Abstract
Objective
To investigate the association between components of the metabolic syndrome (MetS) measured during childhood/adolescence, and adult MetS.
Study design
This investigation focused on members of the Muscatine Study Longitudinal Adult Cohort. Predictor variables were risk factor measurements obtained between 1970 and 1981 when Cohort members participated in school survey examinations. Risk factor measurements obtained between 1982 and 2008 when Cohort members participated in follow-up examinations as young and middle-aged adults were utilized for MetS classification.
Results
33.0% (29.7% of 474 women; 37.0% of 384 men) of Cohort members were classified as having the MetS. The initial MetS classification occurred at ages ranging from 23 to 52 with a mean age of 37.2 years (SD = 7.4). Cohort members with the MetS had significantly higher body mass index (BMI), systolic blood pressure, and triglycerides (TRIG) at the time they participated in the school survey examinations (p < 0.0001). Estimated probabilities of remaining MetS free at age 35 for those whose school survey BMI and TRIG measurements were both < 50th vs. ≥ 75th percentile were strikingly different (0.94 vs. 0.42).
Conclusions
BMI is the strongest childhood predictor of adult MetS. Early identification of at-risk children may reduce the burden of atherosclerotic cardiovascular disease.
The metabolic syndrome (MetS) is a cluster of abnormalities, including central obesity, increased blood pressure, lipid abnormalities, and impaired glucose tolerance,1 all well-documented risk factors for cardiovascular disease. The individual components of the MetS occur together more often than expected by chance and the cluster is associated with increased risk of cardiovascular disease and type 2 diabetes in adults.2 The age-adjusted prevalence of the MetS in U.S. adults as estimated from the Third National Health and Nutrition Examination Survey (NHANES III 1988-1994) was 23.7% for men and women 20 years of age or older; the prevalence increased with age and was highest in Mexican Americans compared with non-Hispanic whites and African Americans.3 The world is currently experiencing a major increase in the prevalence of the MetS that parallels the global obesity epidemic,4 and is likely to result in increased cardiovascular disease (CVD) morbidity and mortality.
Although clinical features of CVD are usually not apparent until the third or fourth decade of life, the atherosclerotic process begins in childhood.5 Components of the metabolic syndrome cluster in children and adolescents and track into young adulthood.6-9 The overall prevalence of the MetS in NHANES III participants 12 to 19 years of age was 4.2% (0.1% of those with a BMI < 85th percentile, 6.8% of those with a BMI between the 85th and 95th percentile, and 28.7% of those with BMI ≥ 95th percentile).10 Depending on the definition used, the prevalence of the MetS in 12 to 19 year old participants of NHANES 99-02 (1999-2002) varied from 2.0 to 9.4% overall, and from 12 to 44% in those who were obese.11 Weiss et al 12 determined that the prevalence of the MetS increased and each component worsened with the severity of obesity in children and adolescents, reaching 49.7% in those who were severely obese (Z-score > 2.5). However, even though clustering of metabolic risk factor components during childhood/adolescence is well-documented, the physiologic changes that occur during adolescence make the clinical classification of the MetS unstable.13
Few studies have examined the same individuals during childhood and followed them to adulthood to evaluate how well pediatric MetS, or components of the MetS, predict adult MetS.14-17 Mattsson et al18 identified childhood obesity, high triglycerides, high insulin, high CRP, family history of hypertension and family history of type 2 diabetes as determinants of adult MetS.
In the present analysis, we utilized longitudinal data from The Muscatine Study to examine the association between childhood/adolescent risk factor measurements and the MetS in adulthood and to predict metabolic syndrome-free survival.
Methods
In 1970, a population-based study was initiated in Muscatine, Iowa to define the distribution of established adult cardiovascular risk factors in school-aged children. Between 1970 and 1981, 11,377 Muscatine school children in grades K through 12 underwent 26,919 examinations in six biennial cross-sectional school surveys.6 The participation rate was approximately 70% for each survey. Between 1982 and 1991, 2,547 individuals who had been examined at least once during childhood participated in follow-up examinations targeted to be near their 23rd, 28th and 33rd birthdays (Young-Adult Follow-up [YAF] Surveys).7,8,19-22 1,620 young adults participated in one and 927 participated In two YAF examinations. There was no defined cohort during the initial 20 years of The Muscatine Study. Any student in the schools at the time a survey was being conducted was eligible to participate, many school survey participants did not reach 23 years of age during the time frame of the YAF examinations so they were never asked to participate, other former school survey participants were beyond the age of 23 when the YAF examinations began and their first YAF examination was at age 28.
Beginning in 1992 a representative subset of YAF survey participants was recruited (the Muscatine Study Longitudinal Adult Cohort, N = 865); since their initial Cohort examination, these individuals have participated in longitudinal computed tomographic and ultrasound examinations to assess subclinical atherosclerotic disease.21-25 The 865 members of the Longitudinal Adult Cohort who participated in 1 to 6 school survey examinations (mean 2.4; 272 in 1 [31.4%]; 574 in 2, 3 or 4 [66.4%]; 19 in 5 or 6 [2.2%]), and have participated in 2 to 8 examinations to date since high school (mean 6.1; 172 in 2, 3 or 4 [19.9%]; 214 in 5 or 6 [24.7%]; 479 in 7 or 8 [55.4%]) are the focus of this analysis. The vast majority of the school survey population was non-Hispanic white, as are the majority of the Cohort members (99%). All of the recruitment and examination procedures used in the schools and for the YAF and Cohort examinations were approved by the Institutional Review Board of the University of Iowa. Written informed consent was obtained from the participants themselves, or from parents/guardians of the children who gave informed assent.
Measurements
The relevant measurements available from the school survey examinations consist of height, weight, resting systolic (SBP) and diastolic (DBP) blood pressure, fasting total cholesterol (CHOL), and total triglycerides (TRIG). Waist circumference (WC), HDL-cholesterol (HDL-C) and fasting glucose (FG), three components of most metabolic syndrome (MetS) definitions,1,2 were not measured during the school survey examinations. The YAF examinations measured height, weight, resting SBP and DBP, fasting CHOL and HDL-C, and total TRIG during the first five years. WC was added to the examinations in the second five years; however, FG was not measured until the Cohort examinations.
School survey and YAF measurements were made using what were standard approaches at the time of the examinations.6-8 All school and YAF survey measurements were converted to age-sex-survey year-specific Z-scores. The mean Z-scores of the 865 Cohort members at the time of their last school survey examination and their last YAF examination are not significantly different from zero, indicating they represent all Muscatine Study school survey and YAF participants.
