Abstract
The nuclear hormone receptor pregnane X receptor (PXR; also called SXR) functions as a xenobiotic sensor to coordinately regulate xenobiotic metabolism via transcriptional regulation of xenobiotic-detoxifying enzymes and transporters. Although many clinically relevant PXR ligands have been shown to affect cholesterol levels, the role of PXR in cholesterol homeostasis and atherosclerosis has not been thoroughly investigated. Here, we report that activation of PXR by feeding the PXR agonist pregnenolone 16α-carbonitrile (0.02%) for 2 weeks to wild-type (WT) mice significantly increased total cholesterol levels and atherogenic lipoproteins VLDL and LDL levels, but had no effect in PXR knockout (PXR−/−) mice. Chronic PXR activation in atherosclerosis prone apolipoprotein E deficient (ApoE−/−) mice was found to decrease HDL levels and increase atherosclerotic cross-sectional lesion area at both the aortic root and in the brachiocephalic artery by 54% (P < 0.001) and 116% (P < 0.01), respectively. PXR activation significantly regulated genes in the liver involved in lipoprotein transportation and cholesterol metabolism, including CD36, ApoA-IV, and CYP39A1, in both WT and ApoE−/− mice. Furthermore, PXR activation can increase CD36 expression and lipid accumulation in peritoneal macrophages of ApoE−/− mice. In summary, PXR activation in WT mice increases levels of the atherogenic lipoproteins VLDL and LDL, whereas in ApoE−/− mice, PXR increases atherosclerosis, perhaps by diminishing levels of the antiatherogenic ApoA-IV and increasing lipid accumulation in macrophages.—Zhou, C., N. King, K. Y. Chen, and J. L. Breslow. Activation of PXR induces hypercholesterolemia in wild-type and accelerates atherosclerosis in apoE deficient mice.
Keywords: xenobiotic metabolism, cholesterol homeostasis, pregnane X receptor, apolipoprotein
The pregnane X receptor (PXR; also known as steroid and xenobiotic receptor, or SXR) is a nuclear hormone receptor activated by xenobiotics as well as diverse sterols and their metabolites (1–3). In the past decade, the role of PXR as a sensor that coordinately regulates xenobiotic clearance in the liver and intestine has been clearly established. The ligand-receptor complex induces expression of genes involved in drug and xenobiotic metabolism, including oxidation [phase I genes; e.g., cytochrome P450s (CYP)], conjugation [phase II genes; e.g., glucuronyl or glutathione transferase (UDP-glucuronosyltransferase and GST, respectively)], and transport [phase III genes; e.g., multidrug resistance 1 (MDR1/ABCB1)] (4–6). For instance, PXR plays a central role in the transcriptional regulation of CYP3A4, which is responsible for the metabolism of more than 50% of clinically used drugs.
Many clinically relevant PXR ligands have been shown to affect lipid levels in patients. For example, treatment with rifampicin, a PXR ligand used in the clinic for the treatment of tuberculosis, can cause hyperlipidemia (7), and in one study, treatment for 6 days increased the ratio of lathosterol to cholesterol, indicating increased cholesterol synthesis (8). Treatment of HIV patients with ritonavir, one of the first commercially available HIV protease inhibitors and a potent PXR activator (9), caused hyperlipidemia and may be associated with increased risk of cardiovascular disease (10–13). Long-term treatment of children with the antiepileptic drugs carbamazipine and phenobarbital, both PXR ligands, increase cholesterol levels (14). Finally, it was recently shown that cafestol, presented in unfiltered brewed coffee and the most potent cholesterol-elevating compound known in the human diet, is a PXR agonist (15). These studies strongly suggest that modulation of PXR activity may directly affect cholesterol homeostasis.
Various endogenous sterol metabolites have also been shown to activate PXR (16). The secondary bile acid lithocholic acid and its 3-keto metabolite efficiently activate PXR (17, 18), as do the bile acid intermediates, 5-cholestanoic acid-3,7,12-triols and 7α-hydroxy-4-cholesten-3-one and 4-cholesten-3-one (19). PXR activation induces CYP3A expression, which can hydoxylate the side chain of sterols and bile acid intermediates, providing an important alternative pathway for sterol clearance (19, 20). PXR also modulates sterol regulatory element binding protein (SREBP)-independent and SREBP-dependent lipogenic pathways. In the former, PXR activates CD36 to increase free fatty acid uptake and hepatic lipid accumulation (21). With regard to SREBP-dependent lipogenic pathways, PXR can induce Insig-1 via a functional binding site in the Insig-1 promoter, resulting in decreased levels of active SREBP-1 and reduced triglyceride synthesis (22). Although these studies showed opposite effects of PXR activation on lipid homeostasis, the overall findings are consistent with a role for PXR in mediating lipid homeostasis at multiple levels.
The identification of the nuclear hormone receptor PXR as a xenobiotic sensor has provided an important tool for studying new mechanisms through which diet, drugs, and chemical exposures might impact human health. Several nuclear hormone receptors, including LXR, peroxisome proliferator-activated receptors, and farnesyl X receptor, have been implicated in the regulation of lipid homeostasis and atherosclerosis; however, the role of PXR has not been thoroughly investigated. Therefore, in this study, mouse models were used to determine the effects of PXR activation on lipid and lipoprotein levels as well as atherosclerosis. PXR activation was achieved by feeding the mouse specific PXR ligand, pregnenolone 16α-carbonitrile (PCN). In wild-type (WT) mice this resulted in increased plasma total, VLDL, and LDL cholesterol levels. In atherosclerosis sensitive apolipoprotein E deficient (ApoE−/−) mice, chronic feeding of PCN increased aortic root and brachiocephalic artery cross-sectional lesion areas and diminished HDL cholesterol levels, but did not elevate total cholesterol. Activation of PXR affected hepatic expression of genes involved in cholesterol metabolism and lipid transportation, including CD36, ApoA-IV, and CYP39A1, in both WT and ApoE−/− mice and increased CD36 expression and lipid accumulation in peritoneal macrophages of ApoE−/− mice.
MATERIALS AND METHODS
Animals
C57BL/6 WT mice and ApoE−/− mice on the C57BL/6 background (stock no. 002052) were purchased from the Jackson Laboratory. PXR−/− mice were originally generated on a mixed C57BL/6/SV129 background in Dr. Ronald Evans’ laboratory at Salk Institute (23) and were kindly provided by Dr. Bruce Blumberg from University of California, Irvine, who had backcrossed these mice with C57BL/6 mice for several generations. In our laboratory, these mice were backcrossed for five additional generations onto the C57BL/6 background using the marker-assisted Microsatellite Genotyping technique and documented to be >99% C57BL/6. All animals were housed in the Rockefeller University Laboratory Animal Research Center under a protocol approved by the Institutional Animal Care and Use Committee in a specific pathogen-free environment in rooms with a light-dark cycle.
Animal feeding study
Six-week-old WT and PXR−/− mice were fed a semisynthetic modified AIN76a diet containing 0.5% cholesterol alone (D0011804, Research Diet, New Brunswick, NJ) or supplemented with PCN at a concentration of 200 mg/kg (0.02%) to provide a dose of approximately 20 mg/kg/day (mpk) (D07051102, Research Diet). The mice were euthanized at 8 weeks of age after a 6 h fast with free access to water. Mice were exsanguinated by left-ventricular puncture, blood collected into EDTA-containing syringes, and plasma prepared by spinning at 16,000 g for 10 min. Liver tissues were collected and stored in RNAlater solution (Ambion).
Blood analysis
Total cholesterol concentrations were determined enzymatically by a colorimetric method (Roche, Indianapolis, IN). Lipoproteins fractions were isolated by sequential ultracentrifugation from 60 μl of plasma at densities (d) of <1.006 g/ml (VLDL), 1.006 ≤ d ≤ 1.063 g/ml (LDL), and d >1.063 g/ml (HDL) in a TL-100 ultracentrifuge (Beckman Coulter). For WT and PXR−/− mice, cholesterol concentrations were determined in all three fractions. For ApoE−/− mice, cholesterol concentrations were determined in the HDL fraction only. Plasma triglyceride levels were also determined enzymatically in the original plasma sample.
