Abstract
We attempted to determine whether intrapulmonary sensory receptors are nourished by the pulmonary or the systemic circulation. Single-unit activity from the cervical vagus nerve was recorded in anesthetized, open chest, mechanically ventilated rabbits, comparing responses to right or left ventricular injection of 2% lidocaine (at 4 mg/kg). Airway mechanosenors [slowly adapting receptor (SARs) and rapidly adapting receptors] were inhibited by lidocaine, whereas chemosensors (C-fiber receptors and high-threshold Aδ-receptors) were stimulated. Furthermore, all types of airway sensors were perfused preferentially by the pulmonary circulation. For example, 14 of the 15 tested SARs ceased discharge at 4.1 ± 0.6 s after lidocaine injection into the right ventricle. The blocking effect lasted 35 ± 6.2 s. In contrast, none of the 15 SARs ceased their activity after lidocaine injection into the left ventricle. Our data show that intrapulmonary sensors are mainly nourished by the pulmonary circulation. Their very short latency indicates that these sensors receive ample blood supply. Thus, intrapulmonary sensors rely on the pulmonary circulation to detect bioactive agents in the blood.
Keywords: lung, vagus nerve, sensory receptor, local anesthetics, airway
the lung and the airways are richly innervated by vagal afferents. The vagus nerve innervates four groups of airway sensory receptors: slowly adapting receptors (SARs), rapidly adapting receptors (RARs), C-fiber receptors (CFRs), and high-threshold Aδ-receptors (HTARs) (6, 14, 20, 32). These receptors participate in defense reflexes, such as coughing and sneezing, and modify respiratory and cardiovascular performance (6, 14). Many studies have focused on the effect of pathological states in activating intrapulmonary sensors and their reflex function (7, 11, 27). Increasing evidence suggests that the airway sensors play important roles in innate immune responses and in pulmonary diseases through neuroimmune interaction (33, 34).
The lungs are the major site for passage and metabolism of bioactive substances that can activate airway sensors. Knowing the source of the blood supply to airway sensors helps with understanding pulmonary sensory mechanisms and thus has both physiological and clinical significance. The lungs are uniquely perfused by two separate circulatory routes: pulmonary and bronchial (3). The pulmonary circulation delivers the entire cardiac output. In addition to gas exchange and maintaining lung fluid balance, pulmonary microvessels act as a source of production, processing, and release of humoral mediators. In contrast, the bronchial circulation carries < 3% of the cardiac output. There is some evidence of bronchopulmonary anastomoses; however, their significance is controversial (18). Although infrequently found in normal lungs, they may increase considerably in certain pathologic disorders (9, 10). Anastomoses are more easily demonstrable in infants than adults (29).
The local anesthetic lidocaine blocks voltage-gated Na+ channels and suppresses action potential generation and propagation of neurons, including airway sensors. In humans, inhalation of lidocaine blocks airway sensors (12, 25, 31); however, whether blocking the sensor alters the breathing pattern is controversial. For example there are variable reports showing it alters breathing pattern at rest (25), during exercise but not at rest (31), or even has no effects during exercise (12). Local anesthetics can also depolarize sensory neurons by activating the transient receptor potential vanilloid 1 channel (TRPV1) (15). TRPV1 is a nonspecific cation channel predominantly expressed by nociceptive sensory neurons. In this study, lidocaine was employed to determine whether intrapulmonary airway sensors are nourished by the pulmonary circulation or the systemic circulation.
METHODS
General.
All procedures complied with ethical standards established by the National Institutes of Health and were IACUC-approved at the University of Louisville. Experiments were performed on 30 New Zealand rabbits (body wt, 2.0–2.4 kg). The rabbits were premedicated with ketamine HCl (50 mg/kg im) and xylazine (5 mg/kg im) and then anesthetized intravenously with 20% urethane (1 g/kg body wt). Both the femoral vein and artery were cannulated for medication delivery and measuring blood pressure through a strain gauge pressure transducer.
