Abstract
The reduced folate carrier (RFC) is a major folate transport system in mammalian cells. RFC is highly expressed in the intestine and believed to play a role in folate absorption. Studies from our laboratory and others have characterized different aspects of the intestinal folate absorption process, but little is known about possible existence of accessory protein(s) that interacts with RFC and influences its physiology and/or cell biology. We investigated this issue by employing a bacterial two-hybrid system to screen a BacterioMatch II human intestinal cDNA library using the large intracellular loop between transmembrane domains 6 and 7 of the human RFC (hRFC) as bait. Our screening has resulted in the identification of dynein light chain road block-1 (DYNLRB1) as an interacting partner with hRFC. Existence of a direct protein-protein interaction between hRFC and DYNLRB1 was confirmed by in vitro pull-down assay and in vivo mammalian two-hybrid luciferase assay and coimmunoprecipitation analysis. Furthermore, confocal imaging of live human intestinal epithelial HuTu-80 cells demonstrated colocalization of DYNLRB1 with hRFC. Coexpression of DYNLRB1 with hRFC led to a significant (P < 0.05) increase in folate uptake. On the other hand, inhibiting the endogenous DYNLRB1 with gene-specific small interfering RNA or pharmacologically with a specific inhibitor (vanadate) led to a significant (P < 0.05) decrease in folate uptake. This study demonstrates for the first time the identification of DYNLRB1 as an interacting protein partner with hRFC. Furthermore, DYNLRB1 appears to influence the function and cell biology of hRFC.
Keywords: folate transport, protein-protein interactions, two-hybrid analysis, accessory proteins
folate is an important B-class vitamin, which plays a fundamental role in one-carbon metabolism and is essential for the synthesis of precursors of nucleic acids (15, 39). Folates are also essential for the conversion of methionine to S-adenosylmethionine, which is required for methylation reactions (4, 39). DNA methylation is an important epigenetic determinant of gene expression (3). Hence, cellular deficiency of this essential micronutrient leads to a disturbance in the normal physiology of the cell that ultimately manifests itself in the form of undesirable clinical abnormalities including neural tube defects (13), megaloblastic anemia (46), cardiovascular abnormalities (5), and cancer (10). In contrast to the adverse effects of folate deficiency, optimization of folate body homeostasis is effective in the prevention of neural tube defects (12, 45) and may also be beneficial in reducing the incidence of cardiovascular diseases (5), Alzheimer disease (38), and colorectal cancer (17).
Unlike bacteria and plants, mammals lack the cellular machinery to synthesize folate (6) and thus must obtain the vitamin from exogenous sources. Three different folate transport systems have been identified in mammalian cells: the folate receptors (1), the reduced folate carrier (RFC) (26, 37), and the proton-coupled folate transporter (PCFT) (31). The normal intestine expresses RFC and PCFT only (31, 33, 44). RFC is widely expressed in a variety of other epithelial and nonepithelial cells (24, 42). The RFC protein is predicted to have 12 transmembrane domains with both the amino and carboxy termini predicted to be oriented intracellularly (7, 14). The RFC protein is also predicted to contain a long intracellular loop between transmembrane domains 6 and 7, which is believed to play a role in function and expression of the carrier protein at the cell membrane (23, 32, 36). Furthermore, the backbone of the RFC polypeptide has been shown to be important for membrane targeting and expression of the protein (25). Moreover, intracellular trafficking of the RFC polypeptide appears to involve distinct trafficking vesicles whose movement is critically dependent on intact microtubules (25).
Recent studies with other membrane transporters have identified an array of interacting proteins with these membrane proteins. These interacting proteins were shown to influence different aspects of the physiology and cell biology of these transporters (11, 16, 40). Little, however, is known about possible existence of interacting proteins with intestinal folate transporters (i.e., RFC and PCFT). Availability of such knowledge is important for better understanding of the normal intestinal folate uptake process. Also, a defect in the function of such an interactor(s) may impact the overall vitamin absorption process. Thus in this study we focused on identifying possible interactor(s) with hRFC using a bacterial two-hybrid system to screen a BacterioMatch II human intestinal cDNA library. Our screening effort has led to the identification of dynein light chain road block-1 (DYNLRB1) as an interacting partner with hRFC. We have confirmed this existence of such a direct interaction between DYNLRB1 and hRFC both in vitro (GST pull-down assay) and in vivo (luciferase assay, coimmunoprecipitation) and also showed that the interaction is important fort the function and cell biology of hRFC.
MATERIALS AND METHODS
All chemicals, reagents, and kits were of analytical or molecular biology grade and were obtained from commercial sources. [3H]folic acid (specific activity: 20 Ci/mmol; radiochemical purity: 98%) was obtained from Moravek Biochemicals (Brea, CA). Anti-DYNLRB1 (anti-km23-1) polyclonal antibodies were kindly provided by Dr. K. M. Mulder (Pennsylvania State University College of Medicine, Hershey, PA). The human-derived intestinal epithelial Caco-2 and HuTu-80 cells and human-derived cervical epithelial HeLa-S3 cells were purchased from American Type Culture Collection (Manassas, VA) and were grown in DMEM or MEM supplemented with 10% FBS. HeLa R5 cell line is a RFC-null mutant and was a generous gift from Dr. I. D. Goldman (Albert Einstein Cancer Research Center, Bronx, New York).
BacterioMatch II human intestinal cDNA library screening.
To search for proteins that may interact with the hRFC, the BacterioMatch two-hybrid system (Stratagene) was used to screen a BacterioMatch II human intestinal cDNA library (Stratagene). A fragment of the hRFC encoding the large intracellular loop between transmembrane domains 6 and 7 (amino acids 204 to 264) was used as bait for screening and was cloned in frame into the SmaI/XhoI sites of the pBT vector to generate pBT-hRFC. cDNA library in the pTRG plasmid was screened with pBT-hRFC as specified by the manufacturer. Briefly, the screening reporter competent cells were cotransformed with pBT-hRFC and pTRG-cDNA library plasmids and transformants were selected on the selective screening medium containing 5 mM of 3-amino-1,2,4-triazole (3-AT). Positive clones were then subjected to further screening on a dual selective screening medium (5 mM 3-AT and streptomycin). To validate the detected protein-protein interaction, competent cells were retransformed by use of pTRG purified from the positive clones and recombinant pBT-hRFC.
