Abstract
Population studies indicate that moderate hyperbilirubinemia is associated with reduced incidence of cardiovascular diseases, including hypertension. Despite this correlative evidence, no studies have directly tested the hypothesis that moderate increases in plasma bilirubin levels can attenuate the development of hypertension. This hypothesis was tested by treating mice with Indinavir, a drug that competes with bilirubin for metabolism by UDP-glucuronosyltransferase 1A1 (UGT1A1). Treatment of mice with Indinavir (500 mg·kg−1·day−1, gavage) resulted in a twofold increase in plasma unconjugated bilirubin levels. Next, we determined the effect of Indinavir-induced changes in plasma bilirubin on the development of ANG II-dependent hypertension. Moderate hyperbilirubinemia was induced 3 days before the implantation of an osmotic minipump that delivered ANG II at a rate of 1 μg·kg−1·min−1. ANG II infusion increased mean arterial pressure (MAP) by 20 mmHg in control mice but by only 6 mmHg in mice treated with Indinavir (n = 6). Similar to Indinavir treatment, direct infusion of bilirubin (37.2 mg·kg−1·day−1 iv) resulted in a twofold increase in plasma bilirubin levels and also attenuated the development of ANG II-dependent hypertension. Moderate hyperbilirubinemia resulted in an increase in plasma nitrate/nitrite levels, which averaged 36 ± 2 vs. 50 ± 7 μM in ANG II vehicle vs. Indinavir-treated mice (n = 5). Moderate hyperbilirubinemia resulted in attenuation of vascular oxidative stress as determined by dihydroethidium staining of aortic segments. These results indicate that moderate hyperbilirubinemia prevents ANG II-dependent hypertension by a mechanism that may involve decreases in vascular oxidative stress.
Keywords: bilirubin; angiotensin II hypertension; uridine 5′-triphosphate-glucuronosyltransferase 1A1, heme oxygenase
bilirubin is one of the by-products of heme catabolism by heme oxygenase (HO). Bilirubin is initially produced as biliverdin, which is rapidly converted by biliverdin reductase (BVR). HO and BVR are ubiquitous enzymes that render all mammalian cell types capable of producing bilirubin; however, the majority of circulating bilirubin is derived from the breakdown of hemoglobin by the reticuloendothelial system. The resultant bilirubin circulates bound to albumin to the liver where its entry into hepatocytes is mediated by the organic anion transporting polypeptide 2 (OATP2). Once in the hepatocyte, bilirubin is glucoronidated by UDP-glucuronyltransferase 1A1 (UGT1A1) and subsequently eliminated in the bile ducts through the multidrug resistance protein 2. The conjugated bilirubin is excreted via the common bile duct in the intestines as one of the components of bile salts.
Recent population studies have revealed that elevated plasma bilirubin levels are associated with reduced incidence of cardiovascular diseases, including hypertension, coronary artery disease, atherosclerosis, and preeclampsia (5, 6, 17, 19). More recent data from a study of Framingham offspring revealed that individuals with reduced UGT1A1 transcription have elevated serum bilirubin and reduced cardiovascular disease events (14). Further epidemiological evidence from the Royal Free Hospital at the University of London reported that untreated hypertensive patients have significantly lower serum bilirubin compared with their normotensive counterparts (19). Experiments in the Gunn rat, which is a model of severe hyperbilirubinemia due to reduced UGT activity, showed an attenuated response to ANG II-dependent hypertension as well as DOCA salt hypertension (16, 20). Although the Gunn rat studies demonstrated the antihypertensive properties of bilirubin, the levels of bilirubin in this model are greater than eightfold higher than in normal rats and much higher than those observed in population studies. Therefore, it is unknown whether moderate hyperbilirubinemia (twofold increase to mimic population studies) can lower blood pressure in ANG II-treated mice (14). In this paper, we used two separate approaches 1) inhibition of UGT1A1 with Indinavir and 2) direct infusion of bilirubin to investigate the effect of moderate hyperbilirubinemia on the development of ANG II hypertension.
METHODS
Animals.
Experiments were performed on 12- to 16-wk-old male C57BL/6J mice obtained from Jackson Laboratories (Bar Harbor, ME). The mice were fed a standard diet containing 0.29% NaCl and were provided water ad libitum. All animal protocols were approved by the Institutional Animal Care and Use Committee at the University of Mississippi Medical Center and performed in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The mice were randomly divided into four treatment groups and were treated with vehicle, Indinavir (500 mg·kg−1·day−1, oral gavage), ANG II (1 μg·kg−1·min−1 sc), and ANG II plus Indinavir. UGT1A1 blockage was started 3 days before exposure to ANG II. ANG II was delivered via an osmotic minipump implanted subcutaneously. ANG II was infused for 12 days. Mice in the bilirubin infusion studies were treated with either bilirubin (37.2 mg·kg−1·day−1 iv) or vehicle (0.1 M NaOH, pH 7.8) in 3 ml isotonic saline. To maintain normal sodium intake, the mice were placed on a sodium-deficient diet (TD-90228; Harlan Teklad). After 5 days, the mice were implanted with ANG II-containing minipumps as above, and blood pressure was measured after 7 days of ANG II infusion.
