Abstract
We performed 2 wk of mechanical overload by synergist ablation on plantaris muscles from a small rodent hibernator, Spermophilus lateralis. While this muscle displays prominent myosin heavy-chain (MyHC) isoform shifts during hibernation, sensitivity to mechanical loading as a stimulus for muscle mass and isoform plasticity has not been demonstrated. Squirrel muscles, whether during hibernation or not, potentially are less sensitive to mechanical unloading, but we hypothesized that increased loading would produce the typical mammalian response of greater plantaris mass and MyHC shifts. Mechanical overload produced a 50% increase in muscle mass but, surprisingly, no changes in MyHC isoform protein or mRNA expression, despite previously observed fast-to-slow MyHC isoform switching during hibernation. Citrate synthase enzyme activity, as well as mRNA expression of creatine kinase and the muscle growth factor myostatin, were all unchanged. The mRNA expression of critical muscle atrophy genes decreased by 50% during hypertrophy, including ubiquitin ligases MuRF1 and MAFbx, and the related transcription factor FOXO-1a. Insulin-like growth factor (IGF-1) and hypoxia-inducible factor (HIF-1α) mRNA expression was elevated by 400% and 150%. Fast-to-slow MyHC isoform shifts appear unnecessary to support the increased recruitment of the plantaris muscle, shifts which are seen in other rodent models. Our results are consistent with muscular activity during interbout arousals as a potential mechanism to preserve muscle mass, but illustrate the primary importance of other seasonal factors besides patterns of muscle activation which must act in concert to alter MyHC isoforms and muscle fiber type during hibernation.
Keywords: myosin heavy-chain isoforms, hibernation, skeletal muscle hypertrophy, FOXO, ubiquitin ligases
hibernating mammals may demonstrate a variety of skeletal muscle adaptations related to their ability to reduce physical activity for many months, with attenuated impacts on muscle function. Skeletal muscles in hibernators are resistant to losses of mass, strength, and oxidative fiber type despite profound changes in weight-bearing activity and metabolism. Hibernators of varying sizes, torpor depths, and life histories (from bats to bears to rodents) may have different mechanisms for emerging from prolonged inactivity with muscle mass and contractile proteins and function remarkably intact (1, 26, 27, 39, 43, 45, 57, 59, 60, 74, 83, 87, 90).
Small, deeply torpid species, like ground squirrels, not only show a resilience in muscle size and contractile protein complement, but demonstrate myosin heavy-chain (MyHC) isoform profiles during hibernation that are not predicted from clinical mammalian studies on inactivity, atrophy, fasting, or exercise. The typical response with inactivity in hindlimbs has been described for decades as atrophic, with commensurate losses of oxidative capacity and slow type 1 MyHC, in a muscle-specific fashion (4, 16, 18, 23, 24, 31, 32, 64, 80, 81). Ground squirrels show several permutations of responses in a highly muscle-type specific manner: some muscle groups atrophy slightly, while others maintain mass, and both can occur with or without accumulation of slower MyHC isoforms (58, 60, 61; M. Nowell, H. Choi, and B. C. Rourke, unpublished observations). Bears and prairie dogs generally show similar muscular resistance to inactivity, and also preserve or increase slower MyHC isoforms in the soleus, biceps femoris, gastrocnemius, and extensor digitorum longus (26, 27, 58, 59, 83).
The reasons for putative skeletal muscle adaptations in the small rodent hibernators may be related both to shivering activity during hibernation and to presumptive activity immediately following emergence. During hibernation, brown adipose tissue is critical to providing heat for rewarming. However, shivering may provide an important contribution to thermogenesis, and muscles might repeatedly have to support this aspect during interbout arousals. Upon emergence, the animals must forage, locate mates, and evade predators, and so, any loss of muscle function or fatigue-resistance may have physiological consequences. Changes in MyHC isoforms indeed occur during hibernation, but the mechanisms and the stimuli for the alterations remain unclear (27, 58–60). Are these changes required for shivering, or are they a byproduct of potentially intensive skeletal muscle activity? Seasonally, if the isoform profiles are sufficient for summer activities, why are they not simply maintained during hibernation, when they clearly change at some point?
The present study specifically tests whether increased mechanical activity plausibly can contribute to plasticity in hindlimb muscle of squirrels, similar to isoform changes occurring during hibernation. We utilize a common rodent model of compensatory hypertrophy, involving the synergistic ablation of hindlimb muscles, which results in profound mechanical overload of the remaining plantaris muscle. To date, no demonstration has been made that muscles of hibernating rodents are even sensitive to changes in mechanical loading, whether increased or decreased. Losses during hibernation instead could be due to fasting and protein catabolism or hibernation triggers unrelated to neuromuscular activity. Anecdotal evidence (B. C. Rourke, unpublished observations) indicates that during seasonally active months, squirrel muscles do not atrophy or alter MyHC isoform profiles in response to reduced activity induced by captivity and cage restriction.
The plantaris muscle of these squirrels is an appropriate choice for this study for several reasons. During hibernation, the plantaris may exhibit atrophy up to 25%, depending on the study; regardless, it consistently shows pronounced shifts from fast to slower MyHC isoforms (60, 61; M. Nowell et al., unpublished observations). These fast-to-slow shifts in other mammalian muscles are characteristic of aerobic training or mechanical overload but would be unusual in their appearance during atrophy or inactivity. We have recordings of plantaris activity during hibernation from chronically implanted electromyography electrodes verifying activation of this muscle at late stages of arousal (U. Pham, H. Choi, and B. C. Rourke, unpublished observations). It is not clear whether the changes in MyHC isoforms during hibernation are required to support shivering, are a byproduct of shivering activity, or are related to another aspect of torpor. Mechanical overload of the squirrel plantaris during nonhibernating periods tests whether MyHC plasticity can be induced by loading, which has special relevance to hibernation physiology. The model we employ is not meant to mimic shivering directly, but is among the strongest stimuli for producing shifts to slower MyHC isoforms in other rodents, explicitly in the plantaris. It simply cannot be assumed that signaling pathways of loading sensitivity are mechanisms shaping fiber type in this species, given other notably unusual muscle responses.
We hypothesized that 2 wk of mechanical overload of the plantaris would result in a 40–50% increase in muscle mass and noticeable shifts in MyHC isoform expression, either as the accumulation of type 1 MyHC or the shifts of type 2b MyHC to slower type 2x MyHC. We report a remarkable absence of these shifts in the plantaris of nonhibernating squirrels, despite a rapid 50% increase in muscle mass, and in contrast to changes during 6 mo of torpor. We further characterize the hypertrophic response through mRNA expression of genes involved in critical pathways controlling muscle mass, such as the ubiquitin ligases MuRF1 and MAFbx, the transcription factors FOXO-1 and FOXO-3, the growth factors IGF-1 and myostatin, and a binding protein, IGF-BP5. We measure mRNA from a molecular marker of hypoxia, hypoxia-inducible factor-1α (HIF-1α), and from muscle creatine kinase. Last, we include fundamental determinations of protein and nucleic acid contents, and citrate synthase activity. Sensitivity to increased skeletal muscle activation and loading, even the profound stimulus of synergist ablation, surprisingly does not appear to be a mechanism directing MyHC isoform shifts in this hibernating species.
MATERIALS AND METHODS
Animal collection and surgical muscle ablation.