Mammalian two-hybrid assay.
To establish protein-protein interactions in vivo in mammalian cells, CheckMate Mammalian Two-hybrid System (Promega, Madison, WI) was used following manufacturer's instructions. A fragment of the hRFC encoding the large intracellular loop between transmembrane domains 6 and 7 (amino acids 204 to 264) was cloned in frame into the BamHI/XbaI sites of pBIND fusion vector to generate Gal4 DNA binding domain fused to hRFC. The full coding sequence of the human DYNLRB1 was cloned in frame into the BamHI/XbaI sites of pACT vector to produce the activation domain of herpes simplex virus type 1 VP16 protein fused to DYNLRB1. pBIND-hRFC and pACT-DYNLRB1 (1 μg each) were cotransfected along with the pG5luc vector into HeLa-S3 cells (90% confluence) by using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. The cells were lysed after 48 h of transfection, and Renilla-normalized Firefly luciferase activity was determined by use of the dual luciferase assay system (Promega).
GST pull-down assay.
The full coding sequence of DYNLRB1 was inserted in frame into BamHI/XhoI sites of the bacterial expression vector pGEX-4T-1 (Invitrogen) to produce GST-DYNLRB1 fusion protein in Escherichia coli. Following the manufacturer's instructions, GST-DYNLRB1 fusion protein and GST were purified from BL-21 E. coli cells harboring recombinant pGEX-4T-1 and pGEX-4T-1, respectively, by using the Bulk GST Purification Module (Amersham Biosciences, Piscataway, NJ). The fusion protein and GST were separated by SDS-PAGE (8%), stained with Coomassie brilliant blue, and further used in GST pull-down assay.
For GST pull-down, Caco-2 cells were lysed with 50 mM Tris·HCl, pH 7.4, containing 100 mM KCl, 1% Triton X-100, 2 mM phenylmethylsulfonyl fluoride, 1 μg/ml aprotinin, and 2.5 μg/ml leupeptin. Cleared (14,000 g) postnuclear extracts (1 mg of protein) were incubated for 3–5 h at 4°C with 50 μg of GST-DYNLRB1 or GST bound to glutathione-Sepharose 4B beads. Beads were washed five times with ice-cold PBS, and bound proteins were eluted with 10 mM glutathione and processed for Western blotting. Western blotting was performed using the anti-hRFC polyclonal antibodies as described by us previously (2).
Coimmunoprecipitation analysis.
Caco-2 cells expressing FLAG-tagged hRFC were used in coimmunoprecipitation analysis. The full coding sequence of hRFC was cloned in frame into SalII/BamHI sites of the pFLAG-CMV-2 expression vector (Sigma) to generate FLAG-tagged hRFC. Caco-2 cells (90% confluence) were transiently transfected with pFLAG-CMV-2-hRFC by use of Lipofectamine 2000 (Invitrogen). Cells were lysed after 48–72 h post transfection in a buffer containing 50 mM Tris·HCl, pH 7.4, containing 100 mM KCl, 1% Triton X-100 (lysis buffer), and protease inhibitors (2 mM phenylmethylsulfonyl fluoride, 1 μg/ml aprotinin, and 2.5 μg/ml leupeptin). Postnuclear extracts (0.1–0.5 mg of protein) from transfected and nontransfected (negative control) cells were incubated with 100 μl anti-FLAG M2 affinity gel (Sigma) suspension overnight at 4°C on rotating wheel. Gels were washed three times with lysis buffer and another three times with lysis buffer without Triton X-100. Final pellet was resuspended in SDS-PAGE sample buffer and analyzed by Western blotting (2) using the anti-FLAG M2 monoclonal antibody (Sigma) at 1:5,000 dilutions and anti-DYNLRB1 (anti-km23-1) polyclonal antibody at 1:1,000 dilutions.
Silencing of DYNLRB1 with siRNA.
Caco-2 cells were transiently transfected with DYNLRB1 gene-specific small interfering RNA (siRNA) or scrambled siRNA (Santa Cruz Biotechnology, Santa Cruz, CA) using Lipofectamine 2,000 (Invitrogen). To check the silencing effect of siRNA on DYNLRB1 mRNA, semiquantitative RT-PCR was performed with total RNA isolated by using the TRIzol reagent from appropriate cell samples after 48 h of siRNA transfection. Briefly, 5 μg of total RNA was reverse transcribed with oligo(dT) primers by using Superscript II (Invitrogen). Samples were then diluted with sterile water, and two different dilutions were used for each PCR with specific primers for hRFC, DYNLRB1, and the housekeeping gene 18S rRNA. For hRFC, the primers were forward, 5′-CACCGACTACCTGCGCTACA-3′; reverse, 5′-GCCATGGTGACGCTGTAGAA-3′. For DYNLRB1, the primers were obtained from Santa Cruz Biotechnology (sc 77167-PR; Santa Cruz, CA). For 18S RNA, the primers were forward, 5′-GGGAGGTAGTGACGAAAAATAACAAT-3′; reverse, 5′-TTGCCCTCCAATGGATCCTC-3′. Conditions for semiquantitative RT-PCR were 95°C for 30 s, annealing at 58°C for 30 s, and extension at 72°C for 1 min (33 cycles). The products were analyzed on 2% agarose gels, the images were captured by use of an Eagle Eye II system, and the amplified RT-PCR products were normalized to amplified 18S rRNA controls as described previously (2).
Folic acid uptake.
Uptake of folic acid by confluent monolayers was performed as described by us previously (2) with some modifications. Briefly, cells were incubated at 37°C for 5 min in Krebs-Ringer (K-R) buffer (in mM: 133 NaCl, 4.93 KCl, 1.23 MgSO4, 0.85 CaCl2, 5 glucose, 5 glutamine, 10 HEPES, and 10 MES, pH 7.4) in the presence of labeled and unlabeled folic acid (final concentration of 2 μM). Reaction was terminated by the addition of 2 ml of ice-cold K-R buffer followed by immediate aspiration. Cells were then rinsed twice with ice-cold buffer, lysed with 1 ml of 1 N NaOH, neutralized with 10 N HCl, and then counted for radioactivity. Protein content of cell digests was determined by using a Bio-Rad kit (Bio-Rad, Richmond, VA).