The Cohort examinations were conducted after a 12-hour overnight fast. Relevant to the analysis reported herein, height, weight, and WC were recorded. Three SBP and DBP measurements were made after a five-minute seated rest. Fasting HDLC, total TRIG, and FG were measured using standard approaches.23-25
Classification of the Metabolic Syndrome
The American Heart Association (AHA) revision1 of the National Heart Lung and Blood Institute (NHLBI) National Cholesterol Education Program (NCEP) definition in the Adult Treatment Panel (ATP) III Report2 was used to determine the MetS status (MetS+; MetS-) of Cohort members at the time of each YAF and Cohort examination. If WC was not measured a criterion of BMI ≥ 30 kg/m2 was substituted. FG measurements were only obtained at some of the Cohort examinations. Two MetS outcome variables were defined for analysis: 1) ever classified with MetS along with age at first classification for each Cohort member based on all available YAF and Cohort examination data; and 2) the MetS status at “age 40” which was determined for a subset (the Age 40 subset) of Cohort members who either had an examination at 40 ± 2 years of age or were classified with MetS based on an examination prior to 40 years of age.
To create the MetS outcome variables, the entire risk factor measurement profile was examined for each Cohort member. One such profile is shown in Table I. This female Cohort member participated in nine examinations between 24 and 49 years of age. She had persistently low HDL-C. At age 43, she satisfied three of the five MetS criteria, at age 45, her blood pressure was lower, she had started taking lipid medication and her HDL-C was higher, however at ages 47 and 48 she satisfied four and five criteria, respectively. She was classified as MetS+ with first classification at 43 years of age for the “ever classified” outcome variable; and as MetS- for the Age 40 outcome variable.
Table I.
Risk factor profile for a female Cohort member with nine Muscatine Study examinations between 24 and 49 years of age
| Age | WC | TRIG | HDL-C | Lipid Medications | SBP | DBP | BP Medications | FG | # of Criteria† | MetS+ |
|---|---|---|---|---|---|---|---|---|---|---|
| 24 | 86 | 40 | 111 | 68 | 1 | No | ||||
| 28 | 73 | 161 | 37 | 111 | 68 | 2 | No | |||
| 34 | 78 | 135 | 35 | No | 120 | 81 | No | 1 | No | |
| 39 | 86 | 103 | 41 | No | 127 | 81 | No | 89 | 1 | No |
| 41 | 85 | 101 | 39 | No | 127 | 79 | No | 90 | 1 | No |
| 43 | 88 | 330 | 33 | No | 131 | 91 | No | 89 | 3 | Yes |
| 45 | 87 | 249 | 54 | Yes | 121 | 81 | No | 2 | No | |
| 47 | 89 | 287 | 44 | Yes | 132 | 87 | No | 4 | Yes | |
| 49 | 94 | 182 | 47 | Yes | 139 | 98 | No | 102 | 5 | Yes |
WC = waist circumference
TRIG = total triglycerides
HDL-C = high-density lipoprotein cholesterol
SBP & DBP = systolic and diastolic (5th phase) blood pressure (BP)
FG = fasting glucose
Risk factors that satisfy the MetS criteria are highlighted the number of metabolic syndrome criteria satisfied1
MetS+ = metabolic syndrome present (+)
Data Analysis
Quantitative variables are described using mean ± standard deviation (SD); categorical variables are described using counts. Age-sex-height-specific blood pressure and age-sex-specific BMI percentiles for all available school survey measurements were determined based on the Fourth Task Force Report26 and the 2000 CDC Growth Charts,27 respectively. The school survey Z-scores for each Cohort member were used to construct a risk factor Z-score vs. time graph. The total area under the curve was calculated using the trapezoidal rule, and divided by the length of time spanned by the evaluations. This produced a mean school survey Z-score that was weighted by the time between measurements - the “risk factor load.”25 School survey risk factor loads were calculated for SBP, DBP and BMI based on the percentiles as well as the Muscatine Study Z-scores. School survey risk factor loads for total cholesterol and triglycerides were calculated based on Z-scores.
Two sets of school survey measurements were used for analysis: 1) the earliest examination for each Cohort member; and 2) the risk factor loads. For predictive value analysis and modeling, school survey measurements for Cohort members were classified based on the 50th, 75th and 90th percentiles of SBP, DBP and BMI; risk factor Z-scores and loads were classified using standard normal distribution values of 0.00, 0.67, and 1.28, respectively, to identify the 50th, 75th and 90th percentiles. Student's t-test was used to compare school survey risk factor measures in adults with and without the MetS; p-values were determined using Satterthwaite's method because of occasional violations of the equal variance hypothesis.
The Kaplan-Meier method was used to estimate the probability of remaining MetS free based on the age at first classification (for those classified with the MetS) or the age at the most recent Cohort examination (for those without the MetS). Estimated survival functions were compared between subsets of the Cohort who were identified based on their school survey risk factor measurements. Multivariable logistic regression models were fitted for the Age 40 subset to investigate the association between MetS and childhood risk factor loads and to develop prediction models. Odds ratios, 95% confidence intervals (CI), and MetS probabilities were estimated.
All data analyses were conducted using procedures from the Statistical Analysis System (SAS, version 9.1.3). A p-value < 0.05 was considered to indicate statistical significance.
Results
Prevalence of the Metabolic Syndrome
Seven of the 865 Cohort members who had been diagnosed with type 1 diabetes were excluded from the analysis. 283 (33.0%) of the remaining 858 Cohort members were classified as having the metabolic syndrome (MetS+) based on a compilation of all their Muscatine Study follow-up examinations from age 20 to the most recent. This included 29.7% of the 474 women, and 37.0% of the 384 men. The age at the time of the examination that resulted in their initial MetS classification ranged from 23 to 52 with a mean of 37.2 years (SD = 7.4). Their earliest school survey examination results are displayed in the left-hand columns of Table II by MetS classification. The mean age at the first school survey examination was approximately 12; however, the Cohort members included in this analysis ranged from 8 to 18 years of age at the time of their first examination (18.6% age 8 or 9; 21.8% age 10 or 11; 21.8% age 12 or 13; 25.3% age 14 or 15, 12.5% age 16+ years). Their risk factor loads are displayed in the left-hand columns of the lower portion of Table II. For both the earliest measurements and the loads, Cohort members who were classified with the MetS at some point during their Muscatine Study follow-up examinations had significantly higher BMI, SBP, DBP and TRIG when they participated in the school survey examinations; their CHOL was not different.
Table II.
