Abstract
The ability to spontaneously recall recently learned information is a fundamental mnemonic activity of daily life, but has received little study using functional neuroimaging. We developed a functional MRI (fMRI) paradigm to study regional brain activity during encoding that predicts free recall. In this event-related fMRI study, ten lists of fourteen pictures of common objects were shown to healthy young individuals and regional brain activity during encoding was analyzed based on subsequent free recall performance. Free recall of items was predicted by activity during encoding in hippocampal, fusiform, and inferior prefrontal cortical regions. Within-subject variance in free recall performance for the ten lists was predicted by a linear combination of condition-specific inferior prefrontal, hippocampal, and fusiform activity. Recall performance was better for lists in which pre-frontal activity was greater for all items of the list and hippocampal and fusi-form activity were greater specifically for items that were recalled from the list. Thus, the activity of medial temporal, fusiform, and prefrontal brain regions during the learning of new information is important for the subsequent free recall of this information. These fronto-temporal brain regions act together as a large-scale memory-related network, the components of which make distinct yet interacting contributions during encoding that predict subsequent successful free recall performance.
Keywords: functional MRI, memory, entorhinal cortex, perirhinal cortex
INTRODUCTION
The ability to spontaneously recall recently learned information is essential in daily life, and often fails with aging and neurodegenerative diseases, particularly Alzheimer’s disease (AD) (Albert, 2002). Human episodic memory performance is typically measured clinically using psychometric tests of free recall, cued recall, and recognition, yet functional neuroimaging studies of memory have employed primarily encoding and recognition memory paradigms. Given the salience of spontaneous recall to humans, surprisingly little work has been reported on the neural or hemodynamic predictors of this cognitive process.
Functional MRI (fMRI) demonstrates that successful recognition memory is predicted by activity during encoding in a distributed set of brain regions, including inferior prefrontal and ventral and medial temporal lobe (MTL) (Brewer et al., 1998; Wagner et al., 1998; Kirchhoff et al., 2000; Sperling et al., 2003; Garoff et al., 2005). The first fMRI study of encoding predictors of free recall reported that hippocampal activation during encoding increased with number of items recalled, but the block design did not enable the disambiguation of activation associated specifically with items that were successfully recalled (Fernandez et al., 1998). An event-related fMRI study of free recall demonstrated greater left perirhinal, left hippocampal, and right entorhinal activity during encoding of verbal stimuli that were successfully recalled (Strange et al., 2002). However, brain coverage was restricted to MTL and ventral fronto-temporal regions. Two recent event-related fMRI studies have identified frontal and medial and ventral temporal activity that predicts free recall; in one of these studies, cerebellar activity also predicted free recall (Brassen et al., 2006), while in the other study, posterior parietal activity also predicted recall (Staresina and Davachi, 2006).
Several important questions remain regarding encoding-related brain activity and subsequent free recall. Although prefrontal-medial temporal interactions have been hypothesized as important for memory function and disorders of memory (Warrington and Weiskrantz, 1982), there is a paucity of functional neuroimaging data directly addressing such interactions (Simons and Spiers, 2003). For example, do prefrontal-MTL interactions at encoding predict the number of items freely recalled from short item lists (analogous in length to some clinical neuropsychological tests)? This issue has never been addressed in part because several previous studies have employed a few relatively long lists of items.
In this study, we developed an event-related paradigm in which fMRI data were acquired during visual encoding of ten relatively short pictorial lists of unique objects and then analyzed with respect to subsequent free verbal recall of object names. Based on previous visual object encoding/recognition fMRI experiments, we hypothesized that greater inferior prefrontal, fusiform, and MTL activity during encoding would predict subsequent free recall (Brewer et al., 1998; Kirchhoff et al., 2000; Sperling et al., 2003). In addition, since we observed within-subject variance in recall across the ten runs, we hypothesized that better intraindividual (within-subject) recall performance on lists of items would be predicted, at least in part, by a greater degree of correlated activity in prefrontal and medial temporal regions during encoding. Because of the hypothesis-driven nature of this study, the primary analysis was performed using a priori ROIs for the regions mentioned above, and this was followed by an exploratory whole-brain analysis (Kirchhoff and Buckner, 2006).
MATERIALS AND METHODS
Participants
Participants were 15 right-handed, native English speakers (10 women, 5 men; ages 20–30 yr, mean = 23.7) who were recruited via local advertisements and received $100 each for participation. Each participant was in good health and free from history of neurologic or psychiatric illness, and was not taking medications with central nervous system pharmacologic activity. All participants gave informed consent, and the study was approved by the Partners Healthcare System Human Research Committee.
Stimuli and Cognitive Task
Color pictures of common objects were obtained from a commercial image service (Hemera Technologies, Gatineau, Quebec, Canada); image backgrounds were masked such that each object appeared against a white background. The test stimuli consisted of 10 lists of 14 pictures each. Each picture was presented for 2.75 s. Using OptSeq (http://surfer.nmr.mgh.harvard.edu/opt-seq/), “jittered” periods of visual fixation lasting between 0.25 and 9 s were pseudo-randomly interspersed between the presentation of each picture to maximize the efficiency of the design matrix (Dale, 1999). The total time for each list presentation was 78 s. Participants were instructed to press a button to indicate whether each object was “natural” or “man-made,” and to try to learn the item for subsequent memory testing. Each test list was balanced for natural and man-made objects, and the sequence of natural and man-made objects was randomized within each list.
Prior to scanning, the procedure was explained and participants completed one training block in a behavioral testing room. Once in the scanner, participants completed another training block to ensure that they could adequately see the stimuli and use the button box. Instructions were presented verbally by the investigators via the scanner intercom system.
During scanning, each of the ten 14-item lists was presented as a 78-s encoding run. The presentation of each list of 14 pictures (each encoding run) was followed immediately by a 15-s distractor task (not scanned) during which subjects were instructed to count (out loud) backward by threes, starting at a number between 70 and 99 (randomly chosen for each run by the investigators). Immediately following the distractor task, the subjects were asked to freely recall (not scanned) the names of as many of the items as possible from the previous list, in any order. Sixty seconds was allowed for free recall. Words were recorded by the investigators as they were stated by the subject, in order, and an item was counted as a free recall “Hit” if it was a specific descriptor of one of the items viewed in the immediately preceding encoding list. Intrusions and perseverations were also recorded. Items that had been presented but for which verbal descriptors were not recalled were categorized as recall “Misses.” Ten such encoding and free recall runs were administered.
All stimuli were presented on a PC laptop using Eprime software (Psychology Software Tools, Pittsburgh, PA). Stimuli were projected into the scanner using a rear mounted LCD projector in conjunction with a mirror mounted on the head coil.