Golden-mantled ground squirrels (Spermophilus lateralis) were live trapped in August 2007 near Redding, CA, under a Department of Fish and Game scientific collecting permit. Mixed sexes were captured, with 13 females included in the present study (7 control, 6 surgical). Because of the confounding influences of testosterone in similar exercise/resistance training protocols involving rodents and humans, four males (2 control, 2 surgical) were considered separately (see results).
Trapped animals were housed in group cages (4–6 animals per cage, ∼1 m3) during a 2-wk quarantine, and held under a light regimen that tracked local sunrise and sunset (Long Beach, CA, latitude 33.76N) at 17–20°C. Animals were maintained on a diet of sunflower seeds, rodent chow, and dog food supplemented with fresh fruit and vegetables. Individuals were weight-matched (155–240 g) and haphazardly assigned to either control or surgical groups.
Surgical animals were anesthetized by ketamine/xylazine intraperitoneal injection (75 and 7.5 mg/kg), and bilateral incisions were made in the hindlimbs from just behind the knee to the calcaneus exposing the tendons. The posterior muscles of the lower leg were bluntly dissected, and the soleus-gastrocnemius complex was removed, leaving the plantaris muscle intact. Very small portions of the lateral and medial gastrocnemius muscles remained and were cauterized as close to their origin as possible. The incisions were sutured closed (Ethilon 5-0; Johnson and Johnson, Langhorne, PA), and the animals were allowed to recover individually in large cages (1 m3). An analgesic (Carprofen, 5 mg/kg) and an antibiotic (Gentamicin) were administered subcutaneously, as well as warm saline. Control animals were similarly anesthetized and injected, and subsequently sorted into cages, as above.
Surgical animals were ambulatory within 24–48 h, and were remarkably mobile, with little observable behavior to distinguish them from controls. All animals were killed under ketamine anesthesia by intracardial pentobarbital sodium injection at 14 days postsurgery, and the plantaris muscles were dissected, weighed, sectioned, and frozen at −80°C. Muscle tissue collections were completed between September 4–7, 2007; this is still a highly active period for the animals, at least 30–40 days prior to onset of torpor. All surgical and animal care protocols were approved by the California State University, Long Beach Institutional Animal Care and Use Committee, and carried out in accordance with National Institutes of Health guidelines.
Muscle morphology.
Wet mass for plantaris muscles was determined individually (right and left legs) before freezing, and subsequent sections were homogenized for a variety of protein and enzyme assays. In all cases, the plantaris provides sufficient tissue to allow cross sections of muscle to be homogenized, sampling across the grain of all muscle fibers. This is routine and ensures that all fibers of the mixed fiber-type plantaris are sampled. These pieces of muscle (∼35 mg) were separately homogenized in 19 vol of ice-cold protein buffer (250 mM sucrose, 100 mM KCl, 5 mM EDTA) for total, myofibrillar, and DNA content analyses, or in 19 vol of ice-cold citrate buffer (175 mM KCl, 2 mM EDTA, 10 mM Tris, pH 7.4) for enzyme assays. Additional pieces of muscle were homogenized for RNA extraction in TriReagent and 1-bromo-3-chloropropane reagent (Molecular Research Center, Cincinnati, OH), precipitated with isopropanol, and rinsed with 75% ethanol for total RNA estimation and cDNA generation. The left muscles were used for all reported molecular data, except where right and left muscles were independently assayed for citrate synthase enzyme activity.
Total protein, myofibrillar protein, and citrate homogenate protein contents were determined through a commercial Bio-Rad kit (Hercules, CA), read in triplicate on a Biotek microplate reader (Winooski, VE), using IgG as a standard. DNA content was determined from total protein homogenates by using a fluorescent microplate reader (Varioskan; Thermo Electron, Waltham MA) and Hoescht dye reagent. Two microliters of homogenate were added to TNE buffer (10 mM Tris, 2 M NaCl, 1 mM EDTA) and Hoescht (50 ml of 1 mg/ml per 100 ml filtered TNE) and read in triplicate by using DNA standards of known concentration. Total RNA content was determined on a UV plate reader at 260 and 280 nm.
Citrate synthase activity was determined spectrophotometrically and incubated at 37°C, based on a method described by Srere (73). Plantaris homogenates were freeze-thawed twice from −80°C to fracture the mitochondrial membrane, then diluted 1:20, and assayed in triplicate during a third thaw. In a microplate, 130 μl Tris (100 mM pH 8.0), 20 μl DTNB (1 mM), and 20 μl freshly prepared acetyl CoA (3 mM, Sigma) were blanked, and then 10 μl were diluted homogenate and 20 μl oxaloacetate (5 mM) were added. The plate was scanned every minute for 6 min at 412 nm, and change in optical density was plotted as a straight line. Activity was calculated initially as micromoles per gram tissue per minute and then corrected for protein concentration in the muscle homogenates.
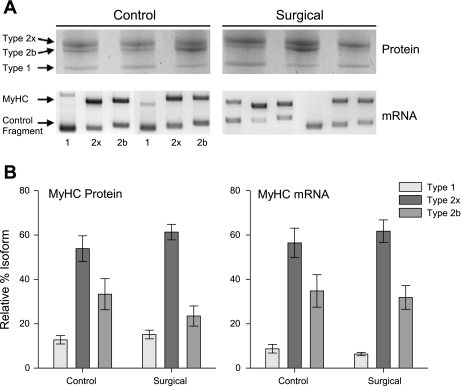
Electrophoretic separation of the MHC isoforms was accomplished by using a method described previously for this species (60, 79). SDS-PAGE gels were run in duplicate, silver stained (Bio-Rad, Hercules, CA) to visualize isoform bands, and analyzed by densitometry (ImageQuant; GE Healthcare, Piscataway, NJ). Plantaris muscles in this species express MyHC isoforms for types 1, 2x, and 2b. Type 2a MyHC is found in this species, but is not commonly expressed in the plantaris or other hindlimb muscles (B. C. Rourke, personal observation).
mRNA expression of muscle genes.
Using total RNA isolated from individual plantaris muscles, 1 μg RNA was reverse transcribed to cDNA (SuperScript III; Invitrogen, Carlsbad, CA). This cDNA served as template for semiquantitative PCR reactions for MyHC isoforms mRNA, and for nine genes expressed in skeletal muscle related to muscle mass, metabolism, and fiber type (Table 1). Primers and methodology for the MyHC genes were designed previously by using cloned sequences obtained from this species (61). The primers for the non-MyHC genes were designed by using available sequences for mammals in GenBank, utilizing regions of complete identity across species. By eye, these regions were selected to bridge ∼180- to 250-bp stretches of the genes (Mega 4.0; Ref. 40), and primers were trimmed to have similar annealing temperatures close to 54°C. This method has worked well in several species of hibernators for which little sequence information is available (59, 60). PCR products were submitted to a sequencing service (MacrogenUSA, Bethesda, MD) for verification of the gene specificity.
Table 1.
