Abstract
This study tested the hypothesis that long-term hypoxia (LTH) results in enhanced fetal corticotrope sensitivity to the ACTH secretagogues, corticotropin-releasing hormone (CRH), and AVP. Ewes were maintained at high altitude (3,820 m) from 40 to 130–131 days of gestation. Upon return to the laboratory, hypoxia was maintained by maternal nitrogen infusion. Vascular catheters were placed in both LTH (n = 4) and normoxic controls (n = 4). Each fetus received a 15-min infusion of either saline, 100 ng/kg of ovine CRH, or 20 ng/kg of AVP/min over 3 consecutive days in a randomized order. Fetal blood samples were collected at 0, 15, 30, 60, and 90 min after the start of infusion and analyzed for ACTH1-39, ACTH precursors, and cortisol. Anterior pituitaries were collected from additional noninstrumented fetuses for analysis of vasopressin receptor 1b (V1b) mRNA and protein. Basal plasma concentrations of both ACTH1-39 and ACTH precursors were higher in LTH fetuses and were not altered by saline infusion. In response to CRH, ACTH1-39 increased in both groups and was higher in the LTH group compared with control (P < 0.05). When analyzed as sum of ACTH1-39 released (Δ0–90 min) above basal, CRH released equal amounts of ACTH1-39 in both groups. In LTH fetuses, AVP evoked a greater ACTH1-39 release (P < 0.05) when analyzed as an increased sum of ACTH1-39 (Δ0–90 min) above basal. Both CRH and AVP elicited a release of ACTH precursors with no differences observed between LTH and control. AVP and CRH elicited significant increases in cortisol, which were higher in response to AVP than CRH. V1b mRNA and protein were elevated in the anterior pituitary of LTH fetuses compared with control. LTH significantly increases pituitary sensitivity to AVP. This enhanced sensitivity may be a mechanism of our previously observed enhanced corticotrope function.
Keywords: cortisol, adrenocorticotropic hormone, hypothalamus
the hypothalamic paraventricular nucleus (PVN) is the source of the two major ACTH secretagogues, corticotropin-releasing hormone (CRH) and AVP (25), thus providing the stimulus for ACTH mediated cortisol production in the adrenal cortex (3, 21). In fetal sheep, the PVN is essential for maintaining ACTH synthesis and release from the anterior pituitary, and as a result, the late-gestation induction of key ACTH-dependent enzymes mediating adrenocortical cortisol biosynthesis, resulting in parturition (6, 16, 19).
Although ACTH is the principal peptide stimulating adrenal cortisol production, the ACTH prohormone, proopiomelanocortin (POMC), and a major POMC-processing intermediate (22 kDa pro-ACTH) have been shown to attenuate ACTH-induced cortisol production in fetal sheep (24). Both POMC and 22 kDa pro-ACTH contain the 39-amino acid ACTH (ACTH1-39) peptide within their sequence, and the ACTH precursors are found in the fetal circulation at ∼10-fold higher concentrations than ACTH1-39 (4, 25). Not only can these ACTH precursors attenuate ACTH-induced adrenal glucocorticoid production in ovine fetal adrenocortical cells in vitro (24), but an increase in the ACTH-to-ACTH precursor ratio during late gestation is consistent with reports of increased biological activity of immunoreactive ACTH in fetal plasma (4, 5, 22). This suggests an enhanced processing of POMC to biologically active ACTH and/or a preferential release of ACTH1-39 relative to ACTH precursors as term gestation approaches.
The relative interactive roles of CRH, AVP, and cortisol in the regulation of POMC expression and processing in the ovine fetus remain relatively undefined. Both CRH and AVP mRNA increase with gestational age (15), and in vitro, both CRH and AVP increase release of both ACTH1-39 and ACTH precursors from sheep fetal pituitaries in a dose-dependent manner (14). Further, when administered to late-gestation fetal sheep, both CRH and AVP increase release of both ACTH1-39 and ACTH precursors, and both neuropeptides enhance the ratio of ACTH1-39 to precursor indicative of enhanced processing of POMC and/or preferential release of ACTH1-39 from mature secretory granules (4). Lesion of the PVN in the late-gestation ovine fetus retards POMC processing in the anterior pituitary and decreases circulating concentrations of ACTH1-39 to below the limit of detection, further supporting a role for hypothalamic neuropeptides in regulation of the ratio of ACTH1-39 to precursors produced in and released from the anterior pituitary (21).
Chronic hypoxia can have profound effects on the fetus. However, the fetus has a tremendous capacity to adapt to the challenges of chronic stress. We previously reported an increase in plasma concentrations of both ACTH1-39 and ACTH precursors in long-term hypoxic (LTH) fetal sheep (18). However, the ratio of ACTH1-39 to precursors in fetal plasma was increased, reflecting our observation of an increase in POMC processing to ACTH in the anterior pituitary in these fetuses. In response to a secondary stressor, the ratio of ACTH1-39-to-ACTH precursors was further enhanced (17), as was the absolute plasma concentration of ACTH1-39 in the LTH fetus. Although we noted an altered expression of the CRH receptor in the anterior pituitary of the LTH fetus, the roles of CRH and AVP in the adaptive changes observed in circulating fetal plasma ACTH1-39 and ACTH precursors remain to be established. The present study was designed to determine the capacity of CRH and AVP in stimulating ACTH1-39 and ACTH precursor release in the late-gestation LTH sheep fetus.
METHODS
Animals
All procedures were conducted with approval of the Loma Linda University Institutional Animal Care and Use Committee. Pregnant ewes were maintained at the Barcroft Laboratory White Mountain Research Station (elevation 3,820 m) beginning at approximately day 40 of gestation until either 130 days of gestation (for surgical instrumentation) or 137–138 days of gestation (for necropsy; term = 146 days). Immediately after arrival at Loma Linda University Medical Center Animal Research Facility (elevation: 346 m), the ewes were implanted with an arterial and nonocclusive tracheal catheter, as previously described (1, 9). The maternal Po2 for LTH group was maintained at ∼60 mmHg by adjusting humidified nitrogen (N2) gas flow through a maternal tracheal catheter. Normoxic control ewes were maintained near sea level (∼300 m) throughout gestation.
Surgical instrumentation animals.
