Abstract
Inhibitors of HMG-CoA reductase (statins) are widely used medications for reduction of cholesterol levels. Statin use significantly reduces risk of cardiovascular disease but has also been associated with lower risk of other diseases and conditions, including dementia. However, some reports suggest that statins also have detrimental effects on the brain. We provide evidence that simvastatin and pravastatin have significantly different effects on expression of genes related to neurodegeneration in astrocytes and neuroblastoma (SK-N-SH) cells in culture. Simvastatin significantly reduced expression of ABCA1 in astrocytes and neuroblastoma cells (by 79% and 97%, respectively; both P < 0.001). Pravastatin had a similar but attenuated effect on ABCA1 in astrocytes (−54%, P < 0.001) and neuroblastoma cells (−70%, P < 0.001). Simvastatin reduced expression of apolipoprotein E in astrocytes (P < 0.01). Furthermore, both statins reduced expression of microtubule-associated protein tau in astrocytes (P < 0.01), while both statins increased its expression in neuroblastoma cells (P < 0.01). In SK-N-SH cells, simvastatin significantly increased cyclin-dependent kinase 5 and glycogen synthase kinase 3β expression, while pravastatin increased amyloid precursor protein expression. Our data suggest that simvastatin and pravastatin differentially affect expression of genes involved in neurodegeneration and that statin-dependent gene expression regulation is cell type specific.—Dong, W., S. Vuletic, and J. J. Albers. Differential effects of simvastatin and pravastatin on expression of Alzheimer’s disease-related genes in human astrocytes and neuronal cells.
Keywords: gene expression, ATP binding cassette transporter A1, apolipoprotein E, phospholipid transfer protein, microtubule-associated protein tau, amyloid precursor protein
Statins are competitive inhibitors of HMG-CoA reductase, a rate-limiting enzyme for cholesterol synthesis, and have been widely used as plasma cholesterol lowering drugs in dyslipidemic patients. Many large-scale clinical intervention trials have demonstrated that statins significantly lower morbidity and mortality, particularly due to reduction in cardiovascular events (1). Furthermore, numerous studies in recent years suggested that prolonged use of statins is associated with lower risk of other diseases, including dementia due to Alzheimer’s disease (AD) (2–6). These findings led to recommendations for significant increase in use of statins and prompted several clinical trials where statins were tested in people who already have AD. However, data published so far suggest that use of statins in AD patients led to mixed outcomes (7–12), somewhat diminishing the initial hope that statins may not only prevent processes leading to AD, but also retard the disease when it is already clinically apparent. Furthermore, a report by Algotsson and Winblad (13) suggested that patients with AD may have increased susceptibility to statin-induced adverse effects.
Additionally, although less often considered as an issue with use of statins, particularly those that cross the blood-brain barrier (BBB) and blood-cerebrospinal fluid (CSF) barrier, is the fact that some patients develop symptoms of dementia as a result of statin therapy, which improve or disappear upon statin withdrawal (14–16). These findings are rarely reported, and many physicians tend to regard patient-reported memory impairment, particularly in the elderly, as an age-related, rather than a therapy-related issue. Despite the fact that memory impairment is rarely reported as an adverse effect of statin therapy, the statin-induced memory problems are real, potentially causing a substantial amount of concern and confusion to the patients. The effects of statins on the brain are far less well understood than their effects on other target organs.
In a previously reported randomized pilot clinical trial in statin-naive hypercholesterolemic patients with no measurable cognitive impairment, we have shown that simvastatin, a lipid-soluble statin that easily crosses the BBB, and pravastatin, a hydrophilic statin, which does not readily cross the BBB, have differential effects on some AD-related markers in human CSF (17, 18). Simvastatin was associated with a statistically significant reduction in CSF levels of tau phosphorylated at Thr-181 (pTauThr181) and an increase in CSF phospholipid transfer protein (PLTP) activity. Levels of CSF apolipoprotein E (apoE) were significantly reduced in patients using pravastatin, and there was a trend for CSF apoE reduction in patients on simvastatin. Due to the different BBB transfer of these two statins, the observed differences may have been due to the intrathecal availability of individual statins or due to different effects of these statins on the brain cells. Therefore, to evaluate direct effects of lipophilic and hydrophilic statins on brain cells, we performed an in vitro study using primary human astrocytes and neuroblastoma (SK-N-SH) cells and assessed the effects of simvastatin and pravastatin on expression of ABCA1, APOE, PLTP, microtubule-associated protein tau (MAPT), and some of the genes related to tau phosphorylation, Disabled 1 (DAB1), cyclin-dependent kinase 5 (CDK5), and glycogen synthase kinase 3β (GSK3β), and amyloid precursor protein (APP), the genes functionally associated with AD.
MATERIALS AND METHODS
Materials
Simvastatin was a gift from Merck (Whitehouse Station, NJ). Pravastatin, mevalonolactone, geranylgeranyl pyrophosphate (GGPP), and farnesyl pyrophosphate (FPP) were obtained from Sigma-Aldrich (St. Louis, MO). Kits used in RNA isolation were obtained from Qiagen (Valencia, CA). Cell culture media were obtained from Lonza (Walkersville, MD), collagen solution (Type I, from calf skin) from Sigma-Aldrich, and FBS with low endotoxin levels from HyClone (Logan, UT). Protein isolation solution, PhosphoSafe, was from Calbiochem/EMD Chemicals (San Diego, CA). Protease inhibitor cocktail, Halt, and growth factors used in tissue culture were obtained from Invitrogen (Carlsbad, CA). Gels, sample, and running buffers were obtained from Bio-Rad (Hercules, CA). Phospholipids for preparation of liposomes used in phospholipid transfer assay were obtained from Sigma-Aldrich, and radioactive phosphatidylcholine from Perkin-Elmer Life Sciences/NEN (Boston, MA). Cholesterol measurement kit, Amplex Red, and the RiboGreen® RNA Quantitation Kit were obtained from Molecular Probes (Eugene, OR).
Activation of simvastatin
Simvastatin needs to be activated by opening of the lactone ring before use in cell culture. We used the protocol provided by Merck. Briefly, eight milligrams of simvastatin (0.019 mM) were dissolved in 0.2 ml of 100% ethanol, with subsequent addition of 0.3 ml of 0.1 N NaOH. The solution was heated at 50°C for 2 h in a sand bath and then neutralized with HCl to pH 7.2. The resulting solution was brought to a final volume (1 ml) with distilled water, and aliquots were stored at −80°C until use.
Preparation of mevalonate from mevalonolactone
One gram of mevalonolactone was mixed with 9 ml of sterile water. Using 1 N NaOH, pH was adjusted to 7.4. Aliquots were stored at −80°C until use.
