Abstract
Cordycepin (3'-deoxyadenosine) has been shown to exhibit many pharmacological activities, including anti-cancer, anti-inflammatory, and anti-infection activities. However, the anti-skin photoaging effects of cordycepin have not yet been reported. In the present study, we investigated the inhibitory effects of cordycepin on matrix metalloproteinase-1 (MMP-1) and -3 expressions of the human dermal fibroblast cells. Western blot analysis and real-time PCR revealed cordycepin inhibited UVB-induced MMP-1 and -3 expressions in a dose-dependent manner. UVB strongly activated NF-κB activity, which was determined by IκBα degradation, nuclear localization of p50 and p65 subunit, and NF-κB binding activity. However, UVB-induced NF-κB activation and MMP expression were completely blocked by cordycepin pretreatment. These findings suggest that cordycepin could prevent UVB-induced MMPs expressions through inhibition of NF-κB activation. In conclusion, cordycepin might be used as a potential agent for the prevention and treatment of skin photoaging.
Keywords: cordycepin, matrix metalloproteinases, NF-κB, skin aging, ultraviolet rays
Introduction
The Cordyceps mushroom, Cordyceps sinensis, is one of the best anti-aging materials in Traditional Chinese Medicine. Cordycepin, which is a nucleoside derivative isolated from Cordyceps, has been reported to exert inhibitory effects on macrophages based on anti-inflammatory property (Jeong et al., 2004). However, the anti-aging effects of cordycepin have not yet been reported.
Skin change is one of the most prominent signs of aging. Skin aging can be divided into intrinsic or chronologic aging, which is the process of senescence that affects all body organs, and extrinsic aging (photoaging), which occurs as a consequence of exposure to environmental factors. One of the most important extrinsic aging factors is sunlight, particularly exposure to UVB irradiation, which causes skin photoaging. It has been well known that chronic exposure of human skin to UVB radiation results in photoaging and induces the production of matrix metalloproteinases (MMPs) (Ho et al., 2005).
MMPs are responsible for the degradation or synthesis inhibition of collagenous extracellular matrix in connective tissues (Scharffetter-Kochanek et al., 2000). Collagen represents the main component of the extracellular matrix of dermal connective tissue, and its concentration decreases in chronoaging and photoaging. MMP-1 preferentially degrades fibrillar collagens, which maintain the tensile strength of fetal membranes, whereas MMP-3 degrades an extremely broad array of extracellular matrix (ECM) substrates and can activate the secreted, zymogenic form of other MMPs (Ogata et al., 1992; Knauper et al., 1996; Jackson et al., 2001). UVB is known to induce the expressions of interstitial collagenase (MMP-1) and stromelysin-1 (MMP-3) in human dermal fibroblasts (HDF) and normal human epidermis in vivo (Brenneisen et al., 1996, 2000, 2002). Among those MMPs, MMP-1 is the most important MMP in the degradation of the extracellular matrix by skin photoaging (Wenk et al., 2004).
NF-κB is a crucial factor for the immunoinflammatory responses and is also implicated in various skin diseases including allergic dermatitis, psoriasis vulgaris, and skin cancer (Bell et al., 2003; Lee et al., 2007; Shin et al., 2007). Hence, although NF-κB is involved in maintaining the skin homeostasis (Pasparakis et al., 2002; Takao et al., 2003), excessive activation is pathogenic. NF-κB is an inducible dimeric transcription factor that belongs to the Rel/NF-κB family of transcription factors. NF-κB consists of two major polypeptides, p65 and p50 (Thanos and Maniatis, 1995). NF-κB is initially located in the cytoplasm in an inactive form complexed with IκB, an inhibitory factor of NF-κB. Various inducers such as IL-1, TNF-α and UV can cause dissociation of this complex, presumably by phosphorylation of IκB, resulting in NF-κB being released from the complex. NF-κB then translocates to the nucleus, where it interacts with specific DNA recognition sites to mediate gene transcription.
In the present study, we evaluated the preventive effects of cordycepin on UVB-induced MMPs expression in HDF. Cordycepin was found to block UVB-induced NF-κB pathway, thereby inhibiting the MMPs expression. These results indicate that cordycepin may be useful as an anti-skin photoaging agent.
