Abstract
Methylmercury (CH3Hg+) is a serious environmental toxicant. Exposure to this metal during pregnancy can cause serious neurological and developmental defects in a developing fetus. Surprisingly, little is known about the mechanisms by which mercuric ions are transported across the placenta. Although it has been shown that 2,3-dimercaptopropane-1-sulfonate (DMPS) and 2,3-dimercaptosuccinic acid (DMSA) are capable of extracting mercuric ions from various organs and cells, there is no evidence that they are able to extract mercury from placental or fetal tissues following maternal exposure to CH3Hg+. Therefore, the purpose of the current study was to evaluate the ability of DMPS and DMSA to extract mercuric ions from placental and fetal tissues following maternal exposure to CH3Hg+. Pregnant Wistar rats were exposed to CH3HgCl, containing [203Hg], on day 11 or day 17 of pregnancy and treated 24 h later with saline, DMPS or DMSA. Maternal organs, fetuses, and placentas were harvested 48 h after exposure to CH3HgCl. The disposition of mercuric ions in maternal organs and tissues was similar to that reported previously by our laboratory. The disposition of mercuric ions in placentas and fetuses appeared to be dependent upon the gestational age of the fetus. The fetal and placental burden of mercury increased as fetal age increased and was reduced by DMPS and DMSA, with DMPS being more effective. The disposition of mercury was examined in liver, total renal mass, and brain of fetuses harvested on gestational day 19. On a per gram tissue basis, the greatest amount of mercury was detected in the total renal mass of the fetus, followed by brain and liver. DMPS and DMSA reduced the burden of mercury in liver and brain while only DMPS was effective in the total renal mass. The results of the current study are the first to show that DMPS and DMSA are capable of extracting mercuric ions, not only from maternal tissues, but also from placental and fetal tissues following maternal exposure to CH3Hg+.
INTRODUCTION
Methylmercury (CH3Hg+) is a reproductive toxicant that is prevalent in the environment. Humans are often exposed to CH3Hg+ through consumption of contaminated water and/or food, such as predatory fish [1]. Of particular concern is the exposure of pregnant women to CH3Hg+ and the potential deleterious effect(s) that this toxicant may have on the developing fetus. CH3Hg+ in maternal blood readily crosses the placenta and accumulates in fetal and placental tissues [2-7]. The neurological system is a major site of CH3Hg+ toxicity. Therefore, it is not surprising that the fetal brain and neurological system are particularly susceptible to the effects of CH3Hg+, with detrimental fetal effects occurring at concentrations that would not negatively affect the mother [8-10]. Prenatal exposure of fetuses to CH3Hg+ has numerous deleterious effects, including reduced cognitive function, dysarthria, strabismus, and cerebral palsy [11-14]. Given the prevalence of CH3Hg+ in the environment and its potential threat to fetal health, it is of significant clinical importance that we have a thorough understanding of the way in which mercuric ions are handled by the placenta.
Despite the clinical importance of this area, little is known about the molecular mechanisms by which CH3Hg+ crosses the placental barrier. Kajiwara et al. [15] showed that CH3Hg+, as a conjugate of cysteine (Cys; Cys-S-CH3Hg), is transported from maternal blood across the placenta by a neutral amino acid carrier, which was hypothesized to be system L. More recently, in vivo studies in Xenopus laevis oocytes showed that Cys-S-CH3Hg is a substrate of both isoforms of system L, Lat1 (Slc7A5) and Lat2 (Slc7A8) [16]. These findings are consistent with the theory that Cys-S-CH3Hg acts as a molecular mimic of one or more endogenous substrates (e.g. methionine) at the docking site of one or more membrane transporters.
The ability of mercuric ions to be extracted from placental and/or fetal tissues has not been examined thoroughly. Although it is well-established that the metal chelators, 2,3-dimercaptopropane-1-sulfonate (DMPS) and 2,3-dimercaptosuccinic acid (DMSA) can reduce the total body burden of CH3Hg+ [17-20], the ability of either chelator to extract mercuric ions from placental or fetal tissues has not been shown. Interestingly, administration of DMPS to pregnant mice exposed orally to CH3Hg+ appeared to protect fetuses from the toxic effects of methylmercury [21]. Similarly, a separate study in rats suggests that DMPS is capable of extracting mercuric ions from fetal and placental tissues following maternal exposure to inorganic mercury [22]. Currently, there is no evidence indicating that either, DMPS or DMSA is capable of extracting mercuric ions from placental and fetal tissues of dams exposed to a form of organic mercury, CH3Hg+.
The metal chelators, DMPS and DMSA, each possess vicinal thiol groups which facilitate the formation of highly stable DMPS and DMSA-S-conjugates of CH3Hg+. In kidney, it is thought that DMSA, and possibly DMPS, are taken up at the basolateral membrane of renal tubular cells by the sodium dicarboxylic transporter (NaC2; Slc13a3) and/or the organic anion transporter 1 (Oat1; Slc22A6), [23,24] following which, they likely form stable conjugates with intracellular mercury. These conjugates appear to be substrates for endogenous transporters, such as the multidrug resistance-associated protein 2 (Mrp2; Abcc2) [25-27], located on the apical plasma membrane of proximal tubular cells [28]. Given that placental trophoblasts are polarized cells similar to proximal tubular cells in the kidney, we hypothesize that DMPS and DMSA are each capable of extracting mercuric ions from fetal and placental tissues in a manner similar to that which occurs in proximal tubular cells. Therefore, the purpose of the current study was to evaluate the ability of DMPS and DMSA to extract mercuric ions from placental and fetal tissues following maternal exposure to CH3HgCl.