Cell culture
Primary human astrocytes from four different donors (gift from Dr. Möller, Department of Neurology, University of Washington, Seattle) were prepared from tissue obtained from legally aborted fetuses (Birth Defects Laboratory, University of Washington, Seattle) using previously described methodology (19). Primary human astrocytes and SK-N-SH neuroblastoma cell line (ATCC) were plated on 6-well plates and grown in DMEM supplemented with 5% or 8% fetal calf serum (HyClone, Logan, UT), respectively, at 37°C, 5% CO2 until 80% confluent. For measurement of gene expression levels at baseline, cells were just washed and RNA was prepared and assayed as outlined below. Baseline gene expression levels in astrocytes were measured in primary human astrocytes obtained from two donors. For experimental purposes, cells were incubated under serum-free conditions. Primary human astrocytes used for the reported results were obtained from four donors. Based on preliminary time-dependent studies, 48-h incubation was used for all of the reported experiments. Based on the dose-response studies, a majority of our experiments were conducted using the following concentrations of active compounds: simvastatin at 5 μM, pravastatin at 10 μM, mevalonate at 50 μM, and GGPP and FPP at 10 μM. Following incubation, cells were extensively washed to remove dead cells and cell debris and prepared for further analyses.
Protein isolation, electrophoresis, and Western blotting
Cells were scraped and cell protein isolated using PhosphoSafe protein isolation solution (EMD Chemicals) in the presence of protease inhibitors (Halt protease inhibitor cocktail; Invitrogen), according to the manufacturer’s instructions and stored at −80°C until use.
Cell proteins were subjected to denaturing SDS-PAGE and Western blot analysis. Samples were loaded on 4–12% gel (Bio-Rad Bis-Tris Criterion XT) for SDS-PAGE and, following electrophoresis, transferred to nitrocellulose membrane using Bio-Rad semidry transfer. Blots were incubated overnight with primary antibodies at 4°C with agitation in 5% milk/TBS-Tween 20. Loading controls were evaluated using β-actin. The blots were incubated with the appropriate TrueBlot secondary antibody for 2 h at room temperature. The membranes were developed using West Femto SuperSignal chemoluminescent kit and scanned using Kodak CF400 imaging system.
Phospholipid transfer activity assay
PLTP-mediated phospholipid transfer activity was assessed using PLTP phospholipid transfer activity assay (20). Ten microliters of conditioned media were assayed for the ability to transfer C14-labeled phospholipid from donor liposome particles to the acceptor particles (plasma HDL without measurable PLTP activity). All values were adjusted for the background transfer, and PLTP activity was calculated as percentage of phospholipid transfer per microliter of conditioned medium per hour (%/µl/h).
RNA isolation
RNA was isolated using the Qiagen RNEasy kit according to the manufacturer’s instructions. To remove trace genomic DNA, samples were treated with DNase and DNA-Shredder kit (Qiagen). Following assessment of 260/280 nm ratio by spectrometry, RNA samples were stored at −80°C until use. Total RNA was quantified on the Mx4000® Multiplex QPCR System (Stratagene, La Jolla, CA) using the RiboGreen® RNA Quantitation Kit (Molecular Probes). All samples obtained from cells grown under chosen experimental conditions (5 µM simvastatin and 10 µM pravastatin) had highly similar total RNA yield (46.8 ± 3 µg/ml), and we did not observe significant cell death under these conditions.
Quantitative PCR
Quantitative PCR was performed on an Mx4000® Multiplex QPCR System with samples loaded in triplicate using 40 ng of total RNA. Quantitative PCR was run in a 10 μl reaction using SYBR® Green PCR Master Mix (Applied Biosystems, Foster City, CA) (5 μl 2× Master Mix, 400 nM each primer, 0.5 units of StrataScript RT, and 0.5 units of RNase Block) with PCR cycling conditions of 48°C for 30 min, 95°C for 10 min, and 40 cycles at 95°C for 15 s, 60°C for 1 min. After each assay, a dissociation curve was run to confirm specificity of all PCR amplicons. Resulting Ct values were converted to nanograms, normalized to S18 mRNA and total RNA, and expressed as the average of triplicate samples ± SD. Pooled sample total RNA was used for standard curves in 1:2 serial dilutions.
Primers were from Operon (Huntsville, AL) and designed using Applied Biosystems Primer Express 2.0 software. The primers and probes used for each gene are listed in Table 1.
TABLE 1.
List of primers used in this study
| Gene | Accession No. | Primer Name | Primer | Amplicon (nt) |
|---|---|---|---|---|
| ABCA1 | NM005502 | hABCA1-3296F | TGGTTCTATGCCCGCTTGA | 176 |
| hABCA1-3471R | ACCTTAGATCCCCCGACAAAG | 176 | ||
| PLTP | NM006227 | hPLTP-289F | TCACAGAGCTGCAACTGACATCT | 81 |
| hPLTP-369R | AGGAGGCATTGGTGATTTGAA | 81 | ||
| APOE | NM000041 | hApoE-145F | AGCAAGCGGTGGAGACAGA | 91 |
| hApoE-235R | AAGCGACCCAGTGCCAGTT | 91 | ||
| MAPT | NM005910 | hMAPT-4038F | CAGGGATTGGGATGAATTGC | 113 |
| variants 1–4 | hMAPT-4150R | TCTGGTCAAGGCTTTGGGAA | 113 | |
| CDK5 | NM004935 | hCDK5-94F | GGAAGGCACCTACGGAACTGT | 101 |
| hCDK5-194R | GCACACCCTCATCATCGTCAT | 101 | ||
| GSK3β | NM002093 | hGSK3B-1526F | TCCACCTGAACAGTCCCGA | 81 |
| hGSK3B-1606R | CGTGACCAGTGTTGCTGAGTG | 81 | ||
| APP | NM201414 | hAPP-373F | GATGCCCTTCTCGTTCCTGA | 86 |
| hAPP-458R | ACGGTGTGCCAGTGAAGATG | 86 | ||
| DAB1 | NM021080 | hDAB1-798F | TACAAAGCCAAATTGATCGGG | 87 |
| hDAB1-884R | GCCCTTGAGTTTCATCATGGA | 87 |
h, human; F, forward; R, reverse.
Cholesterol measurement
Levels of intracellular cholesterol were evaluated using the Amplex Red kit (Molecular Probes) according to the manufacturer’s instructions. Cells were lysed using the MEM-PER kit (Pierce), and cell lysates were evaluated for intracellular cholesterol in the presence of cholesterol oxidase and cholesterol esterase, with appropriate cholesterol standards and positive and negative controls. Samples were incubated for 30 min at 37°C and 60 rpm, cooled for 5 min at room temperature, and read at excitation/emission of 530/590 nm in Bioscan fluorescent plate reader. Data were analyzed using Hidex software, corrected for cell protein, and expressed as picograms of cholesterol per microgram of cell protein (pg/µg).
Statistical analyses
Statistical analyses were performed with Statistica for Windows (StatSoft, Tulsa, OK). Differences were evaluated using Mann-Whitney U-Test and t-test. P values <0.05 or <0.01 (for multiple comparisons) were considered statistically significant.
