Abstract
Although altered T cell function plays a part in immunosenescence, the mechanisms remain uncertain. Here we identify a bona fide age-dependent PD-1+ memory phenotype (MP) CD4+ T cell subpopulation that hardly proliferates in response to T cell receptor (TCR) stimulation and produces abundant osteopontin at the cost of typical T cell lymphokines. These T cells demonstrate impaired repopulation in Rag2−/− mice, but a homeostatic proliferation in γ-ray–irradiated mice. These T cells also reveal a unique molecular signature, including a strong expression of C/EBPα normally expressed in myeloid-lineage cells, with diminished c-Myc and cyclin D1. Transduction of Cebpa in regular CD4+ T cells inhibited the TCR-mediated proliferation with c-Myc and cyclin D1 repression and caused a striking activation of Spp1 encoding osteopontin along with concomitant repression of T cell lymphokine genes. Although these T cells gradually increase in number with age and become predominant at the senescent stage in normal mice, the generation is robustly accelerated during leukemia. In both conditions, their predominance is associated with the diminution of specific CD4+ T cell response. The results suggest that global T cell immunodepression in senescence and leukemia is attributable to the increase in PD-1+ MP CD4+ T cells expressing C/EBPα.
Keywords: immunosenescence, osteopontin
Elderly persons may exhibit a substantial diminution in specific immune response against infection, a reduced efficacy for vaccination, and a proinflammatory trait, a condition known as immunosenescence (1, 2). In T-lineage cells, a prominent effect of aging is thymic involution, resulting in decreased T cell production and export (3). But the total number of peripheral T cells is unaffected by aging in both humans and mice, owing in part to the homeostatic proliferation of memory phenotype (MP) T cells (4–6); consequently, the T cell population shows a progressive shift from naïve to MP cells with age. Such a shift in T cell composition is considered to contribute significantly to immunosenescence. This may result in contraction of the T cell repertoire, leading to an increased incidence of poor responsiveness to new antigens (7). It also is recognized that CD4+ T cells in the elderly are qualitatively altered, including a number of defects in the T cell receptor (TCR)-mediated signaling pathways, reduced immunologic synapse formation with antigen-presenting cells, diminished cognate helper function for B cells, and altered lymphokine production patterns (8–11). These effects may be attributed primarily to the cellular changes in T cells rather than to the host environment (12).
The homeostatic maintenance of MP T cells for prolonged periods may involve multiple factors, including environmental antigens, such as commensal bacteria, low-affinity self-ligands, and homeostatic cytokines (4, 13, 14). The eventual fate of MP T cells remains elusive, however. In most somatic tissue cells, programmed cell differentiation is tightly coupled with the control of cell proliferation, leading to the terminal differentiation with loss of proliferation capacity and limited life span. In myeloid-lineage cells, for instance, a bZIP family transcription factor, C/EBPα, plays a crucial role in controlling the homeostatic differentiation and the quiescence of terminally differentiated cells (15, 16). But expression of Cebpa is repressed during T-lineage cell commitment from hematopoietic progenitors, and mature T cells hardly express C/EBPα (17). How the balance between proliferation and quiescence of MP T cell clones is controlled to maintain the homeostasis for prolonged periods remains unclear.
In the present study, we identified a bona fide age-dependent MP CD4+ T cell population defined by a constitutive expression of PD-1, which is induced only transiently on activation in regular T cells (18). The PD-1+ MP CD4+ T cells hardly proliferate in response to TCR stimulation but produce large amounts of a proinflammatory cytokine, osteopontin (OPN), at the cost of typical T cell lymphokines. We suggest that the functional features of these cells are attributable in part to an unusual expression of C/EBPα. Moreover, in addition to senescence, the generation of equivalent PD-1+ MP CD4+ T cells is robustly accelerated during leukemia. We provide evidence that the predominance of these unique T cells underlies the global depression of T cell immune response both in senescence and during leukemia.
Results
Identification of Age-Dependent PD-1+ MP CD4+ T Cells With Defective TCR-Mediated Proliferation.
