Abstract
Systemic administration of epidermal growth factor (EGF) decreases mortality in a murine model of septic peritonitis. Although EGF can have direct healing effects on the intestinal mucosa, it is unknown whether the benefits of systemic EGF in peritonitis are mediated through the intestine. Here, we demonstrate that enterocyte-specific overexpression of EGF is sufficient to prevent intestinal barrier dysfunction and improve survival in peritonitis. Transgenic FVB/N mice that overexpress EGF exclusively in enterocytes (IFABP-EGF) and wild-type (WT) mice were subjected to either sham laparotomy or cecal ligation and puncture (CLP). Intestinal permeability, expression of the tight junction proteins claudins-1, -2, -3, -4, -5, -7, and -8, occludin, and zonula occludens-1; villus length; intestinal epithelial proliferation; and epithelial apoptosis were evaluated. A separate cohort of mice was followed for survival. Peritonitis induced a threefold increase in intestinal permeability in WT mice. This was associated with increased claudin-2 expression and a change in subcellular localization. Permeability decreased to basal levels in IFABP-EGF septic mice, and claudin-2 expression and localization were similar to those of sham animals. Claudin-4 expression was decreased following CLP but was not different between WT septic mice and IFABP-EGF septic mice. Peritonitis-induced decreases in villus length and proliferation and increases in apoptosis seen in WT septic mice did not occur in IFABP-EGF septic mice. IFABP-EGF mice had improved 7-day mortality compared with WT septic mice (6% vs. 64%). Since enterocyte-specific overexpression of EGF is sufficient to prevent peritonitis-induced intestinal barrier dysfunction and confers a survival advantage, the protective effects of systemic EGF in septic peritonitis appear to be mediated in an intestine-specific fashion.
Keywords: permeability, claudin 2, sepsis, gut, apoptosis
epidermal growth factor (EGF) is a peptide that has been shown to be protective against a variety of gastrointestinal injuries (35). In the intestine, activation of the EGF receptor (EGF-R) following binding by EGF can lead to increased cell survival (8, 17), decreased inflammation (27), and improved epithelial barrier function (16). Systemic administration of EGF attenuates intestinal tissue damage and improves mortality in a variety of animal models of noninfectious inflammation (5, 39) and intestinal injury (7, 23).
Because of its potent effects on cellular turnover in a wide variety of tissues, EGF and EGF-R have been frequently targeted for therapeutic use in a large number of diseases. A federal government registration of clinical trials currently lists over 150 trials involving or targeting EGF and EGF-R (17a). Despite EGF's known beneficial effects on the intestine, the majority of the clinical trials manipulating EGF/EGF-R do not target the intestine. As such, EGF may be used to target intestine-based diseases but may simultaneously have unexpected systemic effects. Alternatively, EGF may be used to target systemic diseases but may have profound effects on the gastrointestinal system.
We have previously shown that systemic administration of EGF after the onset of sepsis confers a survival advantage (10). Sepsis kills 210,000 people each year in the United States (1), and the intestine plays a central role in the pathophysiology of sepsis, where it has been characterized as the “motor” of the systemic inflammatory response (13, 31). Perturbations to the intestinal epithelium in sepsis result in barrier dysfunction (21, 41), increased apoptosis (20, 33, 42), and production of cytokines (40), which may cause distant organ damage leading to multiple organ failure. Systemic EGF prevents peritonitis-induced decreases in intestinal proliferation and peritonitis-induced increases in intestinal epithelial apoptosis (10). However, these finding are associative and do not demonstrate a causal link between EGF's effects on the intestine and its effects on survival. This is especially true in light of EGF's multiple systemic effects and the fact that sepsis is fundamentally a systemic disease (32, 45).
To determine the functional and mechanistic importance of intestine-specific EGF in sepsis, we subjected WT and transgenic mice that overexpress EGF exclusively in enterocytes to peritonitis-induced sepsis. We determined whether enterocyte-specific overexpression of EGF 1) prevented intestinal hyperpermeability and alterations in the tight junction barrier; 2) prevented alterations in villus length, intestinal proliferation, and apoptosis; 3) conferred a survival advantage in sepsis; and 4) altered extraintestinal cytokines and bacterial burden.
MATERIALS AND METHODS
Mice.
FVB/N mice containing nucleotides −1178 to +28 of a rat intestinal fatty acid-binding protein (IFABP) linked to mouse EGF were generated as previously described (24) (a generous gift from Dr. Brad Warner, Washington University, St. Louis, MO). IFABP-EGF mice express EGF exclusively in villus enterocytes. Unmanipulated IFABP-EGF and wild-type (WT) mice appear phenotypically identical. All animal studies were approved by the Washington University Animal Studies Committee and were conducted in accordance with the National Institutes of Health guidelines for the use of laboratory animals.
Peritonitis model.
Mice were subjected to CLP according to the method of Baker et al. (2). A small midline abdominal incision was made under isoflurane anesthesia. The cecum was ligated just distal to the ileocecal valve by a technique that did not result in intestinal obstruction and was then punctured twice with a 23-gauge needle. The cecum was then gently squeezed to extrude a small amount of stool and replaced in the abdomen, which was closed in layers. Sham mice were treated identically, except the cecum was neither ligated nor punctured. All mice were injected subcutaneously with 1 ml of 0.9% saline to account for insensible fluid losses that occurred during surgery. Animals were euthanized at either 24 h for functional studies or followed 7 days for survival. Animals that were euthanized 24 h after CLP received two doses of antibiotic therapy (ceftriaxone 25 mg/kg + metronidazole 12.5 mg/kg, intraperitoneally) at 3 and 15 h postoperatively. Animals that were followed for survival received antibiotics for 2 days.
Systemic EGF levels.
Blood was harvested retroorbitally from anesthetized mice. Serum was obtained by centrifugation and stored at −80°C. EGF was measured by an enzyme-linked immunosorbent assay by using a commercially available kit (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions. All samples were run in duplicate.
Intestinal EGF, TGF-α, and EGF-R expression.
Total RNA was isolated from whole jejunal tissue with the Illustra RNAspin Mini RNA Isolation Kit (GE Healthcare, Piscataway, NJ). The integrity of RNA was verified by electrophoresis on a 1.2% agarose gel containing 2.2 mol/l formaldehyde in 1 × MOPS buffer (40 mmol/l MOPS, pH 7.0, 10 mmol/l sodium acetate; 1 mmol/l EDTA, pH 8.0). Following synthesis of cDNA from 0.5 μg of total RNA, quantitative real-time PCR was used to evaluate gene expression. The mRNA levels of EGF, TGF-α, and EGF-R were detected by use of predeveloped TaqMan primers and probes (Applied Biosystems, Foster, CA) and run on an ABI 7900HT Sequence Detection System (Applied Biosystems). All samples were run in duplicate and normalized to expression of the endogenous control, glyceraldehyde-3-phosphate (GAPDH) (Applied Biosystems). Relative quantification of PCR products was based on the value differences between the target gene and GAPDH by the comparative cycle threshold method.