RESULTS
Baseline expression
Baseline expression levels of AD-related genes, prior to removal of serum supplementation, in primary human astrocytes and SK-N-SH cells are shown in Table 2. Comparison between baseline gene expression and control samples following 48-h incubation in serum-free media suggests that astrocytes and SK-N-SH cells respond differently to serum removal. In astrocytes, serum removal reduced expression of genes involved in removal of cellular lipids, such as ABCA1 and PLTP. Surprisingly, there was no effect of serum removal on expression of APOE. In contrast, removal of serum led to an increase in ABCA1 and PLTP expression in neuroblastoma cells (Table 2). Baseline gene expression in primary human astrocytes was evaluated in astrocytes obtained from two sources. No significant difference in baseline gene expression was found between two astrocytic cultures (data not shown). Spearman correlation coefficients for baseline levels of different genes in primary human astrocytes and SK-N-SH cells suggest that gene expression levels of some genes, for example, APOE and MAPT in astrocytes, are highly correlated and possibly regulated by the same pathway under normal growth conditions (Table 3).
TABLE 2.
Expression of Alzheimer's disease-related genes in primary human astrocytes and SKN-SH cells at baseline and following 48-h incubation in serum-free media
| Astrocytes |
SK-N-SH |
|||
|---|---|---|---|---|
| Gene | Baseline | 48-h Incubation | Baseline | 48-h Incubation |
| ABCA1 | 3.77 ± 0.3 | 1.43 ± 0.4a | 0.51 ± 0.1 | 1.42 ± 0.2a |
| PLTP | 2.46 ± 0.3 | 1.08 ± 0.3a | 0.84 ± 0.1 | 1.15 ± 0.3a |
| APOE | 0.86 ± 0.1 | 0.94 ± 0.2 | NA | NA |
| MAPT | 0.65 ± 0.2 | 1.18 ± 0.2a | 0.75 ± 0.1 | 1.16 ± 0.4a |
| CDK5 | 0.26 ± 0.0 | 0.97 ± 0.3a | 0.90 ± 0.1 | 0.91 ± 0.2 |
| GSK3β | 1.08 ± 0.1 | 0.93 ± 0.1a | 0.88 ± 0.1 | 1.01 ± 0.2a |
| APP | 2.21 ± 0.2 | 1.01 ± 0.2a | 0.75 ± 0.1 | 0.96 ± 0.2a |
| DAB1 | 6.90 ± 0.6 | 0.88 ± 0.2a | 0.13 ± 0.0 | 0.74 ± 0.1a |
Baseline levels in primary human astrocytes were measured in samples obtained from two donors, while 48-h incubation data were obtained from samples obtained from four donors. Data are shown as mean ± SD of gene/18S. NA, not applicable.
P ≤ 0.01 by t-test.
TABLE 3.
Spearman correlations for gene expression levels at baseline in primary human astrocytes (A) and SK-N-SH cells (B)
| A | ABCA1 | PLTP | APOE | MAPT | CDK5 | GSK3β | APP |
|---|---|---|---|---|---|---|---|
| ABCA1 | |||||||
| PLTP | 0.534 | ||||||
| APOE | −0.020 | 0.335 | |||||
| MAPT | −0.293 | 0.323 | 0.615a | ||||
| CDK5 | 0.225 | 0.765a | 0.550 | 0.370 | |||
| GSK3β | 0.486 | 0.645a | 0.439 | 0.103 | 0.514 | ||
| APP | 0.728a | 0.620a | 0.214 | 0.031 | 0.553 | 0.505 | |
| DAB1 | 0.524 | 0.329 | 0.066 | −0.020 | 0.184 | 0.528 | 0.367 |
| B | ABCA1 | PLTP | MAPT | CDK5 | GSK3β | APP | |
| ABCA1 | |||||||
| PLTP | −0.033 | ||||||
| MAPT | −0.100 | 0.483 | |||||
| CDK5 | 0.550 | 0.017 | −0.117 | ||||
| GSK3β | 0.424 | 0.339 | −0.051 | 0.763 | |||
| APP | 0.326 | −0.184 | −0.050 | 0.552 | 0.451 | ||
| DAB1 | 0.550 | 0.067 | −0.217 | 0.883a | 0.644 | 0.435 |
Data are based on gene expression adjusted for 18S (n = 18 for astrocytes and n = 9 for SK-N-SH cells).
P ≤ 0.01.
Dose response
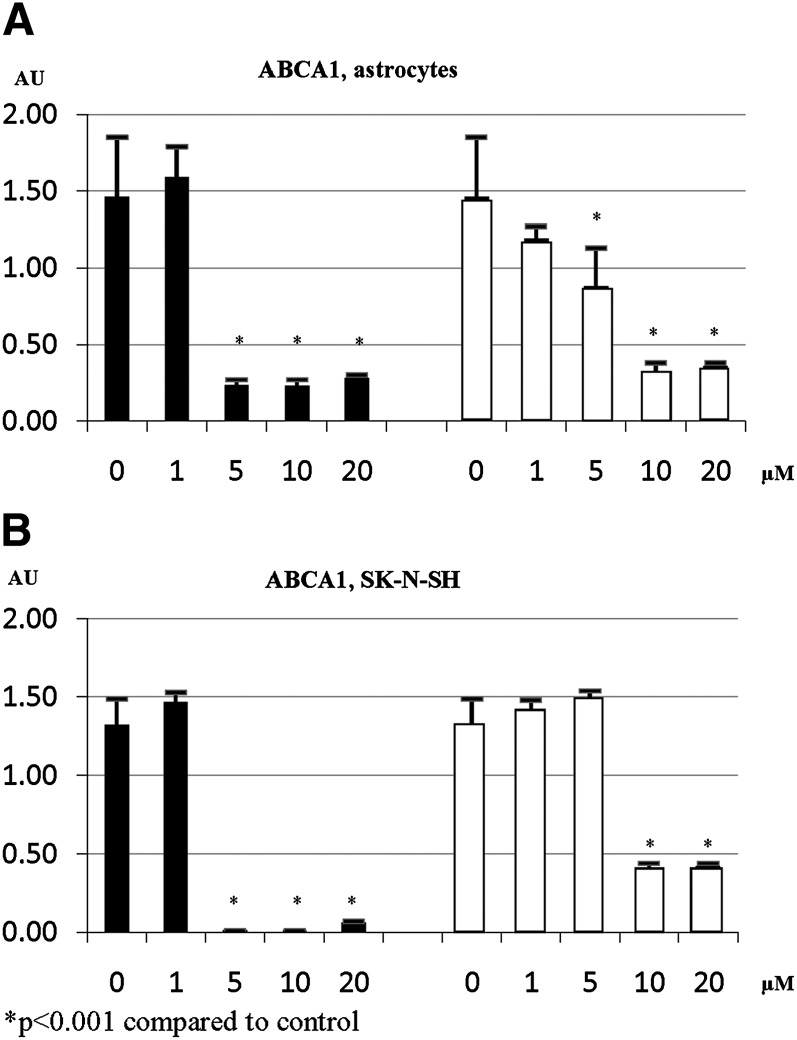
Dose-response studies indicate that a low dose of statins (1 µM) has no statistically significant effect on gene expression in both primary human astrocytes and neuroblastoma cells. For example, simvastatin dose of 5 µM and pravastatin dose of 10 µM achieved maximum effect on ABCA1 gene expression (Fig. 1). Similar results were obtained for other tested genes (data not shown). Therefore, we used a simvastatin dose of 5 µM and a pravastatin dose of 10 µM in our subsequent experiments.
Fig. 1.
