Abstract
Cell polarization is intimately linked to plant development, growth, and responses to the environment. Major advances have been made in our understanding of the signaling pathways and networks that regulate cell polarity in plants owing to recent studies on several model systems, e.g., tip growth in pollen tubes, cell morphogenesis in the leaf epidermis, and polar localization of PINs. From these studies we have learned that plant cells use conserved mechanisms such as Rho family GTPases to integrate both plant-specific and conserved polarity cues and to coordinate the cytoskeketon dynamics/reorganization and vesicular trafficking required for polarity establishment and maintenance. This review focuses upon signaling mechanisms for cell polarity formation in Arabidopsis, with an emphasis on Rho GTPase signaling in polarized cell growth and how these mechanisms compare with those for cell polarity signaling in yeast and animal systems.
Keywords: ROP GTPase, Rho GTPase, cytoskeleton, exocytosis, actin dynamics, polarized cell growth
INTRODUCTION
Cell polarity, broadly defined as asymmetry within a cell, is a fundamental feature of cell function that is tied to developmental and environmental regulation. Polarity formation is initiated by a polarizing signal, which regulates polar distribution of signaling molecules and leads to polarity establishment and maintenance through the cytoskeleton and vesicular trafficking. In plants, cell polarity is fundamental to development, growth, and morphogenesis in every stage of the life cycle. Zygote polarization is required for the first asymmetric division to the embryo axis. Similarly, polarized cell division is essential for cell differentiation and organ initiation and morphogenesis. Polarized cell expansion generates cells with specific shapes that are associated with specialized functions, e.g., polarized and guided pollen tube growth for sperm delivery. Finally, polar localization of auxin-carrier proteins allows directional auxin flow and auxin-gradient formation, processes that are essential for organ and tissue formation and growth.
Given the fundamental importance of cell polarity and its diverse forms, a crucial question in our understanding of this phenomenon is whether there are general principles underlying the various forms of cell polarity in all eukaryotic systems. This question is of particular interest considering the many unique features of plant cells, one of which, the cell wall, is indispensable for polarity formation. Indeed, a paradigm of polarity control centered on Rho GTPase signaling has emerged from investigation of such diverse systems as yeast, worm zygotes, mammalian epithelial cells, plant pollen tubes (PT), and leaf epidermal cells (Figure 1). An equally important question is how diverse systems utilize these general rules to build the respective forms of cell polarity within specific developmental and environmental contexts. The answers to these questions have enhanced our understanding of general principles for cell polarity control and of the molecular mechanisms linking fundamental cell polarity to specific growth and developmental processes in plants. In this review, several plant model systems are discussed to illustrate how polarity control mechanisms in plants compare and contrast with those in animal and fungal systems.
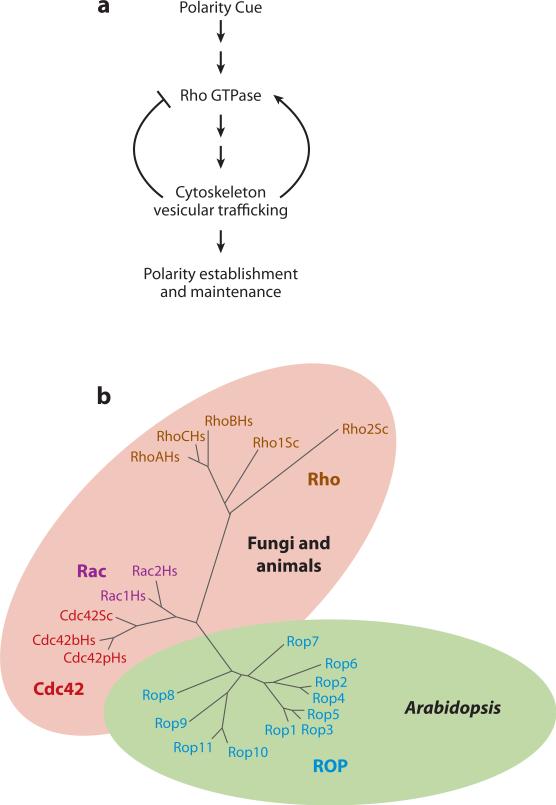
Figure 1.
A unifying principle underlying the formation of cell polarity in eukaryotic cells. (a) A general signaling mechanism for cell polarity formation. (b) A simplified phylogenetic tree showing the major subfamilies of the Rho family small GTPases. All 11 members of the Arabidopsis Rho-related GTPase from plants (ROP) family are shown, but only representative members of the Cdc42, Rac, and Rho subfamilies are included.
MODEL POLARITY SYSTEMS IN PLANTS
Our understanding of the mechanisms underlying plant cell polarity formation has come primarily from several model systems (Figure 2), which offer fascinating biology and are accessible to experimental manipulations. Although the zygote of brown algae was formerly a favorite system (Fowler & Quatrano 1997), it has fallen out of favor because it is not amenable to genetic and molecular studies. Along with the development of Arabidopsis into a model plant, the single-cell pollen or multicellular epidermal systems (Figure 2) from Arabidopsis have been the focus of the investigation of the molecular and cellular biological and genetic bases of cell polarity generation in plants.
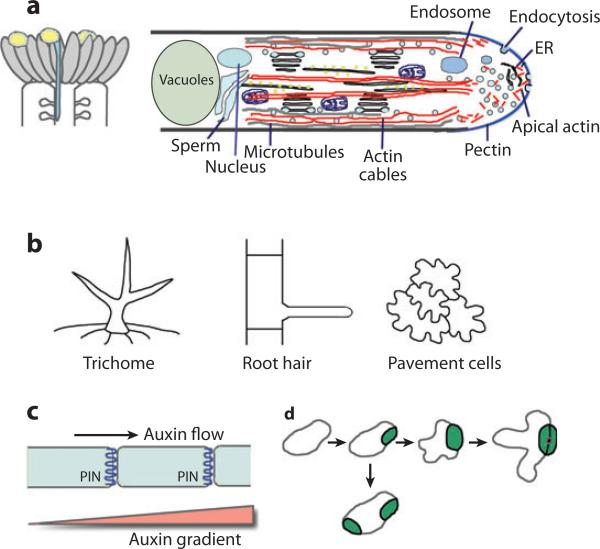
Figure 2.
Model cell polarity systems in plants. (a) Tip growth model: the pollen tube. (Left) A schematic of the top portion of a pistil with growing pollen tubes. (Right) A schematic showing the polarized cellular structure of pollen tubes. (b) Epidermal cells as a model for the study of planar cell polarity and/or the polarity of diffuse growth. (Left) Single-cell leaf trichome. (Middle) Trichoblast and root hairs. (Right) Pavement cells. (c) The polar localization of PINs (PIN-FORMED proteins), which directs auxin flow and produces auxin gradients, is used as a model for the study of asymmetric distribution of molecules within a cell. (d ) Guard cell differentiation as a model for the investigation of the polarity of cell division.
Tip-Growing Cells
Polar growth due to localized vesicle targeting and exocytosis to the growth site is termed tip growth, which occurs in all eukaryotic kingdoms and is required for the generation of highly elongated tubular cells such as fungal hyphae, animal neurons, PT, and root hairs (Figure 2). Although the mechanisms for tip growth in yeast have been extensively studied, it is unclear whether they apply to other tip growth systems, especially those involving rapid growth to an extraordinary length (e.g., neuronal axons, PT, and fungal hyphae). Both PT and root hairs serve as excellent model systems for rapid tip growth. Because root hairs are nonessential, many morphological mutants and concerned genes have been identified. Transcriptome analysis and the candidate gene approach have also identified important genes regulating tip growth. The genes identified by these approaches have provided some important insights into the molecular mechanism of tip growth, including signaling mediated by calcium, reactive oxygen species (ROS), and small GTPases (Bohme et al. 2004; Carol & Dolan 2006; Carol et al. 2005; Foreman et al. 2003; Grierson et al. 1997; Jones et al. 2002, 2006; Molendijk et al. 2001; Parker et al. 2000; Preuss et al. 2006; Schiefelbein & Somerville 1990; Song et al. 2006; Wen et al. 2005).
To deliver sperm to the ovule, the PT must extend rapidly (as fast as 1 cm h−1) and directionally by navigating through many tissues. This directional growth is remarkably similar to neuronal guidance in animals (Kim et al. 2003, 2004; Lord & Russell 2002; Palanivelu & Preuss 2006, Palanivelu et al. 2003). Unlike most other cells from multicellular plants, which dedifferentiate and lose their native polarity upon in vitro culture, cultured pollen maintains its developmental status and polarity. In vitro tubes grow synchronously and uniformly. As the growth of PT is genetically controlled by the haploid genome and involves many pollen-specific genes, lethal mutations or pollen-specific transgenes can be maintained in heterozygous plants, facilitating genetic analysis of the essential genes involved in polarity control. These advantages, combined with the ease with which live imaging can be performed in PT, make PT one of the most exciting systems for polarity studies in higher organisms.
Studies of tip growth mechanisms in PT have focused on two interlinking mechanisms: the oscillating tip–focused cytosolic calcium gradient and tip-localized dynamic Rho signaling. Both of these target the actin cytoskeleton that feedback-regulates them, constituting an intricate signaling network known as LENS (localization-enhancing network, self-sustaining) (Cole & Fowler 2006). Given that there are roles for Rho GTPase, actin dynamics, and probably calcium in tip growth in other systems, LENS may be a useful conceptual framework for understanding the signaling mechanisms underpinning tip growth in various systems.
Morphogenesis of Epidermal Cells
Unlike root hairs and PT, most plant cells expand by diffuse growth, whereby they diffusely or uniformly increase their cell surface while certain regions or positions of the cell differentially expand owing to differential construction or remodeling of localized regions of the cell wall. Therefore, the mechanisms for localized cell-wall construction or modification are the key to the polarity of diffuse growth. These mechanisms have been investigated using several epidermal cell types amenable to microscopic imaging analyses (Figure 2). At least two forms of cell polarity are important for the morphogenesis of epidermal cells: the apical-basal polarity formed perpendicularly to the surface and the planar polarity along the surface. In Arabidopsis leaves, polarized growth from epidermal cells forming a trichome with three branches is controlled by apical-basal polarity. Trichomes are nonessential cells, and their morphology is easily observed, providing an excellent model for genetic analysis of morphogenesis. A large collection of mutants with altered trichome shapes has been isolated, and the molecular analysis of these mutants has demonstrated a critical role for the ARP2/3 actin nucleation complex in the regulation of trichome morphogenesis (Hulskamp et al. 1994, Schellmann & Hulskamp 2005, Smith & Oppenheimer 2005, Szymanski 2005).
Planar cell polarity, which involves coordination between cells within the plane of a cell layer, is essential for development and morphogenesis in animals (e.g., in convergent extension, wherein cells become intercalated to change the shape of early embryos). In plants, planar cell polarity is also critical for the differentiation of certain epidermal cell types (e.g., guard cells) and for the morphogenesis of most epidermal cells, such as trichoblasts in roots and pavement cells (PC) in leaves. Trichoblasts undergo polar diffuse growth to produce a bulge, from which tip-growing root hair forms. Interestingly, the site of the bulge formation, which is invariably adjacent to the basal end of the cross wall of trichoblasts (Figure 2), is regulated by the small-molecule hormone auxin (Fischer et al. 2006, 2007).
PC with a jigsaw-puzzle appearance represent an exciting system for the investigation of polarity involving cell-cell coordination (Fu et al. 2002, 2005; Smith 2003). The development of PC with interdigitating lobes and indentations requires intricate and dynamic polarity formation (Figures 2, 3a) (Fu et al. 2002, 2005). The precise fitting of lobes and indentations among neighboring cells suggests a need for cell-to-cell signaling to spatiotemporally coordinate lobe outgrowth from one cell with the complementary indentation in a neighboring cell. This process bears a striking similarity to animal planar cell polarity, in particular to convergent extension requiring cell-to-cell intercalation (Goto et al. 2005, Klein & Mlodzik 2005, McEwen & Peifer 2001, Price et al. 2006, Settleman 2005). Interestingly, the similarity occurs at the mechanistic level as well. Dynamic gradients of a polarizing signal provide a developmental mechanism that activates the intercalary growth in PC (T. Xu, M. Wen, Y. Fu, J. Friml, J. Chen, M. Wu, A. Jones & Z. Yang, unpublished data); similarly, a gradient of unknown signal is thought to activate planar cell polarity in animals (Klein & Mlodzik 2005). Furthermore, accumulating evidence suggests that Rho GTPase–mediated cytoskeletal reorganization is important for planar cell polarity in animals, as has been shown for PC (Goto et al. 2005, Klein & Mlodzik 2005, McEwen & Peifer 2001, Price et al. 2006, Settleman 2005). Therefore, the study of PC polarity formation may help to shed light onto the mechanistic principles of planar cell polarity in other systems.
