Abstract
Cannabinoids have long been known to be potent inhibitors of intestinal and colonic propulsion. This effect has generally been attributed to their ability to prejunctionally inhibit release of acetylcholine from excitatory motor neurons that mediate, in part, the ascending contraction phase of the peristaltic reflex. In the present study we examined the effect of cannabinoids on the other transmitters known to participate in the peristaltic reflex using a three-compartment preparation of rat colon that allows separation of ascending contraction, descending relaxation, and the sensory components of the reflex. On addition to the orad motor compartment, anandamide decreased and AM-251, a CB-1 antagonist, increased ascending contraction and the concomitant substance P (SP) release. Similarly, on addition to the caudad motor compartment, anandamide decreased and AM-251 increased descending relaxation and the concomitant vasoactive intestinal peptide (VIP) release. On addition to the central sensory compartment, anandamide decreased and AM-251 increased both ascending contraction and SP release orad, and descending relaxation and VIP release caudad. This suggested a role for CB-1 receptors in modulation of sensory transmission that was confirmed by the demonstration that central addition of anandamide decreased and AM-251 increased release of the sensory transmitter, calcitonin gene-related peptide (CGRP). We conclude that the potent antipropulsive effect of cannabinoids is the result of inhibition of both excitatory cholinergic/tachykininergic and inhibitory VIPergic motor neurons that mediate ascending contraction and descending relaxation, respectively, as well as inhibition of the intrinsic sensory CGRP-containing neurons that initiate the peristaltic reflex underlying propulsive motility.
Keywords: endocannabinoid, cannabinoid receptors, enteric nervous system, peristalsis, neuropeptides
endogenous cannabinoids (endocannabinoids) and their receptors are present in the gut tissues where they modulate motility, secretion, and the inflammatory response. Several endocannabinoids are synthesized on demand from fatty acids although the most commonly studied endocannabinoid is anandamide. The endocannabinoids interact mainly with two types of cannabinoid receptors designated CB-1 and CB-2, and in some cases with the TRPV1 (transient receptor potential vanilloid receptor 1 or VR-1) (24, 26, 36). Recently, studies also suggest that there may be an additional receptor that might mediate some actions of endocannabinoids (6, 20, 36).
Endocannabinoids and the CB-1 and CB-2 receptors are present in the gut although much more is known about the localization of the receptors than the endocannabinoids (9, 16, 28). Both CB-1 and CB-2 receptors are present on enteric neurons that are invariably colocalized with choline acetyltransferase (ChAT) but not vasoactive intestinal peptide (VIP) or nitric oxide synthase (NOS) (8, 10, 19, 28, 29, 32). Both Dogiel type I and II enteric neurons have been shown to express CB-1 receptors. The presence of cannabinoid receptors in smooth muscle is controversial although recent evidence suggests the presence of CB-1 receptors on smooth muscle cells (22, 23).
In a variety of species including the human, cannabinoids have long been known to be inhibitory, slowing gut motility as well as secretion, and this has been usually attributed to the most prominent effect of cannabinoids in the gut, that is, CB-1 receptor-mediated presynaptic inhibition of acetylcholine (ACh) release (9, 15, 16, 19, 28, 29, 32, 35). Although this mode of action is well documented, the peristaltic reflex is mediated by a complex array of enteric neurons that make up the reflex circuit. These include intrinsic sensory afferent neurons (IPANs), interneurons, and excitatory and inhibitory motor neurons. The effect of cannabinoids on these components is less well studied even though effects on any of these neurons or their neurotransmitters would also affect the peristaltic reflex.
In the present study we have used a three-compartment preparation of rat colon to examine the effect of the endocannabinoid, anandamide, and cannabinoid receptor antagonists on the ascending contraction and descending relaxation component of the peristaltic reflex and concomitant transmitter release. The results demonstrate that cannabinoids, acting via CB-1 receptors, inhibit the ascending contraction and concomitant substance P (SP) release, and descending relaxation and concomitant VIP release. Additionally, the results also indicate that cannabinoids inhibit calcitonin gene-related peptide (CGRP) release from intrinsic sensory neurons. Thus the potent antipropulsive action of cannabinoids in vivo is likely the result of reduction of all aspects of the peristaltic reflex.
MATERIALS AND METHODS
Measurement of Peristaltic Reflex in Compartmented Flat-Sheet Preparations
The peristaltic reflex was measured in a 3- to 5-cm segment of mid to distal colon of rat, opened to form a flat sheet and pinned mucosal side up in a three-compartment organ bath as described in detail previously (11–13). In these earlier studies using this preparation our laboratory has demonstrated that the reflex is fully neurally mediated being abolished by tetrodotoxin and strongly inhibited by hexamethonium. Additionally, the ascending contraction component was shown to be mediated by cholinergic and tachykininergic motor neurons and the descending relaxation component by VIP-, pituitary adenylate cyclase activating peptide-, and nitric oxide-containing motor neurons. The compartments were isolated by vertical partitions sealed with vacuum grease and containing Krebs-bicarbonate medium containing (in mmol/l) 118 NaCl, 4.8 KCl, 1.2 KH2PO4, 2.5 CaCl2, 1.2 MgSO4, 25 NaH2CO3, and 11 glucose. In addition the medium contained the peptidase inhibitors 10 μmol/l amastatin and 1 μmol/l phosphoramidon and 0.1% bovine serum albumin. All preparations and experimental protocols were approved the Institutional Animal Care and Use Committee of the Virginia Commonwealth University. All peristaltic reflex studies were done using male Sprague-Dawley rats (100–150 g) from Harlan Laboratories (Indianapolis, IN). All colonic pellet propulsion studies were done using male Hartley guinea pigs (100–150 g) from Charles River Laboratories (Wilmington, MA).
Experimental Protocol
Measurement of contractile responses.
The peristaltic reflex was initiated by stroking the mucosa in the central compartment with a fine brush (two, four, six, and/or eight strokes at a rate of 1 stroke/s). Ascending contraction of circular muscle was measured in the orad peripheral compartment and descending relaxation in the caudad peripheral compartment by use of force-displacement transducers attached to the circular muscle layer as described previously (12, 13). After control responses in the absence of test agent were measured, the preparation was washed and allowed to recover for 60 min. At that point, preparations were treated in several different protocols. In some preparations, the cannabinoid agonist anandamide was added to the central compartment and the response to two and six strokes was repeated. Anandamide was tested at 1 nM to 1 μM final concentration. In separate preparations, the protocol was repeated after the different anandamide concentrations were added to one or the other peripheral compartments. In other preparations, a full stroke-response curve was constructed at two to eight strokes after application of the optimally effective concentration of anandamide (1 μM) to central or peripheral compartments separately. In some preparations, the full stroke-response curve in the presence of anandamide was repeated in the additional presence of the CB-1 receptor antagonist AM-251 (0.1 μM), the CB-2 receptor antagonist AM-630 (0.1 μM), or the TRPV1 receptor antagonist resiniferatoxin (0.1 μM) added to either the central or peripheral compartment.
The role of endogenous cannabinoids was tested in a similar manner by additions of the CB-1 receptor antagonist or CB-2 receptor antagonist to the central or peripheral compartments in the absence of anandamide. As described above, the response to two and six strokes was examined with a range of concentrations (1 nM to 1 μM) of the antagonist and a full stroke-response curve was constructed in the presence of the optimally effective concentration of 0.1 μM.
Measurement of transmitter release.
