Abstract
Lysosomal phospholipase A2 (LPLA2) is characterized by increased activity toward zwitterionic phospholipid liposomes containing negatively charged lipids under acidic conditions. The effect of anionic lipids on LPLA2 activity was investigated. Mouse LPLA2 activity was assayed as C2-ceramide transacylation. Sulfatide incorporated into liposomes enhanced LPLA2 activity under acidic conditions and was weakened by NaCl or increased pH. Amiodarone, a cationic amphiphilic drug, reduced LPLA2 activity. LPLA2 exhibited esterase activity when p-nitro-phenylbutyrate (pNPB) was used as a substrate. Unlike the phospholipase A2 activity, the esterase activity was detected over wide pH range and not inhibited by NaCl or amiodarone. Presteady-state kinetics using pNPB were consistent with the formation of an acyl-enzyme intermediate. C2-ceramide was an acceptor for the acyl group of the acyl-enzyme but was not available as the acyl group acceptor when dispersed in liposomes containing amiodarone. Cosedimentation of LPLA2 with liposomes was enhanced in the presence of sulfatide and was reduced by raising NaCl, amiodarone, or pH in the reaction mixture. LPLA2 adsorption to negatively charged lipid membrane surfaces through an electrostatic attraction, therefore, enhances LPLA2 enzyme activity toward insoluble substrates. Thus, anionic lipids present within lipid membranes enhance the rate of phospholipid hydrolysis by LPLA2 at lipid-water interfaces.—Abe, A., and J. A. Shayman. The role of negatively charged lipids in lysosomal phospholipase A2 function.
Keywords: lysosome, GXVPLA2, ceramide, transacylation, cationic amphiphilic drug, amiodarone, esterase
Lysosomes are intracellular organelles that contain several hydrolytic enzymes with acidic pH optima that catalyze the degradation of extracellular as well as intracellular substrates. Lysosomes are critical to several biological functions such as securing nutrition through degradation of cellular components and providing host defense against infection. Several studies have reported that impairments of lysosomal enzyme activity directly or indirectly result in disorders of cellular function or disease. In a recent review of the lysosomal proteome, 75 established lysosomal proteins were listed, of which, deficiencies in 46 were associated with well characterized disease. Thirty-six additional proteins were listed that may also be lysosome associated but are presently less well characterized (1).
Previously, we identified a lysosomal phospholipase A2 (LPLA2) with specificity toward glycerophospholipids in Madin-Darby canine kidney (MDCK) cells (2). LPLA2 is calcium-independent PLA2, localized to lysosomes, has an acidic pH optimum, and transacylates lipophilic primary alcohols (3–5). LPLA2 purified from calf brain is a water-soluble glycoprotein consisting of a single peptide chain with 45 kDa of molecular weight. The primary structure of LPLA2 deduced from DNA sequences coding LPLA2 is highly preserved between mammals, including mouse, rat, human, bovine, and dog (4). LPLA2 belongs to the α/β-hydrolase superfamily and has 49% identity to lecithin-cholesterol acyltransferase. LPLA2 is a secreted enzyme (6). Zymosan-stimulated alveolar macrophages (AMs) release LPLA2, and LPLA2 is taken up by AMs via a mannose receptor and subsequently translocated into acidic compartments. LPLA2 is ubiquitously expressed but is most highly expressed in AM (7). LPLA2-deficient mouse AMs are characterized by the marked accumulation of glycerophospholipid and the formation of lamellar inclusion bodies, which are hallmarks of cellular phospholipidosis (8). Thus, LPLA2 may play a critical role in cellular phospholipid homeostasis in those cells. Finally, cationic amphiphilic drugs (CADs) such as amiodarone (AMD) and D-threo-1-phenyl-2-decanoylamino-3-morpholino-propanol, which interact with negatively charged lipids, inhibit the apparent activity of LPLA2 and result in cellular phospholipidosis in MDCK cells, suggesting a potential role for LPLA2 in some forms of CAD-induced phospholipidosis (9).
Originally, we found that LPLA2 exhibited an increased activity under acidic conditions toward zwitterionic phosphatidylcholine liposomes comprised of negatively charged lipids. By contrast, LPLA2 activity was quite low toward phosphatidylcholine liposomes in the absence of negatively charged lipids (2). A specific anionic lipid, bis(monoacylglycero)phosphate (BMP), is found in acidified cellular compartments, such as endosomes and lysosomes, and has been identified as a stimulator of sphingolipid degradation, as well as an inducer for formation of multivesicular membranes in the luminal space of these compartments (10, 11).
Aside from their association with drug toxicity due to phospholipidosis, CADs may be a beneficial tool to elucidate the interfacial reaction kinetics and mechanism of phospholipases. Mingeot-Leclercq et al. (12) reported that the aminoglycoside gentamicin, which is a CAD and induces a cellular phospholipidosis, binds to negatively charged phospholipids such as phosphatidylinositol and decreases the activity of lysosomal phospholipase A1 and A2 toward a zwitterionic phospholipid, phosphatidylcholine, included in lipid vesicles. This group also found that those enzyme activities were enhanced when the lipid bilayer containing substrate is negatively charged and suggested that gentamicin impairs the activities of the enzymes by decreasing the available negative charges required for optimal activity. More recently, Piret et al. (13) reported that lysosomal phospholipase A1 activity is markedly enhanced by negatively charged lipids incorporated into liposomes containing substrate. However, the enzyme sources used in both reports were crude, making the interpretation of the interfacial reaction mechanism difficult.
In the present study, purified recombinant mouse LPLA2 was used as the enzyme source of LPLA2. The effect of negatively charged lipids on LPLA2 activity was used to probe the molecular mechanisms of the LPLA2 reaction at the lipid-water interface and the basis for the phospholipid accumulation observed with LPLA2 deficiency.
EXPERIMENTAL PROCEDURES
Reagents
1,2-Dioleoyl-sn-glycero-3-phosphocholine (DOPC), 1,1’, 2,2’-tetraoleoyl cardiolipin (TOCL), 1,2-dioleoyl-sn-glycero-3-[phospho-rac-(1-glycerol)] (DOPG), 1,2-dioleoyl-sn-glycero-3-phosphate (DOPA), 1,2-dioleoyl-sn-glycero-3-phosphoinositol (DOPI), 1,2-dioleoyl-sn-glycero-3-phosphoserine (DOPS), 1,2-dioleoyl-sn-glycero-3-phosphoethanol (DOPEt) and 1,2-di-O-octadecenyl-sn-glycero-3-phosphocholine were purchased from Avanti. (S,S) Bis(monooleoylglycero)phosphate sn-(2-oleoyl-3-hydroxy)-glycerol-1-phospho-sn-3′-(2’-oleoyl-1’-hydroxy)-glycerol (BMP) was obtained from Echelon; p-NPB, para-nitro-phenylbutyrate, was from Sigma; purified recombinant mouse LPLA2 tagged with polyhistidine was obtained from Proteos (Kalamazoo, MI); N-acetyl-sphingosine (NAS) was from Matreya; Bicinchoninic acid protein assay reagent was from Pierce; high performance thin layer chromatography (HPTLC) silica gel plates, 10 × 20 cm, were from Merck. Sulfatide was previously prepared in our laboratory (14).