MATERIALS AND METHODS
Animals
Pregnant Wistar rats weighing 250-275 g were purchased from Harlan Laboratories (Indianapolis, IN). There were no significant differences in body weight among the animals used for these studies. Animals were provided a commercial laboratory diet (Tekland 6% rat diet, Harlan Laboratories) and water ad libitum throughout all aspects of experimentation. Animals were handled in accordance with the Guide for the Care and Use of Laboratory Animals as adopted by the National Institutes of Health.
Intravenous Injections
Rats were injected intravenously (i.v.) according to our previously published protocol [25,26,29,30]. Each animal was anesthetized lightly with ether and a small incision was made in the skin in the mid-ventral region of the thigh to expose the femoral vein and artery. The fascia around the femoral vein was trimmed and a non-nephrotoxic dose of CH3HgCl (5 mg/kg in 2 mL normal saline containing 1 μCi of CH3[203Hg] per rat) was administered into the vein. The wound was closed using two 9-mm stainless steel wound clips. CH3[203Hg] (6 - 12 mCi/mg) was generated using a method described previously [26,31].
Experimental Design
Two separate experiments were performed. For the first experiment, 15 pregnant rats were injected (i.v.) with CH3HgCl on day 11 of pregnancy and were sacrificed on day 13 (E13). Animals at this stage of pregnancy were chosen because rapid brain development occurs at this point in the gestational period. For the second experiment, 15 pregnant rats were injected (i.v.) with CH3HgCl on day 17 of pregnancy and were sacrificed on day 19 (E19). These animals were chosen in order to examine the disposition of mercuric ions in fetuses that were near the end of their gestational period, which is 20-21 days for rats. The steps following administration of CH3HgCl were carried out identically in both sets of rats. Following injection with CH3HgCl, each set of rats was divided randomly into three groups of five rats each. Animals were then placed in individual plastic metabolic cages. Twenty-four hours after the injection of CH3HgCl, each of the five rats in group 1 was injected i.v. with a 200 mg/kg dose of DMPS (in 2 mL/kg normal saline). At the same time, each of the five rats in group 2 was injected i.v. with a 200 mg/kg dose of DMSA (in 2 mL/kg normal saline). Each of the remaining five rats (group 3) was injected i.v. with normal saline (2 mL/kg). Forty-eight h after injection with CH3HgCl (either day 13 or day 19), rats were sacrificed.
Collection of Fetuses, Tissues, Organs, Urine and Feces
At the time of sacrifice, pregnant rats were anesthetized with an intraperitoneal overdose of ketamine and xylazine (70/30 mg/kg in 2 mL saline). A 1-mL sample of blood was obtained from the inferior vena cava with a 3-mL syringe and a 20-gauge needle and placed in a polystyrene tube for estimation of [203Hg] content. Total blood volume was estimated to be 6% of body weight.
The liver and kidneys were also removed from each pregnant rat. Each kidney was weighed and cut in half along a transverse plain. A 3-mm transverse slice of the left kidney was utilized for separation of cortex, outer stripe of outer medulla, inner stripe of outer medulla and inner medulla. Each zone of the kidney was weighed and placed in a separate polystyrene tube for estimation of [203Hg] content. The liver was then excised, weighed, and a 1-g section of liver was removed for determination of [203Hg] content. The brain was also removed, weighed, and placed in a glass scintillation vial for estimation of [203Hg] content.
Urine and feces were collected throughout the duration of each experiment. The urine excreted by each animal was collected 24 h and 48 h after injection with CH3HgCl. Subsequently the urine from each animal was mixed and a 1-mL sample was weighed and placed in a polystyrene tube for estimation of [203Hg] content. All of the feces excreted by each animal during each 24-h period were counted to determine accurately the total fecal content of [203Hg]. The content of [203Hg] in each sample collected was determined by counting the samples in a Wallac Wizard 3 automatic gamma counter (Perkin Elmer, Boston, MA).
The uterus of each anesthetized animal was removed and individual fetuses and placentas were extracted. The number of fetuses and placentas harvested from each dam was 11-18. Each placenta was weighed and placed in a polystyrene tube for estimation of [203Hg] content. In addition, each fetus was weighed, decapitated, placed in 3 mL of 80% EtOH in a glass scintillation vial. In experiments where rats were sacrificed on day 19 of pregnancy, individual fetuses were dissected to remove the brain, kidneys and liver. Each organ was weighed and placed in separate polystyrene tubes for the determination of [203Hg] content.
Data Analyses
Data for each experiment were analyzed first with the Kolmogorov-Smirnov test for normality and then with Levene’s test for homogeneity of variances. Data were then analyzed using a 2 × 2 two-way analysis of variance (ANOVA) to assess differences among the means. When statistically significant F-values were obtained with ANOVA, the data were analyzed using Tukey’s post hoc multiple comparison test. A p-value of < 0.05 was considered statistically significant. Each group of animals contained 5 rats, with 11-18 fetuses per rat.
RESULTS
Content of Mercury in Maternal Tissues
Renal Burden of Mercury
The amount of mercury in the total renal mass of pregnant Wistar rats exposed to 5 mg/kg CH3HgCl on day 17 of pregnancy is shown in Figure 1. Approximately 16% of the administered dose was detected in the total renal mass of dams exposed to CH3HgCl and treated subsequently with saline. Treatment of rats with 200 mg/kg 140 DMPS reduced in the renal burden of mercury by approximately 70%. When rats were treated with the same dose of DMSA, the renal burden of mercury was reduced by about 50%.
Figure 1.
