Abstract
Methoxychlor (MXC) reduces fertility in female rodents, decreases antral follicle numbers, and increases atresia through oxidative stress pathways. MXC also inhibits antral follicle growth in vitro. The mechanism by which MXC inhibits growth of follicles is unknown. The growth of follicles is controlled, in part, by cell cycle regulators. Thus, we tested the hypothesis that MXC inhibits follicle growth by reducing the levels of selected cell cycle regulators. Further, we tested whether cotreatment with an antioxidant, N-acetyl cysteine (NAC), prevents the MXC-induced reduction in cell cycle regulators. For in vivo studies, adult cycling CD-1 mice were dosed with MXC or vehicle for 20 days. Treated ovaries were subjected to immunohistochemistry for proliferating cell nuclear antigen (PCNA) staining. For in vitro studies, antral follicles isolated from adult cycling CD-1 mouse ovaries were cultured with vehicle, MXC, and/or NAC for 48, 72 and 96 hrs. Levels of cyclin D2 (Ccnd2) and cyclin dependant kinase 4 (Cdk4) were measured using in vivo and in vitro samples. The results indicate that MXC decreased PCNA staining, and Ccnd2 and Cdk4 levels compared to controls. NAC co-treatment restored follicle growth and expression of Ccnd2 and Cdk4. Collectively, these data indicate that MXC exposure reduces the levels of Ccnd2 and Cdk4 in follicles, and that protection from oxidative stress restores Ccnd2 and Cdk4 levels. Therefore, MXC-induced oxidative stress may decrease the levels of cell cycle regulators, which in turn, results in inhibition of the growth of antral follicles.
Keywords: methoxychlor, ovary, antral follicles, cell cycle
INTRODUCTION
Pesticides are an important part of our farming system, and have an important role in maintaining crop health. Their design requires that they are toxic to pests, and for this reason, no pesticides are considered to be completely safe. The widespread use of pesticides is a concern because humans and wildlife species are exposed to pesticides in the environment. Some of these pesticides act as endocrine disruptors that mimic estrogenic compounds, causing detrimental effects which can negatively impact adult reproductive health. Methoxychlor [1,1,1-trichloro-2,2-bis(4-methoxyphenyl)ethane; MXC] is a pesticide that is considered to be a model endocrine disruptor (Cummings, 1997) because it exerts estrogenic activities (Hall et al., 1997) and is known to affect early ovarian development, folliculogenesis, and adult ovarian function (Armenti et al., 2008; Borgeest et al., 2004).
Although MXC has been considered a potent environmental toxicant for several years (Cummings, 1997), it was not until more recently that MXC was considered to be a reproductive toxicant (Borgeest et al., 2002a; Borgeest et al., 2002b; Borgeest C et al., 2004; Gupta et al., 2006a; Gupta et al., 2006b; Latchoumycandane et al., 2002a; Latchoumycandane et al., 2002b; Latchoumycandane et al., 2002c; Miller et al., 2005; Miller et al., 2006; Schuh et al., 2005). In male sheep and rats, MXC reduces the weight of the testes, prostate, and seminal vesicles and disturbs spermatogenesis (Bal, 1984; Jackson et al., 1975; Tullner et al., 1962). In female rodents, MXC causes ovarian atrophy (Eroschenko et al., 1995) and decreases the ability of ovarian cells to synthesize and secrete steroids (Zachow and Uzumcu, 2006). Developmental exposure to MXC causes reduced litter sizes, irregular estrous cyclicity, decreased progesterone levels, increased luteinizing hormone levels, and abnormal folliculogenesis (Armenti et al., 2008; Zachow and Uzumcu, 2006). Further, postnatal exposure to MXC induces atresia (an apoptotic process) of antral follicles in vivo (Borgeest et al., 2002b; Borgeest et al., 2004) and inhibits growth and increases atresia of antral follicles in vitro (Miller et al., 2005). This is of concern because antral follicles are required for fertility as they are the only follicles that are capable of releasing eggs for fertilization and producing steroid hormones that regulate menstrual/estrous cyclicity and maintain the female reproductive tract (Hirshfield, 1991). Despite the potential adverse reproductive outcomes associated with MXC exposure, little is known about the mechanisms by which MXC inhibits antral follicle growth.
The proliferation and differentiation of granulosa cells is an integral component of follicle growth. Proliferation of granulosa cells begins at a slow and even rate as the follicles are recruited from the primary stage to secondary and antral stages (McGee and Hsueh, 2000). The regulation of growth and proliferation of cells is a tightly controlled process dependent upon cell cycle related factors (Shackelford et al., 2001). Thus, the proposed work was designed to test the hypothesis that MXC inhibits follicle growth by reducing the levels of selected cell cycle related factors. To test this hypothesis, we examined the effect of MXC on antral follicle growth and alterations in the levels of proliferating cell nuclear antigen (PCNA) and expression of selected cell cycle regulators such as cyclin D2 (Ccnd2) and cyclin dependant kinase-4 (Cdk4). We chose to focus on PCNA, Ccnd2, and Cdk4 because these factors are required for the first steps in cell cycle progression, the progression of cell cycle from G1 to S phase. Further, this work was designed to determine if an antioxidant, N-acetyl cysteine (NAC), which is known to protect antral follicles from MXC-induced growth inhibition (Gupta et al., 2006a), also protects antral follicles from MXC-induced changes in cell cycle regulators. This hypothesis is supported by the fact that reactive oxygen species, such as hydrogen peroxide (H2O2) can cause DNA damage, resulting in blockage of cell cycle progression (Shackelford et al., 2001). Further, H2O2 has been found to induce a G1 arrest (Clopton and Saltman, 1995), which can be attenuated by the application of antioxidants (Clopton and Saltman, 1995). Since previous work has shown that MXC induces oxidative stress, DNA damage, and inhibits growth of antral follicles, and that these effects are attenuated by the antioxidant, NAC (Gupta et al., 2006a; Gupta et al., 2006b; Miller et al., 2005), we examined whether NAC treatment restores levels of cell cycle regulators to control levels.
MATERIALS AND METHODS
Chemicals
MXC powder (99%) was purchased from Chemservice (West Chester, PA). For the in vivo experiments, mice were dosed with 16–64 mg/kg/day for 20 days. For the 16 mg/kg dose, 100 mg MXC was mixed with 10 ml sesame oil; for the 32 mg/kg dose, 200 mg MXC was mixed with 10 ml sesame oil; for the 64 mg/kg dose, 400 mg MXC was mixed with 10 ml sesame oil. We concentrated the amount of chemical with each subsequent dose so that the mice received comparable injections based on their weights. This eliminated the need to give a greater volume injection to mice receiving higher doses than those receiving lower doses. On an average, a mouse weighing 25 grams (gms) received 40 µl of sesame oil. The amount of sesame oil was increased by 3 µl per gms increase in mouse weight. For in vitro experiments, stock solutions of MXC were prepared using dimethylsulfoxide (DMSO) (Sigma, St. Louis, MO) as the solvent various concentrations (133, 13.3, and 1.33 mg/ml) that allowed an equal volume to be added to culture wells for each treatment group to control for solvent concentration. Final concentrations of MXC in culture were 1, 10, and 100 µg of MXC per ml. The final concentration of DMSO in culture was 0.075%. NAC was purchased from Sigma-Aldrich Corp; St. Louis, MO. Final concentrations of NAC were 0.1, 1, and 5 mM. We chose NAC because it has been shown to protect from MXC-induced growth inhibition in antral follicles (Gupta et al., 2006a). All the doses mentioned above were based on our previous studies (Borgeest et al., 2004; Gupta et al., 2006a; Gupta et al., 2006b; Miller et al., 2005).
Animals
Cycling female CD-1 (Charles River Laboratories, Charles River, CA) mice (32–35 days old) were housed 4 animals per cage at the University of Illinois at Urbana-Champaign, Veterinary Medicine Animal Facility and provided food and water for ad libitum consumption. Temperature was maintained at 22 ± 1 °C and animals were subjected to 12-hour light-dark cycles. For the in vitro experiments, mice at 39 days old were euthanized, their ovaries collected, and follicles were isolated as described below. For the in vivo experiments, mice (beginning at 39 days old) were dosed with 16, 32, or 64 mg/kg/day of MXC or sesame oil (vehicle) via intraperitoneal injection for 20 continuous days and euthanized when in estrus. Previous studies already determined that 20 days of continuous dosing does not cause overt toxicity, but it does induce ovarian atresia at the 32–64 mg/kg/day doses of MXC (Borgeest et al., 2002b; Borgeest et al., 2004). The Institutional Animal Use and Care Committee at the University of Illinois at Urbana-Champaign approved all procedures involving animal care, euthanasia, and tissue collection.