Results and Discussion
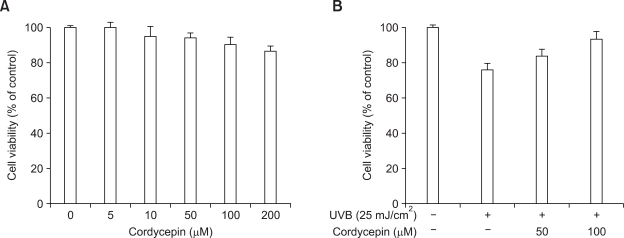
Effect of cordycepin on cell viability of HDF
In order to investigate the cytotoxicity of cordycepin on HDF, the cells were seeded into 96-well culture plates at a density of 1 × 105 cells/well. Effect of cordycepin on cellular toxicity of HDF was analyzed using the MTT assay. Treatment of HDF with indicated concentrations of cordycepin for 24 h did not cause any significant change in cell viability (Figure 2A). Therefore, we performed experiments in optimal non-toxic concentration (50 µM and 100 µM) of cordycepin with no change in morphology. To investigate the cell viability of cordycepin in the presence of UVB. Cells were incubated with the indicated concentration of cordycepin for 24 h in the presence of UVB. Cell viability was determined by cell counting. Cordycepin (50 µM and 100 µM) significantly inhibited the cell toxicity by UVB irradiation (Figure 2B).
Figure 2.
Effect of cordycepin on cell viability in HDF. Cells were cultured in 96-well plates until 70% confluence, then incubated with the indicated concentration of cordycepin for 24 h. MTT assay was used to detect the viability of the cells as detailed in Methods (A). Cells were cultured in 100 mm culture dishes until 70% confluence, then incubated with the indicated concentration of cordycepin for 24 h in the presence of UVB. Cell viability determined by cell count method (B). The optical density value of control was regarded as 100%. Data points are the mean ± SE of three independent experiments.
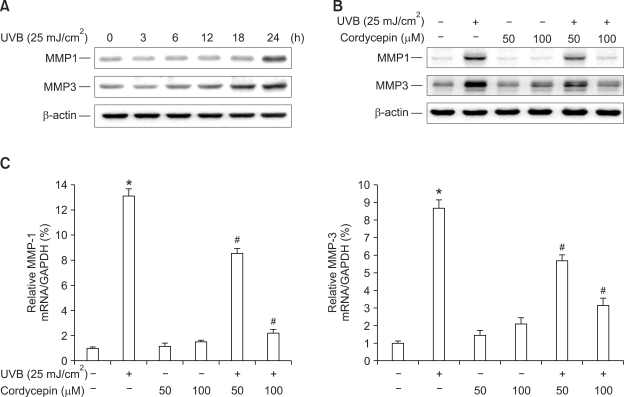
Effect of cordycepin on UVB-induced MMP-1 and -3 expressions in HDF
UV irradiation of cultured HDF in vitro or human skin in vivo induces the expression of MMPs which play important roles in the degradation of extracellular matrix components during skin aging (Brenneisen et al., 2002; Rittie and Fisher, 2002; Chung et al., 2003). Varani et al. (2002) reported that with increasing age, MMP levels rise and collagen synthesis decline for sun-protected human skin in vivo.
In this study, to investigate UVB-induced MMP-1 and -3 expressions, we performed Western blot analysis and real-time PCR in HDF. Western blot analysis revealed that the irradiation of HDF with UVB (25 mJ/cm2) increased the levels of MMP-1 and -3 in time dependent manner (Figure 3A). Treatment with cordycepin (50 µM and 100 µM) completely blocked the up-regulation of MMP-1 and -3 induced by UVB irradiation (Figure 3B). Real-time PCR revealed that UVB irradiation increased the level of MMP-1 and -3 in HDF and cordycepin blocked UVB-induced up-regulation of MMP-1 and -3 in a dose-dependent manner (Figure 3C). As shown, cordycepin itself had no effect on either MMP-1 or -3 in HDF. These results indicate that cordycepin is a potent inhibitor of UVB-induced MMP-1 and -3 expressions in HDF.
Figure 3.
