Abstract
Peroxisome proliferator-activated receptor delta (PPARδ) is involved in regulation of energy homeostasis. Activation of PPARδ markedly increases fecal neutral sterol secretion, the last step in reverse cholesterol transport. This phenomenon can neither be explained by increased hepatobiliary cholesterol secretion, nor by reduced cholesterol absorption. To test the hypothesis that PPARδ activation leads to stimulation of transintestinal cholesterol efflux (TICE), we quantified it by intestine perfusions in FVB mice treated with PPARδ agonist GW610742. To exclude the effects on cholesterol absorption, mice were also treated with cholesterol absorption inhibitor ezetimibe or ezetimibe/GW610742. GW601742 treatment had little effect on plasma lipid levels but stimulated both fecal neutral sterol excretion (∼200%) and TICE (∼100%). GW610742 decreased intestinal Npc1l1 expression but had no effect on Abcg5/Abcg8. Interestingly, expression of Rab9 and LIMPII, encoding proteins involved in intracellular cholesterol trafficking, was increased upon PPARδ activation. Although treatment with ezetimibe alone had no effect on TICE, it reduced the effect of GW610742 on TICE. These data show that activation of PPARδ stimulates fecal cholesterol excretion in mice, primarily by the two-fold increase in TICE, indicating that this pathway provides an interesting target for the development of drugs aiming at the prevention of atherosclerosis.
Keywords: intestine, transintestinal cholesterol efflux, reverse cholesterol transport
The peroxisome proliferator-activated receptor (PPAR) family belongs to the superfamily of nuclear receptors and consists of three known isoforms: PPARα, PPARβ/δ, and PPARγ (1). PPARα modulates various aspects of lipid metabolism (2–5) and is highly expressed in heart, liver, kidney, intestine, brown fat, i.e., organs that display high rates of fatty acid β-oxidation (6). PPARγ is expressed mainly in adipose tissue and affects adipocyte differentiation and energy metabolism, particularly the PPARγ2 isomer (7–10). PPARδ is ubiquitously expressed. High levels of PPARδ transcripts are detected in the brain, kidney, small intestine, and Sertoli cells (11). The role of PPARδ remained elusive for a long time until the successful generation of Pparδ−/− mice. Two groups independently created Pparδ−/− mouse lines (12, 13). Pparδ−/− mice were smaller from the fetal stage onwards and showed an increased prenatal death rate due to placental defects. Brain defects, limited adipose stores, and hyperplasia of the epidermis have been detected in these mice. PPARδ also plays a prominent role in fatty acid oxidation, energy dissipation, and mitochondrial respiration (14, 15). Adipose tissue-specific overexpression of PPARδ in mice protected these mice against high fat-induced obesity. In addition, db/db mice did not develop obesity upon treatment with a PPARδ agonist, GW501516 (15). Overexpression of PPARδ in skeletal muscle of mice led to a remarkable increase in energy endurance capacity (16). PPARδ activation of rat myotubes caused a prominent upregulation of mRNA levels of pyruvate dehydrogenase kinase 4 (PDK4). Treatment with a PPARδ agonist also improved insulin resistance induced by a high-fat diet in mice (14). So, apparently, PPARδ couples fatty acid metabolism with glucose metabolism. Takahashi et al. (17) suggested that the physiological role of PPARδ may be a direct switch from glucose metabolism to fatty acid metabolism.
Besides the roles of PPARδ in fatty acid and glucose homeostasis, PPARδ activation is also suggested to affect reverse cholesterol transport (RCT). PPARδ activation leads to increased levels of HDL cholesterol in primates and humans and enhanced apolipoprotein (Apo)A-I specific cholesterol efflux from THP1 human monocyte cells (18, 19). Van der Veen et al. (20) showed that PPARδ activation resulted in increased fecal neutral sterol output, whereas hepatobiliary cholesterol secretion was unaltered. PPARδ activation in wild-type DBA1 mice led to reduced cholesterol absorption efficiencies. This reduced absorption seemed to be caused by the attenuated expression of the gene encoding the Niemann-Pick C1-like 1 protein (NPC1L1) that mediates cholesterol absorption (21). Although cholesterol absorption was reduced, it could not fully explain the increase in fecal neutral sterol secretion. Recently, our group showed that direct transintestinal cholesterol efflux (TICE) represents a major pathway for the excretion of cholesterol in mice (22). Because PPARδ activation led to an increase in fecal neutral sterol secretion that could not be accounted for by an increase in hepatobiliary cholesterol secretion nor solely by reduction of cholesterol absorption, we hypothesized that PPARδ activation leads to stimulation of TICE.
MATERIALS AND METHODS
Animals and diet
Male FVB mice (2–4 months) were purchased from Harlan (Horst, The Netherlands). Mice ( n = 7 for each group) received a reference diet (20% casein, ref no 4068.02, Arie Blok BV, The Netherlands), a diet containing PPARδ agonist [reference diet supplemented with 0.017% (w/w) GW610742, resulting in an approximate intake of 20 mg/kg/day based on an average food intake of 3 g], a diet containing ezetimibe (reference diet supplemented with Ezetrol, Schering-Plough, Utrecht, The Netherlands; 10 mg/kg/day), or a reference diet supplemented with ezetimibe (10 mg/kg/day) and the PPARδ agonist GW610742 [0.017% (w/w)] for 2 weeks. The specificity of GW610742 has been described previously (20). The ezetimibe, GW610742 and ezetimibe + GW610742 diets were made by mixing ezetimibe and/or GW610742 with powdered reference diet. Reference diet contained 0.006% cholesterol. Food and water were supplied ad libitum. Mice were maintained on a 24 h light-dark cycle and weighed every morning. All experiments were performed with the approval of the local Ethical Committee for Animal Experiments.
Cholesterol intake and output measurements
Daily food intake and fecal neutral sterol excretion of all mice were measured for a period of 3 days. Mice were housed in normal mouse cages, up to five mice per cage, to mimic their natural situation as much as possible. On day 0, the mice and diet were weighed. On day 3, the mice and remaining diet were weighed and feces were collected per cage. Fecal neutral sterols were determined as described below.
