A). huMD-2 (3 ng/well), (B). huMD-2 (300 ng/well) along with the reporter constructs. After overnight recovery, transfected cells were treated with the indicated concentrations of E. coli K235 LPS or S. flexneri 2a LPS or hexaacylataed or pentaacylated lipid A for 5 hr. Luciferase and β-galactosidase activities were measured in cell lysates as described in the Methods. A representative experiment is shown (n = 5). (C). Murine MD-2 rescues pentaacylated signaling by human TLR4. Experiments were set up as described in (A) and (B) using either human or murine MD-2 (100 ng/well) in conjunction with human TLR4 and CD14 constructs. A representative experiment is shown (n = 3).
It has been previously reported that neither tetraacylated (deacylated) LPS (33) nor lipid IVA (8, 34) are stimulatory in human cells, and in fact, act as antagonists of LPS-mediated signaling. In contrast, in murine cells, tetraacylated LPS/lipid A exhibit agonist activity. To determine if the decreased activity of the pentaacylated lipid A that we observed in the HEK293T transfectants was also species-specific, we measured cytokine release in cultures of murine peritoneal macrophages after stimulation with these same hexaacylated or pentaacylated synthetic lipid A preparations. E. coli LPS and S. flexneri 2a LPS were also included as controls. As shown in Table I, the pentaacylated lipid A was active in murine macrophages and induced secretion of IL-1β, TNF-α, IFN-γ, and RANTES to levels similar to those induced by E. coli LPS. Next, we carried out dose response experiments to determine if a difference between the activity of hexaacylated lipid A and pentaacylated lipid A with respect to their ability to induce cytokine secretion in murine macrophages might be detected at a lower concentration than that used in Table I. Table II shows that a minimal concentration of 10 ng/ml is required for either lipid A preparation and that there was no appreciable difference in levels of IL-1β released between the two lipid A preparations. These results, taken together, suggest that murine macrophages respond to synthetic pentaacylated lipid A, in contrast to human HEK293T TLR4/MD-2/CD14 transfectants that do not.
Table I.
Cytokine Release by Murine Macrophage Stimulated with E. coli or S. flexneri 2a LPS or synthetic lipid A speciesa
| Treatment
|
|||||
|---|---|---|---|---|---|
| Cytokine | Media | Hexaacylated Lipid A | Pentaacylated Lipid A | E. coli LPS | S. flexneri 2a LPS |
| IL-1β | ≤7.8b | 66 ± 27 | 43 ± 9 | 27 ± 1 | 45 ± 28 |
| TNF-α | ≤23.8 | 20,747 ± 5,586 | 17,855 ± 389 | 15,405 ± 1,945 | 19,022 ± 2,298 |
| IFN-γ | ≤9.4 | 41 ± 12 | 30 ± 0 | 38 ± 13 | 55 ± 25 |
| RANTES | 473.8 ± 5 | 44,309 ± 5,615 | 39,529.4 ± 562 | 42,000 ± 5,095 | 42,369 ± 2,745 |
Murine peritoneal macrophages were plated at a density of 1 × 106 cells/ml and were allowed to adhere overnight. They were treated for 24 hrs with media, 100 ng/ml of LPS, or 1 μg/ml of lipid A and supernatants harvested for cytokine production detected by ELISA as described in Materials and Methods.
pg/ml
Table II.
Secretion of IL-1β by murine macrophages stimulated with various concentrations of hexaacylated and pentaacylated lipid Aa
| Concentration (ng/ml)
|
||||||
|---|---|---|---|---|---|---|
| 0 | 0.1 | 1 | 10 | 100 | 1000 | |
| Hexaacylated Lipid A | ≤7.8b | ≤7.8 | ≤7.8 | 18.3 | 33.5 | 63.0 |
| Pentaacylated Lipid A | ≤7.8 | ≤7.8 | ≤7.8 | 23.0 | 36.1 | 80.4 |
Murine peritoneal macrophages were plated at a density of 1×106 cells/ml in a 24 well plate; they were allowed to adhere overnight. Macrophage cultures were then treated for 24 hrs with media or lipid A and supernatants assayed for IL-1β by ELISA.
pg/ml
Murine MD-2 has previously been shown to impart species specificity to another TLR4 agonist, Taxol (35), and to rescue responsiveness to lipid IVA (8, 34). To explore the possibility that the species specificity of pentaacylated lipid A activation in HEK293T cells was due to the species of MD-2, HEK293T cells were transfected with mouse or human MD-2 expression constructs, along with human TLR4, human CD14, and reporter constructs, and then treated with medium only, LPS, or lipid A for 6 hr. While LPS preparations and hexaacylated lipid A showed TLR4 activation regardless of the MD-2 species, pentaacylated lipid A showed TLR4 activation only in the presence of mouse MD-2 (Fig. 3C).
