Summary
Reciprocal regulation of tyrosine phosphorylation by protein tyrosine kinases and phosphatases is central to normal immune cell function. Disruption of the equilibrium between protein tyrosine kinase and phosphatase activity can result in immunodeficiency, autoimmunity, or malignancy. Src family kinases play a central role in both immune cell function and disease due to their proximal position in numerous signal transduction cascades including those emanating from integrin, T and B cell antigen receptors, Fc, growth factor, and cytokine receptors. Given that tight regulation of Src family kinase activity is critical for appropriate responses to stimulation of these various signaling pathways, it is perhaps not surprising that multiple protein tyrosine phosphatases are involved in their regulation. Here, we focus on the role of three phosphatases, CD45, CD148, and LYP/PEP, which are critical regulators of src family kinase activity in hematopoietic cells. We review our current understanding of their structures, expression, functions in different hematopoietic cell subsets, regulation, and putative roles in disease. Finally, we discuss remaining questions that must be addressed if we are to have a clearer understanding of the coordinated regulation of tyrosine phosphorylation and signaling networks in hematopoietic cells and how they could potentially be manipulated therapeutically in disease.
Keywords: Phosphatase, CD45, CD148, PTPN22, Pep/Lyp, Immune Regulation, Autoimmunity
Introduction
A key function of all cells is to integrate environmental signals into appropriate cellular responses. Regulated tyrosine phosphorylation plays a central role in this process. This is achieved by the reciprocal actions of protein tyrosine kinases (PTKs) and protein tyrosine phosphatases (PTPs). This equilibrium defines the signal transduction threshold for a given response and is critical for normal immune cell development and function (1-3). For example, precise and coordinated regulation tyrosine phosphorylation is essential for rapid responses to foreign antigens. Conversely, dysregulation of the equilibrium between PTK and PTP function can have pathologic consequences, resulting in immunodeficiency, autoimmunity, or malignancy.
In hematopoietic cells, Src family kinases (SFKs) play a central role in mediating communication between a cell and its environment (4). This is due to the fact that these non-receptor PTKs occupy a proximal position in numerous signaling transduction cascades including those emanating from the T and B cell antigen receptors, Fc receptors, growth factor receptors, cytokine receptors, and integrins. In addition to these positive regulatory roles, SFKs can also function as negative regulators of cellular signaling by phosphorylating immunoreceptor tyrosine-based inhibitory motifs (ITIMs) on inhibitory receptors, resulting in recruitment and activation of inhibitory molecules such as the phosphatases SHP-1 and SH2 containing 5′ inositol phosphatase (SHIP-1).
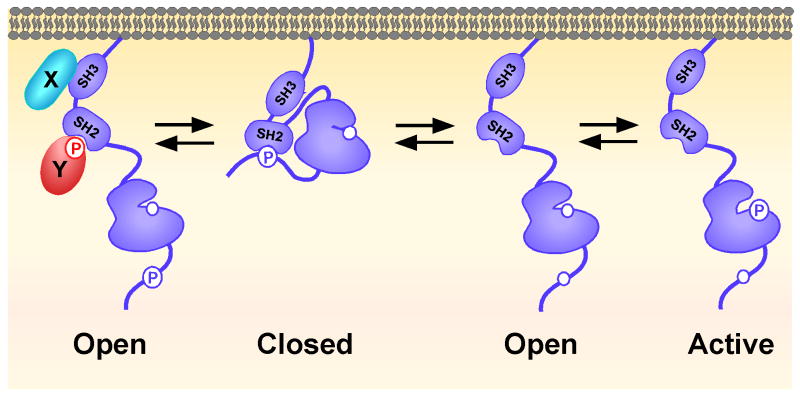
Given that SFKs regulate multiple signaling networks, it is perhaps not surprising that their inappropriate activation has been implicated in both autoimmunity and cancer ((4) and references therein). Thus, a major effort in current immunology and signal transduction research has focused on understanding the regulation of SFK activity. In large part, the relative activity of SFKs is defined by the regulated phosphorylation of two key residues that are highly conserved in all nine SFK family members (Figure 1) (4,5). Transphosphorylation of a site in the activation loop of the catalytic domain is required for full kinase activity. Conversely, phosphorylation of a tyrosine in the carboxy-terminal tail by the ubiquitous PTK C-terminal Src Kinase (Csk) inhibits kinase activity. This is due to an intramolecular interaction between the inhibitory phosphotyrosine and the SFKs own SH2 domain that blocks access to the substrate-binding site. This ‘closed’ conformation is further stabilized by interactions between the adjacent SH3 domain and a linker sequence between the SH2 and kinase domain (6,7). Intermediate between the active (or open) and closed conformations is an unphosphorylated, or ‘primed’ conformation that is capable of rapid activation in response to extracellular stimulation, which leads to clustering of SFKs on the cell surface and permits transphosphorylation of the kinase (Figure 1) (8,9).
Figure 1. SFKs are in a dynamic equilibrium between closed and active conformations.
Interaction of the SH2 domain and the C-terminal negative regulatory phosphotyrosine results in a closed conformation with decreased access to the kinase's catalytic site. Dephosphorylation of this site results in adoption of a more open, or ‘primed’, conformation. This conformation can potentially also be facilitated when the relatively weak interaction between the SH2 domain and the C-terminal phosphotyrosine is overcome by competing interactions of other proteins (denoted in the schematic as X or Y) with the SH2 or SH3 domains of the SFK. Phosphorylation of the catalytic site tyrosine is required for full kinase activity. (Adapted from (2,7)).
Complicating this model is the observation that the interaction between the SH2 domain and the inhibitory tyrosine is a relatively low affinity interaction that can be overcome by interactions of the SFK SH2 or SH3 domain with other proteins (6,7,10). This results in an ‘open’ conformation that can potentially become activated despite persistent phosphorylation of the C-terminal tyrosine. It has been proposed that the status of the catalytic pocket tyrosine dominates in this situation, determining whether or not the kinase is active (6,7,10). While this may explain why the levels of inhibitory tyrosine phosphorylation do not always correlate with kinase activity in some systems, caution must be exercised when interpreting these studies. Current biochemical approaches measure the phosphorylation status of a population and not individual molecules or subcellular pools of kinase. Indeed, while multiple studies support dual phosphorylation of both the C-terminal inhibitory and catalytic pocket activating tyrosines, whether these represent dual phosphorylation of single molecules or distinct kinases in separate pools are unclear. Recent data has shown that the phosphorylation status of SFKs can vary not only between thymocytes at different stages of development but also within different subcellular fractions (11).
Despite these complexities, it is clear that multiple PTPs play a central and essential role in the appropriate regulation of SFKs. Here, we focus on the role of three phosphatases, CD45, CD148, and Lyp/Pep, which are critical regulators of SFKs in hematopoietic cells. We review our current understanding of their structure, expression, functions in different hematopoietic cell subsets, regulation, and putative roles in disease. Finally, we discuss remaining questions that must be addressed if we are to have a clearer understanding of the coordinated regulation of tyrosine phosphorylation and signaling networks in hematopoietic cells and how they could potentially be manipulated therapeutically in disease.
PTPRC; CD45
CD45, encoded by Ptprc, is the prototypical receptor-like PTP on immune cells. It has long captivated the attention of immunologists due to its high levels of expression on all nucleated hematopoietic cells (up to 10% of the cell surface protein) (12), its highly regulated and complex patterns of isoform expression (12,13), and its central role in antigen receptor signaling (13,14). In both mice and humans, CD45 deficiency results in a severe combined immunodeficiency (SCID) phenotype (15-19). More recently, it has been implicated as a potential genetic modifier in autoimmune, infectious, and malignant diseases (20).
CD45 Structure
CD45 is a type 1 glycoprotein containing a large extracellular domain, a single transmembrane domain, and a cytoplasmic portion that consists of a wedge-like structure followed by tandem PTP domains (D1 and D2; only D1 is enzymatically active), and a 79 amino acid C-terminal tail (Figure 2) (12,13). The extracellular domain exists as multiple isoforms due to alternative splicing of three exons (4,5, and 6; designated in the protein structure as A, B, and C). Sequence homology in the extracellular domain is only 35%. However, comparison of the extracellular domain of CD45 from multiple species indicates conserved overall organization. The most N-terminal domain contains the alternatively spliced exons, which have multiple sites for O-linked glycosylation. This glycosylation results in a dramatic increase in the size and charge of the extracellular domain of the higher molecular weight isoforms. These variable domains are followed by a cysteine-rich globular domain and three fibronectin type III domains. Multiple sites for N-linked glycosylation are present in both the cysteine-rich and fibronectin domains. The size, charge, and composition of these glycans depend upon the complement of glycosyltransferases expressed in a cell, which varies with cell lineage, maturation, and activation state (12,13). It is postulated that differential glycosylation of multiple cell surface proteins, including CD45, may influence their interactions with multi-valent lectins and their subsequent organization into defined microdomains on the cell surface (21-24). This in turn may alter access to substrate and thereby modulate responses to cellular stimulation.
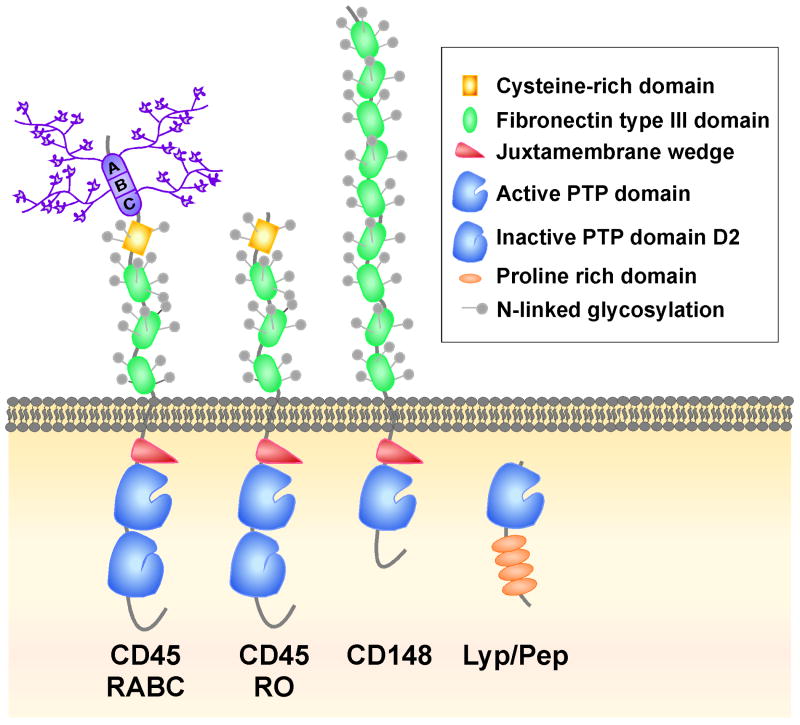
Figure 2. CD45, CD148, and Lyp/Pep structures.
Key functional domains are depicted. Both CD45 and CD148 are RPTPs while Lyp/Pep lacks a transmembrane domain and is cytoplasmic. CD45 exists as multiple isoforms due to alternative splicing of exons 4, 5, and 6 that encode three regions, designated A, B, and C, in the extracellular domain, which contain multiple sites for O-linked glycosylation and sialyation. The largest (RABC) and smallest (RO) isoforms are shown. While CD45 contains two PTP domains (of which only D1 is active), CD148 and Lyp/Pep contain only a single PTP domain.
In contrast to the extracellular domain, the cytoplasmic portion of CD45 is highly conserved between all mammalian species analyzed (12-14). The crystal structure of the cytoplasmic domain, minus the C-terminal 79 amino acids, has been solved to 2.9 angstroms (25). The structures of the phosphatase domains D1 and D2 are quite similar to other phosphatases, consisting of nine highly twisted beta sheets flanked by alpha helices. While functional studies have clearly demonstrated that only the proximal PTP domain (D1) possesses enzymatic activity, both domains are required for optimal phosphatase function in vivo (26). However, the physiologic role of the D2 domain remains incompletely elucidated. The D2 domain contains a unique 19 amino acid insert rich in serine and acidic residues that can be phosphorylated by casein kinase II (27,28). This modification appears necessary for optimal phosphatase activity, although the mechanistic basis for this augmented activity is unclear. Structure-function studies in a T cell line suggest that the D2 domain may also interact with the CD45 substrate Lck, one of the predominant SFKs in T cells, in a phosphotyrosine independent manner, raising the possibility that the D2 domain could also play a role in modulating substrate access and localization (29).
The most highly conserved region within the cytoplasmic portion of CD45 is the juxtamembrane region (30,31). In the crystal structure of the juxtamembrane and D1 domain of a related phosphatase, RPTPα, the juxtamembrane region formed a wedge-like structure consisting of a helix-turn-helix. Interestingly, RPTPα crystallized as a dimer in multiple crystals (30). In each, the wedge of one partner sat in and inhibited access to the catalytic pocket of the partner PTP. This crystal structure suggested a mechanism by which dimerization could inhibit phosphatase activity, thereby providing a novel level of regulation. Functional studies of the putative wedge in CD45 in both cell lines, primary human cells, and mice suggested that the wedge might have a similar regulatory role to that of RPTPα (discussed in detail below) (31,32).
The CD45 crystal structures, which unlike RPTPα consisted of both D1 and D2 domains and crystallized only as monomers, confirmed that the CD45 juxtamembrane domain indeed forms a wedge (25,33). However, the orientation of the CD45 wedge differed from that seen in RPTPa due to inhibitory constraints of the D2 domain. This led the authors to argue that wedge-mediated inhibition of phosphatase activity during dimerization would not be possible for CD45 (25). While this could certainly be the case, additional experimentation will be required to determine whether this is the only conformation that CD45 adopts or whether other more flexible conformations that could facilitate wedge-mediated PTP inhibition during dimerization are possible. Interestingly, the only RPTP crystals that have been isolated as dimers were those in which the D2 domain and C-terminal tail were removed (30). In this regard, it is intriguing that the flexible C-terminal tail of CD45 precluded initial attempts to form a stable crystal.
CD45 Expression
CD45 expression is restricted to all nucleated hematopoietic cells (12). In general, its expression levels increase with cellular maturation (12,13). For example, expression on double positive (DP) thymocytes is two-fold greater than on double negative (DN) thymocytes (34). The pattern of isoform expression on different hematopoietic cell subsets is highly conserved, suggesting that it is physiologically relevant (reviewed in (12,13)). Alternative splicing of exons 4, 5, and 6 (and potentially 7) can generate at least 8 different isoforms at an RNA level; evidence for five of these has been detected at the protein level in humans (13). By convention, the lowest molecular weight (MW) isoform is termed CD45RO and the highest MW isoform is CD45RABC. Protein evidence for existence of the isoforms RB, RAB, and RBC also exists. Naive T cells express primarily the RB isoforms (although lower levels of other isoforms are also expressed). Upon activation, they undergo a ras- and protein kinase C-mediated switch to the low MW isoform RO (35). B cells primarily express the highest MW isoform RABC while myeloid lineage cells generally express the RO isoform until activated, when they switch to RA+ isoforms. The mechanisms governing isoform switching are still incompletely elucidated (36). The global splicing factor polypyriidine tract binding protein-associated splicing factor (PSF) appears to be important in activation induced splicing of CD45 exon 4 (37). In addition, two groups recently identified the heterogeneous ribonucleoprotein hnRNPLL as a key regulator of signal-induced repression of the CD45 variable exon (38,39).
CD45 Substrates
While SFKs are the most definitive CD45 substrates identified to date, multiple additional substrates have been reported including TCRζ, Skap66, Jak family kinases, Dap12, and Pag/Cbp (40-43). However, whether these latter putative interactions are physiologically relevant remains incompletely elucidated. Like most phosphatases, CD45 has relatively low specificity in vitro. Moreover, since SFKs are positioned so proximally in signaling cascades, any of the observed alterations in phosphorylation seen in CD45 deficient mice or cell lines in vivo could be indirect consequences of dysregulated SFK activity. Conversely, genuine CD45-substrates may appear unaffected due to a balanced combination of decreased kinase and phosphatase activity (14). Thus, for the purposes of this review we will focus on the role of CD45 in modulating signaling networks mediated by SFKs.
Studies emerging from CD45 deficient cell lines, mice, and humans indicate a critical role for CD45 as a positive regulator in immunoreceptor mediated signaling by directly regulating SFK activity (Figure 3) (reviewed in (13,14,44-46). It performs this function by opposing Csk and dephosphorylating the SFK negative regulatory tyrosine. While this scenario has been the dogma, recent work indicates that understanding CD45 function in specific hematopoietic cell subtypes is more complex than initially appreciated. Depending upon cell type and activation status, several studies have suggested that CD45 may also negatively regulate SFKs by dephosphorylating the activating catalytic site tyrosine. Interestingly, CD45's relative ability to dephosphorylate these tyrosines appears to differ, with hyperphosphorylation of the negative regulatory tyrosine being most pronounced in CD45 deficient cells. This leaves open the caveat that CD45's impact on the catalytic pocket tyrosine could be either direct or indirect (45). Regardless, an emerging theme is that CD45 can have differential effects on SFKs in different hematopoietic cell lineages and even within the same lineage at different developmental stages or with different activation states.