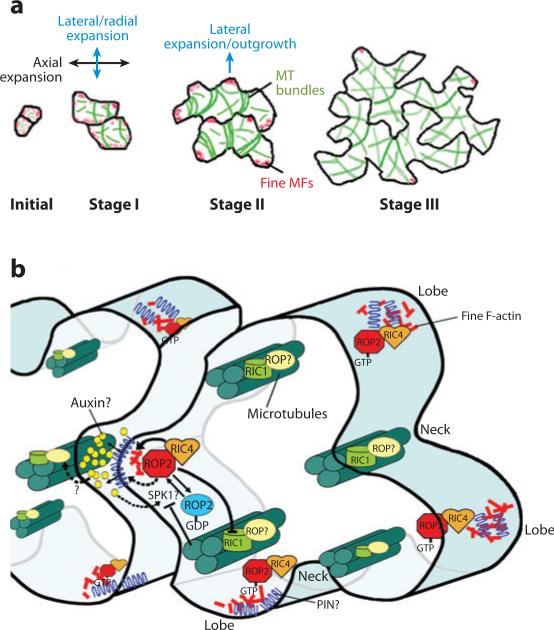
Figure 3.
A model for a signaling network regulating the formation of the jigsaw-puzzle appearance of Arabidopsis leaf pavement cells (PC). (a) The development of PC can be separated into three stages and is associated both with cortical fine actin microfilaments (F-actin) (red patches) and with microtubules (MT) (green lines) (Fu et al. 2002, 2005). Near-square PC initials first elongate slightly to form near-rectangular cells (stage I), which produce alternating small bumps and indentations, generating cells with multiple shallow lobes and indentations (also termed sinuses or necks) (stage II). Reiterative outgrowing and indenting continue, producing highly lobed interlocking PC that often contain secondary lobes (stage III). From Fu et al. (2005) with permission. (b) A model for the ROP GTPase–dependent signaling mechanism for PC morphogenesis. This model includes known components (ROP2, RIC4, F-actin, RIC1, MT, SPK1), speculative factors (auxin and a PIN protein), and their interactions in the ROP (Rho-related GTPase from plants)-signaling network underlying PC morphogenesis. Solid arrows indicate pathways well supported by experiments. Dotted arrows indicate speculative steps/pathways. ER denotes endoplasmic reticulum.
Polar Localization of Auxin Transporters
Cell polarity is often expressed as polar distribution of molecules within the cell; these molecules are not necessarily associated with detectable morphological expression in cell polarity. A good example of this type of polarity is polar localization of auxin transporters in plants, including auxin efflux carriers PIN-FORMED proteins (PINs) and influx carrier AUX1 (see Kleine-Vehn & Friml 2008, in this volume). The function of auxin is associated with its flow, its distribution as a gradient, the maximum of its gradient, and/or its specific concentration within a tissue or cell (Grieneisen et al. 2007, Wisniewska et al. 2006). All these features of auxin distribution and dynamics are primarily regulated by the polar distribution of PINs, with the polar distribution of AUX1 playing a secondary role (Kleine-Vehn & Friml 2008). Different PINs exhibit distinct polar localization patterns within the same cell, and the polarity of a PIN within the same cell is controlled by developmental or environmental signals (Leyser 2005). For example, the apical-to-basal reversal of PIN7 polarity in the basal cell of the two-celled embryo is important for Arabidopsis embryo axis formation (Friml et al. 2003). Therefore, signaling between PINs and polarity signals is required for polar localization of PINs.
Asymmetric Cell Division
As in other multicellular organisms, asymmetric cell division, found in zygotes and various stem cells, generates two unequal daughter cells with distinct fates in plants. Prior to asymmetric division, polarity must be established to allow this unequal distribution of cellular molecules and structures. For instance, in zygotes of higher plants the apical end is enriched with the cytoplasm, whereas the basal end is primarily occupied by a large vacuole. The mechanism for zygote polarization in plants remains poorly understood owing to the difficulties in visualizing the zygote embedded deep in the ovary and in isolating the mutations affecting this process, because they are lethal to the embryo. However, recent genetic studies on the differentiation of guard cells and root stem cells are beginning to unravel regulatory factors, such as transcriptional factor and signaling molecules, that modulate asymmetric cell division in plants (Aida et al. 2004, Bergmann et al. 2004, Hara et al. 2007, Nadeau & Sack 2002, Pillitteri & Torii 2007, Shpak et al. 2005, Wang et al. 2007, Xu et al. 2006).
THE CYTOSKELETON AND VESICULAR TRAFFICKING IN POLARITY CONTROL
A central aspect of the cell polarity paradigm is the significance of the cytoskeleton and its associated vesicular trafficking: Both serve as an essential cellular linkage to molecular pathways by responding to the initial polarity signal and providing spatial information for feedback regulation of the polarity-signaling pathways (Figure 1). Increasing evidence indicates that this paradigm can be extended to the regulation of plant cell polarity formation, although the detailed mechanisms by which the cytoskeleton and vesicular trafficking participate in plant cell polarity formation may vary from those in other systems. As in fungi and animals, both actin microfilaments (F-actin) and microtubules (MT), as well as targeted exocytosis and endocytosis, have been implicated in cell polarity control in plants (Cole & Fowler 2006, Murphy et al. 2005, Smith & Oppenheimer 2005). Given the tight linkage of these cellular events to polarity formation and Rho GTPase signaling, this review includes a discussion of their roles in plant cell polarity. For broader and more detailed descriptions of these cellular events, however, readers are referred to several recent excellent reviews (Campanoni & Blatt 2007, Cole & Fowler 2006, Ehrhardt & Shaw 2006, Murphy et al. 2005, Samaj et al. 2006, Smith & Oppenheimer 2005).
Microtubules
MT act to induce cell polarity formation by targeting and/or locally activating signaling molecules at the polar site in yeast and animal cells (Basu & Chang 2007, Siegrist & Doe 2007). A common signaling molecule regulated by MT in these systems is a Rho family GTPase, which controls actin-based polarity formation (Basu & Chang 2007, Siegrist & Doe 2007). Signaling in the polar site also feedback-regulates MT by stabilizing them. In plants, MT seem to have multiple roles and modes of action in cell polarity, some of which are analogous to polarity control in yeast and animals. Highly ordered paralleled cortical MT form a preprophase band (PPB) that predicts the site and the orientation of cell division, which are critical for asymmetric cell division. The localization of the PPB is determined by an initial polarity signal, which induces the polarization of cells prior to PPB formation. In this sense, the PPB acts to process but not to induce polarity. However, the PPB in turn induces the polarity of cell division, i.e., the position and the orientation of the new cell plate. PPB MT and the associated kinesins POK1 and ROK2 recruit TAN1 to the PPB cortical site, which signals to place and orient the phragmoplast according to the PPB (Muller et al. 2006, Smith et al. 2001, Walker et al. 2007).
Cortical MT modulate the polarity of cell expansion in various plant cells, but their modes of action may vary from one cell type to another. MT induce branching of Arabidopsis trichomes with three to four branches. Stabilizing the MT increases the branch number of wild-type trichomes and induces branching of un-branched mutant trichomes, whereas MT disruption causes unbranched trichomes (Mathur & Chua 2000). MT may regulate the localization of the Golgi apparatus or the targeting of specific molecules to induce trichome branching (Lu et al. 2005).
In diffusely growing cells, well-ordered cortical MT are thought to determine the region of the expanding cell surface; e.g., they are associated with the sinuses but excluded from the expanding lobes of PC (Figure 3). Removal of cortical MT results in isotropic cell expansion, implying that cortical MT may also directly participate in the formation of cell polarity in plants. Ordered MT are believed to guide the deposition of cellulose microfibrils for the restriction of cell expansion (Ehrhardt & Shaw 2006). This mode of MT action is clearly unique to plant cells. In addition, cortical MT modulate the polarity of cell expansion in plants by regulating Rho GTPase signaling (Figure 3) (Fu et al. 2005). This type of MT action in plant cells follows the general theme of the MT induction of cell polarity formation (Basu & Chang 2007, Siegrist & Doe 2007).
Actin Microfilaments
F-actin generally participate in polarity establishment by affecting the biochemical, structural, and biophysical properties of the plasma membrane (PM) at the polar site. Cortical F-actin, which form as patches or dense meshwork by the ARP2/3 actin nucleation complex, directly control PM polarization by regulating both exocytosis and endocytosis in yeast and animals (Basu & Chang 2007, La Carbona et al. 2006, Moseley & Goode 2006). Through vesicular trafficking or direct transport, cortical F-actin target signaling molecules (e.g., Rho GTPases or their activators) to the polar site to promote their own polymerization, forming a positive feedback loop for robust polarity establishment (Charest & Firtel 2006). Cytoplasmic actin bundles, which originate from formin-dependent actin assembly, maintain cell polarity either by targeting post-Golgi vesicles in yeast or by generating the trailing edge in animals.
The picture of F-actin's role in regulating cell polarity in plants is emerging but far from clear. The role of the ARP2/3 complex is limited to shape control in trichomes and in PC (Smith & Oppenheimer 2005). In PC, ARP2/3 knockout mutations do not eliminate but only alter the localization of a lobe-associated actin meshwork that appears as diffuse cortical F-actin (Djakovic et al. 2006, Li et al. 2003). The formation of these ARP2/3–independent diffuse F-actin requires a Rho GTPase–dependent pathway (Fu et al. 2002, 2005).
The ARP2/3–independent F-actin also control tip growth in PT and root hairs. F-actin in the PT extreme apex have been a subject of debate for decades owing mostly to technical difficulties in visualizing the highly dynamic F-actin. Green fluorescent protein (GFP)-tagged mouse talin was used to visualize highly dynamic F-actin at the tip, which rapidly alternate between the cortex of the apical dome and the cortex immediately adjacent to the extreme apex (Fu et al. 2001, Kost et al. 1998). Rapid-freezing methods confirmed abundant presence of the latter but infrequent occurrence of the former (Lovy-Wheeler et al. 2005), probably reflecting snapshots of the dynamic apical F-actin. The existence of the former, however, is clearly supported by the finding that it is activated by a Rho GTPase signaling pathway localized to the extreme tip of the PM (Fu et al. 2001, Gu et al. 2005, Hwang et al. 2005).
The Rho GTPase–mediated dynamics of the apical F-actin is required for the generation or maintenance of PT polarity (Fu et al. 2001, Gu et al. 2005). In PT, evidence suggests that the actin dynamics regulates tip-targeted exocytosis by coordinating vesicle accumulation and docking/fusion (Lee et al. 2008). Rho GTPase–dependent actin assembly is required for vesicle accumulation to the apex, whereas its disassembly is critical for vesicle docking and/or fusion. Apical F-actin have also been implicated in positive feedback activation of Rho GTPases at PT tips, as in yeast and animals (Hwang et al. 2005). Therefore, at least some roles of dynamic F-actin in polarity formation are conserved in plants at the mechanistic level, although it is not clear whether the apical F-actin regulate endocytosis as they do in yeast and animal cells.
Exocytosis
A role for polarized exocytosis in cell polarity formation has been established in various eukaryotic systems (Brennwald & Rossi 2007, France et al. 2006, Mehta et al. 2005, Wedlich-Soldner et al. 2004, Zajac et al. 2005). Polarized exocytosis allows for tip growth in root hairs and PT by supplying membrane and wall materials; for instance, loss-of-function mutations on genes encoding the SEC3 and SEC8 subunits of exocyst, which control polar docking of vesicles to the PM, block tip growth (Cole & Fowler 2006). Polar exocytosis also mediates the targeting of signaling molecules. In yeast, polar activation of signaling molecules such as Cdc42 mediates polar exocytosis, which in turn leads to the molecules’ own polar targeting, generating a positive feedback loop for robust generation of cell polarity (Wedlich-Soldner et al. 2004, Zajac et al. 2005). As mentioned above, the dynamic tip F-actin appear to participate in the positive feedback activation of ROP1 (where ROP refers to Rho-related GTPase from plants) by regulating exocytosis in pollen tubes. Polar localization of ROP GTPases to the PM involves ADP-ribosylation factor (ARF) GTPase–mediated vesicular trafficking (Molendijk et al. 2001; Xu & Scheres 2005a,b).
Knocking out a Rab4Ab effector depolarizes root hair growth (Preuss et al. 2006). Rab4A GTPases are localized to endosomes/trans-Golgi network (TGN)/exocytic vesicles that have accumulated at the tips of root hairs and PT (de Graaf et al. 2005; Lee et al. 2008; Preuss et al. 2004, 2006). Rab4A belongs to a 29-member subfamily of Rab GTPases that are predicted to localize to TGN/post-Golgi vesicles. The unusually large size of this Rab subfamily is consistent with the notion that these Rabs may be involved in the targeting of functionally distinct vesicles (Vernoud et al. 2003, Zhang et al. 2007). Specific molecules carried on RabA4 vesicles may either mark the site of polarity for tip growth or be involved in limiting or maintaining polarity signaling to the tip. In PT, a ROP1-negative regulator required for growth polarity control appears to be carried on tip-localized vesicles ( J. Hwang, V. Vernoud & Z. Yang, unpublished data). Exocytosis-mediated targeting of negative regulators of Rho family GTPases may be a common mechanism for restricting polarity signaling to the polar site, as has been shown for mammalian RICH1 (a CDC42 GTPase–activating protein) in restricting CDC42 signaling to the tight junction (Wells et al. 2006).