Transacylase activity of LPLA2
The reaction mixture consisted of 48 mM sodium citrate (pH 4.5), 10 µg/ml BSA, 38 µM acceptor (NAS) incorporated into phospholipid liposomes (127 µM DOPC), and 14.5 ng/ml recombinant mouse LPLA2 in a total volume of 500 µl. Liposomes were prepared as previously described (2). The reaction was initiated by the addition of the enzyme. The reaction mixture was incubated for 5 min to 30 min at 37°C and terminated by adding 3 ml of chloroform-methanol (2:1) plus 0.3 ml of 0.9% (w/v) NaCl. The mixture was centrifuged for 5 min at room temperature. The resultant lower organic layer was transferred into another glass tube and dried down under a stream of nitrogen gas. The dried lipid was dissolved in 40 µl of chloroform-methanol (2:1) and applied on an HPTLC plate and developed in a solvent system consisting of chloroform-acetic acid (90:10, v/v). The plate was dried and soaked in 8% (w/v) CuSO4, 5H2O, 6.8% (v/v) H3PO4, 32% (v/v) methanol. The uniformly wet plate was briefly dried by a hair dryer and charred for 15 min in a 150°C oven. The plate was scanned and the content of the product (1-O-acyl-NAS) was estimated by NIH-ImageJ 1.37v.
RESULTS
Effect of negatively charged lipid on LPLA2 activity
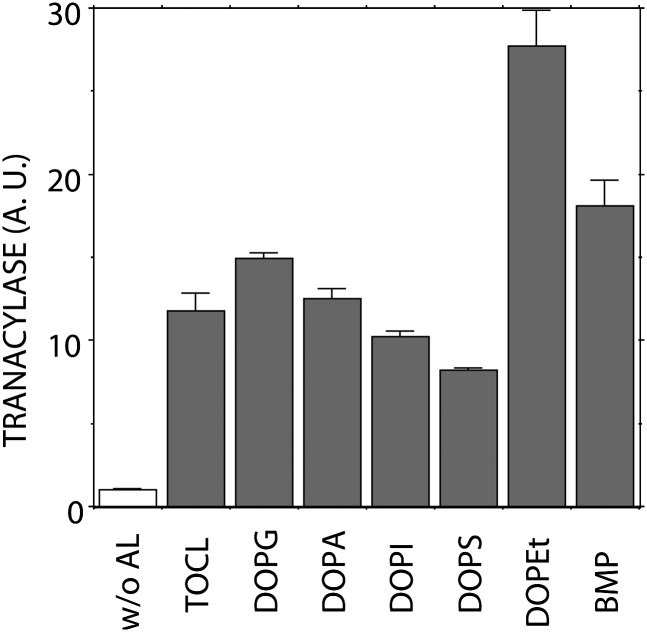
In a preliminary study, several anionic phospholipids, including TOCL, DOPG, DOPA, DOPS, DOPI, DOPEt, and BMP, were studied for their effects on LPLA2 activity (Fig. 1). These phospholipids not only stimulated LPLA2 activity as measured by the formation of 1-O-oleoyl-NAS, but were also observed to be substrates of LPLA2. DOPG, DOPA, and DOPEt were better substrates for LPLA2 than was DOPC when DOPG-NAS (3:1, molar ratio), DOPA-NAS (3:1, molar ratio), and DOPEt-NAS (3:1, molar ratio) liposomes were compared with DOPC-sulfatide-NAS (3:0.3:1, molar ratio) liposomes (data not shown). In the original characterization of LPLA2, we observed that the degradation of zwitterionic phospholipids by LPLA2 was markedly enhanced when sulfatide was incorporated into the phospholipid liposomes (2). To ascertain the effects of anionic lipids on LPLA2 activity, sulfatide, which is resistant to LPLA2 activity, was chosen as a negatively charged lipid. We first studied whether sulfatide incorporated into liposomes enhances the LPLA2 activity (Fig. 2).
Fig. 1.
Effect of anionic phospholipids on LPLA2 activity. The reaction mixture contained 48 mM sodium citrate (pH 4.5), 10 μg/ml BSA, liposomes (127 μM phospholipid), and 14.5 ng/ml of recombinant mouse LPLA2 in 500 μl of total volume. Liposomes consisting of DOPC-NAS (3:1, molar ratio) or DOP-anionic phospholipid-NAS (3:0.3:1, molar ratio) were incubated with the enzyme for 1.25 to 10 min at 37°C. The reaction products were extracted and separated by an HPTLC plate using a solvent system consisting of chloroform-acetic acid (9:1, v/v). The reaction product, 1-O-oleoyl-NAS, was quantified by scanning the plate. Anionic lipid, TOCL, DOPG, DOPA, DOPI, DOPS, DOPEt, and BMP indicate anionic lipid, 1,1′, 2,2′-tetraoleoyl cardiolipin, 1,2-dioleoyl-sn-glycero-3-[phospho-rac-(1-glycerol)], 1,2-dioleoyl-sn-glycero-3-phosphate, 1,2-dioleoyl-sn-glycero-3-phosphoinositol, 1,2-dioleoyl-sn-glycero-3-phosphoserine, 1,2-dioleoyl-sn-glycero-3-phosphoethanol and bis(monooleoylglycero)phosphate (S,S isomer) sn-(2-oleoyl-3-hydroxy)-glycerol-1-phospho-sn-3′-(2′-oleoyl-1′-hydroxy)-glycerol, respectively. For the reaction using DOPC-TOCL-NAS liposomes, the molar ratio of DOPC-TOCL-NAS was 3:0.15:1. Error bars indicate SD. (n = 3).
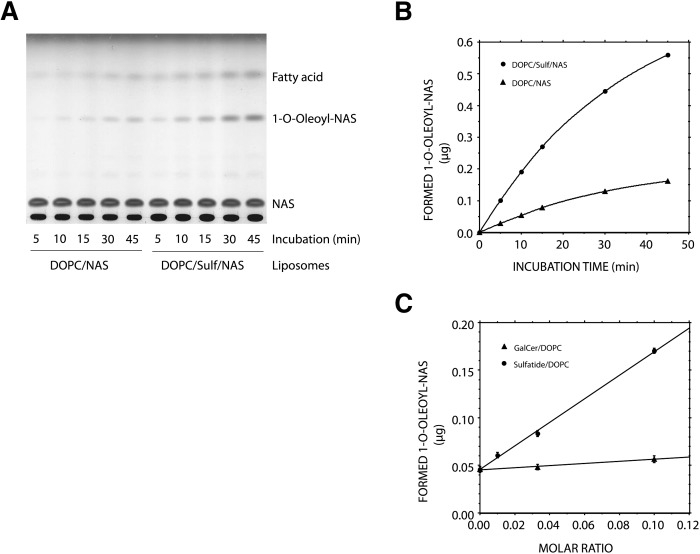
Fig. 2.