Effect of cordycepin on UVB-induced MMP-1 and MMP-3 expressions in HDF. HDF in monolayer were incubated with UVB (25 mJ/cm2) in time dependent manner (A). To investigate effect of cordycepin, cells were stimulated with UVB (25 mJ/cm2) and the indicated concentrations of cordycepin for 24 h (B). The cell lysates were analyzed by Western blotting with anti-MMP-1 and -3. The blot was reprobed with anti-β-actin to confirm equal loading. HDF were pretreated with cordycepin for 1 h and stimulated by UVB (25 mJ/cm2) for 24 h. Total cellular RNA was analyzed by real-time PCR for MMP-1 and -3 (C). Each value represents the mean ± SEM of three independent experiments. *P < 0.01 vs. untreated control, #P < 0.01 vs. UVB.
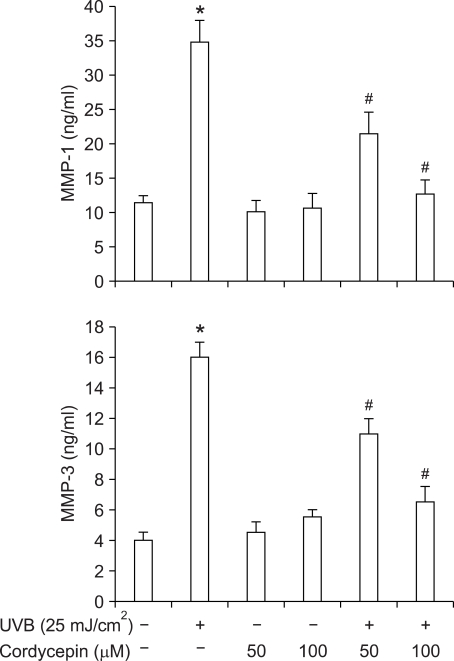
Effect of cordycepin on UVB-induced secretion of MMP-1 and -3
In recent studies, it was suggested that excessive matrix degradation by UV-induced MMPs secreted by various cells (e.g., keratinocytes, fibroblasts, and inflammatory cells) contributes substantially to the connective tissue damage that occurs during photoaging (Fisher et al., 1996; Chung et al., 2001, 2002; Lee et al., 2003). Thus, MMP secretion, which is the hallmark of skin aging, is activated by UVB, and 23 members of the MMP family have been identified in humans to date. Of these, MMP-1 and -3 are particularly important to skin aging because they are responsible for the degradation of collagenous extracellular matrix, and because the levels of MMP-1 and -3 are significantly higher in aged skin. We used ELISA to investigate the effect of cordycepin on UVB-induced MMP secretion. Irradiation of HDF with UVB (25 mJ/cm2) resulted in an increase in the secretion of MMP-1 and -3, respectively (Figure 4). However, cordycepin (50 µM or 100 µM) significantly diminished the UVB-induced MMP-1 and -3 secretions, respectively (Figure 4).
Figure 4.
Inhibition of UVB-induced MMP-1 and -3 secretions from HDF by cordycepin. Cells were stimulated with UVB (25 mJ/cm2) and the indicated concentrations of cordycepin for 24 h. Level of MMP-1 and -3 in culture media was measured using a commercially available ELISA kit as described in Methods. Each value represents the mean ± SEM of three independent experiments. *P < 0.01 vs. untreated control, #P < 0.01 vs. UVB.
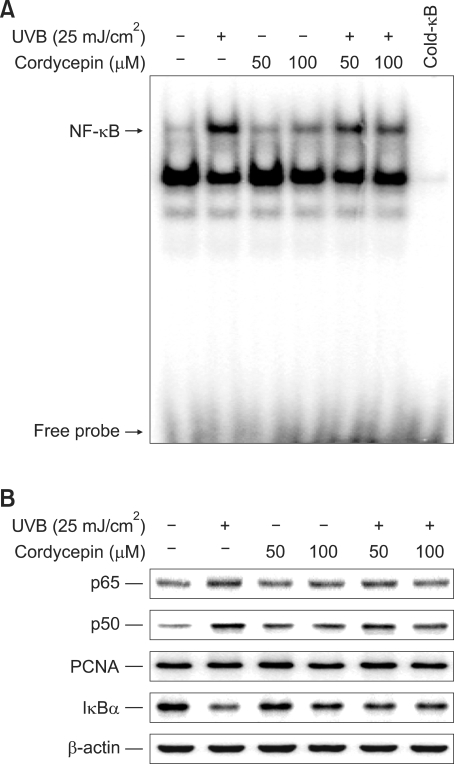
Suppressive effect of cordycepin on UVB-induced NF-κB activation
It is well established that a nuclear transcription factor, NF-κB, is activated upon UV irradiation. (Saliou et al., 1999; Kim et al., 2001; Wenk et al., 2004). In previous study, it is also reported that UVB-mediated skin photoaging is prevented by suppression of NF-κB activation (Adhami et al., 2003; Tanaka et al., 2005, 2007). Thus, inhibition of the NF-κB activation pathway is important for the UVB-mediated skin damage. In fact, NF-κB is known to increase MMP-1 in dermis (Bond et al., 1999; Sun et al., 2002; Chung, 2003). Therefore, we studied the effect of cordycepin on UVB-stimulated translocation of NF-κB from the cytoplasmic compartment to the nucleus and on DNA binding in HDF. UVB-irradiated HDF showed increased binding activity of an NF-κB consensus sequence (Figure 5A), as well as increased p65 and p50 subunit levels in their nuclei (Figure 5B) when compared to unstimulated cells. Additionally, UVB-induced NF-κB activation was significantly suppressed by pretreatment with cordycepin, which