S. flexneri 2a LPS induces diminished TLR4 signaling in HEK293T cells expressing Asp299Gly and Thr399Ile polymorphic TLR4 variants
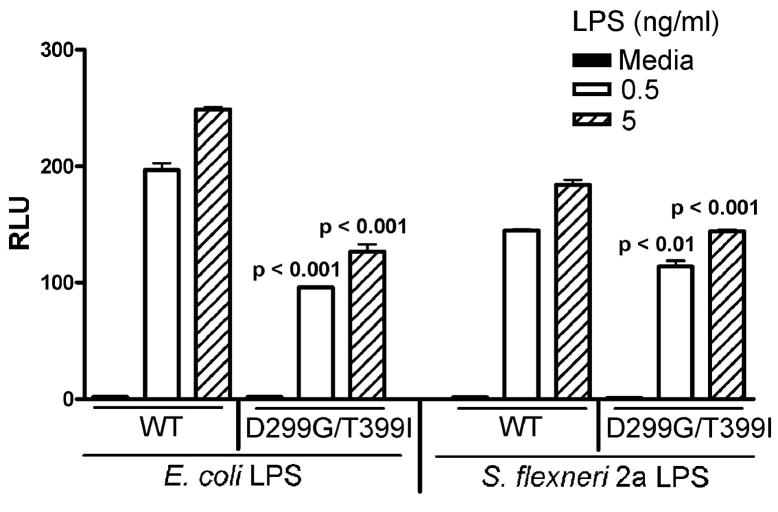
We and others have previously observed that E. coli LPS elicits significantly lower responses in transfectants that express Asp299Gly and/or Thr399Ile polymorphisms, and that this is most striking in cells that express both polymorphisms (i.e., the TLR4 molecules express both SNPs; (3, 10)). Therefore, we next sought to determine if the cosegregating Asp299Gly and Thr399Ile polymorphic variant of TLR4 would exhibit a further diminution of responsiveness to the S. flexneri 2a LPS preparations when compared to WT TLR4 as observed for E. coli LPS (3). Indeed, HEK293T cells transfected with the vector that expresses both SNPs were consistently and significantly less responsive to both S. flexneri 2a as well as E. coli LPS when compared to the WT TLR4 transfectants (p <0.01; Fig. 4).
FIGURE 4.
HEK293T cells expressing Asp299Gly and Thr399Ile polymorphic TLR4 variant showed diminished response to S. flexneri 2a LPS. Two × 105 cells/well HEK293T cells were co-transfected with untagged wild-type TLR4 (300 ng/well) or untagged Asp299Gly and Thr399Ile polymorphic TLR4 expression construct (300 ng/well), huCD14 (30 ng/well), and huMD-2 (3 ng/well) along with the reporter constructs. After overnight recovery, transfected cells were treated with the indicated concentrations of E. coli K235 LPS or S. flexneri 2a LPS for 5 hr and cell lysates were made. Luciferase and β-galactosidase activities were measured in cell lysates as described in the Methods. A representative experiment is shown (n = 5).
Distribution of TLR4 polymorphisms in a normal healthy population
PBMC were purified from blood derived from 73 healthy volunteers recruited from the Greater Baltimore area. Genotyping revealed that only 8 individuals were heterozygous for one or both of the two TLR4 polymorphisms (Table III). Seven individuals were heterozygous for the Asp299Gly variant (carrier frequency: 9.6%; minor allele frequency: 4.8%), whereas 4 were heterozygous for the Thr399Ile polymorphism (carrier frequency: 5.5%; minor allele frequency 2.7%). Three individuals were doubly heterozygous for both Asp299Gly and Thr399Ile polymorphisms, one was heterozygous for only Thr399Ile, and four were heterozygous for Asp299Gly only. None were homozygous for the minor allele. These data are very consistent with many previous reports of allelic frequency in healthy populations (12, 30, 36–38).
Table III.
Summary of genotype analysisa
| Asp299Gly | Volunteers | Thr399Ile | Volunteers |
|---|---|---|---|
| Total Subjects | 73 | Total Subjects | 73 |
| AA (Asp/Asp) | 66 | CC (Thr/Thr) | 69 |
| AG (Asp/Gly) | 7 | CT (Thr/Ile) | 4 |
| GG (Gly/Gly) | 0 | TT (Ile/Ile) | 0 |
|
| |||
| Carrier Frequency (95% CI): | 0.096 (0.039, 0.1876) | Carrier Frequency (95% CI): | 0.055 (0.015, 0.134) |
| Allele (G) Frequency (95% CI): | 0.048 (0.0195, 0.0963) | Allele (I) Frequency (95% CI): | 0.027 (0.008, 0.069) |
Three individuals were doubly heterozygous for Asp299Gly and Thr399Ile polymorphisms, 1 heterozygous for Thr399Ile only, and 4 heterozygous for Asp299Gly only. In total, there were 8 individuals who were heterozygous for one, the other, or both of the two polymorphisms.
