Abstract
The rates of oxidation of glycine and ureagenesis were quantified in the basal state and in response to an intravenous infusion of intralipid with heparin (IL) in healthy subjects (n = 8) and in subjects with nonalcoholic steatohepatitis (NASH) (n = 6). During fasting, no significant difference in weight-specific rate of appearance (Ra) of glycine, glycine oxidation, and urea synthesis was observed. Intralipid infusion resulted in a significant increase in plasma β-hydroxybutyrate in both groups. The correlation between free fatty acids and β-hydroxybutyrate concentration in plasma was 0.94 in NASH compared with 0.4 in controls, indicating greater hepatic fatty acid oxidation in NASH. Intralipid infusion resulted in a significant decrease in urea synthesis and glycine Ra in both groups and did not impact glycine oxidation. The fractional contribution of glycine carbon to serine was lower in subjects with NASH before and after IL infusion. In contrast, the fractional contribution of serine carbon to cystathionine was higher in NASH before and following IL infusion. These results suggest that hepatic fatty acid oxidation is higher in NASH compared with controls and that glycine oxidation and urea synthesis are not altered. An increase in oxidative stress, induced by a higher rate of fatty acid oxidation in NASH, may have caused an increase in the contribution of serine to cystathionine to meet the higher demands for glutathione.
Keywords: glycine cleavage system, glutathione, fatty acid oxidation, ketones, serine, glycine, mitochondrial function, nonalcoholic fatty liver disease
abnormalities in hepatic mitochondrial function have been associated with hepatocellular injury in a number of disorders (5, 22, 29, 30, 37, 38). Specifically in relation to nonalcoholic fatty liver disease (NAFLD), there is growing evidence suggesting a role of mitochondrial dysfunction in the pathophysiology of nonalcoholic steatohepatitis (NASH) in humans and animal models (8, 33, 37, 40, 51). In humans, mitochondrial dysfunction has been based on the demonstration of crystalline inclusions in the mitochondria and the presence of mega mitochondria in liver biopsy, decreased activity of the respiratory chain complex in vitro, evidence of increased generation of reactive oxygen species (ROS) and oxidative stress, and a delay in the regeneration of ATP following a load of oral fructose (5, 6, 35, 44). Perturbations in mitochondrial oxidation in NASH have been suggested by 13C-labeled methionine and octanoate breath tests (3, 32). The impact of NASH on other mitochondrial metabolic pathways such as glycine cleavage system and urea synthesis have not been examined in humans.
Glycine, a dispensable amino acid, participates in a number of metabolic processes in humans and animals as a contributor to the 1-carbon pool, as a precursor for serine, as a component of glutathione, and as a substrate in purine and protein synthesis. In mammals, glycine is metabolized via the glycine cleavage system (EC.2.1.2.10) present in the inner mitochondrial membrane (23). The glycine cleavage system catalyzes the oxidative cleavage of glycine to CO2, NH4+, and a methyl group that is transferred to tetrahydrofolate (Fig. 1). The enzyme is expressed in the liver, kidney, and other tissues in mammals, and its activity is reported to be highest in the liver. Because glycine cleavage system is a hepatic mitochondrial enzyme and NAD is a cofactor for this reaction, mitochondrial dysfunction and change in hepatic redox could impact the activity of this enzyme.
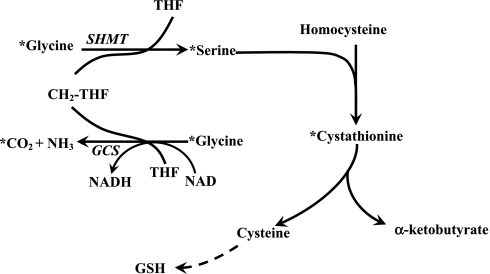
Fig. 1.
The metabolism of glycine in vivo. As shown, glycine is catalyzed by glycine cleavage system (GCS) to yield CO2, ammonia, and formation of N5N10 methylene-tetrahydrofolate (THF). Glycine can also form serine catalyzed by serine hydroxymethyltransferase (SHMT), wherein a methyl group is obtained from N5N10 methylene tetrahydrofolate. Serine can condense with homocysteine to form cystathionine, the precursor for cysteine and α-ketobutyrate. GSH, glutathione. *Represents the pathway for labeling of serine and cystathionine following [1-13C]glycine tracer infusion.
Ureagenesis is a bicompartmental cycle in the liver initiated in the mitochondria and completed in the cytosol. The effect of mitochondrial dysfunction on hepatic ureagenesis has not been examined. We hypothesized that, as a result of hepatic mitochondrial dysfunction in NASH, the rate of ureagenesis may be attenuated.
We also examined the effect of increasing plasma free fatty acids (FFA) on glycine cleavage system and urea synthesis by infusing Intralipid with heparin. Plasma fatty acids are rapidly taken up by the liver, enter the β-oxidation pathway, and are converted to ketones. The hepatic mitochondrial redox state is reduced as a result of an increased generation of NADH and FADH2 during β-oxidation. In addition, fatty acid oxidation increases ROS production by slowing the rate of electron flow through the respiratory chain by interacting with the subunit structure of complex I and by the release of cytochrome c from the inner membrane of the mitochondria (1, 46).
In the present study, we have quantified the rates of oxidation of glycine and of urea synthesis in the basal state in subjects with NASH who had been confirmed by liver biopsy and compared it with healthy controls. The effect of an increase in the concentration of FFA in the plasma on the levels of glutathione in the plasma, glycine cleavage system, urea synthesis, and the incorporation of glycine carbon into serine and cystathionine were quantified.
MATERIALS AND METHODS
Sterile, pyrogen-free [1-13C]glycine and [15N2]urea were obtained from Cambridge Isotope Laboratory (Andover, MA). Intralipid 20% was manufactured by Fresenius, Kabi, Sweden.
Glycine kinetics were quantified in six subjects with NASH and compared with eight, age-matched healthy controls (Table 1). As anticipated, subjects with NASH had a higher body mass index (BMI), greater insulin resistance, as measured by homeostatic model of insulin resistance (HOMA), and had higher plasma alanine aminotransferase and triglyceride levels. NASH was confirmed by liver biopsy.
Table 1.
Study population
| Age, yr | Weight, kg | BMI, kg/m2 | HOMA | ALT, U/dl | Triglycerides, mg/dl | usCRP, mg/l | |
|---|---|---|---|---|---|---|---|
| Controls (n = 8, M/F = 3/5) | 40.1±17.8 (19–68) | 79.7±22.6 (61.9–131.7) | 27.9±6.8 (21.4–43.2) | 0.69±0.23 (0.5–1.0) | 14.8±4.9 (6–22) | 90.8±34.2 (49–134) | 1.33±1.73 (0.1–5.0) |
| NASH (n = 6, M/F =4/2) | 41.3±7.1 (34–52) | 96.7±19.4 (90.7–127.1) | 33.4±4.4 (25.9–39.2) | 3.6±2.36* (1.1–6.8) | 50.8±22.9* (33–92) | 235.5±132.4* (95–479) | 3.55±2.12* (2.2–7.8) |
All applicable data are means ± SD.
