Abstract
Paraoxonases (PONs) are a small family of antioxidant enzymes whose antiatherogenic activity is well known. The aim of the present study was the evaluation of the effects of moderate aerobic training on their expression using a rat model. In order to discriminate between PON1 and PON3 enzymatic activity, we took advantage of some differences in their substrate preferences. PON1 and PON3 enzymatic activities and their protein levels were analyzed in plasma and in liver microsomes, and their mRNA levels in the liver. Exercise training did not affect PON1 expression or enzymatic activity but increased PON3 mRNA, protein levels, and enzymatic activity. Training also induced variations in plasma membrane composition, including an increase in polyunsaturated and a decrease in mono- and di-unsaturated fatty acids. On the other hand, acute exercise inhibited PON activities while increasing PON3 protein content in liver microsomes and reversing the relative composition in mono-, di-, and poly-unsaturated fatty acids, suggesting that physical stress, by altering membrane composition, may impair PON release from liver membranes. In conclusion, we documented, for the first time, the presence of PON3 in rat serum and, notably, found that the upregulation of PON3, rather than PON1, appears to be associated with physical training.
Keywords: arylesterase, carboxylesterase, lactonase, membrane phospholipids, mild exercise training, protein expression, real-time PCR
It is generally accepted that sedentary lifestyle is associated with increased risk of coronary heart disease and stroke and that the relative risk of physical inactivity is similar in magnitude to that of hypertension, hypercholesterolemia, and smoking. In contrast, regular physical activity has been identified as a protective factor against the occurrence and progression of coronary heart disease; it is associated with reduced blood pressure, maintenance of ideal body weight, improvement of lipid profile, and decrease in incidence of type II diabetes (1–3).
Evidence suggests that sustained physical activity has beneficial effects on lipoprotein metabolism, including a decrease in plasma triglyceride levels and an increase in HDL-cholesterol concentration, which bears antioxidant properties and protective effects in the prevention of coronary artery disease (4). HDLs are well known to be antiatherogenic and to protect LDL against oxidation (5). The antioxidant properties of HDLs are due in part to serum paraoxonases (PONs), which are able to degrade a number of biologically active oxidized phospholipids (6).
Most of our present knowledge is limited to PON1 and an emerging body of evidence indicates that this enzyme possesses important antiatherogenic roles. Failure to express PON1 has been implicated as a risk factor in atherosclerosis (7). PON1's protective role against atherosclerosis development was also demonstrated in studies using PON1-deficient mice (6, 8), or mice overexpressing PON1 (9, 10).
PON1 belongs to a family of calcium-dependent esterases that includes PON1, PON2, and PON3 (11). These enzymes catalyze the hydrolysis of a broad spectrum of substrates including organophosphates and aryl esters. PON1 activity has been traditionally measured by employing two different substrates: paraoxon (paraoxonase activity) and phenylacetate (arylesterase activity) (12). However, it recently became apparent that PON1 is also a lactonase with lipophylic lactones as its primary substrates (13). Also, PON2 and PON3 have high lactonase activity but low arylesterase activity and no paraoxonase activity (14–16). Lactones derived from fatty acid oxidation products may constitute the native substrates of PONs (13, 17). Pla et al. (18) showed quantitative differences in the sensitivity of purified PON1 and PON3 to inhibition by cobalt and copper, providing a tool for the development of quicker and easier enzymatic assays capable of separately detecting PON1 and PON3 in a serum sample.
The three members of the PON family have different cell and tissue distributions and their expression is differentially regulated. Although this may suggest distinct physiological roles for each of them (19), much remains unknown. All members of the PON family display antioxidant properties (20) and are mainly synthesized by the liver; however, at least in rabbits and humans, only PON1 and PON3 are found in the serum in association with HDL, where they prevent the oxidative modification of LDL. Their synthesis in the liver provides a location for their insertion into nascent HDL particles (14, 21). However, mouse HDL may not be a good acceptor for both human and mouse PON3; therefore, PON3 remains cell-bound in mouse (22). Microsomal localization for PON3 has been demonstrated in rat liver (23).
James et al. (24) proposed a “desorption” mechanism whereby PON1, inserted into the external membrane layer by its N-terminal hydrophobic signal peptide, may be transferred to HDL during its transient association with the cell membrane. More recently, Shih et al. (22) postulated that PON3 may be released to HDL in humans by a mechanism similar to that of PON1.
The effects of physical exercise on PON activities are far from having been established. Tomás et al. (25) assessed in humans, for the first time, the effects of a single bout of exercise on PON1 activity. The authors showed that training was not associated with a permanent increase in the basal level of PON1 activity, because an increase in PON1 activity could be observed immediately after a single bout of exercise but was followed by a decrease in the next 2 h and a recovery of basal levels in the following 24 h. Another study performed in rats showed a similar effect (26). These data support the idea that acute exercise induces oxidative stress by increasing lipid peroxidation, which in turn decreases PON1 activity (27, 28).
The main aim of the present study was to assess the effect of moderate aerobic training on PON activities and expressions. The rats were subjected to a training pattern similar to that previously described by Marini et al. (29), which was found to protect hearts against ischemia-reperfusion injury as already shown with more intense training procedures. In order to discriminate between the effects of chronic and acute exercise on PON activities and expression, subgroups of untrained and trained rats were subjected to a physically stressing exercise 24 h and again 30 min before euthanization. PON1 and PON3 activities and their protein expression were analyzed in rat plasma as well as in liver microsomes, because PON1 and PON3 are liver microsomal enzymes associated with vesicles derived from the endoplasmatic reticulum (30).
Moreover, the composition of both phospholipids and fatty acids in liver membranes was analyzed, in order to check for possible changes as a consequence of training. In fact, plasma membrane composition may influence PON secretion to the extracellular space, either alone or in association with HDL (31, 32).
MATERIALS AND METHODS
Chemicals
All chemicals were purchased from Sigma-Aldrich S.r.l. (Milano, Italy) unless otherwise indicated. All solutions were made using twice-distilled water.
Animals and experimental design
Male albino Sprague-Dawley rats aged 8 weeks were purchased from Charles River Labs. (Italy), placed in individual cages, and fed a standard diet without limitations; room temperature was kept at 21 ± 2°C; 12 h of light were automatically alternated with 12 h of dark. Food consumption and body weight were evaluated three times a week. After one week of acclimatization, 10 animals were randomly chosen for moderate aerobic exercise training and 10 others assigned to the control groups. Among the trained animals, a subgroup (T) was euthanized after 10 (n = 5) weeks of exercise training, 48 h after the last training session; a second subgroup was chosen for stress exercise (TS) after 10 weeks of exercise training (n = 5). The sedentary controls were assigned to stress (US, n = 5) or nonstress (U, n = 5) protocols before euthanization. All the animals were euthanized at 19 weeks of age.
Training protocol
Rats to be trained (n = 10) ran 1 h a day on a specific treadmill for rats, 3 times a week at 10% incline at increasing speed. The training protocol required a gradually increasing effort for 3 weeks to reach 65% VO2max (29), then maintained constant for a further 7 weeks. Control animals (n = 10) were placed on a nonmoving treadmill during the training sessions. Animal handling, training protocol, and mode of euthanization were approved by the Ethical Committee on the Use of Laboratory Animals of the Health Authority of Milan (Italy) according to the 86/609/CEE guidelines. Training was carried out according to the American Physiological Society guidelines for exercising rodents on treadmills (33).
