Abstract
Maternal obesity affects offspring weight, body composition, and organ function, increasing diabetes and metabolic syndrome risk. We determined effects of maternal obesity and a high-energy diet on fetal pancreatic development. Sixty days prior to breeding, ewes were assigned to control [100% of National Research Council (NRC) recommendations] or obesogenic (OB; 150% NRC) diets. At 75 days gestation, OB ewes exhibited elevated insulin-to-glucose ratios at rest and during a glucose tolerance test, demonstrating insulin resistance compared with control ewes. In fetal studies, ewes ate their respective diets from 60 days before to 75 days after conception when animals were euthanized under general anesthesia. OB and control ewes increased in body weight by ∼43% and ∼6%, respectively, from diet initiation until necropsy. Although all organs were heavier in fetuses from OB ewes, only pancreatic weight increased as a percentage of fetal weight. Blood glucose, insulin, and cortisol were elevated in OB ewes and fetuses on day 75. Insulin-positive cells per unit pancreatic area were 50% greater in fetuses from OB ewes as a result of increased β-cell mitoses rather than decreased programmed cell death. Lambs of OB ewes were born earlier but weighed the same as control lambs; however, their crown-to-rump length was reduced, and their fat mass was increased. We conclude that increased systemic insulin in fetuses from OB ewes results from increased glucose exposure and/or cortisol-induced accelerated fetal β-cell maturation and may contribute to premature β-cell function loss and predisposition to obesity and metabolic disease in offspring.
Keywords: sheep, fetal growth, pancreatic function
recent data from the 1999–2002 National Health and Nutrition Examination Survey (NHANES) show that almost 65% of the adult population in the United States is overweight, defined as having a body mass index > 25 kg/m2, compared with 56% observed in NHANES III, conducted between 1988 and 1994 (32a). Obesity among women of reproductive age ranges from 20 to 34% (8, 10). Furthermore, Boney et al. (8) reported that by 11 years of age, children exposed to maternal obesity were at twice the risk of developing some components of the metabolic syndrome (obesity, systolic or diastolic hypertension, high triglyceride levels, low HDL levels, glucose intolerance), which suggests that obese mothers, even in the absence of gestational diabetes, may have metabolic factors that affect fetal growth and postnatal outcomes. Maternal obesity has been associated with either intrauterine growth restriction (IUGR) or large-for-gestational-age fetuses (12, 33). Both conditions are connected to offspring exhibiting altered insulin secretion and adiposity (27, 43). Studies on Pima Indians (36) indicate that the intrauterine environment plays an important role in development of diabetes in the offspring.
The majority of studies on the effects of maternal nutrition on fetal development have investigated the effects of nutrient restriction and have been conducted in rodents and sheep (13) The “thrifty phenotype” hypothesis proposes that poor in utero nutrition can lead to fetal adaptations that produce permanent changes in insulin and glucose metabolism, increasing the risk of adult obesity, type 2 diabetes, and metabolic syndrome (22). Maternal nutrient restriction alters fetal pancreatic morphology and function (40), as well as insulin homeostasis (22). Studies in human, rat, and sheep fetuses with IUGR indicate that a reduction in pancreatic β-cell mass is a common response to nutrient restriction in fetuses with placental insufficiency and IUGR (37). Studies on maternal obesity in rodents, which are altricial species, show that obesity and maternal high-fat diets can predispose offspring to impaired pancreatic function (3, 7). One recent study has observed threefold increases in triglycerides and histologic correlates of nonalcoholic fatty liver disease in the fetal liver in pregnant Japanese macaques fed a high-fat diet (1). Experimental data are not available on effects of maternal obesity on early pancreatic maturation in precocial species. The sheep has been used extensively as a model for the study of fetal and neonatal metabolic development since it is a precocial, generally monotocous species in which the biomass nurtured by the mother is similar to the human (28, 38). While effects of maternal nutritional deprivation on fetal development have been extensively studied in this ovine model, there are currently no studies in this species that address effects of prepregnancy obesity and overnutrition on early fetal pancreatic development together with information on phenotype of the offspring.
The objective of the present study was to evaluate the impact of maternal obesity and a high plane of nutrition on fetal growth and pancreatic structure and function at midgestation in the sheep. The present study provides evidence for untoward effects of maternal obesity and a high plane of nutrition on fetal pancreatic structure and function and postnatal glucose homeostasis in a monotocous, precocial species.
RESEARCH DESIGN AND METHODS
Animals
All animal procedures were approved by the University of Wyoming Animal Care and Use Committee. From 60 days before conception (first day of mating = day 0) to day 75 of gestation, multiparous ewes (Rambouillet/Columbia cross) were fed either a highly palatable diet, at 100% (control ewes; n = 10) of National Research Council (NRC) recommendations (32b) or 150% [obesogenic (OB) ewes; n = 11] of NRC recommendations for energy based on metabolic body weight (0.75) of individual ewes as previously described (44). All ewes consumed 100% of their diet each day. Only ewes carrying singleton fetuses were utilized in this study. All ewes were weighed at weekly intervals, rations were adjusted for weight gain, and body condition was scored at monthly intervals to evaluate changes in fatness. A Body Condition Score of 1 (emaciated) to 9 (obese) was assigned independently by two trained individuals. Body Condition Score is highly related to carcass lipids and can be used to estimate energy reserves available to ewes (39).
Maternal and fetal blood samples and fetal tissues were obtained at necropsy from control (n = 5) and OB (n = 6) ewes at 75 days of gestation. Immediately before necropsy, each ewe was weighed, and a sample of blood was collected via jugular venipuncture into a chilled nonheparinized vacutainer tube (no additives; Sigma, St. Louis, MO) and serum frozen at −80°C until assayed for insulin, cortisol, and insulin-like growth factor-I (IGF-I). A separate chilled tube (heparin plus sodium fluoride; 2.5 mg/ml; Sigma) was collected, and plasma was frozen at −80°C until assayed for glucose. Ewes were sedated with ketamine (10 mg/kg) and maintained under isofluorane inhalation general anesthesia (2.5%). Immediately following laparotomy, umbilical vein serum and plasma were collected and stored as described for maternal blood. Fetuses were euthanized by exsanguination while still under general anesthesia. Ewes were euthanized with an overdose of pentobarbital sodium (Abbott Laboratories, Abbott Park, IL), and the gravid uterus quickly removed.
Glucose tolerance (described below) was studied in the remaining ewes, control (n = 5) and OB (n = 5), at 75 days gestation, which were maintained on their respective diets throughout the remainder of gestation and allowed to lamb.
