Abstract
Arsenic compounds are classified as toxicants and human carcinogens. Environmental exposure to arsenic imposes a big health issue worldwide. Arsenic elicits its toxic efforts through many mechanisms, including generation of reactive oxygen species (ROS). Nrf2 is the primary transcription factor that controls expression of a main cellular antioxidant response, which is required for neutralizing ROS and thus defending cells from exogenous insults. Previously, we demonstrated a protective role of Nrf2 against arsenic-induced toxicity using a cell culture model. In this report, we present evidence that Nrf2 protects against liver and bladder injury in response to six-weeks of arsenic exposure in a mouse model. Nrf2−/− mice displayed more severe pathological changes in the liver and bladder, compared to Nrf2+/+ mice. Furthermore, Nrf2−/− mice were more sensitive to arsenic-induced DNA hypomethylation, oxidative DNA damage, and apoptotic cell death. These results indicate a protective role of Nrf2 against arsenic toxicity in vivo. Hence, this work demonstrates the feasibility of using dietary compounds that target activation of the Nrf2 signaling pathway to alleviate arsenic-induced damage.
Introduction
Groundwater contaminated with arsenic is the major source of human exposure to arsenic. Chronic arsenic poisoning imposes a global health issue (Tchounwou et al., 2003; Walvekar et al., 2007). Therefore, the World Health Organization (WHO) has set the standard limit for arsenic to 10 parts per billion (ppb) (Kayajanian, 2003). However, approximately 57 million people are drinking groundwater with arsenic concentrations above 10 ppb. Arsenic has caused poisonings in Bangladesh, Bengal, Thailand, Finland, Hungary, Chile, Taiwan, Vietnam, Cambodia, Mexico, Argentina, and China, where geological environments are conducive to generate high amounts of arsenic compounds in groundwater (Smith et al., 2000; Kayajanian, 2003; Tchounwou et al., 2003). Furthermore, many states within the United States also have significant concentrations (up to 50 ppm) of arsenic in the groundwater (Tchounwou et al., 2003; Knobeloch et al., 2006).
The most common species of arsenic in groundwater are arsenate (As(V)) and arsenite (As(III)). Arsenic is able to undergo redox conversion between As(V) and As(III). Following uptake from drinking water, inorganic arsenic species are converted in the liver into methylated arsenic species that are excreted from the bladder. Thus, the bladder is the major target organ that is exposed to methylated arsenic species in populations who consume arseniccontaminated water (Tchounwou et al., 2003; Kenyon et al., 2005). Chronic exposure to arsenic can cause skin, lung and bladder cancers (Hsueh et al., 1995; Brown et al., 1997; Cohen et al., 2000; Smith et al., 2006). A small but measurable increase in the incidence of bladder cancer was associated with exposure to concentration as low as 10 ppm of inorganic arsenic (Chu and Crawford-Brown, 2006). In addition to cancers, epidemiological studies have also established a strong correlation between chronic arsenic exposure and various non-cancer human diseases, such as hyperkeratosis, atherosclerosis, diabetes, and chronic obstructive pulmonary diseases (Tseng, 2002; Tchounwou et al., 2003; Navas-Acien et al., 2006).
The transcription factor, Nrf2, controls a cellular antioxidant response through transcriptional upregulation of an array of downstream genes, such as glutamate cysteine ligase (GCL), heme oxygenase-1 (HO-1), glutathione s-trasferase (GST), multidrug-resistance proteins (MRPs), and NAD(P)H quinone oxidoreductase-1 (NQO1) (Lau et al., 2008; Hayes and McMahon, 2009). Coordinated upregulation of the Nrf2-mediated endogenous antioxidants, phase II detoxifying enzymes, and drug transporters is essential in defending cells or animals from damage by environmental insults. This has been clearly demonstrated in many studies using Nrf2−/− mice. Nrf2−/− mice displayed a greater sensitivity to toxic effects induced by benzo[a]pyrene, diesel exhaust, pentachlorophenol, and bleomycin (Aoki et al., 2001; Ramos-Gomez et al., 2001; Cho et al., 2004; Umemura et al., 2006).
Arsenic exerts its toxicity in part by generation of ROS (Hei et al., 1998; Kitchin and Ahmad, 2003; Liu et al., 2003; Das et al., 2005). Consistent with the role of ROS in arsenic toxicity/carcinogenicity, endogenous sulfhydryl groups and the non-protein sulfhydryl glutathione (GSH) have been reported to play an important role in detoxification of arsenic (Duyndam et al., 2001). The exogenous antioxidant N-acetylcysteine is also able to prevent arsenic-induced toxicity (Liu et al., 2003). Arsenic itself was reported to induce the Nrf2-dependent antioxidant response, although the detailed mechanism of Nrf2 induction by arsenic remains to be explored (He et al., 2006; Wang et al., 2008). Numerous studies have been performed to elucidate events associated with arsenic-induced damage, both in animal and cell culture models. Results from these studies have revealed that arsenic induces multiple biological effects, such as DNA damage, apoptotic cell death, and global DNA hypomethylation, which represent both genotoxic and non-genotoxic mechanisms of action (Huang et al., 1999; Kirkpatrick et al., 2003; Chen et al., 2004). Methylation is a covalent modification of genomic DNA and occurs on cytosines followed by guanines (CpG). DNA methylation appears to influence gene expression through alteration in chromatin structure and in DNA protein interaction. An overall decrease in the content of 5-methylcytosine (5-mc) in DNA has been viewed as an early event in tumor formation in humans and animal models (Lapeyre et al., 1981; Jones and Buckley, 1990; Umemura et al., 1990).