Stress exercise
Trained and control rats were randomly chosen for stress exercise. Twenty-four h before the day of euthanization and again 30 min before euthanization itself, they were individually placed for 30 min in a deep basin filled with lukewarm water and devoid of holding devices, so that they were compelled to swim. They were constantly watched and forced to resume swimming in case they managed to float remaining motionless.
Euthanization
Trained rats were euthanized 48 h after the last training session, stressed rats 30 min after the end of the swimming exercise. In order to avoid diurnal variations in gene expression, all euthanizations were carried out between 10 AM and 12 AM. The euthanization of control rats was alternated with that of trained ones in a one-by-one fashion. All treatments were carried out exactly with the same procedure and timing in the control and in the trained animals, so that any difference in gene expression between the two groups cannot be attributed to differences in animal handling. Rats were anesthetized (100 mg/kg heparinized sodium thiopental); blood was drawn by acupuncture with a heparinized syringe; plasma was separated by centrifugation and immediately frozen; the livers were removed and immediately frozen in liquid nitrogen and stored at −80°C.
Activity gel electrophoresis
Nondenaturing PAGE was performed running 5 µl samples on gradient gels (4% to 30% polyacrylamide gel, 0.75 mm thick) in a vertical slab-gel apparatus (20mA current, 0.025 M Tris/0.192 M glycine 0.5% Triton-X100 running buffer, pH 8.3, 5°C). In order to distinguish between carboxylesterase (CE) and PON activities, serum samples were preincubated 2 h at room temperature with 2.0 mM phenylmethylsulfonyl fluoride (PMSF), a CE inhibitor (17), or with 5 mM EDTA, which inhibits PON. In order to distinguish between PON1 and PON3, plasma samples were preincubated for 15 min at 37°C with 0.1 mM Co(NO3)2, which inhibits PON1 (IC50 = 0.08 mM), or 0.06 mM CuSO4, which inhibits PON3 (IC50 = 0.036 mM) (18). Differential recognition of either CE or PON activity took advantage of the differential ability of the enzymes to cleave either α-naphtylacetate (CE alone) or β-naphtylacetate (both CE and PON). Staining of the gel was performed as described by Li et al. (34). In particular, α-naphtylacetate (50 mg dissolved in 1 ml ethanol added to 100 ml of 50 mM Tris–HCl pH 8 buffer containing 50 mg of solid Fast Blue RR) was used to visualize CE activity without PON activity, whereas staining with β-naphthylacetate (50 mg dissolved in 1 ml ethanol added to 100 ml of 50 mM Tris–HCl pH 8, 10 mM calcium chloride buffer containing 50 mg of solid Fast Blue RR) revealed both PON1 and CE activity. Excess dye was washed out with 50% methanol and 10% acetic acid.
Preparation and solubilization of the microsomal fraction
Microsomal fractions were prepared as previously described (35). Briefly, frozen rat livers (1 g), placed in beakers on ice, were rinsed with ice-cold homogenization buffer (5 mM Tris/HCl (pH 7.4) and 0.25 M sucrose), minced with scissors, and placed in 4 vol. of ice-cold buffer. They were then homogenized (6 strokes at 1100 rev/min) using a mechanically driven Teflon pestle in a glass homogenizer (Potter–Elvehjem-type homogenizer) with 1.02 mm clearance. The homogenate was transferred to a powerdriven close-fitting (0.045 mm clearance) Perspex [poly(methyl methacrylate)]/glass homogenizer and homogenized as before. After diluting the homogenate to 10% (w/v) with the homogenization buffer, nuclei and mitochondria were removed by step centrifugation at 460 g for 10 min and at 12,500 g for 10 min in a Beckman J2–21 refrigerated centrifuge. The postmitochondrial supernatant fraction was then centrifuged at 105,000 g for 60 min in a Beckman 55.2 Ti rotor operated in a Beckman L8–55 refrigerated centrifuge. The microsomal pellet derived from liver tissue was suspended in 2 ml of buffer [5 mM Tris/HCl (pH 7.4)]. PONs were extracted by the addition of Triton X-100. The microsomal fraction was adjusted to 0.75% Triton X-100, disrupted by a glass-glass homogenizer potter, stored at 4°C for 30 min and then centrifuged at 105,000 g for 60 min.
Assay methods
Protein concentrations were measured according to Lowry et al. (36) using BSA as a standard. Paraoxonase activity was determined according to Gan et al. (37), by using as substrate 1.0 mM paraoxon in 50 mM glycine/NaOH (pH 8.0), 1 mM CaCl2, and 1 M NaCl. The generation of p-nitrophenol at 25°C was monitored at 412 nm in a continuously recording spectrophotometer. Enzyme activity was calculated with a molar extinction coefficient of 18,290 M−1 cm−1. One unit of paraoxonase activity produced 1 nM of p-nitrophenol per minute.
Arlyesterase activity was measured by using as substrate phenyl acetate in 20 mM Tris-HCl (pH 8.0) containing 1 mM CaCl2. The rate of hydrolysis was determined spectrophotometrically at 270 nm. Enzyme activity was calculated with a molar extinction coefficient of 1,310M−1 cm−1 (37). One unit of arylesterase activity corresponded to 1 µM of phenyl acetate hydrolyzed per minute.
Lactonase activity was monitored by the increase in UV absorbance at 270 nm and 274 nm, using dihydrocoumarin (DHC) and 2-coumaranone as subtrates, respectively. In a typical experiment, a cuvette contained 1 mM substrate (from a 100 mM stock, dissolved in methanol) in 50 mM Tris/HCl (pH 8.0), 1 mM CaCl2, and 5–20 µl of enzyme in a total volume of 1 ml. The differences in molar extinction coefficients (the difference between the absorption coefficients of the acid formed in the reaction and the lactone) that were used to calculate the rate of hydrolysis were 1295 and 876, M−1 cm−1 for DHC and 2-coumaranone, respectively (38). One unit is defined as 1 μM of each substrate hydrolyzed per milliliter per minute.
It should be noted that phenyl acetate and the two aromatic lactones can be hydrolyzed not only by PONs, but also by CEs, known to be present in rodent plasma. Because PONs are not inhibited by the serine esterase inhibitor PMSF (17), studies in the plasma were performed by incubating the samples at room temperature for 2 hr with PMSF (2 mM final concentration) before the assays. However, CEs that may interfere with PON activity were not present in our preparation of liver microsomes because preliminary experiments, carried out both in the presence and in the absence of PMSF (not shown), demonstrated that they had been efficiently removed during the experimental procedures.
Western blot analysis
Western blot analysis was performed using SDS-PAGE, 12% bis-acrylamide gels. From the plasma and liver microsome fractions, 40 μg were loaded on the gel. After electrophoresis, proteins were transferred for 1 h to nitrocellulose membrane (PIERCE, Rockford, IL) in a mini trans-blot cell (Bio-Rad Italy, Milano, Italy), using transfer buffer pH 7.2. After blotting, the nitrocellulose membranes were treated with specific chromogenic western blot immunodetection kits (Invitrogen Corp., Carlsbad, CA). Blots were blocked for 45 min with concentrated casein solution in buffered saline solution and then incubated with the diluted primary specific antibody for 1 h. Mouse anti-PON1monoclonal antibody (Abcam, Cambridge, UK) was diluted 1:500, and mouse anti-PON3 polyclonal antibody (Santa Cruz Biotechonology, Inc., Santa Cruz, CA) was diluted 1:200. Membranes were washed with an antibody wash solution and incubated for 45 min with alkaline phosphatase-conjugated anti-mouse secondary antibody.
