Abstract
We examined the potential of using native fish species in regulating mosquitoes in the floodplain of the Gambia River, the major source of mosquitoes in rural parts of The Gambia. Fishes and mosquito larvae were sampled along two 2.3-km-long transects, from the landward edge of the floodplain to the river from May to November 2005 to 2007. A semifield trial was used to test the predatory capacity of fish on mosquito larvae and the influence of fish chemical cues on oviposition. In the field, there was less chance of finding culicine larvae where Tilapia guineensis, the most common floodplain fish, were present; however, the presence of anophelines was not related to the presence or absence of any fish species. In semifield trials, both T. guineensis and Epiplatys spilargyreius were effective predators, removing all late-stage culicine and anopheline larvae within 1 d. Fewer culicines oviposited in sites with fish, suggesting that ovipositing culicine females avoid water with fish. In contrast, oviposition by anophelines was unaffected by fish. Our studies show that T. guineensis is a potential candidate for controlling mosquitoes in The Gambia.
Keywords: Anopheles gambiae, fish, mosquitoes, predation, oviposition
The use of fish for controlling mosquitoes was an important tool in the pre-DDT era (Floore 2006, Walker and Lynch 2007). Typically fish were introduced into all potential mosquito breeding habitats, including ricefields, marshes, dams, canals, and ponds (Hadjinicolaou and Betzios 1973, Motabar 1978). However, the introduction of DDT in the mid-1940s led to a significant decrease in the use of biological control (Gabaldon 1969). Nevertheless, after concerns regarding the harmful effects of chemicals on nontarget species and the development of insecticide resistance by mosquitoes (Milam et al. 2000), interest in the biological control of malaria vectors has been rekindled (Killeen et al. 2002, Killeen 2003).
Fish have been used successfully for controlling both culicine and anopheline mosquitoes (Tabibzadeh et al. 1970, Victor et al. 1994) but are used relatively infrequently in sub-Saharan Africa (Walker and Lynch 2007). Mosquito control using fish has focused on a limited number of species, primarily Gambusia affinnis Baird and Girard and Poecilia reticulata Peters, that have traditionally been used for controlling mosquito larvae (Sitaraman et al. 1975, Gall et al. 1980, Cech and Linden 1987, Homsky et al. 1987, Blaustein 1992, Valero et al. 2006, Walton 2007). One of the most important concerns when introducing exotic fish for mosquito control is their impact on native species (Benigno 2001, Hoddle 2004). In Greece, the introduction of G. affinis led to a decline of the endemic fish species Valencia letourneuxi Sauvage (Economidis 1995), with similar findings reported elsewhere (United States, Spain, Australia) (Motabar 1978, Arthington 1991, Garcia-Berthou 1999, Leyse et al. 2004). The problems with introducing exotic species have spurred interest in the use of native species for controlling mosquitoes (Romand 1985; Mancini and Romi 1988; Fletcher et al. 1992, 1993; Frenkel and Goren 1999; Lee 2000; Kusumawathie et al. 2006; Marti et al. 2006; Yildirim and Karacuha 2007).
Using fish for mosquito control can be unpredictable, with several failures reported in the literature (Bence 1988, Blaustein 1992). However, the number of failures may be larger caused by publication bias, over-representing successful trials. The importance of mosquito larvae in the natural diet of fish, exclusions of mosquitoes from aquatic habitats caused by predation, or avoidance by ovipositing female mosquitoes has rarely been studied, with studies often reporting contradictory results. In Colombia, larvae of Anopheles albimanus Wiedemann were found to be negatively associated with fish and predatory invertebrates, such as dragonfly and mayfly nymphs (Marten et al. 1996). This phenomenon is thought to be far more widespread in nature, with predatory fish influencing the distribution of many species of aquatic invertebrates (Wellborn et al. 1996, Maddrell 1998). In contrast, in Pakistan, larvae of Anopheles subpictus Grassi were positively related with the presence of aquatic predators including fish, although the authors were unable to provide an explanation for this (Herrel et al. 2001).
Several aquatic invertebrate taxa have evolved avoidance behaviors to minimize predation risk (Kerfoot and Sih 1987, Tjossem 1990, Resetarits 2001, Abjornsson et al. 2002). This behavior has also been reported in mosquito species, mainly culicines, in response to both invertebrate and vertebrate (mainly fish) predators (Spencer et al. 2002, Kiflawi et al. 2003, Angelon and Petranka 2004, Blaustein et al. 2004, Bond et al. 2005, Munga et al. 2006). Identifying whether mosquitoes detect and avoid ovipositing in habitats containing fish is important because it affects the efficacy of fish to control mosquitoes because gravid female mosquitoes may select alternative breeding sites. Considering the importance of African anopheline mosquitoes in the transmission of malaria and the renewed interest in using fish for mosquito control, it is surprising that no studies have been carried out to determine whether Anopheles gambiae s.l. Giles, the principal vector of malaria in Africa, avoids fish. Although several studies have examined the potential of native fish species to control African malaria vectors, the importance of these species in the ecology of the mosquito has been overlooked (Kumar and Hwang 2006). We therefore set out to test the hypothesis that native fish species can be used for mosquito larval control in West Africa. The following predictions were tested: (1) the absence of aquatic stages of mosquitoes will be associated with the presence of certain floodplain fish species under natural conditions, (2) the diet of these fish species will include mosquitoes, (3) the presence of insectivorous fish will reduce oviposition by female mosquitoes under semifield conditions, and (4) insectivorous fish will be efficient predators of mosquitoes under semifield conditions. The study was carried out in the floodplain of the Gambia River, the major source of anopheline mosquitoes in rural parts of The Gambia (Bøgh et al. 2007, Majambere et al. 2008). This study formed part of a larger project investigating the use of microbial larvicides for controlling malaria vectors and, taking into account the lack of published information on the indirect effects of microbial larvicides on fish communities in lentic systems, allowed us to investigate at a pilot study scale whether this activity affected fish populations in the floodplain.
Materials and Methods
Study Area
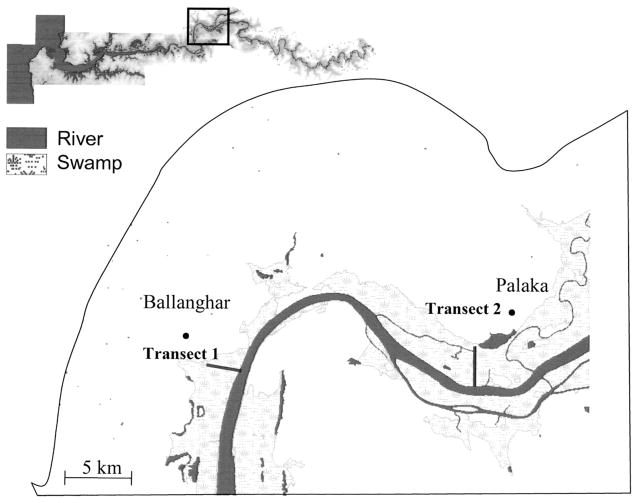
This study was conducted along two transects (Balanghar-Ker Derry village [transect 1], 13°39′ N, 15°23′ W, Palaka village [transect 2] 13°40′ N, 15°13′ W), each ≈2.3 km long, on the floodplain east of Farafenni town (UTM coordinates: 1500200N, 435500E), The Gambia, 193 and 209 km upstream of the estuary mouth, respectively, and approximately at the upper limit of brackish water during the dry season (Fig. 1). The transects were chosen to cross all vegetation zones typical of the floodplains. Rains occur from June to October, with the highest long-term rainfall recorded in August. Baseline data were collected in 2005 when no sites were treated with larvicide. In 2006, transect 1 was treated weekly with Bacillus thuringiensis Berliner variety israelensis, followed by transect 2 in 2007 (Majambere et al., unpublished data).
