Abstract
1,1-Bis(3'-indolyl)-1-(p-anisyl)methane (DIM-C-pPhOCH3) activates the orphan receptor nerve growth factor-induced Bα (NGFI-Bα or Nur77) in cancer cells and, in this study, DIM-C-pPhOCH3 decreased Panc1 pancreatic cancer cell survival and arrested cells in G0/G1. These responses were accompanied by induction of the cyclin-dependent kinase inhibitor p21 in pancreatic cancer cells. Mechanistic studies demonstrated that induction of p21 mRNA and protein by DIM-C-pPhOCH3 was Nur77-dependent but did not depend on Krüppel-like factor-4 which was also induced by DIMC-pPhOCH3. Activation of p21 promoter constructs by DIM-C-pPhOCH3 required the GC-rich proximal region of the promoter, and results of RNA interference studies showed that Nur77-dependent activation of the p21 promoter involved interactions with Sp1 and Sp4 but not Sp3. Interactions of Nur77 with the p21 promoter in Panc1 cells treated with DIM-C-pPhOCH3 were also confirmed in chromatin immunoprecipitation assays. These data show that activation of nuclear Nur77 results in a novel pathway for induction of p21 which is independent of Nur77 response elements but dependent on Sp proteins bound to the GC-rich proximal region of the p21 promoter.
Keywords: Nur77, activation, DIM analog, p21
INTRODUCTION
The nuclear receptor family of transcription factors includes the steroid and thyroid hormone, vitamin D, retinoid and ecdysone receptors, adopted orphan receptors, and orphan receptors with no known ligands (1, 2). NRs influence diverse aspects of normal physiology in multiple tissues; however, despite their functional diversity, this family of proteins exhibits a number of common structural features and molecular mechanisms of gene activation. Nerve growth factor-inducible gene B (NGFI-B) is part of a subfamily of orphan NRs that were initially identified after treatment of PC12 pheochromacytoma cells with NGF (3). Members of this subfamily include Nur77 (NGFI-Bα, TR3), Nurr1 (NGFI-β), and Nor1 (NGFI-γ). Nur77 is expressed in multiple tissues; Nurr1 has been detected in thymus osteoblasts, liver and pituitary gland; and Nor1 is highly expressed in the pituitary gland with low expression in other tissues (4-7). The physiological roles for NGFI-B proteins are not fully understood; however, gene targeting knockout experiments demonstrate several important functions of these proteins which correlate, in part, with other in vitro and in vivo studies (7-11). For example, Nurr1 knockout mice have severe impairments in mid-brain neuronal development and dopamine expression, and these animals die soon after birth (7, 8).
NGFI-B proteins bind to specific promoter DNA response elements as monomers, homodimers, or heterodimers complexed with RXR (12-14). Ligands that bind or activate NFGI-B proteins through AF-2 domains have not been identified; however, 6-mercaptorpurine (6-MP) activates both Nurr1 and Nor1 through their respective N-terminal AF-1 domains (15, 16). 6-MP also activates Nur77-dependent genes including hypoxia-inducible factor 1α in HepG2 liver cancer cells. Nur77 plays an important role in thymocyte-negative selection and in TCR-mediated apoptosis in thymocytes, and overexpression of Nur77 in transgenic mice results in high levels of apoptosis in thymocytes (17, 18). Several studies suggest that Nur77 plays a role in cell death pathways activated by apoptosis-inducing agents (19-28). The mechanisms associated with these responses are drug- and cell context-dependent; however, these responses are primarily due to nuclear export of Nur77. In some cases, Nur77 binds bcl-2 to form a proapoptotic complex (20).
Studies in this laboratory have identified a series of 1,1-bis(3'-indolyl)-1-(p-substituted phenyl)methanes in which the p-trifluoromethyl (DIM-C-pPhCF3), p-methoxy (DIM-C-pPhOCH3), and unsubstituted (DIM-C-pPh) analogs activate Nur77 in colon and pancreatic cancer cells (29, 30). A second compound containing a p-hydroxyl substituent (DIM-C-pPhOH) antagonizes activation of Nur77 by the Nur77-active compounds. DIM-C-pPhOCH3 has been extensively used as a prototype compound which induces apoptosis and inhibits colon and pancreatic cell and tumor growth, and in cell culture studies, the proapoptotic responses are dependent on nuclear Nur77 (29, 30). DIM-C-pPhOCH3 induces G0/G1 to S phase arrest in Panc1 cells and this is accompanied by Nur77-dependent induction of the cyclin-dependent kinase inhibitor p21. Induction of p21 is Krüppel-like factor 4 (KLF4)-independent but is accompanied by recruitment of Nur77 to the GC-rich proximal region of the p21 promoter and interactions of Nur77 with Sp1 and Sp4.
MATERIALS AND METHODS
Cell lines
Panc1 human pancreatic cancer cell lines were obtained from the American Type Culture Collection (Manassas, VA). The L3.6pl cell line was developed at the M. D. Anderson Cancer Center (Houston, TX) and kindly provided by Dr. I. J. Fidler. Panc1 cells were maintained in Dulbecco's modified Eagle's medium nutrient mixture with Ham's F-12 (DMEM/Ham's F-12; Sigma-Aldrich, St. Louis, MO) supplemented with 0.22% sodium bicarbonate, 0.011% sodium pyruvate, 10% fetal bovine serum (FBS), and 10 ml/L 100x antibiotic antimycotic solution (Sigma-Aldrich). L3.6pl cells were maintained in RPMI-1640 medium supplemented with 10% FBS and 10 ml/L 100X antibiotic antimycotic solution. Cells were maintained at 37°C in the presence of 5% CO2.
Plasmids, antibodies and reagents
p21 promoter reporter constructs pWWP, pWWP124, pWWP101, pWWP101-mt3, and pWWP101-mt4 were provided by Dr. Toshiyuki Sakai (Kyoto Prefectural University of Medicine, Kyoto, Japan). pWWP60 was generated by digesting pWWP with SmaI, followed by religation of the purified vector. The Flag-tagged and YFP-tagged full-length Nur77 were constructed by inserting PCR-amplified full-length Nur77 fragments into the EcoRI/BamHI site of p3XFLAG-CMV-10 expression vector (Sigma-Aldrich) and pEYFP-C1 expression vector (BD Biosciences Clontech). The C-substituted DIMs were synthesized in this laboratory as previously described (29), and their identities and purity (>98%) were confirmed by gas chromatography-mass spectrometry. All other chemicals were obtained from Sigma Chemical (St. Louis, MO) unless otherwise indicated. Nur77, p21, and Sp1 antibodies were purchased from Imgenex (San Diego, CA), BD Pharmingen (San Diego, CA), and Upstate (Temecula, CA), respectively. All other antibodies including Sp3 and Sp4 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Reporter lysis buffer and luciferase reagent were supplied by Promega (Madison, WI). β-Galactosidase (β-Gal) reagent was obtained from Tropix (Bedford, MA), and LipofectAMINE 2000 reagent was purchased from Invitrogen (Carlsbad, CA). For RNA interference assays, we used a nonspecific scrambled (siScr) oligonucleotide as described (30, 31). The small inhibitory RNA (siRNA) for Nur77 was identical to the reported oligonucleotide (29, 30) and the siRNA for Sp1, Sp3, and Sp4 were identical to the reported oligonucleotides (32). All the siRNAs were prepared by Dharmacon Research (Lafayette, CO).