Polar localization of PIN and AUX proteins is another example of exocytosis of specialized vesicles that carry specific molecules (Dharmasiri et al. 2006; Geldner et al. 2003; Jaillais et al. 2006, 2007; Kleine-Vehn et al. 2006; Steinmann et al. 1999). Several recent studies clearly demonstrate that different auxin-transporter proteins are targeted to the PM through distinct exocytic routes involving SNX1-dependent and -independent endosome sorting, which are subject to regulation by specific polarity signals (Kleine-Vehn & Friml 2008).
Endocytosis
Endocytosis to retrieve the PM and its associated signaling molecules is a common mechanism for the maintenance of cell polarity in eukaryotes (Engqvist-Goldstein & Drubin 2003, Murphy et al. 2005, Nishimura & Kaibuchi 2007, Pruyne & Bretscher 2000, Yu et al. 2007). In yeast, CDC42 regulates not only polarized exocytosis but also endocytosis in its control of polarity formation. Computational modeling predicts that polarized targeting of PM proteins, their lateral diffusion in the PM, and their internalization by endocytosis are sufficient to generate sustained cell polarity (Marco et al. 2007). Endocytosis has been well documented in plant cells (Dhonukshe et al. 2007, Murphy et al. 2005) and occurs at the tips of PT and root hairs (Lisboa et al. 2007, Monteiro et al. 2005, Ovecka et al. 2005). A recent study in plant cells demonstrated the conservation of clathrin-mediated endocytosis (Dhonukshe et al. 2007), for which phosphatidylinositol 4,5-bisphosphate (PIP2) and its conversion to phosphatidylinositol 4-bisphosphate (PI4P) are required. Both PIP2 and PI4P are localized to the PT-apical PM, and alteration of PIP2 and PI4P levels inhibits the internalization of the PM at PT tips, leading to growth depolarization (Y. Zhao, A. Yan & Z. Yang, unpublished data). Furthermore, expression of the constitutively active form of ROP induces growth depolarization and inhibits endocytosis (Bloch et al. 2005). Interestingly, a screen designed to identify chemicals that affect the polarity of pollen tube tip growth has led to the identification of endocitin, which impacts the endocytosis of specific PM-localized proteins (Roberts et al. 2008). These observations support a role for endocytosis in maintaining polarized cell growth in plants.
Moreover, a large body of evidence shows that PIN proteins are internalized to endosomes by constitutive endocytosis (Dhonukshe et al. 2007, Richter et al. 2007, Teh & Moore 2007). Interestingly, auxin increases PIN localization to the PM by inhibiting endocytosis, forming a positive feedback loop of auxin efflux–signaling PIN localization (Paciorek et al. 2005).
SIGNALING MECHANISMS BEHIND CELL POLARITY FORMATION
How polarizing signals impinge on cytoskeletal reorganization/dynamics and vesicular trafficking is a central concern in polarity studies. A common theme of polarity signaling in plants has emerged from recent studies of polarized cell growth: Rho GTPase signaling networks, composed of multiple coordinate pathways and feedback loops, provide a robust molecular linkage between cytoskeleton and vesicular trafficking and polarity formation. This signaling mechanism not only resonates with the cell polarity paradigm in yeast and animals but also helps to reveal how Rho GTPases can be used to generate cell polarity in diverse systems. Rho functions as a hub in signaling by integrating numerous signals and coordinating multiple downstream pathways (Nibau et al. 2006, Yang & Fu 2007). To control the plant-specific aspects of cell polarity, Rho has evolved to interact with plant-specific partners or variants of conserved regulators.
ROP GTPases and Partners: Plant Reinvention of Conserved Polarity Signaling
In contrast to the multiple subfamilies of the mammalian Rho family (e.g., Cdc42, Rac, Rho, etc.), all plant Rho-like GTPases fall into a single subfamily, ROP (Figure 1) (Brembu et al. 2006, Burridge & Wennerberg 2004, Vernoud et al. 2003, Yang 2002, Yang & Fu 2007, Yang & Watson 1993). Six (ROP1 to ROP6) of the 11 Arabidopsis ROPs are known to regulate cell polarity formation (Gu et al. 2004, Yang 2002). Two classes of negative regulators, guanine nucleotide dissociation inhibitors (RhoGDIs) and GTPase-activating proteins (RhoGAPs), are conserved in plants, but they possess unique regulatory functions such as the Cdc42/Rac–interactive binding (CRIB) motif in RhoGAPs (Brembu et al. 2006, Wu et al. 2000). Interestingly, the conventional RhoGEFs (guanine nucleotide exchange factors) with the Dbl-homology (DH) domain are absent from plants, and only a single homolog of DHR2–RhoGEF, SPK1, is present in Arabidopsis (Basu et al. 2008, Brembu et al. 2006, Meller et al. 2005, Qiu et al. 2002). However, a novel family of plant-specific RhoGEFs known as RopGEF has recently been identified (Berken et al. 2005, Gu et al. 2006). Several pollen-expressed RopGEFs interact physically and functionally with a pollen-expressed receptor-like kinase (RLK), which belongs to a superfamily of receptor ser/thr kinases in plants (Kaothien et al. 2005, Zhang & McCormick 2007). RopGEFs and RLKs may have coevolved to form a plant-specific signaling module to respond to plant-specific extracellular signals (Yang & Fu 2007).
Plants have reinvented a set of Rho effectors, even though their signaling targets (e.g., the cytoskeleton) are conserved. Plant-specific effectors include two classes of novel proteins: a family of ROP-interactive CRIB motif–containing proteins (RICs) and a family of interactor of constitutively active ROP 1 proteins (ICR1 and ICR1-like) (Lavy et al. 2007, Wu et al. 2001, Xu & Scheres 2005a). RIC3 and RIC4 regulate actin dynamics in a manner apparently independent of the ARP2/3 complex (Fu et al. 2005, Gu et al. 2006, Li et al. 2003). Thus, ROP's regulation of actin dynamics in plants may involve a novel mechanism. ICR1 interacts with SEC3, an exocyst component known to be a direct effector of yeast CDC42. ICR1 is required for polarized growth of PC, consistent with a role in the activation of exocyst; however, direct evidence for this role is lacking (Lavy et al. 2007). These variations from the central theme of Rho signaling in cell polarity provide the basis for Rho GTPase control of cell polarity with various functionalities in diverse cell types and organisms. To elaborate further upon this idea, several examples of ROP signaling in cell polarity are discussed in detail below.
ROP GTPase Signaling at the Tip: The Pollen Tube Model
A role for ROPs in cell polarity control in plants was first suggested by a study showing ROP localization to the apical PM of pollen tubes and the essential role of ROPs in tip growth (Lin et al. 1996, Lin & Yang 1997). These initial findings and subsequent studies of ROP signaling have provided us with a road map for addressing key questions about tip growth (Figure 4): (a) What defines the apical PM domain (i.e., tip growth domain) for tip growth? (b) How is the tip growth domain generated and maintained? (c) How does this domain signal to exocytic vesicles to control their targeting and exocytosis to the tip?
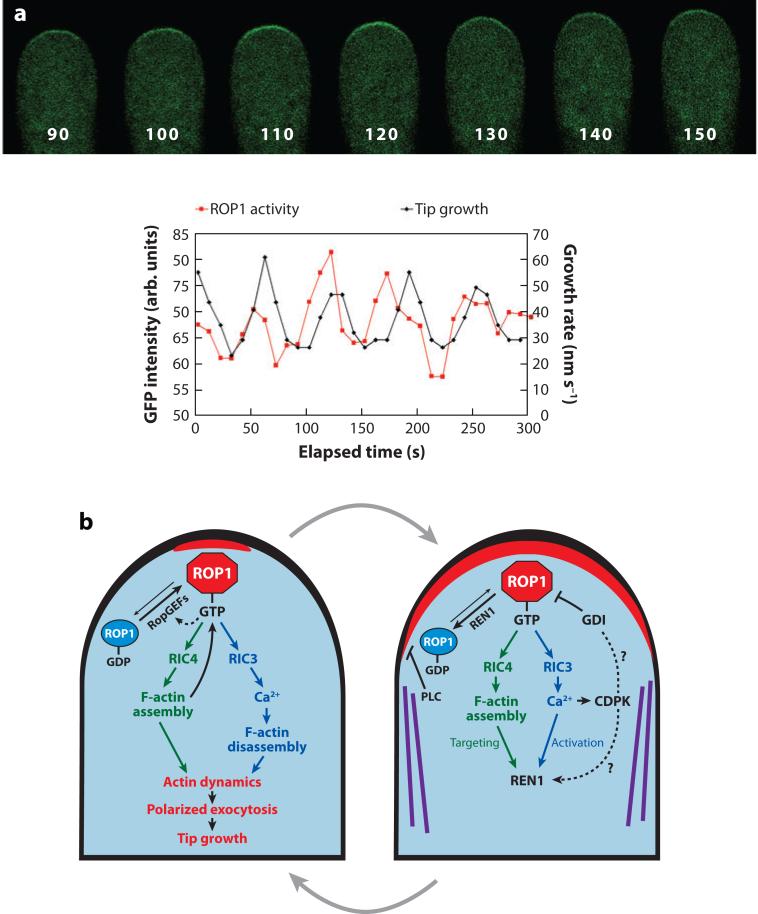
Figure 4.
A model for the generation and maintenance of the dynamic and oscillating apical cap of active ROP1 (where ROP refers to Rho-related GTPase from plants) in pollen tubes. (a) A time series of images showing the localization of a green fluorescent protein (GFP)-based active ROP1 reporter in a tobacco pollen tube transiently expressing this marker, i.e., GFP fused to the N terminus of a RIC4 deletion mutant (Hwang et al. 2005). Numbers at the bottom of each pollen tube image indicate seconds in the time series shown in the graph at the bottom of this figure. (Bottom) Graphs show the oscillation of plasma membrane (PM)-localized GFP-RIC4ΔC. Modified from figure 3 of Hwang et al. (2005). (b) Models for positive feedback–mediated lateral propagation of the apical ROP1 activity and for negative feedback–mediated global ROP1 downregulation by the REN1 GTPase–activating protein (RhoGAP) and lateral inhibition by cortical microtubules (MT) and phospholipase C (PLC). Solid arrows indicate pathways supported by experimental evidence. Dotted arrows indicate speculative pathways lacking experimental support. Abbreviations used: CDPK, calcium-dependent protein kinase; F-actin, actin microfilaments; RIC, ROP-interactive Cdc42/Rac-interactive binding (CRIB) motif–containing protein.
The dynamics of tip-localized ROP activity drives tip growth
Three redundant ROPs (ROP1, −3, and −5), referred to as ROP1 for simplicity, are required for pollen tip growth (Hwang & Yang 2006, Kost et al. 1999, Li et al. 1999). As a positive regulator of tip growth, ROP1 activity is regulated and is critical for the polarity of tip growth (Gu et al. 2003, Hwang & Yang 2006, Kost et al. 1999, Li et al. 1999; J. Hwang, G. Wu, C. Grierson & Z. Yang, unpublished data). Live imaging of active ROP1 reveals the PM distribution of active ROP1 as a dynamic apical cap in normal oscillating PT (Figure 4a). It rises to the maximum size just prior to the growth peak and gradually decreases to the minimum followed by growth rate decreases (Hwang et al. 2005). The maximum of the active ROP1 cap determines the tip growth domain, whereas the dynamics of the apical ROP1 activity drives tip growth ( J. Hwang, G. Wu, C. Grierson & Z. Yang, unpublished data).
The dynamics of the active ROP1 controls tip growth in part by regulating the dynamics of apical F-actin (Fu et al. 2001, Gu et al. 2005, Hwang et al. 2005). Two ROP1 downstream pathways check and balance to control actin dynamics; the RIC4 pathway promotes the assembly of the apical F-actin, and the RIC3-calcium pathway promotes the disassembly of the apical F-actin (Figure 4). The actin dynamics not only modulates tip-targeted exocytosis for tip growth but also is required for the dynamics of the apical ROP1 cap through feedback regulations (Hwang et al. 2005, Lee et al. 2008).