Recently, by using both genetic and biochemical approaches, we demonstrated a protective role of Nrf2 against arsenic-induced toxicity in a cell culture model (Wang et al., 2007). In vivo, even though Nrf2−/− mice have been demonstrated to display increased sensitivity to chemical toxicants and carcinogens (Chan and Kan, 1999; Aoki et al., 2001; Enomoto et al., 2001; Ramos-Gomez et al., 2001; Cho et al., 2002; Yu and Kensler, 2005), currently no study has been carried out to determine the effect of Nrf2 deficiency on arsenic-induced toxicity and carcinogenicity. In this report, we hypothesized that Nrf2−/− mice are more vulnerable to the arsenic-induced toxicity, compared to Nrf2+/+ mice. Our results confirmed that Nrf2−/− mice were more sensitive to arsenic-induced DNA hypomethylation, oxidative DNA damage, and apoptotic cell death. Furthermore, more severe pathological alterations were observed in the liver and bladder of Nrf2−/− mice, compared to Nrf2+/+ mice.
Materials and Methods
Animals and treatments
Nrf2−/− mice were originally generated in Dr. Kan’s laboratory (University of California, San Francisco). Mice were housed in polycarbonate cages (4/cage), provided AIN-76A diet and water ad libitum and maintained on a 12-12 hr light-dark cycle at 22 ± 5 °C and 50 ± 20% relative humidity. At 8 weeks of age, Nrf2+/+ and Nrf2−/− mice (4 mice per group) were treated with 1 ppm, 10 ppm or 100 ppm of sodium arsenite (As(III), Sigma, St Louis, MO, USA) through drinking water for six weeks. All 32 mice survived arsenic treatment.
Tissue collection and Hematoxylin and Eosin (HE) staining
Following six-week treatment, mice were sacrificed and the liver, kidney, lung and bladder were isolated. Tissues were cut into two sections: one section was frozen in liquid nitrogen for total RNA extraction, and another section was fixed in 10% buffered formalin. The bladders were reversed: one section was fixed in formalin, and another was spread onto a flat board and scrubbed by a pestle to loosen up bladder epithelium. The bladder epithelium was then collected in Trizol solution (St Louis, MO, USA) for total RNA extraction. The formalin-fixed tissues were embedded in paraffin and cut into 4 µm sections. Various sections were stained with HE.
DNA methylation in bladder tissue
DNA methylation in bladder epithelium tissues was detected by immunohistochemistry using a monoclonal antibody against 5-methylcytosine (Calbiochem, San Diego, CA, USA). Antigen retrieval of formalin-fixed paraffin-embedded tissue sections was carried out by microwave heating (7 min at the high setting to boil the retrieval solution), followed by 10 min at the low setting (the retrieval solution was maintained at the boiling temperature). The retrieval solution contains 1×TBS with 0.1% Tween 20 (TBS-T) in 1 mol/L sodium citrate. Following antigen retrieval, tissue sections were exposed to 3.5 M HCl for 15 min at room temperature (RT) and washed in TBS-T. Subsequently, tissue sections were treated with 0.3% peroxidase to quench endogenous peroxidase activity. Tissue sections were incubated with 5% normal goat serum for 30 minutes followed by 2 hr incubation with an anti–5-methyl-C monoclonal antibody at 1:100 dilution at RT, followed by 1 hr sequential incubation with a biotinylated goat anti-mouse secondary antibody and ABC kit (Vector Lab, Burlingame, CA, USA) at 1:100 dilution at RT. Finally, tissue sections were developed for 30 seconds using the 3, 3'-diaminobenzidine staining kit (DAKO, Carpinteria, CA, USA), and counterstained with hematoxylin.
Oxidative DNA damage in bladder tissue
A monoclonal antibody against 8-Oxo-7,8-dihydro-2'-deoxyguanosine (8-Oxo-dG) (Trevigen, Gaithersburg, MD) was used for detecting oxidative DNA damage in bladder epithelium. Formalin-fixed paraffin-embedded tissue sections were incubated with proteinase K (10 µg/ml) in PBS for 30 min at 37 °C, and then exposed to 2M HCl for 5 min at RT. Following quench of the endogenous peroxidase activity by 0.3% peroxidase, tissue sections were first incubated with anti-8-Oxo-dG at 1:250 dilution at RT for 2 hrs, followed by sequential incubation with the secondary antibody and ABC kit. Finally, tissue sections were developed and counterstained with hematoxylin.
Apoptotic cell death in bladder tissue
In situ cell death detection kit (Roche, IN, USA) was used for detecting apoptotic cell death in bladder epithelium according to the manufacturer’s instructions. Briefly, tissue sections were pretreated with proteinase K (15 ug/ml) in 10mM Tris/HCl (pH 7.8) at 37 °C for 30 min. After washing 3 times with PBS, tissue sections were incubated with TUNEL reaction mixture for 1 hr at 37 °C in the dark. Tissue sections were then rinsed with PBS 3 times, and analyzed under a fluorescence microscope (Zeiss LSM 510 NLO Meta confocal system). The excitation wavelength is in the range of 450 – 500 nm and the detection wavelength is in the range of 515 – 565 nm.
qRT-PCR analysis
Total RNA from liver tissues and bladder epithelial cells were extracted using Trizol. Equal amounts of RNA (2 µg) were reverse-transcripted into cDNA using the Transcriptor First Strand cDNA synthesis Kit (Roche). Taqman probes were from the universal probe library (Roche) and primers were from Integrated DNA Technologies (Coralville, IA, USA). The Taqman probes used were NQO1(50), HO-1(25), and β-actin(56). The primers used were NQO1: forward (agggttcggtattacgatcc) and reverse (agtacaatcagggctcttctcg), HO-1: forward (ctgctagcctggtgcaaga) and reverse (ccaacaggaagctgagagtga), β-actin: forward (aaggccaaccgtgaaaagat) and reverse (gtggtacgaccagaggcatac). The qPCR conditions were: one cycle of initial denaturation (95°C for 10 min), 40 cycles of amplification (95°C for 10 sec and 60°C for 20 sec), and a cooling period (50°C for 5 sec). The data presented were relative mRNA levels normalized to β-actin, and the value from the Nrf2+/+ control group was set as 1.
Statistics
Two-way Student’s t test was used to determine the significant difference between two samples. Body weight gained and water consumption/week were average values from four different mice within the same group. Pooled mRNAs from four mice within the same group were analyzed by qPCR analysis. qPCR analysis was run in triplicate and data were expressed as mean ± SD.
