Abstract
Repetitive cycles of palatable food access and chronic calorie restriction alter feeding behaviors and forebrain neural systems. The purpose of this study was to determine the behavioral, endocrine, and meal-related hindbrain neural activation in adult male Sprague-Dawley rats exposed to a binge-access feeding schedule. The binge-access schedule consisted of repeated twice-per-week episodes of acute calorie restriction (to one-third of the previous day's intake) followed by 2 h of concurrent access to high-calorie palatable food (sweetened fat: 90% vegetable shortening-10% sucrose) and chow. The binge-access rats consumed more calories during the “binge” period than rats with continuous access to sweetened fat (continuous-access group) or subjected to repeated acute calorie restriction only (chow-restricted group). The binge-access group also exhibited a ∼25% increase in sweetened fat intake from week 1 to week 6. Persistence of the binge phenotype in the binge-access animals was demonstrated 2 wk, but not 4 wk, after ad libitum chow. The binge-access and chow-restricted groups maintained a similar normal body composition and hormonal profiles, whereas the continuous-access animals developed an obese phenotype. Terminal ghrelin levels were significantly higher in the binge-access group than in the continuous-access group. Consumption of a standardized meal resulted in more c-Fos-positive cells along the anterior-posterior nucleus of the solitary tract regions in the binge-access group than in naive controls. These results suggest that repeated cycles of acute calorie restriction followed by palatable food produce physiological alterations that may facilitate overconsumption of a highly palatable food during limited-access periods.
Keywords: bulimia nervosa, binge eating, nucleus of the solitary tract, area postrema
the increased availability of highly palatable foods is one factor strongly implicated in the obesity epidemic of Western cultures (48). The palatability of food is typically a function of its fat and/or sugar composition (31, 79). When given optional access (i.e., free choice) to a fat, sucrose, or mixed-macronutrient solution, chow-fed rodents typically demonstrate a pronounced preference for and increased acceptance of the palatable food (28, 53). Feeding behavior of this type, overconsumption of palatable foods without calorie restriction, has been termed “hedonic hunger” or “hedonically driven eating.” Bingelike intake (i.e., excessive intake in a relatively short amount of time) has been demonstrated in energy-replete rats with intermittent scheduled access to palatable foods (e.g., vegetable shortening, high-fat diet, and sucrose solutions) (7, 9, 19, 26, 28, 59, 60, 87). Scheduled access to palatable food options has been demonstrated to result in a pattern of excess caloric intake on “binge” days and reduced or “compensated” calorie consumption of standard diet on nonbinge days. Such hedonically driven feeding is strongly dependent on temporal cues or habitual patterns of food access (17, 63). Alternatively, the reduced consumption following the bout of “bingeing” could represent diminished reinforcing potency or relative decreased taste hedonics of the less palatable standard diets (21, 46). Exposure of animals to entrained feeding schedules (palatable or standard diets) has been demonstrated to result in anticipatory behaviors, increases in insulin and ghrelin levels, elevated gene expression of hypothalamic neuropeptide Y, and synchronized circadian regulated genes (30, 37, 64–66, 88). Thus optional scheduled access to palatable food results in behavioral and physiological alterations to promote periods of overeating.
Persistent calorie restriction before food access also alters the behavioral and neural response to foods (13, 14, 61). Specifically, chronic calorie restriction (≤85% of ad libitum intake) in rodents has been shown to modulate the opioidergic- and dopaminergic-dependent signaling involved with food reinforcement (5, 6, 10–12, 74). Rats exposed to daily calorie restriction with scheduled optional access (i.e., 12 h of access) to standard chow and sugar solutions displayed an escalating bingelike pattern of sugar consumption (16). Prolonged history of calorie restriction in rats also results in bingelike eating of palatable foods (e.g., Oreo cookies) in response to foot-shock stress (45). In clinical populations, intermittent calorie restriction or persistent dieting is associated with episodic binge eating, and caloric restraint is likely to be involved in the maintenance of bulimia nervosa (BN) (62, 69, 73).
The present studies employ a novel rodent model of binge eating of relevance to human binge-eating behavior. This feeding paradigm incorporates intermittent acute food restriction followed by brief periods of access to a highly palatable sugar-fat mixture. These repeated episodes of acute calorie restriction are in contrast to other binge-feeding paradigms, which use longer periods of calorie restriction for extended periods of times [e.g., 66% calorie restriction for 5 days (8) or 12 h of daily restriction for 8–30 days (15, 16)]. The sweet-fat ration used in this study has a macronutrient profile resembling “forbidden foods,” characteristic of bulimic binges (51). In addition, the binge-access period occurs during the part of the active eating cycle of the rat during which the greatest amounts of food are normally consumed, resembling binge eating disorder and BN patients, who generally binge more during afternoon and evening meal times (27, 51, 75). Furthermore, the binge-access rats in this model are exposed to intermittent restriction days alternating with two binges per week, thereby modeling the intermittent behavior demonstrated in BN patients and paralleling the two-binge-per-week minimum-frequency diagnostic criterion (1) for binge eating in binge-eating disorder and BN (3, 18, 25).
The aim of these experiments was to characterize behavioral, hormonal, and neural consequences of exposure to different dietary and scheduled-access feeding constraints. Three groups of animals were exposed to repeated episodes of acute calorie restriction followed by scheduled access to a palatable food, repeated episodes of acute calorie restriction, or continuous access to a palatable food.
One of the defining features of binge eating is consumption of a meal that is of a larger size (1). Multiple lines of research have suggested that the nucleus of the solitary tract (NTS) is the initial central neural site that integrates meal-related viscerosensory afferent information and peripheral hormonal signals (34, 39, 78, 80). To assess whether a history of repeated episodes of acute calorie restriction followed by scheduled access to a palatable food alters neural responses in the NTS, hindbrain c-Fos immunoreactivity following a standardized meal was also characterized in the groups.
MATERIALS AND METHODS
Animals.
A total of 65 adult male Sprague-Dawley rats (Charles River; 325–350 g initial body wt) were individually housed in stainless steel wire mesh hanging cages and placed on a 12:12-h light-dark schedule (lights off at 1230). All rats were fed ad libitum standard laboratory chow (Global Diet-2018, Harlan Teklad; 3.3 kcal/g: fixed-formula diet of 18% protein and 5% fat) unless otherwise noted. Water was available at all times during the experiment. All procedures were approved by the Institutional Animal Care and Use Committee of the Johns Hopkins University.
Feeding schedules and experimental groups.
The palatable food used in these experiments was “sweetened fat,” which consisted of 90% vegetable shortening (Crisco, a gift from J. M. Smucker; 1.5 g of trans fat) and 10% sucrose; its caloric density was 8.6 kcal/g. To determine whether the animals had an initial difference in their preference for the palatable food, all were preexposed for 24 h to the sweetened fat 3 days before beginning their respective feeding schedules. The rats were divided into three groups with initial statistically similar body weight and sweetened fat preference as follows: continuous-access, binge-access, and chow-restricted groups. The continuous-access group had unlimited optional access to standard chow and jars containing sweetened fat throughout the experiment. Jars containing sweetened fat were refreshed as needed and completely changed out every 3rd day. The chow-restricted and binge-access groups were restricted at the beginning of the dark cycle to 33% of the previous day's chow caloric intake on days 2 and 5 of each week (32). On restriction days, animals consumed the entire restricted amount of chow within the first 4 h. Hence, on the restriction days, this amounted to the equivalent of ∼20 h of food deprivation before the refeeding days. On subsequent days (days 3 and 6) 2 h into the dark cycle (1430 h; total deprivation time ∼22 h), the binge-access groups were given access to standard chow and sweetened fat, whereas the chow-restricted groups were refed chow alone. The binge-access group had access to the jars of sweetened fat only for the first 2 h of each refeeding period. In this fashion, the binge-access group was exposed to a repeated cycle that consisted of three no-restriction days (days 1, 4, and 7), two weekly episodes of calorie restriction (days 2 and 5), and two weekly episodes of scheduled refeeding starting with 2 h of access to an optional palatable food (days 3 and 6). This schedule was chosen to provide the animals with a combination of intermittent days of calorie restriction, palatable food access, and ad libitum standard chow access within a 7-day period.
