Abstract
The purpose of this study was to determine the long-term impact of obesity and related metabolic abnormalities in the absence and presence of hypertension on renal injury and salt-sensitivity of blood pressure. Markers of renal injury and blood pressure salt sensitivity were assessed in 52- to 55-wk-old normotensive melanocortin-4 receptor-deficient (MC4R−/−) mice and lean C57BL/6J wild-type (WT) mice and in 22-wk-old MC4R−/− and WT mice made hypertensive by NG-nitro-l-arginine methyl ester (l-NAME) in the drinking water for 8 wk. Old MC4R−/− mice were 60% heavier, hyperinsulinemic, and hyperleptinemic but had similar mean arterial pressure (MAP) as WT mice (115 ± 2 and 117 ± 2 mmHg) on normal salt diet (0.4% NaCl). A high-salt diet (4.0% NaCl) for 12 days did not raise MAP in obese or lean mice [ΔMAP: MC4R (−/−) 4 ± 2 mmHg; WT, 2 ± 1 mmHg]. Obese MC4R−/− mice had 23% greater glomerular tuft area and moderately increased GFR compared with WT mice. Bowman's space, total glomerular area, mesangial matrix, urinary albumin excretion (UAE), renal TGF-β and collagen expression were not significantly different between old MC4R−/− and WT mice. Renal lipid content was greater but renal macrophage count was markedly lower in MC4R−/− than WT mice. Mild increases in MAP during l-NAME treatment (∼16 mmHg) caused small, but greater, elevations in UAE, renal TGF-β content, and macrophage infiltration in MC4R−/− compared with WT mice without significant changes in glomerular structure. Thus despite long-term obesity and multiple metabolic abnormalities, MC4R−/− mice have no evidence of renal injury or salt-sensitivity of blood pressure. These observations suggest that elevations in blood pressure may be necessary for obesity and related metabolic abnormalities to cause major renal injury or that MC4R−/− mice are protected from renal injury by mechanisms that are still unclear.
Keywords: metabolic syndrome, renal injury, salt sensitivity
obesity per se has not been considered a major contributor of renal disease until recent years, although diabetes mellitus and hypertension, two major consequences of obesity, account for > 70% of end-stage renal disease. Multiple observational studies suggest that the obesity epidemic is associated with a parallel increase in the incidence of kidney disease (11, 13, 21). Moreover, population studies demonstrate a strong correlation between obesity and renal disease even after adjustment for hypertension, diabetes, or preexisting renal disease (9, 16).
Obesity is associated with several functional and structural alterations in the kidney that resemble those seen in diabetic nephropathy. Previous studies, including those from our laboratory in obese dogs, demonstrate that even early in the course of obesity, there is significant glomerular hyperfiltration, glomerular basement membrane thickening, mesangial matrix expansion, and enlargement of the Bowman's capsule (15). These early changes may be precursors to more severe injury, such as focal and segmental glomerulosclerosis, which often occur with prolonged obesity (14, 20).
Although the association between obesity and kidney disease has been previously established, the pathogenic mechanisms involved are still unclear. Multiple metabolic and hemodynamic factors have been proposed to contribute to obesity-associated nephropathy. Some of these factors include insulin resistance, hyperinsulinemia, hyperglycemia, hyperleptinemia, abnormal lipid metabolism, oxidative stress, angiotensin II, inflammation, increased arterial pressure, and glomerular hypertension (1, 2, 5, 7, 8, 10, 27, 41). However, the relative importance of the hemodynamic and the metabolic factors in causing renal injury in obesity has not been determined, mainly because it has been difficult to separate the hemodynamic and metabolic effects of obesity on the kidneys.
Melanocortin-4 receptor-deficient (MC4R−/−) mice develop early-onset obesity because MC4R activation normally suppresses appetite and increases energy expenditure (17). MC4R−/− mice are overweight compared with wild-type (WT) mice as early as 5–6 wk of age (35). We found that at 22 wk of age, MC4R−/− mice exhibited many characteristics of the metabolic syndrome including visceral obesity, insulin resistance, hyperinsulinemia, hyperglycemia, and hyperlipidemia. However, chronic monitoring of blood pressure using telemetry in these mice revealed that they were not hypertensive despite other characteristics of the metabolic syndrome (35). The lack of hypertension in the MC4R−/− mouse may be due to impaired sympathetic nervous system activation that normally mediates obesity-induced hypertension (34, 35). Therefore, the MC4R−/− mouse provide an opportunity to study the impact of obesity and metabolic abnormalities per se on renal injury and salt-sensitivity of blood pressure in the absence of hypertension.
The objective of this study was to investigate the impact of prolonged obesity on kidney function and structure in the absence of hypertension. We also investigated whether obesity and metabolic abnormalities in MC4R−/− mice exacerbate the effects of hypertension on renal function and structure compared with lean WT mice. We therefore studied young (20–22 wk) and old (52–55 wk) MC4R−/− mice to allow prolonged exposure of the kidneys to the metabolic disturbances associated with obesity. Histopathological and molecular markers of renal injury and salt-sensitivity of blood pressure that normally occur with renal damage were assessed as measures of renal structure and function in the MC4R−/− and in control WT mice.
METHODS
All experimental procedures conform to the National Institute of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee, University of Mississippi Medical Center. MC4R−/− mice were generated as previously described from MC4R-deficient breeding pairs obtained from Dr. Roger Cone, Oregon Health Sciences University (34). The mice received food and water ad libitum throughout the study and were placed on a 12:12-h light-dark cycle.
Experimental Protocol for Studies in 52- to 55-wk-old Mice
The 52- to 55-wk-old MC4R−/− mice and age-matched C57BL/6J WT mice (n = 6 per group) were implanted with telemetric pressure transmitters as previously described (model TA11PAC20; Data Sciences International) (35). Ten days after surgery we began monitoring mean arterial pressure (MAP) and heart rate (HR). C57BL/6J mice were used as controls since the MC4R−/− mice were bred into the C57Bl/6J background for more than 10 generations. After 5 days of stable control measurements on a normal salt diet (0.4% NaCl Test Diet), mice were placed on a high-salt diet (4% NaCl diet Test Diet) for 12 days followed by a return to 3 days of normal salt diet for postcontrol measurements. Then 24-h MAP, HR, and food intake were recorded daily.