The generation of the apical cap
ROP1 is first activated at the center of the future apical cap, and then spreads laterally to form the cap until reaching the maximum size (Figure 4a) ( J. Hwang, G. Wu, C. Grierson & Z. Yang, unpublished data). Expression of a constitutively active ROP1 mutant (CA-ROP1) induces dramatic lateral spreading of endogenous active ROP1, producing a stable and dramatically enlarged ROP1 cap that is suppressed by RopGAP1 ( J. Hwang, G. Wu, C. Grierson & Z. Yang, unpublished data). Furthermore, knocking out a RhoGAP produces a CAROP1-like phenotype ( J. Hwang, V. Vernoud & Z. Yang, unpublished data). Finally, the ROP1 or RIC4 overexpression–induced depolarization of ROP1 activity is suppressed by Latrunculin B, which promotes actin depolymerization, implying that F-actin, downstream targets of ROP1 signaling, positively impact ROP1 activation (Fu et al. 2001, Hwang et al. 2005). On the basis of these observations, it was hypothesized that the lateral spreading of ROP1 activation is mediated by positive feedback ( J. Hwang, G. Wu, C. Grierson & Z. Yang, unpublished data). One positive feedback mechanism involves apical F-actin (Hwang et al. 2005), similar to the F-actin-mediated positive feedback activation of Cdc42 in yeast (Wedlich-Soldner et al. 2004). Another positive feedback mechanism may involve RopGEF1, an ROP1 activator localized to the apical cap of PT, which was proposed to be directly activated by active ROP1 (Gu et al. 2006). In animal cells, a key feedback loop is Cdc2/Rac activation of phosphoinositide-3-kinase-mediated accumulation of phosphatidylinositol 3,4,5-triphosphate (PIP3) in the PM, which recruits Cdc42/Rac activators (Charest & Firtel 2006). Although there is no evidence for the presence of PIP3 in plants, roles of PI4P and PIP2 in polarized tip growth in root hairs and PTs have been demonstrated and have been implicated in ROP1 activation in PT (Dowd et al. 2006, Helling et al. 2006, Preuss et al. 2006, Stenzel et al. 2008; Y. Zhao, Y. An & Z. Yang, unpublished data). It would be interesting to determine whether these phosphoinositides participate in the positive feedback regulation of the apical ROP1 activity.
The maintenance and the dynamics of the apical cap through downregulation of ROP1
In wild-type PT, as soon as the apical cap of active ROP1 reaches the maximum, it needs to be reduced to confine the ROP1 activity to the tip growth domain and to maintain the dynamics of ROP1 activity (Figure 4a). The downregulation of ROP signaling may be mediated by either global and lateral inhibitions, or both. It has been proposed that the tobacco NtRopGAP1 acts laterally to restrict ROP activity to the apex on the basis of its subapical localization (Klahre & Kost 2006). Knocking out what appears to be the Arabidopsis ortholog of NtRopGAP1, however, does not cause the spreading of ROP1 to the subapical region, suggesting that this RopGAP does not play a major role in the downregulation of the apical ROP ( J. Hwang, G. Wu, C. Grierson & Z. Yang, unpublished data). However, dramatic lateral spreading of apical ROPs and growth depolarization were induced by knocking out a pleckstrin-homology (PH) domain–containing RhoGAP, REN1 ( J. Hwang, V. Vernoud & Z. Yang, unpublished data). REN1 protein is localized to the tube apex, as expected for a global inhibitor. Therefore, REN1 is the major RhoGAP globally inactivating the apical active ROP1.
Another global inhibitor of ROP1 is RhoGDI, which sequesters Rho in the cytosol to prevent it from being activated by the RhoGEFs. RhoGDIs are localized in the PT cytosol, as expected for a global inhibitor (Klahre et al. 2006). Knocking out RhoGDI2a in Arabidopsis induces tip swelling and lateral spreading of ROP1 from the tip, indicating that it is an important global inhibitor of the apical ROP1 ( J. Hwang, G. Wu, C. Grierson & Z. Yang, unpublished data). A RhoGDI1 knockout also induces ectopic ROP2 localization to the PM, causing growth depolarization in root hairs (Carol et al. 2005).
Apart from global inhibitions, lateral inhibition may also play a role in limiting ROP1 activity to the tip. Cortical MT localized to the subapical region appears to have a minor role in restricting ROP activation to the apex. Stabilization of MT partially suppressed ROP1 overexpression–induced depolarization, whereas MT removal moderately increased lateral spreading of the apical ROP1 activity and growth depolarization (M. Zheng & Z. Yang, unpublished data). Both petunia phospholipase C (PLC1) and tobacco PLC3 are preferentially localized to the subapical region of the PM, and their inhibition induces growth depolarization (Dowd et al. 2006, Helling et al. 2006). Treatments with PLC inhibitors induced lateral spreading of active ROP1 (A. Yan & Z. Yang, unpublished data). These results support a role for PLC in limiting PIP2 and ROP1 activity to the tip.
Calcium: a critical link to feedback loops
As a self-organizing system, the downregulation of the apical ROP1 activity in PT is presumably activated by negative feedback mechanisms. Mathematical modeling predicts that RIC3-dependent calcium, which exhibits as oscillatory tip-high gradients (Holdaway-Clarke et al. 1997, Pierson et al. 1994), is a critical activator of the negative feedback regulation; this has been experimentally validated (A. Yan, G. Xu & Z. Yang, unpublished data). The nature of calcium-signaling targets in the negative feedback regulation is unclear, but mathematical modeling predicts that these targets may be either RhoGAP or an F-actin–disassembling factor, or both (A. Yan, G. Xu & Z. Yang, unpublished data).
Interestingly, calcium also participates in positive feedback regulation of ROS production, which is required for tip growth in root hairs. This growth in turn is catalyzed by the tip-localized RHD2 NADPH oxidase (Takeda et al. 2008). RHD2 appears to act directly downstream of ROP2 in root hair tip growth (Carol & Dolan 2006; Jones et al. 2002, 2007; Molendijk et al. 2001; Wong et al. 2007), and RHD2-mediated ROS activate influxes of tip-localized calcium (Carol & Dolan 2006, Jones et al. 2007), which in turn activates RHD2 to form a positive feedback loop consisting of RHD2, ROS, and calcium (Takeda et al. 2008). A similar ROS-calcium circuit may also regulate tip growth in PT (McInnis et al. 2006, Potocky et al. 2007). Given a role for the RHD2-calcium positive feedback loop in PT tip growth, how could calcium-mediated positive and negative feedback loops coexist in the same system? A simple model is that ROP activation of NADPH oxidase and RIC3 initiates the ROS-calcium positive feedback loop, which then runs independently of ROPs. This causes a calcium burst, which activates a negative feedback loop to downregulate ROPs.
Identification of calcium sensors will be necessary to test this model. A putative calcium sensor involved in feedback loops is calcium-dependent protein kinase (CDPK). Overexpression of a PM-localized petunia PiCDPK1 induced PT growth depolarization and excessive calcium accumulation (Yoon et al. 2006). Interestingly, a dominant negative mutant of PiCDPK1 also caused growth depolarization, whereas a constitutively active form of PiCDPK1 either blocked pollen germination or severely inhibited growth by reducing both the length and the width of PT. This CDPK may be the calcium sensor involved in both feedback loops.
Identifying ROP signaling–mediated calcium channels may also provide insights into feedback loops. A pollen-expressed putative calcium channel, CNGC18, is required for polarized PT growth (Frietsch et al. 2007). CNGC18 is localized to the apical and subapical regions of PT PM and is permeable to calcium (Chang et al. 2007, Frietsch et al. 2007). ROP signaling enhances CNGC targeting to the PM, most likely through ROP-mediated polarized exocytosis, but it does not directly regulate its activity (Chang et al. 2007).
A model for tip growth signaling in pollen tubes and beyond
As discussed above, a model for tip growth signaling centered on ROP1 GTPase has been developed (Figure 4). This model may provide a framework for the understanding of detailed signaling mechanisms for various tip growth systems, e.g., growth of root hairs, fungal hyphae, and neurons. In root hairs, ROP2 probably activates tip growth through F-actin and calcium ( Jones et al. 2002, Molendijk et al. 2001). RhoGDI1 plays a similar role in root hair tip growth as does RhoGDI2a in PT (Carol et al. 2005; J. Hwang, G. Wu, C. Grierson & Z. Yang, unpublished data). Rho family GTPases, actin dynamics, positive feedback loops, and RhoGAP-dependent global inhibition are also important for tip growth in other systems (Alberts et al. 2006; Bidlingmaier & Snyder 2004; Knaus et al. 2007; Ushinsky et al. 2002; Wendland & Philippsen 2000, 2001). It will be interesting to see whether the interlinking feedback loops underlying the dynamics of the apical Rho GTPase cap in pollen represent a general mechanism for the control of tip growth in all systems.
Signaling between ROPs and Microtubules in Polarized Diffuse Growth: The Pavement Cell Model
The striking polarity of PC expansion makes it an ideal system for investigating how polarity is coordinated between cells and is controlled during diffuse growth, which is tightly linked to plant development and morphogenesis. In contrast to tip growth, cortical MT play a predominant role in determining the polarity of diffuse growth. Interestingly, current evidence indicates that polarity of diffuse growth is modulated by signaling mechanisms similar to those for tip growth. This similarity was first suggested by the observation that CA-ROP2 and DN-ROP2 mutants dramatically alter cell shapes in PC (Fu et al. 2002).
The ROP2/4–RIC4 pathway promotes lobe formation
Subsequent studies have shown that functionally redundant ROP2 and ROP4 promote lobe outgrowth (Fu et al. 2005). ROP2/4 are activated locally at the tips of growing lobes, where they promote localized accumulation of RIC4-dependent cortical fine F-actin, similar to the regulation of F-actin by ROP1 in PT tips. In addition, the lobe outgrowth may also require localized exocytosis. ICR1 was recently identified as a putative ROP effector that may link to exocyst recruitment to a polar site (Lavy et al. 2007). A ICR1 knockout generates small PC lacking lobes and indentations. Further studies should determine whether ICR1 is a ROP2/4 effector involved in recruiting exocysts. Active ROP2/4 also need to suppress the organization of ordered cortical MT in the region of outgrowth because ordered MT prevent outgrowth in diffusely growing cells (Figure 3) (Fu et al. 2002, 2005). Similarly, MT are also excluded from the tip growth domain in tip-growing cells, and it would be interesting to see whether active ROPs also play a role in excluding MT from the tip.
The signaling pathway promoting indentation
In the indenting region, RIC1 (a novel MT-associated protein) promotes MT ordering that restricts outgrowth, causing narrow necks (Figure 3) (Fu et al. 2005). Mutations in the CRIB motif of RIC1 eliminate the function of RIC1 in promoting MT ordering, implying that RIC1 may also be activated by a ROP GTPase to promote the organization of cortical MT (Y. Fu, Z.-L. Zheng & Z. Yang, unpublished data). These results seem to suggest a new ROP-RIC-mediated signaling pathway that activates MT ordering in plants (Fu et al. 2005). An increase in radial expansion of hypocotyl cells in the ric1 null mutant suggests that this pathway apparently also restricts radial expansion of elongating cells with simple diffuse growth (Y. Fu, Z.-L. Zheng & Z. Yang, unpublished data). Therefore, knowing which signals regulate this RIC1 signaling pathway may provide important insights into plant morphogenesis and development in general.
Microtubule suppression of outgrowth-promoting ROP signaling: a new paradigm for cell morphogenesis?
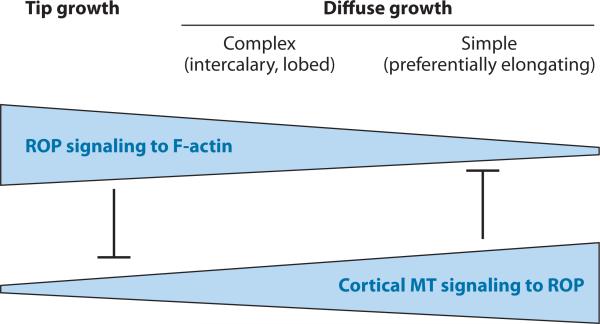
Cortical MT in the indenting region are known to signal back to ROP2/4 in such a way that ROP2/4 activation is repressed by cortical MT in the indenting region (Fu et al. 2005). In tip-growing PT, cortical MT present in the subapical region may also suppress ROP1 activation, probably by lateral inhibition (M. Zheng & Z. Yang, unpublished data). In root hairs, CA-ROP2-induced growth depolarization is enhanced by mutations in a kinesin that affects MT organization (Yang et al. 2007). Therefore, MT-mediated lateral inhibition of tip-localized ROP activation is involved in the regulation of tip growth polarity but is only secondary to the global inhibition. In contrast, cortical MT play a primary role in determining the polarity of diffuse growth, at least in part by suppressing outgrowth-promoting ROPs. This role can be extended to cells with simple diffuse growth, such as differentiating hypocotyls and root cells, in which cortical MT–mediated cell expansion in the direction perpendicular to MT orientation is primary, whereas ROP- and actin-induced cell expansion is secondary or minimal (Fu et al. 2002). On the basis of these observations, I propose that the interregulation between ROP signaling to actin-based polar growth and the polarity determination of cell expansion by cortical MT represents a unifying mechanism governing the polarity of cell expansion in plants, although the degree of significance for each of the two modes varies among tip growth and complex and simple diffuse growth (Figure 5).
Figure 5.
