Abstract
Objectives
Elevated B-type natriuretic peptide (BNP) concentrations are associated with increased morbidity and mortality in ambulatory patients with congestive heart failure (CHF) or acute coronary syndromes. The value of BNP for predicting adverse cardiac surgical outcomes is less certain. We hypothesized that preoperative plasma BNP independently predicts in-hospital postoperative ventricular dysfunction (VnD), hospital length of stay (HLOS) and mortality up to 5 years after primary coronary artery bypass graft (CABG) surgery.
Methods
Prospective longitudinal study of 1023 patients undergoing primary CABG surgery with cardiopulmonary bypass (CPB) at two institutions. VnD was defined as a requirement either for ≥2 inotropes, or new intra-aortic balloon pump or ventricular assist device support after CABG surgery. Mortality was defined as all-cause death within 5 years after surgery. Multivariable analyses were performed to assess the independent role of preoperative BNP in predicting postoperative VnD, HLOS, and up to 5 year postoperative mortality, while controlling for patient demographics, perioperative risk factors, and medications.
Results
Preoperative plasma BNP concentration predicted VnD, HLOS and mortality in univariate and multivariable analyses. Logistic regression identified preoperative BNP as an independent predictor of VnD (odds ratio=1.92; 95% CI=1.12–3.29; P=0.018) after adjusting for preoperative left ventricular ejection fraction, CHF symptom severity and other clinical predictors. Multivariable Cox proportional hazards models identified preoperative BNP as an independent predictor of HLOS (hazard ratio=1.42; 95% CI=1.18–1.72; P=0.0002) and mortality (hazard ratio=1.89; 95% CI=1.08–3.33; P=0.026).
Conclusions
Preoperative plasma BNP concentration independently predicts in-hospital VnD, HLOS, and all-cause mortality up to 5 years after primary CABG surgery.
INTRODUCTION
B-type natriuretic peptide (BNP) is secreted by cardiac ventricular myocytes in response to increased ventricular wall tension and promotes compensatory diuresis, natriuresis and inhibition of the renin-angiotensin-aldosterone axis.1 Elevated plasma BNP independently predicts intermediate to long-term morbidity and mortality in ambulatory patients with congestive heart failure (CHF)2–5 or acute coronary syndromes.6–9 For non-cardiac surgical patients, elevated preoperative BNP independently predicts a composite of in-hospital adverse cardiac events including cardiac death.10 Studies of the utility of preoperative plasma BNP for identifying risk in cardiac surgical patients have been inconsistent, likely due to limitations of small sample sizes or inclusion of diverse cardiac surgical procedures.11–15 We hypothesized that elevated preoperative BNP independently predicts the occurrence of in-hospital postoperative ventricular dysfunction (VnD) and longer term all-cause mortality in patients undergoing primary CABG surgery with cardiopulmonary bypass (CPB), even after adjusting for other established predictors of perioperative risk. We further hypothesized that incorporating preoperative plasma BNP data into established cardiac surgical mortality risk prediction models would significantly improve their ability to predict all-cause mortality up to 5 years after CABG surgery.
METHODS
Study Participants
Patients aged 21–89 years undergoing primary CABG-only surgery with CPB at Brigham and Women’s Hospital, Boston, MA and Texas Heart Institute, St. Luke’s Episcopal Hospital, Houston, TX between August 2001 and June 2005 were enrolled consecutively into a prospective parent study (CABG Genomics Research Study; http://clinicaltrials.gov/show/NCT00281164),16 with the overall aim of identifying relationships between genotypic variation, biomarkers and adverse perioperative outcomes. Institutional Review Board approval and written informed consent was obtained from each patient before enrollment. Exclusion criteria included a preoperative hematocrit <25% or administration of leukocyte-rich blood products within 30 days prior to surgery. Patients undergoing emergency surgery, patients requiring preoperative inotropes, an intraaortic balloon pump (IABP) or a ventricular assist device (VAD), or patients missing preoperative BNP data were prospectively excluded from analysis. Patients with severe renal dysfunction (preoperative hemodialysis or serum creatinine >3 mg/dL) were also excluded from analysis due to concern that perioperative dialysis would variably effect perioperative plasma BNP concentrations.17, 18
Data and Blood Collection
Data were collected for each enrolled patient during primary hospitalization using a study-specific case report form that included patient demographics, perioperative risk factors, medications, perioperative medical and surgical parameters, and postoperative outcomes. To verify accuracy of records, computerized range and logic checking were automatically performed on all records, as well as manual audit of a proportion of records. Postoperative patient survival up to 5 years was assessed annually by mail and telephone questionnaires and by examination of the Social Security mortality index.
Plasma and serum samples obtained preoperatively and on postoperative days (PODs) 1– 5 were stored in vapor-phase liquid nitrogen at −70°C until analysis. Preoperative (day of surgery) plasma BNP and cTnI and postoperative plasma BNP concentrations (PODs 1, 3, and 5) were measured at the Brigham and Women’s Hospital Clinical Laboratory using ADVIA Centaur® BNP and cTnI immunoassays (Siemens Medical Solutions Diagnostics, Tarrytown, NY). Timing choices for postoperative BNP measures were based upon the occurrence of peak postoperative plasma BNP concentrations obtained from a pilot study of 116 CABG Genomics Research Study patients (data not shown).
Definitions
Postoperative VnD was defined as new requirement for ≥2 inotropes or the need for IABP or VAD insertion either intraoperatively after separating from CPB or postoperatively in the intensive care unit (ICU). Intraoperative and postoperative inotropic support was defined as receiving a continuous infusion of amrinone, dobutamine, dopamine (>5 mcg/kg/min), epinephrine, isoproterenol, milrinone, norepinephrine, or vasopressin. A dyspnea score was derived using the New York Heart Association (NYHA) classification, with score 1 = no dyspnea, score 2 = mild impairment of daily functioning, score 3 = substantial functional impairment when not at rest, and score 4 = functional impairment at rest.19 Urgent CABG surgery was defined as surgery occurring within the same hospitalization as the diagnosis of an acute coronary event or coronary artery disease. Stenosis of >50% of the left anterior descending, left circumflex or right coronary arteries or their major branches were quantified based on cardiac catheterization data and scored as regions of coronary arterial disease (1, 2 or 3 regions total). Stenosis of >50% of the left main coronary artery was counted as 2 regions of significant disease. Hospital length of stay (HLOS) included date of surgery and date of discharge as complete days of stay, with a 30 day postoperative follow-up period for this outcome. Extended HLOS was defined as >12 days (90th percentile) after surgery. Mortality was defined as death from any cause during the 5-year follow-up period after surgery.
Statistical Analysis
Statistical analyses were performed using SAS, version 9.1 (SAS Institute, Cary, NC). Using data from a previously conducted pilot study (n=116), required sample size was estimated for >99% power and a type I error rate of 0.05. This stringent power requirement was chosen to allow adequate sample size to assess preoperative BNP concentration as an independent predictor of postoperative VnD after adjusting for multiple other covariates. Accordingly, a minimum of 100 patients having VnD were needed to detect significant differences in baseline BNP concentrations.
