Abstract
It is unclear whether the broad inflammatory response shown in neonatal necrotizing enterocolitis (NEC) is the cause or the effect of tissue injury. Toll-like receptors (TLRs) on intestinal dendritic, mononuclear, and epithelial cells recognize bacterial ligands and damaged tissues, thus activating the inflammatory response. The present study aimed to determine whether active TLR signaling would precede histological injury in NEC. Newborn rat pups were divided into four groups: dam fed, dam fed-hypoxic, formula fed, and formula fed-hypoxic (NEC). The ileal tissues were evaluated for NEC scores at 24, 48, 72, and 120 h. Quantitative real-time reverse transcription-polymerase chain reaction and immunohistochemistry were used to measure and localize intestinal TLRs. Cytokines were assessed by a multispot cytokine array. Among the four groups, ileal injury was seen only after 72 h of formula feeding and hypoxia. We found selective induction of mRNA levels in NEC compared with dam-fed controls for TLR2 > TLR4 > TLR1 = TLR3, TLR7, and TLR9 > TLR6 (P < 0.01); TLR5 was downregulated (P < 0.01). All TLR changes started at 48 h, before any histological evidence of NEC. Both Th1-type cytokines (IFN-γ, IL-1β, TNF-α, and KC/GRO) and Th2-type cytokines (IL-4, IL-5 and IL-13) were significantly increased in NEC but also in nondamaged formula-fed rat ileum. In conclusion, the intestinal expression of TLRs and cytokines precedes histological injury in the experimental NEC.
Keywords: prematurity, hypoxia, breast milk, formula, inflammation
necrotizing enterocolitis (NEC) is the most common gastrointestinal disease of newborn premature infants, affecting ∼10% of very low birth weight infants. The overall mortality for NEC ranges from 10 to 50%, depending on gestational age, but approaches 100% for infants with the most severe form of the disease, characterized by bowel wall necrosis, perforation, peritonitis, bacterial invasion, and sepsis (21). Risk factors for NEC include prematurity, formula feeding, and bacterial colonization of the gastrointestinal tract (21). Certain bacteria identified by culture techniques appear to be associated with outbreaks (19). Other intraluminal microflora, such as Lactobacilli and Bifidobacilli, may be beneficial in preventing NEC (5, 27). Although recent evidence supports a susceptibility to NEC that is linked to gastrointestinal tract immaturity, the mechanisms whereby these factors promote disease are poorly defined. Understanding the defense mechanisms in the premature intestine and their contribution to NEC is therefore of great importance.
Toll-like receptors (TLRs) are pattern recognition receptors that recognize components of intraluminal bacteria (7). TLRs are usually expressed by immune cells such as lymphocytes and phagocytes, but many studies have demonstrated their presence on or in other cells such as epithelial cells that maintain a protective barrier (4, 9). TLRs act as sentinels for the presence of pathogens and are able to activate the innate immune system. They represent the first line of defense against mucosal pathogens by triggering the inflammatory response, while providing protective signals that allow the intestine to tolerate commensal organisms that benefit the host.
Animal models of NEC rely on the induction of pathogenetic events that are similar to those known to be associated with human NEC. One of the earliest descriptions of an animal model of NEC featured the combined treatment of formula by gavage with intermittent episodes of either cold or hypoxic stress in the preterm or early postterm period (3). NEC induction was validated by histopathology. Several other investigators used this model and refined it (6, 14, 31).
Previous studies of bacterial recognition by TLRs in experimental NEC demonstrated that the expression of TLR4, which recognizes the gram-negative lipopolysaccharide (LPS), is increased in the intestine during NEC (24). TLR4-deficient C3H/HeJ mice were protected from the development of NEC (26). Recent studies found that TLR2 mRNA were also increased together with TLR4 mRNA in NEC (25). However, C57BL/6 TLR2-deficient mice, when studied in an intestinal ischemia model, were found to have a dysregulated mucosal innate immune response, with reduced cytokine production and increased injury score (1). Differences in the expression of TLRs may alter a host's response to a commensal or pathogenic microorganism. TLR2 signaling, produced by treatment with Lactobacillus lactis, may provide protective signaling, for example in models of inflammatory colitis (15).
It has been known that TLRs when activated by bacterial ligands signal intracellularly to upregulate the expression of various cytokines in the intestine, including pro- (IL-1β, IL-6, IL-8) and anti-inflammatory cytokines (IL-10) in human and different NEC models (17). Our present studies aimed to determine how either formula or hypoxia (or the combination thereof) affects TLR profiles, cytokine production, and evidence of NEC in a well-established experimental model.
MATERIALS AND METHODS
All the experiments were performed using newborn Sprague-Dawley rat pups (Harlan Laboratories, Indianapolis, IN) weighing 6–10 g and were approved by the Animal Welfare Committee of the University of Texas Health Science Center at Houston (HSC-AWC-07-124).
Experimental design.
Three-day-old Sprague-Dawley rat pups were divided randomly into four groups. 1) Dam-fed (n = 17): rat pups were left with their mothers and were breast fed. 2) Dam-fed-hypoxic (n = 16): dam-fed rat pups were subjected to 10 min of hypoxia (5% oxygen, 95% nitrogen) three times daily in a Modular Incubator Chamber (Billups-Rothenberg, Del Mar, CA) for 3 days. 3) Formula-fed (n = 15): rat pups were separated from their mothers, housed in an incubator, and gavaged with a special rodent formula 200 μl 3 times daily. 4) NEC (formula-fed-hypoxic) (n = 19): rat pups were formula fed followed by hypoxia. The formula consisted of 15 g Similac 60/40 (Ross Pediatrics, Columbus, OH) in 75 ml of Esbilac canine milk replacement (Pet-Ag, Hampshire, IL) (31). The rat pups were euthanized on 72 h. To study the kinetics of changes of TLR expression and cytokine production initiated by formula feeding and hypoxia, we further repeated the same protocol for the four groups, and the rat pups were euthanized on 24 h (n = 24, 6 rats/group), 48 h (n = 24, 6 rats/group), 72 h (n = 24, 6 rats/group), and 120 h (n = 24, 6 rats/group). The incidence of NEC and severity of injury at 72 h were analyzed by addition of those six rats to each group mentioned above 72 h experiment.
Tissue harvest and NEC evaluation.