Fig. 1.
Location of the study sites on the Gambia River floodplain.
Each transect was located at the center of a zone ≈100 km2 in area, where all aquatic sites were treated at weekly intervals from May to November. In 2006 ≈2,200 kg of Bti WDG and 1,200 kg Bti CG were applied in spray zone 1, with 3,200 kg Bti WDG and 3,700 kg Bti CG in 2007 in zone 2. The floodplain in this area was characterized by a stretch of mangroves along the main river channel and some of the larger connecting creeks. Behind the mangroves were continuous areas of mudflats that were often entirely barren because of the prolonged desiccation during the dry season and high content of soluble salts, mainly chlorides and sulfates (Giglioli and King 1966). Rice fields occurred in two belts on the floodplain: one along the landward edge of the floodplain and the other closer to the river. Mudflats that receive periodic flooding in the dry season from the spring tides support the perennial Sesuvium portulacastrum, as well as seasonal Heleocharis spp. and beds of Paspalum spp. (Giglioli and Thornton 1965, Bøgh et al. 2007). The area was therefore characterized by the presence of some salt marsh vegetation but also retains characteristic freshwater flora such as water lilies (Nymphaea spp.) in some habitats, such as semipermanent pools.
Field Mosquito and Fish Sampling
Two transects were sampled monthly from May to November in 2005, 2006, and 2007, beginning ~1 mo before the rains and ending 1 mo after the rains. Each transect started from the beginning of the floodwater and ended at the main river channel in the case of transect 1 and in the thick forest fringing the river in the case of transect 2. Mosquito larvae were sampled by taking 10 dips with a standard mosquito dipper (350 ml capacity dipper; Clarke Mosquito Control Products, Roselle, IL) every 150 m along each transect between 0700 and 1300 hours. Dips were made within 20 m either side of the transect point. The presence or absence of anophelines and culicine larvae at each sampling site was recorded. Late anopheline larvae were transferred to plastic containers with water from the sampling site and transported to the laboratory and allowed to emerge for subsequent identification.
Fish sampling took place along the transects within 20 m of the mosquito-sampling locations using a cast net (diameter: 230 cm, mesh size: 10 mm) and a hand net (25 by 17 cm in area, mesh size: 2 mm). The cast net was used for sampling in open water and areas of sparse vegetation, and the hand net was used to sample smaller fish species and juvenile fish in the shallower vegetated areas (<30 cm). Five cumulative minutes of sweeping were undertaken with the hand-net and three cast net throws were taken within the sampling area. Together, these methods provided effort-standardized sampling throughout the study period along floodplain transects. Fish were preserved in 4% formalin and taken to the laboratory for subsequent identification. Fish were identified to species using Paugy et al. (2003). Each sampling location on the transect was categorised as one of four aquatic habitat types according to the classification scheme of Majambere et al. (2008): floodwater habitats, rice fields, creeks, or pools. Pools were defined as semipermanent, rain fed, and not connected with the rest of the inundated floodplain except during short periods during heavy rainfall.
Diet Analysis
The modified Costello method (Costello 1990) of Amundsen et al. (1996) was used to describe the feeding habits of the different fish species and to identify the insectivores, the feeding guild most likely to prey on mosquito larvae. For each prey item in each fish species, the percentage occurrence (%Fi) and the prey-specific abundance (Pi) were calculated as follows:
where Ni is the number of fish with prey i in their stomach, N is the total number of fish with stomach contents, Si is the stomach contents wet weight comprised of prey i, and ΣSti is the total stomach content weight in only those fish with prey i in their stomach.
Prey abundance values for every prey item were obtained by the product of the percentage occurrence (%Fi) and prey-specific abundance (Pi), which is also represented by the area enclosed by the coordinates of the two axes on a %Fi versus Pi graph. In assigning species to trophic guilds, prey items (starting from the most common one) that totaled 50% of the total prey abundance were used to define the trophic guild of the species. The following trophic guilds were used: (1) omnivores, fish that included both animal and plant material in the items that contributed the first 50% of the prey abundance values; (2) insectivores, this group included both aquatic and terrestrial insect prey items; (3) detritivores, fish that fed primarily on organic detritus that may include mineral material; (4) piscivores, prey items that included other fish; (5) planktivores, this category included zooplankton, mainly cladocerans and crustacean larvae; (6) molluscivores, diet comprising mainly gastropods and bivalves; and (7) herbivores, fish that fed primarily on plant material. Only fish species with a minimum of 20 individuals caught with usable stomach contents (not empty or fully digested) were used in the analysis.
Semifield: Predation Experiment
This experiment was based on a setup that had been used previously for testing the efficacy of microbial larvicides in the field (Fillinger et al. 2003, Majambere et al. 2007). Twenty-five plastic bowls with an upper diameter of 53 cm, a lower one of 40 cm, and a height of 40 cm (70-liter volume) were sunk into an open sunlit area on the MRC field station in Farafenni with the lip protruding 5 cm above the soil. Bowls were set out in a grid 2 m apart, with five rows of five bowls. Overflow holes were created in each bowl, 35 cm from the bottom, to allow excess water to run-off during heavy rains. These holes were 1 cm in diameter and were covered with untreated nylon mosquito netting (mesh: 0.2 mm) to prevent larvae and fish from escaping. A collar of netting (inner diameter: 25 cm, mesh: 1 mm) was placed around the rim of each bowl, directed inward and upward at a 45° angle, to prevent any fish present from jumping out of the bowls.
Each bowl was filled with soil to a depth of 5 and 30 cm of unchlorinated tap water. The soil was thoroughly mixed beforehand to prevent variations in the soil conditions between bowls. Bowls were allowed to be colonized by mosquitoes for 8 d and for late instar larvae to develop. Subsequently, the 25 bowls were ranked in terms of their anopheline larval densities at the end of the 8-d period. The 18 bowls with the highest densities were kept and the rest were emptied. Subsequently, these bowls were separated into six groups of three each with similar anopheline densities. The two treatments, addition of six Epiplatys spilargyreius Duméril (average biomass: 0.45 g/70 liters) or six Tilapia guineensis Bleeker (average biomass: 26.3 g/70 liters) per bowl, and controls, with no fish, were assigned randomly within each one of these six groups.
Epiplatys spilargyreius was selected for this experiment because this species was most frequently found with mosquito larvae in its stomach and was also the most common insectivore species and a surface feeder, whereas T. guineensis was selected because it was the most abundant fish in the floodplain, was a bottom feeder, and is known to readily emigrate and colonize newly available floodplain habitats (Louca et al. 2009a, b). More than 200 individuals of both species were collected from the floodplain. Each fish species was kept in separate stock containers and fed on standard aquarium flake food (Tetramin [Tetra, Hanover, Germany], fed ad libitum) before use in the experiments. Individual fish were used only once during the experiment to avoid the influence of learned behavior on mosquito predation. Fish of similar sizes were used to avoid variations within, but not between, species in the predatory capacity because of size differences. The size range for E. spilargyreius was 3–5 cm total length (TL: tip of snout to margin of caudal fin), which is the size range for adult fish (Paugy et al. 2003), and for T. guineensis, the range was 5–7 cm TL. This represents the most appropriate sizes that can survive adequately in the experimental bowls used for this project but also includes the size at which these fish are commonly found in floodplain habitat.
