Abstract
Vav guanine nucleotide exchange factors (GEFs) modulate changes in cytoskeletal organization through activation of Rho, Rac, and Cdc42 small GTPases. While Vav1 expression is restricted to the immune system, Vav2 and 3 are expressed in several tissues, including highly vascularized organs. Here, we provide the first evidence that Vav2 and Vav3 function within the tumor microenvironment to promote tumor growth, survival, and neovascularization. Host Vav2/3-deficiency reduced microvascular density, as well as tumor growth and/or survival, in transplanted B16 melanoma and Lewis lung carcinoma models in vivo. These defects were due in part to Vav2/3-deficiency in endothelial cells. Vav2/3-deficient endothelial cells displayed reduced migration in response to tumor cells in co-culture migration assays, and failed to incorporate into tumor vessels and enhance tumor volume in tumor-endothelial co-transplantation experiments. These data suggest that Vav2/3 guanine nucleotide exchange factors play a critical role in host-mediated tumor progression and angiogenesis, particularly in tumor endothelium.
Keywords: Vav, guanine nucleotide exchange factor, EphA2, tumor, angiogenesis, mouse, knockout
Introduction
Recruitment of new blood vessels by tumors, primarily through angiogenesis, is crucial for sustained growth, survival, and metastatic dissemination [Reviewed in (1-3)]. Tumor neovascularization facilitates tumor survival, growth, and malignant progression through delivery of oxygen and host nutrients. The process of angiogenesis, or sprouting of new blood vessel branches from pre-existing vasculature, is a complex, multi-step process that includes (i) endothelial cell activation by factors secreted by tumor cells, (ii) degradation of the basement membrane and extracellular matrix by proteases, (iii) proliferation, invasion, and migration of the polarized endothelial cells, (iv) coalescence and lumen formation, and (v) recruitment of perivascular support cells and production of extracellular matrix for stability of the new vessel [Reviewed in (3, 4)]. High level expression of pro-angiogenic factors and/or elevated microvascular density have been correlated with malignant progression and a poor prognosis in patients suffering from several types of cancer, including lung cancer (5-10) and melanoma (11-14). Thus understanding the molecular mechanisms that regulate tumor angiogenesis will enhance our efforts to target this process in the treatment of cancer.
Endothelial cell migration in response to tumor-derived signals is a key component of angiogenesis. Rho family GTPases, including Rho, Rac, and Cdc42, are critical mediators of cellular migration, and have been shown to regulate migration and morphogenesis of cultured endothelial cells [Reviewed in (15, 16)]. Rac, along with Cdc42, regulates endothelial morphogenesis and assembly (17, 18), whereas Rac alone was shown to regulate endothelial assembly and lumen formation in endothelial cells stimulated with vascular endothelial growth factor (VEGF) (19, 20). In addition to VEGF, ephrin-A1 regulates Rac-mediated endothelial cell migration. Ephrins, membrane tethered ligands for the Eph family of receptor tyrosine kinases, regulate angiogenesis during embryonic development, as well as in normal and diseases adult tissues [Reviewed in (21-24)]. In particular, ephrin-A1 and EphA2 have been linked with postnatal angiogenic remodeling and tumor angiogenesis [Reviewed in (21-24)]. We recently demonstrated that EphA2-deficient mice display significantly reduced angiogenic remodeling upon stimulation with ephrin-A1 (25), as well as reduced microvascular density upon transplantation of ephrin-A1 expressing tumor cells (26, 27). In addition, modulating ephrin-A1 expression in tumor cells impacts tumor microvascular density in vivo. Overexpression of ephrin-A1 in mammary adenocarcinoma cells enhanced, while siRNA-mediated downregulation of ephrin-A1 diminished, microvascular density in vivo and tumor-induced endothelial cell migration in vitro (28). Ephrin-A1 stimulates Rac and Cdc42 activation in cultured endothelial cells in an EphA2 receptor-dependent manner (25). Moreover, inhibition of Rac function reduces ephrin-A1-induced endothelial cell migration in vitro (25). Taken together, these data suggest that ephrin-A1/EphA2 activation of Rac is a major mechanism by which this ligand/receptor pair mediates vascular remodeling.
Rho family GTPases are molecular switches that cycle between an inactive, GDP-bound state and an active, GTP-bound conformation. In response to extracellular stimuli, guanine nucleotide exchange factors (GEFs) catalyze the exchange of GDP for GTP to activate the GTPase. GTPases may be inactivated by guanine nucleotide dissociation inhibitors (GDIs) that likely block spontaneous activation, or by GTPase activating proteins (GAPs) that stimulate GTPase activity [Reviewed in (29, 30)]. GEFs serve as links between extracellular stimulation, particularly by receptor tyrosine kinases, and Rho family GTPase activity [Reviewed in (31)]. The Vav family of GEFs, including Vav1, Vav2, and Vav3, are known regulators of GDP-GTP exchange for Rho family GTPases. We recently demonstrated that Vav2 and Vav3 physically associate with EphA2 receptor tyrosine kinase, linking EphA2 with Rac1 activation and endothelial cell assembly and migration in vitro (32). Moreover, Vav2/3-deficient mice display a greatly diminished angiogenic remodeling response to ephrin-A1 stimulation in vivo (32). The role of Vav2 and Vav3 GEFs in tumor angiogenesis, however, remains unclear. Here, we demonstrate that host Vav2/3-deficiency reduces microvascular density and tumor cell growth or survival in two independent mouse tumor models. These defects are, at least in part, intrinsic to loss of Vav2/3 in microvascular endothelium, as primary microvascular endothelial cells isolated from Vav2/3-deficient animals displayed defective tumor cell-induced migration in co-culture assays in vitro. In addition, Vav2/3-deficient endothelial cells failed to incorporate into tumor vessels when co-transplanted with tumor cells in vivo. Taken together, these data suggest that Vav2 and Vav3 GEFs are required for tumor neovascularization, providing insight into the molecular mechanisms that regulate tumor angiogenesis that may be applied to the development of new anti-angiogenic therapies in cancer treatment.