Primers used in reverse-transcriptase PCR
| 5′ Forward Primer | 3′ Reverse Primer | |
|---|---|---|
| Alt 18S | AGGAATTGACGGAAGGGCAC | GTGCAGCCCCGGACATCTAAG |
| Alt 18S competimer | GAATTGACGGAAGGGCACTT | GCAGCCCCGGACATCTAAGAA |
| Classic 18S | TCAAGAACGAAAGTCGGAGG | GGACATCTAAGGGCATCACA |
| Classic 18S competimer | TCAAGAACGAAAGTCGGAGGA | GGACATCTAAGGGCATCACAT |
| Creatine kinase | GAGCACCTGGGCTACGTG | TCCAGCTTCTTCTCCATCTCCA |
| FOXO-1a | AAATGATGAATCCCAGCTCCCA | TTGGTTGGGCAACACATTGTCAA |
| FOXO-3 | CAGTCGGACCCCTTGATGT | GCGCCCTGGGTTTGGTG |
| HIF-1α | TTTTAATACCCTCTGATTTAGCATGT | AGCTCTGAGTAATTCTTCACCC |
| IGF-BP5 | GAGAAGCCGCTGCACGC | GGGTCAGCTTCTTTCTGCGA |
| IGF1 | GCTCTTCAGTTCGTGTGTGG | GTCTTGGGCATGTCAGTGTG |
| MAFbx | AGACCGGCTACTGTGGAAGAG | CCGTGCATGGATGGTCAGTG |
| MURF | GCCATCCTGGACGAGAAGAA | CAGCTGGCAGCCCTTGGA |
| Myostatin | CAAATCCTGAGACTCATCAAACC | ATTTCAATCCTAAGTTGGATTCAGG |
| S. lateralis MHC1 | AGAAGGAGCAGGACACCAGC | CGCCAATGTCACGGCTCTTG |
| S. lateralis MHC2b | As above | CTCTGCAGATTTTATTTCACTGATATAC |
| S. lateralis MHC2x | As above | CCAAAAGTAATAAGTACAAAACAGAGTG |
S. lateralis, Spermophilus lateralis; FOXO, transcription factor; HIF, hypoxia-inducible factor-1α; MAFbx and MURF, ubiquitin ligases; MHC, myosin heavy-chain.
A unique species-specific internal control was designed and used for MyHC mRNA analyses (61), and primer-competimer pairs for ribosomal 18s genes were used for all other controls, similar to a commercial technique (Ambion/Applied Biosystems, Austin, TX). Depending on the interactions of the 18s primers, classic (Ambion) or alternate 18s primers were used, which also amplified products of different length. All PCR reactions were run in duplicate, and were meticulously adjusted to be in the linear range with minimal primer interactions between the gene of interest and the 18s control amplicons. A variety of Taq DNA polymerase enzymes were used to optimize PCR conditions on a primer-specific basis, including Taq and Platinum Taq (Invitrogen), and Qiagen Taq. Actin is not routinely used as a PCR control given the potential for variability in skeletal muscle actin proteins with experimental manipulations, such as growth, atrophy, or season.
PCR products were visualized on 2% agarose gels and stained with Sybr Green fluorescent DNA dye (Invitrogen). Images were analyzed by densitometry (ImageQuant), expressing the mRNA signal as intensity of amplified product corrected for the intensity of the 18s or MyHC control amplicon. This technique of multiprimer PCR reactions allows sensitive detection of differences between groups that vary by as little as 10% or less and gives excellent reproducibility when carefully performed.
ANOVA and descriptive statistics were carried out on Systat 10 (SPSS, Chicago, IL). All data were analyzed from duplicate or triplicate determinations, expressed as the average per individual muscle, and assessed for significance at the P = 0.05 level. Correlations of MyHC protein and mRNA were also performed.
RESULTS
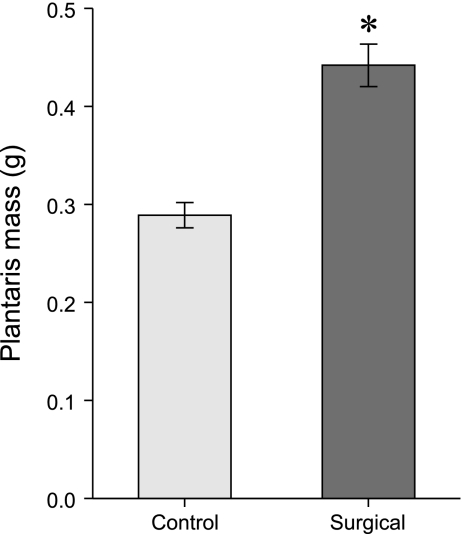
Female ground squirrels used for this study demonstrated a 42% (per animal mass, n = 13, P < 0.009) to 53% (muscle mass alone, P < 0.001) increase in plantaris mass following 14 days of compensatory or mechanical overload (Fig. 1 and Table 2). This is similar to the 40–50% increases over a similar time frame in rats and mice (6, 9, 56, 63 and B. C. Rourke unpublished observations). This response was also seen in males (data not shown, n = 2 each control and overload). A slight, but marginally significant, decrease in total protein in the plantaris of surgical animals is consistent with minor edema and swelling of the muscle. The myofibrillar protein contents were unchanged, which indicates that the mass increase of the muscle is not attributable to edema but rather to hypertrophy and the addition of new contractile elements. The maintenance of RNA content per muscle mass and the increased DNA are also consistent with hypertrophying muscle (Table 2). Citrate synthase activity was unchanged, although the relatively glycolytic plantaris muscle may not be able to increase oxidative capacity in that fashion.
Fig. 1.
Plantaris muscle mass increased by 50%, following 14 days of mechanical overload induced by synergist ablation of the soleus and gastrocnemius muscles in female ground squirrels (n = 13 individuals, 7 control, 6 surgical; 26 muscles total of right and left legs, *P < 0.001). Both right and left muscles hypertrophied similarly, and mass increases remained significant when corrected for individual animal mass (P < 0.009). Data are means ± SE.
Table 2.
Body mass and plantaris muscle parameters
| Sham/Control | Surgical | P Value | |
|---|---|---|---|
| Body mass, g | 212±14.9 | 222±20.5 | 0.699 |
| Left plantaris mass, g | 0.290±0.0186 | 0.433±0.0348 | 0.003 |
| Right plantaris mass, g | 0.287±0.0191 | 0.452±0.0284 | 0.000 |
| Average (left/right) plantaris mass, g | 0.289±0.0128 | 0.442±0.0216 | 0.001 |
| Plantaris mass body mass, g muscle/g | 1.403±0.154 | 1.992±0.125 | 0.009 |
| Total protein, μg/mg muscle | 252.83±9.774 | 212.98±15.969 | 0.050 |
| Myofibrillar protein, μg/mg muscle | 46.11±3.907 | 43.06±1.724 | 0.516 |
| DNA content, ng/mg muscle | 1.725±0.1040 | 2.202±0.1506 | 0.022 |
| RNA content, μg/mg muscle | 0.491±0.0472 | 0.566±0.0655 | 0.363 |
| Citrate synthase activity, units/protein | 0.833±0.0588 | 0.721±0.0503 | 0.169 |
Values are means ± SE.
MyHC protein isoforms were unchanged between control and surgical animals, despite the profound muscle hypertrophy and the anticipated stimulus to increase a shift to more oxidative fiber types (Fig. 2). MyHC mRNA expression, as measured by RT-PCR, closely mirrored protein expression and also showed no shifts. Regression analyses were highly significant for MyHC against MyHC mRNA, whether for control and surgical groups independently or pooled (P < 0.001).
Fig. 2.
Myosin heavy-chain (MyHC) isoform expression. A: representative silver-stained SDS-PAGE gels of MyHC isoform proteins and Sybr Green-stained agarose gels of RT-PCR determined mRNA expression. Squirrel plantaris expresses MyHC types 1, 2x, and 2b. Three simultaneous individual reactions per muscle sample with isoform-specific primers are run for mRNA expression, with an additional synthetic control fragment amplified. B: relative %MyHC isoform expression is unchanged following 14 days of mechanical overload in the plantaris in both protein and mRNA (n = 13, P > 0.05). Data are means ± SE.
