Abstract
We recently reported insulin resistance in adult offspring of obese C57BL/6J mice. We have now evaluated whether parameters of skeletal muscle structure and function may play a role in insulin resistance in this model of developmental programming. Obesity was induced in female mice by feeding a highly palatable sugar and fat-rich diet for 6 wk prior to pregnancy, and during pregnancy and lactation. Offspring of obese dams were weaned onto standard laboratory chow. At 3 mo of age, skeletal muscle insulin signaling protein expression, mitochondrial electron transport chain activity (ETC), muscle fiber type, fiber density, and fiber cross-sectional area were compared with that of offspring of control dams weaned onto the chow diet. Female offspring of obese dams demonstrated decreased skeletal muscle expression of p110β, the catalytic subunit of PI3K (P < 0.01), as well as reduced Akt phosphorylation at Serine residue 473 compared with control offspring. Male offspring of obese dams demonstrated increased skeletal muscle Akt2 and PKCζ expression (P < 0.01; P < 0.001, respectively). A decrease in mitochondrial-linked complex II-III was observed in male offspring of obese dams (P < 0.01), which was unrelated to CoQ deficiency. This was not observed in females. There were no differences in muscle fiber density between offspring of obese dams and control offspring in either sex. Sex-related alterations in key insulin-signaling proteins and in mitochondrial ETC may contribute to a state of insulin resistance in offspring of obese mice.
Keywords: maternal obesity, developmental programming, electron transport chain, insulin signaling, muscle metabolism
the world health organization recognizes obesity as a global pandemic. Of particular concern is the rise in obesity in pregnant women (1, 12, 22, 43), which not only can lead to immediate complications for both mother and infant during pregnancy, but may also have adverse long-term health consequences for the child, including increased risk of developing type 2 diabetes mellitus (T2DM), metabolic syndrome, hypertension, and obesity (8, 11, 39). These observations formed the basis for the developmental “overnutrition hypothesis”. In animal models in which dams have been fed a fat-rich diet, sex differences are frequently observed in the offspring phenotype (17, 24, 25, 35). The molecular mechanisms by which maternal obesity alters the long-term metabolic health of offspring in a sex-specific manner remain to be fully understood.
Rodent models of maternal obesity are a relatively recent development in this field; however, the pace of discoveries has been rapid. We recently developed a murine model of maternal obesity using a diet relevant to that consumed by many obese women that is high in both fats and sugar. We showed that offspring of obese mouse dams are hypertensive and develop increased adiposity with age (42). Hyperinsulinemia was observed at 3 mo of age, suggesting insulin resistance. Male offspring showed more pronounced phenotypic changes than females (42). By 6 mo of age, male offspring of obese dams demonstrated impaired glucose tolerance associated with β-cell failure. We also recently observed insulin resistance as assessed by euglycemic hyperinsulinemic clamp in adult male offspring of obese rats fed an identical diet (36) and have previously reported that following exposure to a high-fat diet during pregnancy and lactation, rat offspring show impaired glucose homeostasis (48) and altered expression of hepatic insulin signaling proteins (10).
Glucose intolerance and insulin resistance in muscle can predict development of T2DM (31, 41). Skeletal muscle is the major site of postprandial glucose disposal and is therefore one of the insulin-sensitive tissues most likely to manifest early signs of insulin resistance. A number of abnormalities have been reported in association with insulin resistance in human muscle, including alterations in muscle fiber composition (37), mitochondrial dysfunction (31), and expression of insulin signaling proteins (14). Previous studies in animal models of placental insufficiency (44, 46) or maternal undernutrition (13, 32) identified changes in these parameters in adult offspring with similar alterations observed in low-birth weight humans (40). However, the consequences of maternal obesity on offspring muscle structure and function, and any relationship with insulin signaling has not been studied to date. We hypothesized that muscle-specific insulin signaling defects, abnormal mitochondrial function, and/or alterations in muscle structure could contribute to insulin resistance in offspring of obese dams.
The aim of this study was therefore to determine whether the expression of insulin-signaling proteins, mitochondrial electron transport chain (ETC) activity, and/or muscle fiber composition are altered in muscle of male and female offspring of obese mice.
MATERIALS AND METHODS
Animal Protocols
All studies were approved by the Local Ethics Committee and were conducted according to Home Office Animals (Scientific Procedures) Act 1986. The model and its metabolic phenotype are described in detail by Samuelsson et al. (42). Briefly, female C57BL/6J mice, 1 wk after a first successful pregnancy were fed either a standard chow RM1 diet or a highly palatable obesogenic diet of chow supplemented with animal lard and condensed milk (Nestle, Croyden, UK) with added mineral mix AIN93G. Both diets were purchased from Special Diet Service UK (Devon, UK). After 6 wk, mice on the obesogenic diet had significantly higher body fat than the controls (42). Both obese and control mice were then mated and maintained on their respective experimental diets throughout pregnancy and lactation. Forty-eight hours after delivery, litters were reduced to six pups (litters lower than four pups were not used), with an equal female to male ratio where possible. At 21 days of age, all pups were weaned onto standard chow (RM1 diet) and maintained on this diet until 3 mo of age when animals in the fed state were killed by rising concentration of CO2. At post mortem, vastus lateralis muscle was removed and snap frozen in liquid nitrogen for molecular and biochemical analyses. For histological analysis, the entire calf muscle group (triceps surae), including soleus muscle was mounted on a cork block and snap frozen in isopentane chilled in dry ice. All tissues were stored at 80°C.