Following a 60-min equilibration period, the medium in each compartment was removed and replaced with fresh medium for a 15-min basal period. At the end of this period, the medium was collected, aliquoted, and stored at −80°C for subsequent measurement of neurotransmitters. Each compartment was refilled with medium, and a mucosal stroking stimuli was applied in the central compartment at a stimulus strength of either four or eight strokes. The stimulus was applied five times at 3-min intervals over a 15-min period. At the end of this period, the medium was collected and stored at −80°C for subsequent measurement of neurotransmitters. After a 30-min equilibration period, during which the bathing medium was changed twice, the basal and mucosal stroking sequence was repeated in the presence of the endocannabinoid anandamide (1 μM), the CB-1 receptor antagonist AM-251 (0.1 μM), or the CB-2 receptor antagonist AM-630 (0.1 μM) added to either the central sensory compartment or the orad or caudad peripheral compartment. When antagonist was added to the central compartment, medium was collected from all three compartments. When it was added to the caudad or orad peripheral compartment, only medium from that compartment was collected.
Separate colonic preparations were used for anandamide and for each antagonist and for additions to the central and to each peripheral compartment. Separate segments were used for measurement of circular muscle activity during peristaltic reflex and for collection of medium for measurement of neurotransmitters.
Measurement of the velocity of propulsion in isolated whole segment of guinea pig colon.
The velocity of propulsion was measured in an isolated whole segment of guinea pig colon by using artificial clay pellets that mimicked fecal pellets, as previously described (18). Isolated colonic segments from guinea pig were used because they provide stable and reproducible rates of propulsion and because the same transmitter systems that operate in the rat peristalsis model have been shown to mediate pellet propulsion in the guinea pig. We and others have been unable to establish a reproducible in vitro model of rat colon in which to study pellet propulsion. Basal velocity was measured by inserting a pellet into the orad end of the segment and calculating the time it took for it to traverse a fixed distance. At 5-min intervals, a second and then a third pellet were inserted and basal velocity was determined from the mean of three values. The sequence was repeated after addition of either AM-251 (0.1 μM) or anandamide (1.0 μM) to the medium. The velocity of propulsion was calculated as millimeters per second.
Measurement of CGRP Release
CGRP was measured by radioimmunoassay using antibody RIK 6006. The limit of detection of the assay was 2.6 fmol/ml and the IC50 was 39.2 ± 5.1 fmol/ml of original sample. The antibody reacts with CGRP but does not cross-react with anandamide, AM-251, AM-630, calcitonin, amylin, SP, neurokinin A, neurokinin B, somatostatin, VIP, Met-enkephalin, or brain-derived neurotrophic factor (BDNF).
Measurement of VIP Release
VIP was measured by radioimmunoassay using antibody RAS 7161. The limit of detection of the assay was 2.6 fmol/ml and the IC50 was 22.2 ± 3.1 fmol/ml of original sample. The antibody reacts with VIP but does not cross-react with anandamide, AM-251, AM-630, secretin, glucagon, pituitary adenylate cyclase activating peptide, CGRP, SP, neurokinin A, neurokinin B, somatostatin, Met-enkephalin, or BDNF.
Measurement of SP Release
SP was measured by radioimmunoassay using antibody RAS 7451. The limit of detection of the assay was 2.6 fmol/ml and the IC50 was 17.3 ± 5.8 fmol/ml of original sample. The antibody reacts with SP but does not cross-react with anandamide, AM-251, AM-630, secretin, glucagon, pituitary adenylate cyclase activating peptide, CGRP, VIP, neurokinin A, neurokinin B, somatostatin, Met-enkephalin, or BDNF.
Data Analysis
Ascending contraction and descending relaxation were measured as grams force. The release of CGRP into the central compartment, VIP into the caudad peripheral compartment, and SP into the orad peripheral compartment during peristalsis was expressed as femtomoles per 100 mg wet tissue weight. Wet tissue weight of the section of the segment in each compartment was determined at the end of the experiment. Values were calculated as means ± SE of measurements obtained in n experiments where separate animals and colonic segments were used for each experiment. Thus n represents the number of experiments and animals for each curve. Statistical significance was evaluated by ANOVA and Student's t-tests (GraphPad Software, San Diego, CA) and P < 0.05 was accepted as the statistically significant level of difference.
Materials
CGRP, VIP, SP, CGRP antiserum RIK 6006, VIP antiserum RAS 7161, SP antiserum RAS 7451, 125I-CGRP, 125I-VIP, and 125I-SP were purchased from Bachem-Peninsula (Torrance, CA). Anandamide, AM-251, and AM-630 were purchased from Tocris (Ellisville, MO). Cannabinoids were dissolved in 10% ethanol and added to the Krebs buffer solution in 20 μl volumes. Addition of this amount of ethanol vehicle alone had no effect on the peristaltic reflex or transmitter release measured in this study. All other agents were dissolved in Krebs buffer. Amastatin, phosphoramidon, resiniferatoxin, and all other chemicals and reagents were purchased from Sigma Chemicals (St. Louis, MO).
RESULTS
Effect of Anandamide on the Peristaltic Reflex
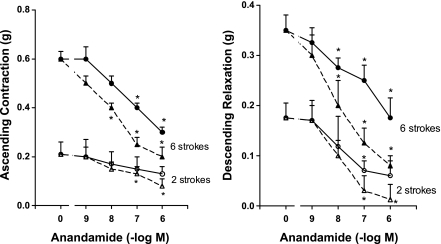
The endocannabinoid, anandamide, significantly decreased both components of the peristaltic reflex (Figs. 1 and 2). The effect of anadamide was concentration dependent in the range of 1 nM to 1 μM when tested against a threshold stimulus of two strokes and a near-maximum stimulus of six strokes (Fig. 1). The maximal inhibition of the response to two and six strokes occurred at 1.0 μM. The inhibition was greater when anandamide was added to the central compartment where the stroke stimulus was applied compared with addition of anandamide to either peripheral motor compartment.
Fig. 1.
Effect of various concentrations of anandamide (1 nM to 1 μM) on the ascending contraction (left) and descending relaxation (right) components of the peristaltic reflex. The control response in the absence of exogenous anandamide is indicated by the zero point. Responses elicited by 2 strokes are indicated by open symbols and responses elicited by 6 strokes are indicated by solid symbols. The effects of anandamide added to the peripheral motor compartments are indicated by circles and solid lines and the effect of anandamide added to the central sensory compartment are indicated by triangles and dashed lines. Separate colons and animals were used for measuring response after addition to central sensory compartment, for addition to orad peripheral motor compartment (ascending contraction), and for addition to caudad peripheral motor compartment (descending relaxation). Values are means ± SE of 3–5 experiments. *Significance of P < 0.05.
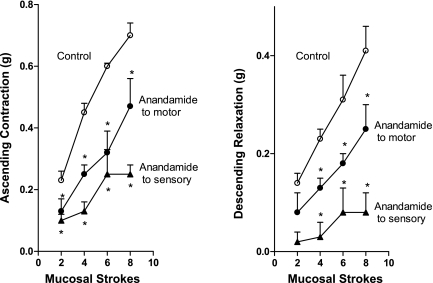
Fig. 2.
Effect of anandamide (1 μM) on ascending contraction (left) and descending relaxation (right) components of the peristaltic reflex. ○, Control responses; •, responses following addition of anandamide to peripheral orad motor compartment for ascending contraction and to peripheral caudad motor compartment for descending relaxation; ▴, responses following addition of anandamide to the central sensory compartment. Separate colons and animals were used for measuring response after addition to central sensory compartment, for addition to orad peripheral motor compartment (ascending contraction), and for addition to caudad peripheral motor compartment (descending relaxation). Values are means ± SE of 3–6 experiments. *Significance of P < 0.05.