At the end of the study, mice were fasted for 6–7 h and anesthetized with isoflurane, and blood samples (200 μl) were collected by cardiac puncture to determine plasma glucose, insulin, and leptin concentrations. Mice were then transcardially perfused with 0.1% PBS, and kidneys and visceral fat (epididymal and retroperitoneal) were collected and weighed. One kidney was frozen in liquid nitrogen for renal transforming growth factor-β (TGF-β) and collagen measurements, and the other kidney was fixed in 10% buffered formalin for histological assessment of glomerular morphology, mesangial matrix, renal lipids, and macrophages. Urinary albumin excretion (UAE) rate and glomerular filtration rate (GFR) were measured in a separate group of 52- to 55-wk-old mice.
Experimental Protocol in 20- to 22-wk-old MC4R−/− and WT Mice
Since the duration of obesity is also a factor that could cause renal changes, we studied younger 20- to 22-wk-old obese MC4R−/− (n = 7) and lean WT mice (n = 8). We assessed several markers of renal injury including UAE, mesangial matrix, and glomerular collagen in the younger mice. Kidney and visceral fat pad weights were also obtained in these mice.
NG-nitro-l-arginine methyl ester Hypertension in 20- to 22-wk-old MC4R−/− and WT Mice
To induce chronic hypertension, NG-nitro-l-arginine methyl ester (l-NAME) (Sigma, St. Louis, MO) was administered to mice at the age of 20–22 wk at a dose of 120 mg·kg−1·day−1 in the drinking water for eight consecutive weeks. MAP and HR were measured by telemetry for three consecutive days during the control period and every 2 wk until the end of the 8th wk. Twenty-four-hour urine samples were collected for three consecutive days during the control period and at the end of the 8th wk of l-NAME treatment for determination of UAE. At the end of the l-NAME treatment, mice were killed and renal tissue was harvested to measure TGF-β in whole kidney and for morphological and histological studies.
UAE and GFR Measurements
Mice were acclimatized for several days in metabolic cages with ad libitum access to food and water. Twenty-four-hour urine samples were collected on three consecutive days for UAE analysis using an ELISA kit (Exocell). The older mice were then prepared for GFR measurements as described previously (n = 6 per group) (34). Briefly, the femoral artery and vein were cannulated for blood collection and infusions, respectively. Four to five hours after surgery, mice were transferred to metabolic cages and maintained on a constant infusion of saline to maintain sodium intake at normal levels (∼ 462 μeq/day) with ad libitum access to sodium-deficient diet and water. The arterial catheter was flushed daily with heparin to prevent clotting. Approximately 7 days after surgery, GFR was measured using a 6- to 7-h fasted plasma sample following a 24-h iv infusion of [125I]iothalamate as previously described (34).
Histological Methods
A coronal cross section containing the hilus of the kidney was removed, fixed in formalin, and embedded in paraffin. Sections (5-μm thick) were mounted on glass slides and stained with hematoxylin and eosin, Methanamine Silver, Picrosirius Red, or Oil Red O. All histological measurements were made by an observer who was blinded to the experimental protocols. Only glomeruli containing visible afferent and efferent arteriolar stalks were used to ensure that all glomeruli were measured in the same plane. Glomerular images at ×40 magnification were digitized using a color video camera attached to a Leica microscope. After digitization, Bowman's space and the glomerular tuft areas in the hematoxylin and eosin-stained sections, mesangial matrix area stained with methanamine silver, and glomerular collagen area stained with Picrosirius Red were traced and measured using Imagepro analysis software. The total area for each glomerulus was calculated as the sum of glomerular tuft and Bowman's space areas. Thirty glomeruli were measured per kidney per animal for estimating glomerular morphology, and 20 glomeruli were measured for mesangial matrix and renal collagen assessment. Mesangial matrix and glomerular collagen areas were normalized to the glomerular tuft area.
For estimating renal lipid accumulation, the area of Oil Red O stain on each image was measured using Imagepro (Universal Imaging). Forty fields, including 10 each of cortical and medullary, and 20 glomerular areas per kidney per animal were analyzed. The total tissue area per field was approximately equal to the total area of the field.
For immunohistochemical analyses of macrophages, sections were deparaffinized and rehydrated, and antigen retrieval was performed by incubating sections in trypsin at 37°C for 20 min. Immunohistochemistry for the macrophage antigen was performed using the Vectastain Elite ABC kit (Vector Laboratories). Sections were incubated in primary antibody (anti-mouse macrophage F4/80 antigen; Serotec, Oxford, UK) at 1:10 dilution in normal rabbit serum overnight. Slides that were simultaneously processed without primary antibody served as negative controls. The area of stained macrophages was measured using Imagepro. Forty fields, including 10 each of cortical and medullary, and 20 glomerular areas per kidney per animal were analyzed. The macrophage area was normalized to the total kidney area measured.
Analytical Methods
Plasma insulin and leptin were determined by ELISA (Linco Insulin ELISA; R&D Leptin ELISA). Plasma glucose was determined by the glucose oxidation method (Beckman Glucose Analyzer 2). Kidney collagen was assessed with the Sircoll assay (Biocolor). Kidney TGF-β was measured by ELISA (Pierce).
Statistical Methods
The data are expressed as means ± SE. Data obtained on a daily basis were analyzed using ANOVA with repeated measures. Comparisons between groups were performed using one-way ANOVA followed by the Bonferroni's post hoc test or Student's t-test where appropriate. Statistical significance was accepted at a level of P < 0.05.
RESULTS
Anthropometric and Metabolic Data
The average body weight of MC4R−/− mice at both age groups was 60% greater compared with age-matched WT mice (Table 1). Parallel to the increase in body weight, MC4R−/− mice had severalfold greater visceral fat compared with WT mice. WT and MC4R−/− mice at 52–55 wk of age were significantly heavier and had greater visceral fat accumulation than at 22 wk of age (Table 1).
Table 1.
Anthropometric and renal characteristics of 20- to 22-wk-old and 52- to 55-wk-old lean wild-type (WT) and obese melanocortin-4 receptor-deficient (MC4R−/−) mice
| Parameters |
20–22 wk old |
52–55 wk old
|
||
|---|---|---|---|---|
| WT | MC4R | WT | MC4R | |
| Food intake, g | 3.5±0.4 | 5.0±0.3* | 4.0±0.2 | 5.8±0.3* |
| Body weight, g | 29±1 | 48±2* | 39±1 | 63±1* |
| Plasma glucose, mM | 9±0.3 | 11±0.2 | 15±0.5 | 14±1.5 |
| Plasma leptin, ng/ml | 3±1 | 47±13* | 18±4 | 82±11* |
| Plasma insulin, ng/ml | 1.33±0.58 | 4.80±1.88* | 0.72±0.12 | 2.4±0.68* |
| Total kidney weight, g | 0.33±0.02 | 0.39±0.02* | 0.46±0.01 | 0.54±0.02* |
| Visceral pad fat | 0.4±0.1 | 3.7±0.2 | 2.1±0.2 | 4.3±0.2* |
Values are means ± SE; n = 6–7 per group.