Functional Imaging
A Siemens (Siemens Medical Solutions, Malvern, PA) 1.5T Magnetom Avanto system equipped with a total imaging matrix (TIM) 12-channel head coil was used to acquire high-resolution T1-weighted anatomical data (MPRAGE: TR/TI/TE 2730/1000/3.31 ms, FOV = 256, FA = 7°, 128 sagittal slices, thickness = 1.33 mm, matrix 192 × 256 (1.3 mm × 1 mm in-plane resolution), and T2*-weighted gradient-echo echo-planar (EPI) functional data (TR = 2,000 ms, TE = 30 ms, 30 oblique coronal slices aligned perpendicularly to the long axis of the left hippocampal formation, foot-to-head phase-encoding direction, 4-mm thickness, 0.4-mm interslice skip, 192 mm FOV, matrix 64 × 64 (3 mm × 3 mm in-plane resolution), 39 acquisitions per run). Four additional volumes were collected and discarded at the beginning of each run to allow for T1 equilibration. An online automatic slice-positioning atlas was used for slice prescription (van der Kouwe et al., 2005). The field of view provided nearly whole brain coverage and data were collected in an occipital-to-frontal direction (Fig. 1).
FIGURE 1. Experimental design.
(A) Schematic of one run of the behavioral paradigm design, illustrating the scanned encoding period (78 s) and unscanned free recall period (60 s). Between these two periods, there is a 15 s counting task (interference). Ten such runs occur per session. (B) Parasagittal section of a subject’s brain demonstrating location of oblique coronal functional image acquisition plane, perpendicular to long axis of left hippocampal formation.
Behavioral Data Analysis
Accuracy and latency of behavioral responses during the semantic decision task during encoding were collected. ANOVA models were used to analyze behavioral data for effects of interest. The first word in each list demonstrated a primacy effect and the last two words demonstrated a recency effect (see Results), and hence the remaining 11 words (the list body) were modeled separately for the first analysis below (Hits vs. Misses), to avoid confounding subsequent memory with primacy and recency effects. The second analysis, focused on intraindividual performance variability, employed all list items to maximize statistical power. These statistical analyses were performed using SPSS 11.0 (Chicago, IL).
Functional MRI Data Analysis
Data were preprocessed using Statistical Parametric Mapping (SPM2; Wellcome Department of Cognitive Neurology, London, UK) for Matlab (The Mathworks, Natick, MA). Functional data were realigned using INRIAlign, a motion-correction algorithm unbiased by local signal changes (Freire and Mangin, 2001; Freire et al., 2002). The data were then normalized to the standard SPM2 EPI template and resampled into 3-mm isotropic resolution in MNI space. Data were then smoothed using a 5-mm Gaussian kernel, and modeled with the canonical hemodynamic response function. A high-pass filter of 84 s was used to remove low-frequency signal (e.g., drifts across run).
The subsequent memory analysis involved sorting encoding trials into bins depending on participants’ behavioral responses in the free recall test. Specifically, trials were divided into two categories depending on whether the items were freely recalled (“Free Recall Hits”) or not (“Free Recall Misses”).
Functional-Anatomic Regional MRI Data Analysis
A functional-anatomic analysis procedure was used to test a priori hypotheses regarding the localization of functional activation within the ventromedial temporal lobe, as well as the inferior prefrontal cortex. Regions of interest (ROI) were derived from each individual subject’s high resolution T1 structural scan using a semiautomated anatomic reconstruction and labeling procedure (Dale et al., 1999; Fischl et al., 1999, 2002) followed by manual editing to define four specific ROIs in each hemisphere of each subject (using Freesurfer software—http://surfer.nmr.mgh.harvard.edu/). ROI labels were visualized using the Freesurfer tkmedit tool in multiple planes, and were modified primarily in the coronal orientation. For cortical ROIs, the Freesurfer tksurfer tool was used to clarify the contiguity of sulcal and gyral anatomy using the cortical surface model of each subject; surface vertex points were then referenced to the surface overlaid on the volume in tkmedit, in which manual ROI editing was performed.
A modified version of previously described protocols was employed to delineate boundaries of ROIs, including hippocampal, parahippocampal (a single ROI containing entorhinal, perirhinal, and posterior parahippocampal regions) (Dickerson et al., 2001, 2004; Goncharova et al., 2001), and fusiform ROIs (Dickerson et al., 2004). In addition, an inferior prefrontal cortical ROI was delineated. In brief, the anatomical criteria used for these ROIs are as follows. For hippocampal formation (CA fields, dentate gyrus, and subiculum), the rostral boundary was the first coronal section in which the rostral hippocampal formation could be clearly visualized ventral to the alveus. The caudal boundary was the last coronal section in which the structure of the tail of the hippocampal formation could be clearly visualized medial to the fornix. The lateral boundary was the cerebrospinal fluid in the temporal horn of the lateral ventricle, and the ventromedial boundary was a 45° placed at the “shoulder” of the parahippocampal gyrus to divide subiculum from entorhinal/parahippocampal cortex. The parahippocampal ROI included entorhinal, perirhinal, and parahippocampal tissue; its rostral boundary was the first coronal section on which the sulcus semiannularis and gyrus ambiens could be visualized; its caudal boundary was the last coronal slice in which the hippocampal formation could be visualized. Its medial boundary was the sulcus semiannularis rostrally and the subiculum caudally after the hippocampal fissure is “open.” The lateral boundary extended to the fundus of the collateral sulcus. The fusiform ROI, a portion of the fusiform gyrus, extended from a rostral boundary at the level of the lateral geniculate nucleus (the first coronal section caudal to the parahippocampal ROI) to a caudal boundary at the same coronal slice as the caudal boundary of the hippocampal formation. The medial and lateral boundaries were the fundi of the collateral and lateral occipito-temporal sulci, respectively. The inferior prefrontal ROI, which included pars opercularis, triangularis, and orbitalis, was bounded by the inferior frontal sulcus (dorsal and rostral boundary), the precentral sulcus (caudal boundary), and the lateral orbital sulcus and/or circular insular sulcus (ventromedial boundary).
For this functional-anatomic analysis, each subject’s motion-corrected echo-planar data were coregistered to that subject’s T1 data. For each subject, each ROI (based on individual subject T1 data) was then transformed to MNI space by applying the same transformation matrix that was applied to the functional data (described above). To obtain a single ROI representing each anatomical region for the entire group of 15 subjects, a mask of the union of the individual ROIs was created, allowing for the maximal extent of each ROI across all subjects. The SPM2 general linear model was applied to the EPI voxels that were identified as belonging to each ROI. Statistical activation maps were obtained for the Free Recall Hits > Free Recall Misses group contrast for the 15 subjects using the union mask for each ROI. In each ROI, activation was considered significant for clusters of three or more contiguous voxels, P < 0.005 uncorrected. This relatively lenient statistical threshold was employed because of the hypothesis-driven approach, and because task-related MTL signal changes can be relatively subtle due in part to susceptibility-related signal losses (Strange et al., 2002; Greicius et al., 2003; Ongur et al., 2005).
In addition, mean time courses of the BOLD signal for each condition (Hits and Misses) were obtained from voxel clusters of interest that exceeded the statistical threshold within each ROI using the SPM MarsBaR toolbox (http://marsbar.sourceforge.net/) to visualize differences in the peak signal and the shape of the hemodynamic response.