Following incision of the abdomen, the small intestine was evaluated visually for typical gross signs of NEC such as intestinal distension, intestinal wall hemorrhage, or necrosis. The gastrointestinal tract was carefully removed. The last 4 cm of terminal ileum was excised. Part of ileum for each animal was immediately embedded in Tissue-Tek OCT-embedding medium (Sakura Finetek, Torrance, CA), frozen in 2-methylbutane (Sigma-Aldrich, St. Louis, MO) cooled with liquid nitrogen, and stored at −80°C until sectioned. Part of ileum for each animal was washed with cold phosphate-buffered saline, pH 7.4 (PBS) and fresh frozen immediately in liquid nitrogen for RNA and protein isolation. Part of each sample was formalin fixed, paraffin embedded, microtome sectioned at 5 μm, and stained with hematoxylin and eosin for histological evaluation.
Pathological changes in intestinal architecture were evaluated via a NEC scoring system developed for use in neonatal rats (31). Histological changes in the ileum were scored by a blinded evaluator on a scale of 0 (normal), 1 (mild, separation of the villous core, without other abnormalities), 2 (moderate, villous core separation, submucosal edema, and epithelial sloughing), and 3 (severe, denudation of epithelium with loss of villi, full-thickness necrosis, or perforation). Animals with histological scores ≥2 were defined as having developed NEC.
TLR mRNA expression by qRT-PCR.
RNA was isolated from frozen tissue samples using TRIzol (Invitrogen, Carlsbad, CA), followed by On-Column DNase digestion (Qiagen, Valencia, CA) according to the manufacturer's protocols. The quantitative real-time reverse transcription-polymerase chain reaction (qRT-PCR) was performed with the rat TLR signaling pathway RT2 profiler PCR array and SYBR Green/ROX qPCR master mix (SABiosciences, Frederick, MD). All qRT-PCR reactions were run at the Quantitative Genomics Core Laboratory (UTHSC-Houston Medical School, Houston, TX) by utilizing a 7700 Detector (Applied Biosystems, Foster City, CA). The threshold cycle (Ct) value for each well was obtained by using the instrument's software. Data analysis by the ΔΔCt method was automatically performed by PCR Array Data Analysis Web Portal provided by SABiosciences. To determine the fold change in gene expression, the normalized expression of each gene of interest (GOI) in the experimental sample was divided by the normalized expression of the same GOI in the control sample. The GOIs assessed by rat TLR signaling pathway RT2 profiler PCR array kit in this study included TLR1 (XM_223421), TLR2 (NM_198769), TLR3 (NM_198791), TLR4 (NM_019178), TLR5 (XM_223016), TLR6 (NM_207604), TLR7 (XM_228909), and TLR9 (NM_198131). Five housekeeping genes (HKG) were used: ribosomal protein, large P1 (Rplp1, NM_001007604); hypoxanthine guanine phosphoribosyl transferase (Hprt, NM_012583); ribosomal protein L13A (Rpl13a, NM_173340); lactate dehydrogenase A (Ldha, NM_017025); and β-actin (Actb, NM_031144). We used the average Ct value of all housekeeping genes that were not influenced by our experimental conditions for normalization with the ΔΔCt method. The calculation was as follows: 2−ΔΔCt = 2−[Ct (GOI)−Ct (HKG)] expt/2−[Ct (GOI) − Ct (HKG)] control. In our study, the fold changes of transcripts of each group were compared with the changes in dam-fed control or formula-fed-hypoxic rats were compared with formula-fed rats. To monitor the quality of tested samples, the controls for genomic DNA, reverse transcription, and positive PCR were examined simultaneously. All the samples passed the tests for quality control.
TLR2 and TLR6 immunofluorescent staining.
Frozen ileal tissues were sectioned (6 μm) on a model CM3050 cryostat (Leica Microsystems, Deerfield, IL), mounted on Superfrost plus microscope slides (Fisher Scientific, Pittsburgh, PA), air-dried overnight, and fixed with cold acetone for 10 min. The tissues were then blocked with 1.5% goat/donkey serum followed by incubation with the primary antibodies: goat anti-TLR2 (D-17, sc-12504), rabbit anti-TLR6 (H-90, sc-30001) (Santa Cruz Biotechnology, Santa Cruz, CA) at 2 μg/ml for 12 h at 4°C. The sections were then washed and incubated with donkey anti-goat-Alexa546 or goat anti-rabbit-Alexa488 Ig G (Molecular Probes, Eugene, OR) at a dilution of 1:500 for 30 min at room temperature. Sections were mounted with Prolong Gold antifade reagent with DAPI (Invitrogen, Carlsbad, CA) and the images were collected via a confocal laser scanning microscope (Zeiss LSM510).
Western immunoblot analysis.
Frozen whole ileal tissues were homogenized in 0.4 ml of lysis buffer containing protease inhibitors with 20 mM Tris·HCl (pH 7.5), 150 mM NaCl, 1 mM Na2 EDTA, 1 mM EGTA, 1% NP-40, 1% sodium deoxycholate, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1 μg/ml leupeptin, 1 μg/ml aprotinin, and 1 mM PMSF. The homogenates were centrifuged at 14,000 g for 10 min at 4°C after incubation on ice for 30 min. The supernatants were removed for detection of protein concentration with Bio-Rad Dc protein assay (Bio-Rad, Hercules, CA). Proteins (50 μg/lane) were immunoblotted with rabbit anti-TLR2 (Imgenex, IMG-545, 2 μg/ml), rabbit anti-TLR4 (Santa Cruz, sc-30002, 2 μg/ml), or rabbit anti-TLR6 (Santa Cruz, sc-30001, 2 μg/ml) for 12 h at 4°C. After incubation with goat anti-rabbit IgG conjugated to horseradish peroxidase (1:5,000; Bio-Rad) for 1 h at room temperature, immunoreactive bands were visualized by chemiluminescence (ECL+; GE Bio-Science) on X-ray film and densitometrically analyzed with Kodak 1D image (Eastman Kodak, Rochester, NY).
Multiplex cytokine measurement.
The weight for each ileal sample collected was recorded for calculation of cytokine production before the ileal tissue lysates were prepared. Each well of the 96-well plate-based assay in MSD Rat Demonstration 7 Spot (Meso Scale Discovery, Gaithersburg, MD) contained antibodies to IFN-γ, IL-1β, TNF-α, keratinocyte-derived chemokine/growth-related oncogene (KC/GRO, also known as cxcl1), IL-4, IL-5, and IL-13. Calibration curves were prepared with a range of 2,500 to 0.6 pg/ml. The assay was run according to the manufacturer's instructions. The results from cytokine measurements were expressed in picograms per gram of tissue.
