Abstract
The intestinal epithelium is in a constant state of renewal. The rapid turnover of cells is fed by a hierarchy of transit amplifying and stem/progenitor cells destined to give rise to the four differentiated epithelial lineages of the small intestine. Doxorubicin (Dox) is a commonly used chemotherapeutic agent that preferentially induces apoptosis in the intestinal stem cell zone (SCZ). We hypothesized that Dox treatment would initially decrease “+4” intestinal stem cell numbers with a subsequent expansion during mucosal repair. Temporal assessment following Dox treatment demonstrated rapid induction of apoptosis in the SCZ leading to a decrease in the number of intestinal stem/progenitor cells as determined by flow cytometry for CD45(−) SP cells, and immunohistochemistry of cells positive for putative +4 stem cell markers β-catSer552 and DCAMKL1. Between 96 and 168 h postinjection, overall proliferation in the crypts increased concomitant with increases in both absolute and relative numbers of goblet, Paneth, and enteroendocrine cells. This regeneration phase was also associated with increases of CD45(−) SP cells, β-catSer552-positive cells, crypt fission, and crypt number. We used Lgr5-lacZ mice to assess behavior of Lgr5-positive stem cells following Dox and found no change in this cell population. Lgr5 mRNA level was also measured and showed no change immediately after Dox but decreased during the regeneration phase. Together these data suggest that, following Dox-induced injury, expansion of intestinal stem cells occurs during mucosal repair. On the basis of available markers this expansion appears to be predominantly the +4 stem cell population rather than those of the crypt base.
Keywords: crypt fission, apoptosis, Paneth cells, goblet cells, Lgr5
the epithelium of the mammalian small intestine is in a constant state of renewal and is replenished every 3–4 days (24). This rapid turnover of cells is fed by a hierarchy of proliferative or transit amplifying cells and progenitor cells destined to give rise to the four cell lineages found in the small intestine including absorptive, goblet, enteroendocrine, and Paneth cells (7). However, the ultimate source of cells is intestinal stem cells (ISC), which are believed to reside in the epithelium in the lower regions of intestinal crypts (7, 32). The current understanding is that two types of ISC populate the bottom of intestinal crypts (2, 37). The first type, which have recently been designated as “crypt base columnar” (CBC) cells, reside among the Paneth cells at the very base of the crypt and are marked by expression of the orphan G protein-coupled receptor Lgr5. These cells are believed to be fast cycling (every 24 h) and resistant to irradiation. The second type, located above the Paneth cells (at approximately cell position 4), has been termed +4 ISC. These cells are believed to cycle slowly or remain quiescent, to retain DNA labeling, and to be highly sensitive to irradiation (2, 37).
Chemotherapeutic agents elicit their anticancer actions by targeting rapidly proliferating cell populations, inducing cell cycle arrest or apoptosis. The actions of these drugs are not tumor specific and are deleterious to normal cells of the intestinal epithelium. Chemotherapeutic-induced cytotoxicity within the gastrointestinal tract is manifested as mucositis, characterized by gross ulcerations of the intestinal mucosa. Several laboratories in the mid-1980s characterized the histological effects of a total of 18 cytotoxic agents on the intestinal mucosa and determined for each drug: 1) the time and dose effects on cell death, 2) the spatial position of target cells within the crypt axis, and 3) the ability of crypts to survive cytotoxic insult. These investigations demonstrated that damage is dose dependent and that cytotoxic agents can affect different regions of the intestinal crypt, altering the clonogenic and/or stem cell populations (21). At moderate doses, damage to these populations of cells can result in a fall in the number of proliferative cells, which leads to a decrease in overall cell number and a reduction of villus height, thus compromising digestive and absorptive capacities. At higher doses there may be a breakdown of mucosal integrity that leads to systemic pathologies. Although these adverse effects of chemotherapeutic drugs are well recognized, scant attention has been given to the fact that the intestinal epithelium is remarkably resistant to chemical damage.
The earlier literature on radiation and cytotoxic damage to the intestinal epithelium indicated that within each crypt resides a three tier clonogenic cell hierarchy including 1) intestinal stem cells (∼4–6 cells), which are highly chemo/radiation sensitive, 2) more damage-resistant immediate daughter cells (∼6 cells), and 3) even more resistant cells (∼26 cells) that can replace lost members of the other tiers (29). This makes a total of ∼36 clonogenic cells capable of maintaining crypt viability. Evidence from careful radiation dose-response studies suggests that only one clonogenic cell is needed to regenerate a crypt and to maintain appropriate crypt numbers by replacing lost crypts (29). Cytotoxic damage kills numerous epithelial cells, thus reducing the size of villi and crypts. The clonogenic cells are called upon to replenish lost or damaged cells to maintain the integrity of the intestinal barrier. Remaining clonogenic cells rapidly divide, and their progeny migrate from the crypt to fill the need for new epithelium. In the case of crypt sterilization (i.e., ablation of all ability for clonogenic cells to rescue the crypt), expansion of intestinal stem cells in surviving crypts results in crypt fission or budding, leading to the creation of new crypts (29).
Despite the broad general knowledge regarding epithelial regeneration following cytotoxic damage, there have been no direct assessments of the behavior of intestinal stem cells and their progeny during this process. Our primary hypothesis was that because doxorubicin (Dox) preferentially induces apoptosis between cell positions 3 and 6 (21), we would observe an initial decrease in +4 ISC number with a subsequent increase during mucosal repair. Our secondary hypothesis was that, by analogy with the response of the intestinal epithelium to the challenge of surgical resection (11, 18), there would be increased allocation to secretory lineages during the regeneration phase. We selected Dox as the cytotoxic agent because of its common use clinically as an antitumor drug and because its general effects on the intestinal epithelium in mouse models are well established (21). In this study, we first detailed the histological changes that occur following Dox treatment, including changes in allocation of the goblet, enteroendocrine, and Paneth cell lineages. Using this timeline of damage and repair processes, we next evaluated the response of intestinal stem/progenitor cells to Dox-induced damage. To accomplish this, we used several hallmarks of changes in intestinal stem/progenitor cell number as follows: 1) counting surviving crypts (19), 2) scoring crypt fission (11), 3) sorting of CD45-negative side population (SP) cells (11, 12), 4) immunohistochemical staining for the putative +4 stem cell markers β-cateninSer552 (16) and DCAMKL1 (25), and 5) analysis of changes in expression of the putative CBC stem cell marker Lgr5 using both LacZ staining in Lgr5-lacZ mice and Lgr5 mRNA expression in wild-type mice (3).
MATERIALS AND METHODS
Animals and experimental procedures.
Adult female C57Bl/6 mice were obtained from Charles River. They were housed in our animal facility under 12-h light-dark cycle and were allowed unlimited access to rodent Lab Chow (no. 5001, Purina Mills, St. Louis, MO) and acidified tap water. Experimental procedures were approved by the Institutional Animal Care and Use Committee of Baylor College of Medicine. Mice were given a single intraperitoneal (IP) injection of Dox (Pharmacia & Upjohn, Kalamazoo, MI) at a dose of 20 mg/kg body wt. This dose has previously been demonstrated to induce intestinal damage in mice (1, 2). Animals were euthanized 6, 24, 48, 72, 96, 120, or 168 h after Dox treatment. Some animals were given an IP injection of 5′-bromo-2′-deoxyuridine (BrdU) and 5′-fluoro-2′-deoxyuridine (12 and 1.2 mg/g body wt, respectively) 2 h prior to euthanasia. In all cases, after euthanasia the small intestine was flushed with ice-cold PBS (pH 7.4) and a piece of middle jejunum was fixed in 10% PBS and embedded in paraffin. In some experiments, a portion of jejunum was collected for use in flow cytometry.