An exploratory whole-brain analysis was performed with similar preprocessing, and results were reported for P < 0.005 (uncorrected) if clusters included five or more contiguous voxels. As has previously been discussed, results from exploratory analyses with these relatively liberal parameters can be interpreted as preliminary results to be investigated further in future studies (Kirchhoff and Buckner, 2006).
Psychophysiologic Interaction Analysis of MTL-Neocortical Functional Connectivity That Predicts Free Recall
To assess the hypothesis that encoding processes leading to successful free recall were associated with a greater degree of “functional connectivity” (correlation of activity) between MTL and neocortical brain regions than was present during the encoding of items that were not recalled, a psychophysiologic interaction (PPI) analysis was performed in SPM (Friston et al., 1997). PPI analysis enables the detection of regionally specific brain activity that is modulated by the interaction between the activity in another brain region(s) and a psychological/behavioral measure. We interpret findings from this analysis as indicating the behavioral condition-specific functional interaction between brain regions (Das et al., 2005; Egner and Hirsch, 2005). Note that, in this case, a single PPI analysis was performed to test the hypothesis that there are hippocampal-neocortical interactions during encoding that predict free recall.
PPI analysis is performed by setting up a design matrix containing three columns of variables: (1) one regressor representing the behavioral measure of interest (the psychologic variable—e.g., Free Recall Hits vs. Misses), (2) one regressor representing the deconvolved timecourse in a particular source volume of interest (the physiologic variable from the source region—e.g., the hippocampal formation), and (3) a regressor representing the cross-product, or PPI, of the other two regressors (the psycho-physiologic interaction term). A statistical parametric map is then computed that shows areas where activation is predicted by the PPI term, and the physiologic and psychologic variables are treated as nuisance regressors. Thus, an effect resulting from this analysis indicates that the correlation between activity in the source region (e.g., the hippocampal formation) and other regions (e.g., prefrontal) during the encoding of items that are subsequently freely recalled (Hits) is significantly greater than that during the encoding of items that are not recalled (Misses).
To perform PPI analysis using SPM2, we extracted the deconvolved timecourse within the left hippocampal ROI from each subject as the physiologic source variable (a 4-mm sphere surrounding the peak voxel of the 5-voxel cluster of significant differential activity in the Hits vs. Misses analysis—see Results section below). The psychologic variable was the contrast of Hits vs. Misses (see Results section below). We then performed PPI analyses to identify the interaction term (first column) using the contrast [1 0 0]. Individual subject contrast images were then entered into a second-level random-effects analysis (using a one-sample t-test), using a mask of the eight a priori ROIs and a threshold of P < 0.005 with a cluster size of 3 voxels, as above.
Regional Brain Activity That Predicts Intraindividual Variability in Free Recall Performance
The analyses described above focused on identifying brain regions that show greater activity for all encoded-and-subsequently-recalled items (Hits) averaged together than for items not recalled (Misses). We observed notable intraindividual (within-subject) variability in free recall performance across the ten encoding-recall lists. We hypothesized that intraindividual differences in recall performance for each list (run) are predicted by differences in prefrontal, medial temporal, or ventral temporal brain activity during encoding. To investigate this hypothesis, a within-subject analysis was performed in SPM2 to determine whether there were correlations between regional brain activity during the encoding of each list and the number of items recalled from each list. Data from all 14 items per list were used in this analysis, rather than items from only the list body. In this analysis, a design matrix was constructed that contained a regressor representing the behavioral conditions of interest (i.e, All vs. Fixation and Hits vs. Misses) and a regressor representing the free recall performance in each of the ten encoding runs (number of items successfully recalled from each list). A statistical parametric map was then computed that showed areas where activation was correlated with recall performance for each individual subject. Individual subject contrast images were then entered into a second-level random-effects analysis (using a one-sample t-test), using a mask of the eight a priori ROIs and a threshold of P < 0.005 with a cluster size of 3 voxels, as above. An effect resulting from this analysis using the All vs. Fixation contrast indicates areas where activity during the encoding of every stimulus during a given list is significantly correlated with recall performance, which would indicate activity in brain regions that support performance-related cognitive processes engaged to a greater degree throughout all items of a list for lists in which recall performance is better (list-specific processes). In contrast, an effect resulting from this analysis using the Hits vs. Misses contrast indicates areas where the differential activity during the encoding of items that are subsequently freely recalled (Hits) vs. encoding of items that are not recalled (Misses) is significantly correlated with recall performance. Thus, this analysis reveals activity in brain regions that support performance-related cognitive processes engaged to a greater degree specifically for the stimuli that are subsequently recalled (vs. stimuli not recalled) for lists in which recall performance is better (item-specific processes).
RESULTS
Behavioral Data
Participants made a correct semantic decision (‘Natural’ vs. ‘Man-made’) for 96.7% (± 3.0%) of the items with a latency of 1,090 ms (± 148 ms). Based on participants’ responses during the free recall periods, encoding data were sorted into Free Recall Hits and Free Recall Misses bins. Neither the participants’ accuracy nor latency on the semantic decision response during encoding differed between items subsequently categorized as Free Recall Hits (97%; 1,082 ms) or Free Recall Misses (97%; 1,095 ms) (all P-values > 0.49). Thus, differential hemodynamic effects observed between these conditions are unlikely to be attributable to differences in stimulus processing time or accuracy during encoding.
As noted above (see Methods), the first word in each list demonstrated a primacy effect and the last two words demonstrated a recency effect; hence the remaining 11 words (the list body) were modeled separately for the first two fMRI analyses. The total number of items freely recalled from the body of the list (excluding list Positions 1, 13, and 14) per subject for the entire scanning session ranged from 25 to 60 (mean = 45.9, SD = 11.6) out of a possible 110 items. There was a difference in free recall by category, with subjects recalling more ‘Natural’ items (25.9 ± 5.6 items) than ‘Man-made’ items (20.0 ± 7.3 items, P < 0.01).
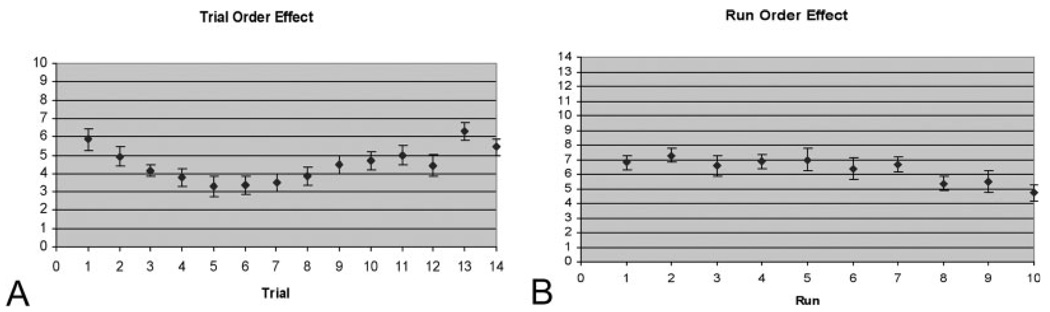
The serial list position recall curve is shown in Figure 2A (average free recall by list position for each of the 10 runs across all 15 subjects, with error bars indicating standard error of the mean). Analysis of these behavioral data indicated both primacy and recency effects. A repeated-measures ANOVA demonstrated a list position by performance interaction [(F (4.4, 57.5) = 14.85; P < 0.001)]. Post hoc testing demonstrated a primacy effect for list Position no. 1 and a recency effect for list Positions 13 and 14 (all P-values 2B shows the mean performance by run for all subjects. Repeated-measures ANOVA demonstrated a run number by performance interaction [(F (4.4, 57.5) = 14.85; P < 0.001)], with post hoc testing revealing slightly reduced mean performance in Runs 8, 9, and 10 compared to the previous 7 runs.