Blots were then visualized using a chromogenic substrate containing 5-bromo-4-chloro-3-indolyl-1-phosphate and nitro blue tetrazolium. Immunopositive bands were semi-quantified by Scion Image for Windows and results were expressed in arbitrary units. Because there is no consensus about which proteins could be considered unchanged following physical exercise, we addressed the problem by always loading the same amount of protein in all lanes and by repeating the Western blots at least three times; the results of repeats were averaged and reported in the Results.
RNA preparation, quality control, and reverse transcription
RNA was prepared and quality controlled as previously described (29). Briefly, TRIZOL™ (Invitrogen s.r.l., Milano, Italy) extraction was followed by controls that assessed the absence of degradation and of contaminating genomic DNA. RNA was then reverse transcripted using an Omniscript Reverse Transcription Kit (Qiagen GmbH, Hilden, Germany), then cDNA was spectrophometrically quantified.
Real-time PCR
The primer sequences for the two genes studied and for the housekeeping gene are given in Table 1, along with the size of their amplification products. Primers were chosen in the 3′ region with the help of the AMPLIFY free software and controlled by melting curve analysis. Real-time PCR was performed in an ABI PRISM 5700 real-time thermal cycler using the SYBR Green kit (Qiagen), as prevously described (29). Relative gene expression was analyzed by the -ΔΔCT method described by Livak and Schmittgen (39).The housekeeping gene used for normalization purposes was RPL13a. Data were expressed in arbitrary units, setting to 100 the amount of the housekeeping gene RPL13a in 12 ng of cDNA to 100.
TABLE 1.
Primer sequence and length of the amplicons of the genes studied with real-time RT-PCR
| UniGene | Gene Name (symbol) | Left Primer Sequence Right Primer Sequence |
Amplicon Length (nt) |
|---|---|---|---|
| Rn 92211 | RPL13a | GATGAACACCAACCCGTCT CCACCATCCGCTTTTTCTTG |
175 |
| Rn 20732 | PON-1 | CATCGGTCTTCCTATCAAA CAAGTCTTCAGCACCCGTCTC |
108 |
| Rn 16469 | PON-3 | CTCTCGTCCACCTGAAAAC CGAAGTCCAGTGAGGGTCCAA |
159 |
Lipid membrane analysis
Lipids were extracted from each liver tissue according to Gaiti et al. (40) and dissolved in chloroform-methanol (2:1, v/v). Fractionation of intact lipid classes was performed by spotting small amounts of the final chloroform-methanol solution on a silica gel layer (300 µm thickness) and developing a two-step thin layer chromatography. The phosphorous content was determined on the total lipid fraction and on the phospholipid classes separated by the two-step thin layer chromatography according to Binaglia et al. (41). The phospholipid fatty acid constitution was determined by scraping into test tubes the spots obtained and extracting them twice with n-hexane; the eluate was evaporated and then methanolyzed at 80°C for 1 h with 1 ml of solution containing 3% H2SO4 in methanol. The tubes were cooled, the content extracted two times with n-hexane, concentrated in N2 and then analyzed by gas chromatography carried out with a gas chromatograph (Carlo Erba HRGC mod. MFC500, Milano, Italy) equipped with a polyalkylenglycol column (35 mt × 0.25mm) and a flame ionization detector. The column temperature was programmed from 170°C to 210°C at 3°C min−1. The carrier gas was helium at a flow rate of 1 ml min−1. This technique allows the separation of fatty acids with a carbon content of 16 to 22 atoms, and the longer chain fatty acids (>22 atoms) are evaluated as a bulk.
Statistical analysis
Data were analyzed using one-way ANOVA and Fisher's least significant difference (LSD) test. Significance was defined as P < 0.05.
RESULTS
Animals
The rats were periodically examined by a veterinarian. Their internal organs, examined by a pathologist on the day of euthanization, appeared to be normal and disease-free.
Activity gels
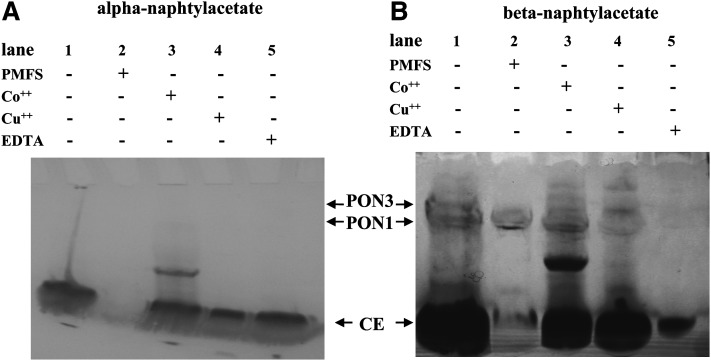
Figure 1 shows the presence of PONs in rat plasma and their absence in EDTA-treated plasma. CE activity stains equally well with alfa- (Fig. 1A) and β-naphthylacetate (Fig. 1B), whereas PON activities are revealed only in gels developed with β-naphthylacetate (Fig. 1B). The CE bands (Fig. 1A) were identified by their resistance to EDTA inhibition (lane 5), and their sensitivity to PMSF inhibition (lane 2). The two bands of PON activities, evidenced with β-naphthylacetate (Fig. 1B), were identified by their sensitivity to EDTA inhibition (lane 5) and their resistance to PMSF inhibition (lane 2). Figure 1 compares the staining pattern of Co(NO3)2 (lane 3) and CuSO4 (lane 4)-treated plasma. In particular, the preincubation with Co2+ or Cu2+, followed by α-naphthylacetate staining (i.e., staining of CEs only) (Fig. 1A), shows that neither Co2+ or Cu2+ affects CE activity. An extra band is present in lanes 3 of both Figs. 1A and 1B, which may be a CE activity that shows up only in the presence of Co2+. In Fig. 1B, where both PON and CE activities are stained, preincubation with Co2+ attenuates the upper band intensity (PON3), whereas preincubation with Cu2+ attenuates the lower band intensity (PON1). This unequivocally demonstrates the presence of PON3 in rat plasma. As expected, both PON activities are inhibited by EDTA (lane 5).
Fig. 1.
Activity gel electrophoresis. Nondenaturing gels stained for CE activity with α-naphthylacetate (A) and for PON and CE activities with β-naphthylacetate (B). Five μl of rat plasma were loaded per lane. In order to distinguish between CE and PON activities, plasma samples were preincubated 2 h at room temperature with 2.0 mM PMFS, a CE inhibitor (A, B Lane 2) or with 5 mM EDTA, which inhibits PON (A, B Lane 5). In order to distinguish between PON1 and PON3, plasma samples were preincubated 15 min at 37°C with 0.1 mM Co(NO3)2 (A, B Lane 3), which inhibits PON1 (IC50 = 0.08 mM) or 0.06 mM CuSO4 (A, B Lane 4), which inhibits PON3 (IC50 = 0.036 mM). Lane 1 (A, B) was loaded with untreated plasma.