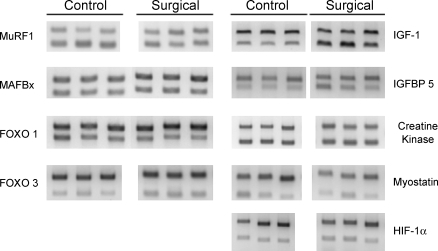
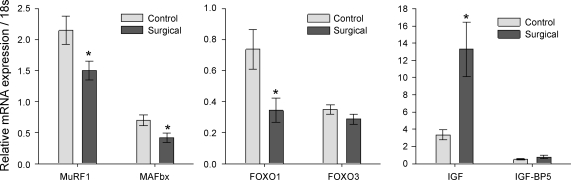
Several important muscle genes related to control of muscle mass show strong responses to the mechanical overload (Figs. 3, 4, and 5; data are normalized to ribosomal 18s mRNA expression). The atrophy-inducing ubiquitin ligases MuRF1 and MAFBx show mRNA expression reduced by 50% (P < 0.05), as protein turnover is slowed during hypertrophy. FOXO-1a, a transcription factor that promotes MAFbx/atrogin, was similarly reduced 50% (P = 0.025), but FOXO-3 was unchanged. The growth factor IGF-1 was increased by three- to fourfold (P = 0.011), and its associated binding protein IGF-BP5 was unchanged.
Fig. 3.
Representative RT-PCR data of 9 muscle genes (the ubiquitin ligases MuRF1 and MAFbx, the transcription factors FOXO-1 and FOXO-3, the growth factors IGF-1 and myostatin, and a binding protein IGF-BP5) analyzed using primers designed by similarity to existing mammalian sequences. Forward and reverse primers were obtained utilizing regions of complete identity in available GenBank sequences and used to amplify cDNA from plantaris muscles. The top band in each lane is amplified from ribosomal 18s primers in a multiplex reaction with primers for the gene of interest, producing the lower band. Data were run in duplicate or triplicate from individual plantaris muscles. HIF-1α, hypoxia inducible factor-1α.
Fig. 4.
Atrophy and growth-related genes. The mRNA expression of atrophy-related ubiquitin ligases MuRF1 and MAFbx is downregulated 50% during the profound hypertrophy of mechanical overload (n = 13, P < 0.05), as is the related transcription factor FOXO1 (*P = 0.025), which promotes MAFbx. Insulin-like growth factor mRNA is also increased 400% as part of the increase in plantaris mass (P = 0.011). Data are means ± SE; expression was normalized to ribosomal 18s.
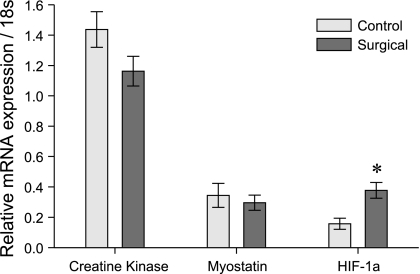
Fig. 5.
Other muscle genes. The mRNA expression of creatine kinase and myostatin is unchanged during mechanical overload, but hypoxia-inducible factor-1α (HIF-1α) is strongly upregulated (n = 13, *P = 0.006). This may reflect the metabolic strain on the plantaris from increased mechanical loading and its relative inability to enhance oxidative mechanisms. Data are means ± SE; expression was normalized to ribosomal 18s.
The mRNA expression of the growth factor myostatin was unchanged in our study. Although creatine kinase mRNA was expected to increase modestly with this type of muscle hypertrophy, no changes were detected. Finally, the mRNA expression of the transcription factor HIF-1α was elevated two- to threefold (P = 0.006). In each of these cases, protein levels and phosphorylation states are important functional considerations that are unaddressed by our mRNA measurements.
DISCUSSION
We observed a rapid 50% increase of plantaris muscle mass following 2 wk of compensatory overload in nonhibernating ground squirrels. We did not observe shifts in MyHC expression, although data from other mammals and our own hibernation studies predicted fast-to-slow conversions (6, 9, 56, 60, 63). While skeletal muscle activity is reasonably confirmed by this study as a possible mediator of atrophy during hibernation, our findings question the ability of ground squirrels to respond to muscle-loading challenges via MyHC transitions. Our data further suggest that other factors, beyond the stimulus to muscles by periodic rewarming bouts, act as primary determinants of MyHC expression during hibernation. We report marked decreases in mRNA expression of the ubiquitin-proteasome pathway genes MuRF1, MAFBx, and FOXO-1, and an increase in IGF-1 mRNA, which are all consistent with muscle hypertrophy and a related reduction in atrophy pathways. The significant increase in HIF-1α mRNA may reveal a limited ability of the plantaris to enhance oxidative metabolism in the face of increased mechanical loading.
MyHC isoform expression is unchanged with hypertrophy.
The details, interactions, and fundamental mechanisms of the control of skeletal muscle mass and fiber type are still being described, because the complexities are rich and incompletely understood (6, 7, 11, 17, 19, 20, 33, 37, 41, 50, 56, 65, 66, 72, 75, 76, 78, 82). A growing collection of studies of muscle in hibernators is contributing a broader and clinically relevant perspective on muscle plasticity (for a review, see Ref. 58; also 10, 14, 15, 25–27, 30, 39, 45, 57, 59, 60, 87). Hibernators may demonstrate adaptations that are not apparent in other mammals and can illustrate how fiber type and muscle mass can be regulated more independently than previously thought. At least for small rodent hibernators, it is not yet clear that multiple, specific, muscle adaptations exist, because the general phenomenon of torpor itself may impact muscle plasticity without altering existing signaling pathways (1, 2, 28, 38, 52, 58–60, 84–86, 90).
The mechanical overload model we employ is capable of generating significant hypertrophy and MyHC isoform switching in rats and mice (6, 9, 56, 63), and was expected to generate both in ground squirrels. In humans and multiple rodent species alike, exercise dramatically increases mRNA for types 1 and 2a MyHC by 50–100%, with smaller changes in proteins (5, 69, 88). Our findings of a 40–50% increase in plantaris muscle mass are very similar to other studies and demonstrate that squirrels are at least sensitive to increased mechanical loading.
The present study addresses the possibility that control of MyHC isoform expression in ground squirrel muscles, distinctly from mass, is not mediated by changes in mechanical loading. The expression of these isoforms still must have consequences for mechanical and energy efficiency, but may have become uncoupled from activity-sensing pathways. This would be a fascinating departure for mammalian muscle and argues that the observed fast-to-slow MyHC transitions occurring during hibernation are more likely to be directed by other control mechanisms. A relevant issue is whether the shivering activity of interbout arousals would be sufficient to induce MyHC shifts, or conversely whether the muscles are altered first to support the shivering activity. Our current data further reinforce how muscle mass and MyHC profiles can be shaped independently, which is a relatively recent but increasingly supported tenet of mammalian muscle physiology.
Despite the apparent strength of the stimulus and the clear increases in mass and contractile proteins the lack of any isoform switching in Fall-active squirrels was surprising. It cannot be argued that the plantaris of this species is simply incapable of such MyHC isoform shifts, because we have shown that type 2b to 2x shifts occur during hibernation (60). The squirrels used in this study were active and killed by the first week of September. They were not transitioning into torpor, which otherwise might be a confounding factor. Most importantly, if the squirrels were in a seasonally transitive state to torpor that should have produced the fast-to-slow shifts that here are so conspicuously absent. Patterns of neuromuscular activation during synergist ablation and rewarming from torpor are different; we do not equate them, but contend that we have produced an energetically and mechanically robust challenge to the plantaris that has not produced MyHC shifts.