FIGURE 2. Serial list position and run position effects.
(A) Serial list position curve for the 15 subjects. Mean free recall performance (± SEM) per list position averaged across all 10 runs within each session and averaged across all subjects, demonstrating primacy (Position 1) and recency (Positions 13 and 14) effects. (B) Serial run position curve for the 15 subjects. Mean free recall performance (± SEM) per run within session averaged across all trial positions within each run and averaged across all subjects, demonstrating reduced performance in Runs 8, 9, and 10.
Because of the primacy and recency effects, the subsequent memory analyses modeled items in these list positions separately, with list Position 1 as a “Primacy” regressor and Positions 13 and 14 as a “Recency” regressor. Because of the small size of these bins, there was inadequate power to perform contrasts of interest for Primacy and Recency, so the subsequent memory contrasts involving Hits and Misses focused on only items in list Positions 2–12. (That is, there were four regressors in the model, and items were assigned to the Hits or Misses bins only if they were in list Positions 2–12.) Although there was a slight fatigue effect, the major findings presented below did not differ when analyses were restricted to only the first seven runs. Thus, to maximize statistical power, analyses were conducted with data from all 10 runs.
Medial and Ventral Temporal and Prefrontal Activity During Encoding Predicts Subsequent Free Recall
Functional-anatomic ROI analyses for a priori regions were performed initially at the individual subject level to optimize localization of activation data with respect to anatomic land-marks. Visualization of individual subject statistical maps over-laid on both mean individual subject EPI and T1 data revealed good correspondence of landmarks, indicating that the quantitative metrics accurately represent BOLD signal effects within the ROIs. Furthermore, group-level clusters of regional brain activation were visualized with respect to each individual subject’s anatomical scans (using inverted transformation matrices), and confirmed that the localization of functional activation clusters at the group level accurately represented the localization of voxels from which the signal was sampled at an individual level.
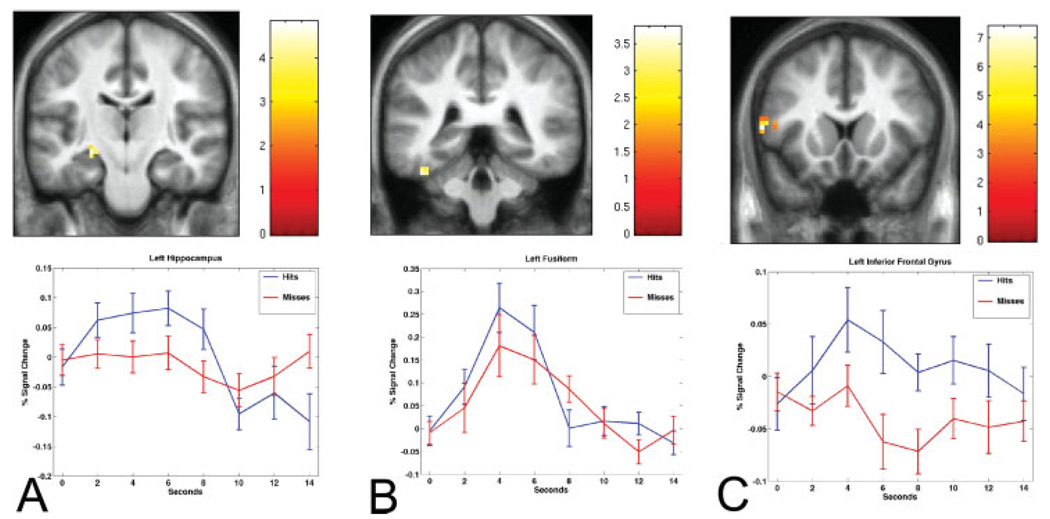
In the group analyses, activation during encoding was significantly greater for items that were subsequently freely recalled (Hits) than for items that were not subsequently recalled (Misses) in the left hippocampal, left fusiform, and left inferior prefrontal regions (P < 0.005; see Table 1 and Fig. 3). In each parahippocampal ROI (left and right), there was a cluster of two voxels that exceeded the P threshold but did not meet the extent criteria of 3 or more contiguous voxels (see Table 1). There were no voxels that exceeded the statistical threshold in the right hippocampal, prefrontal or fusiform ROIs.
TABLE 1.
Activation During Encoding Within A Priori ROIs that Predicts Subsequent Free Recall (Greater for Hits than Misses)
| Brain region | Volume (mm3) | Z score | P uncorrected | MNI coordinates |
|---|---|---|---|---|
| Left hippocampal formation | 135 | 3.64 | 0.0001 | −24, −18, −12 |
| Right hippocampal formation | – | |||
| Left perirhinal cortex | 54a | 3.34 | 0.0006 | −24, 0, −42 |
| Right entorhinal cortex | 54a | 3.40 | 0.0003 | 21, −15, −27 |
| Left fusiform cortex | 189 | 3.10 | 0.0009 | −42, −42, −27 |
| Right fusiform cortex | – | |||
| Left inferior prefrontal cortex | 270 | 4.65 | 0.000002 | −57, 15, 9 |
| Right inferior prefrontal cortex | – |
Volume refers to the size of the cluster of activated voxels (3mm3 voxel size × number of voxels in cluster); P uncorrected refers to the P-value of the voxel with the maximum Z score within the ROI; coordinates refer to the locus of the cluster peak in MNI305 stereotactic space.
These clusters did not meet the minimum a priori extent size of three or more contiguous voxels.
FIGURE 3.
Encoding-related activity that predicts free recall is present in left hippocampal (A), left fusiform (B), and left inferior frontal (C) regions. Brain maps display Hits > Misses statistical parametric map within each ROI mask at P < 0.005, overlaid on average brain template derived from the MPRAGE T1 structural data from the 15 subjects in this study (left hemisphere is displayed on left side of coronal images). Timecourses were derived from functional clusters exceeding statistical threshold within each ROI. Using MarsBaR, % BOLD signal change for each condition (Hits in blue and Misses in red) vs. Fixation was extracted from the functional cluster of voxels within each individual subject’s ROI and displayed with error bars representing the standard error of the mean.