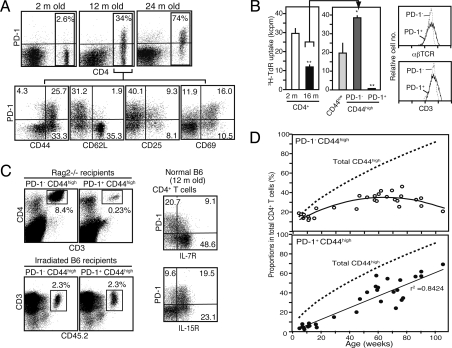
We found that increasing proportions of splenic CD4+ T cells in normal B6 mice constitutively expressed PD-1 as they aged (Fig. 1A, Upper). The PD-1+ CD4+ T cells were confined to a CD44high CD62Llow (MP) population, and most of them exhibited CD69 with little CD25 expression (Fig. 1A, Lower). Purified CD4+ T cells from aged mice showed a significantly diminished TCR-mediated proliferation compared with those from young mice (Fig. 1B). Among the CD4+ T cells in aged mice, however, both the naïve and PD-1− MP populations exhibited a response comparable to that in young mice; in stark contrast, the PD-1+ MP population exhibited no detectable proliferation, despite normal expression of αβTCR/CD3 (Fig. 1B). They showed no anexin V staining, indicating that they were not dying cells in vivo [supporting information (SI) Fig. S1A]. Costimulation with anti-CD28 antibody or IL-2 restored the proliferation only marginally (Fig. S1B). Furthermore, these T cells revealed negligible production of typical T cell lymphokines in response to optimal TCR stimulation (Fig. S1C). PD-1+ MP CD4+ T cells showed a severely impaired repopulation capacity in Rag-2−/− mice (Fig. 1C, Upper Left). Because robust T cell expansion in Rag-2−/− recipients mainly represents the response to exogenous antigens, such as commensal bacteria (13), these T cells are suggested to be defective in TCR-mediated proliferation in vivo as well. On the other hand, PD-1+ MP CD4+ T cells exhibited a homeostatic expansion comparable with the PD-1− T cells in γ-ray–irradiated recipients (Fig. 1C, Lower Left); in agreement with this finding, a significant proportion of PD-1+ CD4+ T cells expressed IL-15R and/or IL-7R (Fig. 1C, Right). Although PD-1+ cells represent a rare population in CD4+ T cells until 6 months of age, their numbers increase linearly throughout later stages, and they eventually become a predominant population at the senescent stage (Fig. 1D). These T cells are found in most lymphoid tissues of aged mice, except for the peripheral blood (Fig. S1D), and the profile of TCR-Vβ chain usage remains unchanged (Fig. S1E). Our DNA microarray analysis (see below) revealed that PD-1+ MP CD4+ T cells overexpressed Cd121b, and that a portion of these T cells selectively expressed CD121b (Fig. S2A). PD-1−/− CD121b+ CD4+ T cells remained defective in TCR-mediated proliferation, suggesting that PD-1 expression might be irrelevant for the effect (Fig. S2A). Stimulation of PD-1+ MP CD4+ T cells with phorbol myristate acetate (PMA) plus ionomycin bypassing TCR stimulation also failed to induce significant proliferation, despite nearly normal ERK activation (Fig. S2B). Finally, PD-1+ MP CD4+ T cells with complete depletion of CD25+ cells showed no TCR-mediated proliferation or inhibitory effect on the proliferation of normal CD4+ T cells (Fig. S2C). These results suggest that the defect in TCR-mediated proliferation may be intrinsic.
Fig. 1.
Age-dependent increase in PD-1+ MP CD4+ T cells with defective TCR-mediated proliferation. (A) Spleen cells from normal B6 mice at various ages were 3-color–analyzed with the indicated antibodies. (B) CD4+ T cells from 2-month-old (open column) and 16-month-old (solid column) mice were cultured in the presence of anti-CD3 antibody for 3 days and pulsed with 3H-TdR (Left). CD4+ T cells from 16-month-old mice were separated into CD44low (light-gray column), PD-1− CD44high (dark-gray column), and PD-1+ CD44high (solid column) populations and cultured similarly (Middle). *P < .05; **P < .01. The latter 2 populations were analyzed for TCRβ and CD3 expression (Right). (C) Sorted PD-1− and PD-1+ CD44high CD4+ T cells from aged mice were transferred into Rag2−/− mice (Upper Left) or γ-ray–irradiated CD45.1 B6 mice (Lower Left) intravenously, and 6 days later the donor T cells (boxed) in the pooled lymphoid tissues were assessed. The percentage of donor cells out of the total and CD4+ T cells in Rag2−/− and irradiated B6 recipients are indicated. Similar results were obtained in 3 recipients. T cells from normal aged B6 mice were 3-color–analyzed with the indicated antibodies (Right). (D) The proportions of total CD44high (dotted lines), PD-1− CD44high (open circles), and PD-1+ CD44high (closed circles) T cells in the CD4+ T cell population at various ages are plotted.
Unique Genetic Signature and Potent OPN Production.