Between days 131 and 133 of gestation, surgery was performed to place fetal and maternal vascular catheters as previously described (1, 9). Following a 5-day recovery period, studies were initiated on each animal over three consecutive days in a randomized order. Each fetus received a 15-min infusion of either saline vehicle, 100 ng/kg estimated body wt/min of ovine CRH (Bachem, Torrance CA), or 20 ng/kg estimated body wt/min of AVP (Bachem). These doses were chosen on the basis of the effectiveness observed in a previous study and the approximate equivalent molar doses of CRH and AVP (25). Mean fetal arterial pressure, heart rate, and amniotic fluid pressure were monitored during each experiment. Fetal blood was collected from the arterial catheter at 0 min (baseline prior to infusion), 15, 60, and 90 min following the start of the infusion. Blood samples were immediately centrifuged; plasma was separated and snap frozen at −70°C, and fetal erythrocytes returned to the fetus. Plasma samples were analyzed for ACTH1-39, ACTH precursors (POMC and 22 kDa pro-ACTH), and cortisol, as described below. Fetal arterial blood samples were also drawn at selected time intervals for determination of fetal blood gas using an automated blood gas analyzer (ABL300; Radiometer, Copenhagen, Denmark).
Tissue collection animals.
Between days 139 and 141 days of gestation, both control and LTH ewes were sedated with pentobarbital sodium, intubated, and maintained under general anesthesia with 1.5–2% halothane in oxygen. Fetuses were then delivered through a midline laparotomy and immediately euthanized by exsanguination. Anterior pituitaries were collected, frozen rapidly in liquid nitrogen, and stored at −80 C until analyzed, as we have previously described (18).
ACTH1-39 ELISA
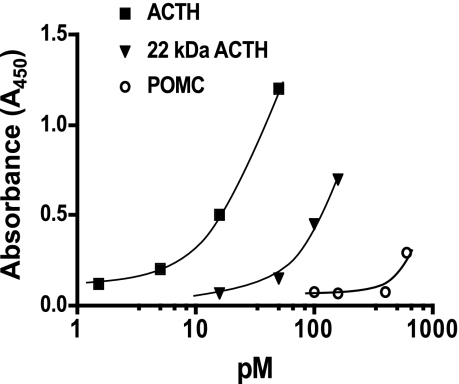
A two-site ELISA was used to determine plasma concentrations of ACTH1-39. This assay uses a biotinylated capture antibody generated against ACTH34-39 and a peroxidase-labeled reporter antibody against ACTH1-24. This assay has ≥98% recovery of ACTH1-39 from ovine fetal plasma and fetal plasma spiked with ACTH1-24 diluted parallel with the standard curve. Recombinant ovine POMC and recombinant ovine 22 kDa pro-ACTH exhibit <1% (POMC) and ∼5% (22 kDa pro-ACTH) cross-reactivity (Fig. 1). The sensitivity of the assay is 2.5 pg/ml for ACTH1-39. Intra-assay coefficient of variation was 2.9%, and inter-assay coefficient of variation was 4.2%.
Fig. 1.
Dose-response curve of the two-site ACTH1-39 ELISA for ACTH1-39, recombinant ovine 22-kDa pro-ACTH and recombinant ovine POMC. 22 kDa of pro-ACTH exhibits ∼10% cross-reactivity, while POMC exhibits ∼1% cross-reactivity.
ACTH Precursor ELISA
Fetal plasma ACTH precursors were measured using a specific two-site ELISA (OCTEIA POMC, American Laboratory Products, Windham, NH). We have previously validated this assay for the measurement of ACTH precursors in fetal sheep plasma (18). This ELISA has a sensitivity of 10 pM and a cross-reactivity against ACTH1-39 of 3.6% and α-MSH of 2.2%. Intra-assay coefficient of variation was 3.2%. Parallelism of the assay was determined for ovine fetal serum spiked with known amounts of recombinant ovine POMC and recombinant ovine 22 kDa pro-ACTH.
Cortisol ELISA
Plasma cortisol was measured by ELISA (EA65; Oxford Biomedical Research, Oxford, MI). Cortisol was extracted from plasma using a previously described extraction procedure (extraction efficiency ∼85%). The sensitivity of this assay is 0.1 ng/ml. This EIA kit has been previously validated for fetal sheep plasma (23). The data are analyzed using four-parameter logistic equation and an assay range of 0.1 (80% bound/free) to 10.0 ng/ml (10% bound/free); the interassay coefficient of variation is less than 10%.
Quantitative Real-Time RT-PCR Analysis of the V1b Receptor
Messenger RNAs for the ovine vasopressin receptor 1b (V1b) and cyclophilin (housekeeping mRNA) were quantified using real-time PCR (qRT-PCR); we have previously described and validated the methods for qRT-PCR for a variety of genes in our laboratory (7, 20). Total RNA was prepared from anterior pituitaries (n = 6 per group for control and LTH fetal sheep) with an RNA preparation kit, as per manufacturers instructions (Qiagen, Valencia, CA). Before reverse transcription, residual genomic DNA was removed from total RNA with DNase I (1 Unit, 60 min at 37°C; Ambion, Austin, TX). The DNase I was subsequently removed from the RNA samples via PCR clean-up columns (Qiagen). An initial denaturation step was performed for 5 min at 95°C prior to first-strand synthesis at 42 C for 50 min. Reverse transcription was then performed using 1 μg total RNA, with oligo dT as the primer and Superscript II as the reverse transcriptase; the reaction was terminated by heating to 70°C for 15 min.