For the hippocampal regions, differential activation between Hits vs. Misses localized—for the group—to the rostral (anterior) left hippocampal formation, at the junction of the uncal and body portions of the long axis of the hippocampal formation. For the parahippocampal regions, differential activation between Hits vs. Misses localized in the group maps to the rostral left perirhinal and caudal right entorhinal cortex. Since these parahippocampal activations did not exceed the a priori extent threshold, they should be interpreted cautiously as trend-level effects.
Consistent with the findings of the targeted a priori ROI analyses, results of whole-brain exploratory analyses for the Hits vs. Misses contrast demonstrated that greater left inferior prefrontal cortex (LIPC), left hippocampal, and left fusiform activity during encoding predicted subsequent free recall (Table 2). Additional regions were also identified, which represent candidates for future study, including two clusters in the left lateral parietal cortex and one in the right precentral gyrus (Table 2). In the left parietal cortex, one region was localized in the gyral crown of the inferior parietal lobule (MNI coordinates −51, −51, 42) and the other was localized near the fundus of the intraparietal sulcus (−27, −60, 39). The localization of these clusters was interpreted visually based on an average T1 volume from the 15 subjects in this study.
TABLE 2.
Exploratory Whole-Brain Analysis of Activation During Encoding that Predicts Subsequent Free Recall (Greater for Hits than Misses, P < 0.005)
| Brain region | Volume (mm3) | Z score | P uncorrected | MNI coordinates |
|---|---|---|---|---|
| Left inferior prefrontal cortex | 189 | 4.65 | 0.000002 | −57, 15, 9 |
| 324 | 3.51 | 0.0001 | −45, 48, 3 | |
| Left hippocampal formation | 135 | 3.64 | 0.0001 | −24, −18, −12 |
| Left fusiform cortex | 162 | 3.10 | 0.001 | −42, −42, −27 |
| Left lateral parietal cortex | 135 | 3.32 | 0.0002 | −27, −60, 39 |
| 216 | 3.32 | 0.0002 | 51, −51, 42 | |
| Right precentral gyrus | 135 | 4.31 | 0.00002 | 39, −18, 66 |
Volume refers to the size of the cluster of activated voxels (3mm3 voxel size × number of voxels in cluster); P uncorrected refers to the P-value of the voxel with the maximum Z score within the ROI; coordinates refer to the locus of the cluster peak in MNI305 stereotactic space.
Prefrontal-Medial Temporal Functional Connectivity Predicts Free Recall
The PPI analysis revealed that correlated activity within a specific set of regions was stronger during the encoding of items that were later recalled. Activity in the left hippocampal region was more highly correlated with activity in LIPC (4 voxel cluster, Z = 2.85, P < 0.002, MNI: −27, −15, −21) and right hippocampal (3 voxel cluster, Z = 3.41, P < 0.0003, MNI: −27, −9, −15) regions during Hits than during Misses. That is, when subjects attempted to learn a list of pictures, a stronger correlation between activity in the left hippocampal formation and activity in the right hippocampal formation and LIPC was present during the encoding of items that were subsequently freely recalled than during encoding of items not recalled.
Medial Temporal, Ventral Temporal, and Prefrontal Activity Predicts Intraindividual Differences in Free Recall Performance
For the All vs. Fixation contrast, the intraindividual (between-run, within-subject) analysis revealed that better free recall performance for any given list was associated with greater activity in the LIPC, right inferior prefrontal cortex (RIPC), and right caudal hippocampal formation during the run. That is, when subjects attempted to learn a list of pictures, greater activity in these prefrontal and medial temporal regions during the viewing of each picture in a given list—regardless of whether that picture was later recalled—predicted better subsequent free recall performance for the names of the pictures in that run (see Table 3 and Fig. 4). The peak activity in the LIPC in this analysis was localized in a more dorsal and caudal area than the peak activity in the Hits vs. Misses analysis above (see Fig. 5). For the Hits vs. Misses contrast, the analysis showed that better free recall performance was associated with greater differential activity in the left hippocampal formation (caudal to the main Hits>Misses finding described above), right caudal hippocampal formation, and left fusiform cortex. This analysis illustrates that better recall performance for a given list was predicted by greater activity in these medial and ventral temporal regions during the viewing of only those items that were successfully recalled compared to items that were not recalled (see Table 3 and Fig. 4).
TABLE 3.
Brain Regions that Exhibit Greater Activity in Association with Better Free Recall Performance (Items Recalled Per Run)
| Brain region | Volume | Z score | P uncorrected | MNI coordinates | R | T |
|---|---|---|---|---|---|---|
| Hits vs. Misses contrast | ||||||
| L. hippocampal formation | 81 | 3.41 | 0.0003 | −27 −9 −15 | 0.23 | 2.73 |
| 108 | 2.85 | 0.002 | −27 −15 −21 | |||
| R. hippocampal formation | 108 | 3.00 | 0.001 | 12 −36 −3 | 0.26 | 3.01 |
| L. fusiform cortex | 81 | 3.18 | 0.001 | −48 −45 −21 | 0.20 | 3.77 |
| All vs. Fixation contrast | ||||||
| L. inferior prefrontal cortex | 108 | 3.21 | 0.001 | −57 9 33 | 0.22 | 3.84 |
| R. inferior prefrontal cortex | 81 | 2.91 | 0.002 | 54 18 12 | 0.24 | 3.38 |
| 108 | 2.84 | 0.002 | 36 33 15 | |||
| R. hippocampal formation | 81 | 3.35 | 0.0004 | 15 −39 3 | 0.29 | 3.38 |
Volume refers to the size (in mm3) of the cluster of activated voxels; P uncorrected refers to the P-value of the voxel with the maximum Z score within the ROI; coordinates refer to the locus of the cluster peak in MNI305 stereotactic space; R indicates the average Pearson r correlation (average of all individual subject r values) between the % signal change in the peak voxel and free recall performance per run; T shows the t value of the one-sample t-test for this correlation for the group as a whole; L indicates left and R indicates right.
FIGURE 4. Dissociation of regional activity during encoding that predicts list-specific free recall performance ability.
(A) During encoding, greater left inferior prefrontal activity for all items—regardless of whether the items are recalled (All vs. Fixation contrast)—predicts better free recall performance on that run (number of hits per run). No such relationship is present in the left hippocampal formation. (B) During encoding, greater left hippocampal activity specifically for items that are later recalled compared to those that are not (Hits vs. Misses contrast)—predicts better free recall performance on that run. No such relationship is present in the left inferior prefrontal cortex. For illustrative purposes, the relative % BOLD signal change between each condition (All – Fixation (A) and Hits – Misses (B)) was extracted from the ROI peak voxel identified in the performance-related analysis described in the text. Dotted lines indicate standard error of the slope of the regression line.
FIGURE 5. Anatomically separate left inferior prefrontal cortical regions make distinct functional contributions during encoding, which predict subsequent free recall.
In the pars opercularis (yellow), activity is greater during the encoding of individual items that are recalled compared to those that are not (Hits vs. Misses; see also Figure 3 and Table 1). In the caudal inferior prefrontal cortex (purple), activity during the encoding of all items of a list (All vs. Fixation) is greater for lists in which subsequent recall performance is better (more items are recalled; see also Fig. 4 and Table 2). For this illustration, suprathreshold functional clusters were rendered on the partially inflated pial surface of the canonical SPM/MNI standard template (colin27) using the Freesurfer tksurfer tool.