Preoperative plasma BNP data were log10 transformed to a normal distribution before analysis. To examine the effects of preoperative BNP concentrations and other perioperative covariates on the incidence of VnD, HLOS, and up to 5 year postoperative mortality, χ2 or Fisher’s exact tests were used for categorical covariates and Wilcoxon-Mann-Whitney rank-sum tests were used for continuous covariates. Mantel-Haenszel χ2 tests were used for ordinal variables. Paired comparisons were made when appropriate. Log-rank tests were used to evaluate univariate associations between perioperative covariates and postoperative survival time. In order to assess the relationship between preoperative BNP and other perioperative covariates with regards to incidence of in-hospital VnD, HLOS, and mortality up to 5 years after surgery, covariates with a two-tailed nominal P <0.15 in univariate analyses and additional demographic variables were entered into a stepwise multivariable logistic regression for analysis of VnD and into Cox proportional hazards models for analysis of HLOS and up to 5 year postoperative mortality. In the HLOS analysis, patients who died within 30 days of surgery were counted as having a HLOS=30 days. Since plasma BNP concentration has been shown to increase with age,20 female gender,20renal dysfunction,18and reduced LVEF, and to decrease with obesity,21 these covariates were adjusted for in all multivariable analyses. Receiver operating characteristic (ROC) curves were used to assess how preoperative BNP concentrations relate to postoperative VnD and mortality. Kaplan-Meier survival curves were constructed when appropriate with Wilcoxan rank tests used to assess significant differences between stratified curves.
In order to assess the value of adding preoperative BNP concentration to established risk stratification models for predicting mortality after CABG surgery, risk scores for each patient were calculated using the Cleveland Clinic,22 EuroScore additive23 and logistic,24 modified Parsonnet25 and New York State (www.health.state.ny.us/nysdoh/consumer/heart/1996-98cabg.pdf) models. Of 19 well known cardiac surgical risk scoring systems, these five risk scores have been shown to best predict one year mortality after CABG surgery.26 We used three statistical approaches (Nagelkerke’s generalized r2, 27 the likelihood ratio test and Akaike information criterion (AIC)28) to assess the benefit of adding preoperative BNP data to the five mortality risk scores in order to predict up to 5 year postoperative mortality. We also used these three statistical approaches to assess the abilities of the multivariable models developed in this study to predict postoperative VnD, HLOS, and mortality with and without inclusion of preoperative BNP. F tests were used to compare generalized r2, and the likelihood ratio test statistic had an asymptotic χ2 distribution.
RESULTS
Of 1162 enrolled into the parent study, 139 were excluded from analysis for one or more of the following prospectively determined exclusion criteria: emergency surgery (n=4), prior CABG (n=1), valve (n=2), or other cardiac (n=15) surgery, off-pump surgery (n=39), preoperative hemodialysis (n=1) or preoperative serum creatinine >3 mg/dL (n=4), preoperative inotropes (n=4), preoperative IABP (n=27) or VAD use (n=1), concurrent cardiac valve surgery (n=50), or missing preoperative BNP concentration (n=12).
Patient Characteristics
Perioperative patient characteristics for the 1023 patients included in the study analysis are shown in Table 1 and are stratified by occurrence of postoperative VnD. Table 2 shows the type of ventricular support required by the VnD patients. Patients with postoperative VnD were significantly more likely to have higher preoperative BNP concentrations as well as preoperative renal insufficiency, recent myocardial infarction determined by patient history, preoperative cTnI > 0.1μg/L, reduced LVEF, higher dyspnea scores, higher pack year smoking histories, preoperative ACE-inhibitors, diuretics, and digoxin and to undergo longer CPB and aortic cross clamp times (Table 1).
Table 1.
Patient characteristics stratified by ventricular dysfunction (VnD) after primary CABG surgery
| Preoperative Characteristics | No-VnD (n = 904) | VnD (n = 119) | P value |
|---|---|---|---|
| Age, years | 63.7 ± 10.2 | 63.8 ± 10.6 | 0.75 |
| Female gender | 169 (18.7%) | 20 (16.8%) | 0.62 |
| Caucasian race | 771 (85.3%) | 102 (85.7%) | 0.90 |
| Institution | 0.38 | ||
| Brigham and Women’s Hospital | 665 (73.6%) | 92 (77.3%) | |
| Texas Heart Institute | 239 (26.4%) | 27 (22.7%) | |
| Diabetes mellitus (n=1022) | 243 (26.9%) | 41 (34.5%) | 0.08 |
| Hypertension (n=1020) | 681 (75.6%) | 92 (77.3%) | 0.68 |
| Hypercholesterolemia (n=1019) | 682 (75.6%) | 93 (79.5%) | 0.36 |
| Obesity, BMI > 30 kg/ m2 | 347 (38.4%) | 49 (41.2%) | 0.56 |
| Current smoker (n=1017) | 114 (12.7%) | 10 (8.5%) | 0.19 |
| Smoking, >30 pack year history (n=972) | 231 (26.7%) | 41 (38.7%) | 0.009 |
| Renal insufficiency, creatinine 1.6–3.0 mg/dL (n=1022) | 44 (4.9%) | 13 (10.9%) | 0.007 |
| Myocardial infarction ≤ 2 weeks preoperatively (n=1022) | 137 (15.2%) | 40 (33.6%) | <0.0001 |
| LVEF % (n=973) | 53.7 ± 11.5 | 43.1 ± 15.8 | <0.0001 |
| Dyspnea score (based on NYHA classification; n=928) | 0.02 | ||
| None or mild | 435 (52.9%) | 48 (45.3%) | |
| Moderate | 301 (36.6%) | 38 (35.9%) | |
| Severe | 86 (10.5%) | 20 (18.9%) | |
| Coronary artery regions with >50% stenosis (n=1008) | 0.14 | ||
| 0–1 region | 50 (5.6%) | 3 (2.5%) | |
| 2 regions | 141 (15.9%) | 17 (14.3%) | |
| 3 regions | 698 (78.6%) | 99 (83.2%) | |
| Mitral insufficiency - moderate or severe (n=975) | 16 (1.9) | 5 (4.4) | 0.09 |
| Past arrhythmia (n=1022) | 79 (8.8%) | 12 (10.1%) | 0.63 |
| Preoperative BNP [median, 10th, 90th percentile], pg/mL | 56.6 | 132.1 | <0.0001 |
| [11.2, 250.7] | [19.0, 696.9] | ||
| Preoperative cTnI > 0.1ug/L (n=982) | 238 (27.4%) | 55 (48.7%) | <0.0001 |
| Preoperative Medications | |||
| ACE-inhibitor (n=1022) | 397 (44.0%) | 71 (59.7%) | 0.001 |
| Diuretic (n=1022) | 165 (18.3%) | 40 (33.6%) | <0.0001 |
| Statin | 686 (75.9%) | 90 (75.6%) | 0.95 |
| Digoxin (n=1022) | 23 (2.6%) | 8 (6.7%) | 0.01 |
| Beta blocker | 679 (75.1%) | 96 (80.7%) | 0.18 |
| Aspirin | 674 (74.6%) | 91 (76.5%) | 0.65 |
| Non-aspirin platelet inhibitor (n=1022) | 176 (19.5%) | 25 (21.0%) | 0.70 |
| Intravenous heparin (n=1022) | 205 (22.7%) | 37 (31.1%) | 0.04 |
| Intravenous nitrate (n=1020) | 104 (11.5%) | 16 (13.5%) | 0.54 |
| Intraoperative Characteristics | |||
| Urgent surgery (n=1021) | 470 (52.1%) | 68 (57.1%) | 0.30 |
| Cardiopulmonary bypass time, minutes | 93.3 ± 37.0 | 107.9 ± 33.3 | <0.0001 |
| Aortic cross clamp time, minutes (n=1007) | 68.4 ± 32.0 | 76.1 ± 29.5 | 0.004 |
| Number of coronary grafts (n=1022) | |||
| 1–2 | 146 (16.2%) | 11 (9.2%) | |
| 3 | 398 (44.1%) | 57 (47.9%) | |
| ≥ 4 | 359 (39.8%) | 51 (42.9%) | 0.14 |
Data are shown as n (%) for dichotomous variables and mean ± standard deviation or median [10th, 90th percentiles] for continuous variables. BMI indicates body mass index; LVEF, left ventricular ejection fraction; NYHA indicates New York Heart Association; ACE indicates angiotensin converting enzyme.
