Abstract
Purpose
This study was designed to determine whether the ability to adversely affect corneal epithelial cell health is a factor common to Pseudomonas aeruginosa keratitis strains and to assess the prevalence of each pathogenic phenotype and genotype in a canine model of naturally-acquired P. aeruginosa ocular infection.
Methods
P. aeruginosa ocular isolates were collected by sampling 100 dogs without disease (six isolates collected) and by sampling dogs with conjunctivitis (two isolates), endophthalmitis (one isolate), active keratitis (12 isolates), and resolved P. aeruginosa keratitis (four isolates). Phenotype was determined in vitro by quantifying corneal epithelial cell invasion by gentamicin survival assays, and cytotoxic activity by Trypan blue exclusion assays. Genotyping was performed for genes encoding the type III secreted effectors.
Results
The ratio of invasive to cytotoxic strains with 95% confidence intervals (CI) was 0.83 (CI, 0.42– 0.99) for conjunctival microflora isolates, 0.80 (CI, 0.54 – 0.94) for ocular infection isolates, and 1.0 (CI, 0.45–1.0) for strains isolated post-resolution of keratitis. Among ocular infection isolates, invasive and cytotoxic strains were significantly (P ≤ 0.02) associated with older and younger dogs, respectively. Visible adverse effects on epithelial cells were significantly (P ≤ 0.03) more frequent for keratitis strains (6/12) than other strains (1/13), but only three of these keratitis strains and the single non-keratitis strain possessed ExoU.
Conclusions
Invasive strains predominated in the dogs of this study. Only keratitis strains had visible adverse effects on epithelial cells without overt cytotoxicity, suggesting virulence strategies affecting live corneal epithelial cell health are selected for among keratitis strains.
The Gram-negative bacillus Pseudomonas aeruginosa is a frequent cause of opportunistic ocular infection.1 P. aeruginosa has evolved a multitude of diverse virulence mechanisms and factors that permit efficient colonization of compromised ocular tissues and subsequent destructive disease.2–5 A diverse array of ocular and adnexal lesions are attributable to P. aeruginosa infection, including blepharitis, conjunctivitis, dacryocystitis, keratitis, scleritis, chorioretinitis, endophthalmitis, and orbital cellulitis.6–10 Ulcerative keratitis associated with P. aeruginosa infection is characterized by extensive dissolution of the corneal stroma and rapid progression of clinical signs.11,12
Historically, P. aeruginosa has been regarded as an extra-cellular pathogen. More recently, however, it was reported that some strains of P. aeruginosa are capable of invading and residing within corneal epithelial cells.13 Intracellular multiplication assays confirm that bacteria remain viable and multiply within the invaded epithelial cells.14 Other studies reported that P. aeruginosa is capable of toxin-mediated killing of epithelial cells.15 When strains of P. aeruginosa that induced acute cytotoxicity were compared with strains that invade epithelial cells, a significant inverse correlation was found between these two properties.16 This demonstrated that there are two distinct phenotypes of P. aeruginosa corneal isolates, one that is acutely cytotoxic and one that can enter corneal epithelial cells and survive intracellularly without killing the host cell. Experimental infection of mouse corneas with P. aeruginosa isolates of known pathogenic phenotype produced distinct corneal pathologies.17 Infection with both cytotoxic and invasive strains produced severe keratitis; however, the predominant pathologic response with cytotoxic strains was corneal edema and the predominant response with invasive strains was corneal ulceration. A strain that was neither cytotoxic nor invasive produced minimal keratitis.
The invasive and cytotoxic phenotypes of P. aeruginosa correspond to distinct genotypes.18 The molecular basis of these phenotypic properties is bacterial effector proteins secreted into host cell cytoplasm by a type III secretion system and controlled by the transcriptional activator ExsA.19 Currently, four bacterial effector proteins have been identified: ExoS, ExoU, ExoT, and ExoY. Among human corneal isolates, both phenotypes have been associated with a classic genotype. P. aeruginosa invasive stains typically possess genes encoding for ExoS, ExoT, and ExoY, but lack the gene for ExoU.18 Cytotoxic strains typically possess genes for ExoU, ExoT, and ExoY, but lack the gene for ExoS.18 The effectors ExoS, ExoY, and ExoT inhibit invasion and ExoU confers cytotoxicity; therefore, the invasive phenotype is considered the default if bacterial effectors are not present.20–23
The purpose of this study was to determine whether the ability to adversely affect corneal epithelial cell health was a factor common to P. aeruginosa ulcerative keratitis strains and to characterize the relationship between P. aeruginosa pathogenic phenotype and ocular disease in a canine model of naturally-acquired P. aeruginosa ocular infection. A specific objective of this study was to determine whether the same two broad classes of P. aeruginosa genotypes are associated with ocular infection in dogs, as has been demonstrated for humans. Exploring the similarities and differences between results of studies examining different P. aeruginosa strain types in spontaneous human infection, induced animal models of infection, and spontaneous animal infection models increases our knowledge of the validity of experimental animal models of ocular infection and their role in P. aeruginosa ocular disease research.
Materials and Methods
Animals and Microbiologic Sample Collection
All protocols were approved by the Animal Care and Use Committee of Cornell University and were conducted in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Conjunctival swabs were collected from 200 canine eyes (100 dogs) meeting the following inclusion criteria: no clinical or historical evidence of external ocular disease, no history of receiving systemic antimicrobials in the preceding 30 days, and no history of receiving topical ophthalmic medications in the preceding 30 days. Samples were collected from dogs representing 27 separate canine breeds. Slit lamp biomicroscopic examination (Kowa SL-14; Kowa Co., Tokyo, Japan) was performed for each eye before sample collection. Samples were collected before the instillation of any ophthalmic solutions. A separate rayon-tipped swab and culture transport system (BBL Culture-Swab Plus; Becton Dickinson, Sparks, MD) was used for each eye. The swab was gently brushed against the dorsal and ventral conjunctival fornices, avoiding contact with eyelid skin and cilia.