Based on the results of this analysis, we hypothesized that free recall performance on a list would be better predicted by a combination of both list-specific prefrontal activity and items-specific hippocampal activity, than by the activity in one of the regions alone. To investigate this hypothesis, a stepwise multiple linear regression analyses was performed in which the independent variables were percent signal change from the peak coordinates of the regions identified in the analysis above (e.g., LIPC average % signal change for all stimuli per run, All vs. Fixation; left hippocampal % signal change for Hits - % signal change for Misses per run, Hits vs. Misses) and the dependent variable was the number of hits per run. Since the LIPC was the region with the highest T value, it was entered first. The remaining regions were subjected to a stepwise analysis to determine which of them would explain significantly greater intraindividual recall performance variance than the LIPC alone. In addition to the left hippocampal region (F = 6.1, P < 0.003), the left fusiform region also entered this model (F = 5.5, P < 0.001; overall model R = 0.32).
This result indicates that intraindividual differences in list-by-list free recall performance were best predicted by a combination of list-specific LIPC activity and item-specific left hippocampal and fusiform activity during encoding. This finding suggests that these brain regions compose a prefrontal-medial temporal-ventral temporal network, the activity in each component of which makes a distinct contribution during learning that is important for subsequent free recall performance.
DISCUSSION
The fMRI data presented here demonstrate that activity within prefrontal, MTL, and fusiform regions during encoding predicts successful subsequent free recall, and that these regions act together as a functionally connected prefrontal-temporal network. Furthermore, the components of this network make distinct contributions during encoding that predict recall performance ability. This paradigm produces robust encoding-related activity in occipitotemporal, MTL, and inferior prefrontal cortex. Greater activity is present during encoding of items that are later freely recalled—compared to items not freely recalled—in several regions, including the left hippocampal formation, left fusiform, and LIPC. Hippocampal-prefrontal regions function together as nodes of a distributed memory-related network (Mesulam, 1990): i.e., left hippocampal activity is more tightly correlated with right hippocampal and LIPC activity during encoding of items that are later freely recalled than those not recalled. Finally, across the ten lists, the presence of a combination of both greater list-specific LIPC activity and greater item-specific hippocampal and fusiform activity during encoding predicts intraindividual (within-subject) variance in the number of items recalled from each list. Thus, optimal free recall performance is subserved by the presence of distinct and interacting prefrontal-temporal processes that take place during learning.
In the MTL, differential encoding-related activity that predicts free recall is present in the left hippocampal formation, and trends are present in left perirhinal, and right entorhinal cortex. These findings are remarkably consistent with those from a previous experiment employing a free recall paradigm similar to the present paradigm, but which involved stimulus lists of visually-presented words (Strange et al., 2002). Activity in the same three MTL regions was predictive of free recall, with peak coordinates very near those identified in the present study. The differential roles of these MTL regions illuminated through studies of recognition memory (e.g., perirhinal cortex may be associated with familiarity-related while hippocampal formation may be associated with recollection-related processes—(Aggleton and Brown, 1999; Murray and Bussey, 1999; Davachi et al., 2003) implies that all of these processes are engaged during encoding that supports free recall, possibly because recall may depend on multiple specific mnemonic operations including recollection and familiarity. However, Staresina and Davachi (Staresina and Davachi, 2006) recently demonstrated that perirhinal activity during encoding was predictive of both recall and associative recognition, suggesting that it supports more than simply familiarity-based recognition processes. In addition to attempting to further investigate process-related contributors to free recall, future work is planned to investigate the possibility of sustained responses, as have been observed in entorhinal cortex (Fernandez et al., 1999) that predict free recall. Furthermore, it would be of interest to investigate specific primacy or recency effects, which have been shown to be associated with electrophysiologic patterns during encoding (Sederberg et al., 2003; Sederberg et al., 2006), but limited statistical power in this paradigm precluded such analyses in the present data.
Free recall is predicted by activity in several non-MTL regions, including left fusiform and LIPC (Brassen et al., 2006; Staresina and Davachi, 2006). Left fusiform activity was observed previously to be a predictor of free recall of initial list items, suggesting a primacy effect (Strange et al., 2002). In the present study, left fusiform activity predicted recall of list-body items, after items showing primacy and recency effects were removed. Since fusiform activity during encoding has been observed to predict subsequent recognition memory (Brewer et al., 1998; Wagner et al., 1998; Kirchhoff et al., 2000; Sperling et al., 2003), it may be that encoding-related visual processes important for recognition memory also support recall (Staresina and Davachi, 2006). It is also possible that, during encoding, deeper high-level visual processing of the perceptual and semantic elements of a visual stimulus influences whether these elements will be retrieved during the search processes involved in free recall. The ability to generate a mental image or otherwise recapitulate prototypical visual-semantic properties of a stimulus may be important for retrieval and the fusiform gyrus appears to be critically involved in this visual memory-related process (Wheeler et al., 2000; Vaidya et al., 2002). In addition, fusiform activity has been observed in picture naming, suggesting that it has a specific lexical-phonologic role in the encoding of namable objects (Kirchhoff et al., 2000).
The LIPC is involved in semantic decision-making (Gabrieli et al., 1998), which was the explicit task asked of the subjects during encoding; activity in this region has been observed previously when similar semantic decisions are made at encoding (Weis et al., 2004). Studies that have specifically manipulated “depth of encoding” processes have demonstrated both greater LIPC engagement during encoding and better subsequent memory performance, although such investigations have typically employed recognition memory tests (Kapur et al., 1994). LIPC activity is also consistently present during covert word generation (Cuenod et al., 1995), in which subjects typically engage while performing the present task. The generation of the name of the visual stimulus in this paradigm likely involves semantic search, response selection, and the phonologic aspects of covert naming, all of which have been associated with LIPC activity in previous studies (Bookheimer, 2002). These semantic and lexical processes may be engaged more deeply during encoding of items that are later recalled compared to those not recalled.
The ability to spontaneously recall previously learned material depends not only on the activity within frontal and temporal regions during encoding, but also on the degree to which activity is correlated between these regions. Such memory-related networks involving MTL regions have been identified in face-name associative encoding/recognition (Sperling et al., 2003) and in visual paired-associate learning (Ongur et al., 2005), but have not been investigated in relation to free recall. The PPI analysis employed in this study suggests that free recall is predicted by the degree of functional connectivity within a bilateral hippocampal-LIPC memory network. At the time of encoding, the activity of these regions is more highly correlated for individual items that will later be recalled than for items not recalled, suggesting that free recall depends upon the convergent engagement of the cognitive processes subserved by prefrontal and hippocampal brain regions.