Induction of inflammatory cytokines by E. coli versus S. flexneri 2a LPS preparations
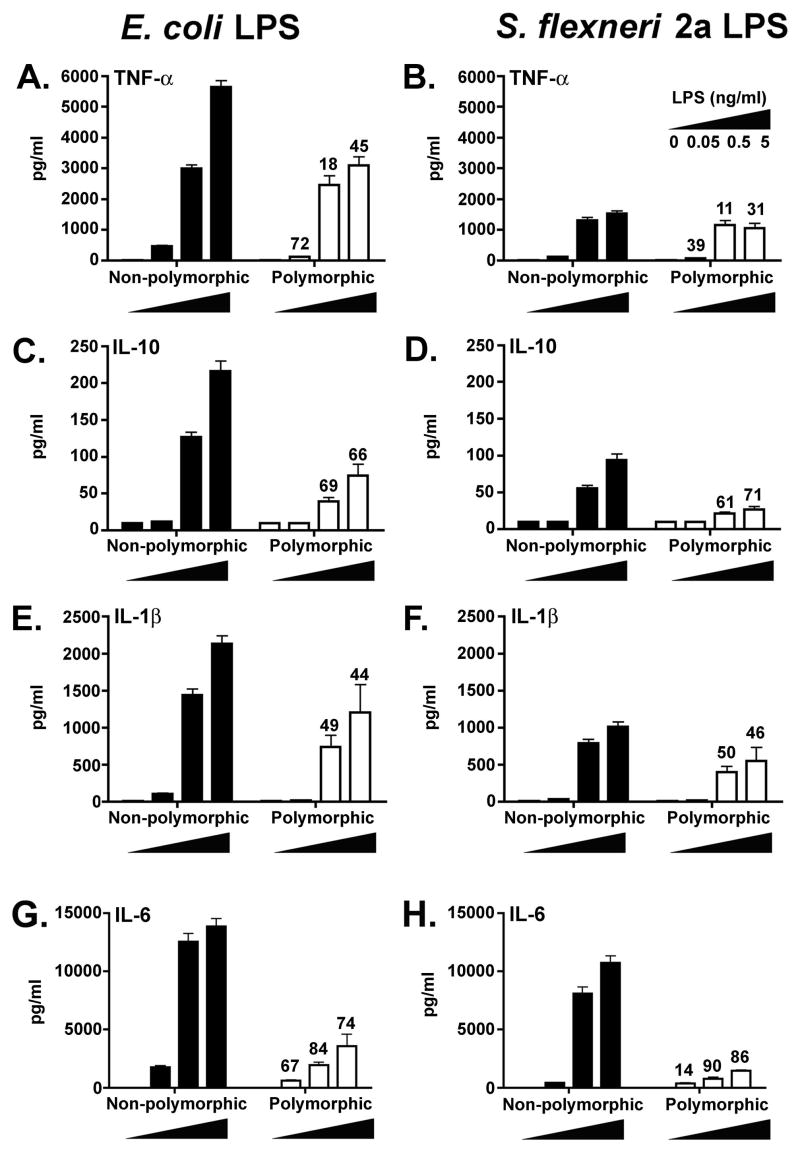
Thus far, all of the comparisons of LPS responsiveness were carried out in the HEK293T transient transfection system. To confirm our findings under more physiological conditions, adherent monocytic cell cultures derived from PBMC from 22 WT or 8 polymorphic donors were stimulated with different concentrations of E. coli LPS or S. flexneri 2a LPS. Analysis of cytokine production revealed that at the same concentrations, E. coli LPS (Fig. 5, panels A, C, E, G) induced higher levels of inflammatory cytokines compared to those elicited by S. flexneri LPS (Fig. 5, panels B, D, F, H). This trend was observed whether the supernatants were derived from WT (non-polymorphic) or polymorphic individuals. For example, at the 5 ng/ml LPS concentration, TNF-α production in response to S. flexneri LPS was 72.8% and 65.7% lower than the levels induced by E. coli LPS in non-polymorphic and polymorphic individuals, respectively (Fig. 5, panels A and B; percentages not shown for clarity). Similar findings were observed for IL-1β, IL-6, and IL-10 (Fig. 5C–H). Although the suppression was readily observed in both WT and polymorphic individuals, highly significant differences (p <0.01) were recorded only in non-polymorphic individuals (n=22). This is likely the result of the relatively low number of polymorphic individuals evaluated (n=8).
FIGURE 5.
Cytokine production by PBMC derived from non-polymorphic and TLR4 polymorphic volunteers stimulated with E. coli or S. flexneri 2a LPS. Mononuclear cells from 22 wild-type and 8 TLR4 polymorphic individuals were stimulated with 0.05, 0.5 or 5 ng/ml of E. coli or S. flexneri 2a LPS as indicated and supernatants collected for cytokine determinations by cytometric bead array assays as described in Materials and Methods. Results are shown as mean pg/ml ± SE for all selected volunteers in each group. Numbers above bars represent the % suppression of cytokine production by cells from polymorphic subjects compared to the corresponding levels observed in supernatants from wild-type (non-polymorphic) individuals, matched by LPS type and concentration.
In addition to the previous findings, polymorphic individuals showed decreased cytokine production in response to both LPS preparations when compared to those measured in non-polymorphic individuals for the same LPS preparation. As shown in Fig. 5, PBMC from polymorphic volunteers secreted lower mean cytokine levels than non-polymorphic subjects, with up to 90% suppression of responsiveness depending on the cytokine and concentration evaluated. These differences were observed for all cytokines evaluated, including the immunosuppressive cytokine, IL-10. Of note, the decrease in TNF-α secreted by PBMC from polymorphic individuals was not as pronounced as observed for the other cytokines (Fig. 5, panel B). This observation may also be attributable to the relatively low number of polymorphic individuals examined and/or the relatively low level of TNF-α that is induced when compared to other cytokines. Overall, these results are consistent with and support those obtained in HEK293T cells transfected with WT or polymorphic TLR4 variants.
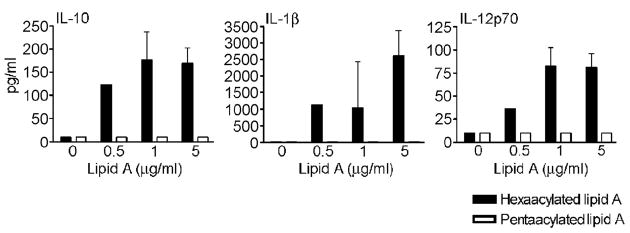
In general, the mean response of PBMC from all volunteers was lower in cells stimulated with S. flexneri 2a LPS, and this was most pronounced at 0.5 and 5 ng/ml LPS (a ~20–80% decrease in cytokine production). These findings were confirmed and extended in two non-polymorphic individuals whose PBMC were stimulated with synthetic hexaacylated vs. pentaacylated lipid A preparations. As shown in Figure 6, while high levels of IL-10, IL-1β, and IL-12 p70 production were observed in response to hexaacylated lipid A, the response to pentaacylated lipid A remained essentially at background levels. Similar results were observed for TNF-α and IL-6 production (data not shown). Taken together, the above observations using cells from non-polymorphic and polymorphic volunteers provide strong evidence to support the contention that both the acylation state of the LPS and the expression of variant TLR4 molecules may combine to alter significantly cytokine production following exposure to LPS.