Palatable food and chow intake during the feeding schedules.
Animals were maintained on each of the three weekly feeding schedules (n = 14 per group) for ≥6 wk. Food intake and spillage were recorded to the nearest 0.1 g and measured separately for the 2-h refeeding period, 20 h following the refeeding period, and 24 h before calorie restriction (for binge-access and chow-restricted groups) throughout the experiment. Intakes for day 7 were not recorded.
Persistence of the bingelike behavior following the binge-access schedule.
A group of binge-access rats (n = 6) were removed from the feeding schedule after 6 wk and placed on ad libitum standard chow. After 2 wk of ad libitum chow feeding (i.e., without calorie restriction or palatable food access), these rats were subjected to 1 day of 33% calorie restriction and, on the subsequent day, were refed chow and sweetened fat for 2 h, and spillage and intakes were recorded. Rats were then again fed ad libitum chow, and this procedure was repeated 2 wk later (4 wk after the initial 6-wk cycle).
Body weight, fat pad weights, and plasma hormone assays.
The remaining eight rats chosen randomly from each group continued their respective schedules for an additional 2 wk. After a total of 8 wk on the feeding schedule, all three groups (n = 8 for each group) were food restricted beginning at the onset of the dark cycle to 33% of the previous day's caloric intake. Since the groups were on different feeding schedules, the uniform 33% calorie restriction before death was employed to eliminate the potential confounding effects of recent food intake. The 8-wk time point was chosen, because it was after significant differences in feeding pattern emerged and would be representative of the maintenance phase of obesity (continuous access) or an eating disorder (binge access). Rats were decapitated on the following day, 2 h into the dark cycle at the time of the expected refeeding for binge-access and chow-restricted groups. The animals were killed in a counterbalanced staggered fashion for each group. Decapitations were performed in a separate room to minimize the stress on the remaining animals. Approximately 4 ml of trunk blood from each rat were collected into an EDTA-containing Vacutainer tube, and 20 μl were removed for blood glucose assay (Freestyle, Abbott Laboratories). The remainder of the blood sample was maintained on ice until centrifugation at 3,000 rpm for 10 min. Standard radioimmunoassay kits (Millipore, St. Charles, MO) were used to determine plasma insulin (sensitivity 0. 1 ng/ml), ghrelin (total; sensitivity 100 pg/ml), leptin (sensitivity 0.5 ng/ml), and corticosterone (sensitivity 25 ng/ml; MP Biomedical, Redding, CA) levels. Epididymal, retroperitoneal, and subcutaneous fat pads were dissected from carcasses and weighed to the nearest 0.1 g. Because these were representative of visceral and subcutaneous fat depots, the sum of these values was expressed as a consistent proportion of estimated body fat. Percent body fat was estimated by multiplying the grams of fat tissue by body weight.
c-Fos immunohistochemistry of the caudal hindbrain.
A separate group of animals was subjected to the above-described feeding schedules for 5 wk. In addition to the binge-access (n = 6), continuous-access (n = 5), and chow-restricted (n = 6) groups, an additional naive group (n = 6) was added to serve as controls for the c-Fos immunohistochemistry. Although the naive group was preexposed to the sweetened fat, they were fed ad libitum standard chow (i.e., not food restricted or given access to the sweetened fat) for 5 wk. At week 6, all groups were subjected to 33% calorie restriction (similar to days 3 and 6 of the binge-access group) and refed a standardized meal on the following day 2 h into the dark cycle. Body weight between each group before the animals received the standardized meal approached significance [F(3,19) = 2.6, P = 0.081]: 464 ± 12 g for the binge-access group, 464 ± 19 g for the chow-restricted group, 537 ± 31 g for the continuous-access group, and 512 ± 18 g for the naïve group. The meal was the average intake of chow and sweetened fat consumed by the continuous-access group during 2 h of refeeding. This amount was chosen to expose the animals to a standardized meal that would be readily consumed by all groups in the time allotted (<20 min, 24 kcal, 2 g of chow and 2 g of sweetened fat). The whole meal was consumed by all the rats. At 90 min after presentation of the standardized meal, rats were deeply anesthetized with Euthasol [1 ml/kg ip, pentobarbital sodium and phenytoin sodium (1 ml/kg); Virbac] and transcardially perfused, via a 16-gauge needle placed in the left ventricle, with ∼200 ml of 0.15 M NaCl followed by ∼150 ml of 4% (wt/vol) paraformaldehyde in PBS. Brains were removed, stored overnight in 4% paraformaldehyde with 25% (wt/vol) sucrose, frozen, and sectioned at 40 μm on a cryostat through the rostrocaudal extent of the NTS and area postrema (AP).
The immunohistochemistry procedure was similar to that previously published by our laboratory (34). Briefly, sections were incubated for 20 h with c-Fos primary antibody (rabbit polyclonal, 1:20,000 dilution; catalog no. PC38, Oncogene) and processed according to standard immunoperoxidase methods (Vectastain ABC reagent, Vector Laboratories), with Ni-3′3′-diaminobenzidine (Vector Laboratories) chromagen incubation used to stain Fos-like products black. To control for staining variability, each immunohistochemistry run contained matched sections from all experimental groups and controls. Quantitative analysis of c-Fos immunoreactivity was done using the IP Laboratory Imaging System (Scanalytics, Vienna, VA) image analysis software. The c-Fos-positive cells were counted bilaterally for each structure by the imaging program by setting minimum and maximum optical density levels. For further normalization of background conditions, software counts were compared with visual counts, and standard optimal settings were applied to all experimental groups and individual runs.
Coronal bilateral sections from four rostrocaudal levels of the NTS and AP were analyzed per animal. The anterior-posterior levels were determined by coordinates from the interaural line following Paxinos and Watson (70). The NTS areas consisted of three anatomically matched sections from caudal (cNTS; −5.6 mm), at the level of the obex, corresponding to the posterior edge of the AP; four anatomically matched sections from medial (mNTS; −5.06 mm) at the maximal extent of the AP; four anatomically matched sections from intermediate (iNTS; −4.3 mm), anterior to the AP, corresponding to the maximal extent of the gelatinous subnucleus of the NTS; and three anatomically matched sections from rostral (rNTS; −3.8 mm) consisting of the area rostral to the gelatinous nucleus and the caudal aspect of the medial vestibular nucleus on the dorsal boundary. This analysis provided a view for the rostral-caudal extent of c-Fos activation. Because the NTS is organized in a viscerotopic fashion, the average number of c-Fos-positive cells was ascertained across these rostral-caudal levels (i.e., caudal, medial, intermediate, and rostral), rather than within individual NTS subnuclei (i.e., dorsolateral, medial, commissural, gelatinous, and central).
Statistical analysis.
Total kilocalorie intakes for 2 h, 20 h, and 24 h for the 6-wk feeding schedules were analyzed using a two-way repeated-measures ANOVA, with feeding groups as the between-subject factor and weeks as the within-subject factor. Separate repeated-measures ANOVAs were performed to determine the contribution of sweetened fat or chow on 6-wk intakes or persistence of the bingelike feeding response. Hormone assays were analyzed with a one-way ANOVA, and quantification of the c-Fos activation was analyzed with a one-way ANOVA at each level of the NTS. Post hoc comparisons were made when appropriate with a Newman-Keuls test, unless otherwise noted. Correlation coefficients and t-test of slope = 0 were used across groups to determine the relationship between body weights and ghrelin levels and total fat mass and leptin levels. All statistical analyses were performed with Statistica 6.0 software (StatSoft), and significance was set at α = 0.05.
RESULTS
Palatable food and chow intake during the feeding schedules.
There were no significant group differences in the initial body weights or the intake of sweetened fat during the 24-h preexposure period. During the preexposure period, all rats demonstrated a preference (kcal) for the sweetened fat over standard chow (65.5 ± 1.1%, range 55.1–84.1%).
