Abstract
Acute ethanol (EtOH) administration impairs circadian clock phase resetting, suggesting a mode for the disruptive effect of alcohol abuse on human circadian rhythms. Here, we extend this research by characterizing the chronobiological effects of chronic alcohol consumption. First, daily profiles of EtOH were measured in the suprachiasmatic nucleus (SCN) and subcutaneously using microdialysis in hamsters drinking EtOH. In both cases, EtOH peaked near lights-off and declined throughout the dark-phase to low day-time levels. Drinking bouts preceded EtOH peaks by ∼20 min. Second, hamsters chronically drinking EtOH received a light pulse during the late dark phase [Zeitgeber time (ZT) 18.5] to induce photic phase advances. Water controls had shifts of 1.2 ± 0.2 h, whereas those drinking 10% and 20% EtOH had much reduced shifts (0.5 ± 0.1 and 0.3 ± 0.1 h, respectively; P < 0.001 vs. controls). Third, incremental decreases in light intensity (270 lux to 0.5 lux) were used to explore chronic EtOH effects on photic entrainment and rhythm stability. Activity onset was unaffected by 20% EtOH at all light intensities. Conversely, the 24-h pattern of activity bouts was disrupted by EtOH under all light intensities. Finally, replacement of chronic EtOH with water was used to examine withdrawal effects. Water controls had photic phase advances of 1.1 ± 0.3 h, while hamsters deprived of EtOH for 2–3 days showed enhanced shifts (2.1 ± 0.3 h; P < 0.05 vs. controls). Thus, in chronically drinking hamsters, brain EtOH levels are sufficient to inhibit photic phase resetting and disrupt circadian activity. Chronic EtOH did not impair photic entrainment; however, its replacement with water potentiated photic phase resetting.
Keywords: suprachiasmatic nucleus, glutamate, microdialysis, drinking rhythms
ethanol (etoh) is highly disruptive to mammalian circadian functions, including sleep (5, 18, 32, 33, 50) and rhythms of melatonin (33, 53), glucocorticoids (2, 49), thyroid-stimulating hormone (13), and body temperature (4, 14, 15, 66). Moreover, disruptions in hypothalamic-pituitary-adrenal axis function are associated with increased EtOH preference in animal models (49), and in humans, this may be a risk factor for developing alcoholism and relapse after abstinence (6, 11, 34, 46, 68). Circadian-based sleep problems are also implicated in the development of alcoholism and in abstinent alcoholics' propensity to relapse (51, 52). In this regard, it is becoming clear that the brain systems involved with circadian regulation are closely and reciprocally tied to those underlying alcohol abuse, since circadian disorders associated with sleep deprivation, shift work, or repeated jet lag can evoke a feedback cycle of circadian rhythm deterioration and reinforcing alcohol self administration (9, 24, 60, 64). A pivotal role of such circadian-related brain systems in the etiology of alcoholism is underscored by recent studies showing a link between circadian clock genes and an increased drive for alcohol consumption (62).
The suprachiasmatic nucleus (SCN) located in the anterior hypothalamus is the master circadian clock in mammals, capable of producing and maintaining physiological and behavioral rhythms (28, 42, 56, 57). Circadian rhythms generated by the SCN are entrained to the daily light-dark cycle via daily phase resetting mediated by glutamate release from the retinohypothalamic tract into the SCN (23, 27, 29). Glutamatergic activation of SCN N-methyl-d-aspartate (NMDA) receptors is essential for light-induced phase shifting to occur (1, 10, 38, 39). In recent reports, we showed that acute EtOH can block such glutamate-induced phase shifts in the SCN in vitro (48) and impair photic phase shifts in vivo (55). These results are consistent with reports that EtOH decreases glutamate release (41, 44) and inhibits NMDA-evoked ion currents (35). Although not all NMDA receptors are inhibited by EtOH (36, 59, 67), the NR2B subunit in the ventrolateral SCN, which is the primary subunit of the NMDA receptor complex involved in photic phase-resetting (43), is EtOH sensitive (3). It is also notable that EtOH-related actions on the NMDA receptor are considered to be central to intoxication, dependence, tolerance, addiction, and neuronal damage during withdrawal (7, 8, 20, 26, 31, 63, 65), and that EtOH's disruption of NMDA receptor-mediated phase resetting of the circadian clock may be a major factor directly or indirectly underlying these problems.
Our past work has been centered on the effects of acute EtOH application on clock regulation. It is known that chronic, free-choice EtOH drinking disrupts circadian phase-resetting responses to light pulses delivered very late in the dark-phase (ZT 21; Ref. 58). However, little is known about the timing of daily EtOH self-administration and the impact of the resultant EtOH pharmacokinetics on photic phase resetting, entrainment, and rhythm stability. The present study was, therefore, undertaken to explore the actions of chronic EtOH consumption on several critical aspects of circadian clock regulation. These included characterizations of 1) the daily pharmacokinetic profiles of EtOH in the SCN and periphery; 2) the in vivo effects of chronic drinking on behavioral activity patterns and photic phase resetting; 3) the ability of the clock to entrain to a range of light intensities; and 4) withdrawal effects on the photic phase-resetting response. Such information is basic to the understanding of neural mechanisms underlying the disruptive effects of alcohol abuse on the human circadian timing system.
MATERIALS AND METHODS
Animals.
Adult, male Syrian hamsters Mesocricetus auratus raised from breeders purchased from Harlan Sprague Dawley (Madison, IL) were used in this study. Animals were maintained in a temperature-controlled vivarium (23°C) under 14:10 light-dark photoperiod (LD) with light intensity of ∼270 lux with food (Prolab 3000, PMI Feeds, St. Louis, MO) and water provided ad libitum. The experiments were approved by the Kent State University Institutional Animal Care and Use Committee and were conducted under the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Microdialysis assessment of SCN and systemic EtOH during chronic drinking.