Statistically different, P < 0.05. BMI, body mass index; HOMA, homeostatic model of insulin resistance; ALT, plasma alanine aminotransferase; Triglycerides, plasma triglycerides; usCRP: ultrasensitive C-reactive protein; NASH, nonalcoholic steatohepatitis.
Healthy controls were recruited by advertisement. A detailed clinical history was obtained on each subject, followed by a physical examination. Hepatic steatosis was excluded by ultrasound examination (performed by the same investigator, S. Dasarathy) and by the presence of normal plasma transaminase levels.
The study protocol was approved by the Institutional Review Board (IRB) of the Cleveland Clinic. Written, informed consent was obtained from all subjects after having the procedure fully explained.
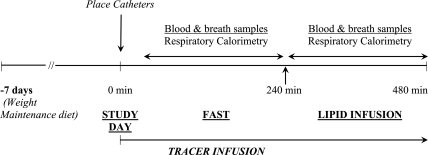
All subjects were placed on a weight maintenance diet containing at least 75 g of protein/day for 7 days before the tracer study. Dietary compliance was monitored by a clinical nutritionist. The study design is displayed in Fig. 2. Tracer kinetic studies were performed in the Clinical Research Unit of the Cleveland Clinic (NIH, CTSA, Case Western Reserve University). Subjects reported to the unit at 7:00 AM, following a 12-h overnight fast. Their weight, height, and vital signs were measured. Two indwelling cannulae were placed in the dorsal vein of each hand, one for tracer infusion and the other to obtain blood samples. The sampling site was kept warm by placing the hand in a thermostat-controlled warm blanket. Accurately weighed amounts of [1-13C]glycine and [15N2]urea were dissolved aseptically in a sterile 0.45% saline solution in a laminar flow hood. Tracer solutions were sterilized by filtering through a 0.22-micron filter (Millex; Millipore, Bedford, MA). The tracers were administered as prime-constant rate infusion for 8 h. Their respective doses were as follows: [1-13C]glycine, prime = 16 μmol/kg, constant rate infusion = 16 μmol·kg−1·h−1; [15N2]urea, prime = 33 μmol/kg, constant rate infusion = 3 μmol·kg−1·h−1. The actual rate of infusion was confirmed by gravimetrically measuring the rate of flow using the same tubing and equipment at the end of the study. The concentration of glycine and urea in the infusate were measured as described in Analytical methods. All tracer solutions were tested in the hospital clinical laboratory and were found to be sterile. Blood samples (6 ml), in EDTA- and heparin-coated tubes, were obtained at time 0, before tracer infusion, and at 30-min intervals for 4 h. Following the basal study (4 h), the response to intravenous lipid infusion was examined. Triglyceride solution (20%) (Intralipid) with heparin (0.2 U/kg) was infused at 40 ml/h to increase the plasma free fatty acid concentration by ∼750 μmol/l. Intralipid contains 50% linolate, 26.5% oleate, 10.5% palmitate, and 3.5% stearate, as stated by the manufacturer. Blood samples were obtained at 20-min intervals throughout the lipid infusion.
Fig. 2.
Study design. Subjects were placed on a weight-maintenance diet (minimum protein intake 75 g/d) for 7 days before the study. On the study day, tracer isotopes [1-13C]glycine and [15N2]urea were infused as prime constant rate infusion for 8 h after an overnight fast. After the initial 4 h of tracer infusion, 20% intralipid with heparin was infused for 4 h. Blood and breath samples were obtained, and respiratory calorimetry measurements were performed as detailed in materials and methods.
Plasma was separated immediately by centrifugation in cold and stored at −80°C. Lipolytic inhibitor was not used. The rates of CO2 production and O2 consumption were measured using an open hood system (Viasys Encore, Cardinal Health, Dublin, OH). Breath samples were obtained by having the subject breathe into an anesthesia bag via a one-way Rudolph valve. An aliquot of the expired air was transferred to the sampling tube for the measurement of 13C enrichment in the expired CO2.
Analytical methods.
13C enrichment of glycine, serine, and cystathionine and 15N enrichment of urea were measured using gas chromatography (GC)-mass spectrometry. Urea and amino acids in the plasma were separated using mixed-bed ion exchange chromatography as described previously (19). [15N2] enrichment of urea was measured as described (48). An N-propyl-n-acetyl ester derivative of glycine and serine was prepared according to the method of Adams (2), with certain modifications (20). The amino acid derivatives were separated on a GC-mass spectrometry system (6890N GC System coupled with 5975 mass selective detector; Agilent Technologies, Santa Clara, CA) using a Supelco Wax-10-fused silica capillary column (30 m × 0.25 mm × 0.25 μm). The injector port temperature and auxiliary temperatures were 250°C. The oven temperature ramp was set as follows: the initial oven temperature was 85°C and was increased to 200°C for 2 min at the rate of 30°C per minute, then to 220°C for 4 min at 30°C per minute, and finally to 250°C for 3 min, at the rate of 50°C per minute. Glycine eluted at 8.5 min and serine at 10.6 min. Positive chemical ionization and selected ion monitoring were used to monitor mass-to-charge ratio (m/z) for ions 160 and 161 representing unlabeled (m0) and 13C-labeled (m1) glycine and m/z 232 and 233 for unlabeled (m0) and labeled (m1) serine. Cystathionine enrichment was quantified as described by Davis and colleagues (7). m/z 678 and 679 were monitored in the negative chemical ionization mode using an Agilent GC-mass spectrometer system. 13C enrichment of expired CO2 was measured using an isotope ratio mass spectrometer (by Metabolic Solutions, Nashua, NH). Plasma concentration of homocysteine, cysteine, and glutathione were measured by high-pressure liquid chromatography (HPLC) (10). Plasma concentration of betaine, dimethylglycine, and choline was measured by LC-MS-MS (14). Amino acid concentration in plasma was measured by HPLC using an OPA derivative and a fluorescence detector as described (20). Plasma FFAs were measured colorimetrically (28), and β-hydroxybutyrate concentration was measured enzymatically using a spectrofluorometer (36). Total, free, and acyl-carnitines in the plasma were measured by HPLC electrospray ionization mass spectrometry (33). Ultrasensitive C-reactive protein (CRP) concentration was measured in the clinical laboratory using a commercial kit. Total antioxidant status in the plasma was measured before and after intralipid infusion using a RANDOX Kit (Randox Laboratories, Crumlin, UK).
Calculations.
The rate of appearance (Ra) of glycine and urea were calculated during the steady state using tracer dilution equations (49) Ra = I × [(EI/EP) − 1], where Ra = rate of appearance, μmol·kg−1·h−1, I is the rate of infusion of tracer (μmol·kg−1·h−1), and EI and EP are the respective enrichments of the tracer infused and that of the tracer in the plasma. The rate of oxidation of glycine was calculated from the appearance of 13C in expired CO2 (19, 21). The contributions of glycine to serine and cystathionine were calculated using precursor-product relationship.
Statistics.
