Abstract
Methionine-choline-deficient (MCD) diets cause steatohepatitis in rodents and are used to study the pathophysiology of fatty liver disease in human beings. The most widely used commercial MCD formulas not only lack methionine and choline but also contain excess sucrose and fat. The objective of this study was to determine whether dietary sucrose in the MCD formula plays a role in the pathogenesis of MCD-related liver disease. We prepared two custom MCD formulas, one containing sucrose as the principal carbohydrate and the other substituting sucrose with starch. Mice fed the sucrose-enriched formula developed typical features of MCD-related liver disease, including hepatic steatosis, hepatocellular apoptosis, alanine aminotransferase elevation, lipid peroxidation, and hepatic inflammation. In contrast, mice fed MCD-starch were significantly protected against liver injury. MCD-sucrose and MCD-starch mice displayed identical diet-related abnormalities in hepatic fatty acid uptake and triglyceride secretion. Hepatic de novo lipogenesis and triglyceride synthesis, however, were 2 times higher in MCD-sucrose mice than MCD-starch mice (P < 0.01). Hepatic lipid analysis revealed accumulation of excess saturated fatty acids in MCD-sucrose mice that correlated with hepatocellular injury. Overall, the results indicate that dietary sucrose is critical to the pathogenesis of MCD-mediated steatohepatitis. They suggest that saturated fatty acids, which are products of de novo lipogenesis, are mediators of hepatic toxicity in this model of liver disease.
Keywords: fatty acid, fatty liver, apoptosis, de novo lipogenesis
Diets devoid of methionine and choline [methionine choline-deficient (MCD)] cause hepatic steatosis and inflammation that mimics nonalcoholic steatohepatitis in human beings (1–3). MCD feeding reportedly induces hepatic steatosis through a dual process involving enhanced uptake of fatty acids by the liver as well as impaired secretion of hepatic triglyceride (TG) (2). Stimulation of fatty acid uptake in MCD-fed animals correlates with hepatic upregulation of fatty acid transport proteins. Suppression of hepatic TG secretion is due to the reduced availability of methionine and choline for phospholipid synthesis, which is critical to the formation of TG-rich VLDL particles (4, 5). Notably, commercial MCD formulas (MP Biomedicals, Harlan Teklad, and Dyets) not only lack methionine and choline but are also enriched in sucrose and fat. These nutrients can themselves stimulate hepatic lipid accumulation (6–8); thus, they may accentuate the hepatic lipid accumulation caused by methionine and choline deprivation alone.
The role of dietary fat as a determinant of liver injury in the MCD model has been the subject of recent investigation. Somewhat unexpectedly, studies have shown that the fat content of the MCD formula can be varied over a wide range (10% to 40% of calories) without any impact on the amount of TG that accumulates in the liver (9, 10). Even more surprising is that dietary fat does not affect MCD-mediated hepatocellular injury based on biochemical or histologic criteria (10). Dietary fat does influence MCD-mediated hepatic lipid peroxidation and inflammatory cytokine induction. As would be expected, MCD formulas enriched in polyunsaturated fat cause the highest levels of lipid peroxidation and provoke hepatic inflammation (10).
The role of dietary sucrose in the pathogenesis of MCD-mediated liver disease is unknown. Sucrose stimulates de novo lipogenesis (11–13) and thus has the potential to contribute to hepatic steatosis and steatohepatitis in the MCD model. The objective of this study was to determine the importance of dietary sucrose to the hepatotoxicity of the MCD formula. We addressed this by feeding mice two different MCD formulas, one that contained sucrose as the principal source of carbohydrate and the other containing starch in place of sucrose. Mice fed the sucrose-based MCD formula developed the characteristic MCD-related features of hepatic steatosis and steatohepatitis. By contrast, mice fed the starch-based MCD formula exhibited some of the features of MCD-fed mice but developed no steatosis or steatohepatitis. The principal difference between the two groups was that the starch-based diet suppressed hepatic lipogenesis and TG synthesis. This was accompanied by reduced hepatic accumulation of saturated fatty acids and significant protection against hepatocellular injury.
MATERIALS AND METHODS
Dietary studies
Adult male C3H/HeOuJ mice (The Jackson Laboratory, Bar Harbor, ME) were fed methionine-choline-sufficient (MCS) or MCD formulas as described in Table 1 for 21 days. All formulas were custom manufactured to include 18% protein, 64% carbohydrate, and 10% fat by weight (Dyets, Bethlehem, PA). Two of the four formulas used sucrose as the principal dietary carbohydrate (590 g/kg; designated MCS-sucrose and MCD-sucrose); the other two used cornstarch in place of sucrose (590 g/kg; designated MCS-starch and MCD-starch). Paired MCS and MCD formulas were matched in all nutrients except l-methionine and choline chloride. Mice were fed the custom formulas ad libitum and had free access to drinking water for the 21-day study period. At the end of the experiment, mice were fasted for 4 h prior to killing in cages designed to prevent coprophagia. All animals received humane care according to guidelines set forth by the US Public Health Service. All experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of California, San Francisco.
TABLE 1.
Composition of the custom MCS and MCD formulas
| Nutrient | MCS-Sucrose | MCS-Starch | MCD-Sucrose | MCD-Starch |
|---|---|---|---|---|
| l-Amino acids (g/kg) | 175.7 | 175.7 | 175.7 | 175.7 |
| l-Methionine (g/kg) | 2.0 | 2.0 | 0.0 | 0.0 |
| Choline chloride (g/kg) | 2.0 | 2.0 | 0.0 | 0.0 |
| Sucrose (g/kg) | 587.9 | 0.0 | 591.9 | 0.0 |
| Dextromaltose (g/kg) | 50.0 | 50.0 | 50.0 | 50.0 |
| Cornstarch (g/kg) | 0.0 | 587.9 | 0.0 | 591.9 |
| Cellulose (g/kg) | 30.0 | 30.0 | 30.0 | 30.0 |
| Corn oil (g/kg) | 100.0 | 100.0 | 100.0 | 100.0 |
| Salt mix (g/kg) | 35.0 | 35.0 | 35.0 | 35.0 |
| Sodium bicarbonate (g/kg) | 7.4 | 7.4 | 7.4 | 7.4 |
| Vitamin mix (g/kg) | 10.0 | 10.0 | 10.0 | 10.0 |
| Total (g/kg) | 1000.0 | 1000.0 | 1000.0 | 1000.0 |
Serum chemistries
Alanine aminotransferase (ALT), glucose, cholesterol, and TG were measured in mouse serum using an ADVIA 1800 autoanalyzer (Siemens Healthcare Diagnostics, Deerfield, IL) in the clinical chemistry laboratory at San Francisco General Hospital. Serum insulin, leptin, and adiponectin were measured by ELISA (Millipore-Linco, St. Charles, MO).
TG and fatty acid analysis
Lipids were extracted from fresh liver tissue using the Folch method (14). Extracts were evaporated under a stream of nitrogen and resuspended in chloroform:methanol (2:1) containing 0.01% butyrated hydroxytoluene. Aliquots were dried and resuspended in 1-butanol containing 0.01% butyrated hydroxytoluene for measurement of total TG (TR0100; Sigma Chemical, St. Louis, MO). Results were reported as milligrams of TG per gram liver.
Fatty acid analysis was performed on flash-frozen liver tissue. Lipid extraction and TrueMass® neutral lipid analysis were performed by Lipomics Technologies (West Sacramento, CA). Tissue samples were subjected to a combination of liquid- and solid-phase extraction procedures to separate neutral lipids from phospholipids, followed by thin-layer chromatography to separate neutral lipid classes and gas chromatography to quantitate individual fatty acids. All samples were processed in the presence of internal standards to monitor extraction efficiency and verify measurement accuracy.
Measurement of hepatic lipid peroxidation
Lipid peroxidation was evaluated by measuring thiobarbituric acid-reactive substances (TBARS) in liver homogenates. Liver tissue was homogenized in 1.15% potassium chloride containing 2 mM deferroxamine; TBARS were measured as described by Jozwik et al. (15). Results were expressed as nanomoles of TBARS per milligram of liver.