Earliest childhood risk factor measurements (mean ± SD) and risk factor loads for Muscatine Study Longitudinal Adult Cohort members, by metabolic syndrome classification
| Complete Cohort (N = 858) | Age 40 Subset (N = 730) | ||||||
|---|---|---|---|---|---|---|---|
| Measurement | MetS-N = 575 | MetS+ N = 283 | p-value | MetS-N = 536 | MetS+ N = 194 | p-value | |
| Earliest School Survey | |||||||
| Age | years | 12.3 ± 2.6 | 12.5 ± 2.7 | > 0.20 | 12.2 ± 2.5 | 12.0 ± 2.6 | > 0.40 |
| BMI | Percentile (%) | 52.9 ± 25.2 | 67.6 ± 24.9 | < 0.0001 | 53.6 ± 25.4 | 71.3 ± 24.2 | < 0.0001 |
| Z-score | -0.19 ± 0.74 | 0.32 ± 1.02 | < 0.0001 | -0.17 ± 0.77 | 0.47 ± 1.07 | < 0.0001 | |
| SBP | Percentile (%) | 64.6 ± 27.8 | 72.2 ± 26.5 | < 0.0001 | 65.5 ± 27.5 | 73.5 ± 26.8 | < 0.0005 |
| Z-score | -0.09 ± 0.96 | 0.22 ± 1.01 | < 0.0001 | -0.06 ± 0.97 | 0.30 ± 1.05 | < 0.0001 | |
| DBP | Percentile (%) | 60.2 ± 26.3 | 65.2 ± 25.3 | < 0.01 | 59.9 ± 26.5 | 65.6 ± 25.5 | < 0.01 |
| Z-score | -0.09 ± 1.00 | 0.11 ± 0.97 | < 0.01 | -0.09 ± 1.00 | 0.16 ± 1.01 | < 0.005 | |
| CHOL | Z-score | -0.01 ± 0.95 | 0.00 ± 1.00 | > 0.80 | -0.04 ± 0.92 | 0.05 ± 1.03 | > 0.30 |
| TRIG | Z-score | -0.06 ± 0.88 | 0.28 ± 1.07 | < 0.0001 | -0.04 ± 0.88 | 0.39 ± 1.15 | < 0.0001 |
| Age | years | 13.8 ± 1.9 | 13.9 ± 2.0 | > 0.50 | 13.8 ± 1.7 | 13.5 ± 2.0 | < 0.10 |
| BMI | Percentile (%) | 54.2 ± 23.1 | 68.6 ± 23.2 | < 0.0001 | 54.7 ± 23.2 | 72.6 ± 22.5 | < 0.0001 |
| Z-score | -0.18 ± 0.70 | 0.37 ± 1.02 | < 0.0001 | -0.17 ± 0.72 | 0.54 ± 1.06 | < 0.0001 | |
| SBP | Percentile (%) | 61.0 ± 24.1 | 69.7 ± 23.1 | < 0.0001 | 61.8 ± 23.7 | 70.8 ± 23.6 | < 0.0001 |
| Z-score | -0.10 ± 0.80 | 0.21 ± 0.85 | < 0.0001 | -0.07 ± 0.80 | 0.28 ± 0.88 | < 0.0001 | |
| DBP | Percentile (%) | 58.7 ± 21.6 | 64.0 ± 21.7 | < 0.001 | 58.7 ± 21.7 | 63.8 ± 21.1 | < 0.005 |
| Z-score | -0.07 ± 0.84 | 0.13 ± 0.82 | < 0.0025 | -0.05 ± 0.84 | 0.16 ± 0.83 | < 0.005 | |
| CHOL | Z-score | -0.01 ± 0.89 | -0.01 ± 0.93 | > 0.90 | -0.04 ± 0.86 | 0.04 ± 0.95 | > 0.20 |
| TRIG | Z-score | -0.09 ± 0.71 | 0.20 ± 0.92 | < 0.0001 | -0.10 ± 0.70 | 0.33 ± 0.99 | < 0.0001 |
SD = standard deviation
Complete Cohort Analysis - includes all 858 Cohort members without type 1 diabetes
Age 40 Analysis - includes 712 Cohort members without type 1 diabetes who had a Muscatine Study examination at age 40 ± 2 years + 18 Cohort members without type 1 diabetes who were classified with MetS before 40 years of age
MetS = metabolic syndrome absent (-) or present (+)
Z-scores are age-sex-survey-specific from the Muscatine Study school surveys conducted between 1970 and 1981
BMI = body mass index (kg/m2)
BMI percentiles - based on the 2000 CDC Growth Charts27
SBP & DBP percentiles - based on the Fourth Task Force Report (age-height-gender-specific)26
Risk factor load = mean school survey Z-score or percentile weighted by the time between measurements
See Table I for additional definitions
Age 40 Subset Analysis: 717 Cohort members participated in a follow-up examination at age 40 ± 2 years (mean 40.3, SD 0.7, minimum 38.1, maximum 41.9); an additional 18 Cohort members were classified as MetS+ based on at least one examination before age 40. Five of the total of 735 had been diagnosed with type 1 diabetes, and those individuals were excluded from the analysis. Therefore, 730 Cohort members (380 women, 52.1%) were included in the Age 40 analysis. 194 of the 730 (26.6%), were classified as having the MetS based on a compilation of all their Muscatine Study follow-up examinations from age 20 up to the closest examination to age 40 ± 2 years; 22.9% of the 380 women, and 30.6% of the 350 men. Their earliest school survey examination results and risk factor loads are displayed in the right-hand columns of Table II by MetS classification. As was seen in the entire cohort, members of the Age 40 subset with MetS had significantly higher BMI, SBP, DBP and TRIG when they participated in the school survey examinations.
Sensitivity, Specificity and Predictive Value
Predictive values for a MetS classification by age 40 associated with 75th and 90th percentile school survey risk factor cutpoints were estimated (Table III). In general, sensitivity was between 15 and 40%, PV+ was between 30 and 60%, specificity was between 80 and 95% and PV- was between 70 and 80%. The highest values were achieved using BMI, followed by TRIG. There was no consistent sex difference, not much difference using Muscatine Study Z-scores vs. percentiles based on national standards, and not much difference using risk factor loads vs. the earliest school survey examination (data not shown).
Table III.