ABCA1 gene expression in primary human astrocytes (A) and SK-N-SH cells (B) in response to different doses of simvastatin (closed bars) or pravastatin (open bars).
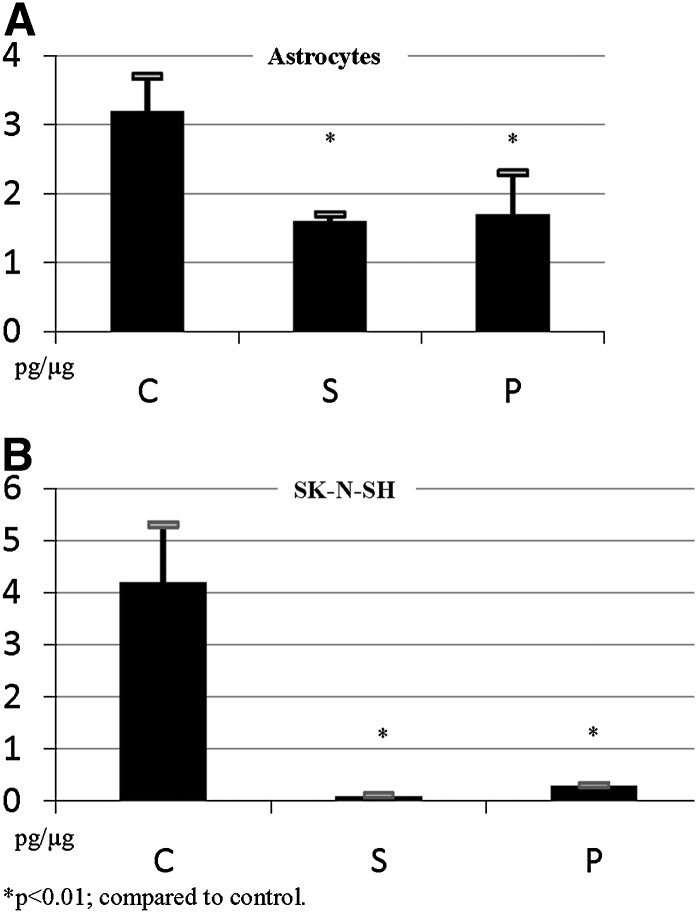
To evaluate biological activity of the chosen statin concentrations, we measured cholesterol levels in cells incubated with simvastatin and pravastatin (Fig. 2). As expected, biological effects of 5 µM of simvastatin and 10 µM of pravastatin on cholesterol levels were very similar. Astrocytes were more resilient to statin effect, with 47–49% reduction in intracellular cholesterol concentration in cells incubated with either statin. The same doses of statins had more profound effect on intracellular cholesterol in SK-N-SH cells, with 92–97% reduction in intracellular cholesterol. These data suggest that the dose of statins used for the majority of our experiments has a similar effect on cholesterol reduction and that reduction of cholesterol in the presence of statins in astrocytes is significantly smaller than in SK-N-SH cells.
Fig. 2.
Effects of statins on levels of intracellular cholesterol in astrocytes and SK-N-SH cells. Primary human astrocytes (A) and human neuroblastoma cells, SK-N-SH (B), were incubated without or with simvastatin (S; 5 μM) and pravastatin (P; 10 μM) for 48 h (n = 9 for each condition). Levels of intracellular cholesterol were measured using the Amplex Red kit (excitation/emission 530/590 nm) and expressed as picograms of cholesterol per microgram of total protein. C, control.
Gene expression and statins
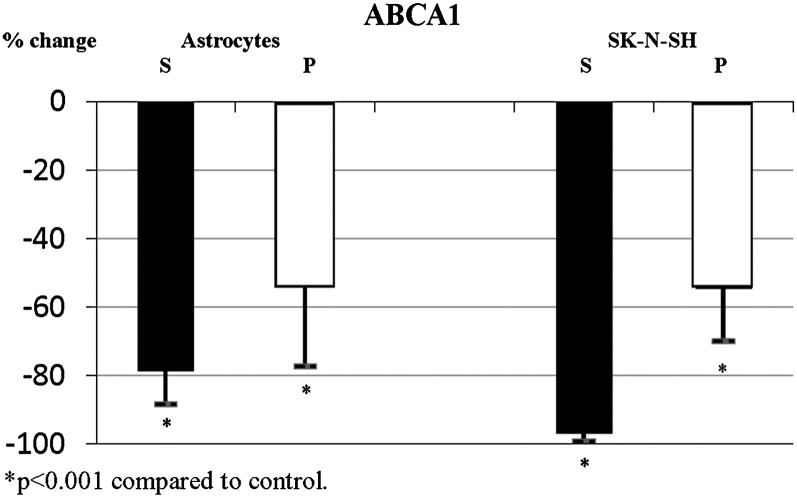
Statins induced significant changes in expression of ABCA1. In the presence of simvastatin, ABCA1 expression was highly significantly reduced in primary human astrocytes and nearly abolished in neuroblastoma cells (Fig. 3). In contrast, pravastatin had a somewhat lesser effect on ABCA1 expression despite a biologically equivalent dose of pravastatin (10 μM), leading to a smaller reduction in ABCA1 expression in both astrocytes and neurons (Figs. 1 and 3; Table 4). Addition of mevalonate to cells exposed to statins partially reversed the statin effects in both cell types. Partial reversal of the simvastatin effect following supplementation with mevalonate suggests that the observed reduction in ABCA1 expression is at least partially related to reduced cholesterol synthesis. Addition of GGPP or FPP to cells exposed to statins had no significant effect on ABCA1 expression in either cell type.
Fig. 3.
Simvastatin and pravastatin significantly reduce expression of ABCA1 in primary human astrocytes and SK-N-SH cells. Expression of ABCA1 in primary human astrocytes and human neuroblastoma cells following incubation with simvastatin (closed bars, S; 5 μM) and pravastatin (open bars, P; 10 μM).
TABLE 4.
Effects of simvastatin (5 μM) and pravastatin (10 μM) on gene expression in primary human astrocytes and SK-N-SH cells
| Gene | Simvastatin | Pravastatin | |
|---|---|---|---|
| Astrocytes | ABCA1 | −78.6 ± 9.8a | −54.25 ± 23.1a |
| PLTP | −31.5 ± 11.8a | −42.9 ± 24.2a | |
| APOE | −23.1 ± 10.2a | −26.9 ± 25.1 | |
| MAPT | −49.5 ± 4.9a | –40.8 ± 3.8a | |
| CDK5 | −6.4 ± 0.7 | −14.6 ± 1.8a | |
| GSK3β | −3.7 ± 15.3 | 3.4 ± 5.0 | |
| APP | −4.3 ± 5.4 | −1.6 ± 9.8 | |
| DAB1 | 4.4 ± 24.7 | −20.72 ± 11.1a | |
| SK-N-SH | ABCA1 | −96.8 ± 2.4a | −69.8 ± 15.5a |
| PLTP | −29.6 ± 6.1a | −2.9 ± 21.2 | |
| MAPT | 40.9 ± 11.1a | 19.3 ± 4.7a | |
| CDK5 | 36.4 ± 11.8a | 1.5 ± 6.4 | |
| GSK3β | 20.4 ± 7.9a | 4.7 ± 9.9 | |
| APP | 16.6 ± 14.7 | 15.2 ± 4.6a | |
| DAB1 | −10.9 ± 34.0 | −2.2 ± 19.3 |
Primary human astrocytes used in these experiments were obtained from four different donors. Data are presented as average percentage change ± SD in at least three consecutive experiments performed in triplicate and assayed in three technical replicates.