Microdialysis was used to characterize the 24-h profile of EtOH in the SCN of freely behaving animals maintained on EtOH (10% vol/vol in their drinking water), as their sole source of fluid for a minimum of 2 wk before experimentation. Procedures for microdialysis of the SCN are described elsewhere (17). Briefly, concentrically designed microdialysis probes were constructed from 26-gauge stainless-steel outer cannula, into which was inserted 32-gauge fused-silica tubing. Hemicellulose dialysis membrane tubing (12 kDa MW cutoff; 230 μm OD) was secured to the outer cannula with epoxy glue. The active dialysis length was 1.0 mm. The microdialysis probe was targeted stereotaxically to the SCN 2 days before experimentation, and the site was confirmed histologically from frozen 10-μm sections stained with cresyl violet after experimentation. Sampling was undertaken over 24 h with a sampling interval of 30 min. The EtOH in microdialysate samples was initially measured using validated gas chromatography procedures (HP-5890A gas chromatograph; internal standard = secondary butanol, initial temperature = 40°C, purge time = 1 min, final temperature = 225°C, final time = 5 min), and later using the Analox AM-1 Alcohol Analyser (Lunenburg, MA). The probe efficiency for EtOH is estimated at ∼12% (55). In a separate trial, simultaneous SCN and subcutaneous microdialysis were carried out in a single animal to compare the 24-h EtOH drinking profile with brain and peripheral EtOH pharmacokinetic profiles. The hamster was maintained on EtOH (20% vol/vol in tap water) over a 2-wk period prior to experimentation, and general circadian locomotor activity was measured using an infrared motion detector interfaced with a computerized data acquisition system (Clocklab; Coulbourn Instruments, Whitehall, PA). Drinking was measured using an in-house fabricated system consisting of a sipper tube inserted into a 1.5 “length of 1”-diameter metal tubing, into which a wireless infrared sensor was aimed directly at the sipper tip. Output from the sensor activated by complete insertion of the animal's head to drink was interfaced with the Clocklab system. For dual microdialysis, two probes were implanted in a single surgery, one targeted to the SCN, as described above, and the second (CMA Microdialysis, North Chelmsford, MA) implanted subcutaneously in the intrascapular region. Collection of both fractions was undertaken over 24 h with a sampling interval of 30 min. EtOH in the microdialysate samples was measured using the Analox AM-1 Alcohol Analyser.
Effect of chronic EtOH consumption on photic phase resetting.
This experiment was designed to examine the effects of chronic EtOH consumption on photic phase advances. Hamsters under LD were individually caged, and their general circadian locomotor activity rhythms were monitored. Animals in the experimental group received 10% or 20% EtOH as their sole source of fluid for at least 2 wk before experimentation. Controls received tap water. Daily EtOH or water consumption was measured for each animal over the course of the experiment. On the day of the experiment, animals in both groups received a 30-min light pulse of strong (270 lux) or weaker (25 lux) intensity at zeitgeber time (ZT) 18.5 (where ZT 12 is designated as the onset of the dark phase). The animals were then released into constant darkness (DD; with EtOH still provided) to assess phase shifting using a modified Aschoff Type II procedure (12). Activity onset was defined as the first 15-min activity bout that 1) exceeded 10% of the maximum rate for the day; 2) was preceded by a period of at least 4 h of inactivity; and 3) was followed by a period of at least 30 min of sustained activity. Phase shifts are calculated as the difference between the projected times of activity onset on the day after stimulation as determined by 1) back extrapolation of the least squares line through activity onsets on days 3–10 after treatment; and 2) extrapolation of the least squares line calculated from activity onset data collected for a minimum of 7 days prior to treatment.
Effects of chronic EtOH on activity and photic entrainment.
Hamsters under LD were individually caged, and their general circadian locomotor activity rhythms were monitored. Animals in the experimental group received 20% EtOH, as their sole source of fluid for a minimum of 2 wk before experimentation. Controls received tap water. Entrainment to LD was assessed over a 4-wk period at each of four successive light levels (270 lux, 25 lux, 5 lux, and 0.5 lux) delivered from overhead fluorescent tubes shielded with aluminum foil in the same cohort of animals. Animals were considered to exhibit stable entrainment to LD when daily activity onsets averaged over 10 days had a rhythm period of 24 h. Assessments of circadian activity were undertaken using activity bout analysis. An activity bout was defined as a period of continuous activity (regardless of duration) separated by at least 10 min of activity quiescence. The quantification of activity bouts during the active period was undertaken by counting the number of bouts between activity onset at lights off and activity offset (defined as the final 10-min period that was preceded by at least 60 min of sustained activity and followed by at least 4 h of activity quiescence). Quantification of activity bouts during the rest period was undertaken by counting the number of bouts occurring between activity offset and activity onset. Total bouts represented the number of activity bouts across the 24-h day. These measurements were averaged over a 1-wk period for each animal for each light intensity.
Effect of EtOH deprivation on photic phase resetting.
This experiment was designed to test whether replacement of EtOH with water after chronic EtOH consumption affects photic circadian clock phase-resetting response. Hamsters under LD were individually caged, and their general circadian locomotor activity rhythms were monitored for a minimum of 2 wk before experimentation. Animals in the experimental group received 20% EtOH as the sole source of fluid for 4 wk. Controls received tap water. After this time, animals in the experimental group received untreated water in lieu of EtOH. After 24, 48, or 72 h of EtOH deprivation, all groups received a 30-min light pulse (25 or 270 lux) at ZT 18.5, and then were released into DD for 2 wk to assess phase-shifting (n = 5 or 6/group).
Statistical analyses.
Differences in behavioral phase shifts were assessed by ANOVA and then when appropriate by a subsequent Student-Newman-Keuls post hoc mean comparison test. The level of significance was set at P < 0.05.
RESULTS
Daily profiles of SCN and peripheral EtOH during chronic drinking.