Tissue Collection
Fetal weights, crown rump lengths, and sex were determined, and the fetal pancreas collected and weighed. Three male and two female fetuses were recovered from control ewes, while three male and three female fetuses were recovered from OB ewes. The hepatic (head) portion of the pancreas was frozen in liquid nitrogen for protein extraction and immunoblotting, and the splenic (tail) portion was placed in a tissue cassette (Tissue Tek; Miles Labs, Elkhart, IN), fixed with 4% (wt/vol) paraformaldehyde in a phosphate buffer (0.12 M; pH 7.4), and paraffin embedded as previously described (42). Weights of selected fetal tissues (liver, lungs, kidneys, adrenals, spleen, gonads, brain, heart, semitendenosus, and longisimus dorsi muscles) were recorded.
Immunohistochemistry
Determination of insulin and glucagon-positive cells.
From each fetus, six 5-μm sections were obtained from paraffin-embedded blocks of control and OB fetal pancreatic tissue (tail portion) maintaining at least 100 μm between sections as previously described by Limesand et al. (26). Paraffin-embedded sections were then deparaffinized and rehydrated by routine methods before the antigen retrieval. Nonspecific antigenic sites were blocked by a 60-min incubation in 1.5% normal goat serum (Vector Laboratories, Burlingame, CA) in PBS with 0.1% Triton X-100 (Union Carbide, Somerset, NJ) and 0.05% Tween 20 (Bio-Rad Laboratories, Hercules, CA) and then for 30 min with FX signal enhancer (Invitrogen, Carlsbad, CA) before the sections were incubated with guinea pig anti-porcine insulin (1:500; Dako, Carpinteria CA) and mouse antiglucagon (1:500; Sigma-Aldrich, St. Louis, MO) antibodies at 4°C overnight. The sections were then labeled with fluorescent secondary antibodies: Rhodamine-labeled goat anti-guinea pig (1:500, Millipore, Billerica, MA) and Alexa-Fluor 488-labeled goat anti-mouse (1:500; Invitrogen, Carlsbad, CA) for 60 min at 22°C. Images were visualized using an Olympus BX50 microscope and captured digitally using a Retiga EXiFast camera. Pictures at ×400 magnification were taken using QED Imaging software (Media Cybernetics, Silver Spring, MD) for 20 randomly selected fields of view for each section. Only fields with 50% or more of the area occupied by islet tissue were evaluated. Numbers of insulin and glucagon-positive cells were counted per unit total pancreatic area for each field of view by two experienced individuals who were blinded to the experimental groups, and the counts averaged.
Histological measurement of β-cell proliferation and mitosis.
Six pancreatic tissue sections from the tail portion (5 μm) were incubated with guinea pig anti-porcine insulin (1:500 dilution; Dako) and rabbit anti-proliferating cell nuclear antigen (PCNA) (1:250 dilution; Santa Cruz Biotechnology, Santa Cruz, CA) for the DNA replication assay or incubated with guinea pig anti-porcine insulin (1:500; Dako) and rabbit anti-phospho-histone H3 (pHH3) (1:400 dilution; Upstate, Lake Placid, NY) for the mitosis assay at 4°C overnight. Sections were then incubated with fluorescent-labeled secondary antibodies: Rhodamine-labeled goat anti-guinea pig for insulin (1:500; Millipore, Billerica, MA) and Alexa-Fluor 488-labeled goat anti-rabbit IgG (1:500) for PCNA and pHH3 by incubating for 60 min at 22°C. Finally, sections were then mounted in Vectashield mounting medium with DAPI (4′,6 diamidino-2-phenylindole; Vector Laboratories) to identify fluorescing cell nuclei. Insulin and PCNA-positive cells (for DNA replication assay) or insulin and pHH3 positive cells (for mitosis assay) were counted as described above.
Histological measurement of β-cell apoptosis.
Pancreatic (tail portion) section dewaxing, rehydrating, and antigen retrieval were conducted as mentioned above. Terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick translation end labeling (TUNEL) was performed with the In Situ Cell Death Detection POD Kit (Roche Molecular Biochemicals, Mannheim, Germany) per manual instruction. The sections were incubated with 50 μl of TUNEL reaction mixture or 50 μl of label solution alone (negative control) for 60 min at 37°C. The sections were then incubated with guinea pig anti-porcine insulin (1:500; Dako), at 4°C overnight, and Rhodamine-labeled goat anti-guinea pig (1:500; Millipore) at 22°C for 60 min. The slides were then mounted in Vectashield mounting medium with DAPI.
Morphometric analysis.
Images were visualized using an Olympus BX50 microscope and captured digitally using a Retiga EXiFast camera. Ten to 20 fields of view were photographed at ×200 magnification using QED Imaging software (Media Cybernetics, Silver Spring, MD) for each section and evaluated for PCNA, pHH3, and TUNEL staining as previously described by Limesand et al. (26). The percentage of PCNA-stained insulin-positive cells were determined by evaluating > 2,000 cells within ≥ 2 pancreatic sections per fetal pancreas, with each section separated from the next by 100 μm. The percentage of pHH3/insulin-positive cells were determined by evaluating > 3,000 cells within ≥ 2 pancreatic sections per fetal pancreas and TUNEL positive cells were determined by evaluating > 2,000 cells within ≥ 2 pancreatic sections per fetal pancreas separated from each other by 100 μm.
Western Blot Analysis for PCNA and pHH3
Pancreatic tissue samples (0.1 g) were homogenized in a polytron homogenizer with 400 μl of ice-cold buffer containing 137 mM NaCl, 50 mM Tris·HCl, 2% SDS, 1% Triton −100 solution, 10% glycerol, 2.5 mM EDTA, 1 mM CaCl2, 1 mM MgCl2, 2 mM Na3VO4, 100 mM NaF, and 1% protease inhibitor cocktail (Sigma), pH 7.4. Each homogenate was mixed with an equal volume of 2× standard SDS sample loading buffer and heated at 95°C for 5 min. Then, 30 μg protein extractions were separated by 10% SDS-PAGE gels, using a Mini-Protean III Gel electrophoresis system (Bio-Rad). After electrophoresis, the resolved proteins on the gel were transferred to nitrocellulose membranes in a transfer buffer containing 20 mM Tris-base, 192 mM glycine, 0.1% SDS, and 20% ethanol. Membranes were incubated in a blocking buffer consisting of 5% nonfat dry milk in TBST (0.1% Tween-20, 50 mM Tris·HCl, pH 7.6, and 150 mM NaCl) for 2 h. Membranes were incubated overnight at 4°C with rabbit anti-PCNS ( Santa Cruz Biotecnology) or rabbit anti-pHH3 (Upstate, Lake Placid, NY) antibodies (1:1,000 dilution in TBST with 5% BSA). After the primary antibody incubation, membranes were washed with TBST three times, for 10 min each. Membranes were incubated with horseradish-peroxidase-conjugated secondary antibody (Cell Signaling, Danvers, MA) for 1 h at room temperature. After three 15-min washes, membranes were visualized using enhanced chemiluminescense Western blot analysis reagents (Amersham Biosciences) and exposure to film (Biomax MR; Kodak, Rochester, NY). The density of bands was quantified by using an Imager Scanner II and ImageQuant TL software (Amersham Bioscience) (45). Each membrane was first used for the detection of PCNA or pHH3, stripped, and reprobed for β-tubulin antibody (Sigma). Band density was normalized according to the β-tubulin content.