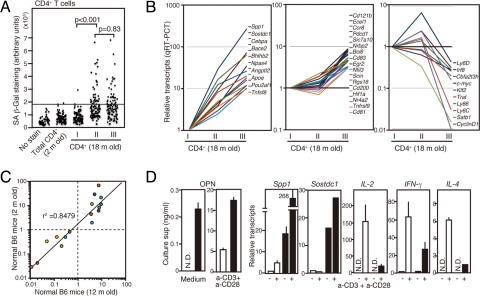
Given the age-dependency, we examined the possibility that PD-1+ MP CD4+ T cells might represent “senescent” T cells, using senescence-associated β-galactosidase (SA-β-Gal) (19). Although CD4+ T cells from 2-month-old mice rarely showed SA-β-Gal staining, a significant proportion of MP, but not naïve, CD4+ T cells from 18-month-old mice revealed positive staining; however, there was no significant difference in the frequencies and the intensities between the PD-1− and PD-1+ MP fractions (Fig. 2A). We then compared the global gene expression profiles by DNA microarray analysis (Table S1). We selected 37 genes from the data set and compared their expression among naïve, PD-1− MP, and PD-1+ MP fractions of CD4+ T cells by qRT-PCR (Fig. 2B). Twenty-seven genes showed markedly increased expression in a PD-1+ MP population, of which 17 genes, including Pdcd1 and Cd121b, were overexpressed rather selectively and 10 genes exhibited decreased expression. PD-1+ MP CD4+ T cells were found only minimally in young mice (see Fig. 1A); however, the genetic signature of these cells highly coincided with those in aged mice (Fig. 2C), indicating that these T cells began to emerge early in life. The most overexpressed gene was Spp1, encoding OPN (also called Eta-1). In agreement, PD-1+, but not PD-1−, MP CD4+ T cells spontaneously secreted significant amounts of OPN. Furthermore, these cells exhibited significantly enhanced OPN secretion on TCR stimulation associated with a robust increase in Spp1 transcripts, whereas the induction of Il-2, Ifn-γ, and Il-4 was significantly compromised (Fig. 2D). These T cells also showed abundant transcripts of Sostdc-1, encoding a secreted antagonist of anti-inflammatory bone morphogenic factor (Fig. 2D).
Fig. 2.
PD-1+ MP CD4+ T cells show a distinctive genetic signature. (A) CD4+ T cells from 2-month-old B6 mice and sorted CD44low (I), PD-1− CD44high (II), and PD-1+ CD44high (III) CD4+ T cell populations from 18-month-old B6 mice were stained for SA-β-Gal. The arbitrary signal units of about 100 cells in each group are plotted. (B) Sorted CD44low (I), PD-1− CD44high (II), and PD-1+ CD44high (III) CD4+ T cell populations from aged mice were analyzed for the expression of 43 genes by qRT-PCR. Relative transcripts in the 3 populations are indicated. (C) PD-1− and PD-1+ CD44high CD4+ T cells sorted from 2-month-old and 12-month-old mice were analyzed for the transcripts of 16 genes by qRT-PCR. The ratios of transcripts (PD-1+/PD-1− CD44high CD4+ T cells) for each gene in the 2 groups are plotted against one another. (D) Sorted PD-1− (open columns) and PD-1+ (solid columns) CD44high CD4+ T cells from aged mice were cultured with or without anti-CD3 plus anti-CD28 antibodies. OPN in the culture supernatants was assessed on day 3 by ELISA. Aliquots of the cells were harvested on day 1, and the transcripts of the indicated genes were assessed by qRT-PCR. ND, not detectable.
Unusual Expression of C/EBPα: A Role in the Unique Functional Features.
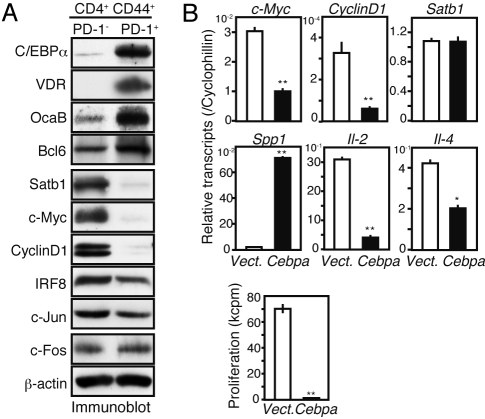
PD-1+ MP CD4+ T cells also showed significantly altered expression of several transcription-related genes (see Fig. 2B). Immunoblot analyses confirmed that in these cells, C/EBPα, VDR, OcaB, and Bcl6 were strongly expressed, whereas Satb1, c-Myc, and cyclin D1 were hardly detectable (Fig. 3A). Because abundant expression of C/EBPα was particularly unexpected, we examined the effects of forced Cebpa expression in regular CD4+ T cells. Young CD4+ T cells were stimulated with anti-CD3 plus anti-CD28 antibodies and infected with Cebpa-containing MIG [murine stem cell virus/internal ribosome entry site/green fluorescent protein (GFP)]. Sorted GFP+ cells exhibited a largely comparable level of Cebpa transcripts with natural PD-1+ MP CD4+ T cells. The Cebpa-expressing CD4+ T cells revealed significant repression of c-Myc, cyclin D1, Il-2, and Il-4, with undetectable proliferation (Fig. 3B). In contrast, these T cells revealed robust activation of Spp1, while Satb1 transcripts were unaffected (Fig. 3B). Although the detection of these proteins was unfeasible because of very limited cell numbers, our findings suggest that C/EBPα expression may be responsible, at least in part, for the unique functional features of PD-1+ MP CD4+ T cells.
Fig. 3.