Insulin Signaling Protein Expression
Vastus lateralis muscle was extracted in ice-cold lysis buffer (n = 6 per group) [50 mmol/l HEPES (pH 8), 150 mmol/l sodium chloride, 1% Triton X100, 1 mmol/l sodium orthovanadate, 30 mmol/l sodium fluoride, 10 mmol/l sodium pyrophosphate, 10 mmol/l EDTA, and a protease inhibitor cocktail]. The total protein content of the lysates was determined using a bicinchoninic acid kit for protein determination (Sigma-Aldrich, Haverhill, UK). Samples were diluted to a standard protein concentration of 2 mg/ml in Laemmli buffer, and 10 μg total protein was subjected to SDS-PAGE. The proteins were transferred to PVDF Immobilon-P (Millipore) membrane and blocked for 1 h (5% nonfat dehydrated milk, 1 × TBS, 0.1% Tween 20). Membranes were incubated overnight with antibody against the insulin receptor β subunit (IRβ), Insulin receptor substrate 1 (IRS-1), phospho-IRS-1 (Ser307), phosphatidyl inositol 3-kinase (PI3 kinase) p85α regulatory subunit, and p110β catalytic subunit, Akt1, Akt2, phospho-Akt (Ser473), protein kinase C zeta (PKCζ), and GLUT4, diluted in TBS-0.1% Tween 20 containing either 5% dried milk or 5% BSA. Protein expression was quantified by spot densitometry using AlphaEase gs 3.3b (AlphaImager). To ensure the linearity of the signal, 20 mg and 10 mg of one sample were loaded onto each gel.
Antibodies and Reagents
Antibodies against IRS-1, phospho-IRS-1 (Ser307), and PI3 kinase p85α were purchased from Upstate Biotechnology (Lake Placid, NY), while Akt1, Akt2, and phospho-Akt (Ser473) were obtained from Cell Signaling Technology, (Beverly, MA). Antibodies against IRβ, PKCζ, and PI3K p110β were from Santa Cruz Biotechnology (Santa Cruz, CA), while GLUT4 was purchased from Abcam (Cambridge, UK). All biochemical reagents unless stated otherwise were purchased from Sigma-Aldrich, UK.
Mitochondrial ETC Activity
Mitochondrial ETC enzyme activity was measured in vastus lateralis muscle. The entire muscle was thawed and immediately homogenized on ice in 1:9 wt/vol sucrose buffer pH 8; 320 mM sucrose, 1 mM EDTA, 10 mM Trizma-base, using a kinematica status mechanical homogenizer (Kinematica, Lucerne, Switzerland) (n = 6 per group). After homogenization samples were snap frozen in liquid nitrogen and stored at −80°C. Aliquots were freeze-thawed twice to lyse cells, and enzymatic activity was assayed at 30°C on a Cary 50 spectrophotometer (Varian, Palo Alto, CA). NADH-Ubiquinone (CoQ) oxidoreductase, EC 1.6.5.3 (complex I), succinate CoQ reductase, EC 1.3.5.1 (complex II), CoQ cytochrome c reductase, EC 1.10.2.2 (complex III), cytochrome c oxidase, EC 1.9.3.1 (complex IV), linked assay succinate-cytochrome c reductase, EC 1.3.5.1 + EC 1.10.2.2 (complex II-III), and citrate synthase EC 4.1.3.7 activity was measured as described previously (45). Total protein content of the homogenate was measured using a commercially available modified Lowry Assay (Bio-Rad, Hertfordshire, UK). Citrate synthase activity was normalized to total protein to check its validity as a normalizing factor and to give an estimate of mitochondrial enrichment (19). Activities of all four complexes were standardized using citrate synthase activity (cs) to compensate for mitochondrial enrichment of the sample.
CoQ9 Analyses
CoQ was measured in vastus lateralis muscle according to Duncan et al. (15). Total CoQ was extracted from freeze thawed vastus lateralis muscle homogenate with organic-phase extraction (hexane: ethanol 5:2) by centrifugation with a known concentration of di-propoxy-CoQ10 analog internal standard (n = 6 per group). CoQ9 was detected using reverse-phase HPLC linked to UV detection (Jasco, Essex, UK). Results were calculated from a CoQ9 standard and normalized to protein and citrate synthase activity to measure total and mitochondrial CoQ content of the sample, respectively (38).