Quantitative real-time PCR
cDNA was prepared from the total RNA of cells using Reverse Transcription System (Promega). Each PCR was carried out in triplicate in a 20-μL volume using SYBR Green Mastermix (Applied Biosystems, Foster City, CA) for 15 min at 95°C for initial denaturing, followed by 40 cycles of 95°C for 30 s and 60°C for 1 min in the Applied Biosystems 7900HT Fast Real-time PCR System. The ABI Dissociation Curves software was used following a brief thermal protocol (95°C for 15 s and 60°C for 15 s, followed by a slow ramp to 95°C) to control for multiple species in each PCR amplification. Values for each gene were normalized to expression levels of TATA-binding protein (TBP). The sequences of the primers used for real-time PCR were as follows: p21 sense 5'-GGC AGA CCA GCA TGA CAG ATT TC-3', antisense 5'-CGG ATT AGG GCT TCC TCT TGG-3'; and TBP sense 5'-TGC ACA GGA GCC AAG AGT GAA-3', antisense 5'-CAC ATC ACA GCT CCC CAC CA-3'. The PCR primers for Nur77 and KLF4 were purchased from Qiagen.
Western blot analysis and immunoprecipitation
Cells (2 × 105) were plated in six-well plates in DMEM/Ham's F-12 media containing 2.5% charcoal-stripped FBS for 16 hr and then treated with different concentrations of the compounds. Cellular lysates were prepared in a lysis buffer containing 50 mM Tris-HCl (pH 7.5), 2 mM ethylenediaminetetraacetic acid, 150 mM NaCl, 0.5% deoxycholate, 0.1% sodium dodecylsulfate (SDS), 1 mM NaF, 1 mM Na3VO4, 1 mM phenyl methyl sulfonyl fluoride, 5 μL/ml Protease inhibitor cocktail (Sigma-Aldrich) and 1% NP-40. The cells were disrupted and extracted at 4°C for 30 min. After centrifugation at 13,000 rpm for 15 min, the supernatant was obtained as the cell lysate. Nuclear extracts were obtained using NE-PER nuclear and cytoplasmic extraction reagents (Pierce Biotechnology, Inc., IL). Protein concentrations were measured using the Bio-Rad protein assay. Aliquots of cellular proteins were electrophoresed on 10 or 12% SDS-polyacrylamide gel electrophoresis (PAGE) and transferred to an PVDF membrane (Bio-Rad, Hercules, CA). The membrane was allowed to react with a specific antibody and detection of specific proteins was carried out by enhanced chemiluminescence (PerkinElmer). Loading differences were normalized using a polyclonal β-actin antibody. For immunoprecipitation, 1 mg of cell lysate were pre-cleared with mouse IgG-agarose (Sigma-Aldrich) for 2 hr with agitation, then the supernatant was incubated overnight with anti-Flag antibody-conjugated agarose beads (Sigma-Aldrich) with agitation. The immunoprecipitates were collected by centrifugation for 1 min at 8,000 × g and washed four times with TBS, then subjected to 8% SDS-PAGE. All immunoprecipitation steps were performed at 2-8°C.
Cell proliferation assay
Cells (1 × 105 per well) were plated in 12-well plates and allowed to attach for 16 hr. The medium was then changed to DMEM/Ham's F-12 medium containing 2.5% charcoal-stripped FBS, and either vehicle (DMSO) or different concentrations of the compound were added. Fresh medium and compounds were added every 48 hr, and cells were then trypsinized and counted after 24, 48, 72 and 96 hr using a Coulter Z1 cell counter (Beckman Coulter Inc., USA).
Fluorescence-activated cell sorting analysis
Cells were treated with either the vehicle (DMSO) or the compound for 48 hr. Cells were trypsinized, centrifuged, and resuspended in staining solution containing 50 μg/mL propidium iodide (PI), 4 mmol/L sodium citrate and 30 units/mL RNase. After incubation at room temperature for 1 hr, cells were analysed on a FACS Vantage SE DiVa made by Becton Dickinson (BD), using BD FACSDiva Software V4.1.1. PI fluorescence was collected through a 610SP bandpass filter, and list mode data were acquired on a minimum of 50,000 single cells defined by a dot plot of PI width versus PI area. Data analysis was performed in BD FACSDiva Software V4.1.1 using PI width versus PI area to exclude cell aggregates.
Subcellular localization assays
Cells on cover slip were fixed in 1% formalin in PBS (pH 7.4) after washing with PBS and permeabilized by immersing the cells in 0.2% Triton X-100 solution in PBS for 10 min. Cells were then incubated with a specific antibody, followed by antirabbit IgG conjugated with FITC or Texas Red (Santa Cruz). For nuclear counterstaining, cells were mounted in mounting medium including DAPI (Vector Lab., CA). Fluorescent images were collected and analyzed using a Zeiss Axioplan2 fluorescence microscope (Carl Zeiss, Jena, Germany).
Transfection and luciferase assay
Cells (1 × 105 cells/well) were plated in 12-well plates in DMEM/Ham's F-12 media supplemented with 5% charcoal-stripped FBS. After 16 hr, various amounts of DNA [i.e., p21 promoter-luciferase reporter constructs (0.1 μg) and pCMV-β-galactosidase reporter plasmid (0.02 μg)] were transfected using LipofectAMINE 2000 reagent (Invitrogen) following the manufacturer's protocol. After transfection for 6 hr, the transfection mix was replaced with complete media containing either vehicle (DMSO) or different concentrations of the compound for 18 hr. Cells were then lysed with 150 μL of 1x reporter lysis buffer, and 30 μL of cell extract was used for luciferase and β-Gal assays. A multifunctional microplate reader (FLUOstar OPTIMA) was used to quantitate luciferase and β-Gal activities, and the luciferase activities were normalized to β-Gal activity.
Transfection of siRNA
Cells (1.5 × 105 cells/well) were plated in 6-well plates in DMEM/Ham's F-12 media supplemented with 5% charcoal-stripped FBS. After 16 hr, the cells were transfected with 100 nM of each siRNA duplex for 7 hr using LipofectAMINE 2000 reagent (Invitrogen) following the manufacturer's protocol. The medium was then changed to DMEM/Ham's F-12 medium containing 5% charcoal-stripped FBS and incubated for 40 hr. After incubation, the cells were treated with either vehicle (DMSO) or different concentrations of the compound and cells were collected for Western blot analysis and quantitative real-time PCR assay.