suggests that cordycepin inhibits MMP-1 and -3 expressions through the inhibition of NF-κB activation. The specificity of the DNA-protein interactions for NF-κB was demonstrated by performing competition assays using a 50-fold excess of unlabeled oligonucleotide (Figure 5A, lane 7). From above, we found that cordycepin blocked UVB-induced NF-κB activation. Under basal condition, the cytoplasmic protein IκB directly binds to p65 and p50 subunits and represses their nuclear translocation (Adhami et al., 2003; Afaq et al., 2003; Kim et al., 2007). Therefore, we determined the alteration of IκBα levels of the cytoplasmic fraction in this study. UVB-irradiated HDF showed a decreased level of IκBα protein in the cytoplasm when compared to a similar fraction in the unstimulated cells, however, the increased IκBα degradation as a result of UVB stimulation was significantly suppressed by treatment with cordycepin (Figure 5B).
Figure 5.
Suppression of UVB-induced DNA binding of NF-κB, translocation of p65 and p50 to the nucleus, and IκBα degradation by cordycepin. Cells were stimulated with UVB (25 mJ/cm2) and the indicated concentrations of cordycepin. Following 3 h of incubation, DNA binding of NF-κB was analyzed by electrophoretic mobility shift analysis (A), and the translocation of p65 and p50 to the nucleus and IκBα degradation in the cytoplasm (B) were determined by Western blotting.
In conclusion, the development of MMP inhibitors is considered to be a promising strategy for skin cancer therapy and photoaging. In recent years, the development of compounds with MMP inhibition activities from natural plants has received a great deal of attention. This study demonstrates the inhibitory effect of cordycepin on the mRNA expression of MMPs. Also, our results have demonstrated that cordycepin is a potent inhibitor of UVB-induced MMPs expressions and blocks strongly the ability of NF-κB signaling pathway in HDF. Therefore, we suggest that cordycepin may be a potential candidate to prevent skin photoaging.
Methods
Materials
Cordycepin, MTT, DMSO and anti-β-actin were obtained from Sigma (St. Louis, MO). Primary antibodies for MMP-1 and -3 were obtained from R&D Systems (Minneapolis, MN). High glucose-containing DMEM, FBS and PBS were obtained from Gibco-BRL (Gaithersburg, ME). Antibody of p50, p65, and IκBα, PCNA, and HRP-conjugated IgG was from Santa Cruz Biochemicals (SantaCruz, CA).
Isolation and culture of HDF
HDF were aseptically isolated from a circumcised neonatal foreskin. The epidermis and dermis were separated by incubation in 0.9 units/ml dispase in medium for 16 h at 4℃. After the epidermis and dermis were mechanically separated, the dermis was minced and attached on the surface of tissue culture flask and fed with DMEM containing 10% FBS for 1-2 weeks. The dermal fibroblasts spreading as radial outgrowth from attached pieces of dermis, were cultured in DMEM with 10% FBS.
UV irradiation
For a UVB irradiation, we used UVB cross-linker (6 × 8 W, 312 nm, Model CL-508M, Vilber lourmat, Paris, France). In brief, serum-starved confluent cells were rinsed twice with PBS, and all irradiations were performed under a thin layer of PBS. Immediately after irradiation, fresh serum-free medium was added to the cells. Responses were measured after an incubation period of 24 h. Mock-irradiated controls followed the same schedule of medium changes without UVB irradiation.