The behavioral results from this task demonstrate notable variability of intraindividual (within-subject) free recall performance across the ten lists. In a block-design study, Fernandez et al. found that MTL activity at encoding correlated with the number of items recalled from word lists (Fernandez et al., 1998). The present data indicate functionally specific contributions within prefrontal and temporal regions to recall performance. For any given list, the presence of greater LIPC activity during the encoding of every item in the list (compared to visual fixation) predicts better recall performance on that list. Performance-related prefrontal activity in this task may reflect a behavioral set (e.g., relating to attention, motivation, or encoding strategy) that is present, while a list is being learned but is not specific to particular list items (i.e., recalled vs. nonrecalled items). In contrast, for any given list, the presence of greater left hippocampal activity specifically during the encoding of items that are later recalled— compared to those that are not—predicts better recall performance. In this case, hippocampal activity reflects item-specific processes that take place at the time of encoding that determine not only whether an item will be recalled but also the number of items that will be recalled. Greater hippocampal activity may subserve a more robust associative binding of various perceptual and semantic attributes of specific items during encoding that then confers better access to these attributes and their associations during the retrieval phase. However, there may be additional types of hippocampal contributions, given that the caudal right hippocampal formation demonstrated greater list-specific activity that predicted recall performance. In addition, left fusiform demonstrated greater item-specific encoding activity that predicted recall performance ability. Since multiple regression analysis demonstrates that intraindividual differences in free recall performance are best explained by a combination of both greater list-specific prefrontal and item-specific hippocampal and fusiform activity, it appears that the involvement—at encoding—of prefrontal attentional/strategic processes and hippocampal and fusiform mnemonic processes is an important determinant of recall performance.
In summary, we provide evidence that the ability to freely recall recently learned information is predicted by activity in a set of prefrontal and temporal lobe regions during the encoding of information. There are likely a host of additional brain processes that take place subsequent to encoding that are important for free recall, such as sustained neural activity during the delay between learning and recall and activity during the recall period itself. Yet the present data support the concept that free recall depends in part on the recruitment at encoding of progressively higher uni- and heteromodal cortical regions involved in perceptual-semantic processing, prefrontal regions involved in phonologic and semantic processing, and MTL regions involved in the integrative association of various features of the percept into a bound representation (Hasselmo and Wyble, 1997; Simons and Spiers, 2003). Furthermore, we provide evidence that prefrontal, MTL, and fusiform regions make distinct contributions during encoding that, together, predict how much information will later be recalled. Thus, dysfunction within any of these regions or in the interactions between regions (i.e., disconnection) may result in memory impairments, as can be seen in focal lesion-related amnesias (Warrington and Weiskrantz, 1982) and Alzheimer’s disease (Hyman et al., 1984). It will be interesting to determine whether a similar set of interacting brain regions is engaged during the free recall phase itself, which has been suggested by recent work showing that encoding-related processes in parahippocampal and fusiform cortex are recapitulated during recall (Polyn et al., 2005).
Acknowledgments
The authors thank Mary Foley, Larry White, and Jill Clark for technical assistance, and Russ Poldrack, Chantal Stern, and Itamar Kahn for helpful discussions.
Grant sponsor: NIA; Grant numbers: K23-AG22509, RO1-AG08441; Grant sponsor: NINDS; Grant number: K23-NS02189; Grant sponsor: NCRR; Grant number: P41-RR14075; Grant sponsor: Mental Illness and Neuroscience Discovery (MIND) Institute.
REFERENCES
- Aggleton JP, Brown MW. Episodic memory, amnesia, and the hippocampal-anterior thalamic axis. Behav Brain Sci. 1999;22:425–444. discussion 444–489. [PubMed] [Google Scholar]
- Albert MS. Memory decline: The boundary between aging and age-related disease. Ann Neurol. 2002;51:282–284. [PubMed] [Google Scholar]
- Bookheimer S. Functional MRI of language: New approaches to understanding the cortical organization of semantic processing. Annu Rev Neurosci. 2002;25:151–188. doi: 10.1146/annurev.neuro.25.112701.142946. [DOI] [PubMed] [Google Scholar]
- Brassen S, Weber-Fahr W, Sommer T, Lehmbeck JT, Braus DF. Hippocampal-prefrontal encoding activation predicts whether words can be successfully recalled or only recognized. Behav Brain Res. 2006;171:271–278. doi: 10.1016/j.bbr.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Brewer JB, Zhao Z, Desmond JE, Glover GH, Gabrieli JD. Making memories: Brain activity that predicts how well visual experience will be remembered. Science. 1998;281:1185–1187. doi: 10.1126/science.281.5380.1185. [DOI] [PubMed] [Google Scholar]
- Cuenod CA, Bookheimer SY, Hertz-Pannier L, Zeffiro TA, Theodore WH, Le Bihan D. Functional MRI during word generation, using conventional equipment: A potential tool for language localization in the clinical environment. Neurology. 1995;45:1821–1827. doi: 10.1212/wnl.45.10.1821. [DOI] [PubMed] [Google Scholar]
- Dale AM. Optimal experimental design for event-related fMRI. Hum Brain Mapp. 1999;8:109–114. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Das P, Kemp AH, Liddell BJ, Brown KJ, Olivieri G, Peduto A, Gordon E, Williams LM. Pathways for fear perception: Modulation of amygdala activity by thalamo-cortical systems. Neuroimage. 2005;26:141–148. doi: 10.1016/j.neuroimage.2005.01.049. [DOI] [PubMed] [Google Scholar]