The experiment was run for a further 12 d. Mosquito sampling took take place daily between 1700 and 1900 hours. Five dips were taken from each bowl using a standard dipper: four from the sides and one from the center. The presence or absence of anopheline and culicine larvae was noted and recorded as early (first and second instar), late (third and fourth instar), or pupae. All larvae were replaced, whereas pupae were removed for subsequent species identifications as described below. Any dead fish were replaced with fish kept in the holding bowl.
Semifield: Oviposition Cues
Nine bowls were placed 4 m apart in a grid arrangement in the same location as described above for the predation experiment. The greater distance between bowls than in the predation experiment was used to reduce the possibility of mosquitoes confusing chemical cues between bowls. Each bowl, including controls had a 20 by 20 by 20-cm netting cage (mesh size: 0.2 mm) suspended in the middle of the bowl, ≈1 cm below the water surface. Three randomly chosen bowls contained six E. spilargyreius placed within the cage, three had six caged T. guineensis, and three without fish served as controls. Dipping for mosquito larvae was carried out as described above for 7 d after first colonization, while the bowls were left open and oviposition continued. Three separate trials were carried out. New bowls were used between trials and experiments to avoid cross-contamination of fish chemical cues.
Mosquito Identification
All pupae were collected from the semifield experiments and late anopheline larvae from the field study were placed in separate individual mosquito cages and allowed to eclode. Adult anophelines were identified morphologically using Gilles and DeMeillon (1968). Culicine mosquitoes were identified as Culex quinquefasciatus Say, Toxorhynchites spp., Aedes vittatus Bigot, and other culicines. Sibling species of the An. gambiae complex were subsequently identified by amplification of ribosomal DNA using polymerase chain reaction (PCR) (Scott et al. 1993).
Statistical Analysis
Data were entered using Epi-Info version, version 3.5.1 (Center for Diseases Control and Prevention, Atlanta, GA). Because of the large number of sites sampled for which zero counts of mosquito larvae were obtained, mosquito data were transformed to presence or absence of larvae. Canonical correspondence analysis (CCA) (Ter Braak 1986) was used to analyze the influence of environmental variables (habitat type, treated/untreated with larvicides, transect, year, distance from the floodplain edge, percentage vegetation cover, sampling month, and presence/absence of each of the four most common insectivore fish species, as well as the commonest fish species, T. guineensis), on the presence/absence of anophelines and culicine larvae. A partial CCA was undertaken with sampling point controlled for as a covariable to account for repeated measures. Only environmental variables explaining a significant additional proportion of variance were used to avoid possible collinearity effects. Forward selection was used to identify significant variables and each variable was tested using Monte Carlo permutation test (999 runs). The CCA multivariate method is a descriptive approach that does not quantify the impact of each of the variables on the mosquitoes individually. Therefore, the impact of each of the variables identified using CCA on the presence/absence of anophelines and culicines was tested using general estimating equations (GEEs) using SPSS version 15 (SPSS Inc., Chicago, IL). This analysis extends generalized linear models (GLMs) to account for repeated measures and clustering of samples as well as allowing for linear and nonlinear models (Horton and Lipsitz 1999). A binomial distribution with a logit link function was used to test the effect of those variables identified by CCA as having a significant impact determining the distribution of positive and negative anophelines and culicine sites on each of those mosquito groups separate. All P values were adjusted to account for the number of comparisons carried out.
For those fish species that significantly influenced the distribution of mosquitoes in the field, separate analyses were carried out using only the hand-net samples, because these were collected from the edges of aquatic habitats where mosquitoes were also sampled, reflecting a better representation of possible interactions between fish and mosquito.
Semifield data were incorporated untransformed in a mathematical model and analyzed using GEE analysis. Bowl identity was accounted for as a repeated-measures variable assuming an exchangeable correlations matrix. For the predation semifield experiment, the presence of anopheline and culicine mosquito was tested against the two fish treatments using a binomial distribution with a logit link function to test the predatory capacity of the two fish species.
In the oviposition experiment, larval densities (number of larvae per dip) were tested against the two fish treatments using a normal distribution and a log link function because a better goodness-of-fit was achieved with this distribution model compared with using a binomial model and testing presence/absence of mosquito. Comparisons between treatments were made only when at least one bowl was colonized by mosquitoes. The percentage reduction in larval mosquito densities was calculated using the formula of Mulla et al. (1971): % reduction = 100 − (C1/T1 × T2/C2) × 100, where C1 and C2 describe the average number of larvae in the control tanks pre- and post-treatment, and T1 and T2 describe the average number of larvae in the treated with fish tanks pre- and post-treatment.
A Poisson probability distribution with a log link function was used to test the impact of spraying with microbial larvicides on the fish species richness (number of fish species caught). The Poisson distribution model is appropriate for counts of animal/plant units and is therefore appropriate for testing species richness (Ter Braak and Smilauer 2002). Catches of the most common six fish species followed a negative binomial distribution; therefore, a negative binomial distribution model, with a negative binomial link, was used to test the impact of microbial larvicide spraying on the abundances of these species.
Ethics
Durham University Ethics Advisory, Gambia Government/Medical Research Council Joint Ethics Committee, and Gambian Fisheries Department granted ethical approval. All fish were handled according to the ethical animal treatment rules (Anon 2003).
Results
Mosquitoes and Fish Feeding Habits
A total of 11,013 fish were caught along the transects between 2005 and 2007 (see species list in Louca et al. 2009b). T. guineensis was the dominant species, comprising >86% of the combined catch with an average density of 7.59 fish/m2 (range: 0 – 518 fish/m2) and an average catch biomass of 296 g/m2 (range: 0 – 1959 g/m2). Because most species were rare, with only a few individuals caught, reliable diet data could only be obtained for 12 species. For E. spilargyreius, the second species tested under semifield conditions, the average density was 0.1 fish/m2 (range: 0 – 1 fish/m2) and average biomass of 0.02 g/m2 (range: 0 – 0.1 g/m2). Fish belonging to several feeding guilds were identified (Table 1), but only three species were collected with mosquitoes in their stomachs. Adult mosquitoes were observed in stomachs of Rhambdalestes septentrionalis Boulenger and anopheline larvae in Ctenopoma kings-leyae Günther, while culicine larvae occurred in E. spilargyreius and R. septentrionalis. The insectivores mostly consumed aquatic Hemiptera, adult stages of terrestrial insects (Diptera, Coleoptera), larval/pupal stages of non-mosquito Diptera, and Odonata nymphs (Table 1).
Table 1.