Histology.
Formalin-fixed paraffin embedded specimens were oriented to provide sections perpendicular to the long axis of the bowel and 4-μm sections were used for evaluating general morphology. Longitudinal sections of crypts or villi were selected for scoring on the basis that a single, continuous layer of epithelium followed from crypt base to villus base and from the crypt-villus junction to the villus tip, respectively. For all quantitative analyses, 20 crypts or villi per animal (n = 6) were randomly selected and scored. For scoring cell position, each crypt was divided in half and cells were numbered sequentially from crypt base to crypt-villus junction, with cell position one being occupied by the first cell at the base of each half crypt, as per convention (21). Apoptosis was scored by immunohistochemistry for cleaved Caspase-3 and by hematoxylin and eosin (H&E) staining on the basis of the presence of one or more pyknotic bodies at a given cell position.
Paneth cells were identified by H&E staining and immunohistochemistry for lysozyme. Goblet cells were identified by separate Alcian blue and periodic acid Schiff (PAS) staining. Enteroendocrine cells were identified by immunohistochemistry for chromogranin A. For each animal, the number of total cells and Paneth cells per crypt were counted to determine the percentage of Paneth cells. Similarly, the number of total cells and Alcian blue-stained goblet cells per half villus were counted to determine the percentage of goblet cells. The number of enteroendocrine cells per crypt and villus were counted along with the total number of crypt and villus cells, respectively, to determine the percentage enteroendocrine cells.
H&E-stained longitudinal tissue sections were utilized to determine the percentage of crypt fission from at least 100 intact crypts per animal at time 0 and at 6, 24, 48, 72, 96, 120 and 168 h following Dox treatment. A crypt undergoing fission was defined as a bifurcating crypt with a bisecting fissure creating two (or sometimes more) flask-shaped bases with a shared single crypt-villus junction. Proliferative index was calculated by dividing the number of BrdU-positive cells per crypt by the total number of cells per crypt. Surviving crypts were quantified by counting crypts that contained at least five BrdU-positive non-Paneth cells. Villus height and crypt depth were measured by using an Axio Imager software on images captured with an Axio Imager A1 microscope and an AxioCam MRC 5 high-resolution camera (Carl Zeiss Microimaging, Thornwood, NY).
Isolation of SP cells.
We have previously demonstrated that a “side population” of cells can be isolated from small intestinal tissue following staining with the DNA-binding dye Hoechst 33342 (12). When cells of bone marrow origin were removed by use of the pan-leukocyte marker CD45, the resulting CD45-negative SP cells were shown to be epithelial and enriched for expression of Msi1, CD133, FGFR3, and Notch1 (12, 15). These findings, together with the fact that the SP fraction was shown localized to the base of intestinal crypts by in situ hybridization of enriched transcripts, led to the conclusion that the CD45(−) SP is enriched for intestinal epithelial stem/progenitor cells (15). Subsequent studies have shown that the number of CD45(−) SP cells is a reasonable surrogate for the number of stem/progenitor cells (11). In the present work, single mucosal cell suspensions were prepared from 5 cm of jejunum harvested at time 0 and at 24, 72, and 168 h after Dox treatment as previously described (12). For these experiments, enzymatic digestion of tissue was performed for 20 min and mechanical disruption was carried out for 15 min. The time required to isolate viable single cells from the intestine and perform flow cytometry limited the number of animals that could be studied to five per day; therefore, groups of mice were harvested 24 h apart to maintain a minimum of n = 5 per group studied. FACS analysis of CD45(−) SP cells was performed by using the Hoechst 33342 staining method reported by Dekaney et al. (12). To control for any day-to-day variation in the SP gate setting, control animals were analyzed with each experiment. Thus the gate for the SP was set by using the control cells. The % SP for the treated groups was then determined based on that gate.
Immunohistochemistry.
For BrdU staining, all slides were deparaffinized, rehydrated, and incubated in 3% hydrogen peroxide in methanol for 15 min at room temperature (RT) to quench endogenous peroxidase activity. Sections were boiled in citrate buffer (pH 6.0) for 25 min, allowed to cool for 10 min, and then denatured in 2 N HCl for 1 h at RT. Mouse anti-BrdU antibody (cat. no. MO744, Dako, Carpinteria, CA) was biotinylated and then applied to sections for 15 min at RT at a 1:100 dilution. Immunodetection was performed by using the Animal Research Kit (ARK, Dako, Carpinteria, CA) according to the manufacturer's directions. For all other immunostaining, slides were deparaffinized, rehydrated, and incubated in 3% hydrogen peroxide for 15 min at RT to quench endogenous peroxidase activity. Sections were boiled in citrate buffer (pH 6.0) for 25 min, allowed to cool to RT. Primary antibodies were applied to each section: 1) rabbit anti-lysozyme (cat. no. NCL-MURAM, Leica Microsystems, Bannockburn, IL) at 1:500 dilution, incubated for 1 h at RT; 2) rabbit anti-chromogranin A (cat. no. 20085, ImmunoStar Hudson, WI) at 1:4,000 dilution, incubated overnight at 4°C; 3) rabbit anti-cleaved caspase-3 (cat. no. 9661, Cell Signaling Technology, Danvers, MA) at 1:200 dilution, incubated for 1 h at RT; 4) rabbit anti-β-cateninSer552 (kindly provided by Dr. Linheng Li, Stowers Institute, Kansas City, MO) at 1:750 dilution, incubated overnight at 4°C; and 5) rabbit anti-DCAMKL1 (cat. no. AP7219b, Abgent, San Diego, CA) at 1:250 dilution for 1 h at RT. Sections were then washed and incubated with biotinylated goat anti-rabbit secondary antibody for 30–60 min at RT. After washing, slides were incubated in Vectastain ABC reagent (Vector Laboratories, Burlingame, CA) for 30 min and then developed in a diaminobenzidine substrate solution.
β-Galactosidase (LacZ) staining protocol.
Adult heterozygous Lgr5-lacZ mice, obtained from Lexicon Pharmaceuticals (The Woodlands, TX), were treated with Dox as outlined above and euthanized at 0, 6, 24, and 168 h after treatment. Jejunal tissue was isolated, flushed with ice-cold PBS, and immediately frozen in optimal cutting temperature compound. Frozen sections were brought to RT, then fixed with 0.2% glutaraldehyde for 5 min and washed in PBS. Next, slides were incubated in X-gal staining solution [2 mM MgCl2, 5 mM K3Fe(CN)6, 5 mM K4Fe(CN)6·3H2O, 1 mg/ml X-gal, in PBS pH 8.0] at 30°C in the dark for ∼16 h. Slides were washed in PBS, counterstained with Nuclear Fast Red, dehydrated, and coverslipped with DPX mounting solution.
Gene expression analysis.
