Abstract
In late-stage atherosclerosis, much of the cholesterol in macrophage foam cells resides within enlarged lysosomes. Similarly, human macrophages incubated in vitro with modified LDLs contain significant amounts of lysosomal free cholesterol and cholesteryl ester (CE), which disrupts lysosomal function similar to macrophages in atherosclerotic lesions. The lysosomal cholesterol cannot be removed, even in the presence of strong efflux promoters. Thus, efflux of sterol is prevented. In the artery wall, foam cells interact with triglyceride-rich particles (TRPs) in addition to modified LDLs. Little is known about how TRP metabolism affects macrophage cholesterol. Therefore, we explored the effect of TRP on intracellular CE metabolism. Triglyceride (TG), delivered to lysosomes in TRP, reduced CE accumulation by 50%. Increased TG levels within the cell, particularly within lysosomes, correlated with reductions in CE content. The volume of cholesterol-engorged lysosomes decreased after TRP treatment, indicating cholesterol was cleared. Lysosomal TG also reduced the cholesterol-induced inhibition of lysosomal acidification allowing lysosomes to remain active. Enhanced degradation and clearance of CE may be explained by movement of cholesterol out of the lysosome to sites where it is effluxed. Thus, our results show that introduction of TG into CE-laden foam cells influences CE metabolism and, potentially, atherogenesis.—Ullery-Ricewick, J. C., B. E. Cox, E. E. Griffin, and W. G. Jerome. Triglyceride alters lysosomal cholesterol ester metabolism in cholesteryl ester-laden macrophage foam cells.
Keywords: atherosclerosis, lysosome, human, lipid
Atherosclerosis is a complex, multifactorial disease. An early event in atherosclerosis is the development of macrophage foam cells. These are primarily formed through the uptake of modified lipoproteins by macrophages within the artery wall (1–4). In late-stage atherosclerotic lesions, a large amount of both free cholesterol (FC) and cholesteryl ester (CE) accumulates in foam cell lysosomes. The presence of extensive amounts of CE suggests an interruption in the normal lysosomal hydrolysis of lipid particle-derived CE, while the accumulation of FC indicates an interruption in the normal removal of FC from lysosomes to other organelles, primarily the plasma membrane (5–7). Many studies indicate that lysosomal sequestration of cholesterol can have consequences for atherosclerotic lesion development (8–15). In addition to a direct effect of accumulating sterol on lysosome function, trapping of sterol in lysosomes can prevent removal of cholesterol by efflux. In fact, the cholesterol and CE in lysosomes remains trapped in lysosomes, even when further uptake of lipoproteins is halted and acceptor concentrations in the media are increased to levels that efflux most of the nonlysosomal CE stores (16). Thus, the sequestration of sterol within lysosomes prevents efflux and limits the availability of cholesterol to other intracellular processes (16). Therefore, factors that influence the removal of lysosomally sequestered sterol could have profound effects on foam cell biology and atherosclerotic lesion development.
Many studies have examined macrophage foam cell metabolism in the presence of various CE-containing particles (2–4). However, triglyceride-rich particles (TRPs), including VLDL, are also present within the atherosclerotic lesion and could have an impact on foam cell cholesterol metabolism (17–21). Surprisingly, though, the influence of TRP on metabolism of CEs has not been extensively studied. We do know that lesion macrophages, primary cultures of human monocyte-derived macrophages, and ex vivo human foam cell macrophages contain triglyceride (TG) (22–25). Moreover, TGs are more metabolically active than CE and represent a dynamic lipid pool with the potential to influence cellular cholesterol ester metabolism. Thus, defining the interaction between TG and intracellular cholesterol pools is critical for a full understanding of atherogenesis.
A key observation linking TRP to CE metabolism is that macrophages hydrolyze CE more efficiently when it is introduced into lysosomes as a mixed CE and TG particle, compared with CE-containing particles alone (26). Additionally, it has been shown that TG can alter the physical state of CE by keeping it more fluid and accessible (26). This is consistent with studies of cytoplasmic CE droplet metabolism, which demonstrate increased activity of lipolytic enzymes in the presence of mixed CE and TG droplets compared with CE alone (27, 28). Furthermore, FAs generated by lysosomal or extralysosomal hydrolysis of TG are known ligands for, and can upregulate, cholesterol homeostatic genes, including liver X receptors and peroxisome proliferator-activated receptors (29–37). Therefore, it is clear that increased levels of TG in cells have the potential to affect macrophage cholesterol ester metabolism.
In this study, we investigate the potential for TG to reestablish lysosomal CE hydrolysis and to enhance the mobilization of the resulting FC out of lysosomes. Since CE cannot be cleared from lysosomes, lysosomal CE hydrolysis is the mandatory first step in cellular metabolism of lipoprotein-derived cholesterol and cellular cholesterol use or efflux. In this report, we show that treatment of macrophages with TRP before, during, or after cholesterol accumulation reduced both lysosomal FC and CE stores and promoted the eventual efflux of sterol from the cells. The alterations in lysosomal CE metabolism occurred, at least in part, through TG’s ability to maintain normal lysosomal activity. Thus, we conclude that modulation of lysosomal CE metabolism, through alterations of cellular TG levels, has profound influences on the ability of foam cells to clear cholesterol. TRP can flux through the atherosclerotic lesion, and our studies indicate that uptake of these particles by macrophage foam cells can influence the ability of foam cells to metabolize the extensive lysosomal CE stores found in late-stage lesions.
MATERIALS AND METHODS
Materials
THP-1 human monocytes/macrophages were purchased from ATCC (Manassas, VA). BSA, EDTA, cholesteryl oleate, trioleate, and cholesteryl methyl ether were purchased from Sigma-Aldrich (St. Louis, MO). Phosphatidylcholine and phosphatidylserine were purchased from Avanti Polar Lipids (Alabaster, AL). FBS was obtained from Atlanta Biologicals (Norcross, GA), and RPMI, l-glutamine, Eagle’s vitamins, streptomycin, and penicillin were purchased from Mediatech (Herndon, VA). All tissue culture plasticware was purchased from Corning (Corning, NY). All other chemical reagents and chemical solvents were obtained from VWR (West Chester, PA).
Lipoprotein isolation and aggregation
Human LDL was isolated from plasma collected from fasted, normocholesterolemic human volunteers who had provided informed consent. Collection of blood followed procedures approved by the Human Subjects Institutional Review Board. LDL (1.006 g/ml < d < 1.063 g/ml) and VLDL (d < 1.006 g/ml) were isolated by sequential ultracentrifugation (16). LDL and VLDL were dialyzed against 0.9% NaCl containing EDTA (0.3 mmol/l) for 72 h, filter-sterilized through a Millipore filter (0.45 µm), and stored under nitrogen at 4°C. Isolated LDL was aggregated by vortex (1 min) followed by sonication with a Branson sonifier (10 min, 50% duty cycle) on ice to break up large aggregates. The resultant aggregates were passed through a 0.45 µm filter to produce small (approximately 30–75 nm) aggregates that induce maximal uptake and lysosomal delivery. Aggregation of LDL and size were confirmed by negative staining with 2% phosphotungstic acid. Measurement of thiobarbituric acid-reactive substances and conjugated diene levels confirmed the absence of oxidation after the aggregation procedure (38, 39).
Preparation of lipid dispersions
CE-rich or TG-rich lipid dispersions (DISP) were prepared under sterile conditions as described by Mahlberg et al. (26). Briefly, phosphatidylcholine (1 mg), and phosphatidylserine (0.1 mg) and either cholesteryl oleate (30 mg, anisotropic) or triolein (10.35 mg, isotropic) were combined in a sterile 50 ml Corex glass tube and dried under nitrogen. RPMI medium (17 ml) supplemented with HEPES (12.5 mM) was then added, and the suspension was heated in an 80°C water bath for 20 min to melt the dried lipids. The solution was sonicated for 20 min using a Branson sonifier (50% duty cycle). Thiobarbituric acid-reactive substance levels showed no oxidation after sonication of DISP. Dispersion size was confirmed by negative staining electron microscopy with 2% phosphotungstic acid (see supplementary Figure I).
Cell culture of THP-1 macrophages
THP-1 macrophages were plated onto 35 mm wells or coverslips at a density of 1.5 × 106 cells and incubated for 3–4 days at 37°C in RPMI containing 10% FBS and 50 ng/ml phorbol ester (TPA) to allow for differentiation into macrophages. Culture media for all incubations was supplemented with HEPES (20 mmol/l), Eagle’s vitamins, l-glutamine (200 mmol/l), streptomycin (100 µg/ml), penicillin (100 IU/ml), and β-mercaptoethanol (0.008 µl/ml). TPA was included in the incubation medium throughout the duration of the experiments. Macrophages were incubated with medium containing 1% FA-free BSA for 24 h before cholesterol loading to minimize excess TG in cells prior to lipid loading.
Lipid loading and analysis
To measure lipid loading, macrophages were incubated for 0–6 days at 37°C in culture medium containing 1% FBS with or without lipid particles, including aggregated LDL (aggLDL), VLDL, CE-rich dispersions, or TG-rich dispersions. In some experiments, only one type particle was employed, while in other experiments, both CE-rich particles (aggLDL or CE dispersions) and TG-rich particles (VLDL or TG dispersions) were used either simultaneously or sequentially. In sequential treatment during pulse-chase experiments, the pulse media was removed after 3 days of treatment, and cells were washed briefly with 1% FBS prior to addition of the chase media for an additional 3 days. Concentrations and specifics of incubation order are described for each experiment. The lipid loading medium was changed every 3–4 days to fresh medium containing the cholesterol or TG loading vehicle.
Loading with aggLDL or VLDL was done using standard culture techniques. In contrast, experiments using DISP were conducted using an inverted culture technique that has been previously described (26). This method maximizes contact of cells with dispersions and the subsequent internalization of the particles (26). For the inverted technique, cells were first plated on glass coverslips on the bottom of 35 mm dishes. After adherence and differentiation, the coverslips were inverted and placed on sterile rings. Loading medium was then added so that the coverslip was submerged and the floating DISP came into contact with the adherent cells.