Diet guilds (both sexes together) are provided for fish species with sufficient sample size (n > 20) sampled in 2005–2006
| Fish species | Diet (n) |
Fish | Molluscs | Terr. arthropods |
Aq. invert. |
Zoopl- ankton |
Fish/invert. eggs |
Fish remains (scavenging) |
Detritus | Macrophytes | Plant seeds |
Diet guild |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elops lacerta | 29 | 78.28 | 21.72 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | P |
| Rhambdalestes septentrionalis | 60 | 0.00 | 0.00 | 41.85 | 31.87 | 21.81 | 1.89 | 0.67 | 1.92 | 0.00 | 0.00 | I |
| Chrysichthys nigrodigitatus | 31 | 4.87 | 7.31 | 0.19 | 81.62 | 0.02 | 0.00 | 5.98 | 0.00 | 0.00 | 0.00 | I |
| Poropanchax normanii | 353 | 1.95 | 0.85 | 3.54 | 14.83 | 5.30 | 0.63 | 0.26 | 69.51 | 2.82 | 0.29 | D |
| Epiplatys spilargyreius | 108 | 0.00 | 0.00 | 36.46 | 39.44 | 7.16 | 0.00 | 0.00 | 16.94 | 0.00 | 0.00 | I |
| Epiplatys bifasciatus | 26 | 11.11 | 0.00 | 66.66 | 11.12 | 11.11 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | I |
| Tilapia guineensis | 1,431 | 0.00 | 0.38 | 0.78 | 3.78 | 1.72 | 0.29 | 2.36 | 86.91 | 3.60 | 0.19 | D |
| Hemichromis bimaculatus | 39 | 0.00 | 4.06 | 4.07 | 79.68 | 0.00 | 4.06 | 0.00 | 4.06 | 0.00 | 4.09 | I |
| Hemichromis fasciatus | 29 | 66.67 | 0.00 | 0.00 | 33.33 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | P |
| Liza grandisquamis | 32 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 97.18 | 2.82 | 0.00 | D |
| Liza falcipinnis | 26 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 100.00 | 0.00 | 0.00 | D |
| Porogobius schlegelli | 39 | 0.00 | 75.00 | 0.00 | 25.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | M |
Percentage abundance of each prey item is provided (%Fi × Pi). The prey item/items that add up to a minimum of 50% of prey abundance and therefore determine the diet guild in which a species belongs are highlighted in bold.
D, detritivores; I, insectivores; P, piscivores; M, molluscivores.
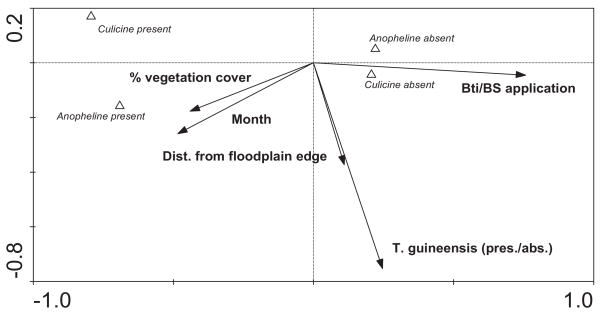
CCA identified five significant variables (percentage vegetation cover, sampling month, distance from floodplain edge, the presence or absence of T. guineensis, and larvicide application) that determined the distribution of sites with or without anophelines or culicines (Fig. 2). The impact of these variables on the presence or absence of anopheline and culicine larvae was further tested using GEE analysis. Larviciding significantly decreased anophelines by 68% and culicines by 74% (Table 2). The presence of T. guineensis was associated with a 38% decrease in culicine mosquito (Table 2). Moreover, anophelines were associated with habitats with higher vegetation cover (Fig. 2; Table 2). Mosquito control using microbial larvicide did not have a significant impact on the fish species richness on the floodplain, and no impact was determined in terms of the abundances of the most common fish species encountered on the floodplain (Table 3).
Fig. 2.
CCA biplot of the distribution of positive and negative sites for anophelines and cilucine larvae (Δ) and significant environmental variables (vector arrows) at P≤0.05. The position of each centroid indicates its association with environmental variables.
Table 2.
Factors associated with the presence and absence of anopheline and culicine larvae during the 2005, 2006, and 2007 rainy seasons
| Factors | Odds ratio | 95% Wald CI |
P | |
|---|---|---|---|---|
| Low | High | |||
| Anophelines | ||||
| Intervention | ||||
| Nonsprayed | 1.00 | – | – | – |
| Sprayed | 0.38 | 0.24 | 0.59 | <0.001 |
| T. guineensis | ||||
| Absent | 1.00 | – | – | – |
| Present | 1.12 | 0.87 | 1.44 | 0.36 |
| Percent vegetation cover | 1.01 | 1.01 | 1.02 | <0.001 |
| Sampling month | 0.96 | 0.92 | 1.00 | 0.05 |
| Distance from floodplain edge | 1.00 | 1.00 | 1.00 | 0.85 |
| Culicines | ||||
| Intervention | ||||
| Nonsprayed | 1.00 | – | – | – |
| Sprayed | 0.26 | 0.17 | 0.38 | <0.001 |
| T. guineensis | ||||
| Absent | 1.00 | – | – | – |
| Present | 0.62 | 0.45 | 0.84 | 0.002 |
| % vegetation cover | 1.01 | 1.00 | 1.02 | 0.03 |
| Sampling month | 1.01 | 0.98 | 1.04 | 0.43 |
| Distance from floodplain edge | 1.00 | 1.00 | 1.00 | 0.95 |
Only those variables identified as significant by the CCA were included in the model. Significant values following Bonferonni correction in bold (Bonferonni significance level, P≤0.025).
CI, confidence interval.
Table 3.
Influence of application of microbial larvicides on fish species richness (no. species) and abundance of the most common fish species
| Factors | Odds ratio | 95% Wald CI |
P | |
|---|---|---|---|---|
| Low | High | |||
| Fish species richness | ||||
| Nonsprayed | 1.00 | – | – | – |
| Sprayed | 0.81 | 0.64 | 1.03 | 0.09 |
| Year | ||||
| 2005 | 1.00 | – | – | – |
| 2006 | 0.96 | 0.75 | 1.21 | 0.72 |
| 2007 | 0.57 | 0.42 | 0.78 | <0.001 |
| Transect | ||||
| 1 | 1.00 | – | – | – |
| 2 | 1.01 | 0.67 | 1.53 | 0.94 |
| T. guineensis | ||||
| Nonsprayed | 1.00 | – | – | – |
| Sprayed | 1.50 | 0.58 | 3.90 | 0.39 |
| R. septentrionalis | ||||
| Nonsprayed | 1.00 | – | – | – |
| Sprayed | 0.99 | 0.75 | 1.32 | 0.98 |
| E. spilargyreius | ||||
| Nonsprayed | 1.00 | – | – | – |
| Sprayed | 0.94 | 0.44 | 2.03 | 0.88 |
| H. bimaculatus | ||||
| Nonsprayed | 1.00 | – | – | – |
| Sprayed | 0.91 | 0.82 | 1.01 | 0.08 |
| H. fasciatus | ||||
| Nonsprayed | 1.00 | – | – | – |
| Sprayed | 1.04 | 0.90 | 1.20 | 0.60 |
| P. normanii | ||||
| Nonsprayed | 1.00 | – | – | – |
| Sprayed | 1.05 | 0.98 | 1.12 | 0.15 |
Bonferonni-corrected significant values in bold (Bonferonni significance level, P ≤ 0.007).
CI, confidence interval.