Intestine perfusion procedures
After 2 weeks of diet, mice were anesthetized intraperitoneally with 0.1 ml FFD [Hypnorm (fentanyl/fluanisone; 1 ml/kg) and diazepam (10 mg/kg)] / 5 g body weight and placed on a heat pad to maintain body temperature. The bile duct was cannulated via the gallbladder to divert biliary cholesterol. Bile flow was determined gravimetrically assuming a density of 1 g/ml. The first 15 min fraction was used to measure biliary cholesterol secretion. Proximal small intestines (first 10 cm) were perfused because, under normal conditions, the highest TICE rate is observed in the proximal part of the small intestine (22). Perfusions were performed as previously described (22). Perfusate fractions were stored at −20°C until further analysis. At the end of the perfusion period, blood was collected by cardiac puncture and plasma was obtained by means of centrifugation (3000 rpm, 5 min). Plasma was stored at −80°C. The perfused intestinal segment was cut into three equal pieces and those pieces were cut in two equal parts. The intestinal segments were snap-frozen in liquid nitrogen. Intestinal segments and plasma were stored at −80°C before further analysis.
Perfusion fluid composition
Perfusions were carried out with a modified Krebs solution (119.95 mM NaCl, 4.8 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4·7H2O, 15 mM HEPES, 1.3 mM CaCl2·2H2O, and 10 mM L-glutamine; final pH 7.4) supplemented with 10 mM taurocholate (TC; Sigma, Zwijndrecht, The Netherlands): 2 mM L- α -phosphatidylcholine (PC; Sigma). TC/PC mixtures were made as follows: TC dissolved in methanol and egg yolk and PC dissolved in chloroform were mixed and solvents were evaporated under a mild stream of nitrogen at 45°C. After evaporation, films were lyophilized overnight. Lyophilized samples were stored under nitrogen gas at −20°C until the day of the intestine perfusions. Before the start of the intestine perfusions, the micelles were dissolved in perfusion buffer (room temperature).
Determination of mRNA levels
Total RNA was isolated from perfused intestinal segments using the Trizol reagent according to the manufacturer's protocol (Invitrogen, Breda, The Netherlands). Purified RNA was treated with RQ1 RNase-free DNase (1 unit/2 µg of total RNA; Promega, Leiden, The Netherlands) and reverse transcribed with SuperScript II Reverse Transcriptase (Invitrogen,) according to the protocols supplied by the manufacturers. Gene expression analysis was performed by use of SYBR green. Quantitative gene expression analysis was performed on a Bio-Rad MyiQ Single-Color Real-Time PCR Detection System (Bio-Rad, Veenendaal, The Netherlands). PCR primers were designed on the basis of Primer Express 1.7 software with the manufacturer's default settings (Applied Biosystems, Foster City, CA) or found by consulting the PrimerBank website (http://pga.mgh.harvard.edu/primerbank) and validated for identical efficiencies (Table 1). Hypoxanthine-guanine phosphoribosyl transferase (HPRT), cyclophilin, and acidic ribosomal phosphoprotein P0 (36B4) were used as standard housekeeping genes.
TABLE 1.
Real-time PCR primers
| Gene | Accession Number | Forward Primer | Reverse Primer | Size |
|---|---|---|---|---|
| 36B4 | NM_007475 | GGACCCGAGAAGACCTCCTT | GCACATCACTCAGAATTTCAATGG | 85 |
| ABCA1 | NM_013454 | GGTTTGGAGATGGTTATACAATAGTTGT | TTCCCGGAAACGCAAGTC | 96 |
| ABCG5 | NM_031884 | TGGCCCTGCTCAGCATCT | ATTTTTAAAGGAATGGGCATCTCTT | 81 |
| ABCG8 | NM_026180 | CCGTCGTCAGATTTCCAATGA | GGCTTCCGACCCATGAATG | 67 |
| Cyclophilin | M60456 | TCGGAGCGCAATATGAAGGT | AAAAGGAAGACGACGGAGCC | 65 |
| HMG-CoA Reductase | M62766 | TCTGGCAGTCAGTGGGAACTATT | CCTCGTCCTTCGATCCAATTT | 69 |
| HPRT | J00423 | TTGCTCGAGATGTCATGAAGGA | AGCAGGTCAGCAAAGAACTTATAG | 91 |
| NPC1L1 | AY437866 | GAGAGCCAAAGATGCTACTATCTTCA | CCCGGGAAGTTGGTCATG | 72 |
| Pdk4 | NM_013743 | GGGGGTGAACTGGTAGATTT | GCACCTTAGCTCTAGGTCA | 191 |
| SCD-1 | NM_009127 | TACTACAAGCCCGGCCTCC | CAGCAGTACCAGGGCACCA | 65 |
| SR-B1 | NM_016741 | GGCTGCTGTTTGCTGCG | GCTGCTTGATGAGGGAGGG | 63 |
| LIMP2 (SR-BII) | NM_007644 | AGAAGGCGGTAGACCAGAC | GTAGGGGGATTTCTCCTTGGA | 159 |
| Rab9 | NM_019773 | TACGCAGATGTGAAAGAGCCT | AAAGTAAGGATAGTCGCCGTTG | 132 |
Lipoprotein analysis
Total cholesterol content of the main lipoprotein classes, VLDL, LDL, and HDL, was determined using high performance gel-filtration chromatography (HPGC) as described previously with some minor modifications (23). In brief, the system contained a PU-980 ternary pump with an LG-980-02 linear degasser, FP-920 fluorescence and UV-975 UV/VIS detectors (Jasco, Tokyo, Japan). An extra PU1500 pump (Jasco) was used for in-line cholesterol PAP enzymatic reagent (Biomerieux, Marcy l'Etoile, France) addition (flowrate; 0.1 ml/min). Plasma lipoprotein separations were performed with a Superose 6 HR 10/30 column (Pharmacia Biotech, Uppsala, Sweden) with TBS pH 7.4 as eluent (flow rate; 0.31 ml/min). Computer analysis of the chromatograms was carried out using Chrompass Chromatographic software, version 1.0 (Jasco).