For quantitation, cellular lipids were extracted by incubation in 2 ml isopropanol containing 5–10 µg of cholesteryl methyl ether as an internal standard. Lysosomal lipids were extracted from isolated lysosomes using the method of Bligh and Dyer (40) with 5 µg of cholesteryl methyl ether as an internal standard. The cholesterol content of the lipid extracts were quantified by gas-liquid chromatography according to the procedure of Ishikawa et al. (41) as modified by Klansek et al. (42). TG content was quantified using a GPO Trinder assay kit from Raichem, according to the manufacturer’s instructions. Cellular proteins and proteins in isolated lysosomes were solublized in 1 N NaOH overnight, and protein content was measured using the method of Lowry et al. (43). Cellular lipid values are reported as micrograms of cholesterol or TG normalized to milligrams of cell protein, while isolated lysosomal lipid values are reported as micrograms of cholesterol or TG normalized to milligrams of lysosomal protein.
Cell viability during loading was assessed by counts of cell number and by protein levels. Experiments were performed in triplicate to assess experimental variability. The mean value for the three measures was used as the value for that experiment for subsequent statistical analysis of multiple experiments.
Microscopy
After lipid loading, microscopy was used to examine the subcellular localization of the accumulated lipids as well as to analyze changes in lysosomal environment. LysoSensor Yellow/Blue DND-160 staining (Molecular Probes, Eugene, OR) was used to determine changes in lysosomal pH (44, 45). This dye fluoresces yellow in an acidic environment, but the fluorescence wavelength shifts toward blue as the environment becomes more alkaline. For staining, cells were washed two times in PBS, and the dye was added to cells at a concentration of 5 µM in medium containing 1% FBS. All images were collected within 10 min after the placement of dye on the cells to avoid artifacts produced by the alkaline properties of the dye. As a positive control, macrophages in which active lysosomes were increased by incubation of macrophage with polystyrene beads rather than lipoproteins were stained with LysoSensor Yellow/Blue DND-160. Images were collected using a Zeiss Axioplan Imaging E fluorescence/bright-field microscope (Zeiss, Oberkochen, Germany) equipped with a Photometrics Coolsnap HQ digital camera with a cooled CCD chip (Roper Scientific, Tucson, AZ). Image analysis was conducted using MetaMorph imaging software (Universal Imaging, Downingtown, PA). To quantify changes in the number of active lysosomes, a grid of points was superimposed over each image. This provided an unbiased selection of vesicles to evaluate. As previously published, vesicles with a pH < 4.8 were classified as active, while those with a pH > 4.8 were considered inactive vesicles (45). A pH of 4.8 was chosen because the lysosomal acid lipase should have no activity above a pH of 4.8, and this value is above the Pka of LysoSensor such that there is a significant blue shift in the fluorescence of the probe. The vesicles in at least 20 cells per condition from three separate experiments were counted.
Negative stain electron microscopy was used to determine the ultrastructural characteristics of the isolated lysosomes and to analyze changes in average lysosome diameter with the various treatments. Lysosomal isolates were absorbed to Formvar-caoted grids for 30 s and then negatively stained for 20 s with 2% phosphotungstic acid, pH 7.0. Digital images were collected using a FEI CM-12 electron microscope operated at 80 keV accelerating voltage and equipped with an AMT cooled CCD camera. To determine the average lysosome diameter, a grid of points was superimposed over the images to select, in an unbiased fashion, the lysosomes for quantification. The diameter of each selected lysosome was computed as the distance between the two most distant points on the lysosome periphery. At least 100 diameters were computed for each condition.
Electron microscopy was also used to assess the distribution of lipid in foam cells between lysosomes and cytoplasmic droplets. Lysosomes and related organelles were identified by the presence of acid phosphatase using a modification of the Gomori lead precipitation method (46, 47). β-Glycerol phosphate was used as a substrate, and the reaction control was incubated in identical medium not containing the enzymatic substrate. After incubation, cells were postfixed in 1% osmium tetroxide, dehydrated, and embedded in epoxy resin. Before further staining, the sections were viewed to verify the enzymatic reaction and then counterstained with uranyl acetate.
Lysosomal isolation and modification
THP-1 macrophages were treated with various lipid particles, as described above, and then isolated as described previously (45). Briefly, the cells were rinsed in cold STE buffer (0.25 M sucrose, 0.01 M Tris-HCl, 1 mM EDTA, and 0.1% ethanol) and scraped into 1 ml/dish of STE buffer containing protease inhibitors (Sigma-Aldrich). The cell suspension was placed in a cell disruption chamber (Kontes) and disrupted using three passes of 20 min each at 150 p.s.i. This method consistently resulted in disruption of >95% of cells but left lysosomes intact. The suspension was centrifuged at 1,500 rpm to separate the postnuclear supernatant from the nuclear pellet. The postnuclear supernatant density was raised to 1.15 g/ml through the addition of sucrose and then applied to a sucrose density gradient ranging from 1.28–1.00 g/ml. The gradient was centrifuged at 19,400 rpm for 4 h at 4°C to separate lysosomal fractions based on their buoyant density. Morphological analysis of the fractions by negative stain electron microscopy (as described above) revealed the presence of a reasonably pure lysosomal population with no apparent structural features of other organelles, including Golgi, rough endoplasmic reticulum, and mitochondria. The purity of the lysosomal populations was assessed further by Western blotting for markers of cellular organelles that might share common morphology to lipid-engorged lysosomes, including early endosomes (EEA1) and lipid droplet associated proteins (perilipin A). Fractions were negative for EEA1 and perilipin and were positive for the lysosomal marker [lysosomal-associated membrane protein (LAMP-1); see supplementary Figure II]. Lysosomal recovery was verified using Western blotting of both the lysosome isolate and the nuclear pellet for LAMP-1 in comparison with whole-cell extracts. LAMP-1 was not detected in the nuclear pellet. The isolated lysosomes were resuspended in buffer containing 150 mM KCl to generate high K+ levels inside the lysosome, which provided a membrane potential during the stimulation of the v-ATPase with ATP. Aliquots were obtained for cholesterol, TG, and protein analyses as described above.