Expression of Cebpa in regular CD4+ T cells recapitulates the functional features of PD-1+ MP CD4+ T cells. (A) Sorted PD-1− and PD-1+ CD44high CD4+ T cells from aged mice were immunoblotted with the indicated antibodies. (B) Sorted CD4+ T cells from young mice were cultured with anti-CD3 plus anti-CD28 antibodies and infected with an empty (open columns) or Cebpa-containing (solid columns) retrovirus (MIG) on day 1. GFP+ T cells were sorted on day 3 and assessed for transcripts of the indicated genes by qRT-PCR (Upper). The cells were cultured in the presence of IL-2 for 2 more days and pulsed with 3H-TdR (Lower). **P < .01; *P < .05.
Rapid and Robust Increase in PD-1+ MP CD4+ T Cells During Leukemia.
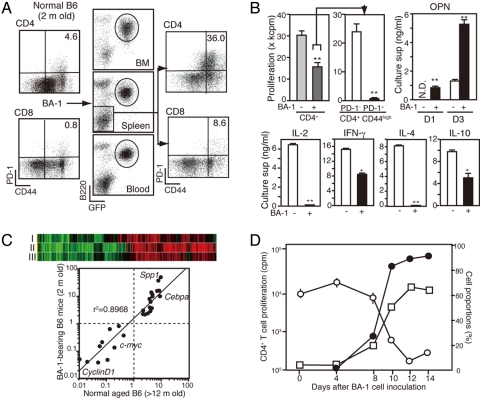
A global diminution of specific T cell response often develops in malignancy. To investigate the possible involvement of PD-1+ MP CD4+ T cells, we transplanted bcr/abl-induced leukemia cells (BA-1) into young B6 mice. Within 2 weeks, as frank leukemia developed, the recipients exhibited a marked increase in PD-1+ MP cells, preferentially in the CD4+ T cell population (Fig. 4A). The CD4+ T cells from leukemic mice showed significantly diminished TCR-mediated proliferation, specifically attributed to the defective response of PD-1+ MP cells (Fig. 4B). Moreover, OPN secretion on TCR stimulation was far greater in PD-1+ MP CD4+ T cells than in PD-1− CD4+ T cells, whereas the production of T cell lymphokines was compromised (Fig. 4B). Similar results were obtained with an independent leukemia cell line. Because PD-L1 was hardly or only marginally expressed on these leukemia cells (Fig. S3A), it does not appear to be relevant to the effects. Spa-1−/− mice, which spontaneously developed various leukemias (20, 21) also exhibited a remarkable increase in PD-1+ MP CD4+ T cells after the development of leukemia (Fig. S3B and C). DNA microarray cluster and quantitative RT-PCR (qRT-PCR) analysis revealed highly coincided gene expression profiles between the PD-1+ MP CD4+ T cells in leukemic and normal aged mice (Fig. 4C). Kinetic analysis indicated that the proportions of PD-1+ MP CD4+ T cells rapidly increased with the systemic spread of leukemia; this was inversely correlated with the diminution of TCR-mediated CD4+ T cell proliferation (Fig. 4D).
Fig. 4.
Senescence-related PD-1+ MP CD4+ T cells are generated robustly during leukemia. (A) Young B6 mice were inoculated intravenously with BA-1 leukemia cells, and the cells of bone marrow, spleen, and blood from mice at 2 weeks posttransplantation were 3-color–analyzed with the indicated antibodies. Circles indicate the leukemia cells. (B) CD4+ T cells from mice pretransplantation (light-gray column) and posttransplantation (dark-gray column) were cultured with anti-CD3 antibody for 3 days to assess DNA synthesis (Upper Left). The CD4+ T cells from leukemia-bearing mice were separated into PD-1− (open column) and PD-1+ (solid columns) CD44high populations and cultured similarly (Upper Middle). The indicated cytokines in the culture supernatants were assessed by ELISA (Upper Right and Lower). **P < .01; *P < .05. (C) DNA microarray data for PD-1− and PD-1+ CD44high CD4+ T cells from normal aged and BA-1–bearing young mice were subjected to cluster analysis with the combinations of PD-1+ [aged]/PD-1− [aged] (I), PD-1+ [leukemic]/PD-1− [aged] (II), and PD-1+ [leukemic]/PD-1− [leukemic] (III) (Upper). The ratios of transcripts (PD-1+/PD-1− populations) of 16 genes in the 2 groups are plotted against one another (Lower). (D) The proportions of leukemia cells in the blood (solid circles) and PD-1+ CD44high cells in the splenic CD4+ T cells (open squares), as well as the TCR-mediated proliferation of CD4+ T cells (open circles), were assessed at various times after the inoculation with BA-1 cells. The mean values of 3 mice are shown.
Emergence of PD-1+ CD4+ T MP Cells Is Correlated With Clonal T Cell Immunodepression in Senescence and During Leukemia.