Table 2.
Type of ventricular support required by 119 ventricular dysfunction (VnD) patients after primary CABG surgery
| Type of Ventricular Support | Number and % of VnD patients | |
|---|---|---|
| Intraoperative | Postoperative | |
| 2 inotropes | 57 (47.9%) | 46 (38.7%) |
| ≥ 3 inotropes | 9 (7.6%) | 24 (20.2%) |
| IABP insertion time | 19 (16.0%) | 12 (10.1%) |
| VAD insertion time | 0 (0%) | 1 (0.8%) |
| Inotrope | ||
| Amrinone | 2 (1.7%) | 2 (1.7%) |
| Milrinone | 14 (11.8%) | 19 (16.0%) |
| Dobutamine | 6 (5.0%) | 6 (5.0%) |
| Isoproterenol | 1 (0.8%) | 2 (1.7%) |
| Dopamine (>5mcg/kg/min) | 19 (16.0%) | 16 (13.4%) |
| Epinephrine | 87 (73.1%) | 75 (63.0%) |
| Norepinephrine | 39 (32.8%) | 42 (35.3%) |
| Vasopressin | 9 (7.6%) | 40 (33.6%) |
IABP indicates intra-aortic balloon pump; VAD indicates ventricular assist device
VnD to Hospital Length of Stay and Postoperative Survival
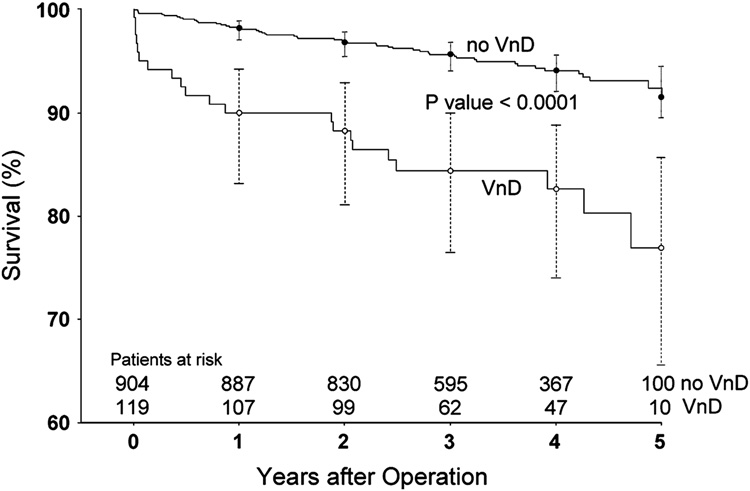
Patients with postoperative VnD had a significantly higher incidence of extended HLOS (OR=6.16, 95% CI = 3.88–9.77; P <0.0001) compared to patients without postoperative VnD. During the up to 5 year postoperative follow-up period, 72 patients (7.0%) died, with mean time between surgery and death being 1.8 ± 1.4 years [range 0–5 years]. As only 10 patients died within 30 days of surgery, we did not assess predictors of short-term postoperative mortality. Mean follow-up for the 951 living patients was 3.6 ± 1.0 years [range 1.7–5 years], with 31.0% and 11.5% of patients having reached the 4 and 5 year follow-up points, respectively. Patients with postoperative VnD had significantly lower survival during the 5-year follow-up period (OR=3.58, 95% CI=2.07–6.21; P <0.0001) compared to patients without postoperative VnD (Figure 1).
Figure 1.
Kaplan-Meier survival curves for all patients up to 5 years after primary CABG surgery according to whether patients experienced postoperative ventricular dysfunction (VnD). 95% confidence intervals are shown for the survival estimates for each year of postoperative follow-up.
Preoperative BNP to Postoperative VnD and Hospital Length of Stay
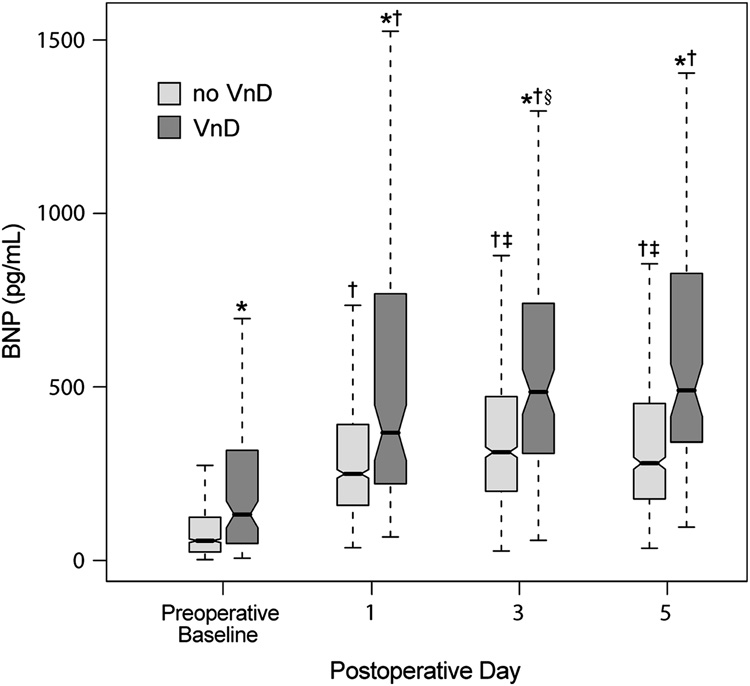
Patients with VnD had significantly higher BNP concentrations at all perioperative time points when compared to patients without VnD (Figure 2). Patients without postoperative VnD had BNP concentrations that peaked on POD 3 and then declined significantly, whereas patients with postoperative VnD had BNP concentrations that remained elevated throughout the observed postoperative period, without significant differences between PODs 3 and 5.