All dogs meeting inclusion criteria presented to the Cornell University Hospital for Animals during the 24-month study period, with naturally-acquired intra- or extraocular infection in which P. aeruginosa was isolated from ocular samples, were included in the study. Inclusion criteria for dogs with ocular infection included no history of receiving systemic or topical ophthalmic antimicrobials in the preceding 30 days. Samples were collected from dogs with ulcerative keratitis, conjunctivitis, and endophthalmitis by corneal scraping, conjunctival swab, or vitreous aspirate, respectively. Conjunctival swabs were also collected, as described for dogs without external ocular disease, from culture-confirmed P. aeruginosa ulcerative keratitis eyes 2 to 3 months after corneal ulcer resolution and the discontinuation of ophthalmic antimicrobials. Dogs examined at the Cornell University Hospital for Animals included both primary and referred cases; however, most ocular infections were either presented primarily or referred before medical intervention.
Clinical Data Collection
Signalment information, determined by physical examination and client supplied information, was recorded for each dog included in the infection group of the study. Complete ocular examination, including slit lamp biomicroscopy, indirect ophthalmoscopy (Heine EN20-01 indirect ophthalmoscope; Heine Optotechnik, Herrsching, Germany), fluorescein staining (Fluor-I-Strips; Schering-Plough Animal Health Co., Union, NJ), and Schirmer I tear testing (Schirmer tear test strips; Schering-Plough Animal Health Co.) were performed on each dog with ocular disease. For eyes with ulcerative keratitis, a corneal ulcer scoring system was used to quantify biomicroscopy findings. The following variables were scored for each eye: corneal ulcer area (defined as area of fluorescein retention relative to total corneal area): 0 = <25%, 1 = 26% to 50%, 2 = 51% to 75%, 3 = >76%; corneal ulcer depth (defined as deepest point of the ulceration relative to total corneal depth): 0 =< 25%, 1 = 26% to 50%, 2 = 51% to 75%, 3 = >76%; anterior chamber reaction: 0 = none, 1 = mild aqueous flare, 2 = moderate-to-marked aqueous flare, 3 = hypopyon; corneal edema area relative to total corneal area: 0 = <25%, 1 = 26% to 50%, 2 = 51% to 75%, 3 = >76%; corneal leukocyte infiltration: 0 = none, 1 = mild, 2 = moderate, 3 = severe; and keratomalacia: 0 = none, 1 = mild, 2 = moderate, 3 = severe.
Bacteria Identification
Direct cultures on solid media (trypticase soy agar with 5% sheep blood, chocolate agar, Levine eosin methylene blue agar, and Columbia colistin-nalidixic acid agar) and an enrichment broth (brain heart infusion broth) were performed for each sample. Direct cultures and enrichment subcultures were incubated at 37°C in 6% CO2 and read at 24 and 48 hours. All microorganism identification and antimicrobial susceptibility determinations were performed with an automated system (Sensititre; Trek Diagnostic Systemic Inc, Cleveland, OH).
Preparation of Bacteria
Bacteria were grown on trypticase soy agar plates overnight at 37°C. Immediately before infection, bacteria were suspended in minimal essential Eagle medium (50% [vol/vol]) in Hams F-12 (BioWhittaker, Walkersville, MD) tissue culture medium (EMEM/Hams F-12) to a final approximate concentration of 5 × 106 CFU/mL. P. aeruginosa clinical isolates 6294 and 6206 were used as positive controls for invasion and cytotoxicity assays, respectively.
Invasion and Cytotoxicity Assays
Rabbit corneal epithelial cells (RCEs) were grown in SHEM medium as previously described16 and seeded onto 96-well flat-bottom microtiter plates until cells reached 50% to 75% confluency. At the time of infection, RCEs were washed with pre-warmed phosphate-buffered saline (PBS) and 100 μL of pre-warmed diluted bacteria was added to each well in triplicates. EMEM/Hams F-12 medium was used as a negative control. Bacterial exposure continued for 3 hours at 37°C, 5.0% CO2. RCEs were washed with pre-warmed PBS and then treated with 100 μL of gentamicin (0.4% [vol/vol]; 200 μg/mL; BioWhittaker) in EMEM/Hams F-12 medium for 1 hour. After another PBS wash, 100 μL of Trypan blue (10% [vol/vol]) in Hams F-12 was added to the cells and incubation continued for 15 minutes. Trypan blue was removed and 100 μL of pre-warmed Hams F-12 medium was added to each well. Brightfield and phase contrast images were taken on an inverted microscope (Olympus IX-70; Olympus America Inc., Center Valley, PA) attached to a video camera (Optronics, Goleta, CA) at ×200 magnification.
To assay for bacterial invasion, the RCEs were lysed in Triton X-100 (0.25% [vol/vol]; LabChem Inc., Pittsburgh, PA) in PBS for 15 minutes. The cells were scraped vigorously to ensure complete lysis. Undiluted and 10−1 dilutions of the lysates were plated in duplicates onto Mac-Conkey agar plates and incubated for 14 to 16 hours at 37°C. Isolates were considered cytotoxic if they exhibited Trypan blue staining with low invasion numbers, invasive if they if exhibited low or no Trypan blue staining and high invasion numbers, and neither phenotype if they exhibited low or no Trypan blue staining and low invasion numbers.
Genotyping of P. aeruginosa
Genotyping of bacterial isolates was performed by the polymerase chain reaction (PCR) on target loci for exotoxins of the type III secretory system in P. aeruginosa. The following primers were used to amplify P. aeruginosa exotoxins: ExoS (sense, 5′-TCA GGT ACC CGG CAT TCA CTA CGC GG-3′; antisense, 5′-TCA CTG CAG GTT CGT GAC GTC TTT CT-3′), ExoT (sense, 5′-AAT CGC CGT CCA ACT GCA TGC G-3′; antisense, 5′-TGT TCG CCG AGG TAC TGC TC-3′), ExoU (sense, 5′-AGC GTT AGT GAC GTG CG-3′; antisense, 5′-GCG CAT GGC ATC GAG TAA CTG-3′), ExoY (sense, 5′-TCC AAG CTT ATG CGT ATC GAC GGT CA-3′; antisense, 5′-CGT ATC GAT CCG AGG GGG GTG TAT CT-3′). All PCR reactions were performed in 50 μL volumes containing 0.2 μM of each primer pair in 10 mM Tris-HCl (pH 9.0), 50 mM KCl, 1.5 mM MgCl2, 200μM of each dNTP, 0.1% TritonX-100, 0.5 U Taq Polymerase (Promega, Madison, WI) and with approximately 105 bacteria. All reactions except for ExoU were performed at 1 cycle at 94°C for 5 minutes, 30 cycles at 94°C for 1 minute, 58°C for 1 minute, 72°C for 1 minute, and 1 cycle at 72°C for 5 minutes. ExoU reactions were performed at an annealing temperature of 55°C. All amplified targets were resolved on 1.2% agarose at 10 V/cm for 30 minutes to give product sizes of 565bp (ExoS), 956bp (ExoT), 1572bp (ExoU), and 749bp (ExoY).