PON expression in plasma
Specific activities.
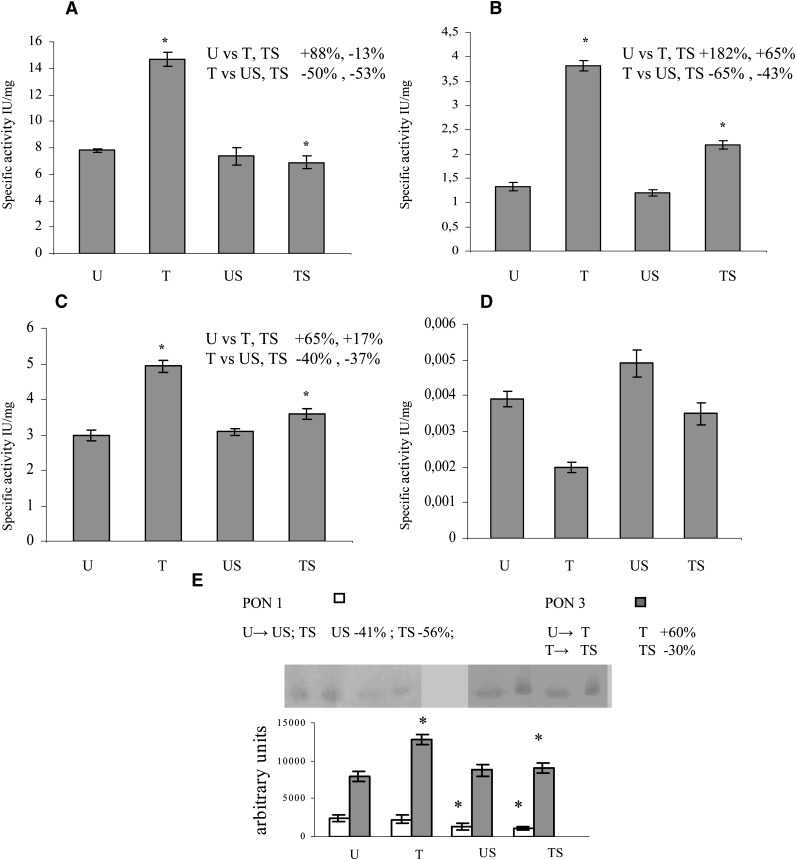
Activity assays were performed with four different substrates, utilizing plasma from the four experimental groups of animals as a source of PONs (Fig. 2A–D). The specific activity is given as mean value ± SD of four assays. Significant increase in arylesteratic activity was found in T rats with respect to the U, US, and TS rats (Fig. 2A). Using DHC and 2-cumaranone as substrates, a significant increase in PON activity was found in T and TS rats with respect to the U and US rats (Fig. 2B, 2C, respectively). In particular, the activity in T and TS rats was higher with the former substrate. In contrast, no significant differences were found among T, U, US, and UT rats using paraoxon as substrate (Fig. 2D).
Fig. 2.
PON expression in plasma. Activity assays were performed in quadruplicate with four different substrates. All samples were incubated at room temperature for 2 hr with PMSF (2 mM final concentration) before the assays, in order to inhibit CEs. (A: phenyl acetate; B: DHC; C: 2-coumaranone; D: paraoxon). The specific activity is given as mean value ± SD. Western blotting analysis was performed in triplicate (E). Data represent the mean value ± SD of multiple evaluations expressed in arbitrary units after analysis by Scion Image for Windows. Panel inserts show immunostaining image for PON1 or PON3. Statistical analysis was performed using one-way ANOVA and Fisher s LSD Test. * P ≤ 0.05 versus the other groups are reported in each insert.
Western blotting.
Western blotting analyses were performed in order to evaluate the amount of plasma PON1 and PON3 proteins from the four experimental groups of animals (Fig. 2E). Data represent the mean value ± SD of three western blots (WBs) analyzed by Scion Image for Windows and expressed in arbitrary units. Plasma PON1 levels were found to be significantly lower in the two groups of stressed rats (US and TS) than in U controls; on the other hand, PON3 levels were significantly higher in the two groups of trained rats (T and TS) than in U controls. However, the TS rats displayed a significant decrease (30%) in PON3 protein when compared with T rats.
PON expression in liver microsomes
Specific activities.
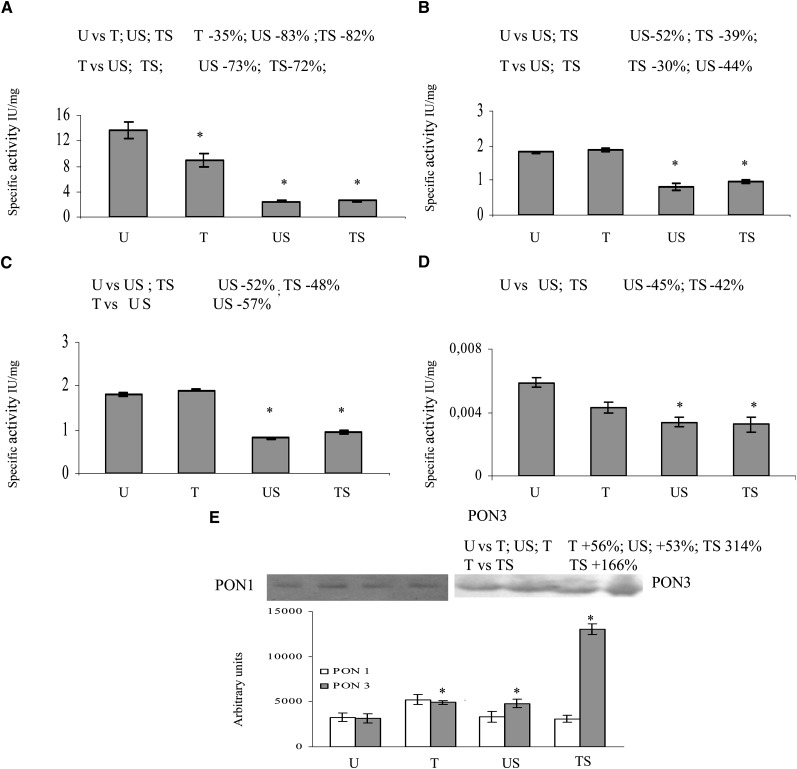
Activity assays were performed with four different substrates, utilizing liver microsomes from the four experimental groups of animals as a source of PONs (Fig. 3A–D). The specific activity is given as mean value ± SD of four assays. The arylesteratic activity (Fig. 3A) was found to significantly decrease in all experimental groups when compared with the U group, whereas the paraoxonase activity and all lactonase activities significantly decreased in the stressed groups (TS and US) (Fig. 3B–D).
Fig. 3.
PON expression in liver microsomes. Activity assays were performed in quadruplicate with four different substrates (A: phenyl acetate; B: DHC; C: 2-coumaranone; D: paraoxon). The specific activity is given as mean value ± SD. Western blotting analysis was performed in triplicate (E). Data represent the mean value ± SD of multiple evaluations expressed in arbitrary units after analysis by Scion Image for Windows. Panel inserts show immunostaining image for PON1 or PON3. Statistical analysis was performed using one-way ANOVA and Fisher s LSD Test. * P < 0.05 versus the other groups are reported in each insert.