Measurement of hepatic fatty acid uptake
Fatty acid uptake by the liver was monitored in vivo using radiolabeled oleic acid as a tracer. After an overnight fast with free access to drinking water, mice were gavaged with 200 μl olive oil containing 2 μCi 1-14C-oleic acid (57 mCi/mmol; GE Healthcare, Piscataway, NJ). Blood was collected at 0, 30, and 240 min to verify absorption. At 240 min, the mice were killed for collection of liver tissue. Radioactivity was measured in serum and liver homogenates by scintillation counting. Uptake was expressed as cpm 14C/liver/4 h.
Measurement of hepatic TG secretion
Hepatic TG secretion was measured by monitoring the rise in serum TG resulting from administration of Triton WR-1339, which blocks lipoprotein lipase (16). After an overnight fast with free access to drinking water, mice were injected with 500 mg/kg Triton WR-1339 [10% (w/v), 5 ml/kg IV]. Blood was collected at 0 and 6 h. TG was measured in serum using an ADVIA autoanalyzer as described above.
Measurement of de novo lipogenesis, hepatic TG synthesis, and glycolytic contribution to hepatic TG-glycerol
De novo lipogenesis (DNL), TG synthesis, and the contribution of glycolysis to TG-glycerol synthesis were measured in the liver in vivo by the use of 2H2O labeling combined with mass isotopomer distribution analysis as described previously (17–19). Mice were injected with saline prepared with 99.8% 2H2O (30 ml/kg IP) and maintained at steady state by administration of drinking water containing 8% 2H2O. After 3 days of heavy water labeling, mice were fasted for 4 h and euthanized. Lipids were extracted from fresh liver tissue by the Folch method (14). TGs were separated by TLC and transesterified by incubation with 3 N methanolic HCl. Fatty acid methyl esters were separated from the glycerol fraction, and both fractions were analyzed by GC-MS.
Methyl palmitate and its isotopes (m/z 270–272 representing M0-M2) were quantified under the selected ion-monitoring mode, and mass isotopomer distribution analysis calculations were based on 22 possible sites for deuterium incorporation (20, 21). The proportion of deuterium-labeled palmitate in hepatic TG was reported as fractional DNL. The absolute amount of palmitate retained in the liver over the 3-day labeling period was calculated by multiplying fractional DNL by the total amount of palmitate in the TG fraction of the liver.
Glycerol was converted to glycerol triacetate by incubation with 2:1 acetic anhydride:pyridine and then analyzed using a DB-225 fused silica column with methane chemical ionization and selected ion monitoring (m/z 159–161 represented M0-M2). The fraction of newly synthesized hepatic TG molecules was determined from the measured body 2H2O enrichment and the experimentally determined number of hydrogen atoms incorporated into the C-H bonds of the glycerol moiety. The absolute amount of newly synthesized TG retained in the liver over the 3-day labeling period was calculated by multiplying fractional TG synthesis by the total amount of TG present in the liver (22).
The number of hydrogen atoms (n) incorporated from 2H2O into the C-H bonds of the liver TG-glycerol moiety was used to determine the metabolic source of liver α-glycerol phosphate (and hence liver TG-glycerol) (23). The relative contribution of glyceroneogenesis to liver TG-glycerol was determined by the equation [(n − 3.5)/1.5], and the relative contribution of glycolysis calculated as [1 – glyceroneogenesis] (23). Data were reported as the percentage of TG-glycerol in the liver originating from glycolysis.
Evaluation of gene expression by real-time quantitative PCR
Total RNA was extracted from mouse liver by homogenization in TRI reagent (Molecular Research Center, Cincinnati, OH), followed by chloroform extraction and ethanol precipitation. RNA was incubated with DNase (Qiagen, Valencia, CA) to remove contaminating DNA; the enzyme was then inactivated and removed according to the manufacturer's specifications (RNeasy; Qiagen). cDNA was synthesized from 1 μg RNA in a reaction mixture containing 2.5 U/μl M-MLV reverse transcriptase (Invitrogen, Carlsbad, CA) and 5 μM random hexamer primers (Invitrogen).
Real-time PCR analysis was performed using an AB Prism 7900 sequence detection system (Applied Biosystems, Foster City, CA). Assays-on-Demand® primer and probe sets (Applied Biosystems) were used for all the genes of interest. The expression of each test gene was normalized to that of mouse β-glucuronidase. Quantitative detection of specific nucleotide sequences was based on the fluorogenic 5′ nuclease assay (24). Relative gene expression was calculated using the method of Livak and Schmittgen (25).
Liver histology and quantitative scoring system
Paraffin sections of liver tissue were stained with hematoxylin and eosin. Slides were blindly evaluated and scored for steatosis, ballooning, and inflammation. Steatosis (0–4): 0 = <5%; 1 = 5–25%; 2 = 25–50%; 3 = 50–75%; 4 = 75–100%. Ballooning (0–3): 0 = absent; 1 = mild (focal involving fewer than three hepatocytes); 2 = moderate (focal involving more than three hepatocytes or multifocal); 3 = severe (multifocal with more than two foci of three or more hepatocytes). Inflammation (0–4): 0 = absent; 1 = minimal (zero to one focus per 20× field); 2 = mild (two foci); 3 = moderate (three foci); 4 = severe (four or more foci). A combined activity score was calculated as the arithmetic sum of all individual scores.
Quantitation of apoptotic cells in mouse liver sections
Apoptotic cells were identified in tissue sections by terminal deoxynucleotide transferase-mediated deoxyuridine triphosphate nick end-labeling (TUNEL) (ApopTag Plus peroxidase in situ apoptosis detection kit; Millipore, Billerica, MA). Sections were counterstained with methyl green for viewing and photography. TUNEL-positive cells were counted in five randomly selected 10× microscopic fields per liver. The results were reported as the average number of positive cells per field.
Statistical methods
Experiments included 4–10 mice per study group. Mean values were compared by two-way ANOVA. P values < 0.05 were considered statistically significant.
RESULTS
The carbohydrate composition of the MCD formula does not affect its influence on body weight, serum glucose or serum lipids
Commercial MCS and MCD formulas (e.g., MP Biomedicals #960439 and Harlan Teklad #90262) typically contain 65% carbohydrate by weight, provided as a 70:30 mixture of sucrose and cornstarch (46% sucrose and 19% starch). In this experiment, we prepared custom MCS and MCD formulas in which nearly the entire carbohydrate fraction (59%) was composed of either pure sucrose or pure cornstarch. A small amount of complex carbohydrate was retained in each formula to permit compounding into pellets (Table 1). Mice fed the sucrose- or starch-enriched formulas exhibited many typical responses to MCS and MCD feeding. Specifically, MCS-fed mice gained weight and MCD-fed mice lost weight, respectively, and exhibited serum leptin levels that paralleled their adipose tissue mass (Table 2). In addition, MCS-fed mice developed hyperglycemia and hyperlipidemia, whereas MCD-fed mice remained normoglycemic and developed hypolipidemia, and MCD-fed mice were more insulin sensitive than MCS controls (1, 26, 27). For the most part, the biochemical abnormalities caused by the MCS and MCD formulas were comparable regardless of their carbohydrate content. The only exception was serum cholesterol, which was diminished to a lesser degree in MCD-starch mice than MCD-sucrose mice. Serum cholesterol was lower in starch-fed control (MCS) mice than sucrose-fed control mice, consistent with previous reports documenting the hypocholesterolemic nature of complex dietary carbohydrate (28–30). Why this same effect was not observed in the MCD groups with different dietary carbohydrate is unknown.
TABLE 2.