Real-time PCR was performed using 50 ng of cDNA (equal to input RNA) per PCR. All PCR were performed in triplicate. Initial qRT-PCRs were performed using serial dilutions of cDNA ranging from 250 to 15.625 ng (250, 125, 62.5, 31.25, and 15.625 ng) to determine that the quantity of cDNA used for analysis of the V1b and cyclophilin mRNA was within the linear range of amplification for each primer. For each primer set, the amplicon was directly sequenced by Sanger dideoxysequencing (Oklahoma Medical Research Foundation Sequencing Core, Oklahoma City, OK) to confirm amplicon identity. SYBR Green (1 × SYBR green master mix; Bio-Rad, Hercules, CA) was utilized as the fluorophore, and PCR was performed using a Bio-Rad iCycler equipped with the real-time optical fluorescent detection system. The V1b primers (forward: 5′-TCCCTGCCCTCTTAACTCCT-3′; reverse: 5′-CTGGAATCCATGGTGGAAGT-3′) were derived from the bovine cDNA sequence (XM_583769) obtained from the National Center for Biotechnology Information. The cylophilin primers have been previously described (18). A three-step PCR was used: 95°C for 45 s, annealing (primer specific ranging between 55–60°C) for 30 s and 72°C extension for 30 s. A total of 35 cycles were performed. A melt curve analysis was conducted on each sample after the final cycle to ensure that a single product was obtained, and agarose gel electrophoresis confirmed that the single PCR product was of the expected size. We used cyclophilin as a “housekeeping” mRNA. The identical first-strand cDNA was used for quantification of specific mRNAs of interest and in the same PCR run as for the gene of interest to circumvent any between-run variation. As previously reported (7, 18, 20), we found that cyclophilin and GAPDH are equally efficacious when used as internal housekeeping mRNAs in our real-time PCR application and, unlike GAPDH, cyclophilin is not subject to glucocorticoid regulation. Control PCR for each primer pair and RNA source included 1) elimination of reverse transcriptase during first-strand cDNA synthesis (assures that PCR product depends upon RNA) and 2) no RNA/cDNA in reverse-transcription reaction (assures that no amplicon contamination has occurred). Primers were utilized that provided 1) a single PCR product (identity confirmed by sequencing), 2) dilution curve of cDNA exhibited a slope of 100% ± 10% “efficiency,” where 100% = Δ3 Ct/log cDNA input (Ct is the threshold PCR cycle at which fluorescence is detected above baseline), and 3) the melt curve analysis post-PCR must demonstrate one product. For quantification purposes, a synthetic single-stranded DNA standard was used to generate a standard curve (100, 10, 1, 0.1, 0.01, and 0.001 pg of standard DNA) for extrapolation of starting cDNA concentrations per reaction. Each standard point was run in duplicate and in the same PCR block as the unknowns. Linear regression was used to quantify starting RNA (cDNA) based on Ct values as extrapolated from the standard curve. The efficiency of the standard and primers was 100% based on the above criteria.
V1b Western Blot Analysis
An ovine V1b antiserum was generated in rabbits using the peptide sequence [NH-SGVLDCWADFRFPWGPR-COOH] corresponding to a region within the 2nd extracellular loop (<70% homology to the bovine V2, V1a, and ovine oxytocin receptors) conjugated to KLH. The preimmune serum from the immunized rabbit was used as a control. Western blot analysis was performed similarly to what has been described for our laboratory (18, 20). Anterior pituitary tissue was homogenized (4°C, 1 ml, 0.25 M Tris·HCl, pH 7.4, 0.01 M sucrose, 0.1% BSA, containing 1 mM pepstatin, 0.4 mM pefablock, 1 μg/ml leupeptin) using a Dounce homogenizer then centrifuged at 500 g for 5 min to pellet the nuclei, and the supernatant was collected. The nuclear pellet was resuspended in the same buffer (500 μl) and centrifuged again, and the supernatants were combined. The supernatant was then centrifuged at 30,000 g at 4°C, and the resulting crude membrane pellet resuspended (50 mM Tris, 5 mM MgCl2, 2 mM EGTA containing 1 mM pepstatin, 0.4 mM pefablock, 1 μg/ml leupeptin). Protein concentrations were determined by the Bio-Rad method. Samples (50 μg of crude membrane protein per anterior pituitary) were electrophoresed by SDS-PAGE (4–10%; Invitrogen, Carlsbad, CA), and the proteins were subsequently electrophoretically transferred to nitrocellulose membranes and subjected to Western blot analysis. The membranes were blocked for 1 h with TBS (10 mM Tris·HCl, pH 7.2, 100 mM saline) containing 0.1% Tween 20 (TTBS) and 10% nonfat dry milk. Membranes were then washed twice in TTBS and incubated with primary antiserum (1:500) or preimmune serum (1:200) prepared in TTBS-5% nonfat dry milk overnight at 4°C. A chemiluminescent detection system (Pierce, Rockford, IL) was used, and blots were exposed to film (Hypermax; Kodak, Rochester, NY) for varying lengths of time. To ensure that equal protein amounts were loaded for each sample, identical SDS-PAGE was performed with the same protein samples from control and LTH anterior pituitaries using an anti-actin antiserum (Santa Cruz Biotechnology, Santa Cruz, CA).
Statistical Analysis
Differences between LTH and control hormone concentrations were analyzed by ANOVA with subsequent Tukey post hoc analysis for individual differences between time points within group and between groups at select time points. Differences in V1b mRNA and protein, as well as area under the curve analysis of plasma hormone concentrations between LTH and control fetuses, were determined by using the Student's t-test. Significance was set at the 0.05 level.
RESULTS
Fetal Physiological Parameters
Fetal Po2 and Pco2 were significantly lower in the LTH fetal sheep compared with control fetuses (Table 1). Infusion of CRH, AVP, or saline did not effect Po2, Pco2, or pH in either group.
Table 1.
Blood gas values for control and LTH fetuses
| Time, min |
Control |
LTH
|
|||||
|---|---|---|---|---|---|---|---|
| Po2, mmHg | Pco2, mmHg | pH | Po2, mmHg* | Pco2, mmHg* | pH | ||
| Saline | 0 | 21.00±0.41 | 56.25±0.95 | 7.34±0.01 | 16.75±0.63 | 46.00±1.96 | 7.36±0.01 |
| 15 | 21.25±0.48 | 55.75±1.25 | 7.34±0.01 | 17.25±0.85 | 44.01±1.92 | 7.35±0.01 | |
| 90 | 21.75±0.48 | 55.25±0.44 | 7.35±0.01 | 18.25±1.03 | 45.02±1.00 | 7.35±0.01 | |
| CRH | 0 | 20.75±0.48 | 54.75±0.48 | 7.25±0.01 | 16.25±0.75 | 44.75±2.67 | 7.35±0.02 |
| 15 | 24.0±0.71 | 50.75±1.32 | 7.36±0.01 | 20.00±1.41 | 42.75±2.68 | 7.36±0.01 | |
| 90 | 21.0±0.41 | 55.5±0.65 | 7.34±0.01 | 15.25±1.44 | 45.00±2.80 | 7.35±0.01 | |
| AVP | 0 | 21.00±0.41 | 53.5±1.04 | 7.35±0.01 | 17.25±0.85 | 45.5±1.67 | 7.34±0.01 |
| 15 | 21.25±0.48 | 53.25±1.70 | 7.35±0.02 | 17.00±0.41 | 44.75±2.34 | 7.34±0.01 | |
| 90 | 21.00±0.41 | 54.00±1.35 | 7.35±0.01 | 17.75±0.75 | 46.00±2.01 | 7.35±0.01 | |
All values represent means ± SE.