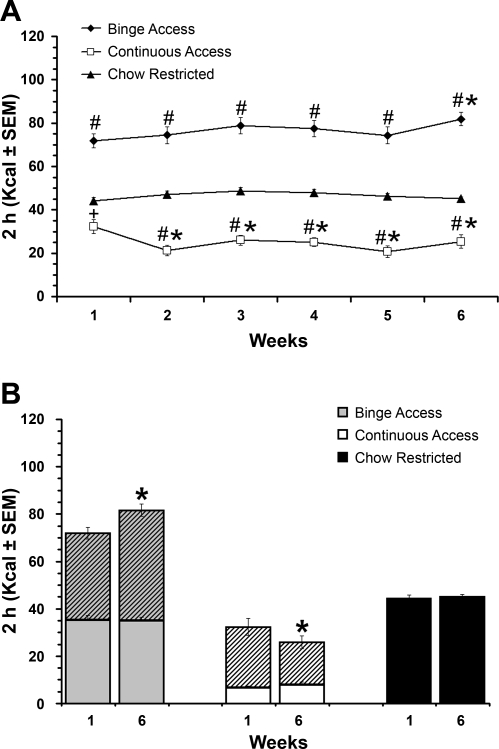
For the 2-h “refeeding” caloric intakes (days 3 and 6), there was a significant group effect [F(2,81) = 218.7, P < 0.001], with all three groups different from each other (P < 0.001). The binge-access group consumed the most calories during this time. There was a significant group × weeks interaction [F(10,405) = 3.5, P < 0.001]. At each weekly time point, the binge-access and continuous-access groups consumed significantly more calories than the chow-restricted group (P < 0.05 or P < 0.01; Fig. 1A). For the binge-access group, post hoc testing revealed significantly greater intake in week 6 than in week 1 (P < 0.05). In the continuous-access group, intake was significantly greater in week 1 than in all other weeks (P < 0.05; Fig. 1A). A separate within-group repeated-measures ANOVA demonstrated a significant increase in calories derived from sweetened fat [F(5,135) = 3.3, P < 0.01] from week 1 to week 3 (not shown) and week 6 (Fig. 1B) in the binge-access groups. Over the same time period, the continuous-access group demonstrated a significant decrease in calories derived from sweetened fat [F(5,135) = 2.5, P < 0.05] from week 1 to weeks 2–6 (P < 0.05; Fig. 1B).
Fig. 1.
Total caloric intakes for the 2-h refeeding period on days 3 and 6 of the 6-wk feeding schedule. A: 6-wk total caloric intake for the 2-h period (n = 14 per group). #P < 0.01; +P < 0.05 vs. chow-restricted at the respective week. *P < 0.05 vs. week 1 within groups. B: over the 6-wk period, the binge-access group consumed significantly more calories derived from sweetened fat at week 6 than week 1, whereas the continuous-access group consumed less over the same time period. *P < 0.05. Hatched bars, calories derived from sweetened fat; solid bars, calories derived from chow.
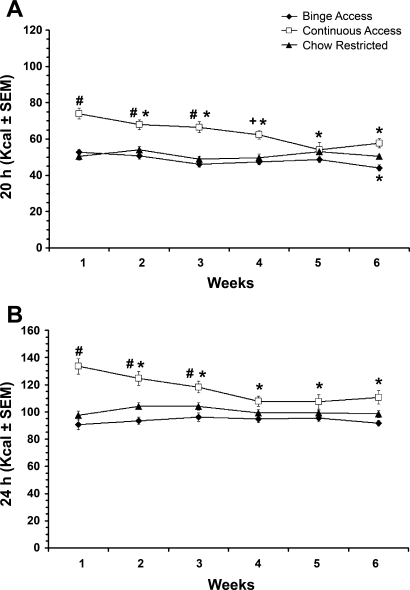
The 20-h caloric intakes following the 2 h of refeeding on days 3 and 6 also differed significantly among groups [F(2,81) = 61.5, P < 0.001], and again all three groups were significantly different from each other (P < 0.001). The continuous-access group consumed the most calories during this period (Fig. 2A). There was a significant weeks effect [F(5,405) = 9.3, P < 0.001] and a significant group × weeks interaction [F(10,405) = 6.2, P < 0.001]. At weeks 1–4, intakes were significantly greater in the continuous-access group than in the chow-restricted group (P < 0.01 or P < 0.05; Fig. 1A). The binge-access group decreased intake between week 1 and week 6 (P < 0.05). The continuous-access group decreased intake from week 1 to weeks 2–6 (P < 0.05 for all; Fig. 2A). For the continuous-access group, the decrease in intake was attributed to decreased intake of sweetened fat [F(5,135) = 7.0, P < 0.001]. The 24-h caloric intakes on “no-restriction” days 1 and 4 were also significantly different among the groups [F(2,81) = 21.1, P < 0.001]. Similar to the 20-h intakes, all three groups were significantly different from each other (P < 0.001), and the continuous-access group consumed the most calories. There was also a significant weeks effect [F(5,405) = 5.4, P < 0.001] and a significant group × weeks interaction [F(10,405) = 6.2, P < 0.001]. Post hoc testing revealed that the continuous-access group decreased intake from week 1 at weeks 2–6 (P < 0.05; Fig. 2B). This effect could be attributed to the decrease in the calories derived from sweetened fat [F(5,135) = 9.4, P < 0.001].
Fig. 2.
Caloric intake for the 20-h period following the 2-h refeeding period on days 3 and 6 and the 24-h feeding period on days 1 and 4 of the 6-wk feeding schedule. A: 6-wk total caloric intake for the 20-h period following the 2-h refeeding. B: 24-h intakes on “no-restriction” (ad libitum) days. #P < 0.01; +P < 0.05 vs. chow-restricted at the respective week. *P < 0.05 vs. week 1 within groups.
Persistence of bingelike behavior following the binge-access schedule.
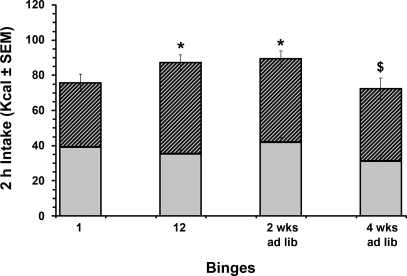
After the 6-wk feeding schedule, a subgroup of binge-access animals (n = 6) were placed on ad libitum chow and retested for the bingelike feeding phenotype after 2 and 4 wk. A repeated-measures ANOVA demonstrated a significant group difference in 2-h kilocalorie intake over this time [F(3,15) = 5.1, P < 0.05]. Differences were observed between the first refeeding (binge 1; 75 ± 4 kcal) and the last refeeding (binge 12; 87 ± 2 kcal) period of the 6-wk cycle (P < 0.05) and between binge 1 and the retest after 2 wk of ad libitum chow (89 ± 4 kcal, P < 0.05). After 4 wk on ad libitum chow, 2-h total kilocalorie intake differed from binge 12 and from that after the 2-wk ad libitum chow retest (P < 0.05), but not from binge 1. However, the differences at the 4-wk retest resulted from a decrease in chow, rather than sweetened fat, consumption from the 2-wk retest time point (P < 0.05) from 41 ± 3 to 31 ± 3 kcal (Fig. 3).
Fig. 3.
Persistence of bingelike feeding after 2 and 4 wk of ad libitum standard chow feeding. Contributions of sweetened fat (hatched bars) and chow (solid bars) to total caloric intake during the 2-h refeeding period (“binge”) for the binge-access group, as well as intakes of first (binge 1) and last (binge 12) individual binges of the 6-wk cycles and after a period of ad libitum chow feeding (i.e., 2 wk and 4 wk) are shown. *P < 0.05, total calories consumed at binge 1 vs. total calories consumed at binge 12 or at the retest after 2 wk of ad libitum chow. $P < 0.05 vs. 2 wk ad lib.
Body weight, fat pad weights, and plasma hormone assays.
Animals (n = 8 for each group) from the 6-wk feeding schedule were maintained on their respective feeding protocols for an additional 2 wk (i.e., 8 wk total). Table 1 illustrates the body weight and fat pad weights at the time of death. After 8 wk, there were significant differences in final body weights [F(2,21) = 13.5, P < 0.01], estimated body fat [F(2,21) = 16.6, P < 0.001], and subcutaneous [F(2,21) = 13.5, P < 0.01], retroperitoneal [F(2,21) = 16.3, P < 0.001], and epididymal [F(2,21) = 14.6, P < 0.005] fat pad weights. In all instances, post hoc tests revealed significant differences between all groups compared with the continuous-access group (P < 0.01) but no differences between the binge-access and chow-restricted groups.