Blood pressure.
Blood pressure was directly measured via microrenathane catheters implanted in the carotid artery using aseptic surgical technique as previously described (28). Surgery was performed 5 days after implantation of the minipumps, and the mice were allowed 2 days to recover from surgery. Mean arterial blood pressure (MAP) was recorded from conscious, freely moving mice for 3 h/day, on days 7 through 12 after initiation of infusion of ANG II, and results are presented as the average of the individual daily recordings over this time period.
Measurement of plasma nitrate/nitrite.
Plasma nitrate was converted to nitrite by nitrate reductase obtained from Escherichia coli, and sodium nitrate was the standard to verify that all nitrate was converted to nitrite. Griess reagent was used for colorimetric measurement of nitrate concentration. Sodium nitrite was used as the standard.
Measurement of plasma liver enzymes.
Mice were treated for 12 consecutive days with Indinavir as previously described. Plasma samples were then collected in EDTA-coated tubes and analyzed in the clinical laboratory services of the University of Mississippi Medical Center.
Western blot and immunohistochemistry.
Western blots for UGT1A1 and OATP2 proteins were performed on liver lysates. Protein (30 μg) was separated on 7.5% SDS-polyacrylamide gels and blotted on nitrocellulose membrane. Membranes were blocked with Odyssey blocking buffer (LI-COR, Lincoln, NE) for 2 h at room temperature and were incubated with goat anti-OATP2 polyclonal (Santa Cruz Biotechnology, Santa Cruz, CA) or rabbit anti-UGT1A1 (Santa Cruz Biotechnology) antibody or anti-endothelial nitric oxide synthase (eNOS) phospho-Ser1177 (Nanotools, Teningen, Germany) overnight at 4°C. The membranes were then incubated with mouse anti-β-actin antibody for 1 h followed by both Alexa 680 donkey anti-rabbit IgG (Molecular Probes) and IRDye 800 donkey anti-mouse IgG (Rockland, Gilbertsville, PA) for 1 h at room temperature and visualized using an Odyssey infrared imager (Li-COR), which allows for the simultaneous detection of two proteins. Densitometry analysis was performed using Odyssey software (LI-COR). Levels of UGT1A1 and OATP2 proteins were expressed as the ratios of the protein to β-actin for each sample.
Aortic superoxide measurement.
Aortic superoxide production was measured by staining the aortic rings with dihydroethidium (DHE) as previously described (2). The aorta was isolated and frozen in tissue-freezing medium and stored at −20°C. Thick (30 μm) frozen sections were then cut and placed on glass slides. The sections were in incubated with DHE (5 μM) at 37°C for 30 min in a light-protected humidified chamber. After 30 min, the slides were washed and coverslipped, and images were obtained and analyzed by a Leica Confocal Microscope (Leica TCS SP2 Microsystems, Heidelberg, Germany). Data were collected from three different sections from four individual mice from each group and averaged.
Statistical analysis.
Means + SE are presented. The significant difference in mean values was evaluated by either a t-test or by Dunnett's paired t-test for multiple comparisons. A value of P < 0.05 was considered to be statistically significant.
RESULTS
UGT1A1 inhibition or direct infusion of bilirubin results in moderate hyperbilirubinemia.
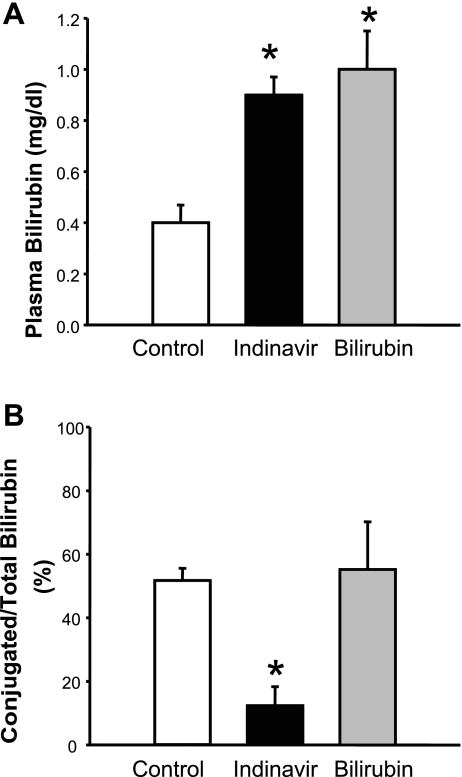
Mice were treated with Indinavir or directly infused with bilirubin for 5 days before plasma samples were collected for measurement of unconjugated and conjugated bilirubin. Both Indinavir treatment and direct infusion of bilirubin resulted in a significant increase in unconjugated bilirubin from 0.48 ± 0.08 to 0.92 ± 0.07 and 1.00 ± 0.2 mg/dl (P < 0.05 vs. control; Fig. 1A). Indinavir treatment resulted in a significant decrease in conjugated bilirubin levels in the plasma from 52 ± 5 to 12 ± 6% (P < 0.05 vs. control; Fig. 1B), whereas direct infusion of bilirubin had no effect on the levels of conjugated bilirubin, averaging 55 ± 15% (Fig. 1B). The dose of Indinavir used was not associated with fulminant hepatotoxicity, since makers of hepatic damage such as alanine aminotransferase, alkaline phosphatase, and total protein and albumin were not significantly changed in the plasma of Indinavir-treated mice compared with control mice. Indinavir treatment was associated with a twofold increase in plasma aspartate aminotransferase (AST) (Table 1).