Protein expression of EGF-R was analyzed by Western blot. Frozen jejunal samples were homogenized with a handheld homogenizer in a 5× volume of ice-cold homogenization buffer (50 mM Tris·HCl, pH 7.4; 150 mM NaCl; 1 mM EDTA; 0.1% SDS; 1% Na-deoxycholic acid; 1% Triton X-100; 50 mM DTT; 50 μg/ml aprotinin; 50 μg/ml leupeptin; 5 mM PMSF; 1 mM NaF; 1 mM Na3VO4) and centrifuged at 10,000 rpm for 5 min at 4°C, and the supernatant was collected. Total protein concentration was quantified by the Bradford protein assay (9). Forty micrograms of protein were added to an equal volume of 2 × Laemmli sample buffer and heated at 95°C for 5 min. Samples were run on polyacrylamide gels (Bio-Rad, Hercules, CA) at 180 V for 45 min, and protein was transferred to Immuno-Blot polyvinylidene difluoride membranes (Bio-Rad) at 80 V for 2 h. Membranes were blocked with 5% nonfat milk in Tris-buffered saline with 0.1% Tween 20 (Sigma) for 1 h at room temperature and then incubated with either rabbit polyclonal anti-EGF-R or β-actin (1:1,000; Cell Signaling Technology) overnight at 4°C. After washing, membranes were incubated for 1 h at room temperature with horseradish peroxidase-conjugated goat anti-rabbit IgG (1:1,000; Cell Signaling Technology). Proteins were visualized via a chemiluminescent system (Pierce, Rockford, IL) and exposed to X-ray film.
Intestinal permeability.
Intestinal permeability was measured in vivo to fluorescein isothiocyanate-conjugated dextran (FD-4, 22 mg/ml, molecular mass 4.4 kDa, Sigma, St. Louis, MO) as previously described (51, 52). Briefly, mice were subjected to CLP or sham laparotomy, food was withdrawn overnight, and mice were gavaged with 0.5 ml of FD-4 at 19 h postsurgery. Blood was collected retroorbitally 5 h after gavage and centrifuged at 3,000 rpm at 4°C for 20 min. Plasma (50 μl) was mixed with an equal volume of sterile phosphate-buffered saline (pH 7.4), and the concentration of FD-4 was determined by fluorospectrometry (NanoDrop 3300, Thermo Scientific, Wilmington, DE) with an excitation wavelength of 470 nm and an emission wavelength of 515 nm by using serially diluted samples as standards.
Gene expression and localization of tight junction components.
Gene expression of intestinal claudins-1, -2, -3, -4, -5, -7, and -8, occludin, and zonula occludens-1 (ZO-1) was determined by quantitative real-time PCR as described above by use of predeveloped TaqMan primers and probes (Applied Biosystems). Protein expression of claudin-2 was evaluated by Western blot as described above. Cellular localization of claudin-2 was evaluated by fluorescent immunohistochemistry. Intestinal sections were deparaffinized, rehydrated, incubated in 3% hydrogen peroxide for 10 min, and heated in Antigen Decloaker (Biocare Medical, Concord, CA) for 45 min for antigen retrieval. Sections were blocked with 20% normal goat serum (Vector Laboratories, Burlingame, CA) and incubated for 3 h at room temperature with rabbit polyclonal anti-claudin-2 (1:200; Abcam, Cambridge, MA). Slides were then incubated in goat anti-rabbit Alexa 594-conjugated secondary antibody (1:200, Invitrogen) for 30 min at room temperature. Diamidino-2-phenylindole was used to fluorescently label nuclei.
Morphological analysis.
Villus length was determined on hematoxylin and eosin (H&E)-stained jejunal sections by measuring the distance in micrometers from the crypt neck to the villus tip using Image J software (National Institutes of Health, Bethesda, MD). A minimum of 12 well-oriented villi from each section were measured by an examiner (J. A. Clark) blinded to its identity.
Intestinal proliferation.
Mice were intraperitoneally injected with 5-bromo-2′-deoxyuridine (BrdU) (5 mg/ml diluted in 0.9% saline; Sigma) 90 min prior to euthanasia to label cells in S phase. Intestinal sections were deparaffinized, rehydrated, and incubated in 1% hydrogen peroxide for 15 min. Slides were then immersed in Antigen Decloaker (Biocare Medical) and heated in a pressure cooker for 45 min. Sections were blocked for 10 min with Protein Block (Dako, Carpinteria, CA) and incubated with rat monoclonal anti-BrdU (1:500; Accurate Chemical & Scientific, Westbury, NY) overnight at 4°C. Sections were then incubated at room temperature with goat anti-rat secondary antibody (1:500; Accurate Chemical & Scientific) for 30 min, followed by streptavidin-horseradish peroxidase (1:500; Dako) for 60 min. Slides were developed with diaminobenzidine and counterstained with hematoxylin. Jejunal S-phase cells in the intestinal epithelium were quantified in 100 contiguous crypts per animal (34).
Intestinal epithelial apoptosis.
Quantification of apoptotic cells in the intestinal epithelium was performed by using two complimentary techniques: active caspase-3 staining and morphological analysis of H&E-stained sections (50). To detect active caspase-3, sections were incubated in 3% hydrogen peroxide for 10 min, heated in Antigen Decloaker (Biocare Medical) for 45 min, blocked with 20% normal goat serum (Vector Laboratories) and then incubated with rabbit polyclonal anti-active caspase-3 (1:100; Cell Signaling Technology, Beverly, MA) overnight at 4°C. Sections were then incubated with goat anti-rabbit biotinylated secondary antibody (1:200; Vector Laboratories) for 30 min at room temperature followed by Vectastain Elite ABC reagent (Vector Laboratories) for 30 min, developed with diaminobenzidine, and counterstained with hematoxylin. For H&E-stained sections, apoptotic cells were identified by using morphological criteria of cell shrinkage with nuclear condensation and fragmentation. Apoptotic intestinal epithelial cells were quantified in 100 contiguous crypts and 50 well-oriented villi per animal.
Bacterial cultures.
The peritoneal cavity was lavaged with 5 ml of sterile 0.9% saline to obtain peritoneal fluid. Blood was collected retroorbitally and serum was obtained following centrifugation at 5,000 rpm for 5 min in serum separator tubes. Both peritoneal fluid and serum were then serially diluted in sterile 0.9% saline and aliquots were cultured on sheep blood agar plates. Samples were incubated overnight at 37°C, and colony counts were enumerated.