A subsample (n = 173) of late-instar anopheline larvae collected along the transects were brought back to the laboratory to dentify them after emergence of the adults. Of these, 41.6% were An. coustani s.l. Laveran, 31.8% were An. gambiae s.l., 11.6% were An. funestus Giles, 9.8% were An. pharoensis Theobald, and 5.2% were An. squamosus Theobald. Those mosquitoes identified as An. gambiae s.l. (n = 55) were further identified to species by PCR analysis, and 76% were identified as An. gambiae s.s., 12% as An. arabiensis Patton, and 12% as An. melas Theobald.
Predation Experiments
The semifield experiments showed that both species of fish had significant impacts on the presence of all categories of anopheline and culicine larvae and pupae (Table 4). Average anopheline densities in the bowls before treatment were 1.01 larvae per dip. For early-instar anophelines, there was a 69% reduction for E. spilargyreius and 96% reduction for T. guineensis at the end of the 12-d period, whereas all late stage culicines and anophelines were consumed within 24 h.
Table 4.
Odds ratios of presence/absence of larvae and pupae from the predation experiment
| Treatment | Odds ratio | 95% Wald CI |
P | |
|---|---|---|---|---|
| Low | High | |||
| Early anophelines | ||||
| Control | 1 | – | – | – |
| T. guineensis | 0.239 | 0.132 | 0.431 | <0.001 |
| E. spilargyreius | 0.204 | 0.113 | 0.368 | <0.001 |
| Late anophelines | ||||
| Control | 1 | – | – | – |
| T. guineensis | 0.032 | 0.008 | 0.126 | <0.001 |
| E. spilargyreius | 0.014 | 0.003 | 0.054 | <0.001 |
| Early culicines | ||||
| Control | 1 | – | – | – |
| T. guineensis | 0.001 | 0.000 | 0.013 | <0.001 |
| E. spilargyreius | 0.011 | 0.003 | 0.045 | <0.001 |
| Late culicines | ||||
| Control | 1 | – | – | – |
| T. guineensis | 0.003 | 0.000 | 0.020 | <0.001 |
| E. spilargyreius | 0.007 | 0.001 | 0.039 | <0.001 |
| Pupae | ||||
| Control | 1 | – | – | – |
| T. guineensis | 0.011 | 0.003 | 0.051 | <0.001 |
| E. spilargyreius | <0.001 | <0.001 | <0.001 | <0.001 |
Each instar was analyzed in a separate model. We adjusted for experimental trial (number of trials: 4) in the model. Bonferonni-corrected significant values in bold (Bonferonni significance level, P ≤ 0.01).
CI, confidence interval.
Of the 715 pupae collected in the predation semifield experiment in 2006, 21.7% were An. gambiae s.l. and the rest were culicines (47.5% Ae. vittatus Bigot, 27.5% Cx. quinquefasciatus Say, 1.3%, Toxorhynchites spp., and 2% other culicines). Of the An. gambiae s.l., 48% were identified as An. gambiae s.s. and 52% as An. arabiensis.
Oviposition Experiment
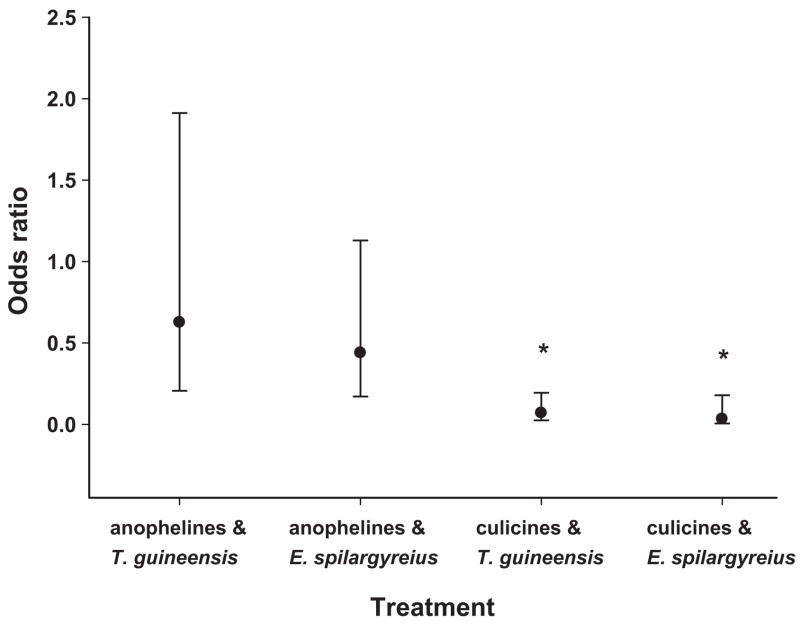
This experiment has shown that ovipositing culicines detected the presence of both E. spilargyreius (97% reduction in mosquito larvae sampled, average density 0.05 larvae per dip) and T. guineensis (93% reduction, average density 0.1 larvae per dip) compared with the controls (average density 1.24 larvae per dip) and avoided ovipositing in sites where they are present. For anophelines, however, our results indicate that they were poorly deterred by the presence of E. spilargyreius (average density 0.13 larvae per dip) and T. guineensis (average density 0.16 larvae per dip) in relation to the controls (average density 0.28 larvae per dip; Fig. 3).
Fig. 3.
Odds ratios of the oviposition experiment for T. guineensis and E. spilargyreius. Experimental round was also included in the analytical model; no differences were observed between rounds for both anophelines and culicines. *Bonferonni corrected significant values in bold; Bonferonni significance level, P ≤ 0.025.
Discussion
A comprehensive fish survey and dietary assessment of the most common species encountered on the floodplain of the Gambia River showed that T. guineensis, a detritivore, dominates the fish communities, constituting >86% of the catches by number. The Gambia River floodplain exhibits low fish diversity compared with other tropical floodplain systems (Welcomme 1979, Cotner et al. 2006, Winemiller et al. 2006). This is partly because of the fact that the Gambia River has an impoverished fish fauna compared with other West African river systems (Leveque et al. 1991) but may also result from the low concentrations of nutrients found in the floodplains (V. Louca, unpublished data). Dominance of the Gambia floodplain fish community by a detritivore is also found in other tropical floodplain systems where detritivore and herbivore species commonly dominate the fish communities (Winemiller 1990). Five fish species were identified as being primarily insectivorous. Of these, E. spilargyreius was the most common, with potentially sufficient numbers to cultivate for mosquito control.
Our analysis showed that the presence of T. guineensis was associated with a reduced likelihood of finding culicine larvae, but not anophelines, whereas the presence of E. spilargyreius showed no significant association with the distribution of mosquito larvae. Because of ts high local densities, it is possible that T. guineensis could have a direct interaction with culicines. Semi-field setups have been shown to accurately predict results obtained in large-scale experiments (deSzalay et al. 1996), and our semifield tests suggest that the field observations of a negative association in local distribution between culicines and T. guineensis may partly be caused by culicines detecting and avoiding ovipositing in habitats where T. guineensis is present. The fact that culicines avoided ovipositing in containers with both species of fish suggests that the semio-chemicals detected by culicine mosquito are more general fish cues and not specific to insectivorous fish, because T. guineensis was identified as a detritivore species.