The histologically verified in vivo microdialysis probe tip locations relative to the SCN are shown in Fig. 1. The 24-h pharmacokinetic profile of EtOH in the SCN extracellular fluid compartment as assessed by microdialysis is presented in Fig. 2. The average amount of EtOH consumed by the hamsters in our experiments was ∼13 g·kg−1·day−1. Levels of EtOH in the SCN were highest just before, and during, the early portion of the dark-phase (ZT 10.5-16), averaging an estimated 13 mM (based on 12% probe efficiency) with a peak concentration of 19 mM (∼250% of the daily mean) at ZT 15 (n = 4). SCN EtOH declined to lower levels (7 mM) for the remainder of the 24-h period. In a separate trial conducted on one animal, the daily profiles of SCN and subcutaneous EtOH were assessed simultaneously using dual microdialysis probes. These were superimposed with daily drinking and locomotor rhythms (Fig. 3). The SCN and subcutaneous peaks of EtOH were closely aligned, with highest levels of both occurring during the early portion of the dark-phase (ZT 13–16), with estimated peak concentrations (20 and 45 mM, respectively) occurring at ZT 15. Levels of EtOH were lowest throughout the day, although there were peaks associated with sporadic drinking bouts during this period. Drinking bouts preceded EtOH peaks by 20 min, and each drinking bout increased brain and peripheral EtOH levels for ∼2 h. As apparent from Fig. 3, the effect of multiple drinking bouts spaced over a few hours (i.e., from ZT 12.5 to ZT 15.5) was cumulative, resulting in prolonged elevations in EtOH levels.
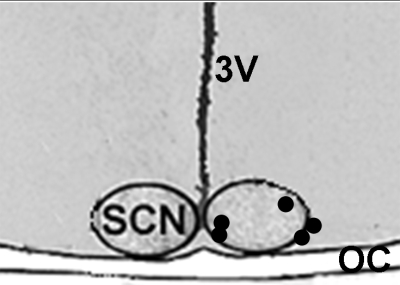
Fig. 1.
Histologically verified microdialysis probe tip locations relative to the SCN used in the ethanol (EtOH) pharmacokinetic analyses.
Fig. 2.
Twenty-four hour pharmacokinetics of EtOH in the suprachiasmatic nucleus (SCN) during forced 10% EtOH drinking. Dots represent means ± SE.
Fig. 3.
Composite 24-h pharmacokinetic profiles of SCN and subcutaneous EtOH superimposed with drinking and general locomotor activity rhythms from an individual hamster during forced 20% EtOH drinking. The black bar (top) represents the dark phase of the light-dark (LD) cycle.
Chronic EtOH inhibition of photic phase-resetting is light intensity dependent.
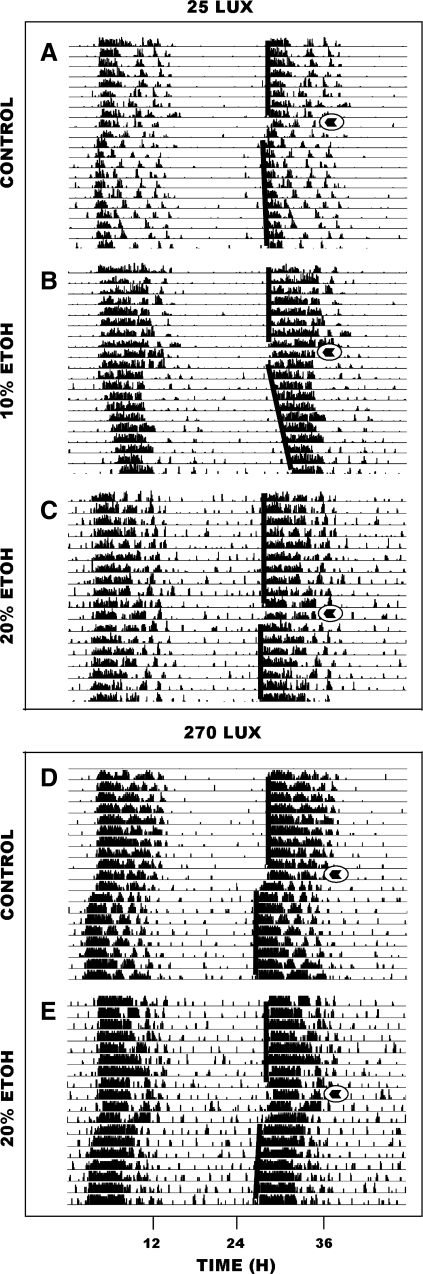
In animals maintained under LD, phase-advance shifts induced by a 30-min light pulse of 270 lux delivered at ZT 18.5 were similar for animals drinking 20% EtOH (n = 6) and water controls: n = 5; 1.4 ± 0.2 vs. 1.7 ± 0.2 h, respectively [F(1,10) = 0.9; P > 0.3] (Fig. 4). Conversely, phase-advance shifts induced by a 30-min pulse of less intense light (25 lux) were signficantly attenuated in animals drinking 20% (n = 5) or 10% EtOH (n = 5) compared with the water controls: n = 10; 0.3 ± 0.1 and 0.6 ± 0.1 h, respectively, vs. 1.2 ± 0.1 h [F(2,17) = 12.5; P < 0.001 vs. water]. Representative actograms of both trials are shown in Fig. 5. An EtOH-only control group was not included, as EtOH itself has no phase-resetting action (55, 58).
Fig. 4.
Long-term EtOH drinking attenuates photic phase-advance responses to a dim (25 lux; left), but not to a bright (270 lux; right) light pulse delivered late in the dark phase [zeitgeber time (ZT) 18.5]. Bars with different letters are significantly different (P < 0.05). Bars represent means ± SE.
Fig. 5.
Representative double-plotted actograms of general locomotor activity showing EtOH inhibition of photic phase-advance responses to dim, but not to bright, light pulses delivered at ZT 18.5. Animals receiving a 25-lux light pulse: A: water-drinker. B: 10% EtOH-drinker. C: 20% EtOH drinker. Animals receiving a 270-lux light pulse: D: water-drinker. E: 20% EtOH drinker. Circled arrowheads denote the time of the light pulses.
Chronic EtOH affects circadian activity but not photic entrainment.