Dot Blotting Assay for Pancreatic Insulin Concentration
Pancreatic tissue was powdered in liquid nitrogen, and then 0.1 g powdered tissue was homogenized in a polytron homogenizer (7-mm diameter generator) with 5 volumes of ice-cold lysis buffer (20 mM Tris pH 7.4, 2% SDS, 1% NP-40, 100 mM NaF, 2 mM Na3VO4), 1% protease inhibitor cocktail (Sigma)]. The homogenates were sonicated and clarified by centrifuging at 12,000 g for 10 min at 4°C. The resulting supernatant was mixed with the same volume of 2× standard SDS sample loading buffer and heated at 95°C for 5 min. Triplicate 2-μl samples from each extracted protein sample were spotted onto a nitrocellulose membrane (0.45 μm pore size, Sartorius 1200; Bio-Rad) and air dried under a hood. Membranes were blocked with 5% nonfat milk powder in TBST (50 mM Tris·HCl, pH 7.6, 150 mM NaCl, 0.05% Tween-20) for 2 h. Blocked membranes were then incubated overnight at 4°C with guinea pig anti-porcine insulin primary antibody (1:1,000, cat. no. A0564; Dako). At the end of the primary antibody incubation, the membranes were washed with TBST three times, for 10 min each. After that, membranes were incubated with horseradish peroxidase-conjugated anti-guinea pig secondary antibody (1:5,000, cat. no. AP193P; Chemicon, Temecula, CA) for 60 min at room temperature. Finally, blots were visualized using enhance chemiluminescense Western blot analysis detection reagents (Amersham Bioscience) and exposure to film (Biomax MR; Kodak). The density of each dot was quantified using an Imager Scanner II (Amersham Bioscience) and ImageQuant TL software (Amersham Bioscience) as previously reported (16).
Membrane Stripping and Reprobing with Actin
For reprobing, membranes were incubated in a stripping solution (100 mM β-mercaptoethanol, 2% SDS, 62.5 mM Tris·HCl pH 6.8) at 50°C for 30 min with shaking. The membranes were then thoroughly washed with TBST and blocked with 5% nonfat dry milk in TBST. Membranes were reprobed for actin using mouse anti-actin at 1:1,000 dilution in TBST with 5% nonfat dry milk, as described above. Each membrane was first used for the detection of insulin, then stripped and reprobed for β-actin.
Glucose and Hormone Analyses
Plasma glucose was analyzed using the Infinity (cat. no. TR15498; ThermoTrace, Melbourne, Australia) colorimetric assay. Samples were run in triplicate. The intra-assay and interassay coefficient of variation (CV). were 3% and 5%, respectively. Serum insulin was measured by RIA (Coat-A-Count, Diagnostic Products, Los Angeles, CA). Intra-assay CV was < 3%, while interassay CV was < 5%, while assay sensitivity was 0.10 ng/ml. Serum cortisol was measured by RIA (Coat-A-Count, Diagnostic Products, Los Angeles, CA) and samples were run in a single assay. Intra-assay CV for cortisol averaged < 3%, and sensitivity was 2.5 ng/ml. Serum IGF-1 (single measurement) was measured on an Immulite 1000 (Diagnostics Products) and validated for sheep serum. The sensitivity of the IGF-1 assay was 20 ng/ml and the intra-assay CV was < 2%.
Determination of Effects of the Model on Maternal Glucose Tolerance
Glucose tolerance tests (GTT) were conducted on pregnant ewes (5 control ewes; 5 OB ewes) at day 75 gestation. All ewes were fasted for a 12-h period and weighed, and jugular veins were catheterized (AbboCath 14 gauge × 5.5 in. long; Abbott Laboratories) under local anesthesia by using aseptic procedures. They were allowed 60-min recovery before initiating a glucose tolerance test (42). Briefly, venous blood samples (3 ml) were drawn and placed into a chilled nonheparinized vacutainer tube containing no additives (Sigma) and a heparin and sodium fluoride tube (2.5 mg/ml; Sigma) to establish baseline values of serum insulin and plasma glucose, respectively, at −15 and −5 min before administration of glucose (0.25 g/kg body wt over 20 s, 50% dextrose solution; Vedco, St. Joseph, MO) via the catheter. Blood samples for insulin and glucose were collected at 2, 5, 10, 15, 30, 60, and 120 min after the injection. Catheters were carefully flushed with saline following the glucose infusion, and each blood sampling. Blood samples were cooled in ice water and centrifuged at 3,000 g for 10 min, and plasma and serum were stored at −80°C until subsequent analysis.
Dual Energy X-Ray Absorptiometry Measurements
To accurately determine bone mass density, lean tissue mass, and fat tissue mass of control and OB ewes at midgestation, and their newborn lambs within 12 h of birth, dual energy X-ray absorptiometry (DEXA; GE Lunar Prodigy 8743; Madison, WI) was utilized as previously described and validated for the sheep (30, 34). Ewes were sedated with ketamine (22.2 mg/kg body wt) immediately prior to performing DEXA scans, and newborn lambs were immobilized by wrapping them tightly in a large towel. All sheep were positioned on the DEXA table on their sides with their forelimbs flexed caudally and hindlimbs flexed cranially so as to remain next to the body, and allow us to scan the entire animal. Measurements made by the DEXA included total tissue mass, lean tissue mass, fat tissue mass, and bone mineral mass in grams. The whole body scan mode was used for all animals and scan times were ∼3 min depending on the length of the animal. A single, blinded, and experienced investigator performed all DEXA scans and quantified lean, fat, and bone mass. Regional analysis was performed on the pregnant ewes to exclude the gravid uterus by use of anatomical landmarks and the region of interest software application, while the entire DEXA image of lambs was placed in the left arm region of the regional grid. DEXA was calibrated, and quality assurance tests were performed daily prior to measurement and according to the manufacture specifications and programmed acceptable limits.