Statistical Analysis
The distribution of P. aeruginosa invasive and cytotoxic phenotype isolates was compared between dogs with ocular infections and dogs without clinical evidence of extraocular disease using the Fisher’s exact test. The distribution of phenotypes for dogs with all types of ocular infections together and dogs with ulcerative keratitis specifically were analyzed separately. For signalment and clinical data analysis, dogs were placed into two groups: dogs with P. aeruginosa invasive phenotype isolates and dogs with P. aeruginosa cytotoxic phenotype isolates. Continuous variables (i.e., age and corneal ulcer scores) were compared with the Wilcoxon rank sum test. Categorical variables (i.e., sex and skull conformation) were compared between the groups using the Fisher’s exact test. For the purpose of analysis, dog breeds were grouped by skull conformation (i.e., brachycephalic versus mesatacephalic/dolichocephalic). Data analyses were performed separately for all dogs with ocular infections and dogs with ulcerative keratitis specifically. The occurrence of in vitro adverse effects on corneal epithelial cell health as observed with light microscopy was compared between keratitis stains and non-keratitis strain using the Fisher’s exact test. Statistical significance was defined by P ≤ 0.05 for all comparisons.
Results
P. aeruginosa was isolated from conjunctival samples of six eyes (3.0%) sampled without clinical evidence of ocular disease. P. aeruginosa was isolated from 15 dogs with ocular infections, including 12 eyes with ulcerative keratitis, two eyes with bacterial conjunctivitis associated with keratoconjunctivitis sicca, and one eye with exogenous infectious endophthalmitis resulting from penetrating ocular trauma. Eight of the dogs with P. aeruginosa ulcerative keratitis were available for repeat sampling 2 to 3 months post-resolution of the corneal lesion and P. aeruginosa was again isolated from four of these eyes. These P. aeruginosa isolates differed by two to six in vitro antimicrobial susceptibilities from their respective initial isolate, but all were susceptible to the antimicrobial used during treatment of the original infection. All dogs with ulcerative keratitis were successfully treated (i.e., globe and vision preservation) with topical ciprofloxacin 0.3% ophthalmic solution administered with (n = 6) or without (n = 6) additional surgical procedures (e.g., lamellar keratoplasty, corneoconjunctival transposition, or conjunctival pedicle graft). Selected in vitro antimicrobial susceptibility determinations for all 25 isolates are listed in Table 1.
Table 1.
In Vitro Antimicrobial Susceptibly Results for 25 Canine Ocular Pseudomonas aeruginosa Isolates
| Antimicrobial | MIC (μg/ml) | Susceptible (No. Isolates) | Intermediate (No. Isolates) | Susceptible or Intermediate (% Isolates) |
|---|---|---|---|---|
| Amikacin | ≤32 | 25 | 0 | 100 |
| Amoxicillin/clavulanic acid | >32 | 0 | 0 | 0 |
| Ampicillin | >16 | 0 | 0 | 0 |
| Bacitracin | ≤2 | 3 | 0 | 12 |
| Cefazolin | >16 | 0 | 0 | 0 |
| Cefoxitin | >16 | 0 | 0 | 0 |
| Cefpodoxime | >16 | 0 | 0 | 0 |
| Cephalothin | >16 | 0 | 0 | 0 |
| Ciprofloxacin | ≤2 | 23 | 1 | 96 |
| Clindamycin | >2 | 0 | 0 | 0 |
| Chloramphenicol | ≤16 | 0 | 6 | 24 |
| Enrofloxacin | ≤1 | 21 | 4 | 100 |
| Erythromycin | <4 | 5 | 0 | 20 |
| Gentamicin | ≤8 | 25 | 0 | 100 |
| Imipenem | ≤8 | 23 | 2 | 100 |
| Marbofloxacin | ≤0.25 | 25 | 0 | 100 |
| Neomycin | ≤8 | 24 | 0 | 96 |
| Orbifloxacin | ≤4 | 19 | 6 | 100 |
| Oxytetracycline | ≤8 | 5 | 1 | 24 |
| Tetracycline | ≤8 | 1 | 5 | 24 |
| Ticarcillin | ≤64 | 25 | 0 | 100 |
| Ticarcillin/clavulanic acid | ≤32 | 25 | 0 | 100 |
| Tobramycin | ≤8 | 23 | 2 | 100 |
| Trimethoprim/sulphamethoxazole | ≤2 | 5 | 0 | 20 |
P. aeruginosa isolates from the conjunctival flora of dogs without clinical extraocular disease were phenotypically invasive (n = 5) and cytotoxic (n = 1; Table 2). Isolates from dogs with ocular infections were phenotypically invasive (n = 12), cytotoxic (n = 2), or displayed neither invasiveness nor cytotoxicity (n = 1). The ratio of the prevalence of invasive to cytoxic strains with 95% confidence intervals (CI) was 0.83 (CI, 0.42– 0.99) for normal conjunctival flora isolates and 0.80 (CI, 0.54 – 0.94) for ocular infection isolates. There was no significant difference in the distribution of each phenotype between dogs with or without ocular infections. All P. aeruginosa isolates from the conjunctival flora of dogs post-resolution of ulcerative keratitis were invasive (n = 4), but only two of these isolates shared the same phenotype and genotype as the original isolate.
Table 2.