Results
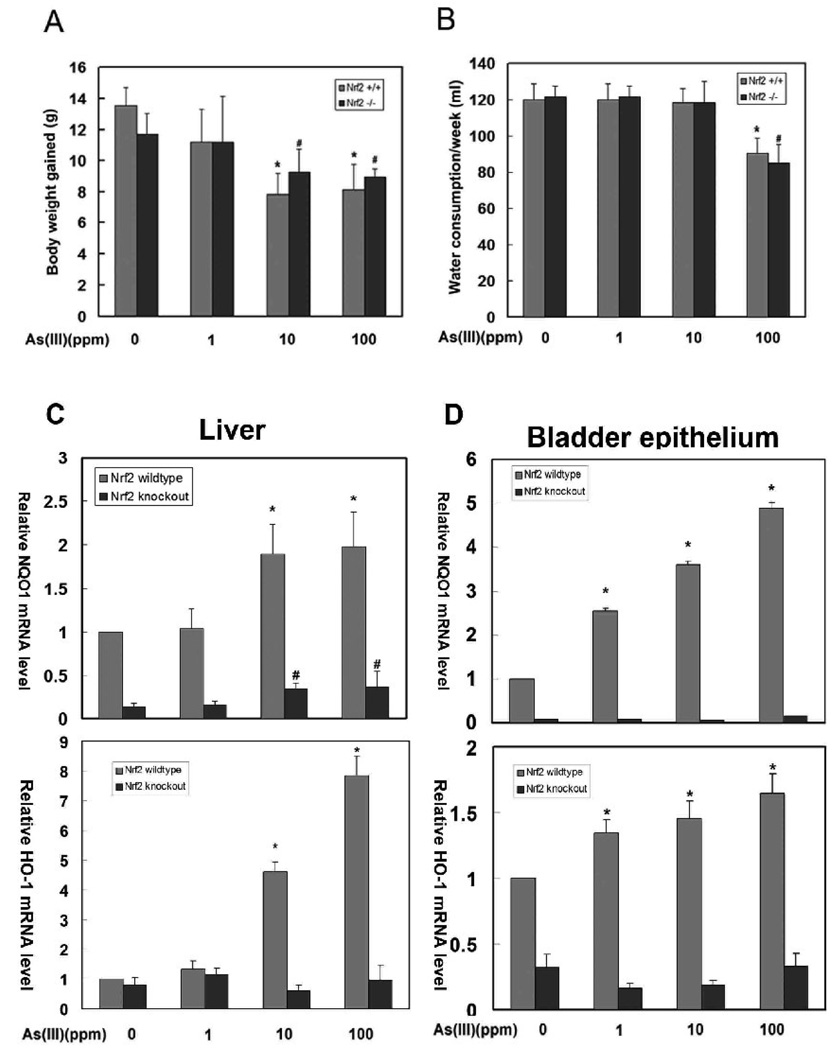
There was no difference in body weight or water consumption between Nrf2+/+ and Nrf2−/− mice
All 32 mice survived the 6-week As(III) treatment, with no obvious systemic toxicity, such as diarrhea, hair loss, or nasal hemorrhaging. Body weight gained during the 6-week period was recorded at the day of harvest. The weight gained was reduced as the doses of As(III) increased (Fig 1A). In addition, no difference in weight gained between Nrf2+/+and Nrf2−/− mice was observed (Fig 1A). The amount of water intake in Nrf2+/+ and Nrf2−/− mice was similar, except that the Nrf2+/+ and Nrf2−/− mice treated with 100 ppm As(III) showed a decrease in water intake (Fig 1B).
Fig 1.
(A) Body weight gained during six-weeks of As(III) exposure. (B) Average water consumption per week. Data represent mean ± standard deviation (SD) of 4 animals per group. (C) NQO1 or HO-1 mRNA expression in the liver or bladder epithelial cells from Nrf2+/+ and Nrf2−/−mice. RNA extraction and real-time PCR were performed as described in the materials and methods. The mRNA from four individual mice within the same group were pooled prior to reverse transcription. qPCR analysis was run in triplicate and data were expressed as mean ± SD. * P<0.01, treated groups compared with the untreated group of Nrf2+/+ mice. # P<0.01, treated groups compared with the untreated group of Nrf2−/− mice.
As(III) activated the Nrf2-dependent antioxidant response in a dose-dependent manner in Nrf2+/+ mice
As(III) has been reported to be an inducer of Nrf2 in vitro. Here, the ability of As(III) to activate the Nrf2 signaling pathway in vivo was tested. mRNA was extracted from both liver tissues and the bladder epithelial cells. qRT-PCR was performed to measure relative transcriptional levels of NQO1 and HO-1, two Nrf2 target genes. As expected, Nrf2+/+ mice had higher basal levels of NQO1 and HO-1 expression, compared to Nrf2−/− mice (Fig. 1C and 1D). As(III) enhanced the transcription of both NQO1 and HO-1, in a dose-dependent manner in Nrf2+/+ (Fig. 1C and 1D). Conversely, Nrf2−/− mice had very low basal and induced levels of NQO1 and HO-1 (Fig. 1C and 1D). In Nrf2−/− mice, As(III) induced NQO1 expression slightly at 10, and 100 ppm in the liver, presumably through an Nrf2-independent mechanism. Interestingly, induction of NQO1 by As(III) is more prominent in bladder epithelial cells than in liver tissues (Compare Fig 1C with 1D, NQO1 panels). In contrast, induction of HO-1 by As(III) was more significant in liver tissues, compared to that in bladder epithelial cells (Fig. 1C and 1D, HO-1 panel). Currently, the reason for this organ-specific induction of a particular gene is unclear.