Table 1.
Body weight and blood parameters after 8 wk on the feeding schedules
| Binge-Access Group | Continuous-Access Group | Chow-Restricted Group | ||||
|---|---|---|---|---|---|---|
| Body wt, g | ||||||
| Initial | 342±10 | 343±12 | 342±10 | |||
| Final | 507±24 | 639±35* | 506±21 | |||
| Estimated body fat, %body wt | ∼11 | ∼22* | ∼11 | |||
| Subcutaneous | 29±4.7 | 92±15* | 32±4.7 | |||
| Retroperitoneal | 15±2.0 | 29±3.4* | 14±1.8 | |||
| Epididymal | 13±1.9 | 23±1.9* | 11±1.3 | |||
| Glucose, mmol/l | 4.5±0.23 | 6.1±0.4* | 4.5±0.18 | |||
| Insulin, ng/ml | 1.1±0.19 | 5.8±1.2* | 1.2±0.21 | |||
| Leptin, ng/ml | 7.8±1.5 | 13.9±1.4* | 8.1±1.6 | |||
| Ghrelin (total), ng/ml | 2.9±0.3 | 1.9±0.2† | 2.5±0.2 | |||
| Corticosterone, ng/ml | 348±68 | 83±11* | 368±60 | |||
Values are means ± SE (n = 8 in each group).
P < 0.05 vs. binge-access and chow-restricted groups.
P < 0.05 vs. binge-access group.
Table 1 also illustrates the blood glucose and hormonal measurements after 8 wk on the feeding regimen. Blood glucose [F(2,21) = 9.7, P < 0.01], insulin [F(2,21) = 14.3, P < 0.001], leptin [F(2,19) = 5.2, P < 0.05], ghrelin [total, F(2,21) = 4.1, P < 0.05], and corticosterone [F(2,21) = 9.2, P < 0.01] were significantly different among groups. Post hoc testing revealed that glucose, insulin, and leptin were significantly elevated in the continuous-access group compared with the binge-access and chow-restricted groups (P < 0.05), but there were no differences between the binge-access and chow-restricted groups. The correlation between leptin levels and total fat mass across groups approached significance (R = 0.386, P = 0.06). The continuous-access group had significantly lower corticosterone levels than the two other groups (P < 0.05), whereas the ghrelin (total) levels were significantly reduced in the continuous-access group compared with the binge-access group (P < 0.05; Table 1). The ghrelin effect was not entirely accounted for by body weight differences across groups, since ghrelin levels were not significantly correlated with body weight (R = −0.305, not significant).
c-Fos immunohistochemistry of the caudal hindbrain.
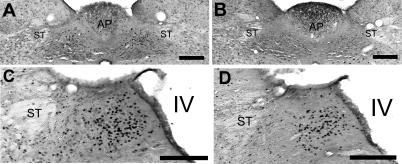
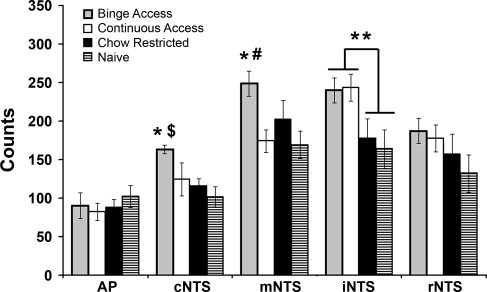
Representative photomicrographs for the mNTS and the iNTS from the binge-access and naive animals are shown in Fig. 4. The number of c-Fos-immunoreactive (positive) cells following a standardized meal differed between groups in three of the five hindbrain regions: cNTS [F(3,19) = 4.2, P < 0.05], mNTS [F(3,19) = 3.7, P < 0.05], and iNTS [F(3,19) = 3.6, P < 0.05]. In the cNTS and mNTS, the binge-access group had more c-Fos-positive cells than the naive group (P < 0.05). In the cNTS, the binge-access group had more c-Fos-positive cells than the chow-restricted group (P < 0.05), whereas in the mNTS, the binge-access group had more c-Fos-positive cells than the continuous-access group (P < 0.05). For the iNTS, planned comparison between groups revealed significantly more c-Fos-positive cells in animals with a history of access to the sweetened fat, binge-access and continuous-access groups, compared with those without, chow-restricted and naive groups (P < 0.01; Fig. 5).
Fig. 4.
Representative coronal micrographs of c-Fos staining (black) of binge-access (A and C) and naive (B and D) rats 90 min after presentation of a standardized meal. Bilateral hindbrain sections from medial (A and B) and unilateral sections from intermediate regions (C and D) of nucleus of the solitary tract are shown. Scale bars, 200 μm. AP, area postrema; ST, solitary tract; IV, 4th ventricle.
Fig. 5.
Average immunoreactive c-Fos counts in hindbrain regions of rats with different histories of feeding schedules in response to a standardized meal. All groups were subjected to 33% calorie restriction (similar to day 2 of binge access) and refed a standardized meal (e.g., 2 g of chow + 2 g of sweetened fat) 2 h into the dark cycle on the following day. The meal was the average intake of chow and sweetened fat consumed by the continuous-access group during the 2-h refeeding (see Fig. 1A). In the caudal NTS (cNTS), there were significantly more c-Fos-positive cells in the binge-access group than in the naive (*P < 0.05) and chow-restricted ($P < 0.05) groups. In the medial NTS (mNTS), there were significantly more c-Fos-positive cells in the binge-access group than in the naive (*P < 0.05) and continuous-access (#P < 0.05) groups. Planned comparisons revealed a significant effect for palatable food access in the intermediate NTS (iNTS): more c-Fos-positive cells in groups with a history of access to the sweetened fat than in those without (**P < 0.01).
DISCUSSION
The aims of this study were to determine the consequences of a binge-access schedule to a palatable food on feeding behavior, hormone profile, and meal-induced hindbrain neural activation in adult male Sprague-Dawley rats. The palatable food used in these experiments combined sweet and fat components with a macronutrient composition similar to that used by others in animal models of binge eating (7, 54). The most prominent differences between this model and others are as follows. The animals were subjected to repeated schedules of an acute calorie restriction (33% of daily intake) before scheduled access to the palatable food. Other binge-eating feeding paradigms in rodents employed longer periods of intermittent calorie restriction (8, 15, 16). In addition, the palatable food access period used in this paradigm occurred during the time phase associated with the greatest food intake (i.e., 2 h into the dark period) (50). These conditions were chosen to maximize the amount of calories consumed during the 2-h access period by engaging calorie-regulating and hedonically driven neural pathways. In this fashion, the binge-access schedule in this study utilized the rebound hyperphagia that typically results from an acute food restriction (∼20 h), the temporal pattern of rodent feeding behaviors, and the innate preference for a palatable food to promote bingelike feeding in adult male rats. The interpretations of this study are limited in determining the exact contribution of each independent dietary variable to the feeding response, and we cannot claim that the binge-access animals are displaying more than an exaggerated hyperphagic response. An additional feeding condition that offered the sweetened fat at the same time and frequency as it was offered to the binge-access group without intermittent calorie restriction would have allowed us to determine the particular contribution of the behavioral and physiological consequences of the scheduled access to sweetened fat. In addition, including a group that had continuous access to the sweetened fat and chow but intermittent acute calorie restriction would offer insight into how intermittent acute calorie restriction influenced the refeeding response. The results of this study are nonetheless beneficial, in that they further our understanding of the consequences of bingelike eating, because the binge-access group demonstrated a caloric intake during a 2-h refeeding with sweetened fat + chow that was ∼170% greater than the subsequent 20-h chow-only intake on binge days and ∼75% of the calories consumed during the 24-h (chow only) non-calorie-restriction days. Because the eating patterns occur in repeated episodes and the calorie amount eaten in a short amount of time (2 h) approximates the rat's daily (24-h) caloric intake, the amount consumed in the measured time period is bingelike. In a laboratory setting, single-course binges (e.g., ice cream) of BN patients are ∼1,300 kcal (84), that is, ∼65% of the recommended daily allowance of caloric intake for humans. In addition, the binge-access group increased their relative preference for sweetened fat during the 2-h binge-access period, as measured by the ratio of sweetened fat to chow calories consumed, by ∼25% across the 6-wk experimental period.