Histology
Soleus muscle was selected for histological analysis of fiber-type distribution because in mice, this muscle has almost an equal proportion of type I and type II fibers (49), whereas vastus lateralis is mainly composed of type II fibers. Therefore, changes in muscle fiber type will be more apparent in soleus muscle. Additionally, soleus muscle is relatively small, so at the midbelly point of analysis, it is possible to analyze all of the muscle fibers in one section. Muscle fiber type was identified by differential ATPase staining after incubation in acid or alkaline buffer, as described previously by Bancroft and Stevens (6). The mounted muscle group was sectioned at 10-μm thickness on a cryostat (Bright Instrument Co., Huntingdon, UK) (n = 4 per group). Sections containing the midbelly of the soleus muscle were collected and transferred to polylysine-coated microscope slides (Thermo Fisher Scientific, Leicestershire, UK) and muscle fiber type identified by differential staining in acidic buffer or alkaline buffer (47). Images were collected using a photomicroscope (Zeiss Germany) and analyzed using Scion Image software (Scion Corporation, Frederick, MD). Muscle fiber type was identified by the stability of myosin ATPase at different pH, which appears as a dark brown/black stain when viewed under a microscope. Fast-type muscle (Type II) myosin is stable at alkaline pH, whereas slow-type muscle (Type 1) is stable at acid pH. Fiber-type ratio was calculated from counts of different muscle type. Fiber density and cross-sectional area were calculated using Scion Image by tracing around perimeters of muscle fibers and calibrated with a scale bar captured at the same magnification.
Statistical Analysis
Data were analyzed from one male and one female per litter; n refers to numbers of litters per group. All data between groups were analyzed using a two-way ANOVA with sex and maternal obesity as the independent variables, followed by Duncan's post hoc test when appropriate (Statistica, Statsoft, Tulsa, OK). Insulin-signaling protein data are represented as mean percentage expression of male control offspring ± SE and mitochondrial measurements as the mean ratio of citrate synthase activity ± SE. For all data sets, a P < 0.05 was considered statistically significant.
RESULTS
Body Weight
Body weight, fat pad mass, and tissue weights have been reported previously in Samuelsson et al. (42).
Insulin-Signaling Protein Expression
IRβ and IRS-1.
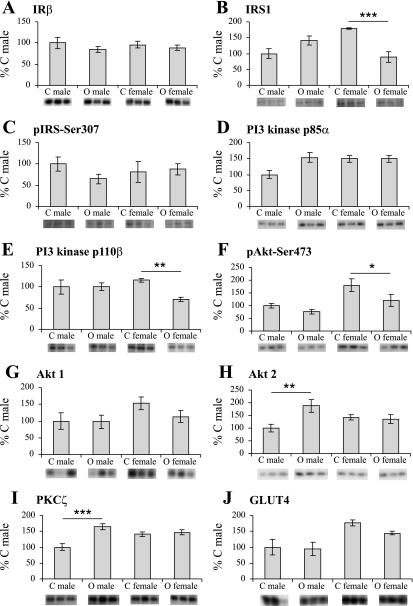
Maternal obesity and sex had no effect on the expression of the insulin receptor in offspring muscle tissue (Fig. 1A). There was also no overall statistically significant effect of maternal obesity on expression of IRS-1 (Fig. 1B). However, there was an interaction between maternal obesity and sex (P < 0.001), reflecting significantly reduced IRS-1 expression in female offspring of obese dams compared with female offspring of control dams (P < 0.001). This effect was not observed in male offspring. Phosphorylation of IRS-1 on Ser307 was not altered in either sex (Fig. 1C).
Fig. 1.
Expression and phosphorylation of key proteins involved in insulin signaling A: Insulin receptor β subunit (IRβ); B: IRS1; C: phospho-IRS-Ser307; D: P13 kinase p85α regulatory subunit; E: P13 kinase p110β catalytic subunit; F: phospho-Akt-Ser473; G: Akt1; and H: Akt2; I: PKCζ; and J: GLUT4. Bars represent mean expression/phosphorylation values for control offspring (C) and offspring of obese dams (O) ± SE, expressed as a percentage of mean male control; n = 6 per group. Images of representative blots of the respective proteins are shown below corresponding bar graphs. *P < 0.05, **P < 0.01, ***P < 0.001 compared with the same sex control.
PI3 kinase.
Maternal obesity and sex had no statistically significant effect on the expression of the PI3K p85α subunit in muscle tissue (Fig. 1D). However, there was a significant effect of maternal diet (P < 0.05) and an interaction between maternal diet and sex (P < 0.05) on the expression of the PI3K p110β subunit (Fig. 1E). This reflected a significant decrease in the expression of p110β in the female offspring of dams fed the obesogenic diet compared with female offspring of control dams (P < 0.01). No such effect was observed in males.
Akt proteins.
The level of phosphorylation of Akt on Ser 473 was higher in females than in males (effect of sex P < 0.01). Importantly, maternal obesity resulted in a significant (P = 0.05) reduction in Akt phosphorylation on Ser 473 (Fig. 1F), an effect that was more pronounced in female offspring. There was no statistically significant effect of maternal obesity or sex on expression of Akt1 (Fig. 1G). However, there was a significant effect of maternal diet (P < 0.05) and an interaction between maternal diet and sex (P < 0.05) on the expression of Akt2 (Fig. 1H). This reflected a significant increase (P < 0.01) in expression of Akt2 in male offspring of obese dams compared with male offspring of control dams. This effect was not observed in females.