Pathogenic Phenotype and Genotype Assay Results for 25 Canine Ocular Pseudomonas aeruginosa Isolates
| Isolate | Lesion/Site | ExoS | ExoU | ExoT | ExoY | Trypan Blue* | Invasion Assay† | Visible Adverse Effects on Epithelial Cell Health |
|---|---|---|---|---|---|---|---|---|
| 1 | Ulcerative keratitis | + | − | + | + | − | 37.5% | Absent |
| 2 | Ulcerative keratitis | + | − | + | + | − | 77.3% | Absent |
| 3 | Ulcerative keratitis | + | − | + | + | − | 62.5% | Absent |
| 4 | Ulcerative keratitis | + | − | + | + | − | 16.9% | Absent |
| 5 | Ulcerative keratitis | − | + | + | + | ++ | 12.7% | Present |
| 6 | Ulcerative keratitis | − | + | + | + | ++++ | 0.04% | Present |
| 7 | Ulcerative keratitis | + | − | + | + | − | 90.8% | Present |
| 8 | Ulcerative keratitis | + | − | + | + | − | 68.2% | Absent |
| 9 | Ulcerative keratitis | + | − | + | + | − | 31.2% | Absent |
| 10 | Ulcerative keratitis | + | − | + | + | − | 37.7% | Present |
| 11 | Ulcerative keratitis | − | + | + | − | − | TNTC | Present |
| 12 | Ulcerative keratitis | − | − | − | − | − | 2.8% | Present |
| 13 | Resolved ulcerative keratitis | + | − | + | + | − | 2.3% | Absent |
| 14 | Resolved ulcerative keratitis | + | − | + | + | − | 80.1% | Absent |
| 15 | Resolved ulcerative keratitis | + | − | + | + | − | 30.3% | Absent |
| 16 | Resolved ulcerative keratitis | + | − | + | + | − | 31.2% | Absent |
| 17 | Conjunctivitis | + | − | + | + | − | 6.5% | Absent |
| 18 | Conjunctivitis | + | − | + | + | − | 1.6% | Absent |
| 19 | Endophthalmitis | + | − | + | + | − | 62.8% | Absent |
| 20 | Normal conjunctival flora | + | − | + | + | − | 4.0% | Absent |
| 21 | Normal conjunctival flora | + | − | + | + | − | 5.0% | Absent |
| 22 | Normal conjunctival flora | + | − | + | + | − | 6.3% | Absent |
| 23 | Normal conjunctival flora | + | − | + | + | − | 11.5% | Absent |
| 24 | Normal conjunctival flora | + | − | + | + | − | 50.9% | Absent |
| 25 | Normal conjunctival flora | + | + | + | + | ++++ | 0.2% | Present |
| 6206 | Standard cytotoxic isolate | − | + | + | + | +++ | 1.3% | Absent |
| 6294 | Standard invasive isolate | + | − | + | + | − | 100% | Absent |
Trypan Blue cytotoxicity assay results presented as cytotoxity scores assigned by estimating the percentage of corneal epithelial cells that retained stain (−, 0%;+, <1%;++, 1% to 5%;+++, 5% to 20%;++++, >20%), normalized to the standard cytotoxic strain 6206.
Invasion assay results presented as % of corneal epithelial cells invaded normalized to standard invasive strain 6294.
The genotype of 21 isolates was congruent with the classic genotype-phenotype pattern reported for human corneal isolates (Table 2). Discordance between phenotype and the classic genotype was identified in four isolates, including one normal conjunctival microflora strain, one conjunctivitis strain, and two ulcerative keratitis strains. The conjunctival microflora strain was phenotypically cytotoxic, but the genotype was unusual as it encoded all four known effectors. The conjunctivitis strain displayed neither phenotype, but a classic invasive genotype. The ulcerative keratitis strains were both phenotypically invasive, but unusual because one had a cytotoxic genotype without ExoY and one encoded none of the type III secretion effectors.
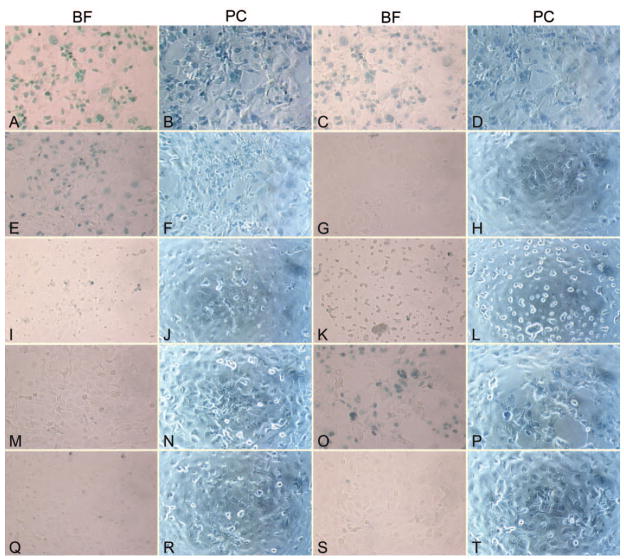
Microscopically visible adverse effects on corneal epithelial cell health (e.g., cell rounding, sloughing from plates, loss) were observed significantly (P ≤ 0.03) more frequently for keratitis strains (6/12) than other strains (1/13; Fig. 1). Of the six keratitis strains adversely affecting cell health, three were invasive, two were cytotoxic, and one was neither genotype (lacked all four known effectors). The one non-keratitis strain affecting cell health was cytotoxic, but unusual in that it encoded all four known effectors. Therefore, only keratitis strains had visible adverse effects on live host cells in the absence of overt cytotoxicity (4/12 keratitis strains vs. 0/13 non-keratitis strains).
Figure 1.
Brightfield (BF) and phase contrast (PC) photomicrographs of Trypan blue stained rabbit corneal epithelial cell cultures infected with Pseudomonas aeruginosa. (A–N) Clinical isolates adversely affecting epithelial cell health in vitro. Epithelial cell rounding, sloughing from plates, and loss is visible. (A, B) Cytoxic ulcerative keratitis strain #5; (C, D) cytotoxic ulcerative keratitis strain #6; (E, F) cytotoxic normal conjunctival flora strain #25; (G, H) invasive ulcerative keratitis strain #7; (I, J) invasive ulcerative keratitis strain #10; (K, L) invasive ulcerative keratitis strain #11; (M, N) invasive ulcerative keratitis strain #12. (O, P) Standard cytotoxic strain 6206. (Q, R) Standard invasive strain 6294. (S, T) Media control without bacteria. Original magnification, ×200. Strain numbers correspond with numbers in Table 2.
Other notable findings included that all normal conjunctival microflora strains encoded ExoS, including an ExoU + strain that was phenotypically cytotoxic. Of the keratitis strains negatively impacting cell health, two strains did not encode ExoU and were both phenotypically and genotypically invasive. The three strains with atypical genotypes had visible adverse effects on corneal epithelial cell health. All strains isolated from ocular infections had at least one of the following effects on corneal epithelial cells: invasion, cytotoxicity, or the induction of abnormal morphology.
