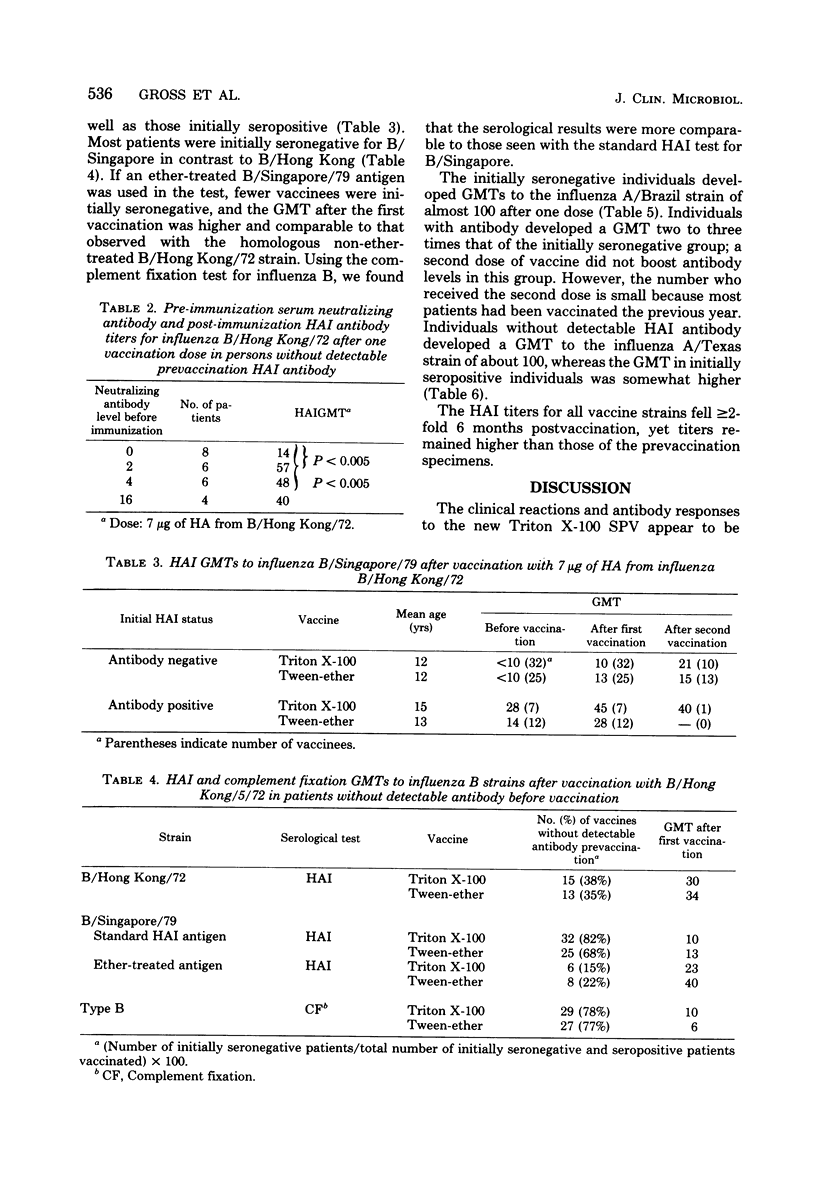
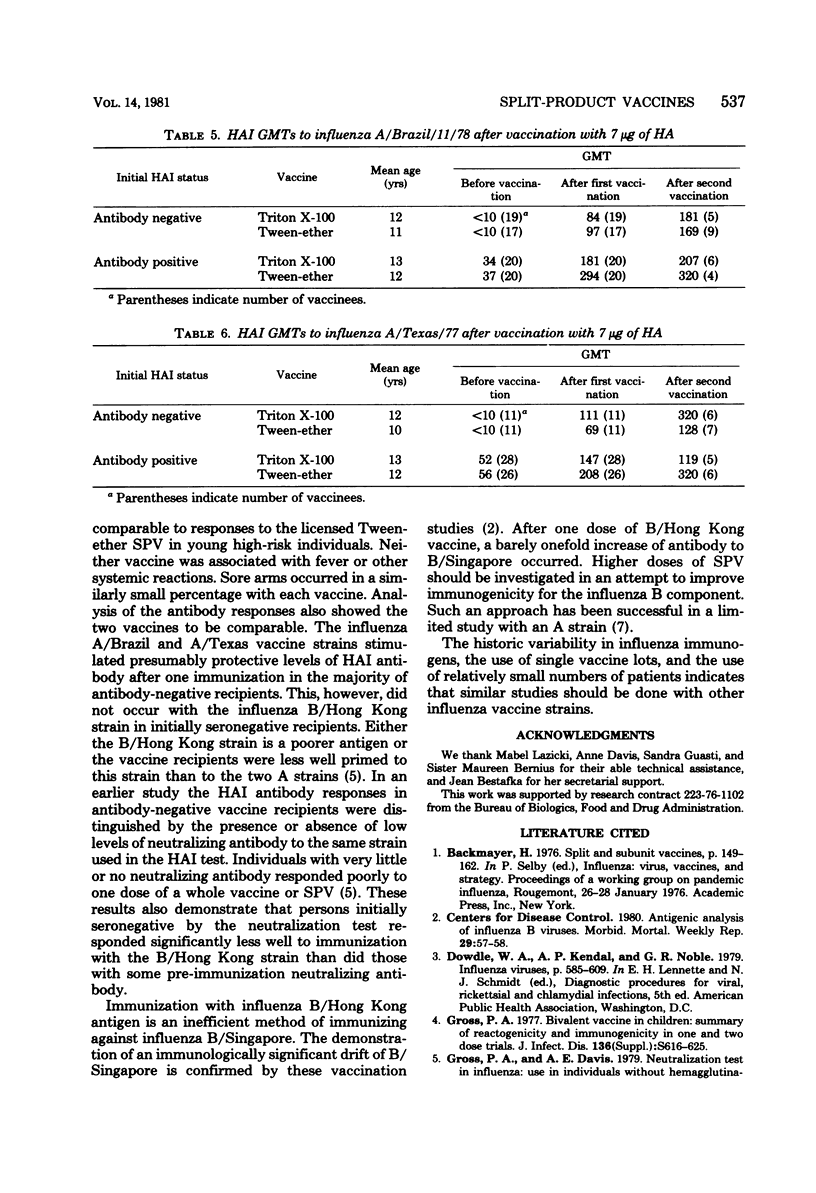
Abstract
Split-product vaccines (SPVs) combine the desirable properties of no systemic reactogenicity and adequate immunogenicity when two doses are given. We compared a new Triton X-100 SPV (Connaught Laboratories, Inc.) with the commercially available Tween-ether SPV (Parke-Davis & Co.) in 76 children and young adults 2 to 25 years old; there were 39 and 37, respectively, in each vaccine group. Both vaccines contained influenza A/Brazil/78, A/Texas/77, and B/Hong Kong/72 (7 microgram of hemagglutinin for each strain); two doses were administered 1 month apart. Among persons seronegative by the hemagglutination inhibition test, the geometric mean antibody titers rose to approximately 100 after the first vaccination for influenza A/Brazil/78 and A/Texas/77. For B/Hong Kong/72, however, seronegative recipients developed lower geometric mean titers of approximately 32 after one immunization. Against the new B/Singapore/79 strain neither SPV stimulated adequate cross-reacting hemagglutination inhibition antibody (geometric mean titers of approximately 10). In conclusion, the new Triton X-100 SPV appears to be comparable to the ether-treated SPV in primed subjects. Further studies in unprimed children should be done to confirm this impression. In addition, it would be advisable to study other dosage regimens in unprimed children with these SPVs.