It is therefore likely that fast-to-slow shifts, already unusual for their appearance during prolonged inactivity and modest atrophy, are prompted by seasonal stimuli and are not generated by the brief but intense activity of interbout arousals. We have additional observations of the plantaris that suggest that these changes occur early in the hibernation season, which also support the supposition that they are not slowly accumulated with shivering activity during Winter (M. Nowell et al., unpublished observations). We hypothesize that activation of the plantaris during late arousal from torpor requires slower MyHC isoforms, which are expressed without activation of activity-sensing pathways. Important support comes from elegant studies which demonstrate that activity-independent mechanisms remain critical in governing muscle fiber type, whether with resistance exercise or inactivity. Daily activity pattern is not correlated with fiber type, which can persist with inactivity (29, 34, 35, 62, 92), and resistance exercise was ineffective at retarding atrophy-related changes (22). It seems more apparent that these and other activity-independent mechanisms (64) are likely in determining fiber type in hibernating species as well.
Molecular pathways of muscle plasticity.
Our survey of the genes involved in controlling muscle mass and fiber type is incomplete, but nevertheless targets several major regulatory factors. The ubiquitin ligases, the FOXO transcription factors, and IGF genes are considered critical and interrelated controllers of muscle atrophy and hypertrophy (8, 21, 42, 49, 70, 89). Their measurement here helps to characterize the mechanisms of muscle mass increase in this species, while contrasting related determinations from hibernation. The IGF-1 axis acts on the FOXO transcription factors, which promotes MAFBx and ubiquitination of contractile proteins, involved in proteasome-mediated breakdown of muscle (37, 46, 55, 65, 68, 71, 91). The ultimate ubiquitination of contractile proteins is slowed in hypertrophy, and was expected in our compensatory overload, as well as potentially in more active muscles and those protected from atrophy (66).
Ubiquitination of proteins is an important, ongoing process even during hibernation (84, 86). FOXO proteins are themselves translocated from nucleus to cytoplasm, and MuRF1 is associated with the contractile architecture under different loading conditions, and so alterations in the mRNA abundance are not required to transduce activity. Nevertheless, we detected significant reductions in the mRNA expression of FOXO-1a, MAFbx, and MuRF1, and associated increases in IGF, while myostatin and FOXO-3 remained the same. Synergist ablation readily induces these changes, and so muscle activity during hibernation, while undeniably different in duration and intensity than chronic overload, plausibly may serve a protective role in limiting atrophy via these pathways. We have previously noted an increase in MAFbx in plantaris during hibernation (60) but have more recent results indicating that MAFbx, MuRF1, and FOXO-1 are in fact reduced, but only toward the very end of hibernation (M. Nowell et al., unpublished observation). Similarly, myostatin was not downregulated in the muscle mass increase of this mechanical overload protocol, but we have observed seasonal decreases by 50% in some muscle types during hibernation. Reduction in myostatin is more typically associated with muscle mass increases or atrophy protection (36, 44, 47, 77). Collectively it does not appear that atrophy avoidance in S. lateralis is simply a reversal of pathways that are upregulated here in muscle growth nor in other rodents (22).
Metabolic markers of hypertrophy in muscle.
The increase in loading, activation, and use of the plantaris with synergist ablation is not trivial. The 50% increase in mass over only 2 wk indicates how dramatically the plantaris must change its recruitment, and potentially, energetic and fatigue characteristics. However, we did not find that citrate synthase activity was elevated, and enzyme activity is low in this muscle compared with other muscles and the heart of this species (B. C. Rourke, unpublished observations). This is not surprising given the predominant fast phenotype and may reveal a limit to the plasticity of the plantaris; mass can change readily in atrophy or hypertrophy and MyHC can remodel during hibernation, but enzyme complements might be less mutable. In a congener, measurements of the gastrocnemius, another mixed fiber-type muscle, also show that mitochondrial oxidative respiration was not changed during hibernation (53).
We thought creatine kinase might be altered with hypertrophy, as fluxes are important in hibernation (67) and mRNA expression is reduced 70% in hibernating squirrels (2). Proteomics analyses also point to important modulations of creatine kinase protein levels, which we have also detected in pilot studies using two-dimensional SDS gel preparations. Our current findings on creatine kinase mRNA suggest that any changes in the plantaris do not require transcriptional control.
The elevated mRNA expression we observed for the hypoxia-related transcription factor HIF-1α is intriguing. Elevations are seen in humans with resistance exercise (13) and in hypoxic skeletal muscle of mice (12), and knockout models cannot improve oxidative capacity (48). Levels in muscle measured by real-time PCR did not change, but protein levels were elevated 60–70% in hibernating squirrels (51). An interesting remark from those findings is that hibernators are not expected to have a systemic hypoxia because of lowered tissue metabolism despite an overall reduced delivery, although certain tissues might be locally hypoxic. The two- to threefold increase we observed in HIF-1α mRNA may be related to the strength of the overload stimulus and the metabolic strain of dramatically increased recruitment. The plantaris itself during overload may be fairly hypoxic, as it might not have the ability to convert additional oxidative capacity readily, as evidenced by our citrate synthase and creatine kinase measurements. Additionally, perfusion of the plantaris may not be sufficient to support increased consumption if capillary density is not similarly altered; VEGF may be a useful molecular marker of this.
Perspectives and Significance
The mechanical overload model applied to ground squirrels reveals yet another surprising characteristic of muscle physiology in this hibernating species: loading can induce an increase in muscle mass without any alteration to MyHC isoforms. This reduces the probability that shivering is directing fast-to-slow MyHC isoform shifts during hibernation, but bolsters arguments that muscle activity may help attenuate loss of mass. Some other neural, hormonal, or seasonal cue must prompt and initiate MyHC isoform profile adaptations for hibernation, with uncertain molecular mechanism but as a probable requirement for thermogenesis. Molecular pathways controlling atrophy are inactivated during the compensatory hypertrophy, and can be contrasted with the prevention of atrophy during hibernation. The plantaris muscle, as one of a group of hindlimb muscles involved in shivering activity, has clearly separated pathways controlling fiber size and type and a seasonal control of MyHC expression that remains unclear. This seasonal regulation of MyHC distinct from loading remains a novel mechanism to be unraveled, newly added as an influences on mammalian muscle contractile protein expression.
GRANTS
Funding was provided by National Institutes of Health Minority Biomedical Research Support SCORE 2 S06 Grant GM-063119 (to B. C. Rourke) and California State University, Long Beach (to P. J. Selpides).
Acknowledgments
Expert surgical assistance was provided by Michael Baker, who was supported by V. J. Caiozzo (National Institutes of Health Grant 46856). Yanett Roman provided additional technical assistance.