Local and systemic cytokine levels.
Cytokine levels in peritoneal lavage fluid and serum were determined by using a cytometric bead array (BD Mouse Inflammation Kit, San Jose, CA) according to manufacturer protocol. All samples were run in duplicate.
Statistics.
Continuous data sets were tested for Gaussian distribution by use of a normality test. If data were found to have Gaussian distributions, multiple group comparisons were performed with one-way analysis of variance followed by the Tukey posttest. If data were found to have non-Gaussian distributions, multiple group comparisons were performed using the Kruskal-Wallis nonparametric one-way analysis of variance by ranks followed by the Dunn's posttest. Survival studies were analyzed by the log-rank test. Data were analyzed with the statistical software program Prism 4.0 (GraphPad Software, San Diego, CA) and are reported as means ± SE. A P value <0.05 was considered statistically significant.
RESULTS
In all experiments, comparisons were made between 1) WT sham mice and WT septic mice, 2) IFABP-EGF sham mice and IFABP-EGF septic mice, and 3) WT septic mice and IFABP-EGF septic mice. With the exception of intestinal EGF gene expression, there were no differences in any measurements between WT and IFABP-EGF sham mice. All animals were euthanized 24 h postoperatively unless otherwise specified.
Systemic and intestinal EGF levels are preserved in septic IFABP-EGF mice.
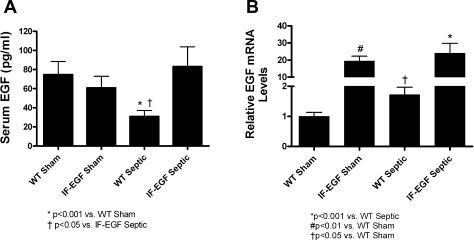
Sepsis induced a 59% decrease in serum EGF levels in WT mice compared with shams. In contrast, sepsis had no effect on serum EGF levels in IFABP-EGF mice since they were similar between IFABP-EGF sham and IFABP-EGF septic mice (Fig. 1A, n = 6–13/group). Serum EGF levels were higher in septic IFABP-EGF mice than septic WT mice but were similar to sham WT and IFABP-EGF mice.
Fig. 1.
Systemic and intestinal EGF levels. Circulating EGF levels (A) were significantly lower in wild-type (WT) septic than WT sham animals but were not statistically different between intestinal fatty acid binding protein (IFABP)-EGF (IF-EGF) sham and IFABP-EGF septic mice. In contrast, intestinal EGF levels (B) were higher in WT septic than WT sham animals. Whereas intestinal EGF levels were higher in IFABP-EGF mice than WT mice, levels of intestinal EGF were not statistically different between IFABP-EGF sham and IFABP-EGF septic mice.
Intestinal EGF mRNA levels were increased more than 20-fold in IFABP-EGF sham mice compared with WT sham mice. Sepsis induced a 1.7-fold increase in intestinal EGF mRNA levels in WT septic mice compared with WT shams. In contrast, intestinal EGF levels were unaffected by sepsis in IFABP-EGF mice since they were similar between IFABP-EGF sham and IFABP-EGF septic mice (Fig. 1B, n = 8–10/group).
Since EGF is not normally expressed at high levels in the mature intestinal epithelium, gene expression of the EGF-R ligand TGF-α was also evaluated. There were no significant differences in intestinal TGF-α mRNA levels between any of the groups (data not shown).
Intestinal EGF-R expression is normalized in septic IFABP-EGF mice.
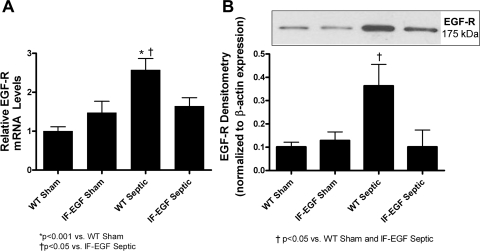
Quantitative evaluation of jejunal EGF-R mRNA (Fig. 2A, n = 8–10/group) and protein levels (Fig. 2B, n = 5–7/group) revealed similar patterns, where intestinal EGF-R levels were significantly higher in WT septic mice compared with WT sham mice. However, overexpression of EGF prevented sepsis-induced increases in intestinal EGF-R expression since IFABP-EGF septic mice had similar levels of EGF-R to both IFABP-EGF sham and WT sham animals.
Fig. 2.
Intestinal EGF receptor (EGF-R) expression. Relative mRNA levels (A) and protein expression (B) of EGF-R were quantified in jejunal tissue via quantitative real-time PCR and Western blot, respectively. Intestinal EGF-R mRNA and protein levels were increased in WT septic mice compared with shams, but enterocyte-specific overexpression of EGF resulted in normalization to sham levels. A representative blot for EGF-R is depicted; densitometry was determined by normalizing expression to β-actin.
Intestinal barrier function is preserved in IFABP-EGF septic mice.
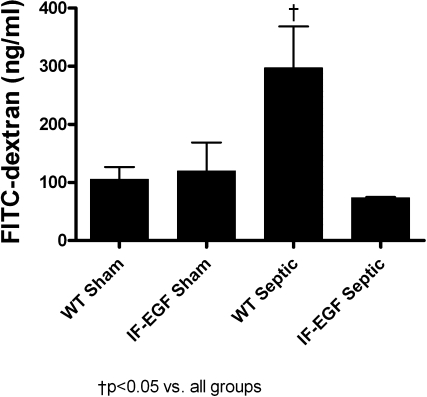
Sepsis induced a threefold increase in intestinal permeability in WT mice compared with shams as measured by the appearance of FD-4 in the bloodstream (Fig. 3, n = 5/group). In contrast, IFABP-EGF mice did not have evidence of sepsis-induced intestinal hyperpermeability, since FD-4 concentrations in septic IFABP-EGF mice were similar to IFABP-EGF sham mice.
Fig. 3.
Intestinal permeability. Intestinal permeability was assayed in vivo by measuring the amount of fluorescein isothiocyanate-conjugated dextran (FD-4) in plasma. WT septic mice had increased intestinal permeability compared with sham animals, whereas sepsis-induced increases in intestinal permeability were prevented in IFABP-EGF septic mice.