Figure 2.
Perioperative plasma B-type natriuretic peptide (BNP) concentrations for all patients stratified according to whether patients did or did not develop postoperative, in-hospital ventricular dysfunction (VnD), with 10th, 25th, 50th, 75th and 90th percentile values shown for each subgroup at each time point.
* signifies P < 0.0001 VnD compared to no VnD. † signifies P < 0.0001 compared to preoperative baseline. ‡ signifies P < 0.0001 compared to previous postoperative day. § signifies P = 0.02 compared to previous postoperative day.
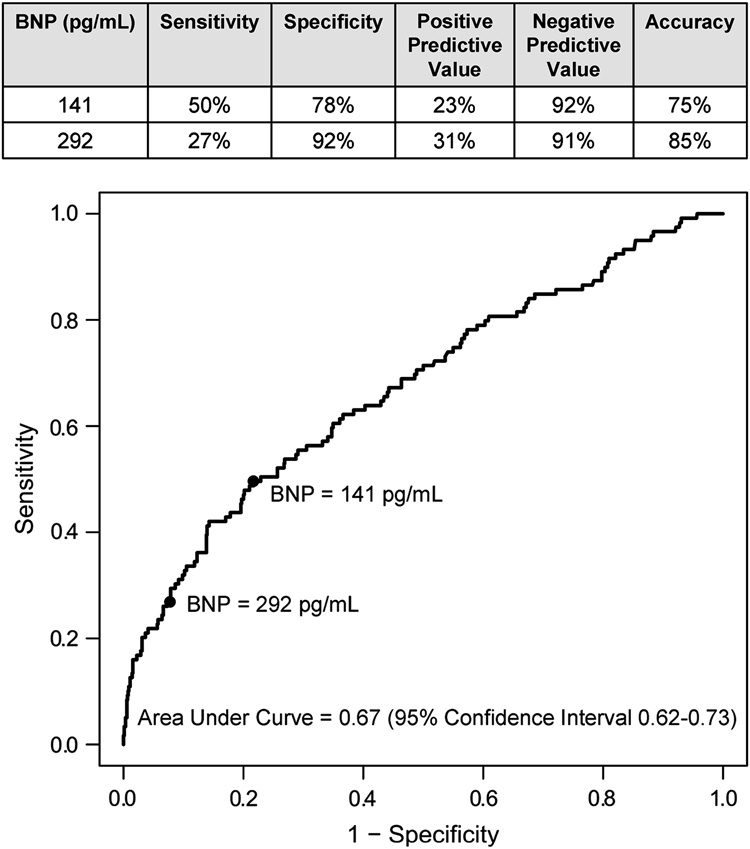
The value of preoperative BNP concentration for predicting postoperative VnD was evaluated after adjusting for the demographic characteristics of age, gender, ethnicity, BMI, institution and other likely clinical predictors of postoperative VnD including preoperative ACE-inhibitor use, heart failure score, renal insufficiency, and the other predictors listed in Table 3. Preoperative BNP concentration independently predicted postoperative VnD (OR=1.92, 95% CI=1.12–3.29; P=0.018). Addition of preoperative BNP to the multivariable model for predicting postoperative VnD (Table 3) significantly improved the model’s predictive ability, as indicated by significant changes in the generalized r2 (P=0.0004) and the likelihood ratio statistic (P=0.016). The 75th (141 pg/mL) and the 90th (292 pg/mL) percentiles of preoperative BNP concentrations had high specificity but lower sensitivity for predicting postoperative VnD as shown in Figure 3.
Table 3.
Multivariable analysis of ventricular dysfunction after primary CABG surgery
| With preoperative BNP in model (n=843*; AIC = 553.05) |
Without preoperative BNP in model (n=843*; AIC = 556.88) |
|||||
|---|---|---|---|---|---|---|
| Predictor | Odds Ratio | 95% Confidence Interval | P value | Odds Ratio | 95% Confidence Interval | P value |
| Log10 preoperative BNP | 1.92 | (1.12, 3.29) | 0.018 | - | - | - |
| Preoperative LVEF (%) | 0.97 | (0.95, 0.99) | 0.0006 | 0.96 | (0.94, 0.98) | <0.0001 |
| CPB time ≥120 minutes | 2.28 | (1.40, 3.72) | 0.001 | 2.35 | (1.45, 3.81) | 0.0006 |
| Preoperative cTnI >0.1 mcg/L | 1.83 | (1.13, 2.96) | 0.013 | 2.11 | (1.33, 3.35) | 0.002 |
| Preoperative Diuretic | 1.70 | (1.02, 2.85) | 0.031 | 1.81 | (1.08, 3.04) | 0.025 |
AIC indicates Akaike information criterion; LVEF indicates left ventricular ejection fraction; CPB indicates cardiopulmonary bypass
180 subjects were missing one or more of the model’s predictor variables and were not included in this analysis.
Figure 3.
Receiver operating characteristic curve describing preoperative plasma B-type natriuretic peptide (BNP) concentrations in relation to in-hospital ventricular dysfunction (VnD) after primary CABG surgery.
Since VnD is significantly associated with both extended HLOS and up to 5 year postoperative mortality, we also assessed the value of preoperative BNP concentration for predicting postoperative HLOS. Elevated preoperative BNP significantly increased the likelihood of requiring longer HLOS (hazard ratio=1.42, 95% CI=1.18–1.72; P=0.0002) after adjusting for demographic characteristics, institution, preoperative medications, and preoperative LVEF, cTnI, renal insufficiency, heart failure score, pack year smoking history, number of diseased coronaries, and CPB time. Addition of preoperative BNP to the multivariable model for predicting postoperative HLOS significantly improved the model’s predictive ability, as indicated by significant changes in the generalized r2 (P=0.0002) and the likelihood ratio statistic (P=0.0002).