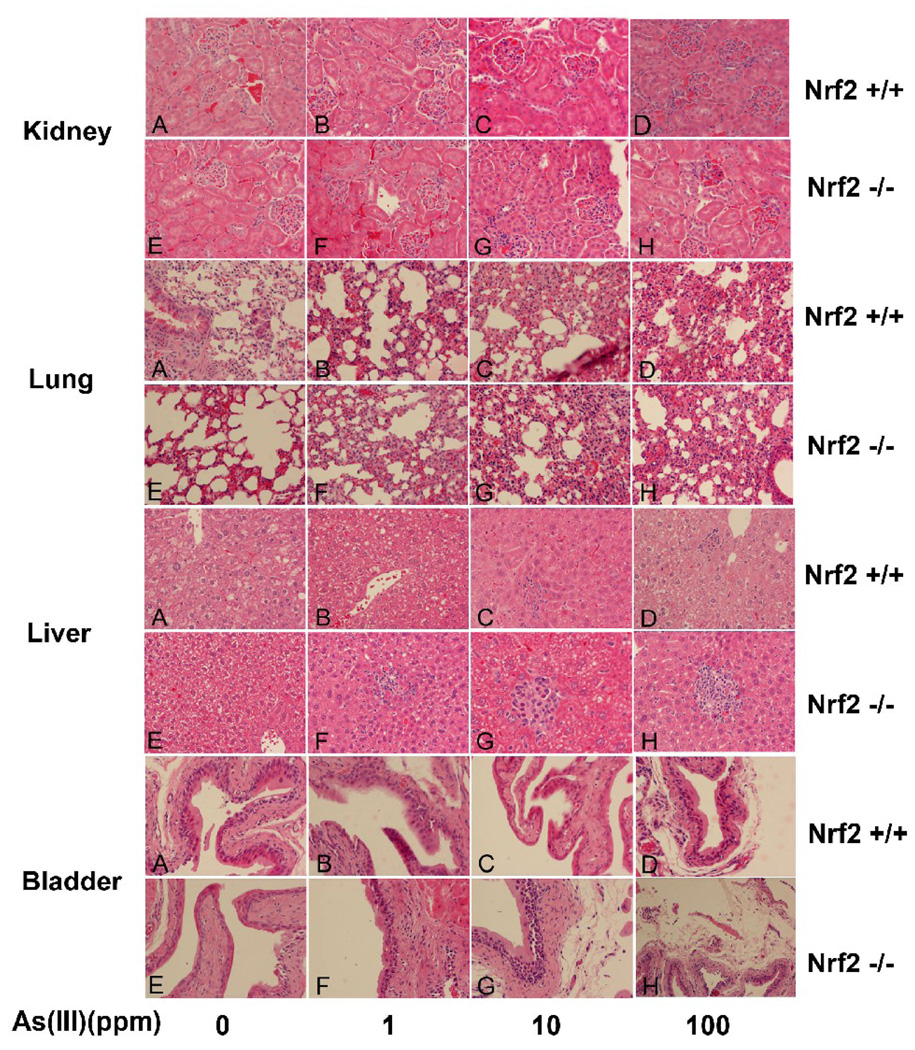
Pathological changes induced by As(III) were more prominent in liver nd bladder tissues from Nrf2−/− mice
The pathological changes in response to As (III) treatments were examined and compared among different groups in four different organs, lung, liver, kidney, and bladder. In the kidney, pathological changes, such as cell proliferation in glomeruli and tubular epithelial cell degeneration, were not observed in either Nrf2+/+ or Nrf2−/− mice. In lung tissues, 1 ppm As(III) did not elicit distinct pathological changes (Fig 2, lung panel, A, B, E and F). However, proliferation of fibroblasts and thickening of alveolar wall were observed in both Nrf2+/+ and Nrf2−/− mice at high doses of As(III), although there was no significant difference between Nrf2+/+and Nrf2−/− groups. (Fig 2, lung panel, compare C and D with A; G and H with E). The liver is a primary defense organ that detoxifies drugs and xenobiotics, which predisposes it to injuries caused by excessive toxic exposure. Uptake of As(III) via drinking water caused severe lesions in the liver. Liver tissues in untreated mice have normal morphology (Fig 2, liver panel, A and E). 1 ppm As(III) induced vacuolar degeneration in Nrf2+/+ mice (Fig 2, liver panel, B), whereas it caused hepatic spotty and lytic necrosis that is accompanied by infiltrated macrophages and lymphocytes in Nrf2−/− mice (Fig 2, liver panel, F). At 10 and 100 ppm As(III), necrosis was induced even in Nrf2+/+ mice (Fig 2, liver panel, C and D). However, the same doses of As(III) elicited severe necrosis in Nrf2−/− mice with more infiltrated inflammatory cells including eosinophils (Fig 2, liver panel, G and H). This indicates progression to liver disease, since eosinophilic infiltration was recently found to be associated with liver steatosis and liver fibrosis (Tarantino et al., 2008). As expected, there was no significant malignant hyperplasia observed in bladder epithelium in either Nrf2+/+ or Nrf2−/− mice following six-weeks of As(III) exposure. In Nrf2+/+ mice exposed to 100 ppm As(III) and Nrf2−/− mice exposed to 10 or 100 ppm, minimal epithelial proliferation in the bladder was observed (Fig 2, bladder panel, D, G, and H). However, 10 or 100 ppm As(III) caused severe interstitial edema and congestion in Nrf2−/− mice (Fig 2, bladder panel, G and H) while only 100 ppm As(III) caused a similar change in Nrf2+/+ mice (Fig 2, bladder panel, D).
Fig 2.
Effects of daily exposure of As(III) on histological changes in the kidney, liver, lung and bladder. The four mice in each group all underwent pathological analysis to avoid individual variation. Panels A, B, C and D show representative photomicrographs of HE staining from individual Nrf2+/+ mice, and panels E, F, G, and H show representive tissues from individual Nrf2−/− mice. Spotty necrosis in the liver and interstitial edema in the bladder were observed in many fields.