P < 0.05 compared with WT mice.
Much of the increase in body weight in the 52- to 55-wk-old MC4R−/− compared with WT mice may have been caused by increased food intake. Daily food intake was ∼45% greater in 52- to 55-wk-old MC4R−/− compared with WT mice (Table 1), and it was not significantly altered by high-salt diet (5.6 ± 0.1 and 3.6 ± 0.1 g, respectively). Plasma leptin and insulin levels were severalfold greater in the old MC4R−/− than in WT mice. However, plasma glucose levels were not significantly different between old MC4R−/− and WT mice at 22 and 52–55 wk of age. Plasma glucose levels in WT mice at 52–55 wk were significantly greater than at 22 wk of age as observed in our previous studies, suggesting that aging contributed to insulin resistance even in WT mice (34).
Arterial Pressure and HR in 52- to 55-wk-old Mice
Normal salt diet.
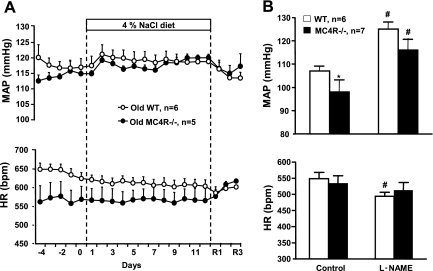
Despite 60% greater body weight in the old MC4R−/− mice, 24-h average MAP in MC4R−/− mice (115 ± 2 mmHg) was not significantly different from that in WT mice (117 ± 2 mmHg) during normal salt diet (Fig. 1A). Similarly, day- and night-time average MAP were not different between MC4R−/− (day: 111 ± 2; night: 121 ± 3 mmHg) and WT mice (day: 113 ± 3; night: 121 ± 2 mmHg). Basal 24-h average HR and day- and night-time HR during normal salt diet in MC4R−/− mice (24-h: 568 ± 36; day: 559 ± 29; night: 576 ± 43 beats/min) were significantly lower than in WT mice (24-h: 635 ± 12; day: 623 ± 10; night: 648 ± 15 beats/min, Fig. 1A).
Fig. 1.
A: 24-h telemetry mean arterial pressure (MAP) and heart rate (HR) in 52- to 55-wk-old wild-type (WT) and melanocortin-4 receptor-deficient (MC4R−/−) mice fed normal salt diet or high-salt diet (n = 5–6 per group). R1-R3 indicates days 1 and 3, respectively, of the recovery period. B: 24-h telemetry MAP and HR in 20- to 22-wk-old WT and MC4R−/− mice during control period and on week 8 of NG-nitro-l-arginine methyl ester (l-NAME) treatment. *P < 0.05 compared with WT mice; #P < 0.05 compared with control values.
High-salt diet.
High-salt diet (4% NaCl) feeding for 12 days did not significantly alter 24-h MAP or HR in MC4R−/− or WT mice (average ΔMAP: MC4R−/−, 4 ± 2 mmHg; WT, 2 ± 1 mmHg, Fig. 1A). Consistent with this observation, the high-salt diet also did not significantly alter day- or night-time MAP and HR in either group of mice.
Renal Function, Molecular and Histological Data in 52- to 55-wk-old Mice
UAE and GFR.
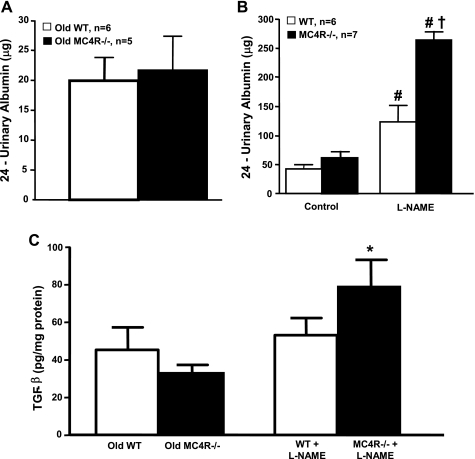
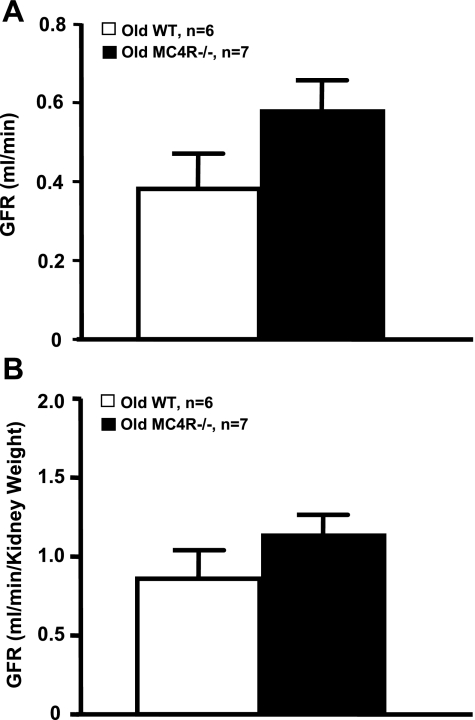
Twenty-four-hour UAE, an early marker of glomerular injury was not significantly different between the 52- to 55-wk-old MC4R−/− and WT mice (Fig. 2A). Another major functional change that usually accompanies obesity is glomerular hyperfiltration. GFR was only moderately elevated in the normotensive obese MC4R−/− group compared with WT mice (Fig. 3A), and the changes were not statistically significant. Even when expressed per gram kidney weight, GFR was not significantly different in MC4R−/− compared with WT mice (Fig. 3B).
Fig. 2.
A: 24-h urinary albumin excretion (UAE) in conscious 52- to 55-wk-old WT and MC4R−/− mice. B: 24-h UAE in 20- to 22-wk-old WT and MC4R−/− mice during control period and after 8 wk of l-NAME treatment. C: transforming grown factor-β (TGF-β) in whole kidney lysates of 52- to 55-wk-old WT and MC4R−/− and in 20- to 22-wk-old WT and MC4R−/− mice after 8 wk of l-NAME treatment. #P < 0.05 compared with control values; *P < 0.05 compared with 52- to 55-wk-old MC4R−/−; †P < 0.05 compared with WT mice treated with l-NAME.
Fig. 3.
A: glomerular filtration rate (GFR) in conscious 52- to 55-wk-old WT and MC4R−/− mice. B: GFR in 52- to 55-wk-old WT and MC4R−/− mice normalized by kidney weight.