Statistics
Data were analyzed as a complete, randomized design using General Linear Model of Statistical Analysis System, 2000. There was no effect of sex on any measurements taken; therefore, data were pooled across sex for analyses. Means separation was performed using LSMEANS. Data are presented as means ± SE and are considered significantly different when P < 0.05, unless otherwise stated. Area under the curve (AUC) was determined for insulin (AUCi) and glucose (AUCg) using the trapezoidal rule with Sigma Plot software (SPSS, Chicago, IL). The first-phase insulin response was calculated in a manner described by Soto et al. (46) as the sum of 2-min and 5-min insulin values after glucose infusion minus the average of baseline (−5 and −15 min) values.
RESULTS
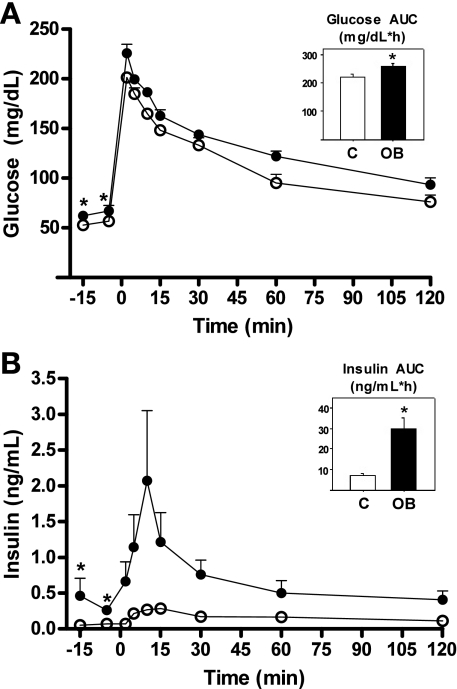
Ewes fed the OB diet developed an obese phenotype with an increase in body weight of ∼30% from diet initiation to mating (71.6 ± 3.23 and 92.8 ± 2.97 kg, respectively; P < 0.05) and an additional ∼13% increase from mating to necropsy (92.8 ± 2.97 and 102.2 ± 2.41 kg, respectively; P < 0.05). In contrast, control ewes exhibited only a modest nonsignificant increase in body weight from diet initiation to necropsy (68.3 ± 2.91 and 72.2 ± 3.27 kg, respectively). Body condition score changes in OB ewes followed those of body weight, increasing from diet initiation to mating (5.0 ± 0.3 and 7.2 ± 0.2, respectively; P < 0.05), and again from mating to day 75 of gestation (7.2 ± 0.2 and 8.3 ± 0.15, respectively; P < 0.05). The Body Condition Score of control ewes remained relatively constant from diet initiation to necropsy (4.7 ± 0.4 and 4.9 ± 0.4, respectively; not significant). When evaluated by DEXA at midgestation (75 ± 2 days of gestation), OB ewes had a markedly higher (P < 0.01) percentage body fat than control ewes (28.6 ± 1.6 vs. 17.7 ± 1.3%, respectively), while bone mineral density was similar across groups averaging 1.164 ± 0.015 g/cm2. At 75 days gestation, clear differences were observed in GTT responses of OB and control ewes. OB ewes demonstrated greater baseline circulating glucose and increased insulin compared with control ewes as well as greater AUCg and AUCi when compared with control ewes (Fig. 1). Insulin-to-glucose ratios before the GTT (average of −15 and −5 min) and at insulin maximum (+10 min) were greater for OB than control ewes averaging 0.56 ± 0.20 vs. 0.11 ± 0.06 (P < 0.05) and 1.09 ± 0.37 vs. 0.16 ± 0.03 (P < 0.05), respectively. Furthermore, first-phase insulin response to the GTT was greater in OB than control ewes (1.37 ± 0.25 vs. 0.21 ± 0.03 ng/ml; P < 0.05).
Fig. 1.
Maternal responses to a glucose challenge (0.25 g/kg of 50% dextrose solution intravenously) on 75 days of gestation to ewes fed 100% (control, ○; n = 5) or 150% [obesogenic group (OB), •; n = 6] of National Research Council recommendations from 60 days before conception to day 75 of gestation. A: glucose concentrations; B: insulin concentrations. Area under curve (AUC) is shown as insets. Values are means ± SE (error bars are too small to show on this scale); *P < 0.05 compared with control.
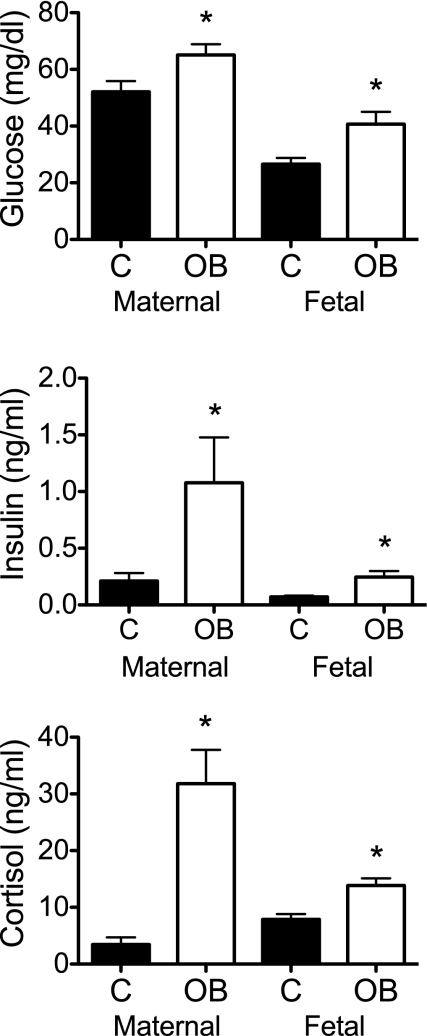
Body weight and crown rump length of OB ewe fetuses were ∼30% greater than control ewe fetuses at day 75 (374 ± 10 g and 24.6 ± 0.2 cm vs. 268 ± 12 g and 22.1 ± 0.2 cm, respectively; P < 0.05). Absolute pancreatic weight of OB ewe fetuses was 236% greater than control ewe fetuses. All other organ weights averaged 141% above control but, except for the pancreas, were proportional to the increased weight in OB ewe fetuses (Table 1). Glucose, insulin and cortisol concentrations were greater in OB compared with control ewes and fetuses on day 75 (Fig. 2). Serum IGF-I concentrations were higher in fetuses from OB ewes compared with control ewes (53.3 ± 1.76 vs. 44.6 ± 0.90 ng/ml; P < 0.05).
Table 1.
