Abstract
We previously demonstrated that endotoxin-induced sepsis results in caspase 8-mediated diaphragmatic dysfunction. The upstream signaling pathways modulating diaphragm caspase 8 activation in response to endotoxin administration are, however, unknown. The purpose of the present study was to test the hypothesis that the JNK (Jun N-terminal Kinase) pathway is activated in the diaphragm during sepsis and contributes to sepsis-induced diaphragm caspase 8 activation. Endotoxin was administered to intact animals to model the effects of sepsis. We first assessed the time course of JNK activation after endotoxin (12 mg/kg ip) administration to mice. We then determined whether JNK inhibitor administration ( 30 μm/kg ip SP600125) could prevent caspase 8 activation and diaphragm weakness in endotoxin-treated mice. Experiments were then repeated comparing the effects of endotoxin on control and transgenic JNK knockout mice. We finally determined whether cytomix (LPS, TNFα, IL1β, and IFN-γ) exposure activated caspase 8 in C2C12 muscle cells and whether caspase 8 activation was attenuated by either chemical inhibition of JNK (30 μM SP600125) or transfection with a dominant negative JNK construct. We found that endotoxin activated diaphragm JNK (P < 0.001) and increased active caspase 8 (P < 0.01). Inhibition of JNK with SP600125 or by use of JNK-deficient animals prevented diaphragm caspase 8 activation (P < 0.01) and prevented diaphragm weakness (P < 0.05). JNK inhibition also prevented caspase 8 activation in cytokine-treated muscle cells (P < 0.001). These data implicate JNK activation as a major factor mediating inflammation-induced skeletal muscle caspase 8 activation and weakness.
Keywords: endotoxin, proteolysis
an objective assessment in 2003 of diaphragm muscle function using the magnetic twitch stimulation technique in critically ill patients unexpectedly discovered that these patients have severe weakness, averaging diaphragm twitch forces that are only 20–25% of those observed in normal subjects (11). This severe weakness predisposes these patients to recurrent respiratory failure after intensive care unit (ICU) discharge and to prolonged post-ICU disability (12). The precise mechanisms by which ICU patients acquire profound respiratory muscle weakness are poorly understood, but many of these patients have infections, and at least some of this weakness is thought to be secondary to the effects of cytokines produced during infections (16). In recent work, we have shown that caspase 3 is activated in the diaphragm in the endotoxin animal model of sepsis and that caspase-mediated protein cleavage contributes to the development of diaphragmatic weakness following endotoxin administration (20). Our work has also shown that diaphragmatic caspase 3 activation following endotoxin administration is a consequence of activation of the caspase 8-dependent, death receptor-linked, extrinsic caspase activation pathway, since selective caspase 8 inhibitors prevent both caspase 3 activation and caspase-mediated diaphragmatic weakness following endotoxin (21).
In many other tissues, the mitogen-activated protein (MAP) kinase signaling pathways modulate the effects of cytokines on tissue function during infections and influence caspase activation pathways (3, 7, 17, 25, 27). In particular, cytokine-mediated Jun N-terminal kinase (JNK) activation has been shown to play a critical role in inducing cell dysfunction and activation of caspase in several organs (3, 26). The role of the JNK kinase pathway in modulating infection-induced diaphragmatic caspase 8 activation and diaphragmatic muscle weakness has never, however, been studied. The purpose of the present group of experiments, therefore, was to test the hypothesis that endotoxin-induced sepsis leads to JNK pathway activation in the diaphragm and that JNK activation modulates sepsis-induced activation of diaphragmatic caspase 8.
METHODS
Mice were employed for these studies, and sepsis was induced by endotoxin injection. Two forms of JNK inhibition (i.e., chemical inhibition with SP600125 and genetic inhibition using transgenic JNK-deficient animals) were tested. Caspase 8 activation was assessed using Western blot analysis techniques. Diaphragm muscle function was also assessed by measuring the forces generated by excised muscle strips. To further examine these issues, we also evaluated the direct effects of the addition of cytokines to a mouse muscle cell line (C2C12 cells) and determined the effects of chemical and genetic inhibitors of JNK (i.e., SP600125 and transfection with a dominant negative JNK construct) on caspase 8 activation in these isolated cells.
Experimental protocols.
All animal experiments were performed using male mice weighing between 25 and 35 g. Mice had unrestrained access to food and water throughout the period of experimentation. Experiments were approved by the University of Kentucky Institutional Animal Care and Use Committee, and animals were monitored throughout experiments by the University of Kentucky animal center staff.
Three sets of studies were performed. In the first set, we determined the time course of diaphragm JNK activation in mice (ICS strain) following endotoxin administration. Animals were killed at 2, 4, 8, and 24 h after 12 mg/kg endotoxin (Escherichia coli lipopolysaccharide from Sigma, St. Louis, MO, injected intraperitoneally in 0.3 ml saline, n = 6/group). These time points were chosen for analysis because our previous experiments (20, 21) demonstrate that diaphragm caspase activation is present as soon as 6–12 h after endotoxin administration. We reasoned that if JNK activation preceded and modulated caspase activation, JNK activity should be present within the first 8 h after endotoxin administration and perhaps within the first 4 h. Saline-injected animals (0.3 ml given intraperitoneally, n = 6) served as controls. To provide hydration, all groups were also injected subcutaneously with 60 ml/kg saline at the time of saline or endotoxin administration. At the time of death, animals were anesthetized with pentobarbital (50 mg/kg); diaphragms were then removed and used to assess JNK activation.