Statistics.
Statistical analysis comparing the fold changes of different mRNAs and protein band intensity between NEC and control groups was performed with one-way ANOVA using GraphPad Prism 4.0 software (GraphPad Software, San Diego, CA). Dunnett's and Tukey's multiple-comparison tests were used for comparison of multiple groups with a control group. Values are expressed as the means ± SE. We considered a P < 0.05 significant.
RESULTS
NEC evaluation.
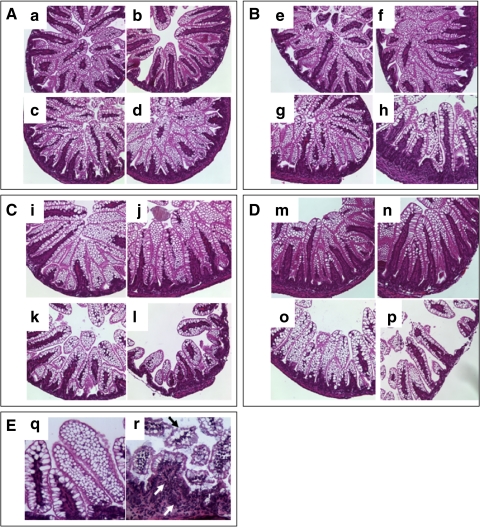
No abnormal ileal histological changes were observed in rat pups from any of the four groups (dam fed, dam fed-hypoxic, formula fed, and formula fed-hypoxic) (n = 48) through the first 48 h of observation (Fig. 1, A and B). At 72 h, no abnormal histological changes were observed in rat pups in the dam-fed groups (with or without hypoxia) (n = 45) (Fig. 1C, i and j). Formula-fed rats (n = 21) had only mild changes (score = 1), manifested by ileal villi that were shorter and more vacuolated than those of dam-fed animals at 72 h (Fig. 1, Ck and Eq). In comparison, of the rat pups given formula in combination with hypoxia (n = 25), none had a normal histological grade, whereas 44% (11/25) had mild histological changes (score = 1), 32% (8/25) had moderate histological changes (score = 2), and 24% (6/25) had severe histological changes (score = 3) (median score = 2.0) (Fig. 1, Cl and Er). No intestinal wall hemorrhage or necrosis was observed grossly. Tissues with histological scores ≥2 were designated positive for NEC. By this criterion, the incidence of NEC was 56% (14/25) at 72 h after formula feeding in combination with hypoxia. The ileal samples of rat pups with formula feeding plus hypoxia at 120 h demonstrated 0 of 6 with normal histological score: 50% (3/6) had mild (score = 1), 33% (2/6) had moderate (score = 2), and 16% (1/6) had severe (score = 3) histological changes (Fig. 1, Dp and Er). The incidence of NEC was thus 50% (3/6) at the 120-h time point. There was no significant difference comparing the incidence of NEC at 72 and 120 h. Formula-fed rats (6/6) had vacuolated villi without other abnormal histological changes (Fig. 1Do). The dam-fed (n = 6) and dam-fed-hypoxia (n = 6) groups at 120 h had no abnormal histological changes (Fig. 1D, m and n). The survival rate of the animals in each of these groups at any time point was 100%.
Fig. 1.
Effect of formula feeding and hypoxia on intestinal morphology. A: intestinal morphology at 24 h (n = 24, 6 rats/group). a: Dam fed. b: Dam fed-hypoxic. c: Formula fed. d: Formula fed-hypoxic. B: intestinal morphology at 48 h (n = 24, 6 rats/group). e: Dam fed. f: Dam fed-hypoxic. g: Formula fed. h: Formula fed-hypoxic. C: intestinal histological morphology at 72 h (n = 91). i: Dam fed (n = 23). j: Dam fed-hypoxic (n = 22). k: formula fed (n = 21). l: Formula fed-hypoxic (n = 25). D: intestinal morphology at 120 h (n = 24, 6 rats/group). m: Dam fed. n: Dam fed-hypoxic. o: Formula fed. p: Formula-fed-hypoxic. E: q and r with high-power image (×200). q: Vacuolated villus cells. r: Necrotizing enterocolitis (NEC) histology with villus core separation (black arrow) and infiltrating inflammatory cells (white arrows). Intestinal segments from dam-fed and dam-fed-hypoxic animals at all time points show normal histology. The villi are tall and healthy. A, B, C, and D are with magnification = ×100. Intestinal segments from formula-fed animals without hypoxic insult show mild changes (score = 1) with shorter and vacuolated villi after 72 h (k, o, and q). Intestinal segments from formula-fed rats subjected to hypoxia displayed NEC histological abnormalities (score ≥ 2) (l, p, and r) after 72 h. The median score was 2.0, with the incidence of NEC 56% (14/25) at 72 h and 50% (3/6) at 120 h.
TLR transcripts in NEC.
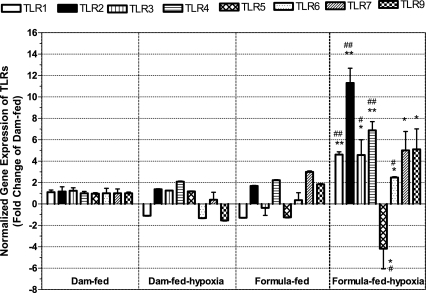
Because TLRs are important pattern recognition molecules that detect motifs of pathogens and host cellular products released during injury, we pursued transcriptional responses of TLRs in the distal ileum of NEC induced by formula feeding and hypoxia and compared them with dam-fed, dam-fed-hypoxic, and formula-fed controls. For this purpose, we examined the mRNA levels of TLR 1–7 and 9 in the intestinal tissues at 72 h feeding time by qRT-PCR (Fig. 2). Up- or downregulation of TLR genes was evaluated according to the fold change of different groups compared with dam-fed controls. We defined a cutoff value of +2-fold as indicating upregulation and −2-fold as indicating downregulation. We found selective induction of mRNA expression, with significant changes in formula-fed-hypoxic (NEC) rats compared with dam-fed rats in the following order: TLR2 (11.3 ± 1.4) > TLR4 > TLR1 (P < 0.001) = TLR3, TLR7, and TLR9 (P < 0.01) > TLR6 (P < 0.01). Only the level of TLR5 was downregulated in formula-fed-hypoxic (NEC) rat pups (−4.2 ± 1.8, P < 0.01). All the changes of TLRs in formula-fed-hypoxic rats were significantly different compared with either dam-fed or dam-fed-hypoxic rat ileum. The mRNA expression of TLR 1–6 in formula-fed-hypoxic rat pups was significantly different from formula-fed rat pups (for TLR1, 2, and 4, P < 0.001; for TLR3, 5, and 6, P < 0.01). Hypoxia treatment of dam-fed pups did not significantly affect TLRs. In formula-fed rat pups, no significant changes in TLR expression were observed.