Western blotting.
Western blotting analyses were performed in order to evaluate the amount of PON1 and PON3 proteins in the liver microsomal fraction from the four experimental groups of animals (Fig. 3E). Data represent the mean value ± SD of three WBs analyzed by Scion Image for Windows and expressed in arbitrary units. Microsomal PON1 levels did not show significant differences among groups; on the other hand, PON3 levels were significantly higher in all experimental groups when compared with the U (control) group, the increase being more relevant in the TS group.
PON mRNA expression in liver
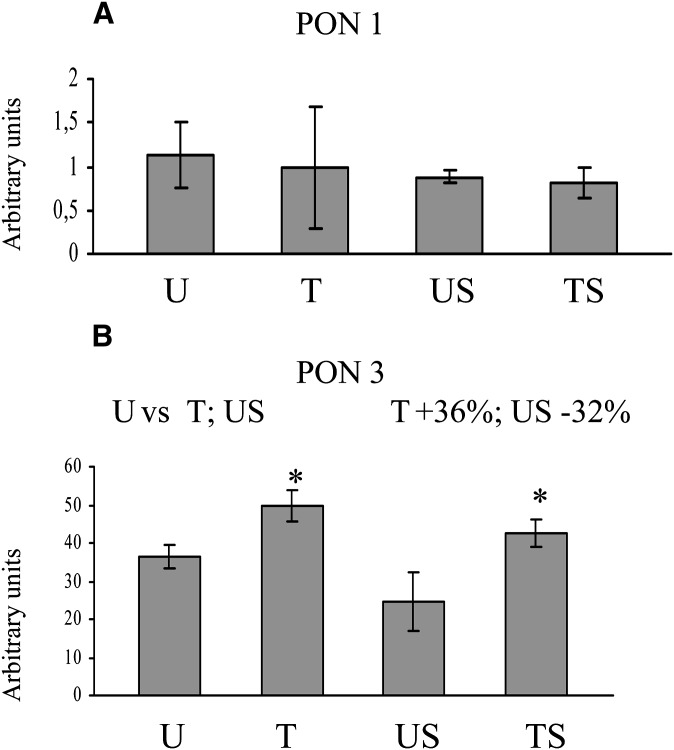
Levels of liver PON mRNAs obtained by real-time quantitative-PCR are shown in Fig. 4. PON1 mRNA levels did not show significant differences among groups (Fig. 4A) PON3 mRNA levels were significantly higher in the T group and significantly lower in the US group when compared with the U group (Fig. 4B).
Fig. 4.
PON mRNA expression. Levels of liver PON mRNAs were obtained by real time quantitative-PCR. Values are mean value ± SD and are expressed in arbitrary units, after background subtraction and normalization by a housekeeping gene (RPL13a). Statistical analysis was performed using one-way ANOVA and Fisher's LSD Test. * P < 0.05 versus the other groups are reported in each insert.
Lipid composition of liver membranes
Table 2 shows liver membrane phospholipid content. Four independent determinations were performed for each group. Data represent the mean values ± SD of total phospholipid content percentage. Phosphatidylethanolamine (PE) and phosphatidylcholine (PC) were found to be the more abundant liver membrane phospholipids, making up 78% of all phospholipids found in control group liver cells. Phosphatidylinositol (PI) was significantly increased in the T group, phosphatidylserine (PS) in the US group and sphingomyelin (SF) in the TS group. The composition of fatty acids linked to PE and PC, the most abundant phospholipids in liver membranes, is shown in Table 3 and is expressed as a percentage of the total amount of fatty acids linked to the correspondent phospholipid head. Each value is the mean ± SD of four independent determinations for each animal of each experimental group. Data show that mono-diunsaturated fatty acids decreased and polyunsaturated fatty acids increased, independently of the phospholipid heads, in T rats when compared with U rats. On the other hand, acute exercise had the opposite effect, as mono-diunsaturated fatty acids increased and polyunsaturated fatty acids decreased in both untrained and trained groups. The increase of mono- and di-unsaturated fatty acids observed in the stressed groups is higher than the corresponding decrease of polyunsaturated fatty acids and may be accounted for by a decrease of long chain fatty acids, which were not analyzed in detail. This is not surprising, because stress is known to cause the oxidative breakdown of longer and polyunsaturated hydrocarbon chains.
TABLE 2.
Liver membrane phospholipid composition (total phospholipid weight %)
| PL | U | T | US | TS | Significant Comparison P < 0.005a |
|---|---|---|---|---|---|
| PE | 27.58 ± 2.1 | 32.74 ± 1.58 | 24.40 ± 2.2 | 28.46 ± 2.45 | |
| PS | 6.04 ± 1.5 | 4.47 ± 1.8 | 9.76 ± 1.4 | 5.82 ± 1.34 | U vs US |
| PC | 49.18 ± 2.3 | 49.53 ± 2.1 | 47.64 ± 1.98 | 45.56 ± 2.33 | |
| SF | 6.84 ± 0.63 | 5.56 ± 0.45 | 7.14 ± 0.98 | 10.21 ± 0.89 | U vs TS |
| PI | 8.26 ± 0.8 | 10.66 ± 0.6 | 9.99 ± 0.9 | 7.33 ± 1.1 | U vs T |
PL, phospholipid; PE, phosphatidylethanolamine; PS, phosphatidylserine; PC, phosphatidylcholine; SF, sphingomyelin; PI, phosphatidylinositol; U, sedentary controls; T, trained animals; US, sedentary controls assigned to Stress; TS, trained animals assigned to Stress. Values are means ± SD of four independent determinations.
Statistical analysis was determined using one-way ANOVA and Fisher's LSD Test.
TABLE 3.
Composition of the fatty acids extracted from liver membranes as % of total amount of fatty acids linked to the correspondent phospholipid head
| PL | Fatty Acid | U | T | US | TS | Significant Comparison P < 0.005a |
|---|---|---|---|---|---|---|
| PC | Saturated | 29.4 ± 6.70 | 31.033 ± 7.11 | 33.88 ± 7.11 | 33.28 ± 4.45 | |
| Mono-unsaturated and di-unsaturated | 34.92 ± 6.40 | 20.13 ± 3.57 | 46.20 ± 8.61 | 49.21 ± 8.90 | U vs T, US, TS T vs US, TS | |
| Polyunsaturated | 19.5 ± 2.21 | 28.87 ± 2.50 | 16.04 ± 3.12 | 11.1 ± 2.71 | U vs T, US, TS T vs TS | |
| PE | Saturated | 29.32 ± 4.22 | 30.33 ± 2.51 | 33.29 ± 3.44 | 33.28 ± 3.32 | |
| Mono-unsaturated and di-unsaturated | 37.24 ± 4.99 | 22.27 ± 2.49 | 50.10 ± 8.15 | 48.92 ± 4.87 | U vs T, US, TS T vs US, TS | |
| Polyunsaturated | 21.88 ± 3.81 | 33.67 ± 4.92 | 13.90 ± 2.53 | 15.32 ± 1.65 | U vs T T vs US, TS |
PL, phospholipid; PC, phosphatidylcholine; PE, phosphatidylethanolamine; U, sedentary controls; T, trained animals; US, sedentary controls assigned to Stress; TS, trained animals assigned to Stress. Values are means ± SD of four independent determinations.