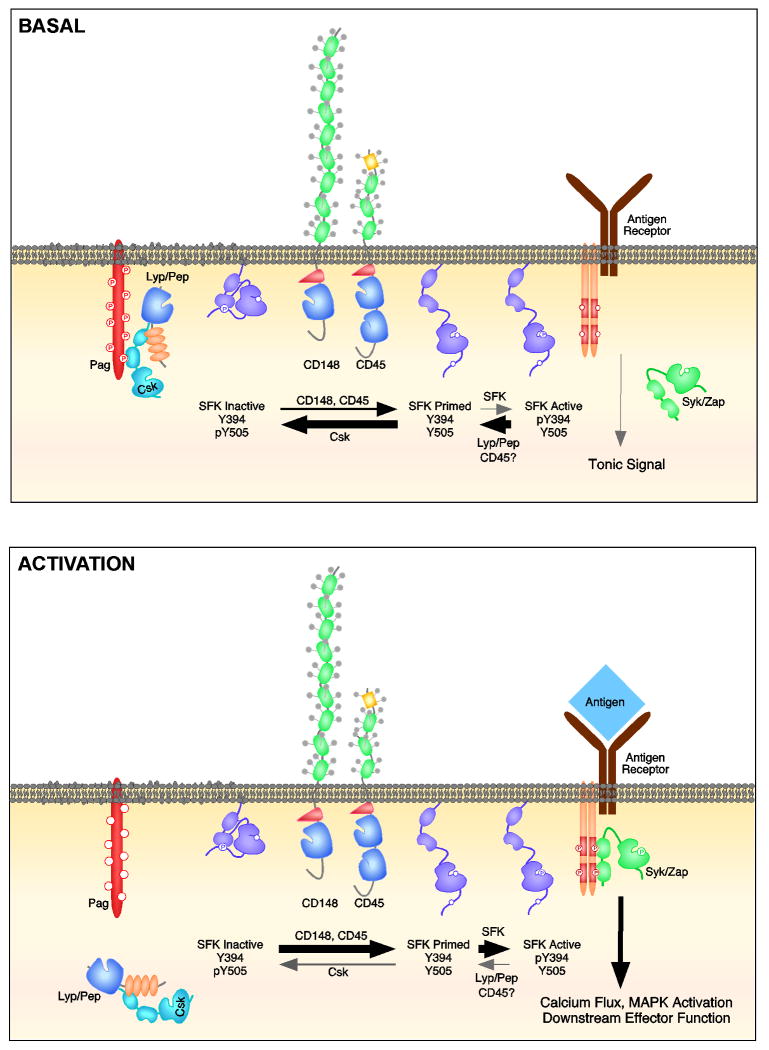
Figure 3. Reciprocal regulation of SFK activation by CD45, CD148, and Lyp/Pep.
SFKs are in a dynamic equilibrium between their inactive and active conformations in both the basal state and during immune cell activation (while B cell activation is depicted, similar mechanisms likely exist in other hematopoietic cell lineages). In the basal state, phosphorylation of the adapter PAG facilitates recruitment of Csk and Lyp/Pep. Csk phosphorylates the negative regulatory tyrosine while Lyp/Pep dephosphorylates the autocatalytic tyrosine. This results in the SFK adopting a closed conformation. By opposing Csk and dephosphorylating the negative regulatory tyrosine, CD45 and CD148 generate a pool of SFKs in an open or ‘primed’ conformation that can be rapidly activated should antigen be encountered. We hypothesize that low levels of SFK transphosphorylation of the autocatalytic site occur in the basal state, which is necessary for tonic signaling and cell survival. This is balanced by the action of PAG/Csk/Pep (and potentially CD45 targeting either directly or indirectly the autocatalytic tyrosine). During antigen encounter and receptor activation, PAG becomes dephosphorylated via mechanisms that are incompletely elucidated (but may involve CD45), Csk and Lyp/Pep dissociate from the cell surface leaving CD45 and CD148 actions on the negative regulatory tyrosine unopposed. Phosphorylation of the autocatalytic tyrosine is now favored. The fully active SFK can phosphorylate the ITAMs of the immunoreceptor, facilitating recruitment of Syk/Zap70 and amplification of downstream signaling cascades.
Here, we review what is known about CD45 biology in these different cellular contexts and point out major gaps in understanding that will serve as fruitful areas for future research. We focus first on its role in the adaptive immune response followed by recent studies highlighting its emerging role in innate immunity. Throughout this discussion it should be kept in mind that biochemical analyses are limited in that they reflect the status of an entire population of cells and are insensitive to the effects of CD45 on the individual pools (e.g., free, raft, CD4 or other protein-associated, and intracellular) of SFKs within a single cell. As discussed below, such approaches also do not account for additional phosphatases, some of which are likely to be redundant, that also modulate SFK phosphorylation status (e.g., PEP, SHP1, CD148). These may co-exist with CD45 in these pools, further complicating interpretation of the data.
CD45 Function in T cells
The majority of studies examining CD45 function have focused on T cells because its absence affects this lineage most profoundly. In three independently generated CD45 deficient mouse lines thymocyte development is partially blocked at β selection and severely blocked at the double positive (DP) selection stage due to the dysfunctional signaling through the preTCR and TCR, respectively (15-17,47). Biochemical analyses of TCR signaling in CD45 deficient thymocytes and cell lines initially highlighted Lck and Fyn, the primary SFK members in T cells, as potential CD45 substrates due to their hyperphosphorylation at the inhibitory tyrosine (8,48-50). The physiologic relevance of this site as a CD45 substrate was confirmed by expression of a Lck transgene containing Phe rather than Tyr at residue 505 (Y505F), which overcame the block in thymic development (47,51). This led to a model proposing that a primary function of CD45 was to counteract the inhibitory effect of Csk on SFKs by dephosphorylating the negative regulatory tyrosine, thereby generating a pool of signal competent or ‘primed’ SFK that could phosphorylate the TCR immunoreceptor tyrosine-based activation motifs (ITAMs) if foreign antigen was encountered.
Complicating this model was the observation that the phenotype of CD45 deficient mice did not phenocopy mice lacking both Lck and Fyn. Lck/Fyn deficient mice have a severe block in thymic development at the double negative (DN) stage while this block is quite mild in CD45 deficient mice (47,52). Instead, CD45 deficient mice exhibit a more profound block at the DP to single positive (SP) transition (15-17). This raises the possibilities that there exists a redundant phosphatase in double negative (DN) thymocytes that could dephosphorylate Lck505 (e.g., CD148) and/or that CD45 functions are more complex than simply dephosphorylating the negative regulatory tyrosine.
It is also hard to reconcile this model with the finding that CD45 deficient macrophages and T cells were abnormally adherent due to augmented SFK dependent integrin signaling (53,54). This hyperresponsiveness was associated with increased phosphorylation of both the autocatalytic and negative regulatory tyrosines, as well as increased kinase activity in in vitro assays. This led to the suggestion that CD45 might also dephosphorylate the catalytic site tyrosine and thereby also negatively regulate SFKs (10,46,55). One caveat to these biochemical analyses is the propensity of SFKs to transphosphorylate themselves when placed in close proximity, as is the nature of these biochemical studies. However, providing genetic evidence for the catalytic site tyrosine as a potential CD45 target (either direct or indirect), expression of non-oncogenic levels of the LckY505F transgene in CD45 null mice resulted in hyperphosphorylation of Lck tyrosine Y394 and thymoma development (56). Together, these data led to the proposal that CD45 could regulate both SFK phosphotyrosines. Whichever dominated would depend upon access to substrate and cellular context.
Recent work by McNeill, Alexander, and colleagues has provided some clarity to this paradox (57). They reconstituted CD45 deficient mice with a series of CD45RO transgenes that resulted in titration of CD45 cell surface expression levels ranging from 1 to 60% of wildtype (wt) levels. Surprisingly, as little as 3% of normal CD45 activity was able to reconstitute T cell development. Even more striking, expression of a transgene at 30% of wt CD45 levels resulted in augmented production of CD4 SP and CD8 SP cells relative to wt. Consistent with increased positive selection, both thymocytes and peripheral T cells with intermediate levels of CD45 expression had augmented phospho-ERK levels in response to TCR stimulation relative to cells with wt levels of CD45.
Analysis of Lck phosphorylation status by phosphoflow cytometry provided potential insight into these observations (57). In CD45 deficient thymocytes, while both phosphotyrosines were hyperphosphorylated, hyperphosphorylation of the negative regulatory tyrosine, Y505, was disproportionately higher than that seen at the autocatalytic tyrosine, Y394. This pattern was maintained in very low expressing transgenics. However, with higher levels of transgene expression, phosphorylation of both sites became more equal. Correspondingly, both thymocytes and peripheral T cells became hyperresponsive to TCR stimulation as evidenced by enhanced phospho-ERK and phospho-AKT induction and increased proliferation rates. Based on these observations, the authors argued that CD45 differentially regulates pTyr-505 and pTyr-394 in Lck. At low CD45 expression levels, pTyr505 dominates but at intermediate levels the negative influence of increased pTyr-505 might be counterbalanced by an enhanced positive contribution from hyperphosphorylation of pTyr-394. This suggests that it takes very little phosphatase activity to generate sufficient primed Lck for initiation of signaling but much more phosphatase activity to inhibit the autocatalytic site (44). They postulate that the normal high expression levels of CD45 may be essential for efficient dephosphorylation of Tyr394 and termination of TCR signaling. An important issue that remains to be addressed experimentally is the mechanistic basis by which CD45 differentially regulates these two phosphotyrosines and whether they are direct or indirect functions of CD45. For example, one could imagine a scenario where altered phosphorylation is an indirect consequence of perturbed activity of feedback loops.
An interesting caveat to these observations is that they only report the quantitative consequences of these transgenes but do not address qualitative differences in the repertoire. Interestingly, mice heterozygous for CD45 deficiency have normal thymocyte numbers. However, enhanced positive selection is unmasked when a TCR transgene is introduced into these mice (58). Based upon this observation, one might predict that the repertoires of the McNeil and Alexander transgenic mice, as well as their relative sensitivity or resistance to autoimmune disease, may well be different. Perhaps a small amount of CD45 is sufficient to reconstitute TCR signals necessary for the production of mature peripheral T cells. However, fine-tuning the level of phosphatase activity may be critical for defining the composition, or quality, of the peripheral T cell repertoire.
We have begun to explore this issue using mice that express a germline point mutation (CD45E613R) in the juxtamembrane wedge of CD45 (Hermiston et al., manuscript submitted). The CD45E613R mutation is predicted to result in constitutive phosphatase activity due to lack of dimerization mediated inhibition of phosphatase activity (32). To control for potential differences in the T cell repertoire, the CD45E613R mice were placed on a Rag1 deficient background and then crossed to mice expressing a MHC II restricted TCR transgene in order to generate animals with a homogeneous TCR repertoire. While thymic development is grossly normal in CD45E613R mice with an endogenous repertoire, augmented positive selection is unmasked in CD45E613R mice expressing a TCR transgene. Consistent with increased signal strength, CD45E613R DP thymocytes demonstrate hyperresponsive calcium and pERK signals in response to TCR stimulation. However, in contrast to McNeill and Alexander's mice where central and peripheral hyperresponsiveness were similar, CD45E613R peripheral T cells are hyporesponsive to TCR stimulation.
Interpreting these results is challenging. Our working hypothesis is that CD45 functions as a rheostat that dials a set point for TCR activation and signal strength. By potentially titrating phosphatase activity, the wedge may contribute to this set point. We postulate that the set point for signal activation may be quite different between central and peripheral T cell compartments. Supporting this notion, evidence for differential signaling networks in thymocytes and peripheral T cells exists 2 (59,60). For example, Fyn appears to play a more important role in peripheral T cells (5,60). This is interesting given that given that while Lck is strongly influenced by the presence or absence of CD45, Fyn appears to be less affected. Interestingly, analysis of PTPα deficient mice indicates selective hyperphosphorylation of Fyn but not Lck, suggesting that different RPTPs may preferentially impact distinct SFKs (61).
CD45 Function in B cells
The phenotypic and functional consequences of CD45 deficiency are less severe in B cells relative to T cells (15-17,33,62-64). Despite this, in contrast to the T cell lineage, CD45 transgenes are unable to reconstitute B cell development and functional responses, for reasons that are not yet clear (13,14) and references therein). Contrasting the block in thymic development, peripheral B cell numbers are actually increased in CD45 deficient mice (15-17,33). Moreover, significant differences in peripheral B cell development exist in CD45 deficient mice. Marginal zone B cells are increased while B1 cell production is inhibited, a developmental pattern consistent with decreased BCR signal strength (33). There also appears to be a block at the T2 transitional to mature follicular B cell step (33,62,64). Taken together, these data argue that CD45 is not just a simple positive regulator of SFKs in B cells. Indeed, if this were the case one would predict that CD45 deficient mice should phenocopy animals lacking Blk, Lyn, and Fyn, the predominant B cell SFKs (65). These mice exhibit a severe block in B cell development at the pro-B cell stage. Functional redundancy with other phosphatases, in particular CD148 (see below), may contribute to these differences.
CD45 deficient peripheral B cells do not signal normally: PI3 kinase, NF-kB, and ERK phosphorylation are decreased upon BCR ligation (64,66,67). Interestingly however, calcium flux is less severely affected relative to CD45 deficient thymocytes. Despite these BCR signaling defects, CD45 deficient mice have normal immunoglobulin levels and normal responses to T-cell dependent and -independent stimuli provided CD45 wt helper T cells are present in trans (16,66,68). This likely reflects the intact response to co-stimulation through CD40 in CD45 deficient cells that appears to overcome the defective BCR signaling (66). Nonetheless, elegant studies employing the hen egg lysozyme (HEL) transgenic system clearly demonstrated that despite these apparently normal functional responses, the B cell repertoire in CD45 deficient mice is qualitatively abnormal (69,70). In wt B cells expressing an anti-HEL BCR transgene, presence of circulating HEL autoantigen results in anergy. However, CD45 deficient B cells expressing the same transgene are positively selected when exposed to soluble HEL. In contrast, exposure of transgenic IgHEL CD45E613R B cells, which demonstrate hyperresponsive BCR-induced signals including augmented pERK and calcium responses, to the same autoantigen results in their deletion (33). Taken together, these data argue that CD45 functions as a rheostat to control the strength of the BCR signaling threshold.
So how are SFKs regulated by CD45? Studies of CD45 deficient B cell lines and mice indicate that Lyn is constitutively hyperphosphorylated at the C-terminal tyrosine (71-73). Despite this, in vitro kinase assays suggest that Lyn is actually hyperactive. A potential molecular explanation for this finding was provided by the observation that the catalytic site tyrosine was also hyperphosphorylated in CD45 deficient B cells, albeit to a lesser degree than the negative regulatory tyrosine (71). It was proposed that this overrides hyperphosporylation of the negative regulatory site. Whether this is a direct or indirect consequence of CD45 deficiency was not addressed. It is also unclear whether this dual hyperphosphorylation (which has not been observed by all groups (67)) is on the same molecule or represents differential phosphorylation of distinct pools of Lyn.
On the surface, augmented kinase activity appears paradoxical as CD45 deficient B cells proliferate poorly in response to BCR stimulation (15-17). It is also perplexing, given that either loss or constitutive activation of Lyn results in autoimmunity in mice (74-76), that CD45 deficient mice are well (even if T cell help is provided in trans). This suggests that stoichiometry may be important and raises the possibility that the levels of increased kinase activity in CD45KO mice are substantially less than that seen in mice expressing constitutively active Lyn (45). The fact that Lyn has both positive and negative regulatory effects in BCR signaling could also contribute to these paradoxical findings. While Lyn's positive effects are redundant with Blk and Fyn, its ability to phosphorylate ITIMs and thereby facilitate the recruitment of negative regulators of BCR signaling such as SHP-1 and SHIP are unique (4). Highlighting the possibility that increased phosphorylation of Lyn's autocatalytic site could be an indirect effect in CD45 deficient mice, one function of SHP-1 is to dephosphorylate this tyrosine and dampen kinase activity (77). Inefficient recruitment of SHP-1 could result in a net increase in catalytic site phosphorylation in Lyn.
Contrasting CD45 deficient B cells, B1 cell production is augmented in CD45E613R mice and CD45E613R follicular B cells are hyperresponsive to BCR stimulation (33). Interestingly, calcium flux is sustained, mirroring that seen in Lyn deficient mice (Gross A, Hermiston M, DeFranco A, and Weiss A, unpublished observation). Evaluation of Lyn phosphorylation in CD45E613R B cells reveals that the autocatalytic tyrosine is hypophosphorylated while the C-terminal tyrosine is hyperphosphorylated, a pattern consistent with Lyn inactivation (33). Given this model, it is perhaps not surprising that the CD45E613R mice phenocopy many aspects of Lyn deficient mice.