Tg mice of OVA-specific TCR (OT-II) at Rag2−/− background developed very few MP T cells and, accordingly, few PD-1+ cells even at age 12 months (Fig. 5A, Upper). The Rag2−/− OT-II cells revealed no diminution of, but rather exhibited an increase in, OVA-specific proliferation compared with those from young mice (Fig. 5A, Lower). Similarly, OT-II/Rag2−/− mice transplanted with BA-1 cells developed few PD-1+ MP CD4+ T cells, with no diminution of OVA-specific T cell response, despite a massive leukemia burden (Fig. 5A). In contrast, aged OT-II Tg mice at a B6 background exhibited a marked increase in PD-1+ MP CD4+ T cells, including Vα2+ OT-II T cells (Fig. 5B, Upper). Here PD-1− OT-II T cells showed significant OVA-specific proliferation, but PD-1+ MP OT-II T cells showed no detectable proliferation (Fig. 5B, Lower). Transplantation of BA-1 cells in young OT-II/B6 mice caused even more prominent increases in PD-1+ MP OT-II T cells, with a profoundly diminished OVA-specific response (Fig. 5B). These results suggest that at the clonal level, the generation of PD-1+ cells occurs concomitantly with the development and expansion of MP CD4+ T cells, and that the increased proliferation of PD-1+ cells underlies T cell immunodepression with age and during leukemia.
Fig. 5.
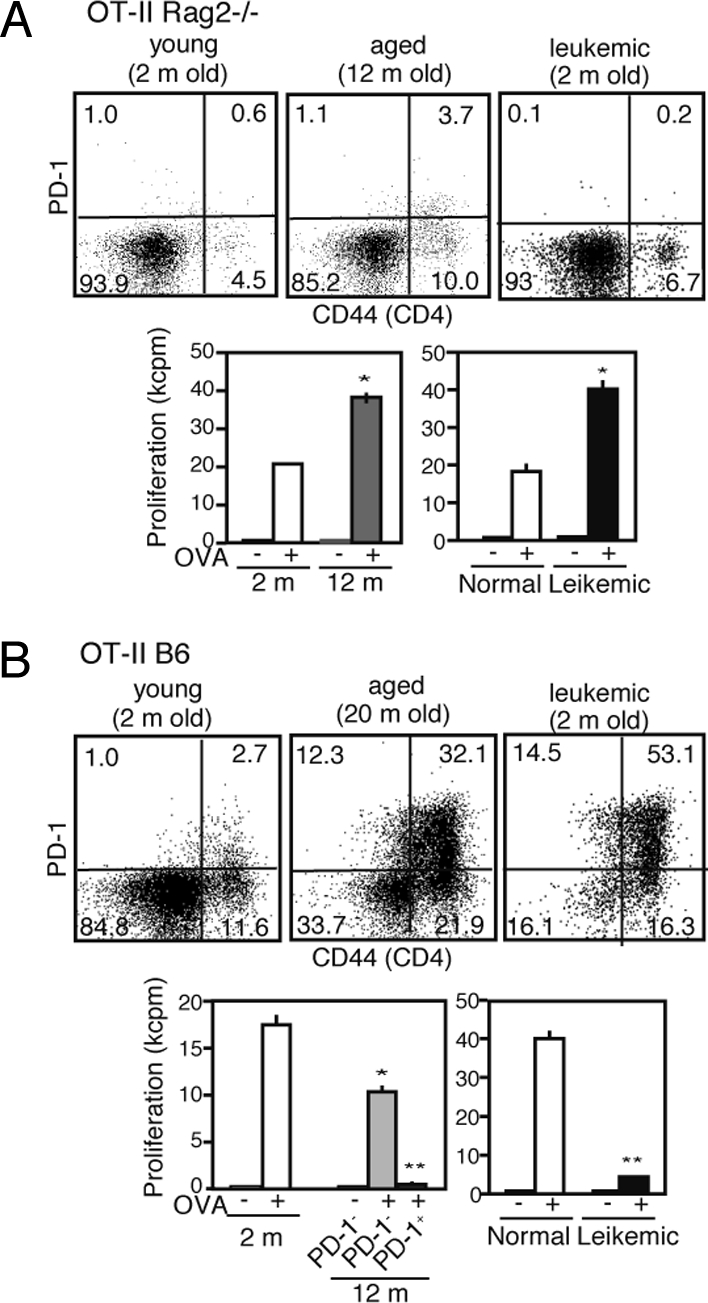
Emergence of PD-1+ CD4+ T MP cells is correlated with clonal T cell immunodepression in senescence and during leukemia. (A) Spleen cells from normal young (open columns), aged (gray columns), and leukemia-bearing young (solid columns) OT-II/Rag2−/− mice were 3-color–analyzed with the indicated antibodies. The CD4+ cells were sorted from each group and cultured in the presence of irradiated B6 spleen cells with or without OVA for 3 days. *P < .01. (B) Identical experiments were performed in OT-II/B6 mice, except that PD-1− (light-gray columns) and PD-1+ (dark-gray columns) CD44high CD4+ T cells were examined separately in the aged mice. *P < .05; **P < .01.