Predictive value of a metabolic syndrome classification by age 40 ± 2 years based on childhood risk factor measurements (N = 730; 26.6% MetS)
| Earliest School Survey | Sensitivity | PV+ | Specificity | PV- | |
|---|---|---|---|---|---|
| ≥ 75th percentile† | BMI | 52.6 | 43.4 | 75.2 | 81.4 |
| SBP | 60.3 | 32.9 | 55.4 | 79.4 | |
| DBP | 46.9 | 31.4 | 62.8 | 76.5 | |
| ≥ 90th percentile† | BMI | 28.9 | 57.7 | 92.4 | 78.2 |
| SBP | 36.6 | 34.8 | 75.2 | 76.6 | |
| DBP | 18.6 | 31.3 | 85.2 | 74.3 | |
| ≥ 75th percentile of Z-scores‡ | BMI | 36.1 | 53.0 | 88.4 | 79.3 |
| SBP | 33.5 | 38.0 | 80.2 | 76.9 | |
| DBP | 29.9 | 33.3 | 78.3 | 75.5 | |
| CHOL | 21.6 | 29.4 | 81.2 | 74.1 | |
| TRIG | 30.9 | 40.8 | 83.8 | 77.0 | |
| ≥ 90th percentile of Z-scores‡ | BMI | 20.6 | 57.1 | 94.4 | 76.7 |
| SBP | 18.0 | 41.7 | 90.9 | 75.4 | |
| DBP | 10.8 | 34.4 | 92.5 | 74.1 | |
| CHOL | 11.3 | 34.9 | 92.4 | 74.2 | |
| TRIG | 15.5 | 42.3 | 92.4 | 75.1 | |
| ≥ 75th percentile of Z-scores‡ | BMI & SBP | 18.0 | 60.3 | 95.7 | 76.3 |
| BMI & TRIG | 18.6 | 73.5 | 97.6 | 76.8 | |
| TRIG & SBP | 9.8 | 50.0 | 96.5 | 74.7 | |
| BMI & SBP & TRIG | 7.7 | 75.0 | 99.1 | 74.8 | |
| School Survey Rick Factor Loads | Sensitivity | PV+ | Specificity | PV- | |
|---|---|---|---|---|---|
| ≥ 75th percentile† | BMI | 55.2 | 47.6 | 78.0 | 82.8 |
| SBP | 50.0 | 34.5 | 65.7 | 78.4 | |
| DBP | 36.1 | 34.0 | 74.6 | 76.3 | |
| ≥ 90th percentile† | BMI | 27.3 | 62.4 | 94.0 | 78.1 |
| SBP | 26.3 | 41.5 | 86.6 | 76.4 | |
| DBP | 9.8 | 32.8 | 92.7 | 74.0 | |
| ≥ 75th percentile of Z-scores‡ | BMI | 37.1 | 55.4 | 89.2 | 79.7 |
| SBP | 32.5 | 40.1 | 82.5 | 77.1 | |
| DBP | 28.9 | 34.6 | 80.2 | 75.7 | |
| CHOL | 23.7 | 32.2 | 81.9 | 74.8 | |
| TRIG | 26.3 | 42.1 | 86.9 | 76.5 | |
| ≥ 90th percentile of Z-scores‡ | BMI | 21.1 | 62.1 | 95.3 | 77.0 |
| SBP | 12.9 | 46.3 | 94.6 | 75.0 | |
| DBP | 4.6 | 27.3 | 95.5 | 73.5 | |
| CHOL | 11.3 | 39.3 | 93.7 | 74.5 | |
| TRIG | 13.4 | 53.1 | 95.7 | 75.3 | |
| ≥ 75th percentile of Z-scores‡ | BMI & SBP | 15.5 | 60.0 | 96.3 | 75.9 |
| BMI & TRIG | 15.5 | 75.0 | 98.1 | 76.2 | |
| TRIG & SBP | 9.8 | 57.6 | 97.4 | 74.9 | |
| BMI & SBP & TRIG | 6.2 | 70.6 | 99.1 | 74.5 | |
Probability of Remaining Metabolic Syndrome Free
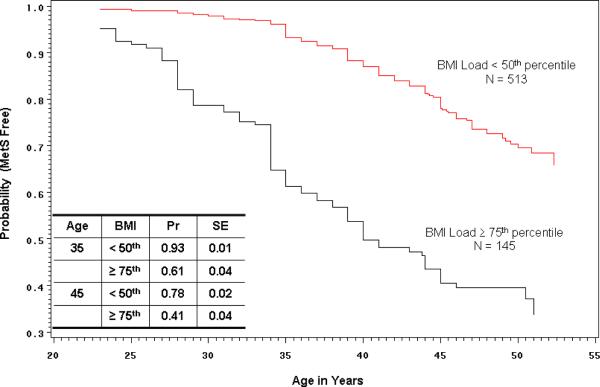
A survival analysis using the Complete Cohort (N = 858) was conducted to estimate the probability of remaining MetS free based on school survey BMI, SBP and TRIG loads. The analysis utilized the “age at first diagnosis of MetS” that was determined for each Cohort member based on all examinations from age 20 to the present to define the time of an “event.” Figure 1 displays the Kaplan-Meier estimates for two Cohort groups based on their school survey BMI load. Those whose BMI load was ≥ 75th percentile (weighted mean Z-score > 0.67) had significantly lower probability (log-rank test p < 0.0001) of remaining MetS free when compared with those whose BMI load was < 50th percentile (weighted mean Z-score < 0.00). In particular, the estimated probability of remaining MetS free at age 35 was 0.93 in the < 50th percentile group and 0.61 in the ≥ 75th percentile group. Similar analyses (data not shown) found that those whose school survey TRIG load was < 50th vs. ≥ 75th percentile had estimated probabilities of remaining MetS free at age 35 of 0.90 (SE = 0.01) vs. 0.69 (SE = 0.04) (log-rank test p < 0.0001), and those whose school survey SBP load was < 50th vs. ≥ 75th percentile had estimated probabilities of remaining MetS free at age 35 of 0.90 (SE = 0.01) vs. 0.72 (SE = 0.03) (log-rank test p < 0.0001).
1.
Kaplan-Meier plot showing the estimated probability of remaining MetS free for Muscatine Study Longitudinal Adult Cohort members whose body mass index (BMI) load based on school survey examinations was < 50th percentile vs. ≥ 75th percentile. The table displays probability and associated standard error estimates for the two BMI groups at 35 and 45 years of age. Based on the log-rank test there is a significant difference between the curves (p < 0.0001).
The association between school survey BMI load and SBP load is the strongest, and that between TRIG load and SBP load is the weakest (BMI and SBP r = 0.36, p < 0.0001; BMI and TRIG r = 0.22, p < 0.0001; TRIG and SBP r = 0.07, p < 0.05). Table IV shows the prevalence of the MetS by school survey risk factor load pattern groups defined using the < 50th and ≥ 75th percentile classification of BMI, TRIG and SBP loads for both the Complete Cohort (N = 858) and the Age 40 subset (N = 730). Among the risk factor load patterns included in Table IV, a school survey BMI load ≥ 75th percentile clearly defines a subset of the Cohort that has a higher prevalence of MetS, especially when one or both of TRIG load and SBP load are ≥ 75th percentile.
Table IV.