In the second set of studies, we determined whether chemical or genetic inhibition of JNK activation could prevent endotoxin-induced caspase 8 activation and diaphragm dysfunction. We first studied (n = 4–5/group, ICS strain): 1) control animals injected with 0.3 ml ip saline; 2) animals injected with endotoxin (12 mg/kg E. coli LPS in 0.3 ml saline ip); 3) animals injected with both 12 mg/kg ip endotoxin and 30 μM/kg ip SP600125, a JNK inhibitor in 0.3 ml saline; and 4) animals injected with 30 μM/kg ip SP600125 in 0.3 ml saline alone. All groups were also injected subcutaneously with 60 ml/kg saline. At 24 h after injections, animals were anesthetized with pentobarbital and killed; diaphragms were removed and used for assessment of force generation and analysis of caspase 8. We also determined whether genetic deficiency of JNK could alter the endotoxin response. The following groups were examined (n = 4–5/group): 1) C57/BL6 control animals injected with 0.3 ml saline ip, 2) C57/BL6 control mice injected with endotoxin 12 mg/kg ip in saline 0.3 ml, 3) JNK knockout transgenic mice (B6.129-Mapk9tm1Flv/J; Jackson Laboratories) injected with 0.3 ml ip saline, and 4) JNK knockout transgenic mice injected intraperitoneally with endotoxin 12 mg/kg in saline 0.3 ml. For C57/BL6 controls we used litter mates of knockout mice that were (+/+) for JNK. Animals were also injected subcutaneously with 60 ml/kg saline. At 24 h, animals were anesthetized with pentobarbital and killed; diaphragms were removed and used for assessment of force generation and determination of caspase 8 levels. In previous experiments examining diaphragmatic caspase activation (20, 21), we found that endotoxin administration induced increases in all of the following: active caspase 8 protein, active caspase 3 protein, caspase 8 function assessed using an activity assay, caspase 3 function assessed using an activity assay, and formation of caspase cleavage-specific spectrin degradation products. Endotoxin did not, however, significantly alter the following: caspase 9 protein, caspase 9 activity, BAX protein levels, and BCL2 protein levels. In this prior work, we also discovered that administration of a caspase 8 inhibitor blocked all indices of caspase activation (20, 21), suggesting that caspase 8 activation is the sentinel event in endotoxin-induced diaphragm caspase pathway activation. For these reasons, we chose to monitor active caspase 8 protein levels in the present study.
In a third set of studies, we determined whether caspase 8 was activated in C2C12 cells in response to incubation with a mixture of cytokines and whether inhibition of JNK prevented caspase activation. Studies were first conducted on the following groups (n = 4–5 plates of cells/group): 1) C2C12 myotubes to which sterile saline was added (35 μl); 2) myotubes treated with a cytokine mixture (termed cytomix, final concentrations in media of 10 μg/ml LPS, 20 ng/ml TNF-α, 50 U/ml IL1-β and 100 U/ml IFN-γ in a volume of 35 μl); 3) myotubes treated with cytomix and 30 μM/kg SP600125, a JNK inhibitor; and 4) myotubes treated with 30 μM/kg SP600125 alone. After 24 h, cells were harvested and assessment made of cell caspase 8 concentrations. We also determined whether genetic inhibition of JNK activation would alter the activation of caspase 8 in C2C12 cells. Studies were conducted on the following groups (n = 4–5 plates of cells/group): 1) control sham-transfected cells treated with saline, 2) sham construct-transfected cells treated with cytomix, 3) cells transfected with a JNK dominant negative construct treated with saline, and 4) JNK dominant negative-transfected cells treated with cytomix. At the end of 24 h after exposure to saline or cytomix, cells were harvested and assessment made of cell caspase 8 concentrations.
JNK and caspase 8 protein identification by Western blot analysis.
Western blot analysis was employed to measure diaphragm and myotube levels of phospho-JNK, total JNK, and caspase 8. We also measured levels of α-tubulin as a loading control. For these assays, muscle samples were homogenized in buffer (10 mM β-glycerophosphate, 50 mM sodium fluoride, 1 mM sodium, 20 mM HEPES, 2 mM EDTA, 250 mM sodium chloride, 2 microgram/ml leupeptin, 2 microgram/ml aprotinin, 1 mM PMSF, 0.5 μg/ml benzamidine, and 1 mM DTT) in a 1 g-to-10 ml buffer ratio, centrifuged at 3,000 g for 10 min, and the supernatant saved and its protein content determined. Supernatant samples of equal protein content were then diluted 1:1 with loading buffer (126 mM Tris·HCL, 20% glycerol, 4% SDS, 1.0% 2-mercaptoethanol, 0.005% bromphenol blue, pH 6.8), placed in boiling water for 5–7 min and then loaded onto Tris glycine polyacrylamide gels. Protein mixtures were separated by electrophoresis (Minicell II; Novex, Carlsbad, CA). Proteins were then transferred to polyvinylidene fluoride membranes and incubated over night at 4°C with primary antibodies to targeted proteins (phospho-JNK and total JNK from Cell Signaling, Danvers, MA, and caspase 8 from Santa Cruz Biotechnology, Santa Cruz, CA). Membranes were then incubated with horseradish peroxidase-conjugated secondary antibodies and antibody binding detected on film using enhanced chemiluminescence (NEN Life Science Products, Boston, MA). Densitometry of filmed gels was performed using a scanner (Microtek, Carson, CA) and UN-SCAN-IT software (Silk Scientific, Orem, UT). After initial determinations, membranes were stripped and reprobed with primary antibodies to α-tubulin (Santa Cruz Biotechnology) to verify equal loading among lanes. We chose α-tubulin for this normalization because previous experiments indicate this protein is not altered in skeletal muscle by sepsis. Densities of the α-tubulin blots were determined using a Microtek scanner; these values were used to normalize densitometry values.
JNK activity assay.