Confocal imaging.
Colocalization of hRFC with DYNLRB1 was performed by live cell confocal imaging following transient transfection of the human intestinal epithelial HuTu-80 cells with hRFC-GFP and DsRed-DYNLRB1 plasmids. Monolayers were grown on glass-bottomed petri dishes (MatTek, Ashland, MA), and imaging was performed with a Nikon C-1 confocal scanner head attached to Nikon inverted phase contrast microscope. Fluorophores were excited by using a 488-nm line from an argon ion laser, and a 543-nm line from a HeNe ion laser-emitted fluorescence was monitored with a 515 ± 30-nm band-pass (GFP) or at 570 ± 50+-nm long-pass (DsRed) filter.
Biotinylation of cell surface proteins.
Biotinylation of cell surface proteins was performed essentially as described by Zimnicka et al. (49) with minor modifications. Briefly, HuTu-80 monolayers were exposed from surface with Sulfo-NHS-SS-biotin (no. 21331, Pierce Biotechnology, Rockford, IL) diluted into a biotinylation buffer (PBS, pH 7.5, supplemented with 10 mM triethanolamine, 2 mM CaCl2, and 150 mM NaCl) to the working concentration of 1 mg/ml, then incubated for 1 h at 4°C in horizontal motion. Cells were then quenched with PBS containing CaCl2, MgCl2, and 100 mM glycine for 20 min at 4°C. Cells were lysed in lysis buffer (150 mM NaCl, 50 mM Tris·HCl, pH 7.4, 5 mM EDTA, 1% Triton X-100). After centrifugation, the supernatant was incubated overnight in streptavidin agarose and then washed three times with lysis buffer. The streptavidin agarose beads were spun down, and sample loading buffer for SDS-PAGE was added. Separated proteins were probed with anti-hRFC antibodies and visualized with enhanced chemiluminescence reagent.
Statistical analysis.
Experimental points of transport studies are means ± SE of multiple separate uptake determinations and were expressed in terms of either femtomoles or picomoles per milligram protein per 5 min. Carrier-mediated folate uptake was determined as described by us previously (2). Statistical analysis was performed by the Student's t-test or one-way ANOVA with statistical significance being set at 0.05. All transfection studies, semiquantitative PCR, and Western blot analysis were performed on at least three separate occasions with comparable results. Data presented are from a representative set of experiments.
RESULTS
Identification of DYNLRB1 as a potential interacting protein partner of hRFC using bacterial two-hybrid assay.
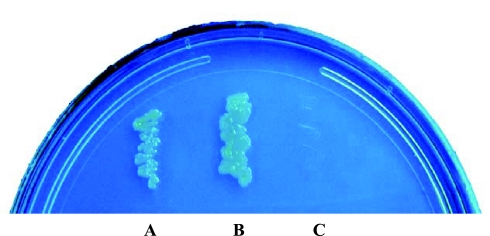
We used the bacterial two-hybrid system to screen a BacterioMatch II human intestinal cDNA library to search for proteins that may interact with the hRFC. The large intracellular loop between transmembranes 6 and 7 (amino acids 204 to 264) of hRFC was used as the bait in screening since it has been reported to play a critical role in function and expression of the RFC at the plasma membrane (23, 32, 36). The BacterioMatch II human intestinal cDNA library (primary size 1.67 × 106; Stratagene) was screened with the generated recombinant pBT-hRFC plasmid. We screened 2.1 × 106 clones and identified 55 positive clones grown on the selective screening medium containing 5 mM of 3-AT. These positive clones were then subjected to further screening on a dual selective screening medium (5 mM 3-AT and streptomycin), and 18 positive clones were identified. To validate the detected protein-protein interactions, we performed retransformation of the reporter strain using pTRG purified from positive clones and recombinant pBT-hRFC. Our results showed the identification of eight clones that reproducibly grow on selective screening medium (5 mM 3-AT) when cotransformed with the bait protein and that failed to grow on selective screening medium when cotransformed with the empty pBT vector. Among the eight identified clones, one clone was found to have a full sequence that is identical to the open reading frame of the dynein road block light chain 1 (DYNLRB1)/km23–1/dynein light chain 2A (DNCL2A) (GenBank accession number NM_014183) (19, 29, 43). The results of a representative verification of the interaction between hRFC and DYNLRB1 are shown in Fig. 1.
Fig. 1.
Protein-protein interaction between human reduced folate carrier (hRFC) and dynein light chain road block-1 (DYNLRB1). Each pair of plasmids (A, pBT-LGF2 + pTRG-Gal 11p; B, pTRG-DYNLRB1 + pBT-hRFC; C, pTRG-DYNLRB1 + pBT-Vector) was cotransformed into BacterioMatch II validation reporter-competent cells. Bacterial transformants were grown on the selective screening medium [5 mM 3-amino-1,2,4-triazole (3-AT)]. The known interaction between LGF2 and Gal 11p (A) serves as a positive control.
hRFC and DYNLRB1 interact in vivo in mammalian two-hybrid luciferase assay.
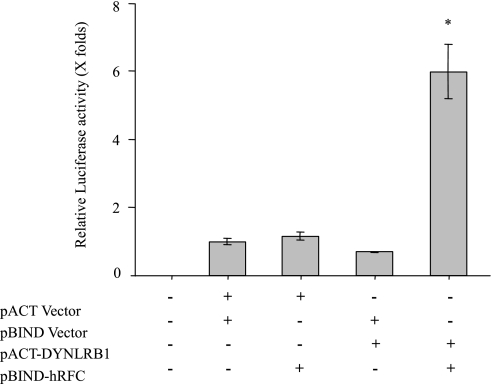
We examined the interaction of hRFC with DYNLRB1 in mammalian cells by using a mammalian two-hybrid system. In this system the interaction between the two test proteins expressed as GAL4 and VP16 fusion constructs results in transcription of the Firefly luciferase gene. Fragment of the hRFC encoding the large intracellular loop between transmembrane domains 6 and 7 (amino acids 204 to 264) was cloned in frame into the pBIND fusion vector to generate a fusion complex with Gal4 DNA binding domain. The full coding sequence of the DYNLRB1 was cloned in frame into the pACT vector to produce the activation domain of herpes simplex virus type 1 VP16 protein fused to DYNLRB1. HeLa S3 cells were cotransfected with pBIND-hRFC and pACT-DYNLRB1 plasmids along with the pG5luc vector, and 48 h posttransfection Renilla-normalized Firefly luciferase activity was determined. Our results (Fig. 2) showed the significant increase (∼6-fold) in luciferase activity of cells cotransfected with hRFC and DYNLRB1 fusion constructs compared with negative controls. Thus DYNLRB1 appears to interact with the hRFC in mammalian cells, which confirms our previous findings in bacterial cells with a bacterial two-hybrid system.