Apolipoprotein composition analysis of plasma lipoproteins
For analysis of apolipoproteins, pooled plasma samples from control and GW610742-treated animals were fractionated by the aforementioned HPGC (flowrate 0.5 ml/min; fractions collected at a 1-min interval). The lipoprotein profile spanned the fractions 3–19. Equal volumes (20 µl) of these fractions were separated on 4–12% gradient gels (Invitrogen, Carlsbad, CA) and the proteins were transferred to nitrocellulose membranes (Protran BA, Whatman, Germany). The blots were blocked in Starting Block buffer (Pierce, Rockford, IL) and probed with rabbit anti-mouse ApoB and ApoA1 (prepared at TNO-Biosciences; kind gift from Jitske de Vries- Van der Weij), and goat anti-mouse ApoE (Santa Cruz, Heerhugowaard, The Netherlands; sc-6384) in block buffer containing 0.05% (v/v) Tween-20, overnight at 4°C. Blots were washed for 30 min in TBS (10 mM Tris-HCl (pH 8.0), 150 mM NaCl) containing 0.01% (v/v) Tween-20. Proteins were detected with an infrared fluorescence detection system and secondary antibodies (Odyssey and IRDye 680 antibody, LI-COR Biosciences, Germany).
Analytical procedures
Lipids of perfusate and bile were extracted using the Bligh and Dyer method (24). Biliary, perfusate, and hepatic cholesterol were measured by a fluorescent method as described previously (25).Total cholesterol concentrations of complete plasma samples were determined using the cholesterol RTU kit from Biomerieux. Triglycerides in plasma and liver homogenates were determined using the Triglycerides Ecoline S+ kit (Diagnostic Systems GmbH, Holzheim, Germany). Biliary and hepatic phospholipids were measured enzymatically by a method described previously (26). Bile acids in bile were measured by a method described earlier by Turley and Dietschy (27). For fecal neutral sterol analysis, 1 day fecal samples were collected, lyophilized, and ground. Samples were prepared for fecal neutral sterol analysis by gas chromatography as described previously (28).
Statistics
All results are presented as mean ± SD. Group means for TICE, as depicted in the figures, were calculated by averaging the outcomes of all mice that got the same treatment. The values for the individual mice, used for the calculation of the group mean, were obtained by averaging the data of the last three perfusion time points. Differences between different groups were determined by one-way ANOVA. Outcomes of P < 0.05 were considered to be significant. Analysis was performed using SPSS.
RESULTS
To establish the effect of PPARδ activation on cholesterol metabolism, four groups of FVB mice received either a semi-purified reference diet, or the same diet supplemented with ezetimibe, or the PPARδ agonist GW610742, or both ezetimibe and GW610742. Mice were given these diets for 2 weeks. After the first week, food intake and fecal neutral sterol output were measured and after the second week, the proximal small intestine was perfused to quantify TICE. Table 2 shows that GW610742 treatment stimulated fecal neutral sterol excretion. Food intake was monitored during the same period as fecal neutral sterol excretion was measured. Surprisingly, the GW610742 treated mice ate 1.7-fold more than control mice did. Interestingly, this effect was abolished in the presence of ezetimibe.
TABLE 2.
Effect of GW610742 on fecal neutral sterol excretion and food intake
| Ref. Diet | GW610742 | Ezetimibe | Ezetimibe + GW610742 | |
|---|---|---|---|---|
| Fecal neutral sterol secretion(µmol/day.100g) | 6.6 ± 0.5 | 18.0 ± 1.2a | 40.9 ± 6.5a | 48.0 ± 3.4 |
| Food intake(g/day.100g) | 15.0 ± 2.4 | 26.1 ± 4.5a | 16.1 ± 1.8 | 15.6 ± 0.9 |
Values are expressed as means ± SD.
Indicates a significant effect of GW610742 in comparison to control treated animals.
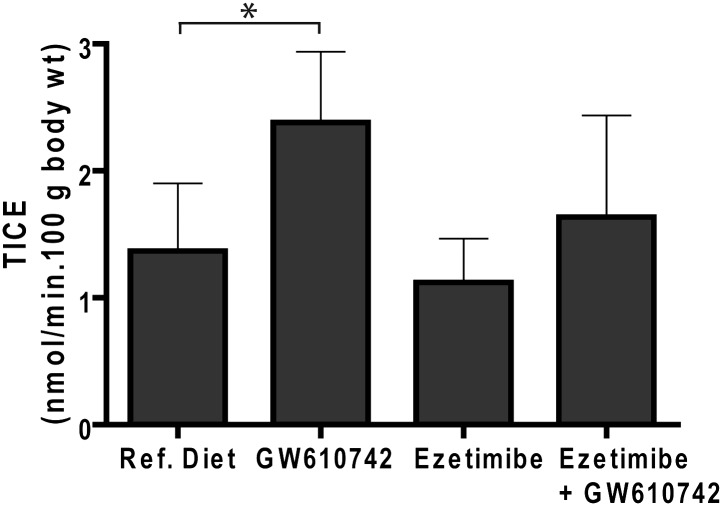
PPARδ activation by GW610742 treatment stimulated TICE significantly (Fig. 1) (2.39 ± 0.56 versus 1.37 ± 0.53 nmol cholesterol/min.100 g body wt, P < 0.05). In combination with ezetimibe, the stimulatory effect of GW610742 on TICE was not significant (1.64 ± 0.80 versus 1.12 ± 0.34 nmol cholesterol/min.100 g body wt). Interestingly, ezetimibe alone did not affect TICE in FVB mice.
Fig. 1.
Effect of GW610742 treatment on intestinal cholesterol efflux. FVB mice (n = 7 per group) received casein diet, a GW610742 diet (0.017% w/w), an ezetimibe diet (10 mg/kg/day), or an ezetimibe + GW610742 diet for 2 weeks. Proximal small intestinal perfusions were performed, using TC/PC (10:2 mM) as cholesterol acceptor. Values are expressed as means SD. * indicates a significant effect of GW610742 in comparison to control treated animals.