Clinical and biochemical data from mice fed MCS and MCD formulas
| MCS-Sucrose | MCS-Starch | MCD-Sucrose | MCD-Starch | |
|---|---|---|---|---|
| Body weight change (%) | 21.8 ± 2.4 | 15.0 ± 2.5 | −29.1 ± 0.7b | −23.4 ± 0.8cd |
| Liver weight/body weight (%) | 4.8 ± 0.2 | 4.3 ± 0.1 | 6.3 ± 0.3b | 4.3 ± 0.5d |
| Adipose weight/body weight (%) | 4.6 ± 0.2 | 4.3 ± 0.3 | 0.5 ± 0.1b | 0.5 ± 0.1c |
| Serum TG (mg/dl) | 158.3 ± 12.2 | 135.9 ± 11.2 | 75.4 ± 2.1b | 84.1 ± 6.7c |
| Serum cholesterol (mg/dl) | 261.7 ± 18.6 | 162.8 ± 5.3a | 85.7 ± 2.8b | 101.6 ± 3.0cd |
| Serum glucose (mg/dl) | 385.6 ± 42.3 | 284.3 ± 11.1 | 173.8 ± 20.0b | 209.2 ± 9.7c |
| Serum insulin (ng/ml) | 1.04 ± 0.28 | 1.07 ± 0.23 | 0.2 ± 0.1b | 0.1 ± 0.0c |
| QUICKI | 0.18 ± 0.0 | 0.19 ± 0.01 | 0.23 ± 0.01b | 0.23 ± 0.0c |
| Serum leptin (ng/ml) | 26.68 ± 3.96 | 16.88 ± 2.65 | 0.3 ± 0.1b | 0.2 ± 0.1c |
| Serum adiponectin (μg/ml) | 4.2 ± 0.2 | 4.3 ± 0.2 | 5.3 ± 0.4b | 4.7 ± 0.3 |
| Hepatic TG (mg/g liver) | 42.9 ± 8.9 | 23.2 ± 3.3 | 115.2 ± 11.3b | 34.4 ± 2.9cd |
| Serum ALT (IU/l) | 56.2 ± 6.2 | 59.6 ± 7.0 | 571.3 ± 74.7b | 93.0 ± 8.8cc |
Values represent mean ± SEM for n = 10. QUICKI, qualitative insulin sensitivity check index = (log insulinpg/ml + log glucosemg/dl)−1.
P < 0.05 for MCS-starch versus MCS-sucrose.
P < 0.05 for MCD-sucrose versus MCS-sucrose.
P < 0.05 for MCD-starch versus MCS-starch.
P < 0.05 for MCD-starch versus MCD-sucrose.
The MCD-sucrose formula, but not MCD-starch, induces steatohepatitis
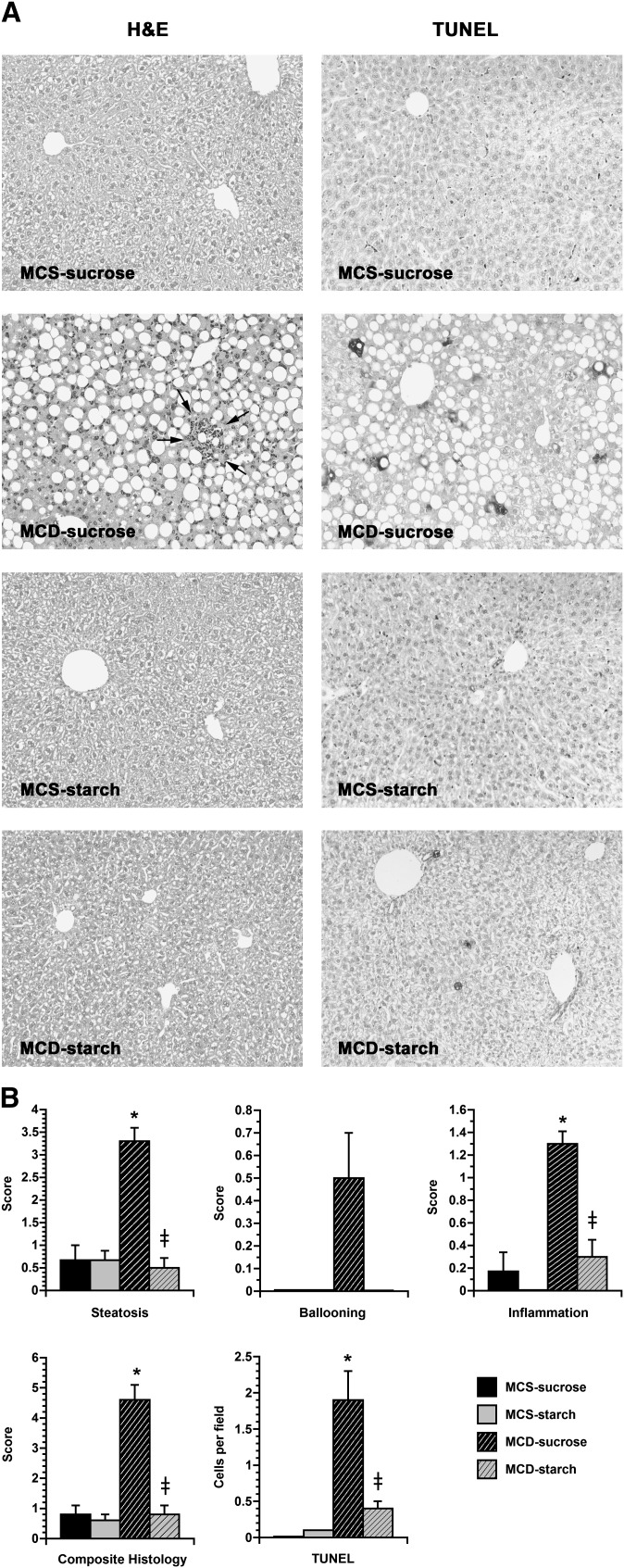
As has been shown previously with commercial MCD formulas containing 46% sucrose (1, 2, 27, 31), our custom MCD formula with 59% sucrose caused steatohepatitis. Mice fed MCD-sucrose displayed several features of hepatic steatosis, including a high liver weight/body weight ratio, elevated hepatic TG content, and prominent fat accumulation by histology (Table 2; Fig. 1). They also exhibited substantial hepatocellular injury, as shown by a markedly elevated serum ALT level as well as histologic ballooning and apoptosis (Table 2, Fig. 1). Liver histology in MCD-sucrose mice also revealed hepatic inflammation. The combined histologic activity score in MCD-sucrose mice was 4.6 ± 0.5 compared with 0.6 ± 0.2 in MCS-sucrose controls (P < 0.0001). In striking contrast to the liver disease that developed in mice fed the MCD-sucrose formula, hepatic abnormalities were much less prominent in mice fed the starch-enriched MCD formula for 21 days. MCD-starch mice accumulated more hepatic fat than MCS-starch controls, but much less than MCD-sucrose mice. Serum ALT was only mildly elevated in MCD-starch mice, and they displayed almost no hepatocellular ballooning, apoptosis, or inflammation, achieving a combined histologic activity score of only 0.8 ± 0.3 (P < 0.0001 vs. MCD-sucrose).
Fig. 1.
Liver histology and scoring in mice fed custom MCS and MCD formulas. A: Photomicrographs illustrate liver sections from mice fed MCS or MCD formulas for 21 days, stained with hematoxylin and eosin or a peroxidase-based TUNEL reagent. Mice fed the two control formulas (MCS-sucrose and MCS-starch) showed mild vacuolization of hepatocytes but no obvious steatosis and no TUNEL-positive cells. Mice fed the MCD-sucrose formula displayed prominent hepatic steatosis and inflammatory foci, as well as scattered TUNEL-positive cells. MCD-starch mice showed no significant steatosis, slight inflammation, and rare TUNEL-positive cells. Original magnification: ×10. B: Histograms depict the quantitative histology scores from mice in the four treatment groups. Steatosis, ballooning, and inflammation were all increased in MCD-sucrose mice compared with MCD-starch mice and MCS controls, yielding a high composite histology score. TUNEL-positive cells were significantly more numerous in MCD-sucrose mice than in the other three treatment groups. Values represent mean ± SEM for n = 10. * P < 0.05 for MCD versus carbohydrate-matched MCS. ‡ P < 0.05 for MCD-starch versus MCD-sucrose.