FIGURE 6.
Cytokine production by PBMC derived from non-polymorphic volunteers stimulated with synthetic hexaacylated vs. pentaacylated lipid A. Cells from two wild-type individuals were stimulated with 0.5, 1 or 5 ng/ml of synthetic hexaacylated (closed bars) or pentaacylated (open bars) lipid A and supernatants collected for cytokine determinations as described in Materials and Methods. Results are shown as mean pg/ml ± SD for both volunteers.
Discussion
The initial interaction of a host with vaccines and/or their adjuvants may significantly impact the overall immunogenicity and efficacy of a vaccine. The innate immune response induced by Gram negative bacterial LPS, a TLR4 agonist, or its derivatives, has been shown to be responsible and necessary for initiation of adaptive immune responses (39, 40). Therefore, when LPS or its derivatives are used as adjuvants, activation of TLR4 may, in turn, enable the acquired immune response to the immunogen through induction of cytokines and up-regulation of key co-stimulatory molecules on antigen presenting cells. Bacterial LPS is a very complex molecule, and its composition varies widely among different species of Gram negative bacteria. Structural variations, such as the extent of acylation, the length of the acyl chains, and degree of phosphorylation within the lipid A moiety, and others have been shown to affect signaling (5, 6, 8), and likely alter the manner in which these molecules interact with the TLR4 receptor complex to initiate signaling (4).
Consistent with our findings, Hajjar et al. reported that a clinical isolate of Ps. aeruginosa from a cystic fibrosis patient, that possesses a predominantly hexaacylated lipid A, was a much more potent stimulus in human THP-1 cells and in HEK293 cells transfected with human TLR4/MD-2 than a laboratory strain that possesses a pentaacylated lipid A; yet, both induced equivalent activity in murine cells. However, in contrast to our findings, Hajjar et al. found that the differential response they observed was independent of the species of MD-2 and was attributable to an 82 amino acid region in the ectodomain of TLR4 that differs between human and mouse. This suggests the possibility that some lipid A species may be preferentially recognized by human TLR4 (i.e., Ps. aeruginosa hexaacylated LPS) while other hexaacylated species may depend more on their interaction with MD-2 (e.g., E. coli). Other structural variations may contribute to these apparently conflicting observations: the E. coli lipid A, although hexaacylated, has a different distribution of amide- and ester-linked fatty acids, as well as different length acyl groups, than the hexaacylated Ps. aeruginosa lipid A used in the study by Hajjar et al. (8). Interestingly, Kim et al. (41) recently reported the crystal structure of murine TLR4-MD-2 complexed with Eritoran (E5564), a synthetic tetraacylated antagonist of LPS and active lipid A (42). MD-2 was found to bind to the concave surface of the N-terminal and central domains of murine TLR4 and the four acyl chains of Eritoran occupied nearly 90% of the solvent-accessible volume of a hydrophobic internal pocket in murine MD-2. How hexaacylated and pentaacylated LPS and lipid A species interact with this hydrophobic pocket in situ, and whether a similar pocket is found in human MD-2, remain to be determined. In addition, Kim et al. used gel filtration chromatography to show that in contrast to tetraacylated Eritoran, LPS led to formation of a TLR4/MD-2 heterotetramer. It is tempting to speculate that hexaacylated vs. pentaacylated lipid A species differ in their efficiencies of aggregation of the TLR4/MD-2 complex, with pentaacylated lipid A behaving more similarly to Eritorin (tetraacylated LPS antagonist).
Huber et al. (43) recently reported that rough forms of LPS derived from S. minnesota or E. coli were much more potent than smooth forms of LPS, suggesting that in addition to other lipid A modifications, the core sugars and/or repeating O-antigen may constrain the TLR4 response to smooth LPS. In addition, they reported that neither lipid A nor rough LPS require CD14 or LBP for signaling, in contrast to smooth LPS. These findings differ from those of Henricson et al. (31) who showed that that lipid A or deep rough mutant LPS preparations are far less stimulatory than the smooth LPS from which they were derived (31). The data presented herein support the findings of Henricson et al. in that the synthetic lipid As were far less stimulatory than smooth LPS species. The reason for these conflicting observations is not obvious, but may be related to variations in the solubility of the LPS preparations and/or the experimental conditions used in these studies (e.g., the absence or presence of serum in the cultures).
In the experiments presented herein, the lipid A was much less potent than intact LPS, and this was most obvious in the HEK293T transfectants where neither the synthetic hexaacylated nor the pentaacylated lipid A preparations activated TLR4 transfectants, even at concentrations as high as 8 μg/ml (Fig. 3A). It has been shown that MD-2 is an essential co-receptor in LPS-induced TLR4 signaling (32). Initially, LBP facilitates the transfer of LPS/lipid A to CD14. In turn, the lipid A is transferred to MD-2 and this complex interacts with TLR4 to initiate activation (44, 45). Increasing the expression of human MD-2 compensated for the failure of hexaacylated lipid A, but not the pentaacylated lipid A, to signal, while murine MD-2 did not differentiate between the two lipid A species and facilitated signaling by both (Figs. 3B and 3C). Our results support earlier predictions that a conically shaped hexaacylated lipid A is biologically active, whereas a cylindrically shaped pentaacylated/tetraacylated lipid A is not active in human cells (6) and perhaps, this reflects the differences in the abilities of these two lipid A species to interact with human MD-2.