The effect of 1.0 μM anandamide was further examined with the full range of stroke stimuli (Fig. 2). When added to the orad peripheral compartment where the ascending contraction was recorded, anandamide inhibited ascending contraction from 48.3 ± 7.5% (P < 0.01) at two strokes to 34.5 ± 9.6% (P < 0.05) at eight strokes but had no effect on descending relaxation recorded simultaneously in the caudad peripheral compartment, suggesting a local effect on excitatory motor neurons. Similarly, when added to the caudad peripheral compartment where the descending relaxation was recorded, anandamide inhibited descending relaxation from 52.7 ± 11.2% (P < 0.05) at two strokes to 33.7 ± 8.7% (P < 0.05) at eight strokes but had no effect on ascending contraction recorded simultaneously in the orad peripheral compartment, suggesting a local effect on inhibitory motor neurons.
At the maximally effect concentration of 1.0 μM, anandamide caused a greater inhibition of the peristaltic reflex throughout the full range of stimuli when added to the central compartment where the mucosal stroking stimulus was applied than when added to either peripheral motor compartment. In this case, addition of anandamide to the central compartment resulted in simultaneous inhibition of both ascending contraction orad and descending relaxation caudad (Fig. 2). The inhibition of ascending contraction ranged from 47.3 ± 7.5% (P < 0.01) at two strokes to 64.4 ± 2.5% (P < 0.01) at eight strokes, and the inhibition of descending relaxation ranged from 95.3 ± 5.5% (P < 0.01) at two strokes to 71.1 ± 8.2% (P < 0.01) at eight strokes. The simultaneous inhibition of ascending contraction and descending relaxation as a result of addition of anandamide to the central compartment suggests an effect on the sensory component of the peristaltic reflex arc.
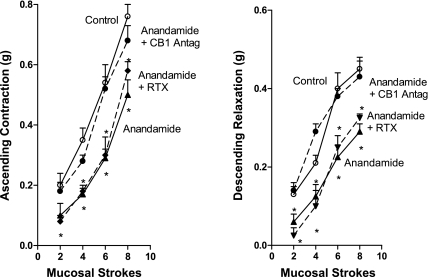
Since cannabinoids can interact with multiple receptors including cannabinoid type 1 and 2 receptors as well as with the TRPV1 receptor, we tested the effect of anandamide in the presence of various receptor antagonists. The inhibitory effect of anandamide (1.0 μM) added to the peripheral motor compartments was fully reversed in the presence of the CB-1 receptor antagonist AM-251 (0.1 μM) (Fig. 3). Addition of the CB-2 antagonist AM-630 (0.1 μM) to the motor compartments did not affect the inhibition induced by anandamide (e.g., two strokes: 40 ± 3% inhibition in the absence and 38 ± 5% inhibition in the presence of AM-630; six strokes: 45 ± 6% inhibition in the absence and 42 ± 3% inhibition in the presence of AM-630). Addition of the TRPV1 antagonist resiniferatoxin (0.1 μM) to the motor compartments also had no effect on the inhibition of either ascending contraction or descending relaxation caused by anandamide (Fig. 3). These results suggested that the inhibitory effects of anandamide on the motor components of ascending contraction and descending relaxation are mediated by an interaction with CB-1 receptors but not CB-2 or TRPV1 receptors.
Fig. 3.
Effect of anandamide (1 μM) added to the peripheral motor compartments on ascending contraction (left) and descending relaxation (right) alone and in the presence of the CB-1 receptor antagonist AM-251 (0.1 μM) and the TRPV1 receptor antagonist resiniferatoxin (RTX; 0.1 μM). ○ and solid line, Control responses to mucosal stroking in the absence of added agents; ▴ and solid line; response in the presence of anandamide alone; • and dashed line; response in the presence of anandamide and AM-251; ▾ or ♦ and dashed line, response in the presence of anandamide and RTX. Separate colons and animals were used for measuring the responses to anandamide plus each antagonist, for addition to orad peripheral motor compartment (ascending contraction), and for addition to caudad peripheral motor compartment (descending relaxation). Values are means ± SE of 3–6 experiments. *Significance of P < 0.05.
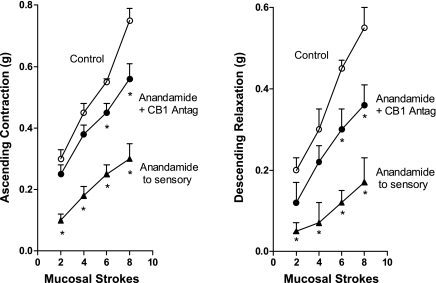
The effect of addition of anandamide to the central compartment in the presence of the cannabinoid receptor antagonists was also examined. The CB-1 receptor antagonist partially reversed the anandamide-induced inhibition of both ascending contraction and descending relaxation (Fig. 4) but the CB-2 receptor antagonist had no effect (data not shown). The response to the lower stroke stimuli (2 and 4 strokes) was fully reversed whereas the response to greater stimuli (6 and 8 strokes) was partially reversed. The TRPV1 receptor antagonist resiniferatoxin alone strongly inhibited the response to mucosal stroking (78–90% inhibition of control response) such that we were unable to test the effects of resiniferatoxin on the inhibition induced by anandamide. These results suggest that the CB-1 receptor but not the CB-2 receptor mediates, in part, the anandamide-induced inhibition of the sensory limb of the peristaltic reflex.
Fig. 4.
Effect of anandamide (1 μM) added to the central sensory compartment on ascending contraction (left) and descending relaxation (right) alone and in the presence of the CB-1 receptor antagonist AM-251 (0.1 μM). ○, Control responses to mucosal stroking in the absence of added agents; ▴, response in the presence of anandamide alone; •, response in the presence of anandamide and AM-251. Values are means ± SE of 4 experiments. *Significance of P < 0.05.
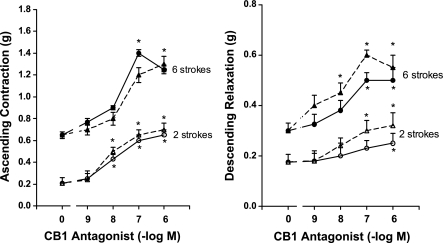
Effect of Cannabinoid Receptor Antagonists on the Peristaltic Reflex
The effect of the CB-1 receptor antagonist AM-251 on the peristaltic reflex was opposite to that described for anandamide (Figs. 5 and 6). The effects of the CB-1 antagonist were concentration dependent in the range of 1 nM to 1 μM when tested against a threshold stimulus of two strokes and a near-maximum stimulus of six strokes (Fig. 5). The maximal augmentation of the response to two and six strokes occurred at 0.1 μM with the augmentation of response in the presence of 1.0 μM being either the same or slightly less than that obtained at 0.1 μM. In contrast to the effects of anandamide, the augmentation was about the same for addition to the central or peripheral compartments with regard to ascending contraction and only slightly more when added to the central compartment with regard to the descending relaxation component (Fig. 5).
Fig. 5.
Effect of various concentrations of the CB-1 antagonist AM-251 (1 nM to 1 μM) on the ascending contraction (left) and descending relaxation (right) components of the peristaltic reflex. The control response in the absence of exogenous anandamide is indicated by the zero point. Responses elicited by 2 strokes are indicated by open symbols and responses elicited by 6 strokes are indicated by solid symbols. The effects of AM-251 added to the peripheral motor compartments are indicated by solid lines and the effect of AM-251 added to the central sensory compartment are indicated by dashed lines. Separate colons and animals were used for measuring response after addition to central sensory compartment, for addition to orad peripheral motor compartment (ascending contraction), and for addition to caudad peripheral motor compartment (descending relaxation). Values are means ± SE of 3–5 experiments. *Significance of P < 0.05.
Fig. 6.