Chromatin immunoprecipitation (ChIP) assay
Panc1 cells (1 × 107 cells) were treated with DMSO, DIM-C-pPhOCH3 (10 μM) for 1, 2 or 6 hr. Cells were then fixed with 1% formaldehyde, and the cross-linking reaction was stopped by addition of 0.125 M glycine. After washing twice with phosphate-buffered saline, cells were scraped and pelleted. Collected cells were hypotonically lysed, and nuclei were collected. Nuclei were then sonicated to desired chromatin length (~500 bp). The chromatin was pre-cleared by addition of protein A-conjugated beads (PIERCE), and then incubation at 4°C for 1 hr with gentle agitation. The beads were pelleted, and the precleared chromatin supernatant was immunoprecipitated with antibodies to IgG, Sp1, Sp3, Sp4, and Nur77 at 4°C overnight. The protein-antibody complexes were collected by addition of protein A-conjugated beads at room temperature for 1 hr, the beads were extensively washed, and protein-DNA crosslinks were reversed. DNA was purified by phenol extract/ethanol precipitation followed by PCR amplification. The p21 primers were 5'-GCT GGC CTG CTG GAA CTC-3' (sense), and 5'-GGC AGC TGC TCA CAC CTC-3' (antisense); and they amplified a 193-bp region of the human p21 promoter, which contains several GC-rich, Sp1 binding sites. The positive control primers were 5'-TAC TAG CGG TTT TAC GGG CG-3' (sense), and 5'-TCG AAC AGG AGG AGC AGA GAG CGA-3' (antisense), and they amplified a 167-bp region of human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene. The negative control primers were 5'-ATG GTT GCC ACT GGG GAT CT-3' (sense), and 5'-TGC CAA AGC CTA GGG GAA GA-3' (antisense), and amplified a 174-bp region of genomic DNA between human GAPDH and CNAP1 genes. PCR products were resolved on a 2% agarose gel in the presence of CYBR gold (1:10,000).
Statistical analysis
The results are expressed as means ± SE and differences between means for two groups were determined by unpaired Student's t test. The minimum significance level was set at P value of ≤0.05 for all analysis. All experiments were performed at least three times.
RESULTS
DIM-C-pPhOCH3 activates Nur77 in pancreatic and colon cancer cells (29, 30) and induces proapoptotic responses. However, in preliminary microarray studies, DIMC-pPhOCH3 also induced p21 expression after treatment for 6 hr (data not shown). Using Panc1 cells as a model, Figure 1A shows that 5 or 10 μM DIM-C-pPhOCH3 induced p21 protein levels and Figure 1B demonstrates the time course induction of p21 mRNA by DIM-C-pPhOCH3. p21 was significantly induced 3-6 hr after treatment and was maximally induced after 18-24 hr. In addition, luciferase activity was also induced in Panc1 cells transfected with pWWP, a construct containing the p21 promoter (-2325 to +8) linked to a luciferase reporter gene. Since p21 is important for cell proliferation and cell cycle progression, the effects of DIM-C-pPhOCH3 on Panc1 cell survival (Fig. 1C) and the % distribution of cells in G0/G1, S and G2/M were determined by cell counting and FACS analysis, respectively (Fig. 1D). Treatment with 5-15 μM DIM-C-pPhOCH3 for 24, 48, 72 and 96 hr decreased cell survival in a time- and concentrationdependent manner, and a significant decrease in cell survival was observed at all concentrations after treatment for 96 hr (Fig. 1C). Treatment of Panc1 cells with 10 μM DIM-C-pPhOCH3 for 24 hr also significantly increased the % cells in G0/G1 and decreased the % cells in S phase (Fig. 1D). These results are consistent with the effects of DIM-C-pPhOCH3 on p21 expression in Panc1 cells.
Figure 1.
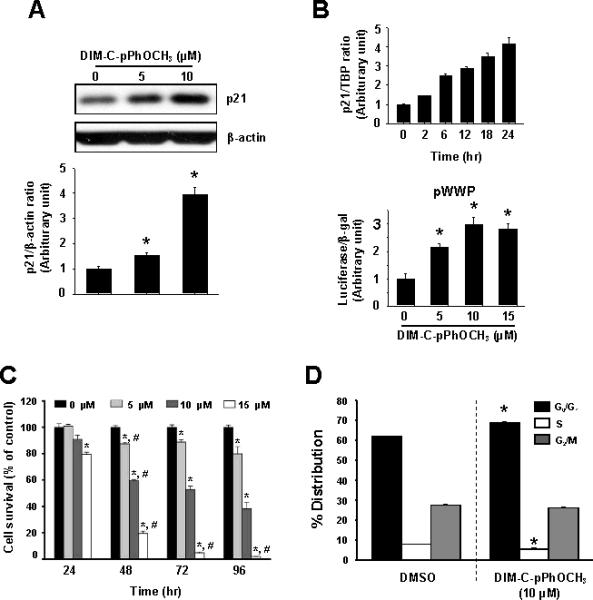
DIM-C-pPhOCH3 induces p21 and decreases Panc1 cell proliferation. Induction of p21 protein (A), mRNA levels, and promoter activity (B). Panc1 cells were treated with 0, 5 and 10 μM DIM-C-pPhOCH3 for 24 hr (A) or 10 μM DIM-C-pPhOCH3 for different times (B), and cellular extracts were analyzed by Western blots or real time PCR as described in the Materials and Methods. For promoter activity, cells were transfected with pWWP, treated with DIM-C-pPhOCH3 and luciferase activity determined as described in the Materials and Methods. (C) Panc1 cell survival. Panc1 cells were treated with DIM-C-pPhOCH3 for 24, 48, 72 and 96 hr and the percentage of cells surviving compared to the DMSO (solvent control set at 100%) were determined as described in the Materials and Methods. (D) FACS analysis. Panc1 cells were treated with 10 μM DIM-C-pPhOCH3 for 48 hr and the distribution of cells in G0/G1, S and G2/M phases were determined as described in the Materials and Methods. Significant (p < 0.05) effects of DIM-C-pPhOCH3 compared to DMSO controls and respective 0 hr controls are indicated by an asterisk and pound symbols, respectively.
Previous studies showed that Nur77-active C-DIMs activated apoptosis in colon and pancreatic cancer cells and Nur77 was detected primarily in the nuclear fraction, whereas studies in other cell lines reported that apoptosis-inducing agents such as the phorbol esters and the retinoid CD437 induced export of nuclear Nur77 (19-21).
Results in Figure 2 illustrate the effects of DMSO (solvent control), 10 μM DIM-C-pPhOCH3, 2 μM CD437 and 200 ng/ml TPA on subcellular localization of Nur77 in Panc1 cells. DAPI staining was used to identify the cell nuclei and the overlay demonstrates colocalization of Nur77 and DAPI staining. In DMSO-treated cells, Nur77 staining was primarily in the nucleus and treatment of Panc1 cells with 10 μM DIM-C-pPhOCH3 did not change the Nur77 staining pattern. In contrast, both CD437 and TPA induced nuclear export of Nur77 out of the nucleus in Panc1 within 3 hr after treatment and this was consistent with previous reports for these compounds in other cancer cell lines (19-21). Thus, induction of p21 by Nur77-active DIM-C-pPhOCH3 is not accompanied by nuclear export of Nur77.
Figure 2.