RNA isolation and quantitative real-time PCR analysis
Total RNA was isolated from tissues using the RNeasy mini kit (Qiagen) according to the manufacturer-supplied protocol. Quantitative real-time PCR (QPCR) was performed using gene specific primers and the SYBR green PCR kit (Applied Biosystems) in an ABI 7900 system (Applied Biosystems) as described before (24, 25). All samples were quantified using the comparative Ct method for relative quantification of gene expression, normalized to GAPDH (26). The primer sets used in this study are shown in supplementary Table I.
SuperArray analysis
The effects of PXR activation on the expression of 84 genes involved in lipoprotein transport and cholesterol metabolism were examined using Mouse Lipoprotein Signaling and Cholesterol Metabolism RT2 Profiler PCR array (SuperArray Bioscience, Catalog no. PAMM-080A) according to the manufacturer’s instructions. Briefly, total RNA was isolated from livers of WT mice fed the control or PCN diet for 2 weeks and cDNA was prepared by using a RT2 PCR array first strand kit. A total volume of 25 µl of PCR mixture, which included 12.5 µl of RT2 Real-Time SYBR Green/ROX PCR master mix from SuperArray Bioscience, 11.5 µl of double-distilled water, and 1 µl of template cDNA, was loaded in each well of the PCR array. PCR cycles were performed according to the manufacturer’s instructions. Data were analyzed using the comparative Ct method with normalization of the raw data to housekeeping genes including β-glucuronidase, hypoxanthine phosphoribosyltransferase 1, heat shock protein β-1, GAPDH, and β-actin.
Cell culture and transfections
The human hepatic cell line, HepG2, was obtained from American Type Culture Collection and cultured in DMEM containing 10% FBS at 37°C in 5% CO2. Mouse primary hepatocytes were isolated from WT and PXR knockout mice as described (27). The hepatocytes were seeded into 6-well plates and maintained in hepatocyte medium (Sigma) for at least 24 h before treatment. Immediately before treatment, the medium was removed, the cells were washed once with PBS and then treated with compounds or DMSO vehicle for 24 h. Transfection assays and Luc and β-galactosidase were performed as described (25, 28). Cells were seeded into 12-well plates overnight and transiently transfected by FuGene 6 (Roche, CA). 24 h post-transfection, the cells were treated as indicated in the figure legends. The cells were lysed 24 h after treatment and β-galactosidase and luciferase assays were performed as described. Reporter gene activity was normalized to the β-galactosidase transfection controls and the results expressed as normalized relative luminescence units per optical density β-galactosidase per minute to facilitate comparisons between plates. Each data point represents the average of experiments done in triplicate ± SEM and was replicated in independent experiments.
Atherosclerosis studies
ApoE−/− mice used for atherosclerosis studies were weaned at 4 weeks of age and fed the same diets with or without 0.02% PCN for 8 weeks. ApoE−/− mice were euthanized at 12 weeks of age after a 6 h fast. They were exsanguinated by left-ventricular puncture and blood was collected into EDTA-containing syringes. The circulation was flushed with PBS, the heart and BCA were removed and stored frozen in Tissue-Tek OCT compound (29).
Quantification of atherosclerosis
To quantify atherosclerosis at the aortic root, OCT-embedded hearts were sectioned and stained with Oil red O as described (29). To quantify atherosclerosis at the brachiocephalic artery, the OCT-compound-embedded vessels were sectioned from distal to proximal at a 10 μm thickness. Atherosclerotic lesions luminal to the internal elastic lamina were quantified in three equidistant oil red O-stained sections 200, 400, and 600 μm proximal from the branching point of the BCA into the carotid and subclavian arteries.
Peritoneal macrophage isolation and Oil red O staining
Mice were injected intraperitoneally with 2 ml of 3% thioglycollate and 4 days later, peritoneal macrophages were collected. After centrifugation at 1000 g for 5 min, the cells were resuspended in DMEM with 10% FBS. Three to five million peritoneal macrophages from each group were allowed to adhere to either cover slips or 10 cm culture dishes. After 4 h, the culture dishes and cover slips were washed three times with PBS to remove nonadherent cells. RNA was isolated from the macrophages on culture dishes and Oil red O staining was performed on macrophages adhering to the covers slips. The expression of PXR was analyzed by RT-PCR using mouse PXR specific primer sets (5′-GACGCTCAGATGCAAACCTT-3′ and 5′- TCTTCTCCGCGCAGCTGCA -3′), which amplify a PCR product of 191 bp.
Statistical analysis
All data are expressed as mean ± SD unless indicated otherwise. Statistically significant differences between two groups were analyzed by t-test for data normally distributed and by the Mann-Whitney test for data not normally distributed using Prism software, version 4.0.
RESULTS
Activation of PXR in WT mice induces hypercholesterolemia
To investigate the role of PXR in lipid homeostasis, we performed a feeding study with C57BL/6 WT and PXR−/− male mice. Six-week-old WT and PXR−/− male mice were fed the semisynthetic AIN76a diet without and with 0.02% PCN for 2 weeks. Total RNA was isolated from livers and QPCR performed to measure PXR target gene expression. As shown in supplementary Fig. I, this resulted in a robust increase in expression of the prototypic PXR target genes CYP3A11 (mouse homolog of CYP3A4) (15-fold), GSTA1 (12-fold), and MDR1a (8-fold). 0.02% PCN feeding did not induce any of these target genes in PXR−/− mice. Therefore, chronic feeding of PCN to WT mice at a dose of 0.02% was shown to efficiently activate PXR in vivo. The same dose had no effect in PXR−/− mice, confirming the key role of PXR in xenobiotic metabolism.
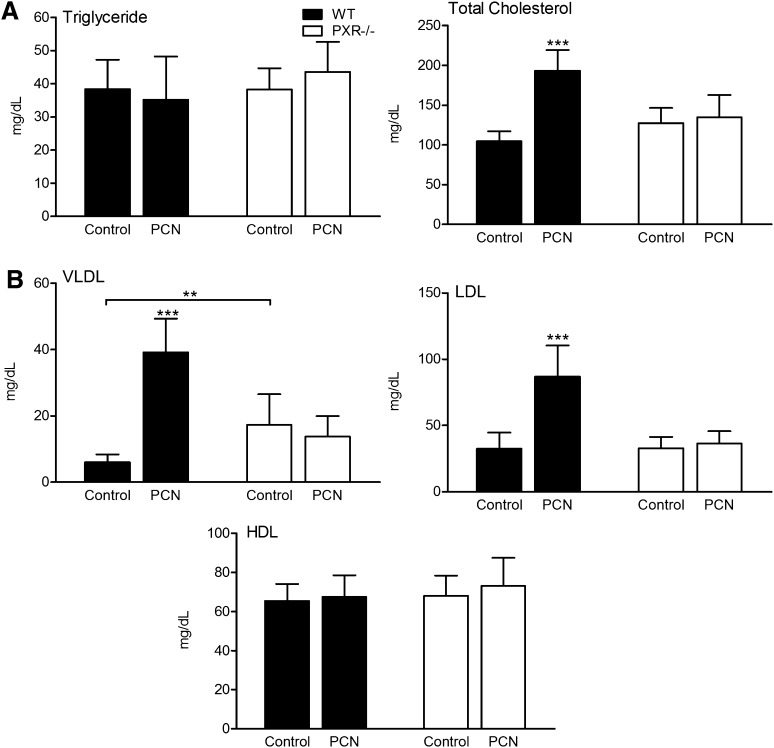
The effect of activation of PXR on plasma lipid and lipoprotein levels was next examined. As shown in Fig. 1A, triglyceride levels were unchanged by PCN feeding in both WT and PXR−/− mice, whereas total cholesterol levels were increased 85% (P < 0.001) in WT mice but unchanged in PXR−/− mice. Fig. 1B shows the effect of PCN feeding on lipoprotein cholesterol levels. VLDL cholesterol levels were increased more than 6-fold (P < 0.001), LDL cholesterol levels increased 167% (P < 0.001), and HDL cholesterol levels were unchanged by feeding WT mice 0.02% PCN. No significant effect was observed in PXR−/− mice by PCN feeding. However, compared with WT mice, PXR−/− mice fed the control diet had increased VLDL cholesterol levels.