Effect of the CB-1 antagonist (Antag) AM-251 (0.1 μM) on ascending contraction (left) and descending relaxation (right) components of the peristaltic reflex. ○, Control responses; •, responses following addition of AM-251 to peripheral orad motor compartment for ascending contraction and to peripheral caudad motor compartment for descending relaxation; ▴, responses following addition of AM251 to the central sensory compartment. Separate colons and animals were used for measuring response after addition to central sensory compartment, for addition to orad peripheral motor compartment (ascending contraction), and for addition to caudad peripheral motor compartment (descending relaxation). Values are means ± SE of 3–6 experiments. *P < 0.05.
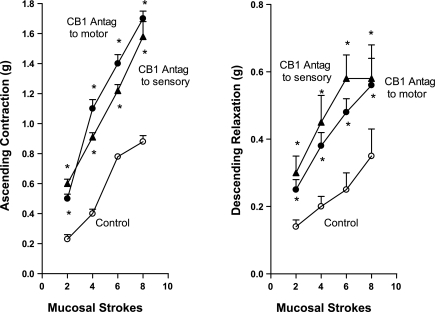
The action of the maximally effective concentration (0.1 μM) was further examined with the full range of stroke stimuli (Fig. 6). When added to the orad peripheral compartment where the ascending contraction was recorded, the CB-1 antagonist AM-251 increased ascending contraction by 112.3 ± 9.6% (P < 0.01) at two strokes to 135.1 ± 25.1% (P < 0.05) at eight strokes but had no effect on descending relaxation recorded simultaneously in the caudad peripheral compartment. Similarly, when added to the caudad peripheral compartment where the descending relaxation was recorded, AM-251 increased descending relaxation by 78.6 ± 3.5% (P < 0.01) at two strokes to 71.7 ± 5.8% (P < 0.01) at eight strokes but had no effect on ascending contraction recorded simultaneously in the orad peripheral compartment.
As noted with the concentration-response curves for two and six strokes, AM-251 caused only slightly more augmentation when added to the central compartment where the mucosal stroking stimulus was applied when all levels of stimulation were examined (Fig. 6). In this case, addition of AM-251 to the central compartment resulted in simultaneous increase in both ascending contraction orad and descending relaxation caudad (Fig. 6). The increase in ascending contraction ranged from 132.8 ± 14.4% (P < 0.01) at two strokes to 79.5 ± 8.3% (P < 0.01) at eight strokes, and the increase in descending relaxation ranged from 108.0 ± 12.1% (P < 0.01) at two strokes to 76.8 ± 8.8% (P < 0.01) at eight strokes.
The ability of the CB-1 antagonist to augment the peristaltic reflex suggests that there is a basal release of endocannabinoids that normally restrains the activity of the sensory as well as motor components of peristaltic reflex arc. Removal of this background restraining tone of endocannabinoids results in an augmentation of the peristaltic reflex.
In contrast to the significant effects of the CB-1 receptor antagonist AM-251 on the peristaltic reflex AM-630 (0.1 μM), the CB-2 receptor antagonist had no effect on ascending contraction or descending relaxation on addition to either the central compartment or either peripheral motor component. The ascending contraction response to mucosal stroking ranged from 89 ± 7 to 115 ± 9% of control in the presence of AM-630 added to either the motor or sensory compartment and the descending relaxation response to mucosal stroking ranged from 92 ± 6 to 121 ± 12% of control in the presence of AM-630 added to either the motor or sensory compartment. These differences from control were not statistically significant.
Effect of Anandamide and Cannabinoid Receptor Antagonists on SP Release
SP was measured in the orad compartment as an indication of activation of the excitatory motor neurons to circular muscle (Fig. 7). Basal release of SP was 8.5 ± 0.5 fmol/100 mg. Addition of anandamide, AM-251, or AM-630 to either the orad peripheral compartment or central compartment had no effect on basal SP release into the orad peripheral compartment (range 7.6 ± 0.9 to 9.3 ± 0.6 fmol/100 mg).
Fig. 7.
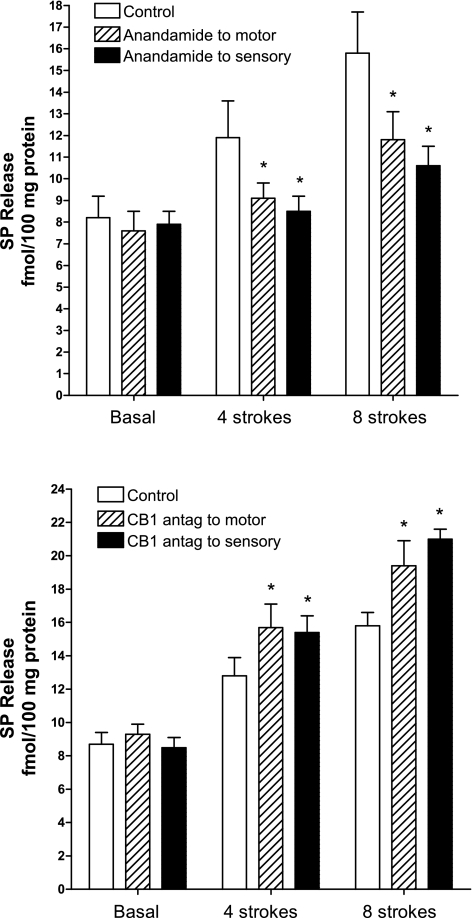
Effect of anandamide (1 μM; top) and AM-251 (0.1 μM; bottom) on substance P (SP) release into the orad peripheral motor compartment. Control release is denoted by open bars, release following addition to peripheral orad motor compartment by hatched bars, and release following addition to the central sensory compartment by solid bars. Separate colons and animals were used for measuring response after addition to central sensory compartment, and for addition to orad peripheral motor compartment. Values are means ± SE of 3–6 experiments. *P < 0.05.
Consistent with the inhibition of the mucosal stroking-induced ascending contraction of circular muscle by anandamide, release of SP was also inhibited by anandamide. Addition of anandamide to the peripheral orad compartment inhibited the stroking-induced increase in SP release by 42.5 ± 7.4% (P < 0.05) at four strokes and 54.4 ± 7.9% (P < 0.01) at eight strokes. Similarly, addition of anandamide to the central compartment also inhibited the stroking-induced increase in SP release into the peripheral orad compartment by 54.4 ± 6.8% (P < 0.01) at four strokes and 70.6 ± 6.5% (P < 0.01) at eight strokes.
Consistent with the augmentation of mucosal stroking-induced ascending contraction of circular muscle by the CB-1 receptor antagonist AM-251, release of SP was also augmented by AM-251. Addition of AM-251 to the peripheral orad compartment augmented the stroking-induced increase in SP release into the peripheral orad compartment by 60.0 ± 14.3% (P < 0.05) at four strokes and 43.2 ± 11.1% (P < 0.05) at eight strokes. Similarly, addition of AM-251 to the central compartment also augmented the stroking-induced increase in SP release into the peripheral orad compartment by 75.1 ± 14.8% (P < 0.05) at four strokes and 77.8 ± 17.1% (P < 0.05) at eight strokes. The CB-2 receptor antagonist AM-630 had no effect on stoking-induced SP release. SP release ranged from 93 ± 5 to 114 ± 7% of control values in the presence of AM-630.
Effect of Anandamide and Cannabinoid Receptor Antagonists on VIP Release
VIP was measured in the caudad compartment as an indication of activation of the inhibitory motor neurons to circular muscle (Fig. 8). Basal release of VIP was 7.2 ± 0.7 fmol/100 mg. Addition of anandamide, AM-251, or AM-630 to either the caudad peripheral compartment or central compartment had no effect on basal VIP release into the caudad peripheral compartment (range 6.8 ± 0.5 to 7.8 ± 0.8 fmol/100 mg).
Fig. 8.