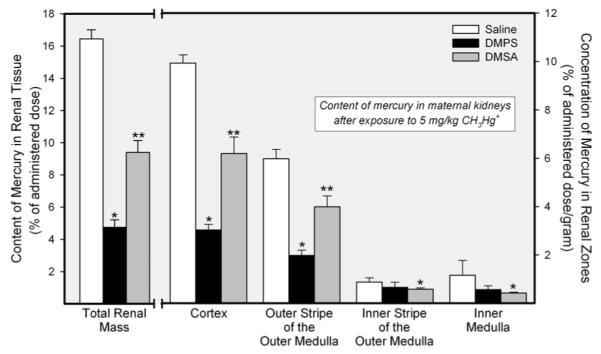
Amount of mercury in renal tissue of pregnant Wistar rats exposed to 5 mg/kg CH3HgCl on day 17 of pregnancy and treated 24 h later with saline (2 mL/kg), DMPS or DMSA (200 mg/kg). Kidneys were harvested 48 h after exposure to CH3HgCl. Data represent mean ± SE of five rats. *, significantly different (p < 0.05) from the corresponding mean for rats treated with saline. **, significantly different (p < 0.05) from the corresponding mean for rats of all other treatment groups.
The disposition of mercuric ions in each of the four renal zones is also shown in Figure 1. The greatest amount of mercury was detected in the cortex, followed by the outer stripe of the outer medulla. The content of mercury in the inner stripe of the outer medulla and in the inner medulla was significantly lower than that in the other two zones. Treatment of dams with DMPS reduced significantly the amount of mercury in the cortex and the outer stripe of the outer medulla. Similarly, DMSA also reduced the amount of mercury in the cortex and outer stripe of the outer medulla. The renal disposition of mercuric ions in pregnant rats exposed to CH3HgCl on day 17 of pregnancy (Figure 1) was not significantly different from that of rats exposed to CH3HgCl on day 11 of pregnancy (data not shown).
Hepatic and Hematological Burden of Mercury
Figure 2 shows the content of mercury in liver and blood of pregnant rats exposed to CH3HgCl on day 17 of pregnancy. Approximately 6% of the administered dose of mercury was detected in the livers of dams exposed to CH3HgCl and treated subsequently with saline. Treatment of rats with either DMPS or DMSA reduced the hepatic burden of mercury by approximately 40%. The content of mercury in blood following exposure of dams to CH3HgCl was nearly 30% of the administered dose. Both, DMPS and DMSA reduced significantly the amount of mercury in blood, with DMPS having the greatest effect. There were no significant differences in the hepatic or hematologic handling of mercuric ions between rats exposed to CH3HgCl on day 17 of pregnancy (Figure 2) and rats exposed to CH3HgCl on day 11 of pregnancy (data not 165 shown).
Figure 2.
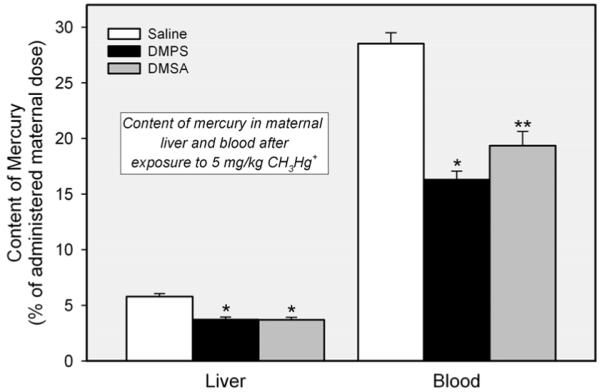
Content of mercury in liver and blood of pregnant Wistar rats exposed to 5 mg/kg CH3HgCl on day 17 of pregnancy and treated 24 h later with saline (2 mL/kg), DMPS, or DMSA (200 mg/kg). Livers and blood were harvested 48 h after exposure to CH3HgCl. Data represent mean ± SE of five rats. *, significantly different (p < 0.05) from the corresponding mean for rats treated with saline. **, significantly different (p < 0.05) from the corresponding mean for rats of all other treatment groups.
Urine and Fecal Excretion of Mercury
The amount of mercury excreted in urine of pregnant rats exposed to CH3HgCl and treated subsequently with DMPS or DMSA was significantly greater than that of rats 170 exposed to CH3HgCl and treated with saline (Figure 3). Treatment of dams with DMPS resulted in a 20-fold increase the urinary excretion of mercury, while treatment with DMSA increased the urinary excretion of mercuric ions by approximately 14-fold.
Figure 3.
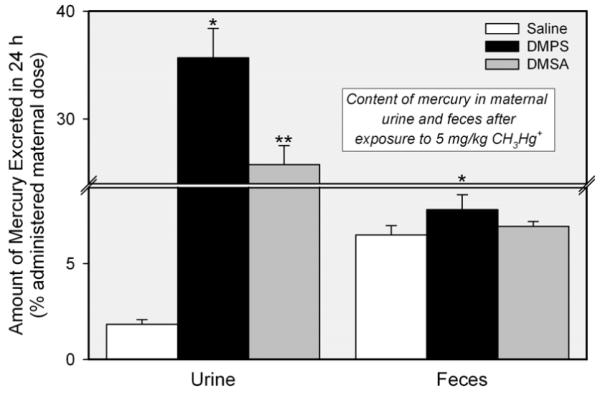
Amount of mercury excreted in urine and feces of pregnant Wistar rats exposed to 5 mg/kg CH3HgCl on day 17 of pregnancy and treated 24 h later with saline (2 mL/kg), DMPS, or DMSA (200 mg/kg). Data represent the amount of mercury in urine and feces collected during the 24 h following treatment with DMPS or DMSA. Data represent mean ± SE of five rats. *, significantly different (p < 0.05) from the corresponding mean for rats treated with saline. **, significantly different (p < 0.05) from the corresponding mean for rats of all other treatment groups.