Surgical procedures.
After a midline incision to expose the trachea and vagus nerve, the trachea was cannulated low in the neck. The lungs were ventilated by a ventilator (model 683; Harvard Apparatus, Holliston, MA). Positive end expiratory pressure was applied by placing the expiratory outlet under 4 cmH2O. Airway pressure was monitored by a Statham pressure transducer (P23) attached to the tracheal tube. The chest was then opened widely through a sternostomy so that the receptive field of the sensory nerve could be grossly located by gently exploring the pleural lung surface with a cotton tip applicator. Airway pressure, blood pressures, and afferent activities were recorded by an Astro-Med thermorecorder (Dash IV).
Recording technique.
The vagus nerve was cut high in the neck. The peripheral end of the nerve was placed on a dissecting plate, desheathed, and flooded with mineral oil. Subsequently, the vagus nerve's filaments were separated with a pair of watchmaker's forceps. The fine filaments were then placed on a pair of platinum electrodes connected to an AC-coupled amplifier. The electrodes were connected to a high-impedance probe (model HIP 511) with the output fed to a Grass (model P511) amplifier. After suitable amplification, the single unit activities were displayed on an oscilloscope and acoustically monitored by an attached loudspeaker. In addition, a voltage analog of impulse frequency was produced by a rate meter (Frederick Haer, Brunswick, ME) at a bin width of 0.1 s. Baseline unit activity was calculated for 1 min, with each unit serving as its own control. Peak response was obtained following injection of lidocaine. Unit activity was expressed as impulses per second.
Sensory receptors were classified according to convention (16). SARs and RARs were identified by their adaptation index (<30% for SARs and >70% for RARs). HTARs were identified by their discharge pattern. They do not behave like SRAs or RARs, but similarly to CFRs, with a conduction velocity of 4–15 m/s (16). CFRs were identified according to their discharge pattern and a conduction velocity of < 1.5 m/s. Sensory receptive fields were identified by the unit response to probing the lung with a cotton applicator (35). The sensors that are identified in the lung were studied. To assess blood supply, we compared the responses of the sensors to right or left intraventricular injection of 2% lidocaine (at 4 mg/kg in 0.4 ml). This dose was determined by a preliminary trial as giving reliable suppression and stimulation of the sensors without eliciting detrimental cardiovascular suppression. The ventricular injections were made through a transcardiac needle. The needle location is verified by the color of blood withdrawn from the heart chamber. The blood drawn from the left ventricle is bright red, while that from the right one is bluish dark. The injection was further confirmed by looking at the time course of the depressor response. The left ventricular injection had a shorter latency than the right one.
RESULTS
Twenty-five mechanosensors and 25 chemosensors were identified. Among the mechanosensors, 15 were SARs and 10 were RARs. Twelve of the 25 chemosensors were HTARs, and 13 were CFRs.
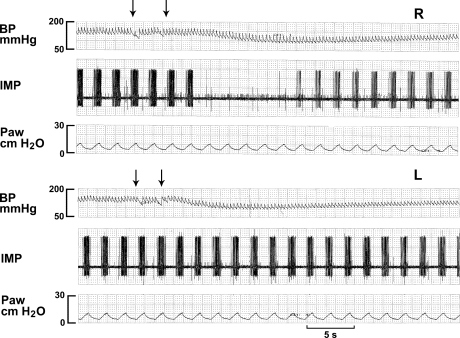
The SARs showed typical increase in activity during lung inflation, with decrease during deflation. Their discharges were regular and predictable. Twelve SARs were high threshold (with a deflation pause in activity); the other three were lower threshold (with a continuous discharge during lung deflation), discharging at 35.6 ± 7.7 and 42.2 ± 11.3 imp/s, respectively. Fourteen of the 15 SARs tested ceased discharge at 4.1 ± 0.6 s after lidocaine injection into the right ventricle (Fig. 1). The blocking effect lasted 35 ± 6.2 s. None of the 15 SARs ceased activity with lidocaine injection into the left ventricle. The difference between cessation rates (14/15 for the right injection vs. 0/15 for the left injection) was statistically significant by χ2 test (P < 0.001). In addition, blood pressure dropped significantly (about 40 mmHg) after injection of lidocaine into both ventricles, confirming effective delivery of the lidocaine. The latency period for the decrease in blood pressure was much shorter for injection into the left ventricle than the right ventricle. The peak responses were 3.95 ± 0.75 s vs. 8.31 ± 0.47 s (n = 15; P < 0.01), suggesting that the depressor effect occurs via the systemic circulation.