Measurement of Granulosa Cell Proliferation In Vivo
Measurements of granulosa cell proliferation in ovaries from MXC-treated mice were performed by immunostaining for PCNA as previously described (Barnett et al., 2007; Masters et al., 2007). PCNA was selected as the proliferation marker for these studies as it has been used successfully and reliably in ovarian tissues by several investigators (Barnett et al., 2007; Masters et al., 2007). Further, cyclin-d/cdk dimerization results into release of several factors required in S phase including PCNA (Lee et al., 1995). Therefore, measurement of PCNA serves as a marker of healthy cell growth. To measure PCNA, ovaries were removed from MXC-treated mice, fixed in Dietrick’s solution, dehydrated in gradient alcohols, cleared in xylene, and embedded in paraffin (VWR Scientific, Baltimore, MD). Sections were cut at 5 µm and subjected to staining for PCNA using a commercially available monoclonal antibody (1:100 dilution; EMD Chemicals Inc., Gibbstown, NJ). The biotinylated streptavidin Histomouse-SP system (Zymed Laboratories, San Francisco, CA) with background blocking was used with 3-amino-9-ethyl carbazole chromogenic visualization and Mayer hematoxylin counterstaining. In all experiments, negative controls (no primary antibody) were run in parallel.
The levels of PCNA staining in MXC-treated mice were quantified using published methods (Barnett et al., 2007; Masters et al., 2007). Specifically, images from stained sections obtained from at least three different animals per treatment group were digitally captured using a Leica DFC 290 camera and analyzed using the ImageJ software (http://rsb.info.nih.gov/nih-image/). Digital images were initially converted to 8-bit grayscale images and then converted to pseudo colored images. Colors were based on relative stain intensity, as defined digitally. Areas with no staining appeared black, while areas with the most intense staining appeared deep orange to red. The PCNA labeling index, expressed as a percentage of positively stained area per follicle, was determined for all follicles in each section (7–20 follicles per section from at least three separate animals per group). Values for PCNA labeling indices were generated by dividing the PCNA positively stained area per follicle by the total area per follicle and multiplying the values by 100. All ovaries were analyzed without knowledge of treatment group to avoid bias.
Measurements of CDK4 and CCND2 Protein Levels
Antral follicles from MXC-treated mice were collected and immediately snap-frozen. Each frozen sample was homogenized in lysis buffer (20 mM Tris-HCl [pH 8.0], 1% NP40, 140 mM NaCl, 2 mM EDTA, 10 mM NaF, 10% glycerol) that contained a protease inhibitor cocktail tablet (Roche Diagnostics, Mannheim, Germany). After homogenization, the amount of protein in each sample was evaluated using a bicinchoninic acid (BCA) protein assay kit (Pierce, Rockford, IL). Protein lysates (10 µg/lane for CDK4 and CCND2) were subjected to western blot analysis using anti-CDK4 (1:2000; Abcam, Cambridge, MA) and anti-CCND2 (1:2000; Abcam, Cambridge, MA) polyclonal antibodies. A HRP-conjugated rabbit polyclonal mouse IgG antibody (1:2000; Abcam, Cambridge, MA) was used as the secondary antibody. Immune complexes were visualized using an enhanced chemiluminescence (ECL) detection kit (Cell Signaling Technologies, Beverly, MA). To ensure that proteins were loaded in equal amounts in each lane, the blots were stripped using the ImmunoPure elution buffer (Pierce, Rockford, IL). The blots were then incubated with β-actin (1:300 dilution; Santa Cruz Biotechnology, Santa Cruz, CA) followed by a HRP-conjugated anti-mouse polyclonal antibody (1:100; Santa Cruz Biotechnology). Scanning densitometry using the ImageJ software was used to compare the protein levels. All values were normalized to β-actin.
In Vitro Follicle Culture
Female CD-1 mice were euthanized on postnatal day (PND) 39 and their ovaries removed. Antral follicles were isolated mechanically from the ovary based on relative size and cleaned of interstitial tissue using fine watchmaker forceps (Gupta RK et al., 2006a; Miller KP et al., 2005). Sufficient numbers of antral follicles for experimental significance were isolated from unprimed mouse ovaries; follicles from 2–4 mice were isolated per day providing approximately 20–40 antral follicles from each mouse. Upon isolation, follicles were placed individually in wells of a 96-well culture plate with unsupplemented α-minimal essential media (α-MEM) prior to treatment. Each experiment contained a minimum of 10–16 follicles per treatment.
Dose response regimens of MXC (1–100 µg/ml) and DMSO controls were individually prepared in supplemented α-MEM. In addition, regimens of MXC 100 µg/ml, MXC 100 µg/ml + NAC (0.1 – 5 mM), and DMSO controls were individually prepared in supplemented α-MEM for experiments involving NAC. An equal volume of chemical was added for each dose to control for the amount of vehicle in each preparation. Follicles were isolated as stated above and follicle culture was performed as described in previous publications (Cortvrindt and Smitz, 2002; Gupta et al., 2006a; Miller et al., 2005).
Analysis of Follicle Growth
Antral follicles were cultured as described above for 96 hrs. Follicle growth was examined at 24 hr intervals by measuring follicle diameter on two perpendicular axes with an inverted microscope equipped with a calibrated ocular micrometer. Antral follicles were considered to be those follicles with diameters of 200 µm or greater (Cortvrindt and Smitz, 2002; Gupta et al., 2006a; Miller et al., 2005), which correlates with histological appearance of these follicles. Follicle diameter measurements were averaged among treatment groups and plotted to compare the effects of chemical treatments on growth over time. Data were presented as percent change over time.
Quantitative Real Time Polymerase Chain Reaction (qPCR)
For in vivo studies, follicles were isolated from MXC-treated mice and snap-frozen at −80°C until real time polymerase chain reaction (qPCR) analysis was performed. For in vitro studies, female CD-1 mouse antral follicles were cultured as described above for 96 hrs. At selected times, the follicles were collected and snap-frozen at −80°C until qPCR analysis. Total RNA was extracted from follicles using the RNeasy Mini Kit (Qiagen, Inc., Valencia, CA) according to the manufacturer’s protocol. Reverse-transcriptase generation of cDNA was performed with 0.3–1 µg of total RNA using an Omniscript RT kit (Qiagen, Inc., Valencia, CA) with random primers according to the manufacturer’s protocols. qPCR was conducted using an MJ Research Chromo4 Real Time PCR machine (MJ Research, Inc., Waltham, MA) and accompanying software according to the manufacturer’s instructions. A standard curve was generated from five serial dilutions of one of the samples, thus allowing analysis of the amount of cDNA in the exponential phase. Primer sequences used were as follows: Ccnd2 (forward) 5′-AGC-TGT-CCC-TGA-TCC-GCA-AG -3′, (reverse) 5′- GTC-AAC-ATC-CCG-CACGTC- TG -3′; Cdk4 (forward) 5′- TGG-CTG-CCA-CTC-GAT-ATG-AAC -3′, (reverse) 5′- CCT-CAG-GTC-CTG-GTC-TAT-ATG -3′ and, β-actin (forward) 5′- GGG-CACAGT- GTG-GGT-GAC-3′, (reverse) 5′- CTG-GCA-CCA-CAC-CTT-CTAC -3′. β-actin was used as an internal standard for each sample. qPCR analysis was performed using 3 µl cDNA, forward and reverse primers (5 pmol) for Ccnd2, Cdk4 or β-actin, in conjunction with a DyNAmo SYBR Green qPCR kit (Finnzymes, c/o Bio-Rad Laboratories, Hercules, CA). An initial incubation of 95°C for 10 min was followed by denaturing at 94°C for 10 s, annealing at 57°C (Ccnd2) or 55°C (Cdk4 and β-actin) for 10 s, and extension at 72°C for 10 s, for 50 cycles, followed by final extension at 72°C for 10 min. A melting curve was generated at 55–90°C to monitor the generation of a single product. The software also generated a standard curve. Final values were calculated and expressed as the ratio Ccnd2:β-actin and Cdk4:β-actin. All experiments were performed in triplicate. We chose to investigate expression of Ccnd2 and Cdk4 expression at time points where follicle growth was observed to be similar (48 hr) and at where it was significantly different (72 and 96 hrs) in MXC treated groups compared to controls. For experiments involving NAC treatment, we used follicles treated with the highest dose of MXC (100 µg/ml) because it is a dose that blocks follicle growth (Gupta et al., 2006a; Miller et al., 2005).
Statistical Analysis
Data were expressed as means ± SEM and comparisons between experimental groups were made using analysis of variance (ANOVA) followed by Tukey’s post hoc comparison. Statistical significance was assigned at p < 0.05.