PKCζ and GLUT4.
There was a significant effect of maternal diet (P < 0.001) and an interaction between maternal diet and sex (P < 0.001) on the expression of PKCζ (Fig. 1I). This reflected a significantly increased expression of PKCζ in male offspring of obese dams compared with male offspring of control dams (P < 0.001). This was not observed in female offspring. GLUT4 expression was increased in female offspring compared with male offspring (effect of sex P < 0.05) (Fig. 1J); however, there was no effect of maternal obesity on GLUT4 expression.
Mitochondrial ETC Enzyme Activity
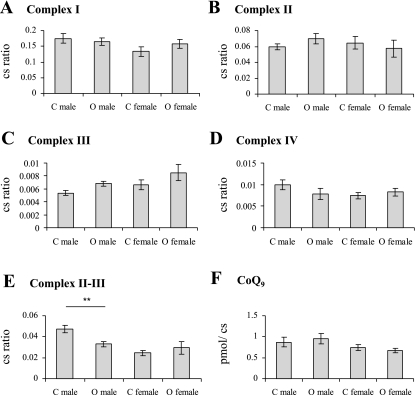
No significant effect of sex or maternal diet was observed for citrate synthase activity. Therefore, citrate synthase was used for normalization of all mitochondrial data, and results are expressed as a ratio of citrate synthase activity. Individual mitochondrial ETC enzyme activities of complex I, complex II, complex III, and complex IV normalized to citrate synthase in muscle were not significantly influenced by sex or maternal obesity (Fig. 2, A–D). However, in the linked complex II-III assay, there was a significant effect of sex (P < 0.01) and an interaction between maternal diet and sex (P < 0.05), which reflected a significant deficit in linked complex II-III activity only in the male offspring of obese dams compared with male control offspring (P < 0.01) (Fig. 2E).
Fig. 2.
Mitochondrial enzyme activities. A–D: individual mitochondrial enzyme activities. E: linked mitochondrial complex II-III enzyme activity. Bars represent mean enzyme activity normalized to citrate synthase (cs). F: mitochondrial CoQ9 content. Bars represent mean CoQ concentration normalized to citrate synthase (cs). Control offspring (C) and offspring of obese dams (O) ± SE; n = 6 per group. **P < 0.01, compared with the same sex control.
Total CoQ9 Content
No significant effect of maternal diet or sex was observed on CoQ9 levels (Fig. 2F).
Muscle Fiber Histology
Muscle fiber type ratio was not influenced by maternal diet or sex. Muscle cross-sectional area and muscle fiber density were also not different between the groups (Table 1).
Table 1.
Muscle fiber histology
|
Type I |
Type II
|
Combined
|
||||
|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | |
| Fiber type ratio | ||||||
| Control | 0.47±0.014 | 0.46±0.021 | 0.53±0.015 | 0.54±0.021 | ||
| Obese | 0.45±0.016 | 0.42±0.044 | 0.55±0.016 | 0.58±0.044 | ||
| Fiber cross-sectional area, μm2 | ||||||
| Control | 1678±122 | 1722±156 | 1681±149 | 1643±220 | 1680±135 | 1683±179 |
| Obese | 1687±15 | 1893±118 | 1442±112 | 1749±213 | 1564±50 | 1821±156 |
| Fiber density fibers, mm2 | ||||||
| Control | 243±18 | 251±30 | 285±35 | 283±27 | 528±49 | 535±56 |
| Obese | 222±19 | 235±23 | 307±38 | 290±32 | 530±28 | 526±54 |
Data are presented as the ratio of fiber type I and II relative to total fiber types in soleus muscle. Cross-sectional area is the area of the individual muscle fibers in transverse section (μm2). Fiber density is the number of fibers in a given area (mm2). Results are shown as means ± SE; n =4 for all groups.
DISCUSSION
The developmental “overnutrition hypothesis” postulates that offspring of obese mothers have a greater propensity for developing obesity, hypertension, and T2DM (11, 39). Some studies in obese women and their children support this hypothesis (21, 29); however, the effects of genetic susceptibility and shared nutritional environment of the mother and child may confound interpretation. Models such as that described here provide a tool to address the role of maternal environment in a controlled manner and so can contribute to our understanding of the developmental programming of metabolic disease.
Defects in muscle structure (33), mitochondrial function (23, 34, 50), and insulin signaling (14) have all been associated with T2DM in man. It is currently unclear which of these defects, if any, arises as a result of maternal diet-induced obesity during gestation and lactation. We hypothesized that offspring of obese dams, which we have shown previously are insulin resistant at 3 mo, would demonstrate muscle insulin signaling defects and that these might be associated with alterations in mitochondrial function and/or muscle structure. At this age, there was an effect of maternal obesity on male offspring body weight, with increases in fat pad mass and a decrease in skeletal muscle weight in both male and female offspring of obese dams (42).