Determination of cell viability
An effect of cordycepin on cell viability of HDF was determined using MTT assay. Briefly, cells of 2 × l04 cells/well were, treated with various concentrations of cordycepin. After incubation for 24 h, cells were washed twice with PBS, MTT (0.5 mg/ml PBS) was added to each well and incubated at 37℃ for 30 min. Formazan crystals formed were dissolved by adding DMSO (100 µl/well) at 570 nm using a microplate reader (Model 3550, BIO-RAD, Richmond, CA)
Western blot analysis
HDF (2 × 106 cells) were irradiated with UVB (25 mJ/cm2) and then treated with cordycepin for 24 h. Cells were lysed with 40 µl of ice-cold M-PER Mammalian Protein Extraction Reagent (Pierce Biotechnology, Rockford, IL). In lysates, the protein concentration was determined using the Bradford method (Bradford, 1976). Samples were separated by SDS-PAGE with 10% acrylamide running and 3% acrylamide stacking gels, and then transferred to hybond-PVDF membranes using a Western blot apparatus. The PVDF membranes were blotted with 1 µg/ml of primary antibodies for MMP-1, MMP-3 p50, p65, PCNA, and β-actin. HRP-conjugated IgG was used as a secondary antibody. The protein expression levels were then determined by analyzing the signals captured on the PVDF membranes using an image analyzer (Las-1000, fuji-film, Japan).
Quantitative real-time PCR assay
Total RNA was extracted from the cells using FastPure RNA Kit (TaKaRa, Shiga, Japan). The concentration and purity of RNA were determined by absorbance at 260/280 nm. cDNA was synthesized from 1 µg total RNA using PrimeScript RT reagent Kit (TaKaRa, Shiga, Japan). The expressions of MMP-1 and -3 mRNA was determined by real-time-PCR using the ABI PRISM 7900 sequence detection system and the SYBR Green (Applied Biosystems, Foster City, CA). The primers were: MMP-1 (NM 002424.2) sense, AGTGACTGGGAAACCAGATGCTGA; antisense, GCTCTTGGCAAATCTGGCCTGTAA and MMP-3 (NM 002422) sense, ATTCCATGGAGCCAGGCTTTC; antisense, CATTTGGGTCAAACTCCAACTGTG and GAPDH (NM 002046) sense, ATGGAAATCCCATCACCATCTT; antisense, CGCCCCACTTGATTTTGG. To control variation in mRNA concentration, all results were normalized to the housekeeping gene, GAPDH. Relative quantitation was performed using the comparative ΔΔCt method according to the manufacture's instructions.
Determination of MMP-1 and -3 secretions by ELISA
HDF were seeded in 100 mm culture dishes at density of 2 × 106 cells per dish, and then irradiated with UVB (25 mJ/cm2) and then treated with cordycepin. Following 24 h of incubation, the culture supernatant was collected and centrifuged at 10,000 × g for 5 min to remove the particulate matter, and stored at -80℃ in fresh tubes. The active MMP-1 in culture supernatants were quantified by fluorescent assay, using the Fluorokine E Human Active MMP-1 Fluorescent Assay Kit (R&D Systems) and MMP-3 in the cell culture supernatants were then determined using Quantikine ELISA kits (R&D Systems), according to the manufacturer's protocol.
Preparation of nuclear extract
HDF (2 × 106 cells) were irradiated with UVB 25 (mJ/cm2) and then treated with cordycepin for 24 h. Cells were immediately washed twice, scraped into 1.5 ml of ice-cold PBS (pH 7.9), and then pelleted at 12,000 × g for 30 s. Cytoplasmic and nuclear extracts were prepared from cells using the NE-PER Nuclear and Cytoplasmic Extraction Reagents (Pierce Biotechnology, Rockford, IL).
Electrophoretic mobility shift assay (EMSA)
The activation of NF-κB was assayed by a gel mobility shift assay using nuclear extracts. An oligonucleotide containing the κ-chain binding site (κB, 5'-CCGGTTAACAGAGGGGGCTTTCCGAG-3') was synthesized and used as a probe for the gel retardation assay. The two complementary strands were then annealed and labeled with [α-32P]dCTP. Labeled oligonucleotides (10,000 cpm), 10 µg of nuclear extracts, and binding buffer (10 mM Tris-HCl, pH 7.6, 500 mM KCl, 10 mM EDTA, 50% glycerol, 100 ng poly (dI·dC), 1 mM DTT) were then incubated for 30 min at room temperature in a final volume of 20 µl. The reaction mixtures were analyzed by electrophoresis on 4% polyacrylamide gels in 0.5×Tris-borate buffer, and the gels were then dried and examined by autoradiography. Specific binding was controlled by competition with a 50-fold excess of cold κB oligonucleotide.