Fig. 1.
Effects of Indinavir treatment and bilirubin infusion on plasma bilirubin levels (A) and conjugated/total bilirubin levels (B). Mice were treated with Indinavir (500 mg·kg−1·day−1, oral gavage) for 5 days or directly infused with 37.2 mg·kg−1·day−1 bilirubin, and then the plasma levels of unconjugated and conjugated bilirubin were determined using a commercially available kit. Both Indinavir treatment and bilirubin infusion resulted in increased unconjugated bilirubin levels in the plasma, whereas the percentage of conjugated bilirubin levels was decreased in Indinavir-treated mice; n = 20 control, 4 Indinavir, 4 bilirubin mice. *P < 0.05 vs. control.
Table 1.
Liver enzyme levels in the plasma of Indinavir-treated mice
| Control | Indinavir | |
|---|---|---|
| Total protein, g/dl | 4.4±0.12 | 3.6±1.1 |
| Albumin, g/d, | 1.9±0.03 | 2.0±0.07 |
| Alkaline phosphatase, IU/l | 69.7±4.9 | 55.3±2.6 |
| Alanine aminotransferase, IU/l | 37.0±1.5 | 41.7±4.6 |
| Aspartate aminotransferase, IU/l | 64.6±0.7 | 137.0±19.9* |
Values are means ± SE. Mice were treated with Indinavir (500 mg·kg−1·day−1) for 12 days before the plasma samples were collected and analyzed.
P < 0.05 compared with control mice.
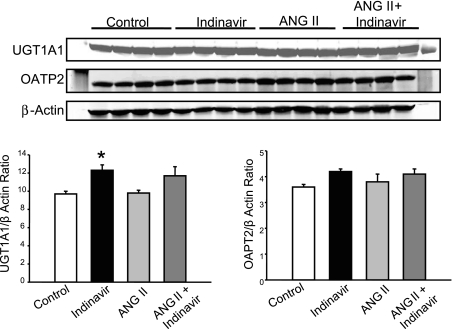
Indinavir treatment induces hepatic UGT1A1 but not OATP2.
To determine if inhibition of UGT1A1 with Indinavir or treatment with ANG II resulted in alteration in the level of proteins responsible for conjugation and transport of bilirubin in the liver, Western blots were performed to measure the levels of UGT1A1 and OATP2 proteins in the liver of control and Indinavir-, ANG II-, and ANG II + Indinavir-treated mice. Indinavir treatment alone significantly increased the levels of UGT1A1 protein in the liver but did not have any effect on the levels of OATP2 protein (P < 0.05 vs. control; Fig. 2). However, Indinavir increased the levels of UGT1A1 protein in the liver of ANG II-treated mice; however, this increase was not statistically significant.
Fig. 2.
Mice were treated with Indinavir (500 mg·kg−1·day−1, oral gavage) for 3 days before ANG II delivery. Indinavir treatment significantly increased the levels of UDP-glucuronosyltransferase 1A1 (UGT1A1) protein in the liver of control but not ANG II-infused mice. Indinavir treatment does not alter organic anion transporting polypeptide 2 (OATP2) protein levels in the liver. *P < 0.05 vs. control, n = 8.
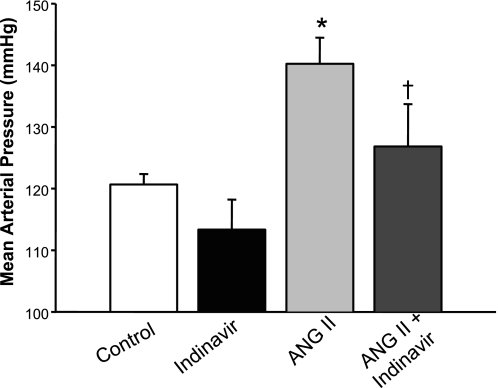
UGT1A1 inhibition attenuates ANG II hypertension.