The fecal content of mercury in rats exposed to CH3HgCl and treated subsequently with saline was approximately 6% of the administered dose (Figure 3). Following treatment with DMPS, the fecal excretion of mercury increased slightly, albeit significantly; fecal excretion remained unchanged following treatment with DMSA. The disposition of mercury was not significantly different between rats exposed to CH3HgCl on day 11 of pregnancy (data not shown) and those exposed on day 17 (Figure 3).
Content of Mercury in Brain
The amount of mercury detected in brains of rats exposed to CH3HgCl was less than 1% of the administered dose (data not shown). Neither DMPS nor DMSA altered significantly the content of mercury in the brain (data not shown).
Content of Mercury in Placental and Fetal Tissues
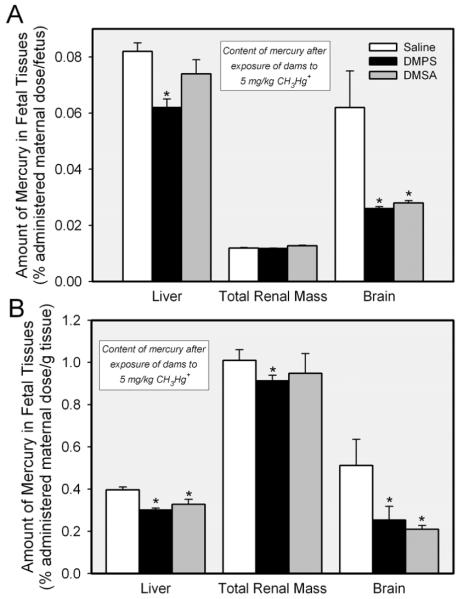
Figure 4 shows the amount of mercury present in placentas and fetuses from dams exposed to CH3HgCl on either day 11 or day 17 of their pregnancies. Fetuses and placentas were harvested on embryonic day 13 (E13) or 19 (E19) of pregnancy. Panel A shows the data obtained from fetuses and placentas harvested from dams exposed to CH3HgCl on day 11 of pregnancy and sacrificed on day 13. At this stage of development, the amount of mercury in each fetus from dams exposed to CH3HgCl and treated subsequently with saline was similar to that detected in the corresponding placenta. Treatment of dams with DMPS reduced the amount of mercury in the placenta by approximately 50%. When rats were treated with DMSA, the placental content of mercury was reduced by about 30%. In the fetus, DMPS did not appear to reduce the fetal burden of mercury; however, treatment of dams with DMSA resulted in a slight, but significant, reduction in the amount of mercury in each fetus. Figure 4B shows data obtained from fetuses and placentas harvested from dams exposed to CH3HgCl on day 17 of pregnancy. Fetuses were harvested on embryonic day 19 (E19). The amount of mercury in each E19 fetus was significantly greater than that of corresponding E13 fetuses. In addition, the amount of mercury in E19 fetuses of each experimental group was significantly greater than that of corresponding placentas. Treatment of dams with either DMPS or DMSA reduced significantly the placental and fetal burden of mercury. Interestingly, DMPS and DMSA appeared to be more effective in reducing the content of mercury of placentas from E13 fetuses than that of placentas associated with E19 fetuses. In contrast, the ability of DMPS and DMSA to reduce the fetal burden of mercury was greater in E19 fetuses than in E13 fetuses.
Figure 4.
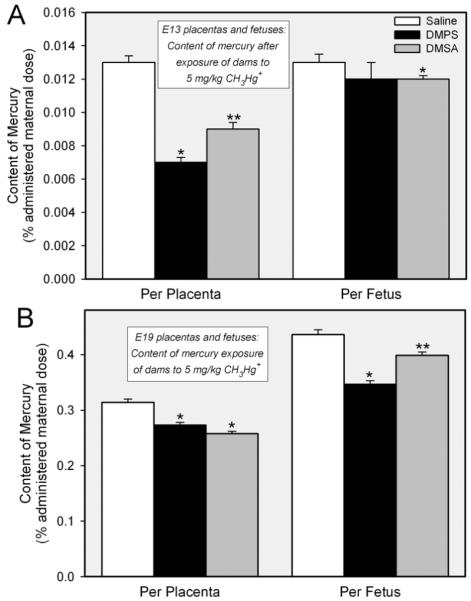
Amount of mercury detected in placentas and fetuses removed from pregnant Wistar rats on embryonic day 13 (E13) or 19 (E19) following exposure to 5 mg/kg CH3HgCl and saline (2 mL/kg), DMPS or DMSA (200 mg/kg). Panel A shows the content of mercury (% of administered maternal dose per placenta or fetus) in E13 placentas and fetuses. Panel B shows the content of mercury (% of administered maternal dose per placenta or fetus) in E19 placentas and fetuses. Placentas and fetuses were harvested 48 h after exposure to CH3HgCl. Data represent mean ± SE of 11-18 fetuses or placentas from five rats. *, significantly different (p < 0.05) from the corresponding mean for rats treated with saline. **, significantly different (p < 0.05) from the corresponding mean for rats of all other treatment groups.