The analysis of insulin-signaling proteins showed that there were no differences in the expression of the insulin receptor in either male or female offspring of obese dams compared with controls. This is consistent with the findings from human studies and animal models of T2DM showing that alterations occur downstream of the insulin receptor (14, 26, 27). Alterations in the expression and phosphorylation of key molecules downstream of the receptor were sex specific.
First, female offspring of obese dams showed a reduced expression of IRS-1. Insulin-dependent phosphorylation of IRS-1 is the initial step in the activation of PI3K. Several studies support the importance of IRS-1 in insulin signaling. siRNA-based gene silencing of IRS proteins has shown that IRS-1, rather than IRS-2, is required for insulin-stimulated Akt phosphorylation and glucose uptake (9). Also, defective insulin action, primarily in muscle, has been reported in IRS-1 knockout mice (5). Second, the p110β subunit of PI3K was also significantly reduced in the female offspring of obese dams. Third, Akt phosphorylation at Serine 473 was decreased in the female offspring of dams fed an obesogenic diet. To determine whether this reduction was caused by decreased substrate availability, we also measured the expression of Akt1 and Akt2 and observed no decrease in expression of either isoforms. Hence, female offspring of obese dams have impaired Akt phosphorylation, which could be due to impaired PI3K activity.
Paradoxically, in male offspring of obese dams, there was a significant increase in the expression of Akt2, the predominant Akt isoform in the muscle (3, 4), which would suggest improved insulin sensitivity. However, Akt phosphorylation on serine 473 tended to be decreased in this group, suggesting that Akt activity is reduced. Thus, an increase in total Akt2 levels may be acting as compensatory mechanism. PKCζ was also overexpressed in male offspring of obese dams. In a rat model of maternal high-fat feeding, 3-mo-old male offspring were shown to have increased adiposity (10) similar to that observed in our model. However, while the pattern of insulin signaling protein expression suggested hepatic insulin resistance in the offspring of the high-fat fed dams, the quadriceps muscle demonstrated a general increase in expression of insulin- signaling proteins. This model of high-fat feeding was, however, not associated with maternal obesity.
Interestingly, females had a higher expression of GLUT 4 compared with males. Because this could affect the overall rate and efficiency of glucose uptake in response to insulin (18, 41), an increased GLUT 4 expression could explain why the male but not female offspring of obese dams becomes glucose intolerant at 6 mo of age (42).
In the mitochondrial ETC activity assays, we found no effect of maternal diet on individual complex activities. Linked complex II-III activity, however, was compromised as a result of maternal obesity in male offspring only. As there was no deficiency in either of the individual complex II or III enzymes, we tested for the loss of CoQ, the essential electron carrier between complex II and III. Although our previous study on a model of undernutrition showed that a deficit in linked complex II-III was due to a loss of CoQ available to mitochondria (45), in this study, total cellular content of CoQ was not influenced by maternal diet and thus is unlikely to be a contributory factor. One possible explanation for this loss in linked activity could be that affinity for substrates in the linked complex II-III assay may be reduced, thus compromising this linked enzyme activity (30). Although we do not understand how the observed sex-specific deficit in linked complex II-III activity will affect ATP, and therefore energy production, electron transport chain uncoupling, and reactive oxygen species production in this model, others have described such sex-specific alterations in mitochondrial activity in association with aging processes (2, 53).
Alterations in muscle fiber type, size, or density have been shown to lead to alterations in glucose disposal by skeletal muscle (33, 37). While muscle fiber number is set during intrauterine development (16, 52), muscle fiber type and size can be changed during postnatal life by environmental influence, or as a compensatory mechanism for an early loss in muscle fiber number (20). Reports from “developmental programming” studies and early commercial meat production studies (7, 54) suggest that both muscle fiber type and muscle fiber number can be altered by intrauterine environment, particularly by changes in maternal diet. Indeed, a recent study in sheep has reported that maternal obesity leads to reduced myogenesis and smaller fiber diameter in fetal skeletal muscle, but it is not known if this persists into adulthood (51). We did not observe any difference in soleus muscle fiber type, size, or density, suggesting that maternal obesity does not have a persistent effect on soleus muscle fiber structure. Muscle fiber structure is therefore also unlikely to be a contributing factor to insulin signaling protein expression changes or mitochondrial abnormalities. However, this does not exclude the possibility that phenotypic alterations may occur later since diabetic and obese patients show altered muscle fiber type, loss of muscle fiber density, and cross-sectional area and therefore overall reduction in muscle mass (28). These subjects may also show a shift to type II (fast twitch) fibers (33), which may then further contribute to disease progression.
Perspectives and Significance
This study evaluated the effects of maternal obesity on programming of metabolic disease, specifically analyzing molecular defects in the insulin-signaling pathway and mitochondrial complex activities of muscle. It constitutes part of an integrated approach, which includes the analysis of these parameters in other metabolically active tissues and also considers the impact of this particular maternal insult on the programming of energy balance and implications for the development of obesity and type 2 diabetes.