Different from control at each time point and for each treatment (P < 0.01).
Fetal Plasma ACTH1-39, ACTH-Precursor and Cortisol Response to Saline Infusion
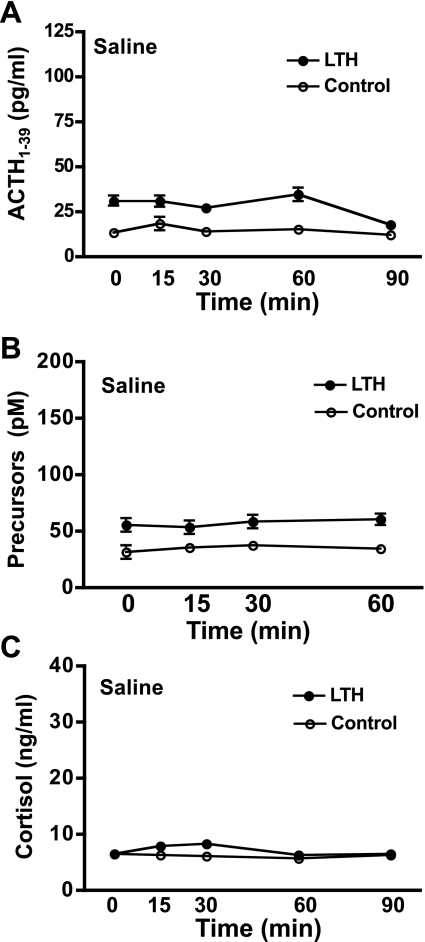
Resting concentrations of ACTH1-39 in fetal plasma were significantly elevated in the LTH compared with control fetuses (ANOVA, P < 0.05; Fig. 2A). Infusion of saline did not elevate ACTH1-39 over the course of the sampling period in either group (Fig. 2A). Similarly, plasma concentrations of ACTH precursors were significantly elevated in LTH fetuses (ANOVA, P < 0.05) and did not change in response to saline infusion (Fig. 2B). Plasma cortisol concentrations were not different between LTH and control fetal sheep throughout the duration of the saline infusion (Fig. 2C). Saline did not affect fetal plasma cortisol levels in either group. These results are consistent with our previous findings for all three hormones (18).
Fig. 2.
Plasma concentrations of ACTH1-39 (A), ACTH precursors (B), and cortisol (C) in long-term hypoxic (LTH; n = 4) and control (n = 4) fetal sheep during infusion of saline as described in methods (means ± SE). Blood samples were collected at the indicated times. As analyzed by ANOVA, basal plasma concentrations of ACTH1-39 and ACTH precursors were significantly elevated in LTH compared with control fetal sheep (P < 0.05). There were no differences in plasma cortisol between the two groups. Saline infusion did not elevate ACTH1–39, ACTH precursors, or cortisol in either group.
Fetal Plasma ACTH1-39, ACTH Precursor and Cortisol Response to Corticotropin-Releasing Hormone
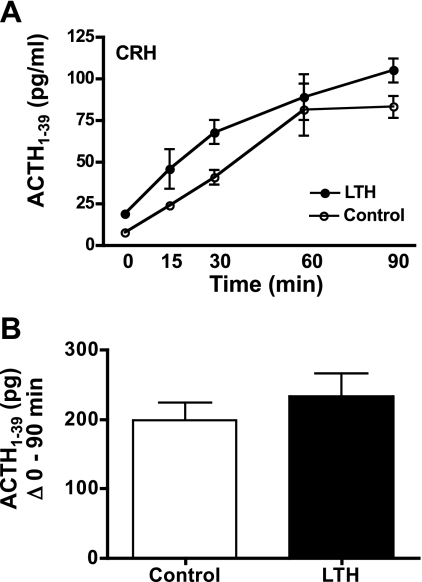
Infusion of CRH resulted in a significant, near-linear increase in fetal plasma ACTH1-39 in both LTH and control fetuses (Fig. 3A) over 90 min. Plasma ACTH1-39 concentrations in LTH fetuses were significantly higher (ANOVA, P < 0.05) compared with control fetuses. However, when analyzed as the sum of ACTH1-39 released from 0 to 90 min above basal concentrations (Student's t-test; Fig. 3B), it can be observed that the amount of ACTH1-39 released in response to CRH was not different between the LTH and control fetuses at any time point. Thus, the higher amounts of plasma ACTH1-39 observed in the LTH fetuses throughout the sampling period reflect the higher basal concentrations observed in these fetuses.
Fig. 3.
A: plasma concentrations of ACTH1-39 in LTH and control fetal sheep during infusion of CRH. CRH infusion was initiated immediately after the preinfusion sample (n = 4 per group; means ± SE). Concentrations of ACTH1-39 were significantly elevated (P < 0.05) in response to CRH in both groups. ACTH1-39 was significantly higher (P < 0.05) in LTH compared with control fetuses prior to and during CRH infusion. B: sum of fetal plasma ACTH1-39 for LTH and control fetuses from 0 (pre-CRH) through 90 min of CRH infusion. There were no differences in total ACTH1-39 released between groups.
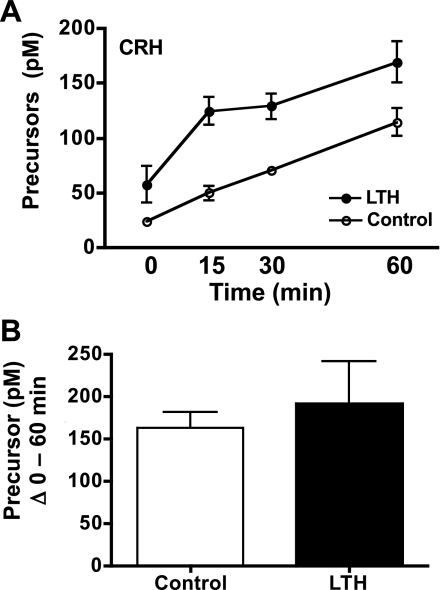
Similar to ACTH1-39, there was a significant increase in plasma concentrations of the ACTH precursors in both LTH and control fetuses in response to CRH that followed a similar near-linear increase over the duration of the sampling period reaching peak levels at 90 min postinitiation of CRH infusion (ANOVA, P < 0.05; Fig. 4A). Plasma concentrations of ACTH precursors in LTH fetuses were significantly higher in the LTH fetuses compared with control over the duration of the sampling period in response to CRH (ANOVA, P < 0.05). However, when analyzed as the sum of ACTH precursors released from 0 through 60 min, similar amounts of ACTH precursors were released in response to CRH in both control and LTH fetal sheep (Student's t-test; Fig. 4B).