Fig. 1.
Comparison of responses of a slowly adapting receptor (SAR) to injections of 2% lidocaine (4 mg/kg) into the right ventricle (top 3 traces, R) and the left ventricle (bottom 3 traces, L). BP, blood pressure; IMP, impulses; SAR activity; Paw, airway pressure. Arrows denote start and end of injection time. Note that the SAR activity ceased after injection of lidocaine into the right ventricle but not the left ventricle. On the other hand, blood pressure decreased earlier in response to the left than to the right injection.
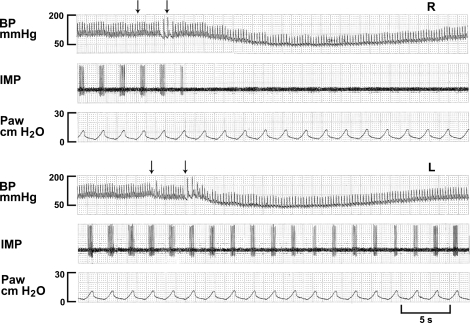
Ten RARs were examined and had irregular discharges with lower frequencies than SARs. Their activity usually occurred during lung inflation; however, three RARs were inactive. The rest had low baseline discharge frequency (1.3 ± 1.4 imp/s, n = 7). Average baseline activity was 0.9 ± 1.2 imp/s (n = 10). RAR peak activity increased to 20.5 ± 18.7, 51.5 ± 37, and 94 ± 26.4 imp/s at lung inflations of 10, 20, and 30 cmH2O, respectively. None of RARs were stimulated by lidocaine. Five had no response to either right or left ventricular injections of lidocaine. The remaining five were suppressed by lidocaine injected into the right side but not the left. Like the SARs, their activity was immediately suppressed at the first or second ventilator cycle after injection and ceased for several breaths before gradually recovering. Like the SAR, the RAR was suppressed earlier before the blood pressure dropped to the nadir (Fig. 2). Therefore, most RARs in the lung are perfused by the pulmonary circulation. It appeared that RARs with relatively high discharge frequency during respiratory cycles were inhibited, but those with lower and sporadic discharges were nonresponders. We cannot determine whether inactive or almost inactive RARs were suppressed.
Fig. 2.
A rapidly adapting receptor (RAR) in response to injection of lidocaine (4 mg/kg) into the right ventricle (top 3 traces, R) and left ventricle (bottom 3 traces, L). Arrows indicate beginning and end of injection. RAR discharge ceased immediately following injection. Blockade of activity preceded the decrease in blood pressure. RAR was also inhibited by left-side injection with a much longer delay. The inhibition occurred after peak response in blood pressure. This suggests that the blockade is at the level of pulmonary circulation.