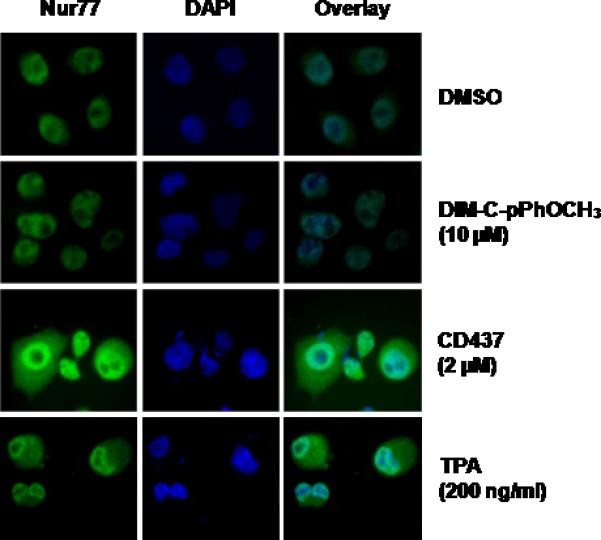
Staining for Nur77 and DAPI. Panc1 cells were treated with DMSO, 10 μM DIM-C-pPhOCH3, 2 μM CD437 or 200 ng/ml TPA for 3 hr, stained and visualized as described in the Materials and Methods.
The role of Nur77 in mediating the induction of p21 by DIM-C-pPhOCH3 was further investigated in Panc1 cells treated with DIM-C-pPhOCH3 alone or in combination with DIM-C-pPhOH, a compound that inhibits activation of Nur77 (29). DIM-C-pPhOH alone decreased basal p21 protein expression and also blocked induction of p21 by DIM-C-pPhOCH3 (Fig. 3A). In addition, induction of p21 by DIM-C-pPhOCH3 was not affected by cotreatment with leptomycin B (LMB; a nuclear export inhibitor) confirming that this response was due to activation of nuclear Nur77 (Fig. 3B). A small inhibitory RNA for Nur77 (siNur77) was also used to confirm the role of the receptor in mediating induction of p21. In Panc1 cells transfected with siNur77, there was a significant decrease in Nur77 protein and this was accompanied by a significant decrease in the induction of p21 by DIM-C-pPhOCH3 (Fig. 3C). siNur77 alone decreased basal expression of p21, suggesting that this cyclin-dependent kinase inhibitor may also be regulated by endogenous Nur77. siNur77 also decreased Nur77 mRNA levels, and Nur77 knockdown inhibited induction of p21 mRNA by DIM-C-pPhOCH3 in Panc1 cells (Fig. 3D).
Figure 3.
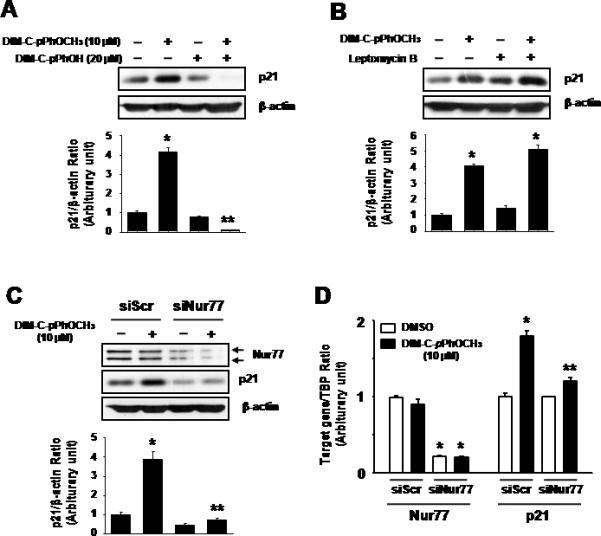
Induction of p21 by DIM-C-pPhOCH3 through activation of nuclear Nur77. Panc1 cells were treated with DMSO or DIM-C-pPhOCH3 alone or in combination with DIM-C-pPhOH (A), LMB (B) or cells transfected with siScr or siNur77 (C). After treatment with the compounds for 24 hr, whole cell lysates were analyzed by western blots as described in the Materials and Methods. Significant (p < 0.05) induction by DIM-C-pPhOCH3 is indicated by an asterisk and inhibition of this response by DIM-C-pPhOH or siNur77 is also indicated (**). (D) Determination of Nur77 and p21 mRNA levels after Nur77 knockdown. Panc1 cells were transfected with siScr or siNur77, treated with DMSO or 10 μM DIM-C-pPhOCH3 for 9 hr, and Nur77 and p21 mRNA levels were determined by real time PCR as described in the Materials and Methods. Significant (p < 0.05) induction of p21 mRNA or decreased Nur77 mRNA (*) and decrease p21 mRNA after transfection with siNur77 is indicated (**). Results are expressed as means ± SE for at least three separate determinations for each treatment group.
We also examined the effects of the Nur77-active DIM-C-pPhOCH3 and DIM-CPh on induction of p21 mRNA levels in L3.6pl pancreatic cancer cells and both compounds were active in this cell line (Fig. 4A). In addition, siNur77 decreased Nur77 in L3.6pl cells and Nur77 knockdown also decreased induction of p21 by DIM-C-pPhOCH3 compared to L3.6pl cells transfected with non-specific siScr (Fig. 4B). Thus, induction of p21 by DIM-C-pPhOCH3 was Nur77-dependent in both Panc1 and L3.6pl cells. Recent studies show that 5,5'-dibromoDIM, a ring substituted DIM, also induces p21 in colon cancer cells and this is KLF4 dependent (31). Nur77-active DIM-C-pPhOCH3 and DIM-C-Ph induced KLF4 mRNA levels in Panc1 cells, however, RNA interference studies with siNur77 show that induction of KLF4 was Nur77-independent and KLF4 knockdown did not affect p21 induction by DIM-C-pPhOCH3 (Supplementary Figure 1). The differences between DIM derivatives and their induction of p21/KLF4 is currently being investigated.
Figure 4.
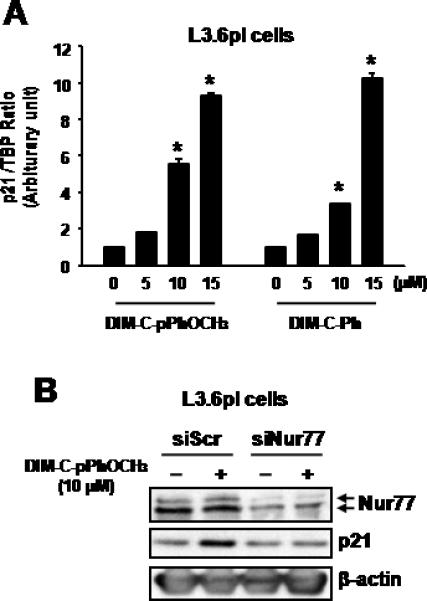
Induction of p21 by DIM-C-pPhOCH3 or DIM-C-Ph in L3.6pl cells. (A) Induction of p21 mRNA. L3.6pl cells were treated with DMSO, DIM-C-pPhOCH3 or DIM-C-Ph, and after 18 hr, p21 mRNA levels were determined as described in the Materials and Methods. Results are expressed as means ± SE (three replicate determinations) and significant (p < 0.05) induction is indicated. L3.6pl cells were transfected with siScr or siNur77 (B) treated with DMSO (0) or DIM-C-pPhOCH3 for 24 hr and whole cell lysates were analyzed by western blots as described in the Materials and Methods.