Preoperative BNP and Postoperative Survival
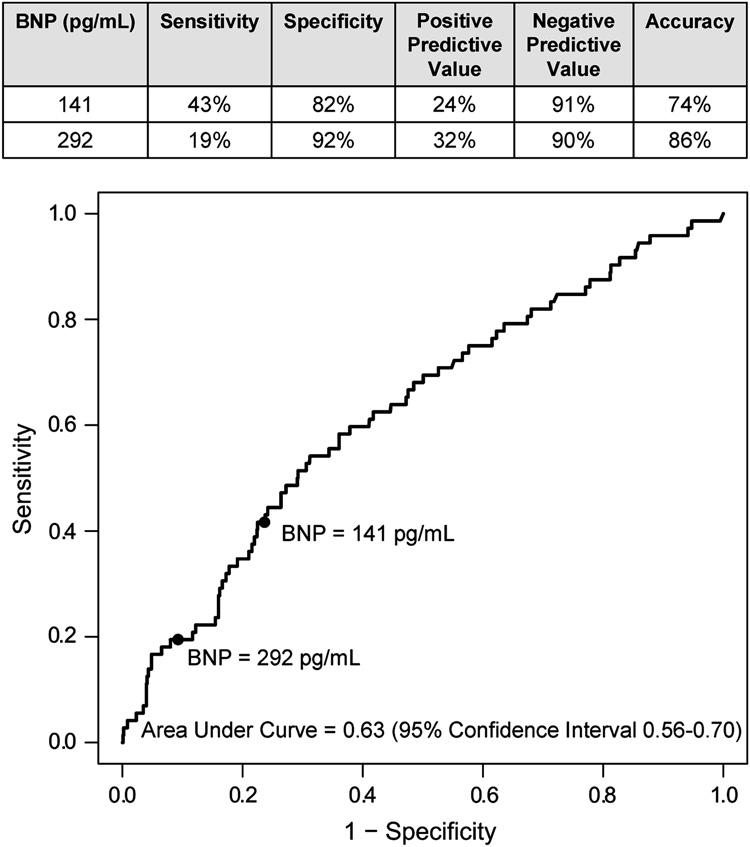
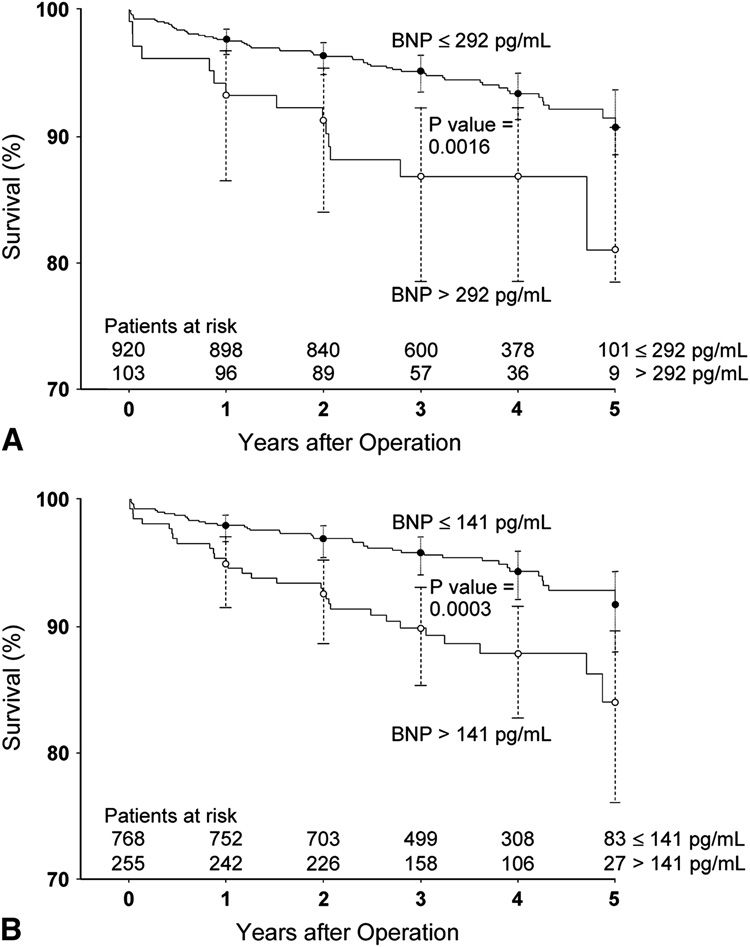
The preoperative BNP concentrations of patients who died during the 5-year postoperative follow-up period (median=111 pg/mL, 10th–90th percentile: 19–473 pg/mL) were significantly higher (P=0.0003) than the preoperative BNP concentrations of those who survived (median=58 pg/mL, 10th–90th percentile: 12–275 pg/mL). Using a proportional hazards regression model with adjustment for gender, ethnicity, institution and the covariates shown in Table 4, preoperative BNP concentration independently predicted postoperative mortality (hazard ratio=1.89, 95% CI=1.08–3.33, P=0.026). Addition of preoperative BNP significantly improved the model’s ability to predict postoperative mortality, as indicated by significant changes in the generalized r2 (P=0.003) and the likelihood ratio statistics (P=0.024). Based on ROC analysis, the 75th and 90th percentiles of preoperative BNP concentrations had high specificity but lower sensitivity for predicting up to 5 year postoperative mortality. The ROC curve and related specificities, sensitivities, positive and negative predictive values, and accuracies for the 75th and 90th percentile BNP cutoffs are shown in Figure 4. Survival was significantly predicted by both the 75th and 90th percentile preoperative BNP cutoffs (Figure 5; P=0.0003 and 0.0016, respectively).
Table 4.
Proportional hazards model of mortality during up to 5 year follow-up after primary CABG surgery
| With preoperative BNP in model (n=924*; AIC = 761.97) |
With preoperative BNP in model (n=924*; AIC = 765.04) |
|||||
|---|---|---|---|---|---|---|
| Predictor | Hazard Ratio | 95% Confidence Interval | P value | Hazard Ratio | 95% Confidence Interval | P value |
| Log10 preoperative BNP | 1.89 | (1.08, 3.33) | 0.026 | - | - | - |
| Age (ten year increment) | 1.71 | (1.30, 2.26) | 0.0001 | 1.84 | (1.40, 2.42) | <0.0001 |
| >30 pack year smoking | 2.38 | (1.39, 4.07) | 0.002 | 2.50 | (1.46, 4.27) | 0.0008 |
| Urgent procedure | 1.85 | (1.05, 3.25) | 0.033 | 1.94 | (1.11, 3.41) | 0.021 |
| BMI > 30 kg/m2 | 2.13 | (1.26, 3.62) | 0.005 | 1.93 | (1.15, 3.25) | 0.013 |
| Preoperative ACE-Inhibitor | 0.58 | (0.34, 0.99) | 0.044 | 0.57 | (0.34, 0.98) | 0.042 |
| LVEF (%) | 0.98 | (0.96, 1.00) | 0.060 | 0.97 | (0.95, 0.99) | 0.005 |
AIC indicates Akaike information criterion; BMI indicates body mass index; ACE indicates angiotensin converting enzyme; LVEF indicates left ventricular ejection fraction
99 subjects were missing one or more of the model’s predictor variables and are not included in this analysis.
Figure 4.
Receiver operating characteristic curve describing preoperative plasma B-type natriuretic peptide (BNP) concentrations in relation to up to 5 year mortality after primary CABG surgery.
Figure 5.
Kaplan-Meier survival curves for all patients up to 5 years after surgery, stratified by preoperative BNP >292 pg/mL versus ≤ 292 pg/mL (A) and by preoperative BNP >141 pg/mL versus ≤ 141 pg/mL (B).
95% confidence intervals are shown for the survival estimates for each year of postoperative follow-up.
Preoperative BNP and Established Risk Models for Mortality after Cardiac Surgery
We used three different statistical assessments of model performance to determine if preoperative BNP data improved the ability of five established cardiac surgical risk models to predict up to 5 year postoperative mortality in our primary CABG patients (Table 5). Mortality prediction from all five risk models improved significantly with the addition of preoperative BNP data, as determined by at least one statistical assessment of model performance. However, the improvement in the logistic Euroscore model was seen only with marginal improvement in one assessment of model performance (generalized r2).