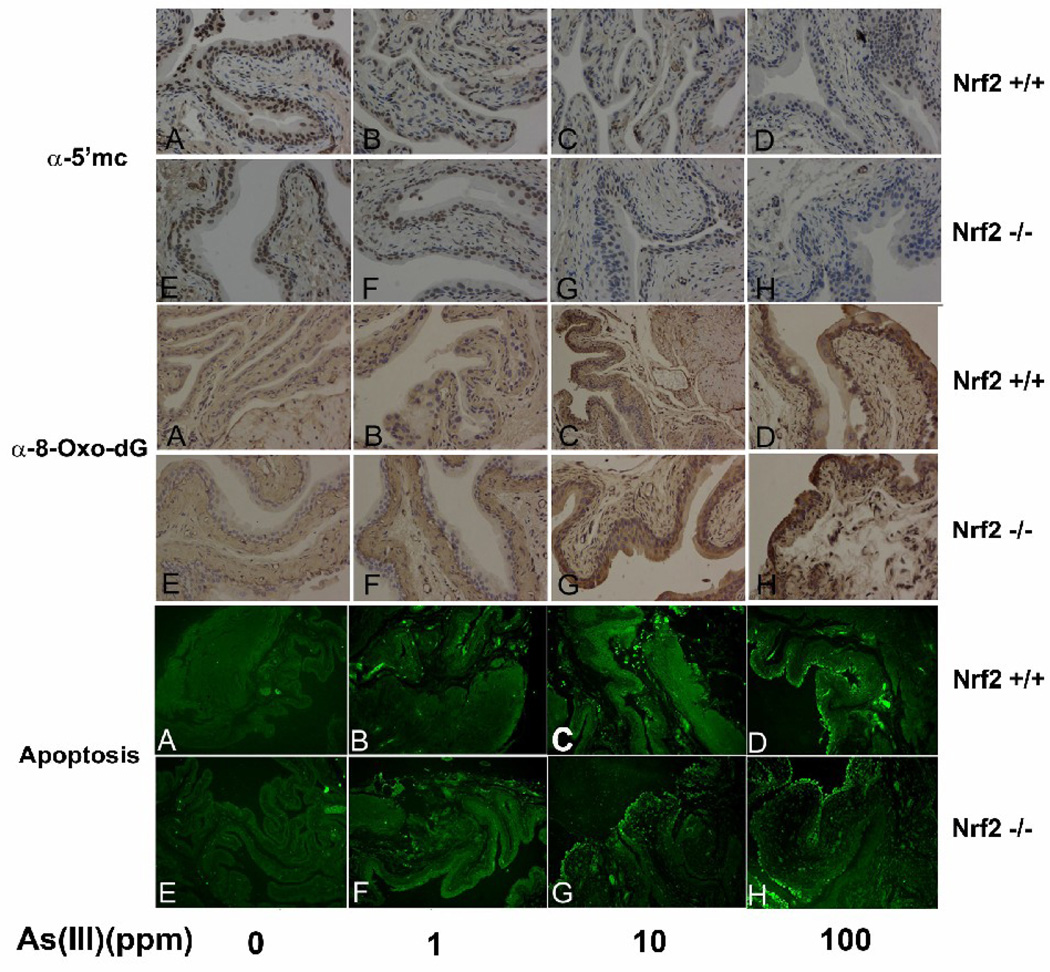
Nrf2−/− mice were more susceptible to As(III)-induced DNA hypomethylation
The DNA methylation status of bladder epithelium was examined by immunohistochemical analysis with an anti 5-methycytosine antibody. In untreated groups, DNA was heavily methylated, indicated by brown nuclear staining (Fig 3, 5’-mC panel, A and E). Following As(III) treatment, the number of positive stained cells, and the intensity of staining decreased in a dose-dependent manner in both Nrf2+/+ or Nrf2−/− mice. These results indicate that As(III) induced global DNA hypomethylation. Furthermore, As(III)-induced hypomethylation is more substantial in Nrf2−/− than in Nrf2+/+ mice as shown by a significant decrease in brown staining in 10 or 100 ppm As-treated Nrf2−/− mice, compared to the same dose treatment in the Nrf2+/+ group (in Fig. 3, 5’-mC panel, compare G with C, and H with D). Collectively, these results indicate that Nrf2−/− mice are more susceptible to As-induced DNA hypomethylation than Nrf2+/+ mice.
Fig 3.
Daily exposure of As(III) caused damage in bladder epithelium. The four mice in each group all underwent immunohistochemical and apoptotic cell death analyses to avoid individual variation. As(III) induced damage was evaluated by levels of DNA methylation detected by an anti-5-methylcytosine antibody (α-5’mc), oxidative DNA damage by an anti-8-Oxo-dG antibody (α-8-Oxo-dG panel), and apoptotic cell death by TUNEL labeling (apoptosis panel).
Nrf2−/− mice were more susceptible to oxidative DNA damage in response to As(III) treatment
8-Oxo-7,8-dihydro-2'-deoxyguanosine (8-oxo-dG) is a mutagenic base modification that is generated by the reaction of reactive oxygen species with DNA and is a quantitative index of oxidative DNA damage (Wainfan and Poirier, 1992). Formation of 8-oxo-dG in bladder tissues of mice from different groups was detected using immunohistochemical analysis with an antibody against 8-oxo-dG. There was no detectable staining in the control and 1 ppm-treated Nrf2+/+ or Nrf2−/− groups (Fig. 3, 8-oxo-dG panel, A, B, E, and F). However, 10 and 100 ppm As(III) induced extensive staining in bladder epithelium in both Nrf2+/+ and Nrf2−/− mice, and higher staining was observed in Nrf2−/− mice, compared to Nrf2+/+ mice (Fig. 3, compare C with G, and D with H).
Nrf2−/− mice were more susceptible to apoptotic cell death induced by As(III)
Apoptotic cell death in bladder epithelium was measured by TUNEL labeling. Similar to the formation of 8-oxo-dG within bladder epithelium, the number of apoptotic epithelial cells increased in response to As(III) in a dose-dependent manner in both Nrf2+/+ and Nrf2−/− mice. There were no apoptotic cells observed in control or 1 ppm As(III)-treated groups (Fig 3, apoptosis panel, A, B, E and F). Apoptotic epithelial cells appeared in response to 10 ppm As(III) only in the Nrf2−/−, but not in the Nrf2+/+ group (Fig 3, apoptosis panel, compare C with G). At 100 ppm As(III), a considerable number of apoptotic epithelial cells were induced in both Nrf2+/+ and Nrf2−/− groups (Fig 3, apoptosis panel, D and H).