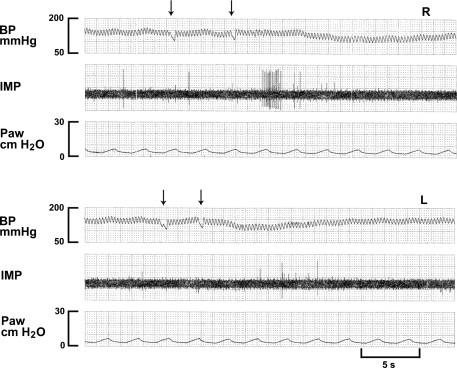
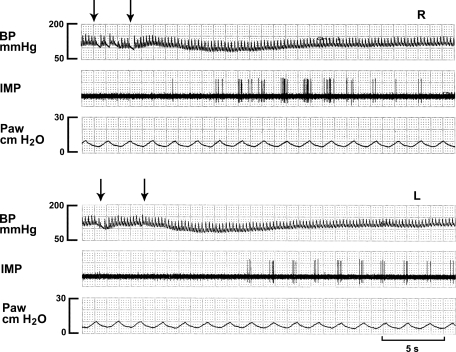
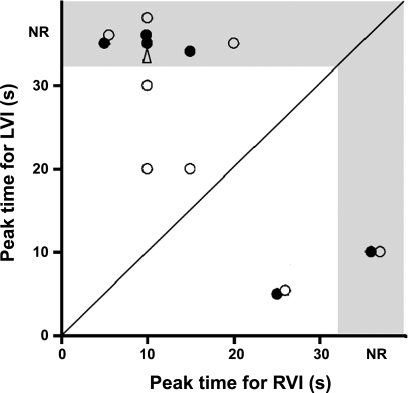
Baseline activity in both CFRs (0.3 ± 0.2 imp/s, n = 13) and HTARs (0.2 ± 0.1 imp/s, n = 12) was sporadic and low (averaging 0.3 ± 0.2 imp/s, n = 25). Their discharge patterns were similar, and there was no clear relation to the ventilation cycle. In marked contrast to RARs and SARs, nociceptors responded to lung inflation but with very low discharge frequencies. For example, peak activities were 5.0 ± 3.8, 10.4 ± 5.1, and 18.8 ± 7.8 imp/s in CFRs, and 1.6 ± 2.2, 8.7 ± 3.1, and 16.3 ± 5.8 imp/s in HTARs at lung inflations of 10, 20, and 30 cmH2O, respectively. This indicates the mechanosensory component is not a major feature of these sensors. Among the 25 nociceptors, 10 were nonresponders, while the other 15 responded to lidocaine. Twelve of the 15 responders (80%) were stimulated by lidocaine injected into the right ventricle (Fig. 3), and five of these 12 nociceptors were also stimulated by the left ventricular injection (Figs. 4 and 5). Two nociceptors were stimulated by the left injection but not the right injection. One of the HTARs was suppressed, instead of stimulated, by lidocaine injection. The 15 responders were illustrated in Fig. 5.
Fig. 3.
A C-fiber receptor (CFR) in response to injection of lidocaine (4 mg/kg) into the right ventricle (top 3 traces, R) and left ventricle (bottom 3 traces, L). Note that this CFR has lower background activity (almost inactive). In contrast to mechanosensors, the CFR is stimulated instead of inhibited following injection of lidocaine (arrows denoting start and end of the injection) into the pulmonary circulation.
Fig. 4.
A high-threshold Aδ-receptor (HTAR) in response to injection of lidocaine (4 mg/kg) into the right ventricle (top 3 traces, R) and left ventricle (bottom 3 traces, L). Note that this HTAR has lower background activity. It is stimulated by both injections into the right and left ventricles (arrows denoting injection period) with a longer latency for left-side injection; this indicates a preferential perfusion by pulmonary circulation.
Fig. 5.
Distribution of the perfusion routes of the 15 nociceptors that responded to left (LVI) or right ventricular injection (RVI) of lidocaine. Each circle represents a specific sensory receptor. •, CFRs; ○, HTARs; ▵, HTAR inhibited by lidocaine. Receptors falling into shaded area are nonresponders (NR) in that route. Receptors falling below the identity line (45-degree line) are preferentially perfused by bronchial circulation, and receptors above the line are preferentially perfused by pulmonary circulation.