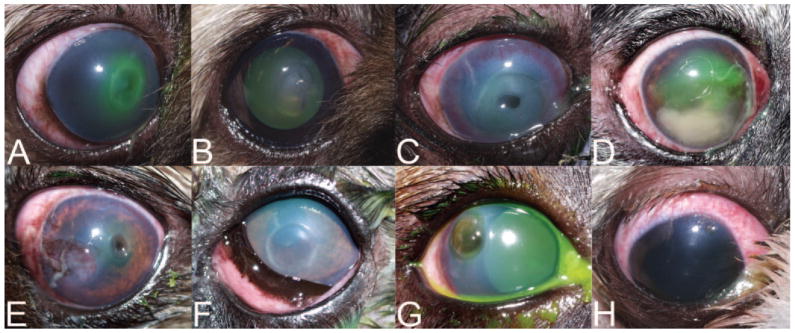
The clinical appearance of corneal lesions in dogs with ulcerative keratitis was highly variable; however, all corneal ulcerations were unilateral, severe, and rapidly progressive (Fig. 2). In all dogs sampled, clinical findings were typical for canine P. aeruginosa ulcerative keratitis.12 No significant differences were found between dogs infected with each phenotype and the clinical biomicroscopic corneal ulcer scoring system characteristics evaluated (data not shown). Dogs with ocular infections included nine brachycephalic and six mesaticephalic/dolichocephalic skull types. Breeds represented included Shih Tzu (n = 5 dogs), Pekingese (n = 3), Jack Russell Terrier (n = 2), mixed breed (n = 2), Pug (n = 1), American Cocker Spaniel (n = 1), and Miniature Schnauzer (n = 1). There were four spayed females, seven intact females, two castrated males, and two intact males. There were no significant differences for sex and skull type between dogs with invasive and cytotoxic isolates. Preexisting ocular disease identified during ophthalmological examination in dogs with ulcerative keratitis included keratoconjunctivitis sicca (n = 4 dogs), medial canthal trichiasis (n = 2), facial nerve paralysis (n = 1), and pigmentary keratitis (n = 1).
Figure 2.
Representative external photographs of eight dogs with spontaneous, naturally-acquired Pseudomonas aeruginosa ulcerative keratitis. (A–F) Dogs infected with invasive phenotype strains; (G, H) dogs infected with cytotoxic phenotype strains.
For dogs with all types of ocular infections, the median age was 65 months (range: 14 to 180) in dogs with the invasive phenotype and the median age was 7 months (all dogs 7 months old) in dogs with the cytotoxic phenotype. This difference in dog ages between the invasive and cytotoxic phenotype groups for dogs with all types of ocular infections was statistically significant (P ≤ 0.02). For dogs with ulcerative keratitis specifically, the median age for dogs with the invasive phenotype was 61.5 months (range, 1–180) and the median age was 7 months (all dogs 7 months old) for dogs with the cytotoxic phenotype. This difference in dog ages between the invasive and cytotoxic phenotype ulcerative keratitis groups was statistically significant (P ≤ 0.03).
Discussion
In contrast to other animal models requiring experimental infection induction,24–26 the dogs of this report are a naturally-occurring P. aeruginosa ulcerative keratitis model. This large-animal model of infection, occurring without experimental corneal wounding or bacterial inoculation, may more closely replicate conditions in natural human P. aeruginosa ulcerative keratitis. P. aeruginosa corneal infections occur spontaneously, subsequent to compromise of anatomic or immunologic corneal protective barriers, with a high frequency in dogs and produce clinical lesions similar to those observed in humans.27–29
The invasive phenotype of P. aeruginosa predominated in the dogs of this study, accounting for 80% of isolates from dogs with ocular infections and 84% of all isolates. The ability to invade and replicate within epithelial cells may protect invasive strains from host immune responses and certain antimicrobials while still stimulating release of inflammatory mediators and inducing tissue damage.14 Invasive strains of P. aeruginosa may also directly kill epithelial cells after prolonged incubation in a bacterial density-dependent manner.30 Aminoglycosides, and other poorly cell-permeable antimicrobials, may be suboptimal therapeutics against invasive strains of P. aeruginosa.31–33 A murine model of P. aeruginosa keratitis has confirmed tobramycin, a non cell-permeable antibiotic, is less effective at eradicating bacteria from corneas infected with invasive strains than ofloxacin, a cell-permeable antibiotic.34 Furthermore, that study demonstrated several aminoglycosides and fluoroquinolones were capable of killing cytotoxic and invasive strains when not cell-associated, but only cell-permeable fluoroquinolones killed invasive strains within corneal epithelial cells.
Invasive and cytotoxic P. aeruginosa strains were isolated in approximately equal proportion from human clinical cases of ulcerative keratitis.35 A significant relationship was present between P. aeruginosa phenotype and patient age, with cytotoxic strains associated with patients <50 years of age and invasive strains associated with patients >50 years of age.35 The authors of that report suggested impairment of immune function with age might reduce the host’s ability to respond to intracellular pathogens, increasing the prevalence of invasive strains in older individuals. Similar to humans with P. aeruginosa ulcerative keratitis, a significant correlation between age and phenotype was found in the ulcerative keratitis group of the present study, with invasive strains associated with older dogs and cytotoxic strains with younger dogs. This finding may reflect age-related immunologic changes similar to those proposed for humans. The canine immune system undergoes a process of age-related compromise in function, with declines in leukocyte proliferation, mitogen stimulation, chemotaxis, phagocytosis, and various other immunologic parameters.36–38 These age-associated changes in immunity are similar between dogs and humans.39,40 Age-related changes of polymorphonuclear cell function impair P. aeruginosa phagocytosis, delay corneal clearance, and increase corneal bacterial loads.41–43
The prevalence of P. aeruginosa isolation from dogs without clinically apparent extraocular disease in this study was comparable to previous reports in which P. aeruginosa was a consistent, but relatively infrequent, inhabitant of the canine extraocular microflora and was isolated from 1% to 14% of eyes.44–50 The relative importance of conjunctival versus environmental sources of P. aeruginosa during initiation of canine ocular infections is unknown, but the similar prevalence of invasive and cytotoxic strains among extraocular microflora and infection isolates suggests that the conjunctival flora may be an important bacterial reservoir and both phenotypes are capable of inducing opportunistic ocular infection during appropriate circumstances. The consistent presence of P. aeruginosa in the normal conjunctival microflora of dogs contrasts with humans where P. aeruginosa is most often reported to be a rare and sporadic isolate in the absence of predisposing extraocular disease.51–53 It is partially on the basis of these reports that, in humans, P. aeruginosa ocular infections are frequently believed to originate from environmental bacterial sources.54–56 In disagreement with most studies of the human extraocular microflora; however, sporadic large human conjunctival microflora surveys have isolated P. aeruginosa from 5.8% to 6.7% of eyes; a prevalence similar to that reported for dogs.57,58 Among individuals or populations where P. aeruginosa is present in the extraocular microflora, the importance of this endogenous source of bacteria in the pathogenesis of ocular infections and the predisposition toward infection resultant from its presence is currently unknown.