Vacuolar-type ATPase activity
Measurement of lysosomal v-ATPase activity was carried out using a modification of a procedure described previously (45, 48). Briefly, isolated lysosomes were placed in a cuvette containing activation buffer and 6.7 µM acridine orange. After achieving a steady spectrophotometric baseline, v-ATPases were primed with MgCl2. After approximately 60 s to allow the baseline to be reestablished, v-ATPases were activated by the addition of ATP (1.4 µM final concentration) and valinomycin (to promote the movement of K+ from inside to out for membrane potential generation). v-ATPase-driven pumping of hydrogen ions into the lysosome lumen, as measured by the quenching of acridine orange fluorescence when excited at 495 nm and recorded at 530 nm, was determined using an SLM Aminco 8100 dual-wavelength spectrophotometer. As a control, lysosomes were activated in the absence of the requisite membrane potential by including valinomycin in the medium.
Statistics
For most analyses, the experiments were repeated at least three separate times. For replicate experiments, a mean, standard deviation, and standard error of the mean were determined. The value used for each separate experiment was the mean value determined from triplicate measures. Two group comparisons used Student’s t-test. For multiple comparisons, following an ANOVA, group comparisons were performed with the multiple comparison honestly significant differences method of Tukey (49). The criterion for significance was set at P < 0.05 for a type I error.
RESULTS
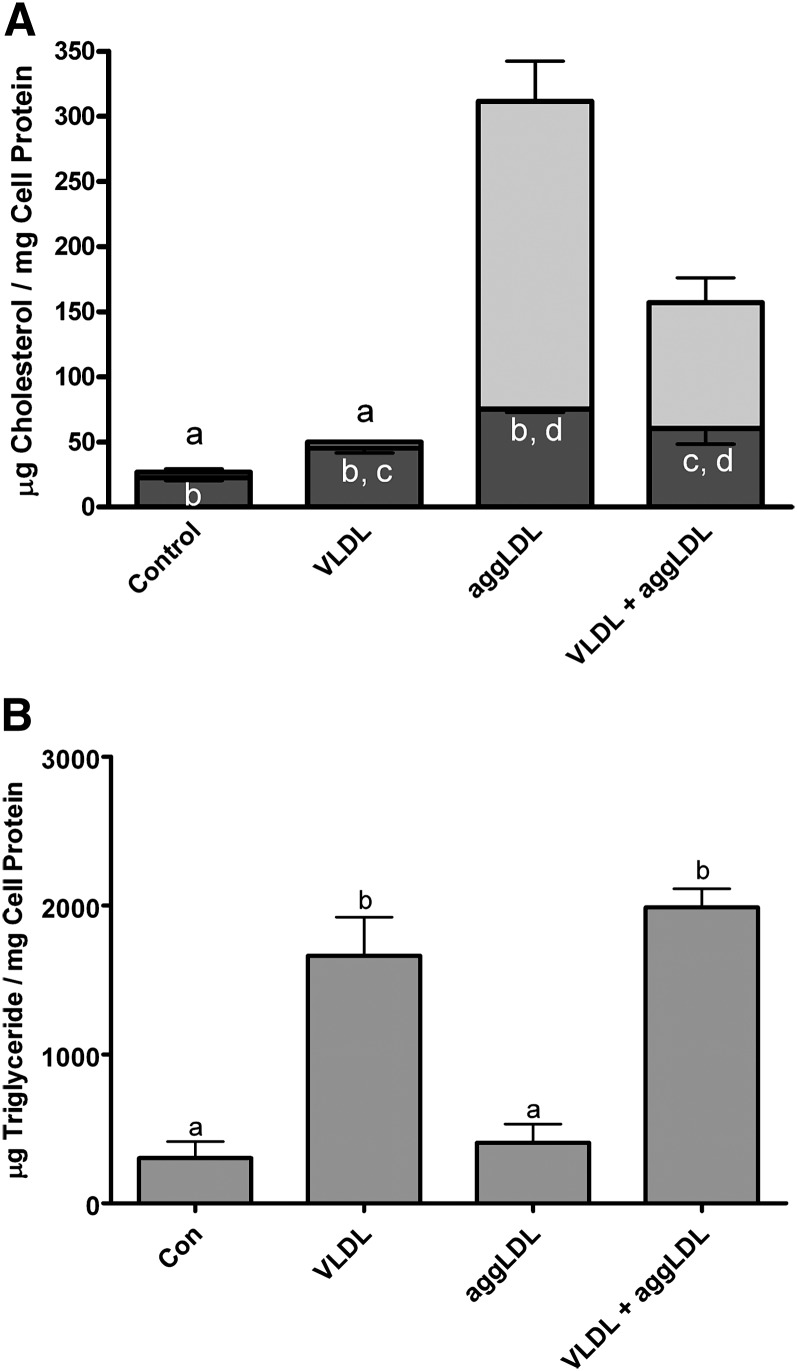
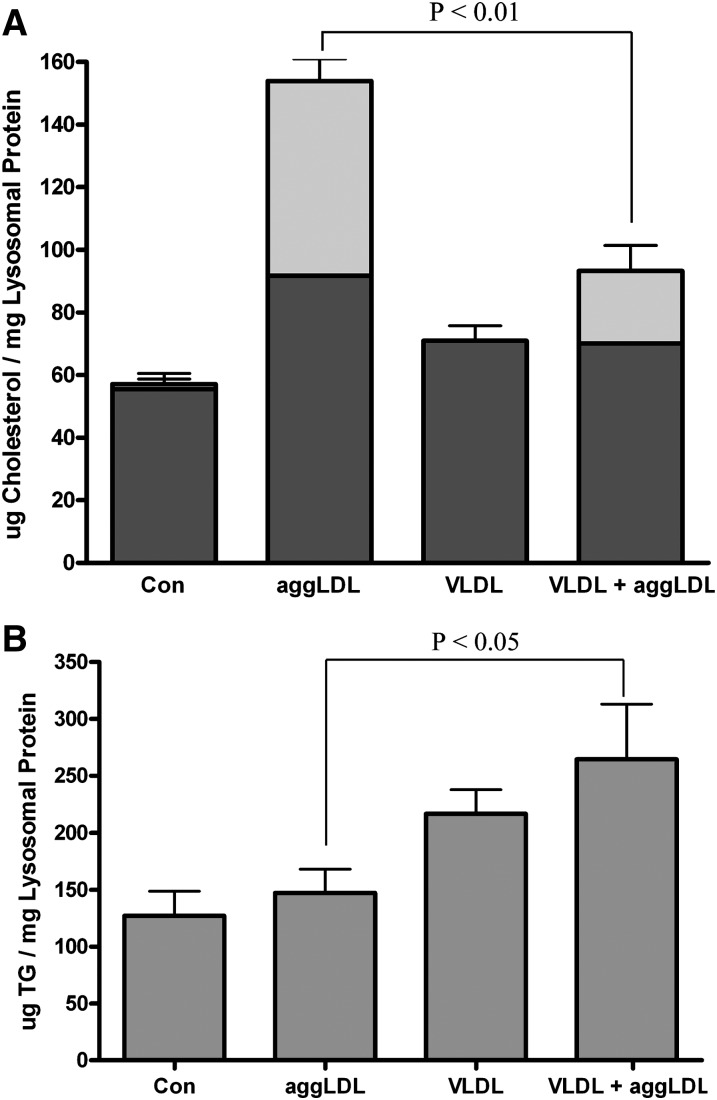
TRPs could potentially influence the foam cell metabolism of cholesterol. This is particularly true of the CEs derived from the uptake of CE-rich particles. These CE-containing particles must first be processed within lysosomes. We have previously shown that this CE hydrolysis is inhibited in heavily cholesterol-laden foam cells due primarily to the accumulation of FC within the lysosome lumen and lysosome membrane (45, 50). The lysosomally sequestered free and esterified cholesterol is trapped and cannot be removed from the lysosome even under conditions that promote the removal of nonlysosomal cholesterol from membranes and intracellular droplets (16). To determine if TRPs affect the lysosomal metabolism of CE, cellular lipid levels were measured in THP-1 macrophages treated with VLDL and aggLDL at the same time. As controls, macrophages were incubated with aggLDL or VLDL alone. Consistent with what we have shown previously, incubation of THP-1 human macrophages with 100 μg aggLDL protein/ml produced a dramatic accumulation of both FC and CE (Fig. 1A). As in previous studies (50), thin section electron microscopy of acid phosphatase-stained samples showed that >75% of the lipid volume was within lipid-engorged lysosomes. In contrast, coincubation of THP-1 with both aggLDL and VLDL resulted in a significant (P < 0.05) reduction the accumulation of CE (Fig. 1A). This correlated with a significant increase in cellular TG (Fig. 1B). Significant TG accumulation was not seen when cells were incubated with aggLDL alone. Thus, the presence of TG, delivered to the cell as a component of VLDL, reduced cholesterol accumulation, specifically CE, from aggLDL in THP-1 macrophages.
Fig. 1.
Accumulation of lipids in THP-1 macrophages incubated with aggLDL and/or VLDL. THP-1 macrophages were treated for 6 days at 37°C in RPMI containing 1% FBS and TPA (50 ng/ml) alone or with 100 μg protein/ml of aggLDL and/or VLDL. The cells were harvested, and the cellular lipid levels were determined as described in Materials and Methods. A: Incubation of THP-1 with aggLDL produced a dramatic increase in total cellular cholesterol seen primarily as a significant increase (P < 0.05) in CE (light-gray portion of bar). Although on average the FC (dark-gray portion of bar) also increased to almost double the control levels, this difference was not statistically significant. Incubation with both aggLDL and VLDL reduced the cellular CE accumulation compared with that seen with aggLDL alone. B: Incubation of cells with VLDL produced a significant (P < 0.05) increase in cellular TG levels compared with control or aggLDL-treated cells both when used alone or in combination with aggLDL. Values are the mean ± SEM for three experiments. Within each panel, bars with the same letter indicate that means were not statistically different. All other comparisons were significantly different (P < 0.05).
The simplest explanation for our results would be that TG-rich and CE-rich lipoproteins compete for uptake. In order to determine the extent to which our observations were the result of competition for uptake, THP-1 cells were treated with I125 labeled aggLDL (50 μg aggLDL protein/ml) in the presence or absence of increasing concentrations of VLDL (0, 10, or 50 μg VLDL protein/ml) for 48 h (see supplementary Figure III). Results show no difference in particle uptake over a range of VLDL concentrations, indicating that VLDL treatment does not reduce uptake of aggLDL. This suggests that the two particles do not compete for uptake and indicates a TRP-specific mechanism by which lysosomal CE metabolism and cellular clearance is enhanced.
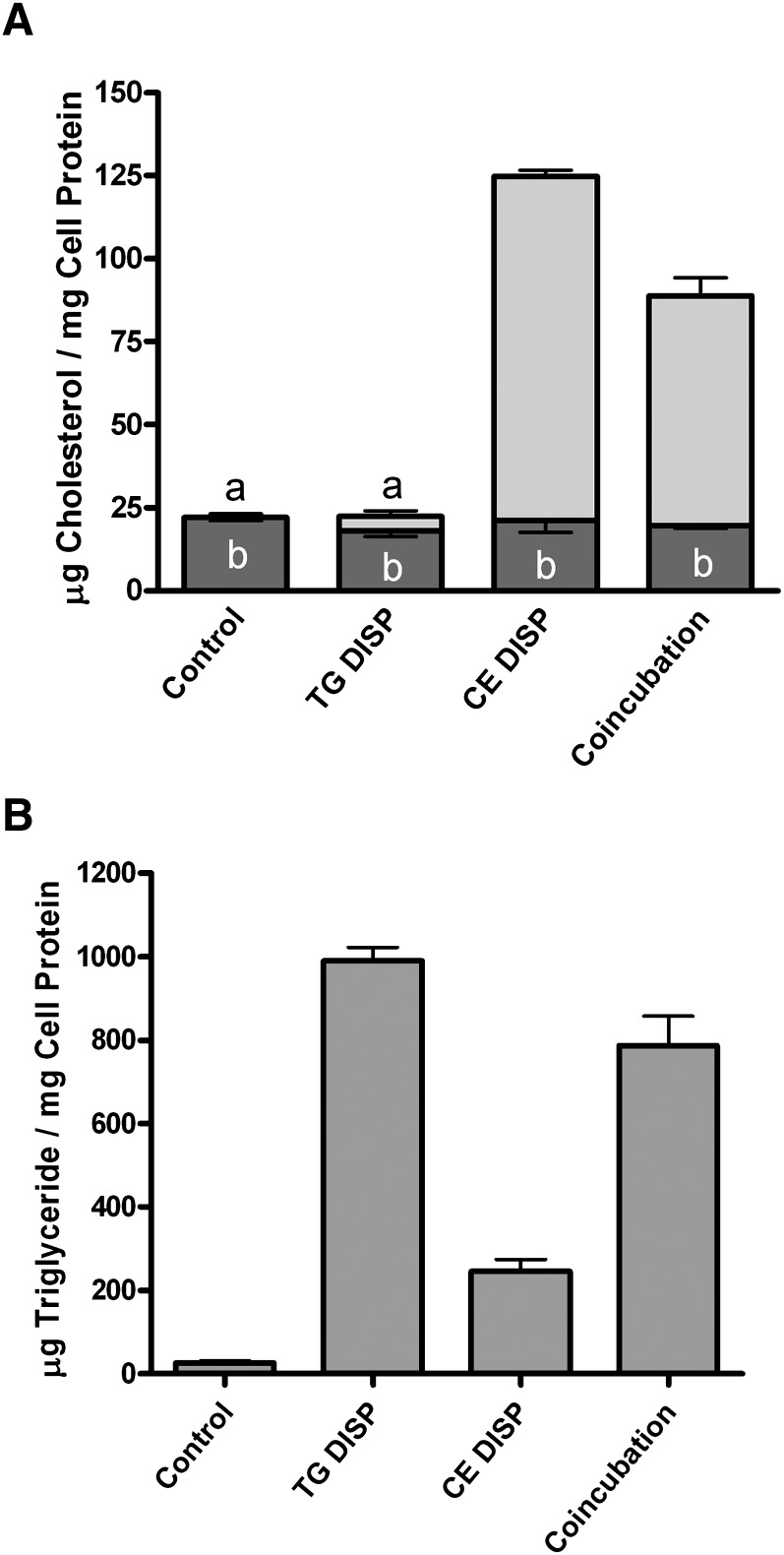
Lipoproteins are complex aggregates of lipids and proteins, both of which can influence the uptake and cellular metabolism of internalized material. To rule out an effect of lipoprotein-derived protein on the process, protein-free lipid dispersions of phospholipid and CE (CE-DISP, 60 μg CE/ml) were substituted for aggLDL, and dispersions of phospholipid and TG (TG-DISP, 50 μg TG/ml) replaced VLDL. TG-DISP significantly reduced (P < 0.05) the CE-DISP-induced accumulation of CE (Fig. 2A). As with lipoproteins, this correlated with a significant increase (P < 0.05) in cellular TG. There was no significant difference in cellular TG levels between incubation with TG-DISP alone and coincubation with TG-DISP and CE-DISP, indicating that uptake of CE-DISP and TG-DISP was not significantly affected by coincubation. Thus, it appears that TG is required for the reduction of cellular cholesterol and is the primary mediator of the effects on cellular sterol metabolism. An even more dramatic reduction in cellular cholesterol concentration was accomplished when cells were first loaded with cholesterol from CE-DISP (60 μg CE/ml) and then chased, after removing CE-DISP from the media, with media containing TG-DISP (50 μg CE/ml). The loading with CE-DISP more than doubled the cellular cholesterol content, and all of the accumulation was as CE. The chase with TG-DISP produced a 60% reduction in cellular total sterol with all of the reduction occurring as loss of CE.