Fig. 2.
Interaction of hRFC and DYNLRB1 in vivo: mammalian 2-hybrid luciferase assay. Plasmids were transfected along with the pG5luc vector into HeLa S3 cells. Cells were lysed after 48 h of transfection, and Renilla-normalized Firefly luciferase activity was determined by using the dual luciferase assay system. Data are presented as means ± SE of at least 3 independent experiments and Firefly luciferase expression given in folds over the background (set arbitrarily at 1). *P < 0.01.
GST-DYNLRB1 fusion protein binds with hRFC in human intestinal epithelial cells (GST pull-down assay).
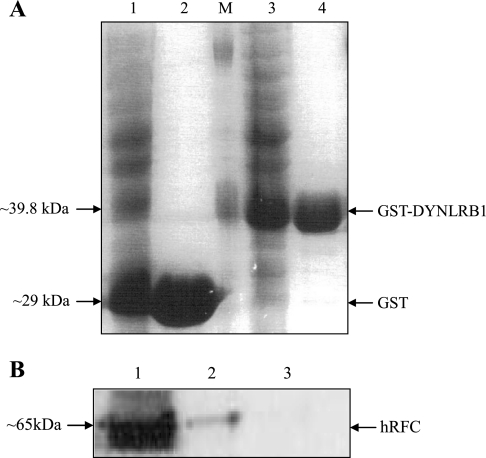
To further confirm the existence of the interaction between hRFC and DYNLRB1 in human intestinal cells, we performed in vitro GST pull-down assay using a GST-fused DYNLRB1 and lysate from the Caco-2 cells. For this, we generated and affinity purified GST-DYNLRB1 fusion protein and GST from BL-21 E. coli cells harboring recombinant pGEX-4T-1 and pGEX-4T-1, respectively (Fig. 3A). Caco-2 cells lysate was incubated with glutathione-Sepharose 4B beads adsorbed with GST-DYNLRB1 or GST alone (negative control). The bound proteins were eluted with glutathione and processed for Western blotting using anti-hRFC antibodies previously generated and characterized in our laboratory (2). The results (Fig. 3B) showed that GST-DYNLRB1 pulled down the hRFC from Caco-2 extract, whereas GST did not bind with the hRFC. These data suggest that DYNLRB1 indeed binds with hRFC in intestinal Caco-2 cells extracts.
Fig. 3.
Pull-down assay of hRFC from human intestinal epithelial Caco-2 cells by GST-DYNLRB1. A: generation and purification of GST-DYNLRB1 fusion protein and GST. Extracts from BL-21 Escherichia coli cells harboring recombinant pGEX-4T-1 (1), purified GST (2), pGEX-4T-1-DYNLRB1 (3), purified GST-DYNLRB1 (4), and marker (M). Protein samples were run on SDS-PAGE and stained with Coomassie blue. B: Caco-2 cells lysate was incubated with glutathione-Sepharose 4B beads adsorbed with GST-DYNLRB1 or GST alone (negative control). Proteins bound to the beads were separated, washed and analyzed by Western Blotting using anti-hRFC antibodies. 1, Caco-2 cell lysate; 2, proteins bound to GST-DYNLRB1; 3, proteins bound to unfused GST.
Interaction between DYNLRB1 and hRFC in vivo in human intestinal epithelial cells (coimmunoprecipitation assay).
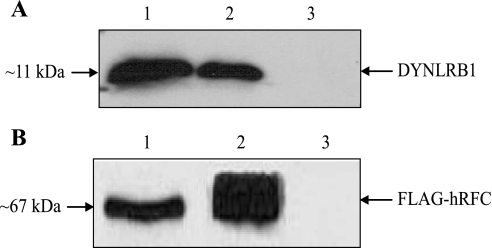
We also studied whether the interaction between DYNLRB1 and hRFC occurred in vivo in Caco-2 cells by coimmunoprecipitation. To this end, we cloned the open reading frame of hRFC into pFLAG-CMV-2 expression vector to generate FLAG-tagged hRFC. Caco-2 cells were transiently transfected with pFLAG-CMV-2-hRFC, and after 48–72 h posttransfection cell extracts were used for coimmunoprecipitation assay. Anti-FLAG M2 affinity gel was incubated with extracts prepared from transfected and nontransfected (negative control) Caco-2 cells, and then the bound proteins (FLAG-hRFC immunoprecipitates) were analyzed by Western blotting using the anti-FLAG M2 monoclonal antibody and anti-DYNLRB1 polyclonal antibodies. The results (Fig. 4) showed that the FLAG-hRFC immunoprecipitate obtained from pFLAG-CMV-2-hRFC transfected cells contains DYNLRB1. In contrast, DYNLRB1 from nontransfected cells was not detected among the proteins bound to anti-FLAG M2 affinity gel. These data suggest that hRFC and DYNLRB1 interact in vivo in human intestinal epithelial Caco-2 cells.
Fig. 4.
DYNLRB1 coimmunoprecipitates with hRFC from human intestinal epithelial Caco-2 cells. Anti-FLAG M2 affinity gel was incubated with Caco-2 cell extracts prepared from pFLAG-CMV-2-hRFC transfected or nontransfected (negative control) cells. Bound proteins were then analyzed by Western blotting using anti-DYNLRB1 polyclonal antibodies (A) and anti-FLAG M2 monoclonal antibody (B). 1, Extract from cells transfected with pFLAG-CMV-2-hRFC; 2, immunoprecipitate obtained from cells transfected with pFLAG-CMV-2-hRFC; 3, immunoprecipitate obtained from nontransfected cells.