Statistical analysis was determined using one-way ANOVA and Fisher's LSD Test.
DISCUSSION
The aim of this study was to provide evidence that moderate aerobic physical exercise could be beneficial for organisms by modulation of PON expressions. While accumulating data that confirmed our hypothesis, we also unexpectedly documented for the first time the presence of PON3 in rat plasma by also finding that the upregulation of PON3 rather than PON1 appears to be associated with physical training. PON expression was studied in the rat model of aerobic exercise training because, at least in humans, PONs are HDL-associated proteins capable of preventing LDL oxidation, which suggests a role for PON proteins as antiatherogenic agents (42). PON1 is the most widely studied member of the family of mammalian enzymes; plasma PON1 has been extensively explored in relation to cardiovascular diseases, whereas few data are present on the hepatic enzyme. PON1 activity has been observed in rat (23, 43) hepatic microsomes; a portion of this enzyme is secreted into the circulation (23), and another portion is stored in the liver (43), where it seems to provide hepatic protection against oxidative stress (44). The presence and the physiological role of PON3 have been recently addressed (12); it was thought that the enzyme is absent in rat plasma, in analogy with the mouse, where PON3 remains cell-bound (22).
We assayed PON enzymatic activities by using four different substrates, because PON1 has paraoxonase and arylesterase activity, whereas its lactonase activity is restricted to lipophylic lactones; on the other hand, PON3 has no paraoxonase activity, low arylesterase activity, and high lactonase activity (higher with DHC than with 2-coumaranone as substrate) (12, 13). Thus, differences between the two enzymes can be appreciated by comparing Figs. 2D and 3D (mainly PON1 activity), on one hand, and Figs. 2B and 3B (mainly PON3 activity), on the other. In fact, should the physical exercise-related variations in enzymatic activity be due to only one PON activity, different substrates would not give differential results. Moreover, the differential inhibition of PON1 and PON3 activity shown in nondenaturing electrophoresis after exposure of extracts to Co2+ and Cu2+, as well as the differential reactivity with monoclonal anti-PON1 or anti-PON3 antibodies in WB provides an independent demonstration of the presence of PON3 in the rat plasma. Because the enzymatic assays performed in plasma samples were carried out in the presence of PMFS, a CE inhibitor, we were also able to distinguish between PONs and CEs, known to be present in the rodent plasma (17).
As a whole, our results suggest that PON1 expression did not change with moderate exercise training, whereas it was downregulated by acute exercise. On the other hand, plasma PON3 protein levels significantly increased after training; acute exercise did not affect protein levels and enzymatic activity of sedentary controls (US), whereas it decreased both protein levels (30%) and enzymatic activity (40%) of trained subjects (TS).
When enzymatic activities were evaluated in liver microsomes, all activities were found to decrease in both groups of stressed rats (TS and US). As for protein levels, PON1 levels were unchanged in all conditions, whereas PON3 protein levels increased. In particular, acute exercise upregulated PON3 in both sedentary and trained rats but the increase was more pronounced in the TS group (six times the U group). The interpretation of these results is somewhat puzzling and requires some speculation.
Help may come from liver membranes phospholipids and their fatty acid composition, which were evaluated in the present study, because they might influence PON expression in plasma (PONs being of hepatic origin). In fact, release of PON from the cell membrane is increased by HDL, which also stabilizes the enzyme. It can be hypothesized that phospholipids (as well as apolipoprotein A-I, the structural peptide of HDL) may also play a role in the release and in the regulation of activity and stability of the enzyme (45). Although the contribution of membrane phospholipids and fatty acid in the release of PONs from liver membranes to HDL requires further study, we suggest that changes in the composition of liver membranes might affect the mechanism involved in PON release and in PON plasmatic expression. In fact, we found that training modifies phospholipid fatty acid composition, increasing the polyunsaturated fraction, whereas acute exercise neutralizes such effect. Thus, the training-associated increase in plasma membrane fluidity, brought about by the increase in polyunsaturated fatty acids, might facilitate PON release to HDL. If PON3, rather than PON1, is upregulated by training, a higher PON3 plasma protein level and activity and a higher liver mRNA and protein concentration will be observed. On the other hand, stress, by reversing the effect on fatty acid composition, might contribute to the retention of PON in the liver membranes. This will result in increased concentration of liver membrane-bound PON3, it being the upregulated enzyme. The observed decrease in all microsomal enzymatic activities may be due to additional effects of stress on the liver. We are aware that some data do not completely fit in this model, however what we have hypothesized is not contradicted by what we observed at the mRNA level. In fact, training did not affect PON1 mRNA synthesis and resulted in PON3 mRNA upregulation. Acute exercise resulted in the decrease of PON3 mRNA, although such decrease did not reach the level of significance in TS subjects.
It is generally accepted that physical activity plays a beneficial role in the prevention of cardiovascular disease (1, 29) and some evidence suggests that sustained physical activity affects lipoprotein metabolism, decreases plasma triglyceride levels, and increases HDL concentration (2) and HDL-associated enzymes such as PON (46). Although PON3 is still largely less studied than PON1, recent reports suggest that PON3 may also possess antiatherogenic capacities. PON3 has been reported to associate with HDL in circulation (25, 30) and to protect against LDL oxidation in vitro (14, 21, 47). Ng et al. (48) demonstrated, for the first time, that elevated levels of human PON3 enhance the cholesterol efflux potential of serum, decrease LDL oxidation, increase the antioxidant properties of HDL, and protect against the progression of atheromatous lesion formation in vivo. Shih et al. (22) generated and characterized transgenic mice overexpressing human PON3 and demonstrated that elevated PON3 expression may play an important role in protection against obesity and atherosclerosis in male mice. The authors concluded that PON3 is an attractive candidate for further studies regarding its involvement in cardiovascular disease and obesity.
In light of these recent findings, we would like to emphasize that the beneficial effects of mild physical activity on the lipid profile (lower total cholesterol, LDL-cholesterol, and triglyceride concentrations and higher concentrations of anti-inflammatory, cholesterol-removing HDL particles) may also be related to the overexpression of PON3 and that PON3, due to its antiatherogenic properties, could play a beneficial role in the prevention of some diseases. The current study is the first demonstration of the presence of PON3 in the rat plasma and of a novel relationship between PON3 overexpression and mild physical activity.
Acknowledgments
The authors are grateful to Francesco Fabi, Gianluca Andrielli, and Giuliana Marinacci for their technical assistance. A.V., M.M., and G.R. are heads of their operative units and share the responsibility of the experimental design.
Footnotes
Abbreviations:
- CE
- carboxylesterase
- DHC
- dihydrocoumarin
- PC
- phosphatidylcholine
- PE
- phosphatidylethanolamine
- PI
- phosphatidylinositol
- PL
- phospholipid
- PMSF
- phenylmethylsulfonylfluoride
- PON
- paraoxonase
- PS
- phosphatidylserine
- SF
- sphingomyelin
- T
- trained animals
- TS
- trained animals assigned to stress
- U
- sedentary controls
- US
- sedentary controls assigned to stress
- WB
- western blot
This study was supported by grants from the Italian “Ministero dell'Università e della Ricerca” (PRIN 2004054720), the “Cariplo” Foundation (2005 Project), and the “Cassa di Risparmio di Perugia” Foundation (2006 Project).