Fig. 4.
A: plasma concentrations of ACTH precursors in LTH and control fetal sheep during infusion of CRH. CRH infusion was initiated immediately after the preinfusion sample (n = 4 per group; means ± SE). Concentrations of ACTH precursors were significantly elevated (P < 0.05) in response to CRH in both groups. ACTH precursors were significantly higher (P < 0.05) in LTH compared with control fetuses prior to and during CRH infusion. B: sum of fetal plasma ACTH-precursors for LTH and control fetuses from 0 (pre-CRH) through 60 min of CRH infusion. There were no differences in total ACTH precursors released between groups.
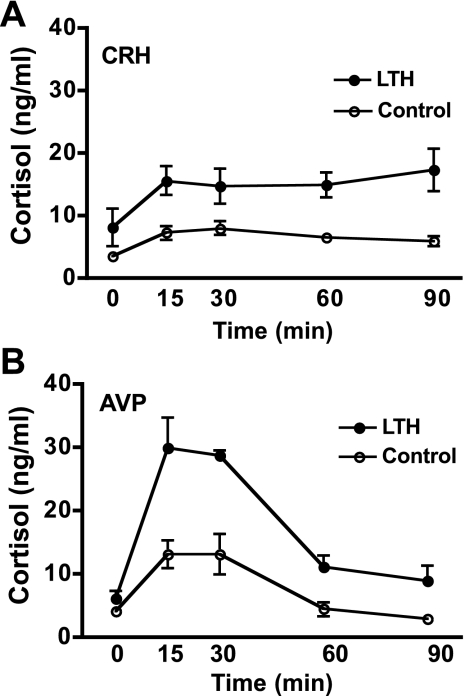
Cortisol concentrations were significantly elevated in response to CRH in both LTH and control fetuses (ANOVA, P < 0.05; Fig. 5A). In control fetuses, peak concentrations of cortisol were attained by 15–30 min postinfusion with a subsequent mild decline by 90 min, while in the LTH fetuses, cortisol concentrations were sustained through 90 min in response to CRH. Concentrations of cortisol were significantly higher in the LTH fetal sheep compared with control from 15 through 90 min postinitiation of CRH infusion (ANOVA, P < 0.05).
Fig. 5.
A: plasma cortisol concentrations achieved in response to CRH infusion in LTH and control fetal sheep (n = 4 per group; means ± SE). Cortisol concentrations were similar prior to CRH infusion (time 0) and significantly increased in response to CRH infusion in both groups (P < 0.05). Cortisol concentrations were significantly higher (P < 0.05) in response to CRH in LTH fetal plasma compared with control for the duration of CRH infusion. B: plasma cortisol concentrations achieved in response to AVP infusion in LTH and control fetal sheep (n = 4 per group; means ± SE). Cortisol concentrations were similar prior to AVP infusion (time 0) and significantly increased in response to AVP infusion in both groups (P < 0.05). Cortisol concentrations were significantly higher (P < 0.05) in response to AVP in LTH fetal plasma compared with control for the duration of AVP infusion.
Fetal Plasma ACTH1-39, ACTH-Precursor, and Cortisol Response to Arginine Vasopressin
Arginine vasopressin elicited a significant elevation in fetal plasma ACTH1-39 in both LTH and control fetuses that reached peak values by 15–30 min then subsequently declined by 60 and 90 min (ANOVA, P < 0.05; Fig. 6A). At the 60-min time point, ACTH1-39 was significantly elevated above baseline only in the LTH fetuses (Fig. 6A). When analyzed as the sum of ACTH1-39 released from 0 through 90 min, significantly greater ACTH1-39 was released in response to AVP in the LTH fetuses (Student's t-test, P < 0.05; Fig. 6B).
Fig. 6.
A: plasma concentrations of ACTH1-39 in LTH and control fetal sheep during infusion of AVP. AVP infusion was initiated immediately after the preinfusion sample (n = 4 per group; means ± SE). Concentrations of ACTH1-39 were significantly elevated (P < 0.05) in response to AVP in both groups. ACTH1-39 was significantly higher (P < 0.05) in LTH compared with control fetuses prior to and at the 15, 30, and 60 min times during AVP infusion. B: sum of fetal plasma ACTH1-39 for LTH and control fetuses from 0 (pre-AVP) through 90 min of AP infusion. AVP elicited a significantly higher total ACTH1-39 release in LTH compared with control fetuses over the sampling period (P < 0.05).
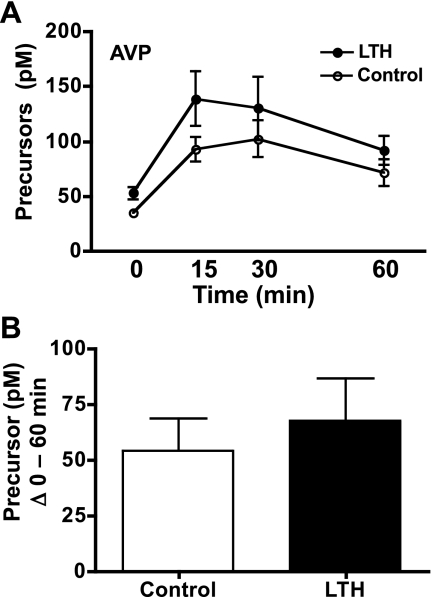
Like ACTH1-39, concentrations of the ACTH precursors in fetal plasma were significantly elevated In response to AVP in both LTH and control fetal sheep, demonstrating a similar response pattern as ACTH1-39 with peak concentrations attained at the 15- and 30-min time points (Fig. 7A). By 60 min, fetal plasma ACTH precursor concentrations were declining and were not significantly different compared with either the time 0, or the 15- and 30-min points in either group. When analyzed as the sum of ACTH precursors released from 0 through 60 min, similar amounts of ACTH precursors were released in response to AVP in both control and LTH fetal sheep (Student's t-test, P < 0.05; Fig. 7B).
Fig. 7.