Hepatic steatosis in MCD-sucrose mice is attributable to de novo lipogenesis
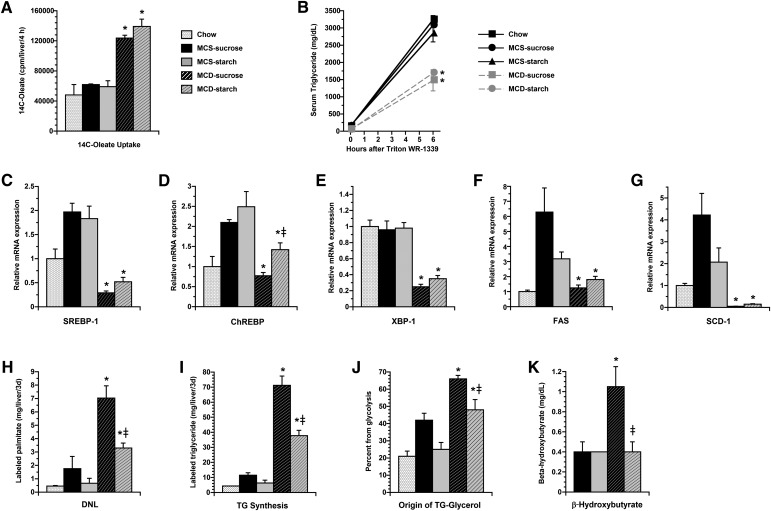
In an effort to explain why the MCD-sucrose and MCD-starch formulas led to such marked differences in hepatic lipid accumulation, we investigated whether the two diets have divergent effects on hepatic lipid transport. As expected, MCD feeding stimulated fatty acid uptake by the liver, but there was no difference in uptake between the MCD-sucrose and MCD-starch groups (Fig. 2A). MCD feeding also suppressed hepatic TG secretion (Fig. 2B); again, there was no difference between the sucrose- or starch-enriched MCD groups. We then explored whether MCS-sucrose and MCS-starch formulas have distinct effects on hepatic lipogenesis. Previous studies have shown that methionine and choline deprivation does not stimulate lipogenic gene expression in the liver, and, in some instances, suppresses lipogenic genes (27). To test whether dietary carbohydrate influences the effect of MCD feeding on lipogenic gene expression, we measured mRNA encoding carbohydrate-responsive element binding protein (ChREBP), sterol regulatory element binding protein-1 (SREBP-1), X-box protein-1 (XBP-1), FAS, and stearoyl-CoA desaturase-1 (SCD-1) in the livers of MCD-fed mice and their respective controls (Fig. 2C–G) (32–34). Each of these genes was less abundant in MCD mice than MCS controls, and some (SREBP-1, XBP-1, and SCD-1) were suppressed even below the levels measured in chow-fed mice. Notably, these genes were expressed similarly in MCD-sucrose mice and MCD-starch mice despite their difference in dietary carbohydrate composition. This was true for all except ChREBP, which paradoxically was less abundant in MCD-sucrose than MCD-starch mice. In contrast to lipogenic gene expression, hepatic DNL, measured as the amount of newly synthesized palmitate accumulating in the liver over a 3-day period, was significantly higher in MCD mice than controls (Fig. 2H). The high values for DNL in MCD-fed mice may be due in part to their defect in hepatic lipid export, which promotes the retention of newly synthesized palmitate in the liver and contributes to an elevation in the measurement over the labeling interval. This does not affect comparisons between MCD-sucrose and MCD-starch mice because both groups share the same abnormality in hepatic lipid secretion. Despite comparable levels of lipogenic gene expression, DNL measured by metabolic labeling was twice as high in MCD-sucrose mice as MCD-starch mice (P < 0.01) (Fig. 2H). Hepatic TG synthesis in MCD and control mice exhibited the same pattern as DNL. MCD-fed mice accumulated more new TG than any of the control mice, again probably due to their impairment in TG secretion. Still, MCD-sucrose mice accumulated twice as much new TG as MCD-starch mice, indicating a true difference in TG synthesis between these two groups (Fig. 2I). MCD-sucrose mice also demonstrated significantly greater utilization of glycolysis than MCD-starch mice in the production of hepatic glycerol, which suggests they are actively using carbohydrate for all aspects of acylglycerol synthesis (Fig. 2J). In aggregate, these results suggest that a significant proportion of fat that accumulates in the livers of MCD livers derives from carbohydrate and that the reduced carbohydrate utilization in MCD-starch mice limits their hepatic lipid accumulation. Finally, to exclude the possibility that MCD-starch mice avoid hepatic steatosis by upregulating fatty acid oxidation, we measured serum levels of β-hydroxybutyrate in the four dietary groups. MCD-starch mice showed no evidence of enhanced fatty oxidation, as their β-hydroxybutyrate levels were well below those in MCD-sucrose mice and no higher than MCS controls (Fig. 2K).
Fig. 2.
Hepatic lipid metabolism in MCS and MCD mice after 21 days on experimental diets. A: Histogram depicts hepatic uptake of 14C-oleate 4 h after oral administration as outlined in Materials and Methods. Uptake was increased in MCD-sucrose and MCD-starch mice compared with control mice, with no difference between the two groups. B: Serum TG levels at 0 and 6 h after IV administration of Triton WR-1339. TG levels increased as expected in control mice but rose much less in MCD mice with no difference between MCD-sucrose and MCD-starch mice. C–G: Hepatic expression of mRNAs encoding SREBP-1, ChREBP, XBP-1, FAS, and SCD-1. All genes were less abundant in MCD-fed mice than MCS controls. There was no difference in expression between MCD-sucrose and MCD-starch except for ChREBP, which was paradoxically lower in MCD-sucrose than MCD-starch mice. H, I: Hepatic DNL and TG synthesis. DNL and TG synthesis were measured in the liver during the final 3 days of experimental feeding as described in Materials and Methods. Both were significantly higher in MCD mice than controls. DNL was reduced by 53% in MCD-starch mice compared with MCD-sucrose mice. TG synthesis was reduced by 46% in MCD-starch mice compared with MCD-sucrose mice. J: Origin of TG-glycerol. The relative contribution of glycolysis to hepatic glycerol synthesis was measured as described in Materials and Methods. MCD-sucrose mice displayed the highest value among all dietary groups, 38% higher than MCD-starch mice. K: Serum β-hydroxybutyrate. Mice were fasted for 4 h prior to blood collection. β-Hydroxybutyrate levels were higher in MCD-sucrose mice than all other treatment groups. Values represent mean ± SEM for n = 4 (A, B, and H–J), n = 5 (C–G), or n = 10 (K). * P < 0.05 for MCD versus carbohydrate-matched MCS. ‡ P < 0.05 for MCD-starch versus MCD-sucrose.