Statistical analysis
Statistical analysis of the data was performed using ANOVA and Duncan's test. Differences with a P < 0.05 were considered statistically significant.
Figure 1.
Chemical structure of cordycepin.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea Government (MEST) (No. 2009-0062917) and (M1052801-0003-05N2801-00310), and the Korea Research Foundation Grant (KRF-2008-C00618), Republic of Korea.
Abbreviations
- HDFs
human dermal fibroblasts
- MMP
matrix metalloproteinase
References
- 1.Adhami VM, Afaq F, Ahmad N. Suppression of ultraviolet B exposure-mediated activation of NF-kappaB in normal human keratinocytes by resveratrol. Neoplasia. 2003;5:74–82. doi: 10.1016/s1476-5586(03)80019-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Afaq F, Ahmad N, Mukhtar H. Suppression of UVB-induced phosphorylation of mitogen-activated protein kinases and nuclear factor kappa B by green tea polyphenol in SKH-1 hairless mice. Oncogene. 2003;22:9254–9264. doi: 10.1038/sj.onc.1207035. [DOI] [PubMed] [Google Scholar]
- 3.Bell S, Degitz K, Quirling M, Jilg N, Page S, Brand K. Involvement of NF-kappaB signalling in skin physiology and disease. Cell Signal. 2003;15:1–7. doi: 10.1016/s0898-6568(02)00080-3. [DOI] [PubMed] [Google Scholar]
- 4.Bond M, Baker AH, Newby AC. Nuclear factor kappaB activity is essential for matrix metalloproteinase-1 and -3 upregulation in rabbit dermal fibroblasts. Biochem Biophys Res Commun. 1999;264:561–567. doi: 10.1006/bbrc.1999.1551. [DOI] [PubMed] [Google Scholar]
- 5.Bradford MM. A rapid, sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 6.Brenneisen P, Oh J, Wlaschek M, Wenk J, Briviba K, Hommel C, Herrmann G, Sies H, Scharffetter-Kochanek K. Ultraviolet B wavelength dependence for the regulation of two major matrix-metalloproteinases and their inhibitor TIMP-1 in human dermal fibroblasts. Photochem Photobiol. 1996;64:877–885. doi: 10.1111/j.1751-1097.1996.tb01851.x. [DOI] [PubMed] [Google Scholar]
- 7.Brenneisen P, Wenk J, Wlaschek M, Krieg T, Scharffetter-Kochanek K. Activation of p70 ribosomal protein S6 kinase is an essential step in the DNA damage-dependent signaling pathway responsible for the ultraviolet B-mediatedincrease in interstitial collagenase (MMP-1) and stromelysin-1 (MMP-3) protein levels in human dermal fibroblasts. J Biol Chem. 2000;275:4336–4344. doi: 10.1074/jbc.275.6.4336. [DOI] [PubMed] [Google Scholar]
- 8.Brenneisen P, Sies H, Scharffetter-Kochanek K. Ultraviolet-B irradiation and matrix metalloproteinases: from induction via signaling to initial events. Ann N Y Acad Sci. 2002;973:31–43. doi: 10.1111/j.1749-6632.2002.tb04602.x. [DOI] [PubMed] [Google Scholar]
- 9.Chung JH, Seo JY, Choi HR, Lee MK, Youn CS, Rhie G, Cho KH, Kim KH, Park KC, Eun HC. Modulation of skin collagen metabolism in aged and photoaged human skin in vivo. J Invest Dermatol. 2001;117:1218–1224. doi: 10.1046/j.0022-202x.2001.01544.x. [DOI] [PubMed] [Google Scholar]
- 10.Chung JH, Seo JY, Lee MK, Eun HC, Lee JH, Kang S, Fisher GJ, Voorhees JJ. Ultraviolet modulation of human macrophage metalloelastase in human skin in vivo. J Invest Dermatol. 2002;119:507–512. doi: 10.1046/j.1523-1747.2002.01844.x. [DOI] [PubMed] [Google Scholar]