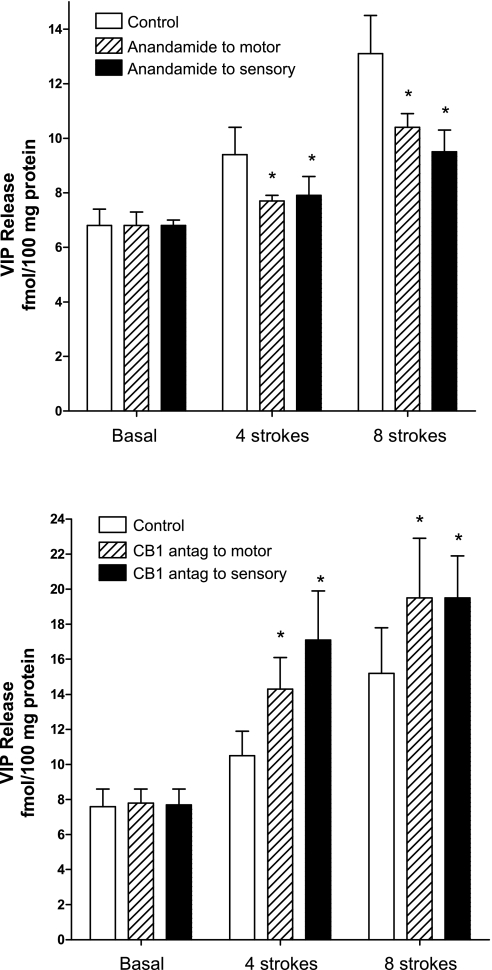
Effect of anandamide (1 μM; top) and AM-251 (0.1 μM; bottom) on vasoactive intestinal peptide (VIP) release into the caudad peripheral motor compartment. Control release is denoted by open bars, release following addition to peripheral caudad motor compartment by hatched bars, and release following addition to the central sensory compartment by solid bars. Separate colons and animals were used for measuring response after addition to central sensory compartment, and for addition to caudad peripheral motor compartment. Values are means ± SE of 3–6 experiments. *P < 0.05.
Consistent with the inhibition of the mucosal stroking-induced descending relaxation of circular muscle by anandamide, release of VIP was also inhibited by anandamide. Addition of anandamide to the peripheral caudad compartment inhibited the stroking-induced increase in VIP release by 63.3 ± 15.4% (P < 0.05) at four strokes and 60.7 ± 8.8% (P < 0.01) at eight strokes. Similarly, addition of anandamide to the central compartment also inhibited the stroking-induced increase in VIP release into the peripheral caudad compartment by 63.4 ± 12.3% (P < 0.05) at four strokes and 55.5 ± 10.1% (P < 0.05) at eight strokes.
Consistent with the augmentation of mucosal stroking-induced descending relaxation of circular muscle by the CB-1 antagonist AM-251, release of VIP was also augmented by AM-251. Addition of AM-251 to the peripheral caudad compartment augmented the stroking-induced increase in VIP release into the peripheral caudad compartment by 128.0 ± 22.5% (P < 0.05) at four strokes and 55.4 ± 12.4% (P < 0.05) at eight strokes. Similarly, addition of AM-251 to the central compartment also augmented the stroking-induced increase in VIP release into the peripheral caudad compartment by 220.4 ± 51.5% (P < 0.05) at four strokes and 62.3 ± 12.6% (P < 0.05) at eight strokes. The CB-2 receptor antagonist AM-630 had no effect on stroking-induced VIP release. VIP release ranged from 88 ± 9 to 108 ± 11% of control values in the presence of AM-630.
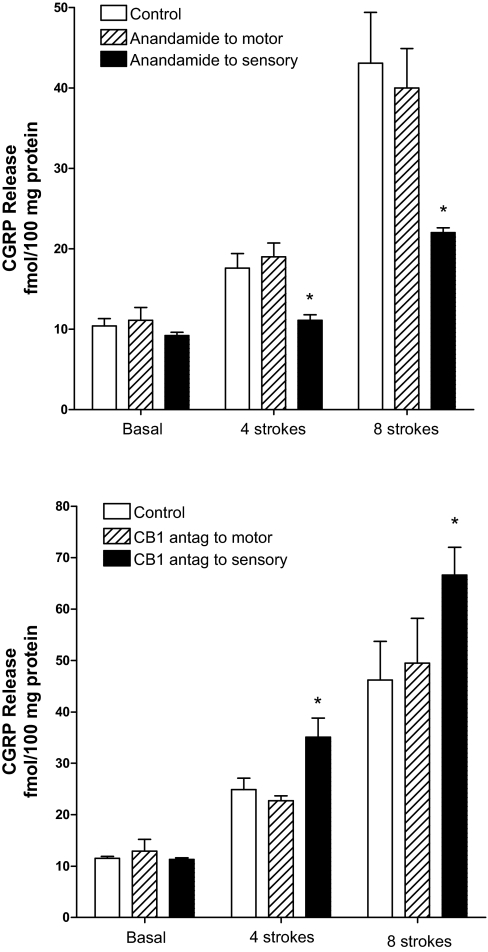
Effect of Anandamide and Cannabinoid Receptor Antagonists on CGRP Release
CGRP was measured in the central compartment as an indication of activation of the sensory neurons of the peristaltic reflex (Fig. 9). Basal release of CGRP was 11.0 ± 0.6 fmol/100 mg. Addition of anandamide, AM-251, or AM-630 to the orad peripheral compartment, the caudad peripheral compartment, or the central compartment had no effect on basal CGRP release into the central compartment (range 9.2 ± 0.4 to 12.9 ± 2.3 fmol/100 mg).
Fig. 9.
Effect of anandamide (1 μM; top) and AM-251 (0.1 μM; bottom) on calcitonin gene-related peptide (CGRP) release into the central sensory compartment. Control release is denoted by open bars, release following addition to peripheral motor compartments by hatched bars, and release following addition to the central sensory compartment by solid bars. Separate colons and animals were used for measuring response after addition to central sensory compartment, and for addition to peripheral motor compartment. Values are means ± SE of 3–6 experiments. *P < 0.05.
Consistent with the inhibition of the mucosal stroking-induced ascending contraction and descending relaxation of circular muscle following addition of anandamide to the central but not peripheral compartments, the release of CGRP was also inhibited following addition of anandamide to the central compartment only. Addition of anandamide to the central compartment inhibited the stroking-induced increase in CGRP release into the central compartment by 70.9 ± 10.4% (P < 0.01) at four strokes and 41.2 ± 10.9% (P < 0.05) at eight strokes.
Consistent with the simultaneous augmentation of mucosal stroking-induced ascending contraction and descending relaxation of circular muscle following addition of the CB-1 antagonist AM-251 to the central but not peripheral compartments, the release of CGRP was also augmented by AM-251. Addition of AM-251 to the central compartment augmented the stroking-induced increase in CGRP release into the central compartment by 78.0 ± 19.15% (P < 0.05) at four strokes and 71.6 ± 16.3% (P < 0.05) at eight strokes. The CB-2 receptor antagonist AM-630 had no effect on stroking-induced CGRP release no matter to which compartment it was added. CGRP release ranged from 101 ± 6 to 112 ± 5% of control values in the presence of AM-630.