Results
Host Vav2/3-deficiency impairs tumor growth and/or survival and microvascular density in vivo
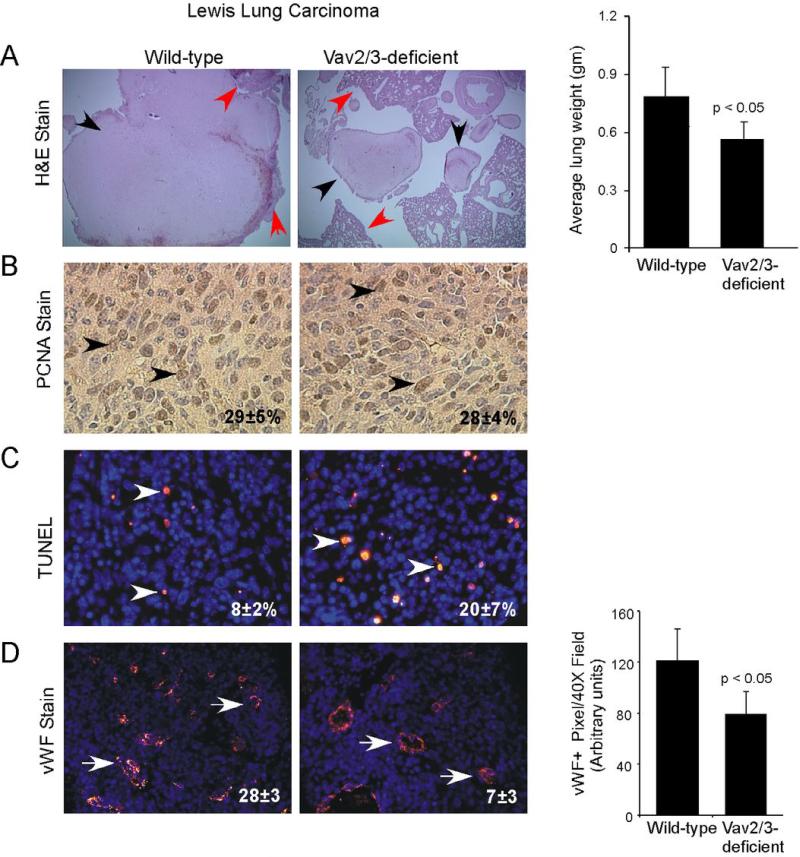
In order to determine the impact of host Vav2/3-deficiency on tumor progression in vivo, we orthotopically transplanted Lewis lung carcinoma (LLC) cells into Vav2/3-deficient and wild-type control animals. Ten days after transplantation, we observed that average lung weights, as a measure of tumor burden, were significantly lower in tissue isolated from Vav2/3-deficient hosts versus wild-type controls (Figure 1A), whereas there was no difference in normal lung weights in non-tumor bearing Vav2/3-deficient mice versus wild-type controls (data not shown). Hematoxylin and eosin stained sections confirmed engraftment of orthotopically transplanted tumor cells into the lung tissue of recipient mice (Figure 1A). While we detected no significant change in tumor cell proliferation (Figure 1B), apoptosis was significantly elevated in lung tumors derived from Vav2/3-deficient versus wild-type animals (Figure 1C). In addition, there was a significant decrease in microvascular density in tumors from Vav2/3-deficient mice versus wild-type controls (Figure 1D). These results are consistent with other studies involving anti-angiogenic therapy in orthotopic LLC tumors, in which increased apoptosis, but not proliferation, was observed (33), suggesting that these conditions favor apoptotic rather than growth defects.
Figure 1. Tumor burden, tumor cell survival, and microvascular density are reduced in Vav2/3-deficient hosts harboring Lewis Lung Carcinoma (LLC) orthotopic allografts in vivo.
(A) Hematoxylin and eosin stained lung tissue sections confirmed engraftment of LLC cells into the lungs of recipient mice (black arrowheads indicate tumor, red arrowheads indicate adjacent normal lung tissue). Tumor burden, as scored by average lung weight, was significantly decreased in Vav2/3-deficient animals harboring orthotopic LLC tumors relative to wild-type control hosts. Data were derived from analysis of 7 to 8 independent allografts/condition from 2 independent experiments. (B) There is no change in tumor cell proliferation between Vav2/3-deficient hosts and wild-type controls, as assessed by quantification of nuclear PCNA expression (arrowheads indicate PCNA+ nuclei). (C) Tumor cell survival, as scored by TUNEL assay, was significantly reduced in Vav2/3-deficient host animals relative to wild-type hosts (arrowheads indicate TUNEL+ nuclei; p<0.05). (D) Microvascular density, as scored by manual counting von Willebrand factor (vWF)+ vessels in tumor sections, was reduced in tumors isolated from Vav2/3-deficient host animals relative to controls (arrows indicate vWF+ vessels; p<0.05). Right panel, reduced microvascular density in tumors isolated from Vav2/3-deficient host animals was confirmed by quantification of vWF+ pixel area in tumor sections.
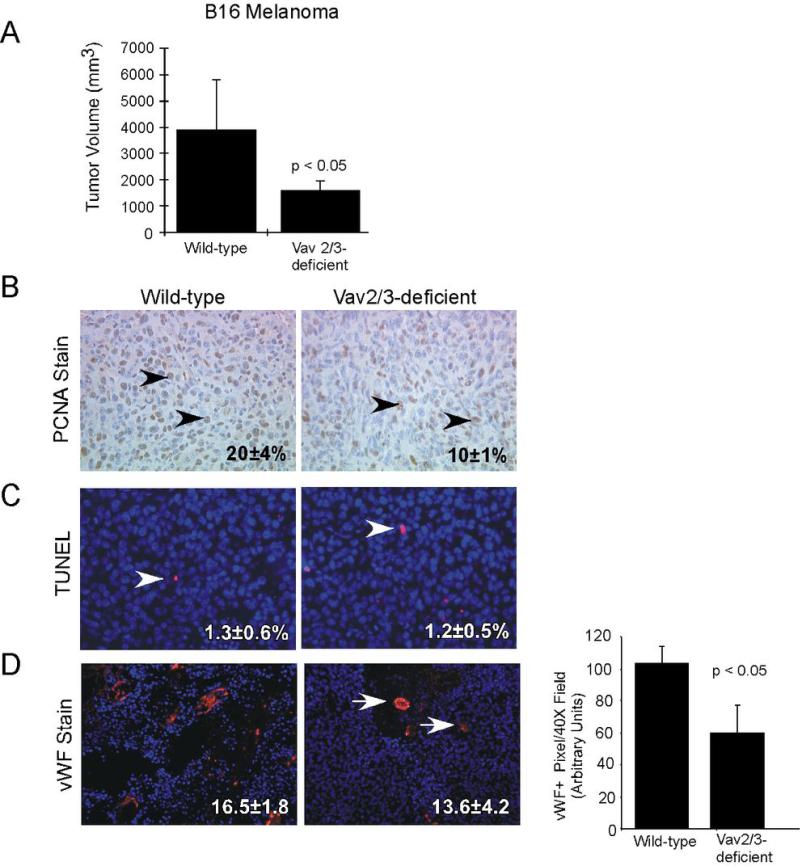
To confirm the impact of host Vav2/3-deficiency on tumor progression, we also assessed tumor volume, growth, survival, and vascularity in an independent allograft model. We injected B16 melanoma cells subcutaneously into Vav2/3-deficient and wild-type control animals. Two weeks after transplantation, we observed that tumor volume was significantly lower in tumors isolated from Vav2/3-deficient hosts relative to wild-type controls (Figure 2A). In this model, there was a significant decrease in peripheral tumor cell proliferation (Figure 2B), but no change in tumor cell apoptosis (Figure 2C) in tumors derived from Vav2/3-deficient animals versus controls. Consistent with data from LLC tumors, we also observed a significant decrease in microvascular density in tumors isolated from Vav2/3-deficient mice relative to wild-type animals (Figure 2D). These data suggest that host expression of Vav2/3 promotes tumor growth and/or survival, as well as neovascularization, in vivo.