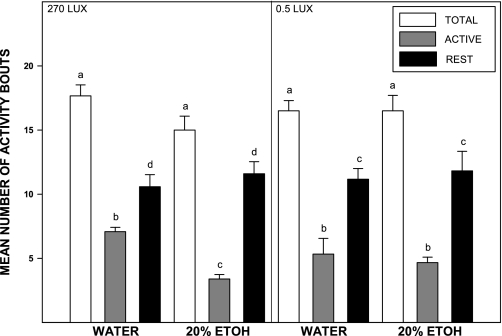
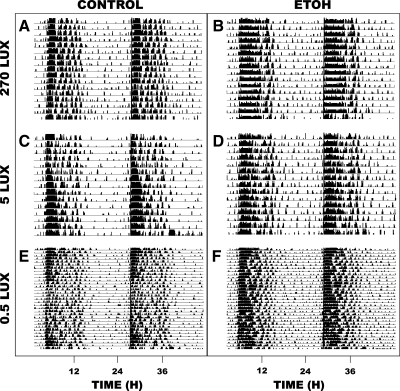
In animals under LD with light intensity at 270 lux, the number of activity bouts throughout the active dark-phase (alpha) was significantly decreased in animals drinking 20% EtOH (n = 5) compared with water controls: n = 4; 3.4 ± 0.7 vs. 7.1 ± 0.6 bouts, respectively [F(1,25) = 58.7; P < 0.01], (Fig. 6). The number of activity bouts during the inactive light phase was unaffected by the EtOH compared with controls: 11.6 ± 1.8 vs. 10.6 ± 1.7 bouts, respectively [F(1,25) = 0.6; P > 0.4]. The 24-h average bout duration was increased in the EtOH vs. water drinkers: 34.4 ± 3.8 vs. 22.3 ± 0.7 min, respectively [F(1,6) = 10.3; P < 0.02], (Fig. 7). In animals under LD with light intensity at 0.5 lux, there was no effect of EtOH drinking on activity bout numbers during the light- or dark-phase [F(1,10) = 0.2 and F(1,10) = 0.3; both P < 0.2]. Stable entrainment to LD, as reflected by a rhythm period of ∼24 h under LD, was unaffected by chronic 20% EtOH at any of the light intensities (270 lux, 5 lux, and 0.5 lux) and was equivalent to entrainment in water controls (Fig. 8). Rhythm periods analyzed for a 2-wk period under these intensities in EtOH (n = 5), and water drinkers (n = 4) ranged from 23.98 to 24.00 h.
Fig. 6.
Disruption of activity bout distribution during long-term 20% EtOH drinking. Under a 270-lux LD cycle (left), EtOH decreases the number of activity bouts during the night. Under a 0.5-lux LD cycle (right), the number of activity bouts is unaffected by EtOH consumption. Bars represent means ± SE. For each light intensity, bars with different letters are significantly different (P < 0.05).
Fig. 7.
Long-term EtOH consumption increases the average duration of activity bouts under 270 lux (left) and 0.5 lux (right) LD cycles. Bars represent means ± SE. For each light intensity, bars with different letters are significantly different (P < 0.05).
Fig. 8.
Representative double-plotted actograms of general locomotor activity at three light intensities. 270 lux: A: water-drinker; B: 20% EtOH-drinker. 5 lux: C: water-drinker; D: 20% EtOH-drinker. 0.5 lux: E: water-drinker; F: 20% EtOH drinker.
Withdrawal from chronic EtOH can increase photic phase resetting.
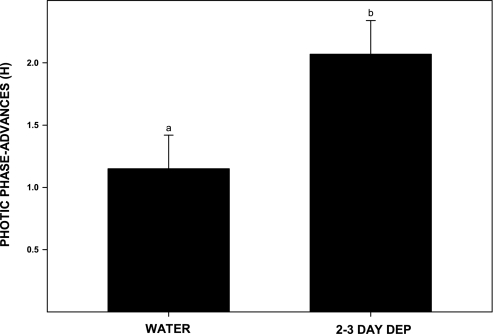
Phase-advance shifts induced by a 30-min light pulse of 270 lux delivered at ZT 18.5 in control animals maintained on water (n = 4) averaged 1.2 ± 0.2. Animals receiving similar light treatment, but withdrawn from 20% EtOH for 1 (n = 6), 2 (n = 6), or 3 days (n = 5), had phase advances that on average were larger than, but not significantly different from, the water controls: 1.3 ± 0.2, 1.9 ± 0.4, and 1.8 ± 0.3 h, respectively [F(3,19) = 1.6; P > 0.2] (Figs. 9 and 10), due to some weakly responding outliers (3/17). Pooling data from the latter two groups, however, revealed an overall enhancement during the 2- to 3-day interval after withdrawal [n = 11; F(1,15) = 4.7; P < 0.05 vs. water controls]. Animals deprived of EtOH for 2–3 days showed an average phase advance of 2.1 ± 0.3 h.
Fig. 9.
EtOH deprivation averaged over a 2- or 3-day interval potentiates phase-advance responses to a 25-lux light pulse at ZT 18.5. Bars represent means ± SE. Bars with different letters are significantly different (P < 0.05).
Fig. 10.
Representative double-plotted actograms of general locomotor activity showing phase-advance responses to a light pulse (25 lux) delivered at ZT 18.5 after 1, 2, and 3 days of EtOH deprivation. A: water-drinker; B: one-day EtOH deprivation; C: two-day EtOH deprivation; and D: three-day EtOH deprivation. Circled arrowheads denote the time of the light pulses.
DISCUSSION
The central pathways implicated in the development of alcoholism are closely and reciprocally linked to those regulating the circadian clock. For example, malaise associated with sleep deprivation, shift work, or repeated jet lag can evoke a destructive, reinforcing cycle of EtOH self-administration and circadian clock desynchrony (9, 24, 60, 64). The clock depends upon photic input for synchrony with the external environment, and this signaling pathway represents a potentially vulnerable target for the disruptive chronobiological effects of alcohol abuse. Evidence supporting this idea comes from recent studies in hamsters and mice in which acute EtOH was shown to act directly in the SCN to strongly inhibit photic phase-resetting responses (48, 55). Here, we extend this work to show that chronic EtOH consumption can disrupt phase-advance shifting in hamsters presented with photic stimuli late in the dark phase, when levels of EtOH measured in the SCN are relatively low. The number and duration of circadian locomotor activity bouts are also affected by this EtOH treatment. These results represent the first lines of evidence that chronic EtOH consumption can affect photic signaling pathways of the hamster circadian clock and also perturb the circadian behavioral activity rhythm.
Chronic EtOH and photic circadian phase regulation.