Discussion
Naturally arising MP CD4+ T cells appear to be composed of heterogeneous populations, only a fraction of which are functional memory T cells (4). In this study, we have identified a distinct population of MP CD4+ T cells that constitutively expresses PD-1 and partially expresses CD121b and hardly proliferates or produces typical T cell lymphokines on TCR stimulation in vitro. PD-1+ MP CD4+ T cells have a severely impaired repopulation capacity in Rag2−/− mice, in which regular T cells exhibit a robust expansion in response to exogenous antigens, particularly commensal bacteria (13). Our results suggest that these T cells have a defective antigen-specific response in vivo, although the possibility of a selective susceptibility to host NK cells, as has been found in certain CD4+ T cells (22), remains to be examined. In contrast, PD-1+ MP CD4+ T cells exhibit repopulation in irradiated B6 recipients comparable to that of PD-1− CD4+ T cells, suggesting that these cells respond to homeostatic cytokines. Thus, PD-1+ CD4+ T cells may represent a unique MP cell population capable of homeostatic proliferation with little contribution to antigen-specific immune response based on the clonal expansion.
Importantly, PD-1+ MP CD4+ T cells steadily increase in number with age in normal mice, becoming a predominant population at the senescent stage. But despite this age-dependent increase, analysis of SA-β-Gal expression argues against the possibility that these cells simply represent a classic cell senescence; rather, the generation of these cells is suggested to be concomitant with the homeostatic proliferation of PD-1− MP CD4+ T cells. The generation of PD-1+ MP CD4+ T cells appears to be associated with a unique genetic reprogramming. A notable molecular feature of these cells is the strong expression of C/EBPα, which is normally expressed in nonlymphoid cells, including myeloid cells (15). C/EBPα exerts a potent antiproliferative effect, either reversibly, by interacting with Cdk inhibitors, or irreversibly, by repressing c-Myc (15, 23). In agreement with this, forced expression of Cebpa in regular CD4+ T cells results in the loss of TCR-mediated proliferation capacity with the repression of c-Myc and cyclin D1. It also induces prominent activation of Spp1 while inhibiting T cell lymphokine gene activation, although the role of endogenous C/EBPα in natural PD-1+ MP CD4+ T cells remains to be confirmed. Interestingly, artificial expression of Cebpa in thymic pre-T cells is reported to redirect their differentiation into myeloid cells with the repression of T cell–specific genes (17). In this respect, PD-1+ MP CD4+ T cells exhibit profound repression of Satb1, a T cell–specific gene crucial to the development and function of T cells (24, 25). Satb1−/− T cells also are reported to show enhanced Pdcd1 expression (25); thus, the constitutive PD-1 expression may be due in part to Satb1 repression. Unlike in “exhausted” CD8+ T cells generated during chronic viral infection (26), our results suggest no relevant involvement of PD-1 in the defective proliferation of PD-1+ MP CD4+ T cells; however, other possible effects of PD-1 ligated by PD-L remain to be investigated.
PD-1+ MP CD4+ T cells with equivalent genetic and functional features to those in senescence are robustly increased during leukemia, suggesting that the senescence-related cellular and genetic changes in CD4+ T cells are rapidly accelerated in malignancy. Human patients with leukemia often exhibit profound T cell immunodepression; a recent report indicates that this may be due in part to the changes in the gene expression of T cells on interaction with leukemia cells (27). Intriguingly, OT-II Rag2−/− mice hardly developed PD-1+ MP CD4+ T cells, and these mice maintained OVA-specific responsiveness with age and during leukemia. In contrast, OT-II B6 mice exhibited a marked increase in the PD-1+ MP cell population similar to normal B6 mice in both conditions, resulting in profoundly diminished OVA responsiveness. The reason for this difference remains to be investigated; it may be that endogenous TCRs expressed on B6 OT-II T cells are required for the development of PD-1+ MP cells. Nonetheless, our results indicate that the increase in PD-1+ MP cell proportion is directly associated with the progression of CD4+ T cell immunodepression in senescence and during leukemia.
OPN is a potent proinflammatory cytokine produced by many cell types, including macrophages, dendritic cells, and activated Th1 cells, that plays a significant role in inflammatory diseases (28, 29). Our results suggest that PD-1+ MP CD4+ T cells also may serve as a potent OPN producer, either spontaneously or inductively via TCR stimulation. The OPN production by these T cells seems to be controlled by C/EBPα, whereas that by Th1 cells is under the regulation of T-bet (30). Recently, T cell–intrinsic OPN was reported to function as an autocrine prosurvival factor for CD4+ T cells in vivo (31); thus, OPN also may contribute to the maintenance of PD-1+ MP CD4+ T cells in vivo. In addition, OPN may affect the growth and invasion of malignant cells, directly or indirectly, by recruiting host inflammatory cells (32, 33). As such, these T cells may contribute to a proinflammatory trait in the elderly, as well as tumor progression. Manipulation of PD-1+ MP CD4+ T cells may provide an avenue for restoring the adverse immune traits in senescence and malignancy.
