Abstract
The enterohepatic recirculation of bile acids (BAs) is important in several physiological processes. Although there has been considerable research on liver regeneration after two-thirds partial hepatectomy (PHx), little is known about how the liver protects itself against BA toxicity during regeneration. In this study, various BAs in plasma and liver, the composition of micelle-forming bile constituents, as well as gene expression of the main hepatobiliary transporters were quantified in sham-operated and PHx mice 24 and 48 h after surgery. PHx did not influence the hepatic concentrations of taurine-conjugated BAs (T-BA) but increased the concentration of glycine-conjugated (G-BA) and unconjugated BAs. Total BA excretion (μg·min−1·g liver wt−1) increased 2.4-fold and was accompanied by a 55% increase in bile flow after PHx. The plasma concentrations of T-BAs (402-fold), G-BAs (17-fold), and unconjugated BAs (500-fold) increased. The mRNA and protein levels of the BA uptake transporter Ntcp were unchanged after PHx, whereas the canalicular Bsep protein increased twofold at 48 h. The basolateral efflux transporter Mrp3 was induced at the mRNA (2.6-fold) and protein (3.1-fold) levels after PHx, which may contribute to elevated plasma BA and bilirubin levels. Biliary phospholipid excretion was nearly doubled in PHx mice, most likely owing to increased mRNA expression of the phospholipid transporter, Mdr2. In conclusion, the remnant liver after PHx excretes 2.5-fold more BAs and three times more phospholipids per gram liver than the sham-operated mouse liver. Upregulation of phospholipid transport may be important in protecting the biliary tract from BA toxicity during PHx.
Keywords: 2/3 partial hepatectomy, liver regeneration, biliary excretion, UPLC-MS/MS
the liver is a functionally versatile organ. It is responsible for the synthesis of several proteins (e.g., albumin and clotting factors), is involved in the coordination of metabolic homeostasis, and stores glycogen and lipid soluble vitamins. In addition, the liver protects the body from toxicants that are absorbed from the intestine or formed endogenously. Severe liver damage can lead to disturbances in these homeostatic mechanisms of liver, which can progress and cause significant morbidity and mortality.
Amphipathic bile acids (BAs) are involved in several physiological and pathophysiological mechanisms in the gastrointestinal tract and liver. BAs, synthesized from cholesterol in liver, are the most important driving force in bile formation and biliary elimination of cholesterol. In the intestine, BAs are required for efficient absorption of dietary fat and fat-soluble vitamins by increasing the surface area of lipid droplets in the small intestine and activating pancreatic lipases (38). In the last decade, BAs have been found to be regulatory hormones that activate the farnesoid X receptor (FXR), activate pathways in which mitogen-activated protein kinase, stress-activated kinase, and lipid kinases are involved, and also serve as ligands for the G protein-coupled receptor TGR5 (50). Activation of FXR by BAs plays an important role in regulating the expression of metabolic target genes and driving homeotrophic liver growth during normal liver regeneration in mice (41).
Tight regulation of BA homeostasis is crucial because increased concentrations of BAs can cause liver toxicity. BAs can damage cell membranes in part because of their detergent properties (59). The biliary tract is protected against the toxic effect of BAs by several mechanisms. Cholangiocytes are protected by the active transport of BAs to plasma via the apical sodium-dependent BA transporter (Asbt) and the basolateral organic solute heteromeric transporter (Ost)α/β (84). The excreted phospholipids also play an important role in protecting the biliary tract (76). Multidrug resistance protein (MDR) 3 (and Mdr2 in rodents) is a phospholipid flipase that translocates phospholipids from the inner to outer membrane of the canaliculi and cholangiocytes. Humans with MDR3 mutations develop type 3 progressive familial intrahepatic cholestasis (FIC-3); a similar phenotype has been reproduced in Mdr2-null mice, manifested by the lack of biliary phospholipid excretion and development of progressive liver disease (19, 68). Genetic defects of aminophospholipid flipase Atp8b1 and Bsep cause similar familial intrahepatic cholestasis, namely FIC-1 and FIC-2 (87). Hepatocyte damage by toxic BAs is thought to be a key event for the progression of cholestatic liver diseases. It has been suggested that when the intracellular concentration of BAs exceeds the cytosolic binding capacity, BAs induce apoptosis and necrosis by damaging mitochondria (64).
To maintain the physiologically appropriate concentrations of BAs in liver, biliary tract, and intestine, BAs are regulated within the enterohepatic circulation by the actions of transporters in the liver and intestine (44). The bile salt export pump (Bsep) is the major canalicular bile salt-transporting protein in liver (29). Intestinal reabsorption of BAs is mediated by Asbt localized in the terminal ileum (36). Organic solute transporter (Ost) α/β heterodimer serves as the ileal basolateral transporter of BAs from enterocytes into blood (62). After their reabsorption, BAs are taken up by hepatocytes from portal blood via the Na+-taurocholate cotransporting polypeptide (Ntcp) and Na+-independent organic anion-transporting polypeptides (Oatps) (46). Only a relatively small fraction (∼5%) of BAs escapes intestinal absorption and vanishes into feces. The fecal BA waste is compensated for by de novo BA biosynthesis in the liver. Therefore, the BA pool remains constant under steady-state conditions.
During liver regeneration, in addition to replacing hepatocytes, the liver must continue normal liver functions such as biliary excretion, albumin and clotting factor synthesis, and protecting itself from harmful endo- and xenobiotics. The proportion of remnant parenchyma cells in S phase increases from 0.1 to 15% within 24 h after two-thirds partial hepatectomy (PHx) (20). Following PHx, the volume and weight of rodent livers are restored within 2 wk. Interestingly, some liver functions such as bile formation and biliary excretory capacity recover rapidly within 2 days after PHx, prior to significant replacement of cellular content (43). The maintenance of bile formation and enterohepatic recirculation of BAs are indispensable for liver regeneration (55). After liver resection in humans, bile flow and BA concentration in bile are good indicators of hepatic regeneration (47).
After PHx, the remnant liver is exposed to a high flux of BAs that could aggravate liver injury. A previous study reported that the concentration of total hepatic BAs does not increase after PHx; however, the profile of individual BAs was not quantified (41).
Whereas there has been considerable research on how the liver regenerates after two-thirds PHx, little is known about the composition and enterohepatic recirculation of BAs, as well as how liver transporters protect the liver against BA toxicity during liver regeneration. Therefore, the present study examined the effects of PHx on BA composition in liver, plasma, and bile, as well as changes in other bile constituents that are important for stable nonaggressive biliary micelle formation. Gene expression, protein levels, and cellular distribution of the most important hepatobiliary transporters were quantified at 24 and 48 h after surgery in PHx and sham-operated male C57Bl/6 mice.
MATERIALS AND METHODS
Chemicals
Oasis-HLB SPE cartridges were purchased from Waters (Milford, MA). Mrp2 (M2III-5, for Western blot) and Mrp3 (M3II-2) antibodies were obtained from George Scheffer (Vrije Universiteit Medical Center, Amsterdam, The Netherlands). Mrp2 (for immunofluorescence), Bsep (K44), and Ntcp (K4) antibodies were generous gifts of Bruno Steiger (University Hospital, Zurich, Switzerland). The Ostα antibody was provided by Ned Ballatori (University of Rochester School of Medicine, Rochester, NY). Oatp1a1, 1a4, and 2b1 antibodies were developed in our laboratory (14). All other chemicals were purchased from Sigma-Aldrich (St. Louis, MO), unless otherwise noted.
Animals
Eight-week-old male C57Bl/6 mice were purchased from Charles River Laboratories (Wilmington, MA). The mice acclimated for at least 1 wk in an American Animal Associations Laboratory Animal Care-accredited facility under standard temperature-, light-, and humidity-controlled environment. Mice had free access to Laboratory Rodent Chow 8604 (Harlan Teklad, Madison, WI) and drinking water. Studies were approved by the University of Kansas Medical Center Institutional Animal Care and Use Committee.
Two-Thirds Partial Hepatectomy and Sham Surgery
PHx of mice was performed by using a modification of the method of Higgins and Anderson (31, 37) by the same surgeon (I. L. Csanaky). Mice were placed into a Plexiglas chamber for closed-circuit induction of anesthesia with an inflow of 3% isoflurane and an oxygen flow rate of 1 l/min. After induction of anesthesia, the head of the mouse was positioned into a face mask that was connected to an anesthesia machine with an inflow of 2% isoflurane and an oxygen flow rate of 1 l/min. Depth of anesthesia was monitored by pinching the footpad of the mice before and throughout surgery. Heart rate and respiratory rate were monitored throughout surgery. The abdomen of the mouse was shaved and swabbed with Betadine and 70% alcohol. A 2-cm upper midline incision was made from the xyphoid caudally. During surgery, the liver was manipulated by moistened Q-tips to avoid injury. The left lateral and left and right segments of the median lobes were removed consecutively; the gallbladder was left intact. This process results in 68% (2/3) hepatectomy in mice. Sham-operated control mice underwent the same surgical procedure without ligation of lobes and without removal of two-thirds of the liver. After two-thirds of the liver was removed, the abdomen was irrigated with 5 ml of sterile saline to remove blood and decrease contamination. The abdominal cavity was closed with an interlocking running stitch with 5-0 silk. The skin was then reapproximated with 9-mm stainless steel wound clips. After closing the abdomen, the incision was again wiped with Betadine. Each mouse was given 3 ml of sterile saline subcutaneously. Surgeries were performed between 8:30 and 11:30 AM.
Bile Collection
At 24 or 48 h after surgery, the partially hepatectomized and sham-operated mice were again anesthetized by isoflurane, and their common bile ducts were cannulated through a high abdominal incision with the shaft of a 30-gauge needle attached to polyethylene PE-10 tubing. Bile samples were collected for 60 min into preweighed 0.6-ml microcentrifuge tubes on ice. The volumes of bile were determined gravimetrically, using 1.0 for specific gravity.
Tissue Collection
From separate groups of mice, 24 or 48 h after PHx, blood was collected from suborbital veins into heparinized tubes; livers from these animals were also collected. The plasma samples were separated by a Microtainer separating tube (BD Biosciences, San Jose, CA). A portion of each liver was frozen in liquid nitrogen and stored at −80°C until further analysis. Sections of livers were also embedded in Optimal Cutting Temperature Compound (Ted Pella, Redding, CA) and kept at −80°C until further use. In the time-course study of alkaline phosphatase (ALP), plasma samples were collected over several time points between 0 min and 14 days.
Biochemical Analysis of Bile, Blood, and Liver Samples
Sample preparation for BA analysis.