Fig. 2.
Toll-like receptor (TLR) mRNA expression levels from the ilea of rat pups with or without exposure to hypoxia and/or formula. RNA was isolated from the ileum of newborn rats subjected to different feeding and/or hypoxia treatment for 72 h. Quantitative RT-PCR analysis was performed to determine the expression of TLRs in dam-fed-hypoxic, formula-fed, or formula-fed-hypoxic group compared with dam-fed control. Data are represented as means ± SE from 3 independent experiments. The cutoff values of fold change compared with the dam-fed control group: > +2 indicating upregulation, < −2 downregulation. Compared with dam-fed controls: *P < 0.01, **P < 0.001. Compared with formula-fed control group: #P < 0.01, ##P < 0.001. Compared with dam-fed controls, all TLR mRNA in the NEC group increased, with the exception of TLR5, which decreased. TLR1, TLR2, and TLR4 increased in NEC ileum compared with formula-fed rat ileum.
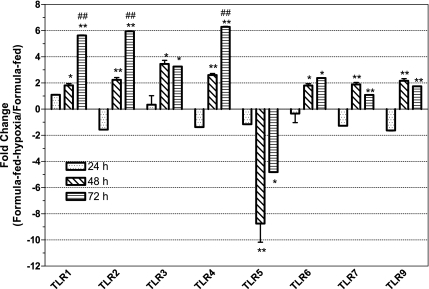
We determined the kinetics of changes of TLR expression initiated by formula feeding and hypoxia. mRNA levels of TLR 1–7 and 9 in the intestinal tissues of formula-fed-hypoxic rats were compared with those of formula-fed rats at different time points (Fig. 3). Because all TLR mRNA levels of either formula-fed or formula-fed-hypoxic rats have not shown the significant changes compared with dam-fed controls at 24 h, we compared the fold changes of the formula-fed-hypoxia to the formula-fed group at 48 and 72 h with those at 24 h, and the fold changes at 72 h with those of at 48 h. We found that formula feeding plus hypoxia induced significant changes of all TLR expression as early as 48 h (P < 0.01) and dramatically increased TLR1, TLR2, and TLR4 at 72 h (P < 0.001, compared with that at 48 h).
Fig. 3.
Evolution of mRNA expression levels of TLRs from the ileum of formula-fed and formula-fed-hypoxic rats over 72 h. RNA was isolated from the ileum of newborn rats subjected to formula feeding alone and formula feeding in combination with hypoxia for 24, 48 and 72 h, respectively. Quantitative RT-PCR (qRT-PCR) analysis was performed to determine the expression level (fold changes) of TLRs comparing formula-fed-hypoxic rats with formula-fed rats. Data are represented as means ± SE from 3 independent experiments. The cutoff values of fold change of formula-fed-hypoxic compared with the formula-fed group: > +2 indicating upregulation, < −2 downregulation. Fold changes at 48 and 72 h compared with those at 24 h: *P < 0.01, **P < 0.001. Fold change at 72 h compared with those at 48 h: #P < 0.01, ##P < 0.001. All mRNA expression levels of TLRs differed significantly at 48 h, when formula-fed-hypoxic rats were compared with formula-fed rats.
Expression and localization of TLR2, TLR4, and TLR6 in NEC.
TLR2, TLR4, and TLR6 recognize their ligands at the cell surface and signal intracellularly after reaction to their specific ligands. However, TLR1 and TLR6 do not elicit signaling on their own; heterodimerization with TLR2 is required for TLR2-mediated responses (30). To further investigate cell surface TLRs, we pursued protein expression level and localization of TLR2, TLR4, and TLR6 by Western blot analysis and immunohistochemistry of the ileal mucosa. Previous studies have demonstrated that TLR3 and TLR7–9 require endosomal maturation, where agonist sensing occurs intracellularly in the lumen of membrane-bound compartments.
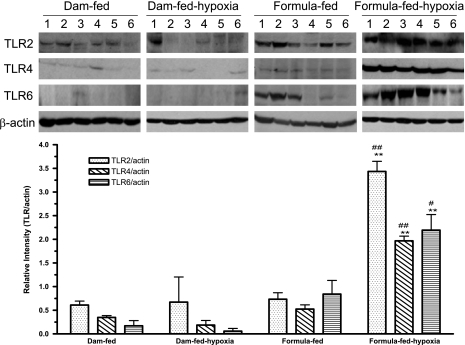
We found increased relative intensities of TLR2, TLR4, and TLR6 proteins in formula-fed-hypoxic (NEC) rat pups compared with the dam-fed, dam-fed-hypoxic, or formula-fed controls (P < 0.001) (Fig. 4). In formula-fed rat pups, no statistic difference of the expression of TLR2 and TLR6 was observed in terms of relative intensities compared with dam-fed controls. Comparing dam-fed-hypoxic rat pups with dam-fed controls, no significant difference was observed with respect to TLR2, 4, or 6 levels.
Fig. 4.
Expression of TLR2, TLR4, and TLR6 proteins in the ileum. Tissue lysates were prepared for Western blot analysis and 50 μg of total protein were loaded and immunoblotted with anti-TLR2, TLR4, and TLR6 antibodies, with β-actin as a loading control. Top: a typical blot; numbers represent number of animals examined in each group. Bottom: relative densitometric values are means ± SE. N = 6 for each groups. All groups compared with dam-fed control: **P < 0.01. Formula-fed-hypoxic compared with formula-fed group: #P < 0.05, ##P < 0.01. Significantly increased relative intensities of TLR2, TLR4, and TLR6 protein were shown in NEC rats compared with either dam-fed or formula-fed controls.