To determine whether alterations in tight junction proteins contributed to intestinal barrier dysfunction in WT septic mice, claudins-1, -2, -3, -4, -5, -7, and -8, occludin, and ZO-1 gene expression were analyzed by real-time PCR (Table 1, n = 7–12/group). Intestinal gene expression of claudin-1, -3, -5, -7, and -8, occludin, and ZO-1 were similar between WT and IFABP-EGF mice, regardless of whether they were shams or septic. Intestinal mRNA levels of claudin-4 were decreased to a similar degree in both WT and IFABP-EGF mice. Intestinal mRNA levels of claudin-2 were increased greater than fourfold in WT septic mice compared with WT shams. In contrast, IFABP-EGF septic mice exhibited normalization of claudin-2 gene expression to sham levels.
Table 1.
Tight junction gene expression
| WT Sham | IFABP-EGF Sham | WT Septic | IFABP-EGF Septic | |
|---|---|---|---|---|
| Claudin-1 | 1.0±0.16 | 0.86±0.17 | 0.94±0.32 | 0.93±0.13 |
| Claudin-2 | 1.0±0.13 | 0.90±0.23 | 4.24±0.96a | 1.21±0.16 |
| Claudin-3 | 1.0±0.07 | 0.99±0.09 | 0.99±0.09 | 0.98±0.06 |
| Claudin-4 | 1.0±0.08 | 1.03±0.15 | 0.55±0.05b | 0.61±0.07c |
| Claudin-5 | 1.0±0.14 | 0.76±0.23 | 0.50±0.07 | 1.02±0.24 |
| Claudin-7 | 1.0±0.13 | 0.91±0.10 | 1.00±0.09 | 0.88±0.10 |
| Claudin-8 | 1.0±0.35 | 0.86±0.36 | 0.61±0.28 | 0.85±0.40 |
| Occludin | 1.0±0.19 | 0.81±0.28 | 0.76±0.19 | 0.70±0.19 |
| ZO-1 | 1.0±0.12 | 1.28±0.24 | 1.46±0.20 | 1.29±0.17 |
Data are expressed as fold change relative to wild-type (WT) sham mice, means ± SE.
P < 0.001 vs. WT Sham and intestinal fatty acid binding protein (IFABP)-EGF Septic mice;
P < 0.001 vs. WT Sham;
P < 0.05 vs. IFABP-EGF Sham.
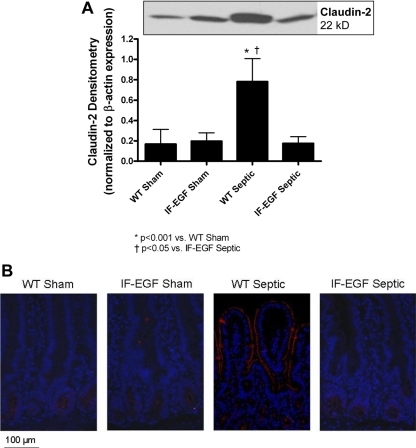
To further evaluate the role of claudin-2, protein expression and localization were analyzed. Similar to the mRNA levels, protein expression of claudin-2 was increased fourfold in WT septic mice compared with WT shams, whereas IFABP-EGF septic mice exhibited normalization of claudin-2 protein expression to sham levels (Fig. 4A, n = 4–6/group). Fluorescent immunohistochemistry revealed not only an increase in claudin-2 expression in WT septic mice but also a change in subcellular localization (Fig. 4B). In sham mice, claudin-2 expression was not detectable on the villi. In contrast, in WT septic mice, claudin-2 was predominantly localized along the apical membrane of the villi. The changes in claudin-2 seen in WT septic mice were not detectable in IFABP-EGF septic mice, which appeared similar to that in sham mice.
Fig. 4.
Expression and localization of claudin-2. Protein expression and localization of claudin-2 were evaluated by Western blot (A) and fluorescent immunohistochemistry (B). Claudin-2 protein levels were increased in WT septic mice compared with shams. In contrast, claudin-2 expression was normalized to sham levels in IFABP-EGF septic mice. A representative blot for claudin-2 is depicted; densitometry was determined by normalizing expression to β-actin. Fluorescent immunohistochemistry revealed that claudin-2 expression was increased and localized along the apical membrane of the villi in WT septic mice. Minimal staining for claudin-2 was observed in either the sham mice or IFABP-EGF septic mice. Representative images for each group are shown. Magnification ×20.
Villus length is normalized in IFABP-EGF septic mice.
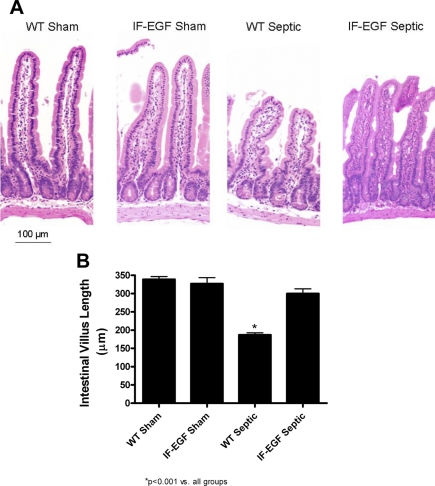
WT septic mice had markedly shorter villi compared with WT sham mice when H&E-stained sections of intestine were qualitatively analyzed (Fig. 5A). In contrast, intestinal morphology of IFABP-EGF septic mice appeared longer than WT septic mice, similar in appearance to sham mice (Fig. 5A). Villus length was then quantified by measuring the distance in μm from the crypt neck to the villus tip (Fig. 5B, n = 10/group). Similar findings were noted as in the morphological analysis, where WT septic mice had significantly shorter villi compared with sham animals, but villus length in IFABP-EGF septic mice was normalized to sham lengths (Fig. 5B).
Fig. 5.
Intestinal villus length. Intestinal morphology (A) was evaluated in hematoxylin and eosin (H&E)-stained intestinal sections. WT septic animals appeared to have markedly shorter villi than sham animals. Villi appeared to be longer in IFABP-EGF septic mice compared with WT septic mice. Magnification ×20. Villus length was quantified in H&E-stained sections of jejunum (B). WT septic mice had significantly shorter villi compared with sham mice. Villus length was preserved in IFABP-EGF septic mice.
Intestinal proliferation and apoptosis are both preserved at basal levels in IFABP-EGF septic mice.
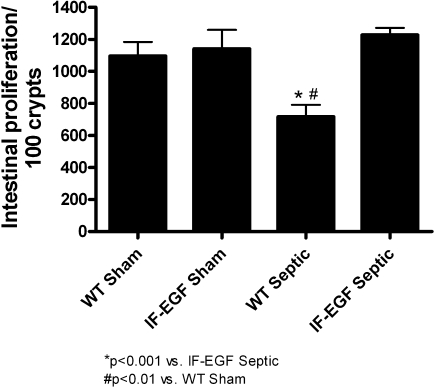
Sepsis induced a marked decrease in intestinal proliferation in WT mice as measured by BrdU-positive cells/100 crypts (Fig. 6, n = 7–12/group). In contrast, the proliferative response in IFABP-EGF mice was not affected by sepsis, since the number of BrdU-positive cells in IFABP-EGF septic mice was similar to that in IFABP-EGF sham mice.