CD45 Function in Natural Killer (NK) Cells
NK cells are increased in a cell-intrinsic manner in CD45 deficient mice, suggesting that CD45 functions in this lineage (78). Two groups have recently explored this possibility by comparing effector functions in CD45 wt and CD45 deficient NK cells (79,80). Upon ligation of ITAM containing activating receptors on NK cells, SFKs initiate a signaling cascade that culminates in both target cell lysis and cytokine production. Surprisingly, CD45 deficiency has differential effects on these effector functions. While both ERK and JNK phosphorylation are severely impaired in CD45 deficient NK cells in response to receptor stimulation, cytotoxicity is only slightly reduced while cytokine and chemokine production were severely inhibited. Hesslein and colleagues propose that this paradox may be explained by differential requirements for MAPK activation in cytoxicity versus cytokine production. Consistent with this notion, the IC50 for the MEK inhibitor PD098059 is much higher for cytotoxicity than for IFNγ production (79).
A second surprising finding from these studies was the observation that basal phosphorylation of multiple phosphoproteins was observed in both freshly isolated and IL2 expanded CD45 deficient NK cells (79). This is a striking contrast to findings in T, B, and myeloid lineage cells where alterations in basal phosphorylation are more limited, primarily manifesting as hyperphosphorylation of the SFKs. This observation raises some intriguing possibilities regarding the function of CD45 in NK cells. It is currently unclear whether CD45 has a net negative or positive impact on SFK activity in NK cells. If CD45 is having a net positive effect on SFKs, the increased basal phosphorylation could reflect increased activity of SFKs on their substrates. In this scenario, the defects in MAPK activation could then be the result of adaptation of cells to elevated constitutive signaling and compensating inhibitory feedback loops. Alternatively, if the net effect of CD45 deficiency on SFKs is an inhibitory one, it is possible that loss of SFK-mediated phosphorylation of inhibitory tyrosine inhibition motifs (ITIMs) results in increased basal phosphorylation due to loss of downstream inhibitory signals. Finally, it is possible that CD45 has unique substrates in addition to SFKs in NK cells and that hyperphosphorylation in the basal state is reflective of this function. Supporting this notion, Mason et. al. have shown that Dap12, the ITAM containing adapter for Ly-49 family of NK cell receptors, is hyperphosphorylated in CD45 deficient NK cells and propose that it may be a direct substrate of CD45 (81). However, whether the hyperphosphorylation of Dap12 was a direct consequence of CD45 deficiency or instead reflected the indirect effects of excessive SFK activity in these cells was not addressed. While further studies will be needed to fully elucidate the function of CD45 in NK cells, these observations do highlight the importance of studying this phosphatase in multiple cell types, as its functions appear to be distinct in different cellular contexts.
CD45 Function in the Myeloid Lineage
Despite high levels of expression, historically there has been a paucity of data, relative to T or B cells, on the function of CD45 in myeloid lineage cells (e.g., neutrophils, monocytes, macrophages, mast cells, and dendritic cells (DCs)). Studies employing antisense oligonucleotides to CD45 or anti-CD45 monoclonal antibodies have implicated CD45 in the phagocyte respiratory burst, neutrophil chemotaxis, and proliferative responses to GM-CSF and IL-3 ((13) and references therein). However, it is unclear whether these antibodies are activating or inhibitory, which makes interpreting these studies difficult.
More enlightening was analysis of cell adhesion in primary CD45-deficient bone marrow derived macrophages (BMDM) (53). In wt cells, CD45 was found to localize to sites of focal contacts, suggesting a role in cell adhesion. Further analysis of CD45 deficient BMDM revealed striking adhesion abnormalities. While CD45 deficient macrophages adhered more rapidly to plastic, they were unable to maintain this adhesion over time. Detachment was found to be due to aberrant integrin mediated signaling due to dysregulation of the SFKs Hck and Lyn, but not Fgr, in CD45 deficient macrophages. Interestingly, detailed biochemical analyses indicated that the initial augmented adherence was due to increased kinase activity in the CD45 deficient cells. Metabolic labeling revealed hyperphosphorylation of both the carboxy terminal and autocatalytic tyrosines in Hck and Lyn. Correlative studies in a cell line using SFKs with a Y to F mutation at the C-terminal tyrosine co-transfected with either catalytically active or inactive CD45 confirmed that the autocatalytic tyrosine was regulated by CD45. While one cannot conclude from these studies whether this was a direct or indirect event, the results support the authors' conclusion that CD45 functions primarily as a negative regulator of SFK-mediated integrin signaling in macrophages. This study was also important in that it demonstrated that SFK members could be differentially regulated within the same cell.
In mast cells, CD45 deficiency results in defective histamine degranulation after IgE receptor cross-linking (82). We have recently revisited this issue and compared the impact of CD45 null and CD45E613R mutations on mast cell function (Growchovy et al., manuscript submitted). We also found that CD45-deficient mast cells had drastically reduced IgE mediated-degranulation and cytokine secretion. In contrast, these effector responses were augmented in CD45E613R mast cells. Surprisingly, despite these dichotomous phenotypes, Lyn was hyperphosphorylated at the inhibitory tyrosine in both sets of cells. A potential explanation for this finding was attenuated interaction between CD45 and Lyn, as well as hyperactivation of the Fyn-regulated PI3 kinase pathway in CD45E613R but not CD45 deficient cells. A second surprising finding from this study was that depending upon the receptor system addressed, CD45 deficient and CD45E613R mast cells also exhibited similar phenotypes such as hyperproliferation in response to IL-3. While the mechanistic basis for these different phenotypes is currently incompletely elucidated, they highlight the complex behavior of this phosphatase.
There has also been a recent flurry of papers beginning to address the function of CD45 in DCs with some intriguing findings (83-85). While production and maturation of DCs is relatively intact in CD45 deficient animals, several groups have noted modest skewing of DC subsets towards the CD11chigh8α population at the expense of CD11chigh11b+ DCs (83,85). There is also a consistent increase in cell surface levels of the co-stimulatory and activation markers CD80, CD86, and MHC II in the basal state. These developmental differences are cell intrinsic as they persist in bone marrow chimeras between CD45 deficient and wt mice (83). However, whether these developmental differences translate to a functional impact remains to be determined.
The more dramatic finding in these studies is the altered responsiveness to toll-like receptor (TLR) ligation in CD45 deficient DCs. While several studies have demonstrated a role for SFKs in TLR responses, how this is mediated mechanistically remains unclear (86,86,87). Interestingly, both positive and negative effects of CD45 were observed in BM and splenic DCs, depending on which TLR receptor was stimulated (83,84). CD45 deficient BMDC and splenic DCs demonstrated increased production of the proinflammatory cytokines TNFα and IL6 and upregulation of activation markers when stimulation with the TLR3 ligand poly(I:C) or the TLR9 ligand CpG }. In contrast, no difference was observed in IFNα production. Together, these data imply that the role of CD45 in TLR responses is more complex than a simple linear pathway emanating from the TLR and could be indirect.
This complexity has been recently examined by Cross, Johnson and colleagues (83). They carefully interrogated responses of DCs to multiple TLR ligands. Suggesting a negative regulatory effect for CD45, they found that TLR2 and 9 stimulation, which both signal via MyD88 dependent pathways, resulted in augmented proinflammatory cytokine secretion, including IFNβ in the case of TLR9, in CD45 deficient DCs. In contrast, TLR3 (MyD88 independent) and TLR4 (MyD88 dependent and independent) stimulation, resulted in decreased IFNβ secretion, suggesting that CD45 plays a positive role in MyD88 independent signaling networks. Interestingly, analysis of LPS responses in CD45 deficient bone marrow derived macrophages (BMDM) showed no difference from wt cells, suggesting that the impact of this phosphatase on the same ligand-receptor pathway may differ in a cell-type specific manner.
Taken together, these results implicate a role for CD45 in regulation of innate immune responses. An important remaining issue is how CD45 impacts these pathways mechanistically. While Beverley's group found alternations in NFkB activation, Johnson's group did not see this (83,84). The latter group found hyperphosphorylation of Hck, Lyn, Fyn, and SHIP in BMDC. Despite dramatic alterations if SFK phosphorylation, analysis of downstream events including MAPK, Akt, and NFκB were similar between mutant and wt BMDC. Further studies are needed to clarify how CD45 influences TLR signaling. Is it via its actions on SFKs, and if so, which ones? Is it directly mediated by the TLR signaling pathway or is it indirect due to paracrine or autocrine effects of cytokines/chemokines? For example, the SFKs Hck and Fgr negatively regulate chemokine signaling by maintaining tonic phosphorylation of the inhibitor receptor PIR-B (86).
Taken together, these results highlight emerging themes from studies of CD45 function in the myeloid lineage: 1) regulation of SFKs by CD45 is complex, 2) different SFKs may be regulated in distinct manners by CD45; and 3) CD45 may have unique effects on different ligand-receptor pathways. One should also keep in mind that a caveat to all of these studies is potential functional redundancy between CD45 and CD148 in the myeloid lineage (see below). Indeed, studies of doubly deficient BMDM indicate that these phosphatases cooperate in regulation of Fc receptor-mediated signaling (67).
Regulation of Homeostasis by CD45
Given its roles in antigen receptor, cytokine, and adhesion signaling, all pathways important for promoting cell survival and death, it is perhaps not surprising that several investigators have suggested that CD45 may regulate apoptosis (21) and references therein). The majority of these studies have relied upon cross-linking anti-CD45 antibodies or lectin binding to induce T cell death. However, the relative non-specific nature of these interactions has made these studies difficult to interpret. More intriguing are studies employing CD45 deficient cell lines that demonstrate increased propensity to apoptosis, suggesting that CD45 is a negative regulator of cell death (88-90). Interestingly, reconstitution of a CD45 deficient cell line with various CD45 mutants indicated that the intracellular domain and active phosphatase activity are dispensable for this phenotype (88). However, other studies have produced conflicting results, suggesting that CD45-mediated upregulation of Fas ligand and phosphoinositide 3-kinase (PI3K)/Akt pathways may be important for CD45-mediated apoptosis (89,91). Finally, aberrant interactions with the extracellular matrix and/or cytoskeleton could also play a role (92). In a cell line study, CD45 was found to promote cell survival by modulating integrin-mediated FAK/ERK signaling (93). In the absence of CD45, cytoskeletal organization was abnormal and postulated to contribute to cell death in this system. An issue with each of these studies is that they employed transformed cell lines that, by definition, are likely to have altered regulation of cell proliferation and death pathways.
Surprising, there is a paucity of data addressing the role of CD45 in regulation of proliferation or cell death in animal models. Huntington et al. demonstrated decreased survival of CD45 deficient B cells at the germinal center stage. This correlated with defective AKT and ERK signaling and poor induction of Bcl-2 and Bcl-XL expression in response to BCR stimulation. This defect could be overcome by enforced expression of Bcl-XL (80). In addition, CD45E613R mice develop a lymphoproliferative disease on multiple genetic backgrounds that is exacerbated in the presence of FAS deficiency (Gupta et al., in press). A challenge to these studies, however, is determining how much of this phenotype is a direct consequence of altered phosphatase activity and how much reflects appropriate responses to an abnormal cytokine milieu.
Regulation of PTPase Activity
Given CD45's pivotal role in signal transduction in multiple cell types, elucidating how it is regulated is critical to our understanding of hematopoietic cell development and function. Ligand/lectin binding, dimerization, differential localization with regulated access to substrate, phosphorylation, and oxidation have all been proposed as possible means of CD45 regulation (13,14). It should be emphasized that these methods are not mutually exclusive and conceivably could be distinct in different cell types and/or activation states.
The highly conserved patterns of isoform expression and overall organization of the CD45 extracellular domain led to the hypothesis that CD45 would be regulated, similar to receptor tyrosine kinases, by differential interactions with protein ligands. In particular, the cysteine-rich motif in the CD45 extracellular domain is analogous to that of the EGFR, where it is involved in binding of EGF (13). However, despite extensive efforts in multiple labs, a bona fide ligand has not been identified. Several glycoproteins, including CD22 (94), galectin-1 (24,95), glucosidase II (96), and the C-type lectin MGL (23), have been shown to bind nonspecifically to T cell glycoproteins, including CD45. Whether these interactions directly modulate CD45 phosphatase activity is presently unclear. One possibility is that they influence phosphatase activity by regulating the localization of CD45 on the cell surface (21,22). For example, by inducing clustering of CD45 one could potentially influencing the extent of receptor dimerization and/or regulate localization of the phosphatase in relation to its substrate (see below).
A second potential means of regulation of phosphatase activity is by spontaneous receptor dimerization. Several groups have identified CD45 dimers physiologically. However, the factors regulating dimerization and its subsequent physiologic consequences have not been fully elucidated (32,97-100). The Weiss lab initially hypothesized that CD45, similar to receptor PTKs, would be positively regulated by ligand-mediated dimerization (101). However, forced dimerization of a chimeric molecule consisting of the extracellular and transmembrane domains of the epidermal growth factor (EGF) receptor and the CD45 cytoplasmic domain surprisingly inhibited CD45 functional activity (101). A potential molecular mechanism for this was suggested by the crystal structures of the juxtamembrane and N-terminal phosphatase domain of a related RPTP, PTPα, which crystallized as a symmetric dimer (30). The juxtamembrane region of each monomer formed a “wedge”-like structure that blocked the catalytic site of its binding partner. The wedge motif is highly conserved in multiple RPTPs, including CD45, suggesting a critical regulatory role. To test its importance, a point mutation was introduced into the tip of the wedge in human CD45 and found to eliminate the inhibitory effects of forced dimerization (31). Introduction of the analogous mutation (CD45E613R) in mice confirmed the physiologic importance of the wedge (32). On a mixed genetic background, the mice developed a lymphoproliferative disease, anti-double stranded DNA antibody formation, and immune complex mediated glomerulonephritis, a phenotype resembling lupus in humans.
While the CD45E613R mice clearly demonstrate that the wedge is physiologically relevant, at least in mice, the mechanistic basis by which it performs its actions is controversial. Since a definitive ligand had not been identified, spontaneous homodimerization, potentially influenced by isoform usage and/or cell surface expression levels, was proposed as an alternative means of regulating CD45 dimerization (32). Indeed, low MW CD45 isoforms were found to spontaneously homodimerize more efficiently than high MW isoforms (100). It was reasoned that this was due to differences in steric hindrance and electrostatic charge between the extracellular domains of these isoforms. Supporting this notion, sialidase treatment or inhibition of O-glycosylation preferentially facilitated the dimerization of the high MW isoforms (which contain more of these modifications). These data led to a model whereby CD45 phosphatase activity would be negatively regulated by the wedge by differential homodimerization of various isoforms.
This model predicts that cells expressing the wedge mutation should be insensitive to the effects of CD45 dimerization, resulting in augmented phosphatase activity and hyperresponsive antigen receptor signals. Evaluation of CD45E613R mice shows this is indeed the case for B cells (33) and thymocytes (Hermiston et al., in press). The finding that B cells were affected by the wedge mutation was unexpected since they express primarily the high MW CD45 isoform. However, even though dimerization of high MW isoforms is less efficient, they do form. Moreover, since expression of CD45 on B cells is highest of all lineages examined, it is possible that this increased expression enables CD45 to overcome potential steric hindrance. Alternatively, B cells could be more sensitive to consequences of dimerization and/or the wedge could function by a means other than inhibiting phosphatase activity during receptor dimerization.
As noted above, challenging the wedge model is structural data demonstrating that steric hindrance between the D1 and D2 domains in the obtained crystals would preclude inhibition of the catalytic site via the wedge during receptor dimerization (25). Can we reconcile the wedge hypothesis with this structural data? The dramatic phenotype of the wedge mutant mice and the biochemical differences in T and B cell hyperresponsiveness indicate that this single point mutation has important physiologic consequences. It is certainly possible that the model is incorrect and that the wedge functions by other means such as altering the spatial or temporal localization of CD45 relative to its substrate or by affecting interactions with other proteins. Conversely, the CD45 crystal structure may represent only one selected conformation that was amenable to crystallization. This does not exclude the possibility that other conformations, including intermolecular dimers, also exist. Perhaps certain physiologic modifications (see below) such as phosphorylation or oxidation induce conformational changes that restore flexibility to the molecule and overcome the steric hindrance. Supporting this possibility, RPTPα dimers are stabilized due to an oxidation-induced conformational change in the D2 domain (102). Additional structural studies will be required to test the dimer model and to understand how the wedge impacts CD45 function.