Results from the predation semifield experiment suggested that predation by fish could also be a factor regulating mosquitoes in the field. Alternatively, it may be that, in the floodplain of the Gambia River, anophelines occupy different spatial niches to theTilapia, with mosquitoes being concentrated close to emergent vegetation on the water’s edge and the fish occupying the open areas away from the shoreline (Wellborn et al. 1996, Maddrell 1998). Moreover, it is possible that anophelines and culicines differ behaviorally and in their microhabitat preferences. The active behavior of culicines might make them more vulnerable to predation by visual predators, like fish, whereas anophelines often reside in vegetation, where they are relatively motionless and parallel to the water surface. The general lack of mosquitoes, especially anopheline mosquitoes in the natural diet of fish, suggests that mosquitoes constitute a minor part in the diet of fish on the Gambia River floodplain and that fish only actively feed on them opportunistically in small aquatic habitats, such as our semifield ponds, where mosquitoes constituted a relatively large proportion of the invertebrate fauna present. This is supported by a number of studies that have shown that, even for established larvivorous fish such as G. affinis, mosquito larvae constitute a minor proportion of their diet under natural conditions (Garcia-Berthou 1999, Specziar 2004, Kumar and Hwang 2006). Despite the presence of E. spilargyreius inhibiting oviposition of culicines and being an active predator under semifield conditions, it did not exclude culicines from habitats in the field. This finding probably results from E. spilargyreius only being encountered at lower densities. In contrast, the oviposition behavior of anopheline mosquitoes was not influenced by fish, and as a result, it seems they do not influence the distribution of anophelines on the floodplain. Moreover, we showed that it only achieves a 69% reduction in early anophelines 2 d after introduction, and this suggests that this species under field conditions is a less efficient predator of anophelines than T. guineensis. Taking into account the high abundance of T. guineensis on the floodplain (recorded mean catch densities of 7.59 fish/m2; range: 0 –518 fish/m2) and their proven ability to influence oviposition in culicine mosquitoes, it is perhaps not surprising to see that they play a regulating role in the distribution of culicines on the floodplain.
Because culicines avoid oviposition in sites with either fish species, when effective control of both anophelines and culicines is needed, it is of vital importance that all possible aquatic habitat sites should be treated to avoid culicine mosquito selecting alternative sites for oviposition, thus minimizing the effectiveness of this mosquito control measure. Nonetheless because adult anophelines do not seem to be able to detect chemical cues in the water from fish, fish may prove to be an efficient control method for reducing anopheline populations in situations where habitats are well defined and long living.
These nonsignificant results from the pilot study indicate that biological control using microbial larvicides did not affect fish species richness and abundance. However, because of the limited sample size, these no-effect results should be interpreted with caution. Moreover, further semifield level testing of the effect of mosquito larvicides on fish growth would be useful.
Tilapia guineensis, a primary animal protein source for people living along the floodplain, is a candidate for use as a mosquito control agent in West Africa because we have shown that (1) it is by far the most common fish in the floodplain, (2) its presence does not influence oviposition by anopheline mosquitoes, and (3) it is a highly efficient predator of both early and late anophelines. Nontheless, these results should be treated with caution because anopheline larvae did not constitute a major part of the diet of this species and therefore they might be inefficient in controlling larvae under natural conditions. With irregular rain patterns from year to year being common in West Africa (Pages and Citeau 1990), the potential for using fish for mosquito control is limited because it can only be effectively implemented in areas with permanent water bodies. Moreover, for T. guineensis, the fish biomass used should be high enough (at least 26.3 g/70 liters of water as used in the semifield study) to ensure that all invertebrates will be consumed and that fish will not preferentially prey on non-mosquito invertebrates. This dependence on permanent water bodies limits the capacity in which fish can be used to control mosquitoes unless these habitats remain flooded for substantial periods for the fish to survive and have an impact in controlling mosquitoes. Nevertheless, fish are likely to be cheaper and provide a more long-term solution to mosquito control compared with larvicides. Thus, fish could be used as part of an integrated control program where fish are used in more permanent sites and microbial larvicides applied to temporary ones. Potential effectiveness of the use of both fish and mosquito larvicides in the same aquatic habitats remains to be tested. Moreover, it must be emphasized that if fish are used for controlling mosquito larvae, it must be well planned because failures with introducing fish for mosquito control can be caused by the limited attention in which these introductions take place. For example, fish might be introduced in habitats that dry up rapidly or people may harvest the fish at the end of the season but not replace them when the habitats are flooded again. Therefore, future projects planning the introduction of fish should take into account these potential problems and plan for a regular monitoring of the fish populations, with reintroduction of fish when needed. Our study suggests that the use of fish for mosquito control in sub-Saharan Africa merits further consideration.
Acknowledgments
We thank the local communities in and around our study areas for help and cooperation throughout the study. This work was supported by the National Institutes of Health (Grant 1 VO1 AI058250–01) and an award of the Freshwater Biological Association Hugh Cary Gilson Memorial Award in 2005. We are grateful to the Medical Research Council’s Laboratories in The Gambia for provision of facilities and support at Farafenni Field Station and the Gambian Fisheries Department for cooperation. We also thank M. Drammeh and B. Salleh for help in the field and two anonymous referees for valuable comments.
References Cited
- Abjornsson K, Bronmark C, Hansson LA. The relative importance of lethal and non-lethal effects of fish on insect colonisation of ponds. Freshwater Biol. 2002;47:1489–1495. [Google Scholar]
- Amundsen PA, Gabler HM, Staldvik FJ. A new approach to graphical analysis of feeding strategy from stomach contents data: modification of the Costello (1990) method. J Fish Biol. 1996;48:607–614. [Google Scholar]
- Angelon KA, Petranka JW. Chemicals of predatory mosquitofish (Gambusiaaffinis) influence selection of oviposition site by culex mosquitoes. J Chem Ecol. 2004;28:797–806. doi: 10.1023/a:1015292827514. [DOI] [PubMed] [Google Scholar]