Effect of Anandamide and Cannabinoid Receptor Antagonists on Propulsion of Fecal Pellets
To confirm that the effects of anandamide and AM-251 on the peristaltic reflex of the static, compartment preparation of rat colon translated to actual peristaltic activity, we examined their effect on pellet propulsion in the intact, isolated colon of guinea pig. The mean velocity of pellet propulsion in the basal state was 1.1 ± 0.1 mm/s. Consistent with the ability of anandamide to inhibit the peristaltic reflex at several sites, anandamide decreased the velocity of pellet propulsion to 0.35 ± 0.03 mm/s (67.8 ± 2.9% inhibition, P < 0.01). Similarly, consistent with the ability of AM-251 to augment the peristaltic reflex at several sites, AM-251 increased the velocity of pellet propulsion to 1.81 ± 0.14 mm/s (63.4 ± 12.4% augmentation; P < 0.01). The CB-2 receptor antagonist AM-630 had no effect on the velocity of pellet propulsion.
DISCUSSION
The present study used a three-compartment preparation of rat colon to examine the effect of a cannabinoid agonist and selective cannabinoid receptor antagonists on each limb of the peristaltic reflex: ascending excitatory limb, descending inhibitory limb, and sensory limb. The results suggest that each of these limbs is inhibited by endocannabinoids and that the effect is mediated by CB-1 receptors since it is reversed by the CB-1 receptor antagonist but not CB-2 or TRPV1 receptor antagonists. The effects on the peristaltic reflex translated to inhibition of colonic pellet propulsion by an endocannabinoid and augmentation by a CB-1 receptor antagonist.
The fact that the CB-1 receptor antagonist itself augmented each of the phases of the peristaltic reflex and also enhanced pellet propulsion strongly suggests that, under basal conditions, endocannabinoids are released and exert a tonic inhibitory tone or restraint on gut motility and the peristaltic reflex. A similar conclusion was reached by Mancinelli et al. (21) using a different CB-1 antagonist SR 141716A, which caused an increase in phasic and tonic activity of murine distal colon. This tonic restraining influence of endocannabinoids would act physiologically to prevent rapid movement of material through the gut, allowing for adequate digestion. It is also likely that removal of this restraint would play a significant role in the enhanced peristalsis that occurs postprandially.
Exogenous cannabinoids have long been known to inhibit gut motility and secretion. Generally, this effect has been attributed to inhibition of the release of ACh from enteric neurons. In support of this notion is the presence of CB-1 and, more recently, CB-2 receptors on enteric neurons that costain for ChAT (8, 10, 19, 28, 29, 32, 33). ACh is one of the primary transmitters released from the excitatory motor neurons innervating the circular muscle layer, suggesting that cannabinoids might inhibit the ascending contraction limb of the peristaltic reflex. Previous studies by others have examined this and shown convincingly that the ascending contraction limb of the peristaltic reflex is inhibited by cannabinoids acting at cholinergic excitatory motor neurons and/or ascending excitatory interneurons (15, 29, 32, 35). Similarly, a variety of CB-1 antagonists have been shown to enhance the ascending limb of the peristaltic reflex, suggesting a restraining influence of endocannabinoids. Most of these studies have used either an electrical stimulation or distension to initiate ascending contraction. Both of these stimulus modalities are rather broad in that electrical stimulation likely activates many neurons that may not be components of the peristaltic reflex. Intraluminal distension is physiological; however, it is applied throughout the isolated segment and does not allow for easy separation of ascending and descending components of the reflex or of the sensory limb. In contrast, in the present study, we have used mucosal stroking as the stimulus for two reasons. First, stroking does not distend the muscle layer, so it is a better approach to mimic the physiological stimulus generated by the passage of intraluminal contents. Secondly, it can be applied to a discrete location thereby allowing separation of the ascending contraction and descending relaxation component of the peristaltic reflex. Thus we used this approach to confirm results obtained in the more diffuse preparation, which suggested that the ascending contraction limb of the peristaltic reflex is inhibited by the endocannabinoid anandamide and, conversely, augmented by the CB-1 receptor antagonist AM-251. This approach also allows us to examine the other components of the reflex simultaneously.
The excitatory motor neurons to circular muscle are well characterized with regard to topography, electrophysiology, and phenotype. Invariably, they have been shown to express and release both ACh and tachykinins (4). It has long been known that the contractile component of the peristaltic reflex and peristalsis is mediated by ACh if the stimulus is of lower strength but that when the stimulus is of greater strength these neurons additionally release tachykinins as mediators of noncholinergic peristaltic contractions (3, 7, 11). In the present study, we measured SP release as a representative tachykinin released by motor neurons in the ascending limb during peristalsis. We postulated that SP release would be inhibited in a similar manner to ACh. As expected, the results show that SP released during the ascending contractile phase of the peristaltic reflex induced by mucosal stroking is inhibited by anandamide and augmented by the CB-1 receptor antagonist. Thus it is likely that part of ability of the cannabinoid to inhibit colonic motility is the result of the combined inhibition of the release of ACh and SP from the excitatory motor neurons. To our knowledge, this is the first direct demonstration of cannabinoid-induced decrease in SP release during colonic peristalsis although there is ample evidence for the presence of CB-1 receptors on enteric excitatory motor neurons and on SP-containing neurons (8, 19, 29) and of cannabinoids to inhibit noncholinergic excitatory contractions (8, 9, 16, 17, 19).
In contrast to the widely accepted effect of cannabinoids on ascending contraction, the effects on descending relaxation are not as frequently studied or consistently identified. Our studies directly measured the descending relaxation of circular muscle and concomitant release of VIP as a marker of inhibitory motor neuron activity following stimulation of colonic peristalsis by a physiological stimulus. The results demonstrate that anandamide inhibits and the CB-1 antagonist augments the descending relaxation phase of the peristaltic reflex. Consistent with this finding, the release of VIP, one of the transmitters released from inhibitory motor neurons, was also inhibited by anandamide and augmented by the CB-1 antagonist. This is the first study to directly examine this component of the peristaltic reflex. In support of this notion, studies by Storr et al. (31) in rat stomach strips suggested that anandamide inhibited electrically induced nonadrenergic, noncholinergic (NANC) relaxations presumably mediated by intrinsic inhibitory neurons. Conversely, in preparations of stomach from mouse and guinea pig, no effect of cannabinoids on NANC relaxation was evident, suggesting that there may be considerable species differences (25, 34). In studies by Heinemann et al. (15) using intraluminal distension of the guinea pig ileum, the antiperistaltic effects of methanandamide were found to be inhibited by apamin and a NOS inhibitor, N-nitro-l-arginine methyl ester, suggesting a facilitatory role of cannabinoids on descending relaxation. These studies were, however, based on measurement of effect on the peristaltic pressure threshold rather than direct examination of the descending relaxation pathway. A recent paper by Kurjak et al. (20) provides new insight into the complex effects of cannabinoids with regard to inhibitory neurotransmission. In this study using synaptosomes prepared from rat ileum, anandamide was shown to act presynaptically to stimulate VIP release and to stimulate NOS activity via a novel noncannabinoid, non-TRPV1 receptor. This is a biphasic effect evident at lower but not higher concentrations of anandamide. It is not clear whether this effect is in operation additionally in the present study such that the net effect of cannabinoids on release of VIP is the combination of both excitatory and inhibitory mechanisms. The lack of effect of CB-2 receptor antagonists alone and the lack of effect of the CB-2 or TRPV1 receptor antagonist on the response to anandamide in the present study, however, suggests that either this presynaptic effect is not activated under the conditions of our study or not in operation in the rat colon. These studies also strongly suggest that the effects of exogenous anandamide and of endogenously released cannabinoids on peristalsis are mediated via the CB-1 receptor.
Immunohistochemical studies of CB-1 receptors almost universally indicate that CB-1 receptors are absent or very sparse on VIP/NOS neurons, including the inhibitory motor neurons that mediate descending relaxation (28–32). Given the findings of the present study that VIP release induced by mucosal stimulation is inhibited by anandamide and augmented by a CB-1 antagonist, we postulate that the action of cannabinoid is not likely to be at the inhibitory motor neuron itself, but rather at an interneuron in the descending pathway. This would most likely be a descending cholinergic interneuron although Sibaev et al. (29) have demonstrated CB-1 receptors on descending 5-HT-containing interneurons.