It is notable that chronic EtOH consumption differentially affected the various circadian timekeeping processes assessed here. In particular, chronic EtOH attenuated the photic phase-advance portion of the hamster photic phase-response curve (PRC) for LD entrainment but had no apparent effect on entrainment of the locomotor activity rhythm to LD, even at very low light intensities. Normal entrainment during EtOH treatment in hamsters has also been reported in other studies (40). One explanation for this lack of effect is that the phase-delay portion of the hamster light PRC is insensitive to acute (55) and chronic EtOH (58; EtOH intake was similar to that in the present study, ∼15 g·kg−1·day−1). Hence, hamsters having a short τ (thus entraining to LD through phase-delay shifts), would theoretically be capable of entraining to LD. Another possibility, based on the masking effect of light on the sleep-activity rhythm, is that locomotor behavior is suppressed by light and stimulated by darkness, producing a seemingly entrained rhythm, irrespective of EtOH-disrupted clock function. Although we cannot rule out a masking effect, the demonstration that normal phase-advance shifts to light pulses at ZT 18.5 occur when pulses are administered soon (1 day) after chronic EtOH deprivation (in contrast to the potentiated shifts seen 2–3 days after EtOH deprivation), may reflect intrinsic pacemaker phase entrainment over the course of EtOH administration.
Related to the differential effects of EtOH on photic entrainment is the present observation that chronic EtOH consumption attenuated phase advances to the lower-intensity light pulse of 25 lux, but not to the higher-intensity pulse of 270 lux. This inhibitory effect of EtOH at the lower-light intensity is consistent with a previous study in hamsters in which chronic drinking reduced phase-advance responses to a 20-lux light pulse (58). The lack of inhibition at 270 lux may have been due to the relatively low concentration of EtOH estimated using microdialysis (∼5 mM) in the SCN at the time of the light pulse (ZT 18.5). This could have been insufficient to overcome a strong phase-resetting signal produced by bright light, but adequate to inhibit a weaker signal produced by the 25-lux exposure. The 5-mM concentration is considerably below the 50-mM EtOH concentration present in the SCN after an acute intraperitoneal 2.0 mg/kg EtOH injection, which blocked phase-advance shifts produced by a 270-lux pulse (55). Moreover, in the mouse SCN slice, the dose required to block glutamatergic (photic-like) phase-advances was 20–50 mM (48). Thus, the attenuation of photic phase-resetting seems proportionately dependent on both the concentration of EtOH in the SCN during chronic drinking and light intensity. This underscores the importance of considering time-of-day fluctuations in drinking and ambient light levels when assessing the neurological effects of EtOH in the circadian system.
Chronic EtOH and behavioral activity patterns.
In humans, alcohol abuse is associated with marked disturbances in clock-driven overt behavioral rhythms (5, 6, 18, 32, 33, 50). In hamsters, our analysis of actogram data over periods of chronic drinking revealed a significant decrease in the number of locomotor bouts during the active period (dark-phase), and an increase in the mean daily bout duration, reflecting a consolidation of locomotor activity, primarily during the active period. Locomotor stimulation in response to low doses of alcohol (and other addictive drugs) is a widely observed phenomenon related to its rewarding properties (see Ref. 47 for review). Consistent with this behavioral effect, we have observed, by combined actogram and drinkometer analyses, that general activity bouts in EtOH drinkers are closely aligned with their drinking rhythms during both the day and the night. This altered locomotor activity may reflect locomotor stimulation due to EtOH consumption, or it may reflect increased motivation to access EtOH.
EtOH withdrawal effects on circadian photic phase resetting.
Chronic EtOH is thought to inhibit SCN NMDA receptor-mediated photic signaling (48, 55, 58), and it is hypothesized that this could lead to a subsequent upregulation of photic phase-resetting upon EtOH withdrawal. However, this response was not evident in groups tested separately after 1, 2, or 3 days of withdrawal. It is known that hamsters do not become physically dependent on EtOH (37) and do not display typical signs of withdrawal, such as handling-induced convulsions. Such seizures are likely based on disinhibition of glutamatergic signaling following cessation of EtOH consumption, and in particular, the upregulation of central glutamate NMDA receptors in response to chronic inhibition by EtOH (30, 61, 65). The hamsters' lack of withdrawal convulsions indicates that this may not occur. On the other hand, the finding that chronic EtOH attenuates glutamate-mediated photic signaling in the SCN is evidence that NMDA receptor response is inhibited by EtOH consumption, and therefore, some degree of withdrawal-related upregulation might exist. This is consistent with observations in the groups withdrawn from EtOH for 2 and 3 days, in which the combined phase-advancing response was ∼60% greater than that of the water controls (but variable due to inclusion of some weakly responding outliers). Notably, by pooling these timepoints (which were not statistically different), a potentiation is evident (P < 0.05 vs. water controls). The reason for the lack of this effect on the first day of EtOH deprivation is unclear, but could relate to a time-lag for complete clearance of EtOH following its long-term consumption. Collectively, these data indicate that although central glutamatergic potentiation during EtOH withdrawal may be too low to produce convulsions in hamsters, it may be sufficient to enhance low-intensity (25 lux) photic phase-resetting in some, but not all, individuals. These observations are also consistent with human studies reporting persistent circadian-related deficits long after alcohol consumption has ceased (5, 6, 34, 54).
Circadian pharmacokinetic profile of SCN EtOH.
This study represents the first characterization of the 24-h profile of brain EtOH assessed using microdialysis concomitantly with circadian locomotor and drinking measurements in freely behaving animals. This pharmacokinetic analysis provides a basis for exploring the effects of EtOH on circadian timing. Here, the daily profile of EtOH in the SCN of chronically drinking hamsters was characterized to compare brain EtOH concentrations in the hamster with other experimental animal models and to provide information on EtOH levels during light pulse administration. The estimated peak level of EtOH (∼19 mM) in the hamster SCN occurred during the early dark-phase (ZT 15). This concentration is comparable to those estimated using microdialysis in the nucleus accumbens of Wistar and Alko Alcohol (AA) rats (∼16 mM and 14 mM, respectively; 45) and the estimated ∼12-mM peak in the mouse nucleus accumbens (25) during voluntary limited access drinking. Interestingly, relatively low levels of EtOH (∼5 mM) are present in the hamster SCN at ZT 18.5, which is near the acrophase of the phase-advancing portion of the photic PRC, and when the phase-advancing light pulses were administered. It is notable that these EtOH levels are sufficient to strongly inhibit photic shifting using lower-intensity 25-lux light pulses as discussed above. Although not related to SCN function per se, similar levels of EtOH enhance dopamine release in the nucleus accumbens (16), confirming that low concentrations of EtOH can effectively disrupt neurophysiological functions throughout the brain.