RESULTS
Effect of In Vivo MXC Treatment on PCNA Levels
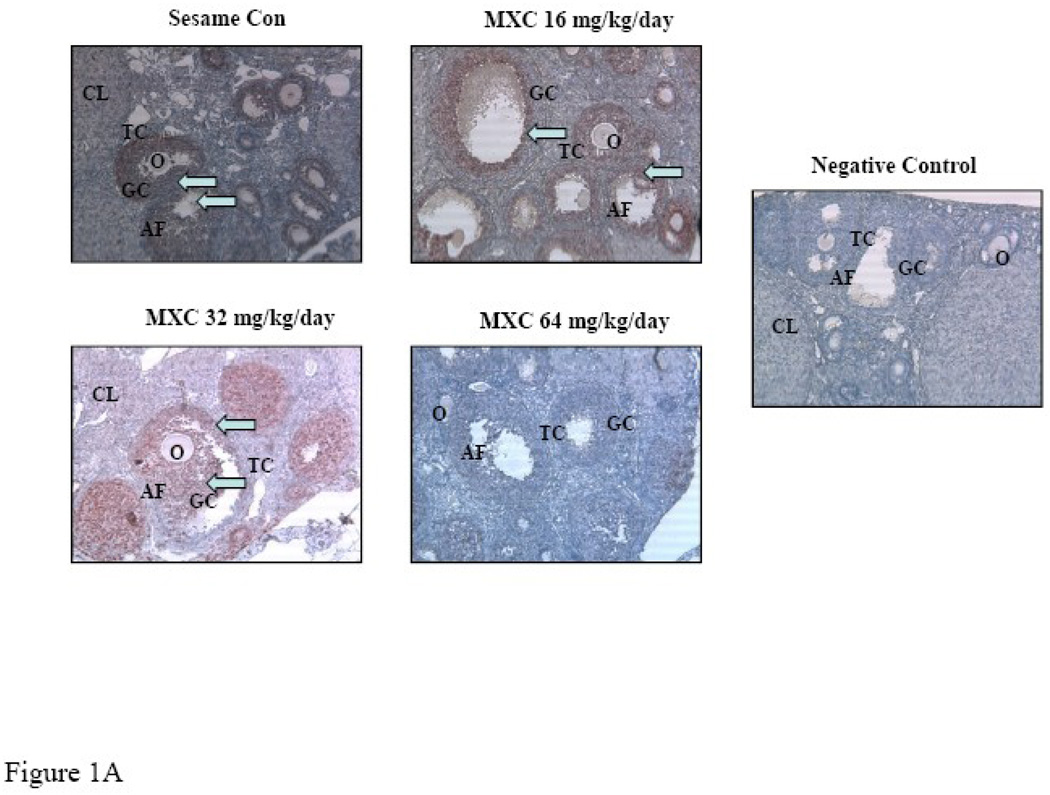
Ovaries collected from mice dosed with sesame oil or MXC 16, 32 or 64 mg/kg/day for 20 days were processed for immunohistochemical staining to detect the presence of PCNA as described in Methods. The control ovaries contained positive red staining in the granulosa cells of antral follicles as well as other smaller follicles (Fig. 1A). The MXC-treated ovaries, however, contained less positive PCNA (red) staining compared to controls (Fig. 1A). Further, increasing MXC doses reduced PCNA staining in all follicle types, but primarily in antral follicles (Fig. 1A). No PCNA staining was observed in the negative controls (Fig. 1A).
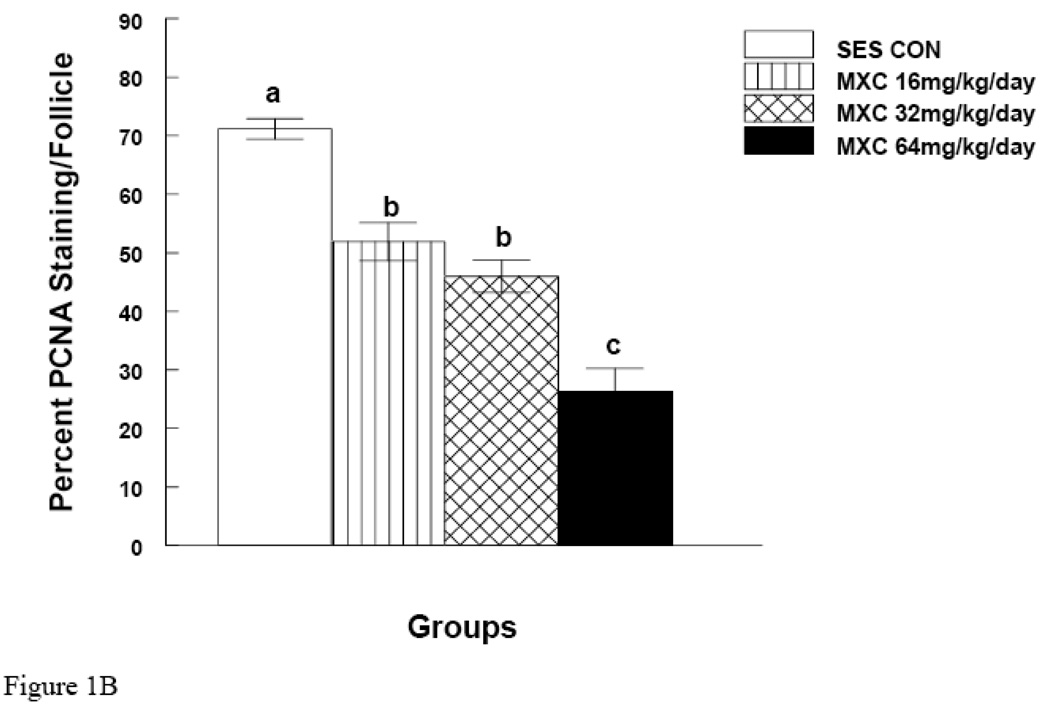
Figure 1. Effect of In Vivo MXC Treatment on PCNA Levels.
Mice were dosed with sesame oil (control) or MXC 16, 32, or 64 mg/kg/day for 20 days. After dosing, ovaries were collected and processed for immunohistochemical staining for the presence of PCNA as described in Methods. For negative controls, sections were incubated without primary antibody. White arrows indicate positive red staining. O=oocyte, CL=corpus luteum, AF=antral follicle, GC=granulosa cells, and TC=theca cells. Three mouse ovaries from three separate dosing experiments were used for PCNA staining (n = 9 mice). Pictures are representative of at least three separate experiments (panel A). Staining was quantified as described in Methods and presented as a bar graph (panel B). Graph represents means ± SE from three separate experiments. Bars with different letters are significantly different from each other (n = 3, p ≤ 0.05).
To quantify MXC-induced changes in PCNA staining, slides were quantified as described in the Methods. The results indicate that MXC significantly decreased PCNA levels in a dose dependant manner (Fig. 1B). MXC treatments of 16, 32, and 64 mg/kg/day decreased PCNA staining by 27 ± 3.2, 35 ± 2.7, and 65 ± 4 % compared to controls (p < 0.05).
Effect of In Vivo MXC Treatment on mRNA Expression of Ccnd2 and Cdk4 in Antral Follicles
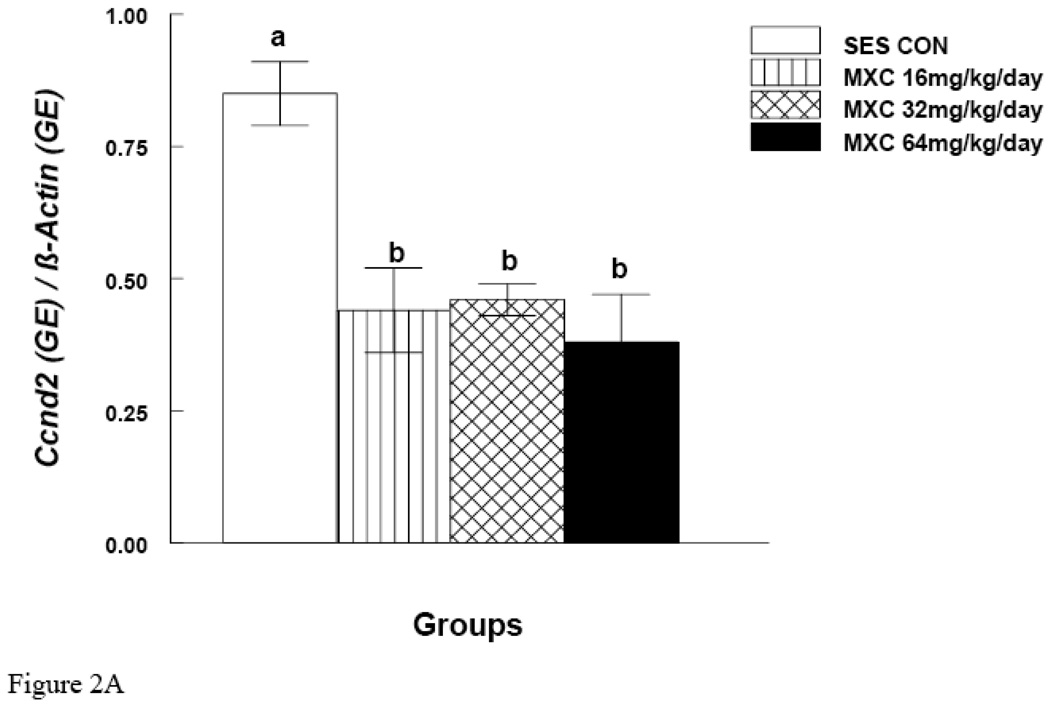
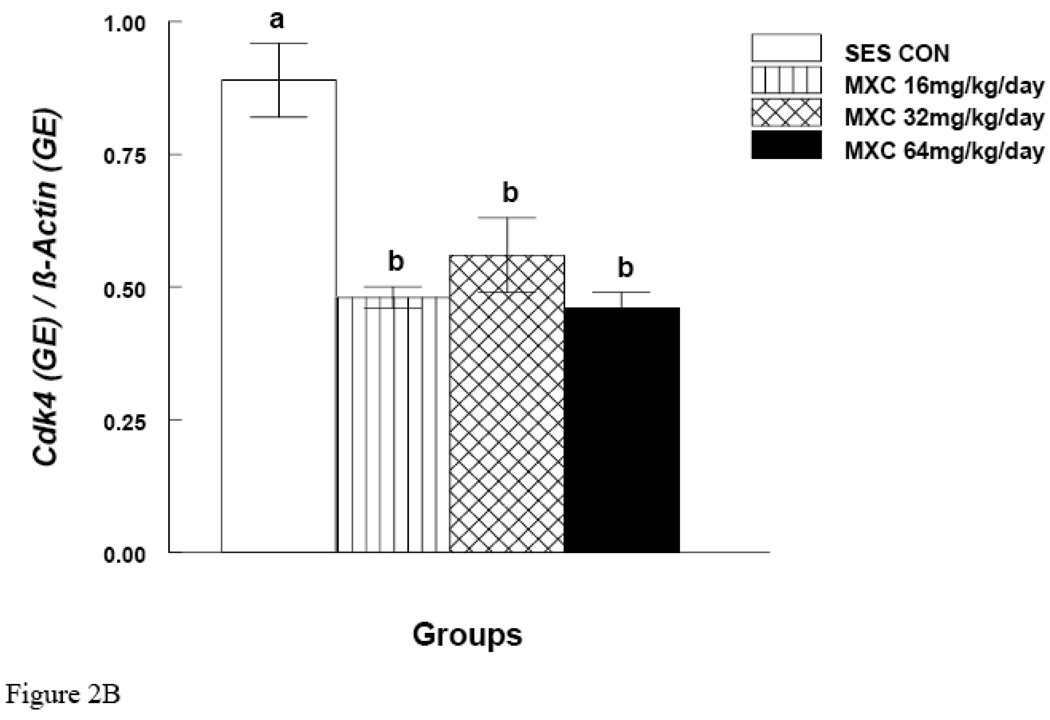
MXC (16, 32 and 64 mg/day) significantly decreased Ccnd2 mRNA expression in antral follicles compared to controls (p < 0.05) (Fig. 2A). MXC (16, 32 and 64 mg/day) also significantly decreased Cdk4 mRNA expression in antral follicles compared to controls (p < 0.05) (Fig. 2B). MXC (16–64 mg/day) did not affect the mRNA expression of the housekeeping gene (β-actin) in any treatment groups.