A: plasma concentrations of ACTH precursors in LTH and control fetal sheep during infusion of AVP. AVP infusion was initiated immediately after the preinfusion sample (n = 4 per group; mean ± SE). Concentrations of ACTH precursors were significantly elevated (P < 0.05) in response to AVP in both groups. ACTH precursors were significantly higher (P < 0.05) in LTH compared with control fetuses prior to and during AVP infusion. B: sum of fetal plasma ACTH precursors for LTH and control fetuses from 0 (pre-AVP) through 60 min of AVP infusion. There were no differences in total ACTH precursors released between groups.
Cortisol concentrations were significantly elevated in response to AVP in both LTH and control fetal sheep (Fig. 5B). Peak concentrations of cortisol were attained at 15 and 30 min in both LTH and control fetuses. Plasma cortisol levels were significantly higher in response to AVP in the LTH fetuses compared with control at the 15- through 90-min time points. Peak concentrations of cortisol were higher in both LTH and control fetuses in response to AVP compared with that attained in response to CRH.
Vasopressin Receptor V1b Expression
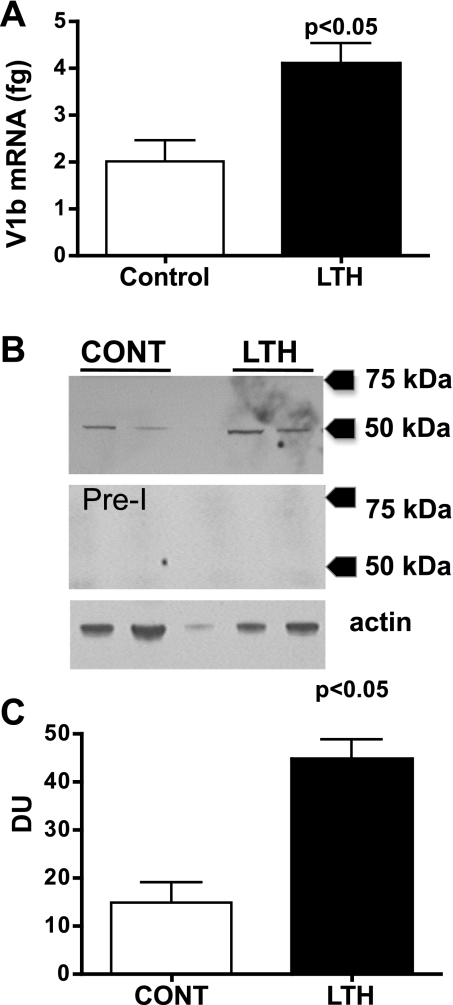
Messenger RNA for the V1b in the anterior pituitaries of LTH fetuses was significantly elevated compared with control fetuses (Student's t-test, P < 0.05; Fig. 8A). Western blot analysis of anterior pituitary protein with the rabbit anti-ovine V1b serum detected a band that migrated at ∼48 kDa, consistent with the predicted molecular weight of various mammalian V1b receptors (Fig. 8B). Western blot analysis of anterior pituitary protein with the preimmune serum did not yield positive signals at the same dilution of serum used for the anti-V1b with the same autoradiographic exposure time (Fig. 8B). Anterior pituitary levels of the V1b protein were higher in the LTH compared with control fetuses (Student's t-test, P < 0.05; Fig. 8C).
Fig. 8.
A: V1b receptor mRNA in anterior pituitaries from LTH and control fetal sheep (n = 6/group; means ± SE) as determined by quantitative RT-PCR. Messenger RNA is expressed in fg V1b mRNA per 100 ng input RNA. B: Western blot analysis for the V1b receptor for two representative samples each from control (CONT) and LTH anterior pituitaries. The middle panel is a Western blot using the preimmune serum from the same rabbit used to generate the anti-ovine V1b antibody. The lower panel is a Western blot for actin demonstrating the same samples. C: densitometry for the V1b Western (n = 4 anterior pituitaries/group). V1b protein was significantly elevated in the anterior pituitaries of LTH fetal sheep compared with control (P < 0.05).
DISCUSSION
In the ovine fetus, the hypothalamo-pituitary-adrenocortical (HPA) axis undergoes a functional maturation during the final weeks of gestation that results in an exponential rise in fetal plasma cortisol (13). The HPA axis in most mammalian species plays a critical role in preparing the late-gestation fetus for the transition to ex utero life by virtue of the maturational actions of adrenal glucocorticoids (10). Similar to the adult, as the fetal HPA axis matures, it plays an important role of maintaining fetal homeostasis in response to intrauterine stressors. Hypoxia, both acute and chronic, is a potent fetal stressor, and the fetus must respond to, or adapt to, hypoxic stress to survive. We have previously shown that the fetal HPA axis undergoes a significant adaptation in response to development under conditions of moderate LTH (1, 9, 18, 20).
The present study confirms our previous observations that basal concentrations of both ACTH1-39 and its precursors are elevated in the LTH fetus (18). Despite the elevation in plasma ACTH1-39, the LTH fetus maintains normal basal plasma cortisol concentrations (1, 9). We previously communicated that LTH fetuses attain significantly higher concentrations of plasma ACTH1-39 in response to a secondary stressor, while ACTH precursors reach levels that are similar to control fetuses (17). This is consistent with significantly higher plasma levels of cortisol being generated in response to a secondary stressor in the LTH fetus. However, the relative contribution of the two major PVN neuropeptides regulating ACTH production and release, CRH and AVP, in the divergent ACTH1-39 and ACTH precursor response in the LTH fetus remained unresolved.
As we show in the present study, fetal plasma concentrations of ACTH1-39 achieved in response to either CRH or AVP were significantly greater in LTH fetuses compared with control fetuses, consistent with the ACTH1-39 release in response to a secondary stressor. However, when analyzed as the sum of the increase in ACTH1-39 above baseline in response to each secretagogue, a divergence in the ACTH1-39 response to AVP and CRH was apparent. Following CRH administration, the amount of ACTH1-39 released above basal levels over the sampling period (15 through 90 min) was not different between the two groups, indicating that the greater concentrations of ACTH1-39 observed in the LTH fetuses throughout the sampling period reflected the higher basal levels of this peptide in these fetuses rather than an increased sensitivity to CRH per se. On the other hand, AVP elicited a significantly greater ACTH1-39 release in the LTH compared with control fetuses, even after accounting for the elevated basal concentrations. Thus, enhanced ACTH1-39 release in response to AVP compared with CRH could reflect a greater contribution of AVP in the increased ACTH1-39 release observed in the LTH fetus following a secondary stressor. In light of our prior observation of an increased processing of POMC to ACTH1-39 in the anterior pituitary of the LTH fetus (18), it would appear that this pool of ACTH1-39 is subject to greater control from AVP, perhaps indicative that AVP-responsive corticotropes are the primary source of the increased processing of POMC. This is consistent with the findings of Liu et al. (12), who showed that in adult sheep, the biosynthesis and secretion of ACTH is predominantly regulated by AVP. The rapid ACTH release in response to AVP (reaching maximal levels within 15 min) may also result from AVP stimulating the release of ACTH from mature secretory granules containing more ACTH relative to precursors. The longer time course for CRH compared with AVP could also arise from the presence of the CRH binding protein (CRH-BP). Although sheep do not have a circulating CRH-BP, it is expressed in the brain and the anterior pituitary of fetal sheep (2, 11, and Myers DA, unpublished observations) and thus may provide a local control of CRH availability. Alternatively, CRH could stimulate the nascent processing of POMC stores to ACTH and the time necessary for this process to occur. The kinetics of ACTH release by these peptides is consistent with previous reports both in adult and fetal sheep.