Effect of negatively charged lipid on LPLA2 activity. The reaction mixture contained 48 mM sodium citrate (pH 4.5), 10 μg/ml BSA, liposomes (127 μM phospholipid), and 14.5 ng/ml of recombinant mouse LPLA2 in 500 μl of total volume. A: Liposomes consisting of DOPC-NAS (3:1, molar ratio) or DOP-sulfatide-NAS (3:0.3:1, molar ratio) were incubated with the enzyme for 5, 10, 15, 30, and 45 min at 37°C. The reaction products were extracted and separated by an HPTLC plate using a solvent system consisting of chloroform-acetic acid (9:1, v/v). The reaction product, 1-O-oleoyl-NAS, quantified by scanning the plate, was plotted against time (B). C: Liposomes consisting of DOPC-galactosylceramide-NAS or DOPC-sulfatide-NAS with a different molar ratio were incubated with the recombinant enzyme at 37°C. The reaction products were extracted and separated as described in A. Error bars indicate SD. (n = 3).
Both 1-O-oleoyl-NAS and fatty acid were produced in a time dependent manner when liposomes consisting of either DOPC-NAS or DOPC-sulfatide-NAS were incubated with LPLA2 under acidic conditions (Fig. 2A). The plot of 1-O-oleoyl-NAS formed against the incubation time indicates that when liposomes containing sulfatide were used, the initial velocity of 1-O-oleoyl-NAS formation by LPLA2 was four times higher compared with the other one (Fig. 2B). The enhancement of LPLA2 activity by sulfatide was linear up to a 10% molar ratio of sulfatide to DOPC (Fig. 2C). Unlike sulfatide, galactosylceramide, the desulfated form of sulfatide, did not enhance LPLA2 activity (Fig. 2C). The subsequent studies were carried out using a 10% molar ratio of sulfatide to DOPC in liposomes.
Effect of ion strength, pH, or CAD on LPLA2 activity
The significant enhancement of LPLA2 activity observed in the presence of anionic lipids is consistent with a role for an electrostatic interaction between the phospholipase and its target membranes. Electrostatic interactions are primary mechanisms for the reversible association between peripheral membrane proteins and lipid membranes. Electrostatic interactions are affected by ionic strength, the net charge of the protein and the membrane, and pH. These potential regulators of LPLA2 activity were further studied.
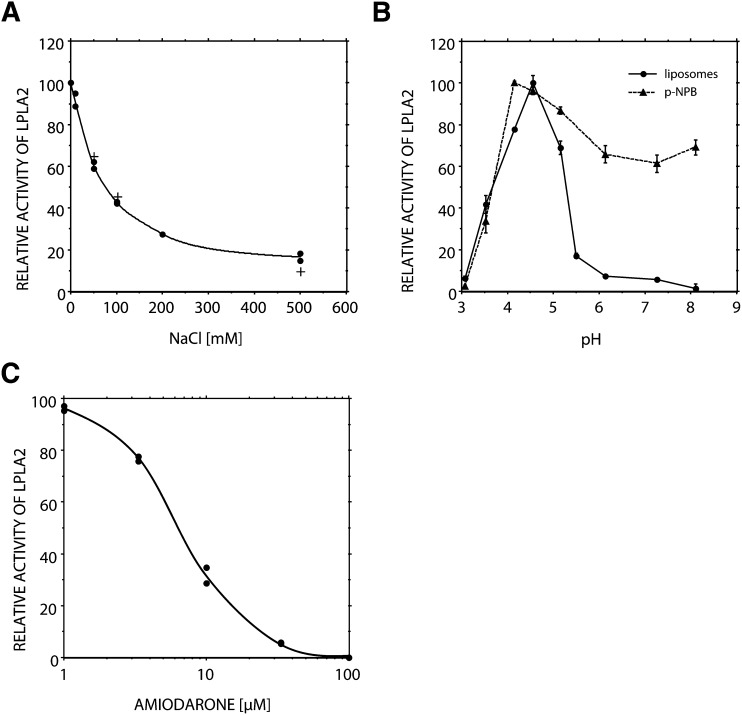
The enhancement of LPLA2 activity by sulfatide was markedly weakened by the addition of NaCl in the reaction mixture (Fig. 3A). The activity of LPLA2 to DOPC/sulfatide/NAS liposomes in the presence of 200 mM NaCl was mostly the same as that to DOPC/NAS liposomes in the absence of NaCl (Fig. 2). The LPLA2 activity of the soluble fraction obtained from MDCK cells transfected with mouse LPLA2 lacking the poly-histidine tag at the C-terminal was similarly affected by the addition of NaCl (Fig. 3A). In addition, the anti-arrhythmic AMD, a CAD that interacts with negatively charged lipids, suppressed the effect of sulfatide in a concentration dependent manner (Fig. 3B). The IC50 of AMD was ∼6 µM.
Fig. 3.
Effect of ion strength, cationic amphiphilic drug, or pH on LPLA2 activity. A: The reaction mixture contained 0-500 mM NaCl, 48 mM sodium acetate (pH 4.5), 10 μg/ml BSA, liposomes (127 μM phospholipid), and either 14.5 ng/ml of recombinant mouse LPLA2 (•) or 7.8 μg protein/ml of the soluble fraction obtained from MDCK cells transfected with mouse LPLA2 (+) in 500 μl of total volume. Before starting the reaction, liposomes consisting of DOPC, sulfatide, and NAS (3:0.3:1, molar ratio) were preincubated for 5 min at 37°C. The reaction was initiated by adding the recombinant LPLA2 or the soluble cell fraction and carried out at 37°C. The reaction products were extracted and separated by an HPTLC plate using a solvent system consisting of chloroform-acetic acid (9:1, v/v). One of the products, 1-O-oleoyl-NAS, quantified by scanning the plate, was plotted against NaCl concentration. B: Liposomes consisting of DOPC sulfatide and NAS were preincubated with a different concentration of cationic amphiphilic drug, amiodarone, for 5 min at 37°C and then incubated with the recombinant LPLA2. C: The reaction was carried out in sodium citrate/sodium phosphate buffer over a pH range. For the transacylase assay of LPLA2 (•), the reaction mixture contained liposomes (127 μM phospholipid) consisting of DOPC, sulfatide, and NAS (3:0.3:1, molar ratio), sodium citrate/sodium phosphate buffer, 10 μg/ml BSA, and 14.5 ng/ml of recombinant mouse LPLA2 in 500 μl of total volume. For the esterase assay of LPLA2 (—), the reaction mixture contained 200 μM p-nitro-phenylbuturate (pNPB), sodium citrate/sodium phosphate buffer, 10 μg/ml BSA, and 14.5 ng/ml of recombinant mouse LPLA2 in 500 μl of total volume. The reaction was initiated by the addition of recombinant LPLA2 and run for 10, 20, 30, and 40 min at 37°C. One hundred μl of the reaction mixture was taken at each time point and mixed with 100 μl of cold 0.2 M Na2CO3. Absorbance of the mixture at 400 nm was measured immediately. Error bars indicate SD. (n = 3).