All data are reported as means ± SD. Quantitative and rating variables were compared by the Student's t-test for data that had a normal distribution. Skewed data were compared using the Mann-Whitney test. Comparisons of data in the same subject during the fasted state and in response to intralipid infusion were done using the paired t-test or the Wilcoxon signed rank test. Association between variables was determined by the Pearson's correlation coefficient. A value of P < 0.05 was considered statistically significant.
RESULTS
Eight healthy controls and six subjects with NASH that had been established by biopsy were studied. The study subjects were matched for age. All subjects with NASH were obese, had high BMI and higher HOMA values, and were not diabetic (Table 1). Their fasting plasma glucose concentration was not different from that of the controls (controls: 82.9 ± 14.1 mg/dl, NASH 95.8 ± 10.1 mg/dl, P = 0.07); however, their plasma insulin concentrations were significantly higher (NASH: 29.5 ± 2.0 μU/ml vs. Controls: 5.3 ± 1.8 μU/ml, P < 0.05). One subject in the healthy control group was obese (BMI: 43.2 kg/m2) but did not have hepatic steatosis on ultrasound examination and had plasma transaminase levels in the normal range. The plasma levels of ultrasensitive CRP were significantly higher in patients with NASH compared with healthy controls (Table 1).
The respiratory calorimetry measurements are displayed in Table 2. There was no difference in the rate of oxygen consumption, CO2 production, and respiratory exchange ratio in the basal state or in response to intralipid infusion between the groups.
Table 2.
Respiratory calorimetry
|
VO2, mmol·kg−1·h−1 |
VCO2, mmol·kg−1·h−1
|
RER
|
||||
|---|---|---|---|---|---|---|
| Fast | Intralipid | Fast | Intralipid | Fast | Intralipid | |
| Controls (n = 8) | 7.04±1.36 | 7.23±1.31 | 5.73±1.18 | 6.04±1.12 | 0.82±0.05 | 0.80±0.04 |
| NASH (n = 6) | 7.46±0.97 | 7.76±0.79 | 6.03±0.39 | 6.01±0.39 | 0.82±0.07 | 0.79±0.06 |
All data are means ± SD. VO2, rate of oxygen consumption; VCO2, rate of production of carbon dioxide; RER: respiratory exchange ratio.
Plasma FFA and β-hydroxybutyrate concentration.
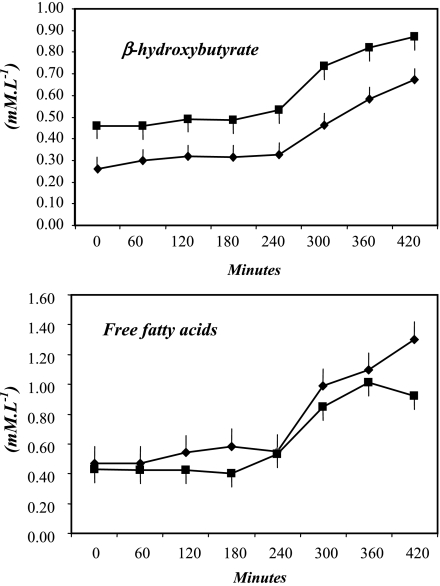
The plasma FFA concentration (mmol/l) were not significantly different between healthy controls and subjects with NASH (controls 0.54 ± 0.17, NASH 0.45 ± 0.1; means ± SD) during the basal period. Infusion of Intralipid resulted in an increase in FFA levels in both groups (Fig. 3). At 4 h of intralipid infusion, the concentration of FFA was not significantly different between the two groups (controls 1.32 ± 0.38, NASH 1.07 ± 0.19).
Fig. 3.
Plasma concentrations of free fatty acids and β-hydroxybutyrate in the basal state (0–240 min) and during intralipid plus heparin infusion (240–480 min). Controls = ⧫, NASH = ▪.
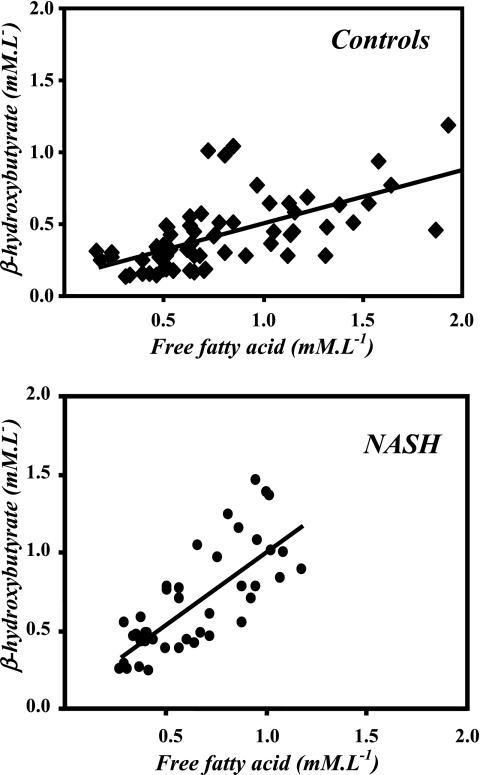
In the basal state, the plasma concentration of β-hydroxybutyrate was not significantly different in subjects with NASH compared with control subjects (control 0.31 ± 0.13 mmol/l, NASH 0.49 ± 0.19 mmol/l, P = 0.06). As a result of infusion of fatty acids, there was a significant increase in plasma β-hydroxybutyrate concentration in both groups (at 480 min, controls 0.73 ± 0.27 μmol/l, NASH 0.98 ± 0.41 μmol/l). A significant correlation between plasma FFA and β-hydroxybutyrate concentrations was evident in both controls and in subjects with NASH (controls: y = 0.38x + 0.13; r2 = 0.43, P < 0.0001; NASH: y = 0.94x + 0.07, r2 = 0.58, P < 0.0001). The greater slope of this correlation in subjects with NASH suggests a higher rate of synthesis of β-hydroxybutyrate (Fig. 4).
Fig. 4.
Relation between plasma levels of free fatty acids and β-hydroxybutyrate in healthy controls (y = 0.38x + 0.13; r2 = 0.43) and patients with nonalcoholic steatohepatitis (NASH) (y = 0.94x + 0.07, r2 = 0.58).
The plasma concentrations of total carnitines, free carnitines, and acylcarnitines were not different in controls and in NASH in the basal state or following intralipid infusion (Table 3). There was a significant decrease in free carnitine levels and a significant increase in acylcarnitine levels following intralipid infusion in both groups.
Table 3.
Effect of intralipids on plasma carnitine concentration
|
Total Carnitine |
Free Carnitine
|
Acylcarnitine
|
||||
|---|---|---|---|---|---|---|
| Fast | IL | Fast | IL | Fast | IL | |
| Controls (n = 7) | 47.6±13.1 | 45.5±12.6 | 37.8±11.2 | 32.2±9.7* | 8.1±2.6 | 11.8±3.4† |
| NASH (n = 6) | 46.2±8.0 | 45.4±6.3 | 37.7±6.8 | 32.5±5.4* | 7.1±2.0 | 10.5±2.4† |
Data are means ± SD, in micromoles per liter. IL = during intralipid infusion at 480 min;
P < 0.01 compared with fast, paired t-test;
P < 0.05 compared with fast, paired t-test.