DISCUSSION
The pulmonary circulation contains a higher concentration of metabolic byproducts than the systemic circulation (8). These bioactive products can stimulate afferents and influence how the central nervous system interprets the chemical state of the lung (6, 14, 17). Our data show that the majority of intrapulmonary sensors, including nociceptors, are nourished by the pulmonary circulation, providing an anatomical basis for detecting endogenous bioactive agents in the lung during disease. These studies support the hypothesis that airway receptors act as biosensors that monitor immune information and can send signals to the brain for lung-brain communication.
Paintal (21) first investigated perfusion of nonmyelinated vagal lung sensors in cats in 1957. These sensors were stimulated following injection of phenyl diguanide into the right atrium (pulmonary circulation) but not into the left atrium (bronchial circulation) (22). In addition, they were immediately suppressed by inhalation of anesthetics, indicating ready accessibility from the surfaces of airways. Therefore, the endings were believed to be located near the alveoli and close to pulmonary capillaries. These sensory receptors were termed juxtacapillary receptors, or J receptors. In 1977, Coleridge and Coleridge (5) identified two groups of nonmyelinated sensors based on the latency of their response following injection of capsaicin into the right or left atrium in dogs; they were named pulmonary and bronchial CFRs. In 1984, Trenchard et. al. (28) classified CFRs in rabbits into three groups: pulmonary, bronchial, and pulmonary-bronchial, on the basis of their responses to cardiovascular injections of phenylbiguanide. Our present results from the nociceptors agree well with Trenchard's observations.
By injecting lidocaine at a minimally effective concentration into the pulmonary and systemic circulation, we demonstrated that the pulmonary circulation perfuses most SARs in the lung. Their activity is blocked by injection into the right ventricle, but not the left. The decrease in blood pressure seems to be caused by action in the systemic circulation. This knowledge is important because it can help to delineate the action of the anesthetic blockade. For example, Aoki et al. (1) reported that intravenous injection of lidocaine suppresses SARs in cats, although they did not specify whether the action was via the pulmonary or systemic circulation. The sensory blockade preceded the decrease in blood pressure, suggesting that the effect is through the pulmonary circulation.
Armstrong and Luck (2) found most SARs are readily stimulated by intravenous injection of veratrine. They believed these sensory receptors are located on the terminal bronchioles near the alveoli because they are readily accessible from the pulmonary circulation. In Ravi's study (23), injection of veratrine into the pulmonary circulation stimulated 83% of high-threshold SARs, but only 25% of low-threshold SARs. Since low-threshold SARs are predominant in central airways while high-threshold SARs are located more peripherally, the result also supports that the majority of intrapulmonary SARs are pulmonary perfused. Accessibility via the systemic or the pulmonary circulation of SARs and RARs was also studied in dogs by Sant'Ambrogio and Sant'Ambrogio (24), who found more centrally located SARs (tracheal) received their blood supply via the systemic circulation, whereas more peripheral SARs (intrapulmonary) were preferentially perfused via the pulmonary circulation. This is in agreement with anatomical studies on airway perfusion (4). However, a mixed dependency between the two vascular routes was observed when sensory endings were located in the intrapulmonary portions of the lobar bronchi. There are two potential explanations for this observation. First, there may be microcirculatory routes shared by the bronchial and pulmonary circulation and these receptors can receive their blood supply from either the pulmonary or systemic circulation. These microcirculatory routes have been anatomically illustrated in previous studies (26). Second, the activity of the receptors observed may be a consequence of the concentration of the bioactive substance injected. If high concentrations are injected into the systemic circulation (left ventricle), then there would be ample concentration available to stimulate pulmonary sensors when it reached the pulmonary circulation through the venous system. Listed in their Table 2 (Ref. 24), two of the three SARs in the lobular bronchi (in the benzoate study) were perfused by bronchial circulation. After eliminating these two SARs and calculating the latency periods for the remaining 19 SARs, the latencies were 4.9 and 15 s following the right and left atrial injection, respectively. Such a long latency after the left-side injection indicates that SAR activation is due to recirculation of the agent into the pulmonary vasculature.