Kidney Morphology, Histological, and Molecular Data
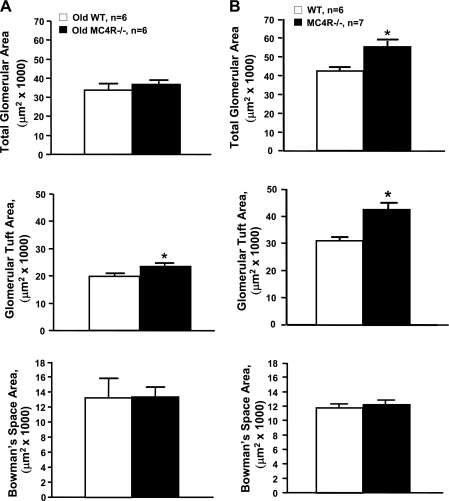
Obese MC4R−/− mice had significantly greater kidney weights compared with lean WT mice (Table 1) and also exhibited slight glomerular hypertrophy. The glomerular tuft area was 23% greater in MC4R−/− than in WT mice. Bowman's space and total glomerular areas, however, were not significantly different between the two groups (Fig. 4A).
Fig. 4.
Glomerular morphology in normotensive 52- to 55-wk-old WT and MC4R−/− mice (A) and in hypertensive 20- to 22-wk-old WT and MC4R−/− mice (B) after 8 wk of l-NAME treatment. *P < 0.05 compared with WT mice.
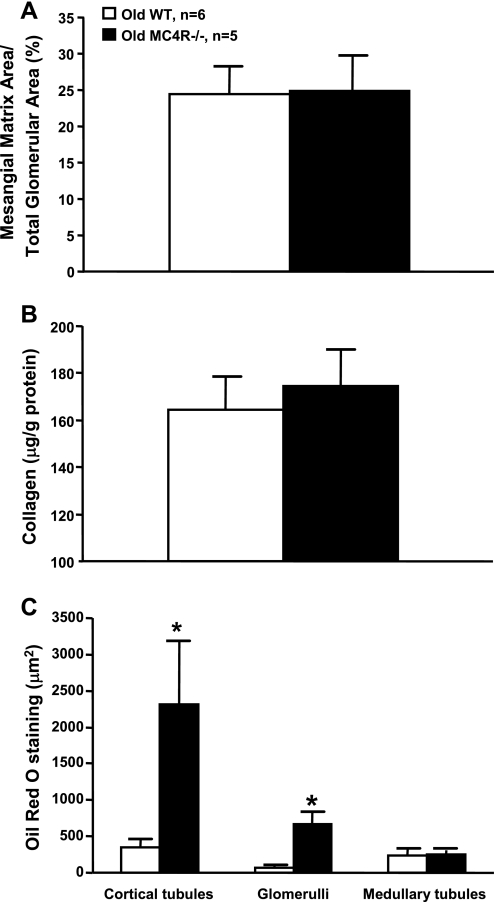
Renal mesangial matrix accumulation, a marker of glomerulosclerosis, was assessed by methanamine silver staining. After analyzing 20 glomeruli per kidney per animal we found no significant differences in mesangial matrix accumulation between MC4R−/− and WT mice (Fig. 5A). Consistent with this observation, there were no significant differences in total collagen extracted from the kidneys of both groups (Fig. 5B).
Fig. 5.
Mesangial matrix (A), whole kidney collagen (B), and renal Oil Red O staining (marker of lipid accumulation) (C) in 52- to 55-wk-old WT and MC4R−/− mice. *P < 0.05 compared with WT mice.
To determine whether prolonged obesity in MC4R−/− mice increased renal lipid accumulation, we performed Oil Red O staining of kidney sections. Histological analysis of these sections revealed increased accumulation of neutral lipids in the glomeruli and cortical tubules in MC4R−/− mice compared with WT mice. However, we found no significant differences in lipid accumulation in the medullary tubules between groups (Fig. 5C).
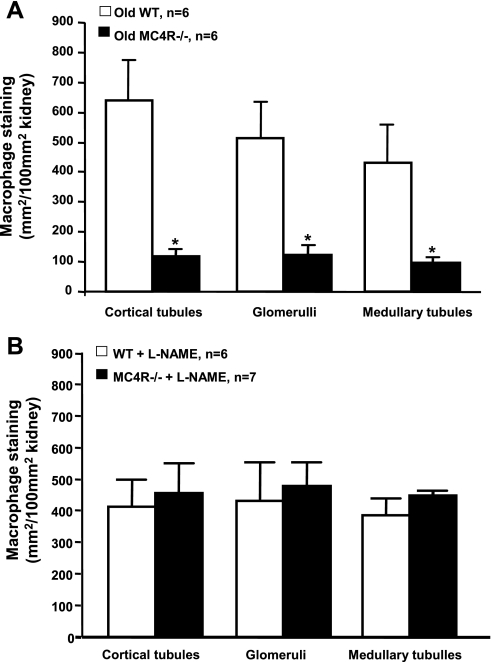
Furthermore, whole kidney expression of TGF-β protein, which is implicated in renal fibrosis by increasing extracellular matrix deposition, was not significantly different between obese MC4R−/− and control WT mice (Fig. 2C). We also evaluated renal macrophage infiltration, which has been proposed to play a major role in lipid-induced renal injury and diabetic nephropathy. Surprisingly, MC4R−/− mice had reduced macrophage infiltration, compared with WT mice, in all measured areas of the kidneys including cortical and medullary tubules and the glomeruli (Fig. 6A).
Fig. 6.
Renal macrophage accumulation in 52- to 55-wk-old WT and MC4R−/− mice (A) and in 20- to 22-wk-old WT and MC4R−/− mice (B) after 8 wk of l-NAME treatment. *P < 0.05 compared with WT mice.
Renal Histological and Molecular Data in 20- to 22-wk-old Mice
Observations in the 20- to 22-wk-old mice were similar to that in the older 52- to 55-wk-old mice (Table 1). Kidney weights were significantly greater in the MC4R−/− compared with WT mice. We found no significant differences in UAE (17 ± 2 vs. 15 ± 2 μg/24-h) mesangial matrix expansion (33 ± 2 vs. 30 ± 1%) or glomerular collagen expression (35 ± 4 vs. 31 ± 4%) between MC4R−/− and WT mice. A noteworthy finding from our previous studies in 20- to 22-wk-old MC4R−/− mice is that they also had no salt-sensitivity of blood pressure despite obesity and associated metabolic abnormalities (35).