Internal standards, as well as bile, plasma, and liver samples were prepared for BA speciation as described and validated by Alnouti et al. (3). Briefly, plasma samples were deproteinized by internal standard containing ice-cold acetonitrile. The supernatants were removed, dried under vacuum, and reconstituted in 50% methanol. The liver samples were homogenized in 50% methanol. Homogenates were mixed with internal standards and ice-cold alkaline-acetonitrile and shaken for 1 h. After shaking, the homogenates were centrifuged, and the resulting supernatants were pooled, evaporated under vacuum, and reconstituted in 50% methanol. The bile samples were diluted 100-fold with deionized water, mixed with internal standards, and loaded onto Oasis-HLB SPE cartridges preconditioned with methanol and water. The loaded cartridges were washed with water and eluted with methanol. The eluates were evaporated under vacuum and reconstituted in 50% methanol.
HPLC and mass spectrometric conditions of BA analysis.
BAs were speciated and quantified by reverse-phase ultraperformance liquid chromatography-mass spectrometry (UPLC-MS/MS). The equipment used and the conditions of the UPLC-MS/MS are described in detail by Alnouti et al. (3). Quantification of the various BAs was based on peak areas of samples and authentic standards [unconjugated BAs: α and β muricholic acids (MCA), cholic acid (CA), ursodeoxycholic acid (UDCA), chenodeoxycholic acid (CDCA), deoxycholic acid (DCA), lithocholic acid (LCA); glycine (G) conjugates: G-CA, G-UDCA, G-CDCA, G-DCA, G-LCA; and taurine (T) conjugates: T-MCA, T-CA, T-UDCA, T-CDCA, T-DCA, and T-LCA].
Biochemical Analysis of Biliary Glutathione Phospholipids, and Cholesterol
Total biliary glutathione (i.e., reduced and oxidized GSH) was assayed by the method of Tietze (75), by use of a glutathione reductase kit (Sigma, St. Louis, MO). Biliary phospholipids were determined by a phospholipase D-choline oxidase method (35), using a kit obtained from Wako Chemicals (Richmond, VA). Biliary cholesterol was assayed by a cholesterol oxidase method, also using a kit obtained from Wako Chemicals.
Biochemical Analysis of Plasma ALT, ALP, and Conjugated Bilirubin
Plasma alanine aminotransferase (ALT) and ALP activity and bilirubin concentrations were determined as biochemical indicators of hepatocellular necrosis, biliary tract injury, and hepatic dysfunction by using ALT, ALP, and direct (conjugated) Bilirubin Reagent Sets (Pointe Scientific, Canton, MI) according to the manufacturer's protocol.
RNA Extraction
Total tissue RNA was extracted by using RNA-Bee reagent (Tel-Test, Friendswood, TX) according to the manufacturer's protocol. Each RNA pellet was redissolved in 0.2 ml of diethyl pyrocarbonate-treated water. RNA concentrations were quantified by ultraviolet absorbance at 260 nm. RNA integrity was confirmed by agarose gel electrophoresis of 5 μg of total RNA and visualization of intact 18S and 28S bands by ethidium bromide staining.
Branched DNA Signal Amplification Assay
mRNA levels were quantified by using the Quantigene 1.0 branched DNA signal amplification kit (Panomics, Fremont, CA) with modifications (49). The probes for Abcg5, Abcg8, Bsep, Cyp7a1, Cyp7b1, Cyp8b1, Cyp27a1, Mdr1a, Mdr1b, Mdr2, Mrp2, Mrp3, Mrp4, Oatp1a1, Oatp1a4, Oatp1b2, Ostα, Ostβ, and Ntcp mouse genes have been reported previously (13, 15, 54). Probe sets for Atp8b1, Fatp5, and bile acid-CoA:amino acid N-acetyltransferase (BAT) (Supplementary Table S1) were designed by ProbeDesigner (version 1.0, Bayer, Emeryville, CA) and synthesized by Integrated DNA Technologies (Coralville, IA).
Total RNA (10 μg/well) was added to each well of a 96-well plate containing capture hybridization buffer (50 μl) and a diluted probe set (50 μl) and allowed to hybridize to the probe set overnight at 53°C. Subsequent washing and hybridization steps were carried out following the manufacturer's protocol. Luminescence of 96-well plates was quantified with a Synergy 2 Microplate reader (BioTek Instruments, Winooski, VT). The luminescence for each well is reported as relative light units (RLU) per 10 μg of total RNA. The results of BA synthetic and conjugating enzymes are shown in Supplemental information for this article available at the American Journal of Physiology Gastrointestinal and Liver Physiology website.
Preparation of Crude Membrane Fractions
Livers were homogenized in sucrose-Tris (0.25 M sucrose, 10 mM Tris·HCl, pH 7.4) containing protease inhibitors and centrifuged at 100,000 g for 60 min at 4°C. The resulting pellet constituted the crude membrane fraction and was resuspended in sucrose-Tris buffer. Protein concentrations were determined using Pierce protein assay reagents according to the manufacturer's recommendation (Pierce Biotechnology, Rockford, IL).
Western Blot Analysis
Proteins were electrophoretically resolved without boiling by use of polyacrylamide gels (4% stacking, 8–12% resolving) and transblotted overnight at 4°C onto Polyvinylidene-Fluoride-Plus membranes (Micron Separations, Westboro, MA). Membranes were blocked for 1 h in 5% nonfat dry milk with 0.5% Tween-20 in phosphate buffered saline (PBS). All primary and secondary antibodies were diluted in 2% nonfat dry milk with 0.5% Tween-20 in PBS. Primary antibody dilutions were as follows: Bsep (K44, 1:3,000), Mrp2 (M2III-5, 1:2,000), Mrp3 (M3II-2, 1:500), Ostα (mA315, 1:1,000), Oatp1a1 (1:2,000), Oatp1a4 (1:2,000), Oatp1b2 (1:2,000), and Ntcp (K4, 1:5,000). Blots were subsequently incubated with a species-appropriate horseradish peroxidase-conjugated secondary antibody for 1 h. Membranes were stripped and reprobed with a dilution of 1:1,000 β-actin antibody (ab8227, Abcam, Cambridge, MA) to confirm equal protein loading. Protein-antibody complexes were detected by use of an enhanced chemiluminescent kit (Amersham Life Science, Arlington Heights, IL) and exposed to X-ray film (Denville Scientific, Metuchen, NJ). Intensities of protein bands were determined by use of the Discovery Series Quantity One 1-D Analysis software (Bio-Rad Laboratories, Hercules, CA).
Indirect Immunofluorescence Staining
Staining for Bsep, Mrp2, Mrp3, and Ntcp was performed as described previously (2). Cryosections (5 μm) were thaw mounted onto Superfrost glass slides (Fisher Scientific) and stored at −80°C with desiccant until use. Tissue sections were fixed with 4% paraformaldehyde for 5 min. Slides were blocked with 5% serum/PBS containing 0.1% Triton X (PBS-Tx) for 1 h and then incubated with primary antibody diluted 1:100 in 5% serum/PBS-Tx for 2 h at room temperature. Sections were subsequently washed in PBS-Tx and incubated for 1 h with goat anti-rat Alexa 488 IgG for Mrp3 detection and goat anti-rabbit Alexa 488 IgG for Bsep, Mrp2, and Ntcp detection (Invitrogen, Carlsbad, CA). Secondary antibodies were diluted 1:200 in 5% goat serum/PBS-Tx. All antibody solutions were filtered through 0.22-μm membrane syringe-driven filter units (Osmonics, Minnetonka, MN) prior to use. Frozen liver sections were stained and imaged under uniform conditions for each antibody. Two tissues were analyzed and found to be similar for each treatment group, and one representative image is presented. Negative controls without primary antibody were also included in the analysis (data not shown). Images were captured on an Olympus BX41 fluorescent microscope with a DP70 camera and DP Controller software (Olympus Optical, Tokyo, Japan). Images were cropped and brightness and contrast were adjusted under uniform conditions in Adobe Photoshop CS2 (San Jose, CA).
Statistical Analysis
Data are presented as means ± SE. Because of the large intragroup variation, the individual values were log-transformed and differences between the PHx and sham control groups were determined by t-test, with significance set at P < 0.05. The changes in concentration of BAs between the sham controls from 24 to 48 h were also determined by t-test with significance set at P < 0.05.
RESULTS
Effects of PHx on Plasma ALP, ALT, and Conjugated Bilirubin After Surgery
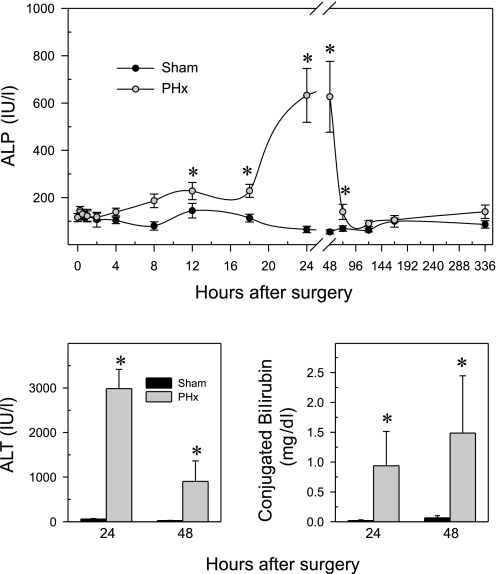
BAs are eliminated mainly via biliary excretion. It could be hypothesized that after PHx biliary excretion remains the major BA elimination route, if the biliary tract remains intact. Serum ALP activity is a good biochemical indicator of biliary tract injury. A time-course study demonstrated that plasma ALP levels started to increase 8 h after PHx, peaked at 24 and 48 h, and returned to basal levels by 72 h (Fig. 1). This phenomenon is similar to that observed in rats after PHx (60, 78). These results suggest that the first 48 h might be critical for biliary tract integrity and biliary excretion to maintain normal concentrations of BAs in liver, especially before the BA pool decreases. Based on these data and the known diurnal changes of BA metabolism (58), time points at 24 and 48 h were selected for subsequent detailed analysis. In addition, these time points are close to the peak time of DNA synthesis (36–40 h) in mice after PHx (82). Lastly, there is minimal apoptosis as assessed by TUNEL staining at 24 and 48 h after PHx (data not shown).
Fig. 1.
Effect of partial hepatectomy (PHx) on plasma alkaline phosphatase (ALP), alanine aminotransferase (ALT), and conjugated bilirubin. In a preliminary ALP time course study, blood samples were collected at several time points between 15 min and 14 days after surgery, whereas to measure ALT and conjugated bilirubin the blood samples were collected at 24 and 48 h after surgery. Data are presented as means ± SE of 5–6 mice, except for the activity of ALP at 24 and 48 h, data represents means ± SE of 11–12 mice, because the preliminary and the final experiment values were combined at these 2 time points after it was determined that they were not statistically different. *Significant difference (P < 0.05) from the respective value of the sham-operated mice.