Total RNA was isolated from jejunal tissue by using the RNeasy Kit (Qiagen, Valencia, CA) according to the manufacturer's directions. Lack of contamination with genomic DNA was verified by running 1 μg of total RNA on a 0.9% agarose gel, as well as by running a control without reverse transcription. Using the TaqMan One-Step RT-PCR Master Mix (Applied Biosystems, Foster City, CA) and Taqman Gene Expression assays for Lgr5 (Mm00438890_m1), 100 ng of total RNA was subjected to real-time RT-PCR. Relative changes in expression levels were calculated by the ΔΔCT method utilizing the 0 h total RNA as the baseline.
Statistical analysis.
All quantitative results are presented as means ± SE. For each animal the 10 observed crypt or villus counts were averaged. All data were subjected to one-way ANOVA with correction for multiple comparisons by the Fisher's procedure. For all comparison, a P value of <0.05 was considered significant.
RESULTS
Doxorubicin induces changes in mucosal morphology.
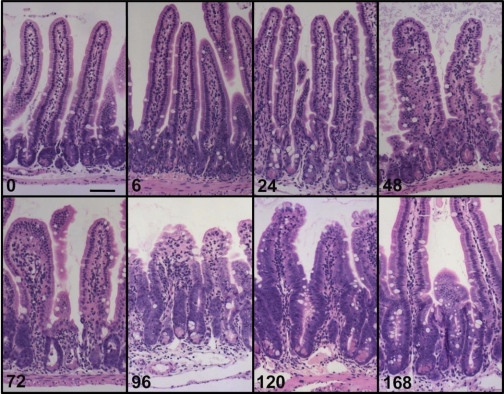
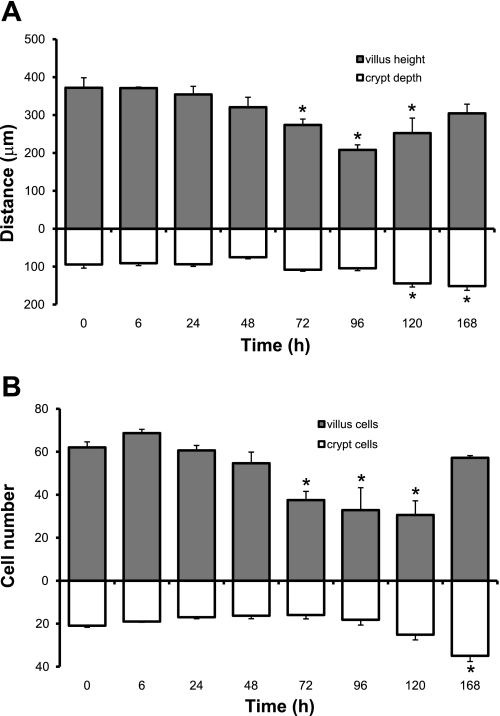
Evaluation of H&E-stained sections revealed changes to the mucosal architecture in response to Dox treatment (Fig. 1). Grossly, very little damage was observed by 6 h postinjection. By 72 h, there was noticeable crypt degeneration. By 96 h villus blunting and loss of crypts became obvious. By 120 h crypts were clearly regenerating and villi were beginning to lengthen. Quantitative analysis showed that villus height significantly decreased between 72 and 120 h postinjection but returned to normal by 168 h (Fig. 2A). This was paralleled by a decline and then return in total villus cell number during the same time span (Fig. 2B). In contrast, crypt depth did not change in response to Dox treatment until 120 and 168 h postinjection when significant increases were observed (Fig. 2A). A concomitant increase in total number of cells per crypt was observed at 168 h postinjection (Fig. 2B).
Fig. 1.
Doxorubicin (Dox) treatment induces changes in intestinal mucosa morphology. Hematoxylin and eosin (H&E)-stained sections were evaluated for changes in the morphology of the intestinal mucosa at various times following Dox treatment. Grossly, very little damage is observed by 6 h postinjection. By 72 h, there is noticeable crypt degeneration. By 96 h villus blunting and loss of crypts becomes obvious. By 120 h crypts are clearly regenerating and villi are beginning to lengthen. Scale bar equals 50 μm.
Fig. 2.
Dox treatment alters crypt and villus morphometry. Measurements were made at 0, 6, 24, 48, 72, 96, 120, and 168 h after Dox treatment. A: villus height and crypt depth. B: cells per villus and cells per crypt. *Statistical significance compared with controls (P < 0.05) (n = 6).
Doxorubicin induces changes in proliferation.
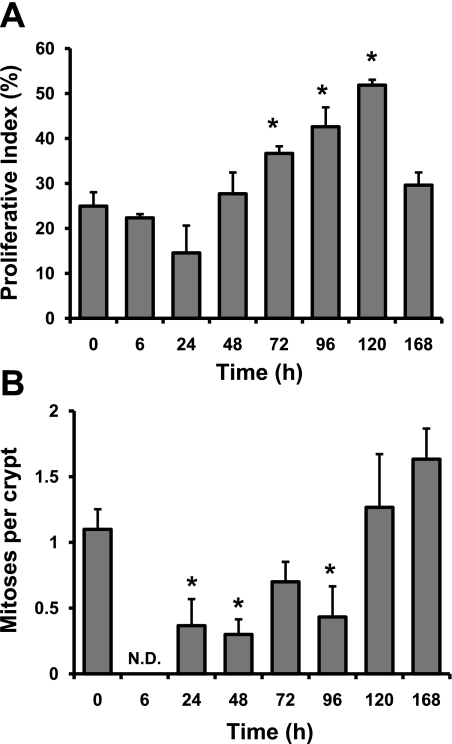
As measured by the percentage of BrdU-positive cells within crypt epithelium after a 2 h pulse of BrdU, Dox treatment showed minimal effects on DNA synthesis within the first 48 h following injection (Fig. 3A). Beginning at 72 h, BrdU labeling increased, peaked at 120 h, and returned to baseline by 168 h postinjection (Fig. 3A). A similar trend was revealed when data were expressed as BrdU-positive cells per crypt (data not shown). In contrast, as seen in Fig. 3B, Dox treatment rapidly altered the number of mitotic figures per crypt with significant decreases seen as early as 6 h following drug administration. In fact, at 6 h we were unable to detect any mitotic figures within the intestinal epithelium (Fig. 3B). Between 24 and 96 h after Dox treatment mitotic figures were observable within the intestinal epithelium, although significantly lower than numbers in control animals. By 120 h, the number of mitotic figures per crypt had returned to control levels.
Fig. 3.
Dox treatment influences proliferation. A: the number of 5′-bromo-2′-deoxyuridine (BrdU)-positive cells was scored at 0, 6, 24, 48, 72, 96, 120, and 168 h after Dox treatment and expressed as a percentage of total number of cells within a given crypt. B: the number of mitoses per crypt was scored at 0, 6, 24, 48, 72, 96, 120, and 168 h after Dox treatment by using H&E-stained sections. *Statistical significance compared with controls (P < 0.05) (n = 3). N.D., data for that time point were not detectible.
Dox alters lineage allocation.