Discussion
Many previous findings suggest an adaptive response exists in mammals, i.e. previous exposure to low toxic reagents can elicit a beneficial effect that reduces damage by subsequent insults. We postulated that the adaptive response is derived from activation of the Nrf2 pathway based on the following observations: (i) resveratrol and epigallocatechin gallate, two known inducers of Nrf2, prevented arsenite-induced transformation of human osteogenic sarcoma cells. (Yang et al., 2005). (ii) tert-butylhydroguinone, an potent inducer of Nrf2, was found to be capable of reversing arsenite-induced gene expression in mouse embryo fibroblasts (Kann et al., 2005). (iii) Interestingly, a recent epidemiological study showed that populations exposed to low dose arsenic from their drinking water had reduced risk for cancer, compared to unexposed populations (Lamm et al., 2004). This “anticarcinogenic” effect of arsenic has also been demonstrated in animal and cell culture models. For example, arsenic inhibited formation of GST-P-positive hepatic foci in rats treated with chemical carcinogens (Pott et al., 1998); lymphocytes from individuals pre-exposed to arsenic-contaminated drinking water were more resistant to subsequent arsenic-exposure (Mahata et al., 2004); 0.1 to 1 µM arsenite exposure provided a protective effect against oxidative stress and DNA damage in human keratinocyte and fibroblast cells (Snow et al., 2005); arsenic or arsenic-containing metal mixtures inhibited malignant transformation of RHEK-1, human keratinocyte cells (Bae et al., 2002); Chronic low dose arsenite exposure enhanced self tolerance in liver epithelial cells (Romach et al., 2000).
Arsenic can elicit both a beneficial Nrf2-dependet antioxidant response and a cell damaging effect. Given the complexity of the cell, it is highly likely that toxic effects in any given situation are dependent on many genes working in concert and not just a single gene. Activation of an array of Nrf2-dependent down-stream genes conditions cells for an adaptive defense response to subsequent toxic/carcinogenic insults. Other pathways that lead to cell death may also be activated. The net outcome in response to arsenic may be dictated by arsenic species, dose, and duration of arsenic exposure. Nevertheless, activation of the ARE-Nrf2-Keap1 pathway represents the initial attempt to counteract deteriorative effects induced by arsenic and to maintain cellular homeostasis. However, with high concentrations or repetitive doses of arsenic, the Nrf2-dependent defense response is outweighed by the deteriorative effects induced by arsenic, ultimately resulting in toxicity.
In this study, we used Nrf2−/− mice to demonstrate that Nrf2 is able to alleviate damage in response to arsenic exposure. Arsenic appeared to be more toxic to Nrf2−/− mice compared to Nrf2+/+ mice, as measured by physiological changes, DNA methylation, oxidative DNA damage, and apoptotic cell death. Arsenic caused detectable physiologic alterations, especially in the liver and bladder in both Nrf2+/+ and Nrf2−/− mice. Although skin damage is frequently observed in the arsenic-exposed population, we did not observe any skin lesions during the course of our study. Two rational reasons can be envisioned: (1) in addition to exposure through drinking water, human exposure to arsenic also includes direct contact with the skin; however, in our study arsenic was solely administered through the digestive system, (2) the duration of arsenic exposure was not long enough to induce skin damage. More importantly, these results indicate a protective role of Nrf2 against arsenic toxicity in vivo. Therefore, we believe that specific activation of the ARE-Nrf2-Keap1 pathway by a reagent with low toxicity should be a great strategy to combat arsenic-induced damage. Our findings will lay the groundwork for dietary and therapeutic interventions against arsenic adverse effects.
Acknowledgments
This study was supported by the NIH grant ES015010 and ACS grant #RSG-07-154-01-CNE to D. D. Zhang, and a NIH center grant ES 006694.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aoki Y, Sato H, Nishimura N, Takahashi S, Itoh K, Yamamoto M. Accelerated DNA adduct formation in the lung of the Nrf2 knockout mouse exposed to diesel exhaust. Toxicol Appl Pharmacol. 2001;173:154–160. doi: 10.1006/taap.2001.9176. [DOI] [PubMed] [Google Scholar]
- Bae DS, Hanneman WH, Yang RS, Campain JA. Characterization of gene expression changes associated with MNNG, arsenic, or metal mixture treatment in human keratinocytes: application of cDNA microarray technology. Environ Health Perspect. 2002;110 Suppl 6:931–941. doi: 10.1289/ehp.02110s6931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KG, Guo HR, Kuo TL, Greene HL. Skin cancer and inorganic arsenic: uncertainty-status of risk. Risk Anal. 1997;17:37–42. doi: 10.1111/j.1539-6924.1997.tb00841.x. [DOI] [PubMed] [Google Scholar]
- Chan K, Kan YW. Nrf2 is essential for protection against acute pulmonary injury in mice. Proc Natl Acad Sci U S A. 1999;96:12731–12736. doi: 10.1073/pnas.96.22.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Li S, Liu J, Diwan BA, Barrett JC, Waalkes MP. Chronic inorganic arsenic exposure induces hepatic global and individual gene hypomethylation:implications for arsenic hepatocarcinogenesis. Carcinogenesis. 2004;25:1779–1786. doi: 10.1093/carcin/bgh161. [DOI] [PubMed] [Google Scholar]