Table 5.
Effect of adding preoperative BNP to known risk models for mortality after CABG surgery *
| AIC | −2 log Likelihood | r2 (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Risk Score | Without BNP | With BNP | Without BNP | With BNP | P value ‡ | Without BNP | With BNP | P value § |
| Cleveland Clinic | 662.556 | 661.683 † | 660.556 | 657.683 | 0.090 | 5.21 | 5.78 † | 0.016 |
| Euroscore (additive) | 667.831 | 666.141 † | 665.831 | 662.141 | 0.055 | 3.95 | 4.69 † | 0.007 |
| Euroscore (logistic) | 659.744 | 659.805 | 657.744 | 655.805 | 0.164 | 5.56 | 5.94 † | 0.048 |
| New York State | 679.132 | 671.661 † | 677.132 | 667.661† | 0.002 | 1.73 | 3.63 † | <0.0001 |
| Modified Parsonnet | 591.846 | 587.316 † | 589.846 | 583.316 † | 0.011 | 2.06 | 3.54† | 0.0003 |
assessed using Proportional Hazards Models with the outcome being all-cause mortality during up to 5 year postoperative follow-up Lower values of AIC and likelihood ratio statistic and higher values of r2 indicate better fit. As there are numerous methods for comparing global measures of fit for predictive models, we chose three well established statistical methods that have penalties for adding variables and for overfitting models.
AIC indicates Akaike Information Criterion
Superior fit in comparison of models with or without inclusion of log10 preoperative BNP concentration
Comparison of nested models under the χ2 statistic with 1 degree of freedom
Comparison of the F-statistic of nested models computed using Nagelkerke’s r2 (%)
DISCUSSION
Significant morbidity is reported in 18% to 43%29 of patients undergoing CABG surgery, and has important implications for health care costs, patient quality of life, and survival. Currently used cardiac surgical risk stratification models demonstrate only a modest ability to predict postoperative mortality.22–26, 29 Consequently, there is a need for models that accurately predict specific major post cardiac surgical morbidity as well as mortality,22, 25, 29 as this may enable targeted implementation of therapeutic interventions. While risk stratification models have traditionally incorporated clinical patient characteristics, there is increasing use of biomarkers in addition to clinical parameters, particularly in ambulatory populations. 6–9 We investigated the benefit of adding preoperative BNP concentration to clinical patient characteristics in order to predict morbidity and mortality after primary CABG surgery.
We found that preoperative BNP concentration independently predicts increased postoperative in-hospital VnD, HLOS, and up to 5 year mortality after primary CABG surgery, after adjustment for clinical predictors. This suggests that preoperative BNP is a useful addition to classically accepted clinical risk factors such as preoperative MI or low LVEF for identifying patients at high risk of perioperative morbidity and mortality. Similar to findings in acute coronary syndrome populations,6–9 we found that preoperative BNP provides prognostic information for CABG surgery patients that is additional to that of preoperative cTnI. Furthermore, to our knowledge, this study is the first to identify a predictive benefit of adding preoperative BNP data to multiple established cardiac surgical risk stratification models.
Most prior studies that have investigated preoperative BNP as a predictor of adverse events after cardiac surgery have been underpowered. Univariate analyses have shown that preoperative BNP predicts adverse outcomes such as postoperative LVEF,11, 12 inotrope requirements,11 mechanical ventricular support,15 postoperative cTnI concentration,13 hospital LOS15 and mortality.13, 15 A prospective study of patients undergoing CABG or valve surgery found that preoperative and POD 1 BNP concentrations predicted postoperative CHF and 1-year survival in univariate analysis, but did not assess preoperative BNP in multivariable analysis. After statistical adjustment for preoperative LVEF, POD 1 BNP concentration no longer predicted postoperative CHF or mortality.14 Another smaller study reported that preoperative BNP predicted 2-year postoperative survival after adjusting for preoperative LVEF, Cleveland Clinic risk score, and postoperative cTnI concentration.13
We identified a preoperative BNP concentration cutoff of >292 pg/mL as being highly specific for development of postoperative VnD and longer-term (up to 5 years) mortality. The utility of a BNP cutoff >292 pg/mL in primary CABG patients is further supported by significantly decreased survival demonstrated in Kaplan-Meier survival curves stratified by the preoperative BNP >292 pg/mL cutoff. The BNP concentration cutoff in the literature for diagnosing ambulatory CHF is approximately 100–200 pg/mL,3, 4 with a 35% increase in relative risk of death for each 100 pg/mL increment in BNP concentration.5 The higher preoperative BNP cutoff of >292 pg/mL, compared to the cutoff for diagnosing CHF in ambulatory patients, is likely secondary to a greater incidence of both preoperative myocardial ischemia and heart failure in CABG surgery patients. The 292 pg/mL preoperative BNP cutoff is similar to, but somewhat lower than the preoperative BNP cutoff of >385 pg/mL identified by Hutfless et al for predicting 1-year mortality in 98 male CABG surgical patients, some of whom underwent concurrent valve surgery.
We used five risk scoring systems initially developed to predict in-hospital or 30 day postoperative cardiac surgical mortality. These five risk models have also been shown to best predict 1-year mortality in CABG surgery populations.26 As we were underpowered to examine only 1-year mortality, we assessed these models’ abilities to predict up to 5-year, all-cause mortality with the addition of preoperative BNP data. Four of these five mortality risk stratification models showed significant improvements in at least two out of three mortality risk prediction measures when preoperative BNP data was added. However, the improvement in the logistic Euroscore model24 was seen only with one assessment of model performance. Overall, these findings support our observation from Cox proportional hazard modeling that preoperative BNP concentration is an important predictor of postoperative mortality. One reason that mortality prediction did not improve substantially with the addition of preoperative BNP data to the Euroscore logistic regression model24 may be that this model is better than the other four models for predicting up to 5 year postoperative mortality in our primary CABG population. Alternatively, the Euroscore logistic regression model may include or more heavily weight covariates that have some co-linearity with preoperative BNP concentration, thus reducing the value of adding preoperative BNP concentration to this model. The Euroscore logistic model’s inclusion of covariates such as reoperative CABG or other previous or concurrent cardiac or thoracic aortic surgeries, emergency surgery, endocarditis and critical preoperative state suggests that further investigation may be warranted to assess the value of preoperative BNP for predicting mortality in cardiac surgical populations that are higher risk than our primary CABG population.
We also observed that plasma BNP concentration declines after POD 3 in patients who did not experience postoperative VnD, but remains elevated at least through POD 5 in patients who experienced VnD. Postoperative BNP concentrations in cardiac14 and vascular30 surgery populations have been shown to predict long-term morbidity and mortality. In ambulatory CHF patients, administering aggressive beta blocker and ACE-inhibitors to reduce plasma BNP concentrations below a 100 pg/mL has been shown to decrease CHF related hospitalizations and mortality.31 Thus, there may be a role for peak postoperative BNP in identifying surgical patients who may benefit from aggressive postoperative therapeutic interventions. Further research is warranted to determine if post cardiac surgical BNP concentrations are useful for assessing cardiac function, targeting postoperative medical therapies, and predicting long-term morbidity and mortality.