Figure 2. Tumor burden, tumor cell proliferation, and microvascular density are reduced in Vav2/3-deficient hosts harboring B16 melanoma allografts in vivo.
(A) Tumor volume was significantly decreased in Vav2/3-deficient animals harboring B16 melanomas relative to wild-type control hosts. Data were derived from analysis of 10 independent allografts/condition from 2 independent experiments. (B) There is a significant reduction in tumor cell proliferation in Vav2/3-deficient hosts relative to controls, as assessed by quantification of nuclear PCNA expression (arrowheads indicate PCNA+ nuclei; p<0.05). (C) Tumor cell survival, as scored by TUNEL assay, was not affected in Vav2/3-deficient animals relative to wild-type controls (C, arrowheads indicate TUNEL+ nuclei). (D) Microvascular density, as scored by manual counting von Willebrand factor+ vessels in tumor sections, was reduced in tumors isolated from Vav2/3-deficient host animals relative to controls (arrows indicate vWF+ vessels; p<0.05). Right panel, reduced microvascular density in tumors isolated from Vav2/3-deficient host animals was confirmed by quantification of vWF+ pixel area in tumor sections.
Vav2/3 function is required for endothelial cell migration in response to tumor cells
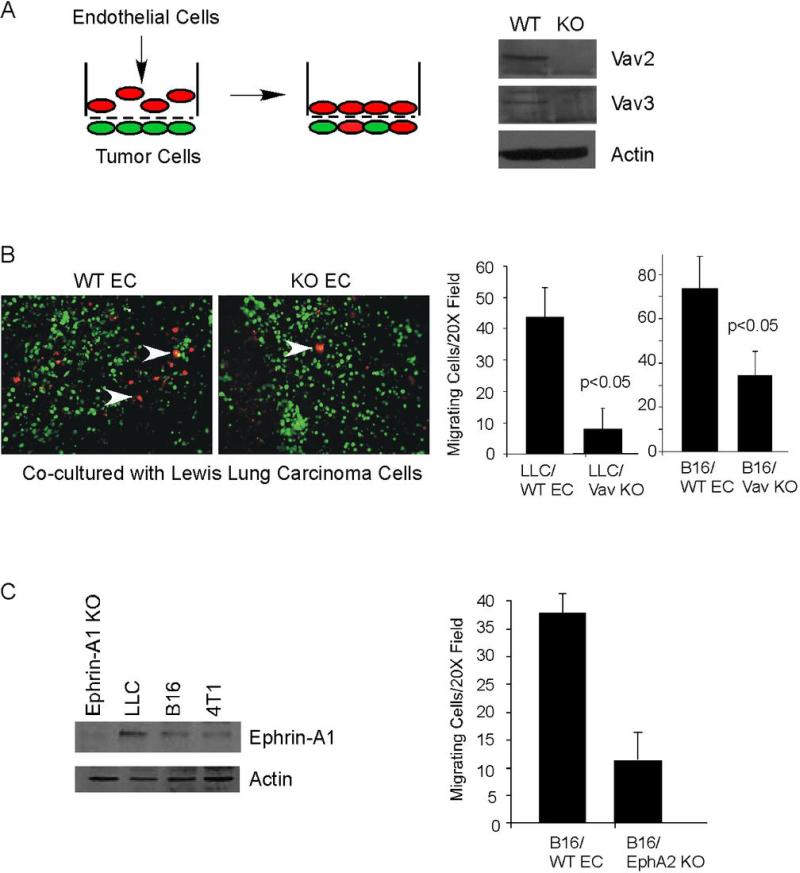
We observed reduced microvascular density in both LLC and B16 tumors in Vav2/3-deficient hosts, suggesting that Vav2/3 deficiency in host endothelium might affect tumor angiogenesis. We performed co-culture migration assays (Schematic, Figure 3A) to assess tumor cell-induced migration of primary endothelial cells derived from mouse lung (25, 27) for Vav2/3-deficient (32) versus control wild-type C57BL/6 animals. Lung microvascular endothelial cells isolated from wild-type or Vav2/3-deficient animals exhibited cobblestone-like morphology (Figure 4A) and over 95% of cells were CD31 positive (data not shown), confirming the endothelial character of these cells. Vav2 and Vav3 protein deficiency in these animals was confirmed by immunoblot of whole spleen extracts harvested at the time of endothelial cell isolation (Figure 3A). Vav2/3-deficient endothelial cells displayed significantly reduced migration in response to LLC and B16 melanoma (Figure 3B) tumor cells, suggesting that the defects in tumor neovascularization are, at least in part, due to Vav2/3-deficiency in endothelium. We have previously shown that Vav2/3 mediates EphA2 receptor signaling in vascular endothelial cells (32). Consistent with our prior studies, both LLC and B16 tumor cells express ephrin-A1 ligand, and EphA2-deficient endothelial cells are defective in migration towards B16 melanoma cells (Figure 3C) (34). These observations indicate that Vav2/3 GEFs also likely act downstream of EphA2 receptor in response to tumor cell-derived signals.
Figure 3. Vav2/3-deficient endothelial cells display reduced migration in response to LLC and B16 tumor cells.
(A) Schematic description of co-culture migration assay. Tumor cells harboring a green fluorescent dye are seeded on the lower surface of a transwell membrane and allowed to attach. Primary endothelial cells harboring a red fluorescent dye are placed in the top chamber and migration in response to tumor cells on the lower surface is measured after 5 hours. Loss of Vav2/3 protein expression in Vav2/3-deficient (KO) donor animals versus control (WT) mice was confirmed by immunoblot analysis of whole spleen extracts harvested at the time of primary endothelial cell isolation. (B) Primary lung microvascular endothelial cells isolated from Vav2/3-deficient mice displayed significantly reduced migration in response to Lewis Lung carcinoma and B16 melanoma tumor cells relative to endothelial cells isolated from wild-type control mice (arrowheads indicate endothelial cells that migrated and intercalated into tumor cell monolayer on the lower surface of the chamber). (C) We confirmed ephrin-A1 expression in B16 and LLC tumor cells by immunoblot analysis. Specificity of the antibody was confirmed by probing lysates from ephrin-A1-deficient cardiac tissue (ephrin-A1 KO). In co-culture migration assays, EphA2-deficient endothelial cells (EphA2 KO) significantly reduced migration in response to B16 melanoma cells plated on the lower surface of transwells relative to wild-type control endothelial cells, suggesting that ephrin-A1 actively promotes EphA2-dependent chemotaxis in this tumor cell model.