Simultaneous measurements of the daily patterns of SCN and subcutaneous EtOH, drinking, and general locomotor activity were undertaken in a separate experiment to correlate EtOH pharmacokinetics with circadian behavior. The data are from an animal that exhibited normal overt behaviors throughout the experiment and are considered representative. Subcutaneous, rather than blood sampling of EtOH, was used as it is less invasive, and follows a similar time course (19). Several points are evident from this trial. First, drinking bouts are temporally matched with clustered locomotor activity bouts. Second, there is approximately a 20-min latency between a drinking episode and rises in tissue EtOH. Third, the intensity and duration of a drinking bout are proportionate to the size of the ensuing EtOH peak, which is consistent with previous studies (21, 22, 25). Fourth, because the temporal profiles of SCN and subcutaneous EtOH are essentially identical, the subcutaneous peaks are 2–3 times as large as those in the SCN. Fifth, the effect of multiple drinking bouts spaced over a few hours is cumulative, resulting in a prolonged presence of EtOH in the brain and periphery. Notably, from this analysis, EtOH is continuously present to varying degrees in these compartments in hamsters given EtOH as the sole fluid source. The highly dynamic nature of the daily EtOH profile also underscores the potential risk of using single blood samples as an index of general blood alcohol concentration in chronically drinking animals.
Perspectives and Significance
Chronic EtOH has adverse effects on circadian clock regulation in the Syrian hamster, with some functions affected to a greater extent (e.g., photic phase-advance shifting) than others (e.g., entrainment to LD). These differences may be related to time-of-day differences in SCN EtOH concentration, differential EtOH effects on the light PRC and/or tolerance. Nevertheless, it is apparent that long-term drinking is disruptive to photic phase-resetting and circadian activity patterns even at relatively low brain EtOH concentrations, and that withdrawal effects, which may not be immediately evident in hamsters, are indeed manifest in the circadian system by persistent disruption of photic phase-resetting. These results underscore the importance of understanding the pharmacokinetics of EtOH when assessing the effects of drinking on circadian timing and other homeostatic functions.
GRANTS
This work was supported by National Institute on Alcohol Abuse and Alcoholism Grant AA-015948 to R. A. Prosser and J. D. Glass.
REFERENCES
- 1.Abe H, Rusak B, Robertson HA. Photic induction of Fos protein in the suprachiasmatic nucleus is inhibited by the NMDA receptor antagonist MK-801. Neurosci Lett 127: 9–12, 1991. [DOI] [PubMed] [Google Scholar]
- 2.Adinoff B, Risher-Flowers D, Dee Jong J, Ravitz B, Bone GHA, Nutt DJ, Roehrich L, Martin PR, Linnoila M. Disturbances of hypothalamic-pituitary-adrenal axis functioning during ethanol withdrawal in six men. Am J Psychiat 148: 1023–1025, 1991. [DOI] [PubMed] [Google Scholar]
- 3.Allgaier C Ethanol sensitivity of NMDA receptors. Neurochem Int 41: 377–382, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Baird TJ, Briscoe RJ, Vallett M, Vanecek SA, Holloway FA, Gauvin DV. Phase-response curve for ethanol: alterations in circadian rhythms of temperature and activity in rats. Pharmacol Biochem Behav 61: 303–315, 1998. [DOI] [PubMed] [Google Scholar]
- 5.Brower KJ Alcohol's effects on sleep in alcoholics. Alcohol Res Health 25: 101–109, 2001. [PMC free article] [PubMed] [Google Scholar]
- 6.Brower KJ, Aldrich MS, Robinson EAR, Zucker RA, Greden JF. Insomnia, self-medication, and relapse to alcoholism. Am J Psychiatry 158: 399–404, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chandler LJ Ethanol and brain plasticity: receptors and molecular networks of the postsynaptic density as targets of ethanol. Pharmacol Ther 99: 311–326, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Chastain G Alcohol, neurotransmitter systems, behavior J Gen Psychol 133: 329–335, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Clark JW, Fixaris MC, Belanger GV, Rosenwasser AM. Repeated light-dark phase-shifts modulate voluntary ethanol intake in male and female HAD1 rats. Alcohol Clin Exp Res 31: 1699–1706, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Colwell CS, Foster RG, Menaker M. NMDA receptor antagonists block the effects of light on circadian behavior in the mouse. Brain Res 554: 105–110, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Costa A, Bono G, Martignoni E, Merlo P, Sances G, Nappi G. An assessment of hypothalamo-pituitary-adrenal axis functioning in non-depressed, early abstinent alcoholics. Psychoneuroendocrinology 21: 263–275, 1996. [DOI] [PubMed] [Google Scholar]
- 12.Daan S, Pittendrigh CS. A functional analysis of circadian pacemakers in nocturnal rodents. IV Entrainment: Pacemaker as a clock. J Comp Physiol 106: 291–331, 1976. [Google Scholar]