P. aeruginosa was isolated from the conjunctival flora of 50% of dogs sampled post-resolution of ulcerative keratitis. This frequency is notably higher than the 3% prevalence of P. aeruginosa within the conjunctival flora of dogs without ocular disease. All isolates collected from dogs after keratitis resolution had different in vitro antimicrobial susceptibility profiles, and only 50% shared the same phenotype and genotype as the original corneal isolate. It is unknown whether P. aeruginosa was present in the conjunctival flora before development of ulcerative keratitis; however, these results suggest that some dogs may be prone to the chronic presence of P. aeruginosa in the extraocular microflora. It does not appear that the same bacterial strain is responsible for this presence, but that bacterial mutation or, more likely, conjunctival recolonization from environmental sources or other endogenous flora is occurring. The reasons for this recolonization are currently unclear, but could include a variety of bacterial, environmental, or host factors.
Discordance between phenotype and the classic genotype was identified in four canine isolates. This may have resulted from production of effectors that were not secreted into host cells or activity of unidentified effectors modulating invasion and cytotoxicity. Only ulcerative keratitis strains had visible adverse effects on corneal epithelial cell health in the absence of overt cytotoxicity, suggesting that virulence strategies that affect live epithelial cells are selected for among ulcerative keratitis strains. The results of this study further suggest P. aeruginosa possesses virulence factors, other than invasiveness and cytotoxicity, which impact cell health. These virulence factors were not identified in the present study, but may include one or more specific proteases or exoenzymes. Study of the canine model of spontaneous P. aeruginosa ocular infection has the potential to advance the understanding of both human and animal infection pathogenesis, improve therapeutic and management approaches for human infection, and contribute to the development of animal models that more closely resemble spontaneous human infection.
Acknowledgments
The authors thank Faith W. Wallace-Gadsden and Maria J. Golchin for assistance with phenotyping and genotyping P. aeruginosa isolates.
Supported by National Institutes of Health Grant R01-EY011221, American College of Veterinary Ophthalmologists Vision for Animals Foundation, and American Society of Veterinary Ophthalmology Dr. Howard Garvin Research Fund.
Footnotes
Disclosure: E.C. Ledbetter, None; J.J. Mun, None; D. Kowbel, None; S.M.J. Fleiszig, None
References
- 1.Lyczak JB, Cannon CL, Pier GB. Establishment of Pseudomonas aeruginosa infection: lessons from a versatile opportunist. Microbes Infect. 2000;2(9):1051–1060. doi: 10.1016/s1286-4579(00)01259-4. [DOI] [PubMed] [Google Scholar]
- 2.Hazlett HD, Moon MM, Singh A, Berk RS, Rudner XL. Analysis of adhesion, piliation, protease production and ocular infectivity of several P. aeruginosa strains. Curr Eye Res. 1991;10(4):351–362. doi: 10.3109/02713689108996341. [DOI] [PubMed] [Google Scholar]
- 3.Hobden JA. Pseudomonas aeruginosa proteases and corneal virulence. DNA Cell Biol. 2002;21(5–6):391–396. doi: 10.1089/10445490260099674. [DOI] [PubMed] [Google Scholar]
- 4.Pillar CM, Hobden JA. Pseudomonas aeruginosa exotoxin A and keratitis in mice. Invest Ophthalmol Vis Sci. 2002;43(5):1437–1444. [PubMed] [Google Scholar]
- 5.Fleiszig SM, Evans DJ. The pathogenesis of bacterial keratitis: studies with Pseudomonas aeruginosa. Clin Exp Optom. 2002;85(5):271–278. doi: 10.1111/j.1444-0938.2002.tb03082.x. [DOI] [PubMed] [Google Scholar]
- 6.Alexandrakis G, Alfonso EC, Miller D. Shifting trends in bacterial keratitis in south Florida and emerging resistance to fluoroquinolones. Ophthalmology. 2000;107(8):1497–1502. doi: 10.1016/s0161-6420(00)00179-2. [DOI] [PubMed] [Google Scholar]
- 7.Farrell PL, Smith RE. Bacterial corneoscleritis complicating pterygium excision. Am J Ophthalmol. 1989;107(5):515–517. doi: 10.1016/0002-9394(89)90496-0. [DOI] [PubMed] [Google Scholar]
- 8.Celik I, Cihangiroglu M, Yilmaz T, Kohle U, Akbulut A. The prevalence of bacteraemia-related retinal lesions in seriously ill patients. J Infect. 2006;52(2):97–104. doi: 10.1016/j.jinf.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 9.Eifrig CW, Scott IU, Flynn HW, Jr, Miller D. Endophthalmitis caused by Pseudomonas aeruginosa. Ophthalmology. 2003;110(9):1714–1717. doi: 10.1016/S0161-6420(03)00572-4. [DOI] [PubMed] [Google Scholar]
- 10.Suneetha N, Battu RR, Thomas RK, Bosco A. Orbital abscess: management and outcome. Indian J Ophthalmol. 2000;48(2):129–134. [PubMed] [Google Scholar]
- 11.Van Horn DL, Davis SD, Hyndiuk RA, Pederson JH. Experimental Pseudomonas keratitis in the rabbit: bacteriologic, clinical, and microscopic observations. Invest Ophthalmol Vis Sci. 1981;20(2):213–221. [PubMed] [Google Scholar]
- 12.Wyman M, Swanson C, Kowalski JJ, Powers JD, Boraski EA. Experimental Pseudomonas aeruginosa ulcerative keratitis model in the dog. Am J Vet Res. 1983;44(6):1135–1140. [PubMed] [Google Scholar]