Antropometric Data and Tissue Weight in 20- to 22-wk-old WT and MC4R−/− Mice Treated with l-NAME
The average body weight of MC4R−/− mice, at the end of l-NAME treatment, was 54% greater compared with age-matched WT mice (Table 2). Food intake was 58% higher in MC4R−/− compared with WT mice, and MC4R−/− mice had threefold greater epididymal fat compared with WT mice. Heart and kidney weights were also higher in MC4R−/− compared with WT mice (Table 2).
Table 2.
Body weight, food intake, heart, kidney, and epididymal fat weights in 20- to 22-wk-old WT and MC4R−/− mice treated with NG-nitro-l-arginine methyl ester
| Parameters | WT | MC4R−/− |
|---|---|---|
| Body weight | 29±1 | 45±3* |
| Food Intake | 3.8±0.2 | 6.0±0.2* |
| Heart | 0.16±0.01 | 0.23±0.01* |
| Total Kidney weight | 0.36±0.01 | 0.48±0.02* |
| Epididymal fat | 0.38±0.06 | 1.27±0.21* |
Data are means ± SE in grams; n = 6–7/group;
P < 0.05 compared to WT mice.
Arterial Pressure and HR in 20- to 22-wk-old WT and MC4R−/− Mice Treated with l-NAME
Twenty-four-hour average baseline MAP before beginning l-NAME treatment was significantly lower in MC4R−/− (98 ± 7 mmHg) than in and WT mice (107 ± 2 mmHg, Fig. 1A). l-NAME treatment caused similar increases in MAP (∼16 mmHg) in both groups. Baseline 24-h average HR in MC4R−/− mice (532 ± 24 beats/min) was not different from HR in WT mice (554 ± 20 beats/min). l-NAME treatment caused a small but significant reduction in HR in WT mice, while HR in MC4R−/− mice remained unchanged (Fig. 1A).
Renal Histological and Molecular Data in 20- to 22-wk-old WT and MC4R−/− Mice Treated with l-NAME
Baseline 24-h UAE was not significantly different between MC4R−/− and WT mice (Fig. 2B). l-NAME treatment caused a significant elevation in UAE in both groups; however, UAE increased to a greater extent in MC4R−/−. Despite the increase in UAE caused by l-NAME, TGF-β protein levels in whole kidney lysates were only slightly, but not significantly, increased in MC4R−/− compared with WT mice (Fig. 2C). No significant differences were observed in renal macrophage infiltration in MC4R−/− compared with WT mice for all measured areas of the kidney, including cortical and medullary tubules and glomeruli (Fig. 5B).
Renal Histological Data in 20- to 22-wk-old WT and MC4R−/− mice Treated with l-NAME
Kidney weight was 33% greater in the MC4R −/− compared with WT mice (Table 2) and also exhibited slight glomerular hypertrophy. Total glomerular area and glomerular tuft area were 29% and 37% larger, respectively, in MC4R−/− compared with WT mice. Bowman's space area, however, was not different between the groups (Fig. 4B).
DISCUSSION
The key finding of this study is that normotensive 52- to 55-wk-old MC4R−/− mice did not develop significant renal injury despite obesity and prolonged exposure to metabolic disturbances such as insulin resistance, hyperinsulinemia, hyperglycemia, hyperlipidemia, and hyperleptinemia, factors that are considered by many investigators to play a major role in the etiology of obesity-associated nephropathy (1, 2, 7, 8, 10, 41). Our observations suggest that obesity and associated metabolic abnormalities may not be a major cause of severe renal disease in the absence of hypertension. Elevations in arterial pressure may be necessary for obesity and related metabolic abnormalities to cause major renal injury.
Although a variety of animal models have been used to investigate obesity-induced renal injury, few studies have attempted to assess the importance of hemodynamic vs. metabolic factors. The obese MC4R−/− mouse is an attractive model to study this issue for several reasons. First, the 52- to 55-wk-old MC4R−/− mouse is a late middle-aged mouse (i.e., the average life span of a mouse is 1.5 to 2 yr) that has been obese for its entire life; previous studies, including those from our lab, indicated that the MC4R−/− mouse has early-onset obesity (17, 35). Second, 52- to 55-wk-old obese MC4R−/− mice have many of the metabolic abnormalities, such as insulin resistance, hyperinsulinemia, hyperleptinemia, and dyslipidemia, which have been suggested to cause renal injury (1, 7, 8, 10, 40). Third, despite metabolic abnormalities and obesity, MC4R−/− mice have normal arterial pressures as assessed by telemetry. Our previous studies suggest that this normotensive state, despite severe obesity, may be due to an impaired sympathetic nervous system activation response to obesity (14, 35). Visceral obesity usually increases adipocyte secretion of leptin, which, in turn, activates hypothalamic proopiomelanocortin neurons causing release of alpha-melanocyte-stimulating hormone (α-MSH), activation of MC4R, increased sympathetic nervous system activity and increased blood pressure (14, 34). Thus, the MC4R may be a key mechanism linking visceral obesity and hypertension (14).
A comparison of the metabolic characteristics of MC4R−/− mice at 52–55 wk of age with those of younger MC4R−/− mice in our previous studies clearly depicts a progression of several features of the metabolic syndrome with age (34). Most of the metabolic changes in the MC4R−/− mice start as early as 8–9 wk of age (17, 34, 35). MC4R−/− mice at 52–55 wk of age are significantly heavier (∼30–50%) compared with 20- to 22-wk-old MC4R−/− mice (34) (Table 1). The weight gain in 52- to 55-wk-old mice was reflected in a doubling of circulating levels of leptin compared with younger MC4R−/− mice (35). Insulin resistance also worsened with age as depicted by significantly greater plasma insulin levels in the 52- to 55-wk-old MC4R−/− mice compared with younger MC4R−/− mice (34). Old WT mice also exhibited metabolic changes such as increases in body weight, plasma glucose, insulin, and leptin levels compared with younger WT mice, suggesting the development of insulin resistance. However, these metabolic changes were much milder than those observed in the MC4−/− mice.
Apart from systemic metabolic changes, the kidneys in old MC4R−/− mice were also exposed to local metabolic changes, such as increased lipid accumulation as assessed by Oil Red O staining. Previous studies have provided evidence that accumulation of lipids in tissues, such as the heart, pancreas, and liver, is associated with organ dysfunction (36, 37). The results from the present study suggest that lipid accumulation in the kidneys of MC4R−/− mice in the absence of increased blood pressure does not appear to cause major organ dysfunction. Whether renal lipid accumulation may exacerbate the detrimental effects of more severe, prolonged increases in blood pressure is unclear and is an important area for further investigation.