We measured JNK activity directly by immunoprecipitation of JNK from diaphragm sample homogenates and subsequent incubation of the immunoprecipitate with a synthetic JNK substrate using the Cell Signaling JNK kinase assay kit. For homogenization of muscle samples to assess JNK activity we employed the lysis buffer provided with the kit and did not use the homogenization buffer used for Western blot analysis.
Measurement of force generation in isolated diaphragm muscle strips.
For assessment of force generation, diaphragm strips were dissected from the left costal diaphragm and mounted vertically in water-jacketed organ baths (Radnoti Glass, Monrovia, CA) containing curarized Krebs-Henseleit solution at 22°C bubbled with 95% O2-5% CO2. The rib end of strips was secured to the bottom of baths by silk ties, and the tendinous end to a Grass FT10 force transducer. Platinum electrodes were placed about strips and connected to an amplifier (Biomedical Technology of America) attached to a Grass S48 stimulator. After a 15-min equilibrium period, muscle length was adjusted to Lo (the length of maximum force generation), stimulation current adjusted to supramaximal levels, and a force frequency curve was constructed by sequentially stimulating strips at 1, 10, 20, 50, 75, 100, 125, and 150 Hz (train duration 800 ms) with a 30-s rest period between adjacent stimulus trains. At the conclusion of force measurements, transducers were calibrated with standard weights, and the force/cross-sectional area was calculated by the method of Close (4).
Cell preparations and transfections.
For cell preparations and transfections, C2C12 myoblasts obtained from American Type Culture Connection, Manassas, VA were grown to 70% confluency in DMEM with 10% fetal bovine serum. To induce differentiation, media was switched to DMEM with 2% horse serum for 5 days.
For transfection experiments, C2C12 myoblasts were first transfected with a genetic construct containing Cre flanked by tamoxifen responsive elements linked to a muscle-specific creatine kinase promoter. This construct was made by ligating an muscle creatine kinase (MCK) promoter [MCK promoter (pMCKG), 4.9 kb, gift of Dr. Suzanne Porszasz-Reisz] to a tamoxifen-inducible Cre element [promoter for tamoxifon inducible Cre (pAN-MCM), from Dr. Michael Reth; this contains a G418 selection cassette]. This resultant MCK-MCM construct was transfected into C2C12 cells using Ambion Xpress transfection reagent, cells containing MCK-MCM were selected by growth in media containing G418, and verification of transfection was performed with PCR using primers for MCK-MCM. Cells were then transfected with a second construct containing a cytomegalovirus (CMV) promoter linked to a floxed stop codon connected, in turn, to a JNK dominant negative gene. This construct was produced by ligating a floxed stop element [floxed stop-topoisomerase vector (LSL-TOPO) from Dr. Tyler Jacks] to a JNK dominant negative element (JNK DN from Dr. Jeffery Schelling, OH; this construct contains a purinomycin selection element). The resultant CMV-LSL-JNK DN was transfected into cells using Ambion Xpress reagent, cells were selected by growth in purinomycin containing media, and verification of transfection was done with PCR with primers to LSL. For sham controls, we used cells transfected with MCK-MCM but not CMV-LSL-JNK DN. For experimental use, transfected cells were exposed to tamoxifen (50 nM) for 3 days to induce Cre activation and then subsequently exposed to either saline or cytomix.
Statistical analysis.
ANOVA was used to compare variables (e.g., force) across groups of animals treated with different agents, with post hoc testing (Tukey) to determine differences between groups. A P value of < 0.05 was taken as indicating statistical significance for all experiments. Data are presented as means ± 1 SE.
RESULTS
Diaphragm JNK activation in response to endotoxin administration.
Western blot analysis revealed increased levels of activated phospho-JNK in diaphragm muscle samples taken from endotoxin-treated septic animals compared with saline-treated controls. The time course of phospho-JNK formation is shown in Fig. 1A for representative muscle samples obtained from a control animal and for animals killed at several points in time (2, 4, 8, and 24 h) after endotoxin administration, with group mean data provided in Fig. 1B. Both the top and bottom phospho-JNK bands (the 44-kDa and 42-kDa isoforms, respectively) were significantly increased at 2 h and 4 h after endotoxin administration (P < 0.05 for each). Total JNK protein was similar for samples from control and septic animals for both the 44- and 42-kDa JNK isoforms at all time points (Figs. 1, A and B).
Fig. 1.
A: time course of JNK activation in representative diaphragm. Samples of Western blot analysis were used to determine protein levels of active phospho-JNK (top) as a function of time after endotoxin administration. Samples were reassessed for total JNK levels (bottom), which serve as a loading control. Phospho-JNK protein levels were increased above control levels between 2 and 4 h after endotoxin administration and then returned to baseline levels. Total JNK was similar for the five time points. B: densitometric analysis was used to quantitate phospho-JNK and total JNK levels over time: data were normalized to the control sample for each blot analyzed. Diaphragm phospho-JNK levels (both 42- and 44-kDa bands) increased significantly at 2 and 4 h after endotoxin administration compared with control levels (P < 0.05). Total JNK levels did not change over time. *Significant statistical difference compared with control levels.
Direct measurement of JNK activity using an in vitro kinase assay also demonstrated a significant increase in JNK activity for JNK proteins immunoprecipitated from diaphragm samples taken from animals killed 2 h after endotoxin administration (Fig. 2). On average, JNK kinase activity was 189% of the control level at 2 h after endotoxin administration (P < 0.03).
Fig. 2.
JNK activity was directly determined by assessing the level of phosphorylation of a synthetic substrate (designated the assay product in the graph). A: kinase assay data for diaphragm samples from controls and at 2 h after endotoxin administration. B: densitometric analysis of assay product levels. Endotoxin administration was associated with a large increase in JNK kinase activity (P < 0.03). *Significant statistical difference compared with control levels.