As reported earlier, GW610742 treatment caused an increase (50% to 60%) in liver weight as compared with the liver weights of the corresponding control mice (Table 3) (20, 29).This increase was not caused by the accumulation of lipids because cholesterol, phospholipids, and triglyceride levels did not change when expressed per mg liver protein (20). GW610742 treatment led to a significant increase in bile flow and bile salt secretion rate but had no effect on biliary cholesterol secretion or phospholipid secretion (Table 4), Ezetimibe alone also induced bile salt secretion but displayed no synergistic effect when given with GW610742.
TABLE 3.
Liver parameters
| Ref. Diet | GW610742 | Ezetimibe | Ezetimibe+ GW610742 | |
|---|---|---|---|---|
| Liver weight (% of body weight) | 4.5 ± 0.2 | 6.7 ± 0.4a | 4.3 ± 0.4 | 6.7 ± 0.4a |
| Hepatic triglycerides (nmol/mg liver) | 9.4 ± 6.2 | 5.2 ± 2.7 | 13.6 ± 5.1 | 8.0 ± 4.4 |
| Hepatic cholesterol (nmol/mg liver) | 7.5 ± 0.7 | 6.1 ± 2.2 | 7.8 ± 1.3 | 7.1 ± 1.9 |
| Hepatic phospholipids (nmol/mg liver) | 24.5 ± 3.0 | 24.4 ± 8.6 | 23.7 ± 2.3 | 29.3 ± 5.5 |
Values are expressed as means ± SD.
Indicates a significant difference between the GW610742 treated animals and their corresponding controls.
TABLE 4.
Bile flow and secretion rates
| Ref. Diet | GW610742 | Ezetimibe | Ezetimibe+ GW610742 | |
|---|---|---|---|---|
| Bile flow (µl/min.g liver) | 0.9 ± 0.6 | 2.7 ± 0.6a | 1.2 ± 0.5 | 2.6 ± 0.8a |
| Bile salts (nmol/min.100g body wt) | 69 ± 28 | 163 ± 79a | 191 ± 74 | 132 ± 79a |
| Cholesterol (nmol/min.100g body wt) | 0.9 ± 0.3 | 0.8 ± 0.4 | 0.9 ± 0.3 | 0.5 ± 0.2a |
| Phospholipids (nmol/min.100g body wt) | 8.3 ± 1.3 | 8.7 ± 4.4 | 12.2 ± 3.9 | 7.7 ± 4.0 |
| Cholesterol / phospholipids ratio | 0.11 ± 0.02 | 0.10 ± 0.04 | 0.08 ± 0.01 | 0.07 ± 0.02 |
Values are expressed as means ± SD.
Indicates a significant difference between the GW610742 treaded animals and their corresponding controls.
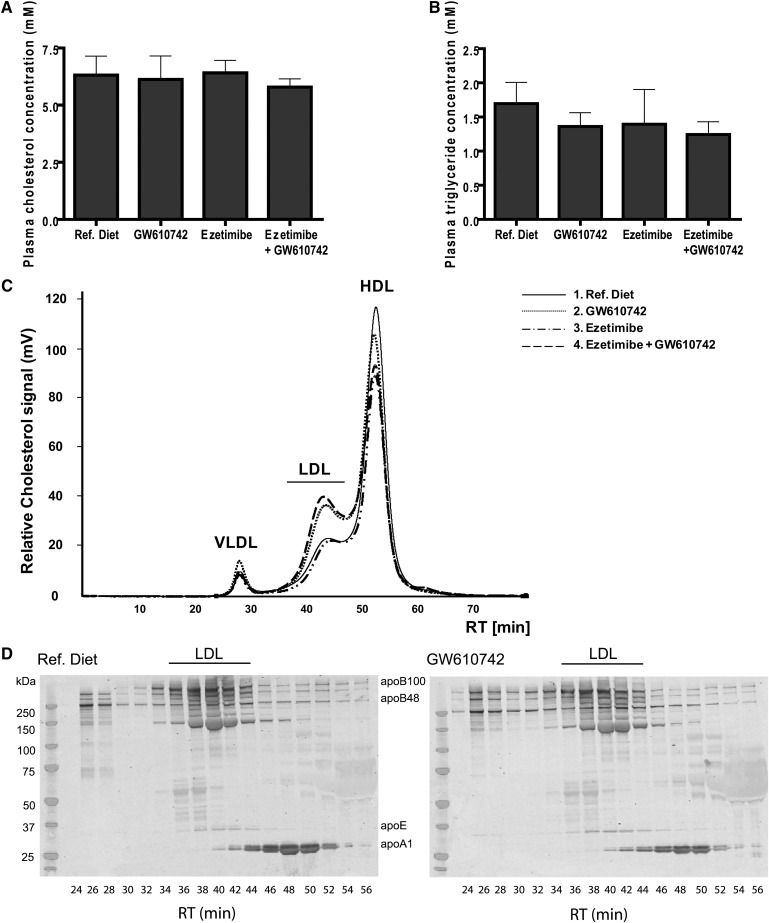
Total plasma cholesterol and triglyceride levels did not change upon GW610742 treatment (Fig. 2A, B). To evaluate whether GW610742 treatment resulted in changes in cholesterol concentrations in the different lipoproteins, HPGC was performed (Fig. 2C). In addition, the apolipoprotein composition of lipoproteins in the plasma of the GW610742 treated and the nontreated mice was determined (Fig. 2D). After fractionating the plasma samples, the presence of ApoA1, ApoB, and ApoE was visualized by Western blotting. GW610742 induced a shift of cholesterol from HDL toward the ApoB-containing LDL-fraction.
Fig. 2.
Plasma analysis of GW610742 treated mice. FVB mice (n = 7 per group) received casein diet, a GW610742 diet (20 mg/kg/day), an ezetimibe diet (10 mg/kg/day) or an ezetimibe + GW610742 diet for 2 weeks. Blood was collected and plasma was obtained by centrifugation. A: Total plasma cholesterol levels and (B) plasma triglyceride levels were measured. C: Lipoproteins were separated by HPGC combined with subsequent in-line total cholesterol determination. D: Fractions obtained by HPGC were subjected to Western blot. Via detection of ApoA1, ApoB, and ApoE, the apolipoprotein composition of the lipoprotein profiles was determined.