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gross P. A., Ennis F. A., Gaerlan P. F., Denson L. J., Denning C. R., Schiffman D. A controlled double-blind comparison of reactogenicity, immunogenicity, and protective efficacy of whole-virus and split-product influenza vaccines in children. J Infect Dis. 1977 Nov;136(5):623–632. doi: 10.1093/infdis/136.5.623. [DOI] [PubMed] [Google Scholar]
- Gross P. A., Ennis F. A., Noble G. R., Gaerlan P. F., Davis W. J., Denning C. E. Influenza vaccine in unprimed children: improved immunogenicity with few reactions following one high dose of split-product vaccine. J Pediatr. 1980 Jul;97(1):56–60. doi: 10.1016/s0022-3476(80)80130-2. [DOI] [PubMed] [Google Scholar]
- Gross P. A. Reactogenicity and immunogenicity of bivalent influenza vaccine in one- and two-dose trials in children: a summary. J Infect Dis. 1977 Dec;136 (Suppl):S616–S625. doi: 10.1093/infdis/136.supplement_3.s616. [DOI] [PubMed] [Google Scholar]
- Webster R. G., Laver W. G. Influenza virus subunit vaccines: immunogenicity and lack of toxicity for rabbits of ether- and detergent-disrupted virus. J Immunol. 1966 Apr;96(4):596–605. [PubMed] [Google Scholar]
- Wright P. F., Dolin R., La Montagne J. R. From the National Institute of Allergy and Infectious Diseases of the National Institutes of Health, the Center for Disease Control, and the Bureau of Biologics of the Food and Drug Administration. Summary of clinical trials of influenza vaccines--II. J Infect Dis. 1976 Dec;134(6):633–638. doi: 10.1093/infdis/134.6.633. [DOI] [PubMed] [Google Scholar]