Day 75 fetal tissue and organ weights from fetuses of ewes fed 100% National Research Council (NRC) recommendations (control ewes; n = 5) or fetuses of ewes fed 150% NRC (OB ewes; n = 6)
| Tissues and Organs | Control ewe, wt, g | OB ewe, wt, g | Control ewe, wt/U fetal wt, % | OB ewe, wt/U fetal wt, % |
|---|---|---|---|---|
| Brain | 7.15±0.23 | 10.12±0.35* | 2.69±0.11 | 2.70±0.07 |
| Kidney | 1.37±0.09 | 2.09±0.09* | 0.51±0.35 | 0.56±0.01 |
| Adrenal | 0.06±0.01 | 0.08±0.02 | 0.02±0.01 | 0.02±0.01 |
| Pancreas | 0.14±0.02 | 0.47±0.03* | 0.05±0.01 | 0.12±0.01* |
| Spleen | 0.32±0.04 | 0.44±0.03* | 0.12±0.01 | 0.12±0.01 |
| Lung | 6.84±0.36 | 9.81±0.32* | 2.55±0.08 | 2.63±0.11 |
| Liver | 15.92±0.99 | 24.26±0.97* | 5.93±0.22 | 6.46±0.15 |
| Testis | 0.07±0.02 | 0.08±0.01 | 0.02±0.01 | 0.02±0.01 |
| Ovary | 0.02±0.01 | 0.03±0.01 | 0.01±0.01 | 0.01±0.01 |
| ST muscle | 0.49±0.01 | 0.65±0.05* | 0.20±0.01 | 0.18±0.01 |
| LD muscle | 2.40±0.16 | 3.46±0.20* | 0.97±0.02 | 0.95±0.04 |
| Heart | 2.56±0.14 | 3.27±0.18* | 0.96±0.05 | 0.87±0.04 |
Values are means ± SE. ST, semitendenosus; LD, longisimus dorsi.
Values within a row and measurement differ (P < 0.05).
Fig. 2.
Concentrations of glucose (mg/dl; A), insulin (ng/ml; B), and cortisol (ng/ml; C) in maternal and fetal blood on day 75 of gestation from control (n = 5) and OB (n = 6) groups. *Significant treatment differences (P < 0.05) within the maternal or fetal compartments.
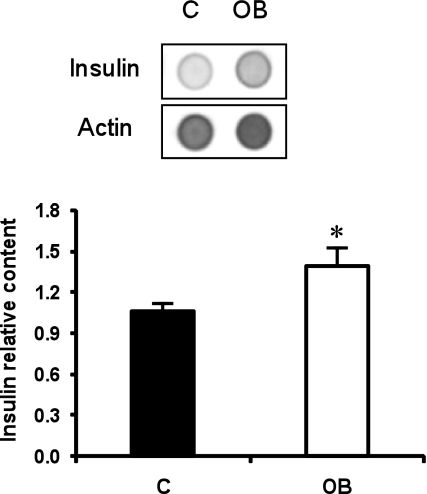
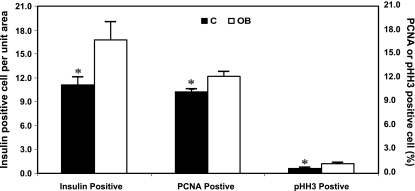
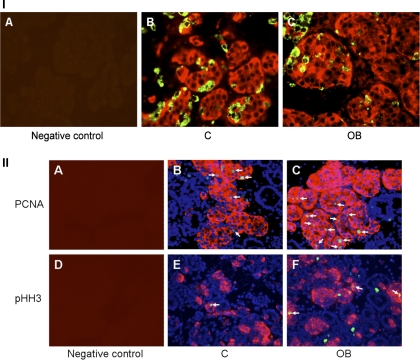
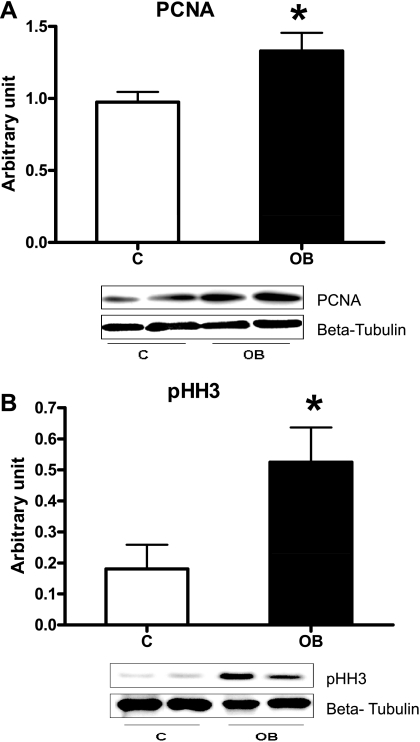
Pancreatic insulin concentration was greater in fetuses from OB ewes than control ewes (Fig. 3). Numbers of insulin-positive cells were 50% greater per field of view of pancreatic islet tissue from fetuses of OB ewes compared with fetuses of control ewes (16.8 ± 2.2 vs. 11.1 ± 1.0 cells; P < 0.05, Figs. 4 and 5A). In contrast, numbers of glucagon-secreting cells per field of view of pancreatic islet tissue were similar between groups (16.0 ± 1.4 vs. 15.3 ± 2.6 cells). No differences in β-cell apoptosis were observed between control (1.41 ± 0.16%, TUNEL+/insulin+ cells) and OB (1.01 ± 0.13%, TUNEL+/insulin+ cells) fetuses (P = 0.2). In contrast, the percentage of β-cells staining positively for PCNA, and pHH3, markers of cells in progress through the cell cycle, were greater in pancreatic tissue of fetuses from OB than control ewes (P < 0.05; Figs. 4 and 5B). Western blot analysis confirmed the increased PCNA and pHH3 content in pancreatic tissue of fetuses of OB vs. control ewes (P < 0.05; Fig. 6).
Fig. 3.
Insulin immunodot blotting in fetal pancreas on day 75 of gestation from control (n = 5) and OB (n = 6) groups. Relative insulin content is the mean ratio of insulin to actin. *Significant difference (P < 0.05) between treatments.
Fig. 4.
Insulin-positive cells per unit area, as well as %insulin-positive cells exhibiting nuclear staining for proliferating cell nuclear antigen (PCNA) or phospho-histone H3 (pHH3) in fetal pancreatic tissue on day 75 of gestation from control (n = 5) and OB (n = 5) ewes. *Significant differences (P < 0.05) between treatments.
Fig. 5.
Immunofluorescent staining of the fetal pancreas. I: pancreatic sections stained with either no first antibody as a negative control (A), or insulin (red) and glucagon (green) in both control (B) and obese (C) groups. II: cell proliferation test using immunofluorescent staining of PCNA and pHH3. Pancreatic sections stained with no first antibody as a negative control (A and D). Insulin (red) and PCNA (green) positive cells stained for counting in both control (B) and OB (C) groups. Insulin (red) and pHH3 (green) cells stained for counting in both control (E) and OB (F) groups. DAPI (blue) indicates general nuclear staining. Arrows indicate PCNA (B and C) and pHH3 (E and F) positive β-cells.