- 13.Fleiszig SM, Zaidi TS, Fetcher EL, Preston MJ, Pier GB. Pseudomonas aeruginosa invades corneal epithelial cells during experimental infection. Infect Immun. 1994;62(8):3485–3493. doi: 10.1128/iai.62.8.3485-3493.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fleiszig SM, Zaidi TS, Pier GB. Pseudomonas aeruginosa invasion of and multiplication within corneal epithelial cells in vitro. Infect Immun. 1995;63(10):4072–4077. doi: 10.1128/iai.63.10.4072-4077.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Apodaca G, Bomsel M, Lindstedt R, et al. Characterization of Pseudomonas aeruginosa-induced MDCK cell injury: glycosylation-defective host cells are resistant to bacterial killing. Infect Immun. 1995;63(4):1541–1551. doi: 10.1128/iai.63.4.1541-1551.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fleiszig SM, Zaidi TS, Preston MJ, Grout M, Evans DJ, Pier GB. Relationship between cytotoxicity and corneal epithelial cell invasion by clinical isolates of Pseudomonas aeruginosa. Infect and Immun. 1996;64(6):2288–2294. doi: 10.1128/iai.64.6.2288-2294.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cole N, Willcox MD, Fleiszig SM, et al. Different strains of Pseudomonas aeruginosa isolated from ocular infections or inflammation display distinct corneal pathologies in an animal model. Curr Eye Res. 1998;7(7):730–735. [PubMed] [Google Scholar]
- 18.Fleiszig SM, Weiner-Kronish JP, Miyazaki H, et al. Pseudomonas aeruginosa-mediated cytotoxicity and invasion correlate with distinct genotypes at the loci encoding exoenzyme S. Infec Immun. 1997;65(2):579–586. doi: 10.1128/iai.65.2.579-586.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evans DJ, Frank DW, Finck-Barbancon V, Wu C, Fleiszig SM. Pseudomonas aeruginosa invasion and cytotoxicity are independent events, both of which involve protein tyrosine kinase activity. Infect Immun. 1998;66(4):1453–1459. doi: 10.1128/iai.66.4.1453-1459.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Finck-Barbancon V, Goranson J, Zhu L, et al. ExoU expression by Pseudomonas aeruginosa correlates with acute cytotoxicity and epithelial injury. Mol Microbiol. 1997;25(3):547–557. doi: 10.1046/j.1365-2958.1997.4891851.x. [DOI] [PubMed] [Google Scholar]
- 21.Hauser AR, Fleiszig SM, Kang PJ, Mostov K, Engel JN. Defects in type III secretion correlate with internalization of Pseudomonas aeruginosa by epithelial cells. Infect Immun. 1998;66(4):1413–1420. doi: 10.1128/iai.66.4.1413-1420.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cowell BA, Chen DY, Frank DW, Vallis AJ, Fleiszig SM. ExoT of cytotoxic Pseudomonas aeruginosa prevents uptake by corneal epithelial cells. Infect Immun. 2000;68(1):403–406. doi: 10.1128/iai.68.1.403-406.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee EJ, Cowell BA, Evans DJ, Fleiszig SM. Contribution of ExsA-regulated factors to corneal infection by cytotoxic and invasive Pseudomonas aeruginosa in a murine scarification model. Invest Ophthalmol Vis Sci. 2003;44(9):3892–3898. doi: 10.1167/iovs.02-1302. [DOI] [PubMed] [Google Scholar]
- 24.Brockman EB, Tarantino PA, Hobden JA, et al. Keratotomy model of Pseudomonas keratitis: gentamicin chemotherapy. Refract Corneal Surg. 1992;8(1):39–43. [PubMed] [Google Scholar]
- 25.Mah FS, Romanowski EG, Kowalski RP, Yates KA, Gordon YJ. Zymar (Gatifloxacin 0.3%) shows excellent gram-negative activity against Serratia marcescens and Pseudomonas aeruginosa in a New Zealand White rabbit keratitis model. Cornea. 1997;26(5):585–588. doi: 10.1097/ICO.0b013e318033a6f2. [DOI] [PubMed] [Google Scholar]
- 26.Alionte LG, Cannon BM, White CD, Caballero AR, O’Callaghan RJ, Hobden JA. Pseudomonas aeruginosa LasA protease and corneal infections. Curr Eye Res. 2001;22(4):266–271. doi: 10.1076/ceyr.22.4.266.5509. [DOI] [PubMed] [Google Scholar]
- 27.Lin CT, Peterson-Jones SM. Antibiotic susceptibility of bacterial isolates from corneal ulcers of dogs in Taiwan. J Small Anim Pract. 2007;48(5):271–274. doi: 10.1111/j.1748-5827.2007.00348.x. [DOI] [PubMed] [Google Scholar]
- 28.Tolar EL, Hendrix DV, Rohrbach BW, Plummer CE, Brooks DE, Gelatt KN. Evaluation of clinical characteristics and bacterial isolates in dogs with bacterial keratitis: 97 cases (1993–2003) J Am Vet Med Assoc. 2006;228(1):80–85. doi: 10.2460/javma.228.1.80. [DOI] [PubMed] [Google Scholar]
- 29.Ledbetter EC, Hendricks LM, Riis RC, Scarlett JM. In vitro fluoroquinolone susceptibility of Pseudomonas aeruginosa isolates from dogs with ulcerative keratitis. Am J Vet Res. 2007;68(6):638–642. doi: 10.2460/ajvr.68.6.638. [DOI] [PubMed] [Google Scholar]
- 30.Zhu H, Thuruthyil SJ, Wilcox MD. Invasive strains of Pseudomonas aeruginosa are able to cause epithelial cell cytotoxicity that is dependent on bacterial cell density. Clin Experiment Ophthalmol. 2000;28(3):201–204. doi: 10.1046/j.1442-9071.2000.00289.x. [DOI] [PubMed] [Google Scholar]
- 31.Solberg CO, Hellum KB. Protection of phagocytosed bacteria against antimicrobial agents. Scand J Infect Dis Suppl. 1978;(14):246–250. [PubMed] [Google Scholar]
- 32.Vaudaux P, Waldvogel FA. Gentamicin antibacterial activity in the presence of human polymorphonuclear leukocytes. Antimicrob Agents Chemother. 1979;16(6):743–749. doi: 10.1128/aac.16.6.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tulkens PM. Intracellular distribution and activity of antibiotics. Eur J Clin Microbiol Infect Dis. 1991;10(2):100–106. doi: 10.1007/BF01964420. [DOI] [PubMed] [Google Scholar]