REFERENCES
- 1.Kurl S., Laukkanen J. A., Niskanen L., Rauramaa R., Tuomainen T. P., Sivenius J., Salonen J. T. 2005. Cardiac power during exercise and the risk of stroke in men. Stroke. 36: 820–824. [DOI] [PubMed] [Google Scholar]
- 2.Haskell W. L., Lee I. M., Pate P. R., Powell K. E., Blair S. N., Franklin B. A., Macera C. A., Heath G. W., Thompson P. D., Bauman A. 2007. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med. Sci. Sports Exerc. 39: 1423–1434. [DOI] [PubMed] [Google Scholar]
- 3.Di Loreto C., Fanelli C., Lucidi P., Murdolo G., De Cicco A., Parlanti N., Ranchelli A., Fatone C., Taglioni C., Santeusanio F., et al. 2005. Make your diabetic patients walk: long-term impact of different amounts of physical activity on type 2 diabetes. Diabetes Care. 28: 1295–1302. [DOI] [PubMed] [Google Scholar]
- 4.Leaf D. A. 2003. The effect of physical exercise on reverse cholesterol transport. Metabolism. 52: 950–957. [DOI] [PubMed] [Google Scholar]
- 5.Parthasarathy S., Barnett J., Fong L. G. 1990. High density lipoprotein inhibits the oxidative modification of low-density lipoprotein. Biochim. Biophys. Acta. 1044: 275–283. [DOI] [PubMed] [Google Scholar]
- 6.Shih D. M., Xia Y. R., Wang X. P., Miller E., Castellani L. W., Subbanagounder G., Cheroutre H., Faull K. F., Berliner J. A., Witztum J. L., et al. 2000. Combined serum paraoxonase knockout/apolipoprotein E knockout mice exhibit increased lipoprotein oxidation and atherosclerosis. J. Biol. Chem. 275: 17527–17535. [DOI] [PubMed] [Google Scholar]
- 7.Durrington P. N., Mackness B., Mackness M. I. 2001. Paraoxonase and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 21: 473–480. [DOI] [PubMed] [Google Scholar]
- 8.Rozenberg O., Rosenblat M., Coleman R., Shih D. M., Aviram M. 2003. Paraoxonase (PON1) deficiency is associated with increased macrophage oxidative stress: studies in PON1-knockout mice. Free Radic. Biol. Med. 34: 774–784. [DOI] [PubMed] [Google Scholar]
- 9.Tward A., Xia Y. R., Wang X. P., Shi Y. S., Park C., Castellani L. W., Lusis A. J., Shih D. M. 2002. Decreased atherosclerotic lesion formation in human serum paraoxonase transgenic mice. Circulation. 106: 484–490. [DOI] [PubMed] [Google Scholar]
- 10.Rozenberg O., Shih D. M., Aviram M. 2005. Paraoxonase 1 (PON1) attenuates macrophage oxidative status: studies in PON1 transfected cells and in PON1 transgenic mice. Atherosclerosis. 181: 9–18. [DOI] [PubMed] [Google Scholar]
- 11.Primo-Parmo S. L., Sorenson R. C., Teiber J., La Du B. N. 1996. The human serum paraoxonase/arylesterase gene (PON1) is one member of a multigene family. Genomics. 33: 498–507. [DOI] [PubMed] [Google Scholar]
- 12.Draganov D. I., La Du B. N. 2004. Pharmacogenetics of paraoxonases: a brief review. Naunyn Schmiedebergs Arch. Pharmacol. 369: 78–88. [DOI] [PubMed] [Google Scholar]
- 13.Draganov D. I., Teiber J. F., Speelman A., Osawa Y., Sunahara R., La Du B. N. 2005. Human paraoxonases (PON1, PON2, and PON3) are lactonases with overlapping and distinct substrate specificities. J. Lipid Res. 46: 1239–1247. [DOI] [PubMed] [Google Scholar]
- 14.Draganov D. I., Stetson P. L., Watson C. E., Billecke S. S., La Du B. N. 2000. Rabbit serum paraoxonase 3 (PON3) is a high density lipoprotein-associated lactonase and protects low-density lipoprotein against oxidation. J. Biol. Chem. 275: 33435–33442. [DOI] [PubMed] [Google Scholar]
- 15.Harel M., Aharoni A., Gaidukov L., Brumshtein B., Khersonsky O., Meged R., Dvir H., Ravelli R. B., McCarthy A., Toker L., et al. 2004. Structure and evolution of the serum paraoxonase family of detoxifying and anti-atherosclerotic enzymes. Nat. Struct. Mol. Biol. 11: 412–419. [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez Esparragon F., Hernandez Trujillo Y., Macias Reyes A., Hernandez Ortega E., Medina A., Rodriguez Perez J. C. 2006. Concerning the significance of paraoxonase-1 and SR-B1 genes in atherosclerosis. Rev. Esp. Cardiol. 59: 154–164. [PubMed] [Google Scholar]
- 17.Teiber J. F., Draganov D. I., La Du B. N. 2003. Lactonase and lactonizing activities of human serum paraoxonase (PON1) and rabbit serum PON3. Biochem. Pharmacol. 66: 887–896. [DOI] [PubMed] [Google Scholar]
- 18.Pla A., Rodrigo L., Hernández A. F., Gil F., Lopez O. 2007. Effect of metal ions and calcium on purified PON1 and PON3 from rat liver. Chem. Biol. Interact. 167: 63–70. [DOI] [PubMed] [Google Scholar]
- 19.Draganov D. I. 2007. Human PON3, effects beyond the HDL: clues from Human PON3 transgenic mice. Circ. Res. 100: 1104–1105. [DOI] [PubMed] [Google Scholar]
- 20.Ng C. J., Wadleigh D. J., Gangopadhyay A., Hama S., Grijalva V. R., Navab M., Fogelman A. M., Reddy S. T. 2001. Paraoxonase-2 is a ubiquitously expressed protein with antioxidant properties and is capable of preventing cell-mediated oxidative modification of low density lipoprotein. J. Biol. Chem. 276: 44444–44449. [DOI] [PubMed] [Google Scholar]
- 21.Reddy S. T., Wadleigh D. J., Grijalva V., Ng C., Hama S., Gangopadhyay A., Shih D. M., Lusis A. J., Navab M., Fogelman A. M. 2001. Human paraoxonase-3 is an HDL-associated enzyme with biological activity similar to paraoxonase-1 protein but is not regulated by oxidized lipids. Arterioscler. Thromb. Vasc. Biol. 21: 542–547. [DOI] [PubMed] [Google Scholar]
- 22.Shih D. M., Xia X. P., Wang X. P., Wang S. S., Bourquard N., Fogelman A. M., Lusis A. J., Reddy S. T. 2007. Decreased obesity and atherosclerosis in human paraoxonase 3 transgenic mice. Circ. Res. 100: 1200–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodrigo L., Gil F., Hernandez A. F., Lopez O., Pla A. 2003. Identification of paraoxonase 3 in rat liver microsomes: purification and biochemical properties. Biochem. J. 376: 261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.James R. W., Deakin S. P. 2004. The importance of high-density lipoproteins for paraoxonase-1 secretion, stability, and activity. Free Radic. Biol. Med. 37: 1986–1994. [DOI] [PubMed] [Google Scholar]