A third possible site of action of cannabinoids with regard to the motor limb of the reflex is a direct postjunctional inhibition of contraction or relaxation of smooth muscle. Although most studies have not demonstrated the presence of cannabinoid receptors on smooth muscle itself by immunohistochemical techniques, studies of isolated smooth muscle cells suggest the presence of inhibitory CB-1 receptors on rabbit gastric smooth muscle as determined by RT-PCR and radioligand binding studies (22, 23). Studies in isolated smooth muscle cells suggest that anandamide can act directly via these smooth muscle CB-1 receptors to inhibit ACh-induced contraction by inhibiting PLC-β activity (22). Similarly, studies in human colonic muscle strips suggest that anandamide can act via a non-CB1/CB2 receptor to inhibit ACh-induced contraction of smooth muscle (30). The same direct effect may also be in operation during descending relaxation since other studies of isolated smooth muscle cells suggest that anandamide acting via CB-1 receptors coupled to Gαi may inhibit adenylate cyclase and thereby attenuate relaxation induced by VIP (23). In the present study we cannot eliminate these postjunctional effects as mediating part of the inhibition of ascending contraction and descending relaxation. It is noteworthy, however, that in either case the postjunctional effect would reinforce the inhibition of response resulting from the prejunctional inhibition of release of ACh, SP, and VIP demonstrated in the present study.
Addition of anandamide and the CB-1 antagonist to the central compartment, where the mucosal stroking was applied, inhibited simultaneously the ascending contraction and descending relaxation components of the peristaltic reflex measured in the peripheral compartments. This suggested an effect mediated by an action on the sensory neuron or intrinsic primary afferent neuron since both components were affected. Consistent with this notion, addition of anandamide to the central compartment inhibited VIP release into the caudad peripheral compartment where descending relaxation was measured and inhibited SP release into the orad peripheral compartment where ascending contraction was measured. The CB-1 antagonist had the opposite effect augmenting release of both VIP and SP in the appropriate peripheral compartment. It is interesting to note that the effects of anandamide and to a lesser extent the CB-1 antagonist were slightly greater when added to the central sensory compartment than when added to the peripheral compartments. This may reflect a dual effect on the sensory neuron as well as on interneurons within this central compartment.
An action on the sensory neuron was confirmed by measurement of the sensory marker neurotransmitter, CGRP. Anandamide inhibited and the CB-1 antagonist augmented CGRP release only when added to the central compartment. The finding that CGRP release was not altered when the cannabinoid agonists and antagonists were added to the peripheral compartments confirms that CGRP release was from the sensory limb of the reflex. We believe that this is the first demonstration of an effect of cannabinoids on the enteric sensory neurons or IPANs although CB-1 receptors have been demonstrated on Dogiel type II neurons, the putative IPANs (19, 29). This notion is further supported by studies in other systems where an effect of cannabinoid on sensory neurons has been examined. For example, cultured primary sensory neurons have been shown to express both CB-1 and TRPV1 receptors; in these sensory neurons, anandamide causes inhibition of CGRP release via the CB-1 receptor and CB-1 receptor antagonists have been shown to augment CGRP release stimulated through the TRPV1 receptor (1, 2). Similarly, CB-1 receptors have been shown to mediate inhibition of CGRP release from sensory neurons of the rat trachea, rat bladder, and rat dorsal root ganglion (5, 14, 27). The role of the TRPV1 receptor in mediating the effects of anandamide on the sensory neurons or CGRP release could not be determined in the present study. The TRPV1 receptor antagonist resiniferatoxin caused a strong inhibition of the response to mucosal stroking when added to the central compartment. This prevented the use of this agent to test whether the inhibitory effects of cannabinoids on the sensory limb of the reflex were mediated by TRPV1 receptors. It also suggests that activation of the sensory limb by mucosal stroking normally involves activation of these receptors. This is consistent with earlier studies (13) demonstrating that acute intraluminal capsaicin abolished the peristaltic reflex elicited by mucosal stimulation. Thus it seems likely that stimulation of the TRPV1 receptor would more likely lead to enhancement of the peristaltic reflex and CGRP release rather than mediate the inhibition of the peristaltic reflex and CGRP release in response to exogenous anandamide identified in the present study.
We did not find evidence for a role of the CB-2 receptor in regulating activity of any of the components of the peristaltic reflex circuit under the normal physiological setting used in the present study. This is not too surprising in that the CB-2 receptor has only recently been demonstrated to be present on enteric neurons and its role appears to be much more apparent in pathophysiological conditions such as inflammatory bowel disease (10, 33).
As shown in the present study and by others (15, 21, 28, 29, 35), the cannabinoids are potent inhibitors of peristaltic propulsion. This has largely been attributed to inhibition of the ascending contraction component as a result of CB-1 receptor-mediated inhibition of acetylcholine release. The present study adds new insight into the mechanism of inhibition of propulsion by demonstrating that, in addition to the action on cholinergic motor neurons, the cannabinoids are potent inhibitors of the descending relaxation component and concomitant VIP release. This coupled with the inhibition of the sensory limb and concomitant CGRP release explains the very potent antiperistaltic activity of cannabinoids. More importantly, the effects of the CB-1 antagonist alone demonstrate the presence of an inhibitory endocannabinoid tone that modulates each component of the peristaltic reflex. This tone would normally exert a restraining influence on sensory and motor components of the reflex and dampen gut motility in the resting state. It is likely that reversal of this inhibitory tone would occur during the postprandial period to allow enhanced peristalsis. Exogenous modulation of this endocannabinoid system presents an attractive site for development of potential therapeutic agents.
GRANTS
This research was supported by Grants DK34153 (J. R. Grider), DK15564 (K. S. Murthy), DK49691 (J. F. Kuemmerle), and DK77917 (L. Y. Qiao) from the National Institute of Diabetes, and Digestive and Kidney Diseases of the National Institutes of Health.