Materials and Methods
See SI Text for details on the materials and methods used in this study.
Mice.
The C57BL/6 (B6), B6 (CD45.1), Rag2−/−, SPA-1−/−, PD-1−/−, and OT-II Tg mice, all at B6 background, were maintained in specific pathogen-free conditions at Kyoto University's Laboratory Animals Center in accordance with University guidelines.
Flow Cytometry and Cell Culture.
Multicolor flow cytometric analysis and cell sorting were performed with FACSCalibur and FACSAria (BD Biosciences). Cell proliferation was assessed by the incorporation of 3H-thymidine, and cytokines were measured by ELISA.
Cell Transfers.
Purified CD4+ T cell subpopulations were injected intravenously into Rag2−/− or γ-ray (6 Gy)-irradiated B6 (CD45.1) mice (3 × 106 cells/head). BA-1 leukemia cells were transplanted intravenously into normal B6 mice (1∼5 × 106 cells/head).
qRT-PCR Analysis.
qRT-PCR was performed with LightCycler SYBR Green I marker kit on a LightCycler instrument (Roche).
SA-β-Gal Staining.
T cells were plated on polyL-lysine–coated cover slips, fixed with glutaraldehyde (0.5% in PBS), washed with Mg2+-containing PBS, and stained with X-Gal in PBS containing K3Fe(CN)6, K4Fe(CN)6, and Mg2+.
Gene Transduction.
Cebpa cDNA, provided by Dr. Iwama (Tsukuba University), was subcloned into retroviral plasmid (pMCs IRES GFP; pMIG), provided by Dr. Kitamura, University of Tokyo. Recombinant retrovirus was produced in Plat-E packaging cells.
DNA Microarray and Clustering Analysis.
Comprehensive DNA microarray analysis was performed with 3D-Gene (Toray Industries). Microarrays were scanned with the ScanArray Lite Scanner (Perkin-Elmer) and analyzed using Cluster 3.0.
Statistical Analysis.
All statistical analyses were performed using the 2-tailed Student t test.
Supplementary Material
Acknowledgments.
We thank T. Sudo and H. Akiyama (Toray New Frontiers Research Laboratories) for assisting with the DNA microarray analysis, K. Aoki and A. Nabetani for assisting with SA-β-Gal staining analysis, and A. Iwama and T. Kitamura for providing the plasmids. This work was supported by grants from the Japanese Ministry of Education, Culture, Science, Sports and Technology and the Takeda Science Foundation.
Footnotes
The authors declare no conflicts of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0908805106/DCSupplemental.
References
- 1.Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nat Immunol. 2004;5:133–139. doi: 10.1038/ni1033. [DOI] [PubMed] [Google Scholar]
- 2.Globerson A, Effros RB. Ageing of lymphocytes and lymphocytes in the aged. Immunol Today. 2000;21:515–521. doi: 10.1016/s0167-5699(00)01714-x. [DOI] [PubMed] [Google Scholar]
- 3.Haynes BF, et al. The role of the thymus in immune reconstitution in aging, bone marrow transplantation, and HIV-1 infection. Annu Rev Immunol. 2000;18:529–560. doi: 10.1146/annurev.immunol.18.1.529. [DOI] [PubMed] [Google Scholar]
- 4.Surh CD, Boyman O, Purton JF, Sprent J. Homeostasis of memory T cells. Immunol Rev. 2006;211:154–163. doi: 10.1111/j.0105-2896.2006.00401.x. [DOI] [PubMed] [Google Scholar]
- 5.Seddon B, Tomlinson P, Zamoyska R. Interleukin 7 and T cell receptor signals regulate homeostasis of CD4 memory cells. Nat Immunol. 2003;4:680–686. doi: 10.1038/ni946. [DOI] [PubMed] [Google Scholar]
- 6.Kondrack RM, et al. Interleukin 7 regulates the survival and generation of memory CD4 cells. J Exp Med. 2003;198:1797–1806. doi: 10.1084/jem.20030735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwab R, et al. Expanded CD4+ and CD8+ T cell clones in elderly humans. J Immunol. 1997;158:4493–4499. [PubMed] [Google Scholar]
- 8.Miller RA, Garcia G, Kirk CJ, Witkowski JM. Early activation defects in T lymphocytes from aged mice. Immunol Rev. 1997;160:79–90. doi: 10.1111/j.1600-065x.1997.tb01029.x. [DOI] [PubMed] [Google Scholar]
- 9.Garcia GG, Miller RA. Single-cell analyses reveal two defects in peptide-specific activation of naive T cells from aged mice. J Immunol. 2001;166:3151–3157. doi: 10.4049/jimmunol.166.5.3151. [DOI] [PubMed] [Google Scholar]
- 10.Eaton SM, et al. Age-related defects in CD4 T cell cognate helper function lead to reductions in humoral responses. J Exp Med. 2004;200:1613–1622. doi: 10.1084/jem.20041395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dayan M, et al. Effect of aging on cytokine production in normal and experimental systemic lupus erythematosus–afflicted mice. Exp Gerontol. 2000;35:225–236. doi: 10.1016/s0531-5565(00)00076-0. [DOI] [PubMed] [Google Scholar]