Functional implications of the interaction between hRFC and DYNLRB1.
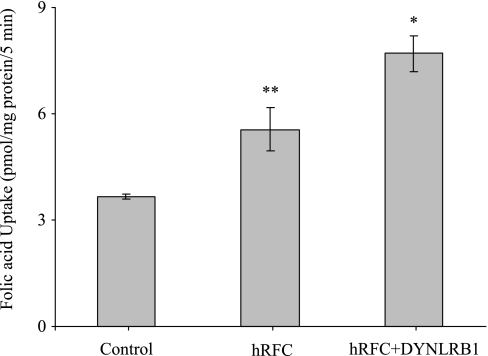
On the basis of the identified physical interaction between DYNLRB1 and hRFC, we investigated the consequences of this interaction on the functionality of hRFC. This was performed by measuring the [3H]folic acid uptake (2 μM; pH 7.4) in HeLa R5 cells transfected with hRFC in the presence and absence of DYNLRB1. The results showed that the coexpression of hRFC with DYNLRB1 leads to a significant (P < 0.05) increase in RFC-mediated folic acid uptake compared with cells transfected with hRFC alone (Fig. 5). Similarly, uptake of folic acid (2 μM; pH 7.4) in the human intestinal epithelial HuTu-80 cells was significantly (P < 0.05) increased with cotransfecting hRFC and DYNLRB1 compared with uptake by the cells transfected with hRFC alone (6.84 ± 0.6 and 5.2 ± 0.2 pmol/mg protein, respectively).
Fig. 5.
Overexpression of DYNLRB1 increases carrier-mediated folic acid uptake in HeLa R5 cells. Cells were transiently cotransfected with hRFC-pFLAG and DYNLRB1-pFLAG. After 48 h of transfection, initial rate of [3H]folic acid (2 μM) uptake was measured by incubating the cells in Krebs-Ringer buffer, pH 7.4 at 37°C for 5 min. Values are means ± SE of 3–4 separate uptake determinations. *P < 0.01, **P < 0.05.
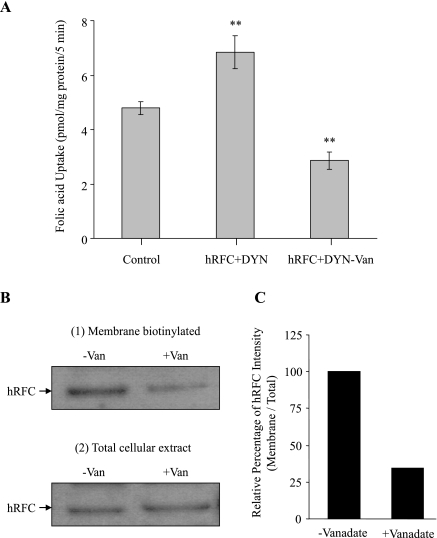
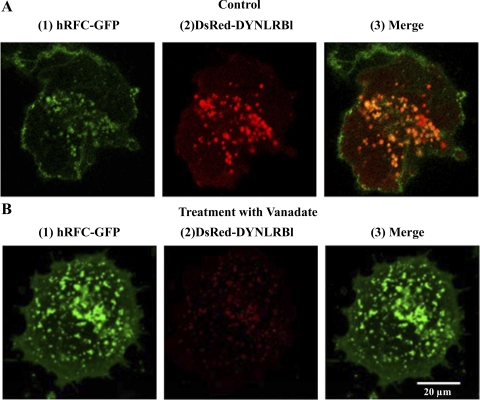
In another approach, we examined the effect of inhibiting the endogenous DYNLRB1 using molecular (gene silencing with use of gene-specific siRNA) and pharmacological (vanadate treatment) approaches on functionality of the endogenous hRFC in intestinal epithelial cells. Results of the gene-knockdown approaches showed a significant (P < 0.05) decrease in the uptake of folic acid (2 μM; pH 7.4) in Caco-2 cells treated with DYNLRB1 siRNA compared with those treated with scrambled siRNA as well as that of control (Fig. 6A). Effectiveness and specificity of the DYNLRB1 knockdown was verified by semiquantitative RT-PCR, which showed a marked reduction in mRNA levels of DYNLRB1 in the cells treated with DYNLRB1 gene-specific siRNA compared with control. On the other hand, no change in hRFC and 18S rRNA expression levels was observed upon DYNLRB1 siRNA treatment (Fig. 6B). In the pharmacological approach, we used vanadate (an agent that disrupts the function of dynein-related proteins; Ref. 28) to study the effect of inhibiting DYNLRB1 on folic acid uptake (2 μM; pH 7.4) in HuTu-80 cells cotransfected with hRFC and DYNLRB1. The results showed significant (P < 0.05) reduction in folic acid uptake in vanadate-treated compared with untreated cells (Fig. 7A). We also examined the effect of DYNLRB1 inhibition on cell surface expression of hRFC in HuTu-80 cells. The results demonstrated that cell surface expression of hRFC to be significantly (P < 0.05) decreased in vanadate-treated compared with untreated control cells (Fig. 7B). Total cellular levels of hRFC, however, were not affected by the vanadate treatment, indicating that vanadate did not affect the total hRFC expression level or processing. We also examined the effect of vanadate on the colocalization of DYNLRB1 with hRFC using live cell confocal imaging. As expected, DYNLRB1 colocalized with hRFC in control untreated HuTu-80 cells (Fig. 8A). However, in vanadate-treated cells, a marked decrease in DYNLRB1 colocalization with hRFC was clearly evident (Fig. 8B).
Fig. 6.
Knockdown of endogenous DYNLRB1 with gene-specific siRNA decreases carrier-mediated folic acid uptake. A: Caco-2 cells were grown in DMEM medium and treated with DYNLRB1 specific small interfering RNA (siRNA). Initial rate (5 min) of [3H]folic acid (2 μM; pH 7.4) uptake was measured after 48 h of siRNA transfection. Values are means ± SE of 3–4 separate uptake determinations. *P < 0.01. B: semiquantitative RT-PCR analysis was performed with mRNA from Caco-2 cells and the specific primers for DYNLRB1, hRFC, and 18S rRNA as described in materials and methods. Results of a representative experiment are shown.