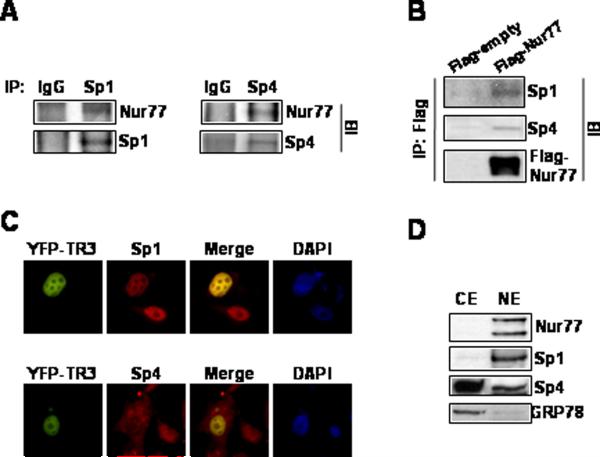
The mechanism of Nur77-dependent induction of p21 was further investigated in Panc1 cells transfected the pWWP construct that contains the -2325 to +8 p21 promoter sequence linked to a luciferase reporter gene and various deletion mutants (Fig. 5A). DIM-C-pPhOCH3 significantly induced luciferase activity in cells transfected with pWWP, however, this promoter sequence does not contain Nur77 binding sites. Comparable induction response was also observed in cells transfected with the pWWP124 deletion mutant which contains 6 well-characterized GC-rich sites in the proximal region of the p21 promoter but does not contain the distal p53 binding site. DIM-C-pPhOCH3 induced luciferase activity in Panc1 cells transfected with pWWP101 (containing GC sites 1 to 4) but not pWWP60 (containing GC sites 1 and 2), indicating that sites 3 and 4 are necessary for the induction response. Panc1 cells were also transfected with pWWP101 and the mutant constructs pWWP101-mt3 and pWWP101-mt4 and treatment with DIM-C-pPhOCH3 induced activity in cells transfected with pWWP101 whereas there was a significant decrease in both basal and inducible activity in cells transfected with the mutant constructs (Fig. 5B). These results imply that Sp proteins such as Sp1 that bind GC-rich motifs and Nur77 may be important for DIM-C-pPhOCH3- dependent activation of pWWP101 and this was confirmed in RNA interference studies which showed that both siSp1 and siNur77 inhibited induction of luciferase activity by DIM-C-pPhOCH3 (Fig. 5B). These results imply that Nur77-dependent induction of p21 may involve Sp proteins that bind GC-rich sites. Previous studies show that Sp1, Sp3 and Sp4 are the major Sp proteins expressed in pancreatic and other cancer cell lines (32). In Panc1 cells transfected with small inhibitory RNAs for Sp1 (siSp1), Sp3 (siSp3), and Sp4 (siSp4), there was a specific decrease in these proteins as previously reported (32). Moreover, siSp1 and siSp4 but not siSp3 decreased induction of p21 by DIM-C-pPhOCH3 (Fig. 5C). ChIP analysis of interactions of Sp proteins and Nur77 with the proximal GC-rich region of the p21 promoter demonstrated that treatment with DIM-C-pPhOCH3 increased recruitment of Nur77 to the promoter (Fig. 5D). In addition, there was a time-dependent increase in Sp1 and Sp4 interactions with the p21 promoter, whereas the constitutive binding of Sp3 was relatively unchanged. In a control experiment, we observed binding of TFIIB to the GAPDH promoter but not to exon I of the CNAP1 gene (data not shown). These results demonstrate that induction of p21 in Panc1 cells is Nur77-dependent and involved novel Nur77 interactions with Sp proteins in the proximal region of the p21 promoter. However, these results do not exclude a role for direct interactions of Nur77 with promoter DNA in more distal regions of the p21 promoter. The interactions of Nur77 with Sp1 and Sp4 were investigated in coimmunoprecipitation studies in Panc1 cells (Fig. 6A) and in cells transfected with Flag-Nur77 followed by coimmunoprecipitation with Flag antibodies (Fig. 6B). Results of both studies demonstrate coimmunoprecipitation of Nur77 with both Sp1 and Sp4. In addition, we also transfected Panc1 cells with EYFP-Nur77 and these cells were also stained with Sp1 and Sp4 antibodies and analysis by immunofluorescence microscopy demonstrated that Nur77 colocalized in the nucleus with both Sp1 and Sp4 (Fig. 6C). Both Nur77 and Sp1 are exclusively localized in nuclear extracts of Panc1 cells, however, Sp4 protein is detected in both the nuclear and cytosolic fraction (Fig. 6B). Thus, the role of Nur77, Sp1, and Sp4 in activation of p21 by DIM-C-pPhOCH3 is accompanied by association of these protein in ChIP, coimmunoprecipitation, and colocalization assays.
Figure 5.
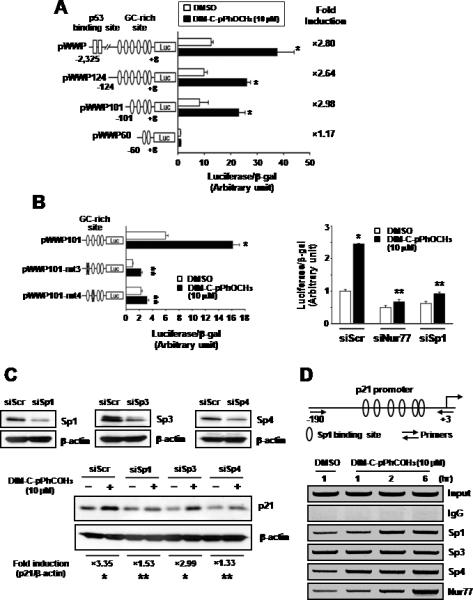
DIM-C-pPhOCH3-dependent activation of the p21 promoter and recruitment of Nur77 to the promoter. DIM-C-pPhOCH3 induced transactivation in cells transfected with pWWP (A) and its mutants (B). Panc1 cells were transfected with pWWP and its mutants, and treated with DMSO or DIM-C-pPhOCH3 (A and B). Cells were also transfected with pWWP101 and siNur77, siSp1, or siScr, and treated with DMSO or DIM-C-pPhOCH3 (B). Luciferase activity (relative to β-galactosidase activity) was determined as described in the Materials and Methods. Significant (p < 0.05) induction by DIM-C-pPhOCH3 (*) is indicated. (C) Sp protein knockdown. Panc1 cells were transfected with siScr, siSp1, siSp3 or siSp4, treated with DMSO or DIM-C-pPhOCH3, and after 24 hr, whole cell lysates were analyzed by western blots as outlined in the Materials and Methods. Results (A and B) are expressed as means ± SE of triplicate experiments and significant (p < 0.05) induction by DIM-C-pPhOCH3 (*) or inhibition of induction by RNA interference (**) are indicated. (D) Time course chromatin immunoprecipitation assay. Panc1 cells were treated with 10 μmol/L DIM-C-pPhOCH3 for 1, 2 and 6 hr, and interactions of Sp1, Sp3, Sp4 and Nur77 with the p21 promoter were determined as described in the Materials and Methods.
Figure 6.