- 11.Chung JH. Photoaging in Asians. Photodermatol Photoimmunol Photomed. 2003;19:109–121. doi: 10.1034/j.1600-0781.2003.00027.x. [DOI] [PubMed] [Google Scholar]
- 12.Chung JH, Hanft VN, Kang S. Aging and photoaging. J Am Acad Dermatol. 2003;49:690–697. doi: 10.1067/s0190-9622(03)02127-3. [DOI] [PubMed] [Google Scholar]
- 13.Fisher GJ, Datta SC, Talwar HS, Wang ZQ, Varani J, Kang S, Voorhees JJ. Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature. 1996;379:335–339. doi: 10.1038/379335a0. [DOI] [PubMed] [Google Scholar]
- 14.Ho JN, Lee YH, Park JS, Jun WJ, Kim HK, Hong BS, Shin DH, Cho HY. Protective effects of aucubin isolated from Eucommia ulmoides against UVB-induced oxidative stress in human skin fibroblasts. Biol Pharm Bull. 2005;28:1244–1248. doi: 10.1248/bpb.28.1244. [DOI] [PubMed] [Google Scholar]
- 15.Jackson C, Nguyen M, Arkell J, Sambrook P. Selective matrixmetalloproteinase (MMP) inhibition in rheumatoid arthritis--targetting gelatinase A activation. Inflamm Res. 2001;50:183–186. doi: 10.1007/s000110050743. [DOI] [PubMed] [Google Scholar]
- 16.Jeong JG, Kim JM, Cho H, Hahn W, Yu SS, Kim S. Effects of IL-1beta on gene expression in human rheumatoid synovial fibroblasts. Biochem Biophys Res Commun. 2004;324:3–7. doi: 10.1016/j.bbrc.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 17.Kim EK, Kwon KB, Koo BS, Han MJ, Song MY, Song EK, Han MK, Park JW, Ryu DG, Park BH. Activation of peroxisome proliferator-activated receptor-gamma protects pancreatic beta-cells from cytokine-induced cytotoxicity via NF kappaB pathway. Int J Biochem Cell Biol. 2007;39:1260–1275. doi: 10.1016/j.biocel.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 18.Kim J, Hwang JS, Cho YK, Han Y, Jeon YJ, Yang KH. Protective effects of (-)-epigallocatechin-3-gallate on UVA- and UVB-induced skin damage. Skin Pharmacol Appl Skin Physiol. 2001;14:11–19. doi: 10.1159/000056329. [DOI] [PubMed] [Google Scholar]
- 19.Knauper V, Lopez-Otin C, Smith B, Knight G, Murphy G. Biochemical characterization of human collagenase-3. J Biol Chem. 1996;271:1544–1550. doi: 10.1074/jbc.271.3.1544. [DOI] [PubMed] [Google Scholar]
- 20.Lee KS, Lee WS, Suh SI, Kim SP, Lee SR, Ryoo YW, Kim BC. Melatonin reduces ultraviolet-B induced cell damages and polyamine levels in human skin fibroblasts in culture. Exp Mol Med. 2003;35:263–268. doi: 10.1038/emm.2003.35. [DOI] [PubMed] [Google Scholar]
- 21.Lee YR, Yu HN, Noh EM, Youn HJ, Song EK, Han MK, Park CS, Kim BS, Park YS, Park BK, Lee SH, Kim JS. TNF-alpha upregulates PTEN via NF-kappaB signaling pathways in human leukemic cells. Exp Mol Med. 2007;39:121–127. doi: 10.1038/emm.2007.14. [DOI] [PubMed] [Google Scholar]
- 22.Ogata Y, Enghild JJ, Nagase H. Matrix metalloproteinase 3 (stromelysin) activates the precursor for the human matrix metalloproteinase 9. J Biol Chem. 1992;267:3581–3584. [PubMed] [Google Scholar]
- 23.Pasparakis M, Courtois G, Hafner M, Schmidt-Supprian M, Nenci A, Toksoy A, Krampert M, Goebeler M, Gillitzer R, Israel A, Krieg T, Rajewsky K, Haase I. TNF-mediated inflammatory skin disease in mice with epidermis-specific deletion of IKK2. Nature. 2002;417:861–866. doi: 10.1038/nature00820. [DOI] [PubMed] [Google Scholar]