Effect of JNK inhibition on diaphragm force generation.
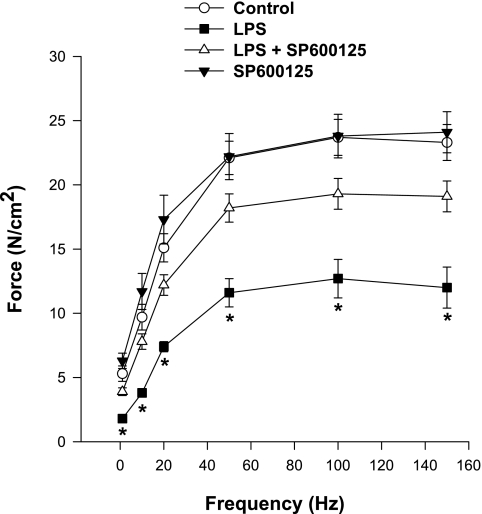
We also found that endotoxin-induced sepsis elicited a marked reduction in diaphragm force generation (Fig. 3). Administration of a specific JNK kinase inhibitor, SP600125 (30 μM/kg) substantially improved force in septic animals, with diaphragms from animals treated with both endotoxin and SP600125 generating forces over the entire stimulation frequency range (1–150 Hz) that were greater than levels achieved by muscles from animals given endotoxin alone. For example, maximum diaphragmatic force generation averaged 23.3 ± 1.4 for controls, 11.9 ± 1.6 for the endotoxin-treated group, 19.1 ± 1.2 for endotoxin plus SP600125-treated animals, and 24.1 ± 1.6 N/cm2 for diaphragms from animals given SP600125 alone (P < 0.001 for comparison of control to endotoxin; P < 0.01 for comparison of endotoxin to endotoxin plus SP600125). In addition, force generation in response to 20-Hz electrical stimulation averaged 15.1 ± 1.1 for controls, 7.4 ± 0.4 for the endotoxin-treated group, 12.2 ± 0.8 for endotoxin plus SP600125-treated animals, and 17.3 ± 1.9 N/cm2 for diaphragms from animals given SP600125 alone (P < 0.03 for comparison of the endotoxin/ SP600125 group to the endotoxin alone group). Lo, the muscle length at which tension was maximal, was similar in the four groups, averaging 1.2 ± 0.1, 1.2 ± 0.1, 1.3 ± 0.1 and 1.2 ± 0.1 cm, respectively, for control, endotoxin, endotoxin/SP600125, and SP600125 groups (not significant).
Fig. 3.
Effect of endotoxin and JNK inhibition on the diaphragm force frequency curve. Force generation was significantly lower at stimulation frequencies from 1–150 Hz for diaphragms from LPS-treated animals (▪) than for control animals (○) (P < 0.001 for all frequencies). Diaphragms from animals given both SP600125 (a JNK inhibitor, ▵) and LPS generated forces significantly higher than diaphragms from animals given LPS alone for all stimulation frequencies tested (P < 0.03). Force generation for muscles taken from animals given SP600125 alone were similar to levels for control animals. *Significant statistical difference between LPS and all other groups.
Effect of JNK inhibition on caspase 8 activation.
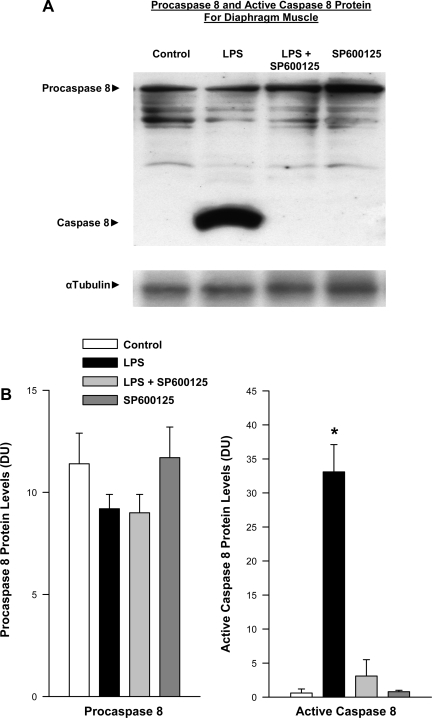
Previous work by our group has shown that endotoxin administration induces significant caspase 8 activation in the diaphragm (20, 21). Specifically, intact procaspase is autocatalytically cleaved to generate a smaller, active caspase molecule in the diaphragm following endotoxin administration. In keeping with these earlier reports, we also observed a significant increase in active caspase 8 protein levels in the diaphragm in the present study following endotoxin administration (Fig. 4, P < 0.001 for comparison of active caspase 8 protein levels in control and endotoxin-treated groups). Administration of the JNK inhibitor SP600125 prevented caspase 8 activation in endotoxin-treated animals, paralleling the effect of this agent to diminish endotoxin-mediated alterations in diaphragm force generation (P < 0.001 for comparison of active caspase 8 protein levels for endotoxin and endotoxin plus SP600125-treated groups).
Fig. 4.
Effect of JNK inhibition on caspase 8 protein levels. A: Western blots stained for procaspase 8 and cleaved. Active caspase 8 protein are shown (top) for representative diaphragm samples. Bottom: blot represents α-tubulin protein levels for these same samples and serves as a loading control. LPS administration elicited a large increase in active cleaved caspase 8 protein compared with the control. Administration of a JNK chemical inhibitor SP600125 prevented this increase in active caspase protein. The sample from the animal given SP600125 alone had caspase levels similar to control. B: mean data examining the effect of SP600125 on diaphragm caspase activation. Densitometry was used to quantitate procaspase 8 and active caspase 8 protein levels for diaphragm samples. Procaspase 8 levels were similar for the four experimental groups (left). In contrast, diaphragm-active caspase 8 protein levels increased dramatically for LPS-treated animals (P < 0.001). Administration of SP600125 to LPS-treated animals prevented this increase in active caspase 8 protein (*P < 0.01 for comparison between LPS and LPS + SP600125 groups).