To determine the effect of moderate hyperbilirubinemia on the development of ANG II hypertension, blood pressures were measured in conscious, freely moving mice 7 days after implantation of osmotic minipumps delivering ANG II. Indinavir treatment by itself did not have any significant effect on blood pressure, with MAP averaging 120 ± 2 vs. 113 ± 5 mmHg in control and Indinavir-treated mice (Fig. 3). ANG II infusion significantly increased MAP to 140 ± 4 mmHg in mice (P < 0.05 vs. control; Fig. 3). Indinavir treatment significantly attenuated the development of ANG II-dependent hypertension, with MAP averaging 126 ± 7 mmHg in this group of mice (P < 0.05 vs. ANG II alone). No significant difference in heart rates was observed between the groups: 532 ± 7 vs. 528 ± 27 vs. 579 ± 6 vs. 583 ± 37 beats/min in control and Indinavir-, ANG II-, and ANG II + Indinavir-treated mice.
Fig. 3.
Moderate hyperbilirubinemia attenuates ANG II-dependent hypertension. Mice were treated with Indinavir (500 mg·kg−1·day−1, oral gavage) for 3 days before the implantation of minipumps that delivered ANG II at 1 μg·kg−1·min−1. Blood pressure was measured in conscious freely moving mice by fluid-filled catheters for three consecutive days beginning 7 days after minipump implantation; n = 7. P < 0.05 vs. control (*) and vs. ANG II (†).
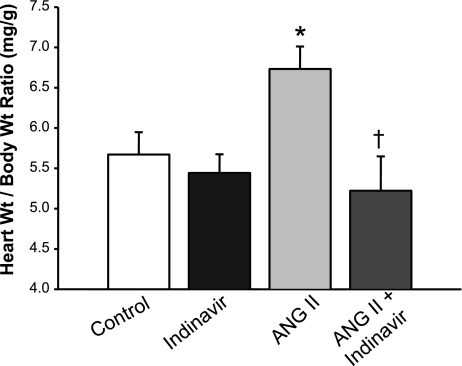
As an additional index of the antihypertensive effects of Indinavir treatment, the heart weight-to-body weight ratio was recorded in each of the groups of mice. Indinavir treatment by itself did not alter the heart weight-to-body ratio (5.4 ± 0.2 mg/g Indinavir vs. 5.6 ± 0.3 mg/g control). ANG II treatment resulted in a significant increase in the heart weight-to-body weight ratio (6.7 ± 0.3 mg/g, P < 0.05 vs. control), and this increase was completely normalized in Indinavir-treated mice (5.2 ± 0.4) (Fig. 4). The various treatments did not have any significant effect on the body weights of the mice.
Fig. 4.
Moderate hyperbilirubinemia attenuates angiotensin II-induced cardiac hypertrophy. Mice were treated with Indinavir (500 mg·kg−1·day−1, oral gavage) for 3 days before the implantation of minipumps that delivered ANG II at 1 μg·kg−1·min−1. Hearts were harvested and weighed after 10 days of ANG II infusion; n = 7. P < 0.05 vs. control (*) and vs. ANG II (†).
Moderate hyperbilirubinemia with direct infusion of bilirubin attenuates ANG II hypertension.
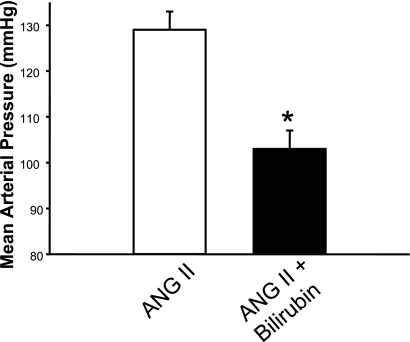
To determine whether the blood pressure-lowering actions of Indinavir treatment were due to moderate hyperbilirubinemia, we performed an additional experiment in which bilirubin was directly infused in mice to mimic the increase in plasma bilirubin observed in Indinavir-treated mice (Fig. 1A). Direct bilirubin infusion resulted in a significant attenuation of ANG II hypertension, decreasing MAP from 129 ± 4 to 104 ± 4 mmHg (P < 0.05 vs. ANG II alone; Fig. 5).
Fig. 5.
Direct infusion of bilirubin attenuates angiotensin II-induced hypertension. Mice were infused with 37.2 mg·kg−1·day−1 bilirubin for 5 days before the implantation of minipumps that delivered ANG II at 1 μg·kg−1·min−1. Blood pressure was measured in conscious freely moving mice by fluid-filled catheters for three consecutive days beginning 7 days after minipump implantation; n = 6. *P < 0.05 vs. ANG II.