We estimated the impact of obesity and associated metabolic abnormalities on renal function, by measuring salt sensitivity of blood pressure, GFR, and UAE rate in conscious mice. In addition, we evaluated glomerular morphology, renal collagen, renal TGF-β expression, and mesangial matrix expansion as structural markers of renal injury. Since the kidney is important in sodium homeostasis and long-term regulation of blood pressure, salt sensitivity of blood pressure provides an assessment of renal dysfunction. Blood pressure in patients with chronic kidney disease is usually very salt sensitive with substantial increases in blood pressure occurring with high-salt intake (39). The 52- to 55-wk-old MC4R−/− mice, however, did not develop a significant increase in arterial pressure during 12 days of a 10-fold increase in salt-intake.
It is possible that compensatory activation of melanocortin-3 receptors (MC3R) may contribute to lack of salt sensitivity of blood pressure in MC4R−/− mice. MC3R activation has been demonstrated to exert natriuresis and lack of MC3R or its endogenous agonist, δ-MSH, result in salt-sensitive hypertension (18). However, the role of MC3R in protecting against salt sensitivity of blood pressure in MC4R-deficient mice is unclear and was not the major objective of the present study.
Multiple lines of evidence, including lack of salt sensitivity of blood pressure and lack of major structural changes indicate that obesity and its associated metabolic abnormalities, in the absence of hypertension, did not cause major renal injury in MC4R−/− mice. Furthermore, 24-h UAE, which increases early in the course of renal injury due to a selective increase in glomerular permeability and glomerular hyperfiltration, was also not increased in MC4R−/− compared with WT mice. Similarly, fibrotic markers of renal injury, namely renal collagen, TGF-β, and mesangial matrix were not significantly different between old MC4R−/− and WT mice.
These findings were closely recapitulated in the 20- to 22-wk-old MC4R−/− mice that, despite obesity, had no significant increases in UAE, glomerular collagen, or mesangial matrix compared with WT mice and had no salt sensitivity of blood pressure (35). Similar observations in both young and old MC4R−/− mice strongly suggest that exposure to obesity and its related metabolic abnormalities over a prolonged period of time failed to cause significant renal injury in normotensive MC4R−/− mice. The only changes observed in the old MC4R−/− mice were moderate increases in kidney weight, GFR, and glomerular tuft size compared with old WT mice. Increases in glomerular tuft size may have contributed to increases in GFR.
The absence of major renal injury in 50- to 52-wk-old obese MC4R−/− mice suggests either that MC4R−/− mice are somehow protected from renal injury or that the metabolic disturbances may not cause significant kidney damage in the absence of hypertension. Reduced renal macrophage infiltration in the MC4R−/− mice compared with WT mice suggests that obesity in MC4R−/− mice was not associated with a renal inflammatory response. Whether the reduced accumulation of macrophages in kidneys of MC4R−/− mice explains the protection of these mice from obesity-induced renal injury, however, is unclear. Although previous studies suggest that inflammation may participate in renal disease progression, this may not be required for initiating obesity-induced renal disease (22, 29). Signs of glomerulosclerosis were observed in obese Zucker rats even before significant inflammation occurred in the kidneys (22).
Another possible explanation for the absence of renal injury in the MC4R−/− mice is that the metabolic disturbances were not severe enough. However, previous studies in other mouse models show that obesity and metabolic abnormalities similar to those seen in the MC4R−/− mice were associated with significant renal injury even after a short period of time. For instance, Levine et al. (23) demonstrated a fivefold increase in UAE, an early marker of renal injury, in only 10- to 11-wk-old db/db mice with plasma glucose levels of ∼14 mM and a body weight of ∼50 g, which is lower than that of the MC4R−/− mice in the present study. Similarly, Cohen et al. (6) found albuminuria in db/db mice as early as 8 wk of age and increased urinary type IV collagen excretion at 12 wk of age, suggesting renal injury. Furthermore, recent studies show that C57Bl/6J mice (which is the background of the MC4R−/− mice used in the present study) developed glomerulosclerosis with significant increases in mesangial matrix, renal collagen, fibronectin, and proteinuria despite developing only moderate obesity (∼48 g) and hyperglycemia (∼12 mM) after only 12 wk on a high-fat diet (19).
Thus, metabolic disturbances, even milder than those observed in the 52- to 55-wk old MC4R−/−, can be associated with severe nephropathy when accompanied by other abnormalities, such as increased blood pressure. Indeed, previous studies using telemetry for blood pressure measurements demonstrate that C57Bl/6J mice after 12–14 wk of a high-fat diet not only developed obesity but also increased arterial pressure (31, 40). Reduced renal vascular resistance in obesity may facilitate a greater transmission of the increase in arterial pressure to the glomerular capillaries making them more susceptible to glomerulosclerosis. Whether db/db mice also have elevated arterial pressure is unclear due to conflicting evidence, but they appear to have increased glomerular capillary pressure. Levine et al. (23) showed, using micropuncture techniques, that db/db mice have increased single-nephron GFR and impaired tubuloglomerular feedback in response to renal hyperperfusion. Similar single-nephron GFR changes were attributed to increased glomerular capillary pressures in the obese Zucker rats that, like db/db mice, have a mutant leptin receptor (28).
A role for hypertensive hemodynamic changes in contributing to renal injury associated with metabolic syndrome is supported by several studies. Previous studies in animals and in humans suggest that hypertension can accelerate and antihypertensive therapy can retard diabetic nephropathy (3, 12, 24, 25). Mauer et al. (24), using the two-kidney one-clip rat model in streptozotocin-treated diabetic rats, showed that glomerular hypertension greatly aggravates glomerulosclerosis in diabetes. Absence of such hypertensive hemodynamic changes in the MC4R−/− mice may have protected them from developing significant renal injury. To test this possibility, we induced mild hypertension in MC4R−/− and WT with l-NAME for 8 wk. We found that l-NAME treatment increased UAE, renal TGF-β expression, and macrophage infiltration to a greater extent in obese MC4R−/− compared with lean WT mice. Although no significant differences were observed for renal macrophage infiltration between WT and MC4R−/− mice treated with l-NAME, the degree of macrophage infiltration in the old normotensive MC4R−/− mice was much less than in young mild-hypertensive MC4R−/− mice, whereas no differences were observed between old normotensive WT and young mild-hypertensive WT mice. These observations indicate that obesity enhances the impact of arterial pressure on renal injury. The l-NAME treatment in our study caused only mild hypertension and small increases in the markers of renal injury investigated in this study. One explanation for lack of more severe renal injury in mice treated with l-NAME may be the fact that we induced only mild increases in blood pressure to more closely mimic the physiological elevations in arterial pressure one observes during weight gain (33). Despite the fact we induced only mild hypertension for 8 wk, our data support the hypothesis that hypertension may be necessary for obesity and related metabolic abnormalities to cause major renal injury.