In contrast to our findings using human TLR4-expressing HEK293T transfectants or human PMBC cultures (Fig. 6), mouse peritoneal macrophages, when treated with hexaacylated or pentaacylated lipid A, secreted similar levels of proinflammatory cytokines (Table I), in a dose-dependent manner for IL-1β secretion (Table II). These results clearly support the hypothesis that the acylation state of lipid A may contribute to species specificity. This was originally observed with tetraacylated LPS or lipid A species in which murine macrophages were responsive, while human cells not only failed to respond, but the response to active LPS was antagonized by the tetraacylated species (33). More recent studies demonstrated that murine MD-2 rescues the response to tetraacylated lipid IVA in a human system (8, 34). Previous studies (35, 46) have shown that activation of TLR4 by Taxol is also species-specific, and can be restored by mouse, but not human, MD-2. Our findings support the hypothesis that murine MD-2 has a greater propensity for interaction with synthetic pentaacylated lipid A than human MD-2 and, perhaps, accounts for the observed differences in human vs. murine responses. It is tempting to speculate that the striking differences in the inflammatory responses observed between mouse and human cells might contribute, at least in part, to the marked host-restriction of Shigella infections to humans and non-human primates. The intensity and characteristics of the inflammatory responses observed in rodent macrophages might allow them to control infection at an early stage, while the relatively meager inflammatory responses of human cells, secondary to the failure of the human TLR4 signaling complex to respond robustly to hypoacylated S. flexneri 2a LPS lipid A, may be insufficient to control the development of disease.
D’Hauteville et al. (16) previously reported that WT S. flexneri serotype 5a possessed a predominantly hexaacylated lipid A (93%), in contrast to our S. flexneri 2a (Figure 1B). Interestingly, deletion of S. flexneri 5a msbB1 or msbB2 genes resulted in decreased levels of hexaacylated lipid A (to 59% and 73%, respectively) and a concomitant increase in pentaacylated lipid A (to 34% and 19%, respectively). In the doubly mutated strain, there was no detectable hexaacylated lipid A, with pentaacylated (86%) and tetraacylated (14%) lipid A species. The extent of histologic damage induced by infection with S. flexneri 5a WT and mutant strains in a rabbit intestinal loop model roughly paralleled the proportion of hexaacylated lipid A recovered from each strain. Although these data have been interpreted to suggest that the level of hexaacylated lipid A may influence the extent to which the intestine will be damaged during infection, it is also possible that a preferential enrichment of inactive or antagonistic tetraacylated lipid A in the doubly mutated strain might also contribute to its attenuation in vivo. At addition, the relative contribution of TNF-α and other inflammatory cytokines to the pathogenesis of S. flexneri in the human gut mucosal microenvironment is not well understood; however, the fact that marked differences have been reported in the degree of lipid A acylation among Shigella flexneri serotypes may help, in part, explain the differences reported in the literature in pathogenicity studies in which different Shigella serotypes have been used.
Arbour et al. (10) demonstrated that inheritance of two SNPs that encode amino acid substitutions in the ectodomain of TLR4 (Asp299Gly and Thr399Ile) were associated with LPS hyporesponsiveness. Recently, we demonstrated that expression of TLR4 constructs that carry the Asp299Gly and/or Thr399Ile extracellular polymorphisms in HEK293T cells result in diminished host responses to three structurally unrelated TLR4-specific agonists: Gram negative LPS, the fusion (F) protein of RSV, and Chlamydia heat shock protein 60 (3). In this study, we have used both transiently transfected HEK293T cells and PBMC to show that these common, naturally occurring TLR4 polymorphisms that affect ~6–10% of the population influence the induction of cytokines in response to both E. coli LPS and S. flexneri 2a LPS preparations. Both LPS preparations induced a significantly lower response in the doubly polymorphic TLR4 variant (Fig. 4). Similar results were obtained with PBMC, where polymorphic individuals showed decreased cytokine production (e.g., TNF-α, IL-1β, IL-6 and IL-10) in response to both LPS preparations when compared to those derived from non-polymorphic individuals for the same LPS preparation (Fig. 5). Therefore, since polymorphic individuals cannot mount an optimal innate immune response to LPS, it is likely that their ability to respond to vaccines in which the LPS is intrinsic to the vaccine itself (e.g., a Shigella vaccine) or a vaccine to which an LPS-based adjuvant has been added, may be compromised.
Collectively, our data provide direct evidence for the prediction that in humans S. flexneri 2a LPS would elicit a weaker TLR4-mediated response than E. coli LPS due to differences in the acylation status of their lipid A moieties (Fig. 1B versus 1A), and that both LPS species elicit a diminished response in cells that express polymorphic TLR4 variants. In both TLR4 transfectants and in monocytic cultures derived from healthy volunteers, we observed similar trends: pentaacylated/tetracylated LPS was consistently less stimulatory than hexaacylated LPS (Fig. 2) and this was more striking when synthetic hexaacylated or pentacylated lipid A molecules were used as stimuli. Moreover, in transfectants or PBMC expressing polymorphic TLR4 variants, the response to either LPS or lipid A was further diminished. In sum, we have provided direct evidence that at least two independent parameters, i.e., the acylation state of the LPS itself and the responsiveness of the vaccinee to the LPS contained within the vaccine, can markedly affect immune responses to LPS and, likely, other vaccine antigens. These are important considerations that could lead to the development of more effective vaccines and adjuvants.