Fig. 6.
Western blot analysis measurement of PCNA (A) and pHH3 (B) protein expression in fetal pancreatic tissue of both control (n = 5) and OB (n = 5) ewes at day 75 of gestation. *Significant differences (P < 0.05) between treatments.
Lambs from control ewes delivered later than OB lambs (145 ± 1 vs. 150 ± 1 days, respectively; P < 0.05). Control ewes produced two male and three female lambs, while OB ewes produced three male and two female lambs. Although birth weights of lambs born to OB ewes tended to be greater (P = 0.14) than those born to control lambs, crown rump lengths were reduced in lambs born to OB vs. control ewes (Table 2). When evaluated by DEXA, lambs from OB ewes had a markedly higher (P < 0.01) percentage body fat than those born to control ewes, while bone mineral density was similar across groups (Table 2).
Table 2.
Weight, size, and dual energy X-ray absorptiometry measurements from lambs born to ewes fed 100% NRC recommendations (control ewes; n = 5) or lambs born to ewes fed 150% NRC (obese ewes; n = 5)
| Control | Obese | |
|---|---|---|
| Birth weight, kg | 5.31±0.49 | 6.28±0.54 |
| Crown rump length, cm | 58.2±1.1c | 53.9±1.1d |
| %Fat | 5.66±0.75a | 13.22±0.71b |
| Bone mineral density, g/cm2 | 0.42±0.02 | 0.37±0.02 |
Means ± SE within a row with different superscripts differ:
means differ P < 0.01;
means differ P < 0.05).
DISCUSSION
As mentioned above, to date the majority of studies in relation to developmental programming during fetal development have been conducted in undernourished experimental models (6, 7) The maternal obesity model that we have developed in this important experimental species is one of excessive intake of the normal diet. Other studies have employed both increased intake and dietary supplementation (6, 7). Both models have value. The increased intake led to elevated maternal glucose and insulin as well as maternal insulin resistance midway through gestation. The increased relative pancreatic weight in OB ewe fetuses was accompanied by increased β-cell numbers produced as a result of increased mitoses rather than decreased programmed cell death and higher pancreatic and fetal plasma insulin concentrations. Fetal plasma insulin concentrations are likely of fetal pancreatic origin since insulin does not cross the placenta (20). In contrast, elevated blood glucose in OB ewe fetuses is likely a direct result of elevated maternal glucose, as glucose does cross the placenta freely (20). To explain the observation that offspring of diabetic mothers tend to exhibit higher birth weights, Pederson (35) proposed a hyperglycemia-hyperinsulism pathway in which higher fetal plasma insulin acts as a major fetal growth-promoting hormone. Chronic fetal hyperinsulinemia induced by maternal diabetes leads to macrosomia, while fetal hypoinsulinemia is associated with fetal growth retardation in a variety of species (21, 31). Ovine fetal pancreatectomy decreases growth rate and birth weight (16, 19). The elevated fetal plasma IGF-I that we observed would constitute another mechanism contributing to increased fetal growth.
We sought to observe the effects of maternal obesity on the fetal pancreas at midgestation rather than a later stage since progenitor cell number in the mouse fetus is set by midgestation, and evidence suggests that the number of cells in this pool dictates final pancreatic size and cell number (41). These authors speculated that pancreatic progenitor cells can only undergo a set number of mitotic divisions during pancreatic development, thus limiting pancreatic size. In agreement with the hypothesis that the period of sensitivity is in the first half of gestation in precocial mammals, Fowden and Comline (16) observed little compensatory growth after excision of a substantial portion of the ovine fetal pancreas between days 113 and 121 of gestation.
Insulin and glucagon are present in fetal sheep pancreatic tissue and systemic blood by 40 and 60 days of gestation, respectively, and rise progressively between mid- and late gestation to plateau near term (18). Glucose stimulates fetal pancreatic insulin secretion in utero and in in vitro experiments with cultured fetal islet cells in several species including sheep (2, 14, 15, 18, 41). Furthermore, fetal insulin levels are positively correlated with blood glucose levels, over a wide range of concentrations (15). Moderate maternal diabetes in rats leads to an increase in fetal pancreatic insulin content, enhanced insulin secretion in response to glucose, and greater islet cell proliferation (14, 25). Increased insulin secretion is observed in fetal sheep when maternal glucose levels are raised by pulsatile glucose infusion (11).
Some of the changes in islet development and β-cell proliferation observed in fetuses from OB ewes may be due to nutritionally-induced alterations in exposure to hormones and growth factors. The ongoing proliferation and developmental differentiation of β-cells, once formed, are highly dependent on IGFs. Both IGF-I and IGF-II are mitogens in the fetal pancreas and lead to an increase in islet cell mass (24). In vitro treatment of the rat clonal β-cell line RIN 1046–38 with IGF-I led to activation of the β-cell-specific transcription factor Pdx-1, and increased mitogenic activity (23). In fetal sheep, insulin is known to increase IGF-I levels in fetal blood (17). Finally, glucocorticoids, which are elevated in the OB fetuses, play an important role in pancreatic development. Breant et al. (9) have shown that glucocorticoid receptors are critical for ensuring pancreatic architecture and survival, as well as β-cell mass expansion during fetal development. Thus the elevated IGF-I and cortisol concentrations in the blood of fetuses from OB vs. control ewes are both likely mechanisms to explain the increased pancreatic growth and β-cell numbers observed in the present study.
The delivery of lambs by the OB ewes earlier than the control ewes and the limited number of offspring evaluated likely contributed to the lack of any significant difference in birth weight between the groups. The cause of the early delivery remains to be determined, but the elevated fetal cortisol may play a role (29). In human pregnancy accompanied by obesity the incidence of cesarean section is high, and thus it is difficult to determine the effect of maternal obesity on length of gestation. Although the mechanisms of parturition are different in primates and ruminants, increased activity of the fetal primate adrenal occurs prior to delivery to increase the availability of androgen as substrate for estrogen synthesis. (32). In addition, a late gestation increase in fetal glucocorticotioid activity is vital to preparation for an independent external existence even in primates, since it is responsible for maturing the fetal lung and preparing other fetal systems for neonatal function (17).
At birth, lambs born to OB ewes had higher body fat than those born to control ewes. Catalano et al. (12) reported that infants of women with mild glucose intolerance have increased body fat compared with infants from women with normal glucose tolerance. Furthermore, these researchers reported that this increase in neonatal adiposity was independent of birth weight, and speculated that this may be an early sign of obesity onset.