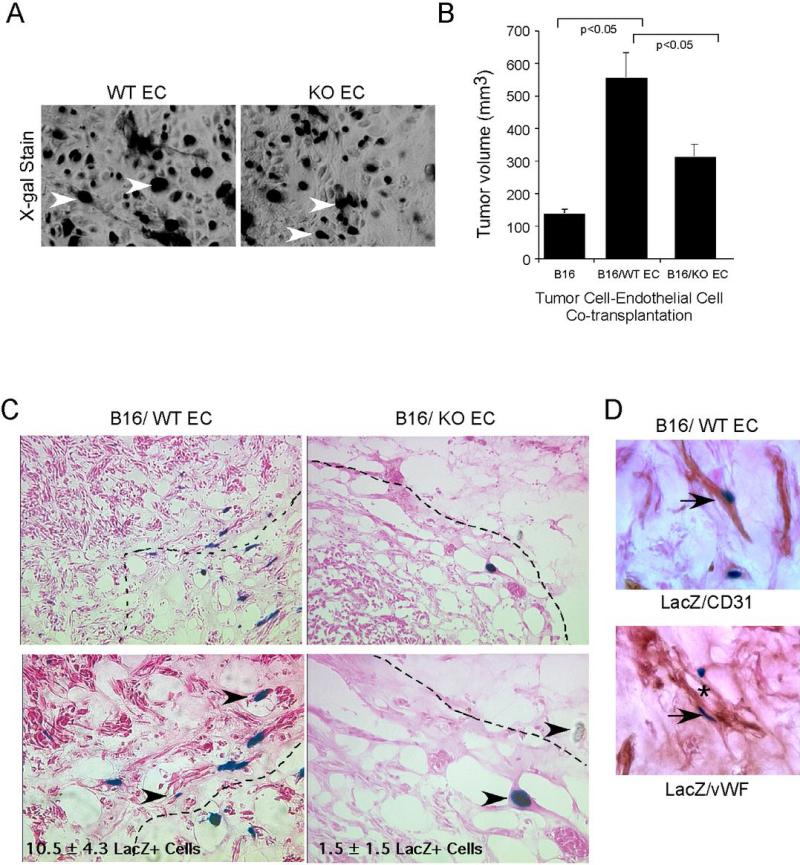
Figure 4. Vav2/3-deficient endothelial cells display reduced incorporation into tumor vessels upon co-transplantation with B16 melanoma cells in vivo.
B16 melanoma cells were mixed with primary lung microvascular endothelial cells isolated from Vav2/3-deficient mice (KO EC) or wild-type controls (WT EC). Prior to co-transplantation, the endothelial cells were infected with adenoviruses expressing β-galactosidase so as to distinguish them from host endothelium. The cells were suspended in growth factor-reduced Matrigel and injected subcutaneously into the dorsal flank of nude mice. Tumors were harvested after 10 days. (A) Endothelial cells isolated from wild-type or Vav2/3-deficient mice exhibit cobblestone-like morphology in culture and have similar numbers of cells with positive nuclear X-gal staining (arrowheads). (B) There is a significant increase in tumor volume in tumors harboring wild-type endothelial cells relative to tumors harboring no exogenous endothelial cells. Tumor volume was also greater in tumors harboring exogenous wild-type endothelial cells relative to tumors harboring Vav2/3-deficient endothelial cells. (C) Endothelial cells from wild-type mice were abundant and incorporated into tumor blood vessels (arrowheads indicate LacZ+ exogenous endothelial cells; dashed lines indicate tumor mass boundaries). In contrast, Vav2/3-deficient endothelial cells were less abundant, remained isolated and did not incorporate into tumor vessels as efficiently (p<0.05). (D) Co-staining with X-gal (blue nuclei) and CD31 or von Willebrand factor (brown) revealed that exogenous LacZ+ cells retain their endothelial cell character in vivo when incorporating into chimeric vessels (* indicates lumen of chimeric vessel).
Vav2/3-deficient endothelial cells fail to incorporate into tumor vasculature and support tumor progression in vivo
To determine if the defects in microvacular density and tumor progression in Vav2/3-deficient hosts are intrinsic to endothelial cells, we performed tumor cell/endothelial cell co-transplantation experiments using primary microvascular endothelial cells derived from Vav2/3-deficient or wild-type control animals. B16 melanoma cells were mixed with wild-type or Vav2/3-deficient primary endothelial cells, resuspended in growth factor-reduced Matrigel, and injected into the subcutaneous dorsal flank of nude female mice as described previously (27). In order to distinguish donor versus host endothelial cells, donor endothelial cells were transduced with adenoviruses harboring β-galactosidase prior to co-transplantation. Within the time frame of adenovirus transduction, over 95% of Vav2/3-deficient or wild-type control endothelial cells stained blue by X-gal, suggesting that transduction and expression of LacZ gene are equivalent in both cell populations (Figure 4A). We observed that co-transplantation with wild-type endothelial cells significantly enhanced tumor volume relative to transplantation of B16 melanoma cells alone after 10 days (Figure 4B). By contrast, co-transplantation with Vav2/3-deficient endothelial cells produced smaller tumor with significantly lower average tumor volume relative to co-transplantation with wild-type endothelial cells (Figure 4B). We observed fewer exogenous, LacZ+ endothelial cells in sections prepared from tumors harboring Vav2/3-deficient endothelial cells relative to tumors harboring wild-type control endothelial cells, both at the periphery and within the tumor mass (Figure 4C; tumor mass indicated by dashed lines). While wild-type control endothelial cells aggregated near tumor cell clusters and incorporated into tumor vessels (Figure 4D, * indicates chimeric vessel lumen), Vav2/3-deficient endothelial cells remained isolated and did not incorporate into tumor vessels as efficiently. We confirmed that exogenous cells retain CD31 and vWF expression in sections co-stained for X-gal (blue) and CD31/vWF (brown; Figure 4D). The majority of these Xgal/CD31 or vWF double positive cells exhibit endothelial morphology, indicating that they are vascular endothelial cells. These data suggest that Vav2/3-deficiency in host endothelium contributes significantly to defective tumor neovascularization in vivo. As small numbers of Xgal-positive donor cells may not be endothelial in origin, it is formally possible that contaminating non-endothelial cells could also contribute to tumor progression in our co-transplantation experiments.