Acknowledgments
The authors wish to thank Ashley S. Beasley and Robert J. Cotter, Johns Hopkins University School of Medicine, for carrying out MS analysis of lipid A preparations.
Footnotes
This work was funded, in part, with federal funds from National Institute of Allergy and Infectious Diseases, NIH, DHHS, under Contract No. N01-AI-30028 (MBS) and grants AI057927 (to MBS), AI18797 (SNV), and GM50870 (NQ).
Abbreviations: SNPs, single nucleotide polymorphisms; WT, Wild-type; hu, human; mu, murine; HEK, human embryonic kidney; TLR4, Toll-like receptor 4.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Rietschel ET, Brade H, Holst O, Brade L, Muller-Loennies S, Mamat U, Zahringer U, Beckmann F, Seydel U, Brandenburg K, Ulmer AJ, Mattern T, Heine H, Schletter J, Loppnow H, Schonbeck U, Flad HD, Hauschildt S, Schade UF, Di Padova F, Kusumoto S, Schumann RR. Bacterial endotoxin: Chemical constitution, biological recognition, host response, and immunological detoxification. Curr Top Microbiol Immunol. 1996;216:39–81. doi: 10.1007/978-3-642-80186-0_3. [DOI] [PubMed] [Google Scholar]
- 2.Vogel SN, Awomoyi AA, Rallabhandi P, Medvedev AE. Mutations in TLR4 signaling that lead to increased susceptibility to infection in humans: an overview. J Endotoxin Res. 2005;11:333–339. doi: 10.1179/096805105X58724. [DOI] [PubMed] [Google Scholar]
- 3.Rallabhandi P, Bell J, Boukhvalova MS, Medvedev A, Lorenz E, Arditi M, Hemming VG, Blanco JC, Segal DM, Vogel SN. Analysis of TLR4 polymorphic variants: new insights into TLR4/MD-2/CD14 stoichiometry, structure, and signaling. J Immunol. 2006;177:322–332. doi: 10.4049/jimmunol.177.1.322. [DOI] [PubMed] [Google Scholar]
- 4.Rietschel ET, Brade H, Brade L, Brandenburg K, Schade U, Seydel U, Zahringer U, Galanos C, Luderitz O, Westphal O, et al. Lipid A, the endotoxic center of bacterial lipopolysaccharides: relation of chemical structure to biological activity. Prog Clin Biol Res. 1987;231:25–53. [PubMed] [Google Scholar]
- 5.Loppnow H, Brade H, Durrbaum I, Dinarello CA, Kusumoto S, Rietschel ET, Flad HD. IL-1 induction-capacity of defined lipopolysaccharide partial structures. J Immunol. 1989;142:3229–3238. [PubMed] [Google Scholar]
- 6.Seydel U, Oikawa M, Fukase K, Kusumoto S, Brandenburg K. Intrinsic conformation of lipid A is responsible for agonistic and antagonistic activity. Eur J Biochem. 2000;267:3032–3039. doi: 10.1046/j.1432-1033.2000.01326.x. [DOI] [PubMed] [Google Scholar]
- 7.Schromm AB, Brandenburg K, Loppnow H, Moran AP, Koch MH, Rietschel ET, Seydel U. Biological activities of lipopolysaccharides are determined by the shape of their lipid A portion. Eur J Biochem. 2000;267:2008–2013. doi: 10.1046/j.1432-1327.2000.01204.x. [DOI] [PubMed] [Google Scholar]
- 8.Hajjar AM, Ernst RK, Tsai JH, Wilson CB, Miller SI. Human Toll-like receptor 4 recognizes host-specific LPS modifications. Nat Immunol. 2002;3:354–359. doi: 10.1038/ni777. [DOI] [PubMed] [Google Scholar]
- 9.Chan S, Reinhold VN. Detailed structural characterization of lipid A: electrospray ionization coupled with tandem mass spectrometry. Anal Biochem. 1994;218:63–73. doi: 10.1006/abio.1994.1141. [DOI] [PubMed] [Google Scholar]
- 10.Arbour NC, Lorenz E, Schutte BC, Zabner J, Kline JN, Jones M, Frees K, Watt JL, Schwartz DA. TLR4 mutations are associated with endotoxin hyporesponsiveness in humans. Nat Genet. 2000;25:187–191. doi: 10.1038/76048. [DOI] [PubMed] [Google Scholar]
- 11.Kiechl S, Lorenz E, Reindl M, Wiedermann CJ, Oberhollenzer F, Bonora E, Willeit J, Schwartz DA. Toll-like receptor 4 polymorphisms and atherogenesis. N Engl J Med. 2002;347:185–192. doi: 10.1056/NEJMoa012673. [DOI] [PubMed] [Google Scholar]
- 12.Lorenz E, Mira JP, Frees KL, Schwartz DA. Relevance of mutations in the TLR4 receptor in patients with gram-negative septic shock. Arch Intern Med. 2002;162:1028–1032. doi: 10.1001/archinte.162.9.1028. [DOI] [PubMed] [Google Scholar]
- 13.Bell JK, Botos I, Hall PR, Askins J, Shiloach J, Segal DM, Davies DR. The molecular structure of the Toll-like receptor 3 ligand-binding domain. Proc Natl Acad Sci U S A. 2005;102:10976–10980. doi: 10.1073/pnas.0505077102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bell JK, Mullen GE, Leifer CA, Mazzoni A, Davies DR, Segal DM. Leucine-rich repeats and pathogen recognition in Toll-like receptors. Trends Immunol. 2003;24:528–533. doi: 10.1016/s1471-4906(03)00242-4. [DOI] [PubMed] [Google Scholar]