A similar bingelike intake of calories has been reported by Corwin and colleagues (20, 28) in adult male and female Sprague-Dawley rats exposed to a three-times-per-week 2-h access to vegetable shortening under non-calorie-deprived conditions. Even though the calories from the fat option (∼40 kcal) in the model described by Corwin and colleagues is similar to the caloric amount of sweetened fat consumed in the present study, the additive calories from chow (∼30 kcal) consumed during the 2-h binge in the present study resulted in a total 2-h intake that was almost twice (∼40 vs. ∼80 kcal) the amount consumed in the paradigm used by Corwin and colleagues. The caloric intake of the binge-access rats in the present study during the 2-h access period approached their 24-h ad libitum caloric intake on nonrestricted days. In the model described by Corwin and colleagues and similar protocols (7, 54), there is an overconsumption of calories on binge days but an underconsumption on nonbinge days. In the present study, we observe a similar pattern of hyperphagia on binge days (∼120 kcal) compared with relative normal caloric intakes on nonbinge days (∼90 kcal). In a similar fashion, we also observed a hyperphagia and hypophagia pattern of intake within binge days. That is, there was an increase in calories during the 2-h binge but a decrease in the following 20 h after the 6-wk period (Figs. 1 and 2). In a recent study by Cottone and colleagues (22), a bingelike feeding response to a sucrose-rich diet was produced in female rats when the preferred diet was preceded by access to a standard diet. In that study, rats were subjected to a daily regimen of 1 h of access to standard chow, 2 h of food deprivation, and 10 min of access to standard chow followed sequentially by 10 min of access to the preferred diet (chow/preferred) or standard chow (chow/chow) for >2 wk. The chow/preferred group displayed an anticipatory negative contrast effect, in that they developed a preferred diet hyperphagia that was dissociable from the standard diet hypophagia (22). Similar to the findings from the present experiment, the bingeing rats also developed a hypophagia of the home-cage standard chow (20 h of access) that was temporally related to the onset of the preferred diet hyperphagia. Even though the 20-h period of hypophagia in this study is likely a compensatory suppression of feeding from overeating, further experiments are needed to determine the relative contributions of hedonic or calorie-regulating mechanisms to this postbinge hypophagic response (40, 44). Whatever the cause, these data demonstrate a shift in the eating pattern during the binge and postbinge feeding periods of rats on the binge-access schedule.
The bingelike feeding response demonstrated over the 6-wk schedule in the binge-access group persisted after a 2-wk period of ad libitum chow intake. This suggests an entrainment of the physiological substrates involved in the feeding response, as suggested by similar examinations of the persistence of bingelike eating used by others. Wojnicki et al. (86) reported that an imposed abstinence from vegetable shortening for 5 wk produced a more pronounced binge intake when the vegetable shortening was reinstated. A comparable persistence of a binge response was reported in rats exposed to longer periods of calorie restriction. Hagan and Moss (43) exposed female Sprague-Dawley rats to 12 restriction-feeding cycles (6–8 days per cycle) consisting of 4–6 days of 75% to 50% calorie restriction followed by 2–4 days of chow refeeding with a palatable food (e.g., vanilla creme cookies). The persistence of a bingelike phenotype after 30 days of ad libitum chow was demonstrated after a 24-h calorie restriction and under spontaneous feeding conditions. In that study, under both conditions, the palatable food was given after 3.5 h of chow access. In the present study, we did not observe a sustained bingelike phenotype after 4 wk of ad libitum chow. Notably, the reduction in total caloric intake at 4 wk was due not to a decrease in the palatable food consumed but, rather, to a decrease in chow intake during the 2-h retest. This suggests that although total calories consumed did not increase in response to reinstatement of the schedule at 4 wk, the relative preference for the palatable food remained intact. In addition, these results suggest that the bingelike phenotype in this model is transient or experience dependent, opening future possibilities for studying the physiological changes that occur at various time points before, during, or after animals are on the binge-access cycle and display or lose the phenotype.
Despite differences in feeding patterns between groups, only the continuous-access group differed in body weight gain or body fat after 8 wk. There were no differences in body weight and composition between the binge-access and chow-restricted groups. Consistent with their increased body weights, the continuous-access group also had higher glucose, leptin, and insulin levels. This finding is in agreement with the findings from other feeding protocols that used continuous access to a palatable food to produce diet-induced obesity in Sprague-Dawley male rats (57, 58). Corticosterone levels were not different between the chow-restricted and binge-access groups, but these levels were nearly fourfold higher than in the continuous-access group. Such elevations are consistent with prior findings of elevated plasma corticosterone levels after acute and chronic food restriction (24, 49). Using adult male Long-Evans rats with 5 wk of continuous access to a sweetened fat mixture, Kinzig and colleagues (54) demonstrated a blunted stress response and higher palatable food consumption in rats with intermittent (2 h of access, 3 days/wk) or scheduled (2 h, 7 days/wk) access to sweetened fat. Long-term intermittent exposure to a physical stressor (i.e., foot shock) is also necessary to elicit a robust bingelike feeding response in a rodent model of binge eating using repeated cycles of calorie restriction, palatable food (i.e., Oreo cookies), and stress (45). Furthermore, baseline corticosterone levels were higher in rats exposed to calorie restriction and foot shock than in rats subjected to restriction or stress alone (2). In our model, rats in the binge-access and chow-restricted groups were exposed to repeated acute calorie restriction twice per week, and their corticosterone levels approximated those following a physical stressor (52, 54, 71).
Elevated plasma ghrelin levels in the binge-access compared with the continuous-access group may have additionally contributed to the increased consumption during the binge-access period. Although ghrelin levels were highest in the binge-access group, they were not significantly different from those in the chow-restricted group. In this study, there was no significant correlation between body weights and ghrelin levels, suggesting that the difference between the binge-access and continuous-access groups was not a result attributed entirely to the weight differences between animals. Higher endogenous ghrelin levels are associated with increased subjective hunger ratings in humans, and exogenously administered ghrelin elicits increases in food intake in humans and rodents (23, 56). The increased salience of the binge meal in the binge-access schedule may have resulted in an entrainment of the “prebinge” ghrelin levels in this group, as has been reported from studies using habitual eating patterns (29, 37). Elevation of plasma cortisol and ghrelin after an overnight fast has also been demonstrated in clinical eating disorder populations (41, 42, 82, 83), although there are some inconsistencies among these studies (68).
To address how a history of the feeding conditions may drive or facilitate the binge response, we examined the hindbrain neural response of all groups to a standardized meal. The meal was considerably smaller than that typically consumed by the binge-access group, and all groups were subjected to 33% calorie restriction on the day before meal presentation to ensure that the entire meal would be consumed by all groups. Elevated c-Fos labeling in response to the test meal was demonstrated in the binge-access group, compared with naive and chow-restricted groups, in regions of the NTS that receive vagal afferent input from the gastrointestinal tract (4, 47). We had expected that a history of bingelike eating might lead to decreased, rather than increased, meal-induced hindbrain activation. The rationale for this expectation was twofold. First, the NTS has been demonstrated to play an important role in mediating controls on meal size. Signals that decrease feeding (e.g., mechano, nutrient, cholecystokinin, and leptin) have been shown to result in NTS neural activation (33, 55, 78). Second, BN patients have increased gastric capacity, a finding that would be expected to result in diminished feedback inhibition on the NTS for a given gastric load, potentially facilitating binge intakes (38). However, this was not the result. The number of c-Fos-positive NTS neurons was increased in the binge-access group.
There are a number of potential explanations for the increased NTS activation in the binge-access group. One possible explanation for the increased c-Fos activation in the binge-access animals could be increased sensory signaling and increased activation due to the enhanced salience of the standardized meal (i.e., 2 g of chow and 2 g of sweetened fat) as a consequence of their binge history. In part, this notion can be supported by robust c-Fos activation in medial and intermediate regions of the NTS after intraoral delivery of a standard volume (7.5 ml) of sweet-tasting sucrose (0.5 M) solution compared with bitter-tasting quinine (1 mM) solution or distilled water (89). Similar to the c-Fos pattern of activation in our study, there were no differences in the rNTS, a region that receives afferent gustatory information, between the intraoral delivery of sucrose and quinine (89).