Fig. 6.
Intestinal proliferation. S-phase cells were quantified in 100 contiguous crypts by 5-bromo-2′-deoxyuridine staining. WT septic mice had significantly decreased intestinal proliferation, whereas IFABP-EGF septic mice exhibited normalization to sham levels.
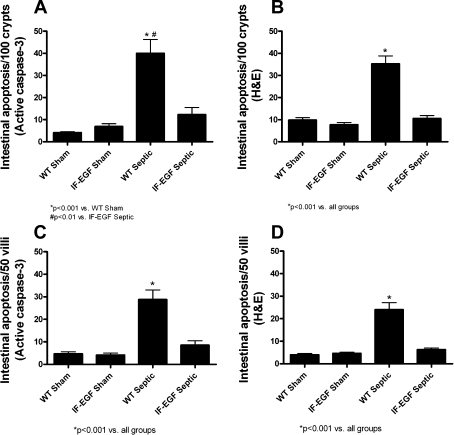
Sepsis also induced a marked increase in intestinal apoptosis in both the crypt and villus epithelium of WT mice as measured by the number of apoptotic cells per 100 crypts (Fig. 7, A and B, n = 10–14/group) and per 50 villi (Fig. 7, C and D, n = 10–14/group) by either active caspase-3 staining or H&E staining. In contrast, the intestinal apoptotic response in IFABP-EGF mice was not affected by sepsis, since the number of apoptotic cells quantified by either active caspase-3 staining or H&E staining in IFABP-EGF septic mice was similar to IFABP-EGF sham mice.
Fig. 7.
Intestinal epithelial apoptosis. Intestinal apoptosis was quantified in crypt (A and B) and villus (C and D) epithelium by active caspase-3 staining and H&E staining. WT septic mice exhibited increased apoptosis compared with shams by both methods. In contrast, IFABP-EGF septic mice had similar levels of apoptosis to sham animals by both methods.
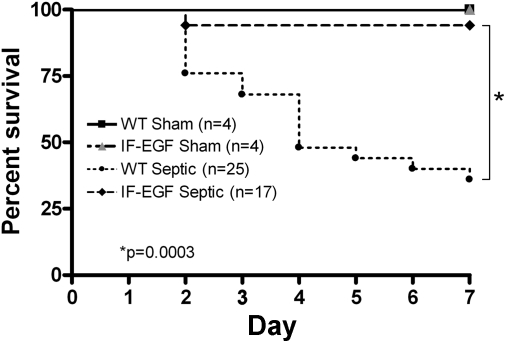
IFABP-EGF mice have a survival advantage in sepsis.
To determine whether enterocyte-specific overexpression of EGF confers a survival advantage in peritonitis-induced sepsis, a separate cohort of mice (n = 50) were subjected to CLP and followed for survival (Fig. 8). WT septic mice had a 64% 7-day mortality, whereas IFABP-EGF septic mice had a 6% 7-day mortality. All sham animals survived.
Fig. 8.
Effect of enterocyte-specific overexpression of EGF on mortality in sepsis. WT and IFABP-EGF mice were subjected to 2 × 23-gauge cecal ligation and puncture. Control animals underwent sham laparotomy. All mice were given antibiotics and followed for survival for 7 days. IFABP-EGF septic mice exhibited significantly improved survival compared with WT septic mice. All sham mice survived.
The local and systemic inflammatory response is similar in WT and IFABP-EGF septic mice.
To determine whether intestine-specific EGF induced secondary extraintestinal effects, a panel of pro- and anti-inflammatory cytokines was assayed in both the peritoneum and serum (Table 2, n = 4–7/group). Proinflammatory cytokines IL-6, MCP-1, and TNF all increased in septic animals. However, cytokine concentrations were similar between WT septic and IFABP-EGF septic mice. In contrast, neither sepsis nor the presence of intestine-specific EGF had a significant effect on the anti-inflammatory cytokine IL-10, although there was a modest increase in IFABP-EGF septic mice compared with IFABP-EGF sham mice in the peritoneal cavity but not in the serum. Of note, peritoneal and serum levels of IFN-γ and IL-12p70 were below the limit of detection for all groups (data not shown).
Table 2.
Cytokine levels in peritoneal fluid and serum
| WT Sham | IFABP-EGF Sham | WT Septic | IFABP-EGF Septic | |
|---|---|---|---|---|
| Peritoneal, pg/ml | ||||
| IL-6 | 27.2±7.3 | 10.7±3.9 | 3,176±1,769a | 3,905±1,789b |
| IL-10 | 21.5±2.7 | 17.5±0 | 83.4±38.6 | 80.4±21.0b |
| MCP-1 | 125.4±26.8 | 117.4±39.0 | 4,872±1,356a | 5,595±1,845c |
| TNF | 8.9±1.1 | 8.8±1.5 | 36.5±10.3a | 40.2±9.8c |
| Serum, pg/ml | ||||
| IL-6 | 30.7±6.3 | 16.4±3.3 | 1,510±502.4d | 1,443±440.8b |
| IL-10 | 17.5±0 | 17.5±0 | 25.0±7.5 | 33.8±10.6 |
| MCP-1 | 79.6±7.4 | 58.1±3.8 | 846.6±191.7a | 1,181±123.4e |
| TNF | 8.0±0.5 | 7.3±0 | 31.67±4.4a | 39.18±10.2c |
Data are expressed as means ± SE.
P < 0.05 vs. WT Sham;
P < 0.01 vs. IFABP-EGF Sham;
P < 0.05 vs. IFABP-EGF Sham,
P < 0.01 vs. WT Sham,
P < 0.001 vs. IFABP-EGF Sham.
A similar trend was noted when examining bacteria levels in both the peritoneal cavity and blood. Less than 10 CFU/ml bacteria were detectable in WT and IFABP-EGF sham mice in both the peritoneal cavity and blood. In contrast, marked increases in bacterial concentration were noted in the peritoneum of WT and IFABP-EGF septic mice (5.7 × 106 ± 2.2 × 106 CFU/ml and 5.0 × 106 ± 2.1 × 106 CFU/ml, respectively) and the blood of the same animals (1.5 × 106 ± 1.2 × 106 CFU/ml and 2.8 × 106 ± 1.9 × 106 CFU/ml, respectively). However, there were no statistically significant differences in culture levels between WT and IFABP-EGF septic mice (P > 0.05 for all, n = 5/group).