Figure 1, bottom depicts plasma ALT activity and conjugated bilirubin 24 and 48 h after PHx and sham operations. Sham operations did not influence ALP (top), ALT (bottom left), or conjugated bilirubin (bottom right) levels, since all of these parameters were within normal ranges (53). PHx markedly increased plasma ALP (11-fold) at 24 and 48 h. ALT, a biochemical indicator of hepatocellular necrosis, was elevated 50-fold by PHx at 24 h. The serum ALT concentrations decreased 70% between 24 and 48 h after PHx; however, it still remained elevated at the later time point. Conjugated bilirubin was elevated 50-fold at both time points after PHx.
Effect of PHx on Hepatic and Plasma Concentrations of Unconjugated-, Glycine-, and Taurine-Conjugated BAs in Mice
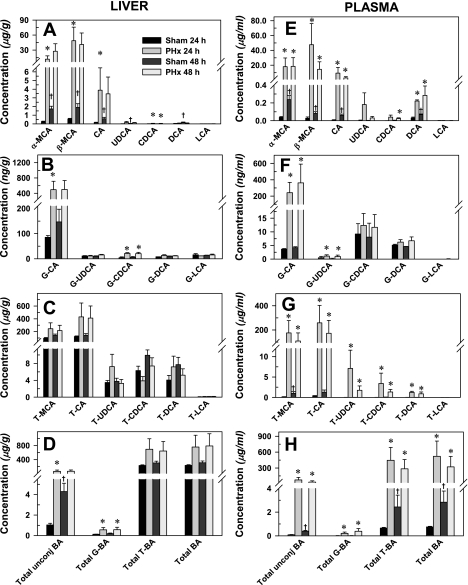
Concentrations of BAs in liver and plasma of sham and partial hepatectomized mice 24 and 48 h after surgery are depicted in Fig. 2. Surprisingly, the concentration in liver of unconjugated BAs, except CDCA and LCA, increased three- to sixfold in sham control mice from 24 to 48 h (Fig. 2A). There was a similar increase in plasma unconjugated BAs (Fig. 2E) and T-MCA (Fig. 2G) of sham control mice at 48 h. In the sham surgery group, the plasma concentration of total BAs increased fourfold from 24 to 48 h (Fig. 2H). Data from historical nonoperated male mice (3) indicate that the concentrations of unconjugated BAs of sham control mice decreased 70–90% at 24 h after sham surgery. The BA concentrations were restored by 48 h in sham control mice.
Fig. 2.
Effect of PHx on hepatic and serum concentrations of unconjugated (A and E), glycine-conjugated (G-; B and F), taurine-conjugated (T-; C and G), and total (D and H) bile acids in mice. Bars represent means ± SE of 5–6 mice. MCA, muricholic acids; CA, cholic acid; UDCA, ursodeoxycholic acid; CDCA, chenodeoxycholic acid; LCA, lithocholic acid. *Statistically significant difference (P < 0.05) from the respective value of the sham-operated mice. †Statistically significant changes (P < 0.05) in sham-operated mice 24 to 48 h.
At 24 h after PHx surgery, the concentrations of α-MCA, β-MCA, CA, and CDCA in the liver increased 43-, 89-, 23-, and 3-fold, respectively (Fig. 3A). Similar to 24 h after PHx, elevated unconjugated BA concentrations were observed at 48 h, although not statistically significant. This is likely due to higher sham values and the large intragroup variation in the PHx mice. The exception is CDCA, which increased threefold at 48 h after PHx.
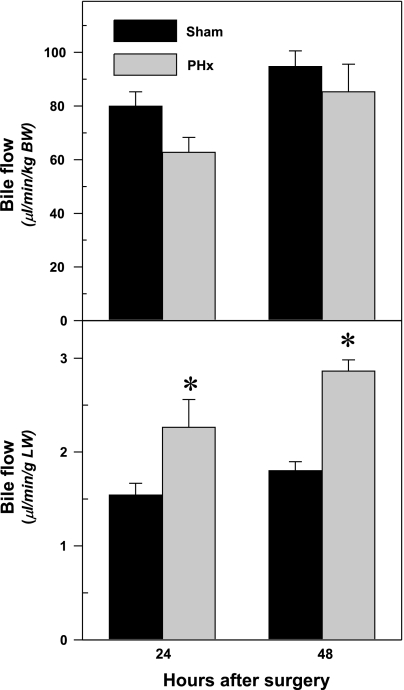
Fig. 3.
Effect of PHx on bile flow per kilogram body weight (top) and bile flow per gram liver weight (bottom) in mice. PHx or sham operations were performed on mice, and bile was collected for 60 min at 24 or 48 h after surgery. Bars represent means ± SE of 5–6 mice. BW, body weight; LW, liver weight. *Significant difference (P < 0.05) from the respective value of the sham-operated mice.
The hepatic concentrations of the glycine conjugates of CA and CDCA were 5.8-fold and 4.5-fold higher, respectively, 24 h after PHx (Fig. 2B). By 48 h after PHx, the concentration of G-CDCA in liver was 3.5-fold higher than in sham-operated mice. Taurine-conjugated BAs were the most abundant BAs in liver, and their concentrations tended to be increased after PHx, but were not statistically significant (Fig. 2C). The total concentration of hepatic BAs, similar to the total concentration of taurine-conjugated BAs, tended to increase, but it was not influenced significantly by PHx (Fig. 2D).
In contrast to liver, not only were the unconjugated and glycine conjugated BAs increased in plasma, but taurine-conjugated BAs were also increased after PHx at both time points (Fig. 2, E–H). PHx increased plasma concentrations of α-MCA, β-MCA, and CA between 200- and 400-fold and of DCA two- to threefold. Similar tendencies were noted in plasma UDCA and CDCA concentrations (Fig. 2E). With the glycine conjugates, PHx increased plasma G-CA (66- and 86-fold, respectively) and G-UDCA (2.2- and 5.6-fold, respectively) at 24 and 48 h after PHx (Fig. 2F). No changes in glycine conjugates of CDCA, DCA, or LCA were observed (Fig. 2F). Plasma levels of taurine-conjugated BAs were elevated 20- to 200-fold by PHx (Fig. 2G). These changes resulted in marked increases in total BA concentrations at both time points (Fig. 2H).
Effects of PHx on Bile Flow in Mice
Figure 3 demonstrates the bile flow per kilogram body weight and the bile flow per gram liver weight in sham and partially hepatectomized mice. The bile flow per body weight (top) did not change, whereas the bile flow per liver weight (bottom) increased 55% at both 24 and 48 h after PHx.
Effect of PHx on Biliary Concentration and Biliary Excretion of Unconjugated, Glycine-, and Taurine-Conjugated BAs
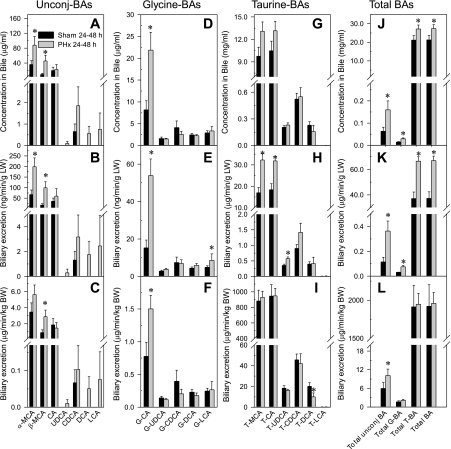
Bile was collected for 60 min at 24 and 48 h after sham and PHx surgeries, and the biliary concentration and excretion of BAs were quantified. The 24- and 48-h data were combined after it was determined that they were not statistically different. The biliary excretion of BAs is expressed as the biliary excretion per liver weight, as well as biliary excretion per body weight. As shown in Fig. 4, PHx primarily increased the concentration and excretion of unconjugated BAs (Fig. 4, A–C) and the glycine conjugates of BAs (Fig. 4, D–F).
Fig. 4.
Effect of PHx on biliary concentration, biliary excretion/body weight, and biliary excretion/liver weight of unconjugated (Unconj-), glycine-conjugated, and taurine-conjugated bile acids (BA). Biliary concentration (A, D, G, J), biliary excretion/liver weight (B, E, H, K), and biliary excretion/body weight (C, F, I, L) of unconjugated-, glycine-, and taurine-conjugated bile acids were quantified in mice following PHx or sham operations. Bile was collected for 60 min 24 or 48 h after surgery. The 24- and 48-h data were combined in sham and PHx groups after it was determined that they were not statistically different. Bars represent means ± SE of 10–12 mice. *Significant difference (P < 0.05) from the respective value of the sham-operated mice.
Unconjugated BAs.
PHx increased α-MCA and β-MCA concentrations in bile (Fig. 4A), as well as their excretion into bile per gram of liver (Fig. 4B). Increases in α-MCA and β-MCA concentrations (3- and 6-fold, respectively) in bile contribute to the enhanced total unconjugated BA concentrations (Fig. 4J) and biliary excretion per gram of liver (Fig. 4K). Thus one gram of liver of PHx mice excreted threefold more unconjugated BAs than per gram of liver from control mice (Fig. 4K). Normalization of biliary excretion to body weight indicates that PHx increases β-MCA (Fig. 4C) and total unconjugated BA excretion (Fig. 4L).
Glycine-conjugated BAs.
PHx increased glycine-conjugated CA (Fig. 4D). The increased concentrations of G-CA in bile resulted in enhanced biliary excretion of G-CA per gram of liver weight (Fig. 4E), biliary excretion of G-CA per body weight (Fig. 4F), and total biliary glycine conjugated BA concentrations (Fig. 4J), as well as the excretion of total G-BA per gram of liver (Fig. 4K). In contrast, the total glycine-conjugated BAs per body weight (Fig. 4L) were unchanged by PHx.
Taurine-conjugated BAs.
Taurine-conjugated BAs constitute the largest proportion of biliary BAs (Fig. 4J). The concentration of taurine-conjugated BAs (Fig. 4G) and their total concentration increased slightly (Fig. 4J) after PHx, but there was no difference in total biliary excretion per body weight (Fig. 4I) of taurine-conjugated BAs between partial hepatectomized and sham-operated mice. In contrast, PHx increased the biliary excretion of T-MCA, T-CA, and T-UDCA per gram of liver by 90, 73, and 60%, respectively (Fig. 4H). Therefore, one gram of liver of partially hepatectomized mice excreted 79% more taurine conjugated BAs than did one gram of liver of sham controls (Fig. 5K).
Fig. 5.
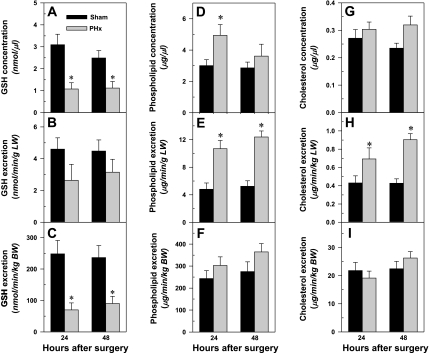
Effect of PHx on biliary concentration, biliary excretion/liver weight, and biliary excretion/body weight of glutathione (A–C), phospholipids (D–F), and cholesterol (G–I) in mice. Bars represent means ± SE of 5–6 mice. *Significant difference (P < 0.05) from the respective value of the sham-operated mice.