- Anonymous. Guidelines for the treatment of animals in behavioural research and teaching. Anim Behav. 2003;65:249–255. doi: 10.1006/anbe.1999.1349. [DOI] [PubMed] [Google Scholar]
- Arthington AH. Ecological and genetic impacts of introduced and translocated freshwater fishes in Australia. Can J Fish Aquat Sci. 1991;48:33–43. [Google Scholar]
- Bence JR. Indirect effects and biological control of mosquitos by mosquitofish. J Appl Ecol. 1988;25:505–521. [Google Scholar]
- Benigno E. Report of the convention on the conservation of European wildlife and natural habitats. Council of Europe; Strasbourg, France: 2001. Identification of non-native freshwater fishes established in Europe and assessment of their potential threats to the biological diversity. [Google Scholar]
- Blaustein L. Larvivorous fishes fail to control mosquitoes in experimental rice plots. Hydrobiologia. 1992;232:219–232. [Google Scholar]
- Blaustein L, Kiflawi M, Eitam A, Mangel M, Cohen JE. Oviposition habitat selection in response to risk of predation in temporary pools: mode of detection and consistency across experimental venue. Oecologia (Berl) 2004;138:300–305. doi: 10.1007/s00442-003-1398-x. [DOI] [PubMed] [Google Scholar]
- Bøgh C, Lindsay SW, Clarke SE, Dean A, Jawara M, Pinder M, Thomas CJ. High spatial resolution mapping of malaria transmission risk in The Gambia, West Africa, using landsat TM satellite imagery. Am J Trop Med Hyg. 2007;76:875–881. [PubMed] [Google Scholar]
- Bond JG, Arredondo-Jimenez JI, Rodriguez MH, Quiroz-Martinez H, Williams T. Oviposition habitat selection for a predator refuge and food source in a mosquito. Ecol Entomol. 2005;30:255–263. [Google Scholar]
- Cech JJ, Linden AL. Comparative larvivorous performances of mosquitofish, Gambusia affinis, and juvenile sacramento blackfish, Orthodon Microlepidotus, in experimental paddies. J Am Mosq Control. 1987;3:35–41. [PubMed] [Google Scholar]
- Costello MJ. Predator feeding strategy and prey importance: a new graphical analysis. J Fish Biol. 1990;36:261–263. [Google Scholar]
- Cotner JB, Montoya JV, Roelke DL, Winemiller KO. Seasonally variable riverine production in the Venezuelan llanos. J N Am Benthol Soc. 2006;25:171–184. [Google Scholar]
- deSzalay FA, Batzer DP, Resh VH. Meso-cosm and macrocosm experiments to examine effects of mowing emergent vegetation on wetland invertebrates. Environ Entomol. 1996;25:303–309. [Google Scholar]
- Economidis PS. Endangered fresh-water fishes of Greece. Biol Conserv. 1995;72:201–211. [Google Scholar]
- Fillinger U, Knols BGJ, Becker N. Efficacy and efficiency of new Bacillus thuringiensis var. israelensis and Bacillus sphaericus formulations against Afrotropical anophelines in Western Kenya. Trop Med Int Health. 2003;8:37–47. doi: 10.1046/j.1365-3156.2003.00979.x. [DOI] [PubMed] [Google Scholar]
- Fletcher M, Teklehaimanot A, Yemane G. Control of mosquito larvae in the port city of Assab by an indigenous larvivorous fish, Aphanius dispar. Acta Trop. 1992;52:155–166. doi: 10.1016/0001-706x(92)90032-s. [DOI] [PubMed] [Google Scholar]
- Fletcher M, Teklehaimanot A, Yemane G, Kassahun A, Kidane G, Beyene Y. Prospects for the use of larvivorous fish for malaria control in Ethiopia: search for indigenous species and evaluation of their feeding capacity for mosquito larvae. J Trop Med Hyg. 1993;96:12–21. [PubMed] [Google Scholar]
- Floore TG. Mosquito larval control practices: past and present. J Am Mosq Control. 2006;22:527–533. doi: 10.2987/8756-971X(2006)22[527:MLCPPA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Frenkel V, Goren M. A spawning cage for eliminating predation on larvae of the killifish Aphanius dispar. North Am J Aquacult. 1999;61:172–174. [Google Scholar]
- Gabaldon A. Global eradication of malaria: changes of strategy and future outlook. Am J Trop Med Hyg. 1969;18:641–56. doi: 10.4269/ajtmh.1969.18.641. [DOI] [PubMed] [Google Scholar]
- Gall GAE, Cech JJ, Garcia R, Resh VH, Washino RK. Mosquito fish: an established predator. Calif Agric. 1980;34:21–22. [Google Scholar]
- Garcia-Berthou E. Food of introduced mosquitofish: ontogenetic diet shift and prey selection. J Fish Biol. 1999;55:135–147. [Google Scholar]
- Giglioli ME, Thornton I. The mangrove swamps of Keneba, Lower Gambia River Basin. 1. Descriptive notes on the climate, the mangrove swamps and the physical composition of their soils. J Appl Ecol. 1965;2:81–103. [Google Scholar]
- Giglioli ME, King DF. Mangrove swamps of Keneba Lower Gambia River Basin. 3. Seasonal variations in chloride and water content of swamp soils with observations on water levels and chloride concentration of free soil water under a barren mud flat during the dry season. J Appl Ecol. 1966;3:1–19. [Google Scholar]
- Gilles MT, DeMeillon B. The Anopheline of Africa south of the Sahara (Ethiopian zoogeographical region) The South African Institute for Medical Research; Johannesburg, South Africa: 1968. [Google Scholar]
- Hadjinicolaou J, Betzios B. Gambusia fish as a means of mosquito control of Anopheles sacharovi in Greece. World Health Organization; Geneva, Switzerland: 1973. [Google Scholar]
- Herrel N, Amerasinghe FP, Ensink J, Mukhtar M, van der Hoek W, Konradsen F. Breeding of Anopheles mosquitoes in irrigated areas of South Punjab, Pakistan. Med Vet Entomol. 2001;15:236–48. doi: 10.1046/j.0269-283x.2001.00312.x. [DOI] [PubMed] [Google Scholar]
- Hoddle MS. Restoring balance: using exotic species to control invasive exotic species. Conserv Biol. 2004;18:38–49. [Google Scholar]
- Homsky D, Goren M, Gasith A. The potential of the fish species Gambusia affinis and Aphanius dispar as a biological control of mosquito larvae in diverse conditions of water quality, as a basis for a new mosquito larvae control system by fishes. Israel J Zool. 1987;34:94–95. [Google Scholar]
- Horton NJ, Lipsitz SR. Review of software to fit generalized estimating equation regression models. Am Stat. 1999;53:160–169. [Google Scholar]
- Kerfoot C, Sih A. Predation: direct and indirect impacts on aquatic communities. University Press of England; London, United Kingdom: 1987. [Google Scholar]
- Kiflawi M, Blaustein L, Mangel M. Predation-dependent oviposition habitat selection by the mosquito Culiseta longiareolata: a test of competing hypotheses. Ecol Lett. 2003;6:35–40. [Google Scholar]
- Killeen GF. Following in Soper’s footsteps: northeast Brazil 63 years after eradication of Anopheles gambiae. Lancet Infect Dis. 2003;3:663–666. doi: 10.1016/s1473-3099(03)00776-x. [DOI] [PubMed] [Google Scholar]
- Killeen GF, Fillinger U, Knols BG. Advantages of larval control for African malaria vectors: low mobility and behavioural responsiveness of immature mosquito stages allow high effective coverage. Malar J. 2002;1:8. doi: 10.1186/1475-2875-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Hwang JS. Larvicidal efficiency of aquatic predators: a perspective for mosquito biocontrol. Zool Stud. 2006;45:447–466. [Google Scholar]
- Kusumawathie PHD, Wickremasinghe AR, Karunaweera ND, Wijeyaratne MJS. Larvivorous potential of fish species found in river bed pools below the major dams in Sri Lanka. J Med Entomol. 2006;43:79–82. doi: 10.1093/jmedent/43.1.79. [DOI] [PubMed] [Google Scholar]