Fig. 1.
Activation of PXR induces hypercholesterolemia in WT mice. Four-week-old male WT and PXR−/− mice fed a normal semisynthetic AIN76a diet (control) or the same diet supplemented with 0.02% PCN (PCN) for 2 weeks. A: The plasma levels of triglyceride and cholesterol and (B) lipoprotein fraction (VLDL, LDL, and HDL) were measured in WT and PXR−/− mice after administration of control or PCN diet for 2 weeks (n = 10–14 per group; **, P < 0.01; ***, P < 0.001). Error bars represent SD.
Activation of PXR affects hepatic genes involved in lipid metabolism
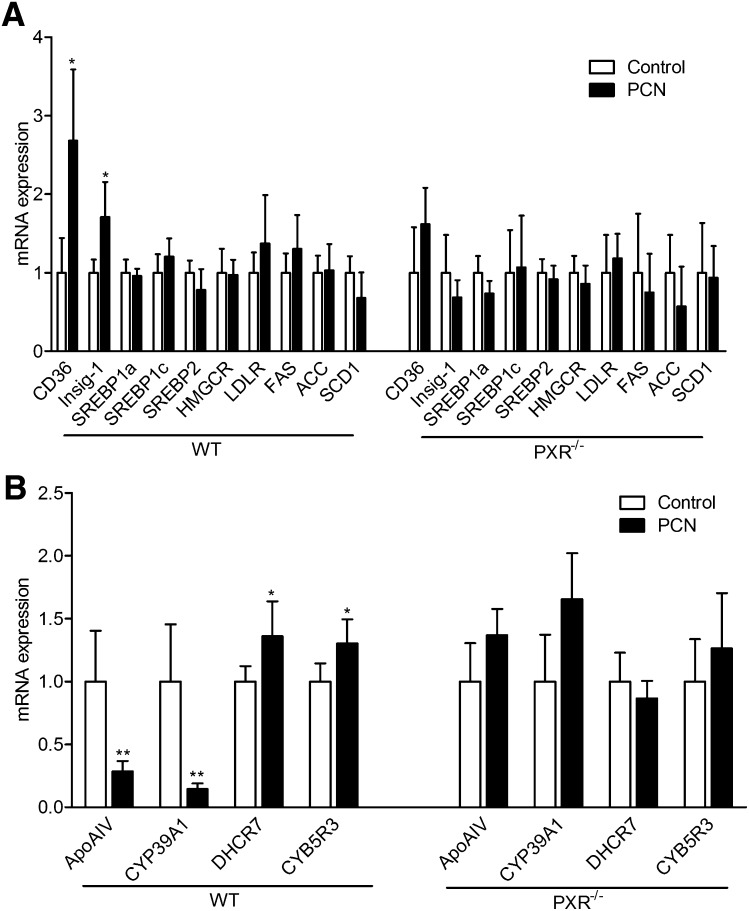
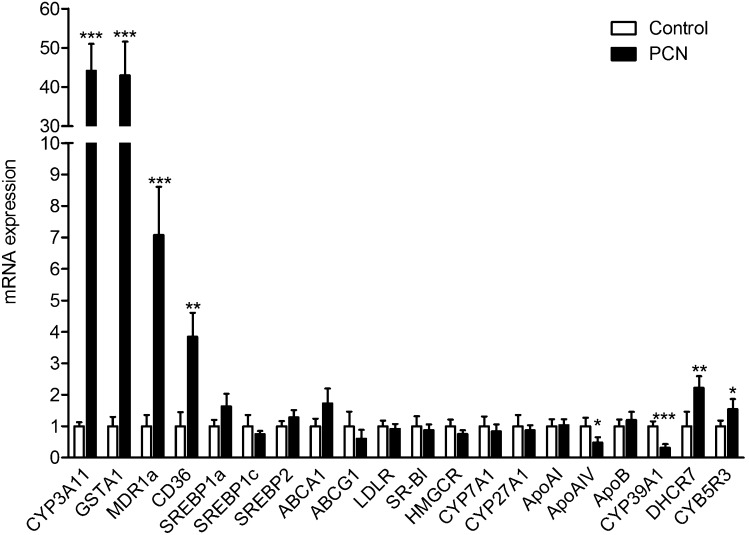
To elucidate possible molecular mechanisms through which chronic PXR activation might induce hypercholesterolemia, expression levels of important genes involved in hepatic lipid metabolism were measured by QPCR and the results shown in Fig. 2A. Of the genes surveyed, CD36 showed significant change, with its expression levels increased 170% (P < 0.05). CD36 is a fatty acid transporter and also a receptor for oxidized LDL. It was previously shown that activation of PXR can increase CD36 expression and induce SREBP independent lipogenesis in the liver (21). Consistent with a previous report (22), Insig-1 expression was also increased by PXR activation (70%, P < 0.05). However, in this analysis, chronic PXR activation did not alter expression at the mRNA level of SREBP1a, SREBP1c, or SREBP2 nor affect their target gene expression, including HMGCR, FAS, ACC, and SCD1. Moreover, many important genes involved in hepatic lipid metabolism, including genes involved in receptor mediated lipoprotein uptake from plasma (LDLR and SR-B1), cholesterol efflux (ABCA1 and ABCG1), and bile acid metabolism (CYP7A1 and CYP27A1), were not affected by PCN treatment in either WT or PXR−/− mice (Fig. 2 and supplementary Fig. II).
Fig. 2.
Activation of PXR regulates hepatic genes involved in lipoprotein transport and cholesterol metabolism. WT and PXR−/− mice were fed a normal semisynthetic AIN76a diet (control) or the same diet supplemented with 0.02% PCN (PCN) for 2 weeks. Total RNA was isolated from the liver. A: The mRNA levels of known PXR target genes and genes involved in lipid homeostasis were measured by QPCR. B: The expression levels of PXR-regulated genes identified by SuperArray experiment were analyzed by QPCR (n = 5 per group; *, P < 0.05; **, P < 0.01). Error bars represent SD.
A more in-depth analysis of expression levels of important genes involved in hepatic lipid metabolism was carried out with the Mouse Lipoprotein Signaling and Cholesterol Metabolism PCR SuperArray (PAMM-080; SABiosciences), which profiles expression of 84 genes. In this experiment, WT mice were fed the AIN76a diet without or with 0.02% PCN for 2 weeks, euthanized, liver total RNA isolated and pooled from five mice on each diet, and analyzed. Significant regulation was found for four of the genes on this SuperArray as shown in Table 1. PXR activation decreased expression of ApoA-IV, a major circulating apolipoprotein encoded in the liver and small intestine in rodents, and CYP39A1, an oxysterol 7α-hydroxylase involved in cholesterol catabolism, and increased expression of CYB5R3 (cytochrome b5 reductase 3) and DHCR7 (7-dehydrocholesterol reductase), two genes mediating cholesterol biosynthesis. Expression levels of these four genes, along with the previously untested ApoA-I, ApoA-II, and ApoE genes were then measured by QPCR using liver total RNA isolated from individual animals (Fig. 2B). This experiment revealed chronic 0.02% PCN feeding in WT but not PXR−/− mice inhibited expression of ApoA-IV 75% (P < 0.01) and CYP39A1 86% (P < 0.01) and increased expression of CYB5R3 30% (P < 0.05) and DHCR7 36% (P < 0.05). Chronic 0.02% PCN feeding did not affect expression of ApoA-I, ApoA-II, and ApoE (supplementary Fig. II).