Localization of components of signaling networks with a cell is a dynamic process. For example, SFKs traffic in and out of lipid rafts, which are platforms for cell signaling (103). Thus, another possible (or contributing) means of CD45 regulation is modification of CD45 localization. Regulating CD45 localization could directly affect phosphatase activity by facilitating dimerization due to increased ‘crowding’ of CD45 on the cell surface. Alternatively or in addition, regulated localization of CD45 could have indirect effects on the pathways it mediates by facilitating or inhibiting its access to substrates (104). For example, the kinetic segregation model proposes that CD45 exclusion from sites of TCR engagement with peptide is a primary event in signal initiation (105). Consistent with this hypothesis, Dustin and colleagues have presented convincing data for the regulated localization of CD45 (106). They find that during T cell activation, CD45 is concentrated in the TCR-rich central supramolecular activation cluster (cSMAC) but excluded from TCR microclusters. Interestingly, TCR microclusters appear to be the site for sustained TCR signaling while the cSMAC is the primary site for termination of TCR signals (107,108). They hypothesize that CD45 plays a pivotal role in both of these processes: First, by generating the pool of primed Lck that diffuses into the microclusters from surrounding CD45-rich domains and second, by inactivating SFKs such as Lck in the cSMAC. Limited biochemical data are consistent with these observations. Studies exploring the consequences of CD45 localization in T cells suggest that raft-excluded CD45 promotes TCR responsiveness while raft or cSMAC localized CD45 antagonizes TCR signals (109,110). However, the relative effects of CD45 on specific SFK phosphotyrosines in these various locations require further study.
Cell lineage, developmental stage, and/or activation state differences in the localization of CD45 relative to its substrate could potentially explain some of the differences in SFK phosphorylation that have been reported. For example, thymocytes, which are about 10 times more sensitive to TCR stimulation than peripheral T cells, do not form a cSMAC. Instead, they form a more decentralized synapse with multifocal signaling clusters (111). A potential consequence of this observation is that lack of coordinated CD45-mediated downregulation of the signal, as is seen in peripheral T cell blasts, could result in more signal per agonist peptide and the enhanced sensitivity of thymocytes. This model could also potentially explain the observed difference in TCR responses between CD45E613R DP thymocytes and peripheral T cells. Unregulated CD45 activity (due to the wedge mutation) in thymocytes could lead to increased TCR responsiveness due to enhanced generation of primed Lck that then localizes to microclusters. However, in the periphery, unregulated phosphatase activity in the cSMAC could result in enhanced downregulation of Lck activity due to augmented dephosphorylation of the autocatalytic tyrosine and peripheral T cell hyporesponsiveness. Further experimentation will be necessary to validate these possibilities.
An important remaining question is how localization of CD45 itself is regulated. Steric exclusion (112), lateral interactions with other proteins (41,113), and differential interactions with the spectrin-ankyrin cytoskeleton (114) are potential mechanisms that warrant further investigation. The latter is particularly interesting. These investigators found that CD45 associated with B1 spectrin and ankyrin and that trafficking of intracellular pools of CD45 and spectrin are coupled (114). Inhibiting spectrin resulted in decreased recruitment of CD45 to the cell surface and had a subsequent negative regulatory effect on TCR signaling. In light of this data, it is interesting to note that cytoskeletal polarization is less efficient in DP thymocytes (115). It will be important to test whether this also affects CD45 trafficking and whether it could influence the difference in signal transduction thresholds between thymocytes and peripheral T cells. Whether this mechanism of regulated localization also functions in B, NK, and/or myeloid cells should also be addressed.
Several other proteins have been suggested to interact with and potentially define the localization of CD45 relative to substrate. The most prominent of these is CD45 associated protein (CD45-AP). This protein was initially identified due to its association with CD45 and can directly interact with CD45 via its transmembrane domain (116). It is thought to stabilize the association of CD45 with Lck and regulate its localization within the cell. Biochemical studies suggest that it may promote or stabilize the association of CD45 with substrates and thus regulate the threshold of T cell activation (117). However, the physiological significance of this interaction is controversial. It has been deleted by three different groups and has a very mild to no appreciable phenotype (118-120). Moreover, we have tested whether its absence can influence the TCR repertoire by breeding the CD45-AP mice to two different TCR transgenes but were not able to identify an impact on TCR development (Hermiston and Weiss, unpublished data).
Further studies aimed at defining the relative access of CD45 to its substrates in different cellular contexts are clearly needed. The fact that CD45 deficient mice do not accurately phenocopy the consequences of SFK deficiency in the same lineage argues that there is either redundancy in phosphatase function and/or that some SFKs are better substrates for CD45 than others. Emerging data from Falahati and Leitenberg indicates that CD45 preferentially interacts with a CD4 associated pool of LCK in DP thymocytes but has minimal effects on the phosphorylation status of non-CD4 associated Lck (11). Interestingly, Lck phosphorylation is also relatively unaffected in DN cells, suggesting that CD4 co-receptor localization of SFK and CD45 may be necessary for optimal PTP-kinase interactions. How this translates to peripheral T cells or to other hematopoietic lineages is currently unclear. It will be important to understand not only the relative localization of CD45 and the relevant SFK, but also the localization of CSK, redundant phosphatases (see below), and adapters (e.g. PAG/Cbp) in order to gain a clearer picture of this localization.
Finally, as alluded to above, protein modifications such as phosphorylation and/or oxidation could modulate CD45 phosphatase activity. Casein kinase II has been shown to phosphorylate the acidic loop of the D2 domain (27,28). Mutation of these serines reduces the ability of CD45 to restore TCR signaling to a CD45-deficient cell line. Transient tyrosine phosphorylation of CD45 has also been reported, although the physiologic significance of this modification is unclear (121,122). Reactive oxygen intermediates have been shown to inhibit phosphatase activity in neutrophils (123). This is intriguing given the role of oxidation in stabilizing dimers in PTP α (102). Further analysis of the potential functional impacts of these modifications is warranted.
CD45 and Disease
Given its central role in regulating SFKs and thus multiple signaling networks essential for immune cell function, a role for CD45 in disease would be logical. However, our understanding of the potential impact of CD45 in disease is currently incomplete. While it is quite clear that absence of CD45 results in SCID, whether CD45 affects susceptibility to other diseases is less definitive. Most work has focused on polymorphic variants that alter CD45 isoform expression and disease susceptibility ((20) and references therein). In at least some ethnic backgrounds, individuals expressing a translationally silent polymorphism, C77G, in exon 4 of CD45 have an increased incidence of multiple sclerosis, autoimmune hepatitis, systemic sclerosis, Langerhans Cell Histiocytosis, hemophagocytic lymphohistiocytosis, and HIV infection. Interestingly, this mutation disrupts an exonic splicing silencer such that these individuals are unable to appropriately produce low MW CD45 isoforms. In contrast, a second polymorphism, A138G, in exon 6 appears to promote exon skipping, resulting in increased expression of the low MW isoform CD45RO. The A138G allele has been associated with a protective effect in hepatitis B infection and autoimmune Graves' thyroiditis. This, and its high allele frequency in approximately 20% of Asians, raised the speculation that it might confer a selective advantage, although this idea has yet to be substantiated.
One interpretation of these observations is that the presence of and/or ability to isoform switch to low MW isoforms somehow enhances immune tolerance and decreases reactivity to self-antigen. While T cells from C77G and A138G individuals appear to have altered TCR threshold and cytokine profiles, it is not clear whether this a direct effect of the CD45 polymorphism or another unrelated process (124,125). Indeed, a challenge in understanding whether these alterations in CD45 expression are causal or simply coincidental in disease has been the paucity of data, despite extensive efforts, demonstrating robust functional differences between the different isoforms. Several groups have generated transgenic mice expressing a single CD45 isoform on a null background (13,14,20,44) and references therein). These transgenic animals revealed the importance of CD45 dosage but did not identify significant differences between different isoforms. Interestingly, all isoforms were capable of rescuing thymic development and peripheral T cell function, even when expressed at very low levels. However, none of these isoforms enabled normal B cell maturation, further emphasizing that CD45 function in these lineages may be quite distinct. Overall, distinct and reproducible isoform-specific functional differences have been modest at best. The fact that these transgenes do not recapitulate wt levels of CD45 expression may contribute to the lack of findings in these studies.
It has also been proposed that other genetic modifiers could cooperate with CD45 in altering sensitivity or resistance to disease. Aberrant CD45 expression has been reported in infantile cholestasis, malnutrition, systemic lupus erythematosus, rheumatoid arthritis, HIV, Alzheimer's disease, and multiple myeloma ((20) and references therein). In mice, we have found that the CD45E613R phenotype is exquisitely sensitive to genetic modifiers (Hermiston M and Weiss A, unpublished observation). On mixed C57Bl/6 (B6)-129 genetic background, these mice develop a lupus like phenotype. However, when backcrossed to B6 or 129 backgrounds, the autoimmune stigmata are absent, despite persistent hyperresponsiveness to antigen receptor signaling at a biochemical level. In contrast, CD45E613R mice on a BALB/c genetic background develop autoantibodies with 100% penetrance by 12 weeks of age. Mapping studies between B6 and BALB/c CD45E613R mice identified contributions from loci on chromosomes 9 and 17 that appear to mediate autoantibody production (Hermiston et al., manuscript in preparation). In addition to this unbiased screen, we have also used a candidate gene approach to determine whether unregulated CD45 phosphatase activity could increase the propensity for autoimmunity. Supporting this notion, CD45E613R mice also carrying MRL/lpr (Gupta, V et al., in press), Pep (Zikherman, J. et al., in press), Aire (Hermiston ML, Lam VC, Anderson M, and Weiss A, unpublished observation), or Dap12 (Hermiston ML, Lam VC, Lanier LL, Weiss A, unpublished observations) (but not C4) mutations on a B6 background (a genetic background that is not associated with substantial autoimmunity for any of these genes) developed significant lymphoproliferation and autoantibody production in the doubly mutant state. Together, these data provide further support for a potential role for CD45 in modulation of susceptibility to autoimmune disease.
Unanswered questions
The questions plaguing the CD45 field have been cited in multiple reviews spanning almost two decades (14,44-46,112). Why is there so much CD45? Why so many isoforms? Why are they expressed in such a precise pattern? Does this receptor have a ligand? How is phosphatase activity regulated? While substantial progress has been made on these fronts, significant work remains. With increasing investigation, the function of this molecule has seemingly become increasingly complex. It is clear that CD45 can serve as both a positive and negative regulator in a cell type- and context-dependent manner. Considering these cell and context dependent scenarios is going to be crucial for understanding the biologic function of this protein. Likewise, as discussed below, exploring the contribution of other PTPs such as CD148 and Lyp/Pep that converge on the same substrates as CD45 will be critical for developing a more complete picture of how this phosphatase regulates immune cell development and function.
PTPRJ; CD148
The variable effects of CD45 deficiency on different hematopoietic cell lineages in vivo are intriguing. Why was the impact on T cell development so profound while the effect on myeloid and B cell development and function much milder? Biochemically, CD45 deficient thymocytes and B cells behave quite differently in response to antigen receptor stimulation. For example, as noted above, calcium flux in CD45 deficient thymocytes is almost completely abolished but largely preserved in CD45 deficient B cells (15,33,64). Several potential explanations could explain these differences. Perhaps in non-T cell lineages CD45 is engaged in different signaling pathways and/or CD45-independent pathways compensate for its absence. Alternatively, perhaps the milder phenotypes in CD45 deficient B and myeloid cells reflect the presence of another PTP that plays a functionally redundant role with CD45. Recent work from the Weiss lab indicate that the RPTP CD148 can indeed subserve this function and regulate SFK activity, together with CD45, in B cells and macrophages (67).
CD148 Structure
While CD148 (encoded by PTPRJ) is also a receptor type protein tyrosine phosphatase, its structure is quite distinct from CD45 (Figure 2). CD148 belongs to the type III family of PTPs. The extracellular domain consists of eight to nine fibronectin domains and 34 potential N-linked glycosylation sites(126). It is not clear if there are different splice forms of CD148. Contrasting the tandem PTP domains of CD45, CD148 has only a single PTP homology domain in the cytoplasm (Figure 2). The PTP domain of CD148 contains a cysteine residue that is critical for its catalytic activity. When the cysteine residue is mutated to serine, the catalytic activity of CD148 is abolished.(127) The structure of the CD148 cytoplasmic domain has been resolved (PBD database). Interestingly, it contains a juxtamembrane wedge structure similar to those seen in both the CD45 and RPTPα crystal structures. It is currently unclear whether CD148 can exist as dimers physiologically or if the wedge plays a regulatory role for this phosphatase. It will be interesting to test these possibilities given the striking structural similarity between the wedge motifs in these different phosphatases.
CD148 Expression
In contrast to the exclusive expression of CD45 in hematopoietic cells, CD148 is widely expressed in many cell types, including epithelial and endothelial cells, fibroblasts, and most hematopoietic cells (128-131). Outside the immune system, CD148 expression has been shown to be upregulated in cells that are cultured at high cell density as well as upon cellular differentiation (132-135). Using monoclonal antibodies to CD148, the expression pattern of CD148 in the murine and human immune systems has been examined (131,136,137). Interestingly, the expression patterns of CD148 are different between mice and humans. In mice, CD148 is expressed primarily in platelets, B cells, and myeloid lineage cells. Expression in the T cell lineage is more restricted. Transient expression has been noted in DN thymocytes (Zhu and Weiss, unpublished data). In peripheral T cells, CD148 expression is induced only after TCR activation (131). In contrast, in humans, CD148 is widely expressed on mature thymocytes, peripheral T cells (with CD8 T cells having higher levels than CD4 T cells), B cells, NK cells, granulocytes, macrophages and certain DC populations. The expression of CD148 on human peripheral T cells is further induced upon TCR stimulation (136).
CD148 Function
Relative to CD45, our understanding of the function of CD148 and its role in governing signaling networks in both immune and non-immune cells is much more limited. Several genetic studies have implicated CD148 as a tumor suppressor. Loss of heterozygosity for CD148 has been reported in colon, lung and breast carcinomas (138,139). In addition, positional cloning of a gene responsible for Susceptibility to Colon Cancer (Scc1) in mice, revealed CD148 as a major candidate (139). Whether or not CD148 may play a similar role in hematologic malignancies is not clear. Interestingly, CD148 has been implicated in at least one autoimmune disease. Cogan's syndrome is a chronic inflammatory disease characterized by sensorineural hearing loss, keratitis, and vasculitis. A screen for autoantigens in this disease revealed antibodies to CD148 (140). These autoantibodies inhibited proliferation of CD148 expressing cells and replicated the features of Cogan's disease when infused into mice. While intriguing, further experimentation will be required to determine whether these antibodies influence CD148 function and are thus causative for this or other autoimmune diseases.
To understand these potential disease associations, it will be important to define the mechanisms by which CD148 regulates signaling cascades within cells. To date, many proteins have been identified as potential CD148 substrates. These include PDGFR, HGFR (c-Met), p120ctn, c-Src (C terminal inhibitory tyrosine), and PI3K (141-146). Unfortunately, the majority of the these putative substrates were found using substrate trapping methodologies in vitro or over-expression approaches in cell lines and have not been rigorously validated in vivo.
The function of CD148 in leukocytes was initially analyzed using forced expression of CD148 in a Jurkat T cell line. These studies indicated that induced expression of CD148 led to selective dephosphorylation of PLCγ-1 and LAT, resulting in downregulation of TCR dependent signaling (127). However, it was not clear whether this was due to direct or indirection actions of the phosphatase, since it was also plausible that the phosphatase could activate a negative feedback loop resulting in activation of other phosphatases within the cell that then down-regulate the TCR response. Consistent with a potential role in termination of the immune response, immunofluorescence microscopy revealed that the extracellular domain of CD148 mediated its exclusion from the immunological synapse, sequestering it from potential substrates (147). The authors proposed that upon T cell-APC disengagement, CD148 could then access and dephosphorylate its relevant substrates, resulting in termination of prolonged TCR signaling.
Several labs attempted to generate CD148 deficient mice to study the role of this RPTP in vivo. The replacement of the CD148 PTP domain with GFP led to early embryonic lethality due to the defects in vasculature development (148). In contrast, Trapasso and Fusco generated CD148 deficient mice by deleting exons 3, 4 and 5 (149). These mice were viable and healthy, leading the authors to conclude that CD148 is dispensable for normal growth and development in mice. However, a detailed analysis of the immune system function was not attempted. Removing the exon encoding the transmembrane domain of CD148 generated a third CD148 mutant mouse line that lacked CD148 phosphatase activity, however a truncated soluble CD148 protein was still expressed (67). These mice were also grossly normal; however, detailed analysis of the hematopoietic lineage of these mice revealed a function for CD148 in the immune system.
Extensive analysis of the T cell lineage, which expresses minimal CD148, failed to reveal any alterations in thymic development or peripheral T cell function. In contrast, the impact of CD148 deficiency on B cells, where considerable CD148 expression is found, was significant (67). B cell development was blocked at the transitional stage, marginal zone B cells were dramatically increased, and there was a paucity of B1 B cells in the peritoneal cavity. This phenotype is almost an exact phenocopy of the B cell phenotype in CD45 deficient mice. Given the phenotypic similarity of CD45 and CD148 deficient B cells, it was hypothesized that these two phosphatases may have overlapping functions in B cells.