As illustrated in Fig. 4, J–L, PHx increased the biliary concentration of total BAs 30%, and the biliary excretion per gram liver increased by 50%, but did not significantly alter the excretion of per body weight.
Effects of PHx on Biliary Concentration and Biliary Excretion of GSH, Phospholipids, and Cholesterol in Mice
PHx reduced biliary GSH concentration (Fig. 5A) ∼65% at both time points. Calculated per gram of liver, there was a trend for reduced GSH excretion in PHx mice, although this was not statistically significant (Fig. 5B). However, the biliary excretion of GSH per body weight decreased 65–70% in the PHx mice (Fig. 5C).
Excretion of phospholipids is critical for reducing the toxic effect of BAs in the biliary tree. Biliary phospholipid concentrations increased 65% at 24 h after PHx (Fig. 5D) and nearly doubled the biliary excretion of phospholipids per gram of liver at 24 and 48 h after PHx (Fig. 5E). However, PHx did not alter the biliary excretion of phospholipids per body weight (Fig. 5F). PHx altered neither the biliary concentration (Fig. 5G) nor the excretion of cholesterol per body weight (Fig. 5I); however, the biliary excretion of cholesterol per gram of liver increased 50–100% after PHx (Fig. 5H).
mRNA Expression of Hepatic Canalicular and Sinusoidal Efflux Transporters After PHx
Canalicular transporters.
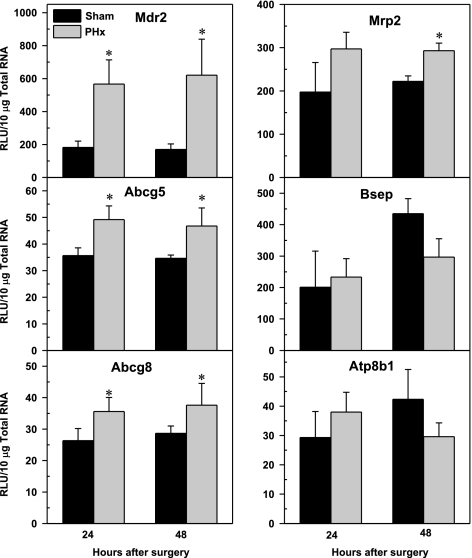
Figure 6 demonstrates that the mRNA level of Mdr2 increased threefold 24 and 48 h after PHx. Cholesterol transporters Abcg5 and Abcg8 were increased 30% in PHx mice compared with shams. A similar pattern was detected for Mrp2, but the increase was only significant at 48 h. PHx did not change the mRNA expression of Bsep or Atp8b1 at 24 or 48 h after surgery. The low mRNA expression of possible BA transporters, Mdr1a and Mdr1b, were also not changed by PHx (data not shown).
Fig. 6.
Effect of PHx on mRNA expression of canalicular transporters Mdr2, Abcg5, Abcg8, Mrp2, Bsep, and Atp8b1 in mouse liver. Bars represent means of relative light unit (RLU)/10 μg total RNA ± SE of 5–6 mice. *Significant difference (P < 0.05) from the respective value of the sham-operated mice.
Sinusoidal efflux transporters.
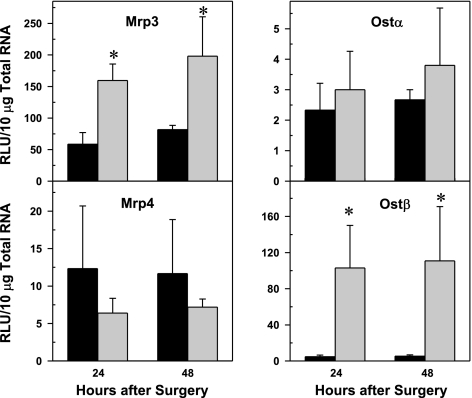
As illustrated in Fig. 7, Mrp3 gene expression was upregulated threefold after PHx. No change in Mrp4 mRNA was observed after PHx. PHx did not alter the gene expression of the bile acid transporter Ostα. In contrast, Ostβ mRNA, the heterodimer partner of Ostα, was 22-fold higher in PHx mice than sham-operated mice.
Fig. 7.
Effect of PHx on mRNA expression of basolateral efflux transporter Mrp3, Mrp4, Ostα, and Ostβ in mouse liver. Bars represent means of relative light unit 10 μg total RNA ± SE of 5–8 mice. *Significant difference (P < 0.05) from the respective value of the sham-operated mice.
Western Blot Analysis of Canalicular and Sinusoidal Hepatic Efflux Transporters After PHx
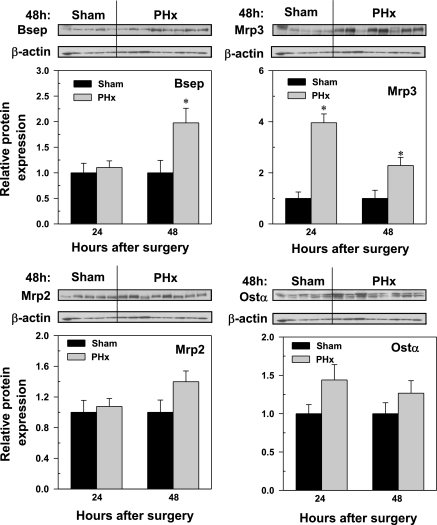
Western blot analyses were performed on transporters for which antibodies were available. Figure 8 depicts the 48-h blots and the relative protein abundance (Fig. 8). Despite unchanged mRNA expression of Bsep, protein levels doubled at 48 h after PHx. PHx did not alter the amount of canalicular Mrp2 protein but increased Mrp3 protein 4- and 2.5-fold at 24 and 48 h, respectively. Because Ostβ was not detectable with the available antibody, only the Ostα Western blot is shown, which indicates no statistically significant changes in the amount of Ostα protein.
Fig. 8.
Bsep, Mrp2, Mrp3, and Ostα protein abundance in livers of mice 24 and 48 h following sham and PHx surgery. Western blots for Bsep (∼170 kDa), Mrp2 (∼190 kDa), Mrp3 (∼180 kDa), and Ostα (∼40 kDa) were performed with use of liver membrane protein fraction (40 μg protein/lane) from sham- and PHx-operated mice 24 and 48 h after surgery. β-Actin (∼45 kDa) blot was used as a protein loading control for each transporter. Bars represent the relative Bsep, Mrp2, Mrp3, or Ostα protein amounts ± SE of 5–8 mice and as representative blots at 48 h. The scales for mean relative protein expression differ for each transport protein. *Statistical difference (P < 0.05) from the respective values of sham-operated mice.
Immunofluorescent Staining of Canalicular and Sinusoidal Hepatic Efflux Transporters After PHx
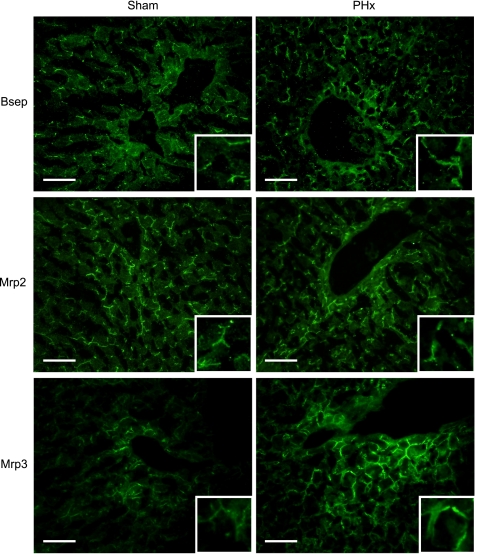
Bsep, Mrp2, and Mrp3 immunofluorescent staining was performed on frozen liver sections to determine patterns of zonal and cellular transporter localization 48 h following sham and PHx surgery. Bsep and Mrp2 immunostaining was localized to the canalicular membrane of hepatocytes in sections from sham and PHx mice (Fig. 9). Compared with sham controls, livers from PHx mice showed slightly more Bsep immunostaining in the periportal region. In contrast, Mrp2 immunostaining was unchanged after PHx. Mrp3 protein was localized to the basolateral membranes of hepatocytes, as evidenced by a distinct honeycomb pattern. Similar to Western blot analysis, limited immunofluorescent staining of Mrp3 was observed in the liver lobule from sham-operated mouse livers. PHx enhanced the immunostaining of Mrp3 on the basolateral membranes of hepatocytes throughout the liver. Mrp3 protein increased most notably in hepatocytes surrounding the central and portal veins.
Fig. 9.
Immunofluorescent analysis of Bsep, Mrp2, and Mrp3 in mouse liver 48 h following sham and PHx surgeries. Indirect immunofluorescence against canalicular Bsep and Mrp2 and sinusoidal Mrp3 (green) was conducted on liver cryosections (5 μm) obtained 48 h following sham and PHx surgery. Representative periportal regions are shown. Portions of images were enlarged and provided as insets. Representative images are shown. Bar, 50 μm.
mRNA expression of Sinusoidal Hepatic Uptake Transporters After PHx
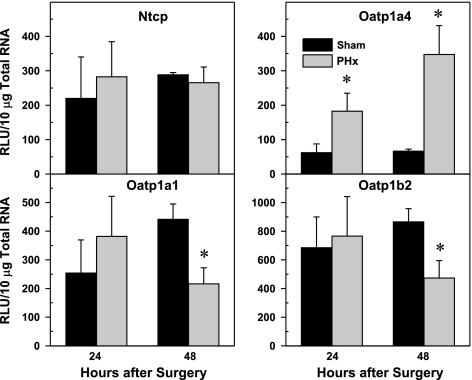
Ntcp and Oatp isoforms constitute the major hepatic uptake transporters for BAs. No changes were observed in Ntcp mRNA at either time point after PHx (Fig. 10). Oatp1a4 mRNA increased 4- and 7-fold at 24 and 48 h, respectively, after PHx surgery. In contrast, Oatp1a1 and Oatp1b2 gene expression decreased ∼50% at 48 h after PHx compared with sham-operated mice.
Fig. 10.
Effect of PHx on expression of basolateral uptake transporters Oatp1a1, Oatp1a4, Oatp1b2, and Ntcp mRNA in mouse liver. Bars represent means of unit (RLU) 10 μg total RNA ± SE of 5–8 mice. *Significant difference (P < 0.05) from the respective value of the sham-operated mice.