TABLE 1.
SuperArray analysis on the hepatic expression of lipid homeostasis-related genes in the liver of WT mice fed control or PCN diet for 2 weeks
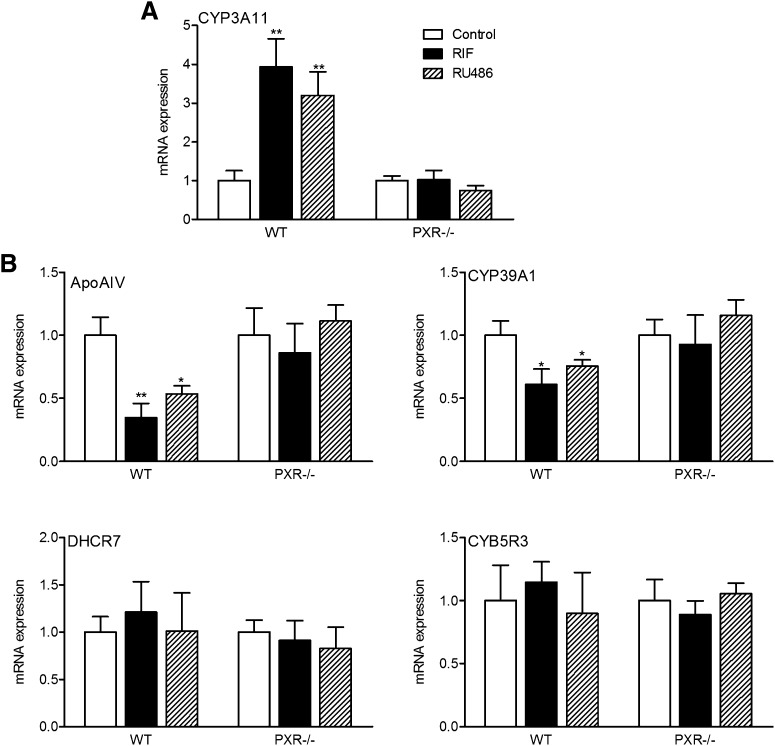
To further study the role of PXR in regulation of these potential PXR target genes, mouse primary hepatocytes were isolated from WT and PXR−/− mice and were treated with two different PXR ligands, PCN and mifepristone (RU486). RU486 is a potent agonist for both mouse and human PXR and can strongly induce PXR reporter activities in PXR-transfected cells (supplementary Fig. III) (6). As expected, both PCN and RU486 significantly induced CYP3A11 expression in primary hepatocytes of WT mice (Fig. 3). mRNAs encoding ApoA-IV and CYP39A1 genes were significantly repressed by PXR activation in primary hepatocytes of WT mice but not in PXR−/− hepatocytes. In contrast, the expression level of CYB5R3 and DHCR7 was not affected by PCN or RU486 treatment in primary hepatocytes of WT or PXR−/− mice, suggesting that those genes are regulated differently in vitro and in vivo.
Fig. 3.
Activation of PXR represses ApoA-IV and CYP39A1 expression in mouse primary hepatocytes. Mouse primary hepatocytes were isolated from WT or PXR−/− mice and were treated with DMSO control or 10 μM PCN or RU486 for 24 h. Total RNAs were isolated and mRNA levels of PXR target gene (A) CYP3A11, and (B) genes identified by SuperArray were determined by QPCR (n = 3; *, P < 0.05; **, P < 0.01). Error bars represent SD.
Chronic activation of PXR accelerates atherosclerosis in ApoE−/− mice and regulates a similar set of genes involved in hepatic lipid metabolism as in WT mice
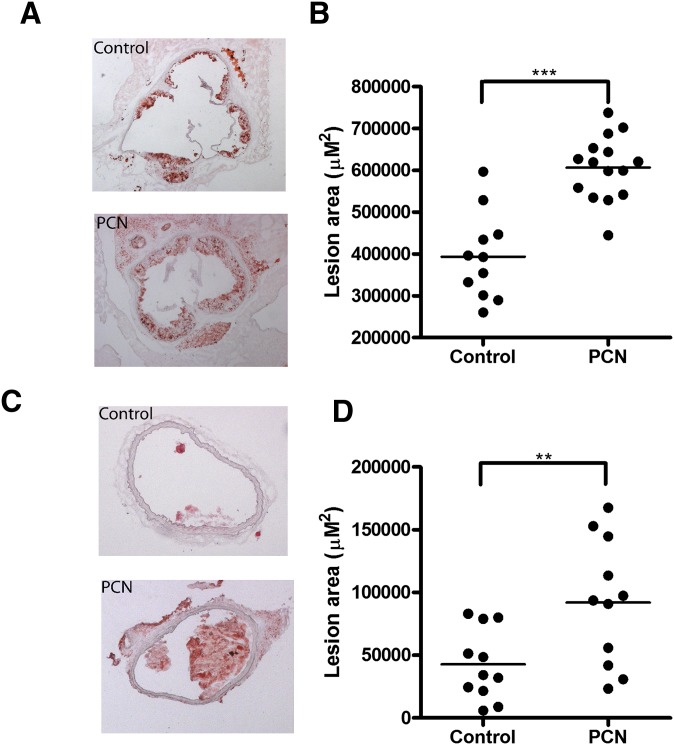
We were prompted to explore a possible role for PXR in atherosclerosis because chronic PXR activation in WT mice increased total, VLDL, and LDL cholesterol levels. The atherosclerosis experiments were done on the sensitizing ApoE−/− background. Male ApoE−/− mice were weaned at 4 weeks of age and fed the AIN76a 0.5% cholesterol diet without (control) or with 0.02% PCN (PCN) for 8 weeks. The mice were euthanized at 12 weeks of age and cross-sectional lesional area determined for both the aortic root and brachiocephalic artery as shown in Fig. 4. Compared with control mice, PCN-fed mice had increased cross-sectional lesion areas at both the aortic root (54%, P < 0.001) and brachiocephalic artery (116%, P < 0.01) (Fig. 4A, B and Fig. 4 C, D, respectively). Feeding ApoE−/− mice PCN for 8 weeks decreased body weight 8% (P < 0.01), did not change triglycerides or total cholesterol, but did decrease HDL cholesterol 51% (P < 0.01) as shown in Table 2. This differed from what was observed in WT mice: PCN feeding for 2 weeks had no effect on body weight, increased total, VLDL, and LDL cholesterol levels, and left HDL cholesterol levels unchanged.
Fig. 4.
Chronic activation of PXR accelerates atherosclerosis in ApoE−/− mice. Four-week-old male ApoE−/− mice were fed a semisynthetic AIN76a diet (control) or the same diet supplemented with 0.02% PCN (PCN) for 8 weeks. A, C: Representative Oil red O-stained section of atherosclerotic lesion area in aortic root (A) and brachiocephalic artery (C) from control or PCN-treated ApoE−/− mice. B, D: Quantitative analysis of the lesion size in the aortic root (B) and brachiocephalic artery (D) from control or PCN treated ApoE−/− mice (n = 11–15 per group; **, P < 0.01; ***, P < 0.001).
TABLE 2.
Body weight, plasma triglyceride, and cholesterol levels in ApoE−/− mice fed control of PCN diet for 8 weeks
| Body Weight | Triglyceride | Total Cholesterol | HDL | |
|---|---|---|---|---|
| g | mg/dl | mg/dl | mg/dl | |
| Control n = 13 | 26.4 ± 1.7 | 57.8 ± 13.4 | 1132 ± 111 | 18.8 ± 9.9 |
| PCN n = 13 | 24.1 ± 1.6** | 63.9 ± 15.1 | 1134 ± 194 | 9.3 ± 2.4** |
**, P < 0.01, significantly different from control group.
Liver gene expression in control and PCN-fed ApoE−/− mice was evaluated by QPCR and the results shown in Fig. 5. As seen before for WT mice, PCN feeding of ApoE−/− mice stimulated expression of the prototypic PXR activated genes, CYP3A11, GSTA1, and MDR1a. Similarly, there was altered expression of liver lipid metabolism genes with CD36, CYB5R3, and DHCR7 increased and ApoA-IV and CYP39A1 decreased. As previously, none of the other liver lipid metabolism genes tested by QPCR had significantly altered expression.