The importance of hypertensive changes in initiating renal injury in obesity is also supported by other observations. Bidani et al. (4) reported that spontaneously reduced ambient blood pressure load may be the reason for the slow development of glomerulosclerosis in the streptozotocin-treated diabetic rat despite being associated with several putative nonhemodynamic mediators of nephropathy for 36–40 wk. Also, Nielsen and Jensen (26) found normal UAE in obese normotensive glucose-tolerant subjects, suggesting lack of renal injury in the absence of hypertension despite obesity. In contrast, the PREVEND study reported that body mass index is associated with microalbuminuria independent of other factors such as hypertension and diabetes (38). However, there are no reports, to our knowledge, demonstrating significant renal injury or glomerulosclerosis in the absence of hypertension in normotensive obese humans.
Although our data support the view that obesity-associated metabolic disturbances alone may not initiate major renal injury in the absence of hypertension, our findings do not exclude the possible contributions of metabolic disturbances and obesity in contributing to nephropathy. It is possible that strain-specific mechanisms in MC4R−/− mice may confer lesser susceptibility for the development of obesity-related renal dysfunction, independent of the fact that these mice are not hypertensive. As discussed previously, MC4R−/− mice have reduced macrophage infiltration, which could be related to lower blood pressure or to other mechanisms that are still unclear.
Previous studies suggest that obesity accelerates preexisting renal injury. For instance, Praga et al. (30) reported in a cross-sectional study of 73 obese patients that obesity increased the risk for developing proteinuria and chronic renal failure after unilateral nephrectomy. Also, Ribstein et al. (32) showed that obesity magnified the effect of hypertension on albuminuria in obese patients, thus raising the possibility of an increased susceptibility of obese hypertensives to renal damage.
Thus, the present study demonstrates that obesity and metabolic disturbances such as hyperglycemia, hyperinsulinemia, hyperleptinemia, and dyslipidemia, even when present together for a prolonged period of time, do not contribute to renal injury in the absence of hypertension in MC4R−/−mice, which could be due to lower macrophage infiltration in the kidney.
Perspectives and Significance
Although, experimental and clinical research suggest that obesity and associated metabolic abnormalities may be risk factors for chronic renal disease, it has been difficult to separate the impact of obesity and hemodynamic changes (e.g., hypertension) in causing renal injury. Our studies indicate that MC4R−/− mice, despite having severe early-onset obesity and other metabolic abnormalities, are not hypertensive and are protected against renal injury as well as the salt sensitivity of blood pressure that often accompanies chronic renal disease. Although it is possible that more severe or more prolonged obesity and metabolic abnormalities could cause renal disease in the presence or absence of hypertension, further studies are needed to test this idea. Also, obese MC4R−/− mice may have additional characteristics besides lower blood pressure that could protect them from renal injury. For example, they do not display the usual accumulation of renal macrophages and inflammation associated with obesity. Further studies are needed to determine whether MC4R−/− deficiency per se prevents the usual inflammatory response associated with obesity or whether this is due to lower blood pressure. It is also important to determine whether the results observed in MC4R−/− mice translate to humans with MC4R−/− deficiency, and whether these individuals are protected against renal disease despite severe obesity.
GRANTS
The authors' research was supported by a National Heart, Lung, and Blood Institute Grant PO1-HL-51971. L. S. Tallam was the recipient of a postdoctoral fellowship from the American Heart Association, and A. A. da Silva is a recipient of Scientist Development Grant from the American Heart Association.
Acknowledgments
We thank Stephanie Evans Peters for assistance with the histology studies.
REFERENCES
- 1.Abrass CK, Spicer D, Raugi GJ. Induction of nodular sclerosis by insulin in rat mesangial cells in vitro: studies of collagen. Kidney Int 47: 25–37, 1995. [DOI] [PubMed] [Google Scholar]
- 2.Abrass CK Lipid metabolism and renal disease. Contrib Nephrol 151: 106–121, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Bakris GL, Williams M, Dworkin L, Elliott WJ, Epstein M, Toto R, Tuttle K, Douglas J, Hsueh W, Sowers J. Preserving renal function in adults with hypertension and diabetes: a consensus approach. National Kidney Foundation Hypertension, and Diabetes Executive Committees Working Group. Am J Kidney Dis 36: 646–661, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Bidani AK, Picken M, Hacioglu R, Williamson G, Griffin KA. Spontaneously reduced blood pressure load in the rat streptozotocin-induced diabetes model: potential pathogenetic relevance. Am J Physiol Renal Physiol 292: F647–F654, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chow F, Ozols E, Nikolic-Paterson DJ, Atkins RC, Tesch GH. Macrophages in mouse type 2 diabetic nephropathy: correlation with diabetic state and progressive renal injury. Kidney Int 65: 116–128, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Cohen MP, Lautenslager GT, Shearman CW. Increased urinary type IV collagen marks the development of glomerular pathology in diabetic d/db mice. Metabolism 50: 1435–1440, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Dengel DR, Goldberg AP, Mayuga RS, Kairis GM, Weir MR. Insulin resistance, elevated glomerular filtration fraction, and renal injury. Hypertension 28: 127–132, 1996. [DOI] [PubMed] [Google Scholar]
- 8.Dunn BR, Zatz R, Rennke HG, Meyer TW, Anderson S, Brenner BM. Prevention of glomerular capillary hypertension in experimental diabetes mellitus obviates functional and structural glomerular injury. J Hypertens Suppl 4: S251–S254, 1986. [PubMed] [Google Scholar]
- 9.Fox CS, Larson MG, Leip EP, Culleton B, Wilson PW, Levy D. Predictors of new-onset kidney disease in a community-based population. JAMA 291: 844–850, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Fujiwara K, Hayashi K, Ozawa Y, Tokuyama H, Nakamura A, Saruta T. Renal protective effect of troglitazone in Wistar fatty rats. Metabolism 49: 1361–1364, 2000. [DOI] [PubMed] [Google Scholar]