Urea kinetics.
A steady-state [15N] enrichment in the plasma urea pool was achieved in all subjects between 2 and 3 h. The 15N enrichment (m2) of urea and the calculated rate of urea synthesis are displayed in Table 4. During fasting, there was no significant difference in the weight-specific or total rate of urea synthesis between the two groups. There was an increase in (m2) enrichment and therefore a decrease in the rate of synthesis of urea, in both healthy controls and in subjects with NASH immediately in response to an increase in plasma FFAs. The magnitude of decrease in urea Ra was similar in both NASH subjects and healthy controls.
Table 4.
Effect of intralipid on urea kinetics
| [15N2]urea, μmol·kg−1·h−1 |
Urea (m2), mol% excess |
Urea Ra, μmol·kg−1·h−1
|
Urea Ra, mmol/h
|
||||
|---|---|---|---|---|---|---|---|
| Fast | IL | Fast | IL | Fast | IL | ||
| Controls (n = 8) | 3.5±1.0 | 1.27±0.60 | 1.44±0.66 | 316.6±132.9 | 249.1±95.7* | 21.8±7.2 | 18.6±4.9* |
| NASH (n = 6) | 3.2±0.2 | 1.18±0.21 | 1.38±0.22 | 257.6±47.8 | 217.8±33.5* | 29.1±9.9 | 24.9±8.8* |
All data are means ± SD. Following a basal study (fast, 0–240 min), intralipid with heparin was infused from 240 to 480 min. IL represents the time during intralipid infusion (440–480 min).
Paired t-test, IL vs. fast, <0.01. I = rate of infusion of [15N2]urea tracer.
Glycine kinetics.
The rate of appearance (Ra) of glycine (on the basis of body weight) after an overnight fast was lower in subjects with NASH compared with the healthy controls (NASH 103.5 ± 10.7 vs. controls 133.1 ± 33.0 μmol·kg−1·h−1; P = <0.05) (Table 5). However, this difference was entirely due to the greater weight of the subjects with NASH so that the total rate of appearance of glycine (mmol/h) was not different in the two groups. Intralipid infusion resulted in a significant decrease in the rate of appearance of glycine (mmol/h) in both healthy controls and in subjects with NASH. The rate of oxidation of glycine, as determined by the rate of appearance of 13C of glycine in expired CO2, was 20–25% of the glycine Ra, or ∼26 μmol·kg−1·h−1 and was not different between the two groups. Intralipid infusion did not effect the rate of oxidation of glycine in either group.
Table 5.
Effect of intralipid on glycine kinetics
|
Ra, μmol·kg−1·h−1 |
Ra, mmol/h
|
Glycine Oxidation, %
|
Rate of Glycine Oxidation, μmol·kg−1·h−1
|
|||||
|---|---|---|---|---|---|---|---|---|
| Fast | IL | Fast | IL | Fast | IL | Fast | IL | |
| Control (n = 8) | 133.1±33.0 | 125.2±32.5* | 10.7±2.1 | 9.8±1.9* | 20.4±5.6 | 22.0±2.5 | 26.4±6.3 | 27.1±5.6 |
| NASH (n = 6) | 103.5±10.7† | 93.7±6.7 | 9.9±1.9 | 9.0±1.6* | 24.3±2.7 | 27.2±2.7 | 26.8±1.8 | 28.3±5.0 |
All data are means ± SD. IL = during intralipid infusion (440–480 min).
P < 0.01 compared with fast, paired t-test;
P < 0.05 compared with controls.
Contribution of glycine C to serine and cystathionine.
The 13C enrichment (m1) of glycine, of serine, and of cystathionine are displayed in Table 6. The contribution of glycine to serine and of serine to cystathionine was estimated by comparing their respective (m1) enrichments. The fractional contribution of glycine to serine was significantly lower in subjects with NASH, both in the basal state and during intralipid infusion (P < 0.03).
Table 6.
Effect of intralipid on serine and cystathionine enrichment during [1-13C]glycine tracer infusion
|
Glycine, m1, mol % excess |
Serine, m1, mol % excess
|
Cystathionine, m1, mol % excess
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Fast | IL1 | IL2 | Fast | IL1 | IL2 | Fast | IL1 | IL2 | |
| Control (n = 8) | 10.9±2.1 | 11.6±2.5 | 11.7±2.6 | 2.2±0.3 | 2.7±0.5 | 2.8±0.5 | 0.6±0.2 | 0.9±0.4 | 0.8±0.3 |
| NASH (n = 6) | 13.5±1.3 | 14.2±1.4 | 14.3±1.2 | 2.3±0.1 | 2.7±0.2 | 2.8±0.2 | 0.7±0.2 | 1.0±0.3 | 1.2±0.2 |
|
Serine from Glycine, % |
Cystathionine from Serine, % | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Controls | 20.3±3.4 | 23.7±3.8* | 24.3±4.1* | 26.7±10.2 | 36.7±13.8* | 34.3±13.4§ | |||
| NASH | 16.9±1.6‡ | 18.7±2.9‡ | 19.1±2.1‡ | 30.1±9.2 | 40.2±10.9§ | 43.5±8.9† | |||
All data are means ± SD. IL1 (early), 360–420 min; IL2 (late), 440–480 min. Statistically different compared with fast
P < 0.005;
P < 0.01;
P < 0.001; statistically different compared with controls
P < 0.03.
The fraction of cystathionine derived from serine was not different in the basal state between the two groups. Intralipid infusion resulted in a significant increase in the fractional contribution of serine to cystathionine in both groups. However, the increase was greater (13.7% vs. 7.2%, P = 0.04) in subjects with NASH, when compared with controls.
Other measurements.
There was no significant difference in the plasma concentration of amino acids during fasting between controls and NASH (Table 7). There was no significant change in plasma amino acids in response to infusion of Intralipid.
Table 7.