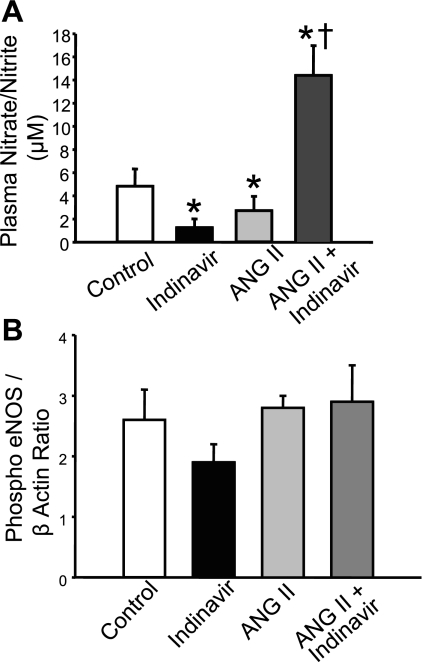
Inhibition of bilirubin metabolism restores plasma nitrate/nitrite levels.
Previous studies have shown that ANG II-dependent hypertension is associated with increases in superoxide production (7, 11, 18). The increased superoxide produced can then interact with nitric oxide (NO) to form peroxynitrate to reduce the bioavailability of NO. To determine the effect of moderate hyperbilirubinemia on levels of NO in the plasma, Nitrate/nitrite (NOx) levels were measured in the plasma of ANG II- and ANG II + Indinavir-treated mice. Indinavir and ANG II treatment resulted in a significant decrease in plasma NOx from 4.8 ± 1.8 in control mice to 1.3 ± 0.8 and 2.7 ± 1.2 in Indinavir- and ANG II-treated mice, respectively (P < 0.05 vs. control; Fig. 6A). However, plasma NOx was significantly increased to 14.3 ± 2.6 in the ANG II + Indinavir-treated mice (P < 0.05 vs. control). The increase in plasma NOx was not associated with changes in vascular phospo-eNOS (Ser1177) levels as determined by Western Blot (Fig. 6B).
Fig. 6.
A: moderate hyperbilirubinemia increases plasma nitrate/nitrite (NOx) in ANG II-infused mice. Indinavir or ANG II treatment alone resulted in a significant decrease in the plasma NOx levels. Combined ANG II plus Indinavir treatment resulted in a significant increase in plasma NOx levels. B: levels of phospho-endothelial nitric oxide synthase (eNOS; Ser1177) in the aorta of control and Indinavir-, ANG II-, and ANG II + Indinavir-treated mice as measured by Western blot. Indinavir treatment had no effect on the levels of phospho-eNOS in the aorta; n = 5. P < 0.05 vs. control (*) and vs. ANG II (†).
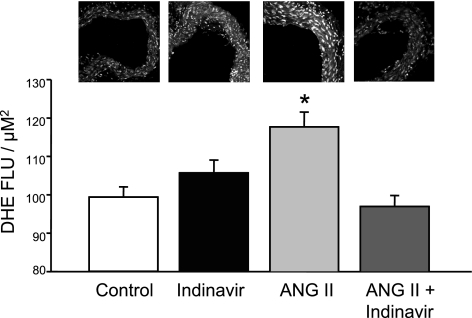
Moderate hyperbilirubinemia attenuates ANG II-dependent increase in vascular oxidative stress.
Previous studies indicated that ANG II acting through the AT1 receptor increases vascular oxidative stress by activation of NADPH oxidase activity. Bilirubin is one of the most potent endogenous antioxidants. We investigated whether moderate hyperbilirubinemia could attenuate ANG II-dependent increases in vascular oxidative stress. Aortic oxidative stress was determined by DHE staining of aortic vascular segments. ANG II infusion resulted in a significant increase in aortic superoxide production compared with control, averaging 99 ± 3 vs. 118 ± 4 fluorescent light units (FLU)/μM2 (P < 0.05 vs. control). Indinavir treatment by itself had no significant effect on aortic superoxide production, averaging 106 ± 3 FLU/μM2; however, Indinavir treatment normalized aortic superoxide levels in ANG II-infused mice, averaging 100 ± 3 FLU/μM (Fig. 7).
Fig. 7.
Moderate hyperbilirubinemia decreases aortic superoxide levels. Superoxide levels were determined using dihydroethidium (DHE) staining of aortic tissue segments. ANG II treatment significantly increased aortic superoxide levels, and this increase was completely attenuated in Indinavir-treated mice; n = 4. FLU, fluorescent light units. *P < 0.05 vs. control.