Fig. 5.
Activation of PXR mediates atherosclerosis-related gene expression in the liver of ApoE−/− mice. Total RNA was extracted from the liver of ApoE−/− mice fed control or PCN diet for 8 weeks. The expression levels of PXR target genes and atherosclerosis-related genes were analyzed by QPCR (n = 5 per group; *, P < 0.05; **, P < 0.01; ***, P < 0.001). Error bars represent SD.
Activation of PXR increases CD36 expression and lipid accumulation in peritoneal macrophages
In addition to liver and intestine, PXR has been shown to be expressed in immune cells such as T cells, B cells, and mononuclear cells, and PXR expression is positively correlated with its target gene MDR1 mRNA in these cells (30–32). One of the PXR target genes, CD36, has been shown to play an important role in atherosclerosis-related processes by mediating uptake of oxidized LDL by macrophages, which leads to foam cell formation (33, 34). To study whether activation of PXR can regulate CD36 expression in macrophages, we isolated peritoneal macrophage from WT and PXR−/− mice 4 days after thioglycollate treatment. Peritoneal macrophages were then treated with DMSO vehicle control or 10 μM PCN for 24 h and total RNAs were isolated for QPCR analysis.
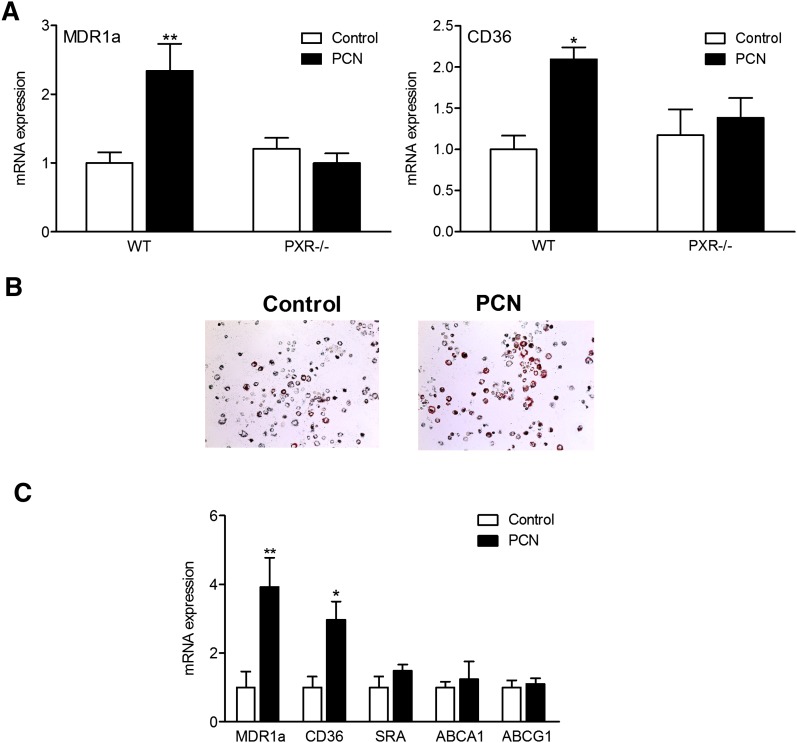
The expression of PXR in peritoneal macrophages was confirmed by RT-PCR analysis (supplementary Fig. IV). PCN treatment significantly increased the expression levels of CD36 and another PXR target gene, MDR1a, in peritoneal macrophages isolated from WT mice but did not affect CD36 or MDR1a expression in PXR−/− macrophages (Fig. 6A).
Fig. 6.
Activation of PXR upregulates CD36 expression in peritoneal macrophage and increases lipid accumulation of ApoE−/− mice. A: Peritoneal macrophages were isolated from WT and PXR−/− mice and treated with DMSO or 10 μM PCN for 24 h. RNA was isolated and mRNA levels were quantified by QPCR (n = 3; *, P < 0.05; **, P < 0.01). B: Peritoneal macrophages were isolated from ApoE−/− mice fed a control or PCN diet for 8 weeks and stained with Oil red O. C: Peritoneal macrophages were isolated from ApoE−/− mice fed a control or PCN diet for 8 weeks. Total RNA was isolated and mRNA levels were quantified by QPCR (n = 3; *, P < 0.05; **, P < 0.01). Error bars represent SD.
To determine whether PXR-mediated upregulation of CD36 expression can increase neutral lipid levels in peritoneal macrophages in ApoE−/− mice, we isolated peritoneal macrophages from ApoE−/− mice fed control or PCN diet for 8 weeks and performed Oil red O staining to assess lipid levels in the cells. As shown in Fig. 6B, neutral lipid levels were increased in peritoneal macrophages of ApoE−/− mice fed PCN diet. Gene expression analysis showed that PCN feeding significantly increased CD36 mRNA levels in the fresh isolated peritoneal macrophages (Fig. 6C). The change in CD36 expression is consistent with increased lipid accumulation in those cells. In contrast, the levels of mRNAs encoding another scavenger receptor SR-A and ABC transporters ABCA1 and ABCG1 were similar in macrophages isolated from ApoE−/− mice fed control or PCN diet. Taken together, these data suggest that the increased CD36 expression and lipid accumulation in macrophages of ApoE−/− mice fed PCN may contribute to the increased atherosclerosis in these mice.
DISCUSSION
It was previously shown that clinically relevant PXR ligands can alter plasma lipid levels in patients and activated PXR can alter the expression of genes involved in lipid homeostasis (4, 7, 11, 12, 14, 21). However, a systematic analysis of chronic PXR activation on plasma lipid levels and atherosclerosis had not been attempted and it was not known what effects, if any, long-term PXR activation might have on cholesterol levels or atherosclerosis in humans or in animal models. In the current study, we show that feeding C57BL/6 WT and ApoE−/− mice the PXR ligand PCN at a dietary concentration of 0.02% PCN for 2 to 8 weeks is well tolerated and efficiently activates expression of the prototypic set of genes involved in drug and xenobiotic metabolism. Moreover, we found that chronic PXR activation alters plasma lipid and lipoprotein levels in WT mice and increases atherosclerosis in ApoE−/− mice.
In WT mice, feeding 0.02% PCN for 2 weeks almost doubled plasma total cholesterol levels with the absolute increase coming roughly equally from both VLDL and LDL cholesterol levels. In contrast, PCN feeding did not affect lipid or lipoprotein levels in PXR−/− mice, indicating that the increased plasma cholesterol levels in WT mice was mediated through PXR activation. Interestingly, in the absence of PCN feeding, PXR−/− mice had increased VLDL cholesterol levels compared with WT mice. The mechanism for this increased VLDL level in PXR−/− mice is currently unknown. Activation of PXR was found to decrease hepatic mRNA levels of the cholesterol catabolic enzyme CYP39A1 and ApoA-IV, both in vivo and in primary hepatocytes (Figs. 2 and 3). CYP39A1 is one of two microsomal enzymes, the other being CYP7B1, that catalyze the 7α-hydroxylation of oxysterols, an early step in the alternative bile acid biosynthesis pathway (35). CYP39A1 is expressed exclusively in liver and not regulated by cholesterol or bile acids. The relationship of the profound decrease in CYP39A1 hepatic mRNA levels in mice fed 0.02% PCN to the observed increase in VLDL and LDL cholesterol is uncertain. It is of interest that hepatic gene expression analysis of congenic mice for the hyplip2 locus, which confers a combined hyperlipidemia phenotype (increased VLDL and LDL), also showed profoundly decreased CYP39A1 mRNA levels (14-fold) (36, 37). ApoA-IV has been proposed to play roles in lipoprotein metabolism, lipid absorption, feeding behavior, and suppression of oxidation, inflammation, and atherosclerosis (38, 39). In plasma, ApoA-IV is found mainly in HDL particles and in the lipoprotein-free fraction. The lipoprotein pattern of the ApoA-IV knockout mouse showed decreased VLDL and HDL cholesterol levels, but the decreased VLDL was shown to be due to decreased hepatic expression of ApoC-III, a triglyceride modulating gene adjacent to the ApoA-IV gene (38). Interestingly, chronic activation of PXR was found to increase levels of two cholesterol biosynthetic enzymes, DHCR7 and CYB5R3, in both WT and ApoE−/− mice. However, DHCR7 and CYB5R3 expression were not affected by PXR ligand treatment in primary hepatocytes, which suggests that these two genes are indirect targets of PXR and the different in vitro and in vivo results may be due to the altered environment.