P < 0.05 represents statistically significant difference between control and statin data, by t-test, that was replicated in each individual experiment.
Incubation of astrocytes with simvastatin led to the expected decrease in APOE expression (Table 4). In contrast, pravastatin had a variable effect on APOE expression in astrocytes. Supplementation of astrocytes with mevalonate reversed the observed simvastatin-induced effects, while GGPP or FPP had variable effects on expression of APOE in primary human astrocytes.
Simvastatin and pravastatin significantly decrease expression of PLTP in primary human astrocytes. A similar effect was observed in PLTP phospholipid transfer activity, with both statins reducing PLTP phospholipid transfer activity by approximately 30% (P < 0.05). In neuroblastoma cells, only simvastatin had a significant effect on PLTP expression (Table 4). Statin effects on PLTP expression in both cell types were reversed by mevalonate supplementation. GGPP and FPP partially reversed simvastatin effect in SK-N-SH cells but had no effect on statin-induced decrease in PLTP expression in astrocytes.
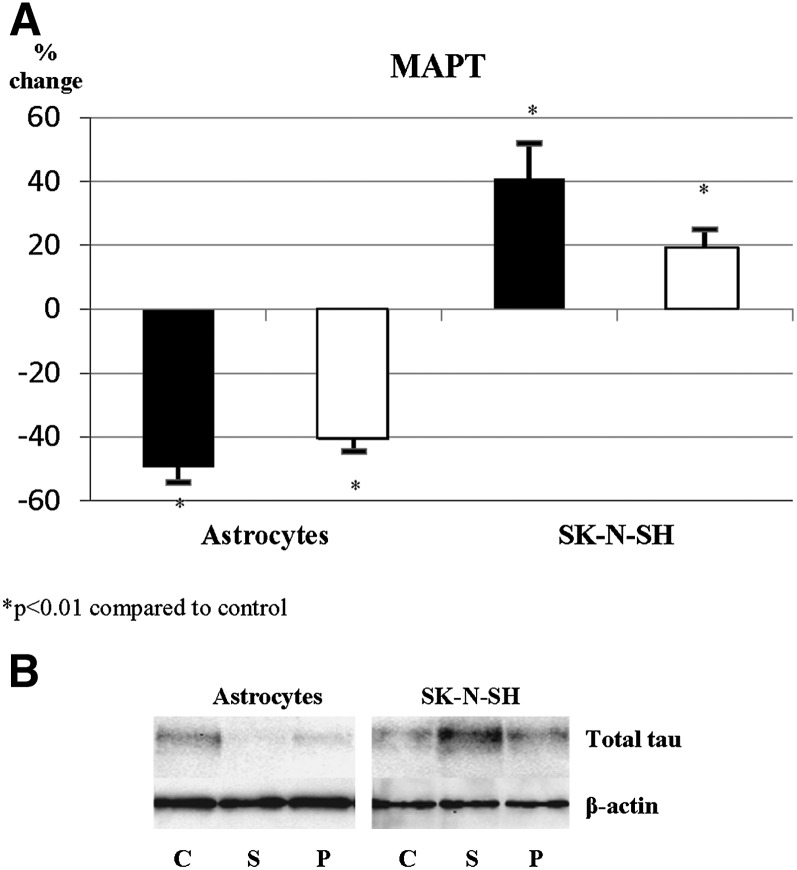
Incubation of astrocytes with simvastatin and pravastatin led to an approximately 60% and 40% decrease in expression of MAPT, respectively. Supplementation of astrocytes with mevalonate, GGPP, and FPP did not reverse the statin effect on MAPT expression in astrocytes, suggesting that the observed reduction is primarily due to a statin effect on pathways other than cholesterol synthesis. In contrast, incubation of neuroblastoma cells with both statins caused an increase in MAPT expression (Fig. 4A). Supplementation of neurons with mevalonate partially reversed the observed effects in neuroblastoma cells, suggesting partial involvement of cholesterol synthesis pathway in regulation of MAPT expression in neuronal cells. These results were confirmed also at the protein level (Fig. 4B), indicating that statins oppositely affect astrocytes and neurons at the level of both gene and protein expression.
Fig. 4.
Statin effect on expression of MAPT differs in primary human astrocytes and SK-N-SH cells. A: MAPT expression levels in primary human astrocytes and SK-N-SH cells incubated with simvastatin (5 μM, closed bars) and pravastatin (10 μM, open bars). B: Total tau protein in primary human astrocytes and SK-N-SH cells.
Furthermore, we evaluated the effects of statins on neuronal expression of DAB1, CDK5, and GSK3β, proteins known to regulate tau phosphorylation in neurons (21, 22). Simvastatin significantly increased expression of CDK5 and GSK3β in neuroblastoma cells but had no significant effect on DAB1. In astrocytes, pravastatin moderately decreased expression of DAB1 and CDK5 (Table 4). Supplementation of mevalonate reversed simvastatin effects on GSK3β but had no effect on expression of CDK5 in neuronal cells.
Evaluation of APP expression in neurons suggested that simvastatin had no effect, while incubation with pravastatin led to a statistically significant increase in APP expression (Table 4). Neither statin affected APP expression in astrocytes. Similar results were seen at the protein level (data not shown).
DISCUSSION
Data from our study indicate that simvastatin and pravastatin had differential effects on expression of genes relevant for AD development and that these effects are dependent not only on the type of statin but also on cell type. Generally, astrocytes were less susceptible to statin effect, probably due to a greater resilience in changes in cholesterol levels in response to statin treatment. Despite the identical concentrations of simvastatin and pravastatin, the effect on expression of ABCA1 in astrocytes was significantly weaker compared with neurons. ABCA1 regulates intracellular levels of cholesterol and phospholipids through regulation of cellular lipid efflux (23). Under the conditions of significantly reduced cholesterol synthesis, reduction in ABCA1 expression would effectively reduce lipid efflux from neurons, thus preserving the existing intracellular cholesterol.
Astrocytes are the main source of lipoproteins in the brain, and ABCA1 is one of the key in vivo determinants of secretion of the lipoproteins containing apoE (24, 25). ApoE is a major apolipoprotein in the brain, and under the normal conditions, all brain apoE is locally synthesized (26–28). Although the effects of statins on APOE expression in astrocytes were relatively modest compared with the effects on ABCA1, reduction in both ABCA1 and APOE in the brain is likely to be detrimental in the long term. ApoE synthesis and secretion in glial cells is one of the critical processes for maintenance of the brain lipid homeostasis. ApoE-containing lipoproteins are the main lipid carriers in the brain, and binding of apoE to its receptors on the neuronal membrane is important not only for holoparticle lipoprotein uptake but also for cell signaling (26, 27, 29). Increasing the level of apoE in the brain generally has beneficial effects on numerous cell and tissue processes, including membrane repair and memory (30–36).