Prevalence of the metabolic syndrome by school survey risk factor load pattern groups
| Complete Cohort (N = 858) | Age 40 Subset (N = 730) | |||||||
|---|---|---|---|---|---|---|---|---|
| BMI load | TRIG load | SBP load | N | % of 858 | MetS+ (%) | N | % of 730 | MetS+ (%) |
| < 50th† | < 50th | < 50th | 202 | 23.6 | 18.8 | 177 | 24.3 | 20.3 |
| ≥ 75th | 35 | 4.1 | 25.7 | 32 | 4.4 | 28.1 | ||
| ≥ 75th | ≥ 50th | 38 | 4.4 | 21.1 | 33 | 4.5 | 24.2 | |
| ≥ 75th | 8 | 0.9 | 25.0 | 7 | 1.0 | 28.6 | ||
| ≥ 75th‡ | < 50th | < 50th | 27 | 3.1 | 37.0 | 23 | 3.2 | 39.1 |
| ≥ 75th | 17 | 2.0 | 52.9 | 14 | 1.9 | 57.1 | ||
| ≥ 75th | < 50th | 9 | 1.1 | 55.6 | 8 | 1.1 | 62.5 | |
| ≥ 75th | 18 | 2.1 | 72.2 | 17 | 2.3 | 76.5 | ||
| Other Patterns | 504 | 58.7 | 37.5 | 419 | 57.4 | 24.8 | ||
23.6% of 513 members of the Complete Cohort with BMI load < 50th percentile, and 26.1% of 436 members of the Age 40 Subset with BMI load < 50th percentile are MetS+
57.9% of 145 members of the Complete Cohort with BMI load ≥ 75th percentile, and 62.3% of 130 members of the Age 40 Subset with BMI load ≥ 75th percentile are MetS+
Figure 2 displays the Kaplan-Meier estimates for four groups of Cohort members identified based on a combination of their school survey BMI and TRIG loads: those whose BMI and TRIG loads were both < 50th percentile; those whose BMI load was < 50th and whose TRIG load was ≥ 75th percentile; those whose BMI load was ≥ 75th and whose TRIG load was < 50th percentile; and those whose BMI and TRIG loads were ≥ 75th percentile. The difference in the estimated probabilities of remaining MetS free at age 35 for those whose BMI and TRIG loads were both < 50th vs. both ≥ 75th percentile is quite striking (0.94 vs. 0.42). The difference in the estimated probabilities of remaining MetS free at age 35 for those whose BMI and SBP loads were both < 50th vs. both ≥ 75th percentile is a bit lower than with BMI and TRIG (data not shown), but still substantial (0.94 vs. 0.57). The probability curve for those whose BMI load was < 50th and whose SBP load was ≥ 75th percentile was not much different from those whose BMI and SBP loads were both < 50th percentile (their estimated probability of remaining MetS free at age 35 was 0.88). A similar analysis based on TRIG and SBP groups (data not shown) found that the probability curves for the middle two groups were nearly identical across the entire age range, and the estimated probabilities in the two extreme groups (both < 50th vs. both ≥ 75th) were 0.93 (SE = 0.02) and 0.59 (SE = 0.08), respectively. The proportion of the TRIG and SBP load < 50th percentile group that also had BMI load < 50th percentile was 74.0%. The proportion of the TRIG and SBP load ≥ 75th percentile group that also had BMI load ≥ 75th percentile was 51.4%.
2.
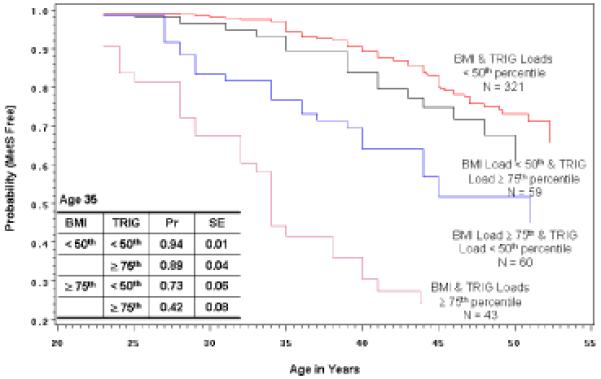
Kaplan-Meier plot showing the estimated probability of remaining MetS free for four groups of Muscatine Study Longitudinal Adult Cohort members: those whose BMI & triglyceride (TRIG) loads were both < 50th percentile; those whose BMI load was < 50th & whose TRIG load was ≥ 75th percentile; those whose BMI load was ≥ 75th & whose TRIG load was < 50th percentile; and those whose BMI & TRIG loads were ≥ 75th percentile. The table displays probability and associated standard error estimates for the four groups at 35 years of age. Based on the log-rank test there is a significant difference among the curves (p < 0.0001).
Prediction of the Metabolic Syndrome at Age 40
Logistic regression analysis models were next fitted using the Age 40 subset to investigate the association between MetS and childhood risk factor loads and to develop prediction models. In sex-adjusted univariable models, BMI, TRIG and SBP loads were each significantly associated with presence of MetS at age 40 (p < 0.0001). The odds ratios associated with a 1 SD increase in BMI load, TRIG load and SBP load were 2.2 (95% CI 1.8, 2.7), 1.6 (95% CI 1.4, 2.0), and 1.5 (95% CI 1.3, 1.8), respectively.
The best fitting multivariable logistic model included BMI and TRIG loads, both p < 0.0001. Sex and SBP load were not significant using a p < 0.05 criterion to retain a predictor in the model. There was no evidence for significant interaction effects (sex × load, load × load) in any multivariable model, for example, the BMI × TRIG load interaction effect had an associated p-value > 0.90. Based on the BMI and TRIG load model, the odds ratios associated with a 1 SD increase were 2.1 (95% CI 1.7, 2.5) and 1.4 (95% CI 1.2, 1.7), respectively. The Hosmer-Lemeshow goodness-of-fit test for this model had an associated p-value > 0.90, and the area under the receiver operating characteristic curve (c-statistic) was 0.73. Figure 3 displays the estimated probability of being classified with MetS by 40 ± 2 years of age as a function of school survey BMI and TRIG loads based on the multivariable model. TRIG load was fixed at three values: the 50th percentile corresponds to a load of 0.00, the 75th percentile to a load of 0.67, and the 90th percentile to a load of 1.28. Based on the multivariable model, individuals whose weighted mean BMI and TRIG loads were both 1 SD below their school-aged peers (BMI & TRIG loads = -1.0) have an estimated probability of MetS at age 40 of 0.08, and those with BMI & TRIG loads = 1.0 have an estimated probability of MetS at age 40 of 0.53.
3.