A schematic model for a unifying concept of the mechanisms underlying the control of cell morphogenesis through tip growth or polarized diffuse growth. I propose that the primary mechanism for the control of cell polarity in tip growth is the tip-localized ROP (Rho-related GTPase from plants) activation of actin dynamics, which promotes targeted exocytosis, whereas microtubule (MT)-mediated lateral inhibition plays a secondary role in polarity control. In contrast, MT-dependent mechanisms play a primary role in the control of cell polarity of diffuse growth, whereas ROP activation of actin microfilaments (F-actin) plays a secondary role in polarity control. Cortical MT–mediated polarity control involves its role in aligning cellulose microfibrils and suppression of the ROP-actin pathway. The ROP-actin pathway and the cortical MT counteract one another to fine-tune cell polarity and cell shape formation.
Auxin: a small molecule acting as a common polarity signal?
The initiation of intercalary growth in Arabidopsis PC is tightly regulated in time and space during leaf development, as growth starts from the tip and progressively moves along the margin and finally toward the middle of leaves/cotyledons. Auxin forms dynamic gradients that are tightly correlated with the spatiotemporal progression of the intercalary growth, suggesting that auxin may be the polarizing signal that initiates the intercalary growth of PC (T. Xu, M. Wen, Y. Fu, J. Friml, J. Chen, M. Wu, A. Jones & Z. Yang, unpublished data). Auxin has also been implicated in the control of the positioning of root hair–forming sites, which appear to depend on ROP2 localization to those sites (Fischer et al. 2006). Therefore, an auxin-ROP2 pathway may also regulate the planar polarity of root hair positioning. Auxin is implicated in the regulation of the polarity of PIN localization, which is also planar in nature (Paciorek et al. 2005). This raises an important question: Is auxin-ROP signaling a common mechanism for cell polarity control in plants?
Given a role for auxin as a polarizing signal, an exciting question is how auxin, a diffusible small molecule, locally controls signaling to establish cell polarity. The answer may lie in polar localization of the auxin efflux carrier proteins, PINs. PIN-mediated/localized auxin efflux may lead to a localized accumulation of extracellular auxin, which in turn may locally activate ROP signaling to establish polarity. Auxin promotes PIN localization to the site of auxin accumulation in root cells (Paciorek et al. 2005). Thus, it is tempting to speculate that auxin may activate ROP2, which may promote polar localization of an auxin efflux carrier to the lobe tip to form a positive feedback loop (Figure 3). In this speculative model, the auxin receptor responsible for ROP2 activation would most likely be an extracellular receptor, which may directly regulate the potential ROP2 activator, SPK1, a RhoGEF whose knockout produces a PC phenotype similar to that of a rop2/rop4 mutant (Basu et al. 2008, Fu et al. 2005, Qiu et al. 2002). PIN-exported auxin, which can diffuse across the cell wall to the indenting region of the neighboring cells, may also activate the ROP-RIC1 pathway as a possible mechanism for coordination between lobe outgrowth and indentation formation (Figure 3).
Polar Localization of Proteins: The PIN-FORMED Protein Model
Endosomal recycling and sorting are critical for polar localization and repolarization of PINs (see Kleine-Vehn & Friml 2008). How a recycled PIN decides to localize to a particular domain of the PM must require a signaling mechanism that integrates the specification of the PM domain with endosomal trafficking. Indeed, the PINOID (PID) ser/thr protein kinase and its antagonist, PP2AA phosphatase, are critical for the control of PIN polarity (Benjamins et al. 2001, Christensen et al. 2000, Friml et al. 2004, Michniewicz et al. 2007). Two important questions are how these kinases and phosphatases are regulated and how they are linked to the initial signal that specifies the cell polarity for PIN localization. Both PID and PP2AA partially colocalize with PIN1 in the PM, suggesting that the signaling most likely occurs there. A likely component in this signaling pathway(s) is 3-phosphoinositide-dependent protein kinase 1 (PDK1), which interacts with, phosphorylates, and activates PID (Zegzouti et al. 2006). An analogous signaling pathway involving PDK1 activation of PKC, a ser/thr kinase related to PID, modulates polarized PM localization of the GLUT4 glucose transporter in mammalian cells (Watson et al. 2004). There are also hints that ROP signaling may regulate PIN polarization ( Jaillais et al. 2007; Li et al. 2005; Xu & Scheres 2005a,b). GFP-ROP2 localization is polarized in a similar manner to PIN2-GFP, and ROP2 overexpression increases PIN2-GFP polar localization (Li et al. 2005). VPS29-mediated endosome sorting is required for PIN1 repolarization to initiate lateral root formation, and the lateral root formation defect in vps29 is epistatic to CA-ROP2 induction of lateral root formation ( Jaillais et al. 2007). Given these results, it will certainly be worthwhile to test the role of ROP signaling in PIN polarization.
Asymmetric Cell Division: The Guard Cell Model
Signaling to cell polarity formation required for asymmetric cell division in plants may be the least understood polarity system. However, a potential important breakthrough in this area is a series of recent discoveries of the signaling mechanisms regulating guard cell differentiation. The first discovery is the identification of the TOO MANY MOUTHS (TMM) gene, which encodes an extracellular leucine-rich repeat (LRR) receptor–like protein (Nadeau & Sack 2002). Loss of TMM function mutations causes a number of stomatal patterning pheno-types including stomata clustering, suggestive of a defect in responding to positional signals. It was later shown that a triple-knockout mutant for three closely related RLKs, ERRECTA and two ERRECTA-like genes (ERL1 and ERL2), exhibits a tmm phenotype and is epistatic to tmm (Shpak et al. 2005). A recent report has identified a secreted peptide (EPF1) that genetically acts in the same pathway as TMM and the ERRECTA family (Hara et al. 2007). Interestingly, EPF1 is only expressed in guard cell precursor cells, whereas both TMM and the ERRECTA family are expressed in their neighboring cells (Hara et al. 2007, Nadeau & Sack 2002, Shpak et al. 2005). Thus, it was proposed that the secreted EPF1 diffuses to the neighbors of the precursor cells to activate the coreceptor of TMM and the ERRECTA family proteins to regulate asymmetric cell division. Other exciting reports show that a mitogen-activated protein kinase (MAPK) cascade, composed of YODA (a MAPKKK), MKK4/MKK5, and MPK3/MPK6, has a function similar to that of TMM, ERRECTA, and EPF1 and appears to act downstream of them (Bergmann et al. 2004, Lukowitz et al. 2004, Wang et al. 2007). The constitutive activation of the MAP cascade causes an apparent defect in asymmetric cell division, turning all guard cells into PC (Lukowitz et al. 2004, Wang et al. 2007). The absence of this cascade apparently also abolishes the asymmetric cell division of the zygote, resulting in the elimination of suspensor cell fate (Lukowitz et al. 2004, Wang et al. 2007). Therefore, this apparent positional signal–mediated pathway seems to control the polarity of cell division in Arabidopsis.
However, there is an alternative hypothesis that this pathway acts downstream of the polarity signaling to regulate cell fate rather than cell polarity per se. In support of the polarity hypothesis, the loss-of-function yoda mutant has a defect in zygote cell elongation, which is likely a defect in zygote polarity establishment (Lukowitz et al. 2004, Wang et al. 2007). Unfortunately, the lack of cell polarity markers for guard cell stem cells or zygotes makes it difficult to directly test the role of this pathway in cell polarity control. Assuming that this signaling pathway regulates the polarity of cell division, some interesting questions remain to be answered. What is the cellular signaling target: polarization of the cytoskeleton, positioning of the nucleus, or polar cell expansion? Is this or another polarity-signaling pathway linked to the control of asymmetric cell division by intrinsic transcriptional factors known to affect asymmetric cell division (Aida et al. 2004, Pillitteri & Torii 2007, Xu et al. 2006)? Do similar pathways regulate asymmetric cell division in other aspects of plant development? What is the connection between RLKs and the MAPK cascade? Given the widespread role of ROPs in cell polarity control and the interaction of RopGEFs with RLKs (see above), it is tempting to speculate that ROP may be involved in bridging this gap.
CONCLUSIONS AND FUTURE PROSPECTS
Investigators have made important breakthroughs in studies of cellular signaling and cell polarity formation, using several plant model systems. In polarized cell growth, localized and dynamic ROP GTPase activity controls polarity establishment through the regulation of the cytoskeleton and vesicular trafficking, providing a framework for addressing many important and interesting questions in cell polarity control. It is yet to be determined whether this signaling paradigm can be extended to other cell polarity systems such as zygote polarization, polar PIN localization, and asymmetric cell division. Cytoskeleton-mediated feedback loops have been implicated in the regulation of localized and dynamic ROP signaling, but it is unclear what the molecular details of the feedback loops are and how they are triggered by an initial polarizing cue. Protein kinases such as plant-specific CDPKs and the conserved PID-related protein kinase family are known to participate in polarity signaling, but it is unknown whether and how they are linked to ROP signaling. PIPs have been implicated in cell polarity regulation in plants, yet their precise role and connection to ROP signaling need to be investigated. Although vesicular trafficking mechanisms, which include processes such as exocytosis and endocytic recycling, are critical for polarity formation, how they are linked to polarity signaling mechanisms remains vague. With existing and expanding genetic tools and molecular and cytological markers combined with proteomic, biochemical, and molecular approaches, exciting insights into these outstanding questions are in sight.
SUMMARY POINTS.
Cell polarity signaling has been studied through several model systems, including (a) single-cell systems such as PT and root hairs and (b) multicellular systems such as PC and PIN protein localization in roots.
As in animal cells, cortical MT are primarily involved in polarity induction, whereas the polarity-signaling targets, F-actin and associated vesicular trafficking, participate in polarity establishment and feedback-regulate polarity signaling.
As in all other eukaryotic cells, the plant subfamily of conserved Rho GTPases, ROP, is the central regulator of cell polarity signaling.
ROP is mediated by both conserved and plant-specific regulators, and it controls the dynamics and organization of both F-actin and MT through plant-specific effector proteins such as RICs.
In the PT single-cell system, the dynamics of the active ROP1 apical cap is essential for polarized cell growth: It modulates polarized exocytosis and is controlled by interlinking positive feedback–mediated lateral propagation and negative feedback–mediated RhoGAP-dependent global inhibition.
In jigsaw-puzzle-shaped PC, the complex polarity of cell expansion is controlled by ROP2 activation of localized F-actin in the lobing domain, which counteracts RIC1 activation of MT arrangement in the indenting domain.
Auxin may be a common polarizing signal, activating ROP signaling pathways in its control of cell polarity.
Polar localization of PINs is mediated by a cascade of PDK1 and PID ser/thr kinases, whereas asymmetric cell division may be controlled by an RLK-MAPK cascade signaling mechanism. Both of these kinase signaling cascades may be linked to ROP GTPase.
ACKNOWLEDGMENTS
I apologize to those whose relevant work cannot be cited in this review owing to space limitations. I thank Chizuko Morita for her preparation of the artwork used in this review. I thank members of my laboratory for stimulating discussions that helped to formulate the conceptual framework of this review. Work in my laboratory is supported by grants from the National Institutes of Health, the National Science Foundation, and the Department of Energy.