Plasma amino acid concentration, μmol/l
|
CONTROL |
NASH
|
|||
|---|---|---|---|---|
| Basal | IL | Basal | IL | |
| Aspartate | 6.00±1.15 | 6.67±1.21 | 7.2±1.15 | 6.8±1.6 |
| Glutamate | 57.14±25.62 | 55.33±22.41 | 70.8±15.2 | 74.7±16.1 |
| Asparagine | 42.57±6.97 | 41.67±5.39 | 38.2±4.7 | 35.5±5.3 |
| Serine | 105.43±24.74 | 110.00±22.67 | 91.7±12.9 | 90.8±14.2 |
| Glutamine | 557.00±100.45 | 570.17±53.63 | 534.5±74.8 | 522.8±71.5 |
| Glycine | 319.29±112.45 | 339.83±118.04 | 228.5±45.6 | 216.8±41.7 |
| Threonine | 126.29±22.26 | 119.50±18.91 | 104.3±21.2 | 95.0±21.1 |
| Citrulline | 40.29±14.91 | 43.00±12.76 | 29.3±5.5 | 28.7±5.6 |
| Histidine | 89.57±15.20 | 85.33±16.24 | 88.7±9.8 | 83.0±9.2 |
| Alanine | 273.14±85.95 | 243.67±49.06 | 316.2±73.2 | 269.8±63.5 |
| Taurine | 105.57±18.95 | 110.67±19.80 | 98.3±18.7 | 95.5±20.9 |
| Tyrosine | 54.29±14.85 | 52.67±11.88 | 61.3±12.7 | 57.5±14.9 |
| Aminobutyric acid | 25.57±10.66 | 29.67±10.07 | 25.8±9.8 | 28.7±11.5 |
| Arginine | 91.86±20.59 | 93.50±8.22 | 85.2±14.9 | 84.7±14.1 |
| Methionine | 22.71±3.64 | 21.67±4.32 | 22.8±3.2 | 22.5±3.6 |
| Valine | 221.14±63.22 | 227.67±45.51 | 260.7±37.3 | 258.5±36.8 |
| Tryptophan | 39.57±11.86 | 34.50±10.09 | 39.0±8.0 | 35.3±7.3 |
| Phenylalanine | 52.43±11.93 | 48.83±9.83 | 55.0±4.6 | 52.7±6.6 |
| Isoleucine | 53.43±12.82 | 62.00±9.70 | 65.3±11.8 | 74.0±15.4 |
| Leucine | 119.57±28.15 | 127.50±20.70 | 142.5±21.5 | 150.5±30.9 |
| Ornithine | 47.14±10.29 | 48.83±3.19 | 43.0±12.4 | 44.3±13.9 |
| Lysine | 189.14±32.05 | 179.67±27.24 | 207.0±18.9 | 206.2±40.4 |
All data are means ± SD.
The concentration of betaine, choline, and dimethylglycine in plasma was similar in subjects with NASH and controls (data not presented). The concentration of glutathione, cysteine, and homocysteine in plasma was also not different between controls and subjects with NASH during the basal state and during intralipid infusion (Table 8). However, there was a significant increase in the concentration of glutathione in the plasma in response to intralipid infusion in subjects with NASH. The total antioxidant status (RANDOX) was not different between controls and NASH before (controls 1.46 ± 0.14 mmol/l; NASH 1.37 ± 0.13 mmol/l) or after (controls 1.65 ± 0.27 mmol/l; NASH 1.43 ± 0.11 mmol/l) intralipid infusion.
Table 8.
Plasma concentration of total glutathione, cysteine, and homocysteine
|
Glutathione, μmol/l |
Cysteine, μmol/l
|
Homocysteine, μmol/l
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Fast | IL1 | IL2 | Fast | IL1 | IL2 | Fast | IL1 | IL2 | |
| Control (n = 8) | 4.2±1.2 | 4.7±1.7 | 4.2±1.3 | 367±52 | 376±45 | 378±49 | 7.7±1.3 | 7.7±1.5 | 7.8±1.4 |
| NASH (n = 6) | 3.9±0.8 | 4.9±0.9* | 4.9±1.0* | 362±47 | 363±50 | 366±45 | 7.1±1.5 | 7.2±1.6 | 7.2±1.5 |
All data are means ± SD. Significantly different than fast,
P < 0.02 paired analysis.
DISCUSSION
In the present study, we have measured the rates of ureagenesis and oxidation of glycine in humans with NASH and examined the response to intralipid administration. We examined subjects with NASH because of the previous data suggesting mitochondrial dysfunction in this disorder (5, 44, 47). We studied age- and sex-matched control subjects; however, matching for weight (obesity) without steatosis was difficult. There was no significant difference in the rates of appearance of glycine, urea, and oxidation of glycine in subjects with NASH compared with the healthy controls. Subjects with NASH showed higher hepatic fatty acid oxidation as evidenced by the correlation between plasma levels of FFA and β-hydroxybutyrate (Fig. 4). In response to fatty acid administration, subjects with NASH increased their plasma glutathione levels and had a higher contribution of serine to cystathionine. The rate of ureagenesis and glycine Ra decreased in response to increase in plasma FFA, whereas the rate of oxidation of glycine remained unchanged in both NASH and healthy controls.
Hepatic fatty acid oxidation.
We infused intralipid with heparin to increase plasma FFAs and consequently increase hepatic fatty acid uptake and oxidation and change the hepatic redox state. Infusion of intralipid with heparin resulted in the anticipated increase in plasma FFA and β-hydroxybutyrate concentration in the blood of both healthy controls and subjects with NASH. Although there was no statistically significant difference in the plasma concentrations of FFAs and β-hydroxybutyrate in the two groups, either in the basal state or during lipid infusion, the relationship (slope 0.94 NASH, 0.38 controls) between the plasma concentration of FFAs and β-hydroxybutyrate was different in the two groups (Fig. 4), suggesting a higher hepatic oxidation of fatty acids in subjects with NASH. Our data are consistent with studies using [13C]octanoate load, showing a high rate of oxidation of the administered fatty acid and with the observation of higher FFA and β-hydroxybutyrate in subjects with NASH by Sanyal et al. (32, 44). These are in contrast to the other data in the literature suggesting a lower rate of fatty acid oxidation and thus contributing to hepatic steatosis (reviewed in Ref. 41). However, these inferences have been drawn from genetic disorders of hepatic fatty acid oxidation, which leads to steatosis, and from other animal models (41).
Intralipid infusion did not cause any significant change in the total antioxidant status in both groups, suggesting an appropriate compensatory response. However, plasma antioxidant status may not necessarily be representative of the liver.
A high hepatic fatty acid oxidation and ketogenesis will result in lower NAD/NADH ratio and consequent lower citric acid cycle flux and greater ROS production. The responses to lipid infusion, urea synthesis, and glycine oxidation, discussed below, are likely to be the consequence of a reduced hepatic redox state and increased oxidative stress.
Urea synthesis.
We quantified the rates of urea synthesis in subjects with NASH because the rate-limiting steps of urea synthesis, i.e., synthesis of carbamoyl phosphate and citrulline, occur in the mitochondria and require ATP. There was no significant difference in the rate of urea synthesis between the two groups during fasting (Table 4). Infusion of intralipid and, consequently, oxidation of fatty acid in the liver were associated with a significant decrease in the rate of urea synthesis, both in healthy controls and in subjects with NASH. The decrease in urea synthesis was evident within 60 min of intralipid infusion in all subjects. A similar decrease in urea synthesis in response to lipids has been reported by others (9, 31, 34). In contrast, one study did not observe any change in urea synthesis in response to intralipid in healthy subjects; however, this may be related to the cross-sectional design of that study (12). The decrease in urea synthesis is unlikely to be related to a reduction in the rate of protein breakdown, resulting in a lower amino acid load to the liver because the observed response was too rapid to be attributable to this mechanism and there was no significant change in plasma concentration of amino acids. Whether the change in urea synthesis was due to decrease in hepatic protein breakdown cannot be excluded. The change in urea synthesis is most likely due to a shift in the hepatic redox state caused by the enhanced rate of fatty acid oxidation (Fig. 4). A lower NAD/NADH ratio could decrease the oxidative deamination of glutamate by glutamate dehydrogenase so that the generation of free ammonia for urea synthesis would be attenuated, resulting in a lower rate of urea synthesis. In addition, the synthesis of aspartate, which provides one of the amino groups incorporated into urea, would be decreased because of the increased conversion of oxalacetate to malate (malate dehydrogenase) that would be favored by the increase in mitochondrial NADH. There was no significant difference in the magnitude of decrease in urea synthesis in either NASH subjects or controls, indicating that the provision of fatty acids controlled the overall fate of hepatic nitrogen disposal by decreasing urea cycle flux.