The disposition of mercury in fetal tissues was determined for E19 fetuses (Figure 5A). Of the organs measured, the greatest percentage of administered maternal dose of mercury was present in the fetal liver (approximately 18% of the total fetal burden, data not shown), followed by fetal brain (15% of total fetal burden, data not shown) and total renal mass (5% of total renal burden, data not shown). When the fetal burden of mercury was factored by organ weight (Figure 5B), the data show that the greatest percentage of administered maternal dose (per g fetal tissue) was detected in the total renal mass, followed by brain and liver. DMPS reduced the amount of mercury (% administered maternal dose/g) in all organs measured while DMSA was found to reduce the amount of mercury in brain and liver only.
Figure 5.
Amount of mercury in organs of fetuses extracted from pregnant Wistar rats exposed to 5 mmol/kg CH3HgCl on day 17 of pregnancy and treated 24 h later with saline (2 mL/kg), DMPS or DMSA (200 mg/kg). Panel A shows the disposition of mercuric ions in organs of each fetus (% administered maternal dose per fetus). Panel B shows the disposition of mercury when factored by the weight of each organ (% administered maternal dose per gram tissue). Fetuses were harvested 48 h (embryonic day 19) after exposure of dams to CH3HgCl. Data represent mean ± SE of 11-18 fetuses and placentas from five rats. *, significantly different (p < 0.05) from the corresponding mean for rats treated with saline.
DISCUSSION
In the current study pregnant Wistar rats exposed intravenously to CH3HgCl and treated subsequently (i.v.) with DMPS or DMSA were used to examine the effects of each chelator on the maternal, fetal, and placental disposition of mercuric ions. The disposition of mercuric ions in kidneys, liver, blood, urine and feces of dams exposed to CH3HgCl was similar to that of our previous study using male Wistar rats [30]. Within the kidneys, the greatest amount of mercury was detected in the cortex, followed by the outer stripe of the outer medulla. It is important to note that proximal tubules, which are the primary sites of renal accumulation of mercury, are located within these two zones of the kidney. DMPS and DMSA extracted mercuric ions from the cortex and outer stripe of outer medulla; this phenomenon is likely related to the presence of Mrp2 in these two zones [28]. Mrp2 has been shown to mediate the transport of DMPS- and DMSA-S-conjugates of mercury and is thought to play an important role in the excretion of mercuric ions in urine [25,26]. Indeed, a corresponding increase in the urinary excretion of mercuric ions was also observed. Treatment with DMPS and DMSA also reduced the hepatic burden of mercury. Similar to that in kidney, this reduction is also probably due to the activity of Mrp2, which is present in the canalicular membrane of hepatocytes [32]. A corresponding increase in the fecal excretion of mercuric ions was observed after treatment with DMPS, but surprisingly, not after treatment with DMSA. The difference in the effectiveness of these two chelators may be due to some degree of intestinal reabsorption of mercuric ions. In blood, levels of mercury were substantial, accounting for approximately 30% of the administered dose. Both, DMPS and DMSA reduced the hematological burden of blood, which may be due to the ability of DMPS- and DMSA-S-conjugates of mercury to be filtered freely at the glomerulus and excreted subsequently into the urine.
In contrast to previous reports that DMSA is more effective than DMPS in chelating CH3Hg+ [17,18], our current data indicate that DMPS was better than DMSA in extracting mercuric ions from maternal tissues following exposure to CH3HgCl. The difference in the effectiveness of DMPS and DMSA may be due to the fact that once absorbed, some of the CH3Hg+ to which dams were exposed may have oxidized to inorganic mercury, which is more effectively chelated by DMPS [17,18].
Our current findings also provide information regarding the distribution of mercuric ions in placental and fetal tissues at two stages of development (E13 and E19). Similar to previous studies [33,34], we found that the fetal burden of mercury increases with fetal age. In addition, the amount of mercury within the placenta also increases as the gestational age of the fetus increases. These increases are likely due to increases in placental size as well as increases in blood flow (and delivery of mercuric ions) to placental and fetal tissues. The current data suggest that the fetus may be exposed to greater levels of mercury as the gestational period progresses, which may increase the susceptibility of the fetus to the toxic effects of mercury. In contrast to previous studies [35,36], our data show that, in E19 fetuses, the burden of mercury is greater in the fetus than in the placenta. These data confirm previous reports indicating that mercuric ions readily cross the placenta and accumulate in fetal tissues [2-7]. In addition, we suggest that once mercuric ions gain access to fetal tissue, they are not readily transported back across the placenta into maternal circulation without the assistance of chelating compounds.
In the current study, we also examined the distribution of mercuric ions in liver, brain, and kidneys of E19 fetuses. Of the administered maternal dose of mercury that accumulated in the fetus, the greatest percentage was detected in the fetal liver. This is not surprising given that the liver is considerably larger than the brain and kidneys at this stage of development. When the percent of administered dose was factored by the weight of the organ, the greatest amount of mercury was detected in the kidneys, followed by brain, and then liver. The kidney and brain have long been recognized as primary sites where mercury accumulates and exerts its toxic effects [37].
Once mercuric ions enter cells, they form strong bonds with intracellular proteins and hence, in a number of organs, very little intracellular mercury is removed without the assistance of a chelating agent. Numerous studies have demonstrated that DMPS and DMSA are effective extractors of mercuric ions [17,18]. However, to our knowledge, there have been no studies examining the ability of these chelators to extract mercuric ions from placental and fetal tissues after maternal exposure to CH3Hg+. In the current study, we demonstrate that both chelators are capable of extracting significant amounts of mercuric ions from placental and fetal tissues at two stages of development. These findings are significant in that treatment of dams with DMPS and DMSA following exposure to CH3Hg+ may reduce or prevent adverse effects in the fetus. The greatest level of extraction of mercuric ions appears to occur in placentas associated with E13 fetuses, with DMPS being more effective than DMSA. Interestingly, the ability of either chelator to extract mercury from placentas appeared to decrease over time. Although more total mercury was extracted from placentas of E19 fetuses, the relative amount of mercury extracted, compared to total fetal burden, was lower than that of placentas from E13 fetuses. In addition, DMSA appeared to be slightly more effective at extracting mercuric ions from placentas of E19 fetuses. This effect was opposite in fetal tissues. DMPS and DMSA extracted more mercury (relative and total amounts) from E19 fetuses than from E13 fetuses. Interestingly, in E13 fetuses, only DMSA was able to reduce significantly the fetal burden of mercury. These findings may be due to a reduced accessibility of DMPS and DMSA to the fetal organs and tissues where mercury accumulates.