Effect of endotoxin administration to JNK knockout animals.
To further assess the role of JNK in mediating diaphragm dysfunction following endotoxin administration, we compared the effects of endotoxin administration to control mice and mice deficient in JNK (Figs. 5 and 6). We found that diaphragm force generation was greatly reduced by endotoxin administration to wild-type mice but that endotoxin elicited much smaller reductions in diaphragm force when given to JNK knockout mice (Fig. 5, P < 0.02 for comparison of forces for wild-type mice treated with endotoxin to forces generated by JNK knockout mice treated with endotoxin for frequencies 20–150 Hz and P < 0.05 for comparison at 10 Hz).
Fig. 5.
Force frequency curves in JNK-deficient animals. Force generation was significantly lower at stimulation frequencies from 1 to 150 Hz for diaphragms from LPS-treated animals (▪) than for control animals (○) (P < 0.01). Diaphragms from JNK-deficient animals that were given LPS (▵) generated forces significantly higher than diaphragms from animals given LPS alone for stimulation frequencies from 10 to 150 Hz (P < 0.05 for each frequency). Force generation for muscles taken from JNK-deficient animals given saline (▴) were similar to levels for control animals. *Significant statistical difference between LPS and control; #Significantly different from all other groups.
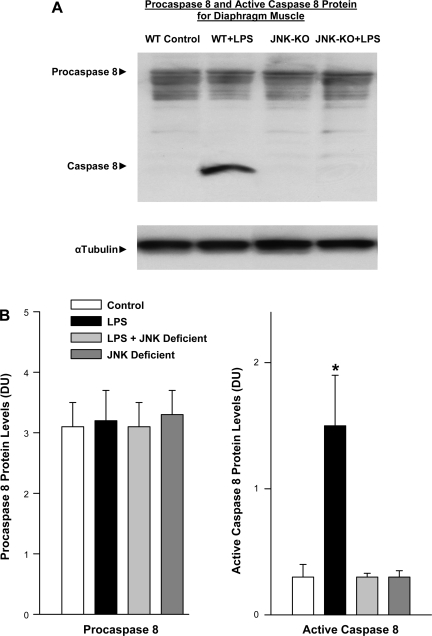
Fig. 6.
Caspase 8 protein levels in JNK-deficient animals. A: Western blots stained for procaspase 8 and cleaved. Active caspase 8 protein (top) are representative diaphragm samples. α-Tubulin protein levels for these same samples (bottom) served as a loading control. LPS administration elicited a large increase in active cleaved caspase 8 protein compared with the control. This increase was not seen in JNK-deficient animals given LPS. The sample from the JNK-deficient animal given saline had caspase levels similar to control. WT, wild type; KO, knockout. B: mean data examining the effect of LPS on diaphragm caspase activation in JNK-deficient animals. Densitometry was used to quantitate procaspase 8 and active caspase 8 protein levels for diaphragm samples. Procaspase 8 levels were similar for the 4 experimental groups (left). In contrast, diaphragm-active caspase 8 protein levels increased dramatically for LPS-treated animals (P < 0.001). Administration of LPS to JNK-deficient animals did not cause an increase in active caspase 8 protein (*P < 0.01 for comparison between LPS and LPS + SP600125 groups).
JNK knockout mice also had diminished activation of diaphragm caspase 8 following endotoxin administration as shown in Fig. 6. On average, wild-type mice given endotoxin had a 364% increase in active caspase 8 protein levels (P < 0.01) compared with controls (Fig. 6B), while JNK knockout mice treated with endotoxin had diaphragm active caspase 8 levels significantly lower than those measured for endotoxin-treated wild-type mice (P < 0.01) and similar to control, saline-treated mice.
Caspase 8 activation in C2C12 myotubes.
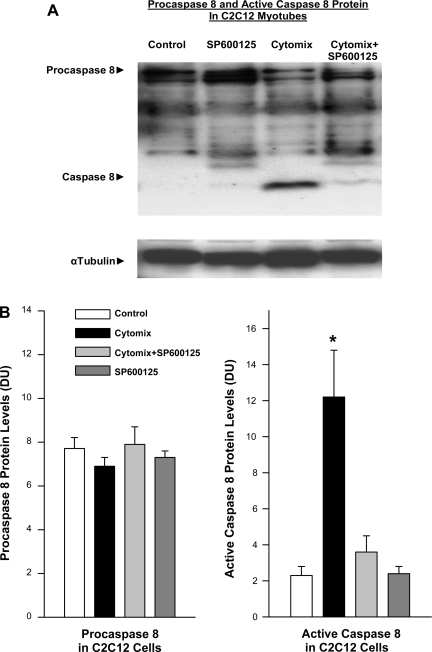
We also examined the effect of incubation of cytokines with isolated C2C12 myotubes and found that 24 h of incubation with a mixture of TNF-α, IL1-β, LPS, and IFN-γ (“cytomix”) evoked significant activation of caspase 8 (Fig. 7). Addition of a JNK inhibitor, SP600125, to cell cultures blocked cytokine induction of caspase 8 activation, as shown for representative experiments in Fig. 7A. On average, addition of cytomix to C2C12 myotubes increased caspase 8 levels by 408%, while myotubes incubated in both SP600125 and cytomix averaged caspase 8 levels equal to control myotubes (Fig. 7B, P < 0.01 for comparison of control to cytomix-treated and P < 0.02 for comparison of cytomix to cytomix plus SP600125-treated groups).
Fig. 7.