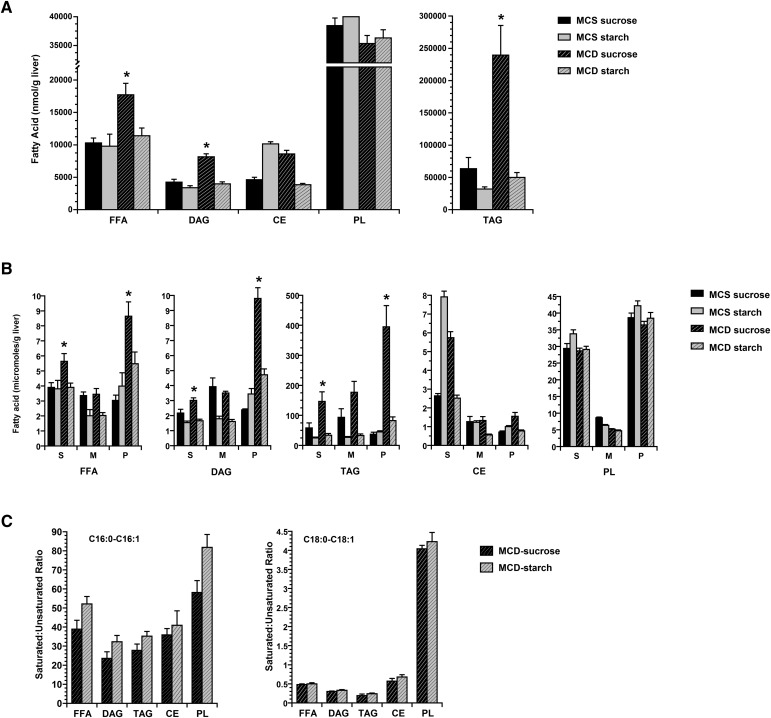
MCD-sucrose mice accumulate more saturated fatty acids in the liver than MCD-starch mice
DNL yields palmitate, a long-chain saturated fatty acid that has been implicated as a mediator of hepatic lipotoxicity (35–38). This is particularly relevant in the MCD model because MCD feeding also suppresses SCD-1, the enzyme that converts long-chain saturated fatty acids to less toxic monounsaturated species (39). The combination of enhanced DNL and suppressed SCD-1 should result in significant accumulation of saturated fatty acid in the liver, an outcome that may be exacerbated even further by the inability of MCD-fed mice to effectively export hepatic TG. This buildup of saturated fatty acid could be the cause of hepatocellular injury in the MCD model. MCD-starch mice are similar to MCD-sucrose mice in SCD-1 expression and hepatic lipid secretion (Fig. 2B, G). DNL in MCS-starch mice, however, is significantly reduced (Fig. 2H). Consequently, MCD-starch mice may exhibit a less saturated hepatic lipid profile than MCD-sucrose mice, which may be an important factor in their protection against liver injury. We analyzed hepatic lipids in MCS and MCD mice and found that MCD-sucrose mice accumulated excess fatty acid in three lipid compartments: FFA, diacylglycerol (DAG), and triacylglycerol (TAG) (Fig. 3A). In contrast, MCD-sucrose mice had diminished levels of hepatic phospholipid, as did MCD-starch mice, consistent with their deficiency in methionine and choline. We then analyzed individual fatty acids in MCS and MCD livers and found that MCD-sucrose mice accumulated excess saturated fatty acid (SFA) in all three lipid compartments with excess total fatty acid (FFA 140%, DAG 140%, and TAG 250%) (Fig. 3B). The individual SFAs that accumulated in these livers were the long-chain species palmitate (C16:0) and stearate (C18:0) (see supplementary Fig. I). Lastly, we calculated the ratio of saturated to monounsaturated long-chain fatty acids in the liver, which increases in mice fed commercial MCD formulas and has been postulated to play a role in MCD-mediated liver injury (27). The saturated-to-monounsaturated fatty acid ratios were no higher in MCD-sucrose mice than MCD-starch mice (Fig. 3C). This suggests that an absolute, rather than relative, increase in long-chain SFAs in the liver coincides with the development of MCD-mediated liver injury.
Fig. 3.
Hepatic lipid analysis in MCS and MCD mice at 21 days. A: Histogram shows the total amount of fatty acid in individual hepatic lipid compartments, including FFA, DAG, TAG, phospholipid, and cholesterol ester. MCD-sucrose mice had significantly more fatty acid than the other three groups in the FFA, DAG, and TAG compartments. B: Saturated (S), monounsaturated (M), and polyunsaturated (P) fatty acid in individual lipid compartments. MCD-sucrose mice had more saturated and polyunsaturated fatty acid in FFA, DAG, and TAG than all other groups of mice. C: Ratio of monounsaturated to polyunsaturated long-chain fatty acids (C16:0-C16:1 and C18:0-C18:1) in MCD-sucrose and MCD-starch mice. Substituting sucrose with starch in the MCD formula had no influence on the ratio. Values represent mean ± SEM for n = 5. * P < 0.05 for MCD-sucrose versus all other dietary groups.
Polyunsaturated fat, oxidative stress, and cytokine induction in MCD-sucrose and MCD-starch mice
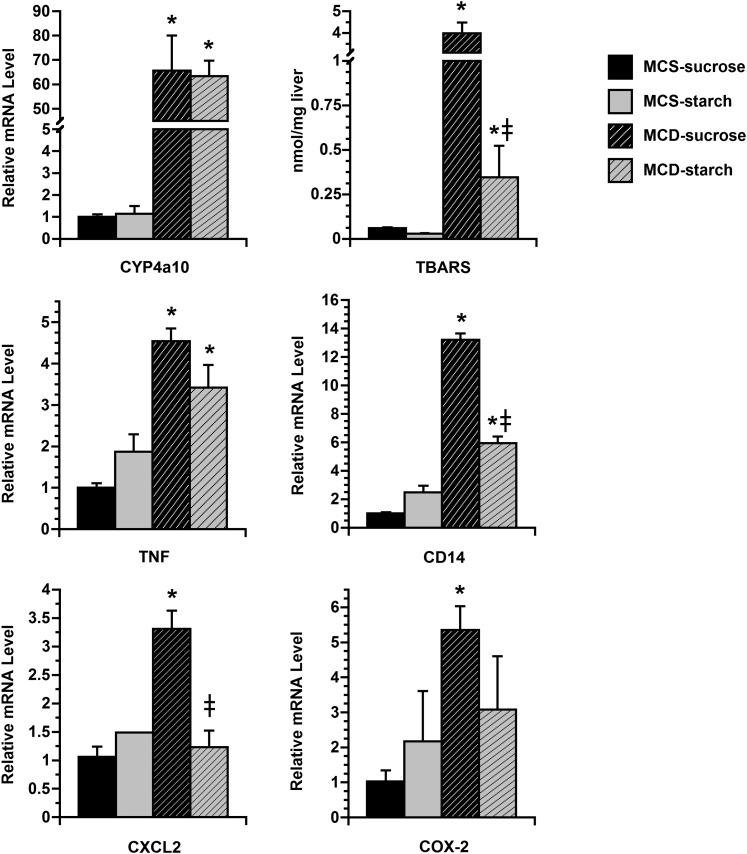
Our fatty acid analysis demonstrated that in addition to SFA, MCD-sucrose mice accumulated excess PUFA in the liver (FFA 160%, DAG 210%, and TAG 476%) (Fig. 3B). The individual PUFA that increased most significantly in MCD-sucrose mice in comparison to the other dietary groups were linoleate (C18:2) and arachidonate (C20:4) (see supplementary Fig. I). These fatty acids could come either from the diet or from adipose tissue, although the diet is more likely, due to the high linoleate content of corn oil used as fat in the MCS and MCD formulas. PUFA may play a role in MCD-related liver disease because of their ability to act as substrates for cytochrome P450-mediated lipid peroxidation. PUFA metabolism causes oxidant stress in the liver, which in turn can stimulate the local production of pro-inflammatory cytokines. To determine the influence of MCD-sucrose and MCD-starch formulas on these processes, we measured the hepatic expression of cytochrome P450 4a10 (CYP4a10) in mice fed MCD and control diets as well as hepatic lipid peroxidation and the expression of several pro-inflammatory genes (Fig. 4). CYP4a10 mRNA was strongly induced in the livers of all MCD mice, without any difference resulting from modulation of dietary carbohydrate. Lipid peroxidation (measured as TBARs), however, was much more evident in MCD-sucrose mice than MCD-starch mice, presumably due to their abundance of hepatic PUFA. Genes involved in inflammation (tumor necrosis factor-α, CD14, CXC chemokine ligand-2, and cyclooxygenase-2) were also upregulated more strongly in the livers of MCD-sucrose mice than MCD-starch mice. When inflammatory gene expression and hepatic lipid peroxidation were compared directly, a linear relationship was observed (P = 0.01–0.09; data not shown), substantiating previous reports that inflammatory gene expression correlates with hepatic lipid peroxidation in MCD-fed mice (10).
Fig. 4.