Association of Nur77 with Sp1 and Sp4. (A) Coimmunoprecipitation. Endogenous Sp1 and Sp4 were immunoprecipitated with each of the antibodies and the immunoprecipitates were analyzed by Western blots using anti-Nur77 antibody as outlined in the Materials and Methods. (B) Cells were transfected with Flag-tagged Nur77 or empty vector, whole cell lysates were immunoprecipitated with Flag antibodies and immunoprecipitates were analyzed by western blots using anti-Sp1 and anti-Sp4 antibodies. (C) Immunoprecipitation with Flag antibodies. Subcellular colocalization of Nur77, Sp1, and Sp4. Cells were transiently transfected with pEYFP-Nur77 and 24 hr after transfection, cells were fixed, incubated with anti-Sp1 or anti-Sp4, and visualized as described in the Materials and Methods. The colocalization of Nur77 with Sp1 and Sp4 are depicted in the merged image. (D) Distribution of Nur77, Sp1, and Sp4 in subcellular fractions. Cytosolic and nuclear extracts were obtained and analyzed by Western blots as described in the Materials and Methods. Sp1, a nuclear protein, also serves as a nuclear protein marker and GRP78, a resident protein of endoplasmic reticulum, serves as a cytosolic protein marker.
DISCUSSION
Nur77 is an orphan receptor and a member of the nuclear receptor superfamily. The role of Nur77 in cancer has been extensively investigated, and the proapoptotic function of this nuclear receptor has paradoxically been associated with drug-induced apoptosis associated with extranuclear Nur77. Zhang and coworkers reported that various proapoptotic drugs including the phorbol ester 12-O-tetradecanoyl-13-phorbol acetate and the adamantyl-derived retinoids 6-[3-(1-adamantyl)-4-hydroxyphenyl]-2-naphthalene (AHPN or CD437) and 5-chloro-AHPN (chlorine substitution on the naphthalene group) induce apoptosis through translocation of Nur77 from the nucleus to the mitochondria in cancer cell lines (19, 20, 24-26). Moreover, results of drug-induced and Nur77 (wild-type/mutant) overexpression studies show that Nur77 specifically binds the antiapoptotic protein bcl2 to form a proapoptotic bcl-2/Nur77 complex (20). Other studies have confirmed drug-induced nuclear export of Nur77 but they do not necessarily detect Nur77 association with mitochondria (21-23). For example, in colon and prostate cancer cells, proapoptotic agents such as 5-fluorodeoxyuridine, TPA, sulindac and short chain fatty acids induce nuclear to cytosolic translocation of Nur77 and the subsequent enhancement of mitochondrial bax and release of cytochrome c (23).
Studies in this laboratory demonstrated that DIM-C-pPh and DIM-C-pPhOCH3 inhibit growth of colon and pancreatic cancer cells and also induce both Nur77-dependent and Nur77-independent apoptosis (29, 30). The Nur77-independent responses in colon cancer cells were primarily observed at higher concentrations of DIM-C-pPhOCH3 and involved induction of ER stress which is a general property of C-DIM compounds (33, 34). Microarray studies in several different cancer cell lines identified a large number of genes associated with growth and apoptosis, and RNA interference experiments in colon cancer cells confirmed that induction of p21 by DIM-C-pPhOCH3 was Nur77-dependent (data not shown) (30). p21 is a critical gene involved in regulating cell proliferation, cell cycle progression, and cell death pathways, and this study focused on the molecular mechanisms of Nur77-dependent induction of p21 by DIM-C-pPhOCH3 in Panc1 cells.
Initial studies demonstrated that DIM-C-pPhOCH3 inhibited Panc1 cell survival (Fig. 1C) and FACS analysis of Panc1 cells in G0/G1, S and G2/M phases of the cell cycle showed that DIM-C-pPhOCH3 induced a G0/G1 to S phase arrest (Fig. 1D). These results are consistent with the induction of p21 mRNA, protein and promoter activity (Figs. 1A and 1B). Results of RNA interference in Panc1 cells transfected with siNur77 confirm that induction of p21 protein and mRNA was Nur77-dependent (Figs. 3B and 3C). DIM-C-pPhOH has previously been characterized as a compound that acts as a Nur77 antagonist (29) and, in this study, DIM-C-pPhOH also inhibited induction of p21 by DIM-C-pPhOCH3 (Fig. 3A). We also observed that p21 was induced DIM-C-pPhOCH3 (and DIM-C-Ph) in L3.6pl pancreatic cancer cells and this response was also inhibited after cotransfection with siNur77 (Fig. 4). Moreover, in Panc1 cells treated with DMSO or 10 μM DIM-C-pPhOCH3 for 3 hr, immunostaining demonstrated that Nur77 remained in the nucleus, whereas treatment with TPA or CD437 for 3 hr resulted in transport of Nur77 out of the nucleus (Fig. 2) as previously reported for these apoptosis-inducing agents (19-21). The Nur77-dependent proapoptotic activity of DIM-C-pPhOCH3 in pancreatic and colon cancer cells was dependent on nuclear Nur77 and was unaffected by LMB, an agent that inhibits nuclear protein export (29, 30). In this study, LMB also did not affect induction of p21 by DIM-C-pPhOCH3 (Fig. 3B), confirming that this response was also dependent on nuclear Nur77.
p21 is induced through multiple p53-dependent and -independent pathways (36, 37), and results in Figure 5A shows that DIM-C-pPhOCH3 activates p21 promoter constructs containing the distal p53 response element (pWWP) or constructs containing only downstream GC-rich motifs (pWWP124 and pWWP101). Since the p21 promoter does not contain binding sites for Nur77 monomers or dimers, the promoter analysis studies suggest that Nur77-dependent activation of p21 by DIM-C-pPhOCH3 is associated with the proximal GC-rich sites (#6-3) in the p21 promoter. Transfection experiments using pWWP101 containing mutations in GC-rich sites 3 or 4 confirm the importance of these cis-elements for basal and inducible activation by DIM-C-pPhOCH3 (Fig. 5B) and RNA interference confirms the importance of Nur77 and Sp1 for activation of pWWP101. Previous studies have shown that other nuclear receptors including the androgen, progesterone and retinoic acid receptors and peroxisome proliferator-activated receptor γ (PPARγ) induce p21 through interactions of the ligand-bound receptors with Sp1 or Sp1/Sp4 (for PPARγ) proteins bound to these sites (32, 38-40). Results of RNA interference and ChIP assays (Figs. 5C and 5D) show that treatment of Panc1 cells with DIM-C-pPhOCH3 enhances Sp1, Sp4 (but not Sp3) and Nur77 binding to the GC-rich region of the p21 promoter, and knockdown of Sp1 or Sp4 but not Sp3 abrogates p21 induction. Moreover, coimmunoprecipitation and colocalization studies demonstrate the association of Nur77 with Sp1 and Sp4 (Fig. 6). Thus, activation of p21 by DIM-C-pPhOCH3 involves Nur77-Sp1/Sp4 (protein-protein) association rather than direct binding of Nur77 with promoter DNA, and this pathway appears to be important not only for Nur77 but also other nuclear receptors that induce p21 expression (32, 38-40). These results do not exclude the possibility that interactions of Nur77 with Sp1 or Sp4 may be indirect and involve other factors, and this is currently being investigated.