Effect of JNK inhibition on caspase 8 protein levels in C2C12 cells. A: Western blots stained for procaspase 8 and cleaved. Top: active caspase 8 protein for representative C2C12 myotube samples. Bottom: α-tubulin protein levels for these same samples and served as a loading control. Cytomix administration elicited a large increase in active cleaved caspase 8 protein compared with the control. Administration of a JNK chemical inhibitor, SP600125, prevented this increase in active caspase protein. Cells treated only with SP600125 alone had caspase levels similar to control. B: mean data examining the effect of SP600125 on C2C12 caspase activation densitometry was used to quantitate procaspase 8 and active caspase 8 protein levels for C2C12 myotube samples. Procaspase 8 levels were similar for the 4 experimental groups (left). In contrast, myotube active caspase 8 protein levels increased dramatically with cytomix administration (P < 0.01). Administration of SP600125 prevented this increase in active caspase 8 protein in cytomix-treated cells (*P < 0.01 for comparison between cytomix and cytomix + SP600125 groups).
Effect of genetic JNK inhibition on caspase activation in response to cytomix in C2C12 cells.
To further determine the role of JNK in modulating muscle caspase activation, we assessed the activation of caspase 8 in C2C12 cells transfected with a dominant negative JNK construct (Fig. 8). Cells transfected with a dominant negative JNK construct had essentially no caspase 8 activation following exposure to a mixture of cytokines, while nontransfected C2C12 cells demonstrated a marked increase in active caspase 8 levels following cytomix exposure (P < 0.001 for comparison of caspase 8 levels for control wild-type C2C12 cells and wild-type C2C12 cells exposed to cytomix, P < 0.001 for comparison of wild-type cells exposed to cytomix and JNK dominant negative cells exposed to cytomix).
Fig. 8.
Effect of dominant negative JNK (JNK DN) transfection on caspase 8 protein levels in C2C12 cells. A: Western blots stained for procaspase 8 and cleaved. Top: active caspase 8 protein for representative C2C12 myotube samples. Bottom: α-tubulin protein levels for these same samples served as a loading control. Cytomix administration elicited a large increase in active cleaved caspase 8 protein compared with the control. Cytomix administration to cells transfected with dominant negative JNK did not manifest an increase in active caspase protein. Dominant negative JNK cells treated with saline had caspase levels similar to control. B: Mean data examining the effect of dominant negative JNK on C2C12 caspase activation. Densitometry was used to quantitate procaspase 8 and active caspase 8 protein levels for C2C12 myotube samples. Procaspase 8 levels were similar for the four experimental groups (left). In contrast, myotube active caspase 8 protein levels increased dramatically with cytomix administration (P < 0.001). Administration of cytomix to dominant negative JNK cells did not cause an increase in active caspase 8 protein (*P < 0.01 for comparison between cytomix in control cells and cytomix in JNK dominant negative cells).
DISCUSSION
The major findings of this study were that endotoxin-induced sepsis elicits an activation of JNK kinase in the diaphragm and that JNK kinase inhibition prevents endotoxin-induced diaphragmatic caspase 8 activation and diaphragm weakness.
Infection-induced diaphragmatic weakness.
A number of different models of infection (pseudomonas-induced pneumonia, cecal ligation perforation-induced sepsis, E. coli injection, endotoxin injection) induce severe reductions in the force generating capacity of the diaphragm and many other respiratory muscles (intercostals, abdominal expiratory muscles) in a variety of animal species (dogs, hamsters, mice, rats) (2, 5, 6, 8, 10, 18). Importantly, studies have shown that infections also produce substantial reductions in respiratory muscle strength in normal humans and patients with preexisting respiratory failure (14, 15). In fact, even the common cold can produce substantial reductions in respiratory muscle strength and can precipitate respiratory failure due to respiratory muscle weakness in patients with preexisting respiratory ailments (14, 15).
The mechanisms by which infection produces these reductions in respiratory muscle strength, however, remain incompletely understood. We have previously shown that caspase activation may be a key factor involved in sepsis-induced diaphragm dysfunction. In this earlier work, we found that endotoxin administration is quickly followed by marked increases in diaphragm caspase activation and that administration of caspase inhibitors largely prevents endotoxin-induced diaphragm dysfunction (20, 21). In this previous work, we found that the extrinsic pathway of caspase activation, involving death receptor-linked activation of caspase 8, was the main pathway of caspase activation. Specifically, we found that endotoxin administration evoked a rapid increase in diaphragm caspase 8 activity and subsequently, an increase in caspase 3 activity (20, 21). In addition, administration of highly selective caspase 8 inhibitors prevented caspase 3 activation (caspase 3 is a downstream effector caspase activated by caspase 8) and also prevented endotoxin-induced muscle weakness (20).
Importantly, generation of active caspase 8 in response to ligand attachment to death receptors is not an all or none phenomenon but is affected by the presence and functional activity of a number of accessory proteins that associate with death receptors. Signaling pathways, including the JNK pathway, have been shown, in turn, to influence death receptor function, presumably by modifying these accessory proteins (3, 25, 26). The role of the JNK pathway in modulating skeletal muscle caspase 8 activation has not, however, been previously evaluated.
The present study extends our previous work and provides the first evidence of JNK activation in skeletal model in an animal model of infection and the first evidence that JNK activation modulates skeletal muscle caspase activity. We found that endotoxin elicited a marked increase in diaphragm JNK activation as evidenced by an increase in both levels of phospho-JNK protein, the active form of this kinase, and an increase in JNK kinase activity measured by detection of phosphorylation of a JNK-specific substrate by diaphragm homogenates. This activation occurred at a relatively early time point, with phospho-JNK protein levels reaching a peak at ∼2–4 h following endotoxin administration. Moreover, these data indicate that JNK activation precedes the development of major diaphragm weakness in this experimental model, since we previously found that that it requires 12 h for diaphragm force to fall significantly after administration of this particular dose of endotoxin to mice (20). We did not explicitly examine the upstream processes by which inflammation induces JNK activation in the diaphragm, but we have previously shown that endotoxin administration induces an increase in diaphragmatic free radical generation, and free radicals are known to induce JNK activation in skeletal muscle (13, 23). As a result, it is quite likely that endotoxin-induced diaphragm JNK activation is free radical dependent.