Markers of oxidant stress and inflammation in the livers of MCS and MCD mice at 21 days. Histograms illustrate hepatic CYP4a10 mRNA expression and TBARS as well as the expression of several inflammatory genes in the liver after 21 days on experimental diets. CYP4a10 expression was induced similarly in MCD-sucrose and MCD-starch mice compared with MCS controls. Coincident with the upregulation of CYP4a10, hepatic TBARS were markedly elevated in MCD-sucrose mice. By contrast, TBARS were not increased in MCD-starch mice. mRNA encoding tumor necrosis factor-α (TNF), CD14, CXC chemokine ligand-2 (CXCL2), and cyclooxygenase-2 (COX-2) were all increased in the livers of MCD-sucrose mice compared with MCS controls. These genes were induced less robustly in MCD-starch mice. Values represent mean ± SEM for n = 5. * P < 0.05 for MCD versus carbohydrate-matched MCS. ‡ P < 0.05 for MCD-starch versus MCD-sucrose.
DISCUSSION
Although methionine and choline deprivation is known to cause a number of disturbances in hepatic lipid metabolism (2, 5, 27), the reason why MCD diets provoke significant liver injury has remained elusive. Emerging data indicate that specific dietary nutrients, when coupled with methionine and choline deprivation, can influence MCD-mediated liver disease. This study demonstrates that MCD-mediated steatohepatitis is absolutely dependent on the presence sucrose in the MCD formula. Dietary sucrose is in fact more important than dietary fat, which can modulate hepatic lipid peroxidation and hepatic inflammation in the MCD model but has no impact on MCD-mediated steatosis or hepatocellular injury (10).
The importance of sucrose to the pathogenesis of MCD-mediated steatohepatitis was established by removing it from the MCD formula. Substituting this simple sugar with complex carbohydrate significantly diminished all the features of MCD-mediated liver disease, including hepatic steatosis, hepatocellular apoptosis, lipid peroxidation, and inflammation. This was true even though the starch-enriched MCD formula still provoked several characteristic features of MCD-fed animals, including weight loss and hypolipidemia. Most importantly, mice fed MCD-starch diet never developed overt hepatic steatosis. In the absence of steatosis, there was little additional evidence of liver injury or inflammation.
Substituting sucrose with starch in the MCD formula did not prevent hepatic steatosis by interrupting any of the derangements in hepatic lipid metabolism that are known to be precipitated by MCD feeding. Specifically, dietary starch did not suppress MCD-mediated stimulation of hepatic fatty acid uptake nor did it reverse MCD-related impairment of hepatic TG secretion. Starch also caused no apparent increase in hepatic fatty acid oxidation that could potentially reduce steatosis. Instead, the principal consequence of including starch in the MCD formula was a reduction in hepatic DNL. Sucrose is a much more potent stimulus to DNL than starch (40–42); thus, the difference in hepatic DNL between MCD-sucrose mice and MCD-starch mice is not surprising. More noteworthy is that the difference was not accompanied by a disparity in lipogenic gene expression between the two dietary groups. This discordance between the metabolic and molecular data indicates that flux through the lipogenic pathway in the liver cannot be predicted solely by gene expression, as noted recently in a study involving adipose tissue (43). Importantly, our metabolic experiments revealed that DNL plays a dominant role in MCD-mediated liver disease. Substituting sucrose with starch in the MCD formula decreased DNL by 53% and hepatic TG synthesis by 55%, resulting in a 66% reduction in hepatic lipid content.
The reduced hepatic DNL in MCD-starch mice coincided with a significant reduction in the amount of long-chain saturated fatty acid present in the liver. This, in turn, was accompanied by a significant diminution of hepatocyte apoptosis and ALT release. These findings point to long-chain saturated fatty acids, produced from dietary sucrose via DNL, as mediators of hepatocyte injury in the MCD model. Long-chain saturated fatty acids are highly toxic to hepatocytes. They kill cells by inducing endoplasmic reticulum stress (38) and activating mitochondrial apoptosis pathways (35–37, 44). Given the prominent hepatocyte apoptosis and the striking ALT elevation that occurred after 21 days of MCD-sucrose feeding, these abnormalities are likely due to saturated fatty acid-induced lipotoxicity. It should be noted that long-chain saturated fatty acids were not the only fatty acids to accumulate in MCD-sucrose livers. PUFAs were also disproportionately increased. Although we cannot entirely exclude a contribution of PUFA to hepatocyte injury based on the current experiments, other studies from our laboratory suggest that this is not the case. We previously employed dietary means to reduce the PUFA content of MCD livers to the levels found in MCS controls. This did not result in any improvement in MCD-mediated ALT release or apoptosis (10). Taken together, these findings suggest that excess SFAs rather than excess PUFAs are responsible for hepatocellular injury in mice fed sucrose-enriched MCD formulas. PUFAs may play a separate role in MCD-mediated liver injury, specifically as inducers of cytokines and inflammation. In this study as well as others, the induction of pro-inflammatory cytokines in the livers of MCD mice parallels hepatic lipid peroxidation, which is directly related to hepatic PUFA content (10). Overall, these findings suggest that dietary sucrose is the driving force behind MCD-mediated hepatic lipid accumulation and liver injury, whereas dietary PUFA regulate MCD-mediated hepatic inflammation. In combination, the two nutrients produce the complete picture of MCD-mediated steatohepatitis.
Although the suppression of DNL in MCD-starch mice can explain why they accumulated less hepatic SFA than MCD-sucrose mice, there is no ready explanation why MCD-starch mice also accumulated less hepatic PUFA. This observation suggests that in MCD-sucrose mice, the accumulation of SFA and PUFA is somehow connected, and both are downstream of DNL. One can envision the following scenario: because the saturated fatty acids produced by DNL in MCD-sucrose livers cannot be desaturated due to low SCD-1 activity, the mice are at high risk of hepatocellular lipotoxicity. Esterification into TG could reduce this toxicity (45), but SFA cannot effectively combine with each other to form TG due to fluidity constraints (46). Unsaturated fatty acid is needed to package the SFA into TG; dietary PUFA can be used for this purpose. If dietary PUFAs are in fact used to shunt newly synthesized SFA into the TG compartment, the end result would be TG that contains excess SFA as well as excess PUFA. This fits our experimental observations but requires further study for confirmation. In support of this theory, hepatic TG synthesis has already been shown to be an important defense against MCD-mediated hepatotoxicity. When mice fed a sucrose-rich MCD formula were prevented from producing hepatic TG by suppressing the enzyme diacylglycerol acyltransferase 2, they developed an unusually severe form of MCD-mediated steatohepatitis characterized by extremely high ALT levels (47).
Noteworthy in our experiments was that MCD-sucrose and MCD-starch mice exhibited markedly different outcomes despite similar degrees of methionine and choline deprivation. This indicates that methionine and choline deprivation by itself is not hepatotoxic, at least over a 3-week interval. Indeed, it is remarkable that hepatic phospholipids in MCD mice remain at near-normal levels despite a complete cutoff of exogenous precursors. This implies the existence of strong homeostatic mechanisms to preserve cellular phospholipids under conditions of stress, which could be achieved by regulation of VLDL secretion, biliary phospholipid secretion, or both (48, 49). Ip et al. (50) made a similar observation previously. They noted that MCD-fed mice treated with a peroxisome proliferator-activated receptor α agonist to stimulate fatty acid oxidation did not develop hepatic steatosis or steatohepatitis. In this situation as well, all features of steatohepatitis were eliminated from MCD-fed animals without influencing the underlying methionine and choline deficiency. Together, these studies confirm that methionine and choline deficiency is necessary, but not sufficient, to promote steatohepatitis in the MCD model. A second insult in the form of excess sucrose represents the essential first step to the development of hepatic steatosis and ultimately more serious liver disease in the MCD model.