The recombinant mouse LPLA2 showed an acidic pH optimum when the enzyme was incubated with liposomes consisting of DOPC/sulfatide/NAS (Fig. 3C). Taniyama et al. (15) reported that LPLA2 has esterase activity. Therefore, we evaluated the pH dependence of the esterase activity of LPLA2 using a small artificial molecule, pNPB, in the absence of liposomes. When this substrate was used, LPLA2 degraded pNPB to p-nitro-phenol (pNP) and butyrate. In this experiment, pNPB was dispersed in the reaction mixture as a monomer. In contrast to the PLA2/transacylase activity by mouse LPLA2, the sizable esterase activity was observed over a wide range of pH (Fig. 3C). In addition, the esterase activity was not significantly inhibited by the addition of AMD or NaCl (data not shown).
LPLA2 reactivity toward pNPB
The catalytic domain of LPLA2 is highly homologous to LCAT (4, 15, 16). Both enzymes are characterized by a catalytic triad. As shown in the mutagenesis study on the PLA2/transacylase activity of LPLA2 (16), the substitution in mouse LPLA2 of the catalytic serine with an alanine residue abolished both the PLA2/transacylase activity and esterase activities of LPLA2 (data not shown). This finding indicates that the PLA2/transacylase reaction and the esterase reaction by LPLA2 occur at the same catalytic site and by the same mechanism.
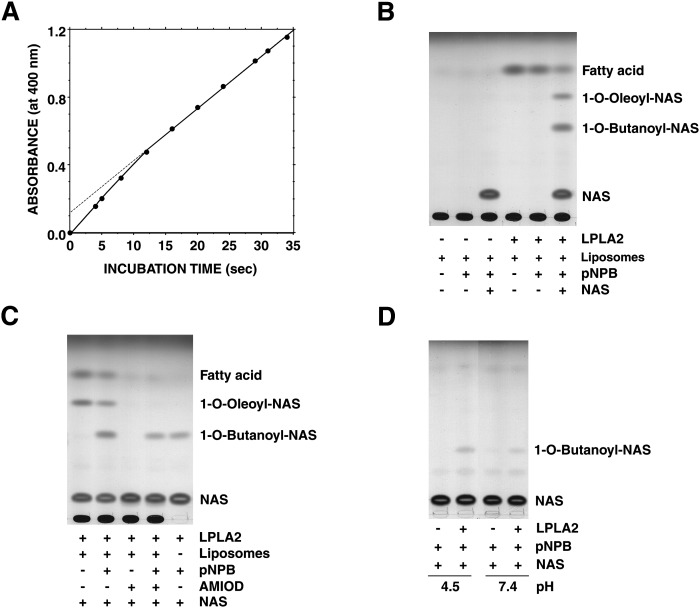
pNPB and its analogs are convenient artificial substrates for studying the enzymatic hydrolysis reaction and an acyl-enzyme formation in the presteady state. The hydrolysis of pNPB by LPLA2 was monitored by following the rate of pNP formation. An initial burst of pNP formation by LPLA2 was observed when pNPB was used as a substrate (Fig. 4A). Also, the magnitude of the initial burst was consistent with a stoichiometry for the binding of the enzyme to the substrate that is one to one.
Fig. 4.
LPLA2 reaction against mono-dispersed substrate. A: pNPB was incubated with recombinant LPLA2 in 200 μl of 0.25 M sucrose, 10 mM Hepes (pH 7.4), and 1 mM EDTA at 24°C. The concentrations of LPLA2 and pNPB in the reaction mixture were 520 μg/ml and 2 mM, respectively. B, C: Liposomes containing DOPC-sulfatide (10:1, molar ratio) were incubated with recombinant enzyme (29 ng/ml) with or without 200 μM pNPB or 33 μM amiodarone in the presence or absence of 10 μM NAS at 37°C in 500 μl Na-citrate (48 mM, pH 4.5). In panel C (right lane), the enzyme was incubated with pNPB and NAS without liposomes. In panel D, the enzyme was incubated with pNPB under both acidic and neutral conditions without liposomes.
When pNPB was added to the reaction mixture containing DOPC/sulfatide liposomes, the release of oleic acid by LPLA2 was significantly inhibited (Fig. 4B). When NAS and pNPB were both present in the reaction mixture in the presence of DOPC/sulfatide liposomes, 1-O-oleoly-NAS, oleic acid, and one additional product were produced by LPLA2 (Fig. 4B and C). The formation of 1-O-oleoly-NAS and the release of oleic acid were clearly inhibited by pNPB (Fig. 4C). The additional product had a slower mobility than 1-O-oleoly-NAS and was degraded under alkaline conditions. One of the resultant products produced by alkaline treatment was NAS (data not shown). Therefore, the additional product was 1-O-butanoyl-NAS.
AMD (33 μM) blocked the deacylation and acylation by LPLA2 in the DOPC/sulfatide/NAS reaction mixture. However, a significant amount of 1-O-butanoyl-NAS was still formed when coincubated with pNPB (Fig. 4C). A comparable level of 1-O-butanoyl-NAS formation occurred when pNPB was incubated with LPLA2 in the presence of NAS without liposomes (Fig. 4C). Unlike the transacylation by LPLA2 in phospholipid/sulfatide/NAS reaction mixture (Fig. 4C), the transacylation to NAS in pNPB/NAS reaction mixture was significantly accelerated by LPLA2 even at neutral pH (Fig. 4D). Under these conditions, the formation of 1-O-butanoyl-NAS at the neutral pH was 50% of that at the acidic pH.