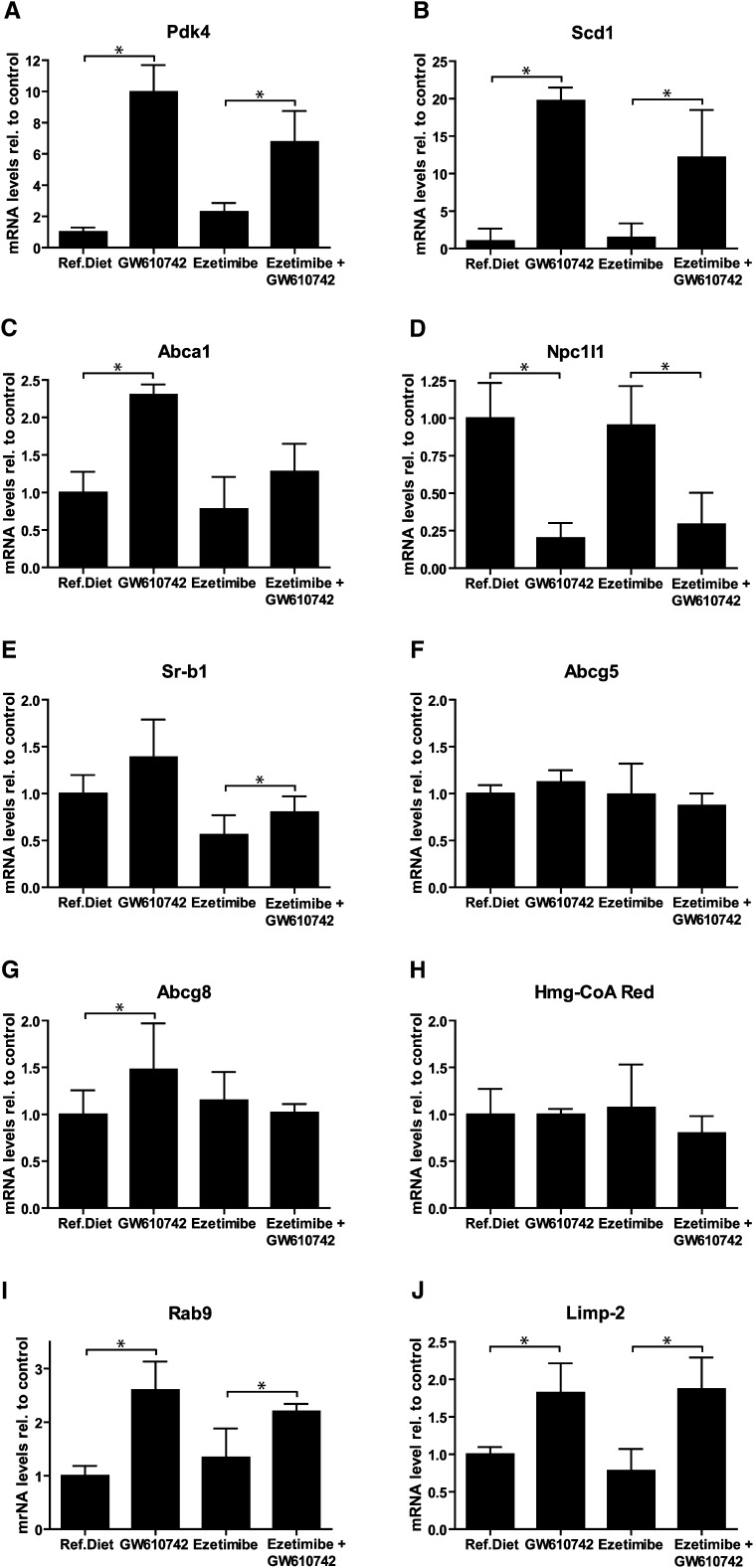
Intestinal gene expression analysis was performed to establish the role of PPARδ activation on the expression of genes that are involved in cholesterol transport as well as known PPARδ target genes (Fig. 3). The classic PPARδ target Pdk4 was induced about 10-fold whereas stearoyl-CoA desaturase 1 expression was induced even more, about 20-fold. The expression of the cholesterol efflux protein Abca1 was increased and Npc1l1 expression was reduced upon PPARδ activation, as described previously (20). Sr-bI expression was not altered upon GW610742 treatment, but ezetimibe significantly decreased Sr-b1 expression. Abcg5, Abcg8, and Hmg-CoA reductase were not affected by GW610742, nor did ezetimibe affect the expression of these genes. Because the NPC1L1 mediated cholesterol transport involves intracellular vesicle trafficking (30), we also determined expression of genes known to be involved in this pathway (31, 32). Interestingly, expression of Rab9 and Limp-2 was increased upon GW610742 treatment.
Fig. 3.
Intestinal gene expression analysis of GW610742 treated mice. FVB mice (n = 7 per group) received casein diet, a GW610742 diet (20 mg/kg/day), an ezetimibe diet (10 mg/kg/day) or an ezetimibe + GW610742 diet for 2 weeks. Proximal small intestinal perfusions were performed, using TC/PC (10:2 mM) as cholesterol acceptor. At the end of the 2 h perfusion period, intestines were collected cut into three equal segments. The middle segment was analyzed. As control genes cyclophilin, 36B4 and HPRT were used. Values are expressed as means SD. * indicates a significant effect of GW610742 in comparison to control treated animals.