Western Blot Analysis of Sinusoidal Hepatic Uptake Transporters After PHx
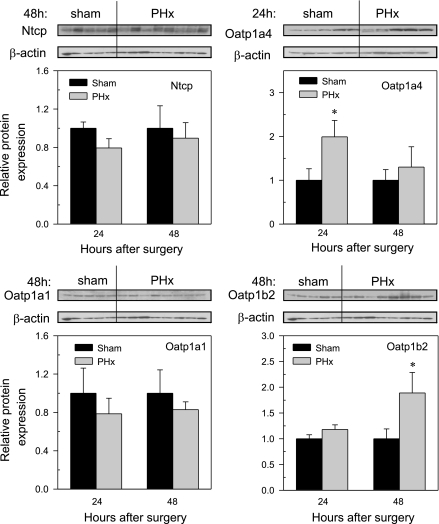
Western blot analysis was performed with available antibodies. Figure 11 depicts the 48-h blots of Oatp1a1, 1b2, and Ntcp and relative protein abundance at 24 and 48 h after PHx. In the case of Oatp1a4, the 24-h blot is shown. The amount of Ntcp protein was not changed by PHx, similar to its mRNA expression. The localization of Ntcp to the basolateral membrane and staining intensity were not altered by PHx (data not shown). There were no significant changes in protein abundance of Oatp1a1 in PHx mice at either 24 or 48 h after surgery. At the 24-h time point, the amount of Oatp1a4 protein was elevated. However, by 48 h the protein expression of Oatp1a4 returned to that seen in levels of sham control mice. Oatp1b2 expression was not changed at 24 h after PHx, but it increased at 48 h.
Fig. 11.
Oatp1a1, Oatp1a4, Oatp 1b2, and Ntcp protein abundance in livers of mice 24 and 48 h following sham and PHx surgery. Western blots for Oatp1a1 (∼70 kDa), Oatp1a4 (∼70 kDa), Oatp1b2 (∼75 kDa), and Ntcp (∼50 kDa) were performed with use of liver membrane protein fractions (40 μg protein/lane) from sham- and PHx-operated mice 24 and 48 h after surgery. β-Actin (∼45 kDa) blot was used as a protein loading control for each transporter. Bars represent the Oatp1a1, Oatp1a4, Oatp1b2, or Ntcp protein expression ± SE of 5–6 mice, and representative blots at 48 h are shown. In the case of Oatp1a4, the 24-h representative blot is shown. The scales for mean relative protein amount differ for each transport protein. *Statistical difference (P < 0.05) from the respective values of sham-operated mice.
DISCUSSION
This study describes the changes in concentration of 18 different BAs in liver, plasma, and bile, and it describes the expression of major hepatobiliary transporters that are important in maintaining the enterohepatic recirculation of BAs, as well as protecting the liver and the biliary tree in mice during the critical first 48 h after PHx in mice. During this critical time frame, when ALP is elevated showing biliary tract injury, the remnant liver needs to maintain the same amount of BAs in recirculation.
Even though the livers are one-third the normal size after PHx, they excreted more than twice the amount of BAs into bile per gram of liver than did sham mice. The total concentrations of BAs in the liver tended to be higher after PHx, but the increase was not statistically significant (Fig. 2D). This result is consistent with previous observations in rats (26) and in mice (41).
Interestingly, after PHx, the concentrations of unconjugated BAs were 16- to 62-fold higher, and the glycine-conjugated BAs in liver were three- to fourfold higher than in sham mice. The importance of this increased concentration of unconjugated and glycine-conjugated BAs in liver after PHx is difficult to ascertain, because the majority of BAs are taurine conjugates. Brand et al. (10) described a two- to threefold increase in the hepatic uptake of the amino acids alanine and glycine via the sodium-coupled amino acid transport system in PHx rats. The increased concentrations of glycine-conjugated BAs in livers of PHx mice might result from increased uptake of glycine. Increased hepatic concentrations of glycine-conjugated BAs might also be explained by enhanced activity in the remnant liver of the enzymes responsible for conjugating BAs. In rats, the BA-conjugating enzymes, namely bile acid-CoA ligase [BAL; in mice fatty acid transporter protein (Fatp) 5 is considered the BAL] and BAT, are targets of FXR signaling (61). However, in mice, the mRNA of BA conjugation enzymes were not induced by PHx (Supplementary Fig. S1).
The hepatic concentration of BAs can be maintained by decreased BA production, increased biliary excretion, decreased basolateral uptake, and increased basolateral efflux. BAs are signaling molecules that activate FXR. During liver regeneration, FXR is thought to orchestrate BA synthesis and transport to restore liver mass (27, 41). After PHx, the remaining liver is exposed to a higher flux of BAs. Activation of FXR in response to BA accumulation causes repression of the key BA synthetic enzyme Cyp7a1 through the small heterodimer protein (SHP). Cyp7a1-mRNA is reduced 50% in mice 24 and 48 h after PHx (Supplementary Fig. S1), as described previously (41). PHx also significantly lowered mRNA expressions of Cyp27a1 and Cyp8b1 (alternative pathway) at 48 h after surgery (Supplementary Fig. S1).
The remnant liver cells after PHx in mice adapt very quickly to the acute challenge and increase the total biliary excretion of BAs close to the normal value. Increased biliary excretion is an important pathway to reduce hepatocellular BA concentrations and protect the liver against BA-induced liver injury. Canalicular secretion of bile is the result of osmotic water flow powered by the active transport of solutes, which are primarily BAs and secondarily GSH. BAs are considered the major solute generating bile flow (5, 8, 69).
Remnant livers of PHx mice compensated for tissue loss and actually increased bile flow (55–60% more bile than sham livers per gram liver). These findings regarding bile flow in mice after PHx are similar to those described for rats after PHx, where the bile flow per gram liver weight was also increased (40, 43, 51, 80). The present study shows that PHx mainly increases biliary concentration and excretion of unconjugated BAs and glycine-BA conjugates (Fig. 4). Biliary concentrations of total unconjugated and total glycine-conjugated BAs increased 3.6- and 2.1-fold, respectively. Nevertheless, the concentration of total taurine-conjugated BAs increased 25% after PHx. However, when biliary excretion is expressed per gram of liver, there is a 50% increase in taurine-conjugated BAs (mainly T-MCA and T-CA) being excreted. The increase in excretion of taurine-conjugated BAs is mainly responsible for the compensated bile formation, because 98% of the biliary BAs are taurine conjugates.
Biliary excretion of BAs is primarily mediated by Bsep and Mrp2. Unconjugated BAs and BA amides are substrates of Bsep, the major canalicular bile acid export pump, whereas sulfated and glucuronidated BAs are substrates of Mrp2 (1). Bsep gene expression is regulated by FXR (23, 83). Previous studies demonstrate increased mRNA expression of the FXR target gene Bsep in mice and rats between 24 and 72 h after PHx (29, 41). Interestingly, in this study, the mRNA expression of Bsep did not change after PHx, similar to that observed in a number of studies with rats (22, 29, 80). Contrary to the unchanged mRNA level of Bsep 48 h after PHx, the amount of Bsep protein increased and was localized to the canalicular membrane (Fig. 8, 9). However, it is difficult to explain what is responsible for the restored bile flow and biliary excretion of BAs at 24 h after PHx. To answer this question, there are several mechanisms that might be responsible for this quick adaptation. Bsep is regulated in a complex way by transcriptional (via FXR) and posttranslational mechanisms, such as the insertion and retrieval of canalicular membrane vesicles (73). Moreover, the regulation of Bsep activity may become more complicated during liver regeneration, since quiescent liver cells might become activated and help to compensate for lost excretory function (43). In physiological conditions, the uptake of BAs into liver occurs primarily by the periportal hepatocytes; however, in the case of high plasma concentrations of BAs, the pericentral hepatocytes also become involved in BA clearance (32, 42, 70). Baumgartner et al. (6) demonstrated a reduction of metabolic zonation with regard to BA handling after liver resection in rats. They found that the velocity of biliary cholate excretion was higher in pericentral cells on day 1 and was more pronounced 5 days after PHx. The reduction of metabolic zonation of liver was described also in regard to carbohydrate-metabolizing enzymes, cholesterol synthesis, and lipid metabolism (4, 11, 12, 79). The change of metabolic zonation of remaining liver lobules helps the hepatectomized animals survive during the early liver regeneration period.
Based on our results and previously mentioned data, it can be hypothesized that the very quick adaptation to maintain bile flow is a complex mechanism, likely due to a combination of higher transport capacity of the existing transporters, activation of quiescent cells, changes in zonation of liver cells, as well as increased insertion into the membrane, decreased retrieval, and increased de novo synthesis of Bsep protein. The participation, sequence, and regulation of these different possible mechanisms are complex, and further research is needed to clarify them during liver regeneration.
Another important canalicular transporter is Mrp2, which participates in transporting sulfate, glucuronide, and GSH conjugates of organic anions, including BAs (39). Contrary to the modest increase in Mrp2 mRNA at 48 h after PHx, the protein level of Mrp2 did not change, which indicates minimal or no role of Mrp2 in the enhanced biliary excretion of BAs (Figs. 6 and 8). In addition to transporting BA conjugates, Mrp2 is crucial in the biliary excretion of GSH. Minimal change in Mrp2 expression is consistent with unchanged GSH excretion per gram liver weight after PHx (Fig. 5). Therefore, PHx mice overall excrete ∼66% less GSH into bile (proportional to their liver mass) than sham-operated mice. The decrease in biliary excretion of GSH in PHx mice is similar to that described in rats (80). The proportional decrease in biliary excretion of GSH is probably an attempt to conserve GSH in the liver. It was shown in rats that maintaining hepatic GSH can facilitate liver regeneration through the restoration of mitochondrial function (30).
In addition to Bsep and Mrp2, Mdr1a and Mdr1b also have the ability to transport BAs, at least in some special cases, such as in Bsep-null mice (48). However, there were no changes in mRNA expression of either Mdr1a or Mdr1b after PHx (data not shown).
Hepatocytes normally eliminate BAs mainly via the bile, but in pathological conditions the liver also effluxes BAs across the sinusoidal membrane into the blood. After PHx, the remnant liver has increased expression of biliary and sinusoidal BA transporters. Basolateral BA efflux in liver cells is mediated by Mrp3, 4, and Ost α/β (28). In this study, there was upregulation of Mrp3 mRNA and protein (Figs. 7–9), with no change in Mrp4 (Figs. 7 and 8). Under normal conditions, Mrp3 is expressed at a very low level in mouse hepatocytes but is upregulated during cholestasis (81). Mrp3 is known as an inducible BA efflux transporter during cholestasis. After induction, Mrp3 increases the elimination of accumulating BAs, as well as conjugated and unconjugated bilirubin, from liver to blood (7). Elevated Mrp3 expression is likely responsible for the postoperative conjugated (direct) hyperbilirubinemia observed after PHx (Fig. 1, bottom right). Recently, the heterodimeric organic solute transporter Ostα/β was identified as a basolateral Na+-independent BA export system. Ostα/β is expressed in the ileum, kidney, cholangiocytes, and at very low levels in murine hepatocytes (18, 62). Interestingly, human hepatocytes highly express Ostα/β (9). Ostβ, but not Ostα, is markedly induced after bile-duct ligation (9), similar to what is shown in this study after PHx (Fig. 7). Zollner et al. (88) showed that CA increases Ostβ mRNA via FXR, with minimal change in Ostα. It can be hypothesized that induction of Ostβ protects the cholangiocytes by their basolateral transport of BAs back into the blood after PHx. Therefore, in addition to the enhanced biliary excretion of BAs, hepatocytes and cholangiocytes protect themselves from high BA concentration by increasing basolateral efflux of BAs via Mrp3 and Ostα/β, respectively.