Discussion
Investigation of signal transduction pathways through which Eph receptor tyrosine kinase and ephrins mediate such diverse biological processes as embryonic patterning, axon guidance, embryonic vascular remodeling, tumor neovascularization, and malignant transformation increasingly center on Rho family GTPases. [Reviewed in (24, 35-38)]. More specifically, interactions between Eph receptor and guanine nucleotide exchange factors such as ephexin (39), Tiam1 (40), kalirin (41), and vascular smooth muscle Rho-GEF (Vsm-RhoGEF) (42) are critical for proper neuronal patterning and vascular remodeling. We, and others, previously reported a novel interaction between Eph receptor tyrosine kinases and Vav GEFs (32, 43). Here, we demonstrate for the first time that Vav2/3 guanine nucleotide exchange factors are required in host tissue, specifically vascular endothelium, for maximal tumor neovascularization in vivo. Host Vav2/3-deficiency resulted in decreased microvascular density in two independent tumor allograft models in vivo, which correlated with decreased tumor cell survival and/or growth relative to allografts from wild-type control animals (Figures 1 and 2). Primary microvascular endothelial cells derived from Vav2/3-deficient animals displayed impaired migration in response to Lewis Lung carcinoma and B16 melanoma tumor cells in co-culture assays (Figure 3). Moreover, Vav2/3-deficient endothelial cells did not enhance tumor volume as robustly or incorporate into tumor vessels as efficiently as wild-type control cells when co-transplanted with B16 melanoma cells in vivo (Figure 4). These data suggest that Vav2 and Vav3 GEFs are key mediators of tumor neovascularization in vivo.
Our previous studies demonstrated that EphA2 receptor tyrosine kinase function in host endothelium is also necessary for tumor-induced endothelial cell migration in vitro and tumor angiogenesis in vivo (27). Moreover, ephrin-A1 expression in tumor cells facilitates tumor angiogenesis in vivo (28). Ephrin-A1 promotes migration and assembly of cultured endothelial cells through activation of Rac1 GTPase (25), and activation of Rac1 by ephrin-A1 was dependent upon EphA2 receptor tyrosine kinase expression, as well as on phosphatidylinositol 3-kinase (PI3-kinase) activity (25). More recently, we observed that Vav2 and Vav3 guanine nucleotide exchange factors physically interact with the intracellular domain of EphA2 receptor (32). Rac1 activation in response to ephrin-A1 was impaired significantly in Vav2/3-deficient primary endothelial cells, as was ephrin-A1 induced assembly into interconnected vascular networks in vitro. These data, coupled with the observation that ephrin-A1 failed to stimulate a subcutaneous angiogenic remodeling response in Vav2/3-deficient mice, suggested that Vav2 and Vav3 GEFs are key mediators of ephrin-A1 induced angiogenesis. As both LLC and B16 melanoma cells express high levels of ephrin-A1, data presented in this study provide a correlation between Vav2/3 GEFs to ephrin-mediated tumor neovascularization.
As ephrins are membrane tethered, however, the mechanism(s) by which these tumor-expressed ligands could communicate with EphA2 receptor on initially distant host endothelium remained unclear. This may be explained by evidence suggesting cooperation between ephrin-A1/EphA2 receptor signaling and the vascular endothelial growth factor (VEGF) pathway. We previously reported that EphA2-deficiency inhibits endothelial cell migration and assembly not only in response to ephrin-A1, but also upon stimulation with VEGF (44). Moreover, inhibition of class A Eph receptor signaling blocks corneal neovascularization in response to both ephrin-A1 and VEGF (45). More recently, we reported that downregulation of ephrin-A1 ligand expression in mouse mammary adenocarcinoma cells also reduced expression of VEGF, and that neutralizing anti-VEGF antibodies inhibited migration of endothelial cells in response to tumor cells overexpressing ephrin-A1 (28). Interestingly, VEGF-induced Rac activation is also dependent upon Vav2 function (46, 47). Moreover, downregulation of Vav2 expression in endothelial cells by siRNA inhibited VEGF-induced migration in vitro (47). Taken together, these data suggest that Vav GEFs may represent a point of convergence between ephrin and VEGF pathways in promoting anigiogenic remodeling. We assessed migration and assembly of EphA2-deficient endothelial cells reconstituted with a mutant receptor (Y587/593EE) that displays impaired binding to Vav2 and Vav3 proteins (48). While VEGF-mediated migration and assembly were impaired in EphA2-deficient control endothelial cells, consistent with previous observations (44), EphA2.Y587/593EE expressing cells displayed levels of migration and assembly comparable to levels observed in wild-type endothelial cells (data not shown). Moreover, expression of Vav2 in EphA2-deficient endothelial cells did not rescue migration and assembly in response to VEGF (data not shown). These data suggest that VEGF does not directly stimulate EphA2 receptor signaling. Given that VEGF treatment upregulates ephrin-A1 expression in endothelial cells (45), we hypothesize that cooperative signaling between VEGF and ephrins in parallel mediates angiogenic remodeling that is dependent upon Vav GEF function. Further investigation is required to investigate this hypothesis in the context of tumor angiogenesis, particularly as many tumors co-express ephrin-A1 and VEGF. Other investigators have reported that inhibition of EphA class signaling by soluble EphA2-Fc impairs angiogenic remodeling in response to basic fibroblast growth factor [FGF; (49)]. Thus, Eph/ephrin signaling may modulate chemotaxis in response to other cytokines, such as FGF2, in tumor angiogenesis.
In summary, we provide the first evidence that Vav2/3 GEFs are required for maximal tumor neovascularization in vivo. Our study provides insight into the molecular mechanisms that regulate tumor angiogenesis that may be applied to the development of new anti-angiogenic therapies in cancer treatment.
Materials and Methods
Reagents
Antibodies used include anti-Vav2 and anti-Vav3 (rabbit polyclonal produced by Y. Hwang), anti-ephrin-A1 (Zymed Laboratories, South San Francisco, CA), anti-EphA2 (Santa Cruz Biotechnology, Santa Cruz, CA), anti-actin (Santa Cruz Biotechnology), anti-von Willebrand factor (vWF; Dako, Carpinteria, CA), biotinylated anti-PCNA (BD Biosciences, San Jose, CA), anti-β-galactosidase (Millipore/Chemicon, Telecuma, CA), anti-Rac1 antibody (BD Biosciences, San Jose, CA), and anti-CD31 (BD Biosciences). Growth factor-reduced Matrigel was purchased from BD Biosciences. Apoptag Red In Situ Apoptosis detection kit was purchased from Millipore/Chemicon. Avidin-peroxidase reagents were from Vector Laboratories (Burlingame, CA), and 3,3’-diaminobenzidine tetrahydrochloride (DAB) substrate kit was from Zymed Laboratories. We purchased CellTracker Green CMFDA and Orange CMTMR dyes from Invitrogen/Molecular Probes (Carlsbad, CA). Costar transwells (8 μm polycarbonate membrane, 6.5 mm inserts) were obtained from Corning, Inc. (NY). Adenoviruses harboring control β-galactosidase were described previously (50). X-gal was purchased from Research Products International Corporation (Prospect, IL). We purchased ephrin-A1-Fc from R&D Systems (Minneapolis, MN).