We have demonstrated that maternal obesity programs sex-specific effects on the expression of the insulin-signaling proteins and mitochondrial complex II-III linked activity in 3-mo-old mice offspring. To our knowledge, this is the first description of programming of these abnormalities by maternal diet-induced obesity. The observed changes in insulin signaling and mitochondrial function may contribute to the hyperinsulinemia observed at this age and thus may represent potential targets for intervention. Further analysis is required to characterize the mechanisms underlying the effects of maternal obesity and the observed programmed reduction in mitochondrial complex activity on the development of increased offspring adiposity.
GRANTS
This study was supported, in part, by a British Heart Foundation Lectureship (to S. E. Ozanne), the Parthenon Trust (M. Martin-Gronert), Tommy Charity (to J. M. McConnell and L. Poston), King's College London Scholarship (P. Shelley) and the Biotechnology and Biological Sciences Research Council (to D. Fernandez-Twinn).
Acknowledgments
We thank Adrian Wayman and Delia Hawkes at the Metabolic Research Laboratories, Institute of Metabolic Sciences, University of Cambridge, for their expert technical assistance. We thank Phillippa Matthews from the Division of Reproduction and Endocrinology King's College London for helpful discussions.
REFERENCES
- 1.ACOG. Committee Opinion number 315, September 2005. Obesity in pregnancy. Obstet Gynecol 106: 671–675, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Ali SS, Xiong C, Lucero J, Behrens MM, Dugan LL, Quick KL. Gender differences in free radical homeostasis during aging: shorter-lived female C57BL6 mice have increased oxidative stress. Aging Cell 5: 565–574, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Altomare DA, Guo K, Cheng JQ, Sonoda G, Walsh K, Testa JR. Cloning, chromosomal localization and expression analysis of the mouse Akt2 oncogene. Oncogene 11: 1055–1060, 1995. [PubMed] [Google Scholar]
- 4.Altomare DA, Lyons GE, Mitsuuchi Y, Cheng JQ, Testa JR. Akt2 mRNA is highly expressed in embryonic brown fat and the AKT2 kinase is activated by insulin. Oncogene 16: 2407–2411, 1998. [DOI] [PubMed] [Google Scholar]
- 5.Araki E, Lipes MA, Patti ME, Bruning JC, Haag B, III, Johnson RS, Kahn CR. Alternative pathway of insulin signalling in mice with targeted disruption of the IRS-1 gene. Nature 372: 186–190, 1994. [DOI] [PubMed] [Google Scholar]
- 6.Bancroft JD, Stevens A. Theory and Practice of Histological Techniques, 2nd ed. Edinburgh, Scotland: Churchill Livingstone, 1982.
- 7.Bayol SA, Simbi BH, Stickland NC. A maternal cafeteria diet during gestation and lactation promotes adiposity and impairs skeletal muscle development and metabolism in rat offspring at weaning. J Physiol 567: 951–961, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics 115: e290–e296, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Bouzakri K, Zachrisson A, Al-Khalili L, Zhang BB, Koistinen HA, Krook A, Zierath JR. siRNA-based gene silencing reveals specialized roles of IRS-1/Akt2 and IRS-2/Akt1 in glucose and lipid metabolism in human skeletal muscle. Cell Metab 4: 89–96, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Buckley AJ, Keseru B, Briody J, Thompson M, Ozanne SE, Thompson CH. Altered body composition and metabolism in the male offspring of high fat-fed rats. Metabolism 54: 500–507, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Catalano PM Obesity and pregnancy—the propagation of a viscous cycle? J Clin Endocrinol Metab 88: 3505–3506, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Chu SY, Kim SY, Bish CL. Prepregnancy obesity prevalence in the United States, 2004–2005. Matern Child Health J doi: 10.1007/s 10995-008-0388-3, 2008. [DOI] [PubMed]
- 13.Costello PM, Rowlerson A, Astaman NA, Anthony FE, Sayer AA, Cooper C, Hanson MA, Green LR. Peri-implantation and late gestation maternal undernutrition differentially affect fetal sheep skeletal muscle development. J Physiol 586: 2371–2379, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cozzone D, Frojdo S, Disse E, Debard C, Laville M, Pirola L, Vidal H. Isoform-specific defects of insulin stimulation of Akt/protein kinase B (PKB) in skeletal muscle cells from type 2 diabetic patients. Diabetologia 51: 512–521, 2008. [DOI] [PubMed] [Google Scholar]
- 15.Duncan AJ, Heales SJ, Mills K, Eaton S, Land JM, Hargreaves IP. Determination of coenzyme Q10 status in blood mononuclear cells, skeletal muscle, and plasma by HPLC with di-propoxy-coenzyme Q10 as an internal standard. Clin Chem 51: 2380–2382, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Dwyer CM, Stickland NC. Supplementation of a restricted maternal diet with protein or carbohydrate alone prevents a reduction in fetal muscle fibre number in the guinea-pig. Br J Nutr 72: 173–180, 1994. [DOI] [PubMed] [Google Scholar]