- 12.Haynes L, et al. Newly generated CD4 T cells in aged animals do not exhibit age-related defects in response to antigen. J Exp Med. 2005;201:845–851. doi: 10.1084/jem.20041933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kieper WC, et al. Recent immune status determines the source of antigens that drive homeostatic T cell expansion. J Immunol. 2005;174:3158–3163. doi: 10.4049/jimmunol.174.6.3158. [DOI] [PubMed] [Google Scholar]
- 14.Lenz DC, et al. IL-7 regulates basal homeostatic proliferation of antiviral CD4+ T cell memory. Proc Natl Acad Sci USA. 2004;101:9357–9362. doi: 10.1073/pnas.0400640101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson PF. Molecular stop signs: Regulation of cell-cycle arrest by C/EBP transcription factors. J Cell Sci. 2005;118:2545–2555. doi: 10.1242/jcs.02459. [DOI] [PubMed] [Google Scholar]
- 16.Zhang P, et al. Enhancement of hematopoietic stem cell repopulating capacity and self-renewal in the absence of the transcription factor C/EBP alpha. Immunity. 2004;21:853–863. doi: 10.1016/j.immuni.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 17.Laiosa CV, et al. Reprogramming of committed T cell progenitors to macrophages and dendritic cells by C/EBP alpha and PU.1 transcription factors. Immunity. 2006;25:731–744. doi: 10.1016/j.immuni.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 18.Okazaki T, Honjo T. The PD-1–PD-L pathway in immunological tolerance. Trends Immunol. 2006;27:195–201. doi: 10.1016/j.it.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Dimri GP, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishida D, et al. Myeloproliferative stem cell disorders by deregulated Rap1 activation in SPA-1–deficient mice. Cancer Cell. 2003;4:55–65. doi: 10.1016/s1535-6108(03)00163-6. [DOI] [PubMed] [Google Scholar]
- 21.Ishida D, et al. Antigen-driven T cell anergy and defective memory T cell response via deregulated Rap1 activation in SPA-1–deficient mice. Proc Natl Acad Sci USA. 2003;100:10919–10924. doi: 10.1073/pnas.1834525100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu L, et al. Regulation of activated CD4+ T cells by NK cells via the Qa-1-NKG2A inhibitory pathway. Immunity. 2007;26:593–604. doi: 10.1016/j.immuni.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iakova P, Awad SS, Timchenko NA. Aging reduces proliferative capacities of liver by switching pathways of C/EBPalpha growth arrest. Cell. 2003;113:495–506. doi: 10.1016/s0092-8674(03)00318-0. [DOI] [PubMed] [Google Scholar]
- 24.Cai S, Lee CC, Kohwi-Shigematsu T. SATB1 packages densely looped, transcriptionally active chromatin for coordinated expression of cytokine genes. Nat Genet. 2006;38:1278–1288. doi: 10.1038/ng1913. [DOI] [PubMed] [Google Scholar]
- 25.Alvarez JD, et al. The MAR-binding protein SATB1 orchestrates temporal and spatial expression of multiple genes during T-cell development. Genes Dev. 2000;14:521–535. [PMC free article] [PubMed] [Google Scholar]
- 26.Barber DL, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 27.Gorgun G, et al. Chronic lymphocytic leukemia cells induce changes in gene expression of CD4 and CD8 T cells. J Clin Invest. 2005;115:1797–1805. doi: 10.1172/JCI24176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang KX, Denhardt DT. Osteopontin: Role in immune regulation and stress responses. Cytokine Growth Factor Rev. 2008;19:333–345. doi: 10.1016/j.cytogfr.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 29.Jansson M, et al. Cutting edge: Attenuated experimental autoimmune encephalomyelitis in eta-1/osteopontin–deficient mice. J Immunol. 2002;168:2096–2099. doi: 10.4049/jimmunol.168.5.2096. [DOI] [PubMed] [Google Scholar]
- 30.Shinohara ML, et al. T-bet–dependent expression of osteopontin contributes to T cell polarization. Proc Natl Acad Sci USA. 2005;102:17101–17106. doi: 10.1073/pnas.0508666102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hur EM, et al. Osteopontin-induced relapse and progression of autoimmune brain disease through enhanced survival of activated T cells. Nat Immunol. 2007;8:74–83. doi: 10.1038/ni1415. [DOI] [PubMed] [Google Scholar]
- 32.El-Tanani MK, et al. The regulation and role of osteopontin in malignant transformation and cancer. Cytokine Growth Factor Rev. 2006;17:463–474. doi: 10.1016/j.cytogfr.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 33.McAllister SS, et al. Systemic endocrine instigation of indolent tumor growth requires osteopontin. Cell. 2008;133:994–1005. doi: 10.1016/j.cell.2008.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.