Fig. 2.
Accumulation of lipids in THP-1 macrophages incubated with TG-DISP and/or CE-DISP. THP-1 macrophages were treated for 48 h at 37°C in RPMI containing 1% FBS and TPA (50 ng/ml) alone or with the specified concentrations of lipid dispersions. Cells were cultured using the inverted cell culture technique as described in Materials and Methods. A: Incubation of THP-1 with 50 μg TG/ml TG dispersions (TG-DISP) alone did not significantly (P < 0.05) alter cellular FC (dark-gray portion of bar) or CE (light-gray portion of bar). In contrast, incubation of cells with 60 μg CE/ml CE dispersions (CE-DISP) significantly increased the CE stores in cells. Coincubation of cells with both CE-DISP and TG-DISP produced a significant (P < 0.05) decrease in CE accumulation. B: Incubation of THP-1 with 50 μg TG/ml TG-DISP either alone or in combination with 60 μg CE/ml CE-DISP produced a significant (P < 0.05) increase in cellular TG compared with control cells or cells treated with CE-DISP alone. Values are the mean ± SEM for three experiments. Within each panel, bars with the same letter indicate that means were not statistically different. All other comparisons were significantly different (P < 0.05).
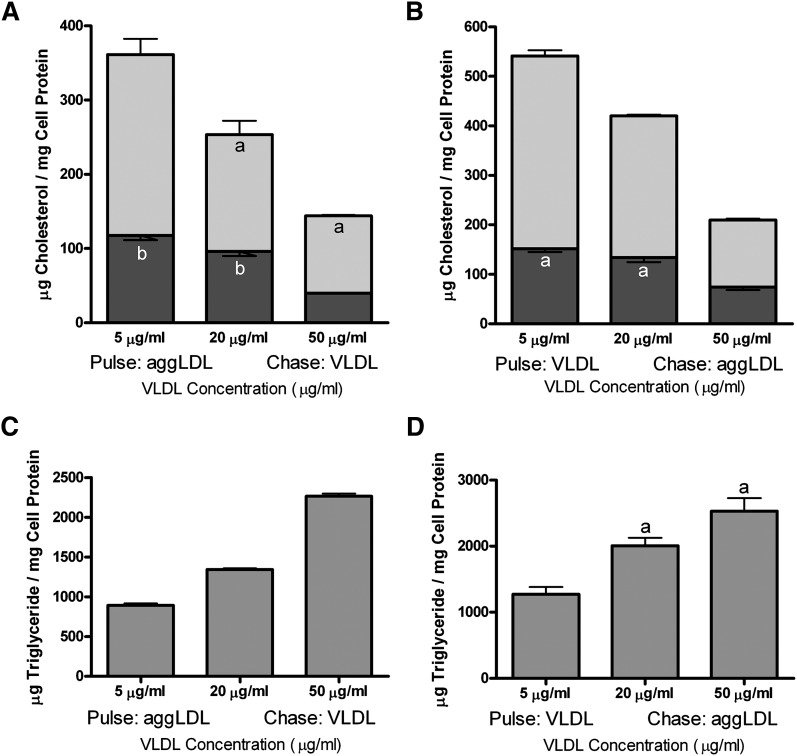
To further confirm that competition for uptake was not the explanation for reduced cellular cholesterol and to more completely define the contribution of intracellular TG concentration on cellular lipid levels, we performed pulse-chase experiments with a constant concentration of aggLDL (50 μg aggLDL protein/ml) and varying concentrations of VLDL (5, 20, and 50 μg VLDL protein/ml). TG treatment reduced cholesterol content in aggLDL-treated THP-1 macrophages in a concentration-dependent manner (Fig. 3A). Importantly, the TG-effect was observed when the cells were incubated with VLDL either following (Fig. 3A) or prior to (Fig. 3B) aggLDL incubation. Moreover, the effect on cellular cholesterol was roughly proportional to cellular TG accumulation (Fig. 3C, D). Therefore, TG reduces cellular cholesterol levels in a manner that is dependent on the cellular concentration of TG. Furthermore, TG can prevent subsequent cellular cholesterol accumulation and can mobilize preexisting stores of cellular CE, including the large volume of sterol that accumulates in cellular lysosomes (50). This is in stark contrast to direct stimulation of extralysosomal cholesterol mobilization, which does not affect the cholesterol trapped within lysosomes (16, 51).
Fig. 3.
Incubation of THP-1 with VLDL decreases cellular FC and CE in a TG dose-dependent manner. A: In cells preloaded with sterol by incubation with 50 μg aggLDL protein/ml, a chase with TG significantly (P < 0.05) reduced total cholesterol accumulation over a range of VLDL concentrations (5, 20, and 50 μg protein/ml) in a dose-dependent manner. B: Preincubation of cells with VLDL also produced a significant (P < 0.05) and dose-dependent reduction in total cellular cholesterol accumulation from subsequent incubation with 50 μg aggLDL protein/ml. C, D: Incubation of THP-1 with increasing concentrations of VLDL produced a stepwise increase in cellular TG levels. Values are the mean ± SEM for three experiments. Within each panel, bars with the same letter indicate that means were not statistically different. All other comparisons were significantly different (P < 0.05).
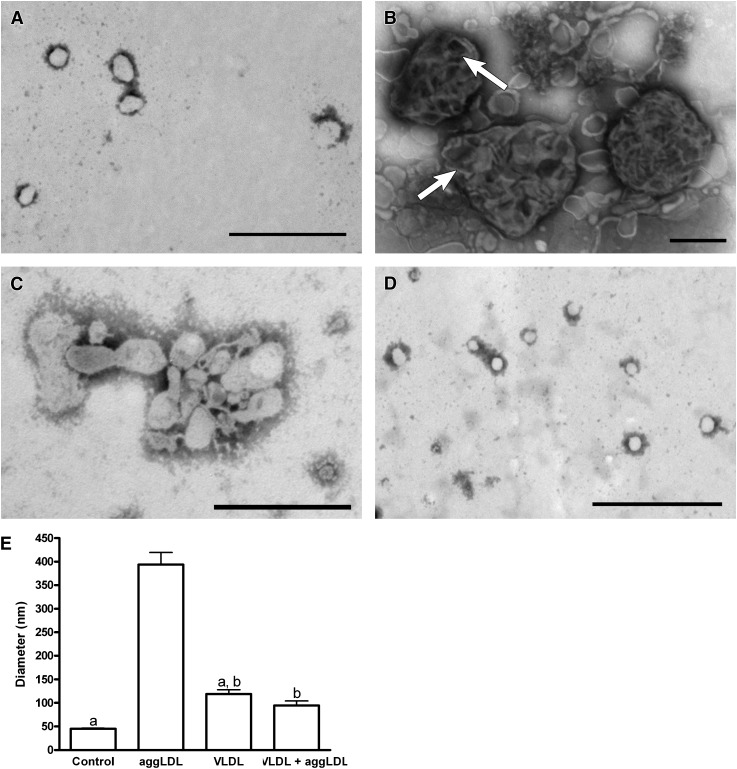
We recently demonstrated that lysosomal accumulation of CE occurring in macrophages incubated with CE-rich particles, including oxidized LDL, aggLDL, and CE-DISP is, in large part, the result of inhibition of lysosomal CE hydrolysis in response to an initial accumulation of excess FC within the lysosome (45, 50, 51). Lysosomal CE hydrolysis is a key rate-limiting step in the cellular clearance of exogenously derived sterol. Thus, the TG-induced enhancement of cellular cholesterol ester metabolism and loss of sterol from the cell suggest that TG alters lysosomal cholesterol ester hydrolysis. To study this further, we isolated lysosomes from cholesterol normal cells and cells under our various treatment conditions. Electron microscopy of negatively stained isolates from various cellular subfractions indicates that the lysosomal fraction contained primarily membrane-limited vesicles having the appearance of lysosomes. Furthermore, these fractions were positive for LAMP-1, a marker for lysosomes/late endosomes (45, 50), and lysosomal acid lipase (LAL), the acid CE hydrolase (see supplementary Figure II). Additionally, these fractions were negative for markers of early endosomes (EEA-1) and cytoplasmic lipid droplets (perilipin A) (see supplementary Figure II). Although the lysosomal fractions from each treatment group contained membrane-limited vesicles, differences in lysosome size and morphology were observed in lysosomes isolated from TG-enriched macrophages compared with those treated with cholesterol-enriched particles. The presence of small LDL-sized particles within the lysosomes isolated from cells incubated with aggLDL (Fig. 4B) is consistent with our previous observations that, under conditions of cholesterol enrichment, CE-rich particles continue to be delivered to the lysosomes, but digestion of the particles is inhibited (45). In contrast to treatment with cholesterol-rich vehicles, cellular TG enrichment results in lysosomes that are much smaller and have relatively homogenous luminal contents, indicative of active lipid particle digestion (Fig. 4C, D). Additionally, coincubation of aggLDL and VLDL results in lysosomes that are similar in size and morphology to control lysosomes (Fig. 4A, D). The quantitative differences in lysosomal diameter are shown in Fig. 4E. These results are consistent with our hypothesis that cellular TG enrichment enhances lysosomal activity and clearance of lipid from the lysosome.
Fig. 4.
THP-1 macrophages treated with TRPs for 6 days had reduced lysosome diameter compared with macrophages that were only cholesterol enriched. A–D: Negative stain electron micrograph of lysosomes isolated from control cells (A), aggLDL-treated cells (100 μg aggLDL protein/ml; B), VLDL-treated cells (100 μg VLDL protein/ml; C), or cells coincubated with aggLDL and VLDL (100 μg aggLDL protein/ml and 100 μg VLDL protein/ml; D). E: The average lysosomal diameter determined from three separate experiments. Lysosomes from control cells were generally small and had a homogenous appearing lumen (A and E). In contrast, lysosomes from aggLDL-treated cells had significantly larger (P < 0.05) lysosomes that had a variety of appearances but often contained a heterogenous mixture of apparently undigested material (arrows in B) within their lumen (B and E). Lysosomes from VLDL-treated cells were small with homogenous lumens similar to those isolated from control cells (C and E). Significantly, when THP-1 were incubated with VLDL in combination with aggLDL, their lysosomes remained small and failed to develop the large, heterogenous appearance of lysosomes isolated from cells incubated with aggLDL alone. Within each panel, bars with the same letter indicate that means were not statistically different. All other comparisons were significantly different (P < 0.05). Magnification for A, C, and D = 40,000×; magnification for B = 25,000×; bar = 500 nm.