In summary, this study demonstrates that DIM-C-pPhOCH3 decreases Panc1 cell survival and inhibits G0/G1 to S phase progression, and this is due, in part, to Nur77-dependent induction of p21. We show that p21 induction by DIM-C-pPhOCH3 in Panc1 cells is KLF4-independent and involves association of Nur77 with Sp1 and Sp4 bound to proximal GC-rich sites in the p21 promoter. This represents a novel pathway for induction of p21 by activation of the orphan receptor Nur77 and demonstrates for the first time that like other nuclear receptors, Nur77 activation can result in transactivation through protein-protein (Nur77-Sp)-DNA association. Current studies are focused on the molecular mechanisms of Nur77-mediated induction of other genes and on development of Nur77-active C-DIMs for cancer chemotherapy.
Supplementary Material
Acknowledgements
The financial assistance of the National Institutes of Health (R01CA124998), the Chonbuk National University, and the Texas A&M AgriLife is gratefully acknowledged.
REFERENCES
- 1.Evans RM. The nuclear receptor superfamily: a rosetta stone for physiology. Mol. Endocrinol. 2005;19:1429–1438. doi: 10.1210/me.2005-0046. [DOI] [PubMed] [Google Scholar]
- 2.Giguere V. Orphan nuclear receptors: from gene to function. Endocr. Rev. 1999;20:689–725. doi: 10.1210/edrv.20.5.0378. [DOI] [PubMed] [Google Scholar]
- 3.Milbrandt J. Nerve growth factor induces a gene homologous to the glucocorticoid receptor gene. Neuron. 1988;1:183–188. doi: 10.1016/0896-6273(88)90138-9. [DOI] [PubMed] [Google Scholar]
- 4.Ryseck RP, Macdonald-Bravo H, Mattei MG, Ruppert S, Bravo R. Structure, mapping and expression of a growth factor inducible gene encoding a putative nuclear hormonal binding receptor. EMBO J. 1989;8:3327–3335. doi: 10.1002/j.1460-2075.1989.tb08494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakai A, Kartha S, Sakurai A, Toback FG, DeGroot LJ. A human early response gene homologous to murine nur77 and rat NGFI-B, and related to the nuclear receptor superfamily. Mol. Endocrinol. 1990;4:1438–1443. doi: 10.1210/mend-4-10-1438. [DOI] [PubMed] [Google Scholar]
- 6.Maruyama K, Tsukada T, Bandoh S, Sasaki K, Ohkura N, Yamaguchi K. Expression of the putative transcription factor NOR-1 in the nervous, the endocrine and the immune systems and the developing brain of the rat. Neuroendocrinology. 1997;65:2–8. doi: 10.1159/000127158. [DOI] [PubMed] [Google Scholar]
- 7.Zetterstrom RH, Solomin L, Jansson L, Hoffer BJ, Olson L, Perlmann T. Dopamine neuron agenesis in Nurr1-deficient mice. Science. 1997;276:248–250. doi: 10.1126/science.276.5310.248. [DOI] [PubMed] [Google Scholar]
- 8.Saucedo-Cardenas O, Quintana-Hau JD, Le WD, Smidt MP, Cox JJ, De Mayo F, Burbach JP, Conneely OM. Nurr1 is essential for the induction of the dopaminergic phenotype and the survival of ventral mesencephalic late dopaminergic precursor neurons. Proc. Natl. Acad. Sci. U. S. A. 1998;95:4013–4018. doi: 10.1073/pnas.95.7.4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeYoung RA, Baker JC, Cado D, Winoto A. The orphan steroid receptor Nur77 family member Nor-1 is essential for early mouse embryogenesis. J. Biol. Chem. 2003;278:47104–47109. doi: 10.1074/jbc.M307496200. [DOI] [PubMed] [Google Scholar]
- 10.Lee SL, Wesselschmidt RL, Linette GP, Kanagawa O, Russell JH, Milbrandt J. Unimpaired thymic and peripheral T cell death in mice lacking the nuclear receptor NGFI-B (Nur77) Science. 1995;269:532–535. doi: 10.1126/science.7624775. [DOI] [PubMed] [Google Scholar]
- 11.Mullican SE, Zhang S, Konopleva M, Ruvolo V, Andreeff M, Milbrandt J, Conneely OM. Abrogation of nuclear receptors Nr4a3 and Nr4a1 leads to development of acute myeloid leukemia. Nat. Med. 2007;13:730–735. doi: 10.1038/nm1579. [DOI] [PubMed] [Google Scholar]
- 12.Wilson TE, Paulsen RE, Padgett KA, Milbrandt J. Participation of non-zinc finger residues in DNA binding by two nuclear orphan receptors. Science. 1992;256:107–110. doi: 10.1126/science.1314418. [DOI] [PubMed] [Google Scholar]
- 13.Wilson TE, Fahrner TJ, Johnston M, Milbrandt J. Identification of the DNA binding site for NGFI-B by genetic selection in yeast. Science. 1991;252:1296–1300. doi: 10.1126/science.1925541. [DOI] [PubMed] [Google Scholar]
- 14.Zetterstrom RH, Solomin L, Mitsiadis T, Olson L, Perlmann T. Retinoid X receptor heterodimerization and developmental expression distinguish the orphan nuclear receptors NGFI-B, Nurr1, and Nor1. Mol. Endocrinol. 1996;10:1656–1666. doi: 10.1210/mend.10.12.8961274. [DOI] [PubMed] [Google Scholar]
- 15.Ordentlich P, Yan Y, Zhou S, Heyman RA. Identification of the antineoplastic agent 6-mercaptopurine as an activator of the orphan nuclear hormone receptor Nurr1. J. Biol. Chem. 2003;278:24791–24799. doi: 10.1074/jbc.M302167200. [DOI] [PubMed] [Google Scholar]
- 16.Wansa KD, Harris JM, Yan G, Ordentlich P, Muscat GE. The AF-1 domain of the orphan nuclear receptor NOR-1 mediates trans-activation, coactivator recruitment, and activation by the purine anti-metabolite 6-mercaptopurine. J. Biol. Chem. 2003;278:24776–24790. doi: 10.1074/jbc.M300088200. [DOI] [PubMed] [Google Scholar]
- 17.Winoto A. Genes involved in T-cell receptor-mediated apoptosis of thymocytes and T-cell hybridomas. Semin. Immunol. 1997;9:51–58. doi: 10.1006/smim.1996.0053. [DOI] [PubMed] [Google Scholar]
- 18.He YW. Orphan nuclear receptors in T lymphocyte development. J. Leukoc. Biol. 2002;72:440–446. [PubMed] [Google Scholar]
- 19.Li H, Kolluri SK, Gu J, Dawson MI, Cao X, Hobbs PD, Lin B, Chen G, Lu J, Lin F, Xie Z, Fontana JA, Reed JC, Zhang X. Cytochrome c release and apoptosis induced by mitochondrial targeting of nuclear orphan receptor TR3. Science. 2000;289:1159–1164. doi: 10.1126/science.289.5482.1159. [DOI] [PubMed] [Google Scholar]