Elevated plasma BAs after PHx (Fig. 2) are likely a result of increased basolateral efflux (Mrp3, Ostα/β), in coordination with reduced hepatic uptake (decreased basolateral surface of liver cells, increased blood flow, and unchanged abundance of Ntcp). Interestingly, elevated plasma BA concentrations are typically used as an indicator of cholestasis. The classical definition of cholestasis is a condition in which the bile flow decreases. It is well known that after PHx marked transient elevations of plasma BA concentrations occur (41, 56). However, the present study demonstrates elevated serum BA concentrations even though there is enhanced bile flow and maintained BA excretion (Figs. 2–4).
BA homeostasis in the liver depends not only on synthesis, biliary excretion, and basolateral efflux, but also on basolateral uptake into hepatocytes. PHx reduces expression of BA and organic anion uptake transporters in rats (22, 29, 31, 80). Surprisingly, in the present work, neither the mRNA expression nor the protein level of Ntcp were altered by PHx (Figs. 10 and 11). Thus there may be a species difference in the regulation of Ntcp. Furthermore, there are conflicting studies demonstrating the ability of BAs to increase, decrease, or not alter Ntcp expression in mice (13, 33, 83). The conflicting effect of BAs on Ntcp expression is likely due to the lack of FXR/RXRα response elements in the mouse Ntcp promoter. BA-mediated regulation of Ntcp may occur through other signaling pathways (28).
Na+-independent BA uptake is mediated by Oatps, namely by Oatp1a1, Oatp1a4, and Oatp1b2, which are localized to the sinusoidal membrane of hepatocytes (63, 65, 86). Although mRNA levels of Oatp1a1 and Oatp1b2 decreased 48 h after PHx, there were minimal changes in protein expression except Oatp1b2, which increased at 48 h. Both Oatp1a4 mRNA and protein expression increased after PHx in mice (Figs. 10 and 11). This elevated expression of Oatp1a4 was opposite to the previous observation in rats (29, 80). Upregulation of Oatp1a4 after PHx is similar to changes observed after bile duct ligation in mice (67). Murine Oatp1a4 is also upregulated after the administration of pregnane X receptor (PXR) ligands (16). The importance of PXR activation in the rate of liver regeneration in mice has been recently recognized (17). Taken together, Oatp1a4 upregulation may be a consequence of PXR activation during liver regeneration in mice. Similar to FXR, PXR is a BA sensor that can be activated by the toxic BA LCA and, to a lesser extent, by DCA and CA (72, 85). PXR coordinately regulates not only transport genes but also biotransformation genes (such as Cyp3a11), which may reduce the toxicity of LCA (71).
The present data suggest that after PHx the increased hepatobiliary efflux of BAs is important in maintaining the amount of BAs excreted into bile, which sustain the intestinal absorption of fatty acids, as well as avoiding hepatotoxicity due to high concentrations of BAs in the liver. The enhanced sinusoidal and biliary excretion of BAs probably contributes to the decrease in liver injury by 48 h after PHx, as indicated by the reduced plasma ALT activity (Fig. 1, bottom left).
BAs can be very toxic to bile canaliculi, because simple BA micelles can solubilize cell membranes of the biliary tract cells. The protection of biliary tract cells against BAs is complex. Cholangiocytes can protect themselves through the upregulation of Ostα/β, as noted earlier. The other local defense mechanism is the Atp8b1 transporter that flips aminophospholipids from the outer to the inner leaflet, increasing the relative content of sphingomyelin and cholesterol in the outer membrane leaflet and consequently increasing the resistance of membranes against the detergent effect of BAs (77). PHx had little effect on Atp8b1, suggesting that after PHx the local defense functions of Atp8b1 are kept intact. The phospholipid flipase Mdr2 is an indirect defense mechanism to protect the biliary tract from injury by flipping phospholipids from the interior to exterior bi-leaflet of the canalicular membrane. After translocation, the phospholipids form mixed micelles with simple BA micelles, thereby reducing the toxicity of BAs (21, 45). As noted in Fig. 1, transient increases in serum ALP occurred between 8 and 72 h after hepatectomy, suggesting biliary tract injury. By 72 h after PHx, serum ALP returned to normal values, suggesting repair of the biliary tract injury. The repair is likely due to enhanced biliary excretion of phospholipids, probably via Mdr2 (Fig. 5). Similar to the present finding in mice, Mdr2, as well as biliary concentration and excretion of phospholipids, also increases in rats 48 h after partial hepatectomy (57, 74), likely in response to BA-activated FXR signaling (25, 34, 52). In addition, enhanced phospholipid synthesis in the remnant liver may also contribute to this protection (24). Therefore, it is concluded that enhanced phospholipid synthesis, upregulation of Mdr2, and higher biliary excretion of phospholipids mitigates early biliary tract injury resulting from BA excretion during liver regeneration. Finally, under normal conditions, the mixed micelles of lecithin (phospholipid) and BAs combine with cholesterol (66). Biliary cholesterol excretion per liver weight increased after PHx, likely owing to higher levels of Abcg5/8 mRNA (Fig. 7).
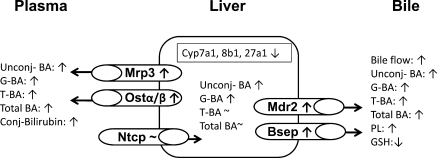
In summary, this work provides new insights into the changes in disposition of 18 different BAs in partially hepatectomized mice (Fig. 12). During the critical first 48 h after PHx, the mouse liver attempts to maintain BA concentrations close to normal values. PHx does not significantly influence the hepatic concentrations of taurine-conjugated BAs and, consequently, the concentrations of total BAs in the liver. However, the glycine and unconjugated BA concentrations increase significantly in liver. To maintain BA concentrations after PHx, the remnant liver, besides decreasing the BA de novo synthesis, increases BA efflux into bile, probably via enhanced Bsep activity. After PHx, hepatocytes also limit the accumulation of BAs by upregulating basolateral efflux transporters, including Mrp3 and Ostα/β. Probably because of the aforementioned induction of transporters, the plasma concentrations of unconjugated, glycine-, and taurine-conjugated BAs increase. In contrast to the increased plasma concentrations of BAs, the remnant mouse liver, similarly to rats, effectively compensates and maintains normal bile flow after PHx. The compensatory maintenance of bile flow is largely due to increased excretion of BAs. The biliary tree is probably protected against the harmful effects of enhanced BA excretion by increased expression of Ostα/β as well as Mdr2, which is responsible for enhanced canalicular excretion of phospholipids.
Fig. 12.
Summary figure depicting the consequences of PHx on bile acid homeostasis and transporters in mice. Arrows represent changes from wild-type mice for a metabolite, enzyme, or transporter. PHx does not significantly influence the hepatic concentrations of taurine-conjugated BAs and, consequently, the concentrations of total BAs in the liver. However, the glycine and unconjugated BA concentrations increase significantly in liver. To maintain BA concentrations after PHx, the remnant liver, besides decreasing bile acid de novo synthesis (Cyp7a, 8b1, 27a1), increases the BA efflux into bile, probably via increasing Bsep activity. After PHx, hepatocytes also limit the accumulation of BAs by upregulating basolateral efflux transporters, including Mrp3 and Ostα/β. Probably owing to this aforementioned induction of transporters, the plasma concentrations of unconjugated, glycine-, and taurine-conjugated BAs increase. The biliary tree is likely protected against the harmful effects of enhanced bile acid excretion by Mdr2, which can be responsible for enhanced canalicular excretion of phospholipids. Conj, conjugated.
GRANTS
This work was supported by National Institute of Health Grants ES09649, ES09716, ES013714, ES07079, and RR021940.
Supplementary Material
Acknowledgments
The authors thank Xiaohong Lei for devoted help with the animal experiments, Dr. Yazen Alnouti for excellent assistance in the bile acid analytical work, and Drs. Ned Ballatori, Bruno Steiger, and George Scheffer for providing specific antibodies.