Changes in the size and morphology of isolated lysosomes suggest enhanced CE hydrolysis and subsequent clearance of lysosomal sterol upon TG enrichment. To confirm this, we examined the effect of TG specifically on lysosomal sterol content. Treatment of cells with TRPs alone or in combination with cholesterol-rich molecules induced an increase in lysosomal TG levels, while treatment with cholesterol-rich molecules alone resulted in increased lysosomal total cholesterol (Fig. 5). However, coincubation of cells with both TRPs and cholesterol-rich molecules resulted in a 33% reduction in lysosomal cholesterol (Fig. 5). As with the analysis on a whole-cell basis, the reduction was almost exclusively in CE. This suggests that the reduction in cellular cholesterol begins with TG-induced changes in lysosomal cholesterol ester hydrolysis.
Fig. 5.
Accumulation of lipids in lysosomes isolated from THP-1 macrophages incubated with aggLDL and/or VLDL. THP-1 macrophages were treated for 6 days at 37°C in RPMI containing 1% FBS and TPA (50 ng/ml) alone or with 100 μg protein/ml of aggLDL and/or VLDL. The cells were harvested following 6 day lipid accumulation and lysosomes were isolated. The lysosomal lipid levels were determined as described in Materials and Methods. A: Incubation of cells with aggLDL produced a significant increase (P < 0.05) in the total cellular cholesterol that was seen primarily as increase in CE. In contrast, incubation of cells with VLDL did not produce an increase in lysosomal sterol. Importantly, coincubation of THP-1 with both VLDL and aggLDL limited significantly reduced the amount of CE found in lysosomes. B: Incubation of THP-1 with aggLDL and VLDL together significantly increased (P < 0.05) the amount of TG accumulating within lysosomes. Values are the mean ± SEM for three experiments.
Conversion of CE to FC is a critical first step in the removal of cholesterol from lysosomes. The hydrolysis of CE is mediated by the enzyme LAL, an acid hydrolase that is only functional at an acidic pH (52). Thus, the observed changes in lysosomal sterol levels are likely the result of effects on LAL. We do not observe changes in the cellular or lysosomal expression of LAL under the various lipid loading conditions (see supplementary Figure IV). Therefore, it is likely that the effects of TG involve changes in the environment in which the enzyme must function. Previously, we have shown that accumulation of excess FC in foam cell lysosomes leads to an inhibition of CE hydrolysis as a result of failure of the lysosomes to maintain the correct acid pH. Thus, the most probably candidate for the TG reversal of the inhibition of CE hydrolysis would be the reestablishment of the acid pH to the lysosome.
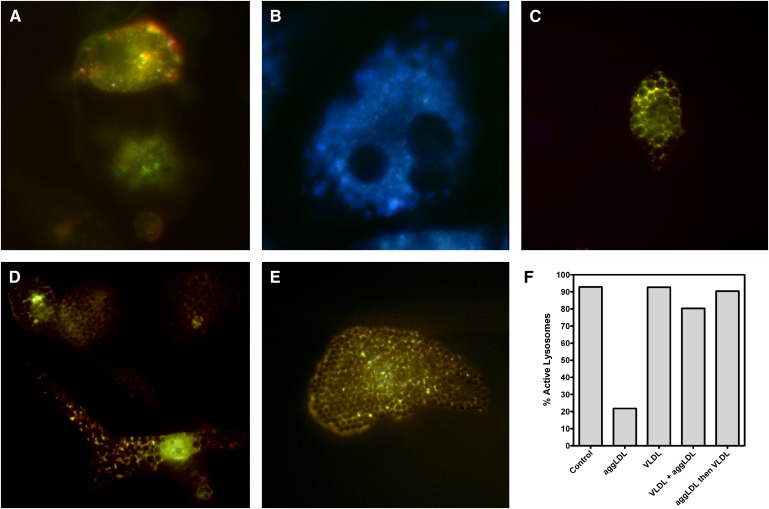
We measured the pH of vesicles within cells under our various treatments using Lysosensor Yellow/Blue DND-160, a pH-sensitive dye (45). LysoSensor Yellow/Blue DND-160 fluoresces yellow in an active lysosomal environment and has a significant blue shift as pH approaches neutrality (44, 53). We classified intracellular vesicles as active if the pH was <4.8 and inactive if the pH was >4.8. This pH is at the upper limit of the accepted normal lysosomal pH and is well above the narrow active pH range of human LAL, which displays peak activity at pH 3.8–4.0 and possesses little activity above pH 4.5 (52). Consistent with our previous studies, the majority of vesicles in untreated macrophages exhibited an active pH (Fig. 6A, F). Additionally, the majority of vesicles in cholesterol-enriched macrophages were inactive (Fig. 6B, F). However, examination of the vesicle pH in TG-enriched macrophage foam cells revealed differences in pH upon treatment with CE-rich compared with TG-rich lipoproteins. In contrast to cholesterol-rich foam cells (Fig. 6B), macrophages enriched with TG via treatment with VLDL did not display an alteration in the number of active vesicles (Fig. 6C, F). In fact, the presence of TG prevented cholesterol-induced lysosome neutralization when TG enrichment occurred, concurrent with aggLDL accumulation (Fig. 6D, F). Moreover, when cells were loaded with sterol via aggLDL, subsequent treatment with VLDL reestablished an active pH to the previously inhibited vesicles (Fig. 6E, F). To confirm that most of the vesicles we analyzed were lysosomes, in separate experiments we used LAMP-1 staining to investigate changes in the number of LAMP-1-positive vesicles under our various treatment conditions. Greater than 75% of vesicles in THP-1 foam cells were LAMP-1 positive, and the number of LAMP-1 positive vesicles did not significantly change (P < 0.05) with treatment. Thus, the shift in percentage of active lysosomes was not the result of changes in lysosome number but rather represents the ability of lysosomes to maintain an active pH. Control cells incubated with polystyrene beads did not show a change in pH, indicating that time duration was not the cause of changes in pH measurement; this was consistent with previous observations (45). Electron microscopy analysis of acid phosphatase-stained cells also indicates that most of the lipid accumulation was in lysosomes and the number of lysosomes was not appreciably altered with our various treatments.
Fig. 6.
LysoSensor Yellow/Blue DND-160 staining of macrophages shows that enrichment of THP-1 macrophages with TG maintains and/or restores active lysosome pH. A: After 6 days in culture, untreated lysosomes maintained an active pH (pH < 4.8), as indicated by a yellow fluorescence pattern. B: After 6 days of incubation with 100 μg aggLDL protein/ml, most lysosomes displayed a predominantly blue fluorescence, indicating an increase in pH to levels above 4.8. At this pH, CE hydrolysis is inhibited. C: Macrophages treated with 100 μg VLDL protein/ml maintained a large population of active lysosomes (pH < 4.8). D: Similarly, when cells were simultaneously incubated with 100 μg aggLDL protein/ml and 100 μg VLDL protein/ml, the lysosomes maintained activity, as evidenced by predominantly yellow fluorescence. E: When macrophages were incubated with 100 μg VLDL protein/ml after the cells were first incubated with 100 μg aggLDL protein/ml, the VLDL treatment was able to restore the pH of the lysosomes back to normal levels. F: Quantification of the percentage of active (pH 4.8) vesicles following the various treatment conditions. Magnification of A–E = 500×.
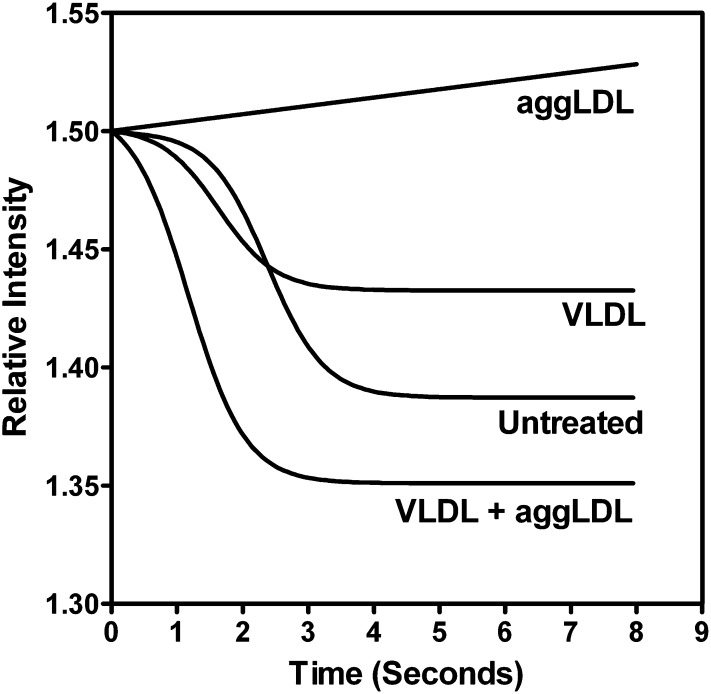
We have previously shown that cholesterol-induced inhibition of lysosomal acidification occurs primarily through the inhibition of the vacuolar ATPase, an integral membrane protein responsible for pumping H+ ions into the lysosomal lumen (45). Loss of v-ATPase function occurred concurrent with the accumulation of unesterified cholesterol (FC) in the lysosome and, specifically, within the lysosomal membrane. Our LysoSensor Yellow/Blue data suggest that TRPs accentuate CE hydrolysis by restoring lysosome pH. We hypothesized that the reduction in pH was due to TG-induced restoration of lysosomal v-ATPase activity. To confirm this, we examined the activity of the lysosomal v-ATPase in isolated lysosomes from cells treated with TG, cholesterol, or both. Quenching of acridine orange fluorescence was our measure of the v-ATPase proton pump activity (45, 48). Consistent with our published results (45), treatment with aggLDL alone (50 μg aggLDL/ml, 6 days) resulted in deficient v-ATPase activity (Fig. 7). However, lysosomes isolated from cells treated with both aggLDL and VLDL (50 μg lipoprotein/ml each, 6 days) exhibited a rapid quenching of the acridine orange, comparable to control lysosomes (Fig. 7), indicating that the v-ATPases remained active. This suggests that TRP can help lysosomes maintain an active pH by suppressing cholesterol’s normal ability to suppress the v-ATPases proton pumping.