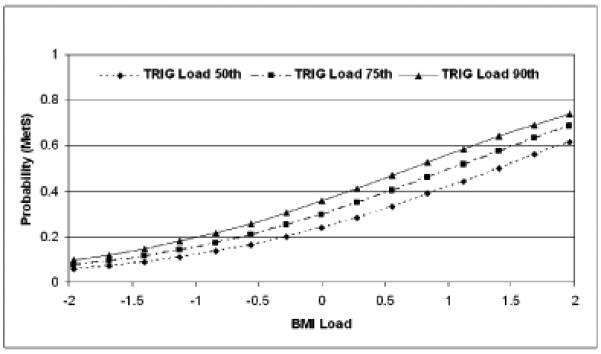
Estimated probability of being classified with MetS in the Age 40 subset as a function of school survey BMI and TRIG loads. The 50th percentile of TRIG load corresponds to a load of 0.00, and the 75th percentile to a load of 0.67, and the 90th percentile to a load of 1.28.
Finally, Table V presents estimated odds ratios and 95% confidence intervals that describe the association between school survey risk factor loads classified as ≥ 75th percentile vs. < 50th percentile in the age 40 subset. The odds of MetS at age 40 for those whose school survey BMI & TRIG loads were both ≥ 75th percentile relative to those whose BMI & TRIG loads were < 50th percentile are 19.5 (95% CI 8.8, 43.3).
Table V.
Association between categorized childhood risk factor loads and the metabolic syndrome in the age 40 subset
| N MetS+ | N MetS- | OR | 95% CI | c | |
|---|---|---|---|---|---|
| BMI Load ≥ 75th relative to < 50th percentile | 142 | 424 | 6.5 | 4.2, 10.0 | 0.68 |
| TRIG Load ≥ 75th relative to < 50th percentile | 136 | 404 | 2.9 | 1.9, 4.4 | 0.60 |
| SBP Load ≥ 75th relative to < 50th percentile | 141 | 399 | 2.6 | 1.7, 3.9 | 0.61 |
| BMI & TRIG ≥ 75th percentile | 97† | 324† | 19.5 | 8.8, 43.3 | 0.72‡ |
| BMI ≥ 75th percentile & TRIG < 50th percentile | 4.2 | 2.2, 8.1 | |||
| SBP ≥ 75th percentile & TRIG < 50th percentile | 1.6 | 0.7, 3.4 | |||
| BMI & TRIG < 50th percentile | 1.0 | reference | |||
| BMI & SBP ≥ 75th percentile | 99† | 316† | 8.8 | 4.5, 16.9 | 0.70‡ |
| BMI ≥ 75th percentile & SBP < 50th percentile | 4.6 | 2.3, 9.2 | |||
| SBP ≥ 75th percentile & BMI < 50th percentile | 1.5 | 0.7, 3.3 | |||
| BMI & SBP < 50th percentile | 1.0 | reference | |||
| TRIG & SBP ≥ 75th percentile | 96† | 307† | 7.2 | 3.3, 15.7 | 0.65‡ |
| TRIG ≥ 75th percentile & SBP < 50th percentile | 2.0 | 1.0, 4.1 | |||
| TRIG ≥ 75th percentile & BMI < 50th percentile | 2.3 | 1.3, 4.2 | |||
| TRIG & SBP < 50th percentile | 1.0 | reference | |||
Discussion
In this analysis of risk factor data accumulated since 1970 for the Muscatine Study Longitudinal Adult Cohort, we found that those who were classified as having the metabolic syndrome (MetS+) as adults had significantly higher mean BMI, SBP, DBP and TRIG at the time of their earliest school survey examination when compared with those who were never classified as having the MetS based on their entire Muscatine Study risk factor measurement profile. We also estimated that the probability of remaining MetS free at age 35 for those whose school survey BMI load was ≥ 75th percentile was only 0.61 compared with a probability of 0.93 for those whose school survey BMI load was < 50th percentile.
The availability of longitudinal examinations of Muscatine Study Cohort members is a major strength of this analysis. Most large cohort studies have not had the luxury of evaluating the early- and middle-adult risk factor profiles that are available for Muscatine Study Cohort members to determine MetS status. On the other hand, our use of measurements from multiple examinations to make that determination may have resulted in higher prevalence estimates. Of the 717 Cohort members with an examination at age 40 ± 2 years, 15 (2.1%) who would have been classified as MetS+ if only the age 40 examination had been used, were classified as MetS- based on the entire set of examinations, and 20 (2.8%) who would have been classified as MetS- if only the age 40 examination had been used, were classified as MetS+ based on the entire set of examinations. Therefore, the prevalence at age 40 would have been nearly the same, however, based on our classification criteria, the misclassification rate would have been 4.9%. Another strength of the Muscatine Study Cohort database is the fact that the school survey examinations were conducted between 1970 and 1981 prior to the more recent dramatic increase in childhood obesity. Therefore, relative to their peers at the time of the school surveys, children whose BMI was in the upper quartile weren't all at the very extreme of the BMI distribution, but represented a range.
There are also limitations. Several of the components of the MetS definition were not obtained at the time of the school survey examinations, and thus we could not investigate the question of whether the MetS during childhood/adolescence predicts the MetS during adulthood. At the time the Muscatine Study Cohort was recruited, members ranged in age from 29 to 43. Therefore, some participated in a YAF examination between 20 and 30 years of age, but not again until they were more than 40 years of age. This may have lead to an underestimate of the age when they might first have been classified as having the MetS. On the other hand, the entire risk factor profile was used, regardless of the frequency or spacing of the examinations, so the age of first classification should have been more precise than in many other studies where classification is usually based on a single examination.
The prevalence of obesity and the MetS in children and adolescents is increasing worldwide. Must et al28 provided one of the first perspectives that can be used to project the potential long-term health effects of the obesity epidemic. In a 55-year follow-up of Harvard Growth Study participants, it was determined that overweight (BMI > 75th percentile on two occasions) during adolescence predicted a broad range of health effects that were independent of adult weight, including morbidity from coronary heart disease and atherosclerosis in both men and women, and mortality from coronary heart disease in men. More recently, Bjørge et al29 investigated the mortality experience of 227,000 Norwegian adolescents initially examined between 1963 and 1975 and followed for a mean of 34.9 years. Risk of death from endocrine, nutritional, and metabolic diseases and from circulatory system diseases was increased in both men and women whose adolescent BMI was ≥ 75th percentile. On the other hand, Lawlor et al30 investigated the risk of ischemic heart disease and stroke in three historical cohorts (the Boyd Orr cohort, the Christ's Hospital cohort and the Glasgow Alumni cohort) and found little evidence that being overweight or obese in childhood was associated with risk of future cardiovascular disease.