Our finding that inhibition of JNK prevented caspase 8 activation in endotoxin-treated animals supports the concept that JNK activation is essential for caspase activation and caspase-mediated reductions in muscle function. In parallel experiments we also demonstrated that cytokine administration to isolated C2C12 cells evoked caspase 8 activation and that inhibition of JNK by use of either a chemical inhibitor or transfection of cells with a genetic JNK inhibitor blocked cytokine-induced caspase 8 activation.
To inhibit JNK activation chemically we employed SP600125, an agent that has been extensively used in both isolated cell lines and intact animals to inhibit JNK with a high level of specificity. This particular agent is very a specific, is potent JNK inhibitor, and has essentially no effect to directly inhibit ERK, p38, or NFκB at the dosage used (1). To inhibit JNK genetically in intact animal studies, we used a transgenic JNK-deficient mouse line that has been extensively used in previous reports and is known to have essentially no JNK activity in all cell lines (27). To inhibit JNK genetically in cells, we transfected muscle cells with a JNK dominant negative gene construct (using the Cre-flox system) that is only expressed when cells are grown in the presence of a trigger compound (i.e., tamoxifen). For these studies we examined the activation of caspase in cells given both tamoxifen (in which the dominant negative JNK is activated) and cytokines with cells given cytokines alone. We found that all methods used to inhibit JNK activation (employment of the chemical inhibitor SP600125 in animals and cells; transgenic knockout of JNK in intact animals; use of a tamoxifen-inducible, dominant negative, genetic JNK construct in cells) completely prevented caspase 8 activation in skeletal muscle.
While it could be argued that employment of JNK inhibitors in intact animals may have nonspecific effects that indirectly alter caspase activation (e.g., by influencing cytokine production by white cells), our C2C12 cell line studies demonstrate that JNK inhibition prevents caspase activation in the absence of systemic factors. This indicates that some specific effect of JNK activation in muscle is required for promoting muscle caspase 8 activation in the presence of cytokines. This may represent either a direct action of JNK to phosphorylate some death receptor component or an effect to influence yet another signaling process that, in turn, affects death receptor complex formation or activation. It seems extremely unlikely that the effect of JNK to modulate caspase activation can be mediated by an action of this pathway to influence muscle cytokine production, since JNK inhibition blocked caspase activation in cell studies even in the presence of high levels of exogenous cytokines (i.e., cytomix containing LPS, TNF-α, IL1-β, and IFN-γ), which should be easily capable of activating death receptors. One would have expected high levels of caspase activation in C2C12 cells during cytomix incubations despite JNK inhibition, if the sole effect of JNK activation was to promote muscle cytokine production.
Potential implications.
It is widely thought that inflammation largely induces muscle weakness and muscle wasting by increasing proteasome-driven muscle protein degradation (9). According to this conventional paradigm, proteolysis leads to protein loss, and protein loss results in the induction of muscle weakness. Emerging data indicates, however, that this classical paradigm may be incorrect. Sepsis reduces muscle force levels long before contractile protein levels fall (22). Moreover, it has been shown that the proteasome system cannot degrade intact myofibrillar proteins (19) and that some additional process must first initiate contractile protein disruption and force reductions.
The present experiment suggests an alternative pathophysiological sequence for inflammation-induced reductions in muscle force. First, endotoxin leads to the production of cytokines that activate muscle receptors (including death receptors). Receptor activation is followed by activation of signaling pathways, including the JNK MAP kinase pathway. JNK activation potentiates caspase 8 activation, which cleaves procaspase 3 to form active caspase 3. Previous work suggests that caspase 3 activation, in turn, may have the capability to cleave contractile proteins, leading to a reduction in contractile protein force generating capacity (24). In keeping with such a possibility, van Hees et al. (24) have shown that caspase 3 activation in skeletal muscle is associated with loss of muscle myosin content and subsequently loss of muscle force generation in an animal model of congestive heart failure.
A complete understanding of the processes that regulate muscle caspase activation may therefore lead to novel treatments to prevent or reverse weakness of the diaphragm and other skeletal muscles in critically ill patients. Such treatments would be expected, in turn, to improve patient functional outcome, reduce ICU and hospital stay, decrease hospital costs, and increase survival from respiratory failure.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants HL-80429, HL-81525, HL-63698, HL-80609, and HL-69821.