- 20.Lin B, Kolluri SK, Lin F, Liu W, Han YH, Cao X, Dawson MI, Reed JC, Zhang XK. Conversion of Bcl-2 from protector to killer by interaction with nuclear orphan receptor Nur77/TR3. Cell. 2004;116:527–540. doi: 10.1016/s0092-8674(04)00162-x. [DOI] [PubMed] [Google Scholar]
- 21.Wu Q, Liu S, Ye XF, Huang ZW, Su WJ. Dual roles of Nur77 in selective regulation of apoptosis and cell cycle by TPA and ATRA in gastric cancer cells. Carcinogenesis. 2002;23:1583–1592. doi: 10.1093/carcin/23.10.1583. [DOI] [PubMed] [Google Scholar]
- 22.Holmes WF, Soprano DR, Soprano KJ. Early events in the induction of apoptosis in ovarian carcinoma cells by CD437: activation of the p38 MAP kinase signal pathway. Oncogene. 2003;22:6377–6386. doi: 10.1038/sj.onc.1206694. [DOI] [PubMed] [Google Scholar]
- 23.Wilson AJ, Arango D, Mariadason JM, Heerdt BG, Augenlicht LH. TR3/Nur77 in colon cancer cell apoptosis. Cancer Res. 2003;63:5401–5407. [PubMed] [Google Scholar]
- 24.Li Y, Lin B, Agadir A, Liu R, Dawson MI, Reed JC, Fontana JA, Bost F, Hobbs PD, Zheng Y, Chen GQ, Shroot B, Mercola D, Zhang XK. Molecular determinants of AHPN (CD437)-induced growth arrest and apoptosis in human lung cancer cell lines. Mol. Cell Biol. 1998;18:4719–4731. doi: 10.1128/mcb.18.8.4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kolluri SK, Bruey-Sedano N, Cao X, Lin B, Lin F, Han YH, Dawson MI, Zhang XK. Mitogenic effect of orphan receptor TR3 and its regulation by MEKK1 in lung cancer cells. Mol. Cell Biol. 2003;23:8651–8667. doi: 10.1128/MCB.23.23.8651-8667.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cao X, Liu W, Lin F, Li H, Kolluri SK, Lin B, Han YH, Dawson MI, Zhang XK. Retinoid X receptor regulates Nur77/TR3-dependent apoptosis [corrected] by modulating its nuclear export and mitochondrial targeting. Mol. Cell Biol. 2004;24:9705–9725. doi: 10.1128/MCB.24.22.9705-9725.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han YH, Cao X, Lin B, Lin F, Kolluri SK, Stebbins J, Reed JC, Dawson MI, Zhang XK. Regulation of Nur77 nuclear export by c-Jun N-terminal kinase and Akt. Oncogene. 2006;25:2974–2986. doi: 10.1038/sj.onc.1209358. [DOI] [PubMed] [Google Scholar]
- 28.Lee KW, Cobb LJ, Paharkova-Vatchkova V, Liu B, Milbrandt J, Cohen P. Contribution of the orphan nuclear receptor Nur77 to the apoptotic action of IGFBP-3. Carcinogenesis. 2007;28:1653–1658. doi: 10.1093/carcin/bgm088. [DOI] [PubMed] [Google Scholar]
- 29.Chintharlapalli S, Burghardt R, Papineni S, Ramaiah S, Yoon K, Safe S. Activation of Nur77 by selected 1,1-Bis(3'-indolyl)-1-(p-substituted phenyl)methanes induces apoptosis through nuclear pathways. J. Biol. Chem. 2005;280:24903–24914. doi: 10.1074/jbc.M500107200. [DOI] [PubMed] [Google Scholar]
- 30.Cho SD, Yoon K, Chintharlapalli S, Abdelrahim M, Pei P, Hamilton S, Khan S, Ramaiah SK, Safe S. Nur77 agonists induce proapoptotic genes and responses in colon cancer cells through nuclear receptor-dependent and independent pathways. Cancer Res. 2007;67:674–683. doi: 10.1158/0008-5472.CAN-06-2907. [DOI] [PubMed] [Google Scholar]
- 31.Cho SD, Chintharlapalli S, Abdelrahim M, Papineni S, Liu S, Guo J, Lei P, Abudayyeh A, Safe S. 5,5'-Dibromo-bis(3'-indolyl)methane induces Kruppel-like factor 4 and p21 in colon cancer cells. Mol. Cancer Ther. 2008;7:2109–2120. doi: 10.1158/1535-7163.MCT-07-2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hong J, Samudio I, Liu S, Abdelrahim M, Safe S. Peroxisome proliferator-activated receptor γ-dependent activation of p21 in Panc-28 pancreatic cancer cells involves Sp1 and Sp4 proteins. Endocrinology. 2004;145:5774–5785. doi: 10.1210/en.2004-0686. [DOI] [PubMed] [Google Scholar]
- 33.Cho SD, Lei P, Abdelrahim M, Yoon K, Liu S, Guo J, Papineni S, Chintharlapalli S, Safe S. 1,1-Bis(3'-indolyl)-1-(p-methoxyphenyl)methane activates Nur77-independent proapoptotic responses in colon cancer cells. Mol. Carcinog. 2008;47:252–263. doi: 10.1002/mc.20378. [DOI] [PubMed] [Google Scholar]
- 34.Lei P, Abdelrahim M, Cho SD, Liu S, Chintharlapalli S, Safe S. 1,1-Bis(3'-indolyl)-1-(p-substituted phenyl)methanes inhibit colon cancer cell and tumor growth through activation of c-Jun N-terminal kinase. Carcinogenesis. 2008;29:1139–1147. doi: 10.1093/carcin/bgn103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rowland BD, Peeper DS. KLF4, p21 and context-dependent opposing forces in cancer. Nat. Rev. Cancer. 2006;6:11–23. doi: 10.1038/nrc1780. [DOI] [PubMed] [Google Scholar]
- 36.Gartel AL, Tyner AL. Transcriptional regulation of the p21WAF1/CIP1 gene. Exp. Cell Res. 1999;246:280–289. doi: 10.1006/excr.1998.4319. [DOI] [PubMed] [Google Scholar]
- 37.Koutsodontis G, Moustakas A, Kardassis D. The role of Sp1 family members, the proximal GC-rich motifs, and the upstream enhancer region in the regulation of the human cell cycle inhibitor p21WAF-1/Cip1 gene promoter. Biochemistry. 2002;41:12771–12784. doi: 10.1021/bi026141q. [DOI] [PubMed] [Google Scholar]
- 38.Liu M, Iavarone A, Freedman LP. Transcriptional activation of the human p21(WAF1/CIP1) gene by retinoic acid receptor. Correlation with retinoid induction of U937 cell differentiation. J. Biol. Chem. 1996;271:31723–31728. doi: 10.1074/jbc.271.49.31723. [DOI] [PubMed] [Google Scholar]
- 39.Owen GI, Richer JK, Tung L, Takimoto G, Horwitz KB. Progesterone regulates transcription of the p21WAF1 cyclin-dependent kinase inhibitor gene through Sp1 and CBP/p300. J. Biol. Chem. 1998;273:10696–10701. doi: 10.1074/jbc.273.17.10696. [DOI] [PubMed] [Google Scholar]
- 40.Lu S, Jenster G, Epner DE. Androgen induction of cyclin-dependent kinase inhibitor p21 gene: role of androgen receptor and transcription factor Sp1 complex. Mol. Endocrinol. 2000;14:753–760. doi: 10.1210/mend.14.5.0461. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.