An increase in the number of AVP-responsive corticotropes and/or an increase V1b receptor expression per cortictrope could contribute to the selective increase in ACTH1-39 release in response to AVP in the LTH compared with control fetuses. Between 100 and 115 days of gestation and 138 and 145 days of gestation, Fora et al. (8) showed that ovine fetal anterior pituitary cells exhibit a decreased ACTH1-39 release in response to CRH, while the release of ACTH1-39 in response to AVP increases. Our results are consistent with LTH, advancing this natural ontogenic gain in AVP responsiveness of corticotropes. Indeed, consistent with the enhanced ACTH1-39 response to AVP, expression (mRNA and protein) of the V1b was greater in the LTH fetal anterior pituitary. We previously noted that although the mRNA for the CRH1 receptor was increased in the LTH anterior pituitary, CRH1 receptor protein was reduced, indicating a potential internalization and a loss of sensitivity to this ACTH secretagogue in response to increased CRH secretion in the LTH fetuses (18). If this is the case, then the adaptive increase in basal ACTH1-39 and ACTH precursors and the increased ACTH1-39 in response to a secondary stress in the LTH fetus likely reflects the increased sensitivity and response to AVP. Clearly, a detailed examination of expression of these peptides in the parvocellular neurons of the PVN of the LTH fetuses is warranted in future studies.
Similar to the absolute plasma concentrations of ACTH1-39 achieved in response to CRH or AVP, concentrations of ACTH precursors attained in response to both CRH and AVP were significantly greater in the LTH fetuses. Overall (sum of ACTH precursors released over the entire sampling period), similar quantities of the precursors were released in the two groups. In response to a secondary stressor, similar maximal levels of the ACTH precursors are achieved between LTH and control fetuses, although when analyzed, relative to the resting levels, the LTH fetus releases less ACTH precursor (18), reflecting the enhanced processing of POMC observed in the anterior pituitary. However, in the earlier study, only one time point postsecondary stress was examined (15 min), thus possibly masking differences in plasma precursors concentrations achieved in the LTH and control fetuses at later time points.
The cortisol response to the two ACTH secretagogues reflected, in general, the pattern of release of ACTH1-39 for each peptide. For both CRH and AVP, cortisol concentrations achieved in the LTH fetuses were greater than control fetuses, in agreement with the greater absolute ACTH1-39 concentrations reached in response to AVP and CRH in these fetuses. Consistent with our prior findings, basal levels of cortisol were not different between LTH and controls, despite the significantly elevated ACTH1-39 observed in the LTH fetuses. The cortisol production in response to AVP in the LTH fetus was substantially greater compared with either the control AVP-infused fetuses or in response to CRH in either group. This could reflect the approximate doubling of the ACTH1-39 to the precursor ratio noted in response to AVP in the LTH fetal sheep. The lower cortisol concentrations achieved in both LTH and control fetuses in response to CRH-induced ACTH release are paradoxical, considering that the level of ACTH1-39 reached in response to CRH is ultimately equal to that observed for AVP. Also, the peak cortisol response following CRH is at 15 min, when ACTH is relatively low, albeit increasing. This may imply that acute activation of cortisol synthesis by ACTH is dependent on the initial rate of ACTH receptor activation rather than on total receptor occupancy, otherwise the level of cortisol produced should be similar to AVP, albeit at a later time point. It is also possible that the greater ACTH precursor release in response to CRH attenuates the adrenal response to ACTH1-39. It is also possible that CRH is having a direct antagonistic action at the adrenal cortex since McDonald and coworkers (16) found that the ovine fetal adrenal cortex is innervated by CRH-ergic fibers.
Perspectives and Significance
In addition to higher resting concentrations of both ACTH1-39 and its precursors, LTH led to higher plasma concentrations of ACTH1-39 and ACTH precursors in response to both CRH and AVP. Of particular relevance, however, the release of ACTH1-39 in response to AVP was selectively enhanced compared with CRH. Thus, AVP resulted in a greater ACTH1-39 to precursor ratio compared with CRH. Because the ACTH precursors have been shown to inhibit cortisol production in ovine fetal adrenal cells, this enhanced ACTH1-39 to precursor response to AVP may be a major factor related to the increase in cortisol noted in LTH fetuses following AVP compared with CRH. Considering our previous report of enhanced processing of POMC to ACTH in the anterior pituitary of the long-term hypoxic LTH fetus, the increased processing of POMC may be occurring in AVP-responsive corticotropes. Of consequence, we found that expression of the V1b receptor was indeed enhanced in the LTH anterior pituitary. Thus, in conclusion, an enhancement of AVP regulation of ACTH release may be a major adaptive mechanism in response to development under conditions of LTH.
GRANTS
This work was supported by National Institutes of Health Grants HD33147 (to D. A. Myers) and HD31226 (C. A. Ducsay).