- Davachi L, Mitchell JP, Wagner AD. Multiple routes to memory: Distinct medial temporal lobe processes build item and source memories. Proc Natl Acad Sci USA. 2003;100:2157–2162. doi: 10.1073/pnas.0337195100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Goncharova I, Sullivan MP, Forchetti C, Wilson RS, Bennett DA, Beckett LA, deToledo-Morrell L. MRI-derived entorhinal and hippocampal atrophy in incipient and very mild Alzheimer’s disease. Neurobiol Aging. 2001;22:747–754. doi: 10.1016/s0197-4580(01)00271-8. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Salat DH, Bates JF, Atiya M, Killiany RJ, Greve DN, Dale AM, Stern CE, Blacker D, Albert MS, Sperling RA. Medial temporal lobe function and structure in mild cognitive impairment. Ann Neurol. 2004;56:27–35. doi: 10.1002/ana.20163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egner T, Hirsch J. The neural correlates and functional integration of cognitive control in a stroop task. Neuroimage. 2005;24:539–547. doi: 10.1016/j.neuroimage.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Fernandez G, Weyerts H, Schrader-Bolsche M, Tendolkar I, Smid HG, Tempelmann C, Hinrichs H, Scheich H, Elger CE, Mangun GR, Heinze HJ. Successful verbal encoding into episodic memory engages the posterior hippocampus: A parametrically analyzed functional magnetic resonance imaging study. J Neurosci. 1998;18:1841–1847. doi: 10.1523/JNEUROSCI.18-05-01841.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez G, Brewer JB, Zhao Z, Glover GH, Gabrieli JD. Level of sustained entorhinal activity at study correlates with subsequent cued-recall performance: A functional magnetic resonance imaging study with high acquisition rate. Hippocampus. 1999;9:35–44. doi: 10.1002/(SICI)1098-1063(1999)9:1<35::AID-HIPO4>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II. Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Freire L, Mangin JF. Motion correction algorithms may create spurious brain activations in the absence of subject motion. Neuroimage. 2001;14:709–722. doi: 10.1006/nimg.2001.0869. [DOI] [PubMed] [Google Scholar]
- Freire L, Roche A, Mangin JF. What is the best similarity measure for motion correction in fMRI time series? IEEE Trans Med Imaging. 2002;21:470–484. doi: 10.1109/TMI.2002.1009383. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Gabrieli JD, Poldrack RA, Desmond JE. The role of left prefrontal cortex in language and memory. Proc Natl Acad Sci USA. 1998;95:906–913. doi: 10.1073/pnas.95.3.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garoff RJ, Slotnick SD, Schacter DL. The neural origins of specific and general memory: The role of the fusiform cortex. Neuropsychologia. 2005;43:847–859. doi: 10.1016/j.neuropsychologia.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Goncharova II, Dickerson BC, Stoub TR, deToledo-Morrell L. MRI of human entorhinal cortex: A reliable protocol for volumetric measurement. Neurobiol Aging. 2001;22:737–745. doi: 10.1016/s0197-4580(01)00270-6. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Boyett-Anderson JM, Eliez S, Schatzberg AF, Reiss AL, Menon V. Regional analysis of hippocampal activation during memory encoding and retrieval: fMRI study. Hippocampus. 2003;13:164–174. doi: 10.1002/hipo.10064. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Wyble BP. Free recall and recognition in a network model of the hippocampus: Simulating effects of scopolamine on human memory function. Behav Brain Res. 1997;89:1–34. doi: 10.1016/s0166-4328(97)00048-x. [DOI] [PubMed] [Google Scholar]
- Hyman BT, Van Hoesen GW, Damasio AR, Barnes CL. Alzheimer’s disease: Cell-specific pathology isolates the hippocampal formation. Science. 1984;225:1168–1170. doi: 10.1126/science.6474172. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Large-scale neurocognitive networks and distributed processing for attention, language, and memory. Ann Neurol. 1990;28:597–613. doi: 10.1002/ana.410280502. [DOI] [PubMed] [Google Scholar]
- Kapur S, Craik FI, Tulving E, Wilson AA, Houle S, Brown GM. Neuroanatomical correlates of encoding in episodic memory: Levels of processing effect. Proc Natl Acad Sci USA. 1994;91:2008–2011. doi: 10.1073/pnas.91.6.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhoff BA, Buckner RL. Functional-anatomic correlates of individual differences in memory. Neuron. 2006;51:263–274. doi: 10.1016/j.neuron.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Kirchhoff BA, Wagner AD, Maril A, Stern CE. Prefrontal-temporal circuitry for episodic encoding and subsequent memory. J Neurosci. 2000;20:6173–6180. doi: 10.1523/JNEUROSCI.20-16-06173.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EA, Bussey TJ. Perceptual-mnemonic functions of the perirhinal cortex. Trends Cogn Sci. 1999;3:142–151. doi: 10.1016/s1364-6613(99)01303-0. [DOI] [PubMed] [Google Scholar]
- Ongur D, Zalesak M, Weiss AP, Ditman T, Titone D, Heckers S. Hippocampal activation during processing of previously seen visual stimulus pairs. Psychiatry Res. 2005;139:191–198. doi: 10.1016/j.pscychresns.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Polyn SM, Natu VS, Cohen JD, Norman KA. Category-specific cortical activity precedes retrieval during memory search. Science. 2005;310:1963–1966. doi: 10.1126/science.1117645. [DOI] [PubMed] [Google Scholar]
- Sederberg PB, Kahana MJ, Howard MW, Donner EJ, Madsen JR. Theta and gamma oscillations during encoding predict subsequent recall. J Neurosci. 2003;23:10809–10814. doi: 10.1523/JNEUROSCI.23-34-10809.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sederberg PB, Gauthier LV, Terushkin V, Miller JF, Barnathan JA, Kahana MJ. Oscillatory correlates of the primacy effect in episodic memory. Neuroimage. 2006;32:1422–1431. doi: 10.1016/j.neuroimage.2006.04.223. [DOI] [PubMed] [Google Scholar]
- Simons JS, Spiers HJ. Prefrontal and medial temporal lobe interactions in long-term memory. Nat Rev Neurosci. 2003;4:637–648. doi: 10.1038/nrn1178. [DOI] [PubMed] [Google Scholar]
- Sperling R, Chua E, Cocchiarella A, Rand-Giovannetti E, Poldrack R, Schacter DL, Albert M. Putting names to faces: Successful encoding of associative memories activates the anterior hippocampal formation. Neuroimage. 2003;20:1400–1410. doi: 10.1016/S1053-8119(03)00391-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staresina BP, Davachi L. Differential encoding mechanisms for subsequent associative recognition and free recall. J Neurosci. 2006;26:9162–9172. doi: 10.1523/JNEUROSCI.2877-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange BA, Otten LJ, Josephs O, Rugg MD, Dolan RJ. Dissociable human perirhinal, hippocampal, and parahippocampal roles during verbal encoding. J Neurosci. 2002;22:523–528. doi: 10.1523/JNEUROSCI.22-02-00523.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya CJ, Zhao M, Desmond JE, Gabrieli JD. Evidence for cortical encoding specificity in episodic memory: Memory-induced re-activation of picture processing areas. Neuropsychologia. 2002;40:2136–2143. doi: 10.1016/s0028-3932(02)00053-2. [DOI] [PubMed] [Google Scholar]
- van der Kouwe AJ, Benner T, Fischl B, Schmitt F, Salat DH, Harder M, Sorensen AG, Dale AM. On-line automatic slice positioning for brain MR imaging. Neuroimage. 2005;27:222–230. doi: 10.1016/j.neuroimage.2005.03.035. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Schacter DL, Rotte M, Koutstaal W, Maril A, Dale AM, Rosen BR, Buckner RL. Building memories: Remembering and forgetting of verbal experiences as predicted by brain activity. Science. 1998;281:1188–1191. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]
- Warrington EK, Weiskrantz L. Amnesia: A disconnection syndrome? Neuropsychologia. 1982;20:233–248. doi: 10.1016/0028-3932(82)90099-9. [DOI] [PubMed] [Google Scholar]
- Weis S, Klaver P, Reul J, Elger CE, Fernandez G. Temporal and cerebellar brain regions that support both declarative memory formation and retrieval. Cereb Cortex. 2004;14:256–267. doi: 10.1093/cercor/bhg125. [DOI] [PubMed] [Google Scholar]
- Wheeler ME, Petersen SE, Buckner RL. Memory’s echo: Vivid remembering reactivates sensory-specific cortex. Proc Natl Acad Sci USA. 2000;97:11125–11129. doi: 10.1073/pnas.97.20.11125. [DOI] [PMC free article] [PubMed] [Google Scholar]