Published studies suggest that neurons do not synthesize sufficient amounts of cholesterol needed for maintenance of their functional and structural stability but depend on lipid delivery from glial cells through glia-derived lipoproteins (37). The reduced lipoprotein delivery from astrocytes due to long-term statin therapy may not be sufficient to maintain structural and functional stability of neurons. Published studies report that astrocyte-derived cholesterol is essential for formation of synapses in neurons and that continuous cholesterol recycling is necessary for structural and functional stability of neurons and for synaptic transmission/long-term potentiation (37, 38). In vulnerable patients, the observed significant reduction in ABCA1 expression combined with the reduction in expression of APOE and PLTP would reduce the level of circulating astrocyte-derived lipoproteins, thus significantly reducing availability of lipids necessary for neuronal membrane repair and for formation, maintenance, and function of synapses (30, 37, 38). Therefore, the long-term effects of statin therapy could lead to transient or permanent cognitive impairment in patients who already have low levels of brain cholesterol. This is primarily an issue in the elderly because brain cholesterol synthesis and levels tend to be reduced in people with advanced age, and cholesterol as well as phospholipid levels are particularly reduced in patients with AD (39–42). While the observed reduction in ABCA1 and APOE may not be the only factors that contribute to a statin-induced cognitive impairment in vulnerable patients, it is likely a contributing factor that should be taken into account when deciding on dosage and type of statin used in individual patients.
Statins reduce ABCA1 expression in both astrocytes and neurons, but the extent of the reduction was different between the two cell types. In contrast, statins significantly reduced MAPT expression in astrocytes but significantly increased its expression in neurons. These findings indicate that the nature of the MAPT expression regulation by statins is cell type dependent. Functional implications of these differences are currently unclear, although it could be argued that an increase in MAPT in neurons would further stabilize the microtubular system, leading to conserved intracellular/axonal distribution of metabolically important molecules under the conditions of reduced cholesterol availability. It should be noted that this differential effect of statins on MAPT expression in astrocytes and neurons would also likely change the relative proportion of astrocyte- to neuron-derived tau that reaches CSF.
The differences between simvastatin and pravastatin on gene expression were not only present in terms of the extent of the relative effect, but in some instances they had a different pattern of action in target cells. For example, under the employed incubation conditions, simvastatin affected expression of GSK3β and CDK5 in neuronal cells, while pravastatin did not. In contrast, pravastatin had an effect on expression of neuronal APP, while simvastatin did not. These findings suggest that these two statins could have significantly different effects on target cells.
Statins are among the most widely used medications in developed countries, and their use has been correlated with a significant reduction not only in cardiovascular disease and death rates, but also in reduction of numerous other diseases and conditions, including diseases of the central nervous system. For example, population studies suggested that statin users have significantly lower risk of dementia, including AD (2, 3), and clinical studies suggested that use of statins may also improve other neurological conditions, such as multiple sclerosis, Parkinson’s disease, and traumatic brain injury (43–47). However, some studies of effects of statins on the brain and brain cells suggested that statins have potential not only to promote health but also to induce harm in the central nervous system. For example, a recent study by Coetsee et al. (48) has shown that statins induce significant DNA damage in neuronal cells in vivo. Other studies reported activation of both pro- and anti-inflammatory pathways, increased cell death, and higher susceptibility to oxidative damage in the brain tissue or brain cells exposed to statins (49–53). These controversial reports on both beneficial and harmful effects of statins in vitro and in vivo suggest that, despite their widespread use, statin effects on the brain need to be better understood. Data from our study support a concept that the use of statins needs to be evaluated on an individual basis and carefully monitored for potentially detrimental effects of statins on the brain.
In summary, our study has shown that effects of statins on expression of genes involved in neurodegenerative processes are statin and cell dependent. Furthermore, our data suggest a potential mechanism involved in development of statin-induced cognitive impairment in vulnerable patients that requires further study.
Acknowledgments
The authors thank Brian van Yserloo from the University of Washington Diabetes Endocrinology Research Center (http://depts.washington.edu/diabetes; supported by the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases Grant P30 DK-17047) for exceptional effort and help with RT-PCR, Dr. Joseph R. Day for technical help, Dr. Thomas Möller for primary human astrocytes used in the study, and Hal Kennedy for help in manuscript preparation. Simvastatin used in this study was a kind gift from the Merck Corporation. Merck was not involved in the study design and conduct, data collection, management, or interpretation of the data.
Footnotes
Abbreviations:
- AD
- Alzheimer's disease
- apoE/APOE
- apolipoprotein E
- APP
- amyloid precursor protein
- BBB
- blood-brain barrier
- CDK5
- cyclin-dependent kinase 5
- CSF
- cerebrospinal fluid
- DAB1
- Disabled 1
- FPP
- farnesyl pyrophosphate
- GGPP
- geranylgeranyl pyrophosphate
- GSK3β
- glycogen synthase kinase 3β
- MAPT
- microtubule-associated protein tau
- PLTP
- phospholipid transfer protein
This work was supported by the National Institutes of Health, National Heart, Lung, and Blood Institute (HL-030086). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Auer J., Eber B. 2001. A clinical focus on statins. Curr. Opin. Investig. Drugs. 2: 382–388. [PubMed] [Google Scholar]
- 2.Jick H., Zornberg G. L., Jick S. S., Seshadri S., Drachman D. A. 2000. Statins and the risk of dementia. Lancet. 356: 1627–1631. [DOI] [PubMed] [Google Scholar]