Under normal conditions, pulmonary circulation operates in a negative pressure environment. Theoretically, positive end expiratory pressure application, mechanical ventilation, and open chest may increase the resistance of pulmonary circulation and thus may affect pulmonary/bronchial blood flow distribution in favor of bronchial perfusion. However, such a potential alteration should not cause a bias in our interpretation that pulmonary circulation is the major perfusion route for intrapulmonary sensors.
HTARs have been identified both in intact animals and in vitro studies (30, 32). HTARs differ from RARs and share many properties with CFRs. Approximately 60% of the nociceptors in this series were stimulated by lidocaine. In a previous report (16), HTARs were significantly stimulated during acute lung injury caused by intravenous injection of oleic acid, yet RAR sensitivity to lung inflation was suppressed during oleic acid-induced injury. The present studies further demonstrate the differences in sensory properties between HTARs and RARs. HTARs were stimulated by lidocaine, whereas RARs were inhibited. Clearly, these myelinated afferents are two distinct populations. We do not know the activation mechanism. If the chemosensor stimulation is due to activation of TRPV1 receptors (15), then it indicates that mechanosensors don't possess TRPV1 receptors. Since not all CFRs and HTARs are activated by lidocaine, the nociceptors must be a heterogeneous group. We do not know why 10 nociceptors did not respond to lidocaine. These sensors may be far away from the capillaries that perfuse them, may not be sensitive to the agent, or may come from different origins since airway nociceptors are reported to originate from the jugular and nodose ganglia (13). Furthermore, lidocaine is an anesthetic. At high concentrations, it should inhibit the sensory neuron, but it may have both stimulatory and inhibitory effects mediated through different mechanisms. Therefore, lidocaine effects should be both time and concentration dependant. However, it is not surprising that the nociceptors responded to lidocaine heterogenously. Similarly, capsaicin stimulates many C-fibers, but not the others.
Since the lung is a major site for passage and metabolism of bioactive substances that can activate airway sensors, knowing the source of the blood supply for those sensors is crucial to understanding their function in immunity. However, the pattern and extent of anastomoses between the circulatory systems differ and vary dramatically by species. For example, the rabbit is more extensively perfused by the pulmonary circulation compared with other species (19). The bronchial artery in the rabbit terminates at the third division of the main stem bronchus. Beyond this point, the parenchymal blood supply is derived from the pulmonary circulation. In view of the significant differences in airway anatomy, blood flow, and receptor distribution, the findings in the present studies preclude direct extrapolation to other species, including humans. However, the results may help to identify the role of airway sensors in disease processes in future studies.
In conclusion, the behaviors of chemosensors, including CFRs and HTARs, are similar. Mechanosensors, such as RARs and SARs, are also much alike, but chemical and mechanical sensors are very different. Chemosensors are stimulated by lidocaine, whereas mechanosensors are inhibited. Since intrapulmonary airway sensors are readily accessible from the pulmonary circulation, any simulatory agent with higher concentration in the pulmonary vs. the systemic circulation during disease conditions will have a greater effect on susceptible sensors.
Perspectives and Significance
Increasing evidence indicates that airway sensors play a crucial role in transmitting immune information from the lung to the central nervous system. Therefore, knowing the source of perfusion for the sensor is important in understanding the pathophysiological mechanisms of these sensors in signal transduction. The present results show that most of the intrapulmonary sensors are perfused by the pulmonary circulation. Furthermore, the behavior of chemosensors (CFRs and HTARs) is different from that of mechanosensors (SARs and RARs). The chemosensors are stimulated by local anesthetics (lidocaine), whereas the mechanosensors are suppressed. Thus, lidocaine is a useful tool to assess the accessibility of the sensors to blood supply. Our results indicate that intrapulmonary sensors are positioned to detect bioactive agents in the pulmonary circulation, providing a neurally mediated lung-brain communication.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grant HL-58727. Dr. Li is a recipient of American Heart Association Fellowship 0825694D.