In summary, our experiments demonstrate that dietary sucrose is essential to the pathogenesis of steatohepatitis in the MCD model of liver disease. Sucrose provokes DNL, which yields saturated fatty acids that become trapped in the liver due to an MCD-related defect in hepatic lipid secretion. The accumulation of excess SFA coincides with hepatocellular injury, manifest as ALT release and histologic apoptosis. Dietary PUFAs also accumulate in the liver, possibly in an effort to reduce SFA-induced lipotoxicity. They contribute to MCD-mediated liver disease by promoting lipid peroxidation and pro-inflammatory cytokine induction. Substituting sucrose with starch in the MCD formula prevents all the features of MCD-mediated liver disease, including steatosis, apoptosis, lipid peroxidation, and cytokine induction. Starch likely reduces hepatocellular injury in MCD-fed mice by reducing hepatic DNL. The mechanism by which it prevents PUFA accumulation and hepatic inflammation is currently unknown. In human beings with nonalcoholic fatty liver disease, as much as 26% of hepatic lipid is produced by DNL (51). This study emphasizes the harmful potential of DNL, particularly when the saturated fatty acids produced by this process cannot be detoxified or exported from the liver. It also demonstrates that eliminating simple sugar from the diet can reduce DNL and its toxic consequences in the liver.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the technical assistance of Gene S. Lee and Miriam Chen.
Footnotes
Abbreviations:
- ALT
- alanine aminotransferase
- ChREBP
- carbohydrate responsive element binding protein
- CYP4a10
- cytochrome P450 4a10
- DNL
- de novo lipogenesis
- DAG
- diacylglycerol
- MCD
- methionine-choline-deficient
- MCS
- methionine choline-sufficient
- SCD-1
- stearoyl-CoA desaturase-1
- SFA
- saturated fatty acid
- SREBP-1
- sterol regulatory element binding protein-1
- TAG
- triacylglycerol
- TBARS
- thiobarbituric acid-reactive substances
- TG
- triglyceride
- TUNEL
- terminal deoxynucleotidyl transferase dUTP nick end labeling
- XBP-1
- X-box protein-1
This work was supported in part by National Institutes of Health Grants R01 DK-068450 (J.J.M.) and T32 DK-007762 (M.K.P.), the Kanzawa Medical Research Foundation (H.O.), and Grant P30 DK-026743 (University of California, San Francisco, Liver Center). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of one figure.
REFERENCES
- 1.Leclercq I. A., Farrell G. C., Field J., Bell D. R., Gonzalez F. J., Robertson G. R. 2000. CYP2E1 and CYP4A as microsomal catalysts of lipid peroxides in murine nonalcoholic steatohepatitis. J. Clin. Invest. 105: 1067–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rinella M. E., Elias M. S., Smolak R. R., Fu T., Borensztajn J., Green R. M. 2008. Mechanisms of hepatic steatosis in mice fed a lipogenic methionine choline-deficient diet. J. Lipid Res. 49: 1068–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schattenberg J. M., Singh R., Wang Y., Lefkowitch J. H., Rigoli R. M., Scherer P. E., Czaja M. J. 2006. JNK1 but not JNK2 promotes the development of steatohepatitis in mice. Hepatology. 43: 163–172. [DOI] [PubMed] [Google Scholar]
- 4.Kulinski A., Vance D. E., Vance J. E. 2004. A choline-deficient diet in mice inhibits neither the CDP-choline pathway for phosphatidylcholine synthesis in hepatocytes nor apolipoprotein B secretion. J. Biol. Chem. 279: 23916–23924. [DOI] [PubMed] [Google Scholar]
- 5.Yao Z. M., Vance D. E. 1988. The active synthesis of phosphatidylcholine is required for very low density lipoprotein secretion from rat hepatocytes. J. Biol. Chem. 263: 2998–3004. [PubMed] [Google Scholar]
- 6.Deng Q. G., She H., Cheng J. H., French S. W., Koop D. R., Xiong S., Tsukamoto H. 2005. Steatohepatitis induced by intragastric overfeeding in mice. Hepatology. 42: 905–914. [DOI] [PubMed] [Google Scholar]
- 7.Feldstein A. E., Canbay A., Guicciardi M. E., Higuchi H., Bronk S. F., Gores G. J. 2003. Diet associated hepatic steatosis sensitizes to Fas mediated liver injury in mice. J. Hepatol. 39: 978–983. [DOI] [PubMed] [Google Scholar]
- 8.Smith S. J., Cases S., Jensen D. R., Chen H. C., Sande E., Tow B., Sanan D. A., Raber J., Eckel R. H., Farese R. V., Jr 2000. Obesity resistance and multiple mechanisms of triglyceride synthesis in mice lacking Dgat. Nat. Genet. 25: 87–90. [DOI] [PubMed] [Google Scholar]
- 9.Larter C. Z., Yeh M. M., Haigh W. G., Williams J., Brown S., Bell-Anderson K. S., Lee S. P., Farrell G. C. 2008. Hepatic free fatty acids accumulate in experimental steatohepatitis: role of adaptive pathways. J. Hepatol. 48: 638–647. [DOI] [PubMed] [Google Scholar]
- 10.Lee G. S., Yan J. S., Ng R. K., Kakar S., Maher J. J. 2007. Polyunsaturated fat in the methionine-choline-deficient diet influences hepatic inflammation but not hepatocellular injury. J. Lipid Res. 48: 1885–1896. [DOI] [PubMed] [Google Scholar]
- 11.Towle H. C. 2005. Glucose as a regulator of eukaryotic gene transcription. Trends Endocrinol. Metab. 16: 489–494. [DOI] [PubMed] [Google Scholar]
- 12.Uyeda K., Repa J. J. 2006. Carbohydrate response element binding protein, ChREBP, a transcription factor coupling hepatic glucose utilization and lipid synthesis. Cell Metab. 4: 107–110. [DOI] [PubMed] [Google Scholar]
- 13.Yamashita H., Takenoshita M., Sakurai M., Bruick R. K., Henzel W. J., Shillinglaw W., Arnot D., Uyeda K. 2001. A glucose-responsive transcription factor that regulates carbohydrate metabolism in the liver. Proc. Natl. Acad. Sci. USA. 98: 9116–9121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Folch J., Lees M., Sloane Stanley G. H. 1957. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226: 497–509. [PubMed] [Google Scholar]
- 15.Jozwik M., Wolczynski S., Jozwik M., Szamatowicz M. 1999. Oxidative stress markers in preovulatory follicular fluid in humans. Mol. Hum. Reprod. 5: 409–413. [DOI] [PubMed] [Google Scholar]
- 16.Bhatia A. J., Wade G. N. 1991. Effects of pregnancy and ovarian steroids on fatty acid synthesis and uptake in Syrian hamsters. Am. J. Physiol. 260: R153–R158. [DOI] [PubMed] [Google Scholar]
- 17.Hellerstein M. K., Christiansen M., Kaempfer S., Kletke C., Wu K., Reid J. S., Mulligan K., Hellerstein N. S., Shackleton C. H. 1991. Measurement of de novo hepatic lipogenesis in humans using stable isotopes. J. Clin. Invest. 87: 1841–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hellerstein M. K., Kletke C., Kaempfer S., Wu K., Shackleton C. H. 1991. Use of mass isotopomer distributions in secreted lipids to sample lipogenic acetyl-CoA pool in vivo in humans. Am. J. Physiol. 261: E479–E486. [DOI] [PubMed] [Google Scholar]
- 19.Hellerstein M. K., Neese R. A. 1999. Mass isotopomer distribution analysis at eight years: theoretical, analytic, and experimental considerations. Am. J. Physiol. 276: E1146–E1170. [DOI] [PubMed] [Google Scholar]
- 20.Jones P. J. 1996. Tracing lipogenesis in humans using deuterated water. Can. J. Physiol. Pharmacol. 74: 755–760. [PubMed] [Google Scholar]
- 21.Lee W. N., Bassilian S., Ajie H. O., Schoeller D. A., Edmond J., Bergner E. A., Byerley L. O. 1994. In vivo measurement of fatty acids and cholesterol synthesis using D2O and mass isotopomer analysis. Am. J. Physiol. 266: E699–E708. [DOI] [PubMed] [Google Scholar]