After submission of this manuscript, de Hann et al. (40) reported that activation of PXR by PCN treatment can also increase plasma cholesterol and VLDL levels in ApoE*3-Leiden (E3L) mice and decrease HDL levels in E3L.CETP transgenic mice. Several hepatic genes involved in HDL metabolism were affected by treatment of PCN at high concentration (0.1%). Another report showed that short term activation PXR by intraperitoneal injection of PCN at 80 mg/kg/day for 3 days can decrease plasma LDL levels but increase plasma triglyceride levels in LDL receptor knockout (LDLR−/−) mice (41). Similar treatment also caused increased plasma triglyceride levels in ApoE−/− mice but the plasma cholesterol and lipoprotein levels were not reported. Interestingly, expression of human PXR in mice antagonizes cholic acid-mediated downregulation of plasma HDL levels and hepatic Apo-AI expression, which was attributed to repressed farnesyl X receptor activity by PXR (42). Albeit that different mechanisms may contribute to PXR-mediated effects on plasma lipid and lipoprotein levels in different animal models, all of this evidence suggests that activation of PXR can affect cholesterol metabolism and may play a role in atherosclerosis.
Several nuclear receptors (LXR, PPARs) that regulate lipid metabolism and modulate inflammation have been extensively studied for their roles in atherosclerosis (43, 44) but such studies have not been done for PXR. We found that feeding ApoE−/− mice AIN76a 0.5% cholesterol diet containing 0.02% PCN for 8 weeks increased atherosclerotic cross-sectional lesion area at the aortic root and the brachiocephalic artery by 54% and 116%, respectively. Therefore, we have concluded that chronic PXR activation is proatherogenic. Interestingly, feeding 0.02% PCN to ApoE−/− mice did not increase plasma total cholesterol levels as it did in WT mice, despite inducing virtually the same changes in hepatic gene expression. Mice have very little ApoB100 and rely almost exclusively on ApoE for LDL receptor mediated clearance of lipoprotein particles from plasma. Thus, if the effects of 0.02% PCN feeding in WT mice on VLDL and LDL cholesterol levels were dependent on altering the ApoE-LDL receptor interaction, as they very well might have been, they would not be seen in ApoE−/− mice. It is also possible that the very high cholesterol levels in ApoE−/− compared with WT mice (∼1100 mg/dl vs. ∼100 mg/dl, respectively, under control conditions) might preclude observing what may be subtle effects on liver lipid synthesis due to the observed hepatic gene expression changes. However, feeding 0.02% PCN to ApoE−/− mice did decrease HDL cholesterol levels, and the profound decrease in hepatic ApoA-IV mRNA (∼85%) is likely involved based on observations in ApoA-IV−/− mice (38). In addition to regulating HDL metabolism, ApoA-IV was shown to be a potent inhibitor of lipid oxidation, and in mouse models, ApoA-IV is antiatherosclerotic (45, 46). Transgenic mice overexpressing mouse and human ApoA-IV were protected against diet-induced atherosclerosis and against atherosclerosis that occurs in ApoE−/− mice on a chow diet (47, 48). Therefore, decreased ApoA-IV production might explain the decreased HDL cholesterol levels and increased atherosclerosis in 0.02% PCN-fed ApoE−/− mice.
The increase in atherosclerosis observed by feeding 0.02% PCN to ApoE−/− mice might also relate to the increased CD36 expression and lipid accumulation in the macrophages. In addition to its role as a fatty acid transporter, CD36 is also a member of the scavenger receptor class B family and might play an important role in atherosclerosis-related processes (33, 34). For example, in vitro CD36 was shown to mediate uptake of oxidized LDL by macrophages, which leads to foam cell formation (49). Although the in vivo role of CD36 in influencing atherosclerosis susceptibility is less clear, Febbraio et al. (50) reported that targeted disruption of CD36 in vivo protects against atherosclerotic lesion development in ApoE−/− mice. PXR has been reported to be expressed in human immune cells and expression of its target genes, such as MDR1, is positively correlated with PXR expression in peripheral blood mononuclear cells (30). Although it has been claimed that PXR is expressed at very low or undetectable levels in bone marrow-derived macrophages (51), we detected PXR expression in thioglycollate-elicited peritoneal macrophages (supplementary Fig. IV). In the current study, we showed that activation of PXR increases CD36 expression in peritoneal macrophages from WT mice and PCN feeding increases lipid accumulation and CD36 expression in ApoE−/− mice. It was previously reported that PXR ligands can induce ABCA1 and ABCG1 expression and stimulate cholesterol efflux in intestine cells but not in other cells, such as liver cells (52). We found that expression levels of ABCA1 and ABCG1 are not affected by PXR activation in macrophages. Therefore, in addition to effects on HDL levels and ApoA-IV expression, another plausible explanation for the increased atherosclerosis observed after 8 weeks of feeding 0.02% PCN is the induction of CD36 expression and its mediated lipid uptake in macrophages.
The precise mechanisms through which PXR modulates lipid metabolism and cholesterol levels in different animal models, as well as in humans, remain to be determined. It would also be interesting to investigate the effects of chronic activation of PXR on lipid levels and atherosclerosis in different animal models, such as LDLR−/− mice. Nevertheless, our study provides critical mechanistic insight for understanding the impact of clinically relevant PXR ligands on lipid levels and, for the first time, showed that chronic activation of PXR increases atherosclerosis in an animal model, which may have direct clinical consequence for patients under long-term treatment with PXR agonist drugs.
In summary, our data show that PXR activation can affect plasmas lipid and lipoprotein levels in animal models and chronic PXR activation increased atherosclerosis in ApoE−/− mice. This study broadens the pharmacological implications of PXR activation beyond xenobiotic response by revealing a potentially important adverse role for chronic PXR activation in lipid homeostasis and atherosclerosis. This will have to be taken into account in the development of new PXR modulators for possible therapeutic uses.
Supplementary Material
Acknowledgments
The authors thank Katie Tsang and Helen Yu for technical assistance, and Drs. Ronald M. Evans and Bruce Blumberg for PXR−/− mice.
Footnotes
Abbreviations:
- Apo
- apolipoprotein
- CYB5R3
- cytochrome b5 reductase 3
- DHCR7
- 7-dehydrocholesterol reductase
- MDR1
- multidrug resistance protein-1
- PCN
- pregnenolone 16α-carbonitrile
- PXR
- pregnane X receptor
- QPCR
- quantitative real-time PCR
- SREBP
- sterol regulatory element binding protein
- SXR
- steroid and xenobiotic receptor
- WT
- wild-type
This work was supported by the National Institutes of Health grant program project Grant P01 HL54591 (Project 1) and Ellison Medical Foundation (AG-SS-2160-08) to J.L.B. Its contents are solely the responsibility of the authors and do not necessarily represent the offi cial views of the National Institutes of Health.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of one table and four fi gures.