- 17.Ferezou-Viala J, Roy AF, Serougne C, Gripois D, Parquet M, Bailleux V, Gertler A, Delplanque B, Djiane J, Riottot M, Taouis M. Long-term consequences of maternal high-fat feeding on hypothalamic leptin sensitivity and diet-induced obesity in the offspring. Am J Physiol Regul Integr Comp Physiol 293: R1056–R1062, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Gaster M, Staehr P, Beck-Nielsen H, Schrøder HD, Handberg A. GLUT4 is reduced in slow muscle fibers of type 2 diabetic patients: is insulin resistance in type 2 diabetes a slow, type 1 fiber disease? Diabetes 50: 1324–1329, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Gegg ME, Beltran B, Salas-Pino S, Bolanos JP, Clark JB, Moncada S, Heales SJ. Differential effect of nitric oxide on glutathione metabolism and mitochondrial function in astrocytes and neurones: implications for neuroprotection/neurodegeneration? J Neurochem 86: 228–237, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Harridge SD Plasticity of human skeletal muscle: gene expression to in vivo function. Exp Physiol 92: 783–797, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Harvey NC, Poole JR, Javaid MK, Dennison EM, Robinson S, Inskip HM, Godfrey KM, Cooper C, Sayer AA. Parental determinants of neonatal body composition. J Clin Endocrinol Metab 92: 523–526, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanagalingam MG, Forouhi NG, Greer IA, Sattar N. Changes in booking body mass index over a decade: retrospective analysis from a Glasgow Maternity Hospital. Brit J Gynecol 112: 1431–1433, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 51: 2944–2950, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Khan IY, Dekou V, Douglas G, Jensen R, Hanson MA, Poston L, Taylor PD. A high-fat diet during rat pregnancy or suckling induces cardiovascular dysfunction in adult offspring. Am J Physiol Regul Integr Comp Physiol 288: R127–R133, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Khan IY, Taylor PD, Dekou V, Seed PT, Lakasing L, Graham D, Dominiczak AF, Hanson MA, Poston L. Gender-linked hypertension in offspring of lard-fed pregnant rats. Hypertension 41: 168–175, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Krook A, Bjornholm M, Galuska D, Jiang XJ, Fahlman R, Myers MG Jr, Wallberg-Henriksson H, Zierath JR. Characterization of signal transduction and glucose transport in skeletal muscle from type 2 diabetic patients. Diabetes 49: 284–292, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Krook A, Kawano Y, Song XM, Efendic S, Roth RA, Wallberg-Henriksson H, Zierath JR. Improved glucose tolerance restores insulin-stimulated Akt kinase activity and glucose transport in skeletal muscle from diabetic Goto-Kakizaki rats. Diabetes 46: 2110–2114, 1997. [DOI] [PubMed] [Google Scholar]
- 28.Krotkiewski M, Seidell JC, Bjorntorp P. Glucose tolerance and hyperinsulinaemia in obese women: role of adipose tissue distribution, muscle fibre characteristics and androgens. J Intern Med 228: 385–392, 1990. [DOI] [PubMed] [Google Scholar]
- 29.Lawlor DA, Cooper AR, Bain C, Davey SG, Irwin A, Riddoch C, Ness A. Associations of birth size and duration of breast feeding with cardiorespiratory fitness in childhood: findings from the Avon Longitudinal Study of Parents and Children (ALSPAC). Eur J Epidemiol 23: 411–422, 2008. [DOI] [PubMed] [Google Scholar]
- 30.Liu J, Atamna H, Kuratsune H, Ames BN. Delaying brain mitochondrial decay and aging with mitochondrial antioxidants and metabolites. Ann N Y Acad Sci 959: 133–166, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science 307: 384–387, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Mallinson JE, Sculley DV, Craigon J, Plant R, Langley-Evans SC, Brameld JM. Fetal exposure to a maternal low-protein diet during mid-gestation results in muscle-specific effects on fibre type composition in young rats. Br J Nutr 98: 292–299, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marin P, Andersson B, Krotkiewski M, Bjorntorp P. Muscle fiber composition and capillary density in women and men with NIDDM. Diabetes Care 17: 382–386, 1994. [DOI] [PubMed] [Google Scholar]
- 34.Morino K, Petersen KF, Dufour S, Befroy D, Frattini J, Shatzkes N, Neschen S, White MF, Bilz S, Sono S, Pypaert M, Shulman GI. Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J Clin Invest 115: 3587–3593, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Napoli C, de Nigris F, Welch JS, Calara FB, Stuart RO, Glass CK, Palinski W. Maternal hypercholesterolemia during pregnancy promotes early atherogenesis in LDL receptor-deficient mice and alters aortic gene expression determined by microarray. Circulation 105: 1360–1367, 2002. [DOI] [PubMed] [Google Scholar]