DISCUSSION
In this study, we show for the first time that increased fecal neutral sterol output in FVB mice upon PPARδ activation by GW610742 is largely due to stimulation of TICE. In an earlier study, Van der Veen et al. (20) demonstrated that treatment of DBA1 mice with GW610742 decreased expression of Npc1l1, contributing to the observed increase in fecal neutral sterol excretion. To control for the influence of NPC1L1, we tested the effect of GW610742 with and without ezetimibe, an established inhibitor of NPC1L1. As shown previously, treatment of mice with ezetimibe alone has a substantial effect on neutral sterol excretion (33). Part can be explained by inhibition of cholesterol absorption, but because biliary cholesterol secretion is not affected, the question arises, from where this cholesterol originates. However, despite the strong increase of fecal neutral sterol excretion, ezetimibe treatment did not affect TICE. On the one hand, this result shows that NPC1L1 does not play an (inhibiting) role in TICE. On the other hand, TICE is not involved in the ezetimibe-induced strong increase in neutral fecal sterol excretion and another pathway must be responsible. In contrast to the effect of ezetimibe, treatment of the mice with GW610742 alone stimulated TICE, which subsequently contributed to the increase of fecal neutral sterol excretion. Because ezetimibe has no effect on TICE, similar results were expected upon concurrent treatment with GW610742. However, GW610742 treatment in the presence of ezetimibe showed no significant increase of TICE. It could be that ezetimibe interferes with intestinal uptake of GW610742 but also a possible intervention of ezetimibe on the PPARδ-activated pathways cannot be ruled out.
GW610742-treated animals ate significantly more than control animals although they did not differ in body weight (not shown). An increased food consumption has also been observed in Ldl receptor knockout (Ldlr−/−) mice treated with PPARδ agonist GW610742 (34). In that study, however, the GW610742-treated Ldlr−/− mice showed a significant increase in body weight as compared with Ldlr−/− mice on a control diet. Muscle-specific PPARδ transgenic animals had enhanced physical activity, however, they did not differ in body weight as compared with wild-type mice (16). An effect of PPARδ activation on energy expenditure might explain why GW610742-treated mice were able to maintain body weight with increased food intake. Surprisingly, the stimulating effect of PPARδ activation by GW610742 treatment on food consumption was not observed when ezetimibe was given at the same time. The recently reported inhibitory effect of ezetimibe on absorption of fatty acids (35) may play a role although we can also not exclude that ezetimibe might attenuate the uptake of GW610742. Additionally, treatment with GW610742 caused an increase in liver weight, which has been reported before and was not caused by accumulation of liver lipids (20). A recent report attributed this induction in liver weight to peroxisome proliferation (29).
PPARδ activation led to a higher bile flow and a higher bile salt secretion rate both with and without ezetimibe treatment (Table 3). This has not been observed previously in DBA1 wild-type mice (20). Cholesterol and other biliary parameters were unaffected upon GW610742 treatment, as observed in DBA1 (20). In addition, total plasma cholesterol and triglycerides levels were unaltered upon PPARδ activation (Fig. 2A) as has been observed previously in Ldlr−/− mice treated with GW610742 (34). In fact, GW610742 treatment in Ldlr−/− mice did not affect plasma lipoprotein profiles. Subsequent cholesterol and apolipoprotein analysis of the lipoprotein profiles in FVB mice revealed a shift from HDL to LDL in both GW610742-treated groups. In contrast, earlier studies in monkeys and humans reported increased HDL cholesterol levels upon PPARδ activation (18, 19). Also in DBA1 mice, PPARδ activation resulted in higher HDL levels (20). Whether our observed change in lipoprotein profiles in FVB mice reflects a strain-specific difference and whether this change in plasma cholesterol distribution might affect TICE need to be investigated.
To get more insight in genes potentially involved in upregulation of TICE, analysis of intestinal gene expression was performed (Fig. 3). Confirming earlier results, the expression of Npc1l1 was decreased in GW610742-treated mice (20). Conversely, expression of Abca1was increased whereas that of Sr-b1 was not affected. Expression of the cholesterol effluxing heterodimer Abcg5/g8 was not influenced. This came as no surprise because we have shown previously that these genes do not control the rate of TICE under the conditions used in this study (22). Ezetimibe treatment decreased the effect of GW610742 on its known target gene Pdk4, suggesting that indeed ezetimibe influences uptake of the agonist. To further investigate the underlying mechanism of PPARδ-induced TICE activation, we also studied the effect of GW610742 treatment on expression of genes that have been linked to the intracellular vesicular transport of cholesterol, namely, Rab8, Rab9, and LIMPII. Rab8 is involved in the regulatory machinery that leads to ABCA1-dependent removal of cholesterol from endocytic circuits (31). Rab9 plays a role in cholesterol trafficking from late endosomes to the trans-Golgi network (32, 36). Also LIMPII, previously referred to as LPG58, has specific functions in intracellular vesicular trafficking (37). Interestingly, LIMPII is the mouse ortholog of human scavenger receptor class B type 2 (Scarb2; MIM* 602257) which has been shown to influence cholesterol homeostasis (38). Intestinal Rab8 expression was unaltered upon PPARδ actvation (data not shown). In contrast, expression of Rab9 and Limp-II was increased significantly. How the proteins encoded by these genes are related to TICE is not yet clear and requires further study.
In earlier studies, PPARδ activation has been shown to increase fecal sterol secretion without affecting the rate of biliary cholesterol secretion (20). The positive effect of PPARδ on cholesterol secretion, which suggests an accelerated RCT, is not confined to mice but has also been demonstrated to occur in primates and humans (18, 19). Here, we show that the effect of PPARδ on neutral sterol excretion is in part mediated by stimulation of TICE. This effect is in line with the stimulatory effect of a high-fat diet on TICE described recently (39). Activation of PPARδ by fatty acid influx into enterocytes may induce TICE. Given the role of RCT in atherosclerosis, we speculate that TICE may be a promising target for the development of novel drugs to prevent or treat cardiovascular diseases.
Footnotes
Abbreviations:
- Apo
- apolipoprotein
- HPGC
- high performance gel-filtration chromatography
- HPRT
- hypoxanthine-guanine phosphoribosyl transferase
- Ldlr−/−
- Ldl receptor knockout
- NPC1L1
- Niemann-Pick C1-like 1
- PC
- phosphatidylcholine
- PPAR
- peroxisome proliferator-activated receptor
- RCT
- reverse cholesterol transport
- TC
- taurocholate
- TICE
- transintestinal cholesterol transport
This study was supported by grant 04-55 from the Dutch Digestive Foundation (MLDS), grant 912-02-063 from the Netherlands Organization for Scientific Research (NWO), and grant T2-110 from TI-Pharma.