To test this hypothesis, CD45 and CD148 doubly deficient mice were generated (67). These mice displayed substantial alterations in B and myeloid lineage development and defective immunoreceptor signaling. In CD45 and CD148 doubly deficient B cells, BCR mediated signaling events, including intracellular calcium mobilization, protein tyrosine phosphorylation, and MAPK activation, were impaired. In macrophages, Fc receptor mediated signaling, as well as phagocytosis and cytokine production, were also found to be dependent on both CD45 and CD148. Further biochemical analysis of CD45 and CD148 doubly deficient B cells and macrophages revealed hyperphosphorylation of the C-terminal inhibitory tyrosine of SFKs. These findings suggested that C-terminal tyrosine of SFKs is a common substrate for both CD45 and CD148 and that decreased activity of SFKs is most likely responsible for at least some of the observed defects (Figure 3). These findings also highlight a previously unappreciated level of functional redundancy between CD45 and CD148.
CD148: Unanswered questions
As a new player in the immune system, CD148 is gradually attracting the attention of immunologists. The overlapping function of the structurally distinct phosphatases CD45 and CD148 in regulating immunoreceptor signaling via dephosphorylation of Src-family kinase inhibitory tyrosine was unexpected. Several intriguing questions remain: First, is there any unique function of CD148, given its distinct structure and expression pattern? We recently have turned our attention to platelets, which are devoid of CD45 but express high levels of CD148. Loss of CD148 in platelets results in hyporesponsiveness of SFKs following collagen and fibrinogen stimulation (Yotis A, Zhu J, Weiss A, Watson SP, unpublished observations). Because CD148 is the only RPTP expressed in platelets, this will be a particularly useful cell type in which to explore its functions. Second, since SFKs are critical regulators of many other signaling pathways, what is the function of CD148 in these pathways, including integrin, G-protein coupled receptor, and growth factor mediated pathways? Finally, since it is a receptor-like PTP, is there any ligand or binding partner of CD148 in vivo? Only future studies can provide deeper insights into these and potentially many other questions on the function of this interesting phosphatase.
PTPN22; Lyp/Pep
Although the inhibitory tyrosine of the SFKs appears to be uniquely regulated in hematopoietic lineages by the phosphatases CD45 and CD148 and the kinase Csk, regulation of the activating tyrosine is less well defined. Multiple unrelated phosphatases subserve this function in a partially redundant fashion (e.g., SHP-1, PTP-PEST, Pep/Lyp) (1). Of particular interest among these is the PTPN22 gene product, Lyp (lymphoid tyrosine phosphatase) and its murine ortholog PEST-domain enriched tyrosine phosphatase (Pep). This locus has been associated with multiple human autoimmune diseases, focusing attention both on the function of Lyp/Pep and the pathway(s) it regulates.
Lyp/Pep Structure
Lyp/Pep is a hematopoietic tyrosine phosphatase that contains an N-terminal phosphatase domain and 4 proline-rich domains, the most C-terminal of which lies within a 20 amino acid conserved region that defines a small family of phosphatases, the so-called PEST group (Figure 2) (3,150,151). This includes Pep as well as the ubiquitously expressed PTP-PEST and HSC-PTP, which is expressed exclusively in hematopoietic stem cells. The function of this conserved C-terminal domain remains unclear. Pep and Lyp are species orthologs but vary considerably in their C-terminal halves (151). There is 89% sequence homology between the phosphatase domains, but only 61% homology for the remaining portions of the proteins.
Two alternative splice isoforms of Lyp have been detected, Lyp1 and 2 (151). The full-length protein is 105Kd, while Lyp2 is a shorter transcript that encodes an 85Kd isoform missing the 3 most C-terminal proline rich sequence (PRS) domains. The protein product of the short isoform has not been characterized and analogous splicing has not been reported for Pep. The in vivo functional significance of this splicing event remains to be determined.
Lyp/Pep Expression
The expression pattern of Pep/Lyp is not completely characterized. Lyp message has been detected in human thymic and lymphoid tissue, including T and B cells as well as myeloid cell lines (151). Lyp protein has been seen in thymocytes and peripheral T cells and appears to be upregulated upon T cell activation (151). We have found Pep protein expression predominantly in the thymus and T cell lineage with little in bone marrow, B cells, or myeloid cells (Zikherman et al., manuscript submitted). Subcellular localization of Pep/Lyp has been studied only with overexpression systems. In these settings, Pep is predominantly cytoplasmic (151,152).
Lyp/Pep Function
Overexpression studies in T cell lines have demonstrated a negative regulatory role for Pep/Lyp in T cell receptor signaling and identified its substrate as the activating loop tyrosine of Lck (153,154). Additional putative targets have been identified by substrate trapping and include TCR signal transduction machinery such as Zap70, CD3ζ, CD3ε, and Vav (155). Since all identified substrates are downstream of Lck and substrate trapping may pull down artifactual interactions, it is less clear if these represent bona fide substrates.
Pep is cytoplasmic and a significant proportion (25-50%) is constitutively associated with Csk (152). Pep is thought to be brought into proximity of its target, CD4-associated Lck, via its association with Csk. Csk is in turn recruited to lipid rafts under basal conditions by the phosphorylated adaptor, PAG (156,157). Upon receptor stimulation, PAG is rapidly dephosphorylated and Csk/Pep are released to the cytoplasm (Figure 3). Both Pep and PTP-PEST have each been shown to cooperatively inhibit TCR signaling together with Csk (153,158). Both the physical association of Pep with Csk and their cooperative inhibition have been demonstrated to depend upon the SH3 domain of Csk and the first proline-rich segment (PRS1) of Pep (152,153,159). This interaction domain has been mapped to an 8-residue stretch of the proline rich segment (613-621 PPPLPERT) (152,159). Interestingly, this minimal domain is well conserved in PRS2 of PTP-PEST and, together with adjacent residues, mediates association with Csk (158). In contrast to Pep, PTP-PEST has a more extensive list of putative interacting partners (e.g. Shc, paxillin, Cas) (160).
A Pep-deficient mouse was generated and confirmed a negative regulatory role for Pep in TCR signaling (161). The phenotype was, however, surprisingly subtle. Mice developed mild-to-moderate lymphadenopathy and splenomegaly on the B6 background along with an expanded effector/memory compartment by 6 months of age. Positive selection was enhanced in the thymus in the context of TCR transgenes. Peripheral T cells were found to be hyper-responsive to TCR stimulation, but only in the effector/memory subset. In contrast, B cell signaling was unperturbed. Although accelerated germinal center formation was observed, the mice remained healthy with no obvious disease. More recently, the expression pattern of the redundant phosphatase PTP-PEST has been refined and it has been shown to be down-regulated in activated/effector T cells, and absent in the Jurkat T cell line (Zikherman and Weiss, unpublished observations). This suggests a mechanism for the selective signaling phenotype observed in the Pep-deficient mice. Analogous to CD45 and CD148, the phenotype of doubly deficient PEP and PTP-PEST mice could prove quite interesting.
Regulation of Lyp/Pep
Recently, a novel relatively selective inhibitor of Lyp has been developed and a low-resolution crystal structure of inhibitor-bound Lyp has been reported (162). The structure revealed contacts not only in the active site (catalytic cysteine 129), but also in an adjacent cluster of residues (S35-K47). This adjacent region could adopt loop or helical conformations depending upon the presence or absence of substrate binding. This contact domain was noted to harbor a putative PKC phosphorylation site at serine 35, which was validated in vitro and in vivo. A S35E mutant of Lyp was generated to model constitutive phosphorylation of this residue. S35E impaired Lyp interaction with both the novel inhibitor and physiological substrates. Finally, in Jurkat overexpression studies, inhibition of T cell signaling by S35E Lyp was impaired. Further work to identify the in vivo significance of this residue remains to be pursued.
Role of Lyp/Pep in Disease
PTPN22 received considerable attention in 2004 when a common human polymorphism, C1858T/R620W, in the gene was identified and noted to be associated with the autoimmune diseases type I diabetes (T1D), systemic lupus erythematosus (SLE), and rheumatoid arthritis (RA) (163-165). Indeed, whole genome association studies in the past two years have demonstrated that this polymorphism constitutes the second strongest common genetic risk factor for RA outside of the major histocompatibility complex (166). Nevertheless, the relative risk conferred by this polymorphism is less than two, implying critical dependence on cooperating genetic loci.
Interestingly, the PTPN22 R620W polymorphism is associated with multiple other autoimmune diseases such as thyroiditis and myasthenia gravis (167). Together with SLE, T1D and RA, this panoply of disease associations places this genetic locus on the short-list of “general” autoimmunity genes alongside such players as the inhibitory T cell co-receptor CTLA-4. Of note, the disease associations of this polymorphism all have autoantibody production as a significant and pathogenic feature (167). Indeed, the R620W allele has been associated with autoantibody production in myasthenia gravis and with autoantibody positive variants of RA (168,169). Additional clues to pathogenesis come from a genetic association study that identified a protective effect of the “risk allele” against tuberculosis (170). This suggests at once both a selective evolutionary pressure and a possible mechanism of action for the polymorphism.
Is the PTPN22 R620W polymorphism a hypo- or hypermorphic allele?
There is a vast human genetic literature about the PTPN22 R620W polymorphism, but a relative paucity of functional data to address what effect this allele has on antigen receptor signaling and by what mechanism. Since R620W is located in the critical proline-rich sequence (PRS1 domain) of Pep that mediates Csk association, it was initially postulated that the human polymorphism generated a hypomorphic allele (163). Indeed, two independent reports identify impaired interaction between R620W and Csk (163,171). However, a single overexpression study conducted in the Jurkat cell line suggested that unexpectedly Lyp R620W might represent a gain-of-function allele (171). Subsequently, a handful of functional studies using human PBMCs from patients or healthy subjects who carry the risk allele, have been published (172). Conflicting results have been reported. The most extensive and well controlled of these studies, performed with healthy subjects heterozygous for the risk allele, identified hyper-responsiveness of memory T and B lymphocytes to antigen-receptor signaling (169,172,173). However, only certain signaling readouts demonstrated this behavior and controlling genetic and environmental heterogeneity in human samples is challenging. Certainly a gain-of-function risk allele might provoke disease by impairing central thymic tolerance, but a hypomorphic allele continues to make sense biochemically and might be more consistent with known genetic association with resistance to tuberculosis.
We have revisited this question by trying to address limitations of prior functional studies such as genetic heterogeneity, and overexpression artifact (Zikherman and Weiss, unpublished observations). We have co-expressed the obligate binding partner Csk in conjunction with wt or R620W Pep in Jurkat cells. By using extremely sensitive flow cytometric assessment of Erk phosphorylation in response to TCR stimulation, we have been able to dramatically limit the degree of overexpression. By gating on co-transfected GFP, we have been able to control for overexpression artifact. Our data suggests that the R620W allele indeed represents a hypomorphic variant. Whether the contrasting earlier Jurkat study reflects differences in expression level or biologically distinct functions of human Lyp and murine Pep remain to be addressed. This has significant implications for pathogenesis, as well as future diagnosis and therapy.
Although spontaneous germinal centers develop in the Pep-deficient mice, no frank autoimmune disease was observed on the B6 background. As further proof of concept, we have taken advantage of Pep deficient mice as a model of the human hypomorphic allele. We have shown that in the context of a susceptible genetic background, Pep deficient T cells drive hyper-responsive CD45E613R B cells to produce autoantibodies. Double mutant mice develop a lupus-like disease characterized by glomerulonephritis and premature mortality, recapitulating the human disease associations of the PTPN22 risk allele. The functional consequences of the R620W polymorphism remain to be definitively established through the generation of a knock-in animal. It is likely that modeling disease even in this context will require additional cooperating genetic loci.
Lyp/Pep: Unanswered questions
While substantial progress has been made in our understanding of the phosphatase Lyp/Pep, significant questions remain. Does Pep have Csk-independent functions and if so, how does it access alternate substrates? What redundant functions are compensated for by PTP-PEST in T cells and how severe a phenotype might be revealed by a double knockout? What other structurally distinct phosphatases can compensate for Pep-deficiency to dephosphorylate the activating tyrosine of the SFKs? What accomplishes this role in B cells where Pep does not appear to be highly expressed? Clearly, regulation of SFK activity has critical implications not only for cancer but also for autoimmune diseases. In light of this fact, greater impetus exists to address remaining questions about Lyp/Pep.
Summary and Prospectus
Can we reconcile the apparent paradoxes regarding SFK regulation that have emerged in the literature over the past 20 years? Certainly, some of these paradoxes can be explained by redundancy. For example, the more pronounced T cell phenotype versus B cell phenotype in CD45 deficient mice can now be understood by the newly found redundancy between CD45 and CD148. However, still perplexing is the observation that CD45 transgenes cannot rescue B cell differentiation in CD45 deficient mice. This might suggest that these SFKs are differentially counter-regulated in B cells versus T cells. Perhaps this reflects Lyn's unique status among SFKs in having well-defined positive and negative roles. Alternatively, trafficking of Csk/Lyp/Pep may be different in non-T cell lineages, an issue that has yet to be addressed in detail.
How do we integrate functional contributions of these different phosphatases? The emerging data indicates that regulation of SFKs is more complex than initially perceived. The data clearly demonstrate that a simple paradigm based on one lineage will be misleading. As discussed, cell type and context are important. Studying these three distinct PTPs and their relative contributions to SFK regulation provides a unique opportunity to examine how evolutionally different cell types have solved similar problems in different ways. For example, T lineage cells appear to rely on segregating substrate access via CD4 while B cells have evolved multiple co-receptors (CD40, CD19, CD22, etc.). It will also be important to evaluate the impact of these PTPs on different signaling pathways within the same cell type (e.g., BCR, Fc, and TCR signaling vs. TLR or integrin mediated signaling). One can imagine distinct outcomes on different signaling nodes based upon differential regulated localization of the PTP and its substrate.
Further work examining the potential dual positive and negative regulatory role of CD45 is also needed. The evaluation of mice expressing intermediate levels of CD45 confirms prior studies that suggested CD45 has negative as well positive roles in TCR signaling. However, it is still unclear how the catalytic and C-terminal tyrosines are differentially regulated by these PTPs, especially in non-T cell lineages or in the context of non-antigen receptor signaling. Perhaps the redundancy of other phosphatases in these signaling pathways mirrors the hyperresponsiveness seen with intermediate CD45 expression levels in T cells. Alternatively or in addition, regulated localization of these kinases and phosphatases in these contexts might drive these differences. Given the paradoxical roles and widespread expression of these phosphatases within multiple hematopoietic lineages, it would be wise to clarify these issues before treating patients with the anti-CD45 antibodies and Lyp inhibitors that are currently being developed.
Acknowledgments
We thank Art Weiss and members of the Weiss and Hermiston Laboratories for helpful discussions and T. Katsumoto for critical reading of the manuscript. The work from our laboratories was supported by K08 CA098418, the Campini, Lurie, Conner, and St. Baldrick's Foundations (M.L.H.), the Arthritis Foundation (J.Z.), and PO1 AI355297 (A.W.).