Several potential limitations of our study deserve consideration. First, this study included primary CABG-only patients undergoing non-emergent surgery with CPB. Consequently, caution should be exercised in extrapolating these findings to other cardiac surgical populations including patients with cardiac valve disease. Secondly, although we adjusted for institution in our multivariable analyses we cannot entirely rule out non-specific biases introduced by institutional variations in intraoperative and postoperative management. Furthermore, we cannot rule out potential bias from individual practice variations introduced by including multiple surgeons in this study. Third, there is no standardized outcome definition for ventricular dysfunction after cardiac surgery by which to guide our study’s VnD outcome definition. Many primary CABG patients at both study institutions do not undergo perioperative monitoring with transesophageal echocardiography or pulmonary artery catheterization. Thus, we elected to define VnD after CABG surgery as a need for two or more inotropes, or new IABP or VAD support in order to best ensure that we were not including patients with normal ventricular function. It is not standard organizational or surgeon based practice at either institution to separate from CPB on prophylactic inotropes. Furthermore, we believe that the significant associations we observed between our VnD outcome and both extended postoperative HLOS and up to 5 year mortality reinforce the importance of our in-hospital postoperative VnD outcome. Fourth, there are multiple available plasma BNP assays, and numeric cut-points identified in this study should be considered as specific to the analysis platform used in this study.
Given the high specificity but lower sensitivity of preoperative BNP concentration cutoffs found both in our study and that of Hutfless et al,15 we believe that preoperative BNP should be used in conjunction with other clinical predictors delineated in the multivariable models that we established for postoperative VnD, HLOS and mortality. Importantly, our results should not be interpreted as meaning that elevated BNP is predictive of poorer perioperative outcomes, in the absence of preoperative cardiovascular compromise. Rather, preoperative BNP may help identify patients who have marginal cardiovascular reserve but ambiguous clinical symptoms. While our results suggest that primary CABG patients with elevated preoperative BNP might benefit from a delay in surgery to allow for better preoperative medical optimization and perioperative planning, this topic needs to be investigated by future studies.
CONCLUSIONS
Our findings support the use of preoperative BNP concentrations along with other clinical predictors to identify patients at risk for in-hospital VnD, increased HLOS, and longer-term (up to 5 years), all-cause mortality after primary CABG surgery with CPB. Additional studies are needed to assess assay specific perioperative BNP cutoffs for risk prediction both in primary CABG patients and other cardiac surgical groups and to evaluate the efficacy of interventions such as delaying surgery for medical optimization based on elevated preoperative BNP concentrations.
ACKNOWLEDGEMENTS
We acknowledge the outstanding contributory efforts of the CABG Genomics research staff: James Gosnell, RN; Kujtim Bodinaku, MD; Jai Madan, MD, MPH; Svetlana Gorbatov, MPH; Juliette Dean, RN; James Chen, RN; Jacques Estephan, RN and Isabella Canderlaria, BS
Funding Sources: Siemens Medical Solutions Diagnostics, Tarrytown, NY provided grant funding and reagents for cTnI and BNP assays. University of Texas, Houston General Clinical Research Center Core Facilities Grant (NCRR M01 02558) provided pilot funding to Drs. Fox and Collard. Dr. Fox is supported by a Society of Cardiovascular Anesthesiologists Research Starter Grant. Dr. Body is supported by NIH grant K23-HL068774.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors maybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Baughman KL. B-type natriuretic peptide -- a window to the heart. N Engl J Med. 2002;347:158–159. doi: 10.1056/NEJMp020057. [DOI] [PubMed] [Google Scholar]
- 2.Maeda K, Tsutamoto T, Wada A, Mabuchi N, Hayashi M, Tsutsui T, et al. High levels of plasma brain natriuretic peptide and interleukin-6 after optimized treatment for heart failure are independent risk factors for morbidity and mortality in patients with congestive heart failure. J Am Coll Cardiol. 2000;36:1587–1593. doi: 10.1016/s0735-1097(00)00912-8. [DOI] [PubMed] [Google Scholar]
- 3.Anand IS, Fisher LD, Chiang YT, Latini R, Masson S, Maggioni AP, et al. Changes in brain natriuretic peptide and norepinephrine over time and mortality and morbidity in the Valsartan Heart Failure Trial (Val-HeFT) Circulation. 2003;107:1278–1283. doi: 10.1161/01.cir.0000054164.99881.00. [DOI] [PubMed] [Google Scholar]
- 4.Harrison A, Morrison LK, Krishnaswamy P, Kazanegra R, Clopton P, Dao Q, et al. B-type natriuretic peptide predicts future cardiac events in patients presenting to the emergency department with dyspnea. Ann Emerg Med. 2002;39:131–138. doi: 10.1067/mem.2002.121483. [DOI] [PubMed] [Google Scholar]
- 5.Doust JA, Pietrzak E, Dobson A, Glasziou P. How well does B-type natriuretic peptide predict death and cardiac events in patients with heart failure: systematic review. BMJ. 2005;330:625–633. doi: 10.1136/bmj.330.7492.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Lemos JA, Morrow DA, Bentley JH, Omland T, Sabatine MS, McCabe CH, et al. The prognostic value of B-type natriuretic peptide in patients with acute coronary syndromes. N Engl J Med. 2001;345:1014–1021. doi: 10.1056/NEJMoa011053. [DOI] [PubMed] [Google Scholar]
- 7.Sabatine MS, Morrow DA, de Lemos JA, Gibson CM, Murphy SA, Rifai N, et al. Multimarker approach to risk stratification in non-ST elevation acute coronary syndromes: simultaneous assessment of troponin I, C-reactive protein, and B-type natriuretic peptide. Circulation. 2002;105:1760–1763. doi: 10.1161/01.cir.0000015464.18023.0a. [DOI] [PubMed] [Google Scholar]
- 8.Mega JL, Morrow DA, De Lemos JA, Sabatine MS, Murphy SA, Rifai N, et al. B-type natriuretic peptide at presentation and prognosis in patients with ST-segment elevation myocardial infarction: an ENTIRE-TIMI-23 substudy. J Am Coll Cardiol. 2004;44:335–339. doi: 10.1016/j.jacc.2004.04.033. [DOI] [PubMed] [Google Scholar]