Cosedimentation of LPLA2 with liposomes
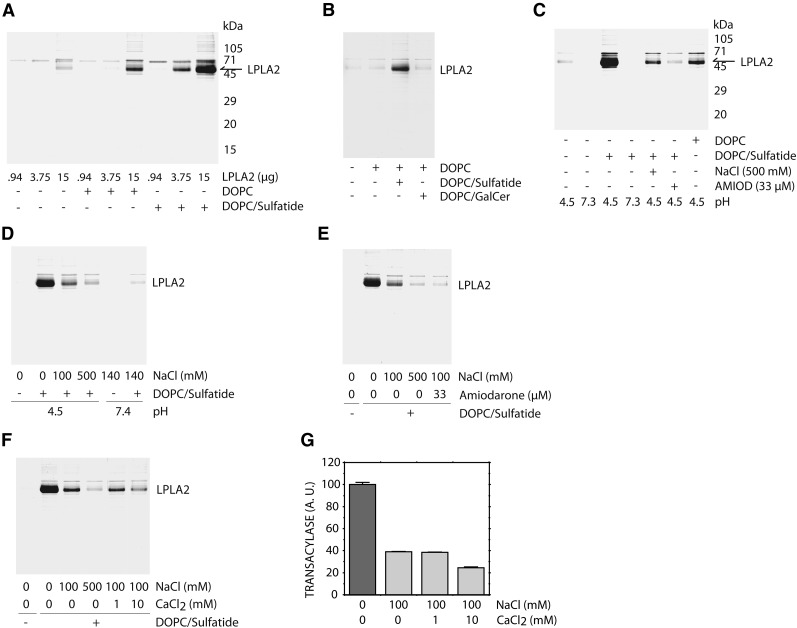
Binding of LPLA2 to the lipid membrane is a key step in the interfacial reaction. In the phospholipase A2 reaction, the binding may be mediated not only by substrates but also by negatively charged lipids. To confirm the binding between LPLA2 and lipid membranes, we applied a simple method, ultracentrifugation. In this study, the recombinant mouse LPLA2 was incubated with or without liposomes for 30 min on ice and then centrifuged at 150,000 g for 1 h at 4°C. After centrifugation, the liposome pellet was dispersed into SDS-PAGE sampling buffer and applied to a 12% separating gel. Following electrophoresis, the proteins were detected by coomassie brilliant blue reaction staining (Fig. 5).
Fig. 5.
Cosedimentation of LPLA2 with liposomes. A: Liposomes consisting of DOPC or DOPC-sulfatide (10:1, molar ratio) were incubated with varying amounts of recombinant mouse LPLA2 in 500 μl of 48 mM Na-citrate (pH 4.5) for 30 min on ice. The reaction mixture was then centrifuged for 1 h at 150,000 g at 4°C. The concentration of DOPC in this assay was 127 μM. The resultant precipitate was briefly washed with cold 50 mM Na-citrate (pH 4.5) and dissolved with 40 μl of SDS-polyacrylamide gel electrophoresis sampling buffer. The sample was separated by using 12% SDS-polyacrylamide gel. After electrophoresis, LPLA2 was detected by staining with CBB. B: Liposomes consisting of DOPC, DOPC-sulfatide (10:1, molar ratio) or DOPC-galactosylceramide (10:1, molar ratio) were incubated with 4 μg of the recombinant LPLA2 and analyzed as described in A. GalCer indicates galactosylceramide. C: Liposomes consisting of DOPC or DOPC-sulfatide (10:1, molar ratio) were incubated with 16 μg of the recombinant LPLA2 with or without 500 mM NaCl or 33 μM amiodarone and analyzed as described in A. D: Liposomes consisting of DOPC-sulfatide (10:1, molar ratio) were incubated with 6.26 μg of the recombinant LPLA2 in 50 mM Na-acetate (pH 4.5) in the presence of 0, 100, or 500 mM NaCl, or in PBS (pH 7.4). E, F: Liposomes consisting of DOPC-sulfatide (10:1, molar ratio) were incubated with 6.26 μg of the recombinant LPLA2 in 50 mM Na-acetate (pH 4.5), 100 mM NaCl with or without 33 μM AMD, 1 mM or 10 mM CaCl2, or 500 mM NaCl. G: LPLA2-transacylase activity was assayed with liposomes consisting of DOPC-sulfatide-NAS (3:0.3:1, molar ratio) that were incubated with 14.5 ng/ml LPLA2 in 50 mM Na-acetate (pH 4.5), 100 mM NaCl, 10 μg/ml BSA with or without 1 mM or 10 mM CaCl2. Error bars indicate SD. (n = 3).
Different amounts of LPLA2 were first incubated with or without liposomes under acidic conditions (Fig. 5A). In the absence of liposomes, some faint bands were found in the 16 µg LPLA2-pellet. In contrast, when using DOPC liposomes without sulfatide, the clear band corresponding to LPLA2 was detected in the 16 µg LPLA2-pellet. When using liposomes containing sulfatide, a denser LPLA2 band was observed. The band was detectable even when using 4 µg LPLA2. However, DOPC/galactosylceramide liposomes were much less effective than DOPC/sulfatide liposomes (Fig. 5B). In the next study, 16 µg of LPLA2 was treated with different conditions and analyzed by using the same method (Fig. 5C). Under neutral conditions, LPLA2 was not detected in the pellet even when LPLA2 was incubated with anionic liposomes. In contrast, when the incubation was performed under acidic conditions, LPLA2 was recovered in the liposome pellets (Fig. 5A). However, the recovery of LPLA2 in the pellet was markedly reduced by the addition of 500 mM NaCl or 33 µM AMD in the reaction mixture. The cosedimentation of LPLA2 and DOPC containing liposomes was subsequently studied under more physiologic ionic strengths. The presence of 100 mM NaCl decreased LPLA2 recovery compared with control conditions (Fig. 5D). The absence of sulfatide in the liposomes resulted in a further loss of LPLA2. The recovery of LPLA2 was also decreased when AMD was incorporated into the liposomes (Fig. 5E). Additionally, the effect was reduced with the addition of 10 mM CaCl2 into the reaction mixture (Fig. 5F, G). A corresponding decrement in LPLA2 transacylase activity was observed under these conditions (Fig. 5G). Thus the cosedimentation of LPLA2 with anionic liposomes demonstrated a good correlation with the enhancement of LPLA2 activity observed in the presence of the anionic liposomes.
When DOPC in DOPC liposomes was replaced with the di-ether-PC, 1,2-di-O-octadecenyl-sn-glycero-3-phosphocholine, the cosedimentation of LPLA2 with the liposomes was not detected (data not shown). Additionally, when 1,2-di-O-octadecenyl-sn-glycero-3-phosphocholine/sulfatide liposomes were used, a higher content of sulfatide was required to observe the cosedimentation of LPLA2 with the liposomes as seen when using DOPC/sulfatide liposomes (data not shown).
DISCUSSION
In general, the catalysis of phospholipids by either phospholipase A1 or phospholipase A2 occurs at the water-membrane interface and may be controlled via two mechanisms. These mechanisms include the binding of the enzyme to the lipid membrane followed by an interaction between the phospholipid substrate and the catalytic site with the release of the products formed by the catalytic reaction. In this study, we have demonstrated that several anionic phospholipids markedly enhance LPLA2 activity toward a zwitterionic phospholipid (Fig. 1). This observation suggested that the negative charge of anionic phospholipids is a target for the binding step and promotes the acceleration of the enzyme reaction. However, LPLA2 hydrolyzed anionic phospholipids as well as zwitterionic phospholipids. This broad substrate specificity made the interpretation of the effect of anionic phospholipids on LPLA2 binding more difficult. In addition, some of the phospholipids that enhance LPLA2 activity do not form lipid bilayers (e.g., cardiolipin and phosphatidic acid) or may destabilize a lipid bilayer based on the molar ratio of the phospholipid (e.g., BMP). In order to study the specific role of charge interactions between LPLA2 and the lipid bilayer, sulfatide, which is stable in the presence of LPLA2, was chosen as a negatively charged lipid and the effect of sulfatide on LPLA2 reaction was investigated.