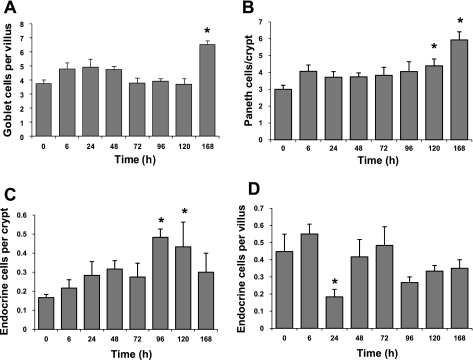
Staining of tissue sections with Alcian blue revealed no changes in the absolute number of goblet cells per half villus until 168 h after Dox injection (Fig. 4A). In contrast, evaluating goblet cells as percent of total cells per half villus demonstrated significant increases compared with control by 72 h and remained elevated out to 168 h after injection (Supplemental Fig. S1A). Similar data were obtained by staining with PAS (not shown). Paneth cell numbers, as evaluated by H&E staining, were significantly increased above controls at 120 and 168 h postinjection (Fig. 4B). In contrast, the percentage of Paneth cells per crypt increased as early as 6 h and remained elevated out to 96 h postinjection but was not different from controls at 120 and 168 h (Supplemental Fig. S1B). To confirm our observations we performed immunohistochemistry for lysozyme and found similar changes in total Paneth cell number and percentage of Paneth cells (data not shown). Using immunohistochemistry for chromogranin A we observed significant increases in enteroendocrine cell number per crypt between 96 and 120 h following Dox treatment (Fig. 4C). A similar trend is seen in the percentage of enteroendocrine cells per crypt (Supplemental Fig. S1C). Very little change in the total number of enteroendocrine cells per villus was observed following Dox treatment with the exception of a significant decrease at 24 h (Fig. 4D). When expressed as a percentage of total crypt cells, enteroendocrine cells showed an increase at 96 and 120 h after Dox treatment (Supplemental Fig. S1D).
Fig. 4.
Dox treatment alters lineage allocation. Total numbers of goblet, Paneth, and enteroendocrine cells were quantified at 0, 6, 24, 48, 72, 96, 120, and 168 h after Dox treatment. A: goblet cells per half villus. B: Paneth cells per crypt. C: enteroendocrine cells per crypt. D: enteroendocrine cells per villus. *Statistical significance compared with controls (P < 0.05) (n = 5–6 for goblet and Paneth cell data; n = 3 for enteroendocrine cell data).
Dox targets intestinal stem cells.
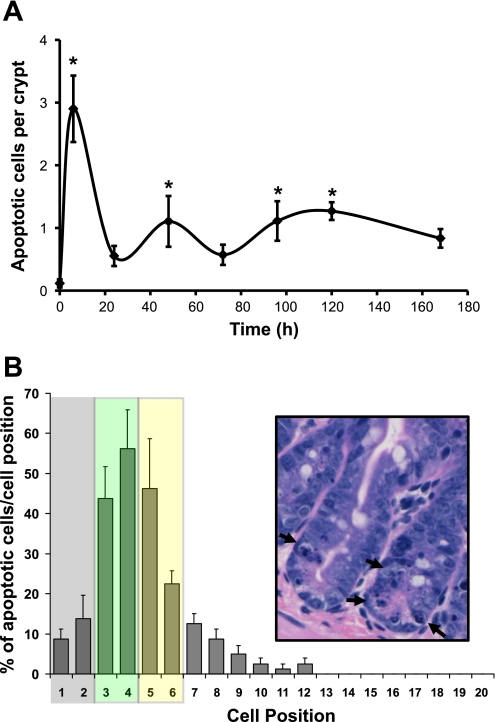
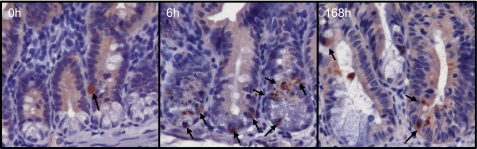
As seen in Fig. 5A, microscopic evaluation of H&E-stained sections revealed that Dox treatment induced 2.9 ± 0.5 apoptotic cells per crypt within 6 h after IP injection compared with 0.11 ± 0.6 apoptotic cell per crypt observed at the 0-h control (a 26-fold increase in apoptosis). By 24 h, the number of apoptotic bodies in crypt epithelium decreased but remained elevated over control levels out to 120 h postinjection (Fig. 5A). Similar to previous reports by Ijiri and Potten (21), we observed that the majority of Dox-induced apoptosis centered around the three to six cell positions within intestinal crypts (Fig. 5B), i.e., the putative location of intestinal stem cells. Typical apoptotic bodies are highlighted by arrows in Fig. 5B, inset. We confirmed our results by immunostaining for cleaved caspase-3. Figure 6 contains representative micrographs showing the baseline staining at 0 h, the increase in staining at 6 h, and the return to near-baseline at 168 h after Dox. When graphed, the changes in the number of cells positive for cleaved caspase-3 mirrored the number of apoptotic cells counted by H&E (data not shown).
Fig. 5.
Dox treatment rapidly induces apoptosis centering on the putative stem cell zone. A: apoptotic cells were scored via H&E staining of jejunal sections collected at 0, 6, 24, 48, 72, 96, 120, and 168 h after Dox treatment (n = 5–6) and expressed as the number of apoptotic cells per crypt. *Statistical significance compared with zero-time controls (P < 0.05). B: the location of apoptotic bodies within particular cell positions along the crypt was recorded at the 6-h time point. Data are presented as the percent of instances a particular cell position contained an apoptotic cell. The stem/progenitor cell zone is indicated by the shaded boxes. Using the nomenclature of Scoville et al. (37), the gray box indicates the area in which crypt base columnar cells (CBCs) reside and the yellow box indicates the area containing the +4 cells. Note that the green box represents potential overlap of these 2 areas. Inset: H&E micrograph of representative crypts containing apoptotic cells (arrows).
Fig. 6.
Dox treatment induces cleavage of caspase-3. Immunohistochemistry for cleaved caspase-3 corroborated our data in Fig. 1A showing increased staining by 6 h after Dox treatment and a return to near baseline by 168 h, compared with 0-h controls. Arrows point to cleaved caspase-3-positive cells.
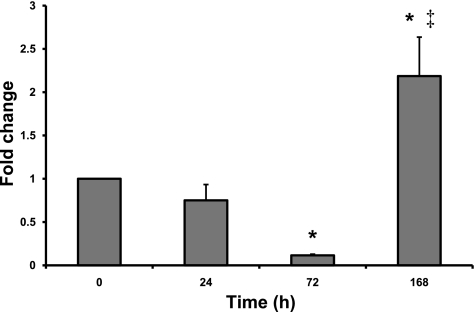
To assess the behavior of intestinal stem/progenitor cells following the apoptotic insult from Dox, we first used flow cytometry to quantitate the number of CD45(−) SP cells in jejunal mucosa harvested at various times after Dox treatment. As explained in materials and methods, the parameter has previously been shown to be an acceptable surrogate for stem cell number (11). As seen in Fig. 7, the percentage of CD45(−) SP cells declined modestly by 24 h then markedly by 72 h (to 10% of the 0 h value). In contrast, by 168 h after injection the percentage of CD45(−) SP cells was 2.5-fold higher than time 0 (Fig. 7).
Fig. 7.
Dox-induced damage results in changes in CD45(−) side population (SP). Flow cytometry was used to evaluate changes in the percentage of CD45(−) SP cells 24, 72, and 168 h following Dox treatment. Data were normalized to zero-time control animals and are presented as means ± SE (n = 5). *Statistical significance compared with zero-time control (P < 0.05). ‡Statistical significance compared with all other time points (P < 0.05).