Figure 2. Effect of In Vivo MXC Treatment on mRNA Expression of Ccnd2 and Cdk4 in Antral Follicles.
Antral follicles were isolated from CD-1 mice dosed with either sesame oil (control) or MXC (16–64 mg/kg/day) for 20 days, and subjected to qPCR for analysis of Ccnd2 (panel A) and Cdk4 (panel B) mRNA levels. All values were normalized to β-actin as a loading control. Graph represents means ± SE from three separate experiments. Bars with different letters are significantly different from each other (n = 20–40 follicles per treatment, p ≤ 0.05).
Effect of In Vivo MXC Treatment on Protein Levels of CCND2 and CDK4 in Antral Follicles
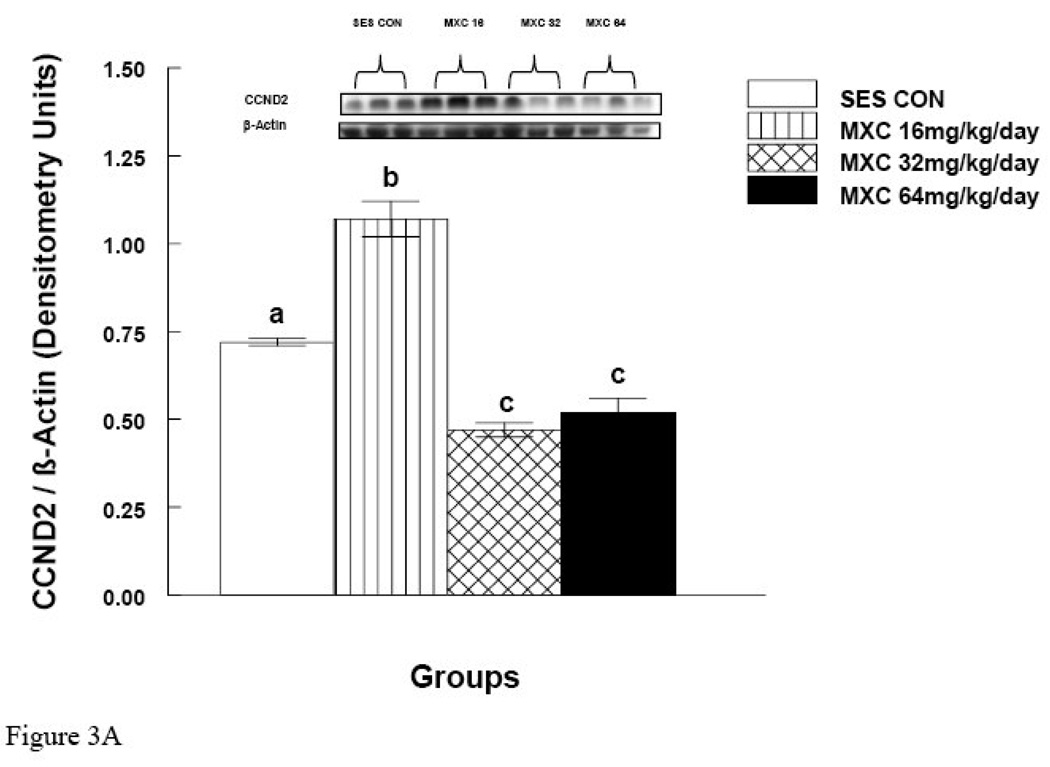
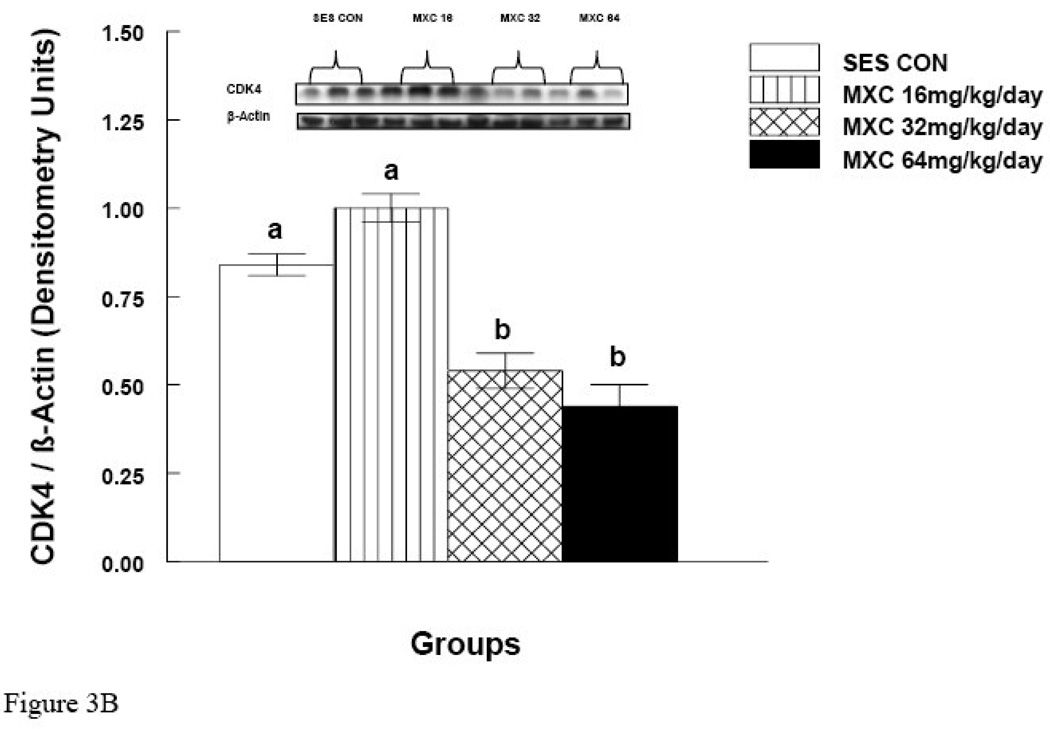
MXC (32 and 64 mg/day) significantly decreased CCND2 protein levels compared to controls (p < 0.05) (Fig. 3A). Surprisingly, however, MXC at 16 mg/day significantly increased the levels of CCND2 protein compared to controls (Fig. 3A). MXC at 16 mg/day did not change CDK4 levels compared to controls (Fig. 3B), but MXC at 32 and 64 mg/day significantly decreased CDK4 protein levels compared to controls (p < 0.05) (Fig. 3B).