- Cho HY, Jedlicka AE, Reddy SP, Kensler TW, Yamamoto M, Zhang LY, Kleeberger SR. Role of NRF2 in protection against hyperoxic lung injury in mice. Am J Respir Cell Mol Biol. 2002;26:175–182. doi: 10.1165/ajrcmb.26.2.4501. [DOI] [PubMed] [Google Scholar]
- Cho HY, Reddy SP, Yamamoto M, Kleeberger SR. The transcription factor NRF2 protects against pulmonary fibrosis. Faseb J. 2004;18:1258–1260. doi: 10.1096/fj.03-1127fje. [DOI] [PubMed] [Google Scholar]
- Chu HA, Crawford-Brown DJ. Inorganic arsenic in drinking water and bladder cancer: a meta-analysis for dose-response assessment. Int J Environ Res Public Health. 2006;3:316–322. doi: 10.3390/ijerph2006030039. [DOI] [PubMed] [Google Scholar]
- Cohen SM, Shirai T, Steineck G. Epidemiology and etiology of premalignant and malignant urothelial changes. Scand J Urol Nephrol Suppl. 2000:105–115. doi: 10.1080/00365590050509869. [DOI] [PubMed] [Google Scholar]
- Das S, Santra A, Lahiri S, Guha Mazumder DN. Implications of oxidative stress and hepatic cytokine (TNF-alpha and IL-6) response in the pathogenesis of hepatic collagenesis in chronic arsenic toxicity. Toxicol Appl Pharmacol. 2005;204:18–26. doi: 10.1016/j.taap.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Duyndam MC, Hulscher TM, Fontijn D, Pinedo HM, Boven E. Induction of vascular endothelial growth factor expression and hypoxia-inducible factor 1alpha protein by the oxidative stressor arsenite. J Biol Chem. 2001;276:48066–48076. doi: 10.1074/jbc.M106282200. [DOI] [PubMed] [Google Scholar]
- Enomoto A, Itoh K, Nagayoshi E, Haruta J, Kimura T, O'Connor T, Harada T, Yamamoto M. High sensitivity of Nrf2 knockout mice to acetaminophen hepatotoxicity associated with decreased expression of ARE-regulated drug metabolizing enzymes and antioxidant genes. Toxicol Sci. 2001;59:169–177. doi: 10.1093/toxsci/59.1.169. [DOI] [PubMed] [Google Scholar]
- Hayes JD, McMahon M. NRF2 and KEAP1 mutations: permanent activation of an adaptive response in cancer. Trends Biochem Sci. 2009;34:176–188. doi: 10.1016/j.tibs.2008.12.008. [DOI] [PubMed] [Google Scholar]
- He X, Chen MG, Lin GX, Ma Q. Arsenic induces NAD(P)H-quinone oxidoreductase I by disrupting the Nrf2 x Keap1 x Cul3 complex and recruiting Nrf2 x Maf to the antioxidant response element enhancer. J Biol Chem. 2006;281:23620–23631. doi: 10.1074/jbc.M604120200. [DOI] [PubMed] [Google Scholar]
- Hei TK, Liu SX, Waldren C. Mutagenicity of arsenic in mammalian cells: role of reactive oxygen species. Proc Natl Acad Sci U S A. 1998;95:8103–8107. doi: 10.1073/pnas.95.14.8103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsueh YM, Cheng GS, Wu MM, Yu HS, Kuo TL, Chen CJ. Multiple risk factors associated with arsenic-induced skin cancer: effects of chronic liver disease and malnutritional status. Br J Cancer. 1995;71:109–114. doi: 10.1038/bjc.1995.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Ma WY, Li J, Dong Z. Arsenic induces apoptosis through a c-Jun NH2-terminal kinase-dependent, p53-independent pathway. Cancer Res. 1999;59:3053–3058. [PubMed] [Google Scholar]
- Jones PA, Buckley JD. The role of DNA methylation in cancer. Adv Cancer Res. 1990;54:1–23. doi: 10.1016/s0065-230x(08)60806-4. [DOI] [PubMed] [Google Scholar]
- Kann S, Estes C, Reichard JF, Huang MY, Sartor MA, Schwemberger S, Chen Y, Dalton TP, Shertzer HG, Xia Y, Puga A. Butylhydroquinone protects cells genetically deficient in glutathione biosynthesis from arsenite-induced apoptosis without significantly changing their prooxidant status. Toxicol Sci. 2005;87:365–384. doi: 10.1093/toxsci/kfi253. [DOI] [PubMed] [Google Scholar]
- Kayajanian G. Arsenic, cancer, and thoughtless policy. Ecotoxicol Environ Saf. 2003;55:139–142. doi: 10.1016/s0147-6513(02)00042-8. [DOI] [PubMed] [Google Scholar]
- Kenyon EM, Del Razo LM, Hughes MF. Tissue distribution and urinary excretion of inorganic arsenic and its methylated metabolites in mice following acute oral administration of arsenate. Toxicol Sci. 2005;85:468–475. doi: 10.1093/toxsci/kfi107. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick DS, Dale KV, Catania JM, Gandolfi AJ. Low-level arsenite causes accumulation of ubiquitinated proteins in rabbit renal cortical slices and HEK293 cells. Toxicol Appl Pharmacol. 2003;186:101–109. doi: 10.1016/s0041-008x(02)00019-4. [DOI] [PubMed] [Google Scholar]
- Kitchin KT, Ahmad S. Oxidative stress as a possible mode of action for arsenic carcinogenesis. Toxicol Lett. 2003;137:3–13. doi: 10.1016/s0378-4274(02)00376-4. [DOI] [PubMed] [Google Scholar]
- Knobeloch LM, Zierold KM, Anderson HA. Association of arsenic-contaminated drinking-water with prevalence of skin cancer in Wisconsin's Fox River Valley. J Health Popul Nutr. 2006;24:206–213. [PubMed] [Google Scholar]
- Lamm SH, Engel A, Kruse MB, Feinleib M, Byrd DM, Lai S, Wilson R. Arsenic in drinking water and bladder cancer mortality in the United States: an analysis based on 133 U.S. counties and 30 years of observation. J Occup Environ Med. 2004;46:298–306. doi: 10.1097/01.jom.0000116801.67556.8f. [DOI] [PubMed] [Google Scholar]
- Lapeyre JN, Walker MS, Becker FF. DNA methylation and methylase levels in normal and malignant mouse hepatic tissues. Carcinogenesis. 1981;2:873–878. doi: 10.1093/carcin/2.9.873. [DOI] [PubMed] [Google Scholar]