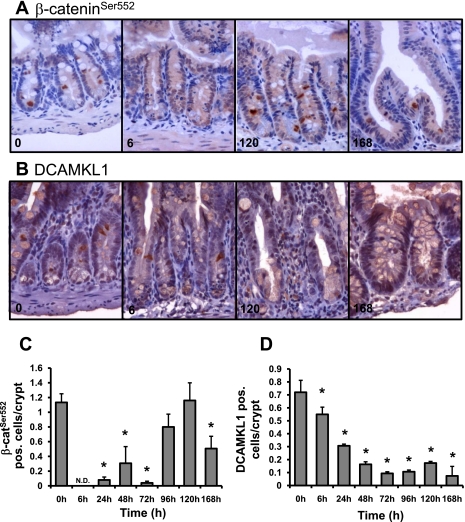
To further document the response of stem/progenitor cells following Dox treatment, we turned to several newly reported markers of these cells. Immunohistochemical evaluation of the putative +4 intestinal stem cell markers β-cateninSer552 and DCAMKL1 revealed significant changes in the expression of both of these antigens (Fig. 8). As shown in Fig. 8, A and C, the number of β-cateninSer552-positive cells dropped to undetectable levels as early as 6 h and remained significantly decreased to 72 h after Dox treatment. By 96 h, the number of β-cateninSer552-positive cells increased to control levels (0 h) but then significantly decreased at 168 h after Dox treatment. In contrast, as shown in Fig. 8, B and D, the number of DCAMKL1-positive cells was significantly decreased as early as 6 h after Dox treatment and remained decreased throughout the rest of the time course. Of note, the early decrease in DCAMKL1 staining appears to have occurred in a graded manner because the number of DCAMKL1-positive cells is significantly less at 24 h compared with 6 h.
Fig. 8.
Dox treatment induces changes in the presence of β-cateninSer552 and DCAMKL1 expressing cells. Immunohistochemistry was used to identify β-cateninSer552 or DCAMKL1-positive cells 0, 6, 24, 48, 72, 96, 120, and 168 h after Dox treatment (n = 3). A: β-cateninSer552 staining at 0, 6, 120, and 168 h revealed changes in the number of positive cells that are expressed in graphic form in C. DCAMKL1 staining (B) at 0, 6, 120, and 168 h revealed changes in the number of positive cells as well, which are quantified in D. *Statistical significance compared with zero-time control (P < 0.05).
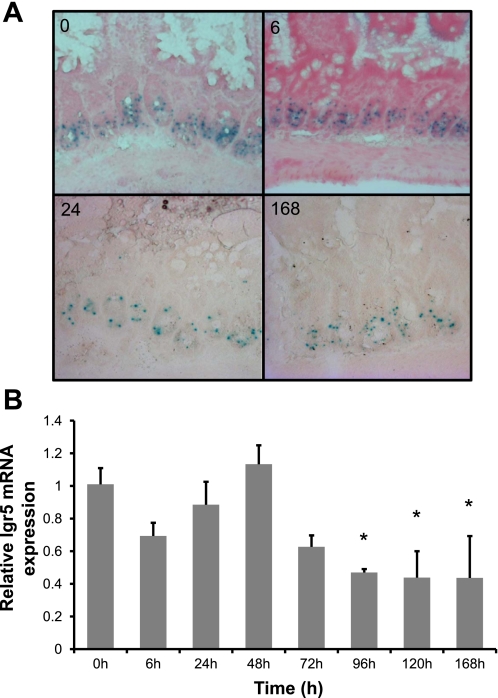
Given the lineage tracing evidence indicating that Lgr5 expressing cells at the crypt base can function as progenitors of all four epithelial lineages (3), it would have been desirable also to stain our sections for this marker. Unfortunately, suitable antibodies for such staining are not currently available. Thus as an alternative approach we utilized Lgr5-lacZ mice to evaluate LacZ expression following Dox treatment. As can be seen in Fig. 9A, blue LacZ staining is present in cells at the base of intestinal crypts. At various times following Dox treatment (6, 24, and 168 h) Lgr5-lacZ-positive cells remained present in the crypt base. In addition, we prepared RNA from tissue pieces adjacent to those used in Figs. 1–4 and performed real-time quantitative RT-PCR for Lgr5 mRNA. The data are shown in Fig. 9B and demonstrate that Lgr5 expression remained unchanged as far out as 72 h after Dox treatment but decreased by 96 h and remained significantly down out to 168 h.
Fig. 9.
Dox treatment has no effect on LacZ activity but induces changes in mRNA expression of the putative intestinal stem cell marker Lgr5. A: staining for LacZ activity using Lgr5-lacZ mice at 0, 6, 24, and 168 h after Dox treatment demonstrates that there is little change in LacZ activity following damage and during epithelial repair. B: using real-time quantitative RT-PCR we evaluated the changes of gene expression for Lgr5 at 0, 6, 24, 48, 72, 96, 120, and 168 h after Dox treatment (n = 3). *Statistical significance compared with zero-time control (P < 0.05).
Dox causes crypt loss followed by crypt fission.
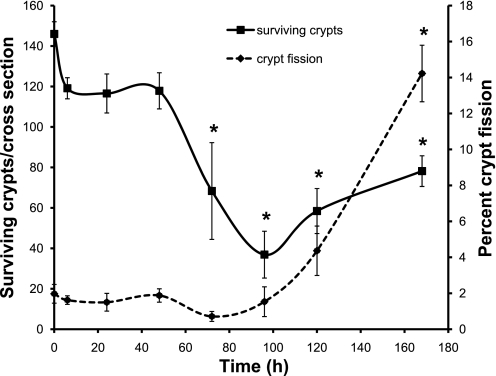
Further evidence of Dox-induced intestinal stem cell damage was observed as a decrease in the number of surviving crypts following Dox injection (Fig. 10). By 72 h postinjection, the number of surviving crypts had significantly decreased to ∼60% of crypt numbers at time 0. Statistically, the number of surviving crypts remained lower than at time 0 from 72 to 168 h postinjection. However, the data show that the number of crypts reached a nadir at 96 h postinjection and began to increase by 120 h.
Fig. 10.
Intestinal crypt regeneration occurs following treatment with Dox. The total number of surviving intestinal crypts per cross section (solid line, primary ordinate) and the percentage of crypt fission (dashed line, secondary ordinate) were calculated in zero-time control and 6, 24, 48, 72, 96, 120, 168 h tissue post-Dox injection (n = 3). *Statistical significance compared with controls (P < 0.05).
During periods of rapid expansion of intestinal stem cells, such as during development, following radiation damage, and in response to intestinal resection, new crypts are formed by the process of crypt fission (6, 8, 11, 30). To assess whether this also occurs during recovery from Dox damage, we determined the percentage of crypt fission occurring in the intestinal mucosa at various times following injection. As can be seen in Fig. 10, through 96 h postinjection the percentage of crypts undergoing fission remained at ∼2% of total crypts. After this time, there was a trend toward increasing crypt fission at 120 h and a marked increase in crypt fission up to ∼14% of total crypts fissioning at 168 h after Dox treatment (Fig. 10). An example of a fissioning crypt at 168 h can be seen in Fig. 8A.