In conclusion, we have demonstrated that by midgestation, maternal obesity induced by a high plane of nutrition, and the accompanying maternal insulin resistance in sheep results in maternal and fetal hyperglycemia and hyperinsulinemia, overgrowth of the pancreatic islets, and accelerated fetal β-cell development due to hyperplasia. Three mechanisms that would drive this increased islet growth are reported: increased nutrient supply, increased fetal plasma growth factors (insulin and IGF-1), and increased fetal cortisol. Postnatally, lambs born to the obese ewes in this study showed only a tendency for an increase in birth weight, but exhibited a marked increase in body fat, which may predispose them to an increased risk of obesity in postnatal life. The marked increase in β-cell proliferation in the fetal pancreas observed with maternal obesity may allow the fetus to adapt its somatic growth to increased nutrient availability in utero. An acceleration of fetal pancreatic growth and cell number, as observed in obese ewes in this study could be expected to alter cellular composition and function of the pancreas in later life. Failure of the pancreas to return to a normal cellular composition and function postnatally could lead to obesity, altered insulin secretion, and diabetes in offspring.
Perspectives and Significance
The United States is experiencing an obesity epidemic increasingly involving women of child-bearing years. Clinical studies show developmental problems in the fetal pancreas in children born to obese pregnant women that lead to abnormalities of fetal growth and body composition. Offspring of obese mothers are more prone to chronic conditions such as obesity and diabetes that greatly impair life-time health. Recent human and animal investigations indicate that the antecedents of these problems are set during fetal life. There is a paucity of knowledge regarding mechanisms whereby maternal obesity and high-energy diets modify fetal pancreatic development and fetal organ insulin sensitivity in precocial species compared with altricial species. Basic research into effects of maternal obesity and high-energy diets on fetal development is limited by lack of an animal model that allows long-term fetal sampling. We have developed such a model in sheep and will use it to determine changes in fetal pancreatic response to glucose and amino acids and fetal insulin sensitivity resulting from maternal obesity and high-energy diets.
GRANTS
This work was supported by National Institutes of Health (NIH) P20-RR-16474-04 and HD-21350.
Acknowledgments
The authors thank Ryan Gustafson and Adam Uthlaut for animal care, and Sarah Hein, Alison Iroz, and Regan Harp for sample collection and processing. Special appreciation is extended to Sarah Hein for preparing tissue sections for immunohistochemistry. Finally, thanks are extended to Christopher Dorozynski for DEXA evaluations of ewes and lambs.
REFERENCES
- 1.Aagaard-Tillery KM, Grove K, Bishop J, Ke X, Fu Q, McKnight R, Lane RH. Developmental origins of disease and determinants of chromatin structure: maternal diet modifies the primate fetal epigenome. J Mol Endocrinol 41: 91–102, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aldoretta PW, Carver TD, Hay WW Jr. Maturation of glucose-stimulated insulin secretion in fetal sheep. Biol Neonate 73: 375–386, 1998. [DOI] [PubMed] [Google Scholar]
- 3.Armitage JA, Gupta S, Wood C, Jensen RI, Samuelsson AM, Fuller W, Shattock MJ, Poston L, Taylor PD. Maternal dietary supplementation with saturated, but not monounsaturated or polyunsaturated fatty acids, leads to tissue-specific inhibition of offspring Na+,K+-ATPase. J Physiol 586: 5013–5022, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armitage JA, Khan IY, Taylor PD, Nathanielsz PW, Poston L. Developmental programming of the metabolic syndrome by maternal nutritional imbalance: how strong is the evidence from experimental models in mammals? J Physiol 561: 355–377, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armitage JA, Taylor PD, Poston L. Experimental models of developmental programming: consequences of exposure to an energy rich diet during development. J Physiol 565: 3–8, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics 115: e290–e296, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Breant B, Gesina E, Blondeau B. Nutrition, glucocorticoids and pancreas development. Horm Res 65, Suppl 3: 98–104, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Callaway LK, Prins JB, Chang AM, McIntyre HD. The prevalence and impact of overweight and obesity in an Australian obstetric population. Med J Aust 184: 56–59, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Carver TD, Anderson SM, Aldoretta PW, Hay WW Jr. Effect of low-level basal plus marked “pulsatile” hyperglycemia on insulin secretion in fetal sheep. Am J Physiol Endocrinol Metab 271: E865–E871, 1996. [DOI] [PubMed] [Google Scholar]
- 12.Catalano PM, Thomas A, Huston-Presley L, Amini SB. Increased fetal adiposity: a very sensitive marker of abnormal in utero development. Am J Obstet Gynecol 189: 1698–1704, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Cripps RL, Martin-Gronert MS, Archer Zoe A, Hales CN, Mercer JG, Ozanne SE. Programming of hypothalamic neuropeptide gene expression in rats by maternal dietary protein content during pregnancy and lactation. Clin Sci (Lond) 117: 85–93, 2009. [DOI] [PubMed] [Google Scholar]
- 14.Dahri S, Reusens B, Remacle C, Hoet JJ. Nutritional influences on pancreatic development and potential links with non-insulin-dependent diabetes (Abstract). Proc Nutr Soc 54: 345–356. 1995. [DOI] [PubMed] [Google Scholar]
- 15.Fowden AL Endocrine regulation of fetal growth. Reprod Fertil Dev 7: 351–363, 1995. [DOI] [PubMed] [Google Scholar]
- 16.Fowden AL, Comline RS. The effects of pancreatectomy on the sheep fetus in utero. Q J Exp Physiol 69: 319–330, 1984. [DOI] [PubMed] [Google Scholar]
- 17.Fowden AL, Forhead AJ. The role of hormones in intrauterine development. In: Fetal Origins Of Cardiovascular And Like Disease, edited by Barker DJP. New York: Decker, 2000, p. 199–228.
- 18.Fowden AL, Hill DJ. Intra-uterine programming of the endocrine pancreas. Br Med Bull 60: 123–142, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Fowden AL, Hughes P, Comline RS. The effects of insulin on the growth rate of the sheep fetus during late gestation. Q J Exp Physiol 74: 703–714, 1989. [DOI] [PubMed] [Google Scholar]
- 20.Freinkel N Banting Lecture 1980: Of pregnancy and progeny. Diabetes 29: 1023–1035, 1980. [DOI] [PubMed] [Google Scholar]
- 21.Gluckman PD, Liggins GC. Regulation of fetal growth. In: Fetal Physiology and Medicine: The Basis of Perinatology, edited by Beard RW and Nathanielsz PW. London: Butterworth, 1984, p. 511–557.