- 24.Rittie L, Fisher GJ. UV-light-induced signal cascades and skin aging. Ageing Res Rev. 2002;1:705–720. doi: 10.1016/s1568-1637(02)00024-7. [DOI] [PubMed] [Google Scholar]
- 25.Saliou C, Kitazawa M, McLaughlin L, Yang JP, Lodge JK, Tetsuka T, Iwasaki K, Cillard J, Okamoto T, Packer L. Antioxidants modulate acute solar ultraviolet radiation-induced NF-kappa-B activation in a human keratinocyte cell line. Free Radic Biol Med. 1999;26:174–183. doi: 10.1016/s0891-5849(98)00212-3. [DOI] [PubMed] [Google Scholar]
- 26.Scharffetter-Kochanek K, Brenneisen P, Wenk J, Herrmann G, Ma W, Kuhr L, Meewes C, Wlaschek M. Photoaging of the skin from phenotype to mechanisms. Exp Gerontol. 2000;35:307–316. doi: 10.1016/s0531-5565(00)00098-x. [DOI] [PubMed] [Google Scholar]
- 27.Shin Y, Yoon SH, Choe EY, Cho SH, Woo CH, Rho JY, Kim JH. PMA-induced up-regulation of MMP-9 is regulated by a PKCalpha-NF-kappaB cascade in human lung epithelial cells. Exp Mol Med. 2007;39:97–105. doi: 10.1038/emm.2007.11. [DOI] [PubMed] [Google Scholar]
- 28.Sun HB, Malacinski GM, Yokota H. Promoter competition assay for analyzing gene regulation in joint tissue engineering. Front Biosci. 2002;7:a169–a174. doi: 10.2741/a751. [DOI] [PubMed] [Google Scholar]
- 29.Takao J, Yudate T, Das A, Shikano S, Bonkobara M, Ariizumi K, Cruz PD. Expression of NF-kappaB in epidermis and the relationship between NF-kappaB activation and inhibition of keratinocyte growth. Br J Dermatol. 2003;148:680–688. doi: 10.1046/j.1365-2133.2003.05285.x. [DOI] [PubMed] [Google Scholar]
- 30.Tanaka K, Hasegawa J, Asamitsu K, Okamoto T. Prevention of the ultraviolet B-mediated skin photoaging by a nuclear factor kappaB inhibitor, parthenolide. J Pharmacol Exp Ther. 2005;315:624–630. doi: 10.1124/jpet.105.088674. [DOI] [PubMed] [Google Scholar]
- 31.Tanaka K, Hasegawa J, Asamitsu K, Okamoto T. Magnolia ovovata extract and its active component magnolol prevent skin photoaging via inhibition of nuclear factor kappaB. Eur J Pharmacol. 2007;565:212–219. doi: 10.1016/j.ejphar.2007.01.095. [DOI] [PubMed] [Google Scholar]
- 32.Thanos D, Maniatis T. NF-kappa B: a lesson in family values. Cell. 1995;80:529–532. doi: 10.1016/0092-8674(95)90506-5. [DOI] [PubMed] [Google Scholar]
- 33.Varani J, Perone P, Fligiel SE, Fisher GJ, Voorhees JJ. Inhibition of type I procollagen production in photodamage: correlation between presence of high molecular weight collagen fragments and reduced procollagen synthesis. J Invest Dermatol. 2002;119:122–129. doi: 10.1046/j.1523-1747.2002.01810.x. [DOI] [PubMed] [Google Scholar]
- 34.Wenk J, Schuller J, Hinrichs C, Syrovets T, Azoitei N, Podda M, Wlaschek M, Brenneisen P, Schneider LA, Sabiwalsky A, Peters T, Sulyok S, Dissemond J, Schauen M, Krieg T, Wirth T, Simmet T, Scharffetter-Kochanek K. Overexpression of phospholipid-hydroperoxide glutathione peroxidase in human dermal fibroblasts abrogates UVA irradiation-induced expression of interstitial collagenase/matrix metalloproteinase-1 by suppression of phosphatidylcholine hydroperoxide-mediated NFkappaB activation and interleukin-6 release. J Biol Chem. 2004;279:45634–45642. doi: 10.1074/jbc.M408893200. [DOI] [PubMed] [Google Scholar]