DISCUSSION
The propensity for Dox treatment to induce mucositis in the intestine is understood; however, the sequela of cellular and morphological changes in the intestinal mucosa that follow is not clear, particularly with respect to intestinal stem cells. We found that following a single IP dose of Dox (20 mg/kg body wt), apoptosis was induced in the intestinal epithelium within 6 h of exposure, similar to what has been previously reported (21). On the basis of our data, the events following induction of apoptosis can be generally divided into two phases: 1) the damage phase, which occurs between 0 and 72 h postinjection and is characterized by loss of intestinal stem/progenitor cells leading to villus blunting and loss of crypts; and 2) the repair phase, which occurs between 72 and 168 h postinjection and is characterized by increases in proliferation, intestinal stem cell/progenitor number, and crypt fission leading to restoration of villus height, crypt depth, and crypt number. The repair phase is also characterized by increases in the numbers and relative proportions of all three members of the secretory lineage of the intestinal epithelium, i.e., goblet, Paneth, and enteroendocrine cells. Since the likely mechanisms and significance of these increases are distinct they will be discussed separately.
Goblet cells on the villi were found to increase in absolute number 168 h after Dox treatment and in the percentage of goblet cells on villi between 72 and 168 h, suggesting that these cells were not lost following Dox treatment. Of note, the latter increase occurred during the repair phase when proliferation was high, crypt and villus size was increasing, and intestinal stem cell numbers were expanding, suggesting preferential allocation to the goblet lineage compared with the dominant absorptive lineage. Although others have reported decreases in goblet cells following intestinal damage (4, 42), it is clear that expression of goblet cell secretory products is increased following damage (9, 43) and that deficiency of goblet cell secretory products, such as intestinal trefoil factor and Muc 2, results in more severe mucosal damage (4, 10). Our findings in this study are similar to what we observed in the adapting intestine following resection (18) and suggest that goblet cells and their secretory products may play a role in the repair process.
The time course of the increased census of Paneth cells suggests a different phenomenon compared with goblet cells. Although the absolute number of Paneth cells was increased only at 120 and 168 h, when expressed as a percentage of total cells per crypt, Paneth cell percentage increased between 6 and 96 h following Dox treatment. Since Paneth cells are long-lived cells (∼21–57 days) (5, 22) that did not display apoptosis after Dox treatment, an increase in their percentage within crypt epithelium during a time of enterocyte loss following apoptosis is to be expected. Conversely, by 120–168 h, as crypts regenerate, the absolute numbers of Paneth cells would be expected to increase while their percentage would return to normal. Just as for goblet cells, regardless of the mechanism, the maintenance of Paneth cells during damage may play an important role in the process of repair. It is well known that antimicrobial peptides including cryptdins (α-defensins), lysozyme, secretory phospholipase A2, and matrilysin (MMP-7) are secreted from granules localized to the apical membrane of Paneth cells (27). Indeed, Paneth cells sense bacteria and release microbicidal secretions into the intestinal lumen upon bacterial stimulation (1), presumably to control the intraluminal bacterial population. Therefore, since others have shown that Dox treatment increases permeability of the intestinal epithelium (40), one plausible role for Paneth cells would be to combat bacterial penetration of the epithelial barrier following Dox treatment. Furthermore, Paneth cells secrete numerous additional products, including TNF-α (23), EGF (33), TGF-β (38), and granulocyte macrophage colony-stimulating factor (14), which may play significant roles in the process of epithelial repair.
The potential impact of changes in enteroendocrine cell number and percentage in crypts and villi during epithelial repair following Dox-induced damage is not clear. Radford and Lobachevsky (34) postulate that enteroendocrine cells contribute to maintenance of a quiescent intestinal stem cell niche. The occurrence of increased enteroendocrine cell number in the crypt at 96 and 120 h following Dox treatment coincides with observed increases in crypt fission and the return of β-cateninSer552-positive cells. This suggests that perhaps enteroendocrine cells are playing a role in returning the +4 cells to a state of relative quiescence. In contrast, a model put forth by Dubé et al. (13) suggests that enteroendocrine cells influence proliferation in the crypt by activating β-catenin signaling. In their model, enteroendocrine cells secrete glucagon-like peptide-2, which binds to receptors on subepithelial myofibroblast stimulating secretion of IGF-I. IGF-I, then, binds to IGF-I receptor on crypt epithelial cells, stimulating proliferation in a β-catenin-dependent manner. In either case, the recent findings that CD45(−) SP cells are enriched for transcripts found in the maturity onset diabetes of the young pathway including Neurog3, NeuroD1, Nkx2–2, Pax4, Pax6, and glucokinase (15) and that Neurog3-positive cells can give rise to all four cell lineages in the small intestinal epithelium (36) suggest that further investigation into the relationship between enteroendocrine cells and intestinal stem cells is needed.
Our data indicate that intestinal stem/progenitor cells are central to the damage induced by Dox treatment and the epithelial repair that follows. Similar to previous studies by Ijiri and Potten (21), we found that induction of apoptosis by Dox localized to the intestinal stem cell zone. The location of the apoptotic response was also similar to what has been reported in the intestine following irradiation (29). Although there is currently no direct method to quantitate intestinal stem cells, several indirect measurements suggest that intestinal stem/progenitor cells are being lost and regenerated following Dox treatment. FACS analysis for side population cells at 72 h following Dox revealed a marked decrease in CD45-negative SP cells, a population of cells previously shown in our laboratory to be enriched for intestinal stem/progenitor cells (12, 15). This decrease was mirrored in the loss of intestinal crypts, suggesting the sterilization of many intestinal crypts, as has been reported after irradiation of the intestine (29). Over time CD45-negative SP cell numbers and crypt numbers increased, correlating with an increase in crypt fission. Together, these data suggest that an expansion of intestinal stem cells occurs as part of the mucosal repair.
Recent work suggests that there may be two populations of intestinal stem cells: the CBCs, which are actively cycling and located at the crypt base among the Paneth cells, and the +4 cells, which are slower cycling, label-retaining cells located at or above cell position 4 relative to the base of the crypt (2, 37). Two transcripts, Lgr5 and Bmi1, are believed to identify the CBCs and +4 cells, respectively (3, 35). More recent work has suggested that these two gene products may not clearly delineate the two aforementioned ISC populations since there are cells at the base of the crypt that express both transcripts (41). A third mRNA transcript, CD133/prominin, is expressed at the base of intestinal crypts in CBC cells that also express Lgr5 mRNA (44). However, CD133/prominin protein expression is not confined to the base of the crypt (39). Lineage tracing experiments have shown that CD133-, Lgr5-, and Bmi1-positive cells can contribute to all four epithelial lineages, although in the case of Bmi1 this occurs only in the proximal region of the small intestine (3, 35, 39, 44). Others have reported that DCAMKL1 and β-cateninSer552 may also be useful markers of +4 intestinal stem cells (16, 25). It should be noted that the latter have not been definitively proven to be intestinal stem cells markers, and the former have not demonstrated that CBCs and +4 cells are two distinct subpopulations of intestinal stem cells. Nonetheless, all of these markers can be valuable tools for evaluating intestinal stem cells.