Fig. 7.
Activation of v-ATPases in isolated lysosomes following TG and/or cholesterol enrichment. Untreated lysosomes exhibited activation of v-ATPase and the pumping of hydrogen ions into the lysosomal lumen when stimulated by the addition of ATP and valinomycin (time 0), as indicated by the decrease in the relative fluorescence intensity of acridine orange. Lysosomes from macrophages that had been treated with 100 μg aggLDL protein/ml exhibited a lack of v-ATPase activity as evidenced by no reduction in the relative fluorescence intensity. In contrast, lysosomes from cells treated with 100 μg VLDL protein/ml, either alone or in combination with 100 μg aggLDL protein/ml, exhibited rapid quenching of acridine orange fluorescence, indicating active v-ATPases. Data are a representative of example chosen from multiple separate experiments.
DISCUSSION
Much of our understanding of atherosclerotic foam cells focuses on the study of cholesterol ester metabolism and accumulation, and most tissue culture models limit themselves to the study of the metabolism of cholesterol-rich particles. However, extracellular areas of atherosclerotic lesions contain a complex milieu with multiple lipid species, each of which could have a distinct influence on foam cell biology. In this study, the influence of TRPs on macrophage cholesterol ester metabolism was examined. TG was introduced through incubation of cells with VLDL or artificial TG-rich dispersions. Treatment with TRPs led to a reduction in total cellular cholesterol, but more importantly, in lysosomal CE macrophage foam cells. This suggests that TG (or a metabolite of TG) is the mediating agent inducing the removal of sequestered sterol from foam cell lysosomes.
Our data and that from previous studies suggest the size and metabolic activity of intracellular TG pools can be an important component of macrophage lipid metabolism. Thus, it is important to define the interaction between TG and intracellular cholesterol pools to fully understand the flux of lipids within foam cells within atherosclerotic lesions. However, despite some provocative reports (28, 54), the role of TRP in foam cell cholesterol metabolism has not been extensively studied. In this report, we demonstrate that cellular TG greatly influences lysosomal cholesterol homeostasis and significantly impacts atherosclerotic macrophage foam cell CE accumulation. Previous research on the interaction of TG-rich and cholesterol-rich lipoproteins has indicated a complex interaction between lipids that can have multiple effects within the macrophage. These potential interactions include the possibility that TG or its metabolites can alter the physical properties of the mixed lipid particles to enhance metabolism, can affect the affinity and activity of the enzymes responsible for lipolysis, and can regulate cholesterol metabolic metabolism and trafficking (26–28). For instance, cellular TG can influence intracellular increase of the rate and efficiency of CE hydrolysis in macrophage lysosomes when the CE resides in a mixed lipid pool containing TG with lysosomes (26). This results, at least in part, from the ability of TG to alter the physical state of CE, keeping it more fluid (26). This physical state effect is not limited to lysosomal hydrolysis, as association of TG with cytoplasmic CE droplets has previously been shown to make CE more susceptible to hydrolysis by neutral cholesterol ester hydrolase in the cytoplasm (27, 28). This is important because the mobilization of cytoplasmic and lysosomal CE stores is a key mechanism for cellular cholesterol clearance (28).
Here, we show that increases in cellular TG drastically reduce cellular and, specifically, lysosomal cholesterol levels by maintaining the activity of foam cell lysosomes. Thus, our results are in line with previous studies demonstrating that TG plays a significant role in altering the metabolism of cholesterol in macrophage foam cells. However, our studies are the first to reveal a reduction in lysosomal cholesterol levels in response to increased cellular and lysosomal TG content. This is not an unimportant finding. In advanced atherosclerotic lesions, it is estimated that >70% of the lipid in a macrophage foam cell is sequestered in lipid-enriched lysosomes (6, 55). Moreover, our previous studies indicate that the cholesterol within lysosomes is trapped and is extremely resistant to treatments that mobilize and efflux other intracellular cholesterol stores (8, 16). We and others have shown that enhancement of efflux promoters, including increases in ATP-binding cassette A1 or the extracellular concentration of free apoproteins or HDL does not reduce lysosomal cholesterol stores (16, 56). Therefore, TG-containing particles appear to be unique in their ability to promote lysosomal cholesterol clearance.
Our studies also suggest that the effect of TG on lysosomal CE metabolism and cholesterol clearance from the lysosome is more complex than just alterations in enzymatic hydrolysis, as evidenced by the influence of TG on lysosomal v-ATPase activity. The lysosomal v-ATPases are critical lysosome integral membrane proteins responsible for pumping H+ into the lysosomal lumen to maintain the acidic pH necessary for the function of lysosomal lipolytic enzymes. It is not clear whether TG directly or indirectly affects the pumps. We have previously shown that the activity of v-ATPases is sensitive to the FC content of the lysosome membrane (45). This is consistent with similar studies that have shown that the macrophage endoplasmic reticulum calcium pump, sarcoplasmic-endoplasmic reticulum calcium ATPase-2b, is also sensitive to changes in membrane fluidity induced by cholesterol content (57). Therefore, it is possible that the TG effect is, at least in part, mediated through lysosome membrane changes that enhance proton pumping, lysosomal acidification, and lipolytic enzyme activity. It is also possible that TG, either directly or through its metabolites, directly influences the removal of cholesterol from lysosomes and into cholesterol efflux pathways. In this regard, while the eggression of cholesterol out of lysosomes is not a well-defined process, it is known that it primarily involves the intercalation of FC into lysosomal membrane and its subsequent removal via formation of FC-enriched vesicles that traffic to and fuse with other cellular membranes (58). Thus, TG-mediated effects on lysosomal membrane properties have the potential to influence this aspect of lysosomal cholesterol clearance. It remains to be seen how exactly lysosomal TG influences lysosomal cholesterol clearance and to what extent membrane alterations are required. However, our study does indicate that the presence of lysosomal TG enhances the metabolism and removal of cholesterol both from the lysosome and, ultimately, from the cell. Further study is required to define the precise mechanism or mechanisms that drive the enhanced clearance. However, our studies to date show that TG can restore lysosome function in foam cells by reestablishing the ability of lysosomes to maintain an acid pH. This leads to increased CE hydrolysis as the initial step in enhanced sterol clearance both from lysosomes and from the cell.
In addition to the potential modulation of the activity of lipolytic enzymes, the metabolic byproducts of TG could affect lysosomal and cellular lipid metabolism. Macrophages have previously been shown to metabolize TG from TRPs to glycerol and FAs through surface hydrolysis and by internalization of TRPs and lysosomal hydrolysis (59). Thus, TG and its metabolic products would be found in lysosomes. The TG pool is metabolized more efficiently than CE (60). This suggests other ways that TG metabolites might impact lysosomal function. For instance, free FAs generated from hydrolysis of TG are known to be influential in cholesterol homeostasis. FAs are key signaling molecules that greatly affect the expression of critical genes controlling cellular cholesterol mobilization (29–37). FAs can act at the level of nuclear receptors to affect the transcription of a number of genes important in cholesterol homeostasis (29–37). In particular, the individual or cooperative upregulation of peroxisome proliferator-activated receptor and liver X receptor expression by FA has been shown to regulate the expression of a number of cholesterol homeostatic genes, including the ATP-binding cassette gene family members A1 and G1, which enhance cholesterol movement and efflux (37). Free FAs might also elicit an effect within the lysosome. Additionally, it is possible that the free FAs generated from the lipolysis of TG within the lysosome may influence lysosomal membrane properties either by direct intercalation into the membrane or by influencing which acyl chains are present on the lysosomal membrane phospholipids. This modification in the FA composition of the lysosomal membrane might improve membrane fluidity and enhance the activity of lysosomal integral membrane proteins, including the lysosomal v-ATPase (61–64). Previously, we have shown that increased membrane cholesterol, which decreases membrane fluidity, can inhibit lysosomal proton pumping (45). Combined, these data suggest several explanations for how TG uptake into lysosomes might influence macrophage foam cell lysosomal cholesterol ester metabolism. It will take additional experimentation to determine which of these possibilities may actually play a role in the TG-induced enhanced lysosomal CE hydrolysis and clearance.
There is an apparent contradiction between published epidemiologic studies suggesting that hypertriglyceridemia (HTG) may increase atherosclerosis, and our current cellular studies, which indicate a role for TG in cholesterol clearance. Two points are worth noting in this regard. First, the epidemiologic evidence is controversial and does not determine whether HTG has a direct or indirect effect on coronary disease (59). A better understanding of how HTG affects cells in the artery wall is required to resolve this aspect of the paradox. An important component of that effort is defining the role of TRP as a modulator of cholesterol ester metabolism and foam cell biology. Second, it remains to be determined if the increased lysosomal cholesterol clearance induced by TG has a positive or negative impact on macrophage foam cell biology and, ultimately, lesion development. Although in most settings removal of foam cell cholesterol is thought to have a positive impact, the massive removal of cholesterol from previously engorged lysosomes may generate high levels of cellular FC that overwhelm the normal homeostatic mechanisms. In this regard, it is known that high FC levels within certain cellular pools are harmful to macrophages (65). Thus, cellular health is regulated not only by the levels, but also the cellular location, of cholesterol. As such, FC is essential for proper cellular growth and membrane stability, but excess cellular FC is cytotoxic (66, 67). The sequestration of cholesterol within the lysosomal compartment may be a protective measure to save the cell from the toxic effects of accumulated FC. Further studies are required to sort out this conundrum.
Lysosomal cholesterol is a major constituent of clinically important atherosclerotic macrophage foam cells. Most importantly, the lysosomally sequestered FC and CE have been shown to be highly resistant to removal, even under conditions that promote extralysosomal cholesterol efflux (16, 68). In this study, we show for the first time that in the presence of TG, cholesterol that had previously been sequestered in foam cell lysosomes is removed from both the lysosome and the cell. Thus, the TG-induced removal of sequestered lysosomal cholesterol allows this sterol pool to be available for cholesterol efflux. Therefore, our data suggest that TG-induced removal of cholesterol from foam cell lysosomes, if properly managed, may prove to have a positive benefit. This may be important in developing therapies for atherosclerotic lesion regression because the removal of cholesterol from macrophage foam cells has previously been shown to reduce lesion area and enhance lesion stability (69).