REFERENCES
- 1.Lemberger T., Desvergne B., Wahli W. 1996. Peroxisome proliferator-activated receptors: a nuclear receptor signaling pathway in lipid physiology. Annu. Rev. Cell Dev. Biol. 12: 335–363. [DOI] [PubMed] [Google Scholar]
- 2.Dreyer C., Krey G., Keller H., Givel F., Helftenbein G., Wahli W. 1992. Control of the peroxisomal beta-oxidation pathway by a novel family of nuclear hormone receptors. Cell. 68: 879–887. [DOI] [PubMed] [Google Scholar]
- 3.Gulick T., Cresci S., Caira T., Moore D. D., Kelly D. P. 1994. The peroxisome proliferator-activated receptor regulates mitochondrial fatty acid oxidative enzyme gene expression. Proc. Natl. Acad. Sci. USA. 91: 11012–11016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodriguez J. C., Gil-Gomez G., Hegardt F. G., Haro D. 1994. Peroxisome proliferator-activated receptor mediates induction of the mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase gene by fatty acids. J. Biol. Chem. 269: 18767–18772. [PubMed] [Google Scholar]
- 5.Tugwood J. D., Issemann I., Anderson R. G., Bundell K. R., McPheat W. L., Green S. 1992. The mouse peroxisome proliferator activated receptor recognizes a response element in the 5′ flanking sequence of the rat acyl CoA oxidase gene. EMBO J. 11: 433–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Issemann I., Green S. 1991. Cloning of novel members of the steroid hormone receptor superfamily. J. Steroid Biochem. Mol. Biol. 40: 263–269. [DOI] [PubMed] [Google Scholar]
- 7.Graves R. A., Tontonoz P., Spiegelman B. M. 1992. Analysis of a tissue-specific enhancer: ARF6 regulates adipogenic gene expression. Mol. Cell. Biol. 12: 3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erranz B., Miquel J. F., Argraves W. S., Barth J. L., Pimentel F., Marzolo M. P. 2004. Megalin and cubilin expression in gallbladder epithelium and regulation by bile acids. J. Lipid Res. 45: 2185–2198. [DOI] [PubMed] [Google Scholar]
- 9.Tontonoz P., Hu E., Devine J., Beale E. G., Spiegelman B. M. 1995. PPAR gamma 2 regulates adipose expression of the phosphoenolpyruvate carboxykinase gene. Mol. Cell. Biol. 15: 351–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braissant O., Foufelle F., Scotto C., Dauca M., Wahli W. 1996. Differential expression of peroxisome proliferator-activated receptors (PPARs): tissue distribution of PPAR-alpha, -beta, and -gamma in the adult rat. Endocrinology. 137: 354–366. [DOI] [PubMed] [Google Scholar]
- 11.Amri E. Z., Bonino F., Ailhaud G., Abumrad N. A., Grimaldi P. A. 1995. Cloning of a protein that mediates transcriptional effects of fatty acids in preadipocytes. Homology to peroxisome proliferator-activated receptors. J. Biol. Chem. 270: 2367–2371. [DOI] [PubMed] [Google Scholar]
- 12.Barak Y., Liao D., He W., Ong E. S., Nelson M. C., Olefsky J. M., Boland R., Evans R. M. 2002. Effects of peroxisome proliferator-activated receptor delta on placentation, adiposity, and colorectal cancer. Proc. Natl. Acad. Sci. USA. 99: 303–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peters J. M., Lee S. S., Li W., Ward J. M., Gavrilova O., Everett C., Reitman M. L., Hudson L. D., Gonzalez F. J. 2000. Growth, adipose, brain, and skin alterations resulting from targeted disruption of the mouse peroxisome proliferator-activated receptor beta(delta). Mol. Cell. Biol. 20: 5119–5128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanaka T., Yamamoto J., Iwasaki S., Asaba H., Hamura H., Ikeda Y., Watanabe M., Magoori K., Ioka R. X., Tachibana K., et al. 2003. Activation of peroxisome proliferator-activated receptor delta induces fatty acid beta-oxidation in skeletal muscle and attenuates metabolic syndrome. Proc. Natl. Acad. Sci. USA. 100: 15924–15929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y. X., Lee C. H., Tiep S., Yu R. T., Ham J., Kang H., Evans R. M. 2003. Peroxisome-proliferator-activated receptor delta activates fat metabolism to prevent obesity. Cell. 113: 159–170. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y. X., Zhang C. L., Yu R. T., Cho H. K., Nelson M. C., Bayuga-Ocampo C. R., Ham J., Kang H., Evans R. M. 2004. Regulation of muscle fiber type and running endurance by PPARdelta. PLoS Biol. 2: e294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takahashi S., Tanaka T., Kodama T., Sakai J. 2006. Peroxisome proliferator-activated receptor delta (PPARdelta), a novel target site for drug discovery in metabolic syndrome. Pharmacol. Res. 53: 501–507. [DOI] [PubMed] [Google Scholar]
- 18.Oliver W. R., Jr., Shenk J. L., Snaith M. R., Russell C. S., Plunket K. D., Bodkin N. L., Lewis M. C., Winegar D. A., Sznaidman M. L., Lambert M. H., et al. 2001. A selective peroxisome proliferator-activated receptor delta agonist promotes reverse cholesterol transport. Proc. Natl. Acad. Sci. USA. 98: 5306–5311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sprecher D. L., Massien C., Pearce G., Billin A. N., Perlstein I., Willson T. M., Hassall D. G., Ancellin N., Patterson S. D., Lobe D. C., et al. 2007. Triglyceride:high-density lipoprotein cholesterol effects in healthy subjects administered a peroxisome proliferator activated receptor delta agonist. Arterioscler. Thromb. Vasc. Biol. 27: 359–365. [DOI] [PubMed] [Google Scholar]