REFERENCES
- 1.Abnous K, Dieni CA, Storey KB. Regulation of Akt during hibernation in Richardson's ground squirrels. Biochim Biophys Acta 1780: 185–193, 2008. [DOI] [PubMed] [Google Scholar]
- 2.Abnous K, Storey KB. Regulation of skeletal muscle creatine kinase from a hibernating mammal. Arch Biochem Biophys 467: 10–19, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Abnous K, Storey KB. Skeletal muscle hexokinase: regulation in mammalian hibernation. Mol Cell Biochem 319: 41–50, 2008. [DOI] [PubMed] [Google Scholar]
- 4.Adams GR, Caiozzo VJ, Baldwin KM. Skeletal muscle unweighting: spaceflight and ground-based models. J Appl Physiol 95: 2185–2201, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Balagopal P, Schimke JC, Ades P, Adey D, Nair KS. Age effect on transcript levels and synthesis rate of muscle MHC and response to resistance exercise. Am J Physiol Endocrinol Metab 280: E203–E208, 2001. [DOI] [PubMed] [Google Scholar]
- 6.Baldwin KM, Haddad F. Effects of different activity and inactivity paradigms on myosin heavy chain gene expression in striated muscle. J Appl Physiol 90: 345–357, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Bassel-Duby R, Olson EN. Signaling pathways in skeletal muscle remodeling. Annu Rev Biochem 75: 19–37, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Bodine S, Latres E, Baumhueter S, Lai VKM, Nunez L, Clarke B, Poueymirou WT, Panaro FJ, Na E, Dharmarajan J, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294: 1704–1708, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Caiozzo VJ, Baker MJ, Baldwin KM. Modulation of myosin isoform expression by mechanical loading: role of stimulation frequency. J Appl Physiol 82: 211–218, 1997. [DOI] [PubMed] [Google Scholar]
- 10.Chen J, Sun M, Liang B, Xu A, Zhang S, Wu D. Cloning and expression of PDK4, FOX01A and DYRK1A from the hibernating greater horseshoe bat (Rhinolophus ferrumequinum). Comp Biochem Physiol B Biochem Mol Biol 146: 166–171, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Chin ER Role of Ca2+/calmodulin-dependent kinases in skeletal muscle plasticity. J Appl Physiol 99: 414–423, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Däpp C, Gassmann M, Hoppeler H, Fluck M. Hypoxia-induced gene activity in disused oxidative muscle. Adv Exp Med Biol 588: 171–188, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Drummond MJ, Fujita S, Takashi A, Dreyer HC, Volpi E, Rasmussen BB. Human muscle gene expression following resistance exercise and blood flow restriction. Med Sci Sports Exerc 40: 691–698, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eddy SF, Morin P Jr, Storey KB. Differential expression of selected mitochondrial genes in hibernating little brown bats, Myotis lucifugus. J Exp Zoolog A Comp Exp Biol 305: 620–630, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Eddy SF, Storey KB. p38(MAPK) regulation of transcription factor targets in muscle and heart of the hibernating bat, Myotis lucifugus. Cell Biochem Funct 25: 759–765, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Edgerton VR, Roy RR, Allen DL, Monti RJ. Adaptations in skeletal muscle disuse or decreased-use atrophy. Am J Phys Med Rehabil 81, Suppl 11: S127–S147, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Franch HA, Price SR. Molecular signaling pathways regulating muscle proteolysis during atrophy. Curr Opin Clin Nutr Metab Care 8: 271–275, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Giger JM, Haddad F, Qin AX, Zeng M, Baldwin KM. Effect of unloading on type I myosin heavy chain gene regulation in rat soleus muscle. J Appl Physiol 98: 1185–1194, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Glass DJ Signalling pathways that mediate skeletal muscle hypertrophy and atrophy. Nat Cell Biol 5: 87–90, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Glass DJ Molecular mechanisms modulating muscle mass. Trends Mol Med 9: 344–350, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Gomes MD, Lecker SH, Jagoe RT, Navon A, Goldberg AL. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc Natl Acad Sci USA 98: 14440–14445, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haddad F, Adams GR, Bodell PW, Baldwin KM. Isometric resistance exercise fails to counteract skeletal muscle atrophy processes during the initial stages of unloading. J Appl Physiol 100: 433–441, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Haddad F, Roy RR, Zhong H, Edgerton VR, Baldwin KM. Atrophy responses to muscle inactivity. I. Cellular markers of protein deficits. J Appl Physiol 95: 781–790, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Haddad F, Roy RR, Zhong H, Edgerton VR, Baldwin KM. Atrophy responses to muscle inactivity. II. Molecular markers of protein deficits. J Appl Physiol 95: 791–802, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Harlow HJ, Lohuis T, Anderson-Sprechner, Beck TD. Body surface temperature of hibernating black bears may be related to periodic muscle activity. J Mammology 85: 414–419, 2004. [Google Scholar]
- 26.Harlow HJ, Lohuis T, Beck TD, Iaizzo PA. Muscle strength in overwintering bears. Nature 409: 997, 2001. [DOI] [PubMed] [Google Scholar]
- 27.Hershey JD, Robbins CT, Nelson OL, Lin DC. Minimal seasonal alterations in the skeletal muscle of captive brown bears. Physiol Biochem Zool 81: 138–147, 2008. [DOI] [PubMed] [Google Scholar]
- 28.Hittel DS, Storey KB. Differential expression of mitochondria-encoded genes in a hibernating mammal. J Exp Biol 205: 1625–1631, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Hodgson JA, Roy RR, Higuchi N, Monti RJ, Zhong H, Grossman E, Edgerton VR. Does daily activity level determine muscle phenotype? J Exp Biol 208: 3761–3770, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Horman S, Hussain N, Dilworth SM, Storey KB, Rider MH. Evaluation of the role of AMP-activated protein kinase and its downstream targets in mammalian hibernation. Comp Biochem Physiol B Biochem Mol Biol 142: 374–382, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Huey KA, Haddad F, Qin AX, Baldwin KM. Transcriptional regulation of the type I myosin heavy chain gene in denervated rat roleus. Am J Physiol Cell Physiol 284: C738–C748, 2003. [DOI] [PubMed] [Google Scholar]