Fig. 7.
Effect of vanadate treatment on carrier-mediated folic acid uptake and on cell surface expression of hRFC. A: HuTu-80 cells cotransfected with hRFC-pFLAG and DYNLRB1-pFLAG were treated with (+) or without (−) vanadate (Van; 100 μM) for 6 h at 37°C. A: initial rate (5 min) of [3H]folic acid (2 μM; pH 7.4) uptake was measured after 24 h of vanadate pretreatment. Values are means ± SE of 3–4 separate uptake determinations. **P < 0.05. B: transfected HuTu-80 cells grown in the presence or absence of vanadate were exposed to sulfo-NHS-SS-biotin for 1 h at 4°C. Cells were then quenched and lysed. Biotinylated proteins were extracted with sample loading buffer for SDS-PAGE and analyzed by Western blotting. The hRFC proteins were detected with anti-hRFC specific antibodies. The biotinylated membranous hRFC levels were normalized to the total amount of cellular hRFC. C: densitometric analysis of cell surface expression of hRFC with or without vanadate treatment. Image and data shown are representative of 3 separate sets of experiments.
Fig. 8.
Effect of vanadate treatment on subcellular distribution of DYNLRB1 and hRFC determined by confocal laser fluorescence microscopy. Full length of DYNLRB1 was inserted into the pDsRed-C1 as a fusion protein (DsRedC1-DYNLRB1) and full length of hRFC was inserted into EGFP-N3 as a fusion protein (hRFC-GFPN3). HuTu-80 cells were grown on glass-bottomed petri dishes and cotransfected with hRFC-GFP (1) and DsRed-DYNLRB1 (2). Transfected cells were treated with vanadate (100 μM) for 6 h at 37°C and imaging was done by confocal microscopy. A(3) shows a merged picture showing colocalization of hRFC and DYNLRB1 as yellow in color.
DISCUSSION
Understanding the cellular and molecular mechanisms involved in the intestinal folate absorption process and their regulation is of significant physiological and nutritional importance. This has been the subject of interest in our laboratory and others for over three decades (reviewed in Refs. 34 and 47). Whereas a significant progress has been made so far in our understanding of the folate uptake systems involved (RFC and PCFT), very little is currently known about possible existence of accessory proteins that may influence the physiology and cell biology of the intestinal folate uptake systems. In this study, we focused on hRFC and used bacterial two-hybrid analysis to screen human intestinal cDNA library. Our effort led to the identification of DYNLRB1 as an interacting partner with hRFC. DYNLRB1 is a small protein that forms an integral part of the cytoplasmic dynein motor protein complex in eukaryotic cells, which binds and links cargo to the motor machinery of the cell (20, 21). The dynein complex plays a role in regulating cell migration, cell division, maintenance of Golgi apparatus integrity, and transport of intracellular vesicles (22, 30). DYNLRB1 has also been reported to interact with TGF-β membrane receptor (41) and with GTPase Rab6 (43). The existence of direct interactions between hRFC and DYNLRB1 observed in our screening was confirmed in vitro by GST-pull-down assay as well as in vivo in mammalian cells by two-hybrid luciferase and coimmunoprecipitation assays. Furthermore, confocal imaging of living human intestinal epithelial cells expressing hRFC-GFP and DsRed-DYNLRB1 showed colocalization of the two proteins, thus providing further support for existence of interaction.
Following establishment of existence of direct interaction between hRFC and DYNLRB1 in vitro and in vivo, we then moved to investigate the effect of such an interaction on physiology and cell biology of hRFC. Physiological (functional) consequences of the interaction between hRFC with DYNLRB1 was examined by coexpressing the two proteins in HeLa R5 cells (a cell line that does not express hRFC; Ref. 9) and in human intestinal epithelial HuTu-80 cells followed by examining the effect of this coexpression on hRFC transport function, i.e., on [3H]folic acid uptake. Uptake studies were performed by using a folic acid concentration of 2 μmol/l at pH 7.4 to eliminate the contributions of the folate receptor (which functions in the nanomolar range; Ref. 27) and PCFT (which functions at acidic pH; Ref. 48) to the uptake process. The results showed that coexpressing hRFC with DYNLRB1 leads to a significant increase in folic acid uptake by both HeLa R5 and HuTu-80 cells compared with cells expressing hRFC alone. These findings clearly suggest a role for DYNLRB1 in the function of hRFC. This was confirmed by the studies in which DYNLRB1 was inhibited by molecular (gene-specific siRNA) and pharmacological (vanadate treatment) means. Knocking down the endogenous DYNLRB1 by gene-specific siRNA (verified by PCR) led to a significant inhibition in folic acid uptake. Similarly, treatment with vanadate led to a significant inhibition in folic acid uptake by cells coexpressing hRFC and DYNLRB1. These findings further confirm a role for DYNLRB1 in hRFC transport physiology.
To understand the role of DYNLRB1 in hRFC cell biology, we examined the effect of inhibiting DYNLRB1 (with vanadate) on membrane expression of hRFC as well as on intracellular colocalization of the two proteins. Effect of DYNLRB1 inhibition on cell surface expression of hRFC in intestinal epithelial cells was investigated by performing membrane biotinylation assay before and after vanadate treatment. The results showed a marked decrease in the levels of hRFC membrane expression in vanadate-treated compared with untreated control cells. This decrease in membrane expression of hRFC may explain the significant decrease in folic acid uptake observed upon inhibition of DYNLRB1. In line with these findings is the observation that DYNLRB1 inhibition also leads to a marked decrease in its colocalization with hRFC. These observations imply a role for DYNLRB1 in the cell biology and membrane expression of hRFC, an effect that ultimately affects the physiology of hRFC. This role for DYNLRB1 in hRFC physiology resembles the role played by another member of the light chain dynein complex (Tctex-1) that interacts with voltage-dependent anion-selective channel 1 and influences its physiology and biology (35). It is interesting to mention here that the expression of DYNLRB1 is upregulated in hepatocellular carcinoma (18) and that overexpression of this protein promotes cell survival and inhibits apoptosis in differentiated PC12 cells (8).
