Abstract
An enzyme-linked immunosorbent assay developed for the demonstration of respiratory syncytial (RS) virus immunoglobulin G antibodies was used for the detection of RS virus in specimens of nasopharyngeal secretions (NPS) obtained from children with acute respiratory disease. Samples of NPS were incubated with a fixed amount of standard serum (human serum antibodies to RS virus) before being added to the enzyme-linked immunosorbent assay test plate. A decrease in the optical density value determined for this standard serum was seen with majority of NPS specimens from which RS virus had been isolated in tissue culture. The reliability and the specificity of this inhibiton test were supported by experiments with purified RS virus and by tests with NPS specimens containing other respiratory viruses.
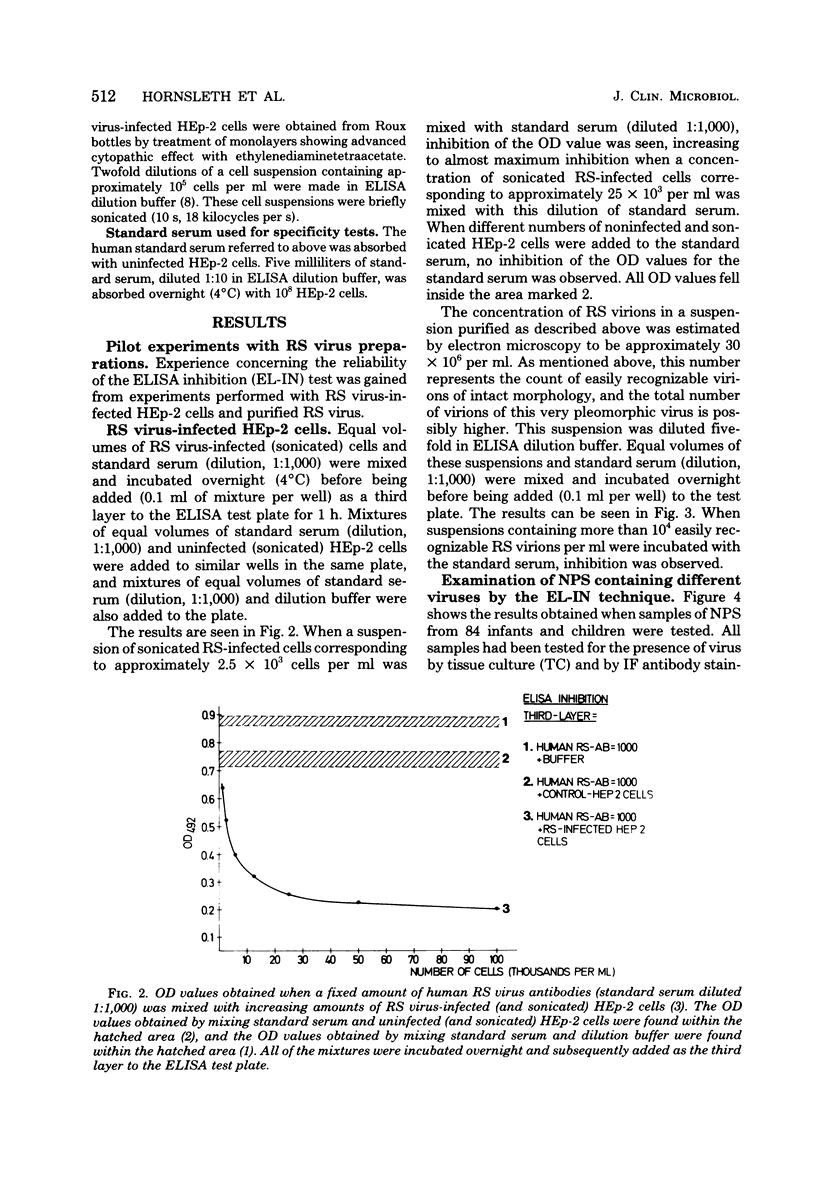
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartz C. R., Conklin R., Steele J. H., Glass S. E. Rotavirus antibody in chickens as measured by enzyme-linked immunosorbent blocking assay. Am J Vet Res. 1980 Jun;41(6):969–971. [PubMed] [Google Scholar]
- Berg R. A., Yolken R. H., Rennard S. I., Dolin R., Murphy B. R., Straus S. E. New enzyme immunoassays for measurement of influenza A/Victoria/3/75 virus in nasal washes. Lancet. 1980 Apr 19;1(8173):851–853. doi: 10.1016/s0140-6736(80)91356-2. [DOI] [PubMed] [Google Scholar]
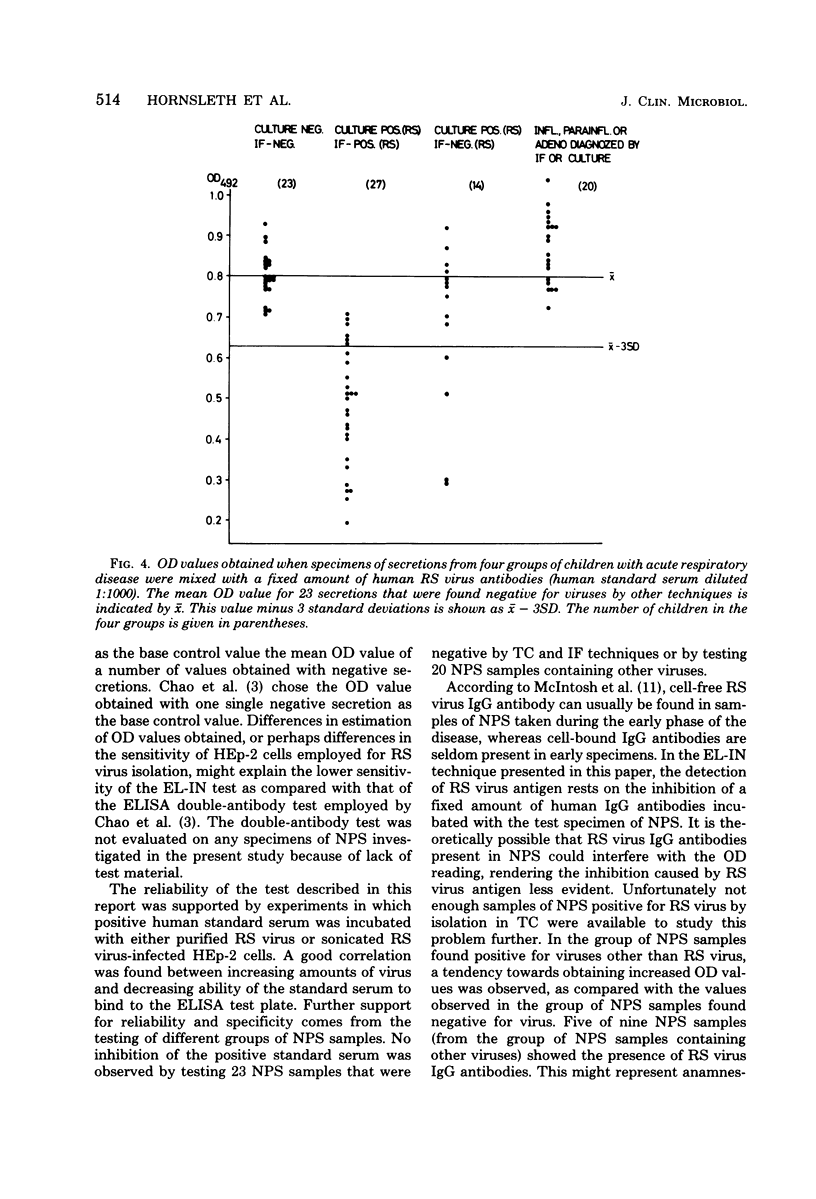
- Chao R. K., Fishaut M., Schwartzman J. D., McIntosh K. Detection of respiratory syncytial virus in nasal secretions from infants by enzyme-linked immunosorbent assay. J Infect Dis. 1979 Apr;139(4):483–486. doi: 10.1093/infdis/139.4.483. [DOI] [PubMed] [Google Scholar]