Acknowledgments
The authors thank Kristen Hoek of the Vanderbilt University Medical Center Editor’s Club for her thorough review of this manuscript. The authors also thank Carla Harris for her assistance with the TG analyses and Cheryl Overton for her assistance with the 125I competition assay.
Footnotes
Abbreviations:
- aggLDL
- aggregated low density lipoprotein
- CE
- cholesteryl ester
- DISP
- lipid dispersions
- FC
- free cholesterol
- LAMP-1
- lysosomal-associated membrane protein
- TG
- triglyceride
- TRP
- triglyceride-rich particle
- TPA
- phorbol ester
Training for J.C.U-R. was supported by a Vascular Biology Training Grant (HL-07751) from the National Institutes of Health, National Heart, Lung, and Blood Institute, and a grant and a predoctoral fellowship (0715387B) from the American Heart Association. Further support for this work came from Grants HL-4914804A2 and HL-086746 from the National Institutes of Health, National Heart, Lung, and Blood Institute, American Heart Association Grant 0855283E, and from the Vanderbilt University Department of Pathology. Electron microscopy was carried out in the Vanderbilt Research Electron Microscopy Core of the Cell Imaging Shared Resource. This resource is partially supported by National Institutes of Health Grants DK-20539, DK-58404, and CA-68485. Its contents are solely the responsibility of the authors and do not necessarily represent the offi cial views of the National Institutes of Health.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of four figures.
REFERENCES
- 1.Brown M. S., Goldstein J. L. 1983. Lipoprotein metabolism in the macrophage. Annu. Rev. Biochem. 52: 223–261. [DOI] [PubMed] [Google Scholar]
- 2.Aviram M. 1993. Modified forms of low density lipoprotein and atherosclerosis. Atherosclerosis. 98: 1–9. [DOI] [PubMed] [Google Scholar]
- 3.Khoo J. C., Miller E., McLoughlin P., Steinberg D. 1988. Enhanced macrophage uptake of low density lipoprotein after self-aggregation. Arteriosclerosis. 8: 348–358. [DOI] [PubMed] [Google Scholar]
- 4.Chao F-F., Blanchette-Mackie E., Chen Y-J., Dickens B., Berlin E., Amende L., Skarlatos S., Gamble W., Resau J., Mergner W., et al. 1990. Characterization of two unique cholesterol-rich lipid particles isolated from human atherosclerotic lesions. Am. J. Pathol. 136: 169–179. [PMC free article] [PubMed] [Google Scholar]
- 5.Jerome W. G., Lewis J. C. 1985. Early atherogenesis in White Carneau pigeons. II. Ultrastructural and cytochemical observations. Am. J. Pathol. 119: 210–222. [PMC free article] [PubMed] [Google Scholar]
- 6.Fowler S., Berberian P., Shio H., Goldfischer S., Wolinsky H. 1980. Characterization of cell populations isolated from aortas of rhesus monkeys with experimental atherosclerosis. Circ. Res. 46: 520–530. [DOI] [PubMed] [Google Scholar]
- 7.Miller B. F., Kothari H. 1969. Increased activity of lysosomal enzymes in human atherosclerotic aortas. Exp. Mol. Pathol. 10: 288–294. [DOI] [PubMed] [Google Scholar]
- 8.Jerome W. G., Lewis J. C. 1990. Early atherogenesis in White Carneau pigeons. Effect of a short-term regression diet. Exp. Mol. Pathol. 53: 223–238. [DOI] [PubMed] [Google Scholar]
- 9.Jerome W. G., Lewis J. C. 1997. Cellular dynamics in early atherosclerotic lesion progression in White Carneau pigeons. Spatial and temporal analysis of monocyte and smooth muscle invasion of the intima. Arterioscler. Thromb. Vasc. Biol. 17: 654–664. [DOI] [PubMed] [Google Scholar]
- 10.Gaton E., Ben-Yshai D., Wolman M. 1976. Experimentally produced hypertension and aortic acid esterase. Arch. Pathol. Lab. Med. 100: 527–530. [PubMed] [Google Scholar]
- 11.Zhang M., Dwyer N., Love D., Cooney A., Comly M., Neufeld E., Pentchev P., Blanchette-Mackie E., Hanover J. 2001. Cessation of rapid late endosomal tubulovesicular trafficking in Niemann-Pick type C1 disease. Proc. Natl. Acad. Sci. USA. 98: 4466–4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reagan J. W., Hubbert M. L., Shelness G. S. 2000. Posttranslational regulation of acid sphingomyelinase in Niemann-Pick type C1 fibroblasts and free cholesterol-enriched chinese hamster ovary cells. J. Biol. Chem. 275: 38104–38110. [DOI] [PubMed] [Google Scholar]
- 13.Pentchev P. G., Kruth H., Comly M., Butler J., Vanier M., Wenger D., Patel S. 1986. Type C Niemann-Pick disease: a parallel loss of regulatory responses in both the uptake and esterification of low density lipoprotein-derived cholesterol in cultured fibroblasts. J. Biol. Chem. 261: 16775–16780. [PubMed] [Google Scholar]
- 14.Blanchette-Mackie E. J., Dwyer N. K., Amende L. M., Kruth H. S., Butler J. D., Sokol J., Comly M. E., Vanier M. T., August J. T., Brady R. O., et al. 1988. Type C Niemann-Pick disease: low density lipoprotein uptake is associated with premature cholesterol accumulation in the Golgi complex and excessive cholesterol storage in lysosomes. Proc. Natl. Acad. Sci. USA. 85: 8022–8026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liscum L., Faust J. 1987. Low density lipoprotein (LDL) mediated suppression of cholesterol synthesis and LDL uptake is defective in Niemann-Pick type C fibroblasts. J. Biol. Chem. 262: 17002–17008. [PubMed] [Google Scholar]
- 16.Yancey P. G., Jerome W. G. 2001. Lysosomal cholesterol derived from mildly oxidized low density lipoprotein is resistant to efflux. J. Lipid Res. 42: 317–327. [PubMed] [Google Scholar]
- 17.Rapp J. H., Lespine A., Hamilton R., Colyvas N., Chaumeton A., Tweedie-Hardman J., Kotite L., Kunitake S., Havel R., Kane J. 1994. Triglyceride-rich lipoproteins isolated by slected-affinity anti-apolipoprotein B immunosorption from human atherosclerotic plaque. Arterioscler. Thromb. 14: 1767–1774. [DOI] [PubMed] [Google Scholar]
- 18.Bates S. R., Murphy P. L., Feng Z. C., Kanazawa T., Getz G. S. 1984. Very low density lipoproteins promote triglyceride accumulation in macrophages. Arteriosclerosis. 4: 103–114. [DOI] [PubMed] [Google Scholar]
- 19.Lindqvist P., Ostlund-Lindqvist A. M., Witztum J. L., Steinberg D., Little J. A. 1983. The role of lipoprotein lipase in the metabolism of triglyceride-rich lipoproteins by macrophages. J. Biol. Chem. 258: 9086–9092. [PubMed] [Google Scholar]
- 20.Gianturco S. H., Ramprasad M. P., Lin A. H., Song R., Bradley W. A. 1994. Cellular binding site and membrane binding proteins for triglyceride- rich lipoproteins in human monocyte-macrophages and THP-1 monocytic cells. J. Lipid Res. 35: 1674–1687. [PubMed] [Google Scholar]
- 21.Evans A. J., Sawyez C. G., Wolfe B. M., Connelly P. W., Maquire G. F., Huff M. W. 1993. Evidence that cholesteryl ester and triglyceride accumulation in J774 macrophages induced by very low density lipoprotein subfractions occurs by different mechanisms. J. Lipid Res. 34: 703–717. [PubMed] [Google Scholar]
- 22.Mattsson L., Johansson H., Ottosson M., Bondjers G., Wiklund O. 1993. Expression of lipoprotein lipase mRNA and secretion in macrophages isolated from human atherosclerotic aorta. J. Clin. Invest. 92: 1759–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mukhin D. N., Orekhov A. N., Andreeva E. R., Schindeler E. M., Smirnov V. N. 1991. Lipids in cells of atherosclerotic and uninvolved human aorta. III. Lipid distribution in intimal sublayers. Exp. Mol. Pathol. 54: 22–30. [DOI] [PubMed] [Google Scholar]
- 24.Garner B., Baoutina A., Dean R., Jessup W. 1997. Regulation of serum-induced lipid accumulation in human monocyte-derived macrophages by interferon-gamma. Correlations with apolipoprotein E production, lipoprotein lipase activity and LDL receptor-related protein expression. Atherosclerosis. 128: 47–58. [DOI] [PubMed] [Google Scholar]
- 25.Cullen P., Fobker M., Tegelkamp K., Meyer K., Kannenberg F., Cignarella A., Benninghoven A., Assmann G. 1997. An improved method for quantification of cholesterol and cholesteryl esters in human monocyte-derived macrophages by high performance liquid chromatography with identification of unassigned cholesteryl ester species by means of secondary ion mass spectrometry. J. Lipid Res. 38: 401–409. [PubMed] [Google Scholar]
- 26.Mahlberg F. H., Glick J. M., Jerome W. G., Rothblat G. H. 1990. Metabolism of cholesteryl ester lipid droplets in a J774 macrophage foam cell model. Biochim. Biophys. Acta. 1045: 291–298. [DOI] [PubMed] [Google Scholar]
- 27.Adelman S. J., Glick J. M., Phillips M. C., Rothblat G. H. 1984. Lipid composition and physical state effects on cellular cholesteryl ester clearance. J. Biol. Chem. 259: 13844–13850. [PubMed] [Google Scholar]
- 28.Zhao B., Fisher B., St Clair R., Rudel L., Ghosh S. 2005. Redistribution of macrophage cholesteryl ester hydrolase from cytoplasm to lipid droplets upon lipid loading. J. Lipid Res. 46: 2114–2121. [DOI] [PubMed] [Google Scholar]
- 29.Gottlicher M., Widmark E., Li Q., Gustafsson J. A. 1992. Fatty acids activate a chimera of the clofibric acid-activated receptor and the glucocorticoid receptor. Proc. Natl. Acad. Sci. USA. 89: 4653–4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Banner C. D., Gottlicher M., Widmark E., Sjovall J., Rafter J. J., Gustafsson J. A. 1993. A systematic analytical chemistry/cell assay approach to isolate activators of orphan nuclear receptors from biological extracts: characterization of peroxisome proliferator-activated receptor activators in plasma. J. Lipid Res. 34: 1583–1591. [PubMed] [Google Scholar]