- 36.Niviot P, Morens C, van Assche FA, Jansen EH, Poston L, Remacle C, Reusens B. Established diet induced obesity (DIO) in female rats leads to offspring hyperphagia. Diabetologia 52: 1133–42, 2009. [DOI] [PubMed] [Google Scholar]
- 37.Oberbach A, Bossenz Y, Lehmann S, Niebauer J, Adams V, Paschke R, Schon MR, Bluher M, Punkt K. Altered fiber distribution and fiber-specific glycolytic and oxidative enzyme activity in skeletal muscle of patients with type 2 diabetes. Diabetes Care 29: 895–900, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Okamoto T, Fukui K, Nakamoto M, Kishi T, Okishio T, Yamagami T, Kanamori N, Kishi H, Hiraoka E. High-performance liquid chromatography of coenzyme Q-related compounds and its application to biological materials. J Chromatogr 342: 35–46, 1985. [DOI] [PubMed] [Google Scholar]
- 39.Oken E, Gillman MW. Fetal origins of obesity. Obes Res 11: 496–506, 2003. [DOI] [PubMed] [Google Scholar]
- 40.Ozanne SE, Jensen CB, Tingey KJ, Storgaard H, Madsbad S, Vaag AA. Low birthweight is associated with specific changes in muscle insulin-signalling protein expression. Diabetologia 48: 547–542, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Petersen KF, Shulman GI. Etiology of insulin resistance. Am J Med 119: S10–S16, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Samuelsson AM, Matthews PA, Argenton M, Christie MR, McConnell JM, Jansen EH, Piersma AH, Ozanne SE, Twinn DF, Remacle C, Rowlerson A, Poston L, Taylor PD. Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance: a novel murine model of developmental programming. Hypertension 51: 383–392, 2008. [DOI] [PubMed] [Google Scholar]
- 43.Sebire NJ, Jolly M, Harris JP, Wadsworth J, Joffe M, Beard RW, Regan L, Robinson S. Maternal obesity and pregnancy outcome: a study of 287,213 pregnancies in London. Int J Obes Relat Metab Disord 25: 1175–1182, 2001. [DOI] [PubMed] [Google Scholar]
- 44.Selak MA, Storey BT, Peterside I, Simmons RA. Impaired oxidative phosphorylation in skeletal muscle of intrauterine growth-retarded rats. Am J Physiol Endocrinol Metab 285: E130–E137, 2003. [DOI] [PubMed] [Google Scholar]
- 45.Shelley P, Tarry-Adkins J, Martin-Gronert M, Poston L, Heales S, Clark J, Ozanne S, McConnell J. Rapid neonatal weight gain in rats results in a renal ubiquinone (CoQ) deficiency associated with premature death. Mech Ageing Dev 128: 681–687, 2007. [DOI] [PubMed] [Google Scholar]
- 46.Simmons RA, Suponitsky-Kroyter I, Selak MA. Progressive accumulation of mitochondrial DNA mutations and decline in mitochondrial function lead to beta-cell failure. J Biol Chem 280: 28785–28791, 2005. [DOI] [PubMed] [Google Scholar]
- 47.Snow DH, Billeter R, Mascarello F, Carpene E, Rowlerson A, Jenny E. No classical type IIB fibres in dog skeletal muscle. Histochemistry 75: 53–65, 1982. [DOI] [PubMed] [Google Scholar]
- 48.Taylor PD, McConnell J, Khan IY, Holemans K, Lawrence KM, Asare-Anane H, Persaud SJ, Jones PM, Petrie L, Hanson MA, Poston L. Impaired glucose homeostasis and mitochondrial abnormalities in offspring of rats fed a fat-rich diet in pregnancy. Am J Physiol Regul Integr Comp Physiol 288: R134–R139, 2005. [DOI] [PubMed] [Google Scholar]
- 49.Timson BF, Bowlin BK, Dudenhoeffer GA, George JB. Fiber number, area and composition of mouse soleus muscle following enlargement. J Appl Physiol 58: 619–624, 1985. [DOI] [PubMed] [Google Scholar]
- 50.Toledo FGS, Kelley DE. Mitochondrial dysfunction in the pathogenesis of insulin resistance associated with obesity, diabetes, and aging. Curr Opin Endocrinol Metab 12: 157–162, 2005. [Google Scholar]
- 51.Tong J, Yang X, Zhu MJ, Ford SP, Nathanielsz P, Du M. Maternal obesity down-regulates myogenesis and β-catenin signaling in fetal skeletal muscle. Am J Physiol Endocrinol Metab 296: E917–E924, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wigmore PM, Stickland NC. Muscle development in large and small pig fetuses. J Anat 137: 235–245, 1983. [PMC free article] [PubMed] [Google Scholar]
- 53.Yan L, Ge H, Li H, Lieber SC, Natividad F, Resuello RR, Kim SJ, Akeju S, Sun A, Loo K, Peppas AP, Rossi F, Lewandowski ED, Thomas AP, Vatner SF, Vatner DE. Gender-specific proteomic alterations in glycolytic and mitochondrial pathways in aging monkey hearts. J Mol Cell Cardiol 37: 921–929, 2004. [DOI] [PubMed] [Google Scholar]
- 54.Zhu MJ, Ford SP, Means WJ, Hess BW, Nathanielsz PW, Du M. Maternal nutrient restriction affects properties of skeletal muscle in offspring. J Physiol 575: 241–250, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]