- 11.Gelber RP, Kurth T, Kausz AT, Manson JE, Buring JE, Levey AS, Gaziano JM. Association between body mass index and CKD in apparently healthy men. Am J Kidney Dis 46: 871–880, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Griffin KA, Abu-Amarah I, Picken M, Bidani AK. Renoprotection by ACE inhibition or aldosterone blockade is blood pressure dependent. Hypertension 41: 201–206, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Hall JE, Crook ED, Jones DW, Wofford MR, Dubbert PM. Mechanisms of obesity-associated cardiovascular and renal disease. Am J Med Sci 324: 127–137, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Hall JE, Henegar JR, Dwyer TM, Liu J, da Silva AA, Kuo JJ, Tallam L. Is obesity a major cause of chronic renal disease? Adv Ren Replace Ther 11: 41–54, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Henegar JR, Bigler SA, Henegar LK, Tyagi SC, Hall JE. Functional and structural changes in the kidney in the early stages of obesity. J Am Soc Nephrol 12: 1211–1217, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Hsu CY, McCulloch CE, Iribarren C, Darbinian J, Go AS. Body mass index and risk for end-stage renal disease. Ann Intern Med 144: 21–28, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, Smith FJ, Campfield LA, Burn P, Lee F. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell 88: 131–141, 1997. [DOI] [PubMed] [Google Scholar]
- 18.Humphreys MH δ-MSH, sodium metabolism, and salt-sensitive hypertension. Am J Physiol Regul Integr Comp Physiol 286: R417–R430, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Jiang T, Wang Z, Proctor G, Moskowitz S, Liebman SE, Rogers T, Lucia MS, Li J, Levi M. Diet-induced obesity in C57BL/6J mice causes increased renal lipid accumulation and glomerulosclerosis via a sterol regulatory element-binding protein-1c-dependent pathway. J Biol Chem 280: 32317–32325, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Kasiske BL, Crosson JT. Renal disease in patients with massive obesity. Arch Intern Med 146: 1105–1109, 1986. [PubMed] [Google Scholar]
- 21.Kramer H, Luke A, Bidani A, Cao G, Cooper R, McGee D. Obesity and prevalent and incident CKD: the Hypertension Detection and Follow-Up Program. Am J Kidney Dis 46: 587–594, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Lavaud S, Poirier B, Mandet C, Belair MF, Irinopoulou T, Heudes D, Bazin R, Bariety J, Myara I, Chevalier J. Inflammation is probably not a prerequisite for renal interstitial fibrosis in normoglycemic obese rats. Am J Physiol Renal Physiol 280: F683–F694, 2001. [DOI] [PubMed] [Google Scholar]
- 23.Levine DZ, Iacovitti M, Robertson SJ, Mokhtar GA. Modulation of single-nephron GFR in the db/db mouse model of type 2 diabetes mellitus. Am J Physiol Regul Integr Comp Physiol 290: R975–R981, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Mauer SM, Steffes MW, Azar S, Sandberg SK, Brown DM. The effects of Goldblatt hypertension on development of the glomerular lesions of diabetes mellitus in the rat. Diabetes 27: 738–744, 1978. [DOI] [PubMed] [Google Scholar]
- 25.Neuringer JR, Brenner BM. Hemodynamic theory of progressive renal disease: a 10-year update in brief review. Am J Kidney Dis 22: 98–104, 1993. [DOI] [PubMed] [Google Scholar]
- 26.Nielsen S, Jensen MD. Relationship between urinary albumin excretion, body composition, and hyperinsulinemia in normotensive glucose-tolerant adults. Diabetes Care 22: 1728–1733, 1999. [DOI] [PubMed] [Google Scholar]
- 27.Noda M, Matsuo T, Nagano-Tsuge H, Ohta M, Sekiguchi M, Shibouta Y, Naka T, Imura Y. Involvement of angiotensin II in progression of renal injury in rats with genetic non-insulin-dependent diabetes mellitus (Wistar fatty rats). Jpn J Pharmacol 85: 416–422, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Park SK, Kang SK. Renal function and hemodynamic study in obese Zucker rats. Korean J Intern Med 10: 48–53, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poirier B, Lannaud-Bournoville M, Conti M, Bazin R, Michel O, Bariety J, Chevalier J, Myara I. Oxidative stress occurs in absence of hyperglycaemia and inflammation in the onset of kidney lesions in normotensive obese rats. Nephrol Dial Transplant 15: 467–476, 2000. [DOI] [PubMed] [Google Scholar]
- 30.Praga M, Hernandez E, Herrero JC, Morales E, Revilla Y, Diaz-Gonzalez R, Rodicio JL. Influence of obesity on the appearance of proteinuria and renal insufficiency after unilateral nephrectomy. Kidney Int 58: 2111–2118, 2000. [DOI] [PubMed] [Google Scholar]
- 31.Rahmouni K, Morgan DA, Morgan GM, Mark AL, Haynes WG. Role of selective leptin resistance in diet-induced obesity hypertension. Diabetes 54: 2012–2018, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Ribstein J, du Cailar G, Mimran A. Combined renal effects of overweight and hypertension. Hypertension 26: 610–615, 1995. [DOI] [PubMed] [Google Scholar]
- 33.Sierra C, de la Sierra A. Early detection and management of the high-risk patient with elevated blood pressure. Vasc Health Risk Manag 4: 289–296, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tallam LS, da Silva AA, Hall JE. Melanocortin-4 receptor mediates chronic cardiovascular and metabolic actions of leptin. Hypertension 48: 58–64, 2006. [DOI] [PubMed] [Google Scholar]
- 35.Tallam LS, Stec DE, Willis MA, da Silva AA, Hall JE. Melanocortin-4 receptor-deficient mice are not hypertensive or salt-sensitive despite obesity, hyperinsulinemia, and hyperleptinemia. Hypertension 46: 326–332, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Unger RH, Orci L. Lipotoxicity diseases of nonadipose tissues in obesity. Int J Obes Relat Metab Disord 24: S28–S32, 2000. [DOI] [PubMed] [Google Scholar]
- 37.Van Herpen NA, Schrauwen-Hinderling VB. Lipid accumulation in non-adipose tissue ad lipotoxicity. Physiol Behav 94: 231–241, 2008. [DOI] [PubMed] [Google Scholar]
- 38.Verhave JC, Hillege HL, Burgerhof JG, Navis G, de Zeeuw D, de Jong PE; Study Group PREVEND. Cardiovascular risk factors are differently associated with urinary albumin excretion in men and women. J Am Soc Nephrol 14: 1330–1335, 2003. [DOI] [PubMed] [Google Scholar]
- 39.Weir MR, Fink JC. Salt intake and progression of chronic kidney disease: an overlooked modifiable exposure? A commentary. Am J Kidney Dis 45: 176–188, 2005. [DOI] [PubMed] [Google Scholar]
- 40.Williams T, Chambers J, Roberts L, Henderson R, Overton J. Diet-induced obesity and cardiovascular regulation in C57BL/6J mice. Clin Exp Pharmacol Physiol 30: 769–778, 2003. [DOI] [PubMed] [Google Scholar]
- 41.Wolf G, Ziyadeh FN. Leptin and renal fibrosis. Contrib Nephrol 151: 175–183, 2006. [DOI] [PubMed] [Google Scholar]