The enhancement of the apparent LPLA2 activity by sulfatide was linear up to a 10% molar ratio of sulfatide to DOPC. However, the further addition of sulfatide (30% molar ratio of sulfatide to DOPC) to DOPC-liposomes did not uniformly result in the optimal effect (data not shown). This observation is consistent with the previously reported observation that phosphatidylcholine containing lipid bilayers are stable in the presence of sulfatide up to 30 mol % of the glycolipid (17). Sulfatide is known to form hexagonal aggregations and promote lipid membrane fusion in aqueous solution (18). Thus, a sufficiently high ratio of sulfatide in liposomes leads to the perturbation of the lipid bilayer structure of the liposomes. We previously reported that LPLA2 activity completely disappeared in the presence of more than critical micelle concentration (CMC) Triton X-100 (3). Thus, LPLA2 may require the lipid bilayer structure to use phospholipid as a substrate with high efficiency.
Sulfatide is an esterified galactosylceramide with a sulfate group at C3 position. Unlike sulfatide, galactosylceramide did not show a significant effect on LPLA2 activity. Therefore, the effect of sulfatide on PLA2/transacylase activity of LPLA2 could be primarily due to the negative charge conferred by the sulfate group of sulfatide molecule. These results suggest that an electrostatic interaction between LPLA2 and liposomes via the sulfate group greatly affects LPLA2 activity at the lipid-water interface but does not rule out other factors in the regulation of the phospholipase.
The enhancement of LPLA2 activity by sulfatide was markedly reduced by the addition of NaCl or AMD to the reaction mixture. An increase of Na+ ion concentration could interfere with an electrostatic interaction at the lipid-water interface in this system. Na+ ion showed a similar effect on LPLA2 activity when the soluble fraction of MDCK cells transfected with mouse LPLA2 lacking the poly-histidine tag was used as the source of enzyme. Thus, the presence of the histidine tag itself did not affect the electrostatic interaction. In addition, the drug AMD, positively charged under physiological conditions, shows a high affinity to phospholipids via hydrophobic as well as hydrophilic interactions (19). Thus, AMD could diminish the negative charge produced by anionic lipids incorporated into liposomes and, as a result, weaken the electrostatic interaction between the LPLA2 and the lipid membranes in this system. The net charge of the liposomes is positive in the presence of 33 µM AMD, increasing the positive charge of the membrane surface. Thus, the electrostatic interaction between LPLA2 and the AMD-containing liposomes is more reduced than that between LPLA2 and the DOPC/NAS-liposomes. Under the former conditions, LPLA2 activity was suppressed.
Recombinant mouse LPLA2 used in the present study showed a pH optimum of about 4.5 when the liposomes containing sulfatide were used as a substrate. Interestingly, this pH dependence curve is quite different from that of calf LPLA2 for a pH range of greater than 5. Although the calf LPLA2 showed a pH optimum of about 4.5, similar to the mouse LPLA2, substantial activity of the calf enzyme was observed up to pH 7 (3). The iso-electric points of mouse LPLA2 and mouse LPLA2 tagged with additional peptide containing six histidine residues estimated from their primary structures are 5.97 and 6.16, respectively. Thus, the recombinant mouse LPLA2 could be an anionic molecule at a pH greater than 6.16. This means that an electrostatic interaction of LPLA2 with anionic lipid membrane surface could be weakened at pH 6.16 and over. On the contrary, the iso-electric point of calf LPLA2 estimated from its primary structure is 8.3. Therefore, the calf LPLA2 may be positively charged even at pH 7. The higher iso-electric point may render the enzyme accessible to anionic lipid membrane surfaces with enzyme activity at pH 7.
LPLA2 has esterase activity. When a small artificial molecule, pNPB, was used as a substrate, the recombinant LPLA2 degraded pNPB to pNP and butyrate. In contrast to the PLA2/transacylase activity of the recombinant LPLA2, the sizable esterase activity was detected over a wide range of pH. In this experiment, pNPB was mostly dispersed in the reaction mixture as a monomer. Taken together, these results indicate that the binding of LPLA2 to lipid membranes via negatively charged lipid through an electrostatic interaction may play a crucial role in enhancing the rate of phospholipid degradation by LPLA2 at lipid-water interfaces.
A site-directed mutagenesis study demonstrated that LPLA2 could use the same catalytic site and reaction mechanism in both PLA2/transacylase and esterase reactions. In the presteady state study using pNPB under neutral conditions, the release of pNP by the enzyme was initially measured as a rapid burst and then at a slower rate. These differences in catalysis rates are consistent with the faster formation of a covalent acyl-enzyme and a slower deacylation step. Extrapolation of the rate of formation of pNP to zero time revealed that one enzyme molecule binds one pNPB molecule and releases one pNP molecule. In this case, the acyl-enzyme is expected to be a butanoyl-LPLA2 intermediate and the butanoyl group is subsequently transferred to a hydroxyl group of water or to a primary lipophilic alcohol, such as NAS, in the reaction system.
NAS is an amphiphilic compound that forms micelles in aqueous solution. The critical micelle concentration and the solubility of NAS is 5 µM and 100 µM, respectively (20). Therefore, some NAS molecules may be dispersed as either a monomer or as a component of micelles in the reaction system employed in this study (38 µM NAS). LPLA2 that recognizes pNPB dispersed as a monomer in aqueous phase forms an acyl-enzyme (butanoyl-LPLA2). NAS dispersed in an aqueous phase may feasibly interact with the acyl-enzyme intermediate. On the contrary, NAS incorporated into liposomes in the presence of AMD would have an extremely low likelihood of interacting with the acyl-enzyme as well as free enzyme in an aqueous phase because the liposomes contain levels of AMD sufficient to cancel the negative charge of the lipid membrane. Under these conditions, AMD would weaken an electrostatic interaction with the enzyme. Taken together, these results provide further support for the view that LPLA2 adsorption to the negatively charged lipid membrane surface through an electrostatic attraction enhances enzyme activity to insoluble substrates.