- 22.Hales CN, Barker DJ. The thrifty phenotype hypothesis. Br Med Bull 60: 5–20, 2001. [DOI] [PubMed] [Google Scholar]
- 23.Hennige AM, Fritsche A, Strack V, Weigert C, Mischak H, Borboni P, Renn W, Haring HU, Kellerer M. PKC zeta enhances insulin-like growth factor 1-dependent mitogenic activity in the rat clonal beta cell line RIN 1046–38. Biochem Biophys Res Commun 290: 85–90, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Hill DJ, Hogg J, Petrik J, Arany E, Han VK. Cellular distribution and ontogeny of insulin-like growth factors (IGFs) and IGF binding protein messenger RNAs and peptides in developing rat pancreas. J Endocrinol 160: 305–317, 1999. [DOI] [PubMed] [Google Scholar]
- 25.Kervran A, Guillaume M, Jost A. The endocrine pancreas of the fetus from diabetic pregnant rat. Diabetologia 15: 387–393, 1978. [DOI] [PubMed] [Google Scholar]
- 26.Limesand SW, Jensen J, Hutton JC, Hay WW Jr. Diminished β-cell replication contributes to reduced β-cell mass in fetal sheep with intrauterine growth restriction. Am J Physiol Regul Integr Comp Physiol 288: R1297–R1305, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Lobelo F Fetal programming and risk of metabolic syndrome: prevention efforts for high-risk populations. Pediatrics 116: 519, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Manikkam M, Thompson RC, Herkimer C, Welch KB, Flak J, Karsch FJ, Padmanabhan V. Developmental programming: impact of prenatal testosterone excess on pre- and postnatal gonadotropin regulation in sheep. Biol Reprod 78: 648–660, 2008. [DOI] [PubMed] [Google Scholar]
- 29.McDonald TJ, Nathanielsz PW. Bilateral destruction of the fetal paraventricular nuclei prolongs gestation in sheep. Am J Obstet Gynecol 1991: 764–771, 1991. [DOI] [PubMed] [Google Scholar]
- 30.Mercier J, Pomar C, Marcoux M, Goulet F, Theriault M, Castonguay FW. The use of dual-energy X-ray absorptiometry to estimate the dissected composition of lamb carcasses. Meat Sci 73: 249–257, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Milner RDG Endocrine control of fetal growth? In: Perinatal Nutrition, edited by Lindblad BS. London: Academic, 1988, p. 45–62.
- 32.Nathanielsz PW Comparative studies on the initiation of labor. Eur J Obstet Gynaecol Reprod Biol 78: 127–132, 1998. [DOI] [PubMed] [Google Scholar]
- 32a.National Center for Health Statistics. Prevalence of overweight and obesity among adults: United States 1999–2002. (Online at http://www.cdc.gov/nchs/products/pubs/pubd/hestats/obese/obse99.htm), 2007.
- 32b.National Research Council. Nutrient Requirements of Sheep. (6th Edition). Washington, DC: National Academy, 1985.
- 33.Nohr EA, Bech BH, Davies MJ, Frydenberg M, Henriksen TB, Olsen J. Prepregnancy obesity and fetal death: a study within the Danish National Birth Cohort. Obstet Gynecol 106: 250–259, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Pearce KL, Ferguson M, Gardner G, Smith N, Greef J, Pethick DW. Dual X-ray absorptiometry accurately predicts carcass composition from live sheep and chemical composition of live and dead sheep. Meat Sci 81: 285–293, 2009. [DOI] [PubMed] [Google Scholar]
- 35.Pederson J Weight and length at birth of infants of diabetic mothers. Acta Endocrinol 6: 330–342, 1954. [DOI] [PubMed] [Google Scholar]
- 36.Pettitt DJ, Aleck KA, Baird HR, Carraher MJ, Bennett PH, Knowler WC. Congenital susceptibility to NIDDM. Role of intrauterine environment. Diabetes 37: 622–628, 199. [DOI] [PubMed]
- 37.Reusens B, Remacle C. Programming of the endocrine pancreas by the early nutritional environment. Intl J Biochem Cell Biol 38: 913–922, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Rozance PJ, Limesand SW, Barry JS, Brown LD, Thorn SR, LoTurco D, Regnault TRH, Friedman JE, Hay WW Jr. Chronic late-gestation hypoglycemia upregulates hepatic PEPCK associated with increased PGC1α mRNA and phosphorylated CREB in fetal sheep. Am J Physiol Endocrinol Metab 294: E365–E370, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanson DW, West TR, Tatman WR, Riley ML, Judkins MB, Moss GE. Relationship of body composition of mature ewes with condition score and body weight. J Anim Sci 71: 1112–1116, 1993. [DOI] [PubMed] [Google Scholar]
- 40.Snoeck A, Remacle C, Reusens B, Hoet JJ. Effect of a low protein diet during pregnancy on the fetal rat endocrine pancreas. Biol Neonate 57: 107–118, 1990. [DOI] [PubMed] [Google Scholar]
- 41.Stanger BZ, Tanaka AJ, Melton DA. Organ size is limited by the number of embryonic progenitor cells in the pancreas but not the liver. Nature 445: 886–891, 2007. [DOI] [PubMed] [Google Scholar]
- 42.Vonnahme KA, Hess BW, Hansen TR, McCormick RJ, Rule DC, Moss GE, Murdoch WJ, Nijland MJ, Skinner DC, Nathanielsz PW, Ford SP. Maternal undernutrition from early- to mid-gestation leads to growth retardation, cardiac ventricular hypertrophy, and increased liver weight in the fetal sheep. Biol Reprod 69: 133–140, 2003. [DOI] [PubMed] [Google Scholar]
- 43.Wei JN, Sung FC, Li CY, Chang CH, Lin RS, Lin CC, Chiang CC, Chuang LM. Low birth weight and high birth weight infants are both at an increased risk to have type 2 diabetes among schoolchildren in Taiwan. Diabetes Care 26: 343–348, 2003. [DOI] [PubMed] [Google Scholar]
- 44.Zhu MJ, Han B, Tong J, Ma C, Kimzey JM, Underwood KR, Xiao Y, Hess BW, Ford SP, Nathanielsz PW, Du M. AMP-activated protein kinase signaling pathways are down regulated and skeletal muscle development impaired in fetuses of obese, over-nourished sheep. J Physiol 586: 2651–2664, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu MJ, Ford SP, Means WJ, Hess BW, Nathanielsz PW, Du M. Maternal nutrient restriction affects properties of skeletal muscle in offspring. J Physiol 575: 241–250, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Soto N, Bazaes RA, Pena V, Salazar T, Avila A, Iniguez G, Ong KK, Dunger DB, Mericq MV. Insulin sensitivity and secretion are related to catch-up growth in small-for-gestational-age infants at age 1 year: results from a prospective cohort. J Clin Endocrinol Metab 88: 3645–3650, 2003. [DOI] [PubMed] [Google Scholar]