Glycine metabolism.
The weight-specific rate of appearance of glycine was lower in subjects with NASH compared with the controls (Table 5). Because the subjects with NASH were all obese and had significantly higher (∼18%) body weight compared with healthy controls, the difference in weight-specific glycine Ra was entirely due to the differences in body weight. Expression of data on the basis of body surface area or estimated lean body mass also did not reveal a significant difference in glycine Ra. The best method for the normalization of glycine kinetics is uncertain. Because the majority of glycine is derived from de novo synthesis, which occurs in all tissues in the body, we elected to report total and weight-specific Ra rather than in relation to lean body mass. Our estimates of glycine Ra are of similar magnitude as reported in the literature (27). We did not correct glycine Ra for the intracellular dilution of the tracer because reliable estimates of intracellular dilution in various compartments are not available. The major sources of glycine in vivo are from protein breakdown and de novo synthesis from other amino acids such as serine and threonine (42, 43). The rate of whole body protein breakdown is suggested to be higher in insulin-resistant states; therefore, an unchanged glycine flux in NASH may be due to lower de novo glycine synthesis. It is important to note that glycine kinetics are unchanged under a variety of metabolic and nutritional perturbations such as malnutrition or in response to a marginal protein intake in healthy adults (11, 16, 17). In response to Intralipid infusion, a significant reduction in glycine Ra occurred in both healthy controls and NASH. This decrease is likely related to a stimulation of gluconeogenesis in the liver by fatty acid oxidation (25, 26). This would increase the utilization of serine for the synthesis of glucose. In addition, because the first step in serine biosynthesis from 3-phosphoglycerate, catalyzed by phosphoglycerate dehydrogenase, uses NAD as a cofactor, a reduced redox state as a result of fatty acid oxidation could lower the rate of synthesis of serine and, consequently, result in a lower glycine Ra. Finally, an increase in the rate of glutathione synthesis as a result of fatty acid-induced oxidative stress (1, 46) could also divert serine into the transulfuration pathway for cysteine synthesis. The increase in the contribution of glycine to serine following intralipid infusion (Table 6) is consistent with such a hypothesis. Therefore, the decrease in glycine Ra in response to an increase in hepatic fatty acid oxidation could be the result of a decrease in production, as well as increased utilization, of serine for gluconeogenesis and cystathionine production; this would decrease the availability of serine for glycine production.
The primary route of glycine disposal is catalyzed by the glycine cleavage system (23) located in the inner mitochondrial membrane in the liver, kidney, and brain. Quantitatively, the activity is estimated to be highest in the liver. The glycine cleavage system catalyzes the oxidative cleavage of glycine to CO2, NH4+, and a methylene group that is transferred to tetrahydrofolate (Fig. 1). In this reaction, NAD is reduced to NADH, which is subsequently reoxidized in the electron transport chain to yield energy. In isolated perfused rat liver (13) and in isolated mitochondria (18), the flux through glycine cleavage system was inhibited by reduction in mitochondrial NAD(H) redox complex. Because a number of studies, both in vivo and in vitro, using a variety of animal models have suggested impaired mitochondrial function in steatosis and steatohepatitis, we hypothesized that, in subjects with NASH, the rate of oxidation of glycine will be attenuated and that it will be further impaired in response to infusion of fatty acids. As shown in Table 5, there was no significant difference in the fraction of glycine Ra that was oxidized to CO2 and the rate of oxidation of glycine in the basal state. In addition, intralipid infusion did not have any significant impact on the appearance of glycine C in CO2, in either controls or in subjects with NASH.
The lack of significant effect of fatty acid on the glycine cleavage system was surprising because fatty acids were being oxidized in the liver (elevated β-hydroxybutyrate levels) at a higher rate in subjects with NASH, and there was a change in other mitochondrial pathways, e.g., decreased urea synthesis. This lack of effect may be due to strict compartmentation of the mitochondrial membrane and matrix NAD/NADH system so that a change in the matrix is not reflected in the mitochondrial membrane (15), the location of the glycine cleavage system. It is also possible, though unlikely, that the changes in the flux acid through the glycine cleavage system were not reflected in expired CO2 during the 4 h of lipid infusion because of relatively slow turnover rate of the whole body bicarbonate pools.
Serine, cystathionine, and glutathione.
Intralipid infusion resulted in an increase in plasma glutathione in subjects with NASH. Because plasma glutathione is mostly derived from the liver, this increase may be due to increased glutathione synthesis as a result of the elevated oxidative stress caused by higher fatty acid oxidation in subjects with NASH. These data are consistent with higher rates of synthesis of cystathionine from serine in the NASH subjects.
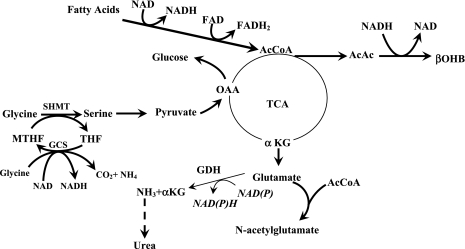
In summary, data from the present study show that two key hepatic mitochondrial pathways, ureagenesis and glycine cleavage system, were not effected by NASH. The relationship between plasma fatty acids and β-hydroxybutyrate levels suggested an increased hepatic fatty acid oxidation in NASH, particularly in response to fatty acid load (Fig. 5). Increased hepatic fatty acid oxidation and the resultant change in hepatic redox state and increased oxidative stress resulted in higher contribution of serine to cystathionine in both groups and higher plasma concentration of plasma glutathione in NASH. These data suggest that subjects with NASH can mount appropriate compensatory responses to oxidative stress and to change in hepatic redox induced by fatty acid load. The present study demonstrates that the delivery of fatty acids to the liver reveals abnormalities in the metabolic capacity of this organ and provides a useful model to study the pathophysiology of NASH. Additionally, the products of β-oxidation may also contribute to the development of hepatic insulin resistance as suggested by Koves et al. (24) for the skeletal muscle.
Fig. 5.