An additional explanation for the increased c-Fos activation in the binge-access animals could be the increased plasma ghrelin levels in the binge-access rats. Recent work examining the orexigenic role of ghrelin has revealed that central and peripheral administration of ghrelin results in increased NTS activation, suggesting a role for the NTS in mediating the feedforward mechanisms of food intake (36, 56, 81). Since we did not include two additional control groups, a group that had repeated access to sweetened fat at the same time and frequency as the binge-access group without intermittent calorie restriction and a group with continuous access to the sweetened fat with intermittent acute calorie restriction, the separate effects of diet and restriction cannot be discounted in the observed meal-induced c-Fos response. The increases in NTS activation in the binge-access group, however, could represent a feedforward mechanism, mediated in part by elevated ghrelin driving the robust intake during the binge. This increased salience is similar to that in response to fourth ventricle administration of a dose of ghrelin, which induces a hyperphagic response and increased c-Fos activation in the NTS (35, 36). Because one of the principal roles of the caudal brain stem in feeding involves the control of meal size, this pattern of neuronal activation in the binge-access group is suggestive of involvement of the NTS in bingelike eating (77). Moreover, the NTS activation may represent the mechanism involved in facilitating the accommodation of the larger meal size of the binge.
Even though the phenotype of the c-Fos-expressing cells was not characterized in the present study, there are reasons to believe that the activated cells are different from catecholamine neurons activated by satiety signals. NTS catecholaminergic [i.e., immunostained for tyrosine hydroxylase; (TH-positive)] c-Fos-positive cells are not significantly activated in rats given a smaller-than-usual (i.e., one-third smaller) volume of a dextrose-sweetened liquid diet compared with unfed rats (76). Rinaman and colleagues (76) observed the highest activation of TH-positive c-Fos-positive cells in animals with standard (unrestricted) access to an unexpected diluted volume of the liquid diet. Also, the c-Fos activation following fourth ventricle administration of ghrelin does not occur in TH-positive cells (36). Additional experiments are required to determine the phenotype of NTS cells that are activated in response to the standardized meal in animals with a history of a binge-access feeding schedule.
Perspectives and Significance
Because of the heterogeneity of onset and multifactorial aspects of BN (or any psychiatric disease), development of an appropriate animal model is difficult. Clinical and preclinical research has focused on whether dieting, dietary restraint, and/or psychological stress are precursor or risk factors to binge eating in this population (67, 72, 85). Rather than examining factors that initiate or lead to an onset of binge eating, we used a restriction/binge model of BN to determine the physiological consequence of this pattern of eating behavior that may facilitate disease maintenance. Data from these experiments demonstrate that repeated acute calorie restriction before access to a highly palatable food resulted in an exaggerated bingelike behavioral response in adult male Sprague-Dawley rats. Accompanying the alteration in palatable food preference are hormonal and hindbrain changes that promote a feedforward feeding response. Other animal models of bingelike intake in rodents have been reported; however, the model used in this study is unique, in that it combines acute calorie restriction with scheduled access to a highly palatable nutrient. These features make the model and its results more relevant to the consequences of human dieting behaviors, including dietary restraint and binge eating, and the clinical syndrome of BN.
GRANTS
This work was supported by National Institutes of Health Grants DK-19302, MH-015330, and DK-078484.
Acknowledgments
The authors thank Dr. Kellie L. K. Tamashiro and Janis Sethness for technical assistance.
REFERENCES
- 1.American Psychiatric Association and American Psychiatric Association Task Force on DSM-IV. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV. Washington, DC: Am. Psychiatric Assoc., 1994.
- 2.Artiga AI, Viana JB, Maldonado CR, Chandler-Laney PC, Oswald KD, Boggiano MM. Body composition and endocrine status of long-term stress-induced binge-eating rats. Physiol Behav 91: 424–431, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avena NM Examining the addictive-like properties of binge eating using an animal model of sugar dependence. Exp Clin Psychopharmacol 15: 481–491, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Barraco R, el-Ridi M, Ergene E, Parizon M, Bradley D. An atlas of the rat subpostremal nucleus tractus solitarius. Brain Res Bull 29: 703–765, 1992. [DOI] [PubMed] [Google Scholar]
- 5.Bello NT, Lucas LR, Hajnal A. Repeated sucrose access influences dopamine D2 receptor density in the striatum. Neuroreport 13: 1575–1578, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bello NT, Sweigart KL, Lakoski JM, Norgren R, Hajnal A. Restricted feeding with scheduled sucrose access results in an upregulation of the rat dopamine transporter. Am J Physiol Regul Integr Comp Physiol 284: R1260–R1268, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Berner LA, Avena NM, Hoebel BG. Bingeing, self-restriction, and increased body weight in rats with limited access to a sweet-fat diet. Obesity (Silver Spring) 16: 1998–2002, 2008. [DOI] [PubMed] [Google Scholar]
- 8.Boggiano MM, Chandler PC. Binge eating in rats produced by combining dieting with stress. Curr Protoc Neurosci Chapter 9: Unit9.23A, 2006. [DOI] [PubMed]
- 9.Byerly MS, Fox EA. High-fat hyperphagia in neurotrophin-4 deficient mice reveals potential role of vagal intestinal sensory innervation in long-term controls of food intake. Neurosci Lett 400: 240–245, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Cabeza de Vaca S, Carr KD. Food restriction enhances the central rewarding effect of abused drugs. J Neurosci 18: 7502–7510, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carr KD Augmentation of drug reward by chronic food restriction: behavioral evidence and underlying mechanisms. Physiol Behav 76: 353–364, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Carr KD, Kim GY, Cabeza de Vaca S. Chronic food restriction in rats augments the central rewarding effect of cocaine and the δ1-opioid agonist, DPDPE, but not the δ2-agonist, deltorphin. II. Psychopharmacology 152: 200–207, 2000. [DOI] [PubMed] [Google Scholar]
- 13.Carr KD, Kutchukhidze N. Chronic food restriction increases Fos-like immunoreactivity (FLI) induced in rat forebrain by intraventricular amphetamine. Brain Res 861: 88–96, 2000. [DOI] [PubMed] [Google Scholar]
- 14.Carroll ME, Meisch RA. The effects of feeding conditions on drug-reinforced behavior: maintenance at reduced body weight versus availability of food. Psychopharmacology 68: 121–124, 1980. [DOI] [PubMed] [Google Scholar]