Despite the limitations noted above we have attempted to define which population of ISC is initially affected by Dox. On the basis of our studies with Lgr5-lacZ mice, it appears that the number of Lgr5-expressing cells is not affected by Dox treatment; however, Lgr5 mRNA expression decreases during the regenerative phase. These data suggest, then, that Lgr5-positive cells, representing CBCs, are not susceptible to Dox-induced damage, similar to what is reported following irradiation (3, 21, 28). In contrast, as early as 6 h after Dox we observed decreases in the number of both DCAMKL1 and β-cateninSer552-positive cells, representing cells at the +4 position, cells that are much more sensitive to irradiation (2, 21, 37). Together, these data support the idea that the +4 intestinal stem cells are immediately affected by Dox treatment, whereas the CBC stem cells are resistant. Additional experiments are needed to delineate the molecular basis of this differential sensitivity.
We have also attempted to dissect out which cells in the intestinal stem cell zone are being regenerated in response to Dox-induced damage (i.e., CBC vs. +4). Our data for Lgr5 show that Lgr5-lacZ-expressing cells do not appear to be affected by Dox treatment. Interestingly, Lgr5 mRNA expression is decreased during regeneration. In contrast, we did find that the number of β-cateninSer552-positive cells in intestinal crypts increased to near control levels by 96 h after Dox treatment, suggesting that cells at the +4 cell position were being regenerated. However, staining for another putative +4 intestinal stem cell marker, DCAMKL1, showed that DCAMKL1-positive cells were infrequent during regeneration compared with control levels. Until we have a clear understanding of the functions of these putative markers it will be difficult to ascertain how changes in expression or secondary modification (in the case of β-cateninSer552) affect intestinal stem/progenitor cells.
The mechanism by which intestinal stem cell numbers are expanded following Dox-induced damage is unclear. One possibility is that the remaining intestinal stem cells (in particular those in the +4 region) undergo symmetric cell division, effectively increasing the intestinal stem cell number. This increase in stem cell number could then drive the expansion of intestinal crypts by crypt fission. This mechanism is believed to occur during intestinal development (6, 8) and following ileocecal resection in mice (11). Alternatively, clonogenic cells, which are part of a hierarchy of crypt cells that can replace lost intestinal stem cells (20, 31), may be recruited following damage. Only one clonogenic cell is needed to regenerate a crypt and to maintain appropriate crypt numbers by replacing lost crypts (29). Recruitment of these cells may then signal the expansion of crypt numbers. In either case, factors such as Wnts (26) and BMP4 (17) play critical roles in controlling intestinal stem cell expansion and crypt fission. De Koning et al. (9) have recently demonstrated that 7 days after Dox treatment BMP4 expression is decreased, possibly allowing for an increase in crypt fission.
In summary, the response of the intestinal mucosa following Dox treatment can be divided into two phases, the damage phase and the repair phase. The damage phase is characterized by induction of apoptosis, which leads to a destruction of the normal mucosal architecture and a loss of intestinal stem cells. In contrast, the repair phase is characterized by an expansion of intestinal stem cell numbers occurring concomitant with an increase in crypt fission. During this phase there is also increased secretory lineage allocation. Secretions from these cells are likely to play critical roles in the process of epithelial repair, which deserves further study. Evidence to date suggests that both the loss of stem/progenitor cells as well as the subsequent expansion primarily involves cells from the +4 region of the crypt. Additional research is needed to fully elucidate the mechanisms that drive intestinal stem cell expansion. Understanding the mechanisms that control these stem cell responses could provide us with potentially valuable treatments for chemotherapy-induced mucositis as well as a better understanding of intestinal stem cell biology.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants P30 DK56338, T32 DK07664, and R01 DK069585.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the Texas Medical Center Digestive Disease Center Cellular and Molecular Morphology Core for tissue processing and histology. The authors thank Kirk McNaughton of the Department of Cell and Molecular Physiology Histology Core at the University of North Carolina for assistance with immunohistochemistry and Dr. Linheng Li for the provision of antibodies to β-cateninSer552. We would also like to thank Mike Cubbage, Chris Threeton, and the Texas Children's Cancer Center Flow Cytometry Laboratory and Dr. Larry Arnold, Joan Kalnitsky, and the UNC Flow Cytometry Facility for assistance with flow cytometry.
REFERENCES
- 1.Ayabe T, Satchell DP, Wilson CL, Parks WC, Selsted ME, Ouellette AJ. Secretion of microbicidal alpha-defensins by intestinal Paneth cells in response to bacteria. Nat Immunol 1: 113–118, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Barker N, van de Wetering M, Clevers H. The intestinal stem cell. Genes Dev 22: 1856–1864, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449: 1003–1007, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Beck PL, Wong JF, Li Y, Swaminathan S, Xavier RJ, Devaney KL, Podolsky DK. Chemotherapy- and radiotherapy-induced intestinal damage is regulated by intestinal trefoil factor. Gastroenterology 126: 796–808, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Bjerknes M, Cheng H. The stem-cell zone of the small intestinal epithelium. I. Evidence from Paneth cells in the adult mouse. Am J Anat 160: 51–63, 1981. [DOI] [PubMed] [Google Scholar]
- 6.Cheng H, Bjerknes M. Whole population cell kinetics and postnatal development of the mouse intestinal epithelium. Anat Rec 211: 420–426, 1985. [DOI] [PubMed] [Google Scholar]
- 7.Cheng H, Leblond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian theory of the origin of the four epithelial cell types. Am J Anat 141: 537–562, 1974. [DOI] [PubMed] [Google Scholar]
- 8.Clarke RM The effect of growth and of fasting on the number of villi and crypts in the small intestine of the albino rat. J Anat 112: 27–33, 1972. [PMC free article] [PubMed] [Google Scholar]
- 9.De Koning BA, Lindenbergh-Kortleve DJ, Pieters R, Buller HA, Renes IB, Einerhand AW. Alterations in epithelial and mesenchymal intestinal gene expression during doxorubicin-induced mucositis in mice. Dig Dis Sci 52: 1814–1825, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Koning BA, Sluis M, Lindenbergh-Kortleve DJ, Velcich A, Pieters R, Buller HA, Einerhand AW, Renes IB. Methotrexate-induced mucositis in mucin 2-deficient mice. J Cell Physiol 210: 144–152, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Dekaney CM, Fong JJ, Rigby RJ, Lund PK, Henning SJ, Helmrath MA. Expansion of intestinal stem cells associated with long-term adaptation following ileocecal resection in mice. Am J Physiol Gastrointest Liver Physiol 293: G1013–G1022, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Dekaney CM, Rodriguez JM, Graul MC, Henning SJ. Isolation and characterization of a putative intestinal stem cell fraction from mouse jejunum. Gastroenterology 129: 1567–1580, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Dubé PE, Rowland KJ, Brubaker PL. Glucagon-like peptide-2 activates beta-catenin signaling in the mouse intestinal crypt: role of insulin-like growth factor-I. Endocrinology 149: 291–301, 2008. [DOI] [PubMed] [Google Scholar]