Chinali et al31,32 evaluated the impact of the MetS on echocardiographically-derived cardiac phenotypes in non-diabetic American Indian adolescents and found that those with the MetS had higher covariate-adjusted left ventricular (LV), left atrial (LA) and aortic root diameters, LV relative wall thickness, and LV mass. The adolescents also had a higher prevalence of LV hypertrophy and LA dilation, which have been shown to be independent risk factors for cardiovascular and cerebrovascular disease, respectively, in adults. The association with the MetS persisted even after adjustment for single metabolic risk factors including obesity and blood pressure. This suggests that the MetS has an adverse effect on risk even during adolescence. Reinehr et al33 investigated the association between MetS and carotid artery intimal-medial thickness (IMT) in a sample of overweight Caucasian children and adolescents. Four different definitions of the MetS were considered, two of which showed weak associations with IMT. Impaired glucose tolerance showed the strongest association; the combination of high waist circumference, hypertension, elevated fasting glucose, which was identified as the best subset of MetS components, was inferior to impaired glucose tolerance.
Wildman et al34 recently assessed the prevalence and correlates of body mass index group status (normal weight, overweight and obese) and cardiometabolic group status (metabolically healthy, metabolically abnormal) among the adult participants of the cross-sectional NHANES 1999-2004. The components of the cardiometabolic classification included blood pressure, triglycerides, HDL cholesterol, fasting glucose, insulin resistance, and hsCRP. They found that 23.5% of normal-weight adults were metabolically abnormal, whereas 51.3% of overweight adults and 31.7% of obese adults were metabolically healthy. In our analysis of the Muscatine Study Cohort we found that 7% of those whose school survey weighted mean BMI load was < 50th percentile were estimated to develop the MetS by the age of 35 years, and 39% of those whose school survey weighted mean BMI load was ≥ 75th percentile were estimated to be MetS free at 35 years of age. These results suggest the importance of assessing risk factors such as blood pressure and lipids, in addition to body size, during childhood and adolescence.
The etiology of the MetS is not known but it likely includes genetic, metabolic, and environmental factors. As suggested in a recent editorial35 we must identify approaches that will allow children to maintain a low-cardiovascular risk status if we are to prevent or delay cardiovascular disease when they become adults.
Acknowledgment
The authors thank the participants, the staff, and the investigators, of The Muscatine Study for their invaluable contributions over the past 38 years.
This analysis was supported by research grant R01 HL061857 (TLB) from the National Heart, Lung and Blood Institute. This manuscript was prepared for the Pediatric Metabolic Syndrome Working Group. Its contents do not necessarily represent the views or policies of the National Institutes of Health.
Glossary
- BMI
Body mass index
- CHOL
Cholesterol
- TRIG
Triglycerides
- HDL
High-density lipoprotein
- SBP
Systolic blood pressure
- DBP
Diastolic blood pressure
- FG
Fasting glucose
- WC
Waist circumference
- YAF
Young-adult follow-up
- NCEP
National Cholesterol Education Program
- NHANES
National Health and Nutrition Examination Survey
- MetS
Metabolic syndrome
- SD
Standard deviation
- SE
Standard error of the estimate
- OR
Odds ratio
- CI
Confidence interval
- ROC
Receiver operating characteristic
- PMSWG
Pediatric Metabolic Syndrome Working Group
Footnotes
Author Disclosures The following authors have no financial arrangement or affiliation with a corporate organization or a manufacturer of a product discussed in this supplement: Trudy L. Burns, MPH, PhD, Elena M. Letuchy, MS, Richard Paulos, BS, John Witt, BS.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1).Grundy SM, Cleeman JI, Daniels SR, Donato KR, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, Spertus JA, Costa F. Diagnosis and management of the metabolic syndrome. An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 2).Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Final Report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 3).Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults. Findings from the Third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–9. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 4).Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–28. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 5).McGill HC, Jr, Geer JC, Strong JP. Natural history of human atherosclerotic lesions. In: Sandler M, Bourne GH, editors. Atherosclerosis and its origin. Academic Press; New York: 1963. pp. 39–65. [Google Scholar]
- 6).Lauer RM, Connor WE, Leaverton PE, Reiter MA, Clarke WR. Coronary heart disease risk factors in school children: the Muscatine Study. J Pediatrics. 1975;86:697–706. doi: 10.1016/s0022-3476(75)80353-2. [DOI] [PubMed] [Google Scholar]
- 7).Clarke WR, Schrott HG, Leaverton PE, Connor WE, Lauer RM. Tracking of blood lipids and blood pressures in school age children: the Muscatine Study. Circulation. 1978;58:626–34. doi: 10.1161/01.cir.58.4.626. [DOI] [PubMed] [Google Scholar]
- 8).Lauer RM, Clarke WR. Childhood risk factors for high adult blood pressure: the Muscatine Study. Pediatrics. 1989;84:633–41. [PubMed] [Google Scholar]
- 9).Webber LS, Srinivasan SR, Wattigney WA, Berenson GB. Tracking of serum lipids and lipoproteins from childhood to adulthood. the Bogalusa Heart Study. Am J Epidemiol. 1991;133:84–99. doi: 10.1093/oxfordjournals.aje.a115968. [DOI] [PubMed] [Google Scholar]
- 10).Cook S, Weitzman M, Auinger P, Nguyen M, Dietz WH. Prevalence of a metabolic syndrome phenotype in adolescents. Arch Pediatr Adolesc Med. 2003;157:821–7. doi: 10.1001/archpedi.157.8.821. [DOI] [PubMed] [Google Scholar]; Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW, Allen K, Lopes M, Savoye M, Morrison J, Sherwin RS, Caprio S. Obesity and the metabolic syndrome in children and adolescents. New Engl J Med. 2004;350:2362–74. doi: 10.1056/NEJMoa031049. [DOI] [PubMed] [Google Scholar]