DISCUSSION
In this study, we report that moderate hyperbilirubinemia due to pharmacological inhibition of hepatic bilirubin conjugation or direct bilirubin infusion can attenuate ANG II-dependent hypertension and cardiac hypertrophy in mice. This observation is in agreement with recent reports that the Gunn rat, a model of severe hyperbilirubinemia, is resistant to the presser effects of ANG II (20). However, it should be noted that the Gunn rat is a model of Crigler-Najjar disease and exhibits plasma bilirubin levels greater than eightfold compared with normal rats (27). In contrast, models in the present study only exhibited a moderate twofold increase in unconjugated plasma bilrubin levels. The increase in bilirubin levels observed in our study is more in line with those observed in patient populations that are protected against cardiovascular disease (14). We also report that moderate hyperbilirubinemia is associated with an increase in plasma NOx levels and a reduction in vascular oxidative stress in ANG II-treated mice.
This is the first study to use pharmacological inhibition of bilirubin metabolism as a model of moderate hyperbilirubinemia to determine if increases in plasma bilirubin levels similar to those observed in patient populations can prevent ANG II-induced hypertension. Indinavir was chosen for these studies because, unlike other drugs known to inhibit bilirubin metabolism, it has not been reported to have any direct beneficial effects on the cardiovascular system. Because Indinavir is a general protease inhibitor, it is possible that the reductions in blood pressure observed in the present study could be due to nonspecific effects of Indinavir to inhibit other proteins. However, our results with direct infusion of bilirubin, which mimicked the increase in plasma unconjugated bilirubin observed in Indinavir-treated mice, demonstrate that the blood pressure-lowering actions of Indinavir are likely due to the moderate hyperbilirubinemia associated with this dose. The effect of Indinavir on blood pressure in humans is controversial. Clinical studies have indicated both increased risk and protection from hypertension in patients on regimens that include Indinavir (1, 3). Unfortunately, none of these studies contained measurements of plasma bilirubin following Indinavir treatment, so the role of alterations in plasma bilirubin in protecting or promoting hypertension in patients is not known.
Indinavir has also been reported to induce urolithiasis in humans; however, none of the mice treated with Indinavir had any signs suggestive of kidney stones (12, 23). We also measured plasma liver enzymes as an index of hepatotoxicity. There were no significant changes in the hepatic profile except for the AST, which was doubled in the Indinavir-treated mice. The increase in AST is unlikely due to hepatotoxicity, since a concomitant increase in the other liver enzymes would be expected. AST is also abundant in skeletal and cardiac myocytes, which could potentially be the source for the increased levels in response to Indinavir treatment. We can conclude that the dose of Indinavir used in the present study was effective in increasing plasma bilirubin levels but did not result in any liver toxicity or the development of kidney stones. However, the data presented are from short-term experiments; the long-term effects of this dose of Indinavir on liver toxicity and the development of kidney stones was not determined in the present study and needs to be fully investigated in future studies. Indinavir treatment alone resulted in a significant increase in the levels of UGT1A1 in the liver, which is in contrast to a previous study that demonstrated an increase in UGT1A1 mRNA but not protein following Indinavir treatment (30). Indinavir treatment did not have any effect on the levels of the bilirubin transporter, OATP2, in the liver.
The mechanism by which moderate increases in plasma bilirubin lower blood pressure in ANG II hypertension is not clearly understood. ANG II elevates blood pressure by two mechanisms. The first is the fast pressor effect produced by high concentrations of ANG II mediated through the phosphoinositide-Ca2+-protein kinase C effector system, resulting in vascular smooth muscle contraction (26). The second is the slow pressor response that is mediated by superoxide (O2•−) produced by NADPH oxidase (9). O2•− reacts with NO to yield peroxynitrite, resulting in endothelial dysfunction through reduction in NO bioavailability (4). Bilirubin is one of the most potent endogenous antioxidants (24). Bilirubin can act as an antioxidant in two ways: 1) by directly scavenging O2•− and 2) by inhibiting NADPH oxidase, which is the major source of O2•− in ANG II hypertension (8, 10, 13). Our data demonstrate that moderate hyperbilirubinemia is capable of decreasing vascular oxidative stress and increasing plasma NOx levels, suggesting a role for increased NO bioavailability in antihypertensive actions of moderate hyperbilirubinemia. Previous studies have indicated that induction of HO-1 attenuates ANG II-dependent hypertension by decreasing oxidative stress (11, 21, 29). The current study provides additional evidence that increased bilirubin might mediate part of the antihypertensive and antioxidant effects of HO-1 induction in these models.