Figure 3. Effect of In Vivo MXC Treatment on Protein Levels of CCND2 and CDK4 in Antral Follicles.
Antral follicles were isolated from CD-1 mice dosed with either sesame oil (control) or MXC (16–64 mg/kg/day) for 20 days, and subjected to western blot analysis of CCND2 (panel A) and CDK4 (panel B) protein levels. All values were normalized to β-actin as a loading control. Each panel is divided into upper and lower halves. The upper half is a representative picture of western blot and the lower half is a graph of showing quantification of the western blot as described in Methods. Graph represents means ± SE from three separate experiments. Bars with different letters are significantly different from each other (n = 20–40 follicles per treatment, p ≤ 0.05).
Effect of In Vitro MXC Treatment on mRNA expression of Ccnd2 and Cdk4 in Antral Follicles
Similar to previous experiments (Gupta et al., 2006a; Miller et al., 2005), MXC (1, 10 and 100 µg/ml) in the current experiments significantly inhibited antral follicle growth at 72 and 96 hrs compared to control follicles. At 72 hrs, the percent change of growth over time was: DMSO = 120.5 ± 1.7; MXC 1 µg/ml = 112.8 ± 1.5; MXC 10 µg/ml = 105.2 ± 1.2; MXC 100 µg/ml = 101.3 ± 0.4. At 96 hrs, the percent change of growth over time was: DMSO = 142.7 ± 3; MXC 1 µg/ml = 123.4 ± 2.0; MXC 10 µg/ml = 107.3 ± 2.1; MXC 100 µg/ml = 99.8 ± 1.0. Further, similar to previous experiments (Gupta et al., 2006a), NAC in these experiments (1 and 5 mM) blocked the effect of MXC-induced growth inhibition. Specifically, MXC (100 µg/ml) + NAC (1 and 5 mM)-treated follicles had similar growth over time compared to DMSO controls. At 72 hrs, the percent change of growth over time was: DMSO = 125.76 ± 1.3; DMSO + NAC 0.1 mM = 126.32 ± 1.4; DMSO + NAC 1 mM = 128.1 ± 1.5; DMSO + NAC 5 mM = 130.4 ± 1.6; MXC 100 µg/ml = 102.7 ± 0.6; MXC 100 µg/ml + NAC 0.1mM = 105.2 ± 0.5; MXC 100 µg/ml + NAC 1mM = 125.1 ± 1.8; MXC 100 µg/ml + NAC 5mM = 125.8 ± 2.0. At 96 hrs, the percent change of growth over time was: DMSO = 145.9 ± 1.2; DMSO + NAC 0.1 mM = 141 ± 1.3; DMSO + NAC 1 mM = 142.4 ± 1.5; DMSO + NAC 5 mM = 144.1 ± 1.5; MXC 100 µg/ml = 101.2 ± 0.7; MXC 100 µg/ml + NAC 0.1mM = 110.1 ± 0.1; MXC 100 µg/ml + NAC 1mM = 139.5 ± 2.1; MXC 100 µg/ml + NAC 5mM = 145.4 ± 0.7.
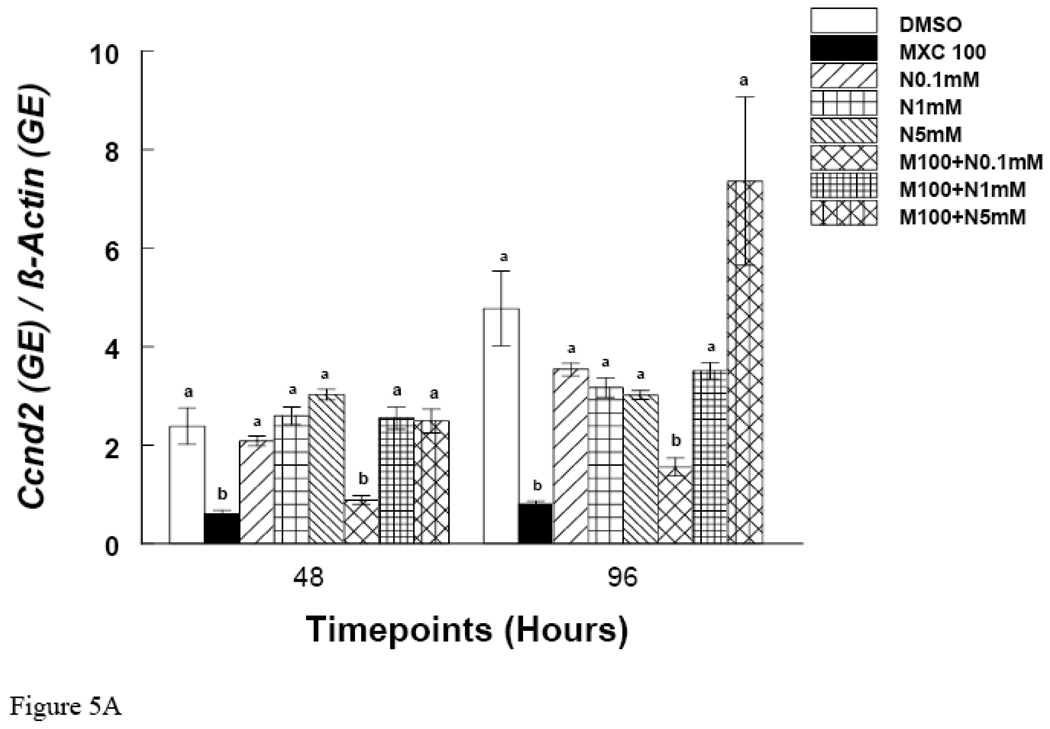
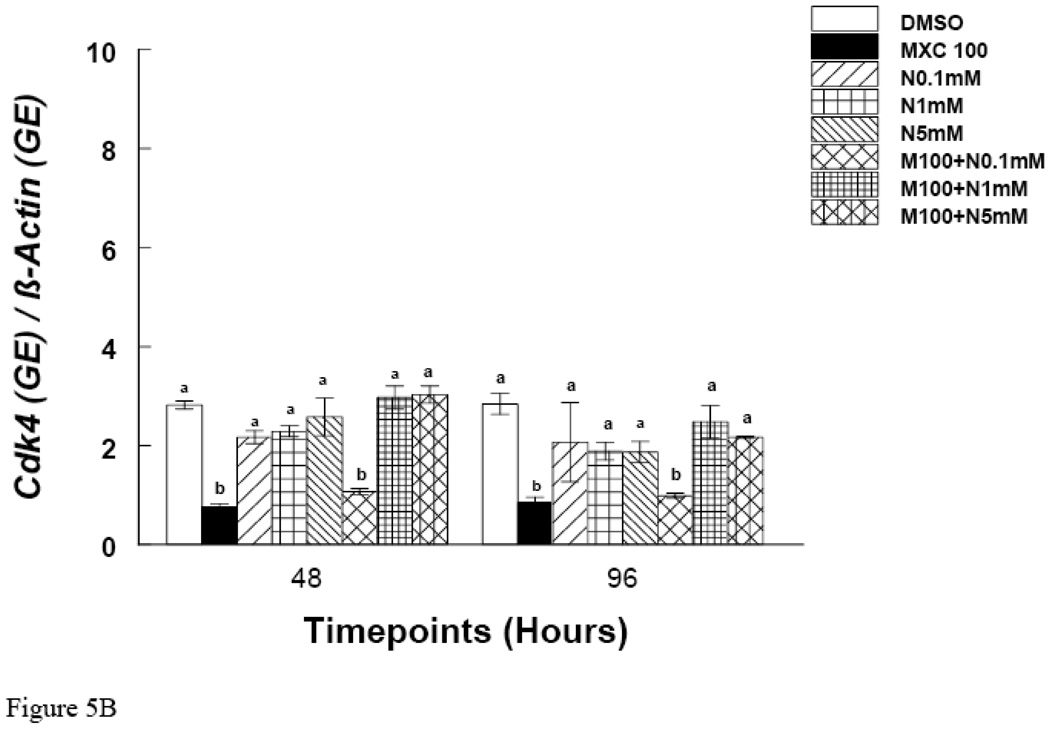
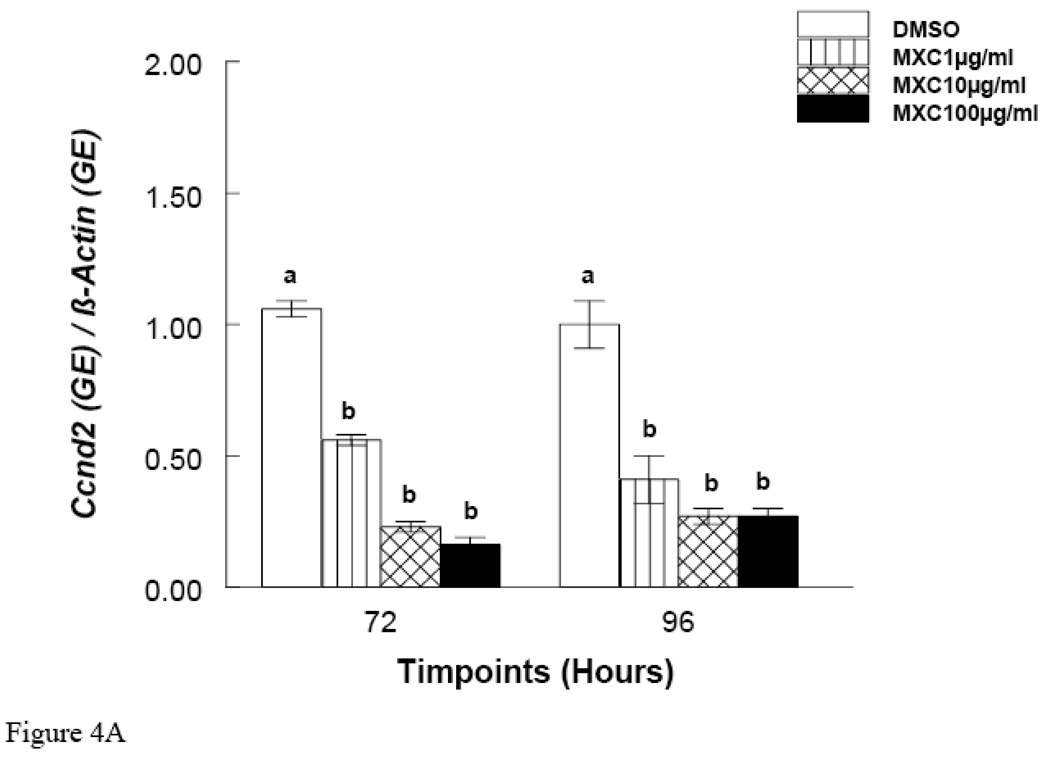
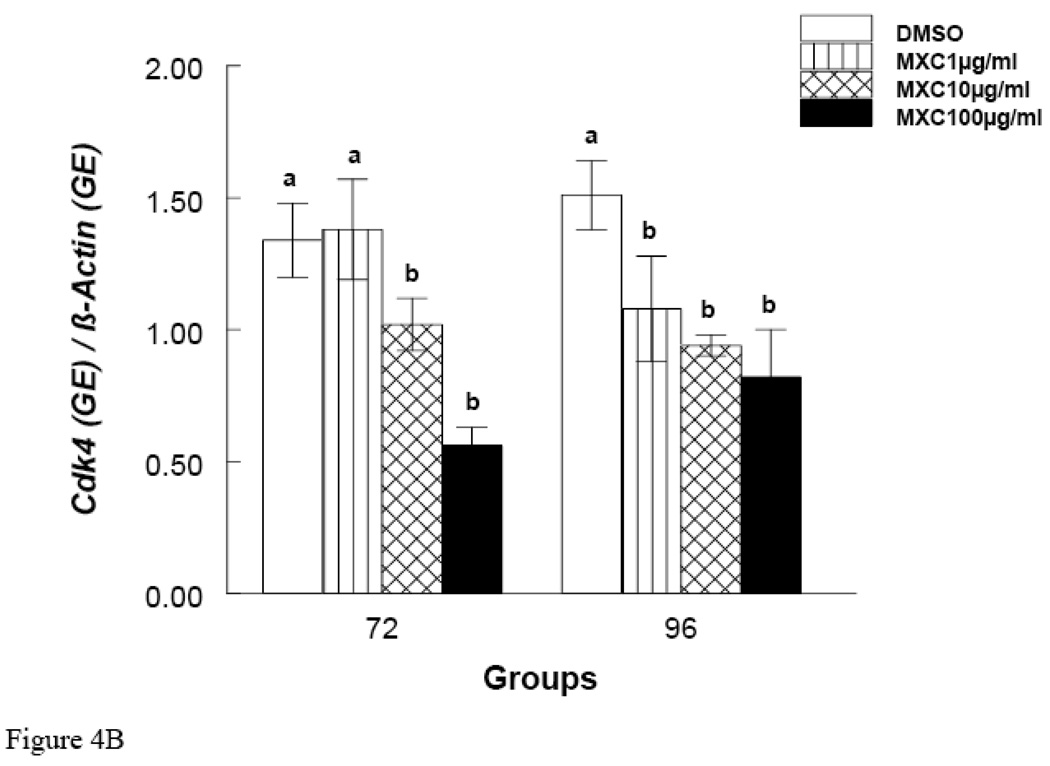
Since MXC treatment did not inhibit growth at 48 hrs, but did at 72 and 96 hrs, these time points were chosen to measure mRNA expression of Ccnd2 and Ckd4. The results show that at the 48 hr time point, MXC (100 µg/ml) reduced Ccnd2 and Cdk4 expression compared to controls (Fig. 5 A and B). Further, at 72 and 96 hrs, MXC (1–100 µg/ml) significantly decreased Ccnd2 expression compared to controls (Fig. 4A). Further, at the 72 hr time point MXC (10 and 100 µg/ml) significantly reduced Cdk4 expression compared to controls (Fig. 4B). At 96 hrs, MXC (1–100 µg/ml) significantly decreased Cdk4 expression compared to controls (Fig. 4B). MXC did not affect β-actin expression in any treatment groups.
Figure 5. Effect of In Vitro NAC and MXC Co-Treatment on Ccnd2 and Cdk4 mRNA Expression.
Antral follicles from CD-1 mice were exposed in vitro to MXC 100 µg/ml and MXC 100 µg/ml + NAC (0.1–5 mM) for 48 and 96 hrs and subjected to qPCR for analysis of Ccnd2 (Panel A) and Cdk4 (Panel B) mRNA levels (n = 10–16 follicles per treatment). All values were normalized to β-actin as a loading control. Graph represents means ± SE from three separate experiments. Bars with different letters are significantly different from each other within the time point (n = 12–16 follicles per treatment, p ≤ 0.05).