- 34.Lee EJ, Truong TN, Mendoza MN, Fleiszig SM. A comparison of invasive and cytotoxic Pseudomonas aeruginosa strain-induced corneal disease response to therapeutics. Curr Eye Res. 2003;27(5):289–299. doi: 10.1076/ceyr.27.5.289.17220. [DOI] [PubMed] [Google Scholar]
- 35.Cowell BA, Weissman BA, Yeung KK, et al. Phenotype of Pseudomonas aeruginosa isolates causing corneal infection between 1997 and 2000. Cornea. 2003;22(2):131–134. doi: 10.1097/00003226-200303000-00010. [DOI] [PubMed] [Google Scholar]
- 36.Massimino S, Kearns RJ, Loos KM, et al. Effects of age and dietary β-carotene on immunological variables in dogs. J Vet Intern Med. 2003;17(6):835–842. doi: 10.1111/j.1939-1676.2003.tb02523.x. [DOI] [PubMed] [Google Scholar]
- 37.Greeley EH, Kealy RD, Ballam JM, Lawler DF, Segre M. The influence of age on the canine immune system. Vet Immunol Immunopathol. 1996;55(1–3):1–10. doi: 10.1016/s0165-2427(96)05563-8. [DOI] [PubMed] [Google Scholar]
- 38.Greeley EH, Ballam JM, Harrison JM, Kealy RD, Lawler DF, Segre M. The influence of age and gender on the immune system: a longitudinal study in Labrador Retriever dogs. Vet Immuno Immunopathol. 2001;82(1–2):57–71. doi: 10.1016/s0165-2427(01)00336-1. [DOI] [PubMed] [Google Scholar]
- 39.Felsburg PJ. Overview of immune system development in the dog: comparison with humans. Hum Exp Toxicol. 2002;21(9–10):487–492. doi: 10.1191/0960327102ht286oa. [DOI] [PubMed] [Google Scholar]
- 40.Blount DG, Heaton PR, Pritchard DI. Changes to levels of DNA damage and apoptotic resistance in peripheral blood mononuclear cells and plasma antioxidant potential with age in Labrador Retriever dogs. J Nutr. 2004;134(8 Suppl):2120S–2123S. doi: 10.1093/jn/134.8.2120S. [DOI] [PubMed] [Google Scholar]
- 41.Polignano A, Tortorella C, Venezia A, Jirillo E, Antonaci S. Age-associated changes in neutrophil responsiveness in a human healthy elderly population. Cytobios. 1994;80(322):145–153. [PubMed] [Google Scholar]
- 42.Antonaci S, Jirillo E, Ventura MT, Garofalo AR, Bonomo L. Non-specific immunity in aging: deficiency of monocyte and polymorphonuclear cell-mediated functions. Mech Ageing Dev. 1984;24(3):357–375. doi: 10.1016/0047-6374(84)90121-0. [DOI] [PubMed] [Google Scholar]
- 43.Kernacki KA, Barrett RP, McClellan SA, Hazlett LD. Aging and PMN response to P. aeruginosa infection. Invest Ophthalmol Vis Sci. 2000;41(10):3019–3025. [PubMed] [Google Scholar]
- 44.Bistner SI, Roberts SR, Anderson RP. Conjunctival bacteria: clinical appearances can be deceiving. Mod Vet Pract. 1969;50(13):45–47. [Google Scholar]
- 45.Urban M, Wyman M, Rheins M, Marraro RV. Conjunctival flora of clinically normal dogs. J Am Vet Med Assoc. 1972;161(2):201–206. [PubMed] [Google Scholar]
- 46.McDonald PJ, Watson DJ. Microbial flora of normal canine conjunctivae. J Small Anim Pract. 1976;17(12):809–812. doi: 10.1111/j.1748-5827.1976.tb06947.x. [DOI] [PubMed] [Google Scholar]
- 47.Hacker DV, Jensen HE, Selby LA. A comparison of conjunctival culture techniques in the dog. J Am Vet Med Assoc. 1979;15(2):223–225. [Google Scholar]
- 48.Gerding PA, Jr, Cormany K, Weisiger R, Kakoma I. Survey and topographic distribution of bacterial and fungal microorganisms in eyes of clinically normal dogs. Canine Pract. 1993;18(2):34–38. [Google Scholar]
- 49.Jones WG. A preliminary report of the flora in health and disease of the external ear and conjunctival sac of the dog. J Am Vet Med Assoc. 1955;127(944):442–443. [PubMed] [Google Scholar]
- 50.Peterson-Jones SM. Quantification of conjunctival sac bacteria in normal dogs and those suffering from keratoconjunctivitis sicca. Vet Comp Ophthalmol. 1997;7(1):29–35. [Google Scholar]
- 51.Singer TR, Isenberg SJ, Apt L. Conjunctival anaerobic and aerobic bacterial flora in paediatric versus adult subjects. Br J Ophthalmol. 1988;72(6):448–451. doi: 10.1136/bjo.72.6.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fleiszig SM, Efron N. Microbial flora in eyes of current and former contract lens wearers. J Clin Microbiol. 1992;30(5):1156–1161. doi: 10.1128/jcm.30.5.1156-1161.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Walker CD, Claoué CMP. Incidence of conjunctival colonization by bacteria capable of causing postoperative endophthalmitis. J R Soc Med. 1986;79(9):520–521. doi: 10.1177/014107688607900907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stapleton F, Dart JK, Seal DV, Matheson M. Epidemiology of Pseudomonas aeruginosa keratitis in contact lens wearers. Epidemiol Infect. 1995;114(3):395–402. doi: 10.1017/s0950268800052109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kenchappa P, Sangwan VS, Ahmed N, et al. High-resolution genotyping of Pseudomonas aeruginosa strains linked to acute post cataract surgery endophthalmitis outbreaks in India. Ann Clin Microbiol Antimicrob. 2005;4(19):1–8. doi: 10.1186/1476-0711-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Prabha V, Singh N, Chopra P. Bacteriological evaluation of conjunctiva, contact lens storage cases, and solutions during contact lens wear. Indian J Pathol Microbiol. 2007;50(1):101–103. [PubMed] [Google Scholar]
- 57.Rajvanshi VS. Bacterial flora of the conjunctiva. J All India Ophthalmol Soc. 1968;16(1):24–28. [PubMed] [Google Scholar]
- 58.Tomar VP, Sharma OP, Joshi K. Bacterial and fungal flora of normal conjunctiva. Ann Ophthalmol. 1971;3(6):669–671. [PubMed] [Google Scholar]