Animal Studies
All animals were housed under pathogen-free conditions, and experiments were performed in accordance with AAALAC guidelines and with Vanderbilt University Institutional Animal Care and Use Committee approval. Vav2/3-deficient animals were generously provided by Dr. Wojciech Swat [Washington University School of Medicine (51)]. Athymic nude-Foxn1 mice (3−4 weeks old) were purchased from Harlan Sprague Dawley, Inc. (Indianapolis, IN).
Cell culture
B16 melanoma, Lewis lung carcinoma (LLC), and 4T1 mouse mammary adenocarcinoma cells were purchased from the American Type Culture Collection (ATCC; Manassas, VA) and maintained in Dulbecco's Modified Eagle's Medium (DMEM; Mediatech, Herndon, VA) supplemented with 10% fetal bovine serum (FBS; Hyclone, Logan, UT), 2 mM L-glutamine, and 5U/ml penicillin-streptymycin (Mediatech). Primary lung microvascular endothelial cells were isolated from wild-type C57BL/6J mice or syngeneic Vav2/3-deficient mice (51) as described previously (27, 32) and maintained in EGM-2 medium (Lonza, Basel, Switzerland) on tissue culture plates coated with 0.1% gelatin (Sigma-Aldrich, St. Louis, MO) solution in phosphate buffered saline (PBS). We confirmed that approximately 98% of cells expressed CD31 by immunofluorescence staining, indicating that the majority of the cells that we isolated are endothelial cells (data not shown). We confirmed loss of Vav2 and Vav3 protein expression in Vav2/3-deficient animals from which primary endothelial cells were derived, as well as intact expression of Vav2/3 in wild-type animals, by probing whole spleen lysates for expression of Vav2 and Vav3.
Tumor allografts
LLCs (2.5 × 105) were resuspended in 100 μl growth factor-reduced Matrigel. The Matrigel mixture was injected orthotopically into the left lateral thorax of syngeneic Vav2/3-deficient or wild-type control animals at the lateral dorsal axillary line to establish lung tumors as described by Wu et al. (52). Lungs were harvested 10 days post-injection and weighed as a measure of tumor burden. Data were derived from analysis of 7 to 8 independent allografts/condition from 2 independent experiments. B16 melanoma cells (5 × 105) were resuspended in 100 μl PBS, and injected subcutaneously into the dorsal flank of Vav2/3-deficient or wild-type control animals. Tumors were harvested 2 weeks post-injection and dimensions measured by digital caliper. Tumor volume was calculated using the following formula: volume = length × width2 × 0.52 (53). Data were derived from analysis of 10 independent allografts/condition from 2 independent experiments.
Tumor cell-endothelial cell co-transplantation assays
Primary lung microvascular endothelial cells isolated from Vav2/3-deficient and wild-type control mice were transduced with 108 pfu/ml adenovirus β-galactosidase 48 hours prior to co-transplantation. Co-transplantation experiments were performed as previously described (27). Briefly, endothelial cells (5 × 105) plus B16 melanoma cells (1 × 105) were suspended in 250 μl growth factor-reduced Matrigel and injected into the subcutaneous dorsal flanks of 5 week old athymic nude-Foxn1 mice. Tumors were collected 10 days post-transplantation and tumor volume assessed as described above. Data are a representation of 10 independent tumors/condition from two independent experiments.
Histology
LLC and B16 tumors were fixed in 10% neutral buffered formalin (Fisher Scientific, Pittsburgh, PA) overnight at 4°C and processed for paraffin embedding. For analysis of microvascular density, sections were stained with anti-vWF antibodies followed by anti-rabbit Cy3 (Jackson ImmunoResearch Laboratories, West Grove, PA) secondary as described previously (27, 28). The sections were counterstained with 4,6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich) to visualize nuclei, and microvascular density was measured in 40X fields photographed using an Olympus BX60 microscope plus digital camera. Microvascular density was quantified by measuring fluorescent pixel area using NIH Image J software as described previously (27, 28), as well as by manual counting of vWF+ vessels. For analysis of proliferation, sections were treated with biotinylated anti-proliferating cell nuclear antigen (PCNA) antibody followed by avidin-peroxidase and DAB substrate. The sections were counterstained with hematoxylin (Fisher Scientific) to visualize nuclei, and proliferation was assessed by calculating the average percent PCNA+ nuclei relative to the total number of nuclei in 40X fields as described previously (26). For analysis of apoptosis, sections were processed for TUNEL assay using the Apoptag Red In Situ Apoptosis detection kit as per manufacturer's instructions. Sections were counterstained with DAPI to visualize nuclei, and apoptosis was assessed by calculating the average percent TUNEL+ nuclei relative to the total number of nuclei in 40X fields as described previously (26). For all quantifications, 4 to 6 random 40X fields were photographed in 3 to 5 independent tumor sections/group.
Tumors harboring exogenous endothelial cells were isolated from nude mice and processed for histology as previously described (25, 27). Cryosections (10 μm) were processed for X-gal staining (25, 27). Sections were counterstained with eosin. LacZ positive, exogenous endothelial cells were counted in four independent 20X fields/section in 5 independent tumors/condition to quantify incorporation of exogenous endothelial cells into the tumor mass. Tumor sections were also co-stained with X-gal and an anti-CD31 antibody (BD Biosciences) or anti-vWF antibody (Dako) as described previously (25, 27).
Endothelial co-culture migration
Co-culture migration assays were performed as described previously (28, 54). Migrating cells were photographed and migration scored by counting the number of tumor cells that migrated and intercalated into the tumor cell monolayer on the lower surface of the transwell after 5 hours incubation. Data are a representation of nine independent samples per condition from three independent experiments.
Acknowledgements
This work was supported by NIH grants CA95004 and CA114301 (J. Chen), NIH grant CA1179151 (D. Brantley-Sieders), and Department of Defense predoctoral fellowships, W81XWH-08-1-029 and W81XWH-08-0250 (to D. Vaught and G. Zhuang, respectively). We thank Dr. Wojciech Swat at Washington University School of Medicine, for providing Vav2/3-deficient mice.