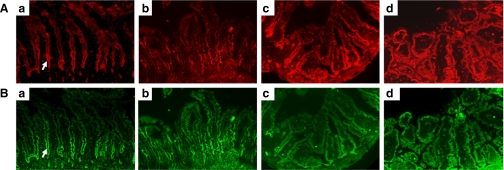
Expression of TLR2 (Fig. 5A) and TLR6 (Fig. 5B) in rat pup intestinal tissues was observed in all dam-fed, dam-fed-hypoxic, formula-fed, and formula-fed-hypoxic animals by immunofluorescence microscopy. In dam-fed, dam-fed-hypoxic, and formula-fed rat intestinal tissues, both TLR2 and TLR6 expression stained with a stronger intensity in the crypt region, compared with in the villi (Fig. 5, a–c). However, in the NEC ileum, strong epithelial TLR2 and TLR6 staining was observed along the entire villus-crypt axis (Fig. 5d).
Fig. 5.
Immunofluorescent localization of TLR2 and TLR6 in ileal tissues. A: TLR2 staining (red). B: TLR6 staining (green). a, Dam fed; b, dam fed-hypoxic; c, formula fed; d, formula fed-hypoxic (NEC). Arrows indicate crypt region. Magnification is ×200. TLR2 and TLR6 were primarily identified in the crypt enterocytes of dam-fed, dam-fed-hypoxic, and formula-fed rats, whereas enterocytes were highly immunoreactive in both crypt and villi regions of NEC ileum.
Production of cytokines associated with Th1 and Th2 responses.
TLRs are activated in NEC, leading to activation of the NF-κB transcription factor (25). This activation in general results in the subsequent upregulation of proinflammatory cytokines and chemokines that is required for protective inflammatory responses but that can also be associated with autoimmune and allergic disorders.
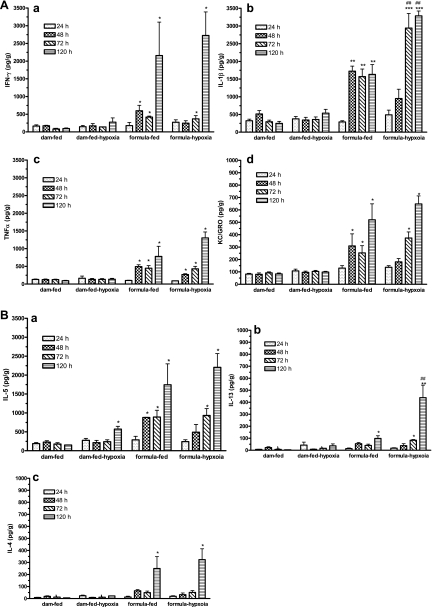
We measured seven cytokines, including Th1 cytokines (IL-1β, IFN-γ, KC/GRO, and TNF-α) and Th2 cytokines (IL-4, IL-5, and IL-13) in the intestinal tissue lysates from rats in the four groups at four different time points. We found that the levels of most cytokines began to increase as early as 48 h, as seen in formula-fed and formula-fed-hypoxic (NEC) rat pups, but remained steady in dam-fed controls (P < 0.05). For the Th1 cytokines, no significant differences were observed between formula-fed-hypoxic and formula-fed groups with respect to the levels of IFN-γ, TNF-α, and KC/GRO. However, IL-1β was increased to a higher level in formula-fed-hypoxic (NEC) intestine (P < 0.01) (Fig. 6A). Hypoxia treatment of dam-fed rats did not change any measured Th1 cytokine level compared with dam-fed rats.
Fig. 6.
Cytokine production by normal, hypoxic, formula-fed, and NEC ileal tissues. Cytokines in ileal tissue lysates at 24, 48, 72, and 120 h exposure to formula feeding and/or hypoxia were assayed by using a MSD rat 7-spot plate (see materials and methods) to compare with dam-fed controls. Each well of the 96-well plate contained antibodies to IL-1β, IFN-γ, KC/GRO (Cxcl1), TNF-α, IL-5, IL-13, and IL-4. A: Th1 cytokines IFN-γ (a), IL-1β (b), TNF-α (c), and KC/GRO (d). B: Th2 cytokines IL-5 (a), IL-13 (b), and IL-4 (c). Data are represented as means ± SE. N = 3. All groups compared with dam-fed control: *P < 0.05; **P < 0.01; ***P < 0.001. Formula-fed-hypoxic group compared with formula-fed group: #P < 0.05, ##P < 0.01. Th1 and Th2 cytokines were produced in the intestines of both formula-fed and formula-fed-hypoxic rats at 48 h. Note that IL-1β (Th1) and IL-13 (Th2) were significantly increased in NEC compared with formula-fed controls.
With respect to the Th2 cytokines, formula-fed pups, compared with dam-fed controls, exhibited levels of IL-5. IL-5 level was also increased in formula-fed-hypoxic (NEC) subjects and in dam-fed-hypoxic subjects only at 120 h. We observed no significant difference between formula-fed and formula-fed-hypoxic rat intestine, with respect to IL-5 level (Fig. 6B).
IL-13 levels were increased after longer exposure to formula feeding (120 h) (P < 0.01) and increased after a longer exposure to formula and hypoxia (120 h) (P < 0.01).
The level of IL-4 showed an increase at 120 h in formula-fed and formula-fed-hypoxic rat intestines compared with dam-fed controls, and there were no significant differences between the two groups. Hypoxia treatment of dam-fed animals did not increase IL-4 production.
DISCUSSION
NEC as a major pediatric problem.
NEC is the leading cause of death and long-term disability from gastrointestinal disease in preterm infants (28). NEC is also most challenging to treat, because the mechanisms leading to the development of NEC are incompletely understood. A longstanding observation is that NEC is an acute inflammatory disease. Currently, different animal models for NEC have corroborated the finding that changes of inflammatory mediator levels are associated with evidence of histological injury in NEC. However, it is unknown whether intestinal immune activation precedes tissue injury. In this study, we investigated the expression profile of Toll-like receptors and the intestinal production of cytokines during the evolution of inflammation using an established neonatal rat model.
New findings arising from this study are the following: 1) NEC heightens intestinal tissue expression of TLRs 1–7 and 9, and NEC alters the localization of expression. 2) Cow milk formula feeding but not hypoxia by itself is also a proinflammatory stimulus, although formula feeding has a lesser effect on general TLR expression. 3) Changes in TLRs and cytokines (both pro- and anti-inflammatory) precede evidence of histological injury.