- Lau A, Villeneuve NF, Sun Z, Wong PK, Zhang DD. Dual roles of Nrf2 in cancer. Pharmacol Res. 2008;58:262–270. doi: 10.1016/j.phrs.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Trimarchi JR, Navarro P, Blasco MA, Keefe DL. Oxidative stress contributes to arsenic-induced telomere attrition, chromosome instability, and apoptosis. J Biol Chem. 2003;278:31998–32004. doi: 10.1074/jbc.M303553200. [DOI] [PubMed] [Google Scholar]
- Mahata J, Ghosh P, Sarkar JN, Ray K, Natarajan AT, Giri AK. Effect of sodium arsenite on peripheral lymphocytes in vitro: individual susceptibility among a population exposed to arsenic through the drinking water. Mutagenesis. 2004;19:223–229. doi: 10.1093/mutage/geh022. [DOI] [PubMed] [Google Scholar]
- Navas-Acien A, Silbergeld EK, Streeter RA, Clark JM, Burke TA, Guallar E. Arsenic exposure and type 2 diabetes: a systematic review of the experimental and epidemiological evidence. Environ Health Perspect. 2006;114:641–648. doi: 10.1289/ehp.8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pott WA, Benjamin SA, Yang RS. Antagonistic interactions of an arsenic-containing mixture in a multiple organ carcinogenicity bioassay. Cancer Lett. 1998;133:185–190. doi: 10.1016/s0304-3835(98)00229-8. [DOI] [PubMed] [Google Scholar]
- Ramos-Gomez M, Kwak MK, Dolan PM, Itoh K, Yamamoto M, Talalay P, Kensler TW. Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in nrf2 transcription factor-deficient mice. Proc Natl Acad Sci U S A. 2001;98:3410–3415. doi: 10.1073/pnas.051618798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romach EH, Zhao CQ, Del Razo LM, Cebrian ME, Waalkes MP. Studies on the mechanisms of arsenic-induced self tolerance developed in liver epithelial cells through continuous low-level arsenite exposure. Toxicol Sci. 2000;54:500–508. doi: 10.1093/toxsci/54.2.500. [DOI] [PubMed] [Google Scholar]
- Smith AH, Lingas EO, Rahman M. Contamination of drinking-water by arsenic in Bangladesh: a public health emergency. Bull World Health Organ. 2000;78:1093–1103. [PMC free article] [PubMed] [Google Scholar]
- Smith AH, Marshall G, Yuan Y, Ferreccio C, Liaw J, von Ehrenstein O, Steinmaus C, Bates MN, Selvin S. Increased mortality from lung cancer and bronchiectasis in young adults after exposure to arsenic in utero and in early childhood. Environ Health Perspect. 2006;114:1293–1296. doi: 10.1289/ehp.8832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow ET, Sykora P, Durham TR, Klein CB. Arsenic, mode of action at biologically plausible low doses: What are the implications for low dose cancer risk? Toxicol Appl Pharmacol. 2005;207:557–564. doi: 10.1016/j.taap.2005.01.048. [DOI] [PubMed] [Google Scholar]
- Tarantino G, Cabibi D, Camma C, Alessi N, Donatelli M, Petta S, Craxi A, Di Marco V. Liver eosinophilic infiltrate is a significant finding in patients with chronic hepatitis C. J Viral Hepat. 2008;15:523–530. doi: 10.1111/j.1365-2893.2008.00976.x. [DOI] [PubMed] [Google Scholar]
- Tchounwou PB, Patlolla AK, Centeno JA. Carcinogenic and systemic health effects associated with arsenic exposure--a critical review. Toxicol Pathol. 2003;31:575–588. doi: 10.1080/01926230390242007. [DOI] [PubMed] [Google Scholar]
- Tseng CH. An overview on peripheral vascular disease in blackfoot disease-hyperendemic villages in Taiwan. Angiology. 2002;53:529–537. doi: 10.1177/000331970205300505. [DOI] [PubMed] [Google Scholar]
- Umemura T, Kuroiwa Y, Kitamura Y, Ishii Y, Kanki K, Kodama Y, Itoh K, Yamamoto M, Nishikawa A, Hirose M. A crucial role of Nrf2 in in vivo defense against oxidative damage by an environmental pollutant, pentachlorophenol. Toxicol Sci. 2006;90:111–119. doi: 10.1093/toxsci/kfj076. [DOI] [PubMed] [Google Scholar]
- Umemura T, Sai K, Takagi A, Hasegawa R, Kurokawa Y. Formation of 8-hydroxydeoxyguanosine (8-OH-dG) in rat kidney DNA after intraperitoneal administration of ferric nitrilotriacetate (Fe-NTA) Carcinogenesis. 1990;11:345–347. doi: 10.1093/carcin/11.2.345. [DOI] [PubMed] [Google Scholar]
- Wainfan E, Poirier LA. Methyl groups in carcinogenesis: effects on DNA methylation and gene expression. Cancer Res. 1992;52:2071s–2077s. [PubMed] [Google Scholar]
- Walvekar RR, Kane SV, Nadkarni MS, Bagwan IN, Chaukar DA, D'Cruz AK. Chronic arsenic poisoning: a global health issue -- a report of multiple primary cancers. J Cutan Pathol. 2007;34:203–206. doi: 10.1111/j.1600-0560.2006.00596.x. [DOI] [PubMed] [Google Scholar]
- Wang XJ, Sun Z, Chen W, Eblin KE, Gandolfi JA, Zhang DD. Nrf2 protects human bladder urothelial cells from arsenite and monomethylarsonous acid toxicity. Toxicol Appl Pharmacol. 2007;225:206–213. doi: 10.1016/j.taap.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XJ, Sun Z, Chen W, Li Y, Villeneuve NF, Zhang DD. Activation of Nrf2 by arsenite and monomethylarsonous acid is independent of Keap1-C151:enhanced Keap1-Cul3 interaction. Toxicol Appl Pharmacol. 2008;230:383–389. doi: 10.1016/j.taap.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Wu J, Zhang R, Zhang P, Eckard J, Yusuf R, Huang X, Rossman TG, Frenkelq K. Caffeic acid phenethyl ester (CAPE) prevents transformation of human cells by arsenite (As) and suppresses growth of As-transformed cells. Toxicology. 2005;213:81–96. doi: 10.1016/j.tox.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Yu X, Kensler T. Nrf2 as a target for cancer chemoprevention. Mutat Res. 2005;591:93–102. doi: 10.1016/j.mrfmmm.2005.04.017. [DOI] [PubMed] [Google Scholar]