REFERENCES
- 1.Blumberg B., Sabbagh W., Jr., Juguilon H., Bolado J., Jr., van Meter C. M., Ong E. S., Evans R. M. 1998. SXR, a novel steroid and xenobiotic-sensing nuclear receptor. Genes Dev. 12: 3195–3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kliewer S. A., Moore J. T., Wade L., Staudinger J. L., Watson M. A., Jones S. A., McKee D. D., Oliver B. B., Willson T. M., Zetterstrom R. H., et al. 1998. An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell. 92: 73–82. [DOI] [PubMed] [Google Scholar]
- 3.Bertilsson G., Heidrich J., Svensson K., Asman M., Jendeberg L., Sydow-Backman M., Ohlsson R., Postlind H., Blomquist P., Berkenstam A. 1998. Identification of a human nuclear receptor defines a new signaling pathway for CYP3A induction. Proc. Natl. Acad. Sci. USA. 95: 12208–12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kliewer S. A., Goodwin B., Willson T. M. 2002. The nuclear pregnane X receptor: a key regulator of xenobiotic metabolism. Endocr. Rev. 23: 687–702. [DOI] [PubMed] [Google Scholar]
- 5.Dussault I., Forman B. M. 2002. The nuclear receptor PXR: a master regulator of “homeland” defense. Crit. Rev. Eukaryot. Gene Expr. 12: 53–64. [DOI] [PubMed] [Google Scholar]
- 6.Zhou C., Verma S., Blumberg B. 2009. The steroid and xenobiotic receptor (SXR), beyond xenobiotic metabolism. Nucl. Recept. Signal. 7: e001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khogali A. M., Chazan B. I., Metcalf V. J., Ramsay J. H. 1974. Hyperlipidaemia as a complication of rifampicin treatment. Tubercle. 55: 231–233. [DOI] [PubMed] [Google Scholar]
- 8.Lutjohann D., Hahn C., Prange W., Sudhop T., Axelson M., Sauerbruch T., von Bergmann K., Reichel C. 2004. Influence of rifampin on serum markers of cholesterol and bile acid synthesis in men. Int. J. Clin. Pharmacol. Ther. 42: 307–313. [DOI] [PubMed] [Google Scholar]
- 9.Dussault I., Lin M., Hollister K., Wang E. H., Synold T. W., Forman B. M. 2001. Peptide mimetic HIV protease inhibitors are ligands for the orphan receptor SXR. J. Biol. Chem. 276: 33309–33312. [DOI] [PubMed] [Google Scholar]
- 10.Carr A., Samaras K., Burton S., Law M., Freund J., Chisholm D. J., Cooper D. A. 1998. A syndrome of peripheral lipodystrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhibitors. AIDS. 12: F51–F58. [DOI] [PubMed] [Google Scholar]
- 11.Carr A., Samaras K., Chisholm D. J., Cooper D. A. 1998. Pathogenesis of HIV-1-protease inhibitor-associated peripheral lipodystrophy, hyperlipidaemia, and insulin resistance. Lancet. 351: 1881–1883. [DOI] [PubMed] [Google Scholar]
- 12.Shafran S. D., Mashinter L. D., Roberts S. E. 2005. The effect of low-dose ritonavir monotherapy on fasting serum lipid concentrations. HIV Med. 6: 421–425. [DOI] [PubMed] [Google Scholar]
- 13.Barbaro G. 2006. Metabolic and cardiovascular complications of highly active antiretroviral therapy for HIV infection. Curr. HIV Res. 4: 79–85. [DOI] [PubMed] [Google Scholar]
- 14.Eiris J. M., Lojo S., Del Rio M. C., Novo I., Bravo M., Pavon P., Castro-Gago M. 1995. Effects of long-term treatment with antiepileptic drugs on serum lipid levels in children with epilepsy. Neurology. 45: 1155–1157. [DOI] [PubMed] [Google Scholar]
- 15.Ricketts M. L., Boekschoten M. V., Kreeft A. J., Hooiveld G. J., Moen C. J., Muller M., Frants R. R., Kasanmoentalib S., Post S. M., Princen H. M., et al. 2007. The cholesterol-raising factor from coffee beans, cafestol, as an agonist ligand for the farnesoid and pregnane X receptors. Mol. Endocrinol. 21: 1603–1616. [DOI] [PubMed] [Google Scholar]
- 16.Xie W., Uppal H., Saini S. P., Mu Y., Little J. M., Radominska-Pandya A., Zemaitis M. A. 2004. Orphan nuclear receptor-mediated xenobiotic regulation in drug metabolism. Drug Discov. Today. 9: 442–449. [DOI] [PubMed] [Google Scholar]
- 17.Staudinger J. L., Goodwin B., Jones S. A., Hawkins-Brown D., MacKenzie K. I., LaTour A., Liu Y., Klaassen C. D., Brown K. K., Reinhard J., et al. 2001. The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc. Natl. Acad. Sci. USA. 98: 3369–3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie W., Radominska-Pandya A., Shi Y., Simon C. M., Nelson M. C., Ong E. S., Waxman D. J., Evans R. M. 2001. An essential role for nuclear receptors SXR/PXR in detoxification of cholestatic bile acids. Proc. Natl. Acad. Sci. USA. 98: 3375–3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dussault I., Yoo H. D., Lin M., Wang E., Fan M., Batta A. K., Salen G., Erickson S. K., Forman B. M. 2003. Identification of an endogenous ligand that activates pregnane X receptor-mediated sterol clearance. Proc. Natl. Acad. Sci. USA. 100: 833–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goodwin B., Gauthier K. C., Umetani M., Watson M. A., Lochansky M. I., Collins J. L., Leitersdorf E., Mangelsdorf D. J., Kliewer S. A., Repa J. J. 2003. Identification of bile acid precursors as endogenous ligands for the nuclear xenobiotic pregnane X receptor. Proc. Natl. Acad. Sci. USA. 100: 223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou J., Zhai Y., Mu Y., Gong H., Uppal H., Toma D., Ren S., Evans R. M., Xie W. 2006. A novel pregnane X receptor-mediated and sterol regulatory element-binding protein-independent lipogenic pathway. J. Biol. Chem. 281: 15013–15020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roth A., Looser R., Kaufmann M., Blaettler S., Rencurel F., Huang W., Moore D. D., Meyer U. A. 2008. Regulatory crosstalk between drug metabolism and lipid homeostasis: Car and Pxr increase Insig-1 expression. Mol. Pharmacol. 73: 1282–1289. [DOI] [PubMed] [Google Scholar]
- 23.Xie W., Barwick J. L., Downes M., Blumberg B., Simon C. M., Nelson M. C., Neuschwander-Tetri B. A., Brunt E. M., Guzelian P. S., Evans R. M. 2000. Humanized xenobiotic response in mice expressing nuclear receptor SXR. Nature. 406: 435–439. [DOI] [PubMed] [Google Scholar]
- 24.Zhou C., Tabb M. M., Sadatrafiei A., Grun F., Blumberg B. 2004. Tocotrienols activate the steroid and xenobiotic receptor, SXR, and selectively regulate expression of its target genes. Drug Metab. Dispos. 32: 1075–1082. [DOI] [PubMed] [Google Scholar]
- 25.Zhou C., Assem M., Tay J. C., Watkins P. B., Blumberg B., Schuetz E. G., Thummel K. E. 2006. Steroid and xenobiotic receptor and vitamin D receptor crosstalk mediates CYP24 expression and drug-induced osteomalacia. J. Clin. Invest. 116: 1703–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Livak K. J., Schmittgen T. D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 27.Zhou C., Tabb M. M., Nelson E. L., Grun F., Verma S., Sadatrafiei A., Lin M., Mallick S., Forman B. M., Thummel K. E., et al. 2006. Mutual repression between steroid and xenobiotic receptor and NF-kappaB signaling pathways links xenobiotic metabolism and inflammation. J. Clin. Invest. 116: 2280–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou C., Poulton E. J., Grun F., Bammler T. K., Blumberg B., Thummel K. E., Eaton D. L. 2007. The dietary isothiocyanate sulforaphane is an antagonist of the human steroid and xenobiotic nuclear receptor. Mol. Pharmacol. 71: 220–229. [DOI] [PubMed] [Google Scholar]