The results of this study indicate that DMPS and DMSA are capable of extracting mercuric ions, not only from maternal tissues, but also from placental and fetal tissues. Although the exact molecular mechanisms involved in this transport remain unclear, we propose that endogenous transport proteins present in the apical and basolateral membranes of placental trophoblasts play important roles in this process. It should be noted that, in biological systems, mercury is bound to one or more thiol-containing biomolecules such as cysteine (Cys) [38]. Therefore, thiol S-conjugates of mercury are the most likely species of mercury transported by membrane carriers. Previous studies have suggested that system L is involved in the transport of CH3Hg+ across the placenta to fetal tissues [15]. Less is known about the mechanisms by which mercuric ions are removed from fetal tissues and are transported back into maternal blood. DMPS has been shown to extract mercuric ions from fetuses and placentas whose mothers were exposed to a form of inorganic mercury, i.e., mercuric chloride [22]. However, to our knowledge, there are no studies examining the effect of DMPS and DMSA on the disposition of mercuric ions in placental and fetal tissues following maternal exposure to a form of organic mercury, i.e., CH3Hg+. In the current study, we showed that DMPS and DMSA, can extract mercuric ions from placental and fetal tissues following maternal exposure to CH3Hg+, which is the most likely form of mercury to cross the placenta. Possible mechanisms for this extraction include Oat4 (Slc22A11) and Mrp2. Mrp2, as well as certain members of the Oat family, Oat1 and Oat3 (Slc22A8), have been shown to mediate the transport of mercuric ions (as Cys S-conjugates) across some types of transporting epithelia [25,26,36,38,39]. Oat4 has been identified in placenta and is localized in the basolateral (fetal-facing) plasma membrane of trophoblasts where it is thought to play a role in the removal of toxic substances from the fetus [40]. In addition, Mrp2 has been identified in the apical plasma membrane of trophoblasts [41]. In renal epithelial cells, Mrp2 appears to play a role in the extraction of mercuric ions [25,26,30,36,39]; it likely has a similar role in placental trophoblasts.
Based on the aforementioned findings, we propose that transportable forms of CH3Hg+ (such as Cys S-conjugates) are transported from maternal to fetal circulation via the system L isoforms, Lat1 and Lat2, present on the apical and basolateral plasma membranes of placental trophoblasts, respectively. Indeed, Cys S-conjugates of CH3Hg+ have been shown to be transportable substrates of these carriers [16]. DMPS or DMSA may enter the trophoblasts via the sodium dicarboxylic carrier, NaC1 (Scl13a2), located on the apical membrane [42], and may be transported into fetal blood via Oat4, which is a bi-directional transporter on the basolateral membrane of trophoblasts. Within fetal tissues, as well as within the intracellular compartment of the trophoblasts themselves, DMPS and DMSA may bond with mercuric ions through a mechanism of thiol competition. The DMPS or DMSA S-conjugates of mercury that are formed in the fetus may be transported from fetal circulation into the trophoblasts via Oat4. These conjugates as well as those formed in the trophoblasts may then be exported into maternal blood via Mrp2, located in the apical plasma membranes of placental trophoblasts. While Mrp2 has been shown to mediate the transport of DMPS- and DMSA S-conjugates of mercury [25,26], there is currently no molecular evidence for a role of Oat4 in this process. Future studies are necessary to examine the role of Oat and other potential membrane transporters in the transplacental movement of mercuric ions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LITERATURE CITED
- 1.Johnson CL. Mercury in the environment: Sources, toxicities, and prevention of exposure. Pedatric annals. 2004;33:437–442. doi: 10.3928/0090-4481-20040701-08. [DOI] [PubMed] [Google Scholar]
- 2.Ask K, Akesson A, Berglund M, Vahter M. Inorganic mercury and methylmercury in placentas of Swedish women. Environ Health Perspect. 2002;110:523–526. doi: 10.1289/ehp.02110523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inouye M, Kajiwara Y. Developmental disturbances of the fetal brain in guinea-pigs caused by methylmercury. Arch Toxicol. 1988;62:15–21. doi: 10.1007/BF00316251. [DOI] [PubMed] [Google Scholar]
- 4.Inouye M, Murao K, Kajiwara Y. Behavioral and neuropathological effects of prenatal methylmercury exposure in mice. Neurobehav Toxicol Teratol. 1985;7:227–232. [PubMed] [Google Scholar]