- Grauballe P. C., Genner J., Meyling A., Hornsleth A. Rapid diagnosis of rotavirus infections: comparison of electron microscopy and immunoelectroosmophoresis for the detection of rotavirus in human infantile gastroenteritis. J Gen Virol. 1977 May;35(2):203–218. doi: 10.1099/0022-1317-35-2-203. [DOI] [PubMed] [Google Scholar]
- Grauballe P. C., Johnsen N. J., Hornsleth A. Rapid diagnosis by immunofluorescence of viral infections associated with the croup syndrome in children. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974 Feb;82(1):41–47. doi: 10.1111/j.1699-0463.1974.tb02290.x. [DOI] [PubMed] [Google Scholar]
- Harmon M. W., Drake S., Kasel J. A. Detection of adenovirus by enzyme-linked immunosorbent assay. J Clin Microbiol. 1979 Mar;9(3):342–346. doi: 10.1128/jcm.9.3.342-346.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornsleth A., Grauballe P. C., Friis B., Genner J., Pedersen I. R. Production of antiserum to respiratory syncytial virus polypeptides: application in enzyme-linked immunosorbent assay. J Clin Microbiol. 1981 Nov;14(5):501–509. doi: 10.1128/jcm.14.5.501-509.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornsleth A., Mordhorst C. H., Pedersen I. R. New method for production of influenza A virus subtype specific antisera: employment of precipitate obtained by crossed immunoelectrophoretic techniques as antigen. J Immunol Methods. 1980;39(1-2):95–100. doi: 10.1016/0022-1759(80)90298-7. [DOI] [PubMed] [Google Scholar]