- Lee DK. Predation efficacy of the fish muddy loach, Misgurnus mizolepis, against Aedes and Culex mosquitoes in laboratory and small rice plots. J Am Mosq Control. 2000;16:258–261. [PubMed] [Google Scholar]
- Leveque C, Paugy D, Teugels G. Annotated check-list of the freshwater fishes of the Nilo-sudan river basins in Africa. Rev Hydrobiol Trop. 1991;24:131–154. [Google Scholar]
- Leyse KE, Lawler SP, Strange T. Effects of an alien fish, Gambusia affinis, on an endemic California fairy shrimp, Linderiella occidentalis: implications for conservation of diversity in fishless waters. Biol Conserv. 2004;118:57–65. [Google Scholar]
- Louca V, Lindsay SW, Lucas MC. Factors triggering floodplain fish emigration: Importance of fish density and food availability. Ecol Fresh Fish. 2009a;18:60–64. doi: 10.1111/j.1600-0633.2008.00323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louca V, Lindsay SW, Majambere S, Lucas MC. Fish community characteristics of the lower Gambia River floodplains: a study in the last major undisturbed West African river. Freshw Biol. 2009b;54:254–271. [Google Scholar]
- Maddrell SHP. Why are there no insects in the open sea? J Exp Biol. 1998;201:2461–2464. doi: 10.1242/jeb.201.17.2461. [DOI] [PubMed] [Google Scholar]
- Majambere S, Lindsay SW, Green C, Kandeh B, Fillinger U. Microbial larvicides for malaria control in The Gambia. Malar J. 2007;6:76. doi: 10.1186/1475-2875-6-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majambere S, Fillinger U, Sayer DR, Green C, Lindsay SW. Spatial distribution of mosquito larvae and the potential for targeted larval control in The Gambia. Am J Trop Med Hyg. 2008;79:19–27. [PubMed] [Google Scholar]
- Mancini L, Romi R. Larvivorous capacity of Barbus pobeguini and possibility of using this in the fight against Anopheles gambiae s.l. Parassitologia. 1988;30:271–7. [PubMed] [Google Scholar]
- Marten GG, Suarez MF, Astaeza R. An ecological survey of Anopheles albimanus larval habitats in Colombia. J Vector Ecol. 1996;21:122–131. [Google Scholar]
- Marti GA, Azpelicueta MDM, Tranchida MC, Pelizza SA, Garcia JJ. Predation efficiency of indigenous larvivorous fish species on Culex pipiens L. larvae (Diptera: Culicidae) in drainage ditches in Argentina. J Vector Ecol. 2006;31:102–106. doi: 10.3376/1081-1710(2006)31[102:peoilf]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Milam CD, Farris JL, Wilhide JD. Evaluating mosquito control pesticides for effect on target and nontarget organisms. Arch Environ Con Toxicol. 2000;39:324–328. doi: 10.1007/s002440010111. [DOI] [PubMed] [Google Scholar]
- Motabar M. Larvivorous fish, Gambusia affinis: a review. World Health Organization; Geneva, Switzerland: 1978. [Google Scholar]
- Mulla MS, Norland RL, Fanara DM, Darwazeh HA, Mckean DW. Control of chironomid midges Diptera-Chironomidae in recreational lakes. J Econ Entomol. 1971;64:300–307. [Google Scholar]
- Munga S, Minakawa N, Zhou GF, Barrack OAJ, Githeko AK, Yan GY. Effects of larval competitors and predators on oviposition site selection of Anopheles gambiae sensu stricto. J Med Entomol. 2006;43:221–224. doi: 10.1603/0022-2585(2006)043[0221:eolcap]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Pages J, Citeau J. Rainfall and salinity of a Sahelian estuary between 1927 and 1987. J Hydrol. 1990;113:325–341. [Google Scholar]
- Paugy D, Leveque C, Teugels G. The fresh and brackish water fishes of West Africa. IRD. I and II. Paris, France: 2003. [Google Scholar]
- Resetarits WJ. Colonization under threat of predation: avoidance of fish by an aquatic beetle, Tropisternus lateralis (Coleoptera: Hydrophilidae) Oecologia (Berl) 2001;129:155–160. doi: 10.1007/s004420100704. [DOI] [PubMed] [Google Scholar]
- Romand R. Feeding biology of Aplocheilichthys normani, Ahl, a small Cyprinodontidae from West Africa. J Fish Biol. 1985;26:399–410. [Google Scholar]
- Scott JA, Brogdon WG, Collins FH. Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am J Trop Med Hyg. 1993;49:520–529. doi: 10.4269/ajtmh.1993.49.520. [DOI] [PubMed] [Google Scholar]
- Sitaraman NL, Karim MA, Reddy GV. Observations on use of Gambusia holbrooki to control to control Anopheles stephensi breeding in wells: results of 2 years study in greater Hyderabad city India. Indian J Med Res. 1975;63:1509–1516. [PubMed] [Google Scholar]
- Specziar A. Life history pattern and feeding ecology of the introduced eastern mosquitofish, Gambusia holbrooki, in a thermal spa under temperate climate, of Lake Heviz, Hungary. Hydrobiologia. 2004;522:249–260. [Google Scholar]
- Spencer M, Blaustein L, Cohen JE. Oviposition habitat selection by mosquitoes (Culiseta longiareolata) and consequences for population size. Ecology. 2002;83:669–679. [Google Scholar]
- Tabibzadeh I, Behbehani G, Nakhai R. Use of gambusia fish in the malaria eradication programme of Iran. Bull WHO. 1970;43:623–628. [PMC free article] [PubMed] [Google Scholar]
- Ter Braak CJF. Canonical correspondence analysis: A new eigenvector technique for multivariate direct gradient analysis. Ecology. 1986;67:1167–1179. [Google Scholar]
- Ter Braak CJF, Smilauer P. Microcomputer Power. Ithaca, NY: 2002. CANOCO reference manual and CanoDraw for Windows user’s guide: software for canonical community ordination (version 4.5) [Google Scholar]
- Tjossem SF. Effects of fish chemical cues on vertical migration behavior of Chaoborus. Limnol Oceanogr. 1990;35:1456–1468. [Google Scholar]
- Valero N, Melean E, Maldonado M, Montiel M, Larreal Y, Espina LM. Larvivorous capacity of the goldfish (Carassius auratus auratus) and the wild guppy (Poecilia reticulata) on larvae of Aedes aegypti in laboratory conditions. Rev Cient-Fac Cien V. 2006;16:414–419. [Google Scholar]
- Victor TJ, Chandrasekaran B, Reuben R. Composite fish culture for mosquito control in rice fields in southern India. Southeast Asian J Trop Med Public Health. 1994;25:522–527. [PubMed] [Google Scholar]
- Walker K, Lynch M. Contributions of Anopheles larval control to malaria suppression in tropical Africa: review of achievements and potential. Med Vet Entomol. 2007;21:2–21. doi: 10.1111/j.1365-2915.2007.00674.x. [DOI] [PubMed] [Google Scholar]
- Walton WE. Larvivorous fish including Gambusia. Biorational control of mosquitoes. J Mosq Cont Assoc Suppl. 2007;23:184–220. doi: 10.2987/8756-971X(2007)23[184:LFIG]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Welcomme RL. Fisheries ecology of floodplain rivers. Longman, London, United Kingdom: 1979. [Google Scholar]
- Wellborn GA, Skelly DK, Werner EE. Mechanisms creating community structure across a freshwater habitat gradient. Annu Rev Ecol Sys. 1996;27:337–363. [Google Scholar]
- Winemiller KO. Spatial and temporal variation in tropical fish trophic networks. Ecol Monogr. 1990;60:331–367. [Google Scholar]
- Winemiller KO, Montoya JV, Roelke DL, Layman CA, Cotner JB. Seasonally varying impact of detritivorous fishes on the benthic ecology of a tropical floodplain river. J N Am Benthol Soc. 2006;25:250–262. [Google Scholar]
- Yildirim O, Karacuha A. A preliminary study on determination of Aphanius chantrei’s feeding behaviour on mosquito larvae. Acta Trop. 2007;102:172–175. doi: 10.1016/j.actatropica.2007.04.016. [DOI] [PubMed] [Google Scholar]