Our data revealed an interesting relationship between Indinavir treatment and plasma NOx levels in the current study. Indinavir or ANG II treatment alone lowered plasma NOx levels compared with control mice. However, Indinavir treatment of mice infused with ANG II resulted in significantly increased plasma NOx levels compared with control and Indinavir- and ANG II-treated mice. The effect of Indinavir on plasma NOx levels in control mice in the present study is consistent with a recent report in which Indinavir treatment caused endothelial dysfunction in a group of normal healthy human subjects (20, 25). Our findings would indicate that a reduction in NO bioavailability may be responsible for endothelial dysfunction in normal individuals treated with Indinavir since, plasma NOx were significantly decreased in mice treated with Indinavir alone. The mechanism by which Indinavir treatment may lower NO bioavailability in normal mice is not known but may be a direct effect of Indinavir independent of the increase in plasma bilirubin levels. Our vascular oxidative stress data excludes oxidative stress as a mechanism for this phenomenon. In ANG II-treated mice, the increase in bilirubin levels may result in a greater decrease in vascular O2•− levels, which are stimulated directly by ANG II and also through increased production of endothelin (15, 18, 22). This decrease in vascular O2•− levels would result in a decrease in peroxynitrite generation and an increase in NO bioavailability.
Perspectives and Significance
Epidemiological data have correlated moderate increases in plasma bilirubin with protection from cardiovascular diseases, including hypertension. Despite this correlative data, there are no studies that have directly demonstrated a blood pressure-lowering action of moderate hyperbilirubinemia or the potential mechanism of how a moderate increase in plasma bilirubin may lower blood pressure. Our data indicate that moderate increases in plasma bilirubin can protect against ANG II-dependent hypertension. Moderate hyperbilirubinemia also resulted in a decrease in vascular oxidative stress, thus increasing plasma NO, suggesting that increases in the bioavailability of NO may mediate the observed antihypertensive effects. Our data also indicate that the beneficial effect of moderate hyperbilirubinemia does not require lifelong exposure to higher bilirubin levels and that inhibition of bilirubin metabolism is a potential target for antihypertensive therapy. Targeting of bilirubin conjugation through chronic UGT1A1 inhibition with Indinavir has potential adverse side effects that may outweigh its antihypertensive benefits; therefore, alternative approaches to inhibit UGT1A1 or increase the levels of unconjugated bilirubin need to be developed.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-038499-16S1 (T. Vera), HL-038499 (J. P. Granger), and HL-88421 (D. E. Stec).
REFERENCES
- 1.Cattelan AM, Trevenzoli M, Sasset L, Rinaldi L, Balasso V, Cadrobbi P. Indinavir and systemic hypertension. AIDS 15: 805–807, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Courtois E, Marques M, Barrientos A, Casado S, Lopez-Farre A. Lead-induced downregulation of soluble guanylate cyclase in isolated rat aortic segments mediated by reactive oxygen species and cyclooxygenase-2. J Am Soc Nephrol 14: 1464–1470, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Crane HM, Van Rompaey SE, Kitahata MM. Antiretroviral medications associated with elevated blood pressure among patients receiving highly active antiretroviral therapy. AIDS 20: 1019–1026, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Didion SP, Sigmund CD, Faraci FM. Impaired endothelial function in transgenic mice expressing both human renin and human angiotensinogen. Stroke 31: 760–765, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Hopkins PN, Wu LL, Hunt SC, James BC, Vincent GM, Williams RR. Higher serum bilirubin is associated with decreased risk for early familial coronary artery disease. Arterioscler Thromb Vasc Biol 16: 250–255, 1996. [DOI] [PubMed] [Google Scholar]
- 6.Hunt SC, Wu LL, Hopkins PN, Williams RR. Evidence for a major gene elevating serum bilirubin concentration in Utah pedigrees. Arterioscler Thromb Vasc Biol 16: 912–917, 1996. [DOI] [PubMed] [Google Scholar]
- 7.Jalil JE, Perez A, Ocaranza MP, Bargetto J, Galaz A, Lavandero S. Increased aortic NADPH oxidase activity in rats with genetically high angiotensin-converting enzyme levels. Hypertension 46: 1362–1367, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Jiang F, Roberts SJ, Datla S, Dusting GJ. NO modulates NADPH oxidase function via heme oxygenase-1 in human endothelial cells. Hypertension 48: 950–957, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Kawada N, Imai E, Karber A, Welch WJ, Wilcox CS. A mouse model of angiotensin II slow pressor response: role of oxidative stress. J Am Soc Nephrol 13: 2860–2868, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Kawamura K, Ishikawa K, Wada Y, Kimura S, Matsumoto H, Kohro T, Itabe H, Kodama T, Maruyama Y. Bilirubin from heme oxygenase-1 attenuates vascular endothelial activation and dysfunction. Arterioscler Thromb Vasc Biol 25: 155–160, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Kelsen S, Patel BJ, Parker LB, Vera T, Rimoldi JM, Gadepalli RS, Drummond HA, Stec DE. Heme oxygenase attenuates angiotensin II-mediated superoxide production in cultured mouse thick ascending loop of Henle cells. Am J Physiol Renal Physiol 295: F1158–F1165, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kohan AD, Armenakas NA, Fracchia JA. Indinavir urolithiasis: an emerging cause of renal colic in patients with human immunodeficiency virus. J Urol 161: 1765–1768, 1999. [DOI] [PubMed] [Google Scholar]