Footnotes
DISCLOSURE STATEMENT
The author is not aware of any biases that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- Aida M, Beis D, Heidstra R, Willemsen V, Blilou I, et al. The PLETHORA genes mediate patterning of the Arabidopsis root stem cell niche. Cell. 2004;119:109–20. doi: 10.1016/j.cell.2004.09.018. [DOI] [PubMed] [Google Scholar]
- Alberts P, Rudge R, Irinopoulou T, Danglot L, Gauthier-Rouviere C, Galli T. Cdc42 and actin control polarized expression of TI-VAMP vesicles to neuronal growth cones and their fusion with the plasma membrane. Mol. Biol. Cell. 2006;17:1194–203. doi: 10.1091/mbc.E05-07-0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu R, Chang F. Shaping the actin cytoskeleton using microtubule tips. Curr. Opin. Cell Biol. 2007;19:88–94. doi: 10.1016/j.ceb.2006.12.012. [DOI] [PubMed] [Google Scholar]
- Basu R, Le J, Zakharova T, Mallery EL, Szymanski DB. A SPIKE1 signaling complex controls actin-dependent cell morphogenesis through the heteromeric WAVE and ARP2/3 complexes. Proc. Natl. Acad. Sci. USA. 2008;105:4044–49. doi: 10.1073/pnas.0710294105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamins R, Quint A, Weijers D, Hooykaas P, Offringa R. The PINOID protein kinase regulates organ development in Arabidopsis by enhancing polar auxin transport. Development. 2001;128:4057–67. doi: 10.1242/dev.128.20.4057. [DOI] [PubMed] [Google Scholar]
- Bergmann DC, Lukowitz W, Somerville CR. Stomatal development and pattern controlled by a MAPKK kinase. Science. 2004;304:1494–97. doi: 10.1126/science.1096014. [DOI] [PubMed] [Google Scholar]
- Berken A, Thomas C, Wittinghofer A. A new family of RhoGEFs activates the ROP molecular switch in plants. Nature. 2005;436:1176–80. doi: 10.1038/nature03883. [DOI] [PubMed] [Google Scholar]
- Bidlingmaier S, Snyder M. Regulation of polarized growth initiation and termination cycles by the polarisome and Cdc42 regulators. J. Cell Biol. 2004;164:207–18. doi: 10.1083/jcb.200307065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch D, Lavy M, Efrat Y, Efroni I, Bracha-Drori K, et al. Ectopic expression of an activated RAC in Arabidopsis disrupts membrane cycling. Mol. Biol. Cell. 2005;16:1913–27. doi: 10.1091/mbc.E04-07-0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohme K, Li Y, Charlot F, Grierson C, Marrocco K, et al. The Arabidopsis COW1 gene encodes a phosphatidylinositol transfer protein essential for root hair tip growth. Plant. J. 2004;40:686–98. doi: 10.1111/j.1365-313X.2004.02245.x. [DOI] [PubMed] [Google Scholar]
- Brembu T, Winge P, Bones AM, Yang Z. A RHOse by any other name: a comparative analysis of animal and plant Rho GTPases. Cell Res. 2006;16:435–45. doi: 10.1038/sj.cr.7310055. [DOI] [PubMed] [Google Scholar]
- Brennwald P, Rossi G. Spatial regulation of exocytosis and cell polarity: yeast as a model for animal cells. FEBS Lett. 2007;581:2119–24. doi: 10.1016/j.febslet.2007.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K, Wennerberg K. Rho and Rac take center stage. Cell. 2004;116:167–79. doi: 10.1016/s0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- Campanoni P, Blatt MR. Membrane trafficking and polar growth in root hairs and pollen tubes. J. Exp. Bot. 2007;58:65–74. doi: 10.1093/jxb/erl059. [DOI] [PubMed] [Google Scholar]
- Carol RJ, Dolan L. The role of reactive oxygen species in cell growth: lessons from root hairs. J. Exp. Bot. 2006;57:1829–34. doi: 10.1093/jxb/erj201. [DOI] [PubMed] [Google Scholar]
- Carol RJ, Takeda S, Linstead P, Durrant MC, Kakesova H, et al. A RhoGDP dissociation inhibitor spatially regulates growth in root hair cells. Nature. 2005;438:1013–16. doi: 10.1038/nature04198. [DOI] [PubMed] [Google Scholar]
- Chang F, Yan A, Zhao L-N, Wu W-H, Yang Z. A putative calcium-permeable cyclic nucleotide–gated channel, CNGC18, regulates polarized pollen tube growth. J. Int. Plant. Biol. 2007;49:1261–70. [Google Scholar]
- Charest PG, Firtel RA. Feedback signaling controls leading-edge formation during chemotaxis. Curr. Opin. Genet. Dev. 2006;16:339–47. doi: 10.1016/j.gde.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Christensen SK, Dagenais N, Chory J, Weigel D. Regulation of auxin response by the protein kinase PINOID. Cell. 2000;100:469–78. doi: 10.1016/s0092-8674(00)80682-0. [DOI] [PubMed] [Google Scholar]
- Cole RA, Fowler JE. Polarized growth: maintaining focus on the tip. Curr. Opin. Plant Biol. 2006;9:579–88. doi: 10.1016/j.pbi.2006.09.014. [DOI] [PubMed] [Google Scholar]
- de Graaf BH, Cheung AY, Andreyeva T, Levasseur K, Kieliszewski M, Wu HM. Rab11 GTPase-regulated membrane trafficking is crucial for tip-focused pollen tube growth in tobacco. Plant Cell. 2005;17:2564–79. doi: 10.1105/tpc.105.033183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmasiri S, Swarup R, Mockaitis K, Dharmasiri N, Singh SK, et al. AXR4 is required for localization of the auxin influx facilitator AUX1. Science. 2006;312:1218–20. doi: 10.1126/science.1122847. [DOI] [PubMed] [Google Scholar]
- Dhonukshe P, Aniento F, Hwang I, Robinson DG, Mravec J, et al. Clathrin-mediated constitutive endocytosis of PIN auxin efflux carriers in Arabidopsis. Curr. Biol. 2007;17:520–27. doi: 10.1016/j.cub.2007.01.052. [DOI] [PubMed] [Google Scholar]
- Djakovic S, Dyachok J, Burke M, Frank MJ, Smith LG. BRICK1/HSPC300 functions with SCAR and the ARP2/3 complex to regulate epidermal cell shape in Arabidopsis. Development. 2006;133:1091–100. doi: 10.1242/dev.02280. [DOI] [PubMed] [Google Scholar]
- Dowd PE, Coursol S, Skirpan AL, Kao TH, Gilroy S. Petunia phospholipase C1 is involved in pollen tube growth. Plant Cell. 2006;18:1438–53. doi: 10.1105/tpc.106.041582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrhardt DW, Shaw SL. Microtubule dynamics and organization in the plant cortical array. Annu. Rev. Plant Biol. 2006;57:859–75. doi: 10.1146/annurev.arplant.57.032905.105329. [DOI] [PubMed] [Google Scholar]
- Engqvist-Goldstein AE, Drubin DG. Actin assembly and endocytosis: from yeast to mammals. Annu. Rev. Cell Dev. Biol. 2003;19:287–332. doi: 10.1146/annurev.cellbio.19.111401.093127. [DOI] [PubMed] [Google Scholar]
- Fischer U, Ikeda Y, Grebe M. Planar polarity of root hair positioning in Arabidopsis. Biochem. Soc. Trans. 2007;35:149–51. doi: 10.1042/BST0350149. [DOI] [PubMed] [Google Scholar]
- Fischer U, Ikeda Y, Ljung K, Serralbo O, Singh M, et al. Vectorial information for Arabidopsis planar polarity is mediated by combined AUX1, EIN2, and GNOM activity. Curr. Biol. 2006;16:2143–49. doi: 10.1016/j.cub.2006.08.091. [DOI] [PubMed] [Google Scholar]
- Foreman J, Demidchik V, Bothwell JH, Mylona P, Miedema H, et al. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature. 2003;422:442–46. doi: 10.1038/nature01485. [DOI] [PubMed] [Google Scholar]
- Fowler JE, Quatrano RS. Plant cell morphogenesis: plasma membrane interactions with the cytoskeleton and cell wall. Annu. Rev. Cell Dev. Biol. 1997;13:697–743. doi: 10.1146/annurev.cellbio.13.1.697. [DOI] [PubMed] [Google Scholar]
- France YE, Boyd C, Coleman J, Novick PJ. The polarity-establishment component Bem1p interacts with the exocyst complex through the Sec15p subunit. J. Cell Sci. 2006;119:876–88. doi: 10.1242/jcs.02849. [DOI] [PubMed] [Google Scholar]
- Frietsch S, Wang YF, Sladek C, Poulsen LR, Romanowsky SM, et al. A cyclic nucleotide–gated channel is essential for polarized tip growth of pollen. Proc. Natl. Acad. Sci. USA. 2007;104:14531–36. doi: 10.1073/pnas.0701781104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friml J, Vieten A, Sauer M, Weijers D, Schwarz H, et al. Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature. 2003;426:147–53. doi: 10.1038/nature02085. [DOI] [PubMed] [Google Scholar]
- Friml J, Yang X, Michniewicz M, Weijers D, Quint A, et al. A PINOID-dependent binary switch in apical-basal PIN polar targeting directs auxin efflux. Science. 2004;306:862–65. doi: 10.1126/science.1100618. [DOI] [PubMed] [Google Scholar]
- Fu Y, Gu Y, Zheng Z, Wasteneys G, Yang Z. Arabidopsis interdigitating cell growth requires two antagonistic pathways with opposing action on cell morphogenesis. Cell. 2005;120:687–700. doi: 10.1016/j.cell.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Fu Y, Li H, Yang Z. The ROP2 GTPase controls the formation of cortical fine F-actin and the early phase of directional cell expansion during Arabidopsis organogenesis. Plant Cell. 2002;14:777–94. doi: 10.1105/tpc.001537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Wu G, Yang Z. ROP GTPase-dependent dynamics of tip-localized F-actin controls tip growth in pollen tubes. J. Cell Biol. 2001;152:1019–32. doi: 10.1083/jcb.152.5.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geldner N, Anders N, Wolters H, Keicher J, Kornberger W, et al. The Arabidopsis GNOM ARF-GEF mediates endosomal recycling, auxin transport, and auxin-dependent plant growth. Cell. 2003;112:219–30. doi: 10.1016/s0092-8674(03)00003-5. [DOI] [PubMed] [Google Scholar]
- Goto T, Davidson L, Asashima M, Keller R. Planar cell polarity genes regulate polarized extracellular matrix deposition during frog gastrulation. Curr. Biol. 2005;15:787–93. doi: 10.1016/j.cub.2005.03.040. [DOI] [PubMed] [Google Scholar]
- Grieneisen VA, Xu J, Maree AF, Hogeweg P, Scheres B. Auxin transport is sufficient to generate a maximum and gradient guiding root growth. Nature. 2007;449:1008–13. doi: 10.1038/nature06215. [DOI] [PubMed] [Google Scholar]
- Grierson CS, Roberts K, Feldmann KA, Dolan L. The COW1 locus of Arabidopsis acts after RHD2, and in parallel with RHD3 and TIP1, to determine the shape, rate of elongation, and number of root hairs produced from each site of hair formation. Plant Physiol. 1997;115:981–90. doi: 10.1104/pp.115.3.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Fu Y, Dowd P, Li S, Vernoud V, et al. A Rho family GTPase controls actin dynamics and tip growth via two counteracting downstream pathways in pollen tubes. J. Cell Biol. 2005;169:127–38. doi: 10.1083/jcb.200409140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Li S, Lord EM, Yang Z. Members of a novel class of Arabidopsis Rho guanine nucleotide exchange factors control Rho GTPase-dependent polar growth. Plant Cell. 2006;18:366–81. doi: 10.1105/tpc.105.036434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Vernoud V, Fu Y, Yang Z. ROP GTPase regulation of pollen tube growth through the dynamics of tip-localized F-actin. J. Exp. Bot. 2003;54:93–101. doi: 10.1093/jxb/erg035. [DOI] [PubMed] [Google Scholar]
- Gu Y, Wang Z, Yang Z. ROP/RAC GTPase: an old new master regulator for plant signaling. Curr. Opin. Plant Biol. 2004;7:527–36. doi: 10.1016/j.pbi.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Hara K, Kajita R, Torii KU, Bergmann DC, Kakimoto T. The secretory peptide gene EPF1 enforces the stomatal one-cell-spacing rule. Genes Dev. 2007;21:1720–25. doi: 10.1101/gad.1550707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helling D, Possart A, Cottier S, Klahre U, Kost B. Pollen tube tip growth depends on plasma membrane polarization mediated by tobacco PLC3 activity and endocytic membrane recycling. Plant Cell. 2006;18:3519–34. doi: 10.1105/tpc.106.047373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdaway-Clarke TL, Feijo JA, Hackett GR, Kunkel JG, Hepler PK. Pollen tube growth and the intracellular cytosolic calcium gradient oscillate in phase while extracellular calcium influx is delayed. Plant Cell. 1997;9:1999–2010. doi: 10.1105/tpc.9.11.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulskamp M, Misra S, Jurgens G. Genetic dissection of trichome cell development in Arabidopsis. Cell. 1994;76:555–66. doi: 10.1016/0092-8674(94)90118-x. [DOI] [PubMed] [Google Scholar]
- Hwang J-U, Yang Z. Small GTPases and spatiotemporal regulation of pollen tube growth. In: Malho R, editor. The Pollen Tube. Springer-Verlag; Heidelberg, Ger.: 2006. pp. 95–116. [Google Scholar]