- 15.Levine MM, Kotloff KL, Barry EM, Pasetti MF, Sztein MB. Clinical trials of Shigella vaccines: two steps forward and one step back on a long, hard road. Nat Rev Microbiol. 2007;5:540–553. doi: 10.1038/nrmicro1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D’Hauteville H, Khan S, Maskell DJ, Kussak A, Weintraub A, Mathison J, Ulevitch RJ, Wuscher N, Parsot C, Sansonetti PJ. Two msbB genes encoding maximal acylation of lipid A are required for invasive Shigella flexneri to mediate inflammatory rupture and destruction of the intestinal epithelium. J Immunol. 2002;168:5240–5251. doi: 10.4049/jimmunol.168.10.5240. [DOI] [PubMed] [Google Scholar]
- 17.McIntire FC, Sievert HW, Barlow GH, Finley RA, Lee AY. Chemical, Physical, Biological Properties of a Lipopolysaccharide from Escherichia coli K-235. Biochemistry. 1967;6:2363–2372. doi: 10.1021/bi00860a011. [DOI] [PubMed] [Google Scholar]
- 18.Westphal O, J Jann. Bacterial lipopolysaccharides: extraction with phenol-water and further application of the procedures. Meth Carbohydr Chem. 1965;5:83–91. [Google Scholar]
- 19.Barzu S, Fontaine A, Sansonetti P, Phalipon A. Induction of a local anti-IpaC antibody response in mice by use of a Shigella flexneri 2a vaccine candidate: implications for use of IpaC as a protein carrier. Infect Immun. 1996;64:1190–1196. doi: 10.1128/iai.64.4.1190-1196.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kusumoto S, Kusunose N, Imoto M, Kamikawa T, Shiba T. Chemical synthesis of endotoxin. Adv Exp Med Biol. 1990;256:3–11. doi: 10.1007/978-1-4757-5140-6_1. [DOI] [PubMed] [Google Scholar]
- 21.Imoto M, Yoshimura H, Yamamoto M, Shimamoto T, Kusumoto S, Shiba T. Total synthesis of Escherichia coli lipid A, the endotoxically active principle of cell-surface lipopolysaccharide. Bull Chem Soc Jpn. 1987;60:2197–2204. [Google Scholar]
- 22.Rietschel ET, Brade L, Schade U, Galanos C, Freudenberg M, Luderitz O, Kusumoto S, Shiba T. Endotoxic properties of synthetic pentaacyl lipid A precursor Ib and a structural isomer. Eur J Biochem. 1987;169:27–31. doi: 10.1111/j.1432-1033.1987.tb13576.x. [DOI] [PubMed] [Google Scholar]
- 23.Chow JC, Young DW, Golenbock DT, Christ WJ, Gusovsky F. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J Biol Chem. 1999;274:10689–10692. doi: 10.1074/jbc.274.16.10689. [DOI] [PubMed] [Google Scholar]
- 24.Medvedev AE, Lentschat A, Kuhns DB, Blanco JC, Salkowski C, Zhang S, Arditi M, Gallin JI, Vogel SN. Distinct mutations in IRAK-4 confer hyporesponsiveness to lipopolysaccharide and interleukin-1 in a patient with recurrent bacterial infections. J Exp Med. 2003;198:521–531. doi: 10.1084/jem.20030701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akashi S, Shimazu R, Ogata H, Nagai Y, Takeda K, Kimoto M, Miyake K. Cutting edge: cell surface expression and lipopolysaccharide signaling via the toll-like receptor 4-MD-2 complex on mouse peritoneal macrophages. J Immunol. 2000;164:3471–3475. doi: 10.4049/jimmunol.164.7.3471. [DOI] [PubMed] [Google Scholar]
- 26.Qureshi N, Takayama K, Ribi E. Purification and structural determination of nontoxic lipid A obtained from the lipopolysaccharide of Salmonella typhimurium. J Biol Chem. 1982;257:11808–11815. [PubMed] [Google Scholar]
- 27.Qureshi N, Takayama K, Heller D, Fenselau C. Position of ester groups in the lipid A backbone of lipopolysaccharides obtained from Salmonella typhimurium. J Biol Chem. 1983;258:12947–12951. [PubMed] [Google Scholar]
- 28.Salkowski CA, Vogel SN. Lipopolysaccharide increases glucocorticoid receptor expression in murine macrophages. A possible mechanism for glucocorticoid-mediated suppression of endotoxicity. J Immunol. 1992;149:4041–4047. [PubMed] [Google Scholar]
- 29.Sztein MB, Wasserman SS, Tacket CO, Edelman R, Hone D, Lindberg AA, Levine MM. Cytokine production patterns and lymphoproliferative responses in volunteers orally immunized with attenuated vaccine strains of Salmonella typhi. J Infect Dis. 1994;170:1508–1517. doi: 10.1093/infdis/170.6.1508. [DOI] [PubMed] [Google Scholar]
- 30.Schmitt C, Humeny A, Becker CM, Brune K, Pahl A. Polymorphisms of TLR4: rapid genotyping and reduced response to lipopolysaccharide of TLR4 mutant alleles. Clin Chem. 2002;48:1661–1667. [PubMed] [Google Scholar]