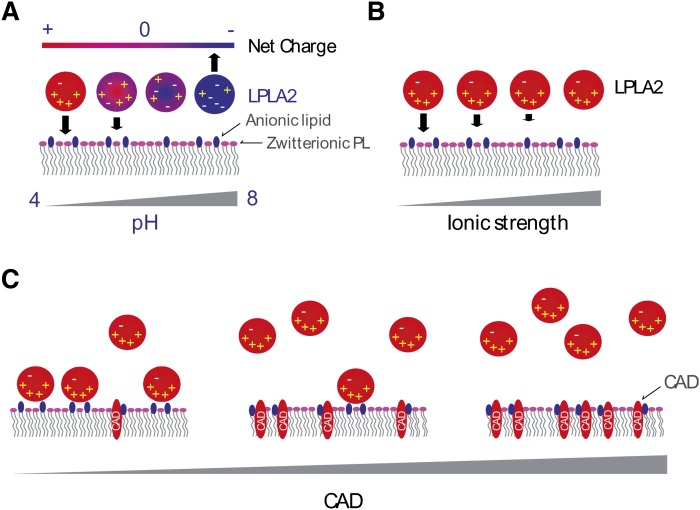
The binding of LPLA2 to the lipid membrane is a crucial step in the lipid-water interfacial reaction. For the peripheral enzymes, binding the membrane bilayer is mediated in part by binding their substrates. The cosedimentation of LPLA2 with DOPC liposomes occurred with a centrifugation at 150,000 g under acidic conditions and was enhanced by the addition of sulfatide, but not galactosylceramide, into the liposomes. The enhancement by sulfatide was markedly reduced by the addition of physiologic concentrations of NaCl or AMD in the reaction mixture. In addition, the binding of LPLA2 to the liposomes with or without sulfatide was completely abolished at the neutral pH. These results indicate that binding between LPLA2 and the anionic membrane surface is mediated in part by binding LPLA2 to its substrates but mainly by binding to negatively charged lipids. In addition, the cosedimentation of LPLA2 with the anionic liposomes showed a good correlation with enhanced LPLA2 activity. Finally, the substitution of di-ether PC showed that the carbonyl groups of DOPC within liposomes contribute to the cosedimentation of LPLA2. These results support the idea that a negatively charged lipid mediates LPLA2-lipid membrane binding through an electrostatic interaction and resulting in the amplification of the apparent LPLA2 activity (Fig. 6).
Fig. 6.
Electrostatic interactions of LPLA2 with a lipid membrane. -, +, and CAD indicate a negative charge, a positive charge, and cationic amphiphilic drug, respectively. Lipids with pink head groups and dark blue head groups denote zwitterionic and anionic phospholipids, respectively. Panels A, B, and C denote the effects of pH, ion strength, and CADs, respectively.
In a recent study, we suggested a potential link between LPLA2 and CAD-induced cellular phospholipidosis (9). In general, CADs are lipophilic amines that accumulate in acidic cellular compartments such as late endosomes and lysosomes and inhibit enzyme activities in these compartments. Some CADs induce a marked accumulation of phospholipid in human and other mammalian tissues and cells (21). This toxicity is termed phospholipidosis. To date, two mechanisms of CAD-induced phospholipidosis have been proposed. The first mechanism is that CADs bind with phospholipids and render them more resistant to phospholipase activity. The second mechanism proposes that CADs interact with phospholipases and limit their ability to affect phospholipid metabolism. However, the molecular mechanism of CAD-induced phospholipidosis remains unknown. The present study using AMD provides an alternative model for some CAD-induced phospholipidosis. In this model, CADs bind to the lipid membrane neutralizing the negative charge on membrane surface neutral and blocking an electrostatic interaction between LPLA2 and the CAD-treated membrane. As a consequence, the phospholipids in the CAD-treated membrane are protected from degradation by LPLA2.
Lysosomes play an important role in the catabolism of both endogenous (autophagy) and exogenous (phagocytosis) phospholipids. The main pathway of phospholipid catabolism is a deacylation process by phospholipase A. As described above, BMP is a specific anionic phospholipid in acidic compartments such as late endosomes and lysosomes in the cell and present in the internal membranes of the acidic compartments but not in the boundary membranes of the acidic compartments (11). BMP is more stable to LPLA2 activity than other anionic phospholipids (data not shown) and stimulates sphingolipid degradation on the internal membranes of the acidic compartment (10, 11). The present data showed that BMP markedly enhances the degradation of DOPC by LPLA2 (Fig. 1). This supports the possibility that BMP also functions as a stimulator for phospholipase A activity by LPLA2 in the acidic compartments. In addition, according to the present results, the apparent LPLA2 activity and its affinity to the zwitterionic phospholipid rich lipid membranes is thought to be much lower than that to the anionic lipid membranes. Topologically, the luminal leaflet of the acidic compartment boundary membrane is thought to be poorer in anionic phospholipids than the cytosolic leaflet. Thus, the binding of LPLA2 to the luminal leaflet of the acidic compartment boundary membrane is weakened, resulting in protection of the boundary membrane from the action by LPLA2. Sandhoff et al. (11) have proposed that the boundary membrane of the acidic compartments is protected from degradation by a thick glycocalix. The boundary membrane of the acidic compartments may be protected from attacking by LPLA2 by these two different properties. On the contrary, materials containing phospholipid membranes sorted to the acidic compartments via autophagy, phagocytotic, and endocytic pathways are brought to the luminal space or incorporated into the membranes of vesicles in the luminal space of the acidic compartments. These internal membranes provide a platform for degradation of the phospholipids sorted to the acidic compartments. When the internal membranes are rich in anionic lipids, they are conceivably accessed by LPLA2 and degraded. Gram-negative bacteria and apoptotic cells are taken up by macrophages and digested in the acidic compartments in those cells. The outer membranes of Gram-positive bacteria are rich in phosphatidylglycerol. The apoptotic cells present phosphatidylserine on their cell membrane surface. Also, mitochondria are incorporated into lysosomes via autophagy and digested. Mitochondria contain cardiolipin, which is a major anionic phospholipid of their inner membranes. Thus, LPLA2 may be associated with the catabolism of these phospholipids in acidic compartments.
The effect of negative charge at anionic lipid membrane surface has been reported for other phospholipase A2s (22). The present study demonstrates that LPLA2 shares some common properties with the type IIA secreted phospholipase A2 with respect to interfacial binding and the effects of anionic phospholipids (23, 24). Similar to type IIA secreted phospholipase A2, not only positively charged amino acid residues but also hydrophobic amino acid residues in LPLA2 molcule are thought to contribute to interactions between LPLA2 and the lipid membrane surface. Determining which specific amino acid residues mediate the lipid-water interfacial reaction by LPLA2 in the control of exogenous and endogenous phospholipid catabolism will provide important information on the role of LPLA2 in cellular biology and drug toxicity.
Footnotes
Abbreviations:
- AM
- alveolar macrophage
- AMD
- amiodarone
- BMP
- bis(monoacylglycero)phosphate
- CAD
- cationic amphiphilic drug
- DOPA
- 1,2-dioleoyl-sn-glycero-3-phosphate
- DOPC
- 1,2-dioctanoyl-sn-glycero-3-phosphocholine
- DOPEt
- 1,2-dioleoyl-sn-glycero-3-phosphoethanol
- DOPG
- 1,2-dioleoyl-sn-glycero-3-[phospho-rac-(1-glycerol)]
- DOPI
- 1,2-dioleoyl-sn-glycero-3-phosphoinositol
- DOPS
- 1,2-dioleoyl-sn-glycero-3-phosphoserine
- GalCer
- galactosylceramide
- HPTLC
- high performance thin layer chromatography
- LPLA2
- lysosomal phospholipase A2
- NAS
- N-acetylsphingosine
- pNP
- p-nitro-phenol
- pNPB
- p-nitro-phenylbutyrate
- TOCL
- 1,1', 2,2'-tetraoleoyl cardiolipin
This work was supported by a Merit Review Award from the Department of Veterans Affairs and a grant from the Michigan Economic Development Corporation.