Effect of fatty acids on hepatic ureagenesis and glycine cleavage system in NASH; greater β-oxidation of fatty acids in response to increased fatty acid uptake results in decreased NAD:NADH ratio and increased generation of reactive oxygen species, consequently attenuating tricarboxylic acid cycle flux and higher β-hydroxybutyrate (βOHB) synthesis. The lower rate of ureagenesis in response to hepatic fatty acid oxidation may be the result of change in redox and its effect on glutamate dehydrogenase (GDH), resulting in lower production of ammonia (detailed in discussion). The greater utilization of serine for gluconeogenesis induced by fatty acid oxidation and for cystathionine synthesis will result in lower Ra of glycine. Interestingly, the change in redox in the mitochondrial matrix did not impact the GCS. AcAc, acetoacetate; αKG, α-ketoglutarate; MTHF, methylene tetrahydrofolate; OAA, oxaloacetic acid; TCA, tricarboxylic acid cycle; AcCoA, acetyl CoA.
GRANTS
The study was supported in part by the National Institutes of Health, National Center for Research Resources, CTSA IULI RR024989, of Case Western Reserve University, by the institutional support (Cleveland Clinic) to S. Kalhan, and by grant DK58620 from the National Institutes of Health to R. Hanson. The metabolic clinics at Cleveland Clinic and MetroHealth Medical Center are part of a multicenter cooperative Clinical Research Network of NASH, sponsored by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK, 5U01 DK61732; A. McCullough, PI).
Acknowledgments
We appreciate the expert support of the nursing staff of the Clinical Research Unit at the Cleveland Clinic. The secretarial support of Mrs. Joyce Nolan is gratefully appreciated.
REFERENCES
- 1.Adam-Vizi V, Chinopoulos C. Bioenergetics and the formation of mitochondrial reactive oxygen species. Trends Pharmacol Sci 27: 639–645, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Adams RF Determination of amino acid profiles in biological samples by gas chromatography. J Chromatogr 95: 189–212, 1974. [DOI] [PubMed] [Google Scholar]
- 3.Armuzzi A, Marcoccia S, Zocco MA, DeLorenzo A, Grieco A, Tondi P, Pola P, Gasbarrini G, Gasbarrini A. Non-invasive assessment of human hepatic mitochondrial function through the 13C-methionine breath test. Scand J Gastroenterol 35: 650–653. 2000. [DOI] [PubMed] [Google Scholar]
- 4.Bradbury MW Lipid metabolism and liver inflammation. I. Hepatic fatty acid uptake: possible role in steatosis. Am J Physiol Gastrointest Liver Physiol 290: G194–G198, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Caldwell SH, Chang CY, Nakamoto RK, Krugner-Higby L. Mitochondria in nonalcoholic fatty liver disease. Clin Liver Dis 8: 595–617, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Cortez-Pinto H, Chatham J, Chacko VP, Arnold C, Rashid A, Diehl AM. Alterations in liver ATP homeostasis in human nonalcoholic steatohepatitis. JAMA 282: 1659–1664, 1999. [DOI] [PubMed] [Google Scholar]
- 7.Davis SR, Stacpoole PW, Williamson J, Kick LS, Quinlivan EP, Coats BS, Shane B, Bailey LB, Gregory JF 3rd. Tracer-derived total and folate-dependent homocysteine remethylation and synthesis rates in humans indicate that serine is the main one-carbon donor. Am J Physiol Endocrinol Metab 286: E272–E279, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Flamment M, Arvier M, Gallois Y, Simard G, Malthiery Y, Ritz P, Ducluzeau PH. Fatty liver and insulin resistance in obese Zucker rats: no role for mitochondrial dysfunction. Biochimie 90: 1407–1413, 2008. [DOI] [PubMed] [Google Scholar]
- 9.Freyse EJ, Giessmann T, Petzke KJ, Knospe S, Engel G, Heinke P, Metges CC, Siegmund W. Effects of fatty acids on hepatic amino acid catabolism and fibrinogen synthesis in young healthy volunteers. Am J Physiol Endocrinol Metab 285: E54–E62, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Garcia AJ, Apitz-Castro R. Plasma total homocysteine quantification: an improvement of the classical high-performance liquid chromatographic method with fluorescence detection of the thiol-SBD derivatives. J Chromatogr B Analyt Technol Biomed Life Sci 779: 359–363, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Gibson NR, Jahoor F, Ware L, Jackson AA. Endogenous glycine and tyrosine production is maintained in adults consuming a marginal-protein diet. Am J Clin Nutr 75: 511–518, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Gormsen LC, Gjedsted J, Gjedde S, Norrelund H, Christiansen JS, Schmitz O, Jorgensen JO, Moller N. Dose-response effects of free fatty acids on amino acid metabolism and ureagenesis. Acta Physiol (Oxf) 192: 369–379, 2008. [DOI] [PubMed] [Google Scholar]
- 13.Hampson RK, Barron LL, Olson MS. Regulation of the glycine cleavage system in isolated rat liver mitochondria. J Biol Chem 258: 2993–2999, 1983. [PubMed] [Google Scholar]
- 14.Holm PI, Ueland PM, Kvalheim G, Lien EA. Determination of choline, betaine, and dimethylglycine in plasma by a high-throughput method based on normal-phase chromatography-tandem mass spectrometry. Clin Chem 49: 286–294, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Hu J, Dong L, Outten CE. The redox environment in the mitochondrial intermembrane space is maintained separately from the cytosol and matrix. J Biol Chem 283: 29126–29134, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jackson AA The glycine story. Eur J Clin Nutr 45: 59–65, 1991. [PubMed] [Google Scholar]
- 17.Jahoor F, Badaloo A, Reid M, Forrester T. Glycine production in severe childhood undernutrition. Am J Clin Nutr 84: 143–149, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Jois M, Ewart HS, Brosnan JT. Regulation of glycine catabolism in rat liver mitochondria. Biochem J 283: 435–439, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalhan SC, Rossi KQ, Gruca LL, Super DM, Savin SM. Relation between transamination of branched-chain amino acids and urea synthesis: evidence from human pregnancy. Am J Physiol Endocrinol Metab 275: E423–E431, 1998. [DOI] [PubMed] [Google Scholar]
- 20.Kalhan SC, Gruca LL, Parimi PS, O'Brien A, Dierker L, Burkett E. Serine metabolism in human pregnancy. Am J Physiol Endocrinol Metab 284: E733–E740, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Kalhan SC Re: “Alternative equations for whole-body protein synthesis and for fractional synthetic rates of proteins” by Ramakrishnan (Metabolism 2007;56:1550–60). Metabolism 57: 871–872, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kass GE Mitochondrial involvement in drug-induced hepatic injury. Chem Biol Interact 163: 145–159, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Kikuchi G, Motokawa Y, Yoshida T, Hiraga K. Glycine cleavage system: reaction mechanism, physiological significance, and hyperglycinemia. Proc Jpn Acad Ser B Phys Biol Sci 84: 246–263, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, Bain J, Stevens R, Dyck JR, Newgard CB, Lopaschuk GD, Muoio DM. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab 7: 45–56, 2008. [DOI] [PubMed] [Google Scholar]