REFERENCES
- 1.Akita H, Suzuki H, Ito K, Kinoshita S, Sato N, Takikawa H, Sugiyama Y. Characterization of bile acid transport mediated by multidrug resistance associated protein 2 and bile salt export pump. Biochim Biophys Acta 1511: 7–16, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Aleksunes LM, Scheffer GL, Jakowski AB, Pruimboom-Brees IM, Manautou JE. Coordinated expression of multidrug resistance-associated proteins (Mrps) in mouse liver during toxicant-induced injury. Toxicol Sci 89: 370–379, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Alnouti Y, Csanaky IL, Klaassen CD. Quantitative-profiling of bile acids and their conjugates in mouse liver, bile, plasma, and urine using LC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci 873: 209–217, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersen B, Zierz S, Jungermann K. Alteration in zonation of succinate dehydrogenase, phosphoenolpyruvate carboxykinase and glucose-6-phosphatase in regenerating rat liver. Histochemistry 80: 97–101, 1984. [DOI] [PubMed] [Google Scholar]
- 5.Balabaud C, Kron KA, Gumucio JJ. The assessment of the bile salt-nondependent fraction of canalicular bile water in the rat. J Lab Clin Med 89: 393–399, 1977. [PubMed] [Google Scholar]
- 6.Baumgartner U, Sellinger M, Ruf G, Jehle L, Ihling C, Farthmann EH. Change of zonal bile acid processing after partial hepatectomy in the rat. J Hepatol 22: 474–480, 1995. [DOI] [PubMed] [Google Scholar]
- 7.Belinsky MG, Dawson PA, Shchaveleva I, Bain LJ, Wang R, Ling V, Chen ZS, Grinberg A, Westphal H, Klein-Szanto A, Lerro A, Kruh GD. Analysis of the in vivo functions of Mrp3. Mol Pharmacol 68: 160–168, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Boyer JL, Klatskin G. Canalicular bile flow and bile secretory pressure. Evidence for a non-bile salt dependent fraction in the isolated perfused rat liver. Gastroenterology 59: 853–859, 1970. [PubMed] [Google Scholar]
- 9.Boyer JL, Trauner M, Mennone A, Soroka CJ, Cai SY, Moustafa T, Zollner G, Lee JY, Ballatori N. Upregulation of a basolateral FXR-dependent bile acid efflux transporter OSTα-OSTβ in cholestasis in humans and rodents. Am J Physiol Gastrointest Liver Physiol 290: G1124–G1130, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Brand HS, Deutz NE, Meijer AJ, Jorning GG, Chamuleau RA. In vivo amino acid fluxes in regenerating liver after two-thirds hepatectomy in the rat. J Hepatol 23: 333–340, 1995. [PubMed] [Google Scholar]
- 11.Chatzipanagiotou S, Nath A, Vogt B, Jungermann K. Alteration in the capacities as well as in the zonal and cellular distributions of pyruvate kinase L and M2 in regenerating rat liver. Biol Chem Hoppe Seyler 366: 271–280, 1985. [DOI] [PubMed] [Google Scholar]
- 12.Cheng HC, Yang CM, Shiao MS. Zonation of cholesterol and glycerolipid synthesis in regenerating rat livers. Hepatology 17: 280–286, 1993. [PubMed] [Google Scholar]
- 13.Cheng X, Buckley D, Klaassen CD. Regulation of hepatic bile acid transporters Ntcp and Bsep expression. Biochem Pharmacol 74: 1665–1676, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng X, Klaassen CD. Critical role of PPAR-alpha in perfluorooctanoic acid- and perfluorodecanoic acid-induced downregulation of Oatp uptake transporters in mouse livers. Toxicol Sci 106: 37–45, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng X, Klaassen CD. Regulation of mRNA expression of xenobiotic transporters by the pregnane x receptor in mouse liver, kidney, and intestine. Drug Metab Dispos 34: 1863–1867, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Cheng X, Maher J, Dieter MZ, Klaassen CD. Regulation of mouse organic anion-transporting polypeptides (Oatps) in liver by prototypical microsomal enzyme inducers that activate distinct transcription factor pathways. Drug Metab Dispos 33: 1276–1282, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Dai G, He L, Bu P, Wan YJ. Pregnane X receptor is essential for normal progression of liver regeneration. Hepatology 47: 1277–1287, 2008. [DOI] [PubMed] [Google Scholar]
- 18.Dawson PA, Hubbert M, Haywood J, Craddock AL, Zerangue N, Christian WV, Ballatori N. The heteromeric organic solute transporter alpha-beta, Ostalpha-Ostbeta, is an ileal basolateral bile acid transporter. J Biol Chem 280: 6960–6968, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Vree JM, Jacquemin E, Sturm E, Cresteil D, Bosma PJ, Aten J, Deleuze JF, Desrochers M, Burdelski M, Bernard O, Oude Elferink RP, Hadchouel M. Mutations in the MDR3 gene cause progressive familial intrahepatic cholestasis. Proc Natl Acad Sci USA 95: 282–287, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Digernes V, Bronstad G, Sand TE, Christoffersen T. The proliferation response of rat liver parenchymal cells after partial hepatectomy. A methodological study comparing flow cytometry of nuclear DNA content and in vivo and in vitro uptake of thymidine. Cell Tissue Kinet 15: 521–528, 1982. [DOI] [PubMed] [Google Scholar]
- 21.Donovan JM, Timofeyeva N, Carey MC. Influence of total lipid concentration, bile salt:lecithin ratio, and cholesterol content on inter-mixed micellar/vesicular (non-lecithin-associated) bile salt concentrations in model bile. J Lipid Res 32: 1501–1512, 1991. [PubMed] [Google Scholar]
- 22.Dransfeld O, Gehrmann T, Kohrer K, Kircheis G, Holneicher C, Haussinger D, Wettstein M. Oligonucleotide microarray analysis of differential transporter regulation in the regenerating rat liver. Liver Int 25: 1243–1258, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Fickert P, Zollner G, Fuchsbichler A, Stumptner C, Pojer C, Zenz R, Lammert F, Stieger B, Meier PJ, Zatloukal K, Denk H, Trauner M. Effects of ursodeoxycholic and cholic acid feeding on hepatocellular transporter expression in mouse liver. Gastroenterology 121: 170–183, 2001. [DOI] [PubMed] [Google Scholar]
- 24.Flock EV, Bollman J, L. Phospholipid synthesis in damaged and regenerating liver. Fed Proc 5: 133–134, 1946. [PubMed] [Google Scholar]
- 25.Frijters CM, Ottenhoff R, van Wijland MJ, van Nieuwkerk CM, Groen AK, Oude Elferink RP. Regulation of mdr2 P-glycoprotein expression by bile salts. Biochem J 321: 389–395, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fukano S, Saitoh Y, Uchida K, Akiyoshi T, Takeda K. Bile acid metabolism in partially hepatectomized rats. Steroids 45: 209–227, 1985. [DOI] [PubMed] [Google Scholar]
- 27.Geier A, Trautwein C. Bile acids are “homeotrophic” sensors of the functional hepatic capacity and regulate adaptive growth during liver regeneration. Hepatology 45: 251–253, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Geier A, Wagner M, Dietrich CG, Trauner M. Principles of hepatic organic anion transporter regulation during cholestasis, inflammation and liver regeneration. Biochim Biophys Acta 1773: 283–308, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Gerloff T, Stieger B, Hagenbuch B, Madon J, Landmann L, Roth J, Hofmann AF, Meier PJ. The sister of P-glycoprotein represents the canalicular bile salt export pump of mammalian liver. J Biol Chem 273: 10046–10050, 1998. [DOI] [PubMed] [Google Scholar]
- 30.Grattagliano I, Lauterburg BH, Portincasa P, Caruso ML, Vendemiale G, Valentini AM, Palmieri VO, Palasciano G. Mitochondrial glutathione content determines the rate of liver regeneration after partial hepatectomy in eu- and hypothyroid rats. J Hepatol 39: 571–579, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Green RM, Gollan JL, Hagenbuch B, Meier PJ, Beier DR. Regulation of hepatocyte bile salt transporters during hepatic regeneration. Am J Physiol Gastrointest Liver Physiol 273: G621–G627, 1997. [DOI] [PubMed] [Google Scholar]
- 32.Groothuis GM, Hardonk MJ, Keulemans KP, Nieuwenhuis P, Meijer DK. Autoradiographic and kinetic demonstration of acinar heterogeneity of taurocholate transport. Am J Physiol Gastrointest Liver Physiol 243: G455–G462, 1982. [DOI] [PubMed] [Google Scholar]
- 33.Guo GL, Lambert G, Negishi M, Ward JM, Brewer HB Jr, Kliewer SA, Gonzalez FJ, Sinal CJ. Complementary roles of farnesoid X receptor, pregnane X receptor, and constitutive androstane receptor in protection against bile acid toxicity. J Biol Chem 278: 45062–45071, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Gupta S, Stravitz RT, Pandak WM, Muller M, Vlahcevic ZR, Hylemon PB. Regulation of multidrug resistance 2 P-glycoprotein expression by bile salts in rats and in primary cultures of rat hepatocytes. Hepatology 32: 341–347, 2000. [DOI] [PubMed] [Google Scholar]
- 35.Gurantz D, Laker MF, Hofmann AF. Enzymatic measurement of choline-containing phospholipids in bile. J Lipid Res 22: 373–376, 1981. [PubMed] [Google Scholar]
- 36.Hagenbuch B, Dawson P. The sodium bile salt cotransport family SLC10. Pflügers Arch 447: 566–570, 2004. [DOI] [PubMed] [Google Scholar]
- 37.Higgins GM, Anderson RM. Experimental pathology of the liver. I. Restoration of the liver of the white rat following partial surgical removal. Arch Pathol 12: 186–202, 1931. [Google Scholar]
- 38.Hofmann AF Bile acids: the good, the bad, and the ugly. News Physiol Sci 14: 24–29, 1999. [DOI] [PubMed] [Google Scholar]
- 39.Homolya L, Varadi A, Sarkadi B. Multidrug resistance-associated proteins: export pumps for conjugates with glutathione, glucuronate or sulfate. Biofactors 17: 103–114, 2003. [DOI] [PubMed] [Google Scholar]
- 40.Hoshino M, Hirano A, Hayakawa T, Kamiya Y, Ohiwa T, Tanaka A, Kumai T, Katagiri K, Miyaji M, Takeuchi T. Comparative studies on bile flow and biliary lipid excretion after bile-acid loading in normal and partially hepatectomized rats. Biochem J 305: 367–371, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang W, Ma K, Zhang J, Qatanani M, Cuvillier J, Liu J, Dong B, Huang X, Moore DD. Nuclear receptor-dependent bile acid signaling is required for normal liver regeneration. Science 312: 233–236, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Jones AL, Hradek GT, Renston RH, Wong KY, Karlaganis G, Paumgartner G. Autoradiographic evidence for hepatic lobular concentration gradient of bile acid derivative. Am J Physiol Gastrointest Liver Physiol 238: G233–G237, 1980. [DOI] [PubMed] [Google Scholar]
- 43.Klaassen CD Comparison of the effects of two-thirds hepatectomy and bile duct ligation on hepatic excretory function. J Pharmacol Exp Ther 191: 25–31, 1974. [PubMed] [Google Scholar]
- 44.Klaassen CD, Lu H. Xenobiotic transporters: ascribing function from gene knockout and mutation studies. Toxicol Sci 101: 186–196, 2008. [DOI] [PubMed] [Google Scholar]
- 45.Komichi D, Tazuma S, Nishioka T, Hyogo H, Une M, Chayama K. Unique inhibition of bile salt-induced apoptosis by lecithins and cytoprotective bile salts in immortalized mouse cholangiocytes. Dig Dis Sci 48: 2315–2322, 2003. [DOI] [PubMed] [Google Scholar]
- 46.Kullak-Ublick GA, Stieger B, Hagenbuch B, Meier PJ. Hepatic transport of bile salts. Semin Liver Dis 20: 273–292, 2000. [DOI] [PubMed] [Google Scholar]
- 47.Kurumiya Y, Nagino M, Nozawa K, Kamiya J, Uesaka K, Sano T, Yoshida S, Nimura Y. Biliary bile acid concentration is a simple and reliable indicator for liver function after hepatobiliary resection for biliary cancer. Surgery 133: 512–520, 2003. [DOI] [PubMed] [Google Scholar]
- 48.Lam P, Wang R, Ling V. Bile acid transport in sister of P-glycoprotein (ABCB11) knockout mice. Biochemistry 44: 12598–12605, 2005. [DOI] [PubMed] [Google Scholar]
- 49.Leazer TM, Klaassen CD. The presence of xenobiotic transporters in rat placenta. Drug Metab Dispos 31: 153–167, 2003. [DOI] [PubMed] [Google Scholar]
- 50.Lefebvre P, Cariou B, Lien F, Kuipers F, Staels B. Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev 89: 147–191, 2009. [DOI] [PubMed] [Google Scholar]
- 51.Leong GF, Pessotti RL, Brauer RW. Liver function in regenerating rat liver. CrPO4 colloid uptake and bile flow. Am J Physiol 197: 880–886, 1959. [PubMed] [Google Scholar]
- 52.Liu Y, Binz J, Numerick MJ, Dennis S, Luo G, Desai B, MacKenzie KI, Mansfield TA, Kliewer SA, Goodwin B, Jones SA. Hepatoprotection by the farnesoid X receptor agonist GW4064 in rat models of intra- and extrahepatic cholestasis. J Clin Invest 112: 1678–1687, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Loeb WF, Quimby FW. The Clinical Chemistry of Laboratory Animals. Oxford, UK: Pergamon, 1989.