REFERENCES
- 1.Lubke T., Lobel P., Sleat D. E. 2008. Proteomics of the lysosome. Biochim Biophys Acta. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abe A., Shayman J. A., Radin N. S. 1996. A novel enzyme that catalyzes the esterification of N-acetylsphingosine. Metabolism of C2-ceramides. J. Biol. Chem. 271: 14383–14389. [DOI] [PubMed] [Google Scholar]
- 3.Abe A., Shayman J. A. 1998. Purification and characterization of 1-O-acylceramide synthase, a novel phospholipase A2 with transacylase activity. J. Biol. Chem. 273: 8467–8474. [DOI] [PubMed] [Google Scholar]
- 4.Hiraoka M., Abe A., Shayman J. A. 2002. Cloning and characterization of a lysosomal phospholipase A2, 1-O-acylceramide synthase. J. Biol. Chem. 277: 10090–10099. [DOI] [PubMed] [Google Scholar]
- 5.Abe A., Hiraoka M., Shayman J. A. 2007. The acylation of lipophilic alcohols by lysosomal phospholipase A2. J. Lipid Res. 48: 2255–2263. [DOI] [PubMed] [Google Scholar]
- 6.Abe A., Kelly R., Kollmeyer J., Hiraoka M., Lu Y., Shayman J. A. 2008. The secretion and uptake of lysosomal phospholipase A2 by alveolar macrophages. J. Immunol. 181: 7873–7881. [DOI] [PubMed] [Google Scholar]
- 7.Abe A., Hiraoka M., Wild S., Wilcoxen S. E., Paine R., 3rd, Shayman J. A. 2004. Lysosomal phospholipase A2 is selectively expressed in alveolar macrophages. J. Biol. Chem. 279: 42605–42611. [DOI] [PubMed] [Google Scholar]
- 8.Hiraoka M., Abe A., Lu Y., Yang K., Han X., Gross R. W., Shayman J. A. 2006. Lysosomal phospholipase A2 and phospholipidosis. Mol. Cell. Biol. 26: 6139–6148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abe A., Hiraoka M., Shayman J. A. 2007. A role for lysosomal phospholipase A2 in drug induced phospholipidosis. Drug Metab. Lett. 1: 49–53. [DOI] [PubMed] [Google Scholar]
- 10.Matsuo H., Chevallier J., Mayran N., Le Blanc I., Ferguson C., Faure J., Blanc N. S., Matile S., Dubochet J., Sadoul R., et al. 2004. Role of LBPA and Alix in multivesicular liposome formation and endosome organization. Science. 303: 531–534. [DOI] [PubMed] [Google Scholar]
- 11.Kolter T., Sandhoff K. 2006. Sphingolipid metabolism diseases. Biochim. Biophys. Acta. 1758: 2057–2079. [DOI] [PubMed] [Google Scholar]
- 12.Mingeot-Leclercq M. P., Laurent G., Tulkens P. M. 1988. Biochemical mechanism of aminoglycoside-induced inhibition of phosphatidylcholine hydrolysis by lysosomal phospholipases. Biochem. Pharmacol. 37: 591–599. [DOI] [PubMed] [Google Scholar]
- 13.Piret J., Schanck A., Delfosse S., Van Bambeke F., Kishore B. K., Tulkens P. M., Mingeot-Leclercq M. P. 2005. Modulation of the in vitro activity of lysosomal phospholipase A1 by membrane lipids. Chem. Phys. Lipids. 133: 1–15. [DOI] [PubMed] [Google Scholar]
- 14.Hara A., Radin N. S. 1979. Simple procedures for the rapid cleavage of ester lipids and for the large-scale isolation from brain of cerebroside sulfate. Anal. Biochem. 100: 364–370. [DOI] [PubMed] [Google Scholar]
- 15.Taniyama Y., Shibata S., Kita S., Horikoshi K., Fuse H., Shirafuji H., Sumino Y., Fujino M. 1999. Cloning and expression of a novel lysophospholipase which structurally resembles lecithin cholesterol acyltransferase. Biochem. Biophys. Res. Commun. 257: 50–56. [DOI] [PubMed] [Google Scholar]
- 16.Hiraoka M., Abe A., Shayman J. A. 2005. Structure and function of lysosomal phospholipase A2: identification of the catalytic triad and the role of cysteine residues. J. Lipid Res. 46: 2441–2447. [DOI] [PubMed] [Google Scholar]
- 17.Cestaro B., Marchesini S., Cervato G., Viani P., Vesely S. 1984. Bilayer-micelle transition in phosphatidylcholine-sulfatide mixtures. Ital. J. Biochem. 33: 381–391. [PubMed] [Google Scholar]
- 18.Wu X., Li Q. T. 1999. Ca2+-induced fusion of sulfatide-containing phosphatidylethanolamine small unilamellar vesicles. J. Lipid Res. 40: 1254–1262. [PubMed] [Google Scholar]
- 19.Nonoyama T., Fukuda R. 2008. Drug-induced phospholipidosis-pathological aspects and its prediction. J. Toxicol. Pathol. 21: 9–24. [Google Scholar]
- 20.Sot J., Goni F. M., Alonso A. 2005. Molecular associations and surface-active properties of short- and long-N-acyl chain ceramides. Biochim. Biophys. Acta. 1711: 12–19. [DOI] [PubMed] [Google Scholar]
- 21.Reasor M. J., Kacew S. 2001. Drug-induced phospholipidosis: are there functional consequences? Exp. Biol. Med. (Maywood). 226: 825–830. [DOI] [PubMed] [Google Scholar]
- 22.Buckland A. G., Wilton D. C. 2000. Anionic phospholipids, interfacial binding and the regulation of cell functions. Biochim. Biophys. Acta. 1483: 199–216. [DOI] [PubMed] [Google Scholar]
- 23.Canaan S., Nielsen R., Ghomashchi F., Robinson B. H., Gelb M. H. 2002. Unusual mode of binding of human group IIA secreted phospholipase A2 to anionic interfaces as studied by continuous wave and time domain electron paramagnetic resonance spectroscopy. J. Biol. Chem. 277: 30984–30990. [DOI] [PubMed] [Google Scholar]
- 24.Bezzine S., Bollinger J. G., Singer A. G., Veatch S. L., Keller S. L., Gelb M. H. 2002. On the binding preference of human groups IIA and X phospholipases A2 for membranes with anionic phospholipids. J. Biol. Chem. 277: 48523–48534. [DOI] [PubMed] [Google Scholar]