REFERENCES
- 1.Adachi K, Umezaki H, Kaushal KM, Ducsay CA. Long-term hypoxia alters ovine fetal endocrine and physiological responses to hypotension. Am J Physiol Regul Integr Comp Physiol 287: R209–R217, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Behan DP, De Souza EB, Lowry PJ, Potter E, Sawchenko P, Vale WW. Corticotropin-releasing factor (CRF) binding protein: a novel regulator of CRF and related peptides. Front Neuroendocrinol 16: 362–382, 1995. [DOI] [PubMed] [Google Scholar]
- 3.Bell ME, Myers TR, Myers DA. Expression of proopiomelanocortin and prohormone convertase-1 and -2 in the late-gestation fetal sheep pituitary. Endocrinology 139: 5135–5143, 1998. [DOI] [PubMed] [Google Scholar]
- 4.Carr GA, Jacobs RA, Young IR, Schwartz J, White A, Crosby S, Thorburn GD. Development of adrenocorticotropin-(1–39) and precursor peptide secretory responses in the fetal sheep during the last third of gestation. Endocrinology 136: 5020–5027, 1995. [DOI] [PubMed] [Google Scholar]
- 5.Castro MI, Valego NK, Zehnder TJ, Rose JC. The ratio of plasma bioactive to immunoreactive ACTH-like activity increases with gestational age in the fetal lamb. J Dev Physiol 18: 193–201, 1992. [PubMed] [Google Scholar]
- 6.Challis JR, Sloboda D, Matthews SG, Holloway A, Alfaidy N, Patel FA, Whittle W, Fraser M, Moss TJ, Newnham J. The fetal placental hypothalamic-pituitary-adrenal (HPA) axis, parturition and post natal health. Mol Cell Endocrinol 185: 135–144, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Ducsay CA, Hyatt K, Mlynarczyk M, Kaushal KM, Myers DA. Long-term hypoxia increases leptin receptors and plasma leptin concentrations in the late-gestation ovine fetus. Am J Physiol Regul Integr Comp Physiol 291: R1406–R1413, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Fora MA, Butler TG, Rose JC, Schwartz J. Adrenocorticotropin secretion by fetal sheep anterior and intermediate lobe pituitary cells in vitro: effects of gestation and adrenalectomy. Endocrinology 137: 3394–3400, 1996. [DOI] [PubMed] [Google Scholar]
- 9.Imamura T, Umezaki H, Kaushal KM, Ducsay CA. Long-term hypoxia alters endocrine and physiologic responses to umbilical cord occlusion in the ovine fetus. J Soc Gynecol Investig 11: 131–140, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Liggins GC The role of cortisol in preparing the fetus for birth. Reprod Fertil Dev 6: 141–150, 1994. [DOI] [PubMed] [Google Scholar]
- 11.Linton EA, Wolfe CD, Behan DP, Lowry PJ. A specific carrier substance for human corticotrophin releasing factor in late gestational maternal plasma which could mask the ACTH-releasing activity. Clin Endocrinol (Oxf) 28: 315–324, 1988. [DOI] [PubMed] [Google Scholar]
- 12.Liu JP, Robinson PJ, Funder JW, Engler D. The biosynthesis and secretion of adrenocorticotropin by the ovine anterior pituitary is predominantly regulated by arginine vasopressin (AVP). Evidence that protein kinase C mediates the action of AVP. J Biol Chem 265: 14136–14142, 1990. [PubMed] [Google Scholar]
- 13.Magyar DM, Fridshal D, Elsner CW, Glatz T, Eliot J, Klein AH, Lowe KC, Buster JE, Nathanielsz PW. Time-trend analysis of plasma cortisol concentrations in the fetal sheep in relation to parturition. Endocrinology 107: 155–159, 1980. [DOI] [PubMed] [Google Scholar]
- 14.Matthews SG, Challis JR. CRH and AVP-induced changes in synthesis and release of ACTH from the ovine fetal pituitary in vitro: negative influences of cortisol. Endocrine 6: 293–300, 1997. [DOI] [PubMed] [Google Scholar]
- 15.Matthews SG, Challis JR. Regulation of CRH and AVP mRNA in the developing ovine hypothalamus: effects of stress and glucocorticoids. Am J Physiol Endocrinol Metab 268: E1096–E1107, 1995. [DOI] [PubMed] [Google Scholar]
- 16.McDonald TJ, Nathanielsz PW. Bilateral destruction of the fetal paraventricular nuclei prolongs gestation in sheep. Am J Obstet Gynecol 165: 764–770, 1991. [DOI] [PubMed] [Google Scholar]
- 17.Myers DA, Bell P, Mlynarczyk M, Ducsay CA. Long term hypoxia alters plasma ACTH 1–39 and ACTH precursors in response to acute cord occlusion in the ovine fetus. J Soc Gynecol Investig 11: 249A, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Myers DA, Bell PA, Hyatt K, Mlynarczyk M, Ducsay CA. Long-term hypoxia enhances proopiomelanocortin processing in the near-term ovine fetus. Am J Physiol Regul Integr Comp Physiol 288: R1178–R1184, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Myers DA, Robertshaw D, Nathanielsz PW. Effect of bilateral splanchnic nerve section on adrenal function in the ovine fetus. Endocrinology 127: 2328–2335, 1990. [DOI] [PubMed] [Google Scholar]
- 20.Myers DA, Hyatt K, Mlynarczyk M, Bird IM, Ducsay CA. Long-term hypoxia represses the expression of key genes regulating cortisol biosynthesis in the near-term ovine fetus. Am J Physiol Regul Integr Comp Physiol 289: R1707–R1714, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Myers DA, McDonald TJ, Nathanielsz PW. Effect of bilateral lesions of the ovine fetal hypothalamic paraventricular nuclei at 118–122 days of gestation on subsequent adrenocortical steroidogenic enzyme gene expression. Endocrinology 131: 305–310, 1992. [DOI] [PubMed] [Google Scholar]
- 22.Ozolins IZ, Antolovich GC, Browne CA, Perry RA, Robinson PM, Silver M, McMillen IC. Effect of adrenalectomy or long term cortisol or adrenocorticotropin (ACTH)-releasing factor infusion on the concentration and molecular weight distribution of ACTH in fetal sheep plasma. Endocrinology 129: 1942–1950, 1991. [DOI] [PubMed] [Google Scholar]
- 23.Reimsnider SK, Wood CE. Does reduction of circulating prostaglandin E2 reduce fetal hypothalamic-pituitary-adrenal axis activity? J Soc Gynecol Investig 12: e13–e19, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz J, Kleftogiannis F, Jacobs R, Thorburn GD, Crosby SR, White A. Biological activity of adrenocorticotropic hormone precursors on ovine adrenal cells. Am J Physiol Endocrinol Metab 268: E623–E629, 1995. [DOI] [PubMed] [Google Scholar]
- 25.Zehnder TJ, Valego NK, Schwartz J, White A, Rose JC. Regulation of bioactive and immunoreactive ACTH secretion by CRF and AVP in sheep fetuses. Am J Physiol Endocrinol Metab 269: E1076–E1082, 1995. [DOI] [PubMed] [Google Scholar]