- 22.Turner S. M., Murphy E. J., Neese R. A., Antelo F., Thomas T., Agarwal A., Go C., Hellerstein M. K. 2003. Measurement of TG synthesis and turnover in vivo by 2H2O incorporation into the glycerol moiety and application of MIDA. Am. J. Physiol. Endocrinol. Metab. 285: E790–E803. [DOI] [PubMed] [Google Scholar]
- 23.Chen J. L., Peacock E., Samady W., Turner S. M., Neese R. A., Hellerstein M. K., Murphy E. J. 2005. Physiologic and pharmacologic factors influencing glyceroneogenic contribution to triacylglyceride glycerol measured by mass isotopomer distribution analysis. J. Biol. Chem. 280: 25396–25402. [DOI] [PubMed] [Google Scholar]
- 24.Ginzinger D. G. 2002. Gene quantification using real-time quantitative PCR: an emerging technology hits the mainstream. Exp. Hematol. 30: 503–512. [DOI] [PubMed] [Google Scholar]
- 25.Livak K. J., Schmittgen T. D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 26.Rinella M. E., Green R. M. 2004. The methionine-choline deficient dietary model of steatohepatitis does not exhibit insulin resistance. J. Hepatol. 40: 47–51. [DOI] [PubMed] [Google Scholar]
- 27.Rizki G., Arnaboldi L., Gabrielli B., Yan J., Lee G. S., Ng R. K., Turner S. M., Badger T. M., Pitas R. E., Maher J. J. 2006. Mice fed a lipogenic methionine-choline-deficient diet develop hypermetabolism coincident with hepatic suppression of SCD-1. J. Lipid Res. 47: 2280–2290. [DOI] [PubMed] [Google Scholar]
- 28.Marckmann P., Raben A., Astrup A. 2000. Ad libitum intake of low-fat diets rich in either starchy foods or sucrose: effects on blood lipids, factor VII coagulant activity, and fibrinogen. Metabolism. 49: 731–735. [DOI] [PubMed] [Google Scholar]
- 29.Pfeuffer M., Barth C. A. 1992. Dietary sucrose but not starch promotes protein-induced differences in rates of VLDL secretion and plasma lipid concentrations in rats. J. Nutr. 122: 1582–1586. [DOI] [PubMed] [Google Scholar]
- 30.Robert L., Narcy A., Rayssiguier Y., Mazur A., Remesy C. 2008. Lipid metabolism and antioxidant status in sucrose vs. potato-fed rats. J. Am. Coll. Nutr. 27: 109–116. [DOI] [PubMed] [Google Scholar]
- 31.Sahai A., Malladi P., Pan X., Paul R., Melin-Aldana H., Green R. M., Whitington P. F. 2004. Obese and diabetic db/db mice develop marked liver fibrosis in a model of nonalcoholic steatohepatitis: role of short-form leptin receptors and osteopontin. Am. J. Physiol. Gastrointest. Liver Physiol. 287: G1035–G1043. [DOI] [PubMed] [Google Scholar]
- 32.Denechaud P. D., Bossard P., Lobaccaro J. M., Millatt L., Staels B., Girard J., Postic C. 2008. ChREBP, but not LXRs, is required for the induction of glucose-regulated genes in mouse liver. J. Clin. Invest. 118: 956–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee A. H., Scapa E. F., Cohen D. E., Glimcher L. H. 2008. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science. 320: 1492–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Postic C., Girard J. 2008. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: lessons from genetically engineered mice. J. Clin. Invest. 118: 829–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barreyro F. J., Kobayashi S., Bronk S. F., Werneburg N. W., Malhi H., Gores G. J. 2007. Transcriptional regulation of Bim by FoxO3A mediates hepatocyte lipoapoptosis. J. Biol. Chem. 282: 27141–27154. [DOI] [PubMed] [Google Scholar]
- 36.Han M. S., Park S. Y., Shinzawa K., Kim S., Chung K. W., Lee J. H., Kwon C. H., Lee K. W., Lee J. H., Park C. K., et al. 2008. Lysophosphatidylcholine as a death effector in the lipoapoptosis of hepatocytes. J. Lipid Res. 49: 84–97. [DOI] [PubMed] [Google Scholar]
- 37.Malhi H., Bronk S. F., Werneburg N. W., Gores G. J. 2006. Free fatty acids induce JNK-dependent hepatocyte lipoapoptosis. J. Biol. Chem. 281: 12093–12101. [DOI] [PubMed] [Google Scholar]
- 38.Wei Y., Wang D., Topczewski F., Pagliassotti M. J. 2006. Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. Am. J. Physiol. Endocrinol. Metab. 291: E275–E281. [DOI] [PubMed] [Google Scholar]
- 39.Ntambi J. M., Miyazaki M. 2004. Regulation of stearoyl-CoA desaturases and role in metabolism. Prog. Lipid Res. 43: 91–104. [DOI] [PubMed] [Google Scholar]
- 40.Hudgins L. C., Hellerstein M. K., Seidman C. E., Neese R. A., Tremaroli J. D., Hirsch J. 2000. Relationship between carbohydrate-induced hypertriglyceridemia and fatty acid synthesis in lean and obese subjects. J. Lipid Res. 41: 595–604. [PubMed] [Google Scholar]
- 41.Parks E. J., Krauss R. M., Christiansen M. P., Neese R. A., Hellerstein M. K. 1999. Effects of a low-fat, high-carbohydrate diet on VLDL-triglyceride assembly, production, and clearance. J. Clin. Invest. 104: 1087–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schwarz J. M., Linfoot P., Dare D., Aghajanian K. 2003. Hepatic de novo lipogenesis in normoinsulinemic and hyperinsulinemic subjects consuming high-fat, low-carbohydrate and low-fat, high-carbohydrate isoenergetic diets. Am. J. Clin. Nutr. 77: 43–50. [DOI] [PubMed] [Google Scholar]
- 43.Turner S. M., Roy S., Sul H. S., Neese R. A., Murphy E. J., Samandi W., Roohk D. J., Hellerstein M. K. 2007. Dissociation between adipose tissue fluxes and lipogenic gene expression in ob/ob mice. Am. J. Physiol. Endocrinol. Metab. 292: E1101–E1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li Z., Berk M., McIntyre T. M., Gores G. J., Feldstein A. E. 2008. The lysosomal-mitochondrial axis in free fatty acid-induced hepatic lipotoxicity. Hepatology. 47: 1495–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Listenberger L. L., Han X., Lewis S. E., Cases S., Farese R. V., Jr., Ory D. S., Schaffer J. E. 2003. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc. Natl. Acad. Sci. USA. 100: 3077–3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Small D. M. 1991. The effects of glyceride structure on absorption and metabolism. Annu. Rev. Nutr. 11: 413–434. [DOI] [PubMed] [Google Scholar]
- 47.Yamaguchi K., Yang L., McCall S., Huang J., Yu X. X., Pandey S. K., Bhanot S., Monia B. P., Li Y. X., Diehl A. M. 2007. Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis. Hepatology. 45: 1366–1374. [DOI] [PubMed] [Google Scholar]
- 48.Li Z., Agellon L. B., Allen T. M., Umeda M., Jewell L., Mason A., Vance D. E. 2006. The ratio of phosphatidylcholine to phosphatidylethanolamine influences membrane integrity and steatohepatitis. Cell Metab. 3: 321–331. [DOI] [PubMed] [Google Scholar]
- 49.Verkade H. J., Havinga R., Shields D. J., Wolters H., Bloks V. W., Kuipers F., Vance D. E., Agellon L. B. 2007. The phosphatidylethanolamine N-methyltransferase pathway is quantitatively not essential for biliary phosphatidylcholine secretion. J. Lipid Res. 48: 2058–2064. [DOI] [PubMed] [Google Scholar]
- 50.Ip E., Farrell G. C., Robertson G. R., Hall P., Kirsch R., Leclercq I. A. 2003. Central role of PPAR-a-dependent hepatic lipid turnover in dietary steatohepatitis in mice. Hepatology. 38: 123–132. [DOI] [PubMed] [Google Scholar]
- 51.Donnelly K. L., Smith C. I., Schwarzenberg S. J., Jessurun J., Boldt M. D., Parks E. J. 2005. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Invest. 115: 1343–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.