References
- 1.Acuto O, Bartolo VD, Michel F. Tailoring T-cell receptor signals by proximal negative feedback mechanisms. Nat Rev Immunol. 2008 Sep;8(9):699–712. doi: 10.1038/nri2397. [DOI] [PubMed] [Google Scholar]
- 2.Hermiston ML, Xu Z, Majeti R, Weiss A. Reciprocal regulation of lymphocyte activation by tyrosine kinases and phosphatases. The Journal of clinical investigation. 2002 Jan;109(1):9–14. doi: 10.1172/JCI14794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vang T, Miletic AV, Arimura Y, Tautz L, Rickert RC, Mustelin T. Protein tyrosine phosphatases in autoimmunity. Annu Rev Immunol. 2008;26:29–55. doi: 10.1146/annurev.immunol.26.021607.090418. [DOI] [PubMed] [Google Scholar]
- 4.Lowell CA. Src-family kinases: rheostats of immune cell signaling. Mol Immunol. 2004 Jul;41(67):631–43. doi: 10.1016/j.molimm.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 5.Palacios EH, Weiss A. Function of the Src-family kinases, Lck and Fyn, in T-cell development and activation. Oncogene. 2004 Oct 18;23(48):7990–8000. doi: 10.1038/sj.onc.1208074. [DOI] [PubMed] [Google Scholar]
- 6.Young MA, Gonfloni S, Superti-Furga G, Roux B, Kuriyan J. Dynamic coupling between the SH2 and SH3 domains of c-Src and Hck underlies their inactivation by C-terminal tyrosine phosphorylation. Cell. 2001 Apr 6;105(1):115–126. doi: 10.1016/s0092-8674(01)00301-4. [DOI] [PubMed] [Google Scholar]
- 7.Nika K, Tautz L, Arimura Y, Vang T, Williams S, Mustelin T. A weak Lck tail bite is necessary for Lck function in T cell antigen receptor signaling. J Biol Chem. 2007 Dec 7;282(49):36000–36009. doi: 10.1074/jbc.M702779200. [DOI] [PubMed] [Google Scholar]
- 8.Sieh M, Bolen JB, Weiss A. CD45 specifically modulates binding of Lck to a phosphopeptide encompassing the negative regulatory tyrosine of Lck. EMBO Journal. 1993;12:315–322. doi: 10.1002/j.1460-2075.1993.tb05659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ostergaard HL, Trowbridge IS. Coclustering CD45 with CD4 and CD8 alters the phosphorylation and kinase activity of p56lck. Journal of Experimental Medicine. 1990;172:347–350. doi: 10.1084/jem.172.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomas ML, Brown EJ. Positive and negative regulation of Src-family membrane kinases by CD45. Immunol Today. 1999 Sep;20(9):406–411. doi: 10.1016/s0167-5699(99)01506-6. [DOI] [PubMed] [Google Scholar]
- 11.Falahati R, Leitenberg D. Changes in the role of the CD45 protein tyrosine phosphatase in regulating Lck tyrosine phosphorylation during thymic development. J Immunol. 2007 Feb 15;178(4):2056–64. doi: 10.4049/jimmunol.178.4.2056. [DOI] [PubMed] [Google Scholar]
- 12.Thomas ML, Lefrancois L. Differential expression of the leucocyte-common antigen family. Immunology Today. 1988;9(10):320–326. doi: 10.1016/0167-5699(88)91326-6. [DOI] [PubMed] [Google Scholar]
- 13.Hermiston ML, Xu Z, Weiss A. CD45: a critical regulator of signaling thresholds in immune cells. Annu Rev Immunol. 2003;21:107–37. doi: 10.1146/annurev.immunol.21.120601.140946. [DOI] [PubMed] [Google Scholar]
- 14.Holmes N. CD45: all is not yet crystal clear. Immunology. 2006 Feb;117(2):145–55. doi: 10.1111/j.1365-2567.2005.02265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mee PJ, Turner M, Basson MA, Costello PS, Zamoyska R, Tybulewicz VL. Greatly reduced efficiency of both positive and negative selection of thymocytes in CD45 tyrosine phosphatase-deficient mice. European journal of immunology. 1999 Sep;29(9):2923–33. doi: 10.1002/(SICI)1521-4141(199909)29:09<2923::AID-IMMU2923>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 16.Byth KF, Conroy LA, Howlett S, Smith AJ, May J, Alexander DR, et al. CD45-null transgenic mice reveal a positive regulatory role for CD45 in early thymocyte development, in the selection of CD4+CD8+ thymocytes, and B cell maturation. J Exp Med. 1996 Apr 1;183(4):1707–18. doi: 10.1084/jem.183.4.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kishihara K, Penninger J, Wallace VA, Kundig TM, Kawai K, Wakeham A, et al. Normal B lymphocyte development but impaired T cell maturation in CD45-Exon6 protein tyrosine phosphatase-deficient mice. Cell. 1993;74:143–156. doi: 10.1016/0092-8674(93)90302-7. [DOI] [PubMed] [Google Scholar]
- 18.Tchilian EZ, Wallace DL, Wells RS, Flower DR, Morgan G, Beverley PC. A deletion in the gene encoding the CD45 antigen in a patient with SCID. J Immunol. 2001 Jan 15;166(2):1308–13. doi: 10.4049/jimmunol.166.2.1308. [DOI] [PubMed] [Google Scholar]
- 19.Kung C, Pingel JT, Heikinheimo M, Klemola T, Varkila K, Yoo LI, et al. Mutations in the tyrosine phosphatase CD45 gene in a child with severe combined immunodeficiency disease. Nat Med. 2000;6:343–345. doi: 10.1038/73208. [DOI] [PubMed] [Google Scholar]
- 20.Tchilian EZ, Beverley PC. Altered CD45 expression and disease. Trends in immunology. 2006 Mar;27(3):146–53. doi: 10.1016/j.it.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 21.Earl LA, Baum LG. CD45 Glycosylation controls T-cell life and death. Immunol Cell Biol. 2008 Oct;86(7):608–615. doi: 10.1038/icb.2008.46. [DOI] [PubMed] [Google Scholar]
- 22.Hernandez JD, Klein J, Van Dyken SJ, Marth JD, Baum LG. T-cell activation results in microheterogeneous changes in glycosylation of CD45. Int Immunol. 2007 Jul;19(7):847–856. doi: 10.1093/intimm/dxm053. [DOI] [PubMed] [Google Scholar]
- 23.van Vliet SJ, Gringhuis SI, Geijtenbeek TB, van Kooyk Y. Regulation of effector T cells by antigen-presenting cells via interaction of the C-type lectin MGL with CD45. Nat Immunol. 2006 Nov;7(11):1200–1208. doi: 10.1038/ni1390. [DOI] [PubMed] [Google Scholar]
- 24.Chen IJ, Chen HL, Demetriou M. Lateral compartmentalization of T cell receptor versus CD45 by galectin-N-glycan binding and microfilaments coordinate basal and activation signaling. J Biol Chem. 2007 Nov 30;282(48):35361–35372. doi: 10.1074/jbc.M706923200. [DOI] [PubMed] [Google Scholar]
- 25.Nam HJ, Poy F, Saito H, Frederick CA. Structural basis for the function and regulation of the receptor protein tyrosine phosphatase CD45. J Exp Med. 2005 Feb 7;201(3):441–52. doi: 10.1084/jem.20041890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Desai DM, Sap J, Silvennoinen O, Schlessinger J, Weiss A. The catalytic activity of the CD45 membrane-proximal phosphatase domain is required for TCR signaling and regulation. The EMBO journal. 1994 Sep 1;13(17):4002–10. doi: 10.1002/j.1460-2075.1994.tb06716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y, Guo W, Liang L, Esselman WJ. Phosphorylation of CD45 by casein kinase 2. Modulation of activity and mutational analysis. The Journal of biological chemistry. 1999 Mar 12;274(11):7454–61. doi: 10.1074/jbc.274.11.7454. [DOI] [PubMed] [Google Scholar]
- 28.Greer SF, Justement LB. CD45 regulates tyrosine phosphorylation of CD22 and its association with the protein tyrosine phosphatase SHP-1. J Immunol. 1999 May 1;162(9):5278–86. [PubMed] [Google Scholar]
- 29.Wang Y, Botvinick EL, Zhao Y, Berns MW, Usami S, Tsien RY, et al. Visualizing the mechanical activation of Src. Nature. 2005 Apr 21;434(7036):1040–5. doi: 10.1038/nature03469. [DOI] [PubMed] [Google Scholar]
- 30.Bilwes AM, den Hertog J, Hunter T, Noel JP. Structural basis for inhibition of receptor protein-tyrosine phosphatase-α by dimerization. Nature. 1996;382:555–559. doi: 10.1038/382555a0. [DOI] [PubMed] [Google Scholar]
- 31.Majeti R, Bilwes AM, N JP, Hunter T, Weiss A. Ligand-induced dimerization regulates receptor protein tyrosine phosphatase function via an inhibitory wedge. Science. 1998;279:88–91. doi: 10.1126/science.279.5347.88. [DOI] [PubMed] [Google Scholar]
- 32.Majeti R, Xu Z, Parslow TG, Olson JL, Daikh DI, Killeen N, et al. An inactivating point mutation in the inhibitory wedge of CD45 causes lymphoproliferation and autoimmunity. Cell. 2000;103:1059–1070. doi: 10.1016/s0092-8674(00)00209-9. [DOI] [PubMed] [Google Scholar]
- 33.Hermiston ML, Tan AL, Gupta VA, Majeti R, Weiss A. The juxtamembrane wedge negatively regulates CD45 function in B cells. Immunity. 2005 Dec;23(6):635–47. doi: 10.1016/j.immuni.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 34.McNeill L, Cassady RL, Sarkardei S, Cooper JC, Morgan G, Alexander DR. CD45 isoforms in T cell signalling and development. Immunol Lett. 2004 Mar 29;92(12):125–134. doi: 10.1016/j.imlet.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 35.Lynch KW, Weiss A. A model system for activation-induced alternative splicing of CD45 pre-mRNA in T cells implicates PKC and Ras. 2000;20:70–80. doi: 10.1128/mcb.20.1.70-80.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lynch KW. Consequences of regulated pre-mRNA splicing in the immune system. Nature reviews. 2004 Dec;4(12):931–40. doi: 10.1038/nri1497. [DOI] [PubMed] [Google Scholar]
- 37.Melton AA, Jackson J, Wang J, Lynch KW. Combinatorial control of signal-induced exon repression by hnRNP L and PSF. Mol Cell Biol. 2007 Oct;27(19):6972–6984. doi: 10.1128/MCB.00419-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oberdoerffer S, Moita LF, Neems D, Freitas RP, Hacohen N, Rao A. Regulation of CD45 alternative splicing by heterogeneous ribonucleoprotein hnRNPLL. Science. 2008 Aug 1;321(5889):686–691. doi: 10.1126/science.1157610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Topp JD, Jackson J, Melton AA, Lynch KW. A cell-based screen for splicing regulators identifies hnRNP LL as a distinct signal-induced repressor of CD45 variable exon 4. RNA. 2008 Oct;14(10):2038–2049. doi: 10.1261/rna.1212008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Furukawa T, Itoh M, Krueger NX, Streuli M, Saito H. Specific interaction of the CD45-protein tyrosine phosphatase with tyrosine phosphorylated CD3 ζ chain. Proc Natl Acad Sci USA. 1994;91:10928–10932. doi: 10.1073/pnas.91.23.10928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu L, Fu J, Shen SH. SKAP55 coupled with CD45 positively regulates T-cell receptor-mediated gene transcription. Mol Cell Biol. 2002 Apr;22(8):2673–2686. doi: 10.1128/MCB.22.8.2673-2686.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Irie-Sasaki J, Sasaki T, Matsumoto W, Opavsky A, Cheng M, Welstead G, et al. CD45 is a JAK phosphatase and negatively regulates cytokine receptor signalling. Nature. 2001 Jan 18;409(6818):349–54. doi: 10.1038/35053086. [DOI] [PubMed] [Google Scholar]
- 43.Davidson D, Bakinowski M, Thomas ML, Horejsi V, Veillette A. Phosphorylation-dependent regulation of T-cell activation by PAG/Cbp, a lipid raft-associated transmembrane adaptor. Mol Cell Biol. 2003 Mar;23(6):2017–2028. doi: 10.1128/MCB.23.6.2017-2028.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zamoyska R. Why is there so much CD45 on T cells? Immunity. 2007 Sep;27(3):421–423. doi: 10.1016/j.immuni.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 45.Huntington ND, Tarlinton DM. CD45: direct and indirect government of immune regulation. Immunol Lett. 2004 Jul 15;94(3):167–74. doi: 10.1016/j.imlet.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 46.Alexander DR. The CD45 tyrosine phosphatase: a positive and negative regulator of immune cell function. Semin Immunol. 2000 Aug;12(4):349–59. doi: 10.1006/smim.2000.0218. [DOI] [PubMed] [Google Scholar]
- 47.Pingel S, Baker M, Turner M, Holmes N, Alexander DR. The CD45 tyrosine phosphatase regulates CD3-induced signal transduction and T cell development in recombinase-deficient mice: restoration of pre-TCR function by active p56(lck) European journal of immunology. 1999 Aug;29(8):2376–84. doi: 10.1002/(SICI)1521-4141(199908)29:08<2376::AID-IMMU2376>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 48.Ostergaard HL, Shackelford DA, Hurley TR, Johnson P, Hyman R, Sefton BM, et al. Expression of CD45 alters phosphorylation of the lck-encoded tyrosine protein kinase in murine lymphoma T-cell lines. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8959–63. doi: 10.1073/pnas.86.22.8959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mustelin T, Pessa-Morikawa T, Autero M, Gassmann M, Andersson LC, Gahmberg CG, et al. Regulation of the p59fyn protein tyrosine kinase by the CD45 phosphotyrosine phosphatase. Eur J Immunol. 1992 May;22(5):1173–8. doi: 10.1002/eji.1830220510. [DOI] [PubMed] [Google Scholar]
- 50.Stone JD, Howlett S, Byth KF, Holmes N, Alexander DR. T-cell antigen receptor signal transduction in CD45-/- thymocytes. Biochemical Society transactions. 1997 May;25(2):302S. doi: 10.1042/bst025302s. [DOI] [PubMed] [Google Scholar]
- 51.Seavitt JR, White LS, Murphy KM, Loh DY, Perlmutter RM, Thomas ML. Expression of the p56lckY505F mutation in CD45-deficient mice rescues thymocyte development. Mol & Cell Biol. 1999;19:4200–4208. doi: 10.1128/mcb.19.6.4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Oers NSC, Lowin-Kropf B, Finlay D, Connolly K, Weiss A. αβ T cell development is abolished in mice lacking both Lck and Fyn protein tyrosine kinases. Immunity. 1996;5:429–436. doi: 10.1016/s1074-7613(00)80499-9. [DOI] [PubMed] [Google Scholar]
- 53.Roach T, Slater S, Koval M, White L, Cahir McFarland ED, Okumura M, et al. CD45 regulates Src family member kinase activity associated with macrophage integrin-mediated adhesion. Curr Biol. 1997 Jun 1;7(6):408–17. doi: 10.1016/s0960-9822(06)00188-6. [DOI] [PubMed] [Google Scholar]
- 54.Shenoi H, Seavitt J, Zheleznyak A, Thomas ML, Brown EJ. Regulation of integrin-mediated T cell adhesion by the transmembrane protein tyrosine phosphatase CD451. J Immunol. 1999;162:7120–7127. [PubMed] [Google Scholar]
- 55.Ashwell JD, D'Oro U. CD45 and Src-family kinases: and now for something completely different. Immunology today. 1999 Sep;20(9):412–6. doi: 10.1016/s0167-5699(99)01505-4. [DOI] [PubMed] [Google Scholar]
- 56.Baker M, Gamble J, Tooze R, Higgins D, Yang FT, O'Brien PCM, et al. Development of T-leukaemias in CD45 tyrosine phosphatase-deficient mutatnt lck mice. EMBO. 2000;19:4644–4654. doi: 10.1093/emboj/19.17.4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McNeill L, Salmond RJ, Cooper JC, Carret CK, Cassady-Cain RL, Roche-Molina M, et al. The differential regulation of Lck kinase phosphorylation sites by CD45 is critical for T cell receptor signaling responses. Immunity. 2007 Sep;27(3):425–37. doi: 10.1016/j.immuni.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 58.Wallace VA, Penninger JM, Kishihara K, Timms E, Shahinian A, Pircher H, et al. Alterations in the level of CD45 surface expression affect the outcome of thymic selection. J Immunol. 1997 Apr 1;158(7):3205–14. [PubMed] [Google Scholar]
- 59.Bosma GC, Custer RP, Bosma MJ. Severe combined immunodeficiency in the mouse. Nature. 1983;301:527–530. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- 60.Zamoyska R, Basson A, Filby A, Legname G, Lovatt M, Seddon B. The influence of the src-family kinases, Lck and Fyn, on T cell differentiation, survival and activation. Immunol Rev. 2003 Feb;191:107–118. doi: 10.1034/j.1600-065x.2003.00015.x. [DOI] [PubMed] [Google Scholar]
- 61.Maksumova L, Le HT, Muratkhodjaev F, Davidson D, Veillette A, Pallen CJ. Protein tyrosine phosphatase alpha regulates Fyn activity and Cbp/PAG phosphorylation in thymocyte lipid rafts. J Immunol. 2005 Dec 15;175(12):7947–7956. doi: 10.4049/jimmunol.175.12.7947. [DOI] [PubMed] [Google Scholar]