In summary, our results demonstrate for the first time the identification of DYNLRB1 as an interacting protein partner with the hRFC in human intestinal epithelial cells and show that this interaction may influence both the cell biology and physiology of hRFC.
GRANTS
This study was supported by grants from the National Institutes of Health (Grants DK-075348 and DK-56061) and Department of Veterans Affairs.
REFERENCES
- 1.Antony AC Folate receptors. Annu Rev Nutr 16: 501–521, 1996. [DOI] [PubMed] [Google Scholar]
- 2.Ashokkumar B, Mohammed ZM, Vaziri ND, Said HM. Effect of folate oversupplementation on folate uptake by human intestinal and renal epithelial cells. Am J Clin Nutr 86: 159–166, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Bai S, Ghoshal K, Datta J, Majumder Yoon SO, Jacob ST. DNA methyltransferase 3b regulates nerve growth factor-induced differentiation of PC12 cells by recruiting histone deacetylase 2. Mol Cell Biol 25: 751–766, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 4.Bailey LB, Moyers S, Gregory JF. Folate. In: Present Knowledge in Nutrition, edited by Bowman BA and Russell RM. Washington, DC: ILSI, 2001, p. 214–229.
- 5.Boushey CJ, Beresford SA, Omenn GS, Motulsky AG. A quantitative assessment of plasma homocysteine as a risk factor for vascular disease. Probable benefits of increasing folic acid intakes. JAMA 274: 1049–1057, 1995. [DOI] [PubMed] [Google Scholar]
- 6.Brzezinska A, Winska P, Balinska M. Cellular aspects of folate and antifolate membrane transport. Acta Biochim Pol 47: 735–749, 2000. [PubMed] [Google Scholar]
- 7.Cao W, Matherly LH. Characterization of a cysteine-less human reduced folate carrier: localization of a substrate-binding domain by cysteine-scanning mutagenesis and cysteine accessibility methods. Biochem J 374: 27–36, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang YW, Jakobi R, McGinty A, Foschi M, Dunn MJ, Sorokin A. Cyclooxygenase 2 promotes cell survival by stimulation of dynein light chain expression and inhibition of neuronal nitric oxide synthase activity. Mol Cell Biol 20: 8571–8579, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chattopadhyay S, Wang Y, Zhao R, Goldman ID. Lack of impact of the loss of constitutive folate receptor alpha expression, achieved by RNA interference, on the activity of the new generation antifolate pemetrexed in HeLa cells. Clin Cancer Res 10: 7986–7993, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Choi SW, Mason JB. Folate and carcinogenesis: an integrated scheme. J Nutr 130: 129–132, 2000. [DOI] [PubMed] [Google Scholar]
- 11.Cubelos B, Gonzalez-Gonzalez IM, Gimenez C, Zafra F. The scaffolding protein PSD-95 interacts with the glycine transporter GLYT1 and impairs its internalization. J Neurochem 95: 1047–1058, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Czeizel AE Primary prevention of neural-tube defects and some other major congenital abnormalities: recommendations for the appropriate use of folic acid during pregnancy. Paediatr Drugs 2: 437–449, 2000. [DOI] [PubMed] [Google Scholar]
- 13.Daly LE, Kirke PN, Molloy A, Weir DG, Scott JM. Folate levels and neural tube defects. Implications for prevention. JAMA 274: 1698–1702, 1995. [DOI] [PubMed] [Google Scholar]
- 14.Ferguson PL, Flintoff WF. Topological and functional analysis of the human reduced folate carrier by hemagglutinin epitope insertion. J Biol Chem 274, 16269–16278, 1999. [DOI] [PubMed] [Google Scholar]
- 15.Herbert V Folic acid. In: Modern Nutrition in Health and Disease (9th ed.), edited by Shills ME, Olson JA, Shike M, and Ross AH. London: Lippincott, Williams & Wilkins, 1999, p. 433–446.
- 16.Ikari A, Nakano M, Kawano K, Suketa Y. Up-regulation of sodium-dependent glucose transporter by interaction with heat shock protein 70. J Biol Chem 451: 133–139, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Jaszewski R, Misra S, Tobi M, Ullah N, Naumoff JA, Kucuk O, Levi E, Axelrod BN, Patel BB, Majumdar AP. Folic acid supplementation inhibits recurrence of colorectal adenomas: a randomized chemoprevention trial. World J Gastroenterol 14: 4492–4498, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang J, Yu L, Huang X, Chen X, Li D, Zhang Y, Tang L, Zhao S. Identification of two novel human dynein light chain genes, DNLC2A and DNLC2B, and their expression changes in hepatocellular carcinoma tissues from 68 Chinese patients. Gene 281: 103–113, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Jin Q, Ding W, Mulder KM. Requirement for the dynein light chain km23-1 in a Smad2-dependent TGF-beta signaling pathway. J Biol Chem 282: 19122–19132, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Kamal A, Goldstein LSB. Principles of cargo attachment to cytoplasmic motor proteins. Curr Opin Cell Biol 14: 63–68, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Karcher RL, Deacon SW, Gelfand VI. Motor-cargo interactions: the key to transport specificity. Trends Cell Biol 12: 21–27, 2002. [DOI] [PubMed] [Google Scholar]
- 22.King SM The dynein microtubule motor. Biochim Biophys Acta 1496: 60–75, 2000. [DOI] [PubMed] [Google Scholar]
- 23.Liu XY, Witt TL, Matherly LH. Restoration of high-level transport activity by human reduced folate carrier/ThTr-1 thiamine transporter chimaeras: role of the transmembrane domain 6/7 linker region in reduced folate carrier function. Biochem J 369: 31–37, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maddox DM, Manlapat A, Roon P, Prasad P, Ganapathy V, Smith SB. Reduced-folate carrier (RFC) is expressed in placenta and yolk sac, as well as in cells of the developing forebrain, hindbrain, neural tube, craniofacial region, eye, limb buds and heart. BMC Dev Biol 3: 6, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marchant JS, Subramanian VS, Parker I, Said HM. Intracellular trafficking and membrane targeting mechanisms of the human reduced folate carrier in mammalian epithelial cells. J Biol Chem 277: 33325–33333, 2002. [DOI] [PubMed] [Google Scholar]