- 14.Fukuzawa H, Sawada M, Kayahara T, Morita-Fujisawa Y, Suzuki K, Seno H, Takaishi S, Chiba T. Identification of GM-CSF in Paneth cells using single-cell RT-PCR. Biochem Biophys Res Commun 312: 897–902, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Gulati AS, Ochsner SA, Henning SJ. Molecular properties of side population-sorted cells from mouse small intestine. Am J Physiol Gastrointest Liver Physiol 294: G286–G294, 2008. [DOI] [PubMed] [Google Scholar]
- 16.He XC, Yin T, Grindley JC, Tian Q, Sato T, Tao WA, Dirisina R, Porter-Westpfahl KS, Hembree M, Johnson T, Wiedemann LM, Barrett TA, Hood L, Wu H, Li L. PTEN-deficient intestinal stem cells initiate intestinal polyposis. Nat Genet 39: 189–198, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He XC, Zhang J, Tong WG, Tawfik O, Ross J, Scoville DH, Tian Q, Zeng X, He X, Wiedemann LM, Mishina Y, Li L. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt-beta-catenin signaling. Nat Genet 36: 1117–1121, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Helmrath MA, Fong JJ, Dekaney CM, Henning SJ. Rapid expansion of intestinal secretory lineages following a massive small bowel resection in mice. Am J Physiol Gastrointest Liver Physiol 292: G215–G222, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Houchen CW, George RJ, Sturmoski MA, Cohn SM. FGF-2 enhances intestinal stem cell survival and its expression is induced after radiation injury. Am J Physiol 39: G249–G258, 1999. [DOI] [PubMed] [Google Scholar]
- 20.Ijiri K, Potten CS. The re-establishment of hypersensitive cells in the crypts of irradiated mouse intestine. Int J Radiat Biol Relat Stud Phys Chem Med 46: 609–623, 1984. [DOI] [PubMed] [Google Scholar]
- 21.Ijiri K, Potten CS. Further studies on the response of intestinal crypt cells of different hierarchical status to eighteen different cytotoxic agents. Br J Cancer 55: 113–123, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ireland H, Houghton C, Howard L, Winton DJ. Cellular inheritance of a Cre-activated reporter gene to determine Paneth cell longevity in the murine small intestine. Dev Dyn 233: 1332–1336, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Keshav S, Lawson L, Chung LP, Stein M, Perry VH, Gordon S. Tumor necrosis factor mRNA localized to Paneth cells of normal murine intestinal epithelium by in situ hybridization. J Exp Med 171: 327–332, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lipkin M Growth and development of gastrointestinal cells. Annu Rev Physiol 47: 175–197, 1985. [DOI] [PubMed] [Google Scholar]
- 25.May R, Riehl TE, Hunt C, Sureban SM, Anant S, Houchen CW. Identification of a novel putative gastrointestinal stem cell and adenoma stem cell marker, doublecortin and CaM kinase-like-1, following radiation injury and in adenomatous polyposis coli/multiple intestinal neoplasia mice. Stem Cells 26: 630–637, 2008. [DOI] [PubMed] [Google Scholar]
- 26.Pinto D, Clevers H. Wnt control of stem cells and differentiation in the intestinal epithelium. Exp Cell Res 306: 357–363, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Porter EM, Bevins CL, Ghosh D, Ganz T. The multifaceted Paneth cell. Cell Mol Life Sci 59: 156–170, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Potten CS Extreme sensitivity of some intestinal crypt cells to X and gamma irradiation. Nature 269: 518–521, 1977. [DOI] [PubMed] [Google Scholar]
- 29.Potten CS A comprehensive study of the radiobiological response of the murine (BDF1) small intestine. Int J Radiat Biol 58: 925–973, 1990. [DOI] [PubMed] [Google Scholar]
- 30.Potten CS Radiation, the ideal cytotoxic agent for studying the cell biology of tissues such as the small intestine. Radiat Res 161: 123–136, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Potten CS, Chadwick C, Ijiri K, Tsubouchi S, Hanson WR. The recruitability and cell-cycle state of intestinal stem cells. Int J Cell Cloning 2: 126–140, 1984. [DOI] [PubMed] [Google Scholar]
- 32.Potten CS, Loeffler M. Stem cells: attributes, cycles, spirals, pitfalls and uncertainties. Lessons for and from the crypt. Development 110: 1001–1020, 1990. [DOI] [PubMed] [Google Scholar]
- 33.Poulsen SS, Nexo E, Olsen PS, Hess J, Kirkegaard P. Immunohistochemical localization of epidermal growth factor in rat and man. Histochemistry 85: 389–394, 1986. [DOI] [PubMed] [Google Scholar]
- 34.Radford IR, Lobachevsky PN. An enteroendocrine cell-based model for a quiescent intestinal stem cell niche. Cell Prolif 39: 403–414, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sangiorgi E, Capecchi MR. Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet 40: 915–920, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schonhoff SE, Giel-Moloney M, Leiter AB. Neurogenin 3-expressing progenitor cells in the gastrointestinal tract differentiate into both endocrine and non-endocrine cell types. Dev Biol 270: 443–454, 2004. [DOI] [PubMed] [Google Scholar]
- 37.Scoville DH, Sato T, He XC, Li L. Current view: intestinal stem cells and signaling. Gastroenterology 134: 849–864, 2008. [DOI] [PubMed] [Google Scholar]
- 38.Seno H, Sawada M, Fukuzawa H, Morita Y, Takaishi S, Hiai H, Chiba T. Enhanced expression of transforming growth factor (TGF) -alpha precursor and TGF-beta1 during Paneth cell regeneration. Dig Dis Sci 46: 1004–1010, 2001. [DOI] [PubMed] [Google Scholar]
- 39.Snippert HJ, van Es JH, van den Born M, Begthel H, Stange DE, Barker N, Clevers H. Prominin-1/CD133 marks stem cells and early progenitors in mouse small intestine. Gastroenterology 136: 2187–2194, 2009. [DOI] [PubMed] [Google Scholar]
- 40.Sun Z, Wang X, Wallen R, Deng X, Du X, Hallberg E, Andersson R. The influence of apoptosis on intestinal barrier integrity in rats. Scand J Gastroenterol 33: 415–422, 1998. [DOI] [PubMed] [Google Scholar]
- 41.Van der Flier LG, van Gijn ME, Hatzis P, Kujala P, Haegebarth A, Stange DE, Begthel H, van den Born M, Guryev V, Oving I, van Es JH, Barker N, Peters PJ, van de Wetering M, Clevers H. Transcription factor achaete scute-like 2 controls intestinal stem cell fate. Cell 136: 903–912, 2009. [DOI] [PubMed] [Google Scholar]
- 42.Verburg M, Renes IB, Meijer HP, Taminiau JA, Buller HA, Einerhand AW, Dekker J. Selective sparing of goblet cells and Paneth cells in the intestine of methotrexate-treated rats. Am J Physiol Gastrointest Liver Physiol 279: G1037–G1047, 2000. [DOI] [PubMed] [Google Scholar]
- 43.Verburg M, Renes IB, Van Nispen DJ, Ferdinandusse S, Jorritsma M, Buller HA, Einerhand AW, Dekker J. Specific responses in rat small intestinal epithelial mRNA expression and protein levels during chemotherapeutic damage and regeneration. J Histochem Cytochem 50: 1525–1536, 2002. [DOI] [PubMed] [Google Scholar]
- 44.Zhu L, Gibson P, Currle DS, Tong Y, Richardson RJ, Bayazitov IT, Poppleton H, Zakharenko S, Ellison DW, Gilbertson RJ. Prominin 1 marks intestinal stem cells that are susceptible to neoplastic transformation. Nature 457: 603–607, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.