- 29.Teupser D., Persky A. D., Breslow J. L. 2003. Induction of atherosclerosis by low-fat, semisynthetic diets in LDL receptor-deficient C57BL/6J and FVB/NJ mice: comparison of lesions of the aortic root, brachiocephalic artery, and whole aorta (en face measurement). Arterioscler. Thromb. Vasc. Biol. 23: 1907–1913. [DOI] [PubMed] [Google Scholar]
- 30.Albermann N., Schmitz-Winnenthal F. H., Z'graggen K., Volk C., Hoffmann M. M., Haefeli W. E., Weiss J. 2005. Expression of the drug transporters MDR1/ABCB1, MRP1/ABCC1, MRP2/ABCC2, BCRP/ABCG2, and PXR in peripheral blood mononuclear cells and their relationship with the expression in intestine and liver. Biochem. Pharmacol. 70: 949–958. [DOI] [PubMed] [Google Scholar]
- 31.Owen A., Chandler B., Back D. J., Khoo S. H. 2004. Expression of pregnane-X-receptor transcript in peripheral blood mononuclear cells and correlation with MDR1 mRNA. Antivir. Ther. 9: 819–821. [PubMed] [Google Scholar]
- 32.Siest G., Jeannesson E., Marteau J. B., Samara A., Marie B., Pfister M., Visvikis-Siest S. 2008. Transcription factor and drug-metabolizing enzyme gene expression in lymphocytes from healthy human subjects. Drug Metab. Dispos. 36: 182–189. [DOI] [PubMed] [Google Scholar]
- 33.Abumrad N. A., el-Maghrabi M. R., Amri E. Z., Lopez E., Grimaldi P. A. 1993. Cloning of a rat adipocyte membrane protein implicated in binding or transport of long-chain fatty acids that is induced during preadipocyte differentiation. Homology with human CD36. J. Biol. Chem. 268: 17665–17668. [PubMed] [Google Scholar]
- 34.Tontonoz P., Nagy L., Alvarez J. G., Thomazy V. A., Evans R. M. 1998. PPARgamma promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell. 93: 241–252. [DOI] [PubMed] [Google Scholar]
- 35.Li-Hawkins J., Lund E. G., Bronson A. D., Russell D. W. 2000. Expression cloning of an oxysterol 7alpha-hydroxylase selective for 24-hydroxycholesterol. J. Biol. Chem. 275: 16543–16549. [DOI] [PubMed] [Google Scholar]
- 36.Moen C. J., Tholens A. P., Voshol P. J., de Haan W., Havekes L. M., Gargalovic P., Lusis A. J., van Dyk K. W., Frants R. R., Hofker M. H., et al. 2007. The Hyplip2 locus causes hypertriglyceridemia by decreased clearance of triglycerides. J. Lipid Res. 48: 2182–2192. [DOI] [PubMed] [Google Scholar]
- 37.Wang X., Gargalovic P., Wong J., Gu J. L., Wu X., Qi H., Wen P., Xi L., Tan B., Gogliotti R., et al. 2004. Hyplip2, a new gene for combined hyperlipidemia and increased atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 24: 1928–1934. [DOI] [PubMed] [Google Scholar]
- 38.Weinstock P. H., Bisgaier C. L., Hayek T., Aalto-Setala K., Sehayek E., Wu L., Sheiffele P., Merkel M., Essenburg A. D., Breslow J. L. 1997. Decreased HDL cholesterol levels but normal lipid absorption, growth, and feeding behavior in apolipoprotein A-IV knockout mice. J. Lipid Res. 38: 1782–1794. [PubMed] [Google Scholar]
- 39.Stan S., Delvin E., Lambert M., Seidman E., Levy E. 2003. Apo A-IV: an update on regulation and physiologic functions. Biochim. Biophys. Acta. 1631: 177–187. [DOI] [PubMed] [Google Scholar]
- 40.de Haan W., de Vries-van der Weij J., Mol I. M., Hoekstra M., Romijn J. A., Jukema J. W., Havekes L. M., Princen H. M., Rensen P. C. 2009. PXR agonism decreases plasma HDL levels in ApoE3-Leiden.CETP mice. Biochim. Biophys. Acta. 1791: 191–197. [DOI] [PubMed] [Google Scholar]
- 41.Hoekstra M., Lammers B., Out R., Li Z., Van Eck M., Van Berkel T. J. 2009. Activation of the nuclear receptor PXR decreases plasma LDL-cholesterol levels and induces hepatic steatosis in LDL receptor knockout mice. Mol. Pharm. 6: 182–189. [DOI] [PubMed] [Google Scholar]
- 42.Masson D., Lagrost L., Athias A., Gambert P., Brimer-Cline C., Lan L., Schuetz J. D., Schuetz E. G., Assem M. 2005. Expression of the pregnane X receptor in mice antagonizes the cholic acid-mediated changes in plasma lipoprotein profile. Arterioscler. Thromb. Vasc. Biol. 25: 2164–2169. [DOI] [PubMed] [Google Scholar]
- 43.Glass C. K. 2006. Going nuclear in metabolic and cardiovascular disease. J. Clin. Invest. 116: 556–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barish G. D., Evans R. M. 2004. PPARs and LXRs: atherosclerosis goes nuclear. Trends Endocrinol. Metab. 15: 158–165. [DOI] [PubMed] [Google Scholar]
- 45.Qin X., Swertfeger D. K., Zheng S., Hui D. Y., Tso P. 1998. Apolipoprotein AIV: a potent endogenous inhibitor of lipid oxidation. Am. J. Physiol. 274: H1836–H1840. [DOI] [PubMed] [Google Scholar]
- 46.Ostos M. A., Conconi M., Vergnes L., Baroukh N., Ribalta J., Girona J., Caillaud J. M., Ochoa A., Zakin M. M. 2001. Antioxidative and antiatherosclerotic effects of human apolipoprotein A-IV in apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 21: 1023–1028. [DOI] [PubMed] [Google Scholar]
- 47.Cohen R. D., Castellani L. W., Qiao J. H., Van Lenten B. J., Lusis A. J., Reue K. 1997. Reduced aortic lesions and elevated high density lipoprotein levels in transgenic mice overexpressing mouse apolipoprotein A-IV. J. Clin. Invest. 99: 1906–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Duverger N., Tremp G., Caillaud J. M., Emmanuel F., Castro G., Fruchart J. C., Steinmetz A., Denefle P. 1996. Protection against atherogenesis in mice mediated by human apolipoprotein A-IV. Science. 273: 966–968. [DOI] [PubMed] [Google Scholar]
- 49.Rahaman S. O., Lennon D. J., Febbraio M., Podrez E. A., Hazen S. L., Silverstein R. L. 2006. A CD36-dependent signaling cascade is necessary for macrophage foam cell formation. Cell Metab. 4: 211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Febbraio M., Podrez E. A., Smith J. D., Hajjar D. P., Hazen S. L., Hoff H. F., Sharma K., Silverstein R. L. 2000. Targeted disruption of the class B scavenger receptor CD36 protects against atherosclerotic lesion development in mice. J. Clin. Invest. 105: 1049–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barish G. D., Downes M., Alaynick W. A., Yu R. T., Ocampo C. B., Bookout A. L., Mangelsdorf D. J., Evans R. M. 2005. A Nuclear Receptor Atlas: macrophage activation. Mol. Endocrinol. 19: 2466–2477. [DOI] [PubMed] [Google Scholar]
- 52.Li T., Chen W., Chiang J. Y. 2007. PXR induces CYP27A1 and regulates cholesterol metabolism in the intestine. J. Lipid Res. 48: 373–384. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.