- 26.Matherly LH Molecular and cellular biology of the human reduced folate carrier. Prog Nucleic Acid Res Mol Biol 67: 131–162, 2001. [DOI] [PubMed] [Google Scholar]
- 27.McMartin KE, Morshed KM, Hazen-Martin DJ, Sens DA. Folate transport and binding by cultured human proximal tubule cells. Am J Physiol Renal Fluid Electrolyte Physiol 263: F841–F848, 1992. [DOI] [PubMed] [Google Scholar]
- 28.Niclas J, Allan VJ, Vale RD. Cell cycle regulation of dynein association with membranes modulates microtubule-based organelle transport. J Cell Biol 133: 585–93, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pfister KK, Fisher EM, Gibbons IR, Hays TS, Holzbaur EL, McIntosh JR, Porter ME, Schroer TA, Vaughan KT, Witman GB, King SM, Vallee RB. Cytoplasmic dynein nomenclature. J Cell Biol 171: 411–413, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pfister KK, Shah PR, Hummerich H, Russ A, Cotton J, Annuar AA, King SM, Fisher EM. Genetic analysis of the cytoplasmic dynein subunit families. PLoS Genet 2: e1, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qiu A, Jansen M, Sakaris A, Min SH, Chattopadhyay S, Tsai E, Sandoval C, Zhao R, Akabas MH, Goldman ID. Identification of an intestinal folate transporter and the molecular basis for hereditary folate malabsorption. Cell 127: 917–928, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Sadlish H, Williams FF, Flintoff WF. Cytoplasmic domains of the reduced folate carrier are essential for trafficking, but not function. Biochem J 364: 777–786, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Said HM, Nguyen TT, Dyer DL, Cowan KH, Rubin SA. Intestinal folate transport: identification of a cDNA involved in folate transport and the functional expression and distribution of its mRNA. Biochim Biophys Acta 1281: 164–172, 1996. [DOI] [PubMed] [Google Scholar]
- 34.Said HM, Seetharam B. Intestinal absorption of water-soluble vitamins. In: Physiology of the Gastrointestinal Tract (4th ed.), edited by Johnson LR. San Diego, CA: Elsevier, 2006, p. 1791–1825.
- 35.Schwarzer C, Barnikol-Watanabe S, Thinnes FP, Hilschmann N. Voltage-dependent anion-selective channel (VDAC) interacts with the dynein light chain Tctex1 and the heat-shock protein PBP74. Int J Biochem Cell Biol 34: 1059–1070, 2002. [DOI] [PubMed] [Google Scholar]
- 36.Sharina IG, Zhao R, Wang Y, Babani S, Goldman ID. Role of the C-terminus and the long cytoplasmic loop in reduced folate carrier expression and function. Biochem Pharmacol 63: 1717–1724, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Sirotnak FM, Tolner B. Carrier-mediated membrane transport of folates in mammalian cells. Annu Rev Nutr 19: 91–122, 1999. [DOI] [PubMed] [Google Scholar]
- 38.Sommer BR, Hoff AL, Costa M. Folic acid supplementation in dementia: a preliminary report. J Geriatr Psychiatry Neurol 16: 156–159, 2003. [DOI] [PubMed] [Google Scholar]
- 39.Stokstad ELR Historical perspective on key advances in the biochemistry and physiology of folates. In: Folic Acid Metabolism in Health and Disease, edited by Picciano MF, Stokstad ELR, and Gregory JF. New York: Wiley, 1990, p. 1–21.
- 40.Sun AQ, Balasubramaniyan N, Liu CJ, Shahid M, Suchy FJ. Association of the 16-kDa subunit c of vacuolar proton pump with the ileal Na-dependent bile acid transporter. J Biol Chem 279: 16295–16300, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Tang Q, Staub CM, Gao G, Jin Q, Wang Z, Ding W, Aurigemma RE, Mulder KM. A novel transforming growth factor-beta receptor-interacting protein that is also a light chain of the motor protein dynein. Mol Biol Cell 13: 4484–4496, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Y, Zhao R, Russell RG, Goldman ID. Localization of the murine reduced folate carrier as assessed by immunohistochemical analysis. Biochim Biophys Acta 1513: 49–54, 2001. [DOI] [PubMed] [Google Scholar]
- 43.Wanschers B, Van de Vorstenbosch R, Wijers M, Wieringa B, King SM, Fransen J. Rab6 family proteins interact with the dynein light chain protein DYNLRB1. Cell Motil Cytoskeleton 65: 183–196, 2008. [DOI] [PubMed] [Google Scholar]
- 44.Weitman SD, Lark RH, Coney LR, Fort DW, Frasca V, Zurawski VR Jr, Kamen BA. Distribution of the folate receptor GP38 in normal and malignant cell lines and tissues. Cancer Res 52: 3396–3401, 1992. [PubMed] [Google Scholar]
- 45.Werler MM, Shapiro S, Mitchell AA. Periconceptional folic acid exposure and risk of occurrent neural tube defects. JAMA 269: 1257–1261, 1993. [PubMed] [Google Scholar]
- 46.Wickramasinghe SN The wide spectrum and unresolved issues of megaloblastic anemia. Semin Hematol 36: 3–18, 1999. [PubMed] [Google Scholar]
- 47.Zhao R, Matherly LH, Goldman ID. Membrane transporters and folate homeostasis: intestinal absorption and transport into systemic compartments and tissues. Expert Rev Mol Med 11: e4, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao R, Min SH, Wang Y, Campanella E, Low PS, Goldman ID. A role for the proton-coupled folate transporter (PCFT-SLC46A1) in folate receptor-mediated endocytosis. J Biol Chem 284: 4267–4274, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zimnicka AM, Maryon EB, Kaplan JH. Human copper transporter hCTR1 mediates basolateral uptake of copper into enterocytes: implications for copper homeostasis. J Biol Chem 282: 26471–26480, 2007. [DOI] [PubMed] [Google Scholar]