- 31.Henricson BE, Perera PY, Qureshi N, Takayama K, Vogel SN. Rhodopseudomonas sphaeroides lipid A derivatives block in vitro induction of tumor necrosis factor and endotoxin tolerance by smooth lipopolysaccharide and monophosphoryl lipid A. Infect Immun. 1992;60:4285–4290. doi: 10.1128/iai.60.10.4285-4290.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Visintin A, Halmen KA, Latz E, Monks BG, Golenbock DT. Pharmacological inhibition of endotoxin responses is achieved by targeting the TLR4 coreceptor, MD-2. J Immunol. 2005;175:6465–6472. doi: 10.4049/jimmunol.175.10.6465. [DOI] [PubMed] [Google Scholar]
- 33.Kovach NL, Yee E, Munford RS, Raetz CR, Harlan JM. Lipid IVA inhibits synthesis and release of tumor necrosis factor induced by lipopolysaccharide in human whole blood ex vivo. J Exp Med. 1990;172:77–84. doi: 10.1084/jem.172.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saitoh S, Akashi S, Yamada T, Tanimura N, Kobayashi M, Konno K, Matsumoto F, Fukase K, Kusumoto S, Nagai Y, Kusumoto Y, Kosugi A, Miyake K. Lipid A antagonist, lipid IVa, is distinct from lipid A in interaction with Toll-like receptor 4 (TLR4)-MD-2 and ligand-induced TLR4 oligomerization. Int Immunol. 2004;16:961–969. doi: 10.1093/intimm/dxh097. [DOI] [PubMed] [Google Scholar]
- 35.Kawasaki K, Gomi K, Nishijima M. Cutting edge: Gln22 of mouse MD-2 is essential for species-specific lipopolysaccharide mimetic action of taxol. J Immunol. 2001;166:11–14. doi: 10.4049/jimmunol.166.1.11. [DOI] [PubMed] [Google Scholar]
- 36.Kiechl S, Wiedermann CJ, Willeit J. Toll-like receptor 4 and atherogenesis. Ann Med. 2003;35:164–171. doi: 10.1080/07853890310008215. [DOI] [PubMed] [Google Scholar]
- 37.Tal G, Mandelberg A, Dalal I, Cesar K, Somekh E, Tal A, Oron A, Itskovich S, Ballin A, Houri S, Beigelman A, Lider O, Rechavi G, Amariglio N. Association between common Toll-like receptor 4 mutations and severe respiratory syncytial virus disease. J Infect Dis. 2004;189:2057–2063. doi: 10.1086/420830. [DOI] [PubMed] [Google Scholar]
- 38.Michel O, LeVan TD, Stern D, Dentener M, Thorn J, Gnat D, Beijer ML, Cochaux P, Holt PG, Martinez FD, Rylander R. Systemic responsiveness to lipopolysaccharide and polymorphisms in the toll-like receptor 4 gene in human beings. J Allergy Clin Immunol. 2003;112:923–929. doi: 10.1016/j.jaci.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 39.Pasare C, Medzhitov R. Toll-dependent control mechanisms of CD4 T cell activation. Immunity. 2004;21:733–741. doi: 10.1016/j.immuni.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 40.Pasare C, Medzhitov R. Toll-like receptors: linking innate and adaptive immunity. Microbes Infect. 2004;6:1382–1387. doi: 10.1016/j.micinf.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 41.Kim HM, Park BS, Kim JI, Kim SE, Lee J, Oh SC, Enkhbayar P, Matsushima N, Lee H, Yoo OJ, Lee JO. Crystal Structure of the TLR4-MD-2 Complex with Bound Endotoxin Antagonist Eritoran. Cell. 2007;130:906–917. doi: 10.1016/j.cell.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 42.Rossignol DP, Lynn M. TLR4 antagonists for endotoxemia and beyond. Curr Opin Investig Drugs. 2005;6:496–502. [PubMed] [Google Scholar]
- 43.Huber M, Kalis C, Keck S, Jiang Z, Georgel P, Du X, Shamel L, Sovath S, Mudd S, Beutler B, Galanos C, Freudenberg MA. R-form LPS, the master key to the activation ofTLR4/MD-2-positive cells. Eur J Immunol. 2006;36:701–711. doi: 10.1002/eji.200535593. [DOI] [PubMed] [Google Scholar]
- 44.Viriyakosol S, Tobias PS, Kitchens RL, Kirkland TN. MD-2 binds to bacterial lipopolysaccharide. J Biol Chem. 2001;276:38044–38051. doi: 10.1074/jbc.M105228200. [DOI] [PubMed] [Google Scholar]
- 45.Kennedy MN, Mullen GE, Leifer CA, Lee C, Mazzoni A, Dileepan KN, Segal DM. A complex of soluble MD-2 and lipopolysaccharide serves as an activating ligand for Toll-like receptor 4. J Biol Chem. 2004;279:34698–34704. doi: 10.1074/jbc.M405444200. [DOI] [PubMed] [Google Scholar]
- 46.Kawasaki K, Akashi S, Shimazu R, Yoshida T, Miyake K, Nishijima M. Mouse toll-like receptor 4. MD-2 complex mediates lipopolysaccharide-mimetic signal transduction by Taxol. J Biol Chem. 2000;275:2251–2254. doi: 10.1074/jbc.275.4.2251. [DOI] [PubMed] [Google Scholar]