- 25.Lam TK, Carpentier A, Lewis GF, van de Werve G, Fantus IG, Giacca A. Mechanisms of the free fatty acid-induced increase in hepatic glucose production. Am J Physiol Endocrinol Metab 284: E863–E873, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Lam TK, van de Werve G, Giacca A. Free fatty acids increase basal hepatic glucose production and induce hepatic insulin resistance at different sites. Am J Physiol Endocrinol Metab 284: E281–E290, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Lamers Y, Williamson J, Gilbert LR, Stacpoole PW, Gregory JF 3rd. Glycine turnover and decarboxylation rate quantified in healthy men and women using primed, constant infusions of [1,2-13C2]glycine and [2H3]leucine. J Nutr 137: 2647–2652, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laurell S, Tibbling G. Colorimetric microdetermination of free fatty acids in plasma. Clin Chim Acta 16: 57–62, 1967. [DOI] [PubMed] [Google Scholar]
- 29.Lee WS, Sokol RJ. Liver disease in mitochondrial disorders. Semin Liver Dis 27: 259–273, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mantena SK, King AL, Andringa KK, Landar A, Darley-Usmar V, Bailey SM. Novel interactions of mitochondria and reactive oxygen/nitrogen species in alcohol mediated liver disease. World J Gastroenterol 13: 4967–4973, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martin-Requero A, Cipres G, Rivas T, Ayuso MS, Parrilla R. Reciprocal changes in gluconeogenesis and ureagenesis induced by fatty acid oxidation. Metabolism 42: 1573–1582, 1993. [DOI] [PubMed] [Google Scholar]
- 32.Miele L, Grieco A, Armuzzi A, Candelli M, Forgione A, Gasbarrini A, Gasbarrini G. Hepatic mitochondrial beta-oxidation in patients with nonalcoholic steatohepatitis assessed by 13C-octanoate breath test. Am J Gastroenterol 98: 2335–2336, 2003. [DOI] [PubMed] [Google Scholar]
- 33.Minkler PE, Stoll MSK, Ingalls ST, Yang S, Kerner J, Hoppel CL. Quantification of carnitine and acylcarnitines in biological matrices by HPLC electrospray ionization mass spectrometry. Clin Chem 54: 1451–1462, 2008. [DOI] [PubMed] [Google Scholar]
- 34.Norrelund H, Nair KS, Nielsen S, Frystyk J, Ivarsen P, Jorgensen JO, Christiansen JS, Moller N. The decisive role of free fatty acids for protein conservation during fasting in humans with and without growth hormone. J Clin Endocrinol Metab 88: 4371–4378, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Perez-Carreras M, Del HP, Martin MA, Rubio JC, Martin A, Castellano G, Colina F, Arenas J, Solis-Herruzo JA. Defective hepatic mitochondrial respiratory chain in patients with nonalcoholic steatohepatitis. Hepatology 38: 999–1007, 2003. [DOI] [PubMed] [Google Scholar]
- 36.Persson B Determination of plasma acetoacetate and D-beta-hydroxybutyrate in new-born infants by an enzymatic fluorometric micro-method. Scand J Clin Lab Invest 25: 9–18, 1970. [DOI] [PubMed] [Google Scholar]
- 37.Pessayre D, Mansouri A, Fromenty B. Nonalcoholic steatosis and steatohepatitis. V. Mitochondrial dysfunction in steatohepatitis. Am J Physiol Gastrointest Liver Physiol 282: G193–G199, 2002. [DOI] [PubMed] [Google Scholar]
- 38.Piccoli C, Scrima R, D'Aprile A, Ripoli M, Lecce L, Boffoli D, Capitanio N. Mitochondrial dysfunction in hepatitis C virus infection. Biochim Biophys Acta 1757: 1429–1437, 2006. [DOI] [PubMed] [Google Scholar]
- 39.Price GM, Halliday D, Pacy PJ, Quevedo MR, Millward DJ. Nitrogen homeostasis in man: influence of protein intake on the amplitude of diurnal cycling of body nitrogen. Clin Sci (Lond) 86: 91–102, 1994. [DOI] [PubMed] [Google Scholar]
- 40.Raffaella C, Francesca B, Italia F, Marina P, Giovanna L, Susanna I. Alterations in hepatic mitochondrial compartment in a model of obesity and insulin resistance. Obesity (Silver Spring) 16: 958–964, 2008. [DOI] [PubMed] [Google Scholar]
- 41.Reddy JK, Rao MS. Lipid metabolism and liver inflammation. II. Fatty liver disease and fatty acid oxidation. Am J Physiol Gastrointest Liver Physiol 290: G852–G858, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Robert JJ, Bier DM, Zhao X, Matthews DE, Young VR. Glucose and insulin effects on de novo amino acid synthesis in young men: studies with stable isotope labeled alanine, glycine, leucine, and lysine. Metabolism 31: 1210–1218, 1982. [DOI] [PubMed] [Google Scholar]
- 43.Robert JJ, Beaufrere B, Koziet J, Desjeux JF, Bier DM, Young VR, Lestradet H. Whole body de novo amino acid synthesis in type I (insulin-dependent) diabetes studied with stable isotope-labeled leucine, alanine and glycine. Diabetes 34: 67–73, 1985. [DOI] [PubMed] [Google Scholar]
- 44.Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, Luketic VA, Shiffman ML, Clore JN. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology 120: 1183–1192, 2001. [DOI] [PubMed] [Google Scholar]
- 45.Schneider ARJ, Kraut C, Lindenthal B, Braden B, Caspary WF, Stein J. Total body metabolism of 13C-octanoic acid is preserved in patients with non-alcoholic steatohepatitis, but differs between women and men. Eur J Gastroenterol Hepatol 17: 1171–1174, 2005. [DOI] [PubMed] [Google Scholar]
- 46.Schonfeld P, Wojtczak L. Fatty acids as modulators of the cellular production of reactive oxygen species. Free Radic Biol Med 45: 231–241, 2008. [DOI] [PubMed] [Google Scholar]
- 47.Serviddio G, Sastre J, Bellanti F, Vina J, Vendemiale G, Altomare E. Mitochondrial involvement in non-alcoholic steatohepatitis. Mol Aspects Med 29: 22–35, 2008. [DOI] [PubMed] [Google Scholar]
- 48.Tserng KY, Kalhan SC. Gas chromatography/mass spectrometric determination of [15-N]urea in plasma and application to urea metabolism study. Anal Chem 54: 489–491, 1982. [DOI] [PubMed] [Google Scholar]
- 49.Tserng KY, Kalhan SC. Calculation of substrate turnover rate in stable isotope tracer studies. Am J Physiol Endocrinol Metab 245: E308–E311, 1983. [DOI] [PubMed] [Google Scholar]
- 50.Turnell DC, Cooper JDH. Rapid assay for amino acids in serum or urine by pre-column derivatization and reversed-phase liquid chromatography. Clin Chem 28/3: 527–531, 1982. [PubMed] [Google Scholar]
- 51.Vendemiale G, Grattagliano I, Caraceni P, Caraccio G, Domenicali M, Dall'Agata M, Trevisani F, Guerrieri F, Bernardi M, Altomare E. Mitochondrial oxidative injury and energy metabolism alteration in rat fatty liver: effect of the nutritional status. Hepatology 33: 808–815, 2001. [DOI] [PubMed] [Google Scholar]