References
- 1.Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990;82:4–6. doi: 10.1093/jnci/82.1.4. [DOI] [PubMed] [Google Scholar]
- 2.Aubry K, Barriere G, Chable-Rabinovitch H, et al. Molecular mechanisms regulating the angiogenic phenotype in tumors: clinical impact in the future. Anticancer Res. 2007;27:3111–3119. [PubMed] [Google Scholar]
- 3.Kesisis G, Broxterman H, Giaccone G. Angiogenesis inhibitors. Drug selectivity and target specificity. Curr Pharm Des. 2007;13:2795–2809. doi: 10.2174/138161207781757033. [DOI] [PubMed] [Google Scholar]
- 4.Quesada AR, Munoz-Chapuli R, Medina MA. Anti-angiogenic drugs: from bench to clinical trials. Med Res Rev. 2006;26:483–530. doi: 10.1002/med.20059. [DOI] [PubMed] [Google Scholar]
- 5.Volm M, Koomagi R, Mattern J. Prognostic value of vascular endothelial growth factor and its receptor Flt-1 in squamous cell lung cancer. Int J Cancer. 1997;74:64–68. doi: 10.1002/(sici)1097-0215(19970220)74:1<64::aid-ijc11>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 6.Fontanini G, Faviana P, Lucchi M, et al. A high vascular count and overexpression of vascular endothelial growth factor are associated with unfavourable prognosis in operated small cell lung carcinoma. Br J Cancer. 2002;86:558–563. doi: 10.1038/sj.bjc.6600130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaya A, Ciledag A, Gulbay BE, et al. The prognostic significance of vascular endothelial growth factor levels in sera of non-small cell lung cancer patients. Respir Med. 2004;98:632–636. doi: 10.1016/j.rmed.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 8.Shimanuki Y, Takahashi K, Cui R, et al. Role of serum vascular endothelial growth factor in the prediction of angiogenesis and prognosis for non-small cell lung cancer. Lung. 2005;183:29–42. doi: 10.1007/s00408-004-2521-4. [DOI] [PubMed] [Google Scholar]
- 9.Ohta Y, Tanaka Y, Watanabe G, Minato H. Predicting recurrence following curative surgery in stage I non-small cell lung cancer patients using an angiogenesis-associated factor. J Exp Clin Cancer Res. 2007;26:301–305. [PubMed] [Google Scholar]
- 10.Yilmaz A, Ernam D, Unsal E, et al. Vascular endothelial growth factor immunostaining correlates with postoperative relapse and survival in non-small cell lung cancer. Arch Med Res. 2007;38:764–768. doi: 10.1016/j.arcmed.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 11.Vlaykova T, Laurila P, Muhonen T, et al. Prognostic value of tumour vascularity in metastatic melanoma and association of blood vessel density with vascular endothelial growth factor expression. Melanoma Res. 1999;9:59–68. doi: 10.1097/00008390-199902000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Straume O, Akslen LA. Expresson of vascular endothelial growth factor, its receptors (FLT-1, KDR) and TSP-1 related to microvessel density and patient outcome in vertical growth phase melanomas. Am J Pathol. 2001;159:223–235. doi: 10.1016/S0002-9440(10)61688-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tas F, Duranyildiz D, Oguz H, et al. Circulating serum levels of angiogenic factors and vascular endothelial growth factor receptors 1 and 2 in melanoma patients. Melanoma Res. 2006;16:405–411. doi: 10.1097/01.cmr.0000222598.27438.82. [DOI] [PubMed] [Google Scholar]
- 14.Vihinen P, Kallioinen M, Vuoristo MS, et al. Serum angiogenin levels predict treatment response in patients with stage IV melanoma. Clin Exp Metastasis. 2007;24:567–574. doi: 10.1007/s10585-007-9093-7. [DOI] [PubMed] [Google Scholar]
- 15.Bryan BA, D'Amore PA. What tangled webs they weave: Rho-GTPase control of angiogenesis. Cell Mol Life Sci. 2007;64:2053–2065. doi: 10.1007/s00018-007-7008-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fryer BH, Field J. Rho, Rac, Pak and angiogenesis: old roles and newly identified responsibilities in endothelial cells. Cancer Lett. 2005;229:13–23. doi: 10.1016/j.canlet.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 17.Connolly JO, Simpson N, Hewlett L, Hall A. Rac regulates endothelial morphogenesis and capillary assembly. Mol Biol Cell. 2002;13:2474–2485. doi: 10.1091/mbc.E02-01-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bayless KJ, Davis GE. The Cdc42 and Rac1 GTPases are required for capillary lumen formation in three-dimensional extracellular matrices. J Cell Sci. 2002;115:1123–1136. doi: 10.1242/jcs.115.6.1123. [DOI] [PubMed] [Google Scholar]
- 19.Soga N, Connolly JO, Chellaiah M, Kawamura J, Hruska KA. Rac regulates vascular endothelial growth factor stimulated motility. Cell Commun Adhes. 2001;8:1–13. doi: 10.3109/15419060109080703. [DOI] [PubMed] [Google Scholar]
- 20.Soga N, Namba N, McAllister S, et al. Rho family GTPases regulate VEGF-stimulated endothelial cell motility. Exp Cell Res. 2001;269:73–87. doi: 10.1006/excr.2001.5295. [DOI] [PubMed] [Google Scholar]
- 21.Pasquale EB. Eph-ephrin bidirectional signaling in physiology and disease. Cell. 2008;133:38–52. doi: 10.1016/j.cell.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 22.Kuijper S, Turner CJ, Adams RH. Regulation of angiogenesis by Eph-ephrin interactions. Trends Cardiovasc Med. 2007;17:145–151. doi: 10.1016/j.tcm.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 23.Zhang J, Hughes S. Role of the ephrin and Eph receptor tyrosine kinase families in angiogenesis and development of the cardiovascular system. J Pathol. 2006;208:453–461. doi: 10.1002/path.1937. [DOI] [PubMed] [Google Scholar]
- 24.Brantley-Sieders DM, Chen J. Eph receptor tyrosine kinases in angiogenesis: from development to disease. Angiogenesis. 2004;7:17–28. doi: 10.1023/B:AGEN.0000037340.33788.87. [DOI] [PubMed] [Google Scholar]
- 25.Brantley-Sieders DM, Caughron J, Hicks D, et al. EphA2 receptor tyrosine kinase regulates endothelial cell migration and vascular assembly through phosphoinositide 3-kinase-mediated Rac1 GTPase activation. J Cell Sci. 2004;117:2037–2049. doi: 10.1242/jcs.01061. [DOI] [PubMed] [Google Scholar]
- 26.Brantley-Sieders DM, Zhuang G, Hicks D, et al. The receptor tyrosine kinase EphA2 promotes mammary adenocarcinoma tumorigenesis and metastatic progression in mice by amplifying ErbB2 signaling. J Clin Invest. 2008;118:64–78. doi: 10.1172/JCI33154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brantley-Sieders DM, Fang WB, Hicks DJ, et al. Impaired tumor microenvironment in EphA2-deficient mice inhibits tumor angiogenesis and metastatic progression. Faseb J. 2005;19:1884–1886. doi: 10.1096/fj.05-4038fje. [DOI] [PubMed] [Google Scholar]