DISCUSSION
This study demonstrates that enterocyte-specific overexpression of EGF prevents peritonitis-induced intestinal injury and confers a significant survival advantage in mice subjected to CLP. Overexpression of enterocyte-specific EGF prevented intestinal hyperpermeability, as well as preventing an increase in claudin-2 expression with altered subcellular localization seen in WT septic mice. IFABP-EGF septic mice also had similar villus length, intestinal proliferation, and epithelial apoptosis to sham animals despite marked abnormalities of each in WT septic mice. EGF can act on a wide variety of cell types, and EGF-R is present in multiple tissues and organs, including vascular smooth muscle, liver, and kidney (18). However, IFABP-EGF septic mice had no detectable differences in peritoneal or systemic cytokines or bacterial burden compared with WT septic mice.
One possible way intestinal EGF could improve survival is by preventing peritonitis-induced intestinal hyperpermeability. Intestinal epithelial permeability is markedly increased in critical illness (26, 43, 56), and barrier failure has been postulated to be a major reason the intestine serves as the “motor” of the systemic inflammatory response. Importantly, EGF has previously been shown to play an important role in maintaining epithelial barrier integrity in other models of intestinal injury. Oral administration of EGF reduces intestinal paracellular permeability in a rat model of necrotizing enterocolitis (15). Additionally, in vitro studies using intestinal monolayers have demonstrated that EGF inhibits increased paracellular permeability (38, 47). Our results showing that enterocyte-specific overexpression of EGF prevented sepsis-induced intestinal hyperpermeability are consistent with these prior studies.
To evaluate the possible mechanisms of increased intestinal permeability in sepsis, tight junction protein expression was analyzed. EGF has been shown to prevent stress-induced hyperpermeability in a variety of cell types by altering expression and subcellular localization of claudin-2, claudin-3, and occludin (3, 4, 14, 46, 48, 49). Additionally, intestinal paracellular permeability is regulated, in part, by the ratio of claudin isoforms within the tight junction complex (25), and the pore-forming protein claudin-2 has been associated with increased paracellular permeability (19). Prior to the present study, claudins have not been evaluated for their roles in intestinal permeability in critical illness. However, decreased intestinal expression and altered subcellular distribution of occludin has been observed following endotoxemia (29), following bile duct ligation (55), and when intestinal epithelial cells are exposed to proinflammatory cytokines in vitro (28, 30). ZO-1 expression has also been found to be altered in hemorrhagic shock (54). We found that sepsis markedly increased levels of claudin-2, and this was associated with a change in subcellular localization. However, consistent with the observation that intestine-specific EGF prevented peritonitis-induced hyperpermeability, IFABP-EGF septic mice did not have any alterations in claudin-2 expression or localization compared with sham mice. This suggests that EGF's effects on intestinal barrier function are mediated, at least in part, via claudin-2. In contrast, EGF has been shown to decrease epithelial permeability by preventing disruption of claudin-4 in vitro (37). However, we found that claudin-4 was decreased in both septic WT and septic IFABP-EGF mice. Furthermore, despite studies suggesting a link between claudin-3 and occludin with either EGF-induced decreases in permeability in other tissues or intestinal hyperpermeability, neither claudin-3 nor occludin was affected by peritonitis or EGF in this study.
IFABP-EGF mice had a >20-fold increase in intestinal EGF gene expression compared with their WT counterparts that was unaffected by peritonitis. Additionally, overexpression of intestinal EGF had a profound effect on sepsis-induced intestinal EGF-R levels. Peritonitis induced a marked increase in intestinal EGF-R levels; however, EGF-R levels were similar in IFABP-EGF septic mice and shams. Under normal conditions, EGF-R activation is under tight regulation where EGF binding leads to rapid receptor internalization and lysosomal degradation (53). Aberrant increases in EGF-R expression or phosphorylation status have been associated with intestinal injury and may reflect dysfunctional receptor trafficking (10, 22), although experiments examining receptor trafficking and phosphorylation status were outside the scope of this study.
Since sepsis is a systemic disease, it was important to examine extraintestinal effects of EGF. This is especially true since oral administration of EGF has been shown to reduce bacterial colonization of the intestinal epithelium by Campylobacter jejuni in an animal model of bacterial enteritis (36). Additionally, intraluminal administration of heparin-binding EGF, an EGF family member, decreases systemic proinflammatory cytokine levels following ischemia-reperfusion injury (44). However, intestine-specific overexpression of EGF had minimal impact on both bacterial burden and cytokine levels following CLP in both blood and the peritoneal cavity. Since the differential mortality rates between WT and IFABP-EGF septic mice do not appear to be related to differences in severity of bacterial infection or inflammatory state, this underscores the importance of the intestinal epithelium in the pathophysiology of sepsis.
Although this study provides important mechanistic insights into the protective role of intestine-specific EGF in sepsis, it has a number of limitations. We did not examine EGF-R phosphorylation during sepsis or perform experiments examining EGF-R trafficking. Additionally, although no major differences were found in either bacterial burden or cytokines in either peritoneal fluid or blood, this does not conclusively preclude the possibility of differences in these parameters. It is possible that if we assayed mice at time points other than 24 h or looked at other cytokines that we would have found differences not detected by our study design. Finally, we were unable to detect any differences between WT and IFABP-EGF sham mice except for intestinal EGF expression. In light of the fact that EGF is known to be a potent mitogen, the absence of basal differences between WT and IFABP-EGF sham mice was surprising. We cannot rule out the possibility that true differences exist between these animals that were not detected within our study design.
Despite these limitations, this study demonstrates that enterocyte-specific overexpression of EGF confers a survival advantage in mice subjected to septic peritonitis. The beneficial effects of EGF are intestine specific and are associated with prevention of peritonitis-induced intestinal hyperpermeability via a claudin-2-mediated mechanism. Systemic EGF represents a potential novel therapeutic agent for the treatment of septic peritonitis, and in large part this appears due to the fact that the intestinal epithelium is a key target of EGF treatment.
GRANTS
This work was supported by funding from the National Institutes of Health (GM66202, GM072808, GM008795, GM082008, P30 DK52574).
Acknowledgments
We thank the Washington University Digestive Diseases Research Morphology Core.