- Hwang JU, Gu Y, Lee YJ, Yang Z. Oscillatory ROP GTPase activation leads the oscillatory polarized growth of pollen tubes. Mol. Biol. Cell. 2005;16:5385–99. doi: 10.1091/mbc.E05-05-0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaillais Y, Fobis-Loisy I, Miege C, Rollin C, Gaude T. AtSNX1 defines an endosome for auxin-carrier trafficking in Arabidopsis. Nature. 2006;443:106–9. doi: 10.1038/nature05046. [DOI] [PubMed] [Google Scholar]
- Jaillais Y, Santambrogio M, Rozier F, Fobis-Loisy I, Miege C, Gaude T. The retromer protein VPS29 links cell polarity and organ initiation in plants. Cell. 2007;130:1057–70. doi: 10.1016/j.cell.2007.08.040. [DOI] [PubMed] [Google Scholar]
- Jones MA, Raymond MJ, Smirnoff N. Analysis of the root-hair morphogenesis transcriptome reveals the molecular identity of six genes with roles in root-hair development in Arabidopsis. Plant J. 2006;45:83–100. doi: 10.1111/j.1365-313X.2005.02609.x. [DOI] [PubMed] [Google Scholar]
- Jones MA, Raymond MJ, Yang Z, Smirnoff N. NADPH oxidase-dependent reactive oxygen species formation required for root hair growth depends on ROP GTPase. J. Exp. Bot. 2007;58:1261–70. doi: 10.1093/jxb/erl279. [DOI] [PubMed] [Google Scholar]
- Jones MA, Shen JJ, Fu Y, Li H, Yang Z, Grierson CS. The Arabidopsis ROP2 GTPase is a positive regulator of both root hair initiation and tip growth. Plant Cell. 2002;14:763–76. doi: 10.1105/tpc.010359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaothien P, Ok SH, Shuai B, Wengier D, Cotter R, et al. Kinase partner protein interacts with the LePRK1 and LePRK2 receptor kinases and plays a role in polarized pollen tube growth. Plant J. 2005;42:492–503. doi: 10.1111/j.1365-313X.2005.02388.x. [DOI] [PubMed] [Google Scholar]
- Kim S, Dong J, Lord EM. Pollen tube guidance: the role of adhesion and chemotropic molecules. Curr. Top. Dev. Biol. 2004;61:61–79. doi: 10.1016/S0070-2153(04)61003-9. [DOI] [PubMed] [Google Scholar]
- Kim S, Mollet JC, Dong J, Zhang K, Park SY, Lord EM. Chemocyanin, a small basic protein from the lily stigma, induces pollen tube chemotropism. Proc. Natl. Acad. Sci. USA. 2003;100:16125–30. doi: 10.1073/pnas.2533800100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klahre U, Becker C, Schmitt AC, Kost B. Nt-RhoGDI2 regulates Rac/ROP signaling and polar cell growth in tobacco pollen tubes. Plant J. 2006;46:1018–31. doi: 10.1111/j.1365-313X.2006.02757.x. [DOI] [PubMed] [Google Scholar]
- Klahre U, Kost B. Tobacco RhoGTPase ACTIVATING PROTEIN1 spatially restricts signaling of Rac/ROP to the apex of pollen tubes. Plant Cell. 2006;18:3033–46. doi: 10.1105/tpc.106.045336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein TJ, Mlodzik M. Planar cell polarization: An emerging model points in the right direction. Annu. Rev. Cell Dev. Biol. 2005;21:155–76. doi: 10.1146/annurev.cellbio.21.012704.132806. [DOI] [PubMed] [Google Scholar]
- Kleine-Vehn J, Dhonukshe P, Swarup R, Bennett M, Friml J. Subcellular trafficking of the Arabidopsis auxin influx carrier AUX1 uses a novel pathway distinct from PIN1. Plant Cell. 2006;18:3171–81. doi: 10.1105/tpc.106.042770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleine-Vehn J, Friml J. Polar targeting and endocytic recycling in auxin-dependent plant development. Annu. Rev. Cell Dev. Biol. 2008;24:447–73. doi: 10.1146/annurev.cellbio.24.110707.175254. [DOI] [PubMed] [Google Scholar]
- Knaus M, Pelli-Gulli MP, van Drogen F, Springer S, Jaquenoud M, Peter M. Phosphorylation of Bem2p and Bem3p may contribute to local activation of Cdc42p at bud emergence. EMBO J. 2007;26:4501–13. doi: 10.1038/sj.emboj.7601873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kost B, Lemichez E, Spielhofer P, Hong Y, Tolias K, et al. Rac homologues and compartmentalized phosphatidylinositol 4,5-bisphosphate act in a common pathway to regulate polar pollen tube growth. J. Cell Biol. 1999;145:317–30. doi: 10.1083/jcb.145.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kost B, Spielhofer P, Chua NH. A GFP-mouse talin fusion protein labels plant actin filaments in vivo and visualizes the actin cytoskeleton in growing pollen tubes. Plant J. 1998;16:393–401. doi: 10.1046/j.1365-313x.1998.00304.x. [DOI] [PubMed] [Google Scholar]
- La Carbona S, Le Goff C, Le Goff X. Fission yeast cytoskeletons and cell polarity factors: connecting at the cortex. Biol. Cell. 2006;98:619–31. doi: 10.1042/BC20060048. [DOI] [PubMed] [Google Scholar]
- Lavy M, Bloch D, Hazak O, Gutman I, Poraty L, et al. A novel ROP/Rac effector links cell polarity, root-meristem maintenance, and vesicle trafficking. Curr. Biol. 2007;17:947–52. doi: 10.1016/j.cub.2007.04.038. [DOI] [PubMed] [Google Scholar]
- Lee Y-J, Szumlanski A, Nielsen E, Yang Z. Rho-GTPase-dependent F-actin dynamics coordinates vesicle targeting and exocytosis during tip growth. J. Cell Biol. 2008;181:1155–68. doi: 10.1083/jcb.200801086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyser O. Auxin distribution and plant pattern formation: How many angels can dance on the point of PIN? Cell. 2005;121:819–22. doi: 10.1016/j.cell.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Li H, Lin Y, Heath RM, Zhu MX, Yang Z. Control of pollen tube tip growth by a ROP GTPase–dependent pathway that leads to tip-localized calcium influx. Plant Cell. 1999;11:1731–42. doi: 10.1105/tpc.11.9.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Xu J, Xu ZH, Xue HW. Brassinosteroids stimulate plant tropisms through modulation of polar auxin transport in Brassica and Arabidopsis. Plant Cell. 2005;17:2738–53. doi: 10.1105/tpc.105.034397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Blanchoin L, Yang Z, Lord EM. The putative Arabidopsis ARP2/3 complex controls leaf cell morphogenesis. Plant Physiol. 2003;132:2034–44. doi: 10.1104/pp.103.028563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Wang Y, Zhu JK, Yang Z. Localization of a Rho GTPase implies a role in tip growth and movement of the generative cell in pollen tubes. Plant Cell. 1996;8:293–303. doi: 10.1105/tpc.8.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Yang Z. Inhibition of pollen tube elongation by microinjected anti-ROP1Ps antibodies suggests a crucial role for Rho-type GTPases in the control of tip growth. Plant Cell. 1997;9:1647–59. doi: 10.1105/tpc.9.9.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisboa S, Scherer GE, Quader H. Localized endocytosis in tobacco pollen tubes: visualisation and dynamics of membrane retrieval by a fluorescent phospholipid. Plant Cell Rep. 2007;27:21–28. doi: 10.1007/s00299-007-0437-1. [DOI] [PubMed] [Google Scholar]
- Lord EM, Russell SD. The mechanisms of pollination and fertilization in plants. Annu. Rev. Cell Dev. Biol. 2002;18:81–105. doi: 10.1146/annurev.cellbio.18.012502.083438. [DOI] [PubMed] [Google Scholar]
- Lovy-Wheeler A, Wilsen KL, Baskin TI, Hepler PK. Enhanced fixation reveals the apical cortical fringe of actin filaments as a consistent feature of the pollen tube. Planta. 2005;221:95–104. doi: 10.1007/s00425-004-1423-2. [DOI] [PubMed] [Google Scholar]
- Lu L, Lee YR, Pan R, Maloof JN, Liu B. An internal motor kinesin is associated with the Golgi apparatus and plays a role in trichome morphogenesis in Arabidopsis. Mol. Biol. Cell. 2005;16:811–23. doi: 10.1091/mbc.E04-05-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukowitz W, Roeder A, Parmenter D, Somerville C. A MAPKK kinase gene regulates extraembryonic cell fate in Arabidopsis. Cell. 2004;116:109–19. doi: 10.1016/s0092-8674(03)01067-5. [DOI] [PubMed] [Google Scholar]
- Marco E, Wedlich-Soldner R, Li R, Altschuler SJ, Wu LF. Endocytosis optimizes the dynamic localization of membrane proteins that regulate cortical polarity. Cell. 2007;129:411–22. doi: 10.1016/j.cell.2007.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur J, Chua NH. Microtubule stabilization leads to growth reorientation in Arabidopsis trichomes. Plant Cell. 2000;12:465–77. doi: 10.1105/tpc.12.4.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen DG, Peifer M. Wnt signaling: the naked truth? Curr. Biol. 2001;11:R524–26. doi: 10.1016/s0960-9822(01)00310-4. [DOI] [PubMed] [Google Scholar]
- McInnis SM, Desikan R, Hancock JT, Hiscock SJ. Production of reactive oxygen species and reactive nitrogen species by angiosperm stigmas and pollen: potential signalling crosstalk? New Phytol. 2006;172:221–28. doi: 10.1111/j.1469-8137.2006.01875.x. [DOI] [PubMed] [Google Scholar]
- Mehta SQ, Hiesinger PR, Beronja S, Zhai RG, Schulze KL, et al. Mutations in Drosophila sec15 reveal a function in neuronal targeting for a subset of exocyst components. Neuron. 2005;46:219–32. doi: 10.1016/j.neuron.2005.02.029. [DOI] [PubMed] [Google Scholar]
- Meller N, Merlot S, Guda C. CZH proteins: a new family of Rho-GEFs. J. Cell Sci. 2005;118:4937–46. doi: 10.1242/jcs.02671. [DOI] [PubMed] [Google Scholar]
- Michniewicz M, Zago MK, Abas L, Weijers D, Schweighofer A, et al. Antagonistic regulation of PIN phosphorylation by PP2A and PINOID directs auxin flux. Cell. 2007;130:1044–56. doi: 10.1016/j.cell.2007.07.033. [DOI] [PubMed] [Google Scholar]
- Molendijk AJ, Bischoff F, Rajendrakumar CS, Friml J, Braun M, et al. Arabidopsis thaliana ROP GTPases are localized to tips of root hairs and control polar growth. EMBO J. 2001;20:2779–88. doi: 10.1093/emboj/20.11.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro D, Castanho Coelho P, Rodrigues C, Camacho L, Quader H, Malho R. Modulation of endocytosis in pollen tube growth by phosphoinositides and phospholipids. Protoplasma. 2005;226:31–38. doi: 10.1007/s00709-005-0102-x. [DOI] [PubMed] [Google Scholar]
- Moseley JB, Goode BL. The yeast actin cytoskeleton: from cellular function to biochemical mechanism. Microbiol. Mol. Biol. Rev. 2006;70:605–45. doi: 10.1128/MMBR.00013-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller S, Han S, Smith LG. Two kinesins are involved in the spatial control of cytokinesis in Arabidopsis thaliana. Curr. Biol. 2006;16:888–94. doi: 10.1016/j.cub.2006.03.034. [DOI] [PubMed] [Google Scholar]
- Murphy AS, Bandyopadhyay A, Holstein SE, Peer WA. Endocytotic cycling of PM proteins. Annu. Rev. Plant Biol. 2005;56:221–51. doi: 10.1146/annurev.arplant.56.032604.144150. [DOI] [PubMed] [Google Scholar]
- Nadeau JA, Sack FD. Control of stomatal distribution on the Arabidopsis leaf surface. Science. 2002;296:1697–700. doi: 10.1126/science.1069596. [DOI] [PubMed] [Google Scholar]
- Nibau C, Wu HM, Cheung AY. RAC/ROP GTPases: “hubs” for signal integration and diversification in plants. Trends Plant Sci. 2006;11:309–15. doi: 10.1016/j.tplants.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Nishimura T, Kaibuchi K. Numb controls integrin endocytosis for directional cell migration with aPKC and PAR-3. Dev. Cell. 2007;13:15–28. doi: 10.1016/j.devcel.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Ovecka M, Lang I, Baluska F, Ismail A, Illes P, Lichtscheidl IK. Endocytosis and vesicle trafficking during tip growth of root hairs. Protoplasma. 2005;226:39–54. doi: 10.1007/s00709-005-0103-9. [DOI] [PubMed] [Google Scholar]
- Paciorek T, Zazimalova E, Ruthardt N, Petrasek J, Stierhof YD, et al. Auxin inhibits endocytosis and promotes its own efflux from cells. Nature. 2005;435:1251–56. doi: 10.1038/nature03633. [DOI] [PubMed] [Google Scholar]
- Palanivelu R, Brass L, Edlund AF, Preuss D. Pollen tube growth and guidance is regulated by POP2, an Arabidopsis gene that controls GABA levels. Cell. 2003;114:47–59. doi: 10.1016/s0092-8674(03)00479-3. [DOI] [PubMed] [Google Scholar]
- Palanivelu R, Preuss D. Distinct short-range ovule signals attract or repel Arabidopsis thaliana pollen tubes in vitro. BMC Plant Biol. 2006;6:7. doi: 10.1186/1471-2229-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker JS, Cavell AC, Dolan L, Roberts K, Grierson CS. Genetic interactions during root hair morphogenesis in Arabidopsis. Plant Cell. 2000;12:1961–74. doi: 10.1105/tpc.12.10.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson ES, Miller DD, Callaham DA, Shipley AM, Rivers BA, et al. Pollen tube growth is coupled to the extracellular calcium ion flux and the intracellular calcium gradient: effect of BAPTA-type buffers and hypertonic media. Plant Cell. 1994;6:1815–28. doi: 10.1105/tpc.6.12.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillitteri LJ, Torii KU. Breaking the silence: Three bHLH proteins direct cell-fate decisions during stomatal development. Bioessays. 2007;29:861–70. doi: 10.1002/bies.20625. [DOI] [PubMed] [Google Scholar]