- 25.Tomás M., Elosua R., Senti M., Molina L., Vila J., Anglada R., Fito M., Covas M. I., Marrugat J. 2002. Paraoxonase1–192 polymorphism modulates the effects of regular and acute exercise on paraoxonase1 activity. J. Lipid Res. 43: 713–720. [PubMed] [Google Scholar]
- 26.Pawlowska D., Moniuszko-Jakoniuk J., Soltys M. 1985. Parathion-methyl effect on the activity of hydrolytic enzymes after single physical exercise in rats. Pol. J. Pharmacol. Pharm. 37: 629–638. [PubMed] [Google Scholar]
- 27.Clarkson P. M. 1995. Antioxidants and physical performance. Crit. Rev. Food Sci. Nutr. 35: 131–141. [DOI] [PubMed] [Google Scholar]
- 28.Aviram M., Rosenblat M., Billecke S., Erogul J., Sorenson R., Bisgaier R. C., Newton R. S., La Du B. N. 1999. Human serum paraoxonase (PON 1) is inactivated by oxidized low density lipoprotein and preserved by antioxidants. Free Radic. Biol. Med. 26: 892–904. [DOI] [PubMed] [Google Scholar]
- 29.Marini M., Lapalombella R., Margonato V., Ronchi R., Samaja M., Scapin C., Gorza L., Maraldi T., Carinci P., Ventura C., et al. 2007. Mild exercise training, cardioprotection and stress gene profile. Eur. J. Appl. Physiol. 99: 503–510. [DOI] [PubMed] [Google Scholar]
- 30.Deakin S., Leviev I., Gomaraschi M., Calabresi L., Franceschini G., James R. W. 2002. Enzymatically active paraoxonase-1 is located at the external membrane of producing cells and released by a high affinity, saturable, desorption mechanism. J. Biol. Chem. 277: 4301–4308. [DOI] [PubMed] [Google Scholar]
- 31.Draganov D. I., Sass K. M., Watson C. E., Bisgaier C. L., Reddy S. T., Teiber J. F., La Du N. B. 2002. The N-terminal sequences of human paraoxonase-1 (PON1) and paraoxonase-3 (PON3) are responsible for their different translocation and secretion. Circulation. 106(II): 123. [Google Scholar]
- 32.Shamir R., Hartman C., Karry R., Pavlotzky E., Eliakim R., Lachter J., Suissa A., Aviram M. 2005. Paraoxonases (PONs) 1, 2, and 3 are expressed in human and mouse gastrointestinal tract and in Caco-2 cell line: selective secretion of PON1 and PON2. Free Radic. Biol. Med. 39: 336–344. [DOI] [PubMed] [Google Scholar]
- 33.American Physiological Society. 2006. Resource Book for the Design of Animal Exercise Protocols. Accessed August 25, 2008, at http://www.the-aps.org/pa/action/exercise/book.pdf.
- 34.Li B., Sedlacek M., Manoharan I., Boopathy R., Duysen E. G., Masson P., Lockridge O. 2005. Butyrylcholinesterase, paraoxonase, and albumin esterase, but not carboxylesterase, are present in human plasma. Biochem. Pharmacol. 70: 1673–1684. [DOI] [PubMed] [Google Scholar]
- 35.Gil F., Pla A., Golzalvo M. G., Hernández A. F., Villanueva E. 1993. Partial purification of paraoxonase from rat liver. Chem. Biol. Interact. 87: 69–75. [DOI] [PubMed] [Google Scholar]
- 36.Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. J. 1951. Protein measurement with Folin phenol reagent. J. Biol. Chem. 193: 265–275. [PubMed] [Google Scholar]
- 37.Gan K. N., Smolen A., Eckerson H. W., La Du B. N. 1991. Purification of human serum paraoxonase/arylesterase. Evidence for one esterase catalyzing both activities. Drug Metab. Dispos. 19: 100–106. [PubMed] [Google Scholar]
- 38.Billecke S., Draganov D., Counsell R., Stetson P., Watson C., Hsu C., La Du B. N. 2000. Human serum paraoxonase (PON1) isozymes Q and R hydrolyze lactones and cyclic carbonate esters. Drug Metab. Dispos. 28: 1335–1342. [PubMed] [Google Scholar]
- 39.Livak K. J., Schmittgen T. D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 40.Gaiti A., Goracci G., De Medio G. E., Porcellati G. 1972. Enzymic synthesis of plasmalogen and O-alkyl glycerolipid by base-exchange reaction in the rat brain. FEBS Lett. 27: 116–120. [DOI] [PubMed] [Google Scholar]
- 41.Binaglia L., Goracci G., Porcellati G., Roberti R., Woelk H. 1973. The synthesis of coline and ethanolamine phosphoglycerides in neuronal and glial cells of rabbit in vitro. J. Neurochem. 21: 1067–1082. [DOI] [PubMed] [Google Scholar]
- 42.Aviram M. 2004. Introduction to the serial review on paraoxonases, oxidative stress, and cardiovascular diseases. Free Radic. Biol. Med. 37: 1301–1303. [DOI] [PubMed] [Google Scholar]
- 43.Gonzalvo M. C., Gil F., Hernandez A. F., Rodrigo L., Villanueva E., Pla A. 1998. Human liver paraoxonase (PON1): subcellular distribution and characterization. J. Biochem. Mol. Toxicol. 12: 61–69. [DOI] [PubMed] [Google Scholar]
- 44.Ferrè N., Camps J., Cabrè M., Paul A., Joven J. 2001. Hepatic paraoxonase activity alterations and free radical production in rats with experimental cirrhosis. Metabolism. 50: 997–1000. [DOI] [PubMed] [Google Scholar]
- 45.Oda M. N., Bielicki J., Berger T., Forte T. M. 2001. Cysteine substitutions in apolipoprotein A-I primary structure modulate paraoxonase activity. Biochemistry. 40: 1710–1718. [DOI] [PubMed] [Google Scholar]
- 46.Navab M., Hama S. Y., Hough G. P., Hedrick C. C., Sorenson R., La Du B. N., Kobashigawa J. A., Fonarow G. C., Berliner J. A., Laks H., et al. 1998. High density associated enzymes: their role in vascular biology. Curr. Opin. Lipidol. 9: 449–456. [DOI] [PubMed] [Google Scholar]
- 47.Rosenblat M., Draganov D., Watson C. E., Bisgaier C. L., La Du B. N., Aviram M. 2003. Mouse macrophage paraoxonase 2 activity is increased whereas cellular paraoxonase 3 activity is decreased under oxidative stress. Arterioscler. Thromb. Vasc. Biol. 23: 468–474. [DOI] [PubMed] [Google Scholar]
- 48.Ng C. J., Bourquard N., Hama S. Y., Shih D., Grijalva V. R., Navab M., Fogelman A. M., Reddy S. T. 2007. Adenovirus-mediated expression of human paraoxonase 3 protects against toprogression of atherosclerosis in apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 27: 1368–1374. [DOI] [PubMed] [Google Scholar]