- 15.Colantuoni C, Rada P, McCarthy J, Patten C, Avena NM, Chadeayne A, Hoebel BG. Evidence that intermittent, excessive sugar intake causes endogenous opioid dependence. Obes Res 10: 478–488, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Colantuoni C, Schwenker J, McCarthy J, Rada P, Ladenheim B, Cadet JL, Schwartz GJ, Moran TH, Hoebel BG. Excessive sugar intake alters binding to dopamine and μ-opioid receptors in the brain. Neuroreport 12: 3549–3552, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Corwin RL, Buda-Levin A. Behavioral models of binge-type eating. Physiol Behav 82: 123–130, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Corwin RL, Hajnal A. Too much of a good thing: neurobiology of non-homeostatic eating and drug abuse. Physiol Behav 86: 5–8, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corwin RL, Wojnicki FH. Binge eating in rats with limited access to vegetable shortening. Curr Protoc Neurosci Chapter 9: Unit9.23B, 2006. [DOI] [PubMed]
- 20.Corwin RL, Wojnicki FH, Fisher JO, Dimitriou SG, Rice HB, Young MA. Limited access to a dietary fat option affects ingestive behavior but not body composition in male rats. Physiol Behav 65: 545–553, 1998. [DOI] [PubMed] [Google Scholar]
- 21.Cottone P, Sabino V, Steardo L, Zorrilla EP. Intermittent access to preferred food reduces the reinforcing efficacy of chow in rats. Am J Physiol Regul Integr Comp Physiol 295: R1066–R1076, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cottone P, Sabino V, Steardo L, Zorrilla EP. Opioid-dependent anticipatory negative contrast and binge-like eating in rats with limited access to highly preferred food. Neuropsychopharmacology 33: 524–535, 2008. [DOI] [PubMed] [Google Scholar]
- 23.Cummings DE Ghrelin and the short- and long-term regulation of appetite and body weight. Physiol Behav 89: 71–84, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Dallman MF Viewing the ventromedial hypothalamus from the adrenal gland. Am J Physiol Regul Integr Comp Physiol 246: R1–R12, 1984. [DOI] [PubMed] [Google Scholar]
- 25.Dallman MF, Pecoraro NC, La Fleur SE. Chronic stress and comfort foods: self-medication and abdominal obesity. Brain Behav Immun 19: 275–280, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Davis JF, Melhorn SJ, Shurdak JD, Heiman JU, Tschop MH, Clegg DJ, Benoit SC. Comparison of hydrogenated vegetable shortening and nutritionally complete high-fat diet on limited access-binge behavior in rats. Physiol Behav 92: 924–930, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Castro JM Eating behavior: lessons from the real world of humans. Nutrition 16: 800–813, 2000. [DOI] [PubMed] [Google Scholar]
- 28.Dimitriou SG, Rice HB, Corwin RL. Effects of limited access to a fat option on food intake and body composition in female rats. Int J Eat Disord 28: 436–445, 2000. [DOI] [PubMed] [Google Scholar]
- 29.Drazen DL, Vahl TP, D'Alessio DA, Seeley RJ, Woods SC. Effects of a fixed meal pattern on ghrelin secretion: evidence for a learned response independent of nutrient status. Endocrinology 147: 23–30, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Drazen DL, Wortman MD, Seeley RJ, Woods SC. Neuropeptide Y prepares rats for scheduled feeding. Am J Physiol Regul Integr Comp Physiol 288: R1606–R1611, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Drewnowski A, Shrager EE, Lipsky C, Stellar E, Greenwood MR. Sugar and fat: sensory and hedonic evaluation of liquid and solid foods. Physiol Behav 45: 177–183, 1989. [DOI] [PubMed] [Google Scholar]
- 32.Elmore DK, de Castro JM. Meal patterns of normal, untreated bulimia nervosa and recovered bulimic women. Physiol Behav 49: 99–105, 1991. [DOI] [PubMed] [Google Scholar]
- 33.Emond M, Ladenheim EE, Schwartz GJ, Moran TH. Leptin amplifies the feeding inhibition and neural activation arising from a gastric nutrient preload. Physiol Behav 72: 123–128, 2001. [DOI] [PubMed] [Google Scholar]
- 34.Emond M, Schwartz GJ, Moran TH. Meal-related stimuli differentially induce c-Fos activation in the nucleus of the solitary tract. Am J Physiol Regul Integr Comp Physiol 280: R1315–R1321, 2001. [DOI] [PubMed] [Google Scholar]
- 35.Faulconbridge LF, Cummings DE, Kaplan JM, Grill HJ. Hyperphagic effects of brainstem ghrelin administration. Diabetes 52: 2260–2265, 2003. [DOI] [PubMed] [Google Scholar]
- 36.Faulconbridge LF, Grill HJ, Kaplan JM, Daniels D. Caudal brainstem delivery of ghrelin induces Fos expression in the nucleus of the solitary tract, but not in the arcuate or paraventricular nuclei of the hypothalamus. Brain Res 1218: 151–157, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frecka JM, Mattes RD. Possible entrainment of ghrelin to habitual meal patterns in humans. Am J Physiol Gastrointest Liver Physiol 294: G699–G707, 2008. [DOI] [PubMed] [Google Scholar]
- 38.Geliebter A, Melton PM, McCray RS, Gallagher DR, Gage D, Hashim SA. Gastric capacity, gastric emptying, and test-meal intake in normal and bulimic women. Am J Clin Nutr 56: 656–661, 1992. [DOI] [PubMed] [Google Scholar]
- 39.Gillespie BR, Burns GA, Ritter RC. NMDA channels control meal size via central vagal afferent terminals. Am J Physiol Regul Integr Comp Physiol 289: R1504–R1511, 2005. [DOI] [PubMed] [Google Scholar]
- 40.Glass MJ, Grace M, Cleary JP, Billington CJ, Levine AS. Potency of naloxone's anorectic effect in rats is dependent on diet preference. Am J Physiol Regul Integr Comp Physiol 271: R217–R221, 1996. [DOI] [PubMed] [Google Scholar]
- 41.Gluck ME Stress response and binge eating disorder. Appetite 46: 26–30, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Gluck ME, Geliebter A, Hung J, Yahav E. Cortisol, hunger, and desire to binge eat following a cold stress test in obese women with binge eating disorder. Psychosom Med 66: 876–881, 2004. [DOI] [PubMed] [Google Scholar]
- 43.Hagan MM, Moss DE. Persistence of binge-eating patterns after a history of restriction with intermittent bouts of refeeding on palatable food in rats: implications for bulimia nervosa. Int J Eat Disord 22: 411–420, 1997. [DOI] [PubMed] [Google Scholar]
- 44.Hagan MM, Rushing PA, Schwartz MW, Yagaloff KA, Burn P, Woods SC, Seeley RJ. Role of the CNS melanocortin system in the response to overfeeding. J Neurosci 19: 2362–2367, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hagan MM, Wauford PK, Chandler PC, Jarrett LA, Rybak RJ, Blackburn K. A new animal model of binge eating: key synergistic role of past caloric restriction and stress. Physiol Behav 77: 45–54, 2002. [DOI] [PubMed] [Google Scholar]
- 46.Hajnal A, Takenouchi K, Norgren R. Effect of intraduodenal lipid on parabrachial gustatory coding in awake rats. J Neurosci 19: 7182–7190, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hamilton RB, Norgren R. Central projections of gustatory nerves in the rat. J Comp Neurol 222: 560–577, 1984. [DOI] [PubMed] [Google Scholar]
- 48.Hetherington MM Cues to overeat: psychological factors influencing overconsumption. Proc Nutr Soc 66: 113–123, 2007. [DOI] [PubMed] [Google Scholar]
- 49.Johansson A, Fredriksson R, Winnergren S, Hulting AL, Schioth HB, Lindblom J. The relative impact of chronic food restriction and acute food deprivation on plasma hormone levels and hypothalamic neuropeptide expression. Peptides 29: 1588–1595, 2008. [DOI] [PubMed] [Google Scholar]
- 50.Johnson RF, Johnson AK. Light/dark cycle modulates food-to-water intake ratios in rats. Physiol Behav 48: 707–711, 1990. [DOI] [PubMed] [Google Scholar]
- 51.Kales EF Macronutrient analysis of binge eating in bulimia. Physiol Behav 48: 837–840, 1990. [DOI] [PubMed] [Google Scholar]
- 52.Kamara K, Eskay R, Castonguay T. High-fat diets and stress responsivity. Physiol Behav 64: 1–6, 1998. [DOI] [PubMed] [Google Scholar]
- 53.Kanarek RB, Aprille JR, Hirsch E, Gualtiere L, Brown CA. Sucrose-induced obesity: effect of diet on obesity and brown adipose tissue. Am J Physiol Regul Integr Comp Physiol 253: R158–R166, 1987. [DOI] [PubMed] [Google Scholar]