Advantages and disadvantages of the animal model.
Our animal model was derived from the extensive work of Ford and colleagues (21, 31) and included the key components: maternal separation, deprivation of optimal enteral nutrition, and artificial feeding with cow milk formula. Our data shows that a hypoxic insult to the breast feeding rat pup did not produce intestinal histological abnormalities. Neither did we identify NEC histological abnormalities in formula-fed animals, although tissue produced abundant cytokines and the villi were shorter than those of dam-fed animals, with a vacuolated morphology. The typical NEC histological abnormalities were observed only in the rat pups after 3 days of formula feeding in combination with hypoxia.
Human NEC usually affects only very low birth weight infants. Also, virtually all human babies that develop NEC are administered parenteral nutrition; however, it was not feasible for us to provide rat pups parenteral nutrition. A potential criticism of this model is that the rat pups were not prematurely delivered. The full-term newborn rat models can be used because the rodent intestine is less developed at birth than the human. In fact, enzyme and villus morphology studies suggested that the preterm infant's gut is developed to about the same extent as a postterm rat pup < 2 wk (before weaning) (16, 33). So the model we fed is representative of the premature human intestine.
The data from previous studies in the same experimental NEC model in formula-fed rats (31) showing that formula-fed rat pups with or without hypoxia lost weight over the study period and displayed morphological changes in the intestinal epithelium. Breast-fed rats nearly doubled their birth weights and showed preservation of the intestinal architecture. We also observed weight loss in formula-fed groups over the study period (data not shown). This may be because the total volume of formula fed to these neonatal rats by gavage was less than that received by breast-fed rats when they stayed with their dams; thus we cannot rule out the development of intestinal inflammation or experimental NEC in rats as a consequence of malnutrition. Although we cannot compare the nutrient intake between dam-fed with formula-fed pups, we can calculate how many calories and whether the calories we gave to rat pups provided their needs. The maintenance energy requirement (MER) for newborn rat pups is ∼130 kcal·kg−1·day−1, whereas the growth energy requirement should be three times higher than MER. The formula we used contains 15 g of Similac (70 kcal) and 75 ml of Esbilac (61.5 kcal), which gives a total in the mixture of 131.5 kcal/75 ml = 1.75 kcal/ml. Each rat pup needs ∼0.8 ml/day of this formula to reach the MER. We gave rat pups 0.2 ml × 3 daily, an amount lower than the MER. Previous studies have reported that malnutrition alone does not cause gut-barrier failure (12, 34). Furthermore, malnutrition produces a severe reduction in the number of gut-associated lymphocytes and Peyer's patches (23), as opposed to inflammation. Several intrinsic factors within breast milk may change gut barrier function and prevent the development of inflammatory changes in the epithelium (13, 14). Thus the combination of malnutrition, elimination of protective breast milk factors, formula feeding, and hypoxia may render the neonatal rat more susceptible to gut barrier disruption and subsequently to NEC.
TLR signaling in NEC.
TLRs are one of the main contributors to pathogen-induced inflammation and ischemia-induced inflammation (2). Previous studies demonstrated that bacteria and TLR4 play significant roles in experimental NEC. Intestinal TLR4 mRNA increased in formula feeding and cold asphyxia stress, correlating with induced inducible nitric oxide (NO) synthase and increased inflammatory cytokine production (24), which occurred in the intestinal epithelium but not in the submucosa. TLR4-mutant C3H/HeJ mice were protected from the development of NEC compared with wild-type C3H/HeJ mice (26). Recent studies found that there was an overexpression of TLR2 and TLR4 in intestinal epithelial cells (IECs) that was correlated with the severity of mucosal damage, together with an increase of apoptotic IECs and markedly impaired proliferation (25). Some studies indicated that TLR2 may provide protective signaling. Evidence was that C57BL/6 TLR2-deficient mice, compared with wild-type mice, have a dysregulated mucosal innate immune response and fail to mount a protective response after ischemia-reperfusion injury (1). TLR2 stimulation in intestinal epithelial cells effectively preserves tight junction (TJ)-associated barrier assembly against stress-induced damage, and inflammatory stress in mice deficient of TLR2 induced early TJ-associated disruption (8). Our studies demonstrated a profound impact of NEC on an array of TLR mRNAs and proteins within the intestinal epithelium, including TLR1, TLR2, TLR3, TLR4, TLR6, TLR7, and TLR9. In addition, these immunological alterations appeared before severe mucosal damage.
The upregulation of differential TLRs at an early stage may serve to protect the intestinal barrier. Fusunyan et al. (18) showed that TLR2 and TLR4 are expressed constitutively on human fetal small intestinal enterocytes, predominantly on the basolateral surface of crypt enterocytes. Our immunofluorescent staining showed that both TLR2 and TLR6 stained with a stronger intensity in the crypt region rather than in the villus tips in intestinal tissues from dam-fed rats with or without hypoxia and formula-fed rats. However, in NEC, the most striking feature was more general immunostaining pattern, with prominent expression of TLR2 and TLR 6 in the villi and the crypts.
One major effect of NEC in the present studies was the marked suppression of TLR5 mRNA expression in formula-hypoxic rats. TLR5 is known to protect the intestine from a variety of microbes via its interaction with flagellin (36). Ligation of TLR5 on the enterocyte basolateral membrane to flagellin of pathogens such as Salmonella typhimurium leads to interleukin-1 receptor kinase 4 activation and subsequent MAPK signaling, NF-κB activation, and chemokine and NO production (36). Knockout of TLR5 results in spontaneous colitis, but only if the host is colonized by commensal flora (37). Exposure of cultured intestinal cells to ligands for TLR5, TLR2, and TLR4 results in cross talk that is complex (35). For example, exposure of cells to flagellin results in upregulation of TLR2 and TLR4 mRNA expression and concomitant sensitization of the cells for subsequent TLR2 activation (by Pam3CSK4, a TLR2 agonist) and TLR4 activation (by LPS, a TLR4 agonist). However, exposure of cells to either Pam3CSK4 or LPS results in downregulation of TLR5 mRNA expression and attenuated subsequent flagellin-mediated NF-κB activation (35). It is possible that there is “biologic logic” underlying the downregulation of the flagellin receptor to reduce inflammation when the newborn intestine is developing inflammation. The newborn does encounter flagellated bacteria early in life, for example certain types of lactobacilli (22) and flagellated pathogens such as Listeria monocytogenes (20).