- KRECH U. The antigenic potency of non-infectious poliomyelitis virus as determined by its liberating effect on active virus neutralized by immune serum. J Exp Med. 1955 Mar 1;101(3):331–339. doi: 10.1084/jem.101.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh K., McQuillin J., Gardner P. S. Cell-free and cell-bound antibody in nasal secretions from infants with respiratory syncytial virus infection. Infect Immun. 1979 Feb;23(2):276–281. doi: 10.1128/iai.23.2.276-281.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyrhönen S. Hunan wart-virus antibodies in patients with genital and skin warts. Acta Derm Venereol. 1978;58(5):427–432. [PubMed] [Google Scholar]
- Sarkkinen H. K., Halonen P. E., Arstila P. P., Salmi A. A. Detection of respiratory syncytial, parainfluenza type 2, and adenovirus antigens by radioimmunoassay and enzyme immunoassay on nasopharyngeal specimens from children with acute respiratory disease. J Clin Microbiol. 1981 Feb;13(2):258–265. doi: 10.1128/jcm.13.2.258-265.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senterfit L. B., Baldridge P. B. Separation and concentration of respiratory syncytial virus (RSV) antigens. J Immunol Methods. 1974 Mar;4(2):349–357. doi: 10.1016/0022-1759(74)90076-3. [DOI] [PubMed] [Google Scholar]
- Yolken R. H., Stopa P. J. Comparison of seven enzyme immunoassay systems for measurement of cytomegalovirus. J Clin Microbiol. 1980 Jun;11(6):546–551. doi: 10.1128/jcm.11.6.546-551.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yolken R. H., Wyatt R. G., Barbour B. A., Kim H. W., Kapikian A. Z., Chanock R. M. Measurement of rotavirus antibody by an enzyme-linked immunosorbent assay blocking assay. J Clin Microbiol. 1978 Sep;8(3):283–287. doi: 10.1128/jcm.8.3.283-287.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]