- 20.van der Veen J. N., Kruit J. K., Havinga R., Baller J. F., Chimini G., Lestavel S., Staels B., Groot P. H., Groen A. K., Kuipers F. 2005. Reduced cholesterol absorption upon PPARdelta activation coincides with decreased intestinal expression of NPC1L1. J. Lipid Res. 46: 526–534. [DOI] [PubMed] [Google Scholar]
- 21.Altmann S. W., Davis H. R., Jr., Zhu L. J., Yao X., Hoos L. M., Tetzloff G., Iyer S. P., Maguire M., Golovko A., Zeng M., et al. 2004. Niemann-Pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science. 303: 1201–1204. [DOI] [PubMed] [Google Scholar]
- 22.van, der Valde A. E., Vrins C. L., van den Oever K., Kunne C., Oude Elferink R. P., Kuipers F., Groen A. K. 2007. Direct intestinal cholesterol secretion contributes significantly to total fecal neutral sterol excretion in mice. Gastroenterology. 133: 967–975. [DOI] [PubMed] [Google Scholar]
- 23.Levels J. H., Pajkrt D., Schultz M., Hoek F. J., van Tol A., Meijers J. C., van Deventer S. J. 2007. Alterations in lipoprotein homeostasis during human experimental endotoxemia and clinical sepsis. Biochim. Biophys. Acta. 1771: 1429–1438. [DOI] [PubMed] [Google Scholar]
- 24.Bligh E. G., Dyer W. J. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37: 911–917. [DOI] [PubMed] [Google Scholar]
- 25.Huang H., Kauan J. W., Guilbault G. G. 1975. Fluorometric enzymatic determination of total cholesterol in serum. Clin. Chem. 21: 1605–1608. [PubMed] [Google Scholar]
- 26.Gurantz D., Laker M. F., Hofmann A. F. 1981. Enzymatic measurement of choline-containing phospholipids in bile. J. Lipid Res. 22: 373–376. [PubMed] [Google Scholar]
- 27.Turley S. D., Dietschy J. M. 1978. Re-evaluation of the 3 alpha-hydroxysteroid dehydrogenase assay for total bile acids in bile. J. Lipid Res. 19: 924–928. [PubMed] [Google Scholar]
- 28.Gerhardt K. O., Gehrke C. W., Rogers I. T., Flynn M. A., Hentges D. J. 1977. Gas-liquid chromatography of fecal neutral steriods. J. Chromatogr. 135: 341–349. [DOI] [PubMed] [Google Scholar]
- 29.Faiola B., Falls J. G., Peterson R. A., Bordelon N. R., Brodie T. A., Cummings C. A., Romach E. H., Miller R. T. 2008. PPAR alpha, more than PPAR delta, mediates the hepatic and skeletal muscle alterations induced by the PPAR agonist GW0742. Toxicol. Sci. 105: 384–394. [DOI] [PubMed] [Google Scholar]
- 30.Ge L., Wang J., Qi W., Miao H. H., Cao J., Qu Y. X., Li B. L., Song B. L. 2008. The cholesterol absorption inhibitor ezetimibe acts by blocking the sterol-induced internalization of NPC1L1. Cell Metab. 7: 508–519. [DOI] [PubMed] [Google Scholar]
- 31.Linder M. D., Uronen R. L., Hölttä-Vuori M., van der Sluijs P., Peränen J., Ikonen E. 2007. Rab8-dependent recycling promotes endosomal cholesterol removal in normal and sphingolipidosis cells. Mol. Biol. Cell. 18: 47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Narita K., Choudhury A., Dobrenis K., Sharma D. K., Holicky E. L., Marks D. L., Walkley S. U., Pagano R. E. 2005. Protein transduction of Rab9 in Niemann-Pick C cells reduces cholesterol storage. FASEB J. 19: 1558–1560. [DOI] [PubMed] [Google Scholar]
- 33.Temel R. E., Tang W., Ma Y., Rudel L. L., Willingham M. C., Ioannou Y. A., Davies J. P., Nilsson L. M., Yu L. 2007. Hepatic Niemann-Pick C1-like 1 regulates biliary cholesterol concentration and is a target of ezetimibe. J. Clin. Invest. 117: 1968–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Graham T. L., Mookherjee C., Suckling K. E., Palmer C. N., Patel L. 2005. The PPARdelta agonist GW0742X reduces atherosclerosis in LDLR(−/−) mice. Atherosclerosis. 181: 29–37. [DOI] [PubMed] [Google Scholar]
- 35.Labonte E. D., Camarota L. M., Rojas J. C., Jandacek R. J., Gilham D. E., Davies J. P., Ioannou Y. A., Tso P., Hui D. Y., Howles P. N. 2008. Reduced absorption of saturated fatty acids and resistance to diet-induced obesity and diabetes by ezetimibe-treated and Npc1l1−/− mice. Am. J. Physiol. Gastrointest. Liver Physiol. 295: G776–G783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choudhury A., Dominguez M., Puri V., Sharma D. K., Narita K., Wheatley C. L., Marks D. L., Pagano R. E. 2002. Rab proteins mediate Golgi transport of caveola-internalized glycosphingolipids and correct lipid trafficking in Niemann-Pick C cells. J. Clin. Invest. 109: 1541–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eskelinen E. L., Tanaka Y., Saftig P. 2003. At the acidic edge: emerging functions for lysosomal membrane proteins. Trends Cell Biol. 13: 137–145. [DOI] [PubMed] [Google Scholar]
- 38.Eckhardt E. R., Cai L., Sun B., Webb N. R., van der Westhuyzen D. R. 2004. High density lipoprotein uptake by scavenger receptor SR-BII. J. Biol. Chem. 279: 14372–14381. [DOI] [PubMed] [Google Scholar]
- 39.van der Velde A. E., Vrins C. L., van den Oever K., Seemann I., Oude Elferink R. P., van Eck M., Kuipers F., Groen A. K. 2008. Regulation of direct transintestinal cholesterol excretion in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 295: G203–G208. [DOI] [PubMed] [Google Scholar]