REFERENCES
- 1.Ahluwalia J, Urban L, Bevan S, Capogna M, Nagy I. Cannabinoid 1 receptors are expressed by nerve growth factor- and glial cell-derived neurotrophic factor-responsive primary sensory neurons. Neuroscience 110: 747–753, 2002. [DOI] [PubMed] [Google Scholar]
- 2.Ahluwalia J, Urban L, Bevan S, Nagy I. Anadamide regulates neuropeptide release from capsaicin-sensitive primary sensory neurons by activating both the cannabinoid 1 receptor and the vanilloid receptor 1 in vitro. Eur J Neurosci 17: 2611–2618, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Bartho L, Holzer P, Donnerer J, Lembeck F. Evidence for the involvement of substance P in the atropine-resistant peristalsis of the guinea pig ileum. Neurosci Lett 32: 69–74, 1982. [DOI] [PubMed] [Google Scholar]
- 4.Brookes SJH, Steele PA, Costa M. Identification and immunohistochemistry of cholinergic and non-cholinergic circular muscle motor neurons in the guinea-pig small intestine. Neuroscience 42: 863–878, 1991. [DOI] [PubMed] [Google Scholar]
- 5.Brooks JW, Thompson SW, Rice AS, Malcangio M. (s)-AMAP inhibits electrically evoked calcitonin gene-related peptide (CGRP) release from the rat dorsal horn: reversal by cannabinoid receptor antagonist SR141716A. Neurosci Lett 372: 85–88, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Brown AJ Novel cannabinoid receptors. Br J Pharmacol 152: 567–575, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Costa M, Furness JB, Pullin CO, Bornstein J. Substance P enteric neurons mediate the non-cholinergic transmission to the circular muscle of the guinea-pig intestine. Naunyn Schmiedebergs Arch Pharmacol 328: 446–453, 1985. [DOI] [PubMed] [Google Scholar]
- 8.Coutts AA, Irving AJ, Mackie K, Pertwee RG, Anavi-Goffer S. Localization of cannabinoid CB1 receptor immunoreactivity in the guinea pig and rat myenteric plexus. J Comp Neurol 448: 410–422, 2002. [DOI] [PubMed] [Google Scholar]
- 9.Duncan M, Davison JS, Sharkey KA. Endocannabinoids and their receptors in the enteric nervous system. Aliment Pharmacol Ther 22: 667–683, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Duncan M, Mouihate A, Mackie K, Keenan CM, Buckley NE, Davison JS, Patel KD, Pittman QJ, Sharkey KA. Cannabinoid CB2 receptors in the enteric nervous system modulate gastrointestinal contractility in lipopolysaccharide-treated rats. Am J Physiol Gastrointest Liver Physiol 295: G78–G87, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grider JR Tachykinins as transmitters of ascending contractile component of the peristaltic reflex. Am J Physiol Gastrointest Liver Physiol 257: G709–G714, 1989. [DOI] [PubMed] [Google Scholar]
- 12.Grider JR CGRP as a transmitter in the sensory pathway mediating peristaltic reflex. Am J Physiol Gastrointest Liver Physiol 266: G1139–G1145, 1994. [DOI] [PubMed] [Google Scholar]
- 13.Grider JR, Jin JG. Distinct populations of sensory neurons mediate the peristaltic reflex elicited by muscle stretch and mucosal stimulation. J Neurosci 14: 2854–2860, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayn MH, Ballesteros I, deMiguel F, Coyle CH, Tyagi S, Yoshimura N, Chancellor MB, Tyagi P. Functional and immunohistochemical characterization of CB1 and CB2 receptors in rat bladder. Urology 72: 1174–1178, 2008. [DOI] [PubMed] [Google Scholar]
- 15.Heinemann A, Shahbazian A, Holzer P. Cannabinoid inhibition of guinea-pig intestinal peristalsis via inhibition of excitatory and activation of inhibitory neural pathways. Neuropharmacology 38: 1289–1297, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Izzo AA, Camilleri M. Emerging role of cannabinoids in gastrointestinal and liver diseases: basic and clinical aspects. Gut 57: 1140–1155, 2008. [DOI] [PubMed] [Google Scholar]
- 17.Izzo AA, Mascolo N, Borrelli F, Capasso F. Excitatory transmission to the circular muscle of the guinea pig ileum: evidence for the involvement of cannabinoid CB1 receptors. Br J Pharmacol 124: 1363–1368, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin JG, Foxx-Orenstein AE, Grider JR. Propulsion in guinea pig colon induced by 5-HT via 5-HT4 and 5-HT3 receptors. J Pharmacol Exp Ther 288: 93–97, 1999. [PubMed] [Google Scholar]
- 19.Kulkarni-Narla A, Brown DR. Localization of CB1-cannabinoid receptor immunoreactivity in the porcine enteric nervous system. Cell Tissue Res 302: 73–80, 2000. [DOI] [PubMed] [Google Scholar]
- 20.Kurjak M, Hamel AM, Allescher HD, Schusdziarra V, Storr M. Differential stimulatory effect of cannabinoids on VIP release and NO synthase activity in synaptosomal fractions from rat ileum. Neuropeptides 42: 623–632, 2008. [DOI] [PubMed] [Google Scholar]
- 21.Mancinelli R, Fabrizi A, Del Monaco S, Azzena GB, Vargiu R, Colombo GC, Gessa GL. Inhibition of peristaltic activity by cannabinoids in the isolated distal colon of mouse. Life Sci 69: 101–111, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Mahavadi S, Zhou H, Murthy KS. Distinctive signaling by cannabinoid CB1 receptors in smooth muscle cells: absence of Gβγ-dependent activation of PLC-β1. Gastroenterology 126: A275, 2004. [Google Scholar]
- 23.Mahavadi S, Huang J, Grider JR, Murthy KS. Cross-inhibition of muscarinic M3 receptor function by cannabinoid CB1 receptors is mediated by ERK1/2-dependent phosphorylation and activation of RGS4. Gastroenterology 128: A609, 2005. [Google Scholar]
- 24.Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 346: 561–564, 1990. [DOI] [PubMed] [Google Scholar]
- 25.Mule F, Amato A, Baldassano S, Serio R. Involvement of CB1 and CB2 receptors in the modulation of cholinergic neurotransmission in mouse gastric preparations. Pharmacol Res 56: 185–192, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Munro S, Thomas KL, Abushaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 365: 61–65, 1993. [DOI] [PubMed] [Google Scholar]
- 27.Nemeth J, Helyes Z, Than M, Jakab B, Pinter E, Szolcsanyi J. Concentration-dependent dual effect of anandamide on sensory neuropeptide release from isolated rat tracheae. Neurosci Lett 336: 89–92, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Pinto L, Izzo AA, Cascio MG, Bisogno T, Hospodar-Scott K, Brown DR, Mascolo N, Marzo VD, Capasso F. Endocannabinoids as physiological regulators of colonic propulsion in mice. Gastroenterology 123: 227–234, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Sibaev A, Yuce B, Kemmer M, Van Nassauw L, Broedk U, Allescher HD, Goke B, Timmermans JP, Storr M. Cannabinoid-1 (CB1) receptors regulate colonic propulsion by acting at motor neurons within the ascending motor pathways in mouse colon. Am J Physiol Gastrointest Liver Physiol 296: G119–G128, 2009. [DOI] [PubMed] [Google Scholar]
- 30.Smid SD, Bjorklund CK, Svensson KM, Heigis S, Revesz A. The endocannabinoids anadamide and 2-arachidonoylglycerol inhibit cholinergic activity in the human colon. Eur J Pharmacol 575: 168–176, 2007. [DOI] [PubMed] [Google Scholar]
- 31.Storr M, Gaffal E, Saur D, Schusdzairra V, Allescher HD. Effect of cannabinoids on neural transmission in rat gastric fundus. Can J Physiol Pharmacol 80: 67–76, 2002. [DOI] [PubMed] [Google Scholar]
- 32.Storr M, Sibaev A, Marsicano G, Lutz B, Schusdiziarra Timmermans JP, Allescher HD. Cannabinoid receptor type 1 modulates excitatory and inhibitory neurotransmission in mouse colon. Am J Physiol Gastrointest Liver Physiol 286: G110–G117, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Storr MA, Yuce B, Andrews CN, Sharkey KA. The role of the endocannabinoid system in the pathophysiology and treatment of irritable bowel syndrome. Neurogastroenterol Motil 20: 857–868, 2008. [DOI] [PubMed] [Google Scholar]
- 34.Todorov S, Pozzoli C, Zamfirova R, Poli E. Prejunctional modulation of non-adrenergic non-cholinergic (NANC) inhibitory responses in the isolated guinea-pig gastric fundus. Neurogastroenterol Motil 15: 678–682, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Yuece B, Sibaev A, Broedl UC, Marsicano G, Goke B, Lutz B, Allescher HD, Storr M. Cannabinoid type 1 receptor modulates intestinal propulsion by an attenuation of intestinal motor responses within the myenteric part of the peristaltic reflex. Neurogastroenterol Motil 19: 744–753, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Wiley JL, Martin BR. Cannabinoid pharmacology: implications for additional cannabinoid receptor subtypes. Chem Phys Lipids 121: 57–63, 2002. [DOI] [PubMed] [Google Scholar]