Inflammation response in experimental NEC.
In various models of inflammation, the activation of TLRs and subsequent signaling events eventually results in secretion of a number of downstream products, especially cytokines and chemokines. The increased expression of TLRs in the intestinal tissues during experimental NEC activated downstream NF-κB pathway (11, 25) and cytokine production. In our study, we found that both Th1 class (generally proinflammatory) cytokines (IL-1β, IFN-γ, KC/GRO, and TNF-α) and Th2 class (generally anti-inflammatory) cytokines (IL-4, IL-5, and IL-13) increased at as early as 48 h after formula feeding alone or formula feeding in combination with hypoxia, at a time when no typical NEC histological abnormalities were observed. Among the cytokines measured at the protein level, IL-1β (Th1) and IL-13 (Th2) were increased to a greater extent in formula-fed-hypoxic rat intestine compared with formula-fed intestine
The concept that cow milk-derived proteins participate in augmenting the cytokine response of peripheral blood lymphocytes is supported by previous studies implicating β-lactoglobulin and casein as contributors to human NEC (10). Several of the cytokines stimulated by milk proteins in NEC patients' lymphocytes were found to be elevated in the ileal tissue in association with cow milk formula feeding (without hypoxia) in the present study. These included IFN-γ, IL-4, and IL-5. Current evidence in neonatal models points to the role of activated inflammatory mediators and an inadequate anti-inflammatory response to the breakdown of mucosal integrity, which may represent the final common pathway in NEC (29). However, a study using immature fetal and mature cell lines and organ culture techniques previously showed that the fetal (immature) human enterocytes react with excessive proinflammatory cytokine production (32). Our present study demonstrated that both Th1 and Th2 cytokines are elaborated in NEC, and they appear to be increased in parallel in terms of time points. Because the neonatal intestine is unlikely to have memory T cells, the Th1 and Th2 responses we observed could reflect activation of cytokine production by the intestinal epithelial cells in vivo.
Summary.
TLRs that are crucial in maintaining intestinal epithelial homeostasis participate in a vigorous signaling process and a heightened inflammatory cytokine output that may contribute to the pathogenesis of NEC. Cow milk formula feeding, by eliciting the elaboration of many cytokines in the mucosa, emerges as a very important initiating factor in this process. The upregulation of TLRs and cytokine expression in the ileum precedes the onset of NEC by at least 48 h. Thus our work furthers the understanding of the pathogenesis of NEC and may facilitate the development of therapeutic approaches for early intervention in this disease. For example, feeding an amino acid formula or a formula with added probiotic organisms with anti-inflammatory characteristics may have preventative effects in this often lethal disease.
GRANTS
This study was supported by the Department of Pediatrics, the University of Texas Health Science Center at Houston-Medical School, and, in part, by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK56338, which supports the Texas Medical Center Digestive Diseases Center.
Acknowledgments
We thank Dr. Gregory L. Shipley from the Quantitative Genomics Core Laboratory, University of Texas Medical School at Houston, TX for assisting in qRT-PCR technique and analysis of transcript profiling results and the Cellular and Molecular Morphology Core, the Texas Medical Center Digestive Disease Center at Houston, TX for performing histological preparations.
REFERENCES
- 1.Aprahamian CJ, Lorenz RG, Harmon CM, Dimmit RA. Toll-like receptor 2 is protective of ischemia-reperfusion-mediated small-bowel injury in a murine model. Pediatr Crit Care Med 9: 105–109, 2008. [DOI] [PubMed] [Google Scholar]
- 2.Arumugam TV, Okun E, Tang SC, Thundyil J, Taylor SM, Woodruff TM. Toll-like receptors in ischemia-reperfusion injury. Shock 32: 4–16, 2009. [DOI] [PubMed] [Google Scholar]
- 3.Barlow B, Santulli TV. Importance of multiple episodes of hypoxia or cold stress on the development of enterocolitis in an animal model. Surgery 77: 687–690, 1975. [PubMed] [Google Scholar]
- 4.Begon E, Michel L, Flageul B, Beaudoin I, Jean-Louis F, Bachelez H, Dubertret L, Musette P. Expression, subcellular localization and cytokinic modulation of Toll-like receptors (TLRs) in normal human keratinocytes: TLR2 up-regulation in psoriatic skin. Eur J Dermatol 17: 497–506, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Bin-Nun A, Bromiker R, Wilschanski M, Kaplan M, Rudensky B, Caplan M, Hammerman C. Oral probiotics prevent necrotizing enterocolitis in very low birth weight neonates. J Pediatr 147: 192–196, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Caplan MS, Kelly A, Hsueh W. Endotoxin and hypoxia-induced intestinal necrosis in rats: the role of platelet activating factor. Pediatr Res 31: 428–434, 1992. [DOI] [PubMed] [Google Scholar]
- 7.Caplan MS, Simon D, Jilling T. The role of PAF, TLR, and the inflammatory response in neonatal necrotizing enterocolitis. Semin Pediatr Surg 14: 145–151, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Cario E, Gerken G, Podolsky DK. Toll-like receptor 2 controls mucosal inflammation by regulating epithelial barrier function. Gastroenterology 132: 1359–1374, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Chen J, Rao JN, Zou T, Liu L, Marasa BS, Xiao L, Zeng X, Turner DJ, Wang JY. Polyamines are required for expression of Toll-like receptor 2 modulating intestinal epithelial barrier integrity. Am J Physiol Gastrointest Liver Physiol 293: G568–G576, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Chuang SL, Hayes PJ, Ogundipe E, Haddad M, MacDonald TT, Fell JM. Cow's milk protein-specific T-helper type I/II cytokine responses in infants with necrotizing enterocolitis. Pediatr Allergy Immunol 20: 45–52, 2009. [DOI] [PubMed] [Google Scholar]
- 11.De Plaen IG, Liu SX, Tian R, Neequaye I, May MJ, Han XB, Hsueh W, Jilling T, Lu J, Caplan MS. Inhibition of nuclear factor-kappaB ameliorates bowel injury and prolongs survival in a neonatal rat model of necrotizing enterocolitis. Pediatr Res 61: 716–721, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Deitch EA, Berg RD. Endotoxin but not malnutrition promotes bacterial translocation of the gut flora in burned mice. J Trauma 27: 161–166, 1987. [DOI] [PubMed] [Google Scholar]