- 32.Huey KA, Roy RR, Haddad F, Edgerton VR, Baldwin KM. Transcriptional regulation of the type I myosin heavy chain promoter in inactive rat soleus. Am J Physiol Cell Physiol 282: C528–C537, 2002. [DOI] [PubMed] [Google Scholar]
- 33.Hunter RB, Kandarian SC. Disruption of either the Nfkb1 or the Bcl3 gene inhibits skeletal muscle atrophy. J Clin Invest 114: 1504–1511, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hyatt JP, Roy RR, Baldwin KM, Edgerton VR. Nerve activity-independent regulation of skeletal muscle atrophy: role of MyoD and myogenin in satellite cells and myonuclei. Am J Physiol Cell Physiol 285: C1161–C1173, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Hyatt JP, Roy RR, Baldwin KM, Wernig A, Edgerton VR. Activity-unrelated neural control of myogenic factors in a slow muscle. Muscle Nerve 33: 49–60, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Ji M, Zhang Q, Ye J, Wang X, Yang W, Zhu D. Myostatin induces p300 degradation to silence cyclin D1 expression through the PI3K/PTEN/Akt pathway. Cell Signal 20: 1452–1458, 2008. [DOI] [PubMed] [Google Scholar]
- 37.Kandarian SC, Jackman RW. Intracellular signaling during skeletal muscle atrophy. Muscle Nerve 33: 155–165, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Knight JE, Narus EN, Martin SL, Jacobson A, Barnes BM, Boyer BB. mRNA stability and polysome loss in hibernating Arctic ground squirrels (Spermophilus parryii). Mol Cell Biol 20: 6374–6379, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koebel DA, Miers PG, Nelson RA, Steffen JM. Biochemical changes in skeletal muscles of denning bears (Ursus americanus). Comp Biochem Physiol B 100: 377–380, 1991. [DOI] [PubMed] [Google Scholar]
- 40.Kumar S, Nei M, Dudley J, Tamura K. MEGA: a biologist-centric software for evolutionary analysis of DNA and protein sequences. Brief Bioinform 9: 299–306, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Latres E, Amini AR, Amini AA, Griffiths J, Martin FJ, Wei Y, Lin HC, Yancopoulos GD, Glass DJ. Insulin-like growth factor-1 (IGF-1) inversely regulates atrophy-induced genes via the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin (PI3K/Akt/mTOR) pathway. J Biol Chem 280: 2737–2744, 2005. [DOI] [PubMed] [Google Scholar]
- 42.Lecker SH, Solomon V, Mitch WE, Goldberg AL. Muscle protein breakdown and the critical role of the ubiquitin-proteasome pathway in normal and disease states. J Nutr 129: 227S–237S, 1999. [DOI] [PubMed] [Google Scholar]
- 43.Lee K, Park JY, Yoo W, Gwag T, Lee LW, Byun MW, Choi I. Overcoming muscle atrophy in a hibernating mammal despite prolonged disuse in dormancy: proteomic and molecular assessment. J Cell Biochem 104: 642–656, 2008. [DOI] [PubMed] [Google Scholar]
- 44.Li ZB, Kollias HD, Wagner KR. Myostatin directly regulates skeletal muscle fibrosis. J Biol Chem 283: 19371–19378, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lohuis TD, Harlow HJ, Beck TDI. Hibernating black bears (Ursus americanus) experience skeletal muscle protein balance during winter anorexia. Comp Biochem Physiol B Biochem Mol Biol 147: 20–28, 2007. [DOI] [PubMed] [Google Scholar]
- 46.Mammucari C, Schiaffino S, Sandri M. Downstream of Akt: FoxO3 and mTOR in the regulation of autophagy in skeletal muscle. Autophagy 4: 524–526, 2008. [DOI] [PubMed] [Google Scholar]
- 47.Martin CI, Johnston IA. The role of myostatin and the calcineurin-signalling pathway in regulating muscle mass in response to exercise training in the rainbow trout Oncorhynchus mykiss Walbaum. J Exp Biol 208: 2083–2090, 2005. [DOI] [PubMed] [Google Scholar]
- 48.Mason S, Johnson RS. The role of HIF-1 in hypoxic response in the skeletal muscle. Adv Exp Med Biol 618: 229–244, 2007. [DOI] [PubMed] [Google Scholar]
- 49.McCall GE, Allen DL, Haddad F, Baldwin KM. Transcriptional regulation of IGF-I expression in skeletal muscle. Am J Physiol Cell Physiol 285: C831–C839, 2003. [DOI] [PubMed] [Google Scholar]
- 50.McCullagh KJ, Calabria E, Pallafacchina G, Ciciliot S, Serrano AL, Argentini C, Kalhovde JM, Lomo T, Schiaffino S. NFAT is a nerve activity sensor in skeletal muscle and controls activity-dependent myosin switching. Proc Natl Acad Sci USA 101: 10590–10595, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morin P, Storey KB. Cloning and expression of hypoxia-inducible factor 1α from the hibernating ground squirrel, Spermophilus tridecemlineatus. Biochim Biophys Acta 1729: 32–40, 2005. [DOI] [PubMed] [Google Scholar]
- 52.Morin P, Storey KB. Evidence for a reduced transcriptional state during hibernation in ground squirrels. Cryobiology 53: 310–318, 2006. [DOI] [PubMed] [Google Scholar]
- 53.Muleme HM, Walpole AC, Staples JF. Mitochondrial metabolism in hibernation: metabolic suppression, temperature effects, and substrate preferences. Physiol Biochem Zool 79: 474–483, 2006. [DOI] [PubMed] [Google Scholar]
- 54.Nelson OL, Robbins CT, Wu Y, Granzier H. Titin isoform switching is a major cardiac adaptive response in hibernating grizzly bears. Am J Physiol Heart Circ Physiol 295: H366–H371, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ni YG, Wang N, Cao DJ, Sachan N, Morris DJ, Gerard RD, Kuro OM, Rothermel BA, Hill JA. FoxO transcription factors activate Akt and attenuate insulin signaling in heart by inhibiting protein phosphatases. Proc Natl Acad Sci USA 104: 20517–20522, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Parsons SA, Millay DP, Wilkins BJ, Bueno OF, Tsika GL, Neilson JR, Liberatore CM, Yutzey KE, Crabtree GR, Tsika RW, Molkentin JD. Genetic loss of calcineurin blocks mechanical overload-induced skeletal muscle fiber type switching but not hypertrophy. J Biol Chem 279: 26192–26200, 2004. [DOI] [PubMed] [Google Scholar]
- 57.Reid WD, Ng A, Wilton R, Milsom WK. Characteristics of diaphragm muscle fibre types in hibernating squirrels. Respir Physiol 101: 301–309, 1995. [DOI] [PubMed] [Google Scholar]
- 58.Rourke BC Myosin isoform dynamics in muscles of hibernating mammal species: lessons and opportunities. In: Hypometabolism in Animals: Hibernation, Torpor and Cryobiology, edited by Lovegrove BG and McKechnie AE. Pitermaritzburg, South Africa: University of KwaZula-Natal, 2008, p. 57–64.