- 13.Kwak JY, Takeshige K, Cheung BS, Minakami S. Bilirubin inhibits the activation of superoxide-producing NADPH oxidase in a neutrophil cell-free system. Biochim Biophys Acta 1076: 369–373, 1991. [DOI] [PubMed] [Google Scholar]
- 14.Lin JP, O'Donnell CJ, Schwaiger JP, Cupples LA, Lingenhel A, Hunt SC, Yang S, Kronenberg F. Association between the UGT1A1*28 allele, bilirubin levels, and coronary heart disease in the Framingham Heart Study. Circulation 114: 1476–1481, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Moreau P, d'Uscio LV, Shaw S, Takase H, Barton M, Luscher TF. Angiotensin II increases tissue endothelin and induces vascular hypertrophy : reversal by ETA-receptor antagonist. Circulation 96: 1593–1597, 1997. [DOI] [PubMed] [Google Scholar]
- 16.Nath KA, d'Uscio LV, Juncos JP, Croatt AJ, Manriquez MC, Pittock ST, Katusic ZS. An analysis of the DOCA-salt model of hypertension in HO-1−/− mice and the Gunn rat. Am J Physiol Heart Circ Physiol 293: H333–H342, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Novotny L, Vitek L. Inverse relationship between serum bilirubin and atherosclerosis in men: a meta-analysis of published studies. Exp Biol Med (Maywood) 228: 568–571, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Ortiz MC, Manriquez MC, Romero JC, Juncos LA. Antioxidants block angiotensin II-induced increases in blood pressure and endothelin. Hypertension 38: 655–659, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Papadakis JA, Ganotakis ES, Jagroop IA, Mikhailidis DP, Winder AF. Effect of hypertension and its treatment on lipid, lipoprotein(a), fibrinogen, and bilirubin levels in patients referred for dyslipidemia. Am J Hypertens 12: 673–681, 1999. [DOI] [PubMed] [Google Scholar]
- 20.Pflueger A, Croatt AJ, Peterson TE, Smith LA, d'Uscio LV, Katusic ZS, Nath KA. The hyperbilirubinemic Gunn rat is resistant to the pressor effects of angiotensin II. Am J Physiol Renal Physiol 288: F552–F558, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Quan S, Yang L, Shnouda S, Schwartzman ML, Nasjletti A, Goodman AI, Abraham NG. Expression of human heme oxygenase-1 in the thick ascending limb attenuates angiotensin II-mediated increase in oxidative injury. Kidney Int 65: 1628–1639, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Rajagopalan S, Laursen JB, Borthayre A, Kurz S, Keiser J, Haleen S, Giaid A, Harrison DG. Role for endothelin-1 in angiotensin II-mediated hypertension. Hypertension 30: 29–34, 1997. [DOI] [PubMed] [Google Scholar]
- 23.Saltel E, Angel JB, Futter NG, Walsh WG, O'Rourke K, Mahoney JE. Increased prevalence and analysis of risk factors for indinavir nephrolithiasis. J Urol 164: 1895–1897, 2000. [PubMed] [Google Scholar]
- 24.Sedlak TW, Snyder SH. Bilirubin benefits: cellular protection by a biliverdin reductase antioxidant cycle. Pediatrics 113: 1776–1782, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Shankar SS, Dube MP, Gorski JC, Klaunig JE, Steinberg HO. Indinavir impairs endothelial function in healthy HIV-negative men (Abstract). Am Heart J 150: 933, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Taubman MB, Berk BC, Izumo S, Tsuda T, Alexander RW, Nadal-Ginard B. Angiotensin II induces c-fos mRNA in aortic smooth muscle Role of Ca2+ mobilization and protein kinase C activation. J Biol Chem 264: 526–530, 1989. [PubMed] [Google Scholar]
- 27.van der Wegen P, Louwen R, Imam AM, Buijs-Offerman RM, Sinaasappel M, Grosveld F, Scholte BJ. Successful treatment of UGT1A1 deficiency in a rat model of Crigler-Najjar disease by intravenous administration of a liver-specific lentiviral vector. Mol Ther 13: 374–381, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Vera T, Kelsen S, Stec DE. Kidney-specific induction of heme oxygenase-1 prevents angiotensin II hypertension. Hypertension 52: 660–665, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang L, Quan S, Nasjletti A, Laniado-Schwartzman M, Abraham NG. Heme oxygenase-1 gene expression modulates angiotensin II-induced increase in blood pressure. Hypertension 43: 1221–1226, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Zucker SD, Qin X, Rouster SD, Yu F, Green RM, Keshavan P, Feinberg J, Sherman KE. Mechanism of indinavir-induced hyperbilirubinemia. Proc Natl Acad Sci USA 98: 12671–12676, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]