REFERENCES
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 29: 1303–1310, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Baker CC, Chaudry IH, Gaines HO, Baue AE. Evaluation of factors affecting mortality rate after sepsis in a murine cecal ligation and puncture model. Surgery 94: 331–335, 1983. [PubMed] [Google Scholar]
- 3.Basuroy S, Seth A, Elias B, Naren AP, Rao R. MAPK interacts with occludin and mediates EGF-induced prevention of tight junction disruption by hydrogen peroxide. Biochem J 393: 69–77, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basuroy S, Sheth P, Mansbach CM, Rao RK. Acetaldehyde disrupts tight junctions and adherens junctions in human colonic mucosa: protection by EGF and l-glutamine. Am J Physiol Gastrointest Liver Physiol 289: G367–G375, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Berlanga J, Lodos J, Lopez-Saura P. Attenuation of internal organ damages by exogenously administered epidermal growth factor (EGF) in burned rodents. Burns 28: 435–442, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Berlanga J, Prats P, Remirez D, Gonzalez R, Lopez-Saura P, Aguiar J, Ojeda M, Boyle JJ, Fitzgerald AJ, Playford RJ. Prophylactic use of epidermal growth factor reduces ischemia/reperfusion intestinal damage. Am J Pathol 161: 373–379, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernal NP, Stehr W, Coyle R, Erwin CR, Warner BW. Epidermal growth factor receptor signaling regulates Bax and Bcl-w expression and apoptotic responses during intestinal adaptation in mice. Gastroenterology 130: 412–423, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Bradford MM A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976. [DOI] [PubMed] [Google Scholar]
- 10.Clark JA, Clark AT, Hotchkiss RS, Buchman TG, Coopersmith CM. Epidermal growth factor treatment decreases mortality and is associated with improved gut integrity in sepsis. Shock 30: 36–42, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clark JA, Coopersmith CM. Intestinal crosstalk: a new paradigm for understanding the gut as the “motor” of critical illness. Shock 28: 384–393, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clark JA, Doelle SM, Halpern MD, Saunders TA, Holubec H, Dvorak K, Boitano SA, Dvorak B. Intestinal barrier failure during experimental necrotizing enterocolitis: protective effect of EGF treatment. Am J Physiol Gastrointest Liver Physiol 291: G938–G949, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Clark JA, Doelle SM, Halpern MD, Saunders TA, Holubec H, Dvorak K, Boitano SA, Dvorak B. Intestinal barrier failure during experimental necrotizing enterocolitis: protective effect of EGF treatment. Am J Physiol Gastrointest Liver Physiol 291: G938–G949, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Clark JA, Doelle SM, Halpern MD, Saunders TA, Holubec H, Dvorak K, Boitano SA, Dvorak B. Intestinal barrier failure during experimental necrotizing enterocolitis: protective effect of EGF treatment. Am J Physiol Gastrointest Liver Physiol 291: G938–G949, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Clark JA, Lane RH, Maclennan NK, Holubec H, Dvorakova K, Halpern MD, Williams CS, Payne CM, Dvorak B. Epidermal growth factor reduces intestinal apoptosis in an experimental model of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol 288: G755–G762, 2005. [DOI] [PubMed] [Google Scholar]
- 17a.ClinicalTrials.gov. A service of the US National Institutes of Health. http://clinicaltrials.gov/ct2/results?term=epidermal±growth±factor [accessed Jan. 7, 2009].
- 18.Cohen S The epidermal growth factor (EGF). Cancer 51: 1787–1791, 1983. [DOI] [PubMed] [Google Scholar]
- 19.Colegio OR, Van IC, Rahner C, Anderson JM. Claudin extracellular domains determine paracellular charge selectivity and resistance but not tight junction fibril architecture. Am J Physiol Cell Physiol 284: C1346–C1354, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Coopersmith CM, Stromberg PE, Dunne WM, Davis CG, Amiot DM, Buchman TG, Karl IE, Hotchkiss RS. Inhibition of intestinal epithelial apoptosis and survival in a murine model of pneumonia-induced sepsis. JAMA 287: 1716–1721, 2002. [DOI] [PubMed] [Google Scholar]
- 21.De-Souza DA, Greene LJ. Intestinal permeability and systemic infections in critically ill patients: effect of glutamine. Crit Care Med 33: 1125–1135, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Dvorak B, Halpern MD, Holubec H, Williams CS, McWilliam DL, Dominguez JA, Stepankova R, Payne CM, McCuskey RS. Epidermal growth factor reduces the development of necrotizing enterocolitis in a neonatal rat model. Am J Physiol Gastrointest Liver Physiol 282: G156–G164, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Dvorak B, Halpern MD, Holubec H, Williams CS, McWilliam DL, Dominguez JA, Stepankova R, Payne CM, McCuskey RS. Epidermal growth factor reduces the development of necrotizing enterocolitis in a neonatal rat model. Am J Physiol Gastrointest Liver Physiol 282: G156–G164, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Erwin CR, Helmrath MA, Shin CE, Falcone RA Jr, Stern LE, Warner BW. Intestinal overexpression of EGF in transgenic mice enhances adaptation after small bowel resection. Am J Physiol Gastrointest Liver Physiol 277: G533–G540, 1999. [DOI] [PubMed] [Google Scholar]
- 25.Furuse M, Furuse K, Sasaki H, Tsukita S. Conversion of zonulae occludentes from tight to leaky strand type by introducing claudin-2 into Madin-Darby canine kidney I cells. J Cell Biol 153: 263–272, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gatt M, Reddy BS, MacFie J. Review article: bacterial translocation in the critically ill—evidence and methods of prevention. Aliment Pharmacol Ther 25: 741–757, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Halpern MD, Dominguez JA, Dvorakova K, Holubec H, Williams CS, Meza YG, Ruth MC, Dvorak B. Ileal cytokine dysregulation in experimental necrotizing enterocolitis is reduced by epidermal growth factor. J Pediatr Gastroenterol Nutr 36: 126–133, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Han X, Fink MP, Delude RL. Proinflammatory cytokines cause NO*-dependent and -independent changes in expression and localization of tight junction proteins in intestinal epithelial cells. Shock 19: 229–237, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Han X, Fink MP, Yang R, Delude RL. Increased iNOS activity is essential for intestinal epithelial tight junction dysfunction in endotoxemic mice. Shock 21: 261–270, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Han X, Uchiyama T, Sappington PL, Yaguchi A, Yang R, Fink MP, Delude RL. NAD+ ameliorates inflammation-induced epithelial barrier dysfunction in cultured enterocytes and mouse ileal mucosa. J Pharmacol Exp Ther 307: 443–449, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Hassoun HT, Kone BC, Mercer DW, Moody FG, Weisbrodt NW, Moore FA. Post-injury multiple organ failure: the role of the gut. Shock 15: 1–10, 2001. [DOI] [PubMed] [Google Scholar]