- 59.Rourke BC, Cotton CJ, Harlow HJ, Caiozzo VJ. Maintenance of slow type I myosin protein and mRNA expression in overwintering prairie dogs (Cynomys leucurus and ludovicianus) and black bears (Ursus americanus). J Comp Physiol [B] 176: 709–720, 2006. [DOI] [PubMed] [Google Scholar]
- 60.Rourke BC, Yokoyama Y, Milsom WK, Caiozzo VJ. Myosin isoform expression and MAFbx mRNA levels in hibernating golden-mantled ground squirrels (Spermophilus lateralis). Physiol Biochem Zool 77: 582–593, 2004. [DOI] [PubMed] [Google Scholar]
- 61.Rourke BC, Qin A, Haddad F, Baldwin KM, Caiozzo VJ. Cloning and sequencing of myosin heavy chain isoform cDNAs in golden-mantled ground squirrels: effects of hibernation on mRNA expression. J Appl Physiol 97: 1985–1991, 2004. [DOI] [PubMed] [Google Scholar]
- 62.Roy RR, Pierotti DJ, Garfinkel A, Zhong H, Baldwin KM, Edgerton VR. Persistence of motor unit and muscle fiber types in the presence of inactivity. J Exp Biol 211: 1041–1049, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Roy RR, Talmadge RJ, Fox K, Lee M, Ishihara A, Edgerton VR. Modulation of MHC isoforms in functionally overloaded and exercised rat plantaris fibers. J Appl Physiol 83: 280–290, 1997. [DOI] [PubMed] [Google Scholar]
- 64.Roy RR, Eldridge L, Baldwin KM, Edgerton VR. Neural influence on slow muscle properties: inactivity with and without cross-reinnervation. Muscle Nerve 19: 707–714, 1996. [DOI] [PubMed] [Google Scholar]
- 65.Sacheck JM, Ohtsuka A, McLary SC, Goldberg AL. IGF-I stimulates muscle growth by suppressing protein breakdown and expression of atrophy-related ubiquitin ligases, atrogin-1 and MuRF1. Am J Physiol Endocrinol Metab 287: E591–E601, 2004. [DOI] [PubMed] [Google Scholar]
- 66.Sandri M, Lin J, Handschin C, Yang W, Arany ZP, Lecker SH, Goldberg AL, Spiegelman BM. PGC-1α protects skeletal muscle from atrophy by suppressing FoxO3 action and atrophy-specific gene transcription. Proc Natl Acad Sci USA 103: 16260–16265, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schaefer S, Carr LJ, Kreutzer U, Jue T. Myocardial adaptation during acute hibernation: mechanisms of phosphocreatine recovery. Cardiovasc Res 27: 2044–2051, 1993. [DOI] [PubMed] [Google Scholar]
- 68.Schneider MR, Wolf E, Hoeflich A, Lahm H. IGF-binding protein-5: flexible player in the IGF system and effector on its own. J Endocrinol 172: 423–440, 2002. [DOI] [PubMed] [Google Scholar]
- 69.Short KR, Vittone JL, Bigelow ML, Proctor DN, Coenen-Schimke JM, Rys P, Nair KS. Changes in myosin heavy chain mRNA and protein expression in human skeletal muscle with age and endurance exercise training. J Appl Physiol 99: 95–102, 2005. [DOI] [PubMed] [Google Scholar]
- 70.Solomon V, Baracos V, Sarraf P, Goldberg AL. Rates of ubiquitin conjugation increase when muscles atrophy, largely through activation of the N-end rule pathway. Proc Natl Acad Sci USA 95: 12602–12607, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Southgate RJ, Neill B, Prelovsek O, El-Osta A, Kamei Y, Miura S, Ezaki O, McLoughlin TJ, Zhang W, Unterman TG, Febbraio MA. FOXO1 regulates the expression of 4E-BP1 and inhibits mTOR signaling in mammalian skeletal muscle. J Biol Chem 282: 21176–21186, 2007. [DOI] [PubMed] [Google Scholar]
- 72.Spangenburg EE, Booth FW. Molecular regulation of individual skeletal muscle fibre types. Acta Physiol Scand 178: 413–424, 2003. [DOI] [PubMed] [Google Scholar]
- 73.Srere P Citrate synthase. Methods Enzymol 13: 3–5, 1969. [Google Scholar]
- 74.Steffen JM, Koebel DA, Musacchia XJ, Milsom WK. Morphometric and metabolic indices of disuse in muscles of hibernating ground squirrels. Comp Biochem Physiol B 99: 815–819, 1991. [DOI] [PubMed] [Google Scholar]
- 75.Stitt TN, Drujan D, Clarke BA, Panaro F, Timofeyva Y, Kline WO, Gonzalez M, Yancopoulos GD, Glass DJ. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell 14: 395–403, 2004. [DOI] [PubMed] [Google Scholar]
- 76.Sugiura T, Abe N, Nagano M, Goto K, Sakuma K, Naito H, Yoshioka T, Powers SK. Changes in PKB/Akt and calcineurin signaling during recovery in atrophied soleus muscle induced by unloading. Am J Physiol Regul Integr Comp Physiol 288: R1273–R1278, 2005. [DOI] [PubMed] [Google Scholar]
- 77.Swanson DL, Sabirzhanov B, VandeZande A, Clark TG. Seasonal variation of myostatin gene expression in pectoralis muscle of house sparrows (Passer domesticus) is consistent with a role in regulating thermogenic capacity and cold tolerance. Physiol and Biochem Zool 82: 121–128. 2009. [DOI] [PubMed] [Google Scholar]
- 78.Talmadge RJ, Otis JS, Rittler MR, Garcia ND, Spencer SR, Lees SJ, Naya FJ. Calcineurin activation influences muscle phenotype in a muscle-specific fashion. BMC Cell Biol 5: 28, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Talmadge RJ, Roy RR. Electrophoretic separation of rat skeletal muscle myosin heavy-chain isoforms. J Appl Physiol 75: 2337–2340, 1993. [DOI] [PubMed] [Google Scholar]
- 80.Templeton GH, Sweeney HL, Timson BF, Padalino M, Dudenhoeffer GA. Changes in fiber composition of soleus muscle during rat hindlimb suspension. J Appl Physiol 65: 1191–1195, 1988. [DOI] [PubMed] [Google Scholar]
- 81.Thomason DB, Herrick RE, Surdyka D, Baldwin KM. Time course of soleus muscle myosin expression during hindlimb suspension and recovery. J Appl Physiol 63: 130–137, 1987. [DOI] [PubMed] [Google Scholar]
- 82.Tidball JG Mechanical signal transduction in skeletal muscle growth and adaptation. J Appl Physiol 98: 1900–1908, 2005. [DOI] [PubMed] [Google Scholar]
- 83.Tinker DB, Harlow HJ, Beck TD. Protein use and muscle-fiber changes in free-ranging, hibernating black bears. Physiol Zool 71: 414–424, 1998. [DOI] [PubMed] [Google Scholar]
- 84.van Breukelen F, Martin SL. Reversible depression of transcription during hibernation. J Comp Physiol [B] 172: 355–361, 2002. [DOI] [PubMed] [Google Scholar]
- 85.van Breukelen F, Martin SL. Molecular adaptations in mammalian hibernators: unique adaptations or generalized responses? J Appl Physiol 92: 2640–2647, 2002. [DOI] [PubMed] [Google Scholar]
- 86.van Breukelen F, Sonenberg N, Martin SL. Seasonal and state-dependent changes of eIF4E and 4E-BP1 during mammalian hibernation: implications for the control of translation during torpor. Am J Physiol Regul Integr Comp Physiol 287: R349–R353, 2004. [DOI] [PubMed] [Google Scholar]
- 87.Wickler SJ, Hoyt DF, van Breukelen F. Disuse atrophy in the hibernating golden-mantled ground squirrel, Spermophilus lateralis. Am J Physiol Regul Integr Comp Physiol 261: R1214–R1217, 1991. [DOI] [PubMed] [Google Scholar]
- 88.Willoughby DS, Nelson MJ. Myosin heavy-chain mRNA expression after a single session of heavy-resistance exercise. Med Sci Sports Exerc 34: 1262–1269, 2002. [DOI] [PubMed] [Google Scholar]
- 89.Witt SH, Granzier H, Witt CC, Labeit S. MURF-1 and MURF-2 target a specific subset of myofibrillar proteins redundantly: towards understanding MURF-dependent muscle ubiquitination. J Mol Biol 350: 713–722, 2005. [DOI] [PubMed] [Google Scholar]
- 90.Yan J, Barnes BM, Kohl F, Marr TG. Modulation of gene expression in hibernating arctic ground squirrels. Physiol Genomics 32: 170–181, 2008. [DOI] [PubMed] [Google Scholar]
- 91.Zhao J, Brault JJ, Schild A, Cao P, Sandri M, Schiaffino S, Lecker SH, Goldberg AL. FoxO3 coordinately activates protein degradation by the autophagic/lysosomal and proteasomal pathways in atrophying muscle cells. Cell Metab 6: 472–483, 2007. [DOI] [PubMed] [Google Scholar]
- 92.Zhong H, Roy RR, Hodgson JA, Talmadge RJ, Grossman EJ, Edgerton VR. Activity-independent neural influences on cat soleus motor unit phenotypes. Muscle Nerve 26: 252–264, 2002. [DOI] [PubMed] [Google Scholar]