- 13.Danel T, Touitou Y. Alcohol decreases the nocturnal peak of TSH in healthy volunteers. Psychopharmacology 170: 213–214, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Danel T, Libersa C, Touitou Y. The effect of alcohol consumption on the circadian control of human core body temperature is time dependent. Am J Physiol Regul Integr Comp Physiol 281: R52–R55, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Devaney M, Graham D, Greeley J. Circadian variation of the acute and delayed response to alcohol: investigation of core body temperature variations in humans. Pharmacol Biochem Behav 75: 881–887, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Doyon WM, York JL, Diaz LM, Samson HH, Czachowski CL, Gonzales RA. Dopamine activity in the nucleus accumbens during consummatory phases of oral ethanol self-administration. Alcohol Clin Exp Res 27: 1573–1582, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Dudley TE, DiNardo LA, Glass JD. Endogenous regulation of serotonin release in the hamster suprachiasmatic nucleus. J Neurosci 18: 5045–5052, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ehler CL, Slawecki CJ. Effects of chronic ethanol exposure on sleep in rats. Alcohol 20: 173–179, 2000. [DOI] [PubMed] [Google Scholar]
- 19.Engleman EA, Ingraham CM, Franklin KM, Keith CM, McClaren JA, Schultz JA, Morzorati SL, O'Connor S, Thielen RJ, Murphy JM, McBride WJ. In vivo time-course changes with ethanol levels sampled with subcutaneous microdialysis. Alcohol Clin Exp Res 32: 435–442, 2008. [DOI] [PubMed] [Google Scholar]
- 20.Fadda F, Rossetti ZL. Chronic ethanol consumption: from neuroadaptation to neurodegeneration. Prog Neurobiol 56: 385–431, 1998. [DOI] [PubMed] [Google Scholar]
- 21.Ford MM, Nickel JD, Finn DA. Treatment with and withdrawal from finasteride alter ethanol intake patterns in male C57BL/6J mice: potential role of endogenous neurosteroids? Alcohol 37: 23–33, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ford MM, Nickel JD, Phillips TJ, Finn DA. Neurosteroid modulators of GABA(A) receptors differentially modulater ethanol intake patterns in male C57BL/6J mice. Alcohol Clin Exp Res 29: 1630–1640, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foster RG, Hankins MW. Non-rod, non-cone photoreception in the vertebrates. Prog Retinal Eye Res 21: 507–527, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Gauvin DV, Baird TJ, Vanecek SA, Briscoe RJ, Vallett M, Holloway FA. Effects of time-of-day and photoperiod phase-shifts on voluntary ethanol consumption in rats. Alcohol Clin Exp Res 21: 817–825, 1997. [PubMed] [Google Scholar]
- 25.Griffin WC, Middaugh LD, Becker HC. Voluntary ethanol drinking in mice and ethanol concentrations in the nucleus accumbens. Brain Res 1138: 208–213, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Heinz A, Schafer M, Higley JD, Krystal JH, Goldman D. Neurobiological correlates of the disposition and maintenance of alcoholism. Pharmacopsychiatry 36: S255–S258, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Hendrikson AE, Wagoner N, Cowan WM. An autoradiographic and electron microscope study of retinohypothalamic connections. Z Zellforsch Mikrosk Anat 135: 1–26, 1972. [DOI] [PubMed] [Google Scholar]
- 28.Holzberg D, Albrecht U. The circadian clock: a manager of biochemical processes within the organism. J Neuroendocrin 15: 339–343, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Johnson RF, Morin LP, Moore RY. Retinohypothalamic projections in the hamster and rat demonstrated using cholera toxin. Brain Res 462: 301–312, 1988. [DOI] [PubMed] [Google Scholar]
- 30.Kalluri HS, Metha AK, Ticku MK. Up-regulation of NMDA receptor subunits in adult brain following chronic ethanol treatment. Mol Brain Res 58: 221–224, 1998. [DOI] [PubMed] [Google Scholar]
- 31.Kelley AE Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron 44: 161–179, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Kubota R, De A, Brown RA, Simasko SM, Krueger JM. Diurnal effects of acute and chronic administration of ethanol on sleep in rats. Alcohol Clin Exp Res 26: 1153–1161, 2002. [DOI] [PubMed] [Google Scholar]
- 33.Kuhlwein W, Hauger RL, Irwin MR. Abnormal nocturnal melatonin secretion and disordered sleep in abstinent alcoholics. Biol Psych 54: 1437–1443, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Landolt HP, Gillin J. Sleep abnormalities during abstinence in alcohol-dependent patients. Aetiol Manag CNS Drugs 15: 413–425, 2001. [DOI] [PubMed] [Google Scholar]
- 35.Lovinger DM, White G, Weight FF. Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science 243: 1721–1724, 1989. [DOI] [PubMed] [Google Scholar]
- 36.Lynch DR, Anegawa NJ, Verdoorn T, Pritchett DB. N-methyl-d-aspartate receptors: different subunit requirements for binding of glutamate antagonists, glycine antagonists, and channel-blocking agents. Mol Pharmacol 45: 540–545, 1994. [PubMed] [Google Scholar]
- 37.MacMillan DE, Ellis FW, Frye GD, Pick JR. Failure of signs of physical dependence to develop in hamsters after prolonged consumption of large doses of ethanol. Pharmacol Biochem Behav 7: 55–57, 1977. [DOI] [PubMed] [Google Scholar]
- 38.Mintz EM, Albers HE. Microinjection of NMDA into the SCN region mimicks the phase shifting effect of light in hamsters. Brain Res 758: 245–249, 1997. [DOI] [PubMed] [Google Scholar]
- 39.Mintz EM, Marvel CL, Gillespie CF, Price KM, Albers HE. Activation of NMDA receptors in the suprachiasmatic nucleus produces light-like phase shifts of the circadian clock in vivo. J Neurosci 19: 5124–5130, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mistlberger RE, Nadeau J. Ethanol and circadian rhythms in the Syrian hamster: effects on entrained phase, reentrainment rate, and period. Pharmacol Biochem Behav 43:159–165, 1992. [DOI] [PubMed] [Google Scholar]
- 41.Moghaddam B, Bolinao ML. Biphasic effect of ethanol on extracellular accumulation of glutamate in the hippocampus and nucleus accumbens. Neurosci Lett 178: 99–102, 1994. [DOI] [PubMed] [Google Scholar]