- 62.Loder F, Mutschler B, Ray RJ, Paige CJ, Sideras P, Torres R, et al. B cell development in the spleen takes place in discrete steps and is determined by the quality of B cell receptor-derived signals. J Exp Med. 1999 Jul 5;190(1):75–89. doi: 10.1084/jem.190.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fleming HE, Milne CD, Paige CJ. CD45-deficient mice accumulate Pro-B cells both in vivo and in vitro. J Immunol. 2004 Aug 15;173(4):2542–2551. doi: 10.4049/jimmunol.173.4.2542. [DOI] [PubMed] [Google Scholar]
- 64.Benatar T, Carsetti R, Furlonger C, Kamalia N, Mak T, Paige CJ. Immunoglobulin-mediated signal transduction in B cells from CD45-deficient mice. J Exp Med. 1996 Jan 1;183(1):329–34. doi: 10.1084/jem.183.1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Saijo K, Schmedt C, Su IH, Karasuyama H, Lowell CA, Reth M, et al. Essential role of Src-family protein tyrosine kinases in NF-kappaB activation during B cell development. Nat Immunol. 2003 Mar;4(3):274–9. doi: 10.1038/ni893. [DOI] [PubMed] [Google Scholar]
- 66.Huntington ND, Xu Y, Puthalakath H, Light A, Willis SN, Strasser A, et al. CD45 links the B cell receptor with cell survival and is required for the persistence of germinal centers. Nat Immunol. 2006 Feb;7(2):190–8. doi: 10.1038/ni1292. [DOI] [PubMed] [Google Scholar]
- 67.Zhu JW, Brdicka T, Katsumoto TR, Lin J, Weiss A. Structurally distinct phosphatases CD45 and CD148 both regulate B cell and macrophage immunoreceptor signaling. Immunity. 2008 Feb;28(2):183–96. doi: 10.1016/j.immuni.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kong YY, Kishihara K, Sumichika H, Nakamura T, Kaneko M, Nomoto K. Differential requirements of CD45 for lymphocyte development and function. Eur J Immunol. 1995 Dec;25(12):3431–6. doi: 10.1002/eji.1830251234. [DOI] [PubMed] [Google Scholar]
- 69.Cyster JG, Healy JI, Kishihara K, Mak TW, Thomas ML, Goodnow CC. Regulation of B-lymphocyte negative and positive selection by tyrosine phosphatase CD45. Nature. 1996 May 23;381(6580):325–8. doi: 10.1038/381325a0. [DOI] [PubMed] [Google Scholar]
- 70.Cyster JG, Goodnow CC. Protein tyrosine phopshatase 1C negatively regulates antigen receptor signaling in B lymphocytes and determines thresholds for negative selection. Immunity. 1995;2:13–24. doi: 10.1016/1074-7613(95)90075-6. [DOI] [PubMed] [Google Scholar]
- 71.Shrivastava P, Katagiri T, Ogimoto M, Mizuno K, Yakura H. Dynamic regulation of Src-family kinases by CD45 in B cells. Blood. 2004 Feb 15;103(4):1425–32. doi: 10.1182/blood-2003-03-0716. [DOI] [PubMed] [Google Scholar]
- 72.Katagiri T, Ogimoto M, Hasegawa K, Arimura Y, Mitomo K, Okada M, et al. CD45 negatively regulates lyn activity by dephosphorylating both positive and negative regulatory tyrosine residues in immature B cells. J Immunol. 1999 Aug 1;163(3):1321–6. [PubMed] [Google Scholar]
- 73.Katagiri T, Ogimoto M, Hasegawa K, Mizuno K, Yakura H. Selective regulation of Lyn tyrosine kinase by CD45 in immature B cells. J Biol Chem. 1995 Nov 24;270(47):27987–90. doi: 10.1074/jbc.270.47.27987. [DOI] [PubMed] [Google Scholar]
- 74.Chan VW, Lowell CA, DeFranco AL. Defective negative regulation of antigen receptor signaling in Lyn-deficient B lymphocytes. Curr Biol. 1998 May 7;8(10):545–53. doi: 10.1016/s0960-9822(98)70223-4. [DOI] [PubMed] [Google Scholar]
- 75.Hibbs ML, Tarlinton DM, Armes J, Grail D, Hodgson G, Maglitto R, et al. Multiple defects in the immune system of Lyn-deficient mice, culminating in autoimmune disease. Cell. 1995 Oct 20;83(2):301–11. doi: 10.1016/0092-8674(95)90171-x. [DOI] [PubMed] [Google Scholar]
- 76.Hibbs ML, Harder KW, Armes J, Kountouri N, Quilici C, Casagranda F, et al. Sustained activation of Lyn tyrosine kinase in vivo leads to autoimmunity. J Exp Med. 2002 Dec 16;196(12):1593–604. doi: 10.1084/jem.20020515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gauld SB, Dal Porto JM, Cambier JC. B cell antigen receptor signaling: roles in cell development and disease. Science. 2002 May 31;296(5573):1641–2. doi: 10.1126/science.1071546. [DOI] [PubMed] [Google Scholar]
- 78.Yamada H, Kishihara K, Kong YY, Nomoto K. Enhanced generation of NK cells with intact cytotoxic function in CD45 exon 6-deficient mice. J Immunol. 1996 Aug 15;157(4):1523–1528. [PubMed] [Google Scholar]
- 79.Hesslein DG, Takaki R, Hermiston ML, Weiss A, Lanier LL. Dysregulation of signaling pathways in CD45-deficient NK cells leads to differentially regulated cytotoxicity and cytokine production. Proc Natl Acad Sci U S A. 2006 May 2;103(18):7012–7. doi: 10.1073/pnas.0601851103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Huntington ND, Xu Y, Nutt SL, Tarlinton DM. A requirement for CD45 distinguishes Ly49D-mediated cytokine and chemokine production from killing in primary natural killer cells. The Journal of experimental medicine. 2005 May 2;201(9):1421–33. doi: 10.1084/jem.20042294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mason LH, Willette-Brown J, Taylor LS, McVicar DW. Regulation of Ly49D/DAP12 signal transduction by Src-family kinases and CD45. J Immunol. 2006 Jun 1;176(11):6615–6623. doi: 10.4049/jimmunol.176.11.6615. [DOI] [PubMed] [Google Scholar]
- 82.Berger SA, Mak TW, Paige CJ. Leukocyte common antigen (CD45) is required for immunoglobulin E-mediated degranulation of mast cells. J Exp Med. 1994 Aug 1;180(2):471–476. doi: 10.1084/jem.180.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cross JL, Kott K, Miletic T, Johnson P. CD45 regulates TLR-induced proinflammatory cytokine and IFN-beta secretion in dendritic cells. J Immunol. 2008 Jun 15;180(12):8020–8029. doi: 10.4049/jimmunol.180.12.8020. [DOI] [PubMed] [Google Scholar]
- 84.Piercy J, Petrova S, Tchilian EZ, Beverley PC. CD45 negatively regulates tumour necrosis factor and interleukin-6 production in dendritic cells. Immunology. 2006 Jun;118(2):250–256. doi: 10.1111/j.1365-2567.2006.02363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Montoya M, Dawes R, Reid D, Lee LN, Piercy J, Borrow P, et al. CD45 is required for type I IFN production by dendritic cells. Eur J Immunol. 2006 Aug;36(8):2150–2158. doi: 10.1002/eji.200535304. [DOI] [PubMed] [Google Scholar]
- 86.Zhang H, Meng F, Chu CL, Takai T, Lowell CA. The Src family kinases Hck and Fgr negatively regulate neutrophil and dendritic cell chemokine signaling via PIR-B. Immunity. 2005 Feb;22(2):235–246. doi: 10.1016/j.immuni.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 87.Hamerman JA, Tchao NK, Lowell CA, Lanier LL. Enhanced Toll-like receptor responses in the absence of signaling adaptor DAP12. Nat Immunol. 2005 Jun;6(6):579–86. doi: 10.1038/ni1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fortin M, Steff AM, Felberg J, Ding I, Schraven B, Johnson P, et al. Apoptosis mediated through CD45 is independent of its phosphatase activity and association with leukocyte phosphatase-associated phosphoprotein. J Immunol. 2002 Jun 15;168(12):6084–6089. doi: 10.4049/jimmunol.168.12.6084. [DOI] [PubMed] [Google Scholar]
- 89.Anand AR, Ganju RK. HIV-1 gp120-mediated apoptosis of T cells is regulated by the membrane tyrosine phosphatase CD45. J Biol Chem. 2006 May 5;281(18):12289–12299. doi: 10.1074/jbc.M511786200. [DOI] [PubMed] [Google Scholar]
- 90.Liu Z, Dawes R, Petrova S, Beverley PC, Tchilian EZ. CD45 regulates apoptosis in peripheral T lymphocytes. Int Immunol. 2006 Jun;18(6):959–966. doi: 10.1093/intimm/dxl032. [DOI] [PubMed] [Google Scholar]
- 91.Desharnais P, Dupere-Minier G, Hamelin C, Devine P, Bernier J. Involvement of CD45 in DNA fragmentation in apoptosis induced by mitochondrial perturbing agents. Apoptosis. 2008 Feb;13(2):197–212. doi: 10.1007/s10495-007-0162-9. [DOI] [PubMed] [Google Scholar]
- 92.Klaus SJ, Sidorenko SP, Clark EA. CD45 ligation induces programmed cell death in T and B lymphocytes. J Immunol. 1996 Apr 15;156(8):2743–2753. [PubMed] [Google Scholar]
- 93.Bijian K, Zhang L, Shen SH. Collagen-mediated survival signaling is modulated by CD45 in Jurkat T cells. Mol Immunol. 2007 Jul;44(15):3682–3690. doi: 10.1016/j.molimm.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 94.Stamenkovic I, Sgroi D, Aruffo A, Sy MS, Anderson T. The B lymphocyte adhesion molecule CD22 interacts with leukocyte common antigen CD45RO on T cells and α2-6 sialyltransferase, CD75, on B cells. Cell. 1991;66:1133–1144. doi: 10.1016/0092-8674(91)90036-x. [DOI] [PubMed] [Google Scholar]
- 95.Walzel H, Schulz U, Neels P, Brock J. Galectin-1, a natural ligand for the receptor-type protein tyrosine phosphatase CD45. Immunol Lett. 1999 Apr 15;67(3):193–202. doi: 10.1016/s0165-2478(99)00012-7. [DOI] [PubMed] [Google Scholar]
- 96.Baldwin TA, Gogela-Spehar M, Ostergaard HL. Specific isoforms of the resident endoplasmic reticulum protein glucosidase II associate with the CD45 protein-tyrosine phosphatase via a lectin-like interaction. J Biol Chem. 2000 Oct 13;275(41):32071–32076. doi: 10.1074/jbc.M003088200. [DOI] [PubMed] [Google Scholar]
- 97.Felberg J, Johnson P. Characterization of recombinant CD45 cytoplasmic domain proteins. Evidence for intramolecular and intermolecular interactions. J Biol Chem. 1998;273:17839–17845. doi: 10.1074/jbc.273.28.17839. [DOI] [PubMed] [Google Scholar]
- 98.Takeda A, Wu JJ, Maizel AL. Evidence for monomeric and dimeric forms of CD45 associated with a 30-kDa phosphorylated protein. J Biol Chem. 1992;267:16651–16659. [PubMed] [Google Scholar]
- 99.Dornan S, Sebestyen Z, Gamble J, Nagy P, Bodnar A, Alldridge L, et al. Differential association of CD45 isoforms with CD4 and CD8 regulates the actions of specific pools of p56lck tyrosine kinase in T cell antigen receptor signal transduction. J Biol Chem. 2002 Jan 18;277(3):1912–8. doi: 10.1074/jbc.M108386200. [DOI] [PubMed] [Google Scholar]
- 100.Xu Z, Weiss A. Negative regulation of CD45 by differential homodimerization of the alternatively spliced isoforms. Nature immunology. 2002 Aug;3(8):764–71. doi: 10.1038/ni822. [DOI] [PubMed] [Google Scholar]
- 101.Desai DM, Sap J, Schlessinger J, Weiss A. Ligand-mediated negative regulation of a chimeric transmembrane receptor tyrosine phosphatase. Cell. 1993;73:541–554. doi: 10.1016/0092-8674(93)90141-c. [DOI] [PubMed] [Google Scholar]
- 102.van der Wijk T, Blanchetot C, Overvoorde J, den Hertog J. Redox-regulated rotational coupling of receptor protein-tyrosine phosphatase alpha dimers. J Biol Chem. 2003 Apr 18;278(16):13968–74. doi: 10.1074/jbc.M300632200. [DOI] [PubMed] [Google Scholar]
- 103.Dustin ML. Hunter to gatherer and back: immunological synapses and kinapses as variations on the theme of amoeboid locomotion. Annu Rev Cell Dev Biol. 2008;24:577–596. doi: 10.1146/annurev.cellbio.24.110707.175226. [DOI] [PubMed] [Google Scholar]
- 104.Douglass AD, Vale RD. Single-molecule microscopy reveals plasma membrane microdomains created by protein-protein networks that exclude or trap signaling molecules in T cells. Cell. 2005 Jun 17;121(6):937–50. doi: 10.1016/j.cell.2005.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Choudhuri K, van der Merwe PA. Molecular mechanisms involved in T cell receptor triggering. Semin Immunol. 2007 Aug;19(4):255–261. doi: 10.1016/j.smim.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 106.Varma R, Campi G, Yokosuka T, Saito T, Dustin ML. T cell receptor-proximal signals are sustained in peripheral microclusters and terminated in the central supramolecular activation cluster. Immunity. 2006 Jul;25(1):117–27. doi: 10.1016/j.immuni.2006.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yokosuka T, Sakata-Sogawa K, Kobayashi W, Hiroshima M, Hashimoto-Tane A, Tokunaga M, et al. Newly generated T cell receptor microclusters initiate and sustain T cell activation by recruitment of Zap70 and SLP-76. Nat Immunol. 2005 Dec;6(12):1253–1262. doi: 10.1038/ni1272. [DOI] [PubMed] [Google Scholar]
- 108.Campi G, Varma R, Dustin ML. Actin and agonist MHC-peptide complex-dependent T cell receptor microclusters as scaffolds for signaling. J Exp Med. 2005 Oct 17;202(8):1031–1036. doi: 10.1084/jem.20051182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.He X, Woodford-Thomas TA, Johnson KG, Shah DD, Thomas ML. Targeting of CD45 protein tyrosine phosphatase activity to lipid microdomains on the T cell surface inhibits TCR signaling. Eur J Immunol. 2002 Sep;32(9):2578–2587. doi: 10.1002/1521-4141(200209)32:9<2578::AID-IMMU2578>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 110.Su MW, Yu CL, Burakoff SJ, Jin YJ. Targeting Src homology 2 domain-containing tyrosine phosphatase (SHP-1) into lipid rafts inhibits CD3-induced T cell activation. J Immunol. 2001 Mar 15;166(6):3975–3982. doi: 10.4049/jimmunol.166.6.3975. [DOI] [PubMed] [Google Scholar]
- 111.Hailman E, Burack WR, Shaw AS, Dustin ML, Allen PM. Immature CD4(+)CD8(+) thymocytes form a multifocal immunological synapse with sustained tyrosine phosphorylation. Immunity. 2002 Jun;16(6):839–848. doi: 10.1016/s1074-7613(02)00326-6. [DOI] [PubMed] [Google Scholar]
- 112.Thomas M. The leukocyte common antigen family. Ann Rev Immunol. 1989;7:339–370. doi: 10.1146/annurev.iy.07.040189.002011. [DOI] [PubMed] [Google Scholar]
- 113.Irles C, Symons A, Michel F, Bakker TR, van der Merwe PA, Acuto O. CD45 ectodomain controls interaction with GEMs and Lck activity for optimal TCR signaling. Nat Immunol. 2003 Feb;4(2):189–197. doi: 10.1038/ni877. [DOI] [PubMed] [Google Scholar]
- 114.Pradhan D, Morrow J. The spectrin-ankyrin skeleton controls CD45 surface display and interleukin-2 production. Immunity. 2002 Sep;17(3):303–315. doi: 10.1016/s1074-7613(02)00396-5. [DOI] [PubMed] [Google Scholar]
- 115.Hailman E, Allen PM. Inefficient cell spreading and cytoskeletal polarization by CD4+CD8+ thymocytes: regulation by the thymic environment. J Immunol. 2005 Oct 15;175(8):4847–4857. doi: 10.4049/jimmunol.175.8.4847. [DOI] [PubMed] [Google Scholar]
- 116.Schraven B, Schoenhaut D, Bruyns E, Koretzky G, Eckerskorn C, Wallich R, et al. LPAP, a novel 32-kDa phosphoprotein that interacts with CD45 in human lymphocytes. J Biol Chem. 1994;269:29102–29111. [PubMed] [Google Scholar]
- 117.Leitenberg D, Falahati R, Lu DD, Takeda A. CD45-associated protein promotes the response of primary CD4 T cells to low-potency T-cell receptor (TCR) stimulation and facilitates CD45 association with CD3/TCR and lck. Immunology. 2007 Aug;121(4):545–54. doi: 10.1111/j.1365-2567.2007.02602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Matsuda A, Motoya S, Kimura S, McInnis R, Maizel AL, Takeda A. Disruption of lymphocyte function and signaling in CD45-associated protein-null mice. The Journal of experimental medicine. 1998 Jun 1;187(11):1863–70. doi: 10.1084/jem.187.11.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ding I, Bruyns E, Li P, Magada D, Paskind M, Rodman L, et al. Biochemical and functional analysis of mice deficient in expression of the CD45-associated phosphoprotein LPAP. Eur J Immunol. 1999 Dec;29(12):3956–3961. doi: 10.1002/(SICI)1521-4141(199912)29:12<3956::AID-IMMU3956>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 120.Kung C, Okumura M, Seavitt JR, Noll ME, White LS, Pingel JT, et al. CD45-associated protein is not essential for the regulation of antigen receptor-mediated signal transduction. European journal of immunology. 1999 Dec;29(12):3951–5. doi: 10.1002/(SICI)1521-4141(199912)29:12<3951::AID-IMMU3951>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 121.Stover DR, Charbonneau H, Tonks NK, Walsh KA. Protein-tyrosine-phosphatase CD45 is phosphorylated transiently on tyrosine upon activation of Jurkat T cells. Proc Natl Acad Sci USA. 1991;88:7704–7707. doi: 10.1073/pnas.88.17.7704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Autero M, Saharinen J, Pessa-Morikawa T, Soula-Rothhut M, Oetken C, Gassmann M, et al. Tyrosine phosphorylation of CD45 phosphotyrosine phosphatase by p50csk kinase creates a binding site for p56lck tyrosine kinase and activates the phosphatase. Mol Cell Biol. 1994;14:1308–1321. doi: 10.1128/mcb.14.2.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fialkow L, Chan CK, Downey GP. Inhibition of CD45 during neutrophil activation. J Immunol. 1997 Jun 1;158(11):5409–5417. [PubMed] [Google Scholar]