- 11).Cook S, Auinger P, Li C, Ford ES. Metabolic syndrome rates in United States adolescents, from the National Health and Nutrition Examination Survey, 1999-2002. J Pediatr. 2008;152:165–70. doi: 10.1016/j.jpeds.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 12).Weiss R, Dziura J, Burgert TS, Tamborlane WW, Taksali SE, Yeckel CW, Allen K, Lopes M, Savoye M, Morrison J, Sherwin RS, Caprio S. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med. 2004;350:2362–74. doi: 10.1056/NEJMoa031049. [DOI] [PubMed] [Google Scholar]
- 13).Goodman E, Daniels SR, Meigs JB, Dolan LM. Instability in the diagnosis of metabolic syndrome in adolescents. Circulation. 2007;115:2316–22. doi: 10.1161/CIRCULATIONAHA.106.669994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Chen W, Srinivasan SR, Li S, Xu J, Berenson GS. Clustering of long-term trends in metabolic syndrome variables from childhood to adulthood in blacks and whites. the Bogalusa Heart Study. Am J Epidemiol. 2007;166:527–33. doi: 10.1093/aje/kwm105. [DOI] [PubMed] [Google Scholar]
- 15).Huang TTK, Nansel TR, Belsheim AR, Morrison JA. Sensitivity, specificity, and predictive values of pediatric metabolic syndrome components in relation to adult metabolic syndrome: the Princeton LRC follow-up study. J Pediatr. 2008;152:185–90. doi: 10.1016/j.jpeds.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Sun SS, Liang R, Huang TTK, Daniels SR, Arslanian SS, Liu K, Grave GD, Siervogel RM. Childhood obesity predicts adult metabolic syndrome: the Fels Longitudinal Study. J Pediatr. 2008;152:191–200. doi: 10.1016/j.jpeds.2007.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Morrison JA, Friedman LA, Wang P, Glueck CJ. Metabolic syndrome in childhood predicts adult metabolic syndrome and type 2 diabetes mellitus 25 to 30 years later. J Pediatr. 2008;152:201–6. doi: 10.1016/j.jpeds.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 18).Mattsson N, Rönnemaa T, Juonala M, Viikari JSA, Raitakari OT. Childhood predictors of the metabolic syndrome in adulthood. The Cardiovascular Risk in Young Finns Study. Annals Med. 2008;40:542–52. doi: 10.1080/07853890802307709. [DOI] [PubMed] [Google Scholar]
- 19).Burns TL, Daniels SR. The Epidemiology of Childhood Blood Pressure. In: Lauer RM, Burns TL, Daniels SR, editors. Pediatric Prevention of Atherosclerotic Cardiovascular Disease. Oxford University Press; New York, NY: 2006. pp. 165–83. [Google Scholar]
- 20).Burns TL, Peyser PA, Donohoue PA. The Epidemiology of Childhood Overweight. In: Lauer RM, Burns TL, Daniels SR, editors. Pediatric Prevention of Atherosclerotic Cardiovascular Disease. Oxford University Press; New York, NY: 2006. pp. 233–53. [Google Scholar]
- 21).Mahoney LT, Burns TL, Bielak LF, Peyser PA. Relationship between Cardiovascular Risk Factors and Coronary Artery Calcification. In: Lauer RM, Burns TL, Daniels SR, editors. Pediatric Prevention of Atherosclerotic Cardiovascular Disease. Oxford University Press; New York, NY: 2006. pp. 63–83. [Google Scholar]
- 22).Davis PH, Dawson JD. Relationship between Cardiovascular Risk Factors and Carotid Artery Intimal-Medial Thickness. In: Lauer RM, Burns TL, Daniels SR, editors. Pediatric Prevention of Atherosclerotic Cardiovascular Disease. Oxford University Press; New York, NY: 2006. pp. 84–105. [Google Scholar]
- 23).Davis PH, Dawson JD, Mahoney LT, Lauer RM. Increased carotid intimamedial thickness and coronary calcification are related in young and middle-aged adults: The Muscatine Study. Circulation. 1999;100:838–42. doi: 10.1161/01.cir.100.8.838. [DOI] [PubMed] [Google Scholar]
- 24).Mahoney LT, Burns TL, Stanford W, Thompson BH, Witt JD, Rost CA, Lauer RM. Usefulness of the Framingham risk score and body mass index to predict early coronary artery calcium in young adults (Muscatine Study) Am J Cardiol. 2001;88:509–15. doi: 10.1016/s0002-9149(01)01728-3. [DOI] [PubMed] [Google Scholar]
- 25).Davis PH, Dawson JD, Riley WA, Lauer RM. Carotid intima-medial thickness is related to cardiovascular risk factors measured from childhood through middle age: The Muscatine Study. Circulation. 2001;104:2815–9. doi: 10.1161/hc4601.099486. [DOI] [PubMed] [Google Scholar]
- 26).Falkner B, Daniels SR, Flynn JT, Gidding S, Green LA, Inglefinger J, Lauer RM, Morgenstern BZ, Portman RJ, Prineas RJ, Rocchini AP, Rosner B, Sinaiko AR, Stettler N, Urbina E, Roccella EJ. The Fourth Report on the Diagnosis, Evaluation and Treatment of High Blood Pressure in Children and Adolescents. Pediatrics. 2004;114(2 Suppl):555–76. [PubMed] [Google Scholar]
- 27). www.cdc.gov/growthcharts/
- 28).Must A, Jacques PF, Dallal GE, Bajema CJ, Dietz WH. Long-term morbidity and mortality of overweight adolescents. A follow-up of the Harvard Growth Study of 1922 to 1935. N Engl J Med. 1992;327:1350–5. doi: 10.1056/NEJM199211053271904. [DOI] [PubMed] [Google Scholar]
- 29).Bjørge T, Engeland A, Tverdal A, Davey Smith G. Body mass index in adolescence in relation to cause-specific mortality: a follow-up of 230,000 Norwegian adolescents. Am J Epidemiol. 2008;168:30–7. doi: 10.1093/aje/kwn096. [DOI] [PubMed] [Google Scholar]
- 30).Lawlor DA, Martin RM, Gunnell D, Galobardes B, Ebrahim S, Sandhu J, Ben-Shlomo Y, McCarron P, Davey Smith G. Association of body mass index measured in childhood, adolescence, and young adulthood with risk of ischemic heart disease and stroke: findings from 3 historical cohort studies. Am J Clin Nutr. 2006;83:767–73. doi: 10.1093/ajcn/83.4.767. [DOI] [PubMed] [Google Scholar]
- 31).Chinali M, de Simone G, Roman MJ, Best LG, Lee ET, Russell M, Howard BV, Devereux RB. Cardiac markers of pre-clinical disease in adolescents with the metabolic syndrome. J Am Coll Cardiol. 2008;52:932–8. doi: 10.1016/j.jacc.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32).Daniels SR. Metabolic syndrome and cardiovascular abnormalities in children. J Am Coll Cardiol. 2008;52:939–40. doi: 10.1016/j.jacc.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 33).Reinehr T, Wunsch R, de Sousa G, Toschke AM. Relationship between metabolic definitions for children and adolescents and intima-media thickness. Atherosclerosis. 2008;199:193–200. doi: 10.1016/j.atherosclerosis.2007.09.041. [DOI] [PubMed] [Google Scholar]
- 34).Wildman RP, Muntner P, Reynolds K, McGinn AP, Rajpathak S, Wylie-Rosett J, Sowers MFR. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering. Prevalence and correlates of 2 phenotypes among the US population (NHANES 1999-2004) Arch Int Med. 2008;168:1617–24. doi: 10.1001/archinte.168.15.1617. [DOI] [PubMed] [Google Scholar]
- 35).Gidding SS. Measuring children's blood pressure matters. Circulation. 2008;117:3163–4. doi: 10.1161/CIRCULATIONAHA.108.787168. [DOI] [PubMed] [Google Scholar]