- 9.Morrow DA, de Lemos JA, Sabatine MS, Murphy SA, Demopoulos LA, DiBattiste PM, et al. Evaluation of B-type natriuretic peptide for risk assessment in unstable angina/non-ST-elevation myocardial infarction: B-type natriuretic peptide and prognosis in TACTICS-TIMI 18. J Am Coll Cardiol. 2003;41:1264–1272. doi: 10.1016/s0735-1097(03)00168-2. [DOI] [PubMed] [Google Scholar]
- 10.Dernellis JM, Panaretou MP. Assessment of Cardiac Risk Before Noncardiac Surgery: Brain Natriuretic Peptide in 1590 Patients. Heart. 2006;92:1645–1650. doi: 10.1136/hrt.2005.085530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saribulbul O, Alat I, Coskun S, Apaydin AZ, Yagdi T, Kiliccioglu M, et al. The role of brain natriuretic peptide in the prediction of cardiac performance in coronary artery bypass grafting. Tex Heart Inst J. 2003;30:298–304. [PMC free article] [PubMed] [Google Scholar]
- 12.Chello M, Mastroroberto P, Perticone F, Cirillo F, Bevacqua E, Olivito S, et al. Plasma levels of atrial and brain natriuretic peptides as indicators of recovery of left ventricular systolic function after coronary artery bypass. Eur J Cardiothorac Surg. 2001;20:140–146. doi: 10.1016/s1010-7940(01)00754-0. [DOI] [PubMed] [Google Scholar]
- 13.Berendes E, Schmidt C, Van Aken H, Hartlage MG, Rothenburger M, Wirtz S, et al. A-type and B-type natriuretic peptides in cardiac surgical procedures. Anesth Analg. 2004;98:11–19. doi: 10.1213/01.ANE.0000093249.35075.F1. [DOI] [PubMed] [Google Scholar]
- 14.Provenchere S, Berroeta C, Reynaud C, Baron G, Poirier I, Desmonts JM, et al. Plasma brain natriuretic peptide and cardiac troponin I concentrations after adult cardiac surgery: association with postoperative cardiac dysfunction and 1-year mortality. Crit Care Med. 2006;34:995–1000. doi: 10.1097/01.CCM.0000206110.94385.C4. [DOI] [PubMed] [Google Scholar]
- 15.Hutfless R, Kazanegra R, Madani M, Bhalla MA, Tulua-Tata A, Chen A, et al. Utility of B-type natriuretic peptide in predicting postoperative complications and outcomes in patients undergoing heart surgery. J Am Coll Cardiol. 2004;43:1873–1879. doi: 10.1016/j.jacc.2003.12.048. [DOI] [PubMed] [Google Scholar]
- 16.Collard CD, Shernan SK, Fox AA, Bernig T, Chanock SJ, Vaughn WK, et al. The MBL2 ‘LYQA secretor’ haplotype is an independent predictor of postoperative myocardial infarction in whites undergoing coronary artery bypass graft surgery. Circulation. 2007;116:I106–I112. doi: 10.1161/CIRCULATIONAHA.106.679530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wahl HG, Graf S, Renz H, Fassbinder W. Elimination of the cardiac natriuretic peptides B-type natriuretic peptide (BNP) and N-terminal proBNP by hemodialysis. Clin Chem. 2004;50:1071–1074. doi: 10.1373/clinchem.2003.030692. [DOI] [PubMed] [Google Scholar]
- 18.Chenevier-Gobeaux C, Claessens YE, Voyer S, Desmoulins D, Ekindjian OG. Influence of renal function on N-terminal pro-brain natriuretic peptide (NT-proBNP) in patients admitted for dyspnoea in the Emergency Department: comparison with brain natriuretic peptide (BNP) Clin Chim Acta. 2005;361:167–175. doi: 10.1016/j.cccn.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 19.Fisher JD. New York Heart Association Classification. Arch Intern Med. 1972;129:836. [PubMed] [Google Scholar]
- 20.Redfield MM, Rodeheffer RJ, Jacobsen SJ, Mahoney DW, Bailey KR, Burnett JC., Jr Plasma brain natriuretic peptide concentration: impact of age and gender. J Am Coll Cardiol. 2002;40:976–982. doi: 10.1016/s0735-1097(02)02059-4. [DOI] [PubMed] [Google Scholar]
- 21.Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Wilson PW, et al. Impact of obesity on plasma natriuretic peptide levels. Circulation. 2004;109:594–600. doi: 10.1161/01.CIR.0000112582.16683.EA. [DOI] [PubMed] [Google Scholar]
- 22.Higgins TL, Estafanous FG, Loop FD, Beck GJ, Blum JM, Paranandi L. Stratification of morbidity and mortality outcome by preoperative risk factors in coronary artery bypass patients. A clinical severity score. JAMA. 1992;267:2344–2348. [PubMed] [Google Scholar]
- 23.Nashef SA, Roques F, Michel P, Gauducheau E, Lemeshow S, Salamon R. European system for cardiac operative risk evaluation (EuroSCORE) Eur J Cardiothorac Surg. 1999;16:9–13. doi: 10.1016/s1010-7940(99)00134-7. [DOI] [PubMed] [Google Scholar]
- 24.Roques F, Michel P, Goldstone AR, Nashef SA. The logistic EuroSCORE. Eur Heart J. 2003;24:881–882. doi: 10.1016/s0195-668x(02)00799-6. [DOI] [PubMed] [Google Scholar]
- 25.Gabrielle F, Roques F, Michel P, Bernard A, de Vicentis C, Roques X, et al. Is the Parsonnet's score a good predictive score of mortality in adult cardiac surgery: assessment by a French multicentre study. Eur J Cardiothorac Surg. 1997;11:406–414. doi: 10.1016/s1010-7940(96)01110-4. [DOI] [PubMed] [Google Scholar]
- 26.Nilsson J, Algotsson L, Hoglund P, Luhrs C, Brandt J. Comparison of 19 pre-operative risk stratification models in open-heart surgery. Eur Heart J. 2006;27:867–874. doi: 10.1093/eurheartj/ehi720. [DOI] [PubMed] [Google Scholar]
- 27.Ash A, Shwartz M. R2: a useful measure of model performance when predicting a dichotomous outcome. Stat Med. 1999;18:375–384. doi: 10.1002/(sici)1097-0258(19990228)18:4<375::aid-sim20>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 28.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 29.Kurki TS, Jarvinen O, Kataja MJ, Laurikka J, Tarkka M. Performance of three preoperative risk indices; CABDEAL, EuroSCORE and Cleveland models in a prospective coronary bypass database. Eur J Cardiothorac Surg. 2002;21:406–410. doi: 10.1016/s1010-7940(02)00007-6. [DOI] [PubMed] [Google Scholar]
- 30.Mahla E, Baumann A, Rehak P, Watzinger N, Vicenzi MN, Maier R, et al. N-Terminal Pro-brain Natriuretic Peptide Identifies Patients at High Risk for Adverse Cardiac Outcome after Vascular Surgery. Anesthesiology. 2007;106:1088–1095. doi: 10.1097/01.anes.0000267591.34626.b0. [DOI] [PubMed] [Google Scholar]
- 31.Jourdain P, Jondeau G, Funck F, Gueffet P, Le Helloco A, Donal E, et al. Plasma brain natriuretic peptide-guided therapy to improve outcome in heart failure: the STARS-BNP Multicenter Study. J Am Coll Cardiol. 2007;49:1733–1739. doi: 10.1016/j.jacc.2006.10.081. [DOI] [PubMed] [Google Scholar]