Figure 4. Effect of In Vitro MXC Treatment on mRNA expression of Ccnd2 and Cdk4 in Antral Follicles.
Antral follicles from CD-1 mice were exposed in vitro to MXC (1–100 µg/ml) for 72 and 96 hrs and subjected to qPCR for analysis of Ccnd2 (Panel A) and Cdk4 (Panel B) mRNA levels. All values were normalized to β-actin as a loading control. Graph represents means ± SE from three separate experiments. Bars with different letters are significantly different from each other within the time point (n = 10–16 follicles per treatment, p ≤ 0.05).
Effect of In Vitro NAC and MXC Co-Treatment on Ccnd2 and Cdk4 mRNA Expression
At 48 and 96 hrs, MXC 100 µg/ml significantly reduced the expression of Ccnd2 (Fig. 5A). Co-treatment of MXC 100 µg/ml with NAC (1 and 5 mM), however, restored Ccnd2 levels to control levels (Fig 5A). Similarly, at 48 and 96 hrs, MXC 100 µg/ml significantly reduced the expression of Cdk4 and co-treatment with NAC (1 and 5 mM) restored Cdk4 levels to control levels (Fig 5B).
DISCUSSION
Collectively, we have shown that MXC reduces PCNA levels and decreases protein levels and mRNA expression of cyclin D-2 and cyclin dependant kinase-4 in vivo. We have also shown that in vitro exposure to MXC inhibits growth of cultured antral follicles and decreases the mRNA expression of Ccnd2 and Cdk4. Further, we have shown that co-treatment with a known antioxidant, NAC, rescues antral follicles from MXC-induced growth inhibition and reduction in mRNA expression of Ccnd2 and Cdk4.
Previous studies indicate that MXC (1–100 µg/ml) inhibits growth of antral follicles in culture (Gupta et al., 2006a; Miller et al., 2005). The proliferation and differentiation of granulosa cells is an integral component of follicle growth. Granulosa and theca cells must proliferate by undergoing cell cycle progression so that follicles can reach the penultimate stage for ovulation (McGee and Hsueh, 2000). The regulation of growth and proliferation of cells is a tightly controlled process dependent upon cell cycle related factors (Shackelford et al., 2001). Cell cycle regulators such as cyclins, cdks, and PCNA are required for cell proliferation (Norbury and Nurse, 1992; Rao and Johnson, 1970). The cell cycle is divided into four distinct stages: G1, S, G2, and M (mitosis) (Howard A and Pelc SR, 1953). Different cyclin/cdk complexes are assembled and activated at different points in the cell cycle (Iliakis, 1995; Morgan, 1995; Nigg, 1995). In the G1 phase of the cycle, a series of molecular events, such as dimerization of CCND2 and CDK4, regulates production of genes required for the S phase of the cell cycle, such as PCNA (Norbury and Nurse, 1992; Rao and Johnson, 1970; Shackelford et al., 2001). Also, G1 phase serves as the first checkpoint in the cell cycle in the event of cellular damage, which gives cell an opportunity to either undergo repair or if the damage is overwhelming, under go apoptosis (Shackelford et al., 2001). Thus, the present study examined whether MXC inhibits growth of antral follicles by altering selected G1 cell cycle regulators: Ccnd2, Cdk4, and PCNA. In our study, we have shown that MXC reduces the levels of Ccnd2 and Cdk4 mRNA and CCND2 and CDK4 proteins. Since Ccnd2 and Cdk4 regulate PCNA levels (Lee HH et al., 1995), this is likely why we see reduced expression of PCNA protein in our MXC treated samples.