REFERENCES
- 1.Aoki M, Harada Y, Namiki A, Ikeda M, Shimizu H. Effects of intravenously administered lidocaine on pulmonary vagal afferents and phrenic nerve activity in cats. J Anesth 6: 395–400, 1992. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong DJ, Luck JC. Accessibility of pulmonary stretch receptors from the pulmonary and bronchial circulations. J Appl Physiol 36: 706–710, 1974. [DOI] [PubMed] [Google Scholar]
- 3.Baile EM The anatomy and physiology of the bronchial circulation. J Aerosol Med 9: 1–6, 1996. [DOI] [PubMed] [Google Scholar]
- 4.Barman SA, Ardell JL, Parker JC, Perry ML, Taylor AE. Pulmonary and systemic blood flow contributions to upper airways in canine lung. Am J Physiol Heart Circ Physiol 255: H1130–H1135, 1988. [DOI] [PubMed] [Google Scholar]
- 5.Coleridge HM, Coleridge JCG. Impulse activity in afferent vagal C-fibres with endings in the intrapulmonary airways of dogs. Respir Physiol 29: 125–142, 1977. [DOI] [PubMed] [Google Scholar]
- 6.Coleridge HM, Coleridge JCG. Reflexes evoked from tracheobronchial tree and lungs. In: Handbook of Physiology. The Respiratory System. Control of Breathing. Bethesda, MD: Am. Physiol. Soc., 1986, sect. 3, vol. II, pt. 1, chapt. 12, p. 395–430. [Google Scholar]
- 7.Dallak MA, Pirie LJ, Davies A. The influence of pulmonary receptors on respiratory drive in a rabbit model of pulmonary emphysema. Respir Physiol Neurobiol 156: 33–39, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Fishman AP The nonrespiratory functions of the lungs. In: Pulmonary Diseases and Disorders, edited by Fishman AP. New York: McGraw-Hill, 1988, p. 205–219.
- 9.Fung YC, Sobin SS. Theory of sheet flow in lung alveoli. J Appl Physiol 26: 472–488, 1969. [DOI] [PubMed] [Google Scholar]
- 10.Gossage JR, Kanj G. Pulmonary arteriovenous malformations. A state of the art review. Am J Respir Crit Care Med 158: 643–661, 1998. [DOI] [PubMed] [Google Scholar]
- 11.Gunawardena S, Ravi K, Longhurst JC, Kaufman MP, Ma A, Bravo M, Kappagoda CT. Responses of C fiber afferents of the rabbit airways and lungs to changes in extra-vascular fluid volume. Respir Physiol Neurobiol 132: 239–251, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Krishnan BS, Stockwell MJ, Clemens RE, Gallagher CG. Airway anesthesia and respiratory adaptations to dead space loading and exercise. Am J Respir Crit Care Med 155: 459–465, 1997. [DOI] [PubMed] [Google Scholar]
- 13.Kwong K, Carr MJ, Gibbard A, Savage TJ, Singh K, Jing J, Meeker S, Undem BJ. Voltage-gated sodium channels in nociceptive versus non-nociceptive nodose vagal sensory neurons innervating guinea pig lungs. J Physiol 586: 1321–1336, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee LY, Pisarri TE. Afferent properties and reflex functions of bronchopulmonary C-fibers. Respir Physiol 125: 47–65, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Leffler A, Fischer MJ, Rehner D, Kienel S, Kistner K, Sauer SK, Gavva NR, Reeh PW, Nau C. The vanilloid receptor TRPV1 is activated and sensitized by local anesthetics in rodent sensory neurons. J Clin Invest 118: 763–776, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin S, Walker J, Xu L, Gozal D, Yu J. Respiratory: behaviours of pulmonary sensory receptors during development of acute lung injury in the rabbit. Exp Physiol 92: 749–755, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Lin SX, Yu J. Effects of arachidonic acid metabolites on airway sensors. Acta Physiologica Sinica 59: 141–149, 2007. [PubMed] [Google Scholar]