- 32.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med 348: 138–150, 2003. [DOI] [PubMed] [Google Scholar]
- 33.Hotchkiss RS, Swanson PE, Freeman BD, Tinsley KW, Cobb JP, Matuschak GM, Buchman TG, Karl IE. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit Care Med 27: 1230–1251, 1999. [DOI] [PubMed] [Google Scholar]
- 34.Husain KD, Stromberg PE, Woolsey CA, Turnbull IR, Dunne WM, Javadi P, Buchman TG, Karl IE, Hotchkiss RS, Coopersmith CM. Mechanisms of decreased intestinal epithelial proliferation and increased apoptosis in murine acute lung injury. Crit Care Med 33: 2350–2357, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jones MK, Tomikawa M, Mohajer B, Tarnawski AS. Gastrointestinal mucosal regeneration: role of growth factors. Front Biosci 4: D303–D309, 1999. [DOI] [PubMed] [Google Scholar]
- 36.Lamb-Rosteski JM, Kalischuk LD, Inglis GD, Buret AG. Epidermal growth factor inhibits Campylobacter jejuni-induced claudin-4 disruption, loss of epithelial barrier function, and Escherichia coli translocation. Infect Immun 76: 3390–3398, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lamb-Rosteski JM, Kalischuk LD, Inglis GD, Buret AG. Epidermal growth factor inhibits Campylobacter jejuni-induced claudin-4 disruption, loss of epithelial barrier function, and Escherichia coli translocation. Infect Immun 76: 3390–3398, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lamb-Rosteski JM, Kalischuk LD, Inglis GD, Buret AG. Epidermal growth factor inhibits Campylobacter jejuni-induced claudin-4 disruption, loss of epithelial barrier function, and Escherichia coli translocation. Infect Immun 76: 3390–3398, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Q, Djuricin G, Nathan C, Gattuso P, Weinstein RA, Prinz RA. The effect of epidermal growth factor on the septic complications of acute pancreatitis. J Surg Res 69: 171–177, 1997. [DOI] [PubMed] [Google Scholar]
- 40.Mainous MR, Ertel W, Chaudry IH, Deitch EA. The gut: a cytokine-generating organ in systemic inflammation? Shock 4: 193–199, 1995. [PubMed] [Google Scholar]
- 41.Neal MD, Leaphart C, Levy R, Prince J, Billiar TR, Watkins S, Li J, Cetin S, Ford H, Schreiber A, Hackam DJ. Enterocyte TLR4 mediates phagocytosis and translocation of bacteria across the intestinal barrier. J Immunol 176: 3070–3079, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Osterberg J, Ljungdahl M, Haglund U. Influence of cyclooxygenase inhibitors on gut immune cell distribution and apoptosis rate in experimental sepsis. Shock 25: 147–154, 2006. [DOI] [PubMed] [Google Scholar]
- 43.Raman KG, Sappington PL, Yang R, Levy RM, Prince JM, Liu S, Watkins SK, Schmidt AM, Billiar TR, Fink MP. The role of RAGE in the pathogenesis of intestinal barrier dysfunction after hemorrhagic shock. Am J Physiol Gastrointest Liver Physiol 291: G556–G565, 2006. [DOI] [PubMed] [Google Scholar]
- 44.Rocourt DV, Mehta VB, Besner GE. Heparin-binding EGF-like growth factor decreases inflammatory cytokine expression after intestinal ischemia/reperfusion injury. J Surg Res 139: 269–273, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Russell JA Management of sepsis. N Engl J Med 355: 1699–1713, 2006. [DOI] [PubMed] [Google Scholar]
- 46.Sheth P, Seth A, Thangavel M, Basuroy S, Rao RK. Epidermal growth factor prevents acetaldehyde-induced paracellular permeability in Caco-2 cell monolayer. Alcohol Clin Exp Res 28: 797–804, 2004. [DOI] [PubMed] [Google Scholar]
- 47.Sheth P, Seth A, Thangavel M, Basuroy S, Rao RK. Epidermal growth factor prevents acetaldehyde-induced paracellular permeability in Caco-2 cell monolayer. Alcohol Clin Exp Res 28: 797–804, 2004. [DOI] [PubMed] [Google Scholar]
- 48.Singh AB, Harris RC. Epidermal growth factor receptor activation differentially regulates claudin expression and enhances transepithelial resistance in Madin-Darby canine kidney cells. J Biol Chem 279: 3543–3552, 2004. [DOI] [PubMed] [Google Scholar]
- 49.Singh AB, Sugimoto K, Dhawan P, Harris RC. Juxtacrine activation of EGFR regulates claudin expression and increases transepithelial resistance. Am J Physiol Cell Physiol 293: C1660–C1668, 2007. [DOI] [PubMed] [Google Scholar]
- 50.Vyas D, Robertson CM, Stromberg PE, Martin JR, Dunne WM, Houchen CW, Barrett TA, Ayala A, Perl M, Buchman TG, Coopersmith CM. Epithelial apoptosis in mechanistically distinct methods of injury in the murine small intestine. Histol Histopathol 22: 623–630, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang L, Srinivasan S, Theiss AL, Merlin D, Sitaraman SV. Interleukin-6 induces keratin expression in intestinal epithelial cells: potential role of keratin-8 in interleukin-6-induced barrier function alterations. J Biol Chem 282: 8219–8227, 2007. [DOI] [PubMed] [Google Scholar]
- 52.Wang Q, Fang CH, Hasselgren PO. Intestinal permeability is reduced and IL-10 levels are increased in septic IL-6 knockout mice. Am J Physiol Regul Integr Comp Physiol 281: R1013–R1023, 2001. [DOI] [PubMed] [Google Scholar]
- 53.Wiley HS Trafficking of the ErbB receptors and its influence on signaling. Exp Cell Res 284: 78–88, 2003. [DOI] [PubMed] [Google Scholar]
- 54.Yang R, Han X, Uchiyama T, Watkins SK, Yaguchi A, Delude RL, Fink MP. IL-6 is essential for development of gut barrier dysfunction after hemorrhagic shock and resuscitation in mice. Am J Physiol Gastrointest Liver Physiol 285: G621–G629, 2003. [DOI] [PubMed] [Google Scholar]
- 55.Yang R, Harada T, Li J, Uchiyama T, Han Y, Englert JA, Fink MP. Bile modulates intestinal epithelial barrier function via an extracellular signal related kinase 1/2 dependent mechanism. Intensive Care Med 31: 709–717, 2005. [DOI] [PubMed] [Google Scholar]
- 56.Yang R, Harada T, Mollen KP, Prince JM, Levy RM, Englert JA, Gallowitsch-Puerta M, Yang L, Yang H, Tracey KJ, Harbrecht BG, Billiar TR, Fink MP. Anti-HMGB1 neutralizing antibody ameliorates gut barrier dysfunction and improves survival after hemorrhagic shock. Mol Med 12: 105–114, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]