- 3.Wolozin B., Kellman W., Ruosseau P., Celesia G. G., Siegal G. 2000. Decreased prevalence of Alzheimer disease associated with 3-hydroxy-3methylglutaryl coenzyme A reductase inhibitors. Arch. Neurol. 57: 1439–1443. [DOI] [PubMed] [Google Scholar]
- 4.Hajjar I., Schumpert J., Hirth V., Wieland D., Eleazer G. P. 2002. The impact of the use of statins on the prevalence of dementia and the progression of cognitive impairment. J. Gerontol. A Biol. Sci. Med. Sci. 57: M414–M418. [DOI] [PubMed] [Google Scholar]
- 5.Rockwood K., Kirkland S., Hogan D. B., MacKnight C., Merry H., Verreault R., Wolfson C., McDowell I. 2002. Use of lipid-lowering agents, indication bias, and the risk of dementia in community-dwelling elderly people. Arch. Neurol. 59: 223–227. [DOI] [PubMed] [Google Scholar]
- 6.Zamrini E., McGwin G., Roseman J. M. 2004. Association between statin use and Alzheimer’s disease. Neuroepidemiology. 23: 94–98. [DOI] [PubMed] [Google Scholar]
- 7.Friedhoff L. T., Cullen E. I., Goeghagen N. S., Buxbaum J. D. 2001. Treatment with controlled-release lovastatin decreases serum concentrations of human beta-amyloid (A beta) peptide. Int. J. Neuropsychopharmacol. 4: 127–130. [DOI] [PubMed] [Google Scholar]
- 8.Fassbender K., Stroick M., Bertsch T., Ragoschke A., Kuehl S., Walter S., Brechtel K., Muehlhauser F., von Bergmann K., Lutjohann D. 2002. Effects of statins on human cerebral cholesterol metabolism and secretion of Alzheimer amyloid peptide. Neurology. 59: 1257–1258. [DOI] [PubMed] [Google Scholar]
- 9.Locatelli S., Lutjohann D., Schimdt H. H., Otto C., Beisiegel U., von Bergmann K. 2002. Reduction of plasma 24S-hydroxycholesterol (cerebrosterol) levels using high-dosage simvastatin in patients with hypercholesterolemia: evidence that simvastatin affects cholesterol metabolism in the human brain. Arch. Neurol. 59: 213–216. [DOI] [PubMed] [Google Scholar]
- 10.Simons M., Schwarzler F., Lutjohann D., von Bergmann K., Beyreuther K., Dichgans J., Wormstall H., Hartmann T., Schulz J. B. 2002. Treatment with simvastatin in normocholesterolemic patients with Alzheimer’s disease: a 26-week randomized, placebo-controlled, double-blind trial. Ann. Neurol. 52: 346–350. [DOI] [PubMed] [Google Scholar]
- 11.Vega G. L., Weiner M. F., Lipton A. M., von Bergmann K., Lutjohann D., Moore C., Svetlik D. 2003. Reduction in levels of 24S-hydroxycholesterol by statin treatment in patients with Alzheimer disease. Arch. Neurol. 60: 510–515. [DOI] [PubMed] [Google Scholar]
- 12.Doraiswamy P. M., Steffens D. C., McQipod D. R. 2004. Statin use and hippocampal volumes in elderly subjects at risk for Alzheimer’s disease: a pilot observational study. Am. J. Alzheimers Dis. Other Demen. 19: 275–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Algotsson A., Winblad B. 2004. Patients with Alzheimer’s disease may be particularly susceptible to adverse effects of statins. Dement. Geriatr. Cogn. Disord. 17: 109–116. [DOI] [PubMed] [Google Scholar]
- 14.Orsi A., Sherman O., Woldeselassie Z. 2001. Simvastatin-associated memory loss. Pharmacotherapy. 21: 767–769. [DOI] [PubMed] [Google Scholar]
- 15.King D. S., Wilburn A. J., Wofford M. R., Harrell T. K., Lindley B. J., Jones D. W. 2003. Cognitive impairment associated with atorvastatin and simvastatin. Pharmacotherapy. 23: 1663–1667. [DOI] [PubMed] [Google Scholar]
- 16.Wagstaff L. R., Mitton M. W., Arvik B. M., Doraiswamy P. M. 2003. Statin-associated memory loss: analysis of 60 case reports and review of the literature. Pharmacotherapy. 23: 871–880. [DOI] [PubMed] [Google Scholar]
- 17.Riekse R. G., Li G., Petrie E. C., Leverenz J. B., Vavrek D., Vuletic S., Albers J. J., Montine T. J., Lee V. M., Lee M., et al. 2006. Effects of statins on Alzheimer’s disease biomarkers in cerebrospinal fluid. J. Alzheimers Dis. 9: 1–8. [DOI] [PubMed] [Google Scholar]
- 18.Vuletic S., Riekse R. G., Marcovina S. M., Peskind E. R., Hazzard W. R., Albers J. J. 2006. Statins of different brain penetrability differentially affect CSF PLTP activity. Dement. Geriatr. Cogn. Disord. 22: 392–398. [DOI] [PubMed] [Google Scholar]
- 19.Yagle K., Lu H., Guizzetti M., Moller T., Costa L. G. 2001. Activation of mitogen-activated protein kinase by muscarinic receptors in astroglial cells: role in DNA synthesis and effect of ethanol. Glia. 35: 111–120. [DOI] [PubMed] [Google Scholar]
- 20.Cheung M. C., Wolfbauer G., Albers J. J. 1996. Plasma phospholipid mass transfer rate: relationship to plasma phospholipid and cholesteryl ester transfer activities and lipid parameters. Biochim. Biophys. Acta. 1303: 103–110. [DOI] [PubMed] [Google Scholar]
- 21.Hiesberger T., Trommsdorff M., Howell B. W., Goffinet A., Mumby M. C., Cooper J. A., Herz J. 1999. Direct binding of Reelin to VLDL receptor and apoE receptor 2 induces tyrosine phosphorylation of disabled-1 and modulates tau phosphorylation. Neuron. 24: 481–489. [DOI] [PubMed] [Google Scholar]
- 22.Wen Y., Planel E., Herman M., Figueroa H. Y., Wang L., Liu L., Lau L. F., Yu W. H., Duff K. E. 2008. Interplay between cyclin-dependent kinase 5 and glycogen synthase kinase 3 beta mediated by neuregulin signaling leads to differential effects on tau phosphorylation and amyloid precursor protein processing. J. Neurosci. 28: 2624–2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oram J. F. 2003. HDL apolipoproteins and ABCA1: partners in the removal of excess cellular cholesterol. Arterioscler. Thromb. Vasc. Biol. 23: 720–727. [DOI] [PubMed] [Google Scholar]
- 24.Hirsch-Reinshagen V., Zhou S., Burgess B. L., Bernier L., McIsaac S. A., Chan J. Y., Tansley G. H., Cohn J. S., Hayden M. R., Wellington C. L. 2004. Deficiency of ABCA1 impairs apolipoprotein E metabolism in brain. J. Biol. Chem. 279: 41197–41207. [DOI] [PubMed] [Google Scholar]
- 25.Wahrle S. E., Jiang H., Parsadanian M., Legleiter J., Han X., Fryer J. D., Kowalewski T., Holtzman D. M. 2004. ABCA1 is required for normal central nervous system apoE levels and for lipidation of astrocyte-secreted apoE. J. Biol. Chem. 279: 40987–40993. [DOI] [PubMed] [Google Scholar]
- 26.Roheim P. S., Carey M., Forte T., Vega G. L. 1979. Apolipoproteins in human cerebrospinal fluid. Proc. Natl. Acad. Sci. USA. 76: 4646–4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pitas R. E., Boyles J. K., Lee S. H., Hui D., Weisgraber K. H. 1987. Lipoproteins and their receptors in the central nervous system. Characterization of the lipoproteins in cerebrospinal fluid and identification of apolipoprotein B, E (LDL) receptors in the brain. J. Biol. Chem. 262: 14352–14360. [PubMed] [Google Scholar]