- 28.Brantley-Sieders DM, Fang WB, Hwang Y, Hicks D, Chen J. Ephrin-A1 facilitates mammary tumor metastasis through an angiogenesis-dependent mechanism mediated by EphA receptor and vascular endothelial growth factor in mice. Cancer Res. 2006;66:10315–10324. doi: 10.1158/0008-5472.CAN-06-1560. [DOI] [PubMed] [Google Scholar]
- 29.Bustelo XR, Sauzeau V, Berenjeno IM. GTP-binding proteins of the Rho/Rac family: regulation, effectors and functions in vivo. Bioessays. 2007;29:356–370. doi: 10.1002/bies.20558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- 31.Schiller MR. Coupling receptor tyrosine kinases to Rho GTPases--GEFs what's the link. Cell Signal. 2006;18:1834–1843. doi: 10.1016/j.cellsig.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 32.Hunter SG, Zhuang G, Brantley-Sieders D, et al. Essential role of Vav family guanine nucleotide exchange factors in EphA receptor-mediated angiogenesis. Mol Cell Biol. 2006;26:4830–4842. doi: 10.1128/MCB.02215-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ning T, Yan X, Lu ZJ, et al. Gene Therapy in Orthotopic Lung Cancer Murine Model with Angiogenesis Inhibitor, Endostatin. Hum Gene Ther. 2008 doi: 10.1089/hum.2008.098. [DOI] [PubMed] [Google Scholar]
- 34.Fang WB, Brantley-Sieders DM, Hwang Y, Ham AJ, Chen J. Identification and functional analysis of phosphorylated tyrosine residues within EphA2 receptor tyrosine kinase. J Biol Chem. 2008;283:16017–16026. doi: 10.1074/jbc.M709934200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poliakov A, Cotrina M, Wilkinson DG. Diverse roles of eph receptors and ephrins in the regulation of cell migration and tissue assembly. Dev Cell. 2004;7:465–480. doi: 10.1016/j.devcel.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 36.Murai KK, Pasquale EB. New exchanges in eph-dependent growth cone dynamics. Neuron. 2005;46:161–163. doi: 10.1016/j.neuron.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 37.Egea J, Klein R. Bidirectional Eph-ephrin signaling during axon guidance. Trends Cell Biol. 2007;17:230–238. doi: 10.1016/j.tcb.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 38.Noren NK, Pasquale EB. Eph receptor-ephrin bidirectional signals that target Ras and Rho proteins. Cell Signal. 2004;16:655–666. doi: 10.1016/j.cellsig.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 39.Shamah SM, Lin MZ, Goldberg JL, et al. EphA receptors regulate growth cone dynamics through the novel guanine nucleotide exchange factor ephexin. Cell. 2001;105:233–244. doi: 10.1016/s0092-8674(01)00314-2. [DOI] [PubMed] [Google Scholar]
- 40.Tolias KF, Bikoff JB, Kane CG, et al. The Rac1 guanine nucleotide exchange factor Tiam1 mediates EphB receptor-dependent dendritic spine development. Proc Natl Acad Sci U S A. 2007;104:7265–7270. doi: 10.1073/pnas.0702044104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Penzes P, Beeser A, Chernoff J, et al. Rapid induction of dendritic spine morphogenesis by trans-synaptic ephrinB-EphB receptor activation of the Rho-GEF kalirin. Neuron. 2003;37:263–274. doi: 10.1016/s0896-6273(02)01168-6. [DOI] [PubMed] [Google Scholar]
- 42.Ogita H, Kunimoto S, Kamioka Y, et al. EphA4-mediated Rho activation via Vsm-RhoGEF expressed specifically in vascular smooth muscle cells. Circ Res. 2003;93:23–31. doi: 10.1161/01.RES.0000079310.81429.C8. [DOI] [PubMed] [Google Scholar]
- 43.Cowan CW, Shao YR, Sahin M, et al. Vav family GEFs link activated Ephs to endocytosis and axon guidance. Neuron. 2005;46:205–217. doi: 10.1016/j.neuron.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 44.Chen J, Hicks D, Brantley-Sieders D, et al. Inhibition of retinal neovascularization by soluble EphA2 receptor. Exp Eye Res. 2006;82:664–673. doi: 10.1016/j.exer.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 45.Cheng N, Brantley DM, Liu H, et al. Blockade of EphA receptor tyrosine kinase activation inhibits vascular endothelial cell growth factor-induced angiogenesis. Mol Cancer Res. 2002;1:2–11. [PubMed] [Google Scholar]
- 46.Gavard J, Gutkind JS. VEGF controls endothelial-cell permeability by promoting the beta-arrestin-dependent endocytosis of VE-cadherin. Nat Cell Biol. 2006;8:1223–1234. doi: 10.1038/ncb1486. [DOI] [PubMed] [Google Scholar]
- 47.Garrett TA, Van Buul JD, Burridge K. VEGF-induced Rac1 activation in endothelial cells is regulated by the guanine nucleotide exchange factor Vav2. Exp Cell Res. 2007;313:3285–3297. doi: 10.1016/j.yexcr.2007.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fang WB, Brantley-Sieders DM, Hwang Y, Ham A, Chen J. Identification and functional analysis of phosphorylated tyrosine residues within EphA2 receptor tyrosine kinase. J Biol Chem. 2008 doi: 10.1074/jbc.M709934200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dobrzanski P, Hunter K, Jones-Bolin S, et al. Antiangiogenic and antitumor efficacy of EphA2 receptor antagonist. Cancer Res. 2004;64:910–919. doi: 10.1158/0008-5472.can-3430-2. [DOI] [PubMed] [Google Scholar]
- 50.Cheng N, Chen J. Tumor necrosis factor-alpha induction of endothelial ephrin A1 expression is mediated by a p38 MAPK- and SAPK/JNK-dependent but nuclear factor-kappa B-independent mechanism. J Biol Chem. 2001;276:13771–13777. doi: 10.1074/jbc.M009147200. [DOI] [PubMed] [Google Scholar]
- 51.Fujikawa K, Miletic AV, Alt FW, et al. Vav1/2/3-null mice define an essential role for Vav family proteins in lymphocyte development and activation but a differential requirement in MAPK signaling in T and B cells. J Exp Med. 2003;198:1595–1608. doi: 10.1084/jem.20030874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu W, O'Reilly MS, Langley RR, et al. Expression of epidermal growth factor (EGF)/transforming growth factor-alpha by human lung cancer cells determines their response to EGF receptor tyrosine kinase inhibition in the lungs of mice. Mol Cancer Ther. 2007;6:2652–2663. doi: 10.1158/1535-7163.MCT-06-0759. [DOI] [PubMed] [Google Scholar]
- 53.Bergers G, Brekken R, McMahon G, et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2:737–744. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brantley DM, Cheng N, Thompson EJ, et al. Soluble Eph A receptors inhibit tumor angiogenesis and progression in vivo. Oncogene. 2002;21:7011–7026. doi: 10.1038/sj.onc.1205679. [DOI] [PubMed] [Google Scholar]