- 42.Moore RY Organization and function of a central nervous system circadian oscillator: the suprachiasmatic nucleus. Fed Proc 42: 2783–2789, 1983. [PubMed] [Google Scholar]
- 43.Moriya T, Horikawa K, Akiyama M, Shibata S. Correlative association between N-methyl-d-aspartate receptor-mediated expression of Period genes in the suprachiasmatic nucleus and phase shifts in behavior with photic entrainment of clock in hamsters. Mol Pharmacol 58: 1554–1562, 2000. [DOI] [PubMed] [Google Scholar]
- 44.Nie Z, Madamba SG, Siggins GR. Ethanol inhibits glutamatergic neurotransmission in nucleus accumbens neurons by multiple mechanisms. J Pharmacol Exp Ther 271: 1566–73, 1994. [PubMed] [Google Scholar]
- 45.Nurmi M, Kiianmaa K, Sinclair JD. Brain ethanol levels after voluntary ethanol drinking in AA and Wistar rats. Alcohol 19: 113–118, 1999. [DOI] [PubMed] [Google Scholar]
- 46.O'Callaghan MJ, Croft AP, Jacquot C, Little HJ. The hypothalamopituitary-adrenal axis and alcohol preference. Brain Res Bull 68: 171–178, 2005. [DOI] [PubMed] [Google Scholar]
- 47.Phillips TJ, Shen EH. Neurochemical bases of locomotion and ethanol stimulant effects. Int Rev Neurobiol 39: 243–282, 1996. [DOI] [PubMed] [Google Scholar]
- 48.Prosser RA, Mangrum CA, Glass JD. Acute ethanol modulates glutamatergic and serotonergic phase shifts of the mouse circadian clock in vitro. Neuroscience 152: 837–848, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rasmussen DD, Boldt BM, Bryant CA, Mitton DR, Larsen SA, Wilkinson CW. Chronic daily ethanol and withdrawal: 1. Long-term changes in the hypothalamo-pituitary-adrenal axis. Alcohol Clin Exp Res 24: 1836–1849, 2000. [PubMed] [Google Scholar]
- 50.Roehrs T, Petrucelli N, Roth T. Sleep restriction, ethanol effects and time of day. Human Psychopharm 11: 199–204, 1996. [Google Scholar]
- 51.Roehrs T, Roth T. Sleep, sleepiness, and alcohol use. Alcohol Res Health 25: 101–109, 2001. [PMC free article] [PubMed] [Google Scholar]
- 52.Roehrs T, Roth T. Sleep, sleepiness, sleep disorders and alcohol use and abuse. Sleep Med Rev 5: 287–297, 2001. [DOI] [PubMed] [Google Scholar]
- 53.Rojdmark S, Wikner J, Adner N, Andersson DEH, Wetterberg L. Inhibition of melatonin secretion by ethanol in man. Metab Clin Exp 42: 1047–1051, 1993. [DOI] [PubMed] [Google Scholar]
- 54.Rosenwasser AM Alcohol, antidepressants, and circadian rhythms. Alcohol Res Health 25: 126–135, 2001. [PMC free article] [PubMed] [Google Scholar]
- 55.Ruby CL, Prosser RA, DePaul MA, Roberts RJ, Glass JD. Acute ethanol impairs photic and nonphotic circadian phase resetting in the Syrian hamster. Am J Physiol Regul Integr Comp Physiol 296: R411–R418, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rusak B Involvement of the primary optic tracts in mediation of light effects on hamster circadian rhythms. J Comp Physiol 118: 165–172, 1977. [Google Scholar]
- 57.Rusak B, Zucker I. Neural regulation of circadian rhythms. Physiol Rev 59: 449–526, 1979. [DOI] [PubMed] [Google Scholar]
- 58.Seggio JA, Logan RW, Rosenwasser AM. Chronic ethanol intake modulates photic and non-photic circadian phase responses in the Syrian hamster. Pharmacol Biochem Behav 87: 297–305, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Simson PE, Criswell HE, Breese GR. Inhibition of NMDA-evoked electrophysiological activity by ethanol in selected brain regions: evidence for ethanol-sensitive and ethanol-insensitive NMDA-evoked responses. Brain Res 607: 9–16, 1993. [DOI] [PubMed] [Google Scholar]
- 60.Smart RG Drinking problems in employed, unemployed, and shift workers. J Occ Med 21: 731–736, 1979. [DOI] [PubMed] [Google Scholar]
- 61.Snell LD, Nunley KR, Lickteig RL, Browning MD, Tabakoff B, Hoffman PL. Regional and subunit specific changes in NMDA receptor mRNA and immunoreactivity in mouse brain following chronic ethanol ingestion. Mol Brain Res 40: 71–78, 1996. [DOI] [PubMed] [Google Scholar]
- 62.Spanagel R, Pendyala G, Abarca C, Zghoul T, Sanchis-Segura C, Magnone MC, Lascorz J, Depner M, Holzberg D, Soyka M, Scheiber S, Matsude F, Lanthrop M, Schuman G, Albrecht U. The clock gene Per2 influences the glutamatergic system and modulates alcohol consumption. Nat Med 11: 35–42, 2005. [DOI] [PubMed] [Google Scholar]
- 63.Stephens DN A glutamatergic hypothesis of drug dependence: extrapolations from benzodiazapine receptor ligands. Behav Pharmacol 6: 425–446, 1995. [PubMed] [Google Scholar]
- 64.Trinkoff AM, Storr CL. Work schedule characteristic and substance abuse. Am J Ind Med 34: 266–271, 1998. [DOI] [PubMed] [Google Scholar]
- 65.Tsai G, Coyle JT. The role of glutamatergic neurotransmission in the pathophysiology of alcoholism. Annu Rev Med 49: 173–184, 1998. [DOI] [PubMed] [Google Scholar]
- 66.Wasielewski JA, Holloway FA. Alcohol's interactions with circadian rhythms. A focus on body temperature. Alcohol Res Health 25: 94–100, 2001. [PMC free article] [PubMed] [Google Scholar]
- 67.Yang X, Criswell HE, Simson P, Moy S, Breese GR. Evidence for a selective effect of ethanol on N-methyl-d-aspartate responses: ethanol affects a subtype of the ifenprodil-sensitive N-methyl-d-aspartate receptors. J Pharmacol Exp Ther 278: 114–124, 1996. [PubMed] [Google Scholar]
- 68.Zimmermann U, Hundt W, Spring K, Grabner A, Holsboer F. Hypothalamic-pituitary-adrenal system adaptation to detoxification in alcohol-dependent patients is affected by family history of alcoholism. Biol Psychiat 53: 75–84, 2003. [DOI] [PubMed] [Google Scholar]