- 5.Ong CN, Chia SE, Foo SC, Ong HY, Tsakok M, Liouw P. Concentrations of heavy metals in maternal and umbilical cord blood. BioMetals. 1993;6:61–66. doi: 10.1007/BF00154234. [DOI] [PubMed] [Google Scholar]
- 6.Suzuki T, Yonemoto J, Satoh H, Naganuma A, Imura N, Kigawa T. Normal organic and inorganic mercury levels in the human feto-placental system. J Appl Toxicol. 1984;4:249–252. doi: 10.1002/jat.2550040507. [DOI] [PubMed] [Google Scholar]
- 7.Yang J, Jiang Z, Wang Y, Qureshi IA, Phil M, Wu XD. Maternal-fetal transfer of metallic mercury via the placenta and milk. Ann Clin Lab Sci. 1997;27:135–141. [PubMed] [Google Scholar]
- 8.Murakami U. Embryo fetotoxic effects of some organic mercury compounds. Annu Rep Res Inst Environ Med, Nagoya Univ. 1971;18:33–43. [PubMed] [Google Scholar]
- 9.Spyker D, Spyker J. Response model analysis for cross-fostering studies: Prenatal versus postnatal effects on offspring exposed to methylmercury dicyandiamide. Toxicol Appl Pharmacol. 1977;40:511–527. doi: 10.1016/0041-008x(77)90077-1. [DOI] [PubMed] [Google Scholar]
- 10.Su M, Okita G. Embryocidal and tetragenic effects of methylmercury in mice. Toxicol Appl Pharmacol. 1976;38:207–216. doi: 10.1016/0041-008x(76)90174-5. [DOI] [PubMed] [Google Scholar]
- 11.Davidson PW, Myers GJ, Weiss B. Mercury exposure and child development outcomes. Pediatrics. 2004;113:1023–1029. [PubMed] [Google Scholar]
- 12.Eto K. Minamata disease. Neuropathol. 2000;20(Suppl):S14–19. doi: 10.1046/j.1440-1789.2000.00295.x. [DOI] [PubMed] [Google Scholar]
- 13.Kazantizis G. Mercury exposure and early effects: an overview. Med Lav. 2002;93:139–147. [PubMed] [Google Scholar]
- 14.Onishchenko N, Tamm C, Vahter M, Hökfelt T, Johnson JA, Johnson DA, Ceccatelli S. Developmental exposure to methylmercury alters learning and induces depression-like behavior in male mice. Toxicol. Sci. 2007;97:428–437. doi: 10.1093/toxsci/kfl199. [DOI] [PubMed] [Google Scholar]
- 15.Kijiwara Y, Yasutake A, Adachi T, Hirayama K. Methylmercury transport across the placenta via neutral amino acid carrier. Arch Toxicol. 1996;70:310–4. doi: 10.1007/s002040050279. [DOI] [PubMed] [Google Scholar]
- 16.Simmons-Willis TA, Koh AS, Clarkson TW, Ballatori N. Transport of a neurotoxicant by molecular mimicry: the methylmercury-L-cysteine complex is a substrate for human L-type large neutral amino acid transporter (LAT) 1 and LAT 2. Biochem J. 2002;367:239–246. doi: 10.1042/BJ20020841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aposhian HV. DMSA and DMPS: water soluble antidotes for heavy metal poisoning. Ann Rev Pharmacol Toxicol. 1983;23:193–215. doi: 10.1146/annurev.pa.23.040183.001205. [DOI] [PubMed] [Google Scholar]
- 18.Aposhian HV, Mairorino RM, Rivera M, Bruce DC, Dart RC, Hurlbut KM, Levine DJ, Zheng W, Fernando Q, Carter D, Aposhian MM. Human studies with the chelating agents DMPS and DMSA. Clin. Toxicol. 1992;30:505–528. doi: 10.3109/15563659209017938. [DOI] [PubMed] [Google Scholar]
- 19.Planas-Bohne F. The effect of 2,3-dimercaptopropane-1-sulfonate and dimercaptosuccinic acid on the distribution and excretion of mercuric chloride in rats. Toxicology. 1981;19:275–278. doi: 10.1016/0300-483x(81)90138-4. [DOI] [PubMed] [Google Scholar]
- 20.Ruprecht J. Scientific Monograph for Dimaval (DMPS) 7th Ed. Heyltex Corporation; Houston TX: 2008. [Google Scholar]
- 21.Gomez M, Sanchez DJ, Colomina MT, Domingo JL, Corbella J. Evaluation of the protective activity of 2,3-dimercaptopropanol and sodium 2,3-dimercaptopropane-1-sulfonate on methylmercury-induced developmental toxicity in mice. Arch Environ Contam Toxicol. 1994;26:64–68. doi: 10.1007/BF00212795. [DOI] [PubMed] [Google Scholar]
- 22.Wannag A, Aaseth J. The effect of immediate and delayed treatment with 2,3-dimercaptopropane-1-sulphonate on the distribution and toxicity of inorganic mercury in mice and in foetal and adult rats. Acta Pharmacol Toxicol. 1980;46:81–88. doi: 10.1111/j.1600-0773.1980.tb02425.x. [DOI] [PubMed] [Google Scholar]
- 23.Burckhardt BC, Drinkuth B, Menzel C, Konig A, Steffgen J, Wright SH, Burckhardt G. The renal Na+-dependent dicarboxylate transporter, NaDC-3 translocates dimethyl- and disulfhydryl-compounds and contributes to renal heavy metal detoxification. J Am Soc Nephrol. 2002;13:2628–2638. doi: 10.1097/01.asn.0000033463.58641.f9. [DOI] [PubMed] [Google Scholar]
- 24.Lungkaphin A, Chatsudthipong V, Evans KK, Groves CE, Wright SH, Dantzler WH. Interaction of the metal chelator DMPS with OAT1 and OAT3 in intact isolated rabbit renal proximal tubules. Am J Physiol Renal Physiol. 2004;2861:F68–76. doi: 10.1152/ajprenal.00075.2003. [DOI] [PubMed] [Google Scholar]