- 18.Malik AB Pulmonary microembolism. Physiol Rev 63: 1114–1207, 1983. [DOI] [PubMed] [Google Scholar]
- 19.McLaughlin RF, Tyler WS, and Canada RO. Subgross pulmonary anatomy of the rabbit, rat, and guinea pig, with additional notes on the human lung. Am Rev Respir Dis 94: 380–387, 1966. [DOI] [PubMed] [Google Scholar]
- 20.Paintal AS Vagal sensory receptors and their reflex effects. Physiol Rev 53: 159–227, 1973. [DOI] [PubMed] [Google Scholar]
- 21.Paintal AS The location and excitation of pulmonary deflation receptors by chemical substances. Q J Exp Physiol Cogn Med Sci 42: 56–71, 1957. [DOI] [PubMed] [Google Scholar]
- 22.Paintal AS Mechanism of stimulation of type J pulmonary receptors. J Physiol 203: 511–532, 1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ravi K Distribution and location of slowly adapting pulmonary stretch receptors in the airways of cats. J Auton Nerv Syst 15: 205–216, 1986. [DOI] [PubMed] [Google Scholar]
- 24.Sant'Ambrogio FB, Sant'Ambrogio G. Circulatory accessibility of nervous receptors localized in the tracheobronchial tree. Respir Physiol 49: 49–73, 1982. [DOI] [PubMed] [Google Scholar]
- 25.Savoy J, Dhingra S, Anthonisen NR. Inhaled lidocaine aerosol changes resting human breathing pattern. Respir Physiol 50: 41–49, 1982. [DOI] [PubMed] [Google Scholar]
- 26.Schraufnagel DE, Pearse DB, Mitzner WA, Wagner EM. Three-dimensional structure of the bronchial microcirculation in sheep. Anat Rec 243: 357–366, 1995. [DOI] [PubMed] [Google Scholar]
- 27.Trenchard D, Gardner D, Guz A. Role of pulmonary vagal afferent nerve fibres in the development of rapid shallow breathing in lung inflammation. Clin Sci 42: 251–263, 1972. [DOI] [PubMed] [Google Scholar]
- 28.Trenchard D, Russell NJ, Raybould HE. Non-myelinated vagal lung receptors and their reflex effects on respiration in rabbits. Respir Physiol 55: 63–79, 1984. [DOI] [PubMed] [Google Scholar]
- 29.Wagenvoort CA, Wagenvoort N. Arterial anastomoses, bronchopulmonary arteries, and pulmobronchial arteries in perinatal lungs. Lab Invest 16: 13–24, 1967. [PubMed] [Google Scholar]
- 30.Widdicombe JG Overview of neural pathways in allergy and asthma. Pulm Pharmacol Ther 16: 23–30, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Winning AJ, Hamilton RD, Shea SA, Knott C, Guz A. The effect of airway anaesthesia on the control of breathing and the sensation of breathlessness in man. Clin Sci (Lond) 68: 215–225, 1985. [DOI] [PubMed] [Google Scholar]
- 32.Yu J Airway mechanosensors. Respir Physiol Neurobiol 148: 217–243, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Yu J Airway receptors and their reflex function. Adv Exp Med Biol 648: 411–420, 2009. [DOI] [PubMed] [Google Scholar]
- 34.Yu J, Lin S, Zhang J, Otmishi P, Guardiola JJ. Airway nociceptors activated by pro-inflammatory cytokines. Respir Physiol Neurobiol 156: 116–119, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Yu J, Zhang J. A single pulmonary mechano-sensory unit possesses multiple encoders in rabbits. Neurosci Lett 362: 171–175, 2004. [DOI] [PubMed] [Google Scholar]