Interestingly, we observed an increase in CCND2 protein levels at the 16 mg/kg dose of MXC and a decrease in CCND2 protein levels at the higher doses of MXC. In contrast, we observed decreased expression of Ccnd2 mRNA at the 16, 32 and 64 mg/kg doses of MXC. It is possible that a lower dose of MXC (16 mg/kg) decreases transcription and increases translation of Ccnd2 mRNA resulting in decreased expression of mRNA and increased levels of proteins, which is what we observed in our current study. A similar result was observed in a previous study, which showed that MXC 16 mg/kg decreases expression of catalase, whereas the same dose does not decrease the enzyme activity level of catalase (Gupta et al., 2006b). A similar increase in CDK4 protein at the 16 mg/kg dose of MXC was not observed. It is possible that the gene Ccnd2 is more susceptible to lower doses of MXC than Cdk4. Nevertheless, CCND2 and CDK4 proteins are both required to dimerize for their action to initiate transcription of genes responsible for S phase of the cell cycle, such as PCNA (Norbury and Nurse, 1992; Rao and Johnson, 1970; Shackelford et al., 2001). We observed an increase in CCND2 levels, but did not observe a similar increase in CDK4 levels with MXC 16 mg/kg compared to controls. We also observed a decrease in PCNA levels with MXC 16 mg/kg compared to controls. It is possible that the dimerized end product of CCND2 and CDK4 is low, which results in decreased transcription of genes for the S phase of the cycle, such as PCNA.
While no studies have reported the effect of MXC on cell cycle regulators in ovarian follicles, there is a study showing that MXC affects cell cycle regulators in the ovarian surface epithelium (OSE) (Symonds et al., 2005). Specifically, it has been shown that MXC increases PCNA, CCND2, and CDK4 levels in the OSE (Symonds et al., 2005). Further, a metabolite of MXC, bis-hydroxy MXC, commonly known as HPTE, has been shown to up-regulate some and down-regulate some cell cycle genes in cultured rat granulosa cells (Harvey et al., 2009). These data are consistent with the finding that MXC causes a proliferative effect in the OSE instead of the apoptotic effect observed in follicles (Borgeest et al., 2002b).
The mechanism by which MXC decreases the levels of cell cycle regulators is not understood. Under normal circumstances, the cell cycle proceeds without interruptions. If cycling cells are damaged, however, they usually have the capacity to pause temporarily in G1, S, or G2 phases, repair the damage, and re-engage the cell cycle. Studies indicate that DNA damage can result in a pause of cell cycle progression and that reactive oxygen species (ROS) induce damage to DNA (Gupta et al., 2006b; Symonds et al., 2008). Several studies have demonstrated that increases in ROS can result in mutations, chromosomal and DNA damage, inhibition of cell division, and tumor promotion (Gupta et al., 2006b; Moody and Hassan, 1982; Symonds et al., 2008). ROS such as peroxides have been found to initiate cell cycle arrest in several eukaryotic cell types, such as Chinese hamster ovary cells, normal human fibroblasts, and Saccharomyces cerevisiae (Clopton and Saltman, 1995; Flattery-O'Brien and Dawes, 1998). Specifically, hydrogen peroxide (H2O2) has been found to induce a G1 arrest (Clopton and Saltman, 1995). Previous studies have also shown that MXC exposure causes ROS production, specifically H2O2, and this in turn results in DNA damage (Gupta et al., 2006b). Our current study shows that MXC exposure decreases PCNA, Ccnd2 and Cdk4 levels. Thus, it is possible that MXC induces oxidative stress by increasing the production of H2O2, which results in oxidative DNA damage. This oxidative DNA damage serves as a checkpoint for the G1 arrest resulting in decreased Ccnd2 and Cdk4 levels. This reduction in Ccnd2 and Cdk4 levels further decreases the production of the PCNA required for the cell cycle progression.
The point at which the cell cycle is paused to give cells an opportunity to repair is referred to as a checkpoint (Hartwell and Weinert, 1989; Elledge, 1996). There are checkpoints at G1, S, and G2 phases of cell cycle (Hartwell and Weinert, 1989; Elledge, 1996; Shackelford et al., 2001). These checkpoints are known to be controlled by tumor suppressor genes, such as p53 (Kastan et al., 1991). When damage to cells is severe, cells may alternatively undergo apoptosis instead of being repaired. Thus, in the event of damage, molecular events in the cell will either activate cyclin/cdk complexes to restart the cell cycle or activate pro-apoptotic genes such as Bcl-2 associated-X (Bax). The tumor suppressor gene p53 is known to control such a switch between the restarting of the cell cycle and apoptosis (Basu and Haldar, 1998). Our previous studies have shown that MXC causes atresia (apoptosis) in antral follicles through the Bax pathway (Borgeest et al., 2004; Miller et al., 2005). We have also previously shown that MXC induces oxidative DNA damage in antral follicular cells (Gupta et al., 2006b). Our current study shows that MXC decreases the expression of cell cycle regulators, Ccnd2 and Cdk4. Thus, it is possible that MXC could be inhibiting the cell cycle pathway and activating the Bax pathway through p53 regulation to induce apoptosis in antral follicles.
Previous studies also indicate that G1 arrest due to ROS production can be attenuated by the application of antioxidants (Clopton and Saltman, 1995). In our current study, we have shown that co-treatment with NAC, a known antioxidant and protectant from MXC-induced follicle growth inhibition (Gupta RK et al., 2006a) helps restore the expression of Ccnd2 and Cdk4 to control levels.
In conclusion, MXC inhibits growth in antral follicles (Gupta et al., 2006a; Miller et al., 2005) and reduces the levels of the cell cycle regulators tested in this study. Further, co-treatment with NAC protects the follicles by alleviating the oxidative stress generated by MXC and thus preventing the reduction in Ccnd2 and Cdk4 levels. Collectively, these data support the hypothesis that MXC inhibits follicle growth by reducing the levels of selected cell cycle regulators. This study has taken us a step further in understanding the mechanism by which MXC inhibits antral follicle growth.
Acknowledgment
Supported by NIH RO1 ES012893-01A2, NIEHS T32 ES07326, NIH HD 46861
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Rupesh K. Gupta, Email: drrupesh@illinois.edu.
Sharon Meachum, Email: smeachum@illinois.edu.
Isabel Hernández-Ochoa, Email: isabelho@illinoiis.edu.
Jackye Peretz, Email: peretz@illinois.edu.