- Potocky M, Jones MA, Bezvoda R, Smirnoff N, Zarsky V. Reactive oxygen species produced by NADPH oxidase are involved in pollen tube growth. New Phytol. 2007;174:742–51. doi: 10.1111/j.1469-8137.2007.02042.x. [DOI] [PubMed] [Google Scholar]
- Preuss ML, Schmitz AJ, Thole JM, Bonner HK, Otegui MS, Nielsen E. A role for the RabA4b effector protein PI-4Kβ1 in polarized expansion of root hair cells in Arabidopsis thaliana. J. Cell Biol. 2006;172:991–98. doi: 10.1083/jcb.200508116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss ML, Serna J, Falbel TG, Bednarek SY, Nielsen E. The Arabidopsis Rab GTPase RabA4b localizes to the tips of growing root hair cells. Plant Cell. 2004;16:1589–603. doi: 10.1105/tpc.021634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts S, Chary SN, Drakakaki G, Li S, Yang Z, Raikhel NV, Hicks GR. Brassinosteroid receptor BRI1 and the auxin transporters PIN2 and AUX1 are sorted via specific endosomes essential for BRI1 signaling. Proc. Natl. Acad. Sci. USA. 2008 doi: 10.1073/pnas.0711650105. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price MH, Roberts DM, McCartney BM, Jezuit E, Peifer M. Cytoskeletal dynamics and cell signaling during planar polarity establishment in the Drosophila embryonic denticle. J. Cell Sci. 2006;119:403–15. doi: 10.1242/jcs.02761. [DOI] [PubMed] [Google Scholar]
- Pruyne D, Bretscher A. Polarization of cell growth in yeast. J. Cell Sci. 2000;113:571–85. doi: 10.1242/jcs.113.4.571. [DOI] [PubMed] [Google Scholar]
- Qiu JL, Jilk R, Marks MD, Szymanski DB. The Arabidopsis SPIKE1 gene is required for normal cell shape control and tissue development. Plant Cell. 2002;14:101–18. doi: 10.1105/tpc.010346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter S, Geldner N, Schrader J, Wolters H, Stierhof YD, et al. Functional diversification of closely related ARF-GEFs in protein secretion and recycling. Nature. 2007;448:488–92. doi: 10.1038/nature05967. [DOI] [PubMed] [Google Scholar]
- Samaj J, Muller J, Beck M, Bohm N, Menzel D. Vesicular trafficking, cytoskeleton and signalling in root hairs and pollen tubes. Trends Plant Sci. 2006;11:594–600. doi: 10.1016/j.tplants.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Schellmann S, Hulskamp M. Epidermal differentiation: trichomes in Arabidopsis as a model system. Int. J. Dev. Biol. 2005;49:579–84. doi: 10.1387/ijdb.051983ss. [DOI] [PubMed] [Google Scholar]
- Schiefelbein JW, Somerville C. Genetic control of root hair development in Arabidopsis thaliana. Plant Cell. 1990;2:235–43. doi: 10.1105/tpc.2.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settleman J. Intercalating Arabidopsis leaf cells: a jigsaw puzzle of lobes, necks, ROPs, and RICs. Cell. 2005;120:570–72. doi: 10.1016/j.cell.2005.02.025. [DOI] [PubMed] [Google Scholar]
- Shpak ED, McAbee JM, Pillitteri LJ, Torii KU. Stomatal patterning and differentiation by synergistic interactions of receptor kinases. Science. 2005;309:290–93. doi: 10.1126/science.1109710. [DOI] [PubMed] [Google Scholar]
- Siegrist SE, Doe CQ. Microtubule-induced cortical cell polarity. Genes Dev. 2007;21:483–96. doi: 10.1101/gad.1511207. [DOI] [PubMed] [Google Scholar]
- Smith LG. Cytoskeletal control of plant cell shape: getting the fine points. Curr. Opin. Plant Biol. 2003;6:63–73. doi: 10.1016/s1369-5266(02)00012-2. [DOI] [PubMed] [Google Scholar]
- Smith LG, Gerttula SM, Han S, Levy J. Tangled1: a microtubule binding protein required for the spatial control of cytokinesis in maize. J. Cell Biol. 2001;152:231–36. doi: 10.1083/jcb.152.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LG, Oppenheimer DG. Spatial control of cell expansion by the plant cytoskeleton. Annu. Rev. Cell Dev. Biol. 2005;21:271–95. doi: 10.1146/annurev.cellbio.21.122303.114901. [DOI] [PubMed] [Google Scholar]
- Song XF, Yang CY, Liu J, Yang WC. RPA, a class II ARFGAP protein, activates ARF1 and U5 and plays a role in root hair development in Arabidopsis. Plant Physiol. 2006;141:966–76. doi: 10.1104/pp.106.077818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmann T, Geldner N, Grebe M, Mangold S, Jackson CL, et al. Coordinated polar localization of auxin efflux carrier PIN1 by GNOM ARF GEF. Science. 1999;286:316–18. doi: 10.1126/science.286.5438.316. [DOI] [PubMed] [Google Scholar]
- Stenzel I, Ischebeck T, Konig S, Holubowska A, Sporysz M, et al. The type B phosphatidylinositol-4-phosphate 5-kinase 3 is essential for root hair formation in Arabidopsis thaliana. Plant Cell. 2008;20:124–41. doi: 10.1105/tpc.107.052852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymanski DB. Breaking the WAVE complex: the point of Arabidopsis trichomes. Curr. Opin. Plant Biol. 2005;8:103–12. doi: 10.1016/j.pbi.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Takeda S, Gapper C, Kaya H, Bell E, Kuchitsu K, Dolan L. Local positive feedback regulation determines cell shape in root hair cells. Science. 2008;319:1241–44. doi: 10.1126/science.1152505. [DOI] [PubMed] [Google Scholar]
- Teh OK, Moore I. An ARF-GEF acting at the Golgi and in selective endocytosis in polarized plant cells. Nature. 2007;448:493–96. doi: 10.1038/nature06023. [DOI] [PubMed] [Google Scholar]
- Ushinsky SC, Harcus D, Ash J, Dignard D, Marcil A, et al. CDC42 is required for polarized growth in human pathogen Candida albicans. Eukaryot. Cell. 2002;1:95–104. doi: 10.1128/EC.1.1.95-104.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernoud V, Horton AC, Yang Z, Nielsen E. Analysis of the small GTPase gene superfamily of Arabidopsis. Plant Physiol. 2003;131:1191–208. doi: 10.1104/pp.013052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker KL, Muller S, Moss D, Ehrhardt DW, Smith LG. Arabidopsis TANGLED identifies the division plane throughout mitosis and cytokinesis. Curr. Biol. 2007;17:1827–36. doi: 10.1016/j.cub.2007.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Ngwenyama N, Liu Y, Walker JC, Zhang S. Stomatal development and patterning are regulated by environmentally responsive mitogen-activated protein kinases in Arabidopsis. Plant Cell. 2007;19:63–73. doi: 10.1105/tpc.106.048298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson RT, Kanzaki M, Pessin JE. Regulated membrane trafficking of the insulin-responsive glucose transporter 4 in adipocytes. Endocr. Rev. 2004;25:177–204. doi: 10.1210/er.2003-0011. [DOI] [PubMed] [Google Scholar]
- Wedlich-Soldner R, Wai SC, Schmidt T, Li R. Robust cell polarity is a dynamic state established by coupling transport and GTPase signaling. J. Cell Biol. 2004;166:889–900. doi: 10.1083/jcb.200405061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells CD, Fawcett JP, Traweger A, Yamanaka Y, Goudreault M, et al. A Rich1/Amot complex regulates the Cdc42 GTPase and apical polarity proteins in epithelial cells. Cell. 2006;125:535–48. doi: 10.1016/j.cell.2006.02.045. [DOI] [PubMed] [Google Scholar]
- Wen TJ, Hochholdinger F, Sauer M, Bruce W, Schnable PS. The roothairless1 gene of maize encodes a homolog of sec3, which is involved in polar exocytosis. Plant Physiol. 2005;138:1637–43. doi: 10.1104/pp.105.062174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendland J, Philippsen P. Determination of cell polarity in germinated spores and hyphal tips of the filamentous ascomycete Ashbya gossypii requires a rhoGAP homolog. J. Cell Sci. 2000;113:1611–21. doi: 10.1242/jcs.113.9.1611. [DOI] [PubMed] [Google Scholar]
- Wendland J, Philippsen P. Cell polarity and hyphal morphogenesis are controlled by multiple Rho-protein modules in the filamentous ascomycete Ashbya gossypii. Genetics. 2001;157:601–10. doi: 10.1093/genetics/157.2.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewska J, Xu J, Seifertova D, Brewer PB, Ruzicka K, et al. Polar PIN localization directs auxin flow in plants. Science. 2006;312:883. doi: 10.1126/science.1121356. [DOI] [PubMed] [Google Scholar]
- Wong HL, Pinontoan R, Hayashi K, Tabata R, Yaeno T, et al. Regulation of rice NADPH oxidase by binding of Rac GTPase to its N-terminal extension. Plant Cell. 2007;19:4022–34. doi: 10.1105/tpc.107.055624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Gu Y, Li S, Yang Z. A genome-wide analysis of Arabidopsis ROP-interactive CRIB motif–containing proteins that act as ROP GTPase targets. Plant Cell. 2001;13:2841–56. doi: 10.1105/tpc.010218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Li H, Yang Z. Arabidopsis ROP GAPs are a novel family of Rho GTPase-activating proteins that require the Cdc42/Rac-interactive binding motif for ROP-specific GTPase stimulation. Plant Physiol. 2000;124:1625–36. doi: 10.1104/pp.124.4.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Hofhuis H, Heidstra R, Sauer M, Friml J, Scheres B. A molecular framework for plant regeneration. Science. 2006;311:385–88. doi: 10.1126/science.1121790. [DOI] [PubMed] [Google Scholar]
- Xu J, Scheres B. Cell polarity: ROPing the ends together. Curr. Opin. Plant Biol. 2005a;8:613–18. doi: 10.1016/j.pbi.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Xu J, Scheres B. Dissection of Arabidopsis ADP-RIBOSYLATION FACTOR 1 function in epidermal cell polarity. Plant Cell. 2005b;17:525–36. doi: 10.1105/tpc.104.028449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Gao P, Zhang H, Huang S, Zheng ZL. A mutation in MRH2 kinesin enhances the root hair tip growth defect caused by constitutively activated ROP2 small GTPase in Arabidopsis. PLoS ONE. 2007;2:e1074. doi: 10.1371/journal.pone.0001074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z. Small GTPases: versatile signaling switches in plants. Plant Cell. 2002;14(Suppl):S375–88. doi: 10.1105/tpc.001065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Fu Y. ROP/RAC GTPase signaling. Curr. Opin. Plant Biol. 2007;10:490–94. doi: 10.1016/j.pbi.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Watson JC. Molecular cloning and characterization of Rho, a Ras-related small GTP-binding protein from the garden pea. Proc. Natl. Acad. Sci. USA. 1993;90:8732–36. doi: 10.1073/pnas.90.18.8732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon GM, Dowd PE, Gilroy S, McCubbin AG. Calcium-dependent protein kinase isoforms in Petunia have distinct functions in pollen tube growth, including regulating polarity. Plant Cell. 2006;18:867–78. doi: 10.1105/tpc.105.037135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu A, Rual JF, Tamai K, Harada Y, Vidal M, et al. Association of Dishevelled with the clathrin AP-2 adaptor is required for Frizzled endocytosis and planar cell polarity signaling. Dev. Cell. 2007;12:129–41. doi: 10.1016/j.devcel.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajac A, Sun X, Zhang J, Guo W. Cyclical regulation of the exocyst and cell polarity determinants for polarized cell growth. Mol. Biol. Cell. 2005;16:1500–12. doi: 10.1091/mbc.E04-10-0896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zegzouti H, Anthony RG, Jahchan N, Bogre L, Christensen SK. Phosphorylation and activation of PINOID by the phospholipid signaling kinase 3-phosphoinositide-dependent protein kinase 1 (PDK1) in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2006;103:6404–9. doi: 10.1073/pnas.0510283103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Hill DR, Sylvester AW. Diversification of the RAB guanine triphosphatase family in dicots and monocots. J. Int. Plant. Biol. 2007;49:1129–41. [Google Scholar]
- Zhang Y, McCormick S. A distinct mechanism regulating a pollen-specific guanine nucleotide exchange factor for the small GTPase ROP in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA. 2007;104:18830–35. doi: 10.1073/pnas.0705874104. [DOI] [PMC free article] [PubMed] [Google Scholar]