- 124.Windhagen A, Sonmez D, Hornig-Do HT, Kalinowsky A, Schwinzer R. Altered CD45 isoform expression in C77G carriers influences cytokine responsiveness and adhesion properties of T cells. Clin Exp Immunol. 2007 Dec;150(3):509–517. doi: 10.1111/j.1365-2249.2007.03508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dawes R, Petrova S, Liu Z, Wraith D, Beverley PC, Tchilian EZ. Combinations of CD45 isoforms are crucial for immune function and disease. J Immunol. 2006 Mar 15;176(6):3417–3425. doi: 10.4049/jimmunol.176.6.3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Schraven B. Cd148. J Biol Regul Homeost Agents. 2000 Jul-Sep;14(3):220–222. [PubMed] [Google Scholar]
- 127.Baker JE, Majeti R, Tangye SG, Weiss A. Protein tyrosine phosphatase CD148-mediated inhibition of T-cell receptor signal transduction is associated with reduced LAT and phospholipase Cgamma1 phosphorylation. Mol Cell Biol. 2001 Apr;21(7):2393–403. doi: 10.1128/MCB.21.7.2393-2403.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Borges LG, Seifert RA, Grant FJ, Hart CE, Disteche CM, Edelhoff S, et al. Cloning and characterization of rat density-enhanced phosphatase-1, a protein tyrosine phosphatase expressed by vascular cells. Circulation research. 1996 Sep;79(3):570–80. doi: 10.1161/01.res.79.3.570. [DOI] [PubMed] [Google Scholar]
- 129.Gaya A, Pirotto F, Palou E, Autschbach F, Del Pozo V, Sole J, et al. CD148, a new membrane tyrosine phosphatase involved in leukocyte function. Leukemia & lymphoma. 1999 Oct;35(34):237–43. doi: 10.3109/10428199909145726. [DOI] [PubMed] [Google Scholar]
- 130.Kuramochi S, Matsuda S, Matsuda Y, Saitoh T, Ohsugi M, Yamamoto T. Molecular cloning and characterization of Byp a murine receptor-type tyrosine phosphatase similar to human DEP-1. FEBS letters. 1996 Jan 2;378(1):7–14. doi: 10.1016/0014-5793(95)01415-2. [DOI] [PubMed] [Google Scholar]
- 131.Lin J, Zhu JW, Baker JE, Weiss A. Regulated expression of the receptor-like tyrosine phosphatase CD148 on hemopoietic cells. J Immunol. 2004 Aug 15;173(4):2324–30. doi: 10.4049/jimmunol.173.4.2324. [DOI] [PubMed] [Google Scholar]
- 132.Balavenkatraman KK, Jandt E, Friedrich K, Kautenburger T, Pool-Zobel BL, Ostman A, et al. DEP-1 protein tyrosine phosphatase inhibits proliferation and migration of colon carcinoma cells and is upregulated by protective nutrients. Oncogene. 2006 Oct 12;25(47):6319–24. doi: 10.1038/sj.onc.1209647. [DOI] [PubMed] [Google Scholar]
- 133.Keane MM, Lowrey GA, Ettenberg SA, Dayton MA, Lipkowitz S. The protein tyrosine phosphatase DEP-1 is induced during differentiation and inhibits growth of breast cancer cells. Cancer Res. 1996 Sep 15;56(18):4236–43. [PubMed] [Google Scholar]
- 134.Trapasso F, Iuliano R, Boccia A, Stella A, Visconti R, Bruni P, et al. Rat protein tyrosine phosphatase eta suppresses the neoplastic phenotype of retrovirally transformed thyroid cells through the stabilization of p27(Kip1) Mol Cell Biol. 2000 Dec;20(24):9236–46. doi: 10.1128/mcb.20.24.9236-9246.2000. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 135.Trapasso F, Yendamuri S, Dumon KR, Iuliano R, Cesari R, Feig B, et al. Restoration of receptor-type protein tyrosine phosphatase eta function inhibits human pancreatic carcinoma cell growth in vitro and in vivo. Carcinogenesis. 2004 Nov;25(11):2107–14. doi: 10.1093/carcin/bgh224. [DOI] [PubMed] [Google Scholar]
- 136.Autschbach F, Palou E, Mechtersheimer G, Rohr C, Pirotto F, Gassler N, et al. Expression of the membrane protein tyrosine phosphatase CD148 in human tissues. Tissue antigens. 1999 Nov;54(5):485–98. doi: 10.1034/j.1399-0039.1999.540506.x. [DOI] [PubMed] [Google Scholar]
- 137.Tangye SG, Phillips JH, Lanier LL, deVries JE, Aversa G. CD148: a receptor-like protein tyrosine phosphatase involved in the regulation of human T cell activation. J Immunol. 1998;161:3249–3255. [PubMed] [Google Scholar]
- 138.Ruivenkamp C, Hermsen M, Postma C, Klous A, Baak J, Meijer G, et al. LOH of PTPRJ occurs early in colorectal cancer and is associated with chromosomal loss of 18q12-21. Oncogene. 2003 May 29;22(22):3472–4. doi: 10.1038/sj.onc.1206246. [DOI] [PubMed] [Google Scholar]
- 139.Ruivenkamp CA, van Wezel T, Zanon C, Stassen AP, Vlcek C, Csikos T, et al. Ptprj is a candidate for the mouse colon-cancer susceptibility locus Scc1 and is frequently deleted in human cancers. Nat Genet. 2002 Jul;31(3):295–300. doi: 10.1038/ng903. [DOI] [PubMed] [Google Scholar]
- 140.Lunardi C, Bason C, Leandri M, Navone R, Lestani M, Millo E, et al. Autoantibodies to inner ear and endothelial antigens in Cogan's syndrome. Lancet. 2002 Sep 21;360(9337):915–21. doi: 10.1016/S0140-6736(02)11028-2. [DOI] [PubMed] [Google Scholar]
- 141.Holsinger LJ, Ward K, Duffield B, Zachwieja J, Jallal B. The transmembrane receptor protein tyrosine phosphatase DEP1 interacts with p120(ctn) Oncogene. 2002 Oct 10;21(46):7067–76. doi: 10.1038/sj.onc.1205858. [DOI] [PubMed] [Google Scholar]
- 142.Kovalenko M, Denner K, Sandstrom J, Persson C, Gross S, Jandt E, et al. Site-selective dephosphorylation of the platelet-derived growth factor beta-receptor by the receptor-like protein-tyrosine phosphatase DEP-1. The Journal of biological chemistry. 2000 May 26;275(21):16219–26. doi: 10.1074/jbc.275.21.16219. [DOI] [PubMed] [Google Scholar]
- 143.Palka HL, Park M, Tonks NK. Hepatocyte growth factor receptor tyrosine kinase met is a substrate of the receptor protein-tyrosine phosphatase DEP-1. The Journal of biological chemistry. 2003 Feb 21;278(8):5728–35. doi: 10.1074/jbc.M210656200. [DOI] [PubMed] [Google Scholar]
- 144.Pera IL, Iuliano R, Florio T, Susini C, Trapasso F, Santoro M, et al. The rat tyrosine phosphatase eta increases cell adhesion by activating c-Src through dephosphorylation of its inhibitory phosphotyrosine residue. Oncogene. 2005 Apr 28;24(19):3187–95. doi: 10.1038/sj.onc.1208510. [DOI] [PubMed] [Google Scholar]
- 145.Persson C, Engstrom U, Mowbray SL, Ostman A. Primary sequence determinants responsible for site-selective dephosphorylation of the PDGF beta-receptor by the receptor-like protein tyrosine phosphatase DEP-1. FEBS letters. 2002 Apr 24;517(13):27–31. doi: 10.1016/s0014-5793(02)02570-x. [DOI] [PubMed] [Google Scholar]
- 146.Tsuboi N, Utsunomiya T, Roberts RL, Ito H, Takahashi K, Noda M, et al. The tyrosine phosphatase CD148 interacts with the p85 regulatory subunit of phosphoinositide 3-kinase. The Biochemical journal. 2008 Jul 1;413(1):193–200. doi: 10.1042/BJ20071317. [DOI] [PubMed] [Google Scholar]
- 147.Lin J, Weiss A. The tyrosine phosphatase CD148 is excluded from the immunologic synapse and down-regulates prolonged T cell signaling. The Journal of cell biology. 2003 Aug 18;162(4):673–82. doi: 10.1083/jcb.200303040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Takahashi T, Takahashi K, St John PL, Fleming PA, Tomemori T, Watanabe T, et al. A mutant receptor tyrosine phosphatase CD148, causes defects in vascular development. Mol Cell Biol. 2003 Mar;23(5):1817–31. doi: 10.1128/MCB.23.5.1817-1831.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Trapasso F, Drusco A, Costinean S, Alder H, Aqeilan RI, Iuliano R, et al. Genetic ablation of Ptprj, a mouse cancer susceptibility gene, results in normal growth and development and does not predispose to spontaneous tumorigenesis. DNA Cell Biol. 2006 Jun;25(6):376–82. doi: 10.1089/dna.2006.25.376. [DOI] [PubMed] [Google Scholar]
- 150.Matthews RJ, Bowne DB, Flores E, Thomas ML. Characterization of hematopoietic intracellular protein tyrosine phosphatases: Description of a phosphatase containing an SH2 domain and another enriched in proline-, glutamic acid-, serine-, and threonine-rich sequences. Mol Cell Biol. 1992;12:2396–2405. doi: 10.1128/mcb.12.5.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cohen S, Dadi H, Shaoul E, Sharfe N, Roifman CM. Cloning and characterization of a lymphoid-specific, inducible human protein tyrosine phosphatase, Lyp. Blood. 1999 Mar 15;93(6):2013–2024. [PubMed] [Google Scholar]
- 152.Cloutier J, Veillette A. Association of inhibitory tyrosine protein kinase p50csk with protein tyrosine phosphatase PEP in T cells and other hemopoietic cells. EMBO J. 1996;15:4909–4918. [PMC free article] [PubMed] [Google Scholar]
- 153.Cloutier JF, Veillette A. Cooperative inhibition of T-cell antigen receptor signaling by a complex between a kinase and a phosphatase. J Exp Med. 1999 Jan 4;189(1):111–21. doi: 10.1084/jem.189.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gjorloff-Wingren A, Saxena M, Williams S, Hammi D, Mustelin T. Characterization of TCR-induced receptor-proximal signaling events negatively regulated by the protein tyrosine phosphatase PEP. Eur J Immunol. 1999 Dec;29(12):3845–54. doi: 10.1002/(SICI)1521-4141(199912)29:12<3845::AID-IMMU3845>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 155.Wu J, Katrekar A, Honigberg LA, Smith AM, Conn MT, Tang J, et al. Identification of substrates of human protein-tyrosine phosphatase PTPN22. J Biol Chem. 2006 Apr 21;281(16):11002–11010. doi: 10.1074/jbc.M600498200. [DOI] [PubMed] [Google Scholar]
- 156.Brdicka T, Pavlistova D, Leo A, Bruyns E, Korinek V, Angelisova P, et al. Phosphoprotein associated with glycosphingolipid-enriched microdomains (PAG), a novel ubiquitously expressed transmembrane adaptor protein, binds the protein tyrosine kinase csk and is involved in regulation of T cell activation. J Exp Med. 2000 May 1;191(9):1591–1604. doi: 10.1084/jem.191.9.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kawabuchi M, Satomi Y, Takao T, Shimonishi Y, Nada S, Nagai K, et al. Transmembrane phosphoprotein Cbp regulates the activities of Src-family tyrosine kinases. Nature. 2000 Apr 27;404(6781):999–1003. doi: 10.1038/35010121. [DOI] [PubMed] [Google Scholar]
- 158.Davidson D, Cloutier JF, Gregorieff A, Veillette A. Inhibitory tyrosine protein kinase p50csk is associated with protein-tyrosine phosphatase PTP-PEST in hemopoietic and non-hemopoietic cells. J Biol Chem. 1997 Sep 12;272(37):23455–23462. doi: 10.1074/jbc.272.37.23455. [DOI] [PubMed] [Google Scholar]
- 159.Gregorieff A, Cloutier JF, Veillette A. Sequence requirements for association of protein-tyrosine phosphatase PEP with the Src homology 3 domain of inhibitory tyrosine protein kinase p50(csk) J Biol Chem. 1998 May 22;273(21):13217–13222. doi: 10.1074/jbc.273.21.13217. [DOI] [PubMed] [Google Scholar]
- 160.Davidson D, Veillette A. PTP-PEST, a scaffold protein tyrosine phosphatase, negatively regulates lymphocyte activation by targeting a unique set of substrates. EMBO J. 2001 Jul 2;20(13):3414–3426. doi: 10.1093/emboj/20.13.3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hasegawa K, Martin F, Huang G, Tumas D, Diehl L, Chan AC. PEST domain-enriched tyrosine phosphatase (PEP) regulation of effector/memory T cells. Science. 2004 Jan 30;303(5658):685–9. doi: 10.1126/science.1092138. [DOI] [PubMed] [Google Scholar]
- 162.Yu X, Sun JP, He Y, Guo X, Liu S, Zhou B, et al. Structure, inhibitor, and regulatory mechanism of Lyp, a lymphoid-specific tyrosine phosphatase implicated in autoimmune diseases. Proc Natl Acad Sci USA. 2007 Dec 11;104(50):19767–19772. doi: 10.1073/pnas.0706233104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Begovich AB, Carlton VE, Honigberg LA, Schrodi SJ, Chokkalingam AP, Alexander HC, et al. A missense single-nucleotide polymorphism in a gene encoding a protein tyrosine phosphatase (PTPN22) is associated with rheumatoid arthritis. Am J Hum Genet. 2004 Aug;75(2):330–337. doi: 10.1086/422827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bottini N, Musumeci L, Alonso A, Rahmouni S, Nika K, Rostamkhani M, et al. A functional variant of lymphoid tyrosine phosphatase is associated with type I diabetes. Nat Genet. 2004 Apr;36(4):337–338. doi: 10.1038/ng1323. [DOI] [PubMed] [Google Scholar]
- 165.Kyogoku C, Langefeld CD, Ortmann WA, Lee A, Selby S, Carlton VE, et al. Genetic association of the R620W polymorphism of protein tyrosine phosphatase PTPN22 with human SLE. Am J Hum Genet. 2004 Sep;75(3):504–507. doi: 10.1086/423790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Plenge RM, Seielstad M, Padyukov L, Lee AT, Remmers EF, Ding B, et al. TRAF1-C5 as a risk locus for rheumatoid arthritis--a genomewide study. N Engl J Med. 2007 Sep 20;357(12):1199–1209. doi: 10.1056/NEJMoa073491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Gregersen PK, Lee HS, Batliwalla F, Begovich AB. PTPN22: setting thresholds for autoimmunity. Semin Immunol. 2006 Aug;18(4):214–223. doi: 10.1016/j.smim.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 168.Karlson EW, Chibnik LB, Cui J, Plenge RM, Glass RJ, Maher NE, et al. Associations between human leukocyte antigen PTPN22, CTLA4 genotypes and rheumatoid arthritis phenotypes of autoantibody status age at diagnosis and erosions in a large cohort study. Ann Rheum Dis. 2008 Mar;67(3):358–363. doi: 10.1136/ard.2007.071662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lefvert AK, Zhao Y, Ramanujam R, Yu S, Pirskanen R, Hammarstrom L. PTPN22 R620W promotes production of anti-AChR autoantibodies and IL-2 in myasthenia gravis. J Neuroimmunol. 2008 Jul 15;197(2):110–113. doi: 10.1016/j.jneuroim.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 170.Gomez LM, Anaya JM, Martin J. Genetic influence of PTPN22 R620W polymorphism in tuberculosis. Hum Immunol. 2005 Dec;66(12):1242–1247. doi: 10.1016/j.humimm.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 171.Vang T, Congia M, Macis MD, Musumeci L, Orru V, Zavattari P, et al. Autoimmune-associated lymphoid tyrosine phosphatase is a gain-of-function variant. Nat Genet. 2005 Dec;37(12):1317–1319. doi: 10.1038/ng1673. [DOI] [PubMed] [Google Scholar]
- 172.Rieck M, Arechiga A, Onengut-Gumuscu S, Greenbaum C, Concannon P, Buckner JH. Genetic variation in PTPN22 corresponds to altered function of T and B lymphocytes. J Immunol. 2007 Oct 1;179(7):4704–4710. doi: 10.4049/jimmunol.179.7.4704. [DOI] [PubMed] [Google Scholar]
- 173.Aarnisalo J, Treszl A, Svec P, Marttila J, Oling V, Simell O, et al. Reduced CD4+T cell activation in children with type 1 diabetes carrying the PTPN22/Lyp 620Trp variant. J Autoimmun. 2008 Aug;31(1):13–21. doi: 10.1016/j.jaut.2008.01.001. [DOI] [PubMed] [Google Scholar]