H. Yao Humphrey, Email: hhyao@illinois.edu.
References
- Armenti AE, Zama AM, Passantino L, Uzumcu M. Developmental methoxychlor exposure affects multiple reproductive parameters and ovarian folliculogenesis and gene expression in adult rats. Toxicol Appl Pharmacol. 2008;233:286–296. doi: 10.1016/j.taap.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bal HS. Effect of methoxychlor on reproductive systems of the rat. Proc Soc Exp Biol Med. 1984;176:187–196. doi: 10.3181/00379727-176-41861. [DOI] [PubMed] [Google Scholar]
- Barnett KR, Tomic D, Gupta RK, Miller KP, Meachum S, Paulose T, Flaws JA. The aryl hydrocarbon receptor affects mouse ovarian follicle growth via mechanisms involving estradiol regulation and responsiveness. Biol Reprod. 2007;76:1062–1070. doi: 10.1095/biolreprod.106.057687. [DOI] [PubMed] [Google Scholar]
- Basu A, Haldar S. The relationship between BcI2, Bax and p53: consequences for cell cycle progression and cell death. Mol Hum Reprod. 1998;4(12):1099–1109. doi: 10.1093/molehr/4.12.1099. [DOI] [PubMed] [Google Scholar]
- Borgeest C, Greenfeld C, Tomic D, Flaws JA. The effects of endocrine disrupting chemicals on the ovary. Front Biosci. 2002a;7:d1941–d1948. doi: 10.2741/borgees. [DOI] [PubMed] [Google Scholar]
- Borgeest C, Miller KP, Gupta R, Greenfeld C, Hruska KS, Hoyer P, Flaws JA. Methoxychlor-induced atresia in the mouse involves Bcl-2 family members, but not gonadotropins or estradiol. Biol Reprod. 2004;70:1828–1835. doi: 10.1095/biolreprod.103.022889. [DOI] [PubMed] [Google Scholar]
- Borgeest C, Symonds D, Mayer LP, Hoyer PB, Flaws JA. Methoxychlor may cause ovarian follicular atresia and proliferation of the ovarian epithelium in the mouse. Toxicol Sci. 2002b;68:473–478. doi: 10.1093/toxsci/68.2.473. [DOI] [PubMed] [Google Scholar]
- Clopton DA, Saltman P. Low-level oxidative stress causes cell cycle-specific arrest in cultured cells. Biochem Biophys Res Commun. 1995;210:189–196. doi: 10.1006/bbrc.1995.1645. [DOI] [PubMed] [Google Scholar]
- Cortvrindt RG, Smitz JE. Follicle culture in reproductive toxicology: a tool for in-vitro testing of ovarian function? Hum Reprod Update. 2002;8:243–254. doi: 10.1093/humupd/8.3.243. [DOI] [PubMed] [Google Scholar]
- Cummings AM. Methoxychlor as a model for environmental estrogens. Crit Rev Toxicol. 1997;27:367–379. doi: 10.3109/10408449709089899. [DOI] [PubMed] [Google Scholar]
- Elledge SJ. Cell cycle checkpoints: preventing an identity crisis. Science. 1996;274:1664–1672. doi: 10.1126/science.274.5293.1664. [DOI] [PubMed] [Google Scholar]
- Eroschenko VP, Abuel-Atta AA, Grober MS. Neonatal exposures to technical methoxychlor alters ovaries in adult mice. Reprod Toxicol. 1995;9:379–387. doi: 10.1016/0890-6238(95)00025-6. [DOI] [PubMed] [Google Scholar]
- Flattery-O'Brien JA, Dawes IW. Hydrogen peroxide causes RAD9-dependent cell cycle arrest in G2 in Saccharomyces cerevisiae whereas menadione causes G1 arrest independent of RAD function. J Biol Chem. 1998;273:8564–8571. doi: 10.1074/jbc.273.15.8564. [DOI] [PubMed] [Google Scholar]
- Gupta RK, Miller KP, Babus JK, Flaws JA. Methoxychlor inhibits growth and induces atresia of antral follicles through an oxidative stress pathway. Toxicol Sci. 2006a;93:382–389. doi: 10.1093/toxsci/kfl052. [DOI] [PubMed] [Google Scholar]
- Gupta RK, Schuh RA, Fiskum G, Flaws JA. Methoxychlor causes mitochondrial dysfunction and oxidative damage in the mouse ovary. Toxicol Appl Pharmacol. 2006b;216:436–445. doi: 10.1016/j.taap.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Hall DL, Payne LA, Putnam JM, Huet-Hudson YM. Effect of methoxychlor on implantation and embryo development in the mouse. Reprod Toxicol. 1997;11(5):703–708. doi: 10.1016/s0890-6238(97)00026-9. [DOI] [PubMed] [Google Scholar]
- Hartwell LH, Weinert TA. Checkpoints: controls that ensure the order of cell cycle events. Science. 1989;246:629–633. doi: 10.1126/science.2683079. [DOI] [PubMed] [Google Scholar]
- Harvey CN, Esmail M, Wang Q, Brooks AI, Zachow R, Uzumcu M. Effect of the methoxychlor metabolite hpte on the rat ovarian granulosa cell transcriptome in vitro. Toxicol Sci. 2009 doi: 10.1093/toxsci/kfp089. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshfield AN. Development of follicles in the mammalian ovary. Int Rev Cytol. 1991;124:43–101. doi: 10.1016/s0074-7696(08)61524-7. [DOI] [PubMed] [Google Scholar]
- Howard A, Pelc SR. Synthesis of deoxyribonucleic acid in normal and irradiated cells and its relation to chromosome breakage. Heredity. 1953;6:261–273. [Google Scholar]
- Iliakis G. Cell cycle regulation in irradiated and nonirradiated cell. Semin Oncol. 1995;24:602–615. [PubMed] [Google Scholar]
- Jackson C, Jr, Lindahl IL, Reynolds P, Sidwell GM. Effects of methoxychlor and malathion on semen characteristics of rams. J Anim Sci. 1975;40:514–517. doi: 10.2527/jas1975.403514x. [DOI] [PubMed] [Google Scholar]
- Kastan MB, Onyekere O, Sidransky D, Volgelstein B, Craig RW. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 1991;51:6304–6311. [PubMed] [Google Scholar]
- Latchoumycandane C, Chitra KC, Mathur PP. The effect of methoxychlor on the epididymal antioxidant system of adult rats. Reprod Toxicol. 2002a;16:161–172. doi: 10.1016/s0890-6238(02)00002-3. [DOI] [PubMed] [Google Scholar]
- Latchoumycandane C, Mathur PP. Effect of methoxychlor on the antioxidant system in mitochondrial and microsome-rich fractions of rat testis. Toxicology. 2002b;176:67–75. doi: 10.1016/s0300-483x(02)00138-5. [DOI] [PubMed] [Google Scholar]
- Latchoumycandane C, Mathur PP. Induction of oxidative stress in the rat testis after short-term exposure to the organachlorine pesticide methoxychlor. Arch Toxicol. 2002c;76:692–698. doi: 10.1007/s00204-002-0388-9. [DOI] [PubMed] [Google Scholar]
- Lee HH, Chiang WH, Chiang SH, Liu YC, Hwang J, Ng SY. Regulation of cyclin D1, DNA topoisomerase I, and proliferating cell nuclear antigen promoters during the cell cycle. Gene Expr. 1995;4:95–109. [PMC free article] [PubMed] [Google Scholar]
- Masters RA, Crean BD, Yan W, Moss AG, Ryan PL, Wiley AA, Bagnell CA, Bartol FF. Neonatal porcine endometrial development and epithelial proliferation affected by age and exposure to estrogen and relaxin. Domest Anim Endocrinol. 2007;33:335–346. doi: 10.1016/j.domaniend.2006.07.002. [DOI] [PubMed] [Google Scholar]
- McGee EA, Hsueh AJ. Initial and cyclic recruitment of ovarian follicles. Endocr Rev. 2000;21:200–214. doi: 10.1210/edrv.21.2.0394. [DOI] [PubMed] [Google Scholar]
- Miller KP, Gupta RK, Flaws JA. Methoxychlor metabolites may cause ovarian toxicity through estrogen-regulated pathways. Toxicol Sci. 2006;93:180–188. doi: 10.1093/toxsci/kfl034. [DOI] [PubMed] [Google Scholar]
- Miller KP, Gupta RK, Greenfeld CR, Babus JK, Flaws JA. Methoxychlor directly affects ovarian antral follicle growth and atresia through Bcl-2- and Bax-mediated pathways. Toxicol Sci. 2005;88:213–221. doi: 10.1093/toxsci/kfi276. [DOI] [PubMed] [Google Scholar]
- Moody CS, Hassan HM. Mutagenicity of oxygen free radicals. Proc Natl Acad Sci, USA. 1982;79:2855–2859. doi: 10.1073/pnas.79.9.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan DO. Principles of CDK regulation. Nature. 1995;374:131–134. doi: 10.1038/374131a0. [DOI] [PubMed] [Google Scholar]
- Nigg EA. Cyclin-dependent protein kinases: key regulators of the eukaryotic cell cycle. Bioessays. 1995;17:471–480. doi: 10.1002/bies.950170603. [DOI] [PubMed] [Google Scholar]
- Norbury C, Nurse P. Animal cell cycles and their control. Annu Rev Biochem. 1992;61:441–468. doi: 10.1146/annurev.bi.61.070192.002301. [DOI] [PubMed] [Google Scholar]
- Rao PN, Johnson RT. Mammalian cell fusion: studies on the regulation of DNA synthesis and mitosis. Nature. 1970;225:159–164. doi: 10.1038/225159a0. [DOI] [PubMed] [Google Scholar]
- Schuh RA, Kristian T, Gupta RK, Flaws JA, Fiskum G. Methoxychlor inhibits brain mitochondrial respiration and increases hydrogen peroxide production and CREB phosphorylation. Toxicol Sci. 2005;88:495–504. doi: 10.1093/toxsci/kfi334. [DOI] [PubMed] [Google Scholar]
- Shackelford RE, Innes CL, Sieber SO, Heinloth AN, Leadon SA, Paules RS. The ataxia telangiectasia gene product is required for oxidative stress-induced g1 and g2 checkpoint function in human fibroblasts. J Biol Chem. 2001;276:21951–21959. doi: 10.1074/jbc.M011303200. [DOI] [PubMed] [Google Scholar]
- Symonds DA, Merchenthaler I, Flaws JA. Methoxychlor and estradiol induce oxidative stress dna damage in the mouse ovarian surface epithelium. Toxicol Sci. 2008;105:182–187. doi: 10.1093/toxsci/kfn100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symonds DA, Tomic D, Miller KP, Flaws JA. Methoxychlor induces proliferation of the mouse ovarian surface epithelium. Toxicol Sci. 2005;83:355–362. doi: 10.1093/toxsci/kfi024. [DOI] [PubMed] [Google Scholar]
- Tullner WW, Edgcomb JH. Cystic tubular nephropathy and decrease in testicular weight in rats following oral methoxychlor treatment. J Pharmacol Exp Ther. 1962;138:126–130. [PubMed] [Google Scholar]
- Zachow R, Uzumcu M. The methoxychlor metabolite, 2,2-bis-(phydroxyphenyl)-1,1,1-trichloroethane, inhibits steroidogenesis in rat ovarian granulosa cells in vitro. Reprod Toxicol. 2006;22:659–665. doi: 10.1016/j.reprotox.2006.04.018. [DOI] [PubMed] [Google Scholar]