- 13.Dickinson EC, Gorga JC, Garrett M, Tuncer R, Boyle P, Watkins SC, Alber SM, Parizhskaya M, Trucco M, Rowe MI, Ford HR. Immunoglobulin A supplementation abrogates bacterial translocation and preserves the architecture of the intestinal epithelium. Surgery 124: 284–290, 1998. [PubMed] [Google Scholar]
- 14.Dvorak B, Halpern MD, Holubec H, Dvorakova K, Dominguez JA, Williams CS, Meza YG, Kozakova H, McCuskey RS. Maternal milk reduces severity of necrotizing enterocolitis and increases intestinal IL-10 in a neonatal rat model. Pediatr Res 53: 426–433, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Foligne B, Nutten S, Steidler L, Dennin V, Goudercourt D, Mercenier A, Pot B. Recommendations for improved use of the murine TNBS-induced colitis model in evaluating anti-inflammatory properties of lactic acid bacteria: technical and microbiological aspects. Dig Dis Sci 51: 390–400, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Fondacaro JD, Nathan P, Wright WE. Methionine accumulation in villi isolated from maturing rat intestine. J Physiol 241: 751–760, 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frost BL, Jilling T, Caplan MS. The importance of pro-inflammatory signaling in neonatal necrotizing enterocolitis. Semin Perinatol 32: 100–106, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fusunyan RD, Nanthakumar NN, Baldeon ME, Walker WA. Evidence for an innate immune response in the immature human intestine: Toll-like receptors on fetal enterocytes. Pediatr Res 49: 589–593, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Gerber AR, Hopkins RS, Lauer BA, Curry-Kane AG, Rotbart HA. Increased risk of illness among nursery staff caring for neonates with necrotizing enterocolitis. Pediatr Infect Dis J 4: 246–249, 1985. [DOI] [PubMed] [Google Scholar]
- 20.Gorski L, Duhe JM, Flaherty D. The use of flagella and motility for plant colonization and fitness by different strains of the foodborne pathogen Listeria monocytogenes. PLoS ONE 4: e5142, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hunter CJ, Upperman JS, Ford HR, Camerini V. Understanding the susceptibility of the premature infant to necrotizing enterocolitis (NEC). Pediatr Res 63: 117–123, 2008. [DOI] [PubMed] [Google Scholar]
- 22.Hynonen U, Westerlund-Wikstrom B, Palva A, Korhonen TK. Identification by flagellum display of an epithelial cell- and fibronectin-binding function in the SlpA surface protein of Lactobacillus brevis. J Bacteriol 184: 3360–3367, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Janu P, Li J, Renegar KB, Kudsk KA. Recovery of gut-associated lymphoid tissue and upper respiratory tract immunity after parenteral nutrition. Ann Surg 225: 707–715, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jilling T, Simon D, Lu J, Meng FJ, Li D, Schy R, Thomson RB, Soliman A, Arditi M, Caplan MS. The roles of bacteria and TLR4 in rat and murine models of necrotizing enterocolitis. J Immunol 177: 3273–3282, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Le Mandat SA, Bonnard A, Barreau F, Aigrain Y, Pierre-Louis C, Berrebi D, Peuchmaur M. Expression of TLR-2, TLR-4, NOD2 and pNF-kappaB in a neonatal rat model of necrotizing enterocolitis. PLoS ONE 2: e1102, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leaphart CL, Cavallo J, Gribar SC, Cetin S, Li J, Branca MF, Dubowski TD, Sodhi CP, Hackam DJ. A critical role for TLR4 in the pathogenesis of necrotizing enterocolitis by modulating intestinal injury and repair. J Immunol 179: 4808–4820, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Lin HC, Su BH, Chen AC, Lin TW, Tsai CH, Yeh TF, Oh W. Oral probiotics reduce the incidence and severity of necrotizing enterocolitis in very low birth weight infants. Pediatrics 115: 1–4, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Lin PW, Nasr TR, Stoll BJ. Necrotizing enterocolitis: recent scientific advances in pathophysiology and prevention. Semin Perinatol 32: 70–82, 2008. [DOI] [PubMed] [Google Scholar]
- 29.Martin CR, Walker WA. Intestinal immune defences and the inflammatory response in necrotising enterocolitis. Semin Fetal Neonatal Med 11: 369–377, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Medvedev AE, Sabroe I, Hasday JD, Vogel SN. Tolerance to microbial TLR ligands: molecular mechanisms and relevance to disease. J Endotoxin Res 12: 133–150, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Nadler EP, Dickinson E, Knisely A, Zhang XR, Boyle P, Beer-Stolz D, Watkins SC, Ford HR. Expression of inducible nitric oxide synthase and interleukin-12 in experimental necrotizing enterocolitis. J Surg Res 92: 71–77, 2000. [DOI] [PubMed] [Google Scholar]
- 32.Nanthakumar NN, Fusunyan RD, Sanderson I, Walker WA. Inflammation in the developing human intestine: a possible pathophysiologic contribution to necrotizing enterocolitis. Proc Natl Acad Sci USA 97: 6043–6048, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sangild PT Gut responses to enteral nutrition in preterm infants and animals. Exp Biol Med (Maywood) 231: 1695–1711, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Steinwender G, Schimpl G, Sixl B, Kerbler S, Ratschek M, Kilzer S, Hollwarth ME, Wenzl HH. Effect of early nutritional deprivation and diet on translocation of bacteria from the gastrointestinal tract in the newborn rat. Pediatr Res 39: 415–420, 1996. [DOI] [PubMed] [Google Scholar]
- 35.Van Aubel RA, Keestra AM, Krooshoop DJ, van Eden W, van Putten JP. Ligand-induced differential cross-regulation of Toll-like receptors 2, 4 and 5 in intestinal epithelial cells. Mol Immunol 44: 3702–3714, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Vijay-Kumar M, Aitken JD, Gewirtz AT. Toll like receptor-5: protecting the gut from enteric microbes. Semin Immunopathol 30: 11–21, 2008. [DOI] [PubMed] [Google Scholar]
- 37.Vijay-Kumar M, Sanders CJ, Taylor RT, Kumar A, Aitken JD, Sitaraman SV, Neish AS, Uematsu S, Akira S, Williams IR, Gewirtz AT. Deletion of TLR5 results in spontaneous colitis in mice. J Clin Invest 117: 3909–3921, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]