- 25.Bridges CC, Joshee L, Zalups RK. Multidrug resistance proteins and the renal elimination of inorganic mercury mediated by 2,3-dimercaptopropane-1-sulfonic acid and meso-2,3-dimercaptosuccinic acid. J Pharmacol Exp Ther. 2008a;324:383–390. doi: 10.1124/jpet.107.130708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bridges CC, Joshee L, Zalups RK. MRP2 and the DMPS- and DMSA-mediated elimination of mercury in TR- and control rats exposed to thiol S-conjugates of inorganic mercury. Toxicol Sci. 2008b;105:211–220. doi: 10.1093/toxsci/kfn107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sugawara N, Lai YR, Sugaware C, Arisono K. Decreased hepatobiliary secretion of inorganic mercury, its deposition and toxicity in the Eisai hyperbilirubinemic rat with no hepatic canalicular organ anion transporter. Toxicology. 1998;126:23–31. doi: 10.1016/s0300-483x(97)00170-4. [DOI] [PubMed] [Google Scholar]
- 28.Schaub TP, Kartenbeck J, Konig J, Vogel O, Witzgall R, Kriz W, Keppler D. Expression of the conjugate export pump encoded by the mrp2 gene in the apical membrane of kidney proximal tubules. J Am Soc Nephrol. 1997;8:1213–1221. doi: 10.1681/ASN.V881213. [DOI] [PubMed] [Google Scholar]
- 29.Zalups RK. Influence of 2,3-dimercaptopropane-1-sulfonate (DMPS) and meso-2,3-dimercaptosuccinic acid (DMSA) on the renal disposition of mercury in normal and uninephrectomized rats exposed to inorganic mercury. J Pharmacol Exp Therap. 1993;267:791–800. [PubMed] [Google Scholar]
- 30.Zalups RK, Bridges CC. MRP2 involvement in renal proximal tubular elimination of methylmercury mediated by DMPS or DMSA. Toxicol Appl Pharmacol. 2009;235:10–17. doi: 10.1016/j.taap.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 31.Rouleau C, Block M. Fast and high yield synthesis of radioactive CH3203Hg(II) Appl Organomet Chem. 1997;2:751–753. [Google Scholar]
- 32.Keppler D, König J, Büchler M. The canalicular multidrug resistance protein, cMRP/MRP2, a novel conjugate export pump expressed in the apical membrane of hepatocytes. Adv Enzyme Regul. 1997;37:321–33. doi: 10.1016/s0065-2571(96)00013-1. [DOI] [PubMed] [Google Scholar]
- 33.Mansour M, Dyer N, Hoffman L, Davis J, Brill A. Placental transfer of mercuric nitrate and methylmercury in the rat. Am J Obstet Gynecol. 1974;119:557–562. doi: 10.1016/0002-9378(74)90220-8. [DOI] [PubMed] [Google Scholar]
- 34.Olson F, Massaro E. Pharmacodynamics of methylmercury in the murine maternal/fetal unit. Toxicol Appl Pharmacol. 1977;39:263–273. doi: 10.1016/0041-008x(77)90159-4. [DOI] [PubMed] [Google Scholar]
- 35.Kelman B, Steinmetz S, Walter B, Sasser L. Fetal distribution of mercury following introduction of methylmercury into porcine maternal circulation. J Toxicol Environ Health. 1982;10:191–200. doi: 10.1080/15287398209530243. [DOI] [PubMed] [Google Scholar]
- 36.Aremu DA, Madejczyk MS, Ballatori N. N-acetylcysteine as a potential antidote and biomonitoring agent of methylmercury exposure. Environ Health Perspect. 2008;116:26–31. doi: 10.1289/ehp.10383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Agency for Toxic Substance and Disease Registry . Toxicological Profile for Mercury. U. S. Department of Health and Humans Services, Public Health Service, Centers for Disease Control; Atlanta, GA: 2007. [Google Scholar]
- 38.Bridges CC, Zalups RK. Molecular and ionic mimicry and the transport of toxic metals. Toxicol Appl Pharmacol. 2005;204:274–308. doi: 10.1016/j.taap.2004.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Madejczyk MS, Aremu DA, Simmons-Willis TA, Clarkson TW, Ballatori N. Accelerated urinary excretion of methylmercury following administration of its antidote N-acetylcysteine requires Mrp2/Abcc2, the apical multidrug resistance-associated protein. J Pharmacol Exp Ther. 2007;322:378–384. doi: 10.1124/jpet.107.122812. [DOI] [PubMed] [Google Scholar]
- 40.Cha SH, Sekine T, Kushuhara H, Yu E, Kim JY, Kim DK, Sugiyama Y, Kanai Y, Endou H. Molecular cloning and characterization of multispecific organic anon transporter 4 expressed in placenta. J Biol Chem. 2000;275:4507–4512. doi: 10.1074/jbc.275.6.4507. [DOI] [PubMed] [Google Scholar]
- 41.St.Pierre MV, Serrano MA, Macias RIR, Dubs U, Hoechli M, Lauper U, Meier PJ, Marin JJG. Expression of members of the multidrug resistance protein family in human term placenta. Am J Physiol Regulatory Integrative Comp Physiol. 2000;279:R1495–R1503. doi: 10.1152/ajpregu.2000.279.4.R1495. [DOI] [PubMed] [Google Scholar]
- 42.Kekuda R, Wang H, Huang W, Pajor AM, Leibach FH, Devoe LD, Prasad PD, Ganapathy V. Primary structure and functional characteristics of a mammalian sodium-coupled high affinity dicarboxylate transporter. J Biol Chem. 1999;274:3422–3429. doi: 10.1074/jbc.274.6.3422. [DOI] [PubMed] [Google Scholar]