- 31.Keller H., Dreyer C., Medin J., Mahfoudi A., Ozato K., Wahli W. 1993. Fatty acids and retinoids control lipid metabolism through activation of peroxisome proliferator-activated receptor-retinoid X receptor heterodimers. Proc. Natl. Acad. Sci. USA. 90: 2160–2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmidt A., Vogel R., Witherup K., Rutledge S., Pitzenberger S., Adam M., Rodan G. 1996. Identification of fatty acid methyl ester as naturally occurring transcriptional regulators of the members of the peroxisome proliferator-activated receptor family. Lipids. 31: 1115–1124. [DOI] [PubMed] [Google Scholar]
- 33.Kliewer S. A., Sundseth S. S., Jones S. A., Brown P. J., Wisely G. B., Koble C. S., Devchand P., Wahli W., Willson T. M., Lenhard J. M., Lehmann J. M. 1997. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors alpha and gamma. Proc. Natl. Acad. Sci. USA. 94: 4318–4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Forman B. M., Chen J., Evans R. M. 1997. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors alpha and delta. Proc. Natl. Acad. Sci. USA. 94: 4312–4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krey G., Braissant O., L’Horset F., Kalkhoven E., Perroud M., Parker M. G., Wahli W. 1997. Fatty acids, eicosanoids, and hypolipidemic agents identified as ligands of peroxisome proliferator-activated receptors by coactivator-dependent receptor ligand assay. Mol. Endocrinol. 11: 779–791. [DOI] [PubMed] [Google Scholar]
- 36.Ou J., Tu H., Shan B., Luk A., DeBose-Boyd R. A., Bashmakov Y., Goldstein J. L., Brown M. S. 2001. Unsaturated fatty acids inhibit transcription of the sterol regulatory element-binding protein-1c (SREBP-1c) gene by antagonizing ligand-dependent activation of the LXR. Proc. Natl. Acad. Sci. USA. 98: 6027–6032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chawla A., Boisvert W. A., Lee C-H., Laffitte B. A., Barak Y., Joseph S. B., Liao D., Nagy L., Edwards P. A., Curtiss L. K. 2001. A PPAR[gamma]-LXR-ABCA1 pathway in macrophages is involved in cholesterol efflux and atherogenesis. Mol. Cell. 7: 161–171. [DOI] [PubMed] [Google Scholar]
- 38.Buege J. A., Aust S. D. 1978. Microsomal lipid peroxidation. Methods Enzymol. 52: 302–310. [DOI] [PubMed] [Google Scholar]
- 39.Esterbauer H., Rotheneder M., Striegel G., Waeg G., Ashy A., Sattler W., Jurgens G. 1989. Vitamin E and other lipophilic antioxidants protect LDL against oxidation. Fat Sci Technol. 8: 316–324. [Google Scholar]
- 40.Bligh E. G., Dyer W. J. 1959. A rapid method for total lipid extraction and purification. Protein measurement with the Folin phenol reagent. Can. J. Biochem. Physiol. 37: 911–917. [DOI] [PubMed] [Google Scholar]
- 41.Ishikawa T. T., MacGee J., Morrison J. A., Glueck C. J. 1974. Quantitative analysis of cholesterol in 5 to 20 ul of plasma. J. Lipid Res. 15: 286–291. [PubMed] [Google Scholar]
- 42.Klansek J. J., Yancey P. G., St. Clair R. W., Fischer R. T., Johnson W. J., Glick J. M. 1995. Cholesterol quantitation by GLC: artifactual formation of short-chain steryl esters. J. Lipid Res. 36: 2261–2266. [PubMed] [Google Scholar]
- 43.Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. J. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193: 265–275. [PubMed] [Google Scholar]
- 44.Diwu Z., Chen C-S., Zhang C., Klaubert D. H., Haugland R. P. 1999. A novel acidotropic pH indicator and its potential application in labeling acidic organelles of live cells. Chem. Biol. 6: 411–418. [DOI] [PubMed] [Google Scholar]
- 45.Cox B. E., Griffin E. E., Ullery J. C., Jerome W. G. 2007. Effects of cellular cholesterol loading on macrophage foam cell lysosome acidification. J. Lipid Res. 48: 1012–1021. [DOI] [PubMed] [Google Scholar]
- 46.Gomori G. 1950. An improved histochemical technic for acid phosphatase. Stain Technol. 25: 81–85. [Google Scholar]
- 47.Jerome W. G., Cash C., Webber R., Horton R., Yancey P. 1998. Lysosomal lipid accumulation from oxidized low density lipoprotein is correlated with hypertrophy of the Golgi apparatus and trans-Golgi network. J. Lipid Res. 39: 1362–1371. [PubMed] [Google Scholar]
- 48.Crider B. P., Xie X-S. 2003. Characterization of the functional coupling of bovine brain vacuolar-type H+ translocating ATPase: effect of divalent cations, phospholipids, and subunit H (SFD). J. Biol. Chem. 278: 44281–44288. [DOI] [PubMed] [Google Scholar]
- 49.Kirk R. E. 1968. Experimental Design: Procedures for the Behavioral Sciences. Brooks/Cole Publishing, Belmont, CA. [Google Scholar]
- 50.Griffin E. E., Ullery J. C., Cox B. E., Jerome W. G. 2005. Aggregated LDL and lipid dispersions induce lysosomal cholesteryl ester accumulation in macrophage foam cells. J. Lipid Res. 46: 2052–2060. [DOI] [PubMed] [Google Scholar]
- 51.Jerome W. G., Cox B. E., Griffin E. E., Ullery J. C. 2008. Lysosomal cholesterol accumulation inhibits subsequent hydrolysis of lipoprotein cholesteryl ester. Microsc. Microanal. 14: 138–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sando G. N., Rosenbaum L. M. 1985. Human lysosomal acid lipase/cholesterol ester hydrolase. J. Biol. Chem. 260: 15186–15193. [PubMed] [Google Scholar]
- 53.Lin H-J., Herman P., Kang J., Lakowicz J. 2001. Fluorescence lifetime characterization of novel low-pH probes. Anal. Biochem. 294: 118–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lada A. T., Willingham M. C., St. Clair R. 2002. Triglyceride depletion in THP-1 cells alters cholesteryl ester physical state and cholesterol efflux. J. Lipid Res. 43: 618–628. [PubMed] [Google Scholar]
- 55.Jerome W. G., Lewis J. C. 1987. Early atherogenesis in White Carneau pigeons. III. Lipid accumulation in nascent foam cells. Am. J. Pathol. 128: 253–264. [PMC free article] [PubMed] [Google Scholar]
- 56.Johnson W. J., Chacko G. K., Phillips M. C., Rothblat G. H. 1990. The efflux of lysosomal cholesterol from cells. J. Biol. Chem. 265: 5546–5553. [PubMed] [Google Scholar]
- 57.Li Y., Ge M., Ciani L., Kuriakose G., Westover E., Dura M., Covey D., Freed J., Maxfield F., Lytton J., et al. 2004. Enrichment of endoplasmic reticulum with cholesterol inhibits sarcoplasmic-endoplasmic reticulum calcium ATPase-2b activity in parallel with increased order of membrane lipids. J. Biol. Chem. 279: 37030–37039. [DOI] [PubMed] [Google Scholar]
- 58.Soccio R. E., Breslow J. L. 2004. Intracellular cholesterol transport. Arterioscler. Thromb. Vasc. Biol. 24: 1150–1160. [DOI] [PubMed] [Google Scholar]
- 59.Ooi T. C., Ooi D. S. 1998. The atherogenic significance of an elevated plasma triglyceride level. Crit. Rev. Clin. Lab. Sci. 35: 489–516. [DOI] [PubMed] [Google Scholar]
- 60.Minor L. K., Mahlberg F. H., Jerome W. G., Lewis J. C., Rothblat G. H., Glick J. M. 1991. Lysosomal hydrolysis of lipids in a cell culture model of smooth muscle foam cells. Exp. Mol. Pathol. 54: 159–171. [DOI] [PubMed] [Google Scholar]
- 61.Burns C. P., Luttenegger D. G., Dudley D. T., Buettner G. R., Spector A. A. 1979. Effect of modification of plasma membrane fatty acid composition on fluidity and methotrexate transport in L1210 murine leukemia cells. Cancer Res. 39: 1726–1732. [PubMed] [Google Scholar]
- 62.Klausner R. D., Kleinfeld A. M., Hoover R. L., Karnovsky M. J. 1980. Lipid domains in membranes. Evidence derived from structural perturbations induced by free fatty acids and lifetime heterogeneity analysis. J. Biol. Chem. 255: 1286–1295. [PubMed] [Google Scholar]
- 63.Izpisúa J. C., Barber T., Cabo J., Hrelia S., Rossi C. A., Parenti Castelli G., Lercker G., Biagi P. L., Bordoni A., Lenaz G. 1989. Lipid composition, fluidity and enzymatic activities of rat liver plasma and mitochondrial membranes in dietary obese rats. Int. J. Obes. 13: 531–542. [PubMed] [Google Scholar]
- 64.Ibrahim A., Natarajan S., Ghafoorunissa R. 2005. Dietary trans-fatty acids alter adipocyte plasma membrane fatty acid composition and insulin sensitivity in rats. Metabolism. 54: 240–246. [DOI] [PubMed] [Google Scholar]
- 65.Tabas I. 2004. Apoptosis and plaque destabilization in atherosclerosis: the role of macrophage apoptosis induced by cholesterol. Cell Death Differ. 11: S12–S16. [DOI] [PubMed] [Google Scholar]
- 66.Seimon T., Tabas I. 2008. Mechanisms and consequences of macrophage apoptosis in atherosclerosis. J. Lipid Res. 50: S382–S387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Feng B., Yao P., Li Y., Devlin C., Zhang D., Harding H., Sweeney M., Rong J., Kuriakose G., Fisher E., et al. 2003. The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nat. Cell Biol. 5: 781–792. [DOI] [PubMed] [Google Scholar]
- 68.Jerome W. G. 2006. Advanced atherosclerotic foam cell formation has features of an acquired lysosomal storage disorder. Rejuvenation Res. 9: 245–255. [DOI] [PubMed] [Google Scholar]
- 69.Zhao B., Song J., Chow W., St Clair R., Rudel L., Ghosh S. 2007. Macrophage-specific transgenic expression of cholesteryl ester hydrolase significantly reduces atherosclerosis and lesion necrosis in Ldlr mice. J. Clin. Invest. 117: 2983–2992. [DOI] [PMC free article] [PubMed] [Google Scholar]