REFERENCES
- 1.Bennett BL, Sasaki DT, Murray BW, O'Leary EC, Sakata ST, Xu W, Leisten JC, Motiwala A, Pierce S, Satoh Y, Bhagwat SS, Manning AM, Anderson DW. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc Natl Acad Sci USA 98: 13681–13688, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Callahan LA, Nethery D, Stofan D, DiMarco A, Supinski GS. Free radical induced contractile protein dysfunction in endotoxin-induced sepsis. Am J Respir Cell Mol Biol 24: 210–217, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Cho SG, Choi EJ. Apoptotic signaling pathways: caspases and stress-activated protein kinases. J Biochem Mol Biol 35: 24–27, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Close RI The dynamic properties of mammalian skeletal muscles. Physiol Rev 52: 129–197, 1972. [DOI] [PubMed] [Google Scholar]
- 5.Divangahi M, Matecki S, Dudley RW, Tuck SA, Bao W, Radzioch D, Comtois AS, Petrof BJ. Preferential diaphragmatic weakness during sustained Pseudomonas aeruginosa lung infection. Am J Respir Crit Care Med 169: 679–686, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Drew JS, Farkas GA, Pearson RD, Rochester DF. Effects of a chronic wasting infection on skeletal muscle size and contractile properties. J Appl Physiol 64: 460–465, 1988. [DOI] [PubMed] [Google Scholar]
- 7.Frost RA, Nystrom GJ, Lang CH. Lipopolysaccharide stimulates nitric oxide synthase-2 expression in mouse skeletal muscle and C2C12 myoblasts via toll-like receptor-4 and c-Jun NH2-terminal kinase pathways. Am J Physiol Cell Physiol 287: C1605–C1615, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Fujimura N, Sumita S, Aimono M, Masuda Y, Schichinohe Y, Narimatsu E, Namiki A. Effect of free radical scavengers on diaphragmatic contractility in septic peritonitis. Am J Respir Crit Care Med 162: 2159–2165, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Hasselgren PO Role of the ubiquitin-proteasome pathway in sepsis-induced muscle catabolism. Mol Biol Rep 26: 71–76, 1999. [DOI] [PubMed] [Google Scholar]
- 10.Hussain SN, Simkus G, Roussos C. Respiratory muscle fatigue: a cause of ventilatory failure in septic shock. J Appl Physiol 58: 2033–2040, 1985. [DOI] [PubMed] [Google Scholar]
- 11.Laghi F, Cattapan SE, Jubran A, Parthasarathy S, Warshawsky P, Choi YS, Tobin MJ. Is weaning failure caused by low-frequency fatigue of the diaphragm? Am J Respir Crit Care Med 167: 120–127, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Laghi F, Tobin MJ. Disorders of the respiratory muscles. Am J Respir Crit Care Med 168: 10–48, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Li YP, Chen Y, John J, Moylan J, Jin B, Mann DL, Reid MB. TNF-α acts via p38 MAPK to stimulate expression of the ubiquitin ligase atrogin1/MAFbx in skeletal muscle. FASEB J 9: 362–370, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mier-Jedrzejowicz M, Brophy C, Green M. Respiratory muscle weakness during upper respiratory tract infections. Am Rev Respir Dis 138: 5–7, 1988. [DOI] [PubMed] [Google Scholar]
- 15.Poponick J, Jacobs I, Supinski G, DiMarco A. Effects of upper respiratory tract infections on respiratory muscle strength in patients with neuromuscular disease. Am J Respir Crit Care Med 156: 659- 664, 1997. [DOI] [PubMed] [Google Scholar]
- 16.Reid MB, Lannergren J, Westerblad H. Respiratory and limb muscle weakness induced by tumor necrosis factor-α. Am J Respir Crit Care Med 166: 479–484, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Saklatvala J, Dean J, Finch A. Protein kinase cascades in intracellular signalling by interleukin-I and tumour necrosis factor. Biochem Soc Symp 64: 63–77, 1999. [PubMed] [Google Scholar]
- 18.Shindoh C, DiMarco A, Nethery D, Supinski G. Effect of PEG-superoxide dismutase on the diaphragmatic response to endotoxin. Am Rev Respir Dis 145: 1350–1354, 1992. [DOI] [PubMed] [Google Scholar]
- 19.Solomon V, Goldberg AL. Importance of the ATP-ubiquitin-proteasome pathway in degradation of soluble and myofibrillar proteins in rabbit muscle extracts. J Biol Chem 271: 26690–26697, 1996. [DOI] [PubMed] [Google Scholar]
- 20.Supinski GS, Callahan LA. Caspase activation contributes to endotoxin-induced diaphragm weakness. J Appl Physiol 100: 1770–1777, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Supinski GS, Ji X, Wang W, Callahan LA. The extrinsic caspase pathway modulates endotoxin-induced diaphragm contractile dysfunction. J Appl Physiol 102: 1649–1657, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Supinski GS, Vanags JA, Callahan LA. Effect of proteasome inhibitors on endotoxin-induced diaphragm dysfunction. Am J Physiol Lung Cell Mol Physiol 296: L994–L1001, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Supinski G Free radical induced respiratory muscle dysfunction. Mol Cell Biochem 179: 99–110, 1998. [DOI] [PubMed] [Google Scholar]
- 24.van Hees HW, van der Heijden HF, Ottenheijm CA, Heunks LM, Pigmans CJ, Verheugt FW, Brouwer RM, Dekhuijzen PN. Diaphragm single-fiber weakness and loss of myosin in congestive heart failure rats. Am J Physiol Heart Circ Physiol 293: H819–H828, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Varfolomeev EE, Ashkenazi A. Tumor necrosis factor: an apoptosis JuNKie? Cell 116: 491–497, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Wang WH, Gregori G, Hullinger RL, Andrisani OM. Sustained activation of p38 mitogen-activated protein kinase and c-Jun N-terminal kinase pathways by hepatitis B virus X protein mediates apoptosis via induction of Fas/FasL and tumor necrosis factor (TNF) receptor 1/TNF-α expression. Mol Cell Biol 24: 10352–10365, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y, Singh R, Lefkowitch JH, Rigoli RM, Czaja MJ. Tumor necrosis factor-induced toxic liver injury results from JNK2-dependent activation of caspase-8 and the mitochondrial death pathway. J Biol Chem 281: 15258–15267, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yan W, Zhao K, Jiang Y, Huang Q, Wang J, Kan W, Wang S. Role of p38 MAPK in ICA/M-1 expression of vascular endothelial cells induced by lipopolysaccharide. Shock 17: 433–438, 2002. [DOI] [PubMed] [Google Scholar]