- 28.Linton M. F., Gish R., Hubl S. T., Butler E., Esquivel C., Bry W. I., Boyles J. K., Wardell M. R., Young S. G. 1991. Phenotypes of apolipoprotein B and apolipoprotein E after liver transplantation. J. Clin. Invest. 88: 270–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoe H. S., Pocivavsek A., Dai H., Chakraborty G., Harris D. C., Rebeck G. W. 2006. Effects of apoE on neuronal signaling and APP processing in rodent brain. Brain Res. 1112: 70–79. [DOI] [PubMed] [Google Scholar]
- 30.Handelmann G. E., Boyles J. K., Weisgraber K. H., Mahley R. H., Pitas R. E. 1992. Effects of apolipoprotein E, very low-density lipoproteins, and cholesterol on the extension of neurites by rabbit dorsal root ganglion neurons in vitro. J. Lipid Res. 33: 1677–1688. [PubMed] [Google Scholar]
- 31.Ignatius M. J., Gebicke-Harter P. J., Skene J. H., Schilling J. W., Weisgraber K. H., Mahley R. W., Shooter E. M. 1986. Expression of apolipoprotein E during nerve degeneration and regeneration. Proc. Natl. Acad. Sci. USA. 83: 1125–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boyles J. K., Zoellner C. D., Anderson L. J., Kosik L. M., Pitas R. E., Weisgraber K. H., Hui D. Y., Mahley R. W., Gebicke-Haerter P. J., Ignatius M. J. 1989. A role for apolipoprotein E, apolipoprotein A-I, and low density lipoprotein receptors in cholesterol transport during regeneration and remyelination of the rat sciatic nerve. J. Clin. Invest. 83: 1015–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Graham D. I., Horsburgh K., Nicoll J. A., Taesdale G. M. 2000. Apolipoprotein E and the response of the brain to injury. Acta Neurochir. Suppl. 73: 89–92. [DOI] [PubMed] [Google Scholar]
- 34.Helkala E. L., Koivisto K., Hanninen T., Vanhanen M., Kervinen K., Kuusisto J., Mykkanen L., Kesaniemi Y. A., Laakso M., Sr. Riekkinen P. 1995. The association of apolipoprotein E polymorphism with memory: a population based study. Neurosci. Lett. 191: 141–144. [DOI] [PubMed] [Google Scholar]
- 35.Gordon I., Grauer E., Genis I., Sehayek E., Michaelson D. M. 1995. Memory deficits and cholinergic impairments in apolipoprotein E-deficient mice. Neurosci. Lett. 199: 1–4. [DOI] [PubMed] [Google Scholar]
- 36.Trommer B. L., Shah C., Yun S. H., Gamkrelidze G., Pasternak E. S., Stine W. B., Manelli A., Sullivan P., Pasternak J. F., LaDu M. J. 2005. ApoE isoform-specific effects on LTP: blockade by oligomeric amyloid-beta 1–42. Neurobiol. Dis. 18: 75–82. [DOI] [PubMed] [Google Scholar]
- 37.Mauch D. H., Nagler J., Schumacher S., Gortiz E. C., Otto A., Pfrieger F. W. 2001. CNS synaptogenesis promoted by glia-derived cholesterol. Science. 294: 1354–1357. [DOI] [PubMed] [Google Scholar]
- 38.Koudinov A. R., Koudinova N. V. 2001. Essential role for cholesterol in synaptic plasticity and neuronal degeneration. FASEB J. 15: 1858–1860. [DOI] [PubMed] [Google Scholar]
- 39.Soderberg M., Edlund C., Kristensson K., Dallner G. 1990. Lipid composition of different regions of the human brain during aging. J. Neurochem. 54: 415–423. [DOI] [PubMed] [Google Scholar]
- 40.Soderberg M., Edlund C., Kristensson K., Dallner G. 1991. Fatty acid composition of brain phospholipids in aging and in Alzheimer’s disease. Lipids. 26: 412–425. [DOI] [PubMed] [Google Scholar]
- 41.Svennerholm L., Bostrom K., Helander C. G., Jungbjer B. 1991. Membrane lipids in the aging human brain. J. Neurochem. 56: 2051–2059. [DOI] [PubMed] [Google Scholar]
- 42.Svennerholm L., Bostrom K., Jungbjer B., Olsson L. 1994. Membrane lipids of adult human brain: lipid composition of frontal and temporal lobe in subjects of age 20 to 100 years. J. Neurochem. 63: 1802–1811. [DOI] [PubMed] [Google Scholar]
- 43.Pantlia A. S., Pantlia M. K., Singh A. K., Singh I. 2008. Inhibition of rho family functions by lovastatin promotes myelin repair in ameliorating experimental autoimmune encephalomyelitis. Mol. Pharmacol. 73: 1381–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bar-On P., Crews L., Koob A. O., Mizuno H., Adame A., Spencer B., Masliah E. 2008. Statins reduce neuronal alpha-synuclein aggregation in in vitro models of Parkinson’s disease. J. Neurochem. 105: 1656–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schuster S., Nadjar A., Guo J. T., Li Q., Ittrich C., Hengerer B., Bezard E. 2008. The 3-hydroxy-3-methylglutaryl-CoA reductase inhibitor lovastatin reduces severity of L-DOPA-induced abnormal involuntary movements in experimental Parkinson’s disease. J. Neurosci. 28: 4311–4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu H., Lu D., Jiang H., Xiong Y., Qu C., Li B., Mahmood A., Zhou D., Chopp M. 2008. Simvastatin-mediated upregulation of VEGF and BDNF, activation of the PI3K/Akt pathway, and increase of neurogenesis are associated with therapeutic improvement after traumatic brain injury. J. Neurotrauma. 25: 130–139. [DOI] [PubMed] [Google Scholar]
- 47.Wang H., Lynch J. R., Song P., Yang H. J., Yates R. B., Mace B., Warner D. S., Guyton J. R., Laskowitz D. T. 2007. Simvastatin and atorvastatin improve behavioral outcome, reduce hippocampal degeneration, and improve cerebral blood flow after experimental traumatic brain injury. Exp. Neurol. 206: 59–69. [DOI] [PubMed] [Google Scholar]
- 48.Coetsee T. N., Pretorius P. J., Terre’blanche G., Bergh J. J. 2008. Investigating the potential neuroprotective effects of statins on DNA damage in mouse striatum. Food Chem. Toxicol. 46: 3186–3192. [DOI] [PubMed] [Google Scholar]
- 49.Konat G. W., Krasowska-Zoladek A., Kraszpulski M. 2008. Statins enhance toll-like receptor 4-mediated cytokine gene expression in astrocytes: implication of Rho proteins in negative feedback regulation. J. Neurosci. Res. 86: 603–609. [DOI] [PubMed] [Google Scholar]
- 50.Tsai H. I., Tsai L. H., Chen M. Y., Chou Y. C. 2006. Cholesterol deficiency perturbs actin signaling and glutamate homeostasis in hippocampal astrocytes. Brain Res. 1104: 27–38. [DOI] [PubMed] [Google Scholar]
- 51.Pannu R., Barbosa E., Singh A. K., Singh I. 2005. Attenuation of acute inflammatory response by atorvastatin after spinal cord injury in rats. J. Neurosci. Res. 79: 340–350. [DOI] [PubMed] [Google Scholar]
- 52.Pahan K., Sheikh F. G., Namboodiri A. M., Singh I. 1997. Lovastatin and phenylacetate inhibit the induction of nitric oxide synthase and cytokines in rat primary astrocytes, microglia, and macrophages. J. Clin. Invest. 100: 2671–2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pavlov O. V., Bobryshev Yu. V., Balabanov Yu. V., Ashwell K. 1995. An in vitro study of the effects of lovastatin on human fetal brain cells. Neurotoxicol. Teratol. 17: 31–39. [DOI] [PubMed] [Google Scholar]