- 54.Kinzig KP, Hargrave SL, Honors MA. Binge-type eating attenuates corticosterone and hypophagic responses to restraint stress. Physiol Behav 95: 108–113, 2008. [DOI] [PubMed] [Google Scholar]
- 55.Ladenheim EE, Emond M, Moran TH. Leptin enhances feeding suppression and neural activation produced by systemically administered bombesin. Am J Physiol Regul Integr Comp Physiol 289: R473–R477, 2005. [DOI] [PubMed] [Google Scholar]
- 56.Lawrence CB, Snape AC, Baudoin FM, Luckman SM. Acute central ghrelin and GH secretagogues induce feeding and activate brain appetite centers. Endocrinology 143: 155–162, 2002. [DOI] [PubMed] [Google Scholar]
- 57.Levin BE, Dunn-Meynell AA. Defense of body weight against chronic caloric restriction in obesity-prone and -resistant rats. Am J Physiol Regul Integr Comp Physiol 278: R231–R237, 2000. [DOI] [PubMed] [Google Scholar]
- 58.Levin BE, Dunn-Meynell AA, Balkan B, Keesey RE. Selective breeding for diet-induced obesity and resistance in Sprague-Dawley rats. Am J Physiol Regul Integr Comp Physiol 273: R725–R730, 1997. [DOI] [PubMed] [Google Scholar]
- 59.Lowe MR, Butryn ML. Hedonic hunger: a new dimension of appetite? Physiol Behav 91: 432–439, 2007. [DOI] [PubMed] [Google Scholar]
- 60.Lowe MR, Levine AS. Eating motives and the controversy over dieting: eating less than needed versus less than wanted. Obes Res 13: 797–806, 2005. [DOI] [PubMed] [Google Scholar]
- 61.Mamczarz J, Bowker JL, Duffy K, Zhu M, Hagepanos A, Ingram DK. Enhancement of amphetamine-induced locomotor response in rats on different regimens of diet restriction and 2-deoxy-d-glucose treatment. Neuroscience 131: 451–464, 2005. [DOI] [PubMed] [Google Scholar]
- 62.Manwaring JL, Hilbert A, Wilfley DE, Pike KM, Fairburn CG, Dohm FA, Striegel-Moore RH. Risk factors and patterns of onset in binge eating disorder. Int J Eat Disord 39: 101–107, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mello NK Schedule-induced polydipsia and oral intake of drugs. Pharmacol Rev 27: 489–498, 1975. [PubMed] [Google Scholar]
- 64.Mendoza J, Angeles-Castellanos M, Escobar C. Differential role of the accumbens Shell and Core subterritories in food-entrained rhythms of rats. Behav Brain Res 158: 133–142, 2005. [DOI] [PubMed] [Google Scholar]
- 65.Mendoza J, Angeles-Castellanos M, Escobar C. Entrainment by a palatable meal induces food-anticipatory activity and c-Fos expression in reward-related areas of the brain. Neuroscience 133: 293–303, 2005. [DOI] [PubMed] [Google Scholar]
- 66.Mendoza J, Graff C, Dardente H, Pevet P, Challet E. Feeding cues alter clock gene oscillations and photic responses in the suprachiasmatic nuclei of mice exposed to a light/dark cycle. J Neurosci 25: 1514–1522, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mitchell JE, Pyle RL, Eckert E, Hatsukami D, Soll E. Bulimia nervosa with and without a history of overweight. J Subst Abuse 2: 369–374, 1990. [DOI] [PubMed] [Google Scholar]
- 68.Monteleone P, Fabrazzo M, Tortorella A, Martiadis V, Serritella C, Maj M. Circulating ghrelin is decreased in non-obese and obese women with binge eating disorder as well as in obese non-binge eating women, but not in patients with bulimia nervosa. Psychoneuroendocrinology 30: 243–250, 2005. [DOI] [PubMed] [Google Scholar]
- 69.Mussell MP, Mitchell JE, Weller CL, Raymond NC, Crow SJ, Crosby RD. Onset of binge eating, dieting, obesity, and mood disorders among subjects seeking treatment for binge eating disorder. Int J Eat Disord 17: 395–401, 1995. [DOI] [PubMed] [Google Scholar]
- 70.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego, CA: Academic, 1998.
- 71.Pecoraro N, Reyes F, Gomez F, Bhargava A, Dallman MF. Chronic stress promotes palatable feeding, which reduces signs of stress: feedforward and feedback effects of chronic stress. Endocrinology 145: 3754–3762, 2004. [DOI] [PubMed] [Google Scholar]
- 72.Pederson Mussell M, Mitchell JE, Fenna CJ, Crosby RD, Miller JP, Hoberman HM. A comparison of onset of binge eating versus dieting in the development of bulimia nervosa. Int J Eat Disord 21: 353–360, 1997. [DOI] [PubMed] [Google Scholar]
- 73.Polivy J, Zeitlin SB, Herman CP, Beal AL. Food restriction and binge eating: a study of former prisoners of war. J Abnorm Psychol 103: 409–411, 1994. [DOI] [PubMed] [Google Scholar]
- 74.Pothos EN, Creese I, Hoebel BG. Restricted eating with weight loss selectively decreases extracellular dopamine in the nucleus accumbens and alters dopamine response to amphetamine, morphine, and food intake. J Neurosci 15: 6640–6650, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Raymond NC, Neumeyer B, Warren CS, Lee SS, Peterson CB. Energy intake patterns in obese women with binge eating disorder. Obes Res 11: 869–879, 2003. [DOI] [PubMed] [Google Scholar]
- 76.Rinaman L, Baker EA, Hoffman GE, Stricker EM, Verbalis JG. Medullary c-Fos activation in rats after ingestion of a satiating meal. Am J Physiol Regul Integr Comp Physiol 275: R262–R268, 1998. [DOI] [PubMed] [Google Scholar]
- 77.Schwartz GJ Biology of eating behavior in obesity. Obes Res 12 Suppl 2: 102S–106S, 2004. [DOI] [PubMed] [Google Scholar]
- 78.Schwartz GJ, Moran TH. Leptin and neuropeptide Y have opposing modulatory effects on nucleus of the solitary tract neurophysiological responses to gastric loads: implications for the control of food intake. Endocrinology 143: 3779–3784, 2002. [DOI] [PubMed] [Google Scholar]
- 79.Sclafani A Carbohydrate taste, appetite, obesity: an overview. Neurosci Biobehav Rev 11: 131–153, 1987. [PubMed] [Google Scholar]
- 80.Sclafani A, Ackroff K, Schwartz GJ. Selective effects of vagal deafferentation and celiac-superior mesenteric ganglionectomy on the reinforcing and satiating action of intestinal nutrients. Physiol Behav 78: 285–294, 2003. [DOI] [PubMed] [Google Scholar]
- 81.Takayama K, Johno Y, Hayashi K, Yakabi K, Tanaka T, Ro S. Expression of c-Fos protein in the brain after intravenous injection of ghrelin in rats. Neurosci Lett 417: 292–296, 2007. [DOI] [PubMed] [Google Scholar]
- 82.Tanaka M, Naruo T, Muranaga T, Yasuhara D, Shiiya T, Nakazato M, Matsukura S, Nozoe S. Increased fasting plasma ghrelin levels in patients with bulimia nervosa. Eur J Endocrinol 146: R1–R3, 2002. [DOI] [PubMed] [Google Scholar]
- 83.Tanaka M, Naruo T, Nagai N, Kuroki N, Shiiya T, Nakazato M, Matsukura S, Nozoe S. Habitual binge/purge behavior influences circulating ghrelin levels in eating disorders. J Psychiatr Res 37: 17–22, 2003. [DOI] [PubMed] [Google Scholar]
- 84.Walsh BT, Kissileff HR, Cassidy SM, Dantzic S. Eating behavior of women with bulimia. Arch Gen Psychiatry 46: 54–58, 1989. [DOI] [PubMed] [Google Scholar]
- 85.Waters A, Hill A, Waller G. Internal and external antecedents of binge eating episodes in a group of women with bulimia nervosa. Int J Eat Disord 29: 17–22, 2001. [DOI] [PubMed] [Google Scholar]
- 86.Wojnicki FH, Johnson DS, Corwin RL. Access conditions affect binge-type shortening consumption in rats. Physiol Behav 95: 649–657, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wojnicki FH, Stine JG, Corwin RL. Liquid sucrose bingeing in rats depends on the access schedule, concentration and delivery system. Physiol Behav 92: 566–574, 2007. [DOI] [PubMed] [Google Scholar]
- 88.Woods SC, Vasselli JR, Kaestner E, Szakmary GA, Milburn P, Vitiello MV. Conditioned insulin secretion and meal feeding in rats. J Comp Physiol Psychol 91: 128–133, 1977. [DOI] [PubMed] [Google Scholar]
- 89.Yamamoto T, Sawa K. Comparison of c-Fos-like immunoreactivity in the brainstem following intraoral and intragastric infusions of chemical solutions in rats. Brain Res 866: 144–151, 2000. [DOI] [PubMed] [Google Scholar]