- 54.Maher JM, Slitt AL, Cherrington NJ, Cheng X, Klaassen CD. Tissue distribution and hepatic and renal ontogeny of the multidrug resistance-associated protein (Mrp) family in mice. Drug Metab Dispos 33: 947–955, 2005. [DOI] [PubMed] [Google Scholar]
- 55.Mizuta A, Chijiiwa K, Saiki S, Kuroki S, Nakamura K, Tanaka M. Differences in biliary lipid excretion after major hepatectomy in obstructive jaundiced rats with preoperative internal, external, or no biliary drainage. Eur Surg Res 34: 291–299, 2002. [DOI] [PubMed] [Google Scholar]
- 56.Nakamura K, Ichimiya H, Nakayama F. Alteration of bile acid metabolism in two-thirds hepatectomized rat. J Gastroenterol Hepatol 7: 121–127, 1992. [DOI] [PubMed] [Google Scholar]
- 57.Nakatsukasa H, Silverman JA, Gant TW, Evarts RP, Thorgeirsson SS. Expression of multidrug resistance genes in rat liver during regeneration and after carbon tetrachloride intoxication. Hepatology 18: 1202–1207, 1993. [PubMed] [Google Scholar]
- 58.Noshiro M, Nishimoto M, Okuda K. Rat liver cholesterol 7 alpha-hydroxylase. Pretranslational regulation for circadian rhythm. J Biol Chem 265: 10036–10041, 1990. [PubMed] [Google Scholar]
- 59.O'Connor CJ, Wallace RG, Iwamoto K, Taguchi T, Sunamoto J. Bile salt damage of egg phosphatidylcholine liposomes. Biochim Biophys Acta 817: 95–102, 1985. [DOI] [PubMed] [Google Scholar]
- 60.Oppenheimer MJ, Flock EV. Alkaline phosphatase levels in plasma and liver following partial hepatectomy. Am J Physiol 429–448, 1947. [DOI] [PubMed]
- 61.Pircher PC, Kitto JL, Petrowski ML, Tangirala RK, Bischoff ED, Schulman IG, Westin SK. Farnesoid X receptor regulates bile acid-amino acid conjugation. J Biol Chem 278: 27703–27711, 2003. [DOI] [PubMed] [Google Scholar]
- 62.Rao A, Haywood J, Craddock AL, Belinsky MG, Kruh GD, Dawson PA. The organic solute transporter alpha-beta, Ostalpha-Ostbeta, is essential for intestinal bile acid transport and homeostasis. Proc Natl Acad Sci USA 105: 3891–3896, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rausch-Derra LC, Hartley DP, Meier PJ, Klaassen CD. Differential effects of microsomal enzyme-inducing chemicals on the hepatic expression of rat organic anion transporters, OATP1 and OATP2. Hepatology 33: 1469–1478, 2001. [DOI] [PubMed] [Google Scholar]
- 64.Rolo AP, Palmeira CM, Holy JM, Wallace KB. Role of mitochondrial dysfunction in combined bile acid-induced cytotoxicity: the switch between apoptosis and necrosis. Toxicol Sci 79: 196–204, 2004. [DOI] [PubMed] [Google Scholar]
- 65.Rost D, Herrmann T, Sauer P, Schmidts HL, Stieger B, Meier PJ, Stremmel W, Stiehl A. Regulation of rat organic anion transporters in bile salt-induced cholestatic hepatitis: effect of ursodeoxycholate. Hepatology 38: 187–195, 2003. [DOI] [PubMed] [Google Scholar]
- 66.Schubert R, Schmidt KH. Structural changes in vesicle membranes and mixed micelles of various lipid compositions after binding of different bile salts. Biochemistry 27: 8787–8794, 1988. [DOI] [PubMed] [Google Scholar]
- 67.Slitt AL, Allen K, Morrone J, Aleksunes LM, Chen C, Maher JM, Manautou JE, Cherrington NJ, Klaassen CD. Regulation of transporter expression in mouse liver, kidney, and intestine during extrahepatic cholestasis. Biochim Biophys Acta 1768: 637–647, 2007. [DOI] [PubMed] [Google Scholar]
- 68.Smit JJ, Schinkel AH, Oude Elferink RP, Groen AK, Wagenaar E, van Deemter L, Mol CA, Ottenhoff R, van der Lugt NM, van Roon MA, van der Valk MA, Offerhaus GJA, Berns AJM, Borst P. Homozygous disruption of the murine mdr2 P-glycoprotein gene leads to a complete absence of phospholipid from bile and to liver disease. Cell 75: 451–462, 1993. [DOI] [PubMed] [Google Scholar]
- 69.Sperber I Secretion of organic anions in the formation of urine and bile. Pharmacol Rev 11: 109–134, 1959. [PubMed] [Google Scholar]
- 70.Stacey NH, Klaassen CD. Uptake of galactose, ouabain and taurocholate into centrilobular and periportal enriched hepatocyte subpopulations. J Pharmacol Exp Ther 216: 634–639, 1981. [PubMed] [Google Scholar]
- 71.Staudinger J, Liu Y, Madan A, Habeebu S, Klaassen CD. Coordinate regulation of xenobiotic and bile acid homeostasis by pregnane X receptor. Drug Metab Dispos 29: 1467–1472, 2001. [PubMed] [Google Scholar]
- 72.Staudinger JL, Goodwin B, Jones SA, Hawkins-Brown D, MacKenzie KI, LaTour A, Liu Y, Klaassen CD, Brown KK, Reinhard J, Willson TM, Koller BH, Kliewer SA. The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc Natl Acad Sci USA 98: 3369–3374, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stieger B, Meier Y, Meier PJ. The bile salt export pump. Pflügers Arch 453: 611–620, 2007. [DOI] [PubMed] [Google Scholar]
- 74.Teeter LD, Estes M, Chan JY, Atassi H, Sell S, Becker FF, Kuo MT. Activation of distinct multidrug-resistance (P-glycoprotein) genes during rat liver regeneration and hepatocarcinogenesis. Mol Carcinog 8: 67–73, 1993. [DOI] [PubMed] [Google Scholar]
- 75.Tietze F Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem 27: 502–522, 1969. [DOI] [PubMed] [Google Scholar]
- 76.Tsuboi K, Tazuma S, Nishioka T, Chayama K. Partial characterization of cytoprotective mechanisms of lecithin against bile salt-induced bile duct damage. J Gastroenterol 39: 955–960, 2004. [DOI] [PubMed] [Google Scholar]
- 77.Ujhazy P, Ortiz D, Misra S, Li S, Moseley J, Jones H, Arias IM. Familial intrahepatic cholestasis 1: studies of localization and function. Hepatology 34: 768–775, 2001. [DOI] [PubMed] [Google Scholar]
- 78.Van Noorden CJ, Vogels IM, Houtkooper JM. Cytophotometric analysis of alkaline phosphatase and 5′-nucleotidase activity in regenerating rat liver after partial hepatectomy. Cell Biochem Funct 6: 53–60, 1988. [DOI] [PubMed] [Google Scholar]
- 79.Van Noorden CJ, Vogels IM, James J. Adaptive sex-dependent changes in the zonation of carbohydrate and lipid metabolism in rat liver lobules after partial hepatectomy. Hepatology 20: 714–724, 1994. [DOI] [PubMed] [Google Scholar]
- 80.Vos TA, Ros JE, Havinga R, Moshage H, Kuipers F, Jansen PL, Muller M. Regulation of hepatic transport systems involved in bile secretion during liver regeneration in rats. Hepatology 29: 1833–1839, 1999. [DOI] [PubMed] [Google Scholar]
- 81.Wagner M, Fickert P, Zollner G, Fuchsbichler A, Silbert D, Tsybrovskyy O, Zatloukal K, Guo GL, Schuetz JD, Gonzalez FJ, Marschall HU, Denk H, Trauner M. Role of farnesoid X receptor in determining hepatic ABC transporter expression and liver injury in bile duct-ligated mice. Gastroenterology 125: 825–838, 2003. [DOI] [PubMed] [Google Scholar]
- 82.Weglarz TC, Sandgren EP. Timing of hepatocyte entry into DNA synthesis after partial hepatectomy is cell autonomous. Proc Natl Acad Sci USA 97: 12595–12600, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wolters H, Elzinga BM, Baller JF, Boverhof R, Schwarz M, Stieger B, Verkade HJ, Kuipers F. Effects of bile salt flux variations on the expression of hepatic bile salt transporters in vivo in mice. J Hepatol 37: 556–563, 2002. [DOI] [PubMed] [Google Scholar]
- 84.Xia X, Francis H, Glaser S, Alpini G, LeSage G. Bile acid interactions with cholangiocytes. World J Gastroenterol 12: 3553–3563, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xie W, Radominska-Pandya A, Shi Y, Simon CM, Nelson MC, Ong ES, Waxman DJ, Evans RM. An essential role for nuclear receptors SXR/PXR in detoxification of cholestatic bile acids. Proc Natl Acad Sci USA 98: 3375–3380, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zollner G, Fickert P, Silbert D, Fuchsbichler A, Stumptner C, Zatloukal K, Denk H, Trauner M. Induction of short heterodimer partner 1 precedes downregulation of Ntcp in bile duct-ligated mice. Am J Physiol Gastrointest Liver Physiol 282: G184–G191, 2002. [DOI] [PubMed] [Google Scholar]
- 87.Zollner G, Trauner M. Mechanisms of cholestasis. Clin Liver Dis 12: 1–26, vii, 2008. [DOI] [PubMed] [Google Scholar]
- 88.Zollner G, Wagner M, Moustafa T, Fickert P, Silbert D, Gumhold J, Fuchsbichler A, Halilbasic E, Denk H, Marschall HU, Trauner M. Coordinated induction of bile acid detoxification and alternative elimination in mice: role of FXR-regulated organic solute transporter-alpha/beta in the adaptive